Abstract
Studies in school-aged children and adults consistently implicate hippocampus, cortical regions, and their interaction as being critical for memory. However, few studies have examined this neural network in younger children (<8 years) although behavioral studies consistently report substantial improvements in memory earlier in life. This study aimed to fill this gap by integrating task-based (i.e., encoding task) and task-free fMRI scans in 4- to 8-year-old children. Results showed that during memory encoding the hippocampus and several cortical regions (e.g., inferior frontal gyrus, IFG) were activated, consistent with findings in older individuals. Novel findings during memory encoding suggested: 1) additional regions (i.e., orbital frontal gyrus, OFG) were recruited, 2) hippocampal activation varied due to age and performance, and 3) differentiation of connectivity between hippocampal subregions and IFG was greater in older versus younger participants, implying increased speicalization with age. Novel findings from task-free fMRI data suggested the extent of functional differentiation along the longitudinal axis of the hippocampus, particularly between hippocampus and OFG, was moderated by both age and performance. Our findings support and extend previous research, suggesting that maturation of hippocampal activity, connectivity, and differentiation may all contribute to development of memory during early childhood.
Keywords: anterior/posterior hippocampus, task-based functional connectivity, hippocampal subfields, psychophysiological interaction analysis, task-free functional connectivity
1. Introduction
The ability to remember the details of events, often termed episodic memory, is important for learning and future planning in our daily life (Schneider, 2010). Based on a large amount of studies on adults and school-aged children (see Ghetti & Bunge, 2012; Ofen, 2012; Tulving, 2002 for reviews), one well-accepted model, known as the component process model of memory (Moscovitch, Cabeza, Winocur, & Nadel, 2016) has been proposed to suggest that hippocampus and its interaction with other cortical regions (e.g., prefrontal cortex, PFC) are the neural networks supporting episodic memory. Additionally, this model has emphasized the regional specificity along the longitudinal axis of hippocampus. Specifically, it has been suggested that anterior hippocampus codes information in term of the general or global relations among entities and posterior hippocampus codes information in term of precise position. This model has been well supported by the studies focusing on the development of episodic memory ability and its underlying neural correlates in school-aged children, through adolescence and into adulthood (> 8 years, Ghetti, DeMaster, Yonelinas, & Bunge, 2010; Ofen, 2012; Ofen et al., 2007). However, the neural mechanisms associated with changes in episodic memory during early childhood (< 8 years) are under investigated despite the fact that behavioral studies suggest that episodic memory shows significant development during this period (Bauer et al., 2012; Drummey & Newcombe, 2002; Riggins, 2014; Riggins & Rollins, 2015; Sluzenski, Newcombe, & Kovacs, 2006). The goal of this investigation was to examine the neural correlates of episodic memory during early childhood using the tools of modern cognitive neuroscience.
Recently, researchers have begun integrating task-based and task-free fMRI methods to study neural networks (Di, Gohel, Kim, & Biswal, 2013; Gabard-Durnam et al., 2016; Jackson, Hoffman, Pobric, & Lambon Ralph, 2016). For example, Gabard-Durnam et al. (2016) used a sequential design following 4- to 18-year-olds over a 2 year period, and reported that age-related changes in amygdala functional connectivity converged on medial PFC and IFG during both task and rest. In addition, they found that the magnitude of amygdala-medial PFC and amygdala-IFG connectivity unidirectionally predicted resting-state functional connectivity 2 years later, supporting the long-term phasic molding hypothesis suggesting the task-free connectivity patterns are shaped by accumulating experiences of phasic stimulus-elicited functional connectivity (Gabard-Durnam et al., 2016). Thus, the similarity and differences between task-related and task-free neural networks can provide a more holistic understanding of human brain function.
To the best of our knowledge, there has been no study integrating task-based and task-free fMRI methods to study the neural correlates of episodic memory in early childhood. However, there are reports of task-based fMRI in adults and school-aged children as well as separate reports of task-free fMRI and memory in adults and young children. We briefly review these separate lines of research, highlighting developmental differences, and then introduce the specific goals and hypotheses of the present study.
1.1. Task-based fMRI studies of memory
Previous task-based fMRI studies examining the encoding of episodic memories in adults and school-aged children have consistently reported that hippocampus is critical for encoding contextual details, however, its contribution to this process differs across development (Ghetti et al., 2010; Ofen, 2012; Ofen et al., 2007; Xue, 2018). For example, Ghetti et al. (2010) found that 14-year-olds and young adults differentially engaged hippocampus for encoding memories with or without contextual details, but 8- and 10- to 11-year-olds did not. In addition to hippocampus, other brain regions such as parietal cortex and PFC have also been suggested to support the encoding of contextual details into episodic memory in school-aged children and adults (see Ghetti & Bunge, 2012; Kim, 2011; Ofen, 2012; Xue, 2018 for reviews). For example, through meta-analyses, Kim (2011) indicated that fusiform, premotor cortex, left inferior frontal gyrus (IFG), and right posterior parietal cortex were engaged in associative encoding in adults.
In addition to activation of separable brain regions, the communication between them has also been shown to be important for memory in school-aged children and adults (Menon, Boyett-Anderson, & Reiss, 2005; Schlichting & Preston, 2016; Tang, Shafer, & Ofen, 2017). For example, Tang et al (2017) used psychophysiological interaction (PPI) analyses in 8–25 year olds revealing that during successful memory formation, functional connectivity between lateral PFC and regions in medial temporal lobe increased with age, but the connectivity between superior PFC and regions within medial temporal lobe decreased with age (see also Menon et al., 2005 ).
1.2. Task-free fMRI studies of memory
It is difficult to collect classical resting-state fMRI data from young children. However, task-free scans (e.g., watching a movie without explicit demands) allows us to measure brain networks in young children. Although there could be differences between classical resting-state and task-free scans, studies on children and adults have consistently indicated that episodic memory is associated with the interaction between hippocampus and cortical regions during resting and/or task-free states(e.g., Riggins, Geng, Blankenship, & Redcay, 2016; Vincent et al., 2006; Wang, LaViolette, et al., 2010; Wang, Negreira, et al., 2010). In adults, functional connectivity during rest from hippocampus to posterior cingulate cortex and precuneus positively predicted memory performance on tasks performed outside the scanner (Wang et al., 2010). In children, functional connectivity during task-free scans from hippocampus to several cortical regions (e.g., precuneus, superior temporal gyrus, middle temporal gyrus) was related to episodic memory in 4- and 6-year-old children (Riggins et al., 2016). However, some of these associations were influenced by age. For example, memory performance was positively related to the connectivity between anterior hippocampus and precuneus in 6-year-old children but negatively related in 4-year-old children. In contrast, the connectivity between posterior hippocampus and right medial temporal gyrus was positively related to memory performance in 4-year-old children but negatively related in 6-year-old children. These results were interpreted within an interactive specialization framework, suggesting that both integration and segregation of cortical networks is important for developmental change (Johnson, 2001). Age-related differences in functional connectivity along the longitudinal axis likely have functional relevance because the relations between hippocampal volume and memory performance have been shown to vary between hippocampal subregions as well as across development (DeMaster, Pathman, Lee, & Ghetti, 2014; Riggins et al., 2018).
1.3. Current study
Despite findings of the importance of the hippocampus, cortical regions, and their connectivity in school-aged children and adults, their role in early childhood remains under-investigated. Thus, the first goal of the current study was to explore the contribution of hippocampus and cortical regions and their interaction during both a memory encoding task and task-free state in early childhood. Based on previous studies showing the heterogeneity of the hippocampus along the longitudinal axis and the hippocampal heterogeneity varies as a function of age (Blankenship, Redcay, Dougherty, & Riggins, 2017; Poppenk, Evensmoen, Moscovitch, & Nadel, 2013), we also explored this potential regional specificity in our analyses of both task and task-free data.
Finally, previous developmental studies in older children suggest that the activity of the regions identified above and the connectivity between these regions can be influenced by both age and performance (Church, Petersen, & Schlaggar, 2010; Duarte, Ranganath, Trujillo, & Knight, 2006; Geng, Canada, & Riggins, 2018; Paz-Alonso, Gallego, & Ghetti, 2013; Sastre, Wendelken, Lee, Bunge, & Ghetti, 2016). For example, Sastre et al. (2016) reported that during memory retrieval, high-performing 10- to 11-year-olds showed whole hippocampus activation similar to low performing adults, but only high performing adults showed activation in the hippocampal head. Therefore, a secondary aim of the present investigation was to explore the influence of age and performance on regions (and connections) identified as contributing to episodic memory.
In summary, the current study sought to identify brain regions engaged in the encoding of contextual details and test whether age and performance at retrieval influenced the activation or the connectivity of these brain regions both during an active memory encoding task and in a task-free state. Based on previous studies, we predicted that the encoding of contextual details would alter activation in the hippocampus, IFG, parietal cortex, occipital cortex, fusiform, and temporal cortex. In addition, we predicted that there would be age- and performance-related differences in the activity of hippocampus during encoding as well as in the connectivity from hippocampus to other cortical regions during encoding task and during task-free state. Regional specificity along the longitudinal axis of hippocampus was expected for these age- and performance-related differences. Finally, an exploratory question was whether age- and performance-related differences would be observed in the activity of other cortical regions as well.
2. Material and Methods
2.1. Participants
Children were recruited from a major metropolitan area through the use of both a University maintained database of families interested in participating in research and the distribution of recruitment flyers. To determine eligibility for the current study, children were screened to ensure they were not more than three weeks premature and had no diagnoses for any neurological conditions, developmental delays, or disabilities or contraindications for MRI.
Participants were part of a larger sample of children participating in a longitudinal study on memory and brain development (n=200). Usability of participants’ scans was determined via objective criteria. A total of 44 children provided useable data for memory task-based analyses (4.19–8.94 years, mean age = 7.12, SD = 1.23, 27 females). Children were excluded due to poor behavioral performance (9), missing data (5), no time to finish or perform the task (129), or too much motion (13). For task-free fMRI data, 110 children provided usable data (4.02–8.96 years, mean age = 6.51, SD = 1.48, 55 females). Children were excluded due to falling asleep (4), too much motion (63), incomplete data (18), or no data (5). For the task and task-free fMRI data analyses, 29 children were included in both analyses (17 females).
2.2. Procedure
The Institutional Review Board at University of Maryland approved all procedures. Parents or guardians provided informed consent for all participants. Children older than 7 years gave written assent, children younger than 7 years provided verbal assent. After participating, children received monetary compensation, a small gift, and a picture of their brain.
Children visited the laboratory twice, approximately 7 days apart (mean = 7.13 days, SD = 2.62). During the first visit, children performed a series of behavioral tests including the encoding part of an episodic memory task (the retrieval part was performed during the second visit). This out-of-scanner episodic memory task was designed based on previous studies and has been extremely successful at identifying age-related differences in children across this age range (i.e., Drummey & Newcombe, 2002; Riggins, 2014; see also Riggins et al., 2018). During the second visit, children participated in the fMRI portion of the study. All participants completed training in a mock scanner before MR data acquisition in order to help children acclimate to the scanner environment and learn stay still. In the scanner, a different memory task was performed, which was adapted from previous fMRI studies examining memory in older children (Ghetti et al., 2010, see details below). The retrieval part of this in-scanner episodic memory task was performed after getting out of the scanner approximately 15 minutes later. The primary differences between in-scanner and out-of-scanner tasks included the type of stimuli (pictorial vs. verbal), encoding-retrieval interval (7 days vs. 15 minutes), presentation time of stimuli (limited vs. unlimited), and whether it was intentional or incidental.
2.3. In-scanner episodic memory task
2.3.1. Training and practice
Participants first completed training and practice blocks/phases outside the scanner to ensure they understood the task. The training session introduced the child to both the encoding and retrieval portions of the task. For encoding, the experimenter first showed a picture of a character alone on the screen and identified the character by name. The characters were well known to children (i.e., The Little Mermaid, SpongeBob, or Mickey Mouse) and one of the characters was selected as a typically female-preferred character, one was a typically male-preferred character, and one was a character typically liked by both males and females. Then the experimenter sequentially presented two items next to the character and verbally labeled each item. The items (animals and objects) determined to be age appropriate were selected from the Bank of Standardized Stimuli. The child was told that it was important to remember both the item and the character. This was done for each of the 3 characters, which resulted in a total of 6 paired items. Immediately following encoding training, the child was sequentially shown each of the 6 old items and 3 new items. For each item, they were asked to identify whether it was old or new. In addition, for items identified as “old”, they were also asked with which character the item had previously been presented (source memory). During this training retrieval period, the experimenter corrected inaccurate responses.
Following training, the child practiced both the encoding and retrieval portions of the paradigm. During encoding practice, each character was paired with 5 different items and children were instructed to observe and remember which items went with which characters. During retrieval practice, inaccurate responses were not corrected. Children were required to make item and source memory judgments on the 15 old items and 5 new items and obtain an accuracy score of 80% or higher before proceeding. If children did not pass with the required accuracy, the experimenter explained the task rules again and participants were asked to complete another practice session with different stimuli.
2.3.2. Encoding (in scanner)
The design of the encoding task in the scanner was the same as the design of the task during training and practice. The only difference was that the encoding task in the scanner engaged more stimuli including 120 stimuli (40 per character block) paired with one of three different character sources. As in the mock scanner, participants were instructed to observe and remember which items went with which characters. No deliberate strategy to accomplish this was recommended. Item presentation order was randomized within block by the presentation software, Eprime (Psychology Software Tools, Pittsburgh, PA). Within each character-block, only one character was presented, item presentation progressed automatically with items presented for 1500 ms and an inter-stimulus interval ranging from 1000–3000 ms, with an average time of 2000 ms.
2.3.3. Retrieval (outside of scanner)
The retrieval portion of the task began approximately 15 minutes after the conclusion of the encoding portion. This delay was to ensure that working memory did not drive performance on the task and to allow for leaving the fMRI data collection room properly and the inclusion of a brief break. There were a total of 160 items (120 old and 40 new items) presented to children during retrieval. Children were instructed to respond “yes” if the item presented was one they had seen during encoding, and “no” if the item presented was new. If children indicated seeing the item previously, they were then asked to indicate to which of the three characters the item belonged. Items were presented on the screen until children identified them as being old or new. If the item was identified as old, the three characters remained on the screen until children indicated which character they believed the item belonged to. Children gave all answers verbally and responses were recorded by the experimenter.
Variables of interest included the following: stimuli accurately recalled as old were further categorized as ‘source correct’ if the child correctly recognized the character with whom the item was presented (these items were labeled as subsequent source correct items during encoding), or ‘source incorrect’ if the child correctly identified an item as old but attributed the item to the incorrect character (these items were labeled as subsequent source incorrect items during encoding). Source memory was computed as the proportion of characters accurately recalled among the recognized items.
2.4. Out-of-scanner episodic memory task
2.4.1. Encoding
During the first visit to the lab, children were taught novel facts (e.g., “A group of rhinos is called a crash”) from one of two different sources, a female adult (“Abby”) and a male-voiced puppet (“Henry”), via digital videos. The children learned 6 facts from each source for a total of 12 facts. Presentation of facts was blocked by source, where children first learned 6 facts from one source followed by 6 facts from the other source, and the order of blocks was randomly assigned across participants. There were 3 lists of facts; each list consisted of unique facts that were similar across lists (e.g., “A group of kangaroos is called a mob” or “A group of goats is called a tribe”). These lists were randomly assigned across participants. Children were told to pay attention to the facts as they would be tested on the facts the following week, but were not told that they would be tested on the source of the facts. Children were asked about each fact to find out if they knew the facts prior to the experiment. Known facts were excluded at testing and additional novel facts from the list from the same source were presented; this rarely occurred. Each source had 8 possible facts to account for the possibility that children would know 1 or 2 of the facts. If a child knew 3 or more facts from one source, the total number of facts the child was tested on was reduced (but this was rare, n = 4).
2.4.2. Retrieval
When children returned to the lab for their second visit, they were tested on their memory for the facts and sources from the first visit. Children were asked to answer 22 trivia questions and to tell the experimenter where they had learned the answers to those trivia questions. They were told that they had learned some of the questions the week before from either “Abby” or “Henry,” some they might have learned outside the laboratory (e.g., from a teacher or parent), and some they may not know. The children had learned 6 of the 22 facts presented from “Abby,” 6 from “Henry,” 5 were facts commonly known by children (e.g., “What color is the sky?”), and 5 were facts that children typically would not know (e.g., “What is the colored part of your eye called?”). Each list of 22 facts had two random presentation orders, and these orders were counterbalanced across participants. If children did not know an answer to a question, they were given five multiple choice options: parents, teacher, girl in the video, puppet in the video, or just knew/guessed.
Source memory was calculated as the proportion of questions for which the child accurately recalled both the fact and the source of the fact (i.e., source memory conditionalized on fact memory) as this is thought to reflect the binding of the fact and source. Additionally, three types of error were computed: children indicated they guessed or always knew the facts, children indicated a person outside the experiment taught them the fact (extra-experimental errors), or children indicated the wrong experimental source taught them the fact (intra-experimental errors). Source memory, extra-experimental error, and intra-experimental error were included for the analyses of brain-behavioral relations.
2.5. Imaging Data Acquisition
Participants were scanned in a Siemens 3.0-T scanner (MAGNETOM Trio Tim System, Siemens Medical Solutions, Erlangen, Germany) using a 32-channel coil. Children first completed the task-free scan, followed by the structural scans (T1 and T2, during which they watched a movie of their choice) and then, if time permitted, the memory task. This order was selected because task-free was our main interest and previous research shows that tasks completed prior to resting scans can influence resting activity (Pyka et al., 2009). During the task-free scan, children were instructed to lie as still as possible with eyes open without any overt task. To minimize motion, Inscapes, a movie designed for collecting fMRI data to reduce potential head motion, was played (Vanderwal, Kelly, Eilbott, Mayes, & Castellanos, 2015). A total of 210 whole-brain task–free fMRI data were collected using a T2*-weighted gradient echo planar imaging sequence (TR 2 s, TE 25 ms, slice thickness 3.5 mm, voxel size 3.0 mm × 3.0 mm × 3.5 mm, voxel matrix 64 × 64, flip angle 70°, field of view 192 mm, 36 slices), duration of 7 minutes and 6 seconds. Structural images were acquired with a T1-weighted magnetization prepared rapid gradient echo sequence: TR 1.9 s; TE 2.32 ms; slice thickness 0.9 mm with no gap; voxel size 0.9×0.9×0.9 mm; voxel matrix 256×256 mm; flip angle 9°; field of volume 230 * 230 mm, duration of 4 minutes and 26 seconds. Finally, task fMRI data were collected while children performed the encoding part of the source memory task using a T2*weighted gradient echo-planar imaging sequence (parameters were the same as that for the above task-free scan).
During the task-free and task fMRI scans, participant head motion was monitored in real-time. If a participant exhibited excessive head motion (>3mm in any direction) during the first half of any run, the scan was restarted and the participant was reminded to stay as still as possible. This re-starting procedure occurred for 16 out of 110 subjects during task-free scan, and to 1 out of 44 subjects during the memory encoding task.
2.6. Data Analysis
2.6.1. Task fMRI data
The preprocessing steps including slice timing correction, motion correction, and smoothing (Gaussian kernel FWHM=5mm) were conducted using DPABI 1.3 (a toolbox for Data Processing & Analysis for Brain Imaging, version 1.3, Yan, Wang, Zuo, & Zang, 2016). The smoothed 4D dataset was then analyzed with FSL MELODIC ICA software (www.fmrib.ox.ac.ukfslmelodic2index.html) to decompose the signal into 40 components (McKeown et al., 1998). An experienced rater viewed each component and categorized it as task-related signal or artifact-related component with the toolbox of FSLeyes (https://zenodo.org/record/1470762#.W-JRgPkzb4Y). With the aim to calculate intra-rater reliability, the rater categorized the components for 10 subjects again in two months. Based on the cut-off proposed by Landis and Koch (Landis & Koch, 1977), the intra-rater reliability was from substantial to excellent (Cohen’s kappa = 0.75–0.90). To calculate inter-rater reliability, another rater categorized the components for 10 subjects independently. The inter-rater reliability was from substantial to excellent (Cohen’s kappa = 0.60–0.90). After removing all artifact-related components, brain extraction and normalization were conducted. Brain extraction was conducted separately in 6 toolboxes including the Advanced Normalization Tools (ANTs), AFNI, FSL, BSE, ROBEX, and SPM8. The voxels extracted by at least four toolboxes were included in the brain mask (Tillman et al., 2018). We used ANTs (Avants et al., 2011) to carry out coregistration and normalization. Statistical analyses were carried out in AFNI (Cox, 1996). For the first level analyses, multiple regression analyses were conducted. The encoding events were convolved based on SPMG 2-parameter gamma variant regression model to create 3 regressors of interest: subsequent source correct items, subsequent source incorrect items, and subsequent forgotten items. All subjects included for statistical analyses had mean framewise displacement (FD) from 0.08 to 0.5 (group mean FD = 0.26, SD = .12). No censoring was carried out in order to preserve as many trials as possible for each condition.
The second level analyses included ROI and whole brain analyses. ROI analyses were conducted using individual seed regions (anterior and posterior hippocampus) that were derived from Freesurfer 5.1 (surfer.nmr.mgh.harvard.edu; Fischl, 2012) and edited using Automatic Segmentation Adapter Tool (ASAT, nitrc.org/projects/segadapter; Yushkevich et al., 2015). The hippocampus was divided into anterior and posterior hippocampus using manual identification of standard anatomical landmarks. The uncal apex served as the border between anterior and posterior hippocampus (Weiss et al., 2005; see also Duvernoy, 2005 and Gloor, 1997). Raters were blind to participant age and sex. Reliability for identification of these landmarks indicated 94.6% agreement within 1 slice and 99.992% agreement within 2 slices. Intra-class correlation coefficients (ICCs) were high and ranged from .897 – .985. Repeated measure ANOVA was conducted with Condition (subsequent source correct versus subsequent source incorrect) and Subregion included as within-subject factors. Age, Performance and their interaction were entered as continuous covariates.
Whole-brain analyses was conducted using 3dttest++ program within AFNI. BOLD signal was compared between subsequent source correct and subsequent source incorrect trials (i.e., subsequent recollection effect). Mean FD, age, performance, and age × performance interaction were included as covariates. The 3dClustSim mixed model autocorrelation function (ACF) indicated that clusters with a minimum of 12 voxel size and puncorrected < .001 were viewed as significant with multiple comparison correction (pcorrected < .05).
In order to further characterize the contribution of hippocampus to contextual information encoding, seed-based psychophysiological interaction (PPI) analyses (Friston et al., 1997) were performed to test the effective connectivity from anterior and posterior hippocampus to the brain regions showing subsequent recollection effects (https://afni.nimh.nih.gov/CD-CorrAna). The steps included extracting the average time series of the ROIs and removing the trend from the seed time series, running deconvolution, obtaining and concatenating the interaction regressor, inspecting data for extreme values (defined as +/− 2.5 SD from mean), and conducting regression analysis. Finally, for each subject, we defined the brain regions (ROIs) showing subsequent recollection effects during the memory encoding task by running leave-1 out procedure (the ROIs for Nth subject was defined by using the data of the other N-1 subjects). The ROIs for each subject were then used to extract the beta value of the interaction regressor for repeated ANOVA analyses, which were performed with Subregion (anterior and posterior hippocampus) and Condition (subsequent source correct versus subsequent source incorrect) as within-subject factors and with Age, Performance, and their interaction as continuous covariates.
2.6.2. Task-free fMRI data
In the analyses, all 210 collected rs-fMRI images were included, as the first 4 volumes were discarded before data collection due to the instability of the initial MRI signal and participant adaptation. Preprocessing included the following steps. First, slice timing, head motion correction, and smoothing (Gaussian kernel FWHM=5mm) were performed using DPABI 1.3. MELODIC ICA was then run on smoothed data to remove artifact-related components using the same procedure as that for task fMRI data. After removing all artifact-related components, brain extraction, normalization, and filtering were conducted. Brain extraction was conducted separately in 6 toolboxes and ANTs was used to carry out coregistration and normalization (the procedure was the same as that for task fMRI data). Statistical analyses were carried out in AFNI (Cox, 1996). Temporal bandpass filtering (0.01–0.1 Hz) and spatial smoothing with a 5 mm full-width-at-half-maximum Gaussian kernel was performed in AFNI to normalized data.
Task-free functional connectivity analyses were conducted in AFNI. First, we scrubbed any volumes with FD ≥ 0.3 mm as well as 1 back and 1 forward volumes in order to minimize the head motion effect. All children included in final statistical analyses had data ≥ 4 minutes in length and mean FD from 0.06 to 0.33 (group mean FD = 0.16, SD = 0.06). The connectivity between the time series of the seed regions (anterior and posterior hippocampus) and those of the whole brain was calculated to generate individual rs-fc maps (r-maps). Subsequently, we used Fisher’s r-to-z transformation to convert r-maps into z-maps to obtain normally distributed values of the connectivity maps. The z values were extracted by using the ROI regions showing subsequent recollection effect at task. Extreme values (define +/− 2.5 SD from mean) were excluded. For each ROI, repeated measures ANOVA was conducted. Subregion (anterior and posterior hippocampus) was entered as within-subject factor. Age, Performance and their interaction were included as continuous covariates.
3. Results
3.1. Behavioral results
The descriptive data for the memory tasks performed in and out of the scanner are presented in Table 1. Consistent with our hypothesis, age was related to source memory performance, intra- and extra-experimental errors and guessed-knew responses on the task performed outside of the scanner, r (108) = 0.40, p < .001; r (108) = 0.57, p < .001; r (108) = − 0.47, p < .001; r (108) = − 0.36, p < .001, respectively. However, counter to this hypothesis, relations between age and source memory performance on the episodic memory task performed in the scanner was not significant (r (42) = 0.25, p = .11). However, the difference between these two correlation coefficients (i.e., correlations between age and the in and out-of scanner task performance) was not significant. The variation in magnitude could be due to the differences in sample size, variations in task design, ages of subjects included (e.g., very few 4-year-old children remained for final analysis for the behavioral task performed in the scanner), or testing environment (i.e., in versus out of scanner).
Table 1.
Descriptive data for the two memory tasks
| Source memory performance | Knew/gues sed error | Intra-experimental error | Extra-experimental error | Hit | False alarm | ||
|---|---|---|---|---|---|---|---|
| In-scanner task* | Mean | 0.53 | N/A | N/A | N/A | 0.5 | 0.08 |
| SD | 0.13 | N/A | N/A | N/A | 0.16 | 0.14 | |
| Minimum | 0.28 | N/A | N/A | N/A | 0.21 | 0 | |
| Maximum | 0.83 | N/A | N/A | N/A | 0.83 | 0.58 | |
| Out-of-scanner task | Mean | 0.26 | 0.27 | 0.12 | 0.08 | 0.59 | 0.04 |
| SD | 0.18 | 0.26 | 0.09 | 0.12 | 0.23 | 0.08 | |
| Minimum | 0 | 0 | 0 | 0 | 0.08 | 0 | |
| Maximum | 0.67 | 1 | 0.29 | 0.5 | 1 | 0.5 | |
Note: N/A = not applicable. For the in-scanner task, data were only used for analyses if children had enough useable trials for analysis of all conditions; thus, average performance on the task is skewed compared to all children in the study who were asked to complete the task.
3.2. fMRI task activation
3.2.1. A priori hippocampal ROI analyses
Individual anterior and posterior hippocampal ROIs (Anterior-Posterior; Figure 1A) were used to extract signal in order to test if there was main effect of Condition (subsequent source correct vs. incorrect trials during encoding) or any interaction involving Age or Performance (during retrieval). We found a main effect of Condition (F (1, 37) = 16.15, p < .001), that was qualified by interactions between Condition × Age × Performance, Condition × Anterior-Posterior× Performance, and Condition × Anterior-Posterior × Age × Performance (F (1, 37) = 10.18, p = .002; F (1, 37) = 10.72, p = .002; F (1, 37) = 8.20, p = .007). Follow-up analyses indicated a main effect of Condition (Anterior: F (1, 37) = 18.30, p < .001; Posterior: F (1, 37) = 11.51, p = .002) and a Condition × Age × Performance interaction (Anterior: F (1, 37) = 11.48, p = .002; Posterior: F (1, 37) = 5.56, p = .024) for anterior and posterior hippocampus separately.
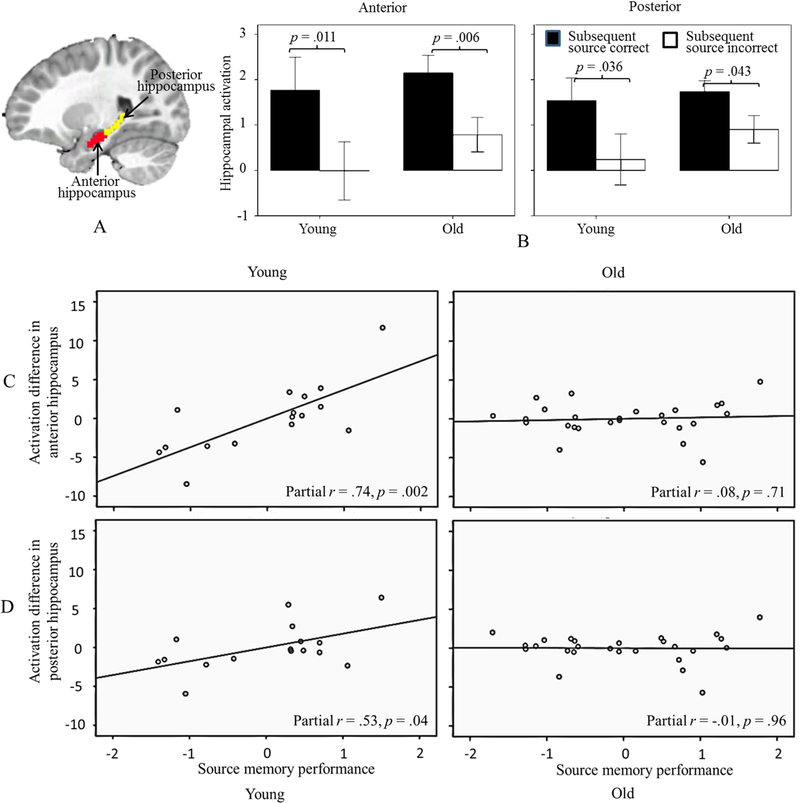
Figure 1.
The Condition × Anterior-Posterior × Age × Performance interaction in hippocampal activation. A) Illustrates subregions used as Regions of Interest (ROIs). B) Illustrates activation for each condition, subregion and age group. C) and D) Illustrate the relation between memory performance and the activation difference between subsequent source correct and incorrect conditions in each age group and subregion. Across all children, differences in activation were apparent for items remembered with correct versus incorrect details. However, within younger children, greater differences in activation between conditions were associated with better performance.
To disentangle the Condition × Age × Performance interactions, we split the subjects into younger and older age groups according to mean age (i.e., 7.12 years): 17 ‘younger’ children (mean age = 5.83 years, age range = 4.19 – 6.83, SD = .81), 27 ‘older’ children (mean age = 7.93 years, age range = 7.21 – 8.94, SD = .59). Older children showed greater activation in subsequent source correct versus subsequent source incorrect trials for both the anterior and posterior hippocampus (F (1, 23) = 8.96, p = .006; F (1, 23) = 4.60, p = .043). However, there was no interaction with Performance. In contrast, in the younger group, we found that there were Condition × Performance interactions for both anterior and posterior hippocampus (F (1, 13) = 15.59, p = .002; F (1, 13) = 5.14, p = .041). Due to the limited sample size, we were unable to further divide young children into low and high performance groups. Thus, we tested how Performance predicted the difference in the activation to the conditions in anterior and posterior hippocampus separately within groups. The results indicated that better performance was related to greater activation differences between subsequent source correct versus subsequent source incorrect trials in both regions in the younger group (anterior: r = 0.74, p = 0.002; posterior: r = 0.53, p = .041).
3.2.2. Whole-brain analyses
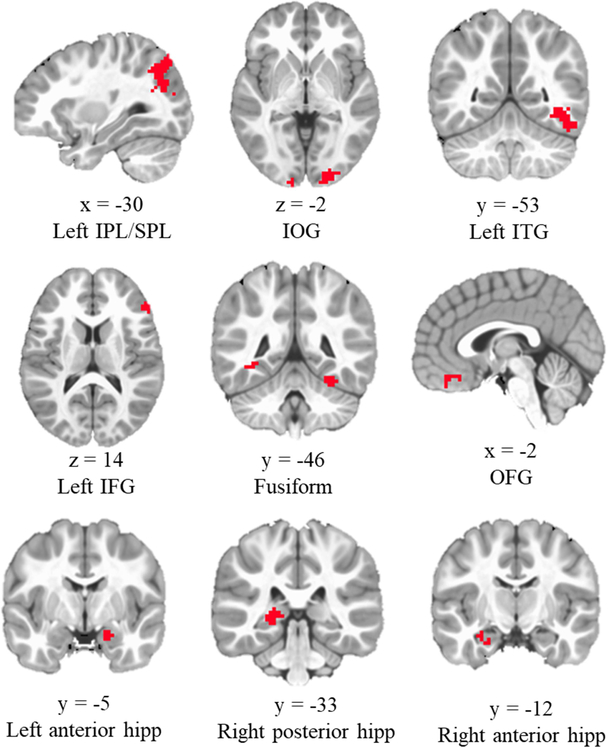
As showed in Figure 2, the analyses indicated 7 brain regions showed greater activation in subsequent source correct versus subsequent source incorrect trials. The 7 regions included bilateral inferior/superior parietal lobule (IPL/SPL; cluster size: left = 182, right = 15; contained regions within middle/superior occipital gyrus), bilateral inferior occipital gyrus (IOG; cluster size: left = 166, right = 36; contained regions within calcarine gyrus), left inferior temporal gyrus (ITG, cluster size = 114), bilateral fusiform (cluster size: left = 48, right = 13), left inferior frontal gyrus (IFG, cluster size = 45), left anterior hippocampus (cluster size =16), right posterior hippocampus (cluster size =29), and orbital frontal gyrus (OFG, cluster size = 25). In contrast, no regions showed greater activation in subsequent source incorrect versus correct trials.
Figure 2.
Brain regions showing greater activation in subsequent source correct versus incorrect trials. IPL/SPL: inferior/superior parietal lobule; IOG: inferior occipital gyrus; ITG: inferior temporal gyrus; IFG: inferior frontal gyrus; hipp: hippocampus; OFG: orbital frontal gyrus. Across all subjects, only greater activation of right anterior hippocmapus in subsequent source correct versus subsquent source incorrect trials was related to better task performance.
There was a region (right anterior hippocampus, Figure 2) showing a significant interaction between Condition and Performance. Better performance was associated with greater activation of right anterior hippocampus (containing regions in parahippocampus) in subsequent source correct versus subsequent source incorrect trials, t = 4.26, p < 0.001. This latter finding was generally consistent with the results from the ROI analyses, which showed a similar pattern, albeit only in younger children.
3.3. Functional connectivity
3.3.1. Task-based functional connectivity
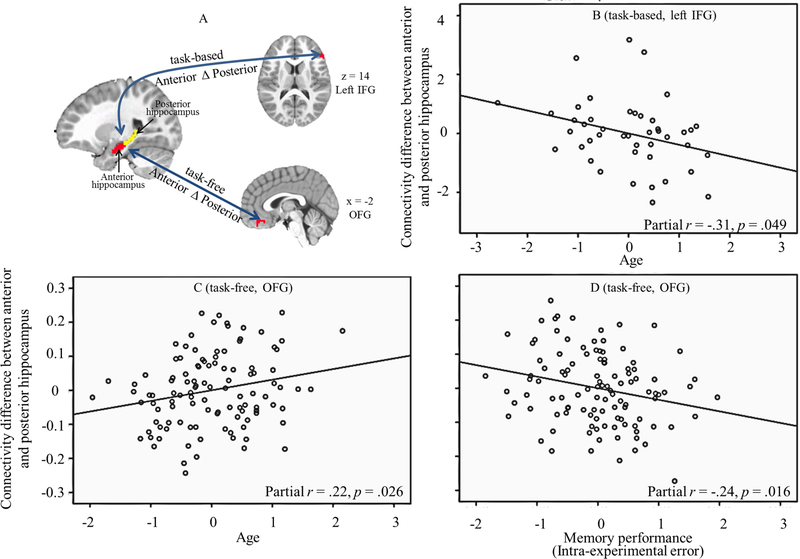
We calculated task-based functional connectivity from bilateral anterior and posterior hippocampus to the six regions (excluding hippocampus) showing main effects of condition (i.e., subsequent recollection effects; subsequent source correct > incorrect condition). Then, we tested how age, performance, and their interaction predicted the functional connectivity. There was an interaction between Condition, Anterior-Posterior, and Age in the connectivity from hippocampus to left IFG (F (1, 39) = 4.10, p = .049). Follow-up analyses indicated that the difference in connectivity between subsequent source correct and incorrect conditions for anterior and posterior hippocampus interacted with Age (F (1, 39) = 4.10, p = .049), indicating that age was positively related to the difference between anterior and posterior hippocampus in their connectivity to left IFG (see Figure 3A). In other words, during the encoding tasks, older participants showed greater differentiation of connectivity between the hippocampal subregions and left IFG.
Figure 3.
Age- and performance-related differences in the connectivity from anterior and posterior hippocampus during encoding and task-free scans. A) illustrates the connectivity from anterior and posterior hippocampus to left IFG (task-based) and OFG (task-free). B) illustrates the difference between anterior and posterior hippocampus in connectivity to IFG was positively related to age. C) illustrates the difference between anterior and posterior hippocampus in connectivity to OFG was positively related to age and D) negatively related to intra-experimental errors.
3.3.2. Task-free functional connectivity
We then examined the effects of Subregion, Age, and Performance on brain activity by calculating functional connectivity from anterior and posterior hippocampus to the six regions (excluding hippocampus) showing greater activation for the items subsequently rememerbered with correct versus incorrect source. The results indicated that posterior hippocampus showed greater connectivity to bilateral IPL/SPL, bilateral IOG, left ITG, fusiform, and left IFG than anterior hippocampus (F (1, 100) = 91.60, p < .001; F (1, 100) = 57.33, p < .001; F (1, 100) = 62.82, p < .001; F (1, 100) = 120.70, p < .001; F (1, 100) = 5.33, p = .023). In contrast, anterior hippocampus showed greater connectivity to orbital frontal gyrus than posterior hippocampus (F (1, 100) = 30.20, p < .001).
Additionally, for OFG, we found Anterior-Posterior × Age (F (1, 100) = 4.95, p = .028) and Anterior-Posterior × Performance (source intra-experimental error) interactions (F (1, 100) = 6.05, p = .016). Then, we calculated the difference between anterior and posterior hippocampus in their connectivity to OFG. Regression analyses indicated that the difference was positively related to age and negatively related to the proportion of intra-experimental errors, such that older children and children with fewer intra-experimental errors showed greater differences between anterior and posterior hippocampus in their connectivity to OFG (illustrated in Figure 3B and 3C). There were no other age- or performance related difference in functional connectivity during task-free scan.
4. Discussion
The goals of the current study were to identify the neural correlates of episodic memory during early childhood and explore whether the findings in this young population would be consistent with the component process model, which suggests that hippocampus and its interaction with other cortical regions make up the core of the neural networks related to episodic memory (Moscovitch, Cabeza, Winocur, & Nadel, 2016). Therefore, we collected fMRI data from children aged 4–8 years during memory encoding and task-free states. Then, the data were analyzed to test age- and performance-related differences in hippocampal activation and connectivity. The findings indicated that, consistent with the component process model (Moscovitch et al., 2016), encoding contextual details activated hippocampus and multiple cortical regions (bilateral IPL/SPL, bilateral IOG, left ITG, left IFG, and fusiform) in young children. In contrast to adult studies, we found that OFG was activated during the successful encoding of contextual details in young children. Other novel findings included age- and performance-related differences in the activation of hippocampus as well as in the interaction between the hippocampus and other cortical regions (specifically, left IFG and OFG). Finally, results revealed functional differentiation along the longitudinal axis of hippocampus is present during early childhood, as were age- and performance-related differences.
Results from the task-based fMRI data indicated that the hippocampus showed greater activation for items that were subsequently remembered with correct versus incorrect source details. This activation difference was greater in anterior versus posterior hippocampus. This finding suggests that in early childhood, there is functional differential along the longitudinal axis of hippocampus, as suggested by the component process model (Moscovitch et al., 2016). Moreover, we found that among children aged 4 to 6 years, better memory performance was related to greater difference in hippocampal activation elicited by the items subsequently remembered with correct versus incorrect source. In other words, for children aged 4 to 6 years, high performers differentially engaged the hippocampus to a greater extent compared to low performers during encoding. However, this finding should be interpreted with caution because there were only 3 4-year-old children and 5 5-year-old children among the 17 children aged 4–6 years. Among children aged 7–8 years, there was no relation between performance and hippocampal activation, suggesting that high and low performers in this group showed no difference in engaging hippocampus. Therefore, the hippocampus, a structure involved in encoding contextual details, might be still maturing during early childhood. The individual differences in such maturation relates to memory ability, particularly between the age of 4 and 6 years. These findings were consistent with behavioral findings in this report and others (Drummey & Newcombe, 2000; Riggins, 2014), suggesting the ability in encoding contextual details is improving during early childhood. Such development might be supported by the maturation and differentiation of the hippocampus.
The finding that all children aged between 4 and 8 years engaged hippocampus for encoding contextual details stands in contrast to a previous study in school-aged children Ghetti et al. (2010), which reported that only 14-years-olds and adults showed the evidence supporting the engagement of hippocampus during memory encoding (i.e., 8- and 10–11-year-old children did not show this evidence). The root of this discrepancy is unknown, but it may be related to differences in sample size, task performance, the design of memory task, or other methodological factors between this study and Ghetti et al., 2010. Therefore, future research would benefit from studies including subjects both younger and older than 8 years to fully understand how hippocampus supports the development of episodic memory across childhood.
Bilateral IPL/SPL also showed activation during encoding. This region, suggested to be a part of the dorsal visual pathway, receives the signal from primary visual regions to represent spatial information (Culham & Kanwisher, 2001; Rizzolatti & Matelli, 2003) and has also been related to memory (Ghetti & Bunge, 2012; Kim, 2011; Ofen et al., 2007). In terms of engagement with dorsal visual system, the encoding task used in the current study did involve spatial information (e.g., as the item and the character were presented side by side), which could be contributing to these effects. However, children were not instructed to use the spatial information to help encode contextual details nor were they specifically tested on their ability in remembering the spatial information. It is also possible that the activation of bilateral IPL/SPL reflects the voluntary allocation of attention during perception because this region has been suggested as a part of the frontoparietal attention system (Cabeza, Ciaramelli, Olson, & Moscovitch, 2008). During encoding, more attention may have been allocated to the items subsequently remembered with correct versus incorrect source details, consistent with a previous finding that sustained attention measured by the activation of posterior parietal cortex during encoding was related to memory performance in adults (Otten, Henson, & Rugg, 2002). More research is needed to test how attention modulates the development of episodic memory in early childhood.
In addition, left IFG, bilateral IOG, left ITG, and fusiform also showed greater activation for the items subsequently remembered with correct versus incorrect source details. These regions are part of the brain system related to high-level perceptual processing in visual memory tasks (Brewer, Zhao, Desmond, Glover, & Gabrieli, 1998; Cansino, Maquet, Dolan, & Rugg, 2002; Kim, 2011; Miyashita, 1993). It is possible that these regions transform visual input into internal representations that could be sent to the hippocampus for consolidation and, ultimately, long-term memory storage, which could be accessed and retrieved into consciousness later. Alternatively, activation in left IFG has been suggested to be related to the organization of multiple pieces of information in working memory for building associations between them (Kim, 2011). Thus, the increased activation of left IFG might suggest complex organization processes were engaged to a greater extent for items subsequently remembered with correct versus incorrect source during encoding.
The OFG also showed activation during the encoding of contextual details that varied as a function of whether those details were remembered. This region is not commonly reported in studies of memory. Therefore, it may reflect that young children (< 8 years) recruit a wider network of brain regions than older children and adults, including regions “outside” of what is commonly thought of as memory regions in older children and adults (see Riggins et al., 2016 for similar findings). An alternative possibility is that because this region receives the outputs of a number of sensory systems such as visual, taste, and somatosensory stimuli (Rolls, 2004) and relates to volitional intention to perform a task (Frey & Petrides, 2002; Ramus, Davis, Donahue, Discenza, & Waite, 2007; Rolls, 2004), the activation of this region during encoding in the current study might reflect the intention of children to encode visual details of the objects or their visual association with the character. However, because previous developmental and adult studies using visual stimuli do not report the activation of OFG during encoding (Ghetti & Bunge, 2012; Kim, 2011; Ofen, 2012), this interpretation seems less likely. Additional studies within this age range are needed to address these and other possibilities.
In addition to the independent activation of brain regions, we also examined connectivity between hippocampus and other cortical regions during both task-based and task-free scans. The results indicated that age was related to the difference between anterior and posterior hippocampus in their connectivity to left IFG during the encoding task. Moreover, age- and performance-related differences were observed between anterior and posterior hippocampus in their connectivity to OFG during task-free state. First, these findings support the component process model in terms of the important role of the interaction between hippocampus and cortical regions in episodic memory and the regional specificity along the longitudinal axis of the hippocampus (Moscovitch et al., 2016; Poppenk et al., 2013). In addition, as it has been suggested that anterior hippocampus codes information in term of the general or global relations among entities and posterior hippocampus codes information in term of precise position (Moscovitch et al., 2016; Poppenk et al., 2013), these findings might suggest that for older or high performing children, OFG may interact more with anterior versus posterior hippocampus to process the stimuli via global relations rather than localized details. However, it should be noted that the effect size for the relations was modest and more research is needed to verify the findings.
Differences were also observed between findings for the task-based and task-free functional connectivity. At least two possible reasons exist. First, Smith et al. (2009) proposed that the connectivity patterns defined using resting-state functional data are organized in functionally-relevant ways because the involved regions typically show co-activation during tasks. This proposal was mainly based on the findings using adult data. In contrast, according to the long-term phasic molding hypothesis proposed by Gabard-Durnam et al. (2016), the task-free connectivity patterns are shaped by accumulating experiences of phasic stimulus-elicited functional connectivity. Therefore, the connectivity patterns between brain regions related to episodic memory might have not stabilized yet during early childhood, which might underlie the discrepancy in functional connectivity characterized during encoding task and during task-free scan in the current study. Second, during the encoding task, brain activation or connectivity may have been influenced by the attributes of the stimuli used in the task. For example, the connectivity between hippocampus and left IFG may be the result of the visual stimuli used in the task. In contrast, the functional connectivity measured in the task-free scan may be more general, not specific to any type of stimuli (Vincent et al., 2006).
Related to this second possibility, during the encoding task, brain activation in ITG and IFG was lateralized to the left hemisphere. Previous studies have suggested that lateralization is related to the type of material used in the study (Kim, 2011). For example, left-lateralized results were mostly found in the studies using verbal materials and slightly left-lateralized or bilaterally balanced results were exhibited in the studies using pictorial material. However, although pictures were mainly used as stimuli in our study, the findings on ITG and IFG were lateralized to left hemisphere. Other studies have suggested that, in addition to the type of stimuli, verbalization or even intrinsic encoding mechanisms affect the lateralization (Menon et al., 2005). It is possible that verbalization might have been used by children to bind the items and build relations between them, which may be part of the reasons for our current findings, which are lateralized to the left hemisphere.
Although the current study made novel contributions to the field, there were limitations that future research could overcome to help understand how brain maturation supports the development of episodic memory across life span. First, this is a cross-sectional study and multiple extraneous factors could contribute to what appear to be age-related differences; only longitudinal designs can be used to characterize developmental change accurately. Another limitation could have been differences in the engagement level during encoding task because previous studies have indicated that attention modulates memory; this also could be addressed in future studies (Chun & Turk-Browne, 2007). In addition, keeping young children still during a task is not as easy as in older children or adults. This difficulty might have influenced our results (e.g., we had fewer 4- and 5-year-old children than older children for task fMRI data analyses; more high performing children were included). Therefore, researchers should continue to think about how to elicit better cooperation from young children with the aim to improve the generalizability of studies in early childhood.
5. Conclusions
In conclusion, the current study showed age- and performance-related differences in hippocampal activity and its connectivity to other cortical regions. These findings provided evidence in support of the component process model, which proposes that the hippocampus and its communication with cortical regions are the core components of the neural networks related to episodic memory (Moscovitch et al., 2016). In addition, differentiation along the longitudinal axis of hippocampus was shown to increase with age and be related to better performance on memory tasks involving encoding and recall of contextual details. In sum, our findings suggest that the maturation of hippocampa1) activity, 2) connectivity and 3) functional differentiation along the longitudinal axis in early childhood are related to age-related differences in memory performance.
Acknowledgments
Thank you to the members of the Neurocognitive Development Lab, especially, Morgan Bordof, Kelsey Canada, Elizabeth Mulligan, Marissa Clark, Lisa Cox, Shane Wise, and Jennifer Sloane for helping with data collection and/or analysis. This work was supported by NICHD under Grant HD079518 and University of Maryland.
Footnotes
Declarations of interest: none
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Avants BB, Tustison NJ, Song G, Cook PA, Klein A, & Gee JC (2011). A reproducible evaluation of ANTs similarity metric performance in brain image registration. NeuroImage, 54(3), 2033–2044. doi: 10.1016/j.neuroimage.2010.09.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer PJ, Doydum AO, Pathman T, Larkina M, Güler OE, & Burch M (2012). It’s all about location, location, location: Children’s memory for the “where” of personally experienced events. Journal of Experimental Child Psychology, 113(4), 510–522. doi: 10.1016/j.jecp.2012.06.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blankenship SL, Redcay E, Dougherty LR, & Riggins T (2017). Development of hippocampal functional connectivity during childhood. Human Brain Mapping, 38(1), 182–201. doi: doi: 10.1002/hbm.23353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brewer JB, Zhao Z, Desmond JE, Glover GH, & Gabrieli JDE (1998). Making Memories: Brain Activity that Predicts How Well Visual Experience Will Be Remembered. Science, 281(5380), 1185–1187. doi: 10.1126/science.281.5380.1185 [DOI] [PubMed] [Google Scholar]
- Cabeza R, Ciaramelli E, Olson IR, & Moscovitch M (2008). The parietal cortex and episodic memory: an attentional account. [Review Article]. Nature Reviews Neuroscience, 9, 613. doi: 10.1038/nrn245910.1038/nrn2459https://www.nature.com/articles/nrn2459#supplementary-informationhttps://www.nature.com/articles/nrn2459#supplementary-information [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cansino S, Maquet P, Dolan RJ, & Rugg MD (2002). Brain Activity Underlying Encoding and Retrieval of Source Memory. Cerebral Cortex, 12(10), 1048–1056. doi: 10.1093/cercor/12.10.1048 [DOI] [PubMed] [Google Scholar]
- Chun MM, & Turk-Browne NB (2007). Interactions between attention and memory. Current Opinion in Neurobiology, 17(2), 177–184. doi: 10.1016/j.conb.2007.03.005 [DOI] [PubMed] [Google Scholar]
- Church JA, Petersen SE, & Schlaggar BL (2010). The “Task B problem” and other considerations in developmental functional neuroimaging. Human Brain Mapping, 31(6), 852–862. doi: 10.1002/hbm.21036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox RW (1996). AFNI: Software for Analysis and Visualization of Functional Magnetic Resonance Neuroimages. Computers and Biomedical Research, 29(3), 162–173. doi: 10.1006/cbmr.1996.0014 [DOI] [PubMed] [Google Scholar]
- Culham JC, & Kanwisher NG (2001). Neuroimaging of cognitive functions in human parietal cortex. Current Opinion in Neurobiology, 11(2), 157–163. doi: 10.1016/S0959-4388(00)00191-4 [DOI] [PubMed] [Google Scholar]
- DeMaster D, Pathman T, Lee JK, & Ghetti S (2014). Structural Development of the Hippocampus and Episodic Memory: Developmental Differences Along the Anterior/Posterior Axis. Cerebral Cortex, 24(11), 3036–3045. doi: 10.1093/cercor/bht160 [DOI] [PubMed] [Google Scholar]
- Di X, Gohel S, Kim E, & Biswal B (2013). Task vs. rest—different network configurations between the coactivation and the resting-state brain networks. [Original Research]. Frontiers in Human Neuroscience, 7(493). doi: 10.3389/fnhum.2013.00493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drummey AB, & Newcombe NS (2002). Developmental changes in source memory. Developmental Science, 5(4), 502–513. doi: 10.1111/1467-7687.00243 [DOI] [Google Scholar]
- Duarte A, Ranganath C, Trujillo C, & Knight RT (2006). Intact recollection memory in high-performing older adults: ERP and behavioral evidence. Journal of Cognitive Neuroscience, 18(1), 33–47. doi: 10.1162/089892906775249988 [DOI] [PubMed] [Google Scholar]
- Fischl B (2012). FreeSurfer. NeuroImage, 62(2), 774–781. doi: 10.1016/j.neuroimage.2012.01.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frey S, & Petrides M (2002). Orbitofrontal Cortex and Memory Formation. Neuron, 36(1), 171–176. doi: 10.1016/S0896-6273(02)00901-7 [DOI] [PubMed] [Google Scholar]
- Friston KJ, Buechel C, Fink GR, Morris J, Rolls E, & Dolan RJ (1997). Psychophysiological and Modulatory Interactions in Neuroimaging. NeuroImage, 6(3), 218–229. doi: 10.1006/nimg.1997.0291 [DOI] [PubMed] [Google Scholar]
- Gabard-Durnam LJ, Gee DG, Goff B, Flannery J, Telzer E, Humphreys KL, … Tottenham N (2016). Stimulus-Elicited Connectivity Influences Resting-State Connectivity Years Later in Human Development: A Prospective Study. The Journal of Neuroscience, 36(17), 4771–4784. doi: 10.1523/jneurosci.0598-16.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geng F, Canada K, & Riggins T (2018). Age- and performance-related differences in encoding during early childhood: insights from event-related potentials. Memory, 26(4), 451–461. doi: 10.1080/09658211.2017.1366526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghetti S, & Bunge SA (2012). Neural changes underlying the development of episodic memory during middle childhood. Developmental Cognitive Neuroscience, 2(4), 381–395. doi: 10.1016/j.dcn.2012.05.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghetti S, DeMaster DM, Yonelinas AP, & Bunge SA (2010). Developmental differences in medial temporal lobe function during memory encoding. The Journal of Neuroscience, 30(28), 9548–9556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson RL, Hoffman P, Pobric G, & Lambon Ralph MA (2016). The Semantic Network at Work and Rest: Differential Connectivity of Anterior Temporal Lobe Subregions. The Journal of Neuroscience, 36(5), 1490–1501. doi: 10.1523/jneurosci.2999-15.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson MH (2001). Functional brain development in humans. [Review Article]. Nature Reviews Neuroscience, 2, 475. doi: 10.1038/3508150910.1038/35081509https://www.nature.com/articles/35081509#supplementary-informationhttps://www.nature.com/articles/35081509#supplementary-information [DOI] [PubMed] [Google Scholar]
- Kim H (2011). Neural activity that predicts subsequent memory and forgetting: A meta-analysis of 74 fMRI studies. NeuroImage, 54(3), 2446–2461. doi: 10.1016/j.neuroimage.2010.09.045 [DOI] [PubMed] [Google Scholar]
- Landis JR, & Koch GG (1977). The Measurement of Observer Agreement for Categorical Data. Biometrics, 33(1), 159–174. doi: 10.2307/2529310 [DOI] [PubMed] [Google Scholar]
- McKeown MJ, Jung T-P, Makeig S, Brown G, Kindermann SS, Lee T-W, & Sejnowski TJ (1998). Spatially independent activity patterns in functional MRI data during the Stroop color-naming task. Proceedings of the National Academy of Sciences, 95(3), 803–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menon V, Boyett-Anderson JM, & Reiss AL (2005). Maturation of medial temporal lobe response and connectivity during memory encoding. Cognitive Brain Research, 25(1), 379–385. doi: 10.1016/j.cogbrainres.2005.07.007 [DOI] [PubMed] [Google Scholar]
- Miyashita Y (1993). Inferior Temporal Cortex: Where Visual Perception Meets Memory. Annual Review of Neuroscience, 16(1), 245–263. doi: 10.1146/annurev.ne.16.030193.001333 [DOI] [PubMed] [Google Scholar]
- Moscovitch M, Cabeza R, Winocur G, & Nadel L (2016). Episodic Memory and Beyond: The Hippocampus and Neocortex in Transformation. Annual Review of Psychology, 67(1), 105–134. doi: 10.1146/annurev-psych-113011-143733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ofen N (2012). The development of neural correlates for memory formation. Neuroscience & Biobehavioral Reviews, 36(7), 1708–1717. doi: 10.1016/j.neubiorev.2012.02.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ofen N, Kao Y-C, Sokol-Hessner P, Kim H, Whitfield-Gabrieli S, & Gabrieli JDE (2007). Development of the declarative memory system in the human brain. [10.1038/nn1950]. Nature Neuroscience, 10(9), 1198–1205. doi: http://www.nature.com/neuro/journal/v10/n9/suppinfo/nn1950_S1.html [DOI] [PubMed] [Google Scholar]
- Otten LJ, Henson RNA, & Rugg MD (2002). State-related and item-related neural correlates of successful memory encoding. [Article]. Nature Neuroscience, 5, 1339. doi: 10.1038/nn967 [DOI] [PubMed] [Google Scholar]
- Paz-Alonso PM, Gallego P, & Ghetti S (2013). Age differences in hippocampus-cortex connectivity during true and false memory retrieval. Journal of the International Neuropsychological Society, 19(10), 1031–1041. doi: 10.1017/S1355617713001069 [DOI] [PubMed] [Google Scholar]
- Poppenk J, Evensmoen HR, Moscovitch M, & Nadel L (2013). Long-axis specialization of the human hippocampus. Trends in Cognitive Sciences, 17(5), 230–240. doi: 10.1016/j.tics.2013.03.005 [DOI] [PubMed] [Google Scholar]
- Pyka M, Beckmann CF, Schöning S, Hauke S, Heider D, Kugel H, … Konrad C (2009). Impact of Working Memory Load on fMRI Resting State Pattern in Subsequent Resting Phases. PLoS ONE, 4(9), e7198. doi: 10.1371/journal.pone.0007198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramus SJ, Davis JB, Donahue RJ, Discenza CB, & Waite AA (2007). Interactions between the Orbitofrontal Cortex and the Hippocampal Memory System during the Storage of Long-Term Memory. Annals of the New York Academy of Sciences, 1121(1), 216–231. doi: doi:10.1196/annals.1401.038 [DOI] [PubMed] [Google Scholar]
- Riggins T (2014). Longitudinal investigation of source memory reveals different developmental trajectories for item memory and binding. Developmental Psychology, 50(2), 449–459. doi: 10.1037/a0033622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riggins T, Geng F, Blankenship SL, & Redcay E (2016). Hippocampal functional connectivity and episodic memory in early childhood. Developmental Cognitive Neuroscience, 19, 58–69. doi: 10.1016/j.dcn.2016.02.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riggins T, Geng F, Botdorf M, Canada K, Cox L, & Hancock GR (2018). Protracted hippocampal development is associated with age-related improvements in memory during early childhood. NeuroImage, 174, 127–137. doi: 10.1016/j.neuroimage.2018.03.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riggins T, & Rollins L (2015). Developmental differences in memory during early childhood: Insights from event-related potentials. Child Development, 86(3), 889–902. doi: 10.1111/cdev.12351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizzolatti G, & Matelli M (2003). Two different streams form the dorsal visual system: anatomy and functions. Experimental Brain Research, 153(2), 146–157. doi: 10.1007/s00221-003-1588-0 [DOI] [PubMed] [Google Scholar]
- Rolls ET (2004). The functions of the orbitofrontal cortex. Brain and Cognition, 55(1), 11–29. doi: 10.1016/S0278-2626(03)00277-X [DOI] [PubMed] [Google Scholar]
- Sastre M, Wendelken C, Lee JK, Bunge SA, & Ghetti S (2016). Age- and performance-related differences in hippocampal contributions to episodic retrieval. Developmental Cognitive Neuroscience, 19, 42–50. doi: 10.1016/j.dcn.2016.01.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlichting ML, & Preston AR (2016). Hippocampal–medial prefrontal circuit supports memory updating during learning and post-encoding rest. Neurobiology of Learning and Memory, 134, 91–106. doi: 10.1016/j.nlm.2015.11.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider W (2010). Memory Development in Childhood. In Goswami U (Ed.), The Wiley-Blackwell Handbook of Childhood Cognitive Development [Google Scholar]
- Sluzenski J, Newcombe NS, & Kovacs SL (2006). Binding, relational memory, and recall of naturalistic events: A developmental perspective. Journal of Experimental Psychology: Learning, Memory, and Cognition, 32(1), 89–100. doi: 10.1037/0278-7393.32.1.89 [DOI] [PubMed] [Google Scholar]
- Smith SM, Fox PT, Miller KL, Glahn DC, Fox PM, Mackay CE, … Beckmann, C. F. (2009). Correspondence of the brain’s functional architecture during activation and rest. Proceedings of the National Academy of Sciences, 106(31), 13040–13045. doi: 10.1073/pnas.0905267106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang L, Shafer AT, & Ofen N (2017). Prefrontal Cortex Contributions to the Development of Memory Formation. Cerebral Cortex, 1–14. doi: 10.1093/cercor/bhx200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tillman RM, Stockbridge MD, Nacewicz BM, Torrisi S, Fox AS, Smith JF, & Shackman AJ (2018). Intrinsic functional connectivity of the central extended amygdala. Human Brain Mapping, 39(3), 1291–1312. doi: 10.1002/hbm.23917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tulving E (2002). Episodic Memory: From Mind to Brain. Annual Review of Psychology, 53(1), 1–25. doi: 10.1146/annurev.psych.53.100901.135114 [DOI] [PubMed] [Google Scholar]
- Vanderwal T, Kelly C, Eilbott J, Mayes LC, & Castellanos FX (2015). Inscapes: A movie paradigm to improve compliance in functional magnetic resonance imaging. NeuroImage, 122, 222–232. doi: 10.1016/j.neuroimage.2015.07.069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent JL, Snyder AZ, Fox MD, Shannon BJ, Andrews JR, Raichle ME, & Buckner RL (2006). Coherent Spontaneous Activity Identifies a Hippocampal-Parietal Memory Network. Journal of Neurophysiology, 96(6), 3517–3531. doi: 10.1152/jn.00048.2006 [DOI] [PubMed] [Google Scholar]
- Wang L, LaViolette P, O’Keefe K, Putcha D, Bakkour A, Van Dijk KRA, … Sperling RA (2010). Intrinsic connectivity between the hippocampus and posteromedial cortex predicts memory performance in cognitively intact older individuals. NeuroImage, 51(2), 910–917. doi: 10.1016/j.neuroimage.2010.02.046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Negreira A, LaViolette P, Bakkour A, Sperling RA, & Dickerson BC (2010). Intrinsic interhemispheric hippocampal functional connectivity predicts individual differences in memory performance ability. Hippocampus, 20(3), 345–351. doi: doi: 10.1002/hipo.20771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue G (2018). The Neural Representations Underlying Human Episodic Memory. Trends in Cognitive Sciences. doi: 10.1016/j.tics.2018.03.004 [DOI] [PubMed] [Google Scholar]
- Yan C-G, Wang X-D, Zuo X-N, & Zang Y-F (2016). DPABI: Data Processing & Analysis for (Resting-State) Brain Imaging. [journal article]. Neuroinformatics, 14(3), 339–351. doi: 10.1007/s12021-016-9299-4 [DOI] [PubMed] [Google Scholar]
- Yushkevich PA, Pluta JB, Wang H, Xie L, Ding S-L, Gertje EC, … Wolk DA (2015). Automated volumetry and regional thickness analysis of hippocampal subfields and medial temporal cortical structures in mild cognitive impairment. Human Brain Mapping, 36(1), 258–287. doi: 10.1002/hbm.22627 [DOI] [PMC free article] [PubMed] [Google Scholar]