Abstract
Background
Scientists use biomarkers to evaluate metal exposures. One biomarker, toenails, is easily obtained and minimally invasive, but less commonly used as a biomarker of exposure. Their utility will depend on understanding characteristics of their variation in a population over time. The objective of our study is to describe the correlation of toenail metal levels many years apart among participants in the VA Normative Aging Study (NAS).
Methods
Toenail clippings from 825 participants of the NAS from year 1992 to 2014 were analyzed for lead (Pb), Arsenic (As), Cadmium (Cd), Manganese (Mn), and Mercury (Hg). We utilized linear mixed models to assess correlation between toenail metal concentrations in multiple toenail samples from the same subject collected years apart and identified the optimal covariance pattern by likelihood ratio tests and Akaike’s information criterion (AIC). Correlations among different metals were described using Spearman correlations.
Results
The average number of times toenail samples were collected from each subject ranged from 1.63 (Hg) to 2.04 (As). The average number of years between toenails collected per subject ranged from 4.73 (SD=2.44) (Mn) to 5.35 (SD=2.69) (Hg). Metal concentrations had slightly different correlation patterns over time, although for all metals correlations decreased with increasing time between samples. Estimated correlations over a 3-year span were highest for toenail Pb (0.68) and Hg (0.67), while As, Cd, and Mn had lower correlations of 0.49, 0.44, and 0.47, respectively. Even across a six-year span, the lowest correlation was 0.35 (Cd).
Conclusion
Our results suggest that Pb, As, Cd, Mn, and Hg levels from toenail clippings can reasonably reflect exposures over several years in elderly men in the NAS. Even across six years, toenail metal levels were generally well correlated among NAS participants. As such, they may be useful as biomarkers of exposure in epidemiological studies of similar populations.
Introduction
Researchers have utilized human blood, bone, hair, and urine to assess people’s exposures to contaminants from the environment. One biomarker of exposure that is less frequently used but could potentially be useful in epidemiology studies is toenails. Toenails are easily obtained, minimally invasive, and more easily stored than urine and blood making them potentially advantageous in many studies. In addition, toenails from people wearing shoes are likely to have less external contamination, or have less contamination from hygiene products such as selenium containing shampoos, than hair (1, 2). Disadvantages of utilizing other biomarkers such as urine and blood include the requirement of sterile collection containers and specific training (e.g., phlebotomy). In addition, blood metals may only reflect recent exposure and hence are not good biomarkers for longer-term exposures. Toenails likely capture a somewhat longer exposure window.
Some studies have examined the association between external exposures and toenail metal concentrations. In general population samples, selenium in toenails has been found to reflect selenium intake from dietary supplements and meals (3–6). Other studies evaluating arsenic in toenails show positive correlation with drinking water and soil arsenic concentrations (7–9). Toenails grow at a rate of roughly 1.00–1.50mm/month (0.05 mm/day), so the direct exposure window estimated from a sample of clippings from all ten toenails covers several weeks to months about 6–12 months prior to clipping (4, 1016). Based on such findings, toenail metal concentrations from samples collected at one time point have been used to evaluate the relationship between exposures to metals and many health outcomes including blood pressure (17) and cancer (18, 19).
Despite prior work using toenails as biomarkers of exposure, few studies have examined the correlation of these measures over time. One study found wide variability in Pb concentrations in toenail samples collected 4 months apart (20). A study evaluating trace elements in toenails among women in the U.S. found a correlation between two measurements of As and Hg taken six-years apart to be 0.54 and 0.56, respectively (1). Other metals like Pb, Cd, or Mn were not considered. Hinners et al. (21) found that toenails collected at three different occasions over the span of one year had Hg correlations ranging from 0.75 (1st and 3rd visits, range 266 to 350 days apart) to 0.92 (1st and 2nd visits, range 100 to 189 days apart). However, few studies evaluating metal concentrations in toenails have multiple toenail samples per subject collected over many years. Thus, there is still little data on how toenail metal correlations change over many years. In addition, how well one toenail sample reflects exposures outside the 6–12 months prior to clipping is not well understood.
Understanding how metal levels in toenails change over many years for an individual in the general population is important to know to understand the time-period of exposure reflected by measurement in a single toenail sample. This is critical when using toenail measurements as exposure biomarkers in epidemiological studies, particularly if samples are only available from a single time point. Our study utilizes toenail data from an ongoing longitudinal study, the VA Normative Aging Study (NAS), to understand how toenail metal concentrations change over time for an individual. We utilized multiple toenail samples per participant collected over many years to examine the correlations of metals in toenails over time.
Methods
Study Population
The NAS is an ongoing longitudinal study of men in the greater Boston metropolitan area with no known chronic medical conditions at the time of recruitment (22). Participants were World War II and Korean War veterans and non-veterans living the Boston area. The NAS started in 1961 and enrolled 2280 healthy men who were evaluated at clinical visits every 3–5 years. Between 1992 and 2014, 825 of 1286 still active NAS participants provided toenail clippings at several different clinical visits (maximum of 6 visits with toenail clippings per person among the 825 men). If possible, toenails were collected from all toes and placed in small paper envelopes. Samples were stored in locked facilities at room temperature. All participants gave written informed consent. The Institutional Review Boards of the Brigham and Women’s Hospital, the Harvard T.H. Chan School of Public Health, and the Boston VA Medical Center approved this research.
Metals Analysis
We analyzed lead (Pb), arsenic (As), cadmium (Cd), manganese (Mn), and mercury (Hg) in the toenails. Toenail clippings from all ten toes were collected and pre-cleaned by sonicating for 15 minutes in approximately 10 milliliters (mL) of 1% Triton X-100 solution to remove extraneous contaminants. The samples were rinsed with distilled deionized water and dried at 60°C for 24 hours in a drying oven. If the weight of the whole toenail sample was greater than 0.1 grams (g), a portion of the toenail sample was used for mercury analysis. The rest of the sample was dissolved in HNO3 acid for 48 hours and diluted to 5 mL with deionized water. Samples that weighed less than 0.1 g were acid-digested for Pb, As, Cd, and Mn only.
Lead, arsenic, cadmium, and manganese.
Samples dissolved in HNO3 acid were analyzed by inductively coupled plasma-mass spectrometry (ICP-MS). Analysis of the most recent 300 toenail samples (years range 1998 to 2014) utilized the Agilent 8800 ICP-MS Triple Quad (Agilent technologies, Inc., Delaware, USA). The other samples were analyzed using Perkin Elmer Elan DRC II ICP-MS (PerkinElmer, Inc., Connecticut, USA). We used the same sample preparation method for all samples presented in this paper. Machines used to analyze samples were checked for comparability and accuracy by comparing the results of measuring the certified reference materials in one machine against the other. We found no adjustments were needed for between instrument variability.
For Pb, Cd, Mn, and As analysis, quality control (QC) measures included analysis of the National Institute of Standard and Technology Standard Reference Material 1643e (trace elements in water, Gaithersburg, MD) and a 1.0 nanogram per milliliter (ng/mL) mixed element (Pb, Cd, Mn, As) standard solution for the initial calibration verification standards, continuous calibration standards, and procedural blanks. Certified reference material GBW 07601 (human hair; Institute of Geophysical and Geochemical Exploration, Langfang, Hebei Province, People’s Republic of China) was used as the QC sample. We used a large preparation of GBW 07601 (2.0 grams per liter (g/L)) and a preparation of pooled toenail sample to monitor inter-day and intra-day variations. Certified reference material NIES CRM-13 (Analysis of National Institute for Environmental Studies certified reference material 13 (human hair; Ibaraki, Japan)) was used as the QC standard.
Recovery of the QC from the in-house preparation of the solution of pooled toenail sample digested in acid was 90%−110% with 95% precision for the four metals. Recovery of NIES CRM-13 was 90–110% for Pb, As, and Cd. Recovery of GBW 07601 was 99% for Mn. The between-assay coefficient of variation between days was 2.94% for the four metals.
Limit of detection (LOD) for the QC blanks with analytical solution for the four metals (micrograms per milliliter (µg/mL)): Pb 0.00001, As 0.00001, Cd 0.000001, Mn 0.00002. The LOD for the sample itself varied according to sample weight, and equaled the LOD for the analytical solution multiplied by the dilution factor. Since sample weight varied from 0.009 g to 0.760 g, the LOD ranged from 0.000001 µg/g (Cd) to 0.00253 µg/g (Mn). The average LOD among the four metals was 0.000126 µg/g.
Mercury.
Mercury analysis occurred only if there was sufficient sample left after analyzing for the other metals. Mercury assays utilized the Direct Mercury Analyzer 80 (Milestone Inc., Monroe, CT, USA), and samples were analyzed using a matrix-matched calibration curve derived from different weights of certified reference material GBW 07601. Recovery of the NIES CRM-13 was 99%. Quality control included daily calibration verification of high and low ends of the calibration curve, procedural blank, and analysis of NIES CRM-13. Mercury recovery was 90–110%, with >90% precision. The average (lowest, highest) LOD for Hg was 0.00607 µg/g (0.00171, 0.0355 µg/g). Similar to the previous metals, the range of LOD was due to different sample weights.
Blood lead.
Blood samples collected from 1992 to 2008 were analyzed for lead. Whole blood samples were collected in lead-free tubes containing edentate calcium disodium (EDTA) and analyzed at ESA Laboratories, Inc., Chelmsford, Massachusetts. After digestion with nitric acid and centrifuge, the laboratory analyzed the supernatant with Zeeman background-corrected graphite furnace atomic absorption, which was calibrated with national Bureau of Standards blood lead standards materials. All glassware were soaked overnight in 20% nitric acid and rinsed with distilled water to prevent lead contamination.
Diet
Dietary data, including supplement use, were obtained via a semi-quantitative food frequency questionnaire administered at each study visit (23). Manganese supplement intake was indicated by the subject on the questionnaire (answering “Manganese” to the question “Are there other supplements that you take on a regular basis?”) and was dichotomized (yes/no) in the analysis. Consumption of whole grains (combining wheat, rice, oats, and corn) on the questionnaire was grams consumed per day at time of survey and dichotomized into >0 grams and 0 grams in the analysis. Consumption of canned tuna in the food frequency questionnaire was categorized into eating zero servings, 1–3 servings/month, 1/week, 2–4/week, 5–6/week, 1/day, 2–3/day, 4–5/day, and 6+/day. Tuna consumption in our analysis was dichotomized into ≤ 1/week and > 1/week.
Statistical Methods
Because toenail metal concentrations were right skewed, we log(10)-transformed them for analyses. For the small number of samples that had levels below the limit of detection (LOD) (e.g., Pb, As, and Mn levels) we substituted the LOD for that measurement divided by the square root of two. There were no toenail Hg measurements below the LOD, while toenail Pb had one measurement below the LOD, As had two, and Mn had one. We took the same substitution approach with Cd despite a larger number of Cd values below the LOD (106, 5.8%). For one of these the LOD was missing so we substituted the lowest positive toenail Cd measurement in the data set as the LOD. Toenail Cd levels had many more below LOD values than other metals due to many more very low Cd levels measured in the toenails.
We evaluated different methods for handling the 106 negative toenail Cd measurements. These included adding the absolute value of the most negative measurement to all toenail Cd measurements (Kurtosis=86.824) or adding the absolute value of the most negative measurement plus one (Kurtosis=112.665). Both of these methods resulted in log toenail Cd distributions that were less normally distributed compared to log values of the LOD divided by the square root of 2 substitution method (Kurtosis=2.880). Other transformations such as taking the square, square root, cube, cube root, and the inverse of these for toenail Cd values also resulted in inferior normal distributions.
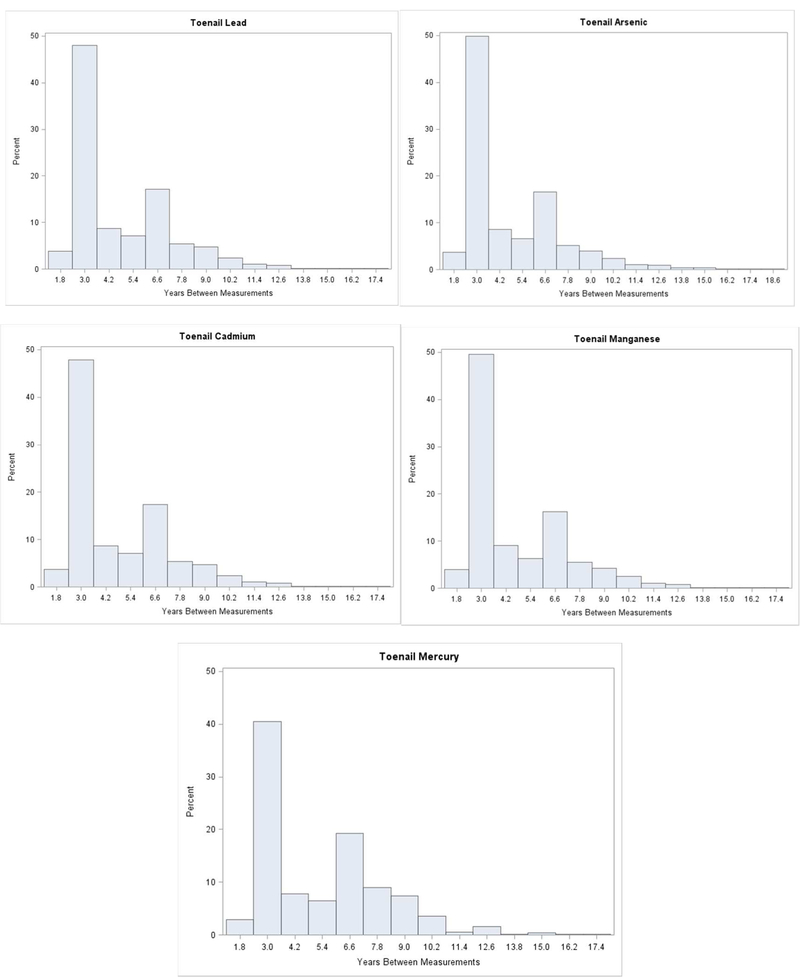
We applied linear mixed models to assess correlation between metal concentrations across multiple toenail samples per subject collected years apart. Because NAS study visits are not always equally spaced, and because toenails are not always collected, the intervals between individuals’ toenail samples vary. The distribution of years between repeated toenail samples from the same subject is shown in Figure 1. The linear mixed model statistical approach considers all the pairwise correlations between the many toenail samples from all NAS subjects, and the varying time intervals between them, and determines the optimal covariance pattern to describe the overall function of change in correlation over time for each metal separately; either using likelihood ratio tests for nested covariance pattern models or Akaike’s information criterion (AIC). A hybrid covariance structure combining an exponential and a compound symmetry structure—describing a decreasing correlation among toenails collected further apart superimposed on a baseline non-zero correlation, as previously described by Fitzmaurice (24), provided the best overall fit for each metal. This hybrid covariance structure is then used to predict the correlation between toenail concentrations of a given metal at any time interval regardless of the actual interval between two toenail samples for any individual. In addition, we applied Spearman correlations to evaluate correlation between metal levels at the first visit with donated toenails (e.g., correlation of toenail Pb and As).
Figure 1.
Distribution of years between measurements for toenail metals among NAS participants, 1992 to 2014
For variables that are strong predictors of some of the metals, we considered correlations between subjects’ multiple toenail samples separately among those who never reported the strong predictor and those who did at one or more study visits. In determining the correlation of toenail metals in multiple samples among subjects who reported a strong predictor at least once we utilized all that subject’s visits with toenail data. The strong predictors considered were use of Mn supplements, consuming whole grains, and eating canned tuna more than once per week. Student’s t-test were used to compare difference in means. Significance was defined as an alpha level of <0.05.
Code availability
We utilized SAS software (Version 9.4, SAS Institute Inc., Cary, North Carolina) for data analysis. Code for analyses is available via contacting the corresponding author.
Results
Demographics
At the first visit where toenail clippings were donated, participants were on average 70 years old, almost all white, and just under 4 out of 5 were overweight or obese (Table 1). Nearly all subjects were never or former smokers, with 95% of smokers quitting by 1990. About 9 out of 10 of those who reported consumption of canned tuna did so up to once per week. Clinical visits were fewest during the winter.
Table 1.
Characteristics of participants at their first visit with donated toenails, 1992 to 2014.
| Characteristic | n | Mean | S.D |
|---|---|---|---|
| Age (years) | 825 | 70.7 | ± 7.0 |
| Smoking (pack-years) | 824 | 21.6 | ± 26.5 |
| Years of education | 803 | 14.9 | ± 3.0 |
| Alcohol intake (g/day) | 112 | 11.7 | ± 15.9 |
| Year of visit | 825 | 1998.6 | ± 3.9 |
| Manganese from food (mg) | 732 | 4.5 | ± 2.4 |
| Whole grain amount in diet (g) | 347 | 26.0 | ± 21.5 |
| Blood Lead (µg/dL) | 759 | 4.5 | ± 2.6 |
|
BMI (kg/m2) |
n |
% |
|
| <25 | 174 | 21.1 | |
| 25–29 | 439 | 53.2 | |
| ≥30 | 212 | 25.7 | |
| Smoking Status | |||
| Never Smoker | 255 | 31.0 | |
| Former Smoker | 530 | 64.4 | |
| Current Smoker | 38 | 4.6 | |
| Race/ethnicity | |||
| Non-Hispanic white | 806 | 97.9 | |
| Non-Hispanic black | 12 | 1.5 | |
| Hispanic white | 4 | 0.5 | |
| Hispanic black | 1 | 0.1 | |
| Season of first clinical visit | |||
| Spring | 209 | 25.4 | |
| Summer | 233 | 28.3 | |
| Fall | 242 | 29.4 | |
| Winter | 139 | 16.9 | |
| Canned tuna consumption | |||
| never,<1/month | 182 | 23.7 | |
| 1–3/month | 277 | 36.1 | |
| 1/week | 215 | 28.0 | |
| >1/week | 93 | 12.1 | |
Metal Measurement Occasions
Individual participants provided toenail clippings on up to six separate study visits. Each subject on average had about two samples analyzed for each toenail metal (Table 2). The distribution of years between toenail samples (Figure 1) had an average per subject that ranged from 4.73 (SD=2.44) years for Mn to 5.35 (SD=2.69) for Hg (Table 2). Most toenail samples had Pb, As, Cd, and Mn measured although, for a small few, not all four of those metals were measured leading to slight differences in years between measurements for the different metals. The average years between the first and last sample per subject ranged from 8.21 (SD=4.15) for Hg to 10.08 (SD=4.82) for Pb. Toenail Hg had a more pronounced difference than the other four metals because mercury analysis occurred only if there was sufficient sample left after analyzing for the other metals. Around 46% of measurements were taken roughly three years apart and about 18% taken roughly 6.5 years apart corresponding to typical intervals between NAS study visits. The distribution of years between measurements among the five toenail metals were similar except for Hg (Figure 1), which showed the frequency of years between measurements to occur less around 3 and more around 6.5 years compared with the other metals.
Table 2.
Toenail metal measurements among NAS participants, 1992 to 2014
| People | # of samples | Mean # of samples per subject | Mean Years Btw samples | S.D | Median Years btw. Samples | Mean years btw. 1st and last sample | S.D | Median years btw. 1st and last sample | |
|---|---|---|---|---|---|---|---|---|---|
| Lead | 790 | 1818 | 2.03 | 4.81 | 2.45 | 3.48 | 10.08 | 4.82 | 10.41 |
| Arsenic | 804 | 1866 | 2.04 | 4.79 | 2.57 | 3.34 | 9.96 | 4.81 | 10.43 |
| Cadmium | 789 | 1812 | 2.03 | 4.82 | 2.46 | 3.49 | 10.06 | 4.79 | 10.41 |
| Manganese | 766 | 1757 | 2.02 | 4.73 | 2.44 | 3.35 | 9.82 | 4.63 | 10.46 |
| Mercury | 711 | 1266 | 1.63 | 5.35 | 2.69 | 4.20 | 8.21 | 4.15 | 8.46 |
Metal Concentrations
The correlations between the toenail metal concentrations at the first collection are shown in Supplemental Table 2 along with correlations with blood Pb. At the first sample collection, toenail Pb had the widest range of concentration levels (min 0.007, max 32.52 µg/g) and toenail As had the narrowest range (min 0.0004, max 1.70 µg/g). Toenail Pb had the highest interquartile range (IQR) of concentration levels while toenail Cd had the lowest (Table 3).
Table 3.
Number of participants (n), median, and inter-quartile range (IQR) of metal concentrations at first donated toenail sample.
| Lead | Arsenic | Cadmium | Manganese | Mercury | |
|---|---|---|---|---|---|
| n | 790 | 804 | 789 | 766 | 711 |
| Median, (µg/g) | 0.323 | 0.071 | 0.018 | 0.270 | 0.252 |
| IQR | 0.541 | 0.063 | 0.033 | 0.398 | 0.339 |
Participants reporting consumption of Mn supplements had a higher first donated sample mean toenail Mn level than those not taking supplements (p-value=0.04) (Supplemental, Table 1). A significant difference was found between those reporting consumption of whole grains and those not. Whole grain consumers had a higher first donated sample mean toenail Mn level (p-value=0.0002) and higher mean toenail As level (p-value=0.0009) than non-consumers. In addition, those that reported consuming canned tuna more than once per week had a significantly higher first donated sample mean toenail Hg level (pvalue<0.0001) then those reporting less tuna consumption.
Over 95% of toenail metal measurements came from former or never smokers (Supplemental Table 4 to 8). By 1991, over 95% of NAS participants who smoked had quit, leaving 30 to 35 current smokers (depending on metal) with at least one visit with donated toenails (toenail collection began in 1992). Among these, only 18 had more than one visit. Therefore, we did not describe correlations of subjects’ toenail metals stratified by smoking status, but present median levels of metals by order of donated toenail samples (Supplemental Table 4 to 8). At the first donated sample, former smokers had slightly higher median metal levels than never smokers, except for Cd, which was the same. At first donated sample, current smokers had the lowest median metal levels for Pb, Cd, and Hg but had the highest median level for Mn. The geometric mean (GM) of blood Pb levels at the first visit was 3.9 ug/dL, [95% CI 3.7–4.0] (calculated from Table 1 blood Pb data).
Correlations of Toenail Metal Measurements over Time
The estimated within person correlation patterns of toenail metal concentrations over time from the hybrid covariance models were slightly different for the different metals, although for all metals correlations decreased with increasing time between samples (Figure 2). Correlations over time were virtually the same for toenail As and Mn. Toenail Cd correlations started just slightly lower than As and Mn at the 1-year interval, but the correlation dropped off slightly more steeply over time. The decline in correlation over time for Hg was similar to Cd, but started at a much higher correlation at the 1-year interval. Toenail Pb had a similar correlation at the 3-year interval as Hg, but the correlation dropped off more steeply over time. Interestingly, blood Pb (3463 samples, median 4.00, IQR 3.00, min 0.29, max 35.00 µg/dL) collected during the same study period had a higher correlation than toenail Pb at the 1-year interval, similar correlation at the 3 year interval, and a lower correlation and steeper drop-off after the 3year interval (Figure 2). When we considered known high levels of exposure to metals—taking supplements with Mn, consuming whole grains (Mn, As), or eating canned tuna more than once per week (Hg)—we found that the correlation of those metals across multiple samples collected over time was higher at shorter intervals, but then decreased to either the same or lower correlation than those not taking supplements, not consuming whole grains or eating canned tuna less frequently at the seven-year interval (Table 4, Figure 3).
Figure 2.
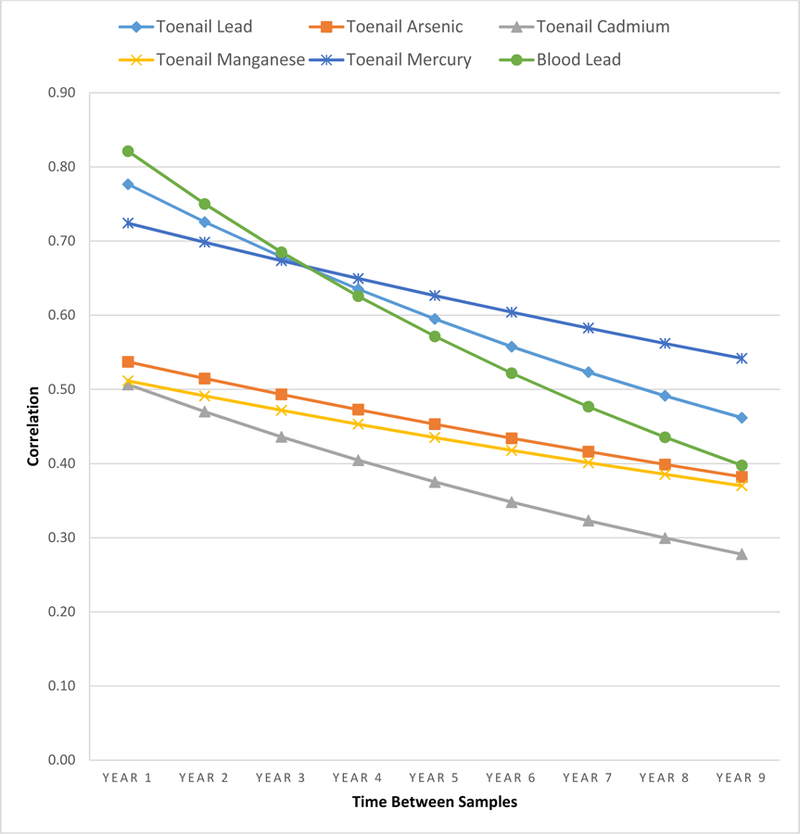
Estimated correlations of toenail metal levels by years between measurement occasions via hybrid covariance structure, 1992 to 2014. (Blood lead shown for comparison)
Table 4.
Estimated correlation of measures of toenail manganese (Mn), arsenic (As), and mercury (Hg) at hypothetical sample collection intervals 1 to 7 years apart stratified by Mn in supplements, consumption of whole grains, and frequency of consumption of canned tuna.
| Correlation | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Toenail Metal | n | Visits | 1 Year Apart | 2 Years | 3 years | 4 years | 5 years | 6 years | 7 years | |
| No Mn supplements | Mn | 565 | 1196 | 0.50 | 0.48 | 0.46 | 0.44 | 0.43 | 0.41 | 0.40 |
| Taking Mn supplements | Mn | 201 | 561 | 0.75 | 0.60 | 0.50 | 0.44 | 0.40 | 0.38 | 0.36 |
| Reported no consumption of whole grains | Mn | 211 | 258 | 0.40 | 0.40 | 0.40 | 0.40 | 0.40 | 0.40 | 0.40 |
| Reported consumption of whole grains | Mn | 555 | 1499 | 0.54 | 0.51 | 0.49 | 0.46 | 0.44 | 0.42 | 0.40 |
| Reported no consumption of whole grains | As | 247 | 316 | 0.60 | 0.49 | 0.46 | 0.45 | 0.45 | 0.45 | 0.45 |
| Reported consumption of whole grains | As | 577 | 1550 | 0.54 | 0.52 | 0.50 | 0.47 | 0.45 | 0.43 | 0.42 |
| Canned tuna consumption 1/week or less | Hg | 525 | 917 | 0.73 | 0.70 | 0.68 | 0.66 | 0.65 | 0.63 | 0.61 |
| Canned tuna consumption >1/week | Hg | 186 | 349 | 0.83 | 0.72 | 0.64 | 0.57 | 0.51 | 0.47 | 0.44 |
n= participants: Having at least one visit with taking supplements, consumption of whole grains, or consuming more than 1 can of tuna per week comprised the “taking Mn supplements”, “reported consumption of whole grains”, or “canned tuna consumption >1/week” groups. Those that never took supplements, consumed whole grains, or consumed more than 1 can of tuna per week comprised the “no mn supplements”, “reported no consumption of whole grains”, or “canned tuna consumption 1/week or less groups”.
Visits= visits with toenail metal
Figure 3.
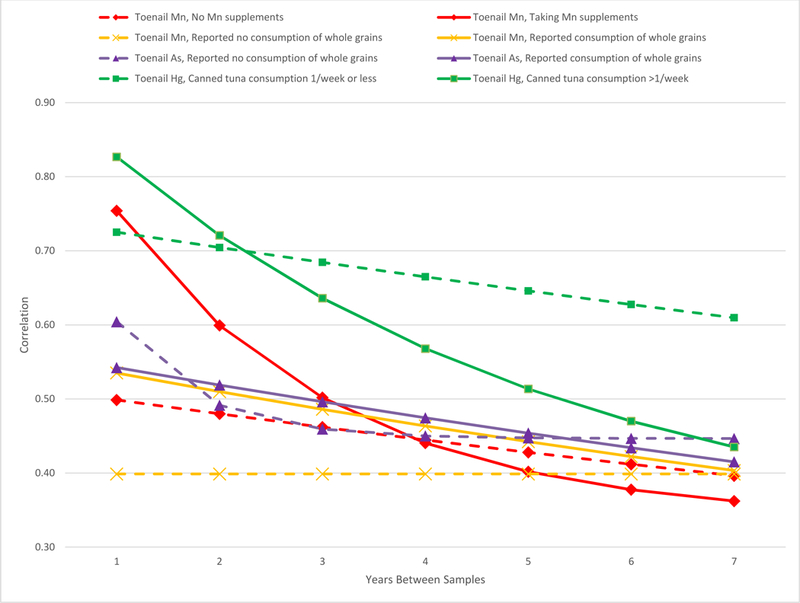
Estimated correlation of repeated measures of toenail manganese (Mn), arsenic (As), and mercury (Hg) taken 1 to 7 years apart stratified by Mn in supplements, consumption of whole grains, and frequency of consumption of canned tuna. (The correlation for measurements taken one year apart was estimated by the hybrid covariance structure since no actual measurements were taken 1-year apart.)
Discussion
The objective of our study was to describe the correlations of metal concentrations among multiple toenail samples per participant collected over time in the NAS. We found the estimated correlation of metals in toenail samples collected three-years apart (the most common duration between visits) ranged from 0.44 (Cd) to 0.68 (Pb) (Figure 2). These correlations steadily decreased with increasing time intervals between samples. Importantly, for toenail Pb and Hg, the correlations remained above 0.5 out to 7 years, while for Mn and Cd, correlations fell to that level after a year, and for As, by 3 years. Hence, toenail Pb and Hg measurements capture longer windows of exposure.
Our study of 825 participants and 1927 total toenail samples found Pb to have the highest within-person correlation across a three-year period. One study of toenails with subjects’ samples collected 4 months apart found wide variability in Pb concentrations (20). A study evaluating trace elements in toenails among women in the U.S. found a correlation between two measurements of As and Hg taken six-years apart to be 0.54 and 0.56, respectively (1), which are similar to the estimated correlations for samples 6 years apart of 0.43 and 0.59 we found for As and Hg, respectively. Hinners et al. (21) found toenails collected at three different occasions over the span of one year had toenail Hg correlations ranging from 0.75 (1st and 3rd visits, range 266 to 350 days apart) to 0.92 (1st and 2nd visits, range 100 to 189 days apart). Our study found a similar toenail Hg correlation (0.72) for a 1-year interval.
We included blood Pb in our study because it is the standard for, and a well-studied biomarker of, exposure to Pb when investigating Pb exposure and health outcomes (25–30). The geometric mean (GM) of blood Pb levels in our study cohort at the first visit (3.9 ug/dL, [95% CI 3.7–4.0], calculated from Table 1 blood Pb data) was similar to the U.S. general population living in cities with ≥ 1 million people from 1988–1991 (GM 3.9 ug/dL [95% CI 3.6–4.3]) (25). There was a significant, but modest positive correlation between blood Pb and toenail Pb levels at first visit with toenail sample (Supplemental, Table 2). Our results found a high correlation across multiple samples per subject for both blood Pb and toenail Pb (Figure 2). However, toenail Pb had higher correlation across multiple samples per subject than blood Pb when samples were taken >3 years apart (Figure 2), which suggests that a single toenail sample may capture long term exposures better than blood. This finding is supported by studies reporting toenail Pb reflecting longer exposure durations than blood Pb (10–14).
Our findings of higher toenail Mn levels among those taking Mn supplements compared to those not taking supplements seems intuitive. A study examining 49 toenail samples from welders found that those with higher dietary intake of Mn did have higher toenail Mn levels (16). Also, a study of welders in Thailand found higher levels of toenail Mn among those with higher levels of blood Mn compared to those with lower blood Mn levels (31). Our results of significantly higher average toenail Mn levels among those reporting consumption of whole grains compared to those not reporting consumption are also consistent with research indicating whole grains as a source of Mn exposure (32, 33). A search in PubMed and Web of Science did not find studies evaluating how whole grain consumption affects toenail Mn concentration correlations over time.
Strong evidence exists for consumption of fish such as tuna and other saltwater fish as a primary source of Hg exposure (34, 35). A study conducted among 579 males from the Health Professionals Follow-up Study found the primary exposure route for Hg, measured via toenails, was consumption of tuna and saltwater fish, with a correlation of 0.31 (p<0.05) between toenail Hg and tuna consumption (36). We found a higher correlation across toenails collected less than 2 years apart among those who consumed more tuna, although the correlation dropped to lower than that of non-consumers over greater intervals (Table 4 and Figure 3).
A dominant, consistent single exposure source should reduce variation in toenail concentrations over time and increase the correlation between toenail samples collected at different times because that source would dominate the contribution to biomarker concentrations even in the face of fluctuations in other lower level exposures. However, if that dominant exposure source does not remain constant over time, then the correlation in biomarker concentrations would likely drop steeply since there would be very large differences in biomarker concentration between samples collected when the dominant source was present vs. absent. The strong predictors of some of our metals that we considered are dietary. Diet is generally found to be relatively stable (37, 38), but dietary habits have been found to be more likely to vary as the interval between assessments lengthens (39, 40). These dietary patterns are consistent with the higher level of correlation between toenail metal concentrations over shorter intervals, but then the same or lower correlations over longer intervals, among those ever reporting the dominant exposure sources we explored compared with those that did not report these sources.
We posit toenail Pb has a greater correlation over longer time intervals than other toenail metals largely because Pb in bone acts as a constant source of endogenous exposure to the blood, and thus eventually to toenails. Bone is a sink for Pb in the body, which can then be released later in life as bone resorption occurs (29, 41). As older men who lived during the time of leaded gasoline use in the US, NAS participants’ greatest “exposure” to Pb after the era of leaded gasoline is likely from their bones and thus would be relatively consistent over the time period of our study.
Toenail Hg showed greater correlation over time compared to As, Cd, and Mn, which could be due to fish consumption. About 9 out of 10 of those who reported consumption of canned tuna in our cohort did so up to once per week. Studies have suggested that nails and toenails are relevant biomarkers of exposure to organic mercury (e.g., methyl-Hg) since it can bind to keratin (42, 43).
Our results suggest that toenails could be utilized in addition to other well-studied biomarkers such as hair, blood, and urine in assessing exposure to metals, specifically Pb, As, Cd, Mn, and Hg. Compared with hair and fingernails, toenails in general are less prone to contamination from external sources such as dirt and soaps among people wearing shoes (1, 2). In addition, toenails are more isolated than blood and urine from other metabolic activities in the body, and generally represent longer exposure periods than blood or urine, although urinary Cd is an exception (1, 26, 44, 45). Collecting toenail clippings is painless, less invasive than drawing blood samples, and more convenient to store (1). Therefore, utilizing toenails may be more convenient and less expensive to collect, store, and transport compared to blood (46).
Limitations to our study include low Cd levels that resulted in almost 6% negative readings. Our statistical approach requires a normal distribution necessitating log-transformation and thus some substitution of the negative values. Setting all negative values to zero would have falsely increased correlations as it would have reduced the variability in those measurements. Because each measurement had a separate LOD, our substitution approach maintained some variability, although our estimates still could have been slightly over-estimated because of this. There are limits to the generalizability of our findings because correlations over time will depend on external exposures in the specific population being examined. To the extent that other populations have different exposure patterns than the NAS, the correlations could be different. However, our findings were similar to other studies when similar intervals were considered (1, 21). Another limitation is our study population only evaluated males residing in the Boston metropolitan area.
Conclusion
Our results suggest that Pb, As, Cd, Mn, and Hg levels from toenail clippings can reasonably reflect exposures over several years in the NAS population—a population of elderly men residing in the Boston metropolitan area. Specifically, toenail metal levels provide good estimates up to 6 years (Cd had lowest correlation of 0.35 at measures 6 years apart). Understanding the decrease in correlation over time for the different metals can be used with statistical approaches to account for error in estimating prior exposures in epidemiologic studies of health outcomes (47).
Supplementary Material
Acknowledgements
We thank all the participants and dedicated staff of the VA Normative Aging Study.
The VA Normative Aging Study is supported by the Cooperative Studies Program/ERIC, Department of Veterans Affairs, and is a component of the Massachusetts Veterans Epidemiology Research and Information Center (MAVERIC). We also thank Anna Kosheleva at the Harvard T.H. Chan School of Public Health for her help as the data manager.
Support for this research was provided by the U.S. Centers for Disease Control and Prevention, National Institute of Occupational Health and Safety, Education and Research Center training grant T42 OH008416 (Alexander Wu), National Institutes of Health, National Institute of Environmental Health Sciences grant ES05257 and P30 00002 (Marc Weisskopf), and National Institutes of Health R01 ES015172 (Chitra Amarasiriwardena, Joel Schwartz) and R01 ES027747 (Joel Schwartz).
Footnotes
Conflict of Interest
The authors declare they have no competing financial interests.
References
- 1.Garland M, Morris JS, Rosner BA, Stampfer MJ, Spate VL, Baskett CJ, et al. Toenail trace element levels as biomarkers: reproducibility over a 6-year period. Cancer Epidemiol Biomarkers Prev 1993;2(5):493–7. [PubMed] [Google Scholar]
- 2.LeBlanc A, Dumas P, Lefebvre L. Trace element content of commercial shampoos: impact on trace element levels in hair. Sci Total Environ 1999;229(1–2):121–4. [DOI] [PubMed] [Google Scholar]
- 3.Hunter, Morris J, Chute C, Kushner E, Colditz G, Stampfer M, et al. Predictors of Selenium Concentration in Human Toenails. American Journal of Epidemiology 1990;132(1):114. [DOI] [PubMed] [Google Scholar]
- 4.Longnecker PM, Stram OD, Taylor RP, Levander AO, Howe AM, Veillon YC, et al. Use of Selenium Concentration in Whole Blood, Serum, Toenails, or Urine as a Surrogate Measure of Selenium Intake. Epidemiology 1996;7(4):384–90. [DOI] [PubMed] [Google Scholar]
- 5.Ovaskainen ML, Virtamo J, Alfthan G, Haukka J, Pietinen P, Taylor PR, et al. Toenail selenium as an indicator of selenium intake among middle-aged men in an area with low soil selenium. The American journal of clinical nutrition 1993;57(5):662. [DOI] [PubMed] [Google Scholar]
- 6.Swanson CA, Longnecker MP, Veillon C, Howe M, Levander OA, Taylor PR, et al. Selenium intake, age, gender, and smoking in relation to indices of selenium status of adults residing in a seleniferous area. American journal of clinical nutrition 1990;52(5):858–62. [DOI] [PubMed] [Google Scholar]
- 7.Hinwood AL, Sim MR, Jolley D, de Klerk N, Bastone EB, Gerostamoulos J, et al. Hair and toenail arsenic concentrations of residents living in areas with high environmental arsenic concentrations. (Environmental Medicine). Environ Health Perspect 2003;111(2):187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Karagas MR, Stukel TA, Morris JS, Tosteson TD, Weiss JE, Spencer SK, et al. Skin Cancer Risk in Relation to Toenail Arsenic Concentrations in a US Population-based Case-Control Study. American Journal of Epidemiology 2001;153(6):559–65. [DOI] [PubMed] [Google Scholar]
- 9.Karagas MR, Tosteson TD, Blum J, Klaue B, Weiss JE, Stannard V, et al. Measurement of Low Levels of Arsenic Exposure: A Comparison of Water and Toenail Concentrations. American Journal of Epidemiology 2000;152(1):84–90. [DOI] [PubMed] [Google Scholar]
- 10.Levit EK, Scher RK. Basic science of the nail unit. In: Freinkel RK, Woodley D, editors. The biology of the skin New York: Parthenon PubGroup; 2001. p. 101–10. [Google Scholar]
- 11.Slotnick MJ, Nriagu JO. Validity of human nails as a biomarker of arsenic and selenium exposure: A review. Environ Res 2006;102(1):125–39. [DOI] [PubMed] [Google Scholar]
- 12.Steven Morris J, Stampfer MJ, Willett W. Dietary selenium in humans toenails as an indicator. Biol Trace Elem Res 1983;5(6):529–37. [DOI] [PubMed] [Google Scholar]
- 13.Weller RB, Hunter HJA, Mann MW. Wiley: Clinical Dermatology, 5th Edition. 5th ed2014 2014. Oxford, United Kingdom. [Google Scholar]
- 14.Grashow R, Zhang J, Fang SC, Weisskopf MG, Christiani DC, Cavallari JM. Toenail metal concentration as a biomarker of occupational welding fume exposure. J Occup Environ Hyg 2014;11(6):397–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sakamoto M, Chan HM, Domingo JL, Oliveira RB, Kawakami S, Murata K. Significance of fingernail and toenail mercury concentrations as biomarkers for prenatal methylmercury exposure in relation to segmental hair mercury concentrations. Environ Res 2015;136:289–94. [DOI] [PubMed] [Google Scholar]
- 16.Laohaudomchok W, Lin X, Herrick RF, Fang SC, Cavallari JM, Christiani DC, et al. Toenail, blood, and urine as biomarkers of manganese exposure. J Occup Environ Med 2011;53(5):506–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mordukhovich I, Wright RO, Hu H, Amarasiriwardena C, Baccarelli A, Litonjua A, et al. Associations of toenail arsenic, cadmium, mercury, manganese, and lead with blood pressure in the normative aging study. Environ Health Perspect 2012;120(1):98–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hunter DJ, Morris JS, Stampfer MJ, Colditz GA, Speizer FE, Willett WC. A prospective study of selenium status and breast cancer risk. JAMA 1990;264(9):1128–31. [PubMed] [Google Scholar]
- 19.Maasland DHE, Schouten LJ, Kremer B, van den Brandt PA. Toenail selenium status and risk of subtypes of head-neck cancer: The Netherlands Cohort Study. Eur J Cancer 2016;60:83–92. [DOI] [PubMed] [Google Scholar]
- 20.Gulson BL. Nails: concern over their use in lead exposure assessment. Science of the Total Environment 1996;177(1):323–7. [Google Scholar]
- 21.Hinners T, Tsuchiya A, Stern A, Burbacher T, Faustman E, Mariën K. Chronologically matched toenail-Hg to hair-Hg ratio: temporal analysis within the Japanese community (U.S.). Environ Health 2012;11:81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bell B, Rose CL, Damon A. The Normative Aging Study: An Interdisciplinary and Longitudinal Study of Health and Aging. The International Journal of Aging and Human Development 1972;3(1):5–17. [Google Scholar]
- 23.Willett W, Sampson L, Stampfer M, Rosner B, Bain C, Witschi J, et al. Reproducibility and Validity of a Semiquantitative Food Frequency Questionnaire. American Journal of Epidemiology 1985;122(1):51–65. [DOI] [PubMed] [Google Scholar]
- 24.Fitzmaurice GM. Applied longitudinal analysis Hoboken, N.J.: Wiley-Interscience; 2004. 2004. xix+506 p. [Google Scholar]
- 25.Pirkle JL, Brody DJ, Gunter EW, Kramer RA, Paschal DC, Flegal KM, et al. The decline in blood lead levels in the United States. The National Health and Nutrition Examination Surveys (NHANES). JAMA 1994;272(4):284–91. [PubMed] [Google Scholar]
- 26.Barbosa F, Tanus-Santos JE, Gerlach RF, Parsons PJ. A critical review of biomarkers used for monitoring human exposure to lead: advantages, limitations, and future needs. Environ Health Perspect 2005;113(12):1669–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bouchard MF, Bellinger DC, Weuve J, Matthews-Bellinger J, Gilman SE, Wright RO, et al. Blood lead levels and major depressive disorder, panic disorder, and generalized anxiety disorder in US young adults. Arch Gen Psychiatry 2009;66(12):1313–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cheng Y, Schwartz J, Sparrow D, Aro A, Weiss ST, Hu H. Bone Lead and Blood Lead Levels in Relation to Baseline Blood Pressure and the Prospective Development of Hypertension The Normative Aging Study. American Journal of Epidemiology 2001;153(2):164–71. [DOI] [PubMed] [Google Scholar]
- 29.Rhodes D, Spiro A, Aro A, Hu H. Relationship of bone and blood lead levels to psychiatric symptoms: the normative aging study. J Occup Environ Med 2003;45(11):1144–51. [DOI] [PubMed] [Google Scholar]
- 30.Schwartz J, Tsaih SW, Korrick S Fau - Schwartz J, Schwartz J Fau - Lee ML, Lee Ml Fau - Amarasiriwardena C, Amarasiriwardena C Fau - Aro A, et al. Lead, blood pressure, and cardiovascular disease in men and women. Environ Health Perspect 1991;91:71–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wongwit W, Kaewkungwal J, Chantachum Y, Visesmanee V. Comparison of biological specimens for manganese determination among highly exposed welders. Southeast Asian J Trop Med Public Health 2004;35(3):764–9. [PubMed] [Google Scholar]
- 32.Centers for Disease Control and Prevention. Manganese - ToxFAQs. Prepared by the Agency for Toxic Substances and Disease Registry. 2012. CAS# 7439-96-5.
- 33.Linus Pauling Institute OSU. Manganese: Sources Linus Pauling Institute. Oregon, USA: 2014. [Google Scholar]
- 34.Centers for Disease Control and Prevention. Mercury - ToxFAQs. Prepared by the Agency for Toxic Substances and Disease Registry. 1999. CAS# 7439-97-6.
- 35.NRC. Toxicological Effects of Methylmercury Washington (DC): National Academies Press (US); 2000. 2000. [PubMed] [Google Scholar]
- 36.Joshi A, Douglass CW, Kim H-D, Joshipura KJ, Park MC, Rimm EB, et al. The relationship between amalgam restorations and mercury levels in male dentists and nondental health professionals. J Public Health Dent 2003;63(1):52–60. [DOI] [PubMed] [Google Scholar]
- 37.Dekker LH, Boer JM, Stricker MD, Busschers WB, Snijder MB, Nicolaou M, et al. Dietary patterns within a population are more reproducible than those of individuals. The Journal of nutrition 2013;143(11):1728–35. [DOI] [PubMed] [Google Scholar]
- 38.Jankovic N, Steppel MT, Kampman E, de Groot LC, Boshuizen HC, Soedamah-Muthu SS, et al. Stability of dietary patterns assessed with reduced rank regression; the Zutphen Elderly Study. Nutrition journal 2014;13:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Movassagh EZ, Baxter-Jones ADG, Kontulainen S, Whiting SJ, Vatanparast H. Tracking Dietary Patterns over 20 Years from Childhood through Adolescence into Young Adulthood: The Saskatchewan Pediatric Bone Mineral Accrual Study. Nutrients 2017;9(9):990 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Weismayer C, Anderson JG, Wolk A. Changes in the stability of dietary patterns in a study of middle-aged Swedish women. The Journal of nutrition 2006;136(6):1582–7. [DOI] [PubMed] [Google Scholar]
- 41.Sharma B, Singh S, Siddiqi NJ. Biomedical Implications of Heavy Metals Induced Imbalances in Redox Systems. BioMed Research International 2014;2014:1–26. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 42.Bjorkman L, Lundekvam BF, Laegreid T, Bertelsen BI, Morild I, Lilleng P, et al. Mercury in human brain, blood, muscle and toenails in relation to exposure: an autopsy study. Environ Health 2007;6:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Keil A, Richardson D. Reassessing the Link between Airborne Arsenic Exposure among Anaconda Copper Smelter Workers and Multiple Causes of Death Using the Parametric g-Formula. Environmental Health Perspectives (Online) 2017;125(4):608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hopps HC. The biologic bases for using hair and nail for analyses of trace elements. Science of the Total Environment 1977;7(1):71–89. [DOI] [PubMed] [Google Scholar]
- 45.Willett W Nutritional Epidemiology: Issues and Challenges. Int J Epidemiol 1987;16(2):312–7. [DOI] [PubMed] [Google Scholar]
- 46.Davis MA, Signes-Pastor AJ, Argos M, Slaughter F, Pendergrast C, Punshon T, et al. Assessment of human dietary exposure to arsenic through rice. Sci Total Environ 2017;586:1237–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rosner B, Spiegelman D, Willett WC. Correction of logistic regression relative risk estimates and confidence intervals for random within-person measurement error. American journal of epidemiology 1992;136(11):1400. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.