Abstract
The use of propranolol for the treatment of subglottic haemangioma has become hugely popular due to its effectiveness and safety profile. We report a case of 7-month-old boy who presented with stridor and histopathology suggestive of subglottic haemangioma following microlaryngoscopy and bronchoscopy (MLB). However, he did not respond to propranolol treatment. This could be due to an older age of propranolol commencement. In general, early commencement of propranolol is necessary when diagnosis of symptomatic infantile haemangioma is made to achieve maximal improvement in symptoms and prevent further proliferation. There should be a high index of suspicion for subglottic haemangioma in children presenting with chronic biphasic stridor, with early MLB and diagnosis. This will allow early treatment, giving the best chance to avoid our situation.
Keywords: ear, nose and throat; congenital disorders
Background
Infantile haemangioma is the most common vascular tumour of infancy, with an incidence of approximately 5 per cent.1 These vascular lesions are characterised by a programmed life cycle of proliferation followed by apoptotic involution.1 Subglottic haemangioma is a rare form of infantile haemangioma and make up only 1.5% of all congenital laryngeal abnormalities.2 Despite its self-limiting natural course, subglottic haemangioma can impair the child’s quality of life and even be life threatening.3 It presents as a well-recognised but rare cause of stridor with or without cutaneous haemangioma. However, due to the possibilities of more common differential diagnoses such as croup or laryngomalacia, it remains a challenge to be diagnosed in a timely manner. This is especially so for those without cutaneous manifestations. Awake nasolaryngoscopy in infants is able to visualise the supraglottic and glottis regions to exclude causes of stridor such as laryngomalacia and vocal cord abnormalities. Microlaryngoscopy and bronchoscopy (MLB) performed under general anaesthesia is needed to visualise the subglottic area and provide a definitive diagnosis of subglottic haemangioma. Hence, a high index of suspicion is needed to diagnose subglottic haemangioma in infants presenting with stridor of unknown cause during initial consultation.
The effectiveness of propranolol on cutaneous infantile haemangioma was first described by Léauté-Labrèze et al in 2008.4 Since then, the use of propranolol for the treatment of subglottic haemangioma has become hugely popular due to ‘its effectiveness and safety profile as compared with more traditional methods of treatment such as systematic and intralesional steroids, vincristine, bleomycin or interferon’.5 ‘Laser and surgical excision are now reserved for unresponsive for acutely life-threatening haemangiomas’.5 Despite this, the effectiveness of propranolol has not been consistent in all reported cases of subglottic haemangioma. Treatment failures in the forms of propranolol resistance or rebound growth have been reported in a recent systematic review.5 The mechanism of action by which propranolol reduce the size of haemangioma is yet unknown with theories including actions mediated by vasoconstriction, down-regulation of growth factors or apoptosis.6 Factors influencing the varying responsiveness of subglottic haemangioma to propranolol are also yet to be determined. We report a case of a 7-month-old boy with subglottic haemangioma who did not respond satisfactorily to propranolol and oral corticosteroid and is now being considered for surgical intervention.
Case presentation
A 7-month-old boy was referred to our ENT department with respiratory stridor and mild sternal recession. Mother of the child described noisy breathing since 4 weeks of age, associated with feeding difficulties that were improving. There were no cyanotic episodes and no significant birthing history.
On examination, there was evidence of pectus excavatum and biphasic stridor was heard when the patient was lying supine. His tonsils were small and symmetrical, nasal airway was patent and his voice was normal. There were also no signs of vascular staining or cutaneous birthmarks. Flexible awake nasolaryngoscopy was performed which showed no evidence of laryngomalacia and vocal cord abduction was normal.
Investigations
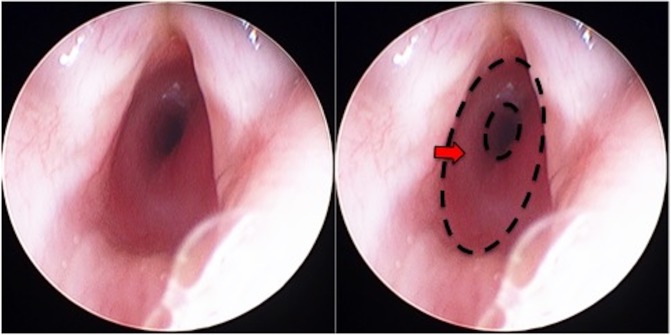
MLB was performed which demonstrated a classical mucosal blush and ‘bean-like’ swelling in the posterior sub glottis, equivalent to grade II Cotton-Myer classification (obstruction of 51% to 70% of lumen) (figure 1). Histological examination following surgical excision showed prominent capillary-calibre vascular channels accompanied by lymphoid cells. Immunohistochemical staining for endothelial glucose transporter 1 (GLUT-1) was positive within the vascular channels. These findings, together with clinical findings, are suggestive of subglottic haemangioma.
Figure 1.
MLB demonstrating subglottic haemangioma (Left: original image; Right: area of subglottic stenosis highlighted by dotted outline and arrow). MLB, microlaryngoscopy and bronchoscopy.
Differential diagnosis
Biphasic stridor in a 7-month-old boy suggests multiple differential diagnoses, including glottic webs, vocal cord palsy and severe laryngomalacia. However, these were unlikely given normal flexible nasoendoscopy findings. Differential diagnoses for subglottic swelling include anterior or posterior glottic stenosis, either congenital or acquired.
Treatment
The patient was commenced on oral propranolol, at 8 months of age, started at 1 mg/kg/day and titrated to 2 mg/kg/day with pulse rate and blood pressure monitoring for an intended duration of 12 months according to the Great Ormond Street Hospital protocol for the treatment of subglottic haemangioma.7 No improvement in stridor was noted within the first 24 hours after commencing on propranolol. A repeat MLB was scheduled at a 3 month interval.
Outcome and follow-up
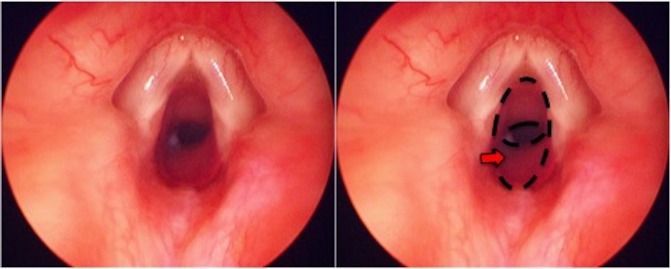
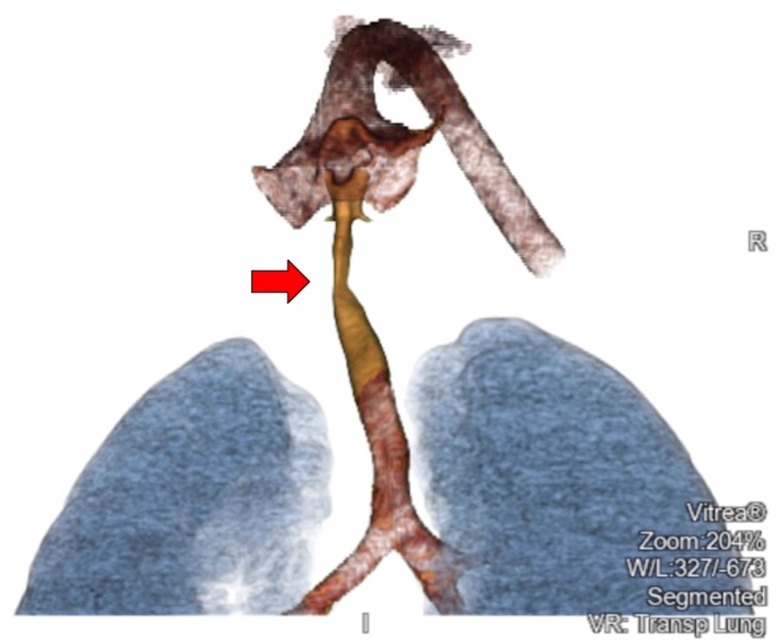
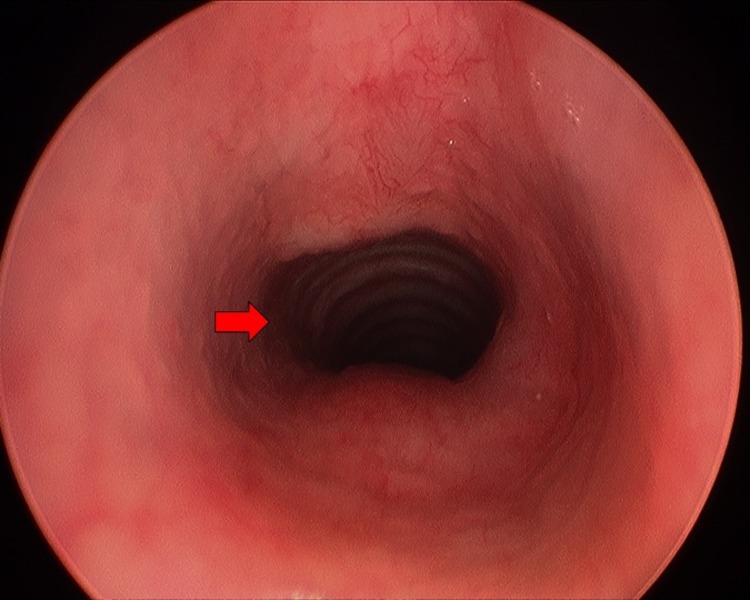
Repeat MLB at a 3 month interval showed little regression in subglottic haemangioma and prednisolone was started concurrently (titrated to 3 mg/kg/day). The prednisolone dose is in accordance with the recommended dosing for cutaneous haemangiomas.8 Followed up MLB at 9.5 months after the commencement of propranolol showed slight improvement in subglottic stenosis (figure 2) and propranolol was weaned off over a 2 week period. A CT scan was performed to exclude any external causes of compression (figure 3). Unfortunately, the patient’s symptoms were frequently exacerbated by concurrent respiratory tract infections that led to reduced exercise tolerance. He has also developed a more significant pectus excavatum due to an increased work of breathing. As such, surgical intervention with excision of the soft tissue mass was carried out. The excised fibrofatty remnant was sent for histology and immunohistochemical staining. The operation was successful and a repeat MLB showed no recurrence of subglottic stenosis (figure 4).
Figure 2.
Repeat MLB following 9.5 months of treatment with propranolol (Left: original image; Right: area of subglottic stenosis highlighted by dotted outline and arrow). MLB, microlaryngoscopy and bronchoscopy.
Figure 3.
CT thorax with contrast (area of subglottic stenosis highlighted by arrow).
Figure 4.
Repeat MLB following surgical excision of subglottic haemangioma (patent subglottic area highlighted by arrow). MLB, microlaryngoscopy and bronchoscopy.
Discussion
While knowledge of the effectiveness of propranolol for subglottic haemangioma has been limited due to a lack of reported cases, paediatric cases of infantile haemangioma treated with propranolol are well documented. In children treated for infantile haemangioma, there is a trend of poorer response to propranolol as the age at commencement increases from 2 months to 18 months of age.9 Another study found a similar trend when comparing children who were started on propranolol before and after 5 months of age.10 This was attributed to the fact that infantile haemangioma tend to proliferate rapidly in the first 3 to 6 months after birth.11 It is generally accepted that once the diagnosis of symptomatic infantile haemangioma is made, early commencement of propranolol is desirable to have a maximal improvement in symptoms and prevent further proliferation. An additional explanation for the ineffectiveness of late treatment may be that those that persist for longer than 1 year without any signs of involution may be inherently resistant to propranolol treatment. In our case, the lack of external signs of disease made early diagnosis difficult. We advocate a strong index of suspicion for subglottic haemangioma in patients presenting with chronic biphasic stridor, with early MLB and diagnosis. This will allow early treatment, giving the best chance to avoid our situation.
We found a lack of guidance on the dose of prednisolone for the treatment of subglottic haemangioma. In this case, we have titrated prednisolone to a maximum dose of 3 mg/kg/day in accordance to findings from a systematic review for the treatment of cutaneous haemangiomas.8 This review found that prednisolone dose of 2 to 3 mg/kg/day was effective in 75% of cases with side effects reported in 16%. Whereas a dose of >3 mg/kg/day showed a 94% response rate with greater side effects reported at 51%. Less than 2 mg/kg/day of prednisolone was associated with fewer responses and adverse effects, but a rebound rate of 70%.
Concurrent use of systemic steroids at the time of propranolol initiation has been suggested to decrease the efficacy of propranolol therapy.5 12 However, this data was drawn from children who had concurrent propranolol and steroids at the initial treatment. In our case, systemic steroid was started when initial therapy with 3 months of propranolol showed little regression of subglottic swelling on MLB. More research is still needed to understand the interaction between systemic propranolol and steroid for the treatment of subglottic haemangioma.
We looked at all the reported cases of subglottic haemangioma treated with propranolol in a recent systematic review5 to observe for any relationship between the age of commencement of propranolol and its resistance. Unfortunately, we found that there is a lack of reporting of the age of commencement of propranolol. Of the 49 cases of subglottic haemangioma identified, age of commencement of propranolol was only reported in seven of them (14.3%). While age of initial presentation was often reported, we cannot assume that it corresponds to the age of commencement of propranolol as subglottic haemangioma can only be confidently diagnosed following MLB.
Nevertheless, there are only six reported cases of treatment failures of subglottic haemangioma to propranolol to our knowledge.5 We contribute here a case of resistance to propranolol in a 7-month-old boy.
Learning points.
High index of suspicion for subglottic haemangioma in children presenting with stridor of unknown cause.
Microlaryngoscopy and bronchoscopy should be arranged as soon as possible for these patients to exclude a diagnosis of subglottic haemangioma.
Children diagnosed with subglottic haemangioma should be started on oral propranolol as soon as possible.
The age of commencement of propranolol should be reported in published cases of subglottic haemangioma to allow for identification of an association between age of commencement and response to propranolol.
Footnotes
Contributors: ZL: manuscript preparation and editing. YHY: manuscript preparation and editing. CJ: manuscript editing and operating surgeon. KT: manuscript editing, operating surgeon and supervisor.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Patient consent for publication: Next of kin consent obtained.
References
- 1. Munden A, Butschek R, Tom WL, et al. Prospective study of infantile haemangiomas: incidence, clinical characteristics and association with placental anomalies. Br J Dermatol 2014;170:907–13. 10.1111/bjd.12804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Wu L, Wu X, Xu X, et al. Propranolol treatment of subglottic hemangiomas: a review of the literature. Int J Clin Exp Med 2015;8:19886–90. [PMC free article] [PubMed] [Google Scholar]
- 3. Vlastarakos PV, Papacharalampous GX, Chrysostomou M, et al. Propranolol is an effective treatment for airway haemangiomas: a critical analysis and meta-analysis of published interventional studies. Acta Otorhinolaryngol Ital 2012;32:213–21. [PMC free article] [PubMed] [Google Scholar]
- 4. Léauté-Labrèze C, Dumas de la Roque E, Hubiche T, et al. Propranolol for severe hemangiomas of infancy. N Engl J Med 2008;358:2649–51. 10.1056/NEJMc0708819 [DOI] [PubMed] [Google Scholar]
- 5. Schwartz T, Faria J, Pawar S, et al. Efficacy and rebound rates in propranolol-treated subglottic hemangioma: A literature review. Laryngoscope 2017;127:2665–72. 10.1002/lary.26818 [DOI] [PubMed] [Google Scholar]
- 6. Canadas KT, Baum ED, Lee S, et al. Case report: Treatment failure using propanolol for treatment of focal subglottic hemangioma. Int J Pediatr Otorhinolaryngol 2010;74:956–8. 10.1016/j.ijporl.2010.05.012 [DOI] [PubMed] [Google Scholar]
- 7. Bajaj Y, Kapoor K, Ifeacho S, et al. Great Ormond Street Hospital treatment guidelines for use of propranolol in infantile isolated subglottic haemangioma. J Laryngol Oto 2013;127:295–8. 10.1017/S0022215112003192 [DOI] [PubMed] [Google Scholar]
- 8. Bennett ML, Fleischer AB, Chamlin SL, et al. Oral corticosteroid use is effective for cutaneous hemangiomas: an evidence-based evaluation. Arch Dermatol 2001;137:1208–13. [DOI] [PubMed] [Google Scholar]
- 9. Phillips RJ, Penington AJ, Bekhor PS, et al. Use of propranolol for treatment of infantile haemangiomas in an outpatient setting. J Paediatr Child Health 2012;48:902–6. 10.1111/j.1440-1754.2012.02521.x [DOI] [PubMed] [Google Scholar]
- 10. Andersen IG, Rechnitzer C, Charabi B. Effectiveness of propanolol for treatment of infantile haemangioma. Dan Med J 2014;61:A4776. [PubMed] [Google Scholar]
- 11. Léauté-Labrèze C, Prey S, Ezzedine K. Infantile haemangioma: part I. Pathophysiology, epidemiology, clinical features, life cycle and associated structural abnormalities. J Eur Acad Dermatol Venereol 2011;25:1245–53. 10.1111/j.1468-3083.2011.04102.x [DOI] [PubMed] [Google Scholar]
- 12. Hardison S, Wan W, Dodson KM. The use of propranolol in the treatment of subglottic hemangiomas: a literature review and meta-analysis. Int J Pediatr Otorhinolaryngol 2016;90:175–80. 10.1016/j.ijporl.2016.09.012 [DOI] [PubMed] [Google Scholar]