Abstract
Notch pathway signaling is known to promote gastric stem cell proliferation, and constitutive pathway activation induces gastric tumors via mTORC1 activation in mouse genetic models. The purpose of this study was to determine whether human gastric adenocarcinomas are similarly dependent on Notch and mTORC1 signaling for growth. Gene expression profiling of 415 human gastric adenocarcinomas in The Cancer Genome Atlas, and a small set of locally obtained gastric cancers showed enhanced expression of Notch pathway components, including Notch ligands, receptors and downstream target genes. Human gastric adenocarcinoma tissues and chemically induced mouse gastric tumors both exhibited heightened Notch and mTORC1 pathway signaling activity, as evidenced by increased expression of the NOTCH1 receptor signaling fragment NICD, the Notch target HES1, and the mTORC1 target phosphorylated S6 ribosomal protein. Pharmacologic inhibition of either Notch or mTORC1 signaling reduced growth of human gastric cancer cell lines, with combined pathway inhibition causing a further reduction in growth, suggesting that both pathways are activated to promote gastric cancer cell proliferation. Further, mTORC1 signaling was reduced after Notch inhibition suggesting that mTOR is downstream of Notch in gastric cancer cells. Analysis of human gastric organoids derived from paired control and gastric cancer tissues also exhibited reduced growth in culture after Notch or mTOR inhibition. Thus, our studies demonstrate that Notch and mTOR signaling pathways are commonly activated in human gastric cancer to promote cellular proliferation. Targeting these pathways in combination might be an effective therapeutic strategy for gastric cancer treatment.
Introduction
Although gastric cancer (GC) incidence has recently declined in the United States, it remains a significant human health problem. It is currently the 5th most common cancer and the 3rd leading cause of cancer-related deaths worldwide [1]. Despite the morbidity associated with GC, few effective treatment options exist due to factors such as late-stage diagnosis [2], drug resistance [3], metastasis [4], and a general lack of understanding regarding the molecular mechanisms involved in GC initiation and disease progression. Recent genetic profiling approaches, such as those completed by analysis of The Cancer Genome Atlas (TCGA) in 2014, not only revealed the molecular complexity of human GC but also began to identify key oncogenes and signaling pathways that may be involved in human GC development [5]. However, a deeper functional understanding of how these pathways support GC cell growth is paramount to understanding if and how they could be targeted for GC treatment.
Basic developmental signaling pathways are attractive candidates for targeted treatment of a variety of cancers because of their role in regulating stem cell function to maintain tissue homeostasis. The Notch signaling pathway, for example, regulates cellular proliferation and differentiation in a variety of gastrointestinal tract tissues, including the stomach (reviewed in [6], [7]). We have recently shown that in both mouse and human stomach, Notch is required for gastric stem cell proliferation, effects that are mediated through the NOTCH1 and NOTCH2 receptors [8], [9]. Additionally, constitutive Notch activation in mouse LGR5+ antral stem cells leads to increased stem cell activity and tissue expansion via gland fission, which induces hyper-proliferative, undifferentiated antral polyps [10]. Interestingly, these polyps exhibit activation of the mammalian target of rapamycin (mTOR) signaling pathway, suggesting that Notch may cooperate with this signaling pathway to promote gastric epithelial cell hyperproliferative disorders.
The mTOR signaling pathway is involved in numerous processes, such as cell growth, proliferation and survival (reviewed in [11], [12]). mTOR signaling occurs through two protein complexes: mTOR complex 1 (mTORC1), which regulates cell growth in response to nutrient and growth factor signaling, and mTOR complex 2 (mTORC2), which promotes cellular proliferation and survival. Our previous findings in the Notch-activated mouse stomach suggest that Notch may be upstream of mTOR in a Notch-mTOR signaling cascade. Mouse gastric polyps induced by genetic activation of Notch signaling also exhibited mTORC1 activation, and polyp formation was attenuated by treatment with the mTORC1 inhibitor rapamycin [10]. These findings suggest that mTORC1 may be a key effector of dysregulated Notch pathway signaling in the stomach.
It has been previously demonstrated in the hematopoietic system that Notch signaling can activate the mTOR pathway [13], [14], and that mTOR inhibition can upregulate Notch [15], suggesting that Notch and mTOR signaling cross-talk occurs in other tissues. How those interactions might translate to initiation and promotion of GC remains to be elucidated. Activation of Notch and mTOR signaling have each been previously associated with human GC. Correlative studies demonstrating a link between NOTCH1 and NOTCH2 signaling and GC morbidity have been reported [16], [17], [18]. Increased mTOR signaling in GC, either via activating mutations in phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha (PIK3CA), and/or loss of the tumor suppressor phosphatase-tensin homolog deleted from chromosome 10 (PTEN), have also been reported [19]. Although these previous studies identified Notch as a potential pathway that plays a role in gastric tumorigenesis, they did not include a comprehensive analysis of Notch pathway component expression in gastric adenocarcinomas, and, importantly, did not examine Notch and mTOR function to regulate cancer cell proliferation.
In this study we examined mouse and human gastric tumors to show that both Notch and mTOR pathways are activated. Functional analysis demonstrated that GC cell growth is dependent on both pathways, with combined pathway inhibition leading to the most significant suppressive effect on GC cell proliferation. Further we show that mTOR is downstream of Notch. We also demonstrate that both Notch and mTOR are required to support growth of human gastric organoids derived from non-cancer and GC tissues. Our study highlights the importance that intersecting signaling pathways may have in regulating gastric cell function during normal homeostasis, and for tumorigenesis.
Material and Methods
Mice
Adult, in house bred, C57BL/6 mice of both sexes, ages 2 months or greater, were used for experiments. Mice were housed under specific pathogen free conditions in automated watered and ventilated cages on a 12-hour light/dark cycle. Prior to tissue collection, animals were fasted overnight with ad libitum access to water. To measure cellular proliferation, mice were injected with 5-ethynyl-2′-deoxyuridine (EdU, 25 mg/kg, Invitrogen) 2 hours prior to tissue collection, as previously described [10]. All mouse procedures were approved by the Institutional Animal Care and Use Committee of the University of Michigan.
To induce gastric tumors, mice were treated with N-nitroso-N-methylurea (MNU, 240 ppm in drinking water, Sigma) or vehicle (0.5% EtOH in drinking water) in bottles protected from light, as previously described [20]. Mice received MNU or vehicle (prepared fresh 3 times per week) for 1 week, followed by 1 week of normal drinking water, repeated for a total of 10 weeks (5 cycles on/off MNU). Stomachs were harvested from mice 9–12 months post-cessation of MNU or vehicle treatment.
Human Tissue Analysis
Surgical resections from GC patients were obtained under Institutional Review Board-approved protocols (see Supplementary Table 1 for patient demographic information). GC and adjacent non-cancer tissue were utilized for histology and immunostaining for Notch and mTOR pathway signaling, gene expression analysis by measuring mRNA abundance of Notch pathway components, or for generation of gastric organoid cultures, as detailed in Supplementary Table 1.
Tissue Collection and Histological Analysis
Mouse and human gastric tissues were fixed and paraffin-embedded, as previously described [10], [21]. Immunostaining was carried out as previously described [9], [22], using antibodies to cleaved Notch1 (NICD), HES1, and phosphorylated S6 ribosomal protein (pS6), as described in Supplementary Table 2. Staining was visualized with appropriate secondary antibodies (1:400, Invitrogen), as previously described [10]. For NICD immunostaining, signal was visualized using the Tyramide Signal Amplification Superboost kit (Invitrogen #B40943), according to manufacturer's instructions. Digital imaging was performed using a Nikon E800 fluorescence microscope.
TCGA Database Analysis
Gene expression data of Notch pathway components and target genes in human gastric adenocarcinoma and adjacent normal tissue were obtained from TCGA (doi:10.7908/C11G0KM9) using the FireBrowse portal (http://firebrowse.org/; Broad Institute of MIT and Harvard, Cambridge, Massachusetts, USA). The methods of sample acquisition and mRNA sequencing for this database, and the molecular classification of GC were previously reported [5]. mRNA expression of Notch receptors (NOTCH1, NOTCH2, NOTCH3, NOTCH4), ligands (DLL1, DLL3, DLL4, JAG1, JAG2) and target genes (HES1, HES2, HES3, HES4, HES5, HES6, HES7, HEY1, HEY2, HEYL) obtained by RNAseq analysis was compared between gastric adenocarcinoma and normal gastric tissue. For associations of Notch pathway components and GC subtype, all gastric adenocarcinomas were stratified into four previously identified molecular subgroups [5]: 1) chromosomal instability (CIN), 2) microsatellite instability (MSI), 3) Epstein–Barr virus (EBV)-positive, and 4) genomically stable (GS), using data recently reported by the TCGA Research Network [23].
Human Gastric Organoid Culture
Human gastric organoids were established from gastric adenocarcinoma and adjacent non-cancer tissue, which were cultured as previously described [9] (Supplementary Table 1). For Notch and mTOR pathway regulation of human gastric organoid growth, organoids were treated 24 hours after passage with vehicle (DMSO), the gamma-secretase inhibitor N-[N-(3,5-difluorophenacetyl-L-alanyl)]-(S)-phenylglycine-t-butyl ester (DAPT, 1–20 μM, EMD4Biosciences), the mTORC1 inhibitor rapamycin (250 nM, LC Laboratories), the pan-mTOR inhibitor Torin1 (250 nM, Tocris Bioscience), or a combination of inhibitors. Inhibitors were renewed in culture media every other day for 5 days, and organoids were harvested the following day for imaging and growth analysis. To measure organoid cell proliferation, EdU (10 μM) was added to the culture media for 2 hours prior to harvesting organoids for whole-mount staining, as previously described [8]. Organoid experiments were performed in technical triplicates, in established organoid cultures that had been passaged at least 3 times prior to inhibitor treatment.
Morphometrics
Morphometric analysis of human gastric organoid size was performed using ImageJ software (1.51 s, Wayne Rasband, National Institutes of Health). The area of at least 75 organoids per treatment group was measured.
Cell Lines
Human GC cell lines AGS, MKN45 and NCI-N87 were cultured as previously described [24]. AGS and NCI-N87 were authenticated by American Type Culture Collection (ATCC) short-tandem repeat profiling (September 2018). For analysis of Notch and mTOR pathway regulation of proliferation, cells were seeded in 96-well plates at 1x103 cells/well in quadruplicate, cultured overnight and treated with vehicle (DMSO), the Notch inhibitor DAPT (20–40 μM), the mTORC1 inhibitor rapamycin (250 nM), or DAPT + rapamycin, renewed daily. Proliferation was measured using the CellTiter 96 Aqueous One Solution Cell Proliferation Assay (Promega #G3580), according to manufacturer's instructions. Cells were incubated with the CellTiter reagent for 2 hours prior to measuring cell proliferation via absorbance at 490 nm using a Perkin Elmer Wallac Victor3 1420 plate reader/luminometer.
Western Blot Analysis
MKN45 cells were seeded in a 6 well plate at 5×103 cells/well in triplicate, cultured overnight and treated with vehicle (DMSO) or 30 μM DAPT, which was renewed daily for 5 days. Cells were collected and lysed to obtain whole-cell extracts and immunoblotting was performed as previously described [25]. Briefly, after cell lysis, solubilized protein concentrations were quantified by Bio-Rad protein assay (#5000006) as per manufacturer's instructions. Proteins were resolved on 10% SDS-polyacrylamide gels and transferred to polyvinylidene difluoride membrane (PVDF), blocked with 3% dry milk, followed by immunoblotting with primary antibodies (pAKT, tAKT, p4EBP1, t4EBP1, pS6, tS6, β-actin). Antibody source and concentrations used are listed in Supplementary Table 3. Blots were imaged using a Bio-Rad Chemidoc Gel Imaging System and protein bands were quantified using ImageJ software.
Gene Expression Analysis
Total RNA from human tissue was isolated using Trizol (Invitrogen), followed by column purification and DNase I treatment, as previously described [9]. RNA was isolated from GC cell lines 6 days post-passage by harvesting cells in lysis buffer (RLT, Qiagen) with β-mercaptoethanol (10 μL/mL), followed by column purification and DNase I treatment, as previously described [10]. cDNA was prepared as previously described [10]. RT-qPCR was performed as previously described [22], using the primer sets listed in Supplementary Table 4 to measure the mRNA abundance of Notch receptors and ligands. Data was normalized to GAPDH (cell lines) or ACTB (human gastric tissue).
Statistical Analysis
Quantitative data sets were analyzed using GraphPad Prism software. Data are presented as mean ± SEM and analyzed using Student's t-test, one- or two-way ANOVA with Sidak post hoc test. P < .05 was considered significant.
Results
Notch and mTOR are Activated in Mouse Gastric Tumors
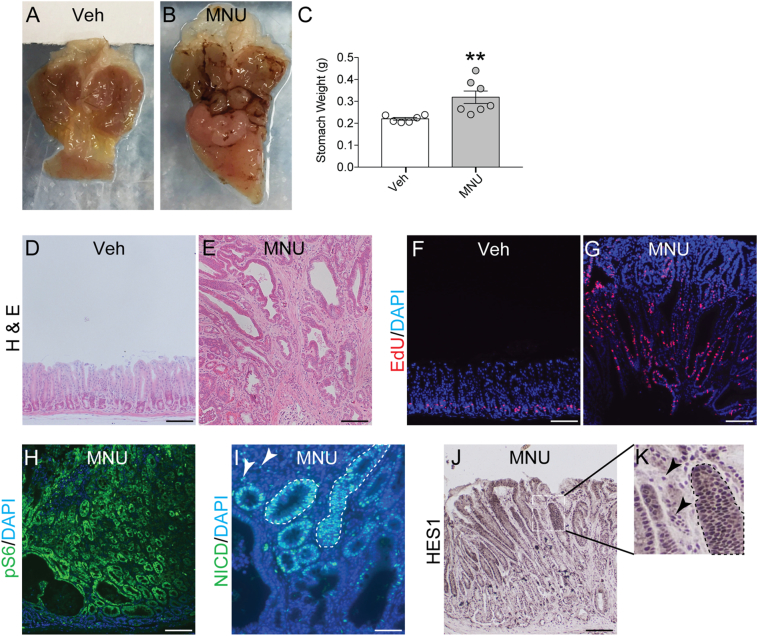
We previously demonstrated in mouse genetic models that constitutive Notch pathway activation in LGR5+ gastric stem cells led to increased stem cell proliferation and the development of antral polyps, which showed mTOR pathway activation [10]. Here we used the chemical carcinogen MNU to induce gastric tumors in mice and asked whether these chemically induced gastric tumors also exhibit Notch and mTOR pathway activation. Compared to vehicle-treated mice, MNU-treated mice developed multiple tumors that were primarily localized to the gastric antrum and antral/duodenal boundary (Figure 1, A and B). Such tumors caused a significant increase in overall stomach weight in MNU vs. vehicle-treated mice (Figure 1C). Histologically, tumors contained both epithelial and stromal cells, and exhibited a dramatic increase in epithelial cell proliferation, compared to control tissue (Figure 1, D–G). Tumors also demonstrated activation of the mTOR signaling pathway, as shown by strong expression of the mTORC1 signaling target pS6 (Figure 1H). Additionally, MNU-induced gastric tumors exhibited activation of the Notch signaling pathway, evidenced by increased expression of the NOTCH1 receptor signaling fragment NICD and the Notch target gene HES1 (Figure 1, I–K). These findings suggest that gastric tumors may depend on both Notch and mTOR signaling for promoting growth. Interestingly, NICD and HES1 staining showed that Notch pathway activation was localized to both stromal and epithelial cell compartments (Figure 1, I–K).
Figure 1.
Notch and mTOR pathway activation in mouse gastric tumors induced by N-nitroso-N-methylurea (MNU). (A-B) Gross morphology of stomachs isolated from control (Veh) or MNU-treated mice. (C) Stomach wet weight in control and MNU-treated mice 9–12 months post-treatment. (D&E) Histology of control antrum and an MNU-induced antral tumor assessed by H&E staining. (F&G) Epithelial cell proliferation was assessed by EdU incorporation (red), with DAPI nuclear stain (blue). (H-K) mTOR and Notch pathway activity in MNU-induced tumors was determined via immunostaining for pS6 (H), NICD (I) or Hes1 (J&K). Examples of epithelial and stromal Notch activity are indicated by the dotted outlines or arrowheads, respectively. DAPI or hematoxylin was used as nuclear counterstain. Data are presented as mean ± SEM (n = 6–7 mice/group). **P < .01 vs. control. Scale bars: 100 (D-H, J) or 50 μm (I).
Notch and mTOR are Activated in Human GC
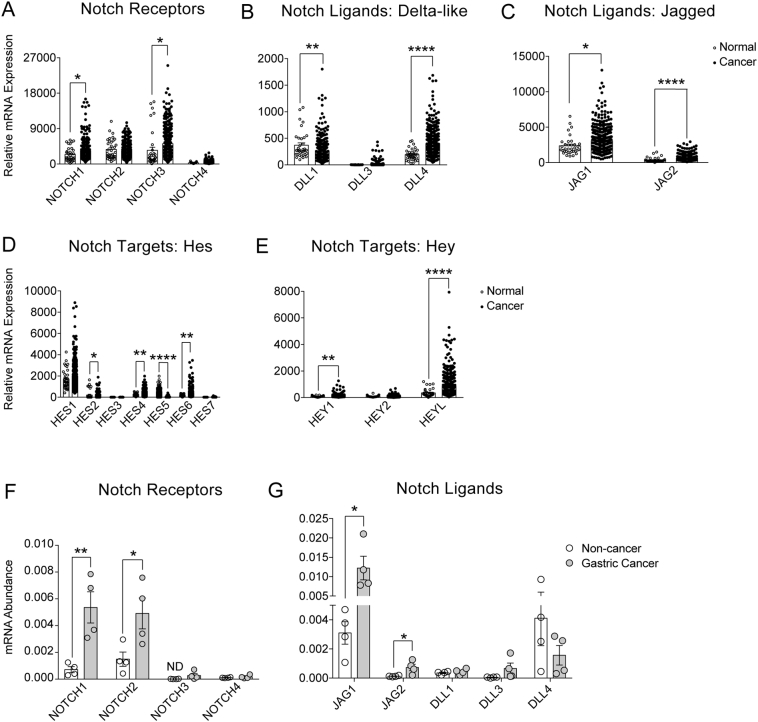
To examine Notch pathway activation in human GC, we first analyzed RNA sequencing data compiled in the gastric adenocarcinoma TCGA database [5]. Analysis of 35 normal and 415 gastric adenocarcinomas revealed significant Notch pathway activation in GC, with upregulation of receptors NOTCH1 and NOTCH3 (Figure 2A), ligands DLL1, DLL4, JAG1 and JAG2 (Figure 2, B and C) and target genes HES2, HES4–6, HEY1 and HEYL (Figure 2, D and E). We further analyzed the GC TCGA database to ask whether increased expression of these Notch pathway components was particularly associated with any of the 4 molecular subgroups of GC: CIN, MSI, EBV, or GS (Supplementary Figure 1). Of note, GCs from the EBV subgroup were not associated with increased NOTCH1 or NOTCH3 expression, whereas GCs from the CIN, MSI and GS subgroups had increased NOTCH1, and CIN and GS also showed increased NOTCH3. All 4 subgroups demonstrated increased expression of the Notch ligand DLL4. Interestingly, DLL1 was either decreased or unchanged among the GC subgroups. The Notch ligand JAG1 was also increased in all subgroups except for CIN, whereas JAG2 was upregulated in all subgroups except for EBV. Thus, although there are some variations among the subgroups, increased expression of Notch components is commonly observed.
Figure 2.
Increased Notch pathway component expression in human gastric adenocarcinoma. (A-E) Normalized RNAseq data from The Cancer Genome Atlas (TCGA) database examining 35 normal stomach and 415 gastric adenocarcinoma tissue samples. Gene expression analysis of (A) Notch receptors, (B&C) Notch ligands and (D&E) Notch target genes in normal (white circles) and gastric adenocarcinoma (black circles) samples. Analysis of the 4 molecular subtypes of gastric adenocarcinomas is presented in Supplementary Figure 1. (F&G) RT-qPCR analysis of Notch receptors (F) and Notch ligands (G) in paired non-cancer (white circles) and gastric cancer (gray circles) full-thickness tissue obtained from Michigan Medicine patients. See Supplementary Table 1 for information related to patient samples. Data are presented as mean ± SEM. *P < .05, **P < .01, ****P < .0001 vs. normal or non-cancer.
We further analyzed expression of Notch receptors and ligands in human GC by analyzing a small number of paired non-cancer and GC tissues obtained locally (Supplementary Table 1). Consistent with the larger-scale TCGA database analysis, we detected increased expression of Notch pathway components, with significant increases in expression of receptors NOTCH1 and NOTCH2, and ligands JAG1 and JAG2 (Figure 2, F and G). We have previously demonstrated that during homeostasis, Notch regulation of mouse and human gastric stem cell proliferation occurs via NOTCH1 and NOTCH2 [8], [9]. Our current analysis suggests that these receptors may also play a role in increased cell proliferation associated with gastric adenocarcinoma.
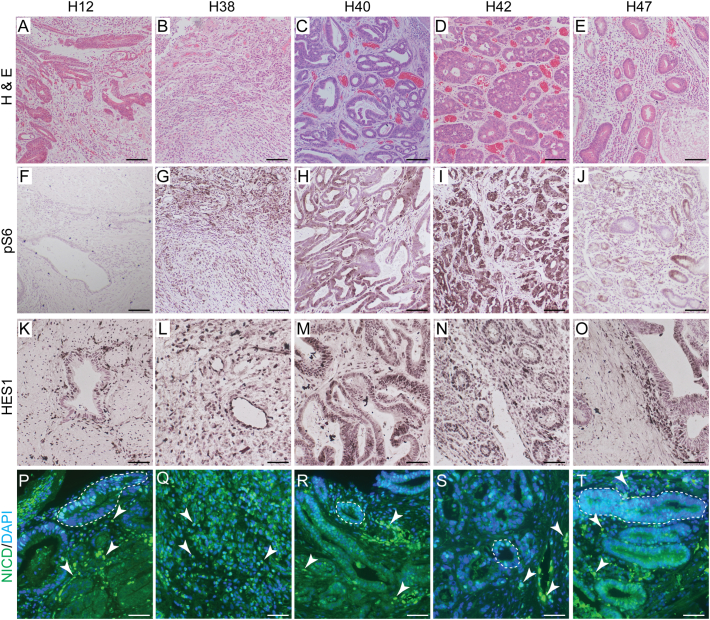
Because we previously found that activation of Notch signaling in mice increased mTORC1 signaling, we next wanted to examine activity of both signaling pathways in human GC tissue. Among the 5 primary gastric tumors analyzed, all exhibited disorganized glandular architecture (Figure 3, A–E) compared to matched non-cancer controls (Supplementary Figure 2), with significant stromal cell expansion as a large component of the tumor tissue. Importantly, all tumors demonstrated Notch pathway activation in both epithelial and stromal cells, as shown by widespread expression of the Notch target HES1 (Figure 3, K–O), and the Notch signaling fragment NICD (Figure 3, P–T). Additionally, 4 out of 5 tumors showed strong levels of the mTORC1 target pS6 (H38, H40, H42, H47), while one showed minimal (H12) pS6 expression (Figure 3, F–J), highlighting the molecular differences in GC that occurs at the individual patient level. Taken together, these data further suggest that both Notch and mTORC1 pathways are commonly activated in human gastric adenocarcinoma.
Figure 3.
Notch and mTOR pathway activation in human gastric adenocarcinoma tissue. (A-E) Histology of 5 primary gastric adenocarcinomas was assessed by H&E staining. Comparative histology of adjacent non-cancer tissue from the same patients is presented in Supplementary Figure 2. (F-J) mTOR pathway activity was detected via pS6 immunohistochemistry (brown). (K-T) Notch pathway activity was detected by immunostaining for (K-O) Hes1 (brown) or (P–T) NICD (green). (A-O) Hematoxylin or (P-T) DAPI was used as nuclear counterstains. Epithelial and stromal Notch activity are indicated by the dotted lines or arrowheads, respectively. Scale bars: (A-J) 100 or (K-T) 50 μm.
Notch and mTOR Promote GC Cell Line Proliferation
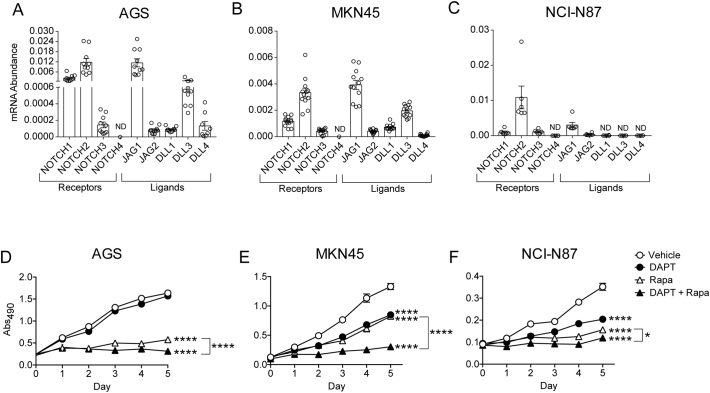
We measured the expression of Notch receptors and ligands in the human GC cell lines AGS, MKN45 and NCI-N87. Analysis of mRNA abundance demonstrated broad expression of Notch pathway components, with NOTCH1 and NOTCH2 having highest receptor expression (Figure 4, A–C), consistent with our previous studies in both mouse and human stomach [8], [9]. The JAG1 ligand was the predominant ligand expressed in all GC cell lines analyzed, with varying expression of JAG2 and DLL ligands also observed. DLL ligand expression was not detected in NCI-N87 cancer cells (Figure 4C), highlighting the genetic variation that occurs among gastric tumors [5].
Figure 4.
Notch and mTOR pathway signaling are both required for human gastric cancer cell growth. (A-C) Gene expression analysis of Notch pathway components in AGS (A), MKN45 (B), and NCI-N87 (C) human gastric cancer cell lines by RT-qPCR. (D-F) Cell growth was measured using a colorimetric assay kit in human gastric cancer cell lines AGS (D), MKN45 (E) and NCI-N87(F), after treatment with vehicle (Veh; white circles), the Notch inhibitor DAPT (30 μM; black circles), the mTORC1 inhibitor rapamycin (Rapa; 250 nM; white triangles), or a combination of both inhibitors (black triangles). Data are presented as mean ± SEM, with n = 6–12 technical replicates (A-C) or n = 4 technical replicates (D-F) for each cell line. *P < .05, ****P < .0001 vs. Day 5 vehicle, or as indicated on graph. ND = not detected.
To test the functional significance of Notch and mTOR pathway expression in human GC, we next treated human GC cell lines with either DAPT (Notch inhibition), rapamycin (mTORC1 inhibition) or both inhibitors in combination, and measured cell growth over 5 days with a colorimetric assay. We found that both Notch and mTORC1 inhibition alone showed an overall reduction in cancer cell growth (Figure 4, D–F), with the exception of AGS, which in this experiment, was not sensitive to Notch inhibition (Figure 4D). To determine if a higher dose of DAPT was required to inhibit AGS cell growth we performed a dose response analysis, comparing AGS to MKN45 cells. A higher DAPT dose was required to inhibit AGS cell growth (Supplementary Figure 3), which may relate to the higher level of expression of Notch components in this GC line (Figure 4). Thus, all three GC cell lines exhibited slower growth when Notch was inhibited. Combined pathway inhibition with DAPT and rapamycin produced the largest reduction in cancer cell growth, compared to inhibiting either pathway alone. This was observed in all three GC cell lines (Figure 4, D–F). These findings highlight the importance of these two signaling pathways to promote GC cell proliferation.
mTORC1 Signaling is Dependent on Notch Signaling
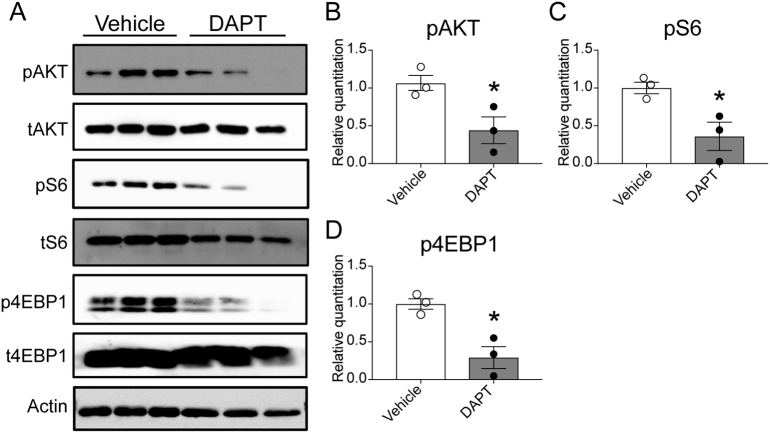
Our previously published mouse genetic studies suggested that mTORC1 might be activated by Notch signaling [10]. We tested this concept in the human GC cell line MKN45, measuring mTORC1 signaling in cells treated with vehicle or DAPT. mTORC1 activity was assessed by Western blot analysis of phosphorylation of targets AKT, S6 and 4EBP. This analysis showed a decrease in the amount of target phosphorylation in DAPT-treated cells (Figure 5A). Quantitation confirmed that pAKT, pS6 and p4EBP were significant reduced, demonstrating that mTORC1 activity is Notch dependent (Figure 5, B–D). This finding suggests that mTORC1 activation is downstream of Notch signaling in GC cells.
Figure 5.
mTORC1 activity is dependent on Notch signaling in gastric cancer cells. MKN45 cells were treated daily with DAPT (30 μM) or vehicle for 5 days and cells were collected for protein extraction. (A) Western blot analysis for mTORC1 pathway targets, measuring phosphorylated (p) or total (t) protein, with β-actin loading control. (B-D) Quantification of phospho-AKT/total-AKT (B), phosphor-S6/total S6 (C) and phospho-4EBP/total-4EBP. Data are presented as mean ± SEM, with 3 independent cultures. *P < .05 vs. vehicle by student's t-test.
Notch and mTOR Promote Human Gastric Organoid Growth
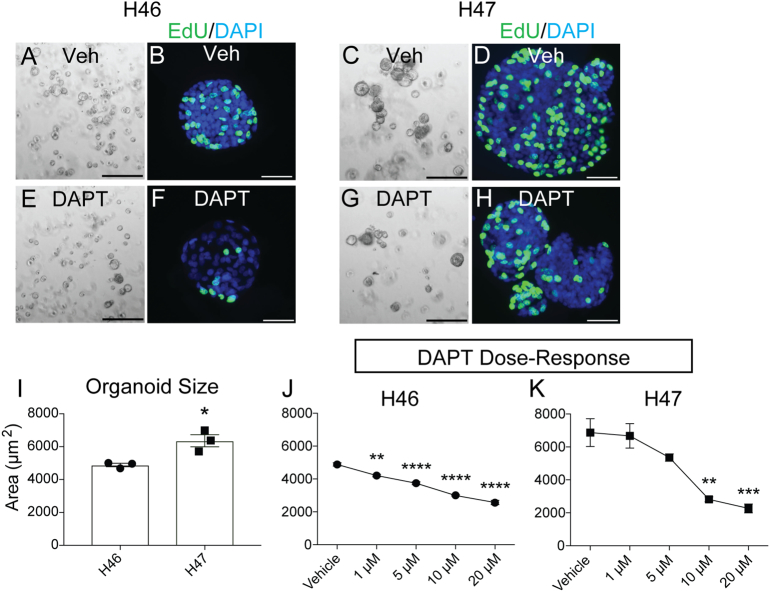
We next examined Notch regulation of human GC cell growth in an organoid model. Organoid lines were established from paired non-cancer (H46) and GC (H47) tissue from the same patient (Supplementary Table 1). GC organoids appeared to grow larger than non-cancer organoids (Figure 6, A and C). Measurement of organoid size confirmed that H47 GC organoids were approximately 40% larger than H46 non-cancer organoids (Figure 6I), suggesting enhanced cellular proliferation. Increased proliferation in GC organoids was confirmed by imaging EdU incorporation (Figure 6, B and D). Treatment of both non-cancer and GC-derived organoid cultures with DAPT revealed a significant reduction in organoid size and cell proliferation (Figure 5, E–H), compared to vehicle-treated organoids (Figure 6, A–D). Interestingly, GC-derived organoids required 10-fold increased concentration of DAPT to inhibit growth (Figure 5, J and K). Growth inhibition was observed in H46 with 1 μM DAPT (Figure 6J), while significant inhibition of H47 growth required 10 μM DAPT (Figure 6K). This result suggests that GC organoids have enhanced Notch signaling, which follows our earlier observation of increased Notch ligand and receptor expression (Figure 2, F and G), and high Notch signaling (Figure 3) observed in primary GC tissue.
Figure 6.
Notch regulates growth of human gastric cancer-derived organoids. Human gastric organoids from paired non-cancer (H46) and gastric cancer (H47) tissue isolated from the same patient were treated with Veh or DAPT (10 μM), renewed every other day for 5 days. Morphology of non-cancer (A, E) and gastric cancer (C, G)-derived organoids after 5 days of treatment. Cell proliferation was assessed by EdU incorporation (green) in non-cancer (B,F) and gastric cancer (D,H) organoids. (I) Morphometric analysis of organoid size in Veh-treated H46 non-cancer and H47 gastric cancer organoids. (J,K) Dose response of non-cancer and gastric cancer organoid growth in various doses of DAPT, as indicated. Data are presented as mean ± SEM, with n = 3 technical replicates for each organoid line. *P < .05, **P < .01, ***P < .001, ****P < .0001 vs. vehicle. Scale bars: (A,C,E,G) 500 μm or (B,D,F,H) 50 μm.
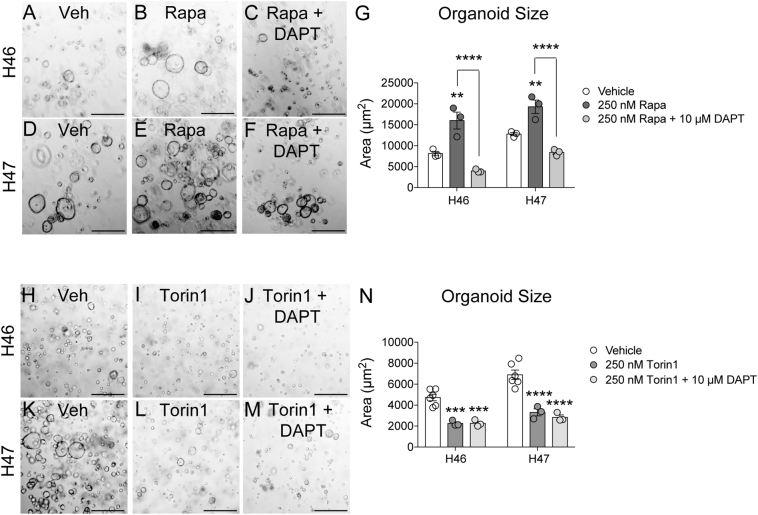
We next examined mTOR pathway regulation of human gastric organoid growth. Surprisingly, treatment of both H46 and H47 organoids with the mTORC1 inhibitor rapamycin significantly increased organoid growth (Figure 7, A–G). This contrasts with the reduced cell growth observed in rapamycin-treated GC cell lines (Figure 4). Combined pathway inhibition by treatment with both DAPT and rapamycin effectively reduced organoid growth, suggesting that Notch is the dominant pathway supporting gastric cell proliferation.
Figure 7.
mTOR signaling is required for human gastric cancer organoid growth. (A-G) Human gastric organoids from paired (A-C) non-cancer (H46) and (D-F) gastric cancer (H47) tissues were treated with (A&D) vehicle, (B&E) the mTORC1 inhibitor rapamycin (250 nM), or (C&F) 10 μM DAPT +250 nM rapamycin, renewed every other day for 5 days. (G) Morphometric analysis of organoid size after 5 days of inhibitor treatment. (H-N) Paired non-cancer (H-J) and gastric cancer (K-M) tissues were treated with (H&K) vehicle, (I&L) the pan-mTOR inhibitor Torin1 (250 nM), or (J&M) 10 μM DAPT +250 nM Torin1, renewed every other day for 5 days. (N) Morphometric analysis of organoid size after 5 days of inhibitor treatment. Data are presented as mean ± SEM, with n = 3–6 technical replicates for each organoid line. **P < .01, ***P < .001, ****P < .0001 vs. vehicle or as indicated on graph. Scale bars: 500 μm.
We next treated gastric organoid cultures with the pan-mTOR inhibitor Torin1 to block both mTORC1 and mTORC2 signaling. In contrast to the enhanced growth observed after rapamycin treatment, mTOR inhibition with Torin1 significantly reduced growth of both non-cancer (H46) and GC (H47) organoids (Figure 7), thus suggesting that mTOR does promote GC organoid growth, potentially via both mTORC1 and mTORC2. Combined Notch and mTOR inhibition significantly reduced organoid growth to levels observed with mTOR inhibition alone (Figure 6J, M–N).
Together our functional studies in GC cell lines and organoids suggest that GC cell proliferation is promoted by both Notch and mTOR signaling, that mTORC1 is downstream of Notch signaling, and that use of a pan-mTOR inhibitor may be more effective in reducing cellular proliferation than targeting mTORC1 alone.
Discussion
In this study, we report that both Notch and mTOR signaling pathways are activated in GC to promote cancer cell proliferation. Elevated Notch pathway and mTOR pathway activity were detected in mouse gastric tumors induced by the chemical carcinogen MNU. Thus, activation of these two pathways appears to be a component of tumorigenesis in the mouse stomach. We also report for the first time a complete analysis of Notch ligand and receptor mRNA expression in hundreds of human gastric adenocarcinomas via analysis of the TCGA stomach cancer data base, in a small collection of locally obtained GC samples, and in 3 independent human GC cell lines. This analysis demonstrated that expression of specific Notch pathway components is increased in human GC, including both Notch ligands and receptors. We also tested functional dependence of human GC cells on Notch and mTOR pathway signaling by treatment of GC cell lines and GC patient-derived human gastric organoids with pathway specific inhibitors. Overall our results show that both Notch and mTOR pathways are activated in mouse gastric tumors and human GC tissues, that mTORC1 activation is Notch-dependent, and that both signaling pathways promote GC cell growth and cellular proliferation.
Our observation that both Notch and mTOR pathways are activated in mouse and human gastric tumors suggest that these pathways may be involved in tumorigenesis, since both pathways play important roles in regulating cellular proliferation. Notch is required for LGR5+ gastric antral stem cell function in mouse, regulating stem cell proliferation via NOTCH1 and NOTCH2 [8], [9], [10]. Our previous studies in mouse demonstrated functional interactions between Notch and mTOR [10]. Treatment of adult mice with a gamma-secretase inhibitor to inhibit Notch signaling led to reduced pS6 staining, suggesting reduced mTORC1 pathway activity. Conversely, activation of Notch in mouse LGR5+ antral stem cells using a genetic approach, resulted in increased expression of pS6, particularly in NICD-driven gastric tumors. Together these findings suggest that these two pathways interact to promote gastric proliferation. Indeed, treatment of Notch-activated mice with the mTORC1 inhibitor rapamycin significantly attenuated NICD-induced tissue expansion via suppression of hyperproliferation and antral gland fission [10], suggesting that Notch activation dysregulates gastric epithelial cells via activation of mTORC1, and that these two pathways cooperate to induce and sustain gastric hyperproliferative cell growth in mouse. The current study suggests similar interactions between Notch and mTOR in human GC.
mTOR is well-established as an important pathway promoting cell growth and proliferation. However, in the normal mouse stomach, inhibition of mTORC1 signaling with rapamycin does not perturb gastric epithelial cell homeostasis [10], suggesting that this pathway is not required for maintenance of normal stem cell proliferation in the stomach. This contrasts with Notch signaling, which is required for maintenance of the gastric stem cell pool [10]. It has previously been reported that mTOR is activated in mouse models of gastric cancer [26] as well as in human gastric cancer [27], [28], [29], [30]. It is possible that gastric stem cells are highly sensitive to dysregulation of this pathway during tumorigenesis, even though the contribution of mTOR signaling to normal gastric epithelial cell homeostasis appears to be minimal, at least in the mouse [10]. Interestingly, the EBV subgroup of human GC is highly associated with PIK3CA mutations, which are known to lead to increased mTOR signaling [5]. Additionally, our functional studies in human GC cell lines suggest that GC cell growth is more sensitive to mTOR inhibition than Notch inhibition.
Our findings suggest that human GC cellular proliferation may be sustained by a complex interaction between Notch and mTOR signaling. In addition to observing that both the Notch and mTOR pathways are activated in human GC, we used GC cell lines and patient-derived gastric organoids to demonstrate that GC cell proliferation in culture is dependent on both Notch and mTOR signaling. Inhibition of either Notch or mTORC1 alone in human GC cell lines resulted in reduced cell growth, with combined pathway inhibition further inhibiting growth, suggesting that Notch and mTOR coordinately promote GC cell proliferation, and highlighting the dependence of GC cells on both pathways for sustained growth. Further, our findings suggest that Notch signaling enhances mTORC1 signaling in human GC. Our functional analysis is consistent with previous reports showing activation of both pathways in human gastric cancer [16], [17], [18], [19], [28], [29], [30]. How these pathway interactions lead to dysregulated cell proliferation to support tumorigenesis, and the potential therapeutic targeting of these pathways in combination, remain important future areas of investigation.
The development of gastric organoid culture technology has provided a powerful experimental tool to probe pathway regulation of cultured stem cells in an epithelial-specific setting [31], [32], [33], [34]. Our data from human gastric organoids suggest that both Notch and mTOR are required to support growth of human gastric stem cells derived from non-cancer and GC tissues. Both pan-Notch and pan-mTOR inhibition reduced non-cancer and GC organoid growth, highlighting the importance of both pathways for regulating human gastric stem/progenitor cell proliferation.
Paradoxically, mTORC1 inhibition with rapamycin stimulated human gastric organoid growth. mTORC1 participates in a negative-feedback loop in which it promotes cell growth and survival and inhibits apoptosis via suppression of PI3K signaling and mTORC2 [35]. It is known that prolonged mTORC1 inhibition with rapalogs such as rapamycin can relieve this inhibition and consequentially activate PI3K/Akt signaling, leading to enhanced cell proliferation (reviewed in [11], [36]). This may explain the paradoxical increased organoid growth after rapamycin treatment. Combined therapy targeting both Notch and mTOR (mTORC1 and mTORC2) signaling may be an effective approach to slow growth of gastric adenocarcinomas.
Conclusions
Expression of Notch pathway ligands, receptors and downstream targets are increased in human gastric adenocarcinomas. Accordingly, increased Notch signaling was confirmed in gastric cancer tissues by detection of nuclear NICD, with signaling detected in both epithelial and stromal cell compartments. Similar to previous studies in mouse, Notch activation in human gastric cancers was associated with increased mTOR signaling. Functional analysis in cultured human gastric cancer cell lines and in gastric cancer organoids demonstrated that both signaling pathways act to promote cellular proliferation. Thus, Notch and mTOR appear to be important signaling pathways promoting growth of gastric adenocarcinomas.
Acknowledgments
Acknowledgments
We would like to thank Gail Gifford for technical assistance with organoid establishment, Jason Spence and the Translational Tissue Modeling Laboratory at the University of Michigan for generation of L-WRN conditioned media for organoid culture, and the Tissue Procurement staff in the University of Michigan Department of Pathology for preparation of human gastric tissue. We would also like to thank the patients who generously agreed to provide tissue samples for this study.
Funding
E.S.H. was supported by AACR-Debbie's Dream Foundation 16–20-41-DEMI and NIH K01-DK111710. N.R. was supported by an AASLD Pinnacle Award. G.W. was supported by an Undergraduate Research Excellence Summer Fellowship through The American Physiological Society. The research was funded by an NIH P01-DK06041 project award to L.C.S., and core support from the Michigan Gastrointestinal Research Center Grant NIH P30-DK34933, and the University of Michigan Cancer Center Support Grant NCI P30-CA6592.
Author contributions
E.S.H. obtained funding, designed and performed experiments, analyzed data and wrote the manuscript. N.R. analyzed data and wrote the manuscript. Y.M.S., S.S., T.M.K, and G.W. performed experiments and analyzed data. L.C.S. obtained funding, designed experiments and wrote the manuscript. All authors critically reviewed the manuscript.
Footnotes
Declarations of Interest: None.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.neo.2019.05.002.
Appendix A. Supplementary data
Supplementary material
References
- 1.Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]; Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A (2015). Global cancer statistics, 2012 CA Cancer J Clin 65, 87-108. [DOI] [PubMed]
- 2.Abbas M, Faggian A, Sintali DN, Khan GJ, Naeem S, Shi M, Dingding C. Current and future biomarkers in gastric cancer. Biomed Pharmacother. 2018;103:1688–1700. doi: 10.1016/j.biopha.2018.04.178. [DOI] [PubMed] [Google Scholar]; Abbas M, Faggian A, Sintali DN, Khan GJ, Naeem S, Shi M, Dingding C (2018). Current and future biomarkers in gastric cancer Biomed Pharmacother 103, 1688-1700. [DOI] [PubMed]
- 3.Yao Y, Ni Y, Zhang J, Wang H, Shao S. The role of Notch signaling in gastric carcinoma: molecular pathogenesis and novel therapeutic targets. Oncotarget. 2017;8:53839–53853. doi: 10.18632/oncotarget.17809. [DOI] [PMC free article] [PubMed] [Google Scholar]; Yao Y, Ni Y, Zhang J, Wang H, Shao S (2017). The role of Notch signaling in gastric carcinoma: molecular pathogenesis and novel therapeutic targets Oncotarget 8, 53839-53853. [DOI] [PMC free article] [PubMed]
- 4.Shimizu D, Kanda M, Kodera Y. Emerging evidence of the molecular landscape specific for hematogenous metastasis from gastric cancer. World J Gastrointes Oncol. 2018;10:124–136. doi: 10.4251/wjgo.v10.i6.124. [DOI] [PMC free article] [PubMed] [Google Scholar]; Shimizu D, Kanda M, Kodera Y (2018). Emerging evidence of the molecular landscape specific for hematogenous metastasis from gastric cancer World J Gastrointes Oncol 10, 124-136. [DOI] [PMC free article] [PubMed]
- 5.Cancer Genome Atlas Research N Comprehensive molecular characterization of gastric adenocarcinoma. Nature. 2014;513:202–209. doi: 10.1038/nature13480. [DOI] [PMC free article] [PubMed] [Google Scholar]; Cancer Genome Atlas Research N (2014). Comprehensive molecular characterization of gastric adenocarcinoma Nature 513, 202-209. [DOI] [PMC free article] [PubMed]
- 6.Demitrack ES, Samuelson LC. Notch as a Driver of Gastric Epithelial Cell Proliferation. Cell Mol Gastroenterol Hepatol. 2017;3:323–330. doi: 10.1016/j.jcmgh.2017.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]; Demitrack ES, Samuelson LC (2017). Notch as a Driver of Gastric Epithelial Cell Proliferation Cell Mol Gastroenterol Hepatol 3, 323-330. [DOI] [PMC free article] [PubMed]
- 7.Demitrack ES, Samuelson LC. Notch regulation of gastrointestinal stem cells. J Physiol. 2016;594:4791–4803. doi: 10.1113/JP271667. [DOI] [PMC free article] [PubMed] [Google Scholar]; Demitrack ES, Samuelson LC (2016). Notch regulation of gastrointestinal stem cells J Physiol 594, 4791-4803. [DOI] [PMC free article] [PubMed]
- 8.Demitrack ES, Gifford GB, Keeley TM, Horita N, Todisco A, Turgeon DK, Siebel CW, Samuelson LC. NOTCH1 and NOTCH2 regulate epithelial cell proliferation in mouse and human gastric corpus. Am J Physiol Gastrointest Liver Physiol. 2017;312:G133–G144. doi: 10.1152/ajpgi.00325.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]; Demitrack ES, Gifford GB, Keeley TM, Horita N, Todisco A, Turgeon DK, Siebel CW, Samuelson LC (2017). NOTCH1 and NOTCH2 regulate epithelial cell proliferation in mouse and human gastric corpus Am J Physiol Gastrointest Liver Physiol 312, G133-G144. [DOI] [PMC free article] [PubMed]
- 9.Gifford GB, Demitrack ES, Keeley TM, Tam A, La Cunza N, Dedhia PH, Spence JR, Simeone DM, Saotome I, Louvi A. Notch1 and Notch2 receptors regulate mouse and human gastric antral epithelial cell homoeostasis. Gut. 2017;66:1001–1011. doi: 10.1136/gutjnl-2015-310811. [DOI] [PMC free article] [PubMed] [Google Scholar]; Gifford GB, Demitrack ES, Keeley TM, Tam A, La Cunza N, Dedhia PH, Spence JR, Simeone DM, Saotome I, Louvi A, et al. (2017). Notch1 and Notch2 receptors regulate mouse and human gastric antral epithelial cell homoeostasis Gut 66, 1001-1011. [DOI] [PMC free article] [PubMed]
- 10.Demitrack ES, Gifford GB, Keeley TM, Carulli AJ, VanDussen KL, Thomas D, Giordano TJ, Liu Z, Kopan R, Samuelson LC. Notch signaling regulates gastric antral LGR5 stem cell function. EMBO J. 2015;34:2522–2536. doi: 10.15252/embj.201490583. [DOI] [PMC free article] [PubMed] [Google Scholar]; Demitrack ES, Gifford GB, Keeley TM, Carulli AJ, VanDussen KL, Thomas D, Giordano TJ, Liu Z, Kopan R, Samuelson LC (2015). Notch signaling regulates gastric antral LGR5 stem cell function EMBO J 34, 2522-2536. [DOI] [PMC free article] [PubMed]
- 11.Saxton RA, Sabatini DM. mTOR signaling in growth, metabolism, and disease. Cell. 2017;168:960–976. doi: 10.1016/j.cell.2017.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]; Saxton RA, Sabatini DM (2017). mTOR Signaling in Growth, Metabolism, and Disease Cell 168, 960-976. [DOI] [PMC free article] [PubMed]
- 12.Sengupta S, Peterson TR, Sabatini DM. Regulation of the mTOR complex 1 pathway by nutrients, growth factors, and stress. Mol Cell. 2010;40:310–322. doi: 10.1016/j.molcel.2010.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]; Sengupta S, Peterson TR, Sabatini DM (2010). Regulation of the mTOR complex 1 pathway by nutrients, growth factors, and stress Mol Cell 40, 310-322. [DOI] [PMC free article] [PubMed]
- 13.Chan SM, Weng AP, Tibshirani R, Aster JC, Utz PJ. Notch signals positively regulate activity of the mTOR pathway in T-cell acute lymphoblastic leukemia. Blood. 2007;110:278–286. doi: 10.1182/blood-2006-08-039883. [DOI] [PMC free article] [PubMed] [Google Scholar]; Chan SM, Weng AP, Tibshirani R, Aster JC, Utz PJ (2007). Notch signals positively regulate activity of the mTOR pathway in T-cell acute lymphoblastic leukemia Blood 110, 278-286. [DOI] [PMC free article] [PubMed]
- 14.Palomero T, Sulis ML, Cortina M, Real PJ, Barnes K, Ciofani M, Caparros E, Buteau J, Brown K, Perkins SL. Mutational loss of PTEN induces resistance to NOTCH1 inhibition in T-cell leukemia. Nat Med. 2007;13:1203–1210. doi: 10.1038/nm1636. [DOI] [PMC free article] [PubMed] [Google Scholar]; Palomero T, Sulis ML, Cortina M, Real PJ, Barnes K, Ciofani M, Caparros E, Buteau J, Brown K, Perkins SL, et al. (2007). Mutational loss of PTEN induces resistance to NOTCH1 inhibition in T-cell leukemia Nat Med 13, 1203-1210. [DOI] [PMC free article] [PubMed]
- 15.Shepherd C, Banerjee L, Cheung CW, Mansour MR, Jenkinson S, Gale RE, Khwaja A. PI3K/mTOR inhibition upregulates NOTCH-MYC signalling leading to an impaired cytotoxic response. Leukemia. 2013;27:650–660. doi: 10.1038/leu.2012.285. [DOI] [PubMed] [Google Scholar]; Shepherd C, Banerjee L, Cheung CW, Mansour MR, Jenkinson S, Gale RE, Khwaja A (2013). PI3K/mTOR inhibition upregulates NOTCH-MYC signalling leading to an impaired cytotoxic response Leukemia 27, 650-660. [DOI] [PubMed]
- 16.Yeh TS, Wu CW, Hsu KW, Liao WJ, Yang MC, Li AF, Wang AM, Kuo ML, Chi CW. The activated Notch1 signal pathway is associated with gastric cancer progression through cyclooxygenase-2. Cancer Res. 2009;69:5039–5048. doi: 10.1158/0008-5472.CAN-08-4021. [DOI] [PubMed] [Google Scholar]; Yeh TS, Wu CW, Hsu KW, Liao WJ, Yang MC, Li AF, Wang AM, Kuo ML, Chi CW (2009). The activated Notch1 signal pathway is associated with gastric cancer progression through cyclooxygenase-2 Cancer Res 69, 5039-5048. [DOI] [PubMed]
- 17.Hsu KW, Hsieh RH, Huang KH, Fen-Yau LA, Chi CW, Wang TY, Tseng MJ, Wu KJ, Yeh TS. Activation of the Notch1/STAT3/Twist signaling axis promotes gastric cancer progression. Carcinogenesis. 2012;33:1459–1467. doi: 10.1093/carcin/bgs165. [DOI] [PubMed] [Google Scholar]; Hsu KW, Hsieh RH, Huang KH, Fen-Yau LA, Chi CW, Wang TY, Tseng MJ, Wu KJ, Yeh TS (2012). Activation of the Notch1/STAT3/Twist signaling axis promotes gastric cancer progression Carcinogenesis 33, 1459-1467. [DOI] [PubMed]
- 18.Bauer L, Takacs A, Slotta-Huspenina J, Langer R, Becker K, Novotny A, Ott K, Walch A, Hapfelmeier A, Keller G. Clinical significance of NOTCH1 and NOTCH2 expression in gastric carcinomas: an immunohistochemical study. Front Oncol. 2015;5:1–8. doi: 10.3389/fonc.2015.00094. [DOI] [PMC free article] [PubMed] [Google Scholar]; Bauer L, Takacs A, Slotta-Huspenina J, Langer R, Becker K, Novotny A, Ott K, Walch A, Hapfelmeier A, Keller G (2015). Clinical Significance of NOTCH1 and NOTCH2 Expression in Gastric Carcinomas: An Immunohistochemical Study Front Oncol 5, 1-8. [DOI] [PMC free article] [PubMed]
- 19.Tapia O, Riquelme I, Leal P, Sandoval A, Aedo S, Weber H, Letelier P, Bellolio E, Villaseca M, Garcia P. The PI3K/AKT/mTOR pathway is activated in gastric cancer with potential prognostic and predictive significance. Virchows Arch. 2014;465:25–33. doi: 10.1007/s00428-014-1588-4. [DOI] [PubMed] [Google Scholar]; Tapia O, Riquelme I, Leal P, Sandoval A, Aedo S, Weber H, Letelier P, Bellolio E, Villaseca M, Garcia P, et al. (2014). The PI3K/AKT/mTOR pathway is activated in gastric cancer with potential prognostic and predictive significance Virchows Arch 465, 25-33. [DOI] [PubMed]
- 20.Yamachika T, Nakanishi H, Inada K, Tsukamoto T, Shimizu N, Kobayashi K, Fukushima S, Tatematsu M. N-methyl-N-nitrosourea concentration-dependent, rather than total intake-dependent, induction of adenocarcinomas in the glandular stomach of BALB/c mice. Jpn J Cancer Res. 1998;89:385–391. doi: 10.1111/j.1349-7006.1998.tb00575.x. [DOI] [PMC free article] [PubMed] [Google Scholar]; Yamachika T, Nakanishi H, Inada K, Tsukamoto T, Shimizu N, Kobayashi K, Fukushima S, Tatematsu M (1998). N-methyl-N-nitrosourea concentration-dependent, rather than total intake-dependent, induction of adenocarcinomas in the glandular stomach of BALB/c mice Jpn J Cancer Res 89, 385-391. [DOI] [PMC free article] [PubMed]
- 21.Lopez-Diaz L, Hinkle KL, Jain RN, Zavros Y, Brunkan CS, Keeley T, Eaton KA, Merchant JL, Chew CS, Samuelson LC. Parietal cell hyperstimulation and autoimmune gastritis in cholera toxin transgenic mice. Am J Physiol Gastrointest Liver Physiol. 2006;290:G970–G979. doi: 10.1152/ajpgi.00461.2005. [DOI] [PubMed] [Google Scholar]; Lopez-Diaz L, Hinkle KL, Jain RN, Zavros Y, Brunkan CS, Keeley T, Eaton KA, Merchant JL, Chew CS, Samuelson LC (2006). Parietal cell hyperstimulation and autoimmune gastritis in cholera toxin transgenic mice Am J Physiol Gastrointest Liver Physiol 290, G970-979. [DOI] [PubMed]
- 22.Keeley TM, Samuelson LC. Cytodifferentiation of the postnatal mouse stomach in normal and Huntingtin-interacting protein 1-related-deficient mice. Am J Physiol Gastrointest Liver Physiol. 2010;299:G1241–G1251. doi: 10.1152/ajpgi.00239.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]; Keeley TM, Samuelson LC (2010). Cytodifferentiation of the postnatal mouse stomach in normal and Huntingtin-interacting protein 1-related-deficient mice Am J Physiol Gastrointest Liver Physiol 299, G1241-1251. [DOI] [PMC free article] [PubMed]
- 23.Cancer Genome Atlas Research N Integrated genomic characterization of oesophageal carcinoma. Nature. 2017;541:169–175. doi: 10.1038/nature20805. [DOI] [PMC free article] [PubMed] [Google Scholar]; Cancer Genome Atlas Research N (2017). Integrated genomic characterization of oesophageal carcinoma Nature 541, 169-175. [DOI] [PMC free article] [PubMed]
- 24.Al Menhali A, Keeley TM, Demitrack ES, Samuelson LC. Gastrin induces parathyroid hormone-like hormone expression in gastric parietal cells. Am J Physiol Gastrointest Liver Physiol. 2017;312:G649–G657. doi: 10.1152/ajpgi.00366.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]; Al Menhali A, Keeley TM, Demitrack ES, Samuelson LC (2017). Gastrin induces parathyroid hormone-like hormone expression in gastric parietal cells Am J Physiol Gastrointest Liver Physiol 312, G649-G657. [DOI] [PMC free article] [PubMed]
- 25.Xue X, Ramakrishnan S, Anderson E, Taylor M, Zimmermann EM, Spence JR, Huang S, Greenson JK, Shah YM. Endothelial PAS domain protein 1 activates the inflammatory response in the intestinal epithelium to promote colitis in mice. Gastroenterology. 2013;145:831–841. doi: 10.1053/j.gastro.2013.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]; Xue X, Ramakrishnan S, Anderson E, Taylor M, Zimmermann EM, Spence JR, Huang S, Greenson JK, Shah YM (2013). Endothelial PAS domain protein 1 activates the inflammatory response in the intestinal epithelium to promote colitis in mice Gastroenterology 145, 831-841. [DOI] [PMC free article] [PubMed]
- 26.Thiem S, Pierce TP, Palmieri M, Putoczki TL, Buchert M, Preaudet A, Farid RO, Love C, Catimel B, Lei Z. mTORC1 inhibition restricts inflammation-associated gastrointestinal tumorigenesis in mice. J Clin Invest. 2013;123:767–781. doi: 10.1172/JCI65086. [DOI] [PMC free article] [PubMed] [Google Scholar]; Thiem S, Pierce TP, Palmieri M, Putoczki TL, Buchert M, Preaudet A, Farid RO, Love C, Catimel B, Lei Z, et al. (2013). mTORC1 inhibition restricts inflammation-associated gastrointestinal tumorigenesis in mice J Clin Invest 123, 767-781. [DOI] [PMC free article] [PubMed]
- 27.Bellacosa A, Kumar CC, Di Cristofano A, Testa JR. Activation of AKT kinases in cancer: implications for therapeutic targeting. Adv Cancer Res. 2005;94:29–86. doi: 10.1016/S0065-230X(05)94002-5. [DOI] [PubMed] [Google Scholar]; Bellacosa A, Kumar CC, Di Cristofano A, Testa JR (2005). Activation of AKT kinases in cancer: implications for therapeutic targeting Adv Cancer Res 94, 29-86. [DOI] [PubMed]
- 28.Lang SA, Gaumann A, Koehl GE, Seidel U, Bataille F, Klein D, Ellis LM, Bolder U, Hofstaedter F, Schlitt HJ. Mammalian target of rapamycin is activated in human gastric cancer and serves as a target for therapy in an experimental model. Int J Cancer. 2007;120:1803–1810. doi: 10.1002/ijc.22442. [DOI] [PubMed] [Google Scholar]; Lang SA, Gaumann A, Koehl GE, Seidel U, Bataille F, Klein D, Ellis LM, Bolder U, Hofstaedter F, Schlitt HJ, et al. (2007). Mammalian target of rapamycin is activated in human gastric cancer and serves as a target for therapy in an experimental model Int J Cancer 120, 1803-1810. [DOI] [PubMed]
- 29.Murayama T, Inokuchi M, Takagi Y, Yamada H, Kojima K, Kumagai J, Kawano T, Sugihara K. Relation between outcomes and localisation of p-mTOR expression in gastric cancer. Br J Cancer. 2009;100:782–788. doi: 10.1038/sj.bjc.6604915. [DOI] [PMC free article] [PubMed] [Google Scholar]; Murayama T, Inokuchi M, Takagi Y, Yamada H, Kojima K, Kumagai J, Kawano T, Sugihara K (2009). Relation between outcomes and localisation of p-mTOR expression in gastric cancer Br J Cancer 100, 782-788. [DOI] [PMC free article] [PubMed]
- 30.Yu G, Wang J, Chen Y, Wang X, Pan J, Li G, Jia Z, Li Q, Yao JC, Xie K. Overexpression of phosphorylated mammalian target of rapamycin predicts lymph node metastasis and prognosis of chinese patients with gastric cancer. Clin Cancer Res. 2009;15:1821–1829. doi: 10.1158/1078-0432.CCR-08-2138. [DOI] [PubMed] [Google Scholar]; Yu G, Wang J, Chen Y, Wang X, Pan J, Li G, Jia Z, Li Q, Yao JC, Xie K (2009). Overexpression of phosphorylated mammalian target of rapamycin predicts lymph node metastasis and prognosis of chinese patients with gastric cancer Clin Cancer Res 15, 1821-1829. [DOI] [PubMed]
- 31.Barker N, Huch M, Kujala P, van de Wetering M, Snippert HJ, van Es JH, Sato T, Stange DE, Begthel H, van den Born M. Lgr5(+ve) stem cells drive self-renewal in the stomach and build long-lived gastric units in vitro. Cell Stem Cell. 2010;6:25–36. doi: 10.1016/j.stem.2009.11.013. [DOI] [PubMed] [Google Scholar]; Barker N, Huch M, Kujala P, van de Wetering M, Snippert HJ, van Es JH, Sato T, Stange DE, Begthel H, van den Born M, et al. (2010). Lgr5(+ve) stem cells drive self-renewal in the stomach and build long-lived gastric units in vitro. Cell Stem Cell 6, 25-36. [DOI] [PubMed]
- 32.Bartfeld S, Bayram T, van de Wetering M, Huch M, Begthel H, Kujala P, Vries R, Peters PJ, Clevers H. In vitro expansion of human gastric epithelial stem cells and their responses to bacterial infection. Gastroenterology. 2015;148:126–136. doi: 10.1053/j.gastro.2014.09.042. [DOI] [PMC free article] [PubMed] [Google Scholar]; Bartfeld S, Bayram T, van de Wetering M, Huch M, Begthel H, Kujala P, Vries R, Peters PJ, Clevers H (2015). In vitro expansion of human gastric epithelial stem cells and their responses to bacterial infection. Gastroenterology 148, 126-136. [DOI] [PMC free article] [PubMed]
- 33.Sato T, Vries RG, Snippert HJ, van de Wetering M, Barker N, Stange DE, van Es JH, Abo A, Kujala P, Peters PJ. Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche. Nature. 2009;459:262–265. doi: 10.1038/nature07935. [DOI] [PubMed] [Google Scholar]; Sato T, Vries RG, Snippert HJ, van de Wetering M, Barker N, Stange DE, van Es JH, Abo A, Kujala P, Peters PJ, et al. (2009). Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche. Nature 459, 262-265. [DOI] [PubMed]
- 34.VanDussen KL, Marinshaw JM, Shaikh N, Miyoshi H, Moon C, Tarr PI, Ciorba MA, Stappenbeck TS. Development of an enhanced human gastrointestinal epithelial culture system to facilitate patient-based assays. Gut. 2015;64:911–920. doi: 10.1136/gutjnl-2013-306651. [DOI] [PMC free article] [PubMed] [Google Scholar]; VanDussen KL, Marinshaw JM, Shaikh N, Miyoshi H, Moon C, Tarr PI, Ciorba MA, Stappenbeck TS (2015). Development of an enhanced human gastrointestinal epithelial culture system to facilitate patient-based assays Gut 64, 911-920. [DOI] [PMC free article] [PubMed]
- 35.Saltiel AR, Kahn CR. Insulin signalling and the regulation of glucose and lipid metabolism. Nature. 2001;414:799–806. doi: 10.1038/414799a. [DOI] [PubMed] [Google Scholar]; Saltiel AR, Kahn CR (2001). Insulin signalling and the regulation of glucose and lipid metabolism. Nature 414, 799-806. [DOI] [PubMed]
- 36.Meng D, Frank AR, Jewell JL. Vol. 145. 2018. mTOR signaling in stem and progenitor cells Development; pp. 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]; Meng D, Frank AR, Jewell JL (2018). mTOR signaling in stem and progenitor cells Development 145, 1-16. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material