Abstract
Signaling through the dual leucine zipper-bearing kinase (DLK) is required for injured neurons to initiate new axonal growth; however, activation of this kinase also leads to neuronal degeneration and death in multiple models of injury and neurodegenerative diseases. This has spurred current consideration of DLK as a candidate therapeutic target, and raises a vital question: in what context is DLK a friend or foe to neurons? Here, we review our current understanding of DLK’s function and mechanisms in regulating both regenerative and degenerative responses to axonal damage and stress in the nervous system.
Introduction
An overarching question is whether mechanisms that are required for the wiring of neuronal circuits during development can be re-utilized to stimulate repair after damage or to restore function after loss in disease. In contrast to development, the capacity to repair mature neuronal circuits following damage, and, in many circumstances, the inability to repair, is linked to the activation of damage response pathways in the nervous system. Injury response signaling mediated by the dual leucine zipper-bearing kinase (DLK) is critical for neurons to initiate new axonal growth in the peripheral nervous system (PNS). However, this same kinase enhances neuronal death and degeneration in a growing number of models for neuronal injury, stress and neurodegenerative diseases. These dichotomous responses, along with other recent observations discussed in this review, can be reconciled into a unified view in which DLK regulates and coordinates stress response signaling in neurons [1].
In particular, DLK signaling appears specifically tuned to stressors that impair or damage axons (Figures 1, 2 and Table 1). These stressors include mechanical transection (Figure 1), which leads to activation of DLK signaling in all neurons and model organisms examined thus far [2–7]. They also include more chronic forms of stress associated with genetic mutations and drugs that hinder the microtubule cytoskeleton and axonal transport within neurons (Table 1 and Figure 2). Since axons often extend over great distances, reaching lengths of over 1000 times the diameter of the neuron’s cell body [8], the integrity of the axon and the ability to transport organelles and proteins within it is a point of vulnerability for neurons. Such impairments within an axon can effectively silence a neuron from communicating with its post-synaptic targets, so it is logical that neurons should have mechanisms to monitor the state of their axon. In this review we will discuss how DLK’s signaling mechanisms and functions appear to be intimately linked to the process of axonal transport.
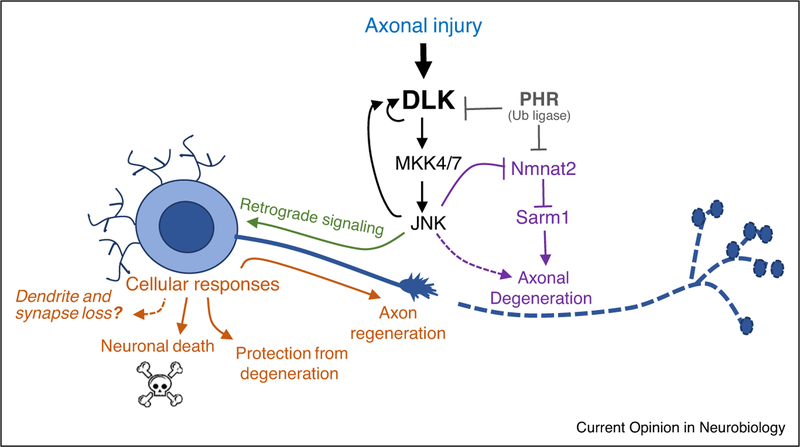
Figure 1.
DLK regulates multiple responses to axonal damage. DLK signaling becomes activated following axonal injury and regulates multiple cellular responses (in orange): neuronal death [4,7], axonal regeneration [2,3,5,6] and/or protection from degeneration [69], depending upon the context (Table 1). Whether DLK promotes loss of dendrites and synaptic inputs is hypothesized based on discussed data [25,29,30,29–31••], but remains to be determined. The distal part of the axon, which becomes removed from the cell body undergoes Wallerian degeneration. This is also influenced by DLK signaling [64,69,103]. In addition, DLK and downstream signaling components crosstalk with other factors that influence axonal degeneration, the NMNAT enzyme and Sarm1 NADylase [18,65,75•,101].
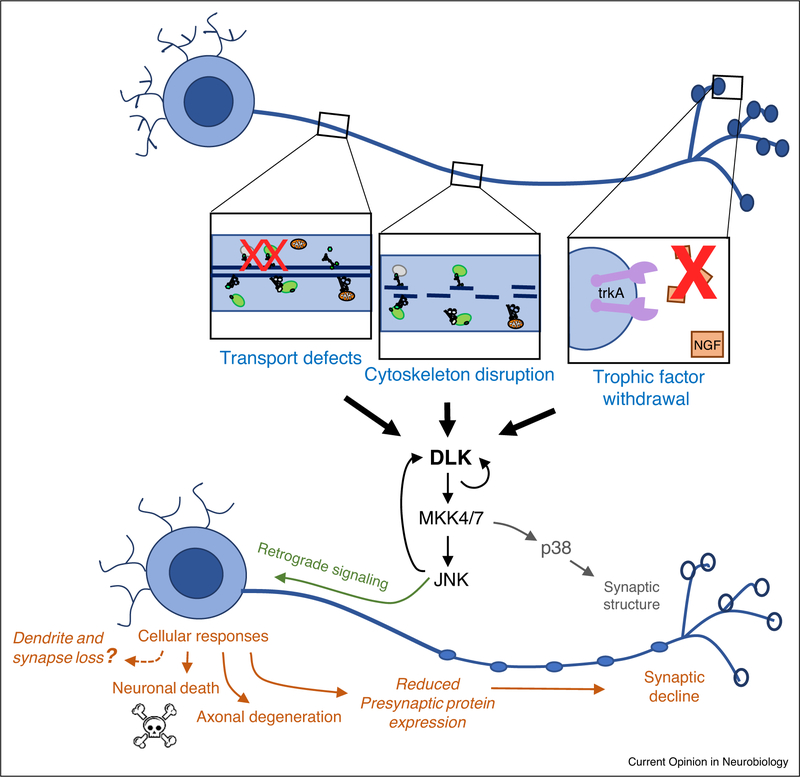
Figure 2.
Examples of axonal stress that lead to activation of DLK. Defects in axonal transport [24••], disruption of cytoskeleton within axons [47–49], and inhibition of trophic factor signaling [14,41,44•] all result in the activation of DLK signaling. Downstream responses (in orange) include reduced expression levels of presynaptic proteins [24••] and yet unknown signals that impair postsynaptic receptor function and synaptic homeostasis mechanisms [27•]. Over time these responses are expected to promote synaptic decline and loss.
Table 1.
Functions ascribed to DLK signaling in different paradigms and model systems
| Function | Context |
|---|---|
| Axonal regeneration in PNS | DLK is required for axonal regeneration following laser axotomy in C. elegans GABA motoneurons [6] and ALM and PLM touch neurons [2,95], and in D. melanogaster larval motoneuron [3] and sensory neurons [35]. Following sciatic nerve injury in mice, DLK is required in motoneurons for reinnervation of motoneuron endplates [5]. DRG neurons deleted for DLK fail to undergo enhanced regeneration stimulated by a conditioning injury [5,104]. |
| Axonal regeneration in CNS | DLK is required for PTEN−/− induced regeneration is the mouse optic nerve [4]. |
| Wallerian degeneration of injured axons | Modest defects in Wallerian degeneration have been observed for DLK mutants in D. melanogaster olfactory neurons [64], cultured embryonic DRGs [64,65], and in the mouse sciatic nerve [64]. Combined knockout of DLK with other components of MAPK signaling leads to a strong inhibition of Wallerian degeneration of RGC axons in the optic nerve [65]). |
| Chemotherapy-induced axonal degeneration | DLK-deficient mouse DRG axons are protected after vincristine exposure [64]. Loss of DLK in taxol treated Drosophila axons prevents degeneration [67]. |
| Resistance to axonal degeneration | DLK activation following a conditioning lesion in larval PNS motoneurons protects axons from degeneration following subsequent injuries [69]. |
| Neuronal remodeling in response to cytoskeletal stress | Growth of C. elegans in the presence of microtubule destabilizing agent colchicine causes changes in the levels of many touch receptor proteins via DLK-1 signaling [50]. Genetic perturbations in microtubules causes synaptic remodeling of C. elegans GABA dorsal D-Type (DD) neurons via DLK-1 signaling [55]. D. melanogaster mutations in alpha-spectrin and ankyrin, which should chronically impair cytoskeleton, cause retraction and loss of presynaptic boutons; genetic manipulations in upstream and downstream components of the DLK signaling pathway modify these phenotypes [49]. |
| Neuronal death | Mouse embryonic DRGs deleted for DLK fail to undergo cell death following trophic factor withdrawal [14,44•]. Mouse retinal ganglion cells (RGCs) deleted for DLK fail to undergo cell death following optic nerve injury [4,7,105] and in a cellular model of stress/glaucoma [7,]. DLK inhibition in adult mice is also protective against cell death in models of excitoxicity[61], subarachnoid hemorrhage [59], and 6-OHDA-induced dopaminergic cell death [60]. |
| Neural degeneration in disease models | In two different mouse models of Alzheimer’s Disease (AD) and a mouse model of ALS, conditional deletion of DLK in the adult nervous system shows neuroprotective phenotypes [31••]. These include reduced loss of axons and NMJ synapses and reduced inflammation in the spinal cord of SOD1G93A mice; reduced memory impairment and dendritic spine loss in PS2APP mice, and reduced neuron loss in TauP301L mice. Neither A-beta nor Tau pathology was affected by DLK knockout, suggesting a downstream role for DLK in promoting degeneration [31••]. In D. melanogaster, heterozygous mutations in DLK/Wnd rescue premature lethality in a TDP-43 overexpression model of ALS, however homozygous mutations enhances lethality in this model [83]. |
| Synaptic decline | DLK activation in both C. elegans and D. melanogaster leads to defects in the structure of presynaptic terminals [19,20]. Electrophysiology recordings at D. melanogaster NMJ synapses indicate that DLK signaling activation in motoneurons induces both presynaptic reductions in synaptic vesicle release and post-synaptic responses to neurotransmitter [24••,27•]. |
| Developmental axonal outgrowth and neuronal migration | DLK-deficient mice show defects in neocortical radial migration and reduced axon tracts in the anterior commissure, internal capsule, and corpus callosum [12]. Dissociated cortical neurons knocked down for DLK have reduced axonal growth [13]. Double mutants of DLK with JNK1 have severe defects in axon formation [16]. |
| Neuroinflammation | Inhibition of DLK leads to reduced microglial responses in a mouse model of neuropathic pain [106•] and in a mouse model of ALS [31••] |
As a mitogen-activated protein kinase kinase kinase (MAP3K), DLK functions as an upstream regulator of MAP Kinase signaling by activating the MAP2Ks MKK7 and MKK4, and the stress activated kinases JNK and p38 [9,10] (Figure 1). In mammals, DLK (MAP3K12) has a sister kinase, MAP3K13 (LZK), which has some partially overlapping biochemical activities and roles [9,11•]. Worms (Caenorhabditis elegans) and flies (Drosophila melanogaster) each have a single orthologue of equivalent homology to both DLK and LZK, named DLK-1 and Wallenda. Since these kinases share similar functions with DLK in the nervous system, we refer to all of these related kinases as ‘DLK’ in this review.
Developmental roles versus stress response
Some roles in nervous system development, including developmental neuronal cell death in sensory and motor neurons, neuronal migration, axon formation, and axon outgrowth have been documented for DLK and LZK [12–16], particularly when disrupted in combination with other components of JNK signaling [16] (Table 1). More dramatic defects in developmental wiring of the nervous system have been linked to lost regulation of DLK: DLK protein is held in check by a highly conserved ubiquitin ligase, Pam/Highwire/Rpm-1 (PHR) [17–20]. This restraint appears to be important for some axon guidance decisions [17,21], axon termination at correct locations [22,23], assembly of presynaptic machinery [19,20,24••], and elaboration of dendrite branches [25]. Hence restraint verses activity of DLK appears to be important at specific time points in nervous system development.
In contrast to development, in which only mild axon outgrowth defects have been noted for loss of dlk function in sensory and motor axons [12–15,26], DLK becomes activated in all types of neurons and axonal damage paradigms examined thus far in multiple model organisms [2–7], and is required for both regenerative and degenerative responses to axonal damage (Table 1). Many of the developmental defects associated with unrestrained DLK regulation may actually mimic responses made by neurons to axonal injury. For instance, recent studies using the Drosophila larval neuromuscular junction (NMJ) suggest that activation of DLK signaling promotes synaptic decline [24••,27•], which also occurs at disconnected synapses following injury [28]. Another well known response to axonal injury is a reduction in the injured neuron’s dendritic tree and in the synaptic inputs received by the injured neuron [29,30]. Whether DLK promotes post-developmental changes in dendrite architecture remains to be examined, however recent findings that DLK mediates a reduction in synaptic spines in a mouse model of Alzheimer’s Disease [31••] suggests this possibility.
Considering DLK’s major role in damage responses, and that its most striking requirement during development is for programmed neuron cell death [14], one may speculate that DLK’s function and restraint is relevant for developmental transitions in which neurons inherently experience conditions of cellular stress. For instance, limited levels of neurotrophic factors, or major rearrangements in neuronal cytoskeleton required for neuronal migration, may be considered ‘stressful’ for neurons. Also, Li et al. found that DLK signaling restrains the expression levels of presynaptic proteins to match the timing of synaptic maturation and growth [24••]. Premature expression of these abundant structural components of the synapse fully ready to transport and implement these molecules may also result in cellular stress.
DLK regulates retrograde responses to axonal damage and trophic factor withdrawal
A large body of work supports a unified view that DLK regulates an axon-to-nucleus signaling cascade that monitors the state of the axon and becomes activated in response to axonal damage. Endogenous DLK associates with vesicles [3], and live imaging studies of GFP-DLK transgenes suggest these vesicles are transported both anterogradely and retrogradely in axons [3,32]. DLK function is required cell autonomously for nuclear responses induced by axonal injury, including the activation of specific transcription factors [2,4,5,7,11•,14,33••]. These include phosphorylated STAT3, which is thought to be retrogradely transported in peripheral nerves from axons to the nucleus [5], and also transcriptional reporters for JNK signaling [3]. Mutations that disrupt retrograde axonal transport, including mutations in dynein and dynactin [3] and a known cargo for retrograde transport, JNK interacting protein JIP3, inhibit cell body responses downstream of DLK [14,34]. Importantly, DLK’s actions and signaling mechanisms appear specifically tuned to axonal damage and not dendrite damage: in contrast to axonal regeneration, DLK is not required for the regrowth of dendrites following injury [35,36•]. In addition, certain cell body responses to axonal injury induced by DLK are not induced by dendritic injury [37–39].
DLK was first discovered to play an essential role in the ability of axons to initiate new axonal growth following injury in the PNS [2,3,5,6,40]. However, following CNS injury in the optic nerve, DLK signaling initiates a cell death program [4,7]. Death downstream of DLK can be induced by other signals, including trophic factor withdrawal [14], which is known to rely upon retrograde transport and whose response can be probed specifically in axons using compartmentalized cultures [42,43]. Strikingly, DLK is essential for this classic form of developmental apoptosis in embryonic dorsal root ganglion (eDRG) neurons [14]. Moreover, DLK signaling can originate from the axonal compartment following NGF withdrawal: biochemical indications of DLK and JNK activation can be detected in extracts isolated from axons [44•], and inhibition of DLK and/or JNK solely in the axonal compartment can inhibit the appearance of downstream signaling markers in the cell body [14,44•]. These studies demonstrate compellingly DLK’s ability to initiate compartmentalized signaling within axons.
Links between DLK signaling, cytoskeleton and axonal transport
Intracellular transport within axons becomes acutely blocked at sites of axonal damage, and it can also become impaired or diminished in the presence of cellular stressors (Figure 2), such as chemotherapeutic agents that disrupt the cytoskeleton [45] or accumulations of misfolded proteins in neurodegenerative disease models [46]. There is a striking correlation between conditions that impair axonal transport and conditions that activate DLK signaling: DLK signaling becomes activated in invertebrate and vertebrate PNS neurons that are treated with cytoskeletal destabilizing agents [47,48•,49,50], or with genetic mutations in the cytoskeletal components spectroplakin, TCP1, Tau, or spectrin [6,47,51•]. Activation also occurs in mutations that impair the kinesin Unc-104 (homologous to Kif1A), which is a major carrier of synaptic vesicle precursors in axons [24••]. Mutations that inhibit DLK signaling rescue the synaptic defects associated with mutations in the kinesin unc-104 [24••]. Other genetic interaction studies in invertebrate peripheral neurons suggest that DLK mediates changes in neuronal morphology caused by mutations that impair cytoskeletal structure [47,52–55]. Hence DLK signaling appears responsible for both neuronal plasticity and for major pathologies associated with defects in cytoskeleton and axonal transport.
Many previous studies have suggested that JNK signaling may directly regulate kinesin and dynein motors and their cargos [56,57]. However, Li et al. found that DLK signaling tunes the expression levels of presynaptic proteins, which are major cargoes for transport in axons by the Unc-104 kinesin [24••]. The restraint of presynaptic protein levels by DLK signaling when axonal transport is impaired may function as a negative feedback loop to reduce stress by decreasing the amount of cargo for transport, thereby minimizing build-up. These findings suggest that DLK can function as both a sensor and effector to regulate intracellular transport within axons.
DLK signaling contributes to neurodegenerative disease
The degenerative responses induced by DLK are gaining increased attention for their roles in a growing number of neurodegenerative diseases. These include glaucoma, where functional genomic screens have identified DLK and LZK as key mediators of retinal ganglion cell (RGC) death [7,11•]. In addition, recent studies have suggested that DLK knockout or inhibition can delay pathology in multiple models of Amyotrophic Lateral Sclerosis (ALS) and Alzheimer’s disease (AD) [31••,58]. DLK inhibition is also protective in other models of neuronal death, including models of subarachnoid hemorrhage [59], 6-OHDA-induced dopaminergic cell death [60] and excitotoxicity [61], further increasing interest in DLK as a potential therapeutic target.
These findings imply that DLK signaling can be activated in contexts beyond simple axonal injury. It is also now apparent that the fundamental role of DLK signaling is not simply to increase axonal regeneration, despite its importance in regeneration paradigms. The dichotomous roles in regeneration and degeneration may be unified into an underlying biological function to stimulate pathways that allow the nervous system to react to axonal damage and cellular stress. Similar to other stress pathways (including ER stress and DNA damage) transient activation of stress pathways enables recovery, however chronic activation leads to cell death [62,63].
DLK signaling influences axonal integrity
An overarching theme for DLK signaling roles relates to the integrity of axons and trafficking within axons. It is striking that the multiple scenarios of DLK signaling summarized in Figures 1 and 2 also share a common resulting phenotype of axonal degeneration. Disruption of DLK together with other components of MAPK signaling leads to strong inhibition of axonal degeneration following axotomy [64,65], trophic factor withdrawal [14,41,66•] and chemotherapy-induced axon degeneration [64,67]. We therefore consider here our current understanding of the mechanistic relationships between DLK signaling and axonal degeneration.
Since DLK signaling may be initiated locally in axons and can regulate global (transcriptional/translational) responses in neurons, its influence upon axonal integrity and degeneration is likely multi-pronged, involving both local mechanisms in axons and global mechanisms downstream of retrograde signaling [68]. The ‘global’ responses downstream of retrograde signaling are simplest to consider first. Following trophic factor withdrawal in mouse DRGs, DLK and downstream MAPK signaling induce the expression of pro-apoptotic proteins Bax, Puma and caspases, some of which stimulate axonal degeneration following their induction in the cell body [66•]. A strikingly opposite protective response has been observed in fly motoneurons, where activation of DLK, either by ectopic expression or axonal injury, leads to a global response that increases the resiliency of both axons and dendrites to degenerate in subsequent injuries [38,69]. These responses may serve a biological purpose for neurons that have been injured to have increased resiliency to subsequent damage. In contrast, the pro-degenerative actions downstream of trophic factor deprivation may allow for pruning of axonal branches.
Together with downstream MAPK signaling effectors, DLK signaling also acts locally in distal axons to influence axonal degeneration. This may be most clearly considered for Wallerian degeneration of distal axons that become separated from cell bodies following acute axonal injury (pictured in Figure 1). Wallerian degeneration involves cell autonomous ‘self-destruction’ events that occur locally in axons independent of classical cell death machinery [70,71]. Acute inhibition of JNK in axotomized axons is sufficient to delay axonal degeneration [64], suggesting a local role for DLK/JNK signaling in promoting axon destruction.
What is this local role in axons? A key driver of Wallerian degeneration is the TIR-domain protein Sarm1, which functions as a NADase enzyme, degrading the essential metabolite NAD+ [72,73]. Sarm1 function is antagonized by the NAD+ biosynthetic enzyme NMNAT2 [74,75•], which, due to its short half-life in axons, must be continuously transported in axons from the cell body [76]. Yang et al. observed that genetic inhibition of MAPK signaling could blunt degeneration induced by ectopic activation of Sarm1 in DRG explants, and proposed a role for MAPK in promoting degeneration downstream of Sarm1 [65]. However Walker et al. more firmly identified an upstream role with the finding that MAPK signaling enhances the stability/turnover of NMNAT2 in both mouse DRG and fly motoneurons [75•]. Connections between DLK and NMNAT2 are also noted via their shared regulation by the PHR ubiquitin ligase [18–20,69], which is discussed further below in section 8. We acknowledge inherent challenges to distinguishing local from global effects of DLK signaling, which likely intersect to influence axonal integrity.
Stress responses regulated by DLK
Given the many cellular responses to DLK activation discussed above, surprisingly little is currently documented about the cellular pathways controlled by DLK. The known pathways thus far all share features of roles in stress response. Studies in worms have suggested that DLK signaling leads to increased mitochondrial transport and density in axons after injury [77], and that DLK signaling stimulates the expression of poly(ADP-ribose) glycohydrolases (PARGs) [78], which are linked to a growing number of genotoxic and metabolic stress signaling pathways[79].A recent study using mouse models of axonal stress in both the PNS and CNS found that DLK is a critical regulator of the Integrated Stress Response (ISR) pathway [33••]. ISR appears to influence translational responses in cells: while global translation is inhibited, genes with upstream Open Reading Frames such as ATF4 can be selectively induced. These findings are interesting in light of other data linking ISR to neuronal loss in models of neurodegenerative diseases[80,81],as well as studies linking DLK to translational mechanisms of regulation [2,82].
In addition to cell-autonomous stress responses, DLK signaling may also promote responses by non-neuronal cell types. A recent study in flies suggested that signaling downstream of DLK (via p38) may increase neuroinflammation in a TDP-43 overexpression model of neurodegenerative disease [83], while conditional knockout of DLK in a ALS mouse model reduced the appearance of activated microglia [31••]. A recent study found that DLK controls the expression of neuroinflammatory chemokines and is required for microgliosis and neuropathic pain [106•] Future studies are needed to determine whether these pathways are controlled by DLK in different cell types and model organisms and to understand their mechanisms in axonal stress responses.
Mechanisms for restraint and activation of DLK signaling
Essential for the current model that DLK gates responses to axonal stress is that its mechanism is tightly tuned to axonal damage and restrained in healthy/undamaged neurons. One important mechanism of control is at the level of protein stability and turnover. Genetic perturbations in multiple components of ubiquitin ligase complexes and deubiquitinating enzymes result in elevated DLK levels and chronically activated DLK signaling [19,20,84,85]. Moreover, overexpression of DLK in neurons, and even ectopic expression of DLK in non-neuronal cell types, is sufficient to activate downstream signaling [10,20,41]. This is thought to be mediated by its capacity to dimerize via leucine zipper domains and phosphorylate itself [9]. Once activated, downstream signaling via JNK stimulates DLK phosphorylation at additional sites and a decrease in DLK’s turnover rate [41]. This feed-forward relationship may enable neurons to kick-start DLK signaling in response to a local damage event in axons. How does DLK become activated? A growing number of conditions, kinases and some phosphatases have been implicated in its regulation [23,41,84,86•,87–95], and activated DLK is heavily phosphorylated across multiple sites [41,86•]. However, the molecular mechanisms that link various stressors in axons (in Figures 1 and 2) to DLK activation are still poorly understood. Recent work has indicated that Protein Kinase A (PKA) is an important mediator of DLK’s activation following axonal injury [86•], while Ste20 Kinases MAP4K4, MINK1 and TNIK promote DLK’s activation in axons following trophic factor withdrawal [44•]. Whether these different stressors use overlapping or distinct mechanisms is not yet known. C. elegans DLK-1 contains a domain shared with MAP3K13/LZK that gates signaling activation in response to elevated calcium [96]. However application of microtubule destabilizing agents to axons leads to activation of DLK signaling independently of calcium [48•]. Hence it is likely that multiple distinct mechanisms regulate DLK activation in neurons.
DLK’s retrograde signaling functions require that DLK is physically present to become activated in axons. A conserved site for palmitoylation allows DLK to associate with vesicles that are transported in axons, and palmitoylation is essential for DLK’s signaling ability [97]. Since defects in axonal transport and the cytoskeleton lead to DLK activation, is DLK transport directly linked to its activation mechanism? It is intriguing that a major negative regulator of DLK, the PHR ubiquitin ligase, localizes to presynaptic terminals [98–100], hence may promote destruction of DLK at synapses (Figure 3). It is also intriguing that PHR regulates axonal degeneration via an additional target, the protective enzyme NMNAT2 [18,101] and Figure 2). PHR’s regulation of DLK is best documented in the context of synapse development, where PHR’s regulation of DLK becomes apparent with a timing that coincides with termination of axonal outgrowth and the initiation of synaptogenesis [20,102]. Since axonal damage inherently disrupts synaptic connections in axons, whether PHR influences DLK’s activation mechanisms following axonal damage remains an interesting future question.
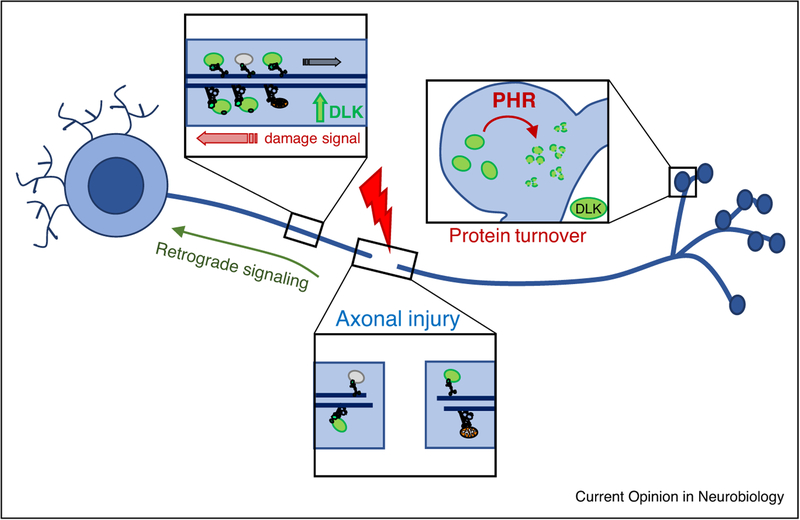
Figure 3.
Regulation of DLK. DLK associates with vesicles that are transported in axons (indicated in green) [3,32]. DLK protein is regulated by ubiquitin ligases, including the highly conserved synaptic protein PHR (Pam/Highwire/Rpm-1), which regulates DLK during synaptic development [19,20].
Conclusion
We propose that a higher order function for DLK signaling may be to promote a damage-response state in neurons that enables plasticity in neuronal circuits. In this state, the ultimate response may be strongly influenced by the circumstance of the damage. In some contexts, such as PNS injury, neurons may be supported for growth and inhibited for death. However in other contexts, in order to incur the least damage or the best adaptation within a neuronal circuit, it may be more advantageous for the damaged neuron to degenerate and be removed. As an evolutionarily conserved sensor of axonal stress and injury, DLK’s regulation and modes of action are tightly coordinated with the integrity of the axonal cytoskeleton and transport machinery. As a critical mediator of injury responses and neurodegeneration pathways, future work is needed to understand the cellular responses that DLK regulates and the mechanisms that control its activation in the nervous system.
Acknowledgements
The Collins lab is supported by a grant from the National Institute of Health, R01NS069844. We thank Aaron DiAntonio, Dion Dickman, Roman Giger, Ashley Kalinski and Claire Le Pichon for helpful comments on the manuscript.
References and recommended reading
Papers of particular interest, published within the period of review, have been highlighted as
• of special interest
•• of outstanding interest
- 1.Farley MM, Watkins TA: Intrinsic neuronal stress response pathways in injury and disease. Annu Rev Pathol 2018, 13:93–116. [DOI] [PubMed] [Google Scholar]
- 2.Yan D, Wu Z, Chisholm AD, Jin Y: The DLK-1 kinase promotes mRNA stability and local translation in C. elegans synapses and axon regeneration. Cell 2009, 138:1005–1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Xiong X, Wang X, Ewanek R, Bhat P, DiAntonio A, Collins CA: Protein turnover of the Wallenda/DLK kinase regulates a retrograde response to axonal injury. J Cell Biol 2010, 191:211–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Watkins TA, Wang B, Huntwork-Rodriguez S, Yang J, Jiang Z, Eastham-Anderson J, Modrusan Z, Kaminker JS, Tessier-Lavigne M, Lewcock JW: DLK initiates a transcriptional program that couples apoptotic and regenerative responses to axonal injury. Proc Natl Acad Sci U S A 2013, 110:4039–4044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shin JE, Cho Y, Beirowski B, Milbrandt J, Cavalli V, DiAntonio A: Dual leucine zipper kinase is required for retrograde injury signaling and axonal regeneration. Neuron 2012, 74:1015–1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hammarlund M, Nix P, Hauth L, Jorgensen EM, Bastiani M: Axon regeneration requires a conserved MAP kinase pathway. Science 2009, 323:802–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Welsbie DS, Yang Z, Ge Y, Mitchell KL, Zhou X, Martin SE, Berlinicke CA, Hackler L Jr, Fuller J, Fu J et al. : Functional genomic screening identifies dual leucine zipper kinase as a key mediator of retinal ganglion cell death. Proc Natl Acad Sci U S A 2013, 110:4045–4050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Matsuda W, Furuta T, Nakamura KC, Hioki H, Fujiyama F, Arai R, Kaneko T: Single nigrostriatal dopaminergic neurons form widely spread and highly dense axonal arborizations in the neostriatum. J Neurosci 2009, 29:444–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nihalani D, Merritt S, Holzman LB: Identification of structural and functional domains in mixed lineage kinase dual Leucine Zipper-bearing kinase required for complex formation and stress-activated protein kinase activation. J Biol Chem 2000, 275:7273–7279. [DOI] [PubMed] [Google Scholar]
- 10.Fan G, Merritt SE, Kortenjann M, Shaw PE, Holzman LB: Dual leucine zipper-bearing kinase (DLK) activates p46SAPK and p38mapk but not ERK2. J Biol Chem 1996, 271:24788–24793. [DOI] [PubMed] [Google Scholar]
- 11.•.Welsbie DS, Mitchell KL, Jaskula-Ranga V, Sluch VM, Yang Z, Kim J, Buehler E, Patel A, Martin SE, Zhang P-W et al. : Enhanced functional genomic screening identifies novel mediators of dual Leucine Zipper kinase-dependent injury signaling in neurons. Neuron 2017, 94:1142–1154.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study found that DLK’s sister kinase LZK works cooperatively with DLK to promote cell death in an in vitro glaucoma model. Through large scale genomic screening the authors found that this death pathway involves the function of transcription factors SOX11, MEF2A JUN and ATF2.
- 12.Hirai S-I, Cui DF, Miyata T, Ogawa M, Kiyonari H, Suda Y, Aizawa S, Banba Y, Ohno S: The c-Jun N-terminal kinase activator dual leucine zipper kinase regulates axon growth and neuronal migration in the developing cerebral cortex. J Neurosci 2006, 26:11992–12002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eto K, Kawauchi T, Osawa M, Tabata H, Nakajima K: Role of dual leucine zipper-bearing kinase (DLK/MUK/ZPK) in axonal growth. Neurosci Res 2010, 66:37–45. [DOI] [PubMed] [Google Scholar]
- 14.Ghosh AS, Wang B, Pozniak CD, Chen M, Watts RJ, Lewcock JW: DLK induces developmental neuronal degeneration via selective regulation of proapoptotic JNK activity. J Cell Biol 2011, 194:751–764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen M, Geoffroy CG, Wong HN, Tress O, Nguyen MT, Holzman LB, Jin Y, Zheng B: Leucine Zipper-bearing Kinase promotes axon growth in mammalian central nervous system neurons. Sci Rep 2016, 6:31482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hirai S-I, Banba Y, Satake T, Ohno S: Axon formation in neocortical neurons depends on stage-specific regulation of microtubule stability by the dual leucine zipper kinase-c-Jun N-terminal kinase pathway. J Neurosci 2011, 31:6468–6480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lewcock JW, Genoud N, Lettieri K, Pfaff SL: The ubiquitin ligase Phr1 regulates axon outgrowth through modulation of microtubule dynamics. Neuron 2007, 56:604–620. [DOI] [PubMed] [Google Scholar]
- 18.Babetto E, Beirowski B, Russler EV, Milbrandt J, DiAntonio A: The Phr1 ubiquitin ligase promotes injury-induced axon self-destruction. Cell Rep 2013, 3:1422–1429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nakata K, Abrams B, Grill B, Goncharov A, Huang X, Chisholm AD, Jin Y: Regulation of a DLK-1 and p38 MAP kinase pathway by the ubiquitin ligase RPM-1 is required for presynaptic development. Cell 2005, 120:407–420. [DOI] [PubMed] [Google Scholar]
- 20.Collins CA, Wairkar YP, Johnson SL, DiAntonio A: Highwire restrains synaptic growth by attenuating a MAP kinase signal. Neuron 2006, 51:57–69. [DOI] [PubMed] [Google Scholar]
- 21.Shin JE, DiAntonio A: Highwire regulates guidance of sister axons in the Drosophila mushroom body. J Neurosci 2011, 31:17689–17700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Borgen M, Rowland K, Boerner J, Lloyd B, Khan A, Murphey R: Axon termination, pruning, and synaptogenesis in the giant fiber system of Drosophila melanogaster is promoted by highwire. Genetics 2017, 205:1229–1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Feoktistov AI, Herman TG: Wallenda/DLK protein levels are temporally downregulated by Tramtrack69 to allow R7 growth cones to become stationary boutons. Development 2016, 143:2983–2993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.••.Li J, Zhang YV, Asghari Adib E, Stanchev DT, Xiong X, Klinedinst S, Soppina P, Jahn TR, Hume RI, Rasse TM et al. : Restraint of presynaptic protein levels by Wnd/DLK signaling mediates synaptic defects associated with the kinesin-3 motor Unc-104. Elife 2017:6. [DOI] [PMC free article] [PubMed] [Google Scholar]; This paper showed that DLK signaling becomes activated in mutations that impair the kinesin Unc-104, and that DLK signaling is actually responsible for many of the synaptic defects that associated with Unc-104’s function. DLK signaling reduces the total expression levels of presynaptic proteins that rely upon Unc-104 for transport, hence may reduce stress caused by impaired transport.
- 25.Wang X, Kim JH, Bazzi M, Robinson S, Collins CA, Ye B: Bimodal control of dendritic and axonal growth by the dual leucine zipper kinase pathway. PLoS Biol 2013, 11:e1001572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bloom AJ, Miller BR, Sanes JR, DiAntonio A: The requirement for Phr1 in CNS axon tract formation reveals the corticostriatal boundary as a choice point for cortical axons. Genes Dev 2007, 21:2593–2606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.•.Goel P, Dickman D: Distinct homeostatic modulations stabilize reduced postsynaptic receptivity in response to presynaptic DLK signaling. Nat Commun 2018, 9:1856. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study investigated the effects of elevated DLK signaling upon homeostatic plasticity mechanisms at the Drosophila larval NMJ, and detailed non-autonomous effects in postsynaptic muscles in response to DLK signaling activation in motoneurons. These include diminished neurotransmitter receptor levels in the postsynaptic compartment. Observations in this paper suggest that DLK activation leads to alterations in trans-synaptic signaling mechanisms, whose identity is currently not known.
- 28.Mishra B, Carson R, Hume RI, Collins CA: Sodium and potassium currents influence Wallerian degeneration of injured Drosophila axons. J Neurosci 2013, 33:18728–18739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Purves D: Functional and structural changes in mammalian sympathetic neurones following interruption of their axons. J Physiol 1975, 252:429–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Navarro X, Vivo M, Valero-Cabre A: Neural plasticity after peripheral nerve injury and regeneration. Prog Neurobiol 2007, 82:163–201. [DOI] [PubMed] [Google Scholar]
- 31.••.Le Pichon CE, Meilandt WJ, Dominguez S, Solanoy H, Lin H, Ngu H, Gogineni A, Sengupta Ghosh A, Jiang Z, Lee S-H et al. : Loss of dual leucine zipper kinase signaling is protective in animal models of neurodegenerative disease. Sci Transl Med 2017:9. [DOI] [PubMed] [Google Scholar]; This paper presented data from human patients suggesting that signaling downstream of DLK may be activated in genetically diverse cases of inherited and sporadic forms of ALS and AD. Moreover, they showed that conditional genetic or pharmacologic inhibition of DLK in multiple mouse models of ALS and AD could delay the onset of disease phenotypes.
- 32.Holland SM, Collura KM, Ketschek A, Noma K, Ferguson TA, Jin Y, Gallo G, Thomas GM: Palmitoylation controls DLK localization, interactions and activity to ensure effective axonal injury signaling. Proc Natl Acad Sci U S A 2016, 113:763–768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.••.Larhammar M, Huntwork-Rodriguez S, Jiang Z, Solanoy H, Sengupta Ghosh A, Wang B, Kaminker JS, Huang K, Eastham-Anderson J, Siu M et al. : Dual leucine zipper kinase-dependent PERK activation contributes to neuronal degeneration following insult. Elife 2017:6. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study found that genes associated with the Integrated Stress Response (ISR) pathway become induced in a DLK-dependent manner in multiple injury paradigms. Larhammar et al showed that DLK induces ATF4 expression and activation of ISR-associated kinase PERK, and that ISR inhibition could delay neuronal loss following optic nerve crush.
- 34.Klinedinst S, Wang X, Xiong X, Haenfler JM, Collins CA: Independent pathways downstream of the Wnd/DLK MAPKKK regulate synaptic structure, axonal transport, and injury signaling. J Neurosci 2013, 33:12764–12778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stone MC, Albertson RM, Chen L, Rolls MM: Dendrite injury triggers DLK-independent regeneration. Cell Rep 2014, 6:247–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.•.Chung SH, Awal MR, Shay J, McLoed MM, Mazur E, Gabel CV: Novel DLK-independent neuronal regeneration in Caenorhabditis elegans shares links with activity-dependent ectopic outgrowth. Proc Natl Acad Sci U S A 2016, 113:E2852–60. [DOI] [PMC free article] [PubMed] [Google Scholar]; Axonal regeneration after axotomy required DLK-1, however concomitant injury to dendrites in conjunction with the axon resulted in DLK-1-independent and MAPK-independent regeneration of sensory neurons, suggesting the existence of multiple regeneration mechanisms. Because dendrite injury is required for this, the mechanism may potentially be shared with dendrite regeneration, which does not require DLK [35].
- 37.Stone MC, Nguyen MM, Tao J, Allender DL, Rolls MM: Global up-regulation of microtubule dynamics and polarity reversal during regeneration of an axon from a dendrite. Mol Biol Cell 2010, 21:767–777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chen L, Stone MC, Tao J, Rolls MM: Axon injury and stress trigger a microtubule-based neuroprotective pathway. Proc Natl Acad Sci U S A 2012, 109:11842–11847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hao Y, Collins C: Intrinsic mechanisms for axon regeneration: insights from injured axons in Drosophila. Curr Opin Genet Dev 2017, 44:84–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Itoh A, Horiuchi M, Bannerman P, Pleasure D, Itoh T: Impaired regenerative response of primary sensory neurons in ZPK/DLK gene-trap mice. Biochem Biophys Res Commun 2009, 383:258–262. [DOI] [PubMed] [Google Scholar]
- 41.Huntwork-Rodriguez S, Wang B, Watkins T, Ghosh AS, Pozniak CD, Bustos D, Newton K, Kirkpatrick DS, Lewcock JW: JNK-mediated phosphorylation of DLK suppresses its ubiquitination to promote neuronal apoptosis. J Cell Biol 2013, 202:747–763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Campenot RB: NGF uptake and retrograde signaling mechanisms in sympathetic neurons in compartmented cultures In Cell Biology of the Axon. Edited by Koenig E Berlin, Heidelberg: Springer; 2009:141–158. [DOI] [PubMed] [Google Scholar]
- 43.Mok S-A, Lund K, Campenot RB: A retrograde apoptotic signal originating in NGF-deprived distal axons of rat sympathetic neurons in compartmented cultures. Cell Res 2009, 19:546–560. [DOI] [PubMed] [Google Scholar]
- 44.•.Larhammar M, Huntwork-Rodriguez S, Rudhard Y, Sengupta-Ghosh A, Lewcock JW: The Ste20 family kinases MAP4K4, MINK1, and TNIK converge to regulate stress-induced JNK signaling in neurons. J Neurosci 2017, 37:11074–11084. [DOI] [PMC free article] [PubMed] [Google Scholar]; Using compartmentalized chambers this paper demonstrated that DLK activation occurs locally in axons following NGF withdrawal from the axonal compartment.
- 45.Nicolini G, Monfrini M, Scuteri A: Axonal transport impairment in chemotherapy-induced peripheral neuropathy. Toxics 2015, 3:322–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Millecamps S, Julien J-P: Axonal transport deficits and neurodegenerative diseases. Nat Rev Neurosci 2013, 14:161–176. [DOI] [PubMed] [Google Scholar]
- 47.Valakh V, Walker LJ, Skeath JB, DiAntonio A: Loss of the spectraplakin short stop activates the DLK injury response pathway in Drosophila. J Neurosci 2013, 33:17863–17873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.•.Valakh V, Frey E, Babetto E, Walker LJ, DiAntonio A: Cytoskeletal disruption activates the DLK/JNK pathway, which promotes axonal regeneration and mimics a preconditioning injury. Neurobiol Dis 2015, 77:13–25. [DOI] [PMC free article] [PubMed] [Google Scholar]; Using compartmentalized cultures this paper showed that pharmacological agents that disrupt either microtubule or actin cytoskeleton in axonal compartments lead to activation of DLK signaling. The cell-permeable calcium chelator BAPTA-AM had no effect upon this activation mechanism.
- 49.Massaro CM, Pielage J, Davis GW: Molecular mechanisms that enhance synapse stability despite persistent disruption of the spectrin/ankyrin/microtubule cytoskeleton. J Cell Biol 2009, 187:101–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bounoutas A, Kratz J, Emtage L, Ma C, Nguyen KC, Chalfie M: Microtubule depolymerization in Caenorhabditis elegans touch receptor neurons reduces gene expression through a p38 MAPK pathway. Proc Natl Acad Sci U S A 2011, 108:3982–3987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.•.Voelzmann A, Okenve-Ramos P, Qu Y, Chojnowska-Monga M, Del Caño-Espinel M, Prokop A, Sanchez-Soriano N: Tau and spectraplakins promote synapse formation and maintenance through Jun kinase and neuronal trafficking. Elife 2016:5. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study found that JNK signaling regulates the expression levels of the Unc-104 kinesin, however Liet al. found that DLK and JNK signaling are restrained by Unc-104 [24••]. Together the two studies suggest an intimate relationship between DLK/JNK signaling, cytoskeleton and the Unc-104 kinesin.
- 52.Chen C-H, Lee A, Liao C-P, Liu Y-W, C-L Pan: RHGF-1/PDZRhoGEF and retrograde DLK-1 signaling drive neuronal remodeling on microtubule disassembly. Proc Natl Acad Sci U S A 2014, 111:16568–16573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Marcette JD, Chen JJ, Nonet ML: The Caenorhabditis elegans microtubule minus-end binding homolog PTRN-1 stabilizes synapses and neurites. Elife 2014, 3:e01637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Richardson CE, Spilker KA, Cueva JG, Perrino J, Goodman MB, Shen K: PTRN-1, a microtubule minus end-binding CAMSAP homolog, promotes microtubule function in Caenorhabditis elegans neurons. Elife 2014, 3:e01498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kurup N, Yan D, Goncharov A, Jin Y: Dynamic microtubules drive circuit rewiring in the absence of neurite remodeling. Curr Biol 2015, 25:1594–1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Verhey KJ: Motor proteins: trafficking and signaling collide. Curr Biol 2007, 17:R804–R806. [DOI] [PubMed] [Google Scholar]
- 57.Liu J-J: Regulation of dynein-dynactin-driven vesicular transport. Traffic 2017, 18:336–347. [DOI] [PubMed] [Google Scholar]
- 58.Patel S, Meilandt WJ, Erickson RI, Chen J, Deshmukh G, Estrada AA, Fuji RN, Gibbons P, Gustafson A, Harris SF et al. : Selective inhibitors of dual Leucine zipper kinase (DLK, MAP3K12) with activity in a model of Alzheimer’s disease. J Med Chem 2017, 60:8083–8102. [DOI] [PubMed] [Google Scholar]
- 59.Yin C, Huang G-F, Sun X-C, Guo Z, Zhang JH: DLK silencing attenuated neuron apoptosis through JIP3/MA2K7/JNK pathway in early brain injury after SAH in rats. Neurobiol Dis 2017, 103:133–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chen X, Rzhetskaya M, Kareva T, Bland R, During MJ, Tank AW, Kholodilov N, Burke RE: Antiapoptotic and trophic effects of dominant-negative forms of dual leucine zipper kinase in dopamine neurons of the substantia nigra in vivo. J Neurosci 2008, 28:672–680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Pozniak CD, Ghosh AS, Gogineni A, Hanson JE, Lee S-H, Larson JL, Solanoy H, Bustos D, Li H, Ngu H et al. : Dual leucine zipper kinase is required for excitotoxicity-induced neuronal degeneration. J Exp Med 2013, 210:2553–2567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Pakos-Zebrucka K, Koryga I, Mnich K, Ljujic M, Samali A, Gorman AM: The integrated stress response. EMBO Rep 2016, 17:1374–1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Fribley A, Zhang K, Kaufman RJ: Regulation of apoptosis by the unfolded protein response. Methods Mol Biol 2009, 559:191–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Miller BR, Press C, Daniels RW, Sasaki Y, Milbrandt J, DiAntonio A: A dual leucine kinase-dependent axon self-destruction program promotes Wallerian degeneration. Nat Neurosci 2009, 12:387–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yang J, Wu Z, Renier N, Simon DJ, Uryu K, Park DS, Greer PA, Tournier C, Davis RJ, Tessier-Lavigne M: Pathological axonal death through a MAPK cascade that triggers a local energy deficit. Cell 2015, 160:161–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.•.Simon DJ, Pitts J, Hertz NT, Yang J, Yamagishi Y, Olsen O, Teić Mark M, Molina H, Tessier-Lavigne M: Axon degeneration gated by retrograde activation of somatic pro-apoptotic signaling. Cell 2016, 164:1031–1045. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study revealed that DLK and MAPK signaling promote axonal degeneration following NGF withdrawal by inducing a transcriptional response in the cell body. This response involves expression of the Bcl-family member Puma, whose activation in the cell body is sufficient to promote axonal degeneration.
- 67.Bhattacharya MR, Gerdts J, Naylor SA, Royse EX, Ebstein SY, Sasaki Y, Milbrandt J, DiAntonio A: A model of toxic neuropathy in Drosophila reveals a role for MORN4 in promoting axonal degeneration. J Neurosci 2012, 32:5054–5061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Geden MJ, Deshmukh M: Axon degeneration: context defines distinct pathways. Curr Opin Neurobiol 2016, 39:108–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Xiong X, Collins CA: A conditioning lesion protects axons from degeneration via the Wallenda/DLK MAP kinase signaling cascade. J Neurosci 2012, 32:610–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Conforti L, Gilley J, Coleman MP: Wallerian degeneration: an emerging axon death pathway linking injury and disease. Nat Rev Neurosci 2014, 15:394–409. [DOI] [PubMed] [Google Scholar]
- 71.Gerdts J, Summers DW, Milbrandt J, DiAntonio A: Axon self-destruction: new links among SARM1, MAPKs, and NAD+ metabolism. Neuron 2016, 89:449–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Gerdts J, Brace EJ, Sasaki Y, DiAntonio A, Milbrandt J: SARM1 activation triggers axon degeneration locally via NAD+ destruction. Science 2015, 348:453–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Essuman K, Summers DW, Sasaki Y, Mao X, DiAntonio A, Milbrandt J: The SARM1 toll/interleukin-1 receptor domain possesses intrinsic NAD+ cleavage activity that promotes pathological axonal degeneration. Neuron 2017, 93:1334–1343. e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Gilley J, Orsomando G, Nascimento-Ferreira I, Coleman MP: Absence of SARM1 rescues development and survival of NMNAT2-deficient axons. Cell Rep 2015, 10:1974–1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.•.Walker LJ, Summers DW, Sasaki Y, Brace EJ, Milbrandt J, DiAntonio A: MAPK signaling promotes axonal degeneration by speeding the turnover of the axonal maintenance factor NMNAT2. Elife 2017:6. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study demonstrated evolutionary conserved role for JNK/MAPK signaling in promoting the turnover of the protective NAD+ biosynthethic enzyme NMNAT2.
- 76.Milde S, Gilley J, Coleman MP: Axonal trafficking of NMNAT2 and its roles in axon growth and survival in vivo. Bioarchitecture 2013, 3:133–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Han SM, Baig HS, Hammarlund M: Mitochondria localize to injured axons to support regeneration. Neuron 2016, 92:1308–1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Byrne AB, McWhirter RD, Sekine Y, Strittmatter SM, Miller DMIII, Hammarlund M: Inhibiting poly(ADP-ribosylation) improves axon regeneration. eLife Sci 2016, 5:e12734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Luo X, Kraus WL: On PAR with PARP: cellular stress signaling through poly(ADP-ribose) and PARP-1. Genes Dev 2012, 26:417–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Page G, Rioux Bilan A, Ingrand S, Lafay-Chebassier C, Pain S, Perault Pochat MC, Bouras C, Bayer T, Hugon J: Activated double-stranded RNA-dependent protein kinase and neuronal death in models of Alzheimer’s disease. Neuroscience 2006, 139:1343–1354. [DOI] [PubMed] [Google Scholar]
- 81.Moreno JA, Radford H, Peretti D, Steinert JR, Verity N, Martin MG, Halliday M, Morgan J, Dinsdale D, Ortori CA et al. : Sustained translational repression by eIF2a-P mediates prion neurodegeneration. Nature 2012, 485:507–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kim JH, Wang X, Coolon R, Ye B: Dscam expression levels determine presynaptic arbor sizes in Drosophila sensory neurons. Neuron 2013, 78:827–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Zhan L, Xie Q, Tibbetts RS: Opposing roles of p38 and JNK in a Drosophila model of TDP-43 proteinopathy reveal oxidative stress and innate immunity as pathogenic components of neurodegeneration. Hum Mol Genet 2015, 24:757–772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Baker ST, Opperman KJ, Tulgren ED, Turgeon SM, Bienvenut W, Grill B: RPM-1 uses both ubiquitin ligase and phosphatase-based mechanisms to regulate DLK-1 during neuronal development. PLoS Genet 2014, 10:e1004297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Brace EJ, Wu C, Valakh V, DiAntonio A: SkpA restrains synaptic terminal growth during development and promotes axonal degeneration following injury. J Neurosci 2014, 34:8398–8410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.•.Hao Y, Frey E, Yoon C, Wong H, Nestorovski D, Holzman LB, Giger RJ, DiAntonio A, Collins C: An evolutionarily conserved mechanism for cAMP elicited axonal regeneration involves direct activation of the dual leucine zipper kinase DLK. Elife 2016:5. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study found that the cAMP effector kinase PKA functions as a direct activator of DLK in both flies and mammalian neurons by stimulating phosphorylation of DLK’s activation loop. This regulation is required for axonal regeneration. Hence two previously known mediators of axonal regeneration directly intersect via a shared signaling pathway. Future work is needed to determine whether PKA mediates DLK activation in other contexts, including those that lead to neuronal death.
- 87.Huang Y-WA, Zhou B, Wernig M, Südhof TC: ApoE2, ApoE3, and ApoE4 differentially stimulate APP transcription and Ab secretion. Cell 2017, 168:427–441.e21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Fukuyama K, Yoshida M, Yamashita A, Deyama T, Baba M, Suzuki A, Mohri H, Ikezawa Z, Nakajima H, Hirai S et al. : MAPK upstream kinase (MUK)-binding inhibitory protein, a negative regulator of MUK/dual leucine zipper-bearing kinase/leucine zipper protein kinase. J Biol Chem 2000, 275:21247–21254. [DOI] [PubMed] [Google Scholar]
- 89.Börchers S, Babaei R, Klimpel C, Duque Escobar J, Schröder S, Blume R, Malik MNH, Oetjen E: TNFa-induced DLK activation contributes to apoptosis in the beta-cell line HIT. Naunyn Schmiedebergs Arch Pharmacol 2017, 390:813–825. [DOI] [PubMed] [Google Scholar]
- 90.Wong C-O, Palmieri M, Li J, Akhmedov D, Chao Y, Broadhead GT, Zhu MX, Berdeaux R, Collins CA, Sardiello M et al. : Diminished MTORC1-dependent JNK activation underlies the neurodevelopmental defects associated with lysosomal dysfunction. Cell Rep 2015, 12:2009–2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Daviau A, Di Fruscio M, Blouin R: The mixed-lineage kinase DLK undergoes Src-dependent tyrosine phosphorylation and activation in cells exposed to vanadate or platelet-derived growth factor (PDGF). Cell Signal 2009, 21:577–587. [DOI] [PubMed] [Google Scholar]
- 92.Wu C-C, Wu H-J, Wang C-H, Lin C-H, Hsu S-C, Chen Y-R, Hsiao M, Schuyler SC, Lu FL, Ma N et al. : Akt suppresses DLK for maintaining self-renewal of mouse embryonic stem cells. Cell Cycle 2015, 14:1207–1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Robitaille K, Daviau A, Lachance G, Couture J-P, Blouin R: Calphostin C-induced apoptosis is mediated by a tissue transglutaminase-dependent mechanism involving the DLK/JNK signaling pathway. Cell Death Differ 2008, 15:1522–1531. [DOI] [PubMed] [Google Scholar]
- 94.Shen W, Ganetzky B: Autophagy promotes synapse development in Drosophila. J Cell Biol 2009, 187:71–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Chen L, Wang Z, Ghosh-Roy A, Hubert T, Yan D, O’Rourke S, Bowerman B, Wu Z, Jin Y, Chisholm AD: Axon regeneration pathways identified by systematic genetic screening in C. elegans. Neuron 2011, 71:1043–1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Yan D, Jin Y: Regulation of DLK-1 kinase activity by calcium-mediated dissociation from an inhibitory isoform. Neuron 2012, 76:534–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Holland SM, Collura KM, Ketschek A, Noma K, Ferguson TA, Jin Y, Gallo G, Thomas GM: Palmitoylation controls DLK localization, interactions and activity to ensure effective axonal injury signaling. Proc Natl Acad Sci U S A 2016, 113:763–768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Wu C, Wairkar YP, Collins CA, DiAntonio A: Highwire function at the Drosophila neuromuscular junction: spatial, structural, and temporal requirements. J Neurosci 2005, 25:9557–9566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Zhen M, Huang X, Bamber B, Jin Y: Regulation of presynaptic terminal organization by C. elegans RPM-1, a putative guanine nucleotide exchanger with a RING-H2 finger domain. Neuron 2000, 26:331–343. [DOI] [PubMed] [Google Scholar]
- 100.Schaefer AM, Hadwiger GD: Nonet ML: rpm-1, a conserved neuronal gene that regulates targeting and synaptogenesis in C. elegans. Neuron 2000, 26:345–356. [DOI] [PubMed] [Google Scholar]
- 101.Xiong X, Hao Y, Sun K, Li J, Li X, Mishra B, Soppina P, Wu C, Hume RI, Collins CA: The Highwire ubiquitin ligase promotes axonal degeneration by tuning levels of Nmnat protein. PLoS Biol 2012, 10:e1001440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Borgen MA, Wang D, Grill B: RPM-1 regulates axon termination by affecting growth cone collapse and microtubule stability. Development 2017, 144:4658–4672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Yang J, Weimer RM, Kallop D, Olsen O, Wu Z, Renier N, Uryu K, Tessier-Lavigne M: Regulation of axon degeneration after injury and in development by the endogenous calpain inhibitor calpastatin. Neuron 2013, 80:1175–1189. [DOI] [PubMed] [Google Scholar]
- 104.Frey E, Valakh V, Karney-Grobe S, Shi Y, Milbrandt J, DiAntonio A: An in vitro assay to study induction of the regenerative state in sensory neurons. Exp Neurol 2015, 263:350–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Fernandes KA, Harder JM, John SW, Shrager P, Libby RT: DLK-dependent signaling is important for somal but not axonal degeneration of retinal ganglion cells following axonal injury. Neurobiol Dis 2014, 69:108–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.•.Wlaschin JJ, Gluski JM, Nguyen E, Silberberg H, Thompson JH, Chesler AT, Le Pichon CE: Dual leucine zipper kinase is required for mechanical allodynia and microgliosis after nerve injury. Elife 2018:7. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study found that DLK controls the expression of multiple pain associated genes, including the cytokine Csf1. Genetic or pharmacological inhibition of DLK reduced microgliosis at DRG terminals in the spinal cord and strongly reduced pain responses.