Abstract
For over 40 years, food-matrix certified reference materials (CRMs) have been available for determination of trace element content, and a wide variety of materials are available from most producers of CRMs. However, the availability of food-matrix CRMs for organic nutrients has been more limited. The European Commission (EC) Bureau Communautaire de Référence (BCR) and the National Institute of Standards and Technology (NIST) introduced food-matrix CRMs with values assigned for vitamins and other organic nutrients such as fatty acids and carotenoids in the 1990s. The number of organic nutrients for which values were assigned has increased significantly in the past decade, and the approach and analytical methods used for assignment of the certified values have also evolved. Recently, dietary supplement-matrix CRMs such as multivitamin tablets with values assigned for vitamins and carotenoids, and fish and plant oils with values assigned for fatty acids have appeared. The development, evolution, and improvement of food- and dietary supplement-matrix CRMs for determination of vitamins, carotenoids, and fatty acids are described, with emphasis on CRMs made available in the past 10 years. Recent food and dietary supplement CRMs for the determination of organic nutrients include infant formula, multivitamin tablets, milk and egg powders, breakfast cereal, meat homogenate, blueberries, soy flour, fish and plant oils, dry cat food, and protein drink powder. Many of these food- and supplement-matrix CRMs have values assigned for over 80 organic and inorganic nutrients, toxic elements, proximates, and contaminants. The review provides a critical assessment of the challenges and evolving improvements in the production and the analytical methods used for value assignment of these CRMs. The current status and future needs for additional food- and dietary supplement-matrix CRMs for organic nutrients are also discussed.
Keywords: Certified reference materials (CRMs), Cholesterol, Dietary supplements, Fatty acids, Nutrients, Standard reference materials (SRMs), Vitamins
Introduction
In 1964, H.J.M. Bowen ground and homogenized 91 kg of kale leaves and distributed 100 g samples to numerous analysts for determination of elemental composition, thereby creating a “standard plant material” and the first widely distributed biological-matrix reference material [1–3]. The supply of this material, known as Bowen’s Kale, existed for over 20 years with data available for over 60 elements; 20 elements were determined repeatedly with sufficient accuracy to be denoted as “recommended” values, and 17 elements determined with somewhat less confidence were classified as “indicated” values [2]. In 1971, the U.S. National Bureau of Standards (NBS), now the National Institute of Standards and Technology (NIST), issued Standard Reference Material (SRM®) 1571 Orchard Leaves as the first biological-matrix certified reference material (CRM) with certified values for the content of 19 major, minor, and trace elements based on the concept of using results from multiple, independent analytical methods [4, 5]. Even though Bowen’s Kale could be considered as a food matrix, the intent was to provide a biological-matrix material for trace element characterization rather than specifically a food-matrix reference material. During the 1970s and early 1980s, NIST issued six additional biological-matrix CRMs that could be considered as food matrices, i.e., spinach leaves, bovine liver, wheat flour, rice flour, oyster tissue, and non-fat milk powder. As with Bowen’s Kale, the intent was not specifically to produce food-matrix CRMs, but to provide dry, powdered, homogeneous, and stable biological-matrix reference materials characterized for trace elements of nutritional, toxicological, and technological (i.e., rare-earth elements) interest.
During this same period, other institutions and organizations produced biological-matrix CRMs, including food matrices, for the determination of trace elements. During the late 1980s, the European Commission (EC) Bureau Communautaire de Référence (Community Bureau of Reference) (BCR) initiated the development of CRMs in several food matrices for the determination of trace element content (e.g., wheat and rye flours, milk powder, bovine muscle and liver, pig kidney, and haricots verts) [6, 7], and these efforts were extended to vitamins in the early 1990s [8]. These CRM efforts continued after 1994 at the Institute for Reference Materials and Measurements (IRMM) in Geel, Belgium and since 2016 known as the Joint Research Centre (JRC) Directorate F – Health, Consumer and Reference Materials. The International Atomic Energy Agency (IAEA) has provided CRMs for trace element content in biological/food matrices since the late 1980s (e.g., fish flesh, milk powder, mixed human diet, rye flour) [9], and the National Research Council Canada (NRCC) has developed marine tissue (seafood) CRMs for trace elements since the early 1980s (e.g., dogfish tissue, dogfish liver, lobster hepatopancreas) [10]. More recently, the National Metrology Institute of Japan (NMIJ), Korea Institute of Standards and Science (KRISS), National Metrology Institute of China (NIMC), LGC in the United Kingdom, and the National Metrology Institute Australia (NMIA) have also produced CRMs for trace elements in food-related matrices.
In the mid-1980s, Agriculture Canada led an effort to produce a series of 12 agricultural materials to serve as RMs for elemental content [11, 12] including corn stalk and kernel, bovine muscle powder, whole egg powder, microcrystalline cellulose, wheat gluten, corn starch, corn bran, whole milk powder, durum wheat flour, hard red spring wheat, and soft winter wheat flour. These RMs were characterized for content of trace elements through an interlaboratory characterization campaign involving a large number oflaboratories and using a variety of analytical techniques. Since Agriculture Canada did not have a distribution mechanism, these RMs were handled by NIST. In 1999, six of the most popular of these RMs were updated with reference values assigned for proximates, selected individual fatty acids, and water-soluble vitamins (egg and milk powder only) based only on measurements at contract laboratories. Ihnat summarized the development of these food-matrix RMs in a review in 2001 [13]. In 2009, these RMs were transferred to NRCC for updating and distribution, and eight of these materials were reissued in 2015 [14] as RMs with values assigned for trace elements only.
In 1990, NIST issued SRM 1548 Total Diet [15], which was prepared from foods obtained as part of the U.S. Food and Drug Administration’s (FDA) Total Diet Study, now known as the “market basket study” [16], in which foods are collected from various regions of the U.S. to monitor the food supply for pesticides, toxins, and some nutrients. The foods used to prepare SRM 1548 were combined in proportions that were representative of the U.S. adult dietary intake. The food mixture was freeze-dried and certified for content of 14 major and trace nutritional elements and proximates, and as such, SRM 1548 was the first NIST food-matrix SRM intended specifically to address food measurements rather than trace elements in a biological matrix. By the mid-1990s, there were a number of food-matrix CRMs available from IAEA, BCR, and NIST for determination of elemental nutrients and other trace element composition; however, few materials were available with values assigned for proximates or organic nutrients.
In the U.S., several significant regulations have influenced the development of food- and dietary supplement-matrix CRMs for determination of organic nutrients. The Infant Formula Act of 1980 (IFA) [17], and later amendments in 1986, have made infant formula the most highly regulated food in the U.S. by establishing minimum and maximum allowable content of protein, fat, 15 vitamins and organic nutrients, and 11 nutritional elements (minerals). The Nutrition Labeling and Education Act of 1990 (NLEA) requires that all processed foods distributed in the U.S. must have “Nutrition Facts” labels that specify the amount contained per serving of total fat, saturated fat, cholesterol, sodium, total carbohydrate, dietary fiber, sugars, total protein, vitamin A, vitamin C, calcium, and iron [18]. In 2011, FDA enacted the Food Safety Modernization Act (FSMA) with the aim of ensuring that the U.S. food supply is safe by shifting the focus from responding to contamination to prevention [19]. Closely following the model of NLEA, the Dietary Supplement Health and Education Act (DSHEA) [20], which became law in 1994, defines a dietary supplement as “any product that is intended to supplement the diet and that contains one or more of the following: vitamins, minerals, herbs or botanicals, amino acids metabolites or extracts.” DSHEA created the framework to regulate dietary supplement products as foods and put the burden of proof for safety on the FDA. In 2007, FDA implemented current Good Manufacturing Procedures (cGMPs) for manufacturers of dietary supplements, which defines quality as “…consistently meet(ing) the established specifications for identity, purity, strength and composition and limits on contaminants” [21].
To assist food and dietary supplement manufacturers in establishing compliance with these regulations, NIST has focused on providing food- and dietary supplement-matrix CRMs with values assigned for the regulated nutrients as well as other nutritional components of interest. Similar food labeling regulations exist in most countries (e.g., EC directives [22] and China regulations [23]). Compliance with these international regulations on the content of nutrients implies that sufficiently accurate methods of analysis are available. Food- and dietary supplement-matrix CRMs offer an important tool to assess the accuracy of analytical methods and to monitor quality and comparability of measurements.
This critical review documents the historical development and the evolution of food- and dietary supplement-matrix CRMs for the determination of vitamins and other organic nutrients (carotenoids and fatty acids) produced by National Metrology Institutes (NMIs) including NIST, KRISS, and NIMC and other national organizations such as EC JRC. The review highlights the need and rationale for the development of food-matrix CRMs for organic nutrients and provides a discussion of future needs and recommendations on further advances to improve these materials. This review does not include food-matrix CRMs with values assigned only for elemental content, nor does it include CRMs for food safety concerns such as contaminants (e.g., mycotoxins, drug residues, or additives) or botanical dietary supplements with values assigned for marker compounds that are not nutrients. The information in this review will be of particular interest to analytical chemists involved in the determination of nutrients in food and use these materials to validate methods and provide quality assurance. The evolution of these food-matrix CRMs from biological matrices intended for trace element determination only to food-specific matrices for both organic and inorganic nutrients will be of interest to analytical chemists in general, who will gain an appreciation of the significant chemical metrology efforts invested by the CRM producers to provide these CRMs with accurate, traceable composition values with low associated measurement uncertainties.
Reference material definitions and background
ISO Guide 30 defines a reference material (RM) as a “material, sufficiently homogeneous and stable with respect to one or more specified properties, which has been established to be fit for its intended use in a measurement process” [24]. A certified reference material (CRM) is defined as a “reference material characterized by a metrologically valid procedure for one or more specified properties, accompanied by a reference material certificate that provides the value of the specified property, its associated uncertainty, and a statement of metrological traceability” [24]. ISO Guide 30 denotes a “matrix reference material” as a “reference material that is characteristic of a real sample” [24]. Therefore, CRMs discussed in this review are matrix reference materials since they represent real food sample matrices. The “property value” corresponds to “a quantity representing a physical, chemical, or biological property of an RM.” In the cases discussed in this review, the property values are chemical composition generally expressed as mass fraction (mg/kg) of organic nutrients and elements. ISO Guide 30 defines only two types of values assigned for a RM or CRM, i.e., a certified value and an indicative value. A certified value is a “value, assigned to a property of a reference material (RM) that is accompanied by an uncertainty statement and a statement of metrological traceability, identified as such in the RM certificate,” and an indicative value (alternate names include information value or informative value) is defined as a “value of a quantity or property, of a reference material, which is provided for information only” [24], which means that it cannot be used as a reference in a metrological traceability chain.
SRMs are CRMs issued by NIST, which meet additional NIST-specific certification criteria. SRM® is a registered trademark and only refers to NIST-issued CRMs. NIST has not used the term “indicative” for values that were not at the level of certified values. For the NIST CRMs issued until the mid-1990s, the assigned values were denoted either as certified or noncertified values. From the mid-1990s, noncertified values evolved to be called reference values. In 2000, NIST summarized their process for assignment of values to CRMs for chemical composition [5]. Three categories of assigned values were identified (certified, reference, and information), and these categories were linked to seven different modes or approaches used for value assignment, which is also related to the degree of confidence in the assigned value [5]. A certified value has the highest level of confidence in accuracy. A reference value is a noncertified value that is a best estimate of the true value and is provided with associated uncertainties that may not include all sources of uncertainty. Finally, an information value is a value that may be of interest to the CRM user, but insufficient information is available to assess the uncertainty. Most CRM producers including JRC-Geel, KRISS, and NIMC use the term indicative or information value for any value not denoted as a certified value. Some CRM producers have adopted the use of the term reference rather than indicative values (e.g., NRCC). In this review, we will use the term reference/indicative when describing reference values assigned to NIST CRMs to denote that reference values are indicative values in the international reference material vocabulary.
Food composition triangle
In the early 1990s, AOAC INTERNATIONAL developed a food-matrix organizational system based on the macro composition of food matrices to demonstrate the applicability of an analytical method to a variety of foods [25, 26]. The AOAC Food Composition Triangle consists of nine sectors based on the protein, fat, and carbohydrate content (100% of each component provide the vertices of the triangle) with the intent that the selection of one or two food products in a particular sector would represent the attributes of the majority of foods in that sector for analytical method validation (see Fig. 1) [25, 26]. Wolf and Andrews [27] extrapolated this concept “to select one or two food matrices representing each sector, for development of a series of reference materials representing all foods.” During the 1990s and early 2000s, NIST adopted this strategy to develop a number of food-matrix CRMs with values assigned for proximates and organic and element nutrients to fill the various sectors of the AOAC Food Composition Triangle as described in several publications [28–33]. Other CRM producers have focused primarily on food safety specific food-matrix CRMs and only a limited number of food-matrix CRMs have been produced for organic nutrient content by other CRM producers.
Fig. 1.
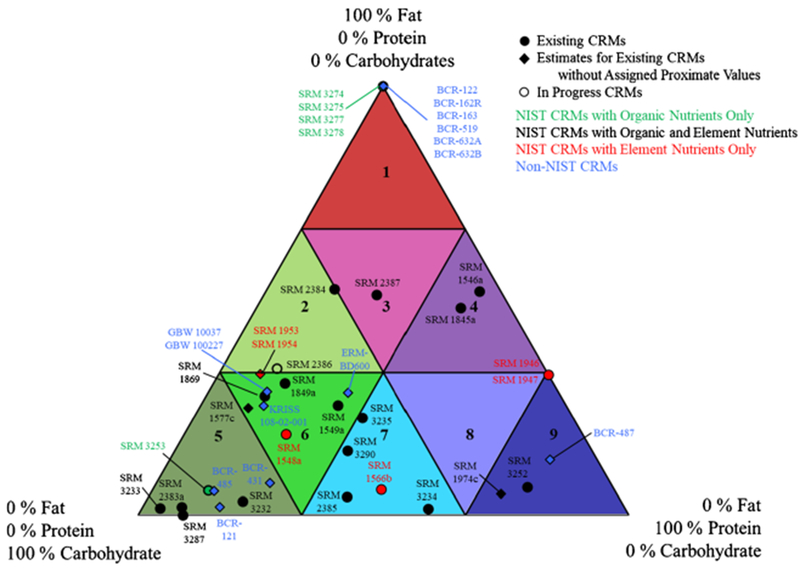
AOAC Food Composition Triangle with food-matrix CRMs located based on protein, fat and carbohydrate content. Symbol code: ● Existing CRMs, ◆ Estimate of position for existing CRMs, and ∘ In progress CRMs. Color Code: Green—NIST SRMs with organic nutrients only; Black—NIST SRMs with organic and element nutrients; Red—NIST SRMs with element nutrients only; and Blue—Non-NIST CRMs. The following CRMs are included: (Sector 1) SRM 3274 Botanical Oils, SRM 3275 Fish Oils, SRM 3277 Krill Oil, SRM 3278 Edible Oil, BCR-122 Margarine, BCR-162R Soya-Maize Oil Blend, BCR-163 Beef-Pork Fat Blend, BCR-519 Anhydrous Butter Fat, BCR-632A Pure Butter Fat, and BCR-632B Adulterated Butter Fat; (Sector 2) SRM 2384 Baking Chocolate and SRM 2386 Avocado Powder; (Sector 3) SRM 2387 Peanut Butter; (Sector 4) SRM 1546a Meat Homogenate and SRM 1845a Whole Egg Powder; (Sector 5) SRM 2383a Baby Food Composite, SRM 3232 Kelp Powder, SRM 3233 Fortified Breakfast Cereal, SRM 3253 Yerba Mate, SRM 3287 Blueberry (Fruit), BCR-121 Wholemeal Flour, BCR-431 Brussels Sprouts, and BCR-485 Mixed Vegetables; (Sector 6) SRM 1548a Typical Diet, SRM 1549a Whole Milk Powder, SRM 1577c Bovine Liver, SRM 1849a Infant/Adult Nutritional Formula, SRM 1869 Infant/Adult Nutritional Formula II, SRMs 1953/1954 Human Milk, ERM-BD600 Milk Powder, GBW 10037 Infant Formula, GBW 100227 Infant Formula, and KRISS 108-02-003 Infant Formula; (Sector 7) SRM 1566b Oyster Tissue, SRM 2385 Slurried Spinach, SRM 3234 Soy Flour, SRM 3235 Soy Milk, and SRM 3290 Dry Cat Food; (Sector 9) SRMs 1946/1947 Fish Tissue, SRM 1974c Mussel Tissue, and SRM 3252 Protein Drink Mix
First CRMs for organic nutrients
NIST
An early attempt to provide a CRM for organic nutrients was the development of SRM 1563 cholesterol and fat-soluble vitamins in coconut oil, issued in 1987 with cholesterol and vitamins (ergocalciferol and tocopheryl acetate) added to the coconut oil matrix [34]. The first NIST food-matrix CRM with a value assigned for an endogenous organic nutrient was SRM 1845 cholesterol in egg powder issued in 1989. The first two NIST food-matrix CRMs with values assigned for proximates and selected endogenous organic nutrients were issued in 1996, SRM 1544 fatty acids and cholesterol in a frozen diet composite and SRM 1846 infant formula. SRM 1544 was significant for several reasons: (1) it was the first frozen food-matrix CRM, as prior materials were all freeze-dried, (2) it was a natural (non-fortified) composite of foods rather than a single food material, and (3) it was the first NIST food-matrix CRM with values assigned for individual fatty acids. SRM 1846 infant formula was issued to address directly the measurement needs established by the IFA regulations; however, it was also the first food-matrix CRM intended to address the broad scope of NLEA with values assigned for vitamins, nutritional elements, and proximates [35]. In 1997 SRM 2383 baby food composite was issued as a custom-designed mixture of foods selected to provide measurable content of carotenoids and vitamins [36, 37]. SRM 1546 meat homogenate was issued in 1999 to provide a meat-matrix, high in both fat and protein content. Three food-matrix CRMs were developed in the early 2000s to fill voids in the food triangle, SRM 2384 baking chocolate in sector 2, SRM 2387 peanut butter in sector 3 [38], and SRM 2385 slurried spinach in sector 7, with all three CRMs having values assigned for vitamins and nutritional elements [33]. During this same period, two fresh water fish tissue CRMs, SRM 1946 Lake Superior fish tissue [39] and SRM 1947 Lake Michigan fish tissue, were issued and were intended primarily for the determination of contaminants, i.e., polychlorinated biphenyls (PCBs), polybrominated diphenyl ethers (PBDEs), pesticides, and toxic elements. However, since fish tissue is also a food matrix, these CRMs were also characterized for proximates, individual fatty acid content including nutritionally important omega-3 fatty acids, and for several elements of nutritional interest. By 2003, NIST CRMs were available in (or on the borders of) all nine sectors of the food composition triangle [33], and these materials represented the first generation of NIST food-matrix CRMs for organic nutrients (Table 1).
Table 1.
First generation of NIST food-matrix CRMs for vitamins and organic nutrients
| Nutrients with values assigneda |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| SRM | Name | Issue date | COA revisedb | WSVs | FSVs | Carotenoids | Fatty acids | Elements | Other constituentsc | Totald |
| 1563 | Fat-soluble vitamins and cholesterol in coconut oil | 1987 | 2 | Cholesterol | 3 | |||||
| 1845 | Whole egg powder | 1989 | Cholesterol | 1 | ||||||
| 1846 | Infant formula | 1996 | 1998e | 4 (6) | (4) | (11) | 1 (9) [2] | Inositol | 5 (38) [2] = 45 | |
| 1544 | Fatty acids and cholesterol in frozen diet composite | 1996 | 6 (8) | (3) | Cholesterol | 7 (17) = 24 | ||||
| 2383 | Baby food composite | 1997 | 2002 | (7) [2] | 4 (9) [1] | 5 (11) | (15) [10] | (10) [3] | Cholesterol; amino acids (15); fiber | 9 (56) [18] = 83 |
| 1546 | Meat homogenate | 1999 | 2008 | (6) [1] | 7 (6) | 6 (5) [3] | Cholesterol; amino acids (18); sucrose | 14 (43) [6] = 63 | ||
| 2384 | Baking chocolatef | 2002 | 2015g | (2) | (3) | 11 (4) | 2 (7) | Caffeine; theobromine, theophylline; catechins (3); procyanidins | 18 (27) = 45 | |
| 2387 | Peanut buttef | 2003 | 2004, 2015g | (4) | 3 | 12 (5) | 9 | Amino acids (18); aflatoxins (4) | 24 (37) = 61 | |
| 2385 | Slurried spinachf | 2003 | 2018 | (1)h | (3)h | 7 (2) | fiber | 7 (11)= 18 | ||
| 1946 | Lake superior fish tissuef | 2003 | 2012 | 9 (12) [4] | 3 (9) [2] | Methyl-Hg; PCBs (42); pesticides (17); PBDEs (10) | 67 (44) [6] = 117 | |||
| 1947 | Lake Michigan fish tissuef | 2007 | 2017 | (13) | 8 | Methyl-Hg; PCBs (45), pesticides (17); PBDEs (9); Hg isotopes (9) | 63 (51) [6] = 120 | |||
Numbers indicate certified, (reference/indicative) and [information] values. See Certificate of Analysis (COA) for SRMs at http://www.nist.gov/srm/index.cfm. For SRMs no longer available, see Historical Archival SRM Certificates at http://www.nist.gov/srm/index.cfm
Major revisions with value assignment of additional constituents
All SRMs in Table 1 have reference values assigned for proximates (moisture, solids, ash, fat, protein, and carbohydrates) and calories (calculated based on fat, carbohydrate, and protein content)
Total number of certified, (reference/indicative), and [information] values assigned including proximates and calories
Revisions for SRM 1846 in 1998 added values for iodine and fatty acids; revisions in 2001, 2004, and 2006 changed certified values to reference values or removed values due to instability
SRM is still available
Updated information included in Table 3
In 2018 update, values for two carotenoids and vitamin B2 were removed based on decision not to maintain these analytes in this matrix. Number of WSVs and carotenoids are for original COA
BCR/JRC-IRMM/JRC-Geel
In the 1980s, the EC program to develop CRMs was organized under the name Bureau Communautaire de Reference (BCR); in 1994 the program name was changed to Standards, Measurement and Testing (SMT) Programme [40]. The BCR and SMT programs were operated by EC scientific officers in Brussels with no laboratory facilities; all technical work was performed by external laboratories within the EC selected for their expertise. CRMs were distributed by EC JRC at Geel, Belgium, known until 1992 as the Central Bureau for Nuclear Measurements (CBNM), and known as IRMM until 2016, and now again designated as the European Commission’s JRC-Geel site where the reference material production is under Directorate F – Health, Consumer and Reference Materials. In the early 2000s the SMT program was replaced by reference materials development under the auspices of JRC-IRMM and now in JRC Unit F.6. The BCR program initiated the development of food-matrix CRMs for vitamins [8] in the early 1990s, expanding on their efforts to provide food-matrix CRMs for elemental content. Several food-matrix CRMs were prepared in the early 1990s specifically for organic nutrients as shown in Table 2, including milk powder, mixed vegetables, pig liver, wheatmeal flour, Brussels sprouts, and margarine [41, 42]. These food-matrix CRMs had certified values for a number of water-soluble vitamins (WSVs) and fat-soluble vitamins (FSVs), and all these CRMs are still available with the exception of the milk powder (BCR-421) which has been replaced by ERM-BD-600. The Certificate of Analysis for each material has been updated by JRC-Geel to meet recent guidelines, but the nutrient values are still based on the original measurements.
Table 2.
JRC-Geel food-matrix CRMs for vitamins and organic nutrients
| CRM | Name | Issue date | COA revision | Nutrients with values assigned | Values assigneda |
|---|---|---|---|---|---|
| BCR-163 | Beef-pork fat blend | 1993 | 2013 | Cholesterol; fatty acids as methyl esters | 8 [23] |
| BCR-519 | Anhydrous butter fat | 1997 | 2013 | Cholesterol; triglycerides | 17 [1] |
| BCR-122 | Margarine | 1997 | 2010 | FSVs (D and E) | 2 |
| BCR-431 | Brussels sprouts | 1997 | 2007 | WSVs | 2 |
| BCR-421 | Milk powder (discontinued) | 1998 | 2007 | FSVs; WSVs | 9 |
| BCR-485 | Mixed vegetables | 1998 | 2013 | WSVs and carotenoids | 9 (1) [3] |
| BCR-487 | Pig liver | 1998 | 2013 | WSVs | 5 (1) |
| BCR-121 | Wholemeal flour | 1998 | 2007 | WSVs | 3 |
| BCR-632A | Pure butter fat | 2002 | 2013 | Cholesterol; triglycerides | 16 |
| BCR-632B | Adulterated butter fat | 2002 | 2013 | Cholesterol; triglycerides | 17 |
| BCR-162R | Soya-maize oil blend | 2007 | – | Cholesterol, fatty acids, sterols | 5 (3) [11] |
| ERM-BD600 | Milk powder | 2011 | – | FSVs; WSVs | 7 (2) [3] |
Certificate of Analysis (COA) for each CRM is available at https://crm.jrc.ec.europa.eu
Number of certified values; (number of indicative values); [additional information]
The JRC-Geel food-matrix CRMs have values assigned for two to nine vitamins, primarily WSVs (see Electronic Supplementary Material (ESM) Table S1). BCR-485 mixed vegetables, which was prepared from sweet corn, carrots, and tomatoes to provide suitable levels of the carotenoids lutein, β-carotene, and lycopene, respectively, has the most values assigned for both WSVs (see ESM, Table S1) and FSVs and carotenoids (see ESM, Table S2). During this same period, three butter fat CRMs were developed for determination of fatty acid methyl esters and/or triglycerides: BCR-519 anhydrous butter fat, BCR-632A pure butter fat, and BCR-632B adulterated butter fat [43]. The CRMs listed in Table 2 (except ERM-BD600) represent the first-generation food-matrix CRMs from the BCR/IRMM/JRC-Geel.
Second generation of NIST food-matrix CRMs
In 2009, NIST issued SRM 1849 infant/adult nutritional formula and SRM 3280 multivitamin/multielement tablets, which marked the beginning of a new generation of NIST CRMs with values assigned for a broader scope of organic nutrients and for a significantly greater number of constituents. The NIST CRMs issued during the past decade are summarized in Table 3 consisting of six new matrices (multivitamin tablets, blueberries, soy flour, fortified breakfast cereal, protein drink mix, and dry cat food), four renewals of previous matrices (three infant/adult nutritional formulas, baby food composite, and meat homogenate), two reissues of matrices that were previously only certified for trace elements (whole milk and whole egg powders), and two existing first-generation CRMs that were reanalyzed and significantly updated (baking chocolate and peanut butter). The second-generation NIST food-matrix CRMs have a significantly greater number of assigned values (ranging from 44 to 105 values) for organic nutrients (WSVs, FSVs, carotenoids, cholesterol, and fatty acids) and nutrient elements. However, the amount of food material in a CRM unit may not be sufficient to allow measurement of all the analytes/properties with assigned values.
Table 3.
Recent NIST food- and dietary supplement-matrix CRMs for vitamins and organic nutrients
| SRM | Name | Issue date (COA update) | Nutrients with values assigneda |
|||||
|---|---|---|---|---|---|---|---|---|
| WSVs | FSVs | Fatty acids | Elements | Other constituentsb | Totalc | |||
| 1849 | Infant/adult nutritional formula | 2009 | 7 (3) | 7 | 16 (6) | 13 (1) | Cholesterol; amino acids (19); nucleotides (4) | 44 (41) [1] = 86 |
| 3280 | Multivitamin/multielement tablets | 2009 (2011) | 10 | 1 (2) | 18 (9) | Carotenoids (4) | 31 (13) = 44 | |
| 3287 | Blueberries | 2010 (2016) | 4 | 8(1) | Organic acids (8); sugars (2); fiber; amino acids (16) | 13 (37) = 50 | ||
| 1849a | Infant/adult nutritional formula I (milk-based) | 2011 (2015) | 10 (2) | 6 (1) | 20 (11) | 14 | Cholesterol; amino acids (19); nucleotides (4); myo-inositol | 51 (45) [5] = 101 |
| 2383a | Baby food composite | 2012 (2015) | 9 (2) | 6 (3) | 12 (3) [4] | Sugars (5); fiber | 27 (19) [4] = 50 | |
| 3234 | Soy flour | 2013 (2017) | 2 (1) | 8 (1) | Isoflavones (5); fiber; amino acids (18) | 10 (32) = 42 | ||
| 1549a | Whole milk powder | 2013 (2016) | 8 (4) | 11 (9) | 10 (5) | Cholesterol; amino acids (17) | 40 (41) = 81 | |
| 3233 | Fortified breakfast cereal | 2013 (2017) | 5 (2) | 1 | 12 (3) [4] | Sugars (5); amino acids (17); fiber (4) | 18 (33) [4] = 55 | |
| 1546a | Meat homogenate | 2014 (2017) | 5 (9) | 1 (1) | 17 (24) | 9 (7) | Cholesterol; amino acids (19) | 33 (66) = 99 |
| 1845a | Whole egg powder | 2014 (2017) | 6 (6) | 2 (3) | 13 (12) | 12 (9) | Cholesterol; amino acids (18) | 34 (54) = 88 |
| 2384 | Baking chocolate | 2002 (2015) | (9) | 12 (4) | 10 (1) | Caffeine; theobromine, theophylline; catechins (3); procyanidins; fiber | 28 (22) = 50 | |
| 2387 | Peanut butter | 2003 (2015) | (10) | 2 (1) | 12 (5) | 9 | Amino acids (18); aflatoxins (4) | 23 (44) = 67 |
| 3252 | Protein drink mix | 2015 | 10 (6) | (3) | 4 (27) | 13 (6) | Cholesterol; amino acids (16); fiber | 28 (67) = 95 |
| 3290 | Dry cat food | 2015 (2017) | 13 (4) | (2) | 15 (22) | 17 (3) [2] | Amino acids (19) | 45 (58) [2] = 105 |
| 3235 | Soy milk | 2017 | 2 (2) | 3 (2) | (11) | 8 (1) | Amino acids (16); total sugars | 13 (39) = 52 |
| 1869 | Infant/adult nutritional formula II (milk/whey/soy-based) | 2018 | 10 (5) | (16) | 19 (14) | 14 | Cholesterol; amino acids (20); sugars (6); nucleotides (5); myo-inositol | 47 (71) = 118 |
Numbers indicate certified, (reference/indicative), and [information] values. See Certificate of Analysis (COA) for SRMs at http://www.nist.gov/srm
All SRMs in Table 3 have reference values assigned for proximates (moisture, solids, ash, fat, protein, and carbohydrates) and calories (calculated based on fat, carbohydrate, and protein content)
Total number of certified, (reference/indicative), and [information] values assigned including proximates and calories
New matrices were selected based on several factors including requests from other U.S government agencies and the desire to fill under-populated sectors of the food composition triangle. Three of the recent materials (multivitamin tablets [44], blueberries [45], and soy flour [46]) were requested by the National Institutes of Health, Office of Dietary Supplements (NIH-ODS) as part of an effort to develop dietary supplement-related CRMs [31, 33, 47]. To address the need for food-matrix CRMs in less-populated sectors, a dry cat food (SRM 3290) and a protein drink mix (SRM 3252) were developed, which are positioned in sectors 7 and 9, respectively. The decision to develop the protein drink mix was based on customer use of a previously issued (in 2006) ephedra-containing protein powder (SRM 3244) that was characterized for content of 11 vitamins and 19 elements as well as ephedrine alkaloids and caffeine [48, 49]. SRM 3244 was later discontinued when ephedra-containing products were banned for use in the U.S. Both the milk powder (SRM 1549a) and the egg powder (SRM 1845a) were reissues of popular, early food-matrix CRMs that were certified for trace elements or cholesterol only. When these CRMs were reissued, they were certified for both organic and element nutrients to expand their usefulness and to broaden the scope of food matrices available for organic nutrients.
The most recent NIST food-matrix CRM issued is SRM 1869 infant/adult nutritional formula II, which was produced to complement SRM 1849a with a different base composition and with some additional nutrients included. The marketplace for infant and adult nutritional formulas includes materials with not only varying composition of nutrients but also different base materials. The most common infant formulas are based on bovine milk (whey and casein) as the protein source, vegetable oils as a fat source, and lactose as a carbohydrate source; however, some infant formulas are based on soybean protein, hydrolyzed milk proteins, or concentrated milk proteins. SRM 1849a is a milk-based formula, whereas the new SRM 1869 was prepared from a mixture of milk protein concentrate, soy protein, and whey. In addition, SRM 1869 contains vitamin D2, fluoride, and carotenoids, which are not in the formulation of the existing SRM 1849a. SRM 1869 was issued with 47 certified and 71 reference values; however, many of the reference values (e.g., all of the FSVs, see Table 3) are based only on results from collaborating laboratories and the manufacturer, and these values will be upgraded to certified values soon with NIST measurements, thereby significantly increasing the number of certified values in this latest food-matrix CRM.
The most significant trends over the 30 years ofNIST food-matrix CRM development have been the increasing number of values assigned and the increasing average number of values assigned per CRM as illustrated in Fig. 2 and Fig. 3, respectively. As illustrated in Fig. 3, the average number of total values assigned remained relatively constant until the appearance of the second generation of food matrix CRMs in 2009. As the number of certified values has increased, there has been a corresponding decrease in the number of reference values assigned as reference values have been upgraded to certified values as the analytical capabilities have increased and improved.
Fig. 2.
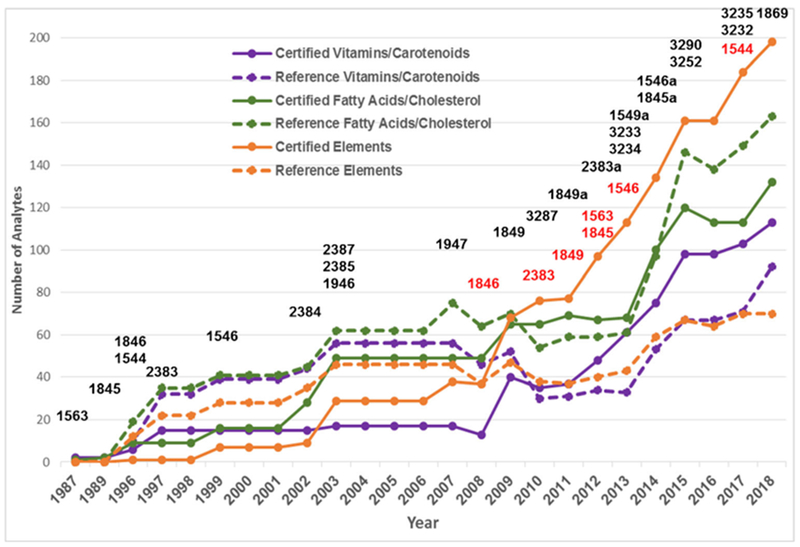
Evolution of NIST Food-Matrix CRMs over three decades with respect to number of assigned values forvitamins/carotenoids, fatty acids/cholesterol, and trace elements. Legend: Solid purple line = number of certified values for vitamins/carotenoids, dashed purple line = number of reference values for vitamins/carotenoids, solid green line = number of certified values for fatty acids/carotenoids, dashed green line = number of reference values for fatty acids/cholesterol, solid orange line = number of certified values for trace elements, dashed orange line = number of reference values for trace elements. For trace element content, only SRMs that have both organic and element nutrients are listed. SRM numbers are listed above the year in which they were issued (black) or discontinued (red)
Fig. 3.
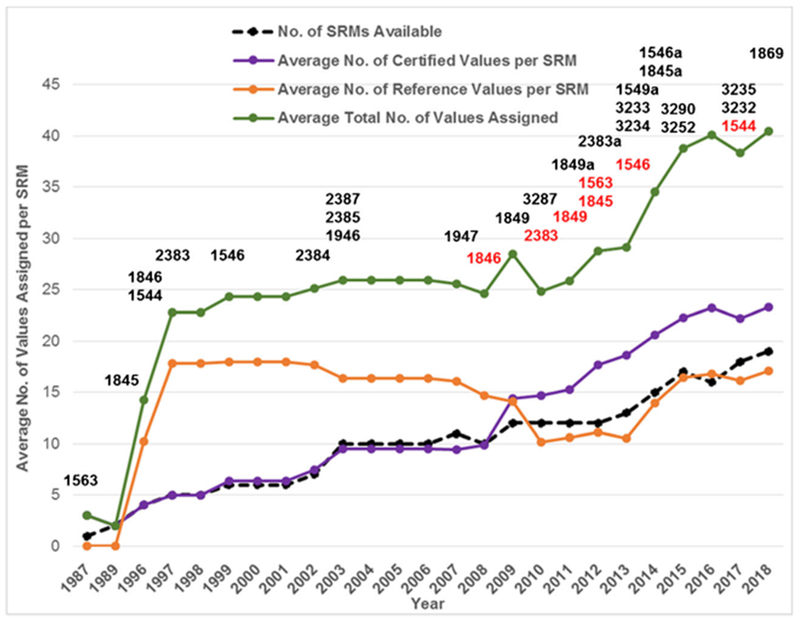
Evolution of NIST Food-Matrix CRMs over three decades with respect to number of SRMs and average number of assigned values per SRM for vitamins/carotenoids, fatty acids/cholesterol, and trace elements. Legend: Solid black line = cumulative number of food-matrix SRMs, solid purple line = average number of certified values per SRM, solid orange line = average number of reference values per SRM, and solid green line = average total number of values assigned. SRM numbers are listed above the year in which they were issued (black) or discontinued (red)
Value assignment of food and dietary supplement CRMs for organic nutrients
The value assignment process for CRMs characterized for chemical content has generally followed one of three strategies using (1) results from multiple laboratories using a variety of analytical techniques, (2) multiple analytical techniques at one laboratory, or (3) one higher-order method. These three approaches, which have evolved over four decades of CRM development for chemical composition, are now formalized in an ISO guide for the production of reference materials [50]. The first two strategies were implemented early for the assignment of values for elemental content, where multiple independent analytical techniques were widely available. Using results from interlaboratory studies has been the general approach for assigning values to CRMs at Agriculture Canada, IAEA, and BCR/JRC-IRMM/JRC-Geel. Ihnat [51] provided a synopsis of different approaches to certification of RMs based on the xperiences with food/agricultural-matrix materials for trace elements. The BCR CRMs shown in Table 2 were certified for vitamin content using multiple laboratories (typically 10 to 15 laboratories) that had demonstrated their measurement capabilities in preliminary exercises. This approach has been refined by JRC-Geel to include now only laboratories that provide sufficient evidence of demonstrated capabilities, e.g., participation in proficiency testing, use of validated methods, and accreditation to ISO 17025 or working in accordance with ISO 17025 for the relevant measurements. The mass fractions of WSVs and FSVs in JRC-Geel food-matrix CRMs, assigned using the interlaboratory study approach, are shown in Tables S1 and S2, respectively (see ESM).
The approach of using two or more independent analytical techniques has been the primary strategy used at NIST for assignment of chemical composition values to food-matrix CRMs. The implementation of this approach at NIST has been discussed by Epstein [4] for trace element characterization and by Wise et al. [52] for organic contaminants, with particular emphasis on environmental matrices. NMIJ, KRISS, and NIMC have adopted similar approaches based on combining results from two or more independent methods to assign certified values for trace elements in food-matrix CRMs.
The value assignment of CRMs for organic nutrients at NIST has involved variations of all three strategies, and the approach has evolved significantly over the past 30 years with the development of new, improved analytical methods and capabilities. For the first-generation NIST food-matrix CRMs (see Table 1), the certified values for organic nutrients were assigned typically using measurements from one method at NIST and results from collaborating laboratories (usually multiple laboratories as part of an interlaboratory exercise). Reference/indicative values were often assigned using only the results from collaborating laboratories when NIST did not have the measurement capabilities for some nutrients at the time. Examples of the value assignment approach for several first-generation NIST food-matrix CRMs have been reported [35–38, 53–55]. The major group of collaborating laboratories contributing to value assignment for the NIST food-matrix CRMs has been the Grocery Manufacturers Association (GMA), formerly the National Food Processors Association. The GMA Food Industry Analytical Chemists Committee (FIACC), which represents food industry and contract laboratories, has included candidate CRMs in its biannual interlaboratory comparison exercises among 10 to 40 member laboratories for the determination of nutrients in food samples. The values for proximates in the CRMs listed in Table 1, and for current NIST food-matrix CRMs, were assigned using results from the GMA FIACC exercises. For the CRMs in Table 1, the limited numbers of certified values assigned for vitamins and carotenoids were generally based on combining NIST measurements using liquid chromatography (LC) with ultraviolet (UV) or fluorescence (FL) detection and results from a GMA FIACC exercise. Certified values for a limited number of individual fatty acids were assigned based on NIST measurements using gas chromatography with flame ionization detection (GC-FID) combined with results from a GMA FIACC exercise [33]. Only three of the first-generation CRMs listed in Table 1 with values for WSVs and FSVs are still available (peanut butter, chocolate, and spinach), and for these three CRMs, the majority of the values assigned for vitamins and/or carotenoids were denoted as reference/indicative values when first issued [33], and these CRMs have been recently reanalyzed and the values updated as described later.
The third approach for value assignment, i.e., using a single higher-order method, has in recent years become more widely used for determination of organic nutrients. The determination of cholesterol in SRM 1845 cholesterol in whole egg powder, released in 1989, was performed using a definitive (primary) method based on isotope dilution (ID) GC-MS [56]. Measurement of vitamins has also progressed toward higher-order methods, mainly using ID LC with mass spectrometry (ID LC-MS) or tandem mass spectrometry (ID LC-MS/MS) using native compounds that are isotopically enriched with stable isotopes as internal standards for quantification. The benefits of using ID LC-MS and ID LC-MS/MS approaches for value assignment of vitamin content have been described elsewhere [57]. Advancements in mass spectrometry have improved sensitivity and selectivity for organic nutrients, and the availability of isotopically labeled compounds has made ID approaches feasible. The ID MS approach (with LC or GC) has become the preferred approach in value assignment of organic nutrients in food-matrix CRMs by NIST, KRISS, and NIMC (see discussion below).
Recent value assignment of organic nutrients in food and dietary supplement CRMs at NIST
The second generation of NIST food-matrix CRMs was initiated with the development of SRM 1849 infant/adult nutritional formula and SRM 3280 multivitamin/multielement tablets. The value assignment approach for these materials was significantly enhanced by the implementation of ID LC-MS, and later ID LC-MS/MS methods resulting in values assigned for additional vitamins. The development of the multivitamin/multielement tablet CRM provided both the motivation and the opportunity to develop ID LC-MS and ID LC-MS/MS methods for a greater number of WSVs and FSVs. The goal for the development of the multivitamin/multielement tablet CRM (SRM 3280) was to assign values for the content of all vitamins and carotenoids (12 to 15 compounds) and nutritional elements (18 elements) typically listed on the Supplement Facts panel for multivitamin/multielement products [44, 58]. Until this time, NIST methods for the determination of vitamins and carotenoids were primarily based on LC-UV or LC-FL; these methods, however, often lacked the specificity required for complex food matrices, particularly in non-fortified matrices. For the certification of SRM 3280, ID LC-MS and ID LC-MS/MS methods were developed for 11 of the 13 vitamins measured [44, 57]. Many of these methods were the first reports of ID LC-MS and ID LC-MS/MS methods for vitamins in supplements or food matrices [57–60].
SRM 1849 infant/adult nutritional formula also represented a significant increase in the number of values assigned for organic nutrients in a food-matrix CRM. The first infant formula CRM (SRM 1846) [35] was issued in 1996 with certified values assigned for only 4 WSVs and 2 FSVs, and reference/indicative values for an additional 29 nutrient constituents including 10 vitamins, which were assigned as reference/indicative values primarily based on measurements from collaborating laboratories with no measurements at NIST. In addition to the ID LC-MS and ID LC-MS/MS methods described above for the vitamins, in the late 2000s two independent GC methods, GC-FID and GC-MS, were implemented for determination of fatty acids in food. As a result, SRM 1849 was issued with certified values assigned for 43 nutrients including all WSVs and FSVs typically fortified in foods, individual fatty acids, and nutritional elements, with reference/indicative values assigned for an additional 42 constituents including additional vitamins and elements, proximates, amino acids, and nucleotides [61]. Measurements from collaborating laboratories were still an important contribution to the value assignment process; however, the increased capabilities to provide two independent methods for most of the vitamins and the fatty acids resulted in a significant increase in the number of values denoted as certified in SRM 1849 compared to SRM 1846. Because of the increased demand for SRM 1849 (in part because of the increased number of nutrients with values assigned), a replacement material, SRM 1849a, was issued in 2011, and the current Certificate of Analysis for SRM 1849a contains certified values for 51 constituents and reference/indicative values for 45 constituents of interest to the food testing community.
Value assignment for SRM 3280, SRM 1849, and SRM 1849a illustrates the extensive application of the multiple analytical methods approach used at NIST for organic nutrients in food and dietary supplement-matrix CRMs and will be presented in detail as an example of this approach. For these three CRMs, results from multiple analytical techniques at NIST, multiple analytical techniques at a collaborating laboratory (USDA), results from two interlaboratory studies, i.e., the European Committee for Standardization (CEN) and GMA, and results from analyses by the manufacturer of the infant formula materials were used to assign the certified and reference values [44,61]. The certified values and the multiple methods used to assign these values for SRM 3280, SRM 1849, and SRM 1849a are summarized in Table 4. For most of the vitamins, ID LC-MS or ID LC-MS/MS methods were used for at least one set of results. Vitamin C was the only vitamin with a certified value assigned for which no LC-MS measurements were made, i.e., only LC-UV measurements and results from collaborating laboratories were used [62]. After combining from two to seven sets of results, the certified values for the WSVs and FSVs had relative expanded uncertainties ranging typically from 5 to 10% for the two infant/adult nutritional formula CRMs and from 10 to 15% for the multivitamin CRM. (Note: All percent relative uncertainties report in parentheses for NIST certified values are expanded uncertainties. For detailed information on the uncertainties associated with NIST SRMs, please refer to the Certificate of Analysis [63] for the specific SRM). For the certification of SRM 1849a, values for vitamins were assigned based on the combination of one set of NIST measurements (typically ID LC-MS) with results from an interlaboratory study and/or from the manufacturer.
Table 4.
Analytical methods used for value assignment of vitamins in three NIST food and dietary supplement CRMs
| Vitamins and carotenoids | SRM 3280 Multivitamin/multielement tablets |
SRM 1849 Infant adult/nutritional formula |
SRM 1849a Infant adult/nutritional formula |
||||
|---|---|---|---|---|---|---|---|
| Mass fraction (mg/kg)a | Methods | Mass fraction (mg/kg)a | Methods | Mass fraction (mg/kg)a | Methods | ||
| Thiamine HCl | B1 | 1060 ± 120 | 1–5 | 15.8 ± 1.3 | 1–4, 6, 11 | 12.57 ± 0.98 | 2, 6, 11 |
| Riboflavin | B2 | 1320 ± 170 | 1, 2, 6, 7 | 17.4 ± 1.0 | 1, 3, 4, 6, 7, 11 | 20.37 ± 0.52 | 2, 6, 11 |
| Niacinamide | B3 | 14,100 ± 230 | 1–5 | 97.5 ± 2.3 | 1–4, 6, 11 | 109 ± 10 | 2, 6, 11 |
| Pantothenic acid | B5 | 7300 ± 960 | 2, 3, 5, 6 | 64.8 ± 2.2 | 2, 3 | 68.2 ± 1.9 | 2, 6, 11 |
| Pyridoxine HCl | B6 | 1810 ± 170 | 1–3, 5, 8 | 14.2 ± 1.5 | 1–3,6,8,11 | 13.46 ± 0.93 | 2, 6, 11 |
| Biotin | B7 | 23.4 ± 3.2 | 2, 3, 5, 6, 9 | 1.92 ± 0.25 | 2, 3, 6, 11, 12 | 1.99 ± 0.13 | 2, 6, 11 |
| Folic Acid | B9 | 394 ± 22 | 3, 5, 6, 12 | 2.11 ± 0.13 | 3, 6, 11, 12 | 2.293 ± 0.062 | 6, 11, 12 |
| Cyanocobalamin | B12 | 4.8 ± 1.0 | 5, 6, 10 | 0.040 ± 0.008 | 6, 11 | 0.0482 ± 0.0085 | 6, 11 |
| Ascorbic Acid | C | 42,200 ± 3700 | 1, 3, 5, 6 | 1060 ± 30 | 6, 11 | 784 ± 65 | 1, 6, 11 |
| Choline | 882 ± 88 | 6, 11 | 1090 ± 110 | 2, 6, 11 | |||
| Retinol | A | 444 ± 46 | 2 | 16.4 ± 1.3b | 1, 2, 6, 11, 12 | 7.68 ± 0.23 | 2, 6, 11 |
| trans-β-Carotene | 420 ± 100 | 1, 5 | |||||
| cis-β-Carotene | 72 ± 7 | 1 | |||||
| Total-β-Carotene | 514 ± 87 | 1, 5 | |||||
| Lutein | 205 ± 50 | 1, 5, 6 | |||||
| Ergocalciferol | D2 | 8.6 ± 2.6 | 2 | ||||
| Cholecalciferol | D3 | 0.251 ± 0.027 | 2, 3, 6, 11 | 0.111 ± 0.017 | 2, 6, 11 | ||
| α-Tocopherol | E | 21,400 ± 3500c | 1, 2, 5, 6 | 369 ± 16 | 1, 6, 13 | 219 ± 16 | 6, 13 |
| Phylloquinone | K1 | 22.8 ± 2.2 | 2, 5, 6 | 2.20 ± 0.18 | 2, 6, 11 | 1.06 ± 0.17 | 2, 6, 11 |
1 LC-UV (NIST), 2 ID LC-MS (NIST), 3 LC-MS (USDA), 4 LC-absorbance (USDA), 5 CEN interlaboratory results, 6 GMA interlaboratory results, 7 LC-MS (NIST), 8 LC-fluorescence (USDA), 9 LC-evaporative light scattering detection (NIST), 10 LC-ICP-MS (NIST), 11 Manufacturer’s analyses, 12 ID LC-MS/MS (NIST), 13 LC-fluorescence (NIST)
Certified values in italic type; reference/indicative values in normal type. See Certificate of Analysis for SRMs at http://www.nist.gov/srm/index.cfm
Mass fractions are reported on dry-mass basis for SRM 3280 and on as-received basis for SRM 1849 and 1849a
Retinol added as retinyl palmitate; value expressed as retinol equivalents
α-Tocopherol was added as α-tocopherol acetate; value expressed as α-tocopherol equivalents. For 1849, value includes naturally occurring α-tocopherol
Evolving vitamin B value assignment
The most recent improvement in the value assignment approach for food-matrix CRMs at NIST has been the development and implementation of an ID LC-MS/MS method for the simultaneous determination of five of the B vitamins [64] in both fortified and non-fortified food-matrix CRMs. For recent CRMs, many of the certified values have been assigned based only on the ID LC-MS/MS results with confirmation using results from collaborating laboratories. The development of ID LC-MS and ID LC-MS/MS methods is described [64] for the simultaneous determination of five B vitamins in eight of the recent food-matrix CRMs listed in Table 3. Over the 5-year period in which these eight CRMs were issued, the value assignment process for the B vitamins evolved significantly. For the first two CRMs (infant/adult nutritional formula and fortified breakfast cereal), the certified values were assigned by combining ID LC-MS measurements at NIST with results from the GMA interlaboratory study and/or the manufacturer. For the blueberries and baby food composite, the ID LC-MS results were used alone with confirmation of the values by the collaborating laboratory results. The milk powder CRM represented a transition from ID LC-MS to ID LC-MS/MS with the values assigned by combining results from both techniques. For the final three CRMs (soy flour, meat homogenate, and egg powder), the certified values were generally based on the ID LC-MS/MS measurements with confirmation by the median result from collaborating laboratories. Two of the first-generation food-matrix CRMs in Table 1, SRM 2384 baking chocolate and SRM 2387 peanut butter, were reanalyzed for determination of the B vitamins using the ID LC-MS/MS method [64] to provide updated values. The mass fractions of various WSVs and nutrients for fortified food and dietary supplement CRMs are summarized in Table 5; mass fractions for WSVs in selected non-fortified food-matrix SRMs are summarized in Table 6. Additional NIST food-matrix CRMs with WSV values assigned are summarized in Table S3 in the ESM.
Table 5.
Mass fractions of the water-soluble vitamins and nutrients in selected fortified NIST food-matrix CRMs
| Mass fraction (mg/kg, dry-mass basis) |
||||||
|---|---|---|---|---|---|---|
| Vitamins | SRM 1849a Infant/adult nutritional formula I |
SRM 1869 Infant/adult nutritional formula II |
SRM 3233 Fortified breakfast cereal |
SRM 3252 Protein drink mix |
SRM 3290 Dry cat food |
|
| Thiamine | B1 | 12.57 ± 0.98 | 13.36 ± 0.32 | 60.2 ± 9.4 | 12.3 ± 1.6 | 26.5 ± 3.0 |
| Riboflavin | B2 | 20.37 ± 0.52 | 13.6 ± 1.5 | 28.7 ± 2.8 | 39.8 ± 5.6 | |
| Niacinamide | B3 | 109 ± 10 | 98.4 ± 2.2 | 799 ± 27 | 269.7 ± 4.4 | 27.99 ± 0.22 |
| Niacin | B3 | 16.67 ± 0.35 | 7.33 ± 0.26 | 181.0 ± 3.1 | ||
| Total vitamin B3a | B3 | 99.5 ± 4.4 | 822 ± 39 | 287 ± 21 | 218 ± 21 | |
| Pantothenic acid | B5 | 68.2 ± 1.9 | 64.9 ± 6.6 | 540 ± 40 | 150 ± 12 | 54 ± 11 |
| Pyridoxine | B6 | 13.46 ± 0.93 | 13.09 ± 0.32 | 29.2 ± 1.6 | 27.38 ± 0.24 | |
| Pyridoxal | B6 | 0.877 ± 0.025 | ||||
| Total Vitamin B6b | B6 | 81.9 ± 9.0 | 29.1 ± 2.7 | 30.1 ± 3.2 | ||
| Biotin | B7 | 1.99 ± 0.13 | 1.89 ± 0.24 | 4.43 ± 0.19 | 1.42 ± 0.23 | |
| Folic acid | B9 | 2.293 ± 0.062 | 2.239 ± 0.086 | 15.1 ± 1.2 | 7.6 ± 1.9 | 6.0 ± 1.0 |
| 5-MTHF | B9 | 0.0839 ± 0.0031 | ||||
| Cyanocobalamin | B12 | 0.0482 ± 0.0085 | 0.0435 ± 0.0065 | 0.108 ± 0.026 | 0.095 ± 0.025 | |
| Ascorbic acid | C | 784 ± 65 | 897 ± 43 | 940 ± 100 | 100.5 ± 1.4 | |
| Choline | 1090 ± 110 | 1612 ± 64 | 1328 ± 17 | 2627 ± 12 | ||
| Carnitine | 136 ± 14 | 103.5 ± 4.5 | 4.76 ± 0.12 | 59.2 ± 1.3 | ||
Certified values in italic type; reference/indicative values in normal type. See Certificate of Analysis for SRMs at http://www.nist.gov/srm/index.cfm
Measured as the sum of niacinamide and niacin, which was mathematically converted to niacinamide using the relative molecular masses
Measured as the sum of pyridoxal, pyridoxamine, and pyridoxine, which were mathematically converted to pyridoxine using the relative molecular masses
Table 6.
Mass fractions of water-soluble vitamins in selected non-fortified NIST food-matrix CRMs
| Vitamins | Mass fraction (mg/kg, as-received basis) |
||||||
|---|---|---|---|---|---|---|---|
| SRM 1546a Meat homogenate |
SRM 1549a Whole milk powder |
SRM 1845a Whole egg powder |
SRM 2383a Baby food composite |
SRM 2384 Baking chocolate |
SRM 2387 Peanut butter |
||
| Thiamine | B1 | 0.90 ± 0.48a,b | 1.365 ± 0.029b | 1.87 ± 0.68 | 0.768 ± 0.011b | 1.59 ± 0.27a,b | 0.563 ± 0.012a,b |
| Riboflavin | B2 | 0.35 ± 0.10a | 10.6 ± 1.9 | 16.51 ± 0.58a | 0.56 ± 0.15 | 2.58 ± 0.36a | 0.179 ± 0.012a |
| Niacinamide | B3 | 38.18 ± 0.74a | 5.91 ± 0.39 | 1.421 ± 0.014a | 3.59 ± 0.06a | 1.35 ± 0.16a | 3.006 ± 0.068a |
| Niacin | B3 | 0.401 ± 0.022a | 1.79 ± 0.04a | 10.9 ± 1.8a | 38.50 ± 0.97a | ||
| Total vitamin B3 | B3 | 41.0 ± 4.8c | 5.36 ± 0.10a,c | 11.6 ± 2.0c | 41.2 ± 1.0c | ||
| Pantothenic acid | B5 | 4.58 ± 0.59a | 33.7 ± 2.7 | 64.6 ± 96 | 1.64 ± 0.02a | 3.19 ± 0.51a | 8.53 ± 0.18a |
| Total vitamin B5 | B5 | 6.4 ± 2.4d | 2.75 ± 0.42i | ||||
| Pyridoxine | B6 | 0.044 ± 0.12a | 0.052 ± 0.002a,d | 0.129 ± 0.020a,d | 0.1171 ± 0.0049a,d | ||
| Pyridoxal | B6 | 1.72 ± 0.16 | 0.1329 ± 0.0052 | 0.0976 ± 0.0026a,e | |||
| Pyridoxamine | B6 | 0.272 ± 0.054a | 0.259 ± 0.0231 | 0.0472 ± 0.0025a | 0.159 ± 0.002a,f | 0.102 ± 0.017a,f | 0.0852 ± 0.0036ba,f |
| Total vitamin B6 | B6 | 0.318 ± 0.063e | 1.97 ± 0.16f | 0.1798 ± 0.0059f | 0.271 ± 0.003a,g | 0.231 ± 0.034g | |
| Total vitamin B6 | B6 | 1.83 ± 0.69g | 4.2 ± 1.2g | 0.710 ± 0.003j | 0.3006 ± 0.0087h | ||
| Biotin | B7 | 0.152 ± 0.016h | 0.75 ± 0.31h | ||||
| Folic acid | B9 | 1.300 ± 0.069i | |||||
| 5-MTHF | B9 | 0.211 ± 0.014j | 0.838 ± 0.044j | ||||
| Cyanocobalamin | B12 | 0.0055 ± 0.0016k | 0.032 ± 0.002 | ||||
| Ascorbic acid | C | 41.9 ± 2.5 | |||||
| Choline | 536.4 ± 9.8 | 998 ± 63 | 16,400 ± 3800 | ||||
| Carnitine | 92.0 ± 1.4 | 173.1 ± 8.6 | 6.15 ± 0.56 | ||||
Certified values are in italic type; reference/indicative values are in normal type. See Certificate of Analysis for SRMs at http://www.nist.gov/srm/index.cfm
Value represents the free (unbound) form of the vitamin
Reported as thiamine ion (relative molecular mass 265.36 g/mol), not chloride or chloride hydrochloride
Niacinamide and niacin were measured individually; niacin was mathematically converted to niacinamide by multiplication by the ratio of the relative molecular masses
Reported as total vitamin B5 from interlaboratory exercise using microbiological assays
Pyridoxamine and pyridoxine measured individually; pyridoxamine was mathematically converted to pyridoxine by the ratio of the relative molecular masses
Pyridoxamine and pyridoxal measured individually; pyridoxamine was mathematically converted to pyridoxal by the ratio of the relative molecular masses
Reported as total vitamin B6 from interlaboratory exercise using microbiological assays
Reported as total biotin from interlaboratory exercise using microbiological assays
Reported as total folate from interlaboratory exercise using microbiological assays
Value ± SD reported in Camara et al. [65]
Reported as total vitamin B12 from interlaboratory exercise using microbiological assays
The ID LC-MS and ID LC-MS/MS methods described by Phillips [64] provided quantitative results for eight forms of five B vitamins (thiamine, riboflavin, niacin, niacinamide, pantothenic acid, pyridoxine, pyridoxal, and pyridoxamine). The ID LC-MS/MS analysis of the whole egg powder (SRM 1845a) and baking chocolate (SRM 2384) are illustrated in Fig. S1 in the ESM. The multiple forms of B3 (niacin and niacinamide) and B6 (pyridoxine, pyridoxal, and pyridoxamine) can be determined individually in food matrices; however, they are often reported as total B3 or total B6, respectively. For example, in the case of total B6, pyridoxine, pyridoxal, and pyridoxamine are each measured individually and pyridoxal and pyridoxamine are mathematically converted to pyridoxine. Because of the multiple forms of B3 and B6, it may be difficult to compare values reported on the Certificates of Analysis for different CRMs unless they are reported in the same manner.
The evolution of the value assignment process at NIST is illustrated in Fig. 4 for vitamin B3 in the four infant formula CRMs over the past 30 years. In the first infant formula material, SRM 1846, issued in 1996, the certified value for niacin was assigned based on the combination of NIST measurements using LC-UV with the results from collaborating laboratories. The collaborating laboratory value was a combination of individual results from seven laboratories (many using microbiological assays) and the mean of 20 laboratories participating in a GMA study (various methods used) with a relative uncertainty of 22% [standard deviation (SD) of measurements]. The NIST LC-UV measurements had a relative uncertainty of 5.0% (SD). The two sets ofresults were in good agreement, and the final certified value for niacinamide was 63.3 mg/kg ± 7.6 mg/kg (12%). For SRM 1849 infant/adult nutritional formula, issued in 2009, the value assignment was based on seven different data sets using multiple methods as shown in Fig. 4 (and Table 4) including LC-UV and ID LC-MS. Because the large number of results from different methods were in excellent agreement, the certified value for niacinamide of 97.5 mg/kg ± 2.3 mg/kg had one of the lowest relative expanded uncertainties (2.4%) associated with any of the certified values for vitamins in SRM 1849. For SRM 1849a, only two sets ofresults were used to assign the certified value, i.e., ID LC-MS measurements at NIST and results from the manufacturer’s analyses. The ID LC-MS measurements (103.1 mg/kg ± 1.3 mg/kg) were combined with the manufacturer’s mean (113.1 mg/kg ± 4.8 mg/kg) to assign a certified value of 108 mg/kg ± 10 mg/kg. Because fewer data sets were used to assign the certified value in SRM 1849a, the uncertainty is larger than the uncertainty associated with niacin value in SRM 1849. Perhaps the ID LC-MS value could be considered as a more accurate assessment of the true value with a lower associated uncertainty; however, results from collaborating laboratories (using multiple methods including microbiological, LC-FL, and LC-MS) and results of analyses from two other infant formula manufacturers (which were not used in the value assignment process) provided results similar to the higher manufacturer’s value. The decision was made to combine only the ID LC-MS and manufacturer’s measurements to provide an appropriate assessment of the true value with a wider uncertainty. For the most recent infant/adult nutritional formula, SRM 1869, issued in 2018, NIST used ID LC-MS/MS as the primary measurement technique combined with results from two interlaboratory studies and the manufacturer which provided a certified value with the lowest relative expanded uncertainty (2.2%) among the four infant/adult formula CRMs. Other recent food-matrix CRMs have been value assigned for vitamin B3 using only the ID LC-MS/MS method [64] with confirmation by collaborating laboratories, and the resulting certified values generally have low relative expanded uncertainties (< 2%, e.g., see SRM 1546a, SRM 2383a, SRM 3252, and SRM 3290 in Tables 5 and 6).
Fig. 4.
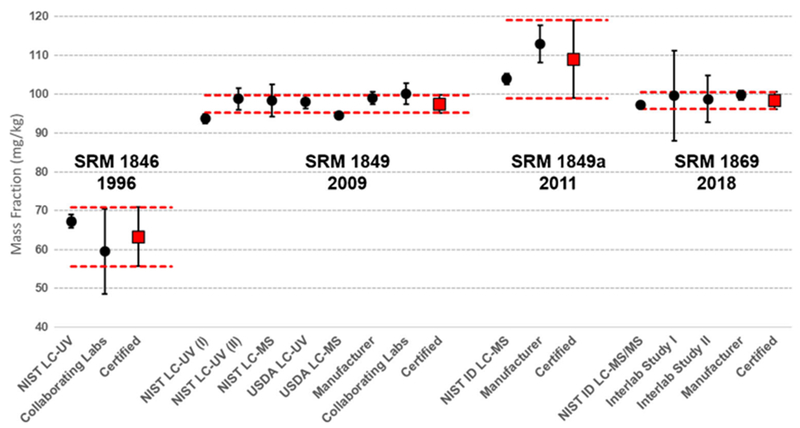
Evolution of value assignment for vitamin B3 (niacin) in four infant\adult nutritional formula CRMs and the analytical techniques used for value assignment. Error bars on certified values represent the expanded uncertainty (U95); error bars on individual measurement methods represent the standard deviation of the measurement set
Several other examples for improved vitamin B3 values are the recent re-issue of SRM 2383 baby food composite and SRM 1546 meat homogenate and the reanalysis of SRM 2384 baking chocolate and SRM 2387 peanut butter. For both SRM 2383 and SRM 1546, the mass fraction for niacin was assigned only as a reference/indicative value based on the results of the GMA interlaboratory studies with values of 18.1 mg/kg ± 2.2 mg/kg (12%) and 36.3 mg/kg ± 3.8 mg/kg (10%), respectively. The recent SRM 2383a and SRM 1546a have certified values for niacinamide of 3.59 mg/kg ± 0.06 mg/kg (1.7%) and 38.18 mg/kg ± 0.74 mg/kg (1.9%) based only on ID LC-MS/MS results [64]. When originally issued, both SRM 2384 and SRM 2387 had reference/indicative values assigned for niacin of 12.1 mg/kg ± 2.0 mg/kg and 142 mg/kg ± 6 mg/kg, respectively, based only on results from interlaboratory exercises. Based on recent ID LC-MS/MS analyses, reference/indicative values for niacin, niacinamide, and total vitamin B3 have been assigned for SRM 2384 and SRM 2387 (see Table 6). The new reference/indicative value for total vitamin B3 in SRM 2384 (11.6 mg/kg ± 2.0 mg/kg) is in agreement with the original value (12.1 mg/kg ± 2.0 mg/kg); however, for the peanut butter SRM, the new reference/indicative value (41.3 mg/kg ± 1.0 mg/kg) is only 30% of the original value (142 ± 6 mg/kg), which was assigned based on collaborating laboratories using exclusively microbiological methods that respond to niacin, niacinamide, and nicotinamide adenine dinucleotide (NAD). A high concentration of NAD in the peanut butter may be the cause of this discrepancy. Speciation of the forms of vitamin B3 is a significant advancement that provides additional information to food industry laboratories about the source of this nutrient in food products.
In a similar manner as illustrated for vitamin B3, the value assignment of other WSVs (thiamine, riboflavin, pantothenic acid, and pyridoxine) has also evolved and significantly improved as illustrated by the reanalysis of the peanut butter and chocolate CRMs. For the peanut butter CRM, ID LC-MS/MS values for thiamine (0.614 mg/kg ± 0.18 mg/kg) and pantothenic acid (9.48 mg/kg ± 0.23 mg/kg) are consistent with the original reference/indicative values of 0.66 mg/kg ± 0.13 mg/kg and 10.8 mg/kg ± 3.2 mg/kg, respectively. A reference/indicative value for riboflavin of 1.21 mg/kg ± 0.16 mg/kg was available when the baking chocolate CRM was originally issued based only on results from an interlaboratory exercise; the reanalysis using ID LC-MS/MS provided an updated reference/indicative value of2.61 mg/kg ± 0.36 mg/kg. However, the original reference/indicative value for total vitamin B6 as pyridoxine was 4.66 mg/kg ± 0.62 mg/kg, whereas the updated reference/indicative value is 0.3193 mg/kg ± 0.0092 mg/kg. The original value was based on measurements from collaborating laboratories using microbiological methods exclusively. Microbiological approaches for the determination of vitamin B6 include an acid hydrolysis step to remove glycosidic linkages and therefore may represent a true total B6 measurement. Reports indicate that more than half of the vitamin B6 in peanut butter is in the form of glycosides, which may account for the discrepancy between the ID LC-MS/MS results and the collaborating laboratory results using microbiological assays. The availability of ID LC-MS/MS methods provides a different measurement of vitamin B6, which may not provide results comparable to the microbiological assay. However, for LC-MS/MS the measurand is known, whereas for microbiological assays, the measurand may not be well defined.
Vitamin B12 determination is another example of the evolution of analytical methods used for value assignment of vitamins in CRMs. The first CRM with a value assigned for vitamin B12 was BCR-487 Pig Liver (see ESM, Table S1). The value was assigned based on results from an interlaboratory study using microbiological assays. The first NIST CRMs with values assigned for vitamin B12 were the infant/adult nutritional formula (SRM 1849) and the multivitamin/multielement tablet (SRM 3280) both issued in 2009; the values were based on results from interlaboratory studies (and the manufacturer’s analyses for SRM 1849) both using microbiological assays. The vitamin B12 value in SRM 3280 was assigned as a reference/indicative value at 4.9 mg/kg ± 1.9 mg/kg; however, this value was later updated to a certified value using additional results from a newly developed method based on the measurement of the cobalt in the cyanocobalamin using LC with inductively coupled plasma (ICP) MS. The LC-ICP/MS result was 4.51 mg/kg ± 0.38 mg/kg (SD, n = 10) and when combined with the microbiological assay results from the interlaboratory study provided a certified value of 4.8 mg/kg ± 1.0 mg/kg. Recently, Raju et al. [66] reported an LC-ICP/MS method for vitamin B12 and used this method to analyze SRM 3280 as a control sample obtaining a result of 4.38 mg/kg ± 0.05 mg/kg (SD, n = 2). The current certified value, which still includes results from microbiological assays, should be updated using only results from the two LC-ICP/MS methods to represent a value based on chemical methods of analysis only.
The evolution of the analytical methods for the B vitamins, particularly the use of ID-LC-MS/MS, has significantly increased the number of vitamins with certified values assigned in NIST CRMs. For example, for the first-generation CRMs (Table 1), only SRM 1846 had certified values for WSV (4 values), whereas all the second-generation CRMs (Table 3) have certified values for WSV with most having certified values assigned for 8 to 13 vitamins. In addition, the uncertainties associated with the certified values have decreased significantly with recent CRMs having uncertainties as low as 2 to 4% for many of the vitamins indicating that we have greater confidence in the accuracy and reliability of these values. Similar trends for number of analytes and lower uncertainties are observed also for recent KRISS CRMs as described below.
CRMs for cholesterol and fatty acids
Although not always considered as “good” nutrients, sufficient quantities of cholesterol and fatty acids or triglycerides are required through dietary intake or supplementation. The earliest CRM for determination of cholesterol in food was SRM 1845 cholesterol in whole egg powder, which was issued in 1989 with the mass fraction of cholesterol determined using an ID GC-MS method [56]. The earliest food-matrix CRM from BCR for organic nutrients, issued in 1993, was intended for determination of fatty acids and cholesterol, BCR-163 pork-beef fat blend, with values assigned for fatty acid methyl esters (FAMEs), sterols, and cholesterol [67] (see Table 2). Four additional BCR food-matrix CRMs were issued from 1997 through 2007 with values assigned for fatty acids or triglycerides and cholesterol, three of which are butter fat materials that have values assigned for long chain triglycerides (C24 to C54). The first NIST food-matrix CRM with values assigned for fatty acids was SRM 1544, a frozen diet composite specifically developed for measurement of fatty acids and cholesterol. Of the other first-generation NIST CRMs listed in Table 1, all but the spinach material, which is not a source of fat, had values assigned for selected fatty acids, and three CRMs had values assigned for cholesterol (infant formula, meat homogenate, and baby food composite). Fatty acid values are available for 11 of the 16 recent food-matrix CRMs listed in Table 3. Values for selected fatty acids in five food-matrix CRMs are summarized in Table 7. Additional CRMs with assigned values for fatty acids are summarized in Table S4 (see ESM).
Table 7.
Mass fractions of cholesterol and selected fatty acids in non-fortified food-matrix CRMs
| Mass fraction (g/100 g, as-received basis) (as free fatty acids) |
|||||
|---|---|---|---|---|---|
| Fatty acids | Common name | SRM 1546a Meat homogenate |
SRM 1549a Whole milk powder |
SRM 1845a Whole egg powder |
BCR-163a Beef-pork fat blend |
| Cholesterol (mg/g) | 0.717 ± 0.022 | 0.981 ± 0.071 | 17.67 ± 0.29 | 134 ± 5 | |
| Total fat (sum of fatty acids) | 18.96 ± 0.40b | 26.98 ± 0.66 | 43.4 ± 1.4 | ||
| Dodecanoic acid (C12:0) | Lauric | 0.0153 ± 0.0011 | 0.764 ± 0.085 | ||
| Tetradecanoic acid (C14:0) | Myristic | 0.245 ± 0.023 | 2.48 ± 0.19 | 0.1094 ± 0.0048 | 2.29 ± 0.04 |
| (Z)-9-Tetradecenoic acid (C14:1) | Myristoleic | 0.0118 ± 0.0028 | 0.286 ± 0.038 | 0.0185 ± 0.0008 | |
| Hexadecanoic acid (C16:0) | Palmitic | 4.63 ± 0.53 | 6.65 ± 0.45 | 8.22 ± 0.26 | 25.96 ± 0.30 |
| (Z)-9-Hexadecanenoic acid (C16:1 n-7) | Palmitoleic | 0.618 ± 0.078 | 0.385 ± 0.025 | 0.831 ± 0.023 | 2.58 ± 0.16 |
| Octadecanoic acid (C18:0) | Stearic | 2.18 ± 0.32 | 2.57 ± 0.18 | 2.802 ± 0.095 | 18.29 ± 0.17 |
| (Z)-9-Octadecenoic acid (C18:1 n-9) | Oleic | 8.09 ± 0.40 | 4.83 ± 0.50 | 11.0 ± 1.4 | 38.3 ± 0.4 |
| (Z)-11-Octadecenoic acid (C18:1 n-7) | Vaccenic | 0.324 ± 0.017 | 0.153 ± 0.026 | 0.532 ± 0.015 | |
| (Z,Z)-9,12-Octadecadienoic acid (C18:2 n-6) | Linoleic | 3.32 ± 0.42 | 0.659 ± 0.057 | 5.43 ± 0.12 | 7.05 ± 0.017 |
| (Z,Z,Z)-9,12,15 Octadecatrienoic acid (C18:3 n-3) | α-Linolenic | 0.133 ± 0.020 | 0.132 ± 0.010 | 0.1643 ± 0.0047 | 0.86 ± 0.14 |
| (Z,Z,Z)-6,9,12-Octadecatrienoic acid (C18:3 n-6) | γ-linolenic | 0.0107 ± 0.0022 | 0.0452 ± 0.0018 | ||
| Eicosanoic acid (C20:0) | Arachidic | 0.0329 ± 0.0009 | 0.0049 ± 0.0021 | ||
| (Z)-11-Eicosenoic acid (C20:1 n-9) | Gondoic | 0.1322 ± 0.0044 | 0.045 ± 0.021 | ||
| Tetracosanoic acid (C24:0) | Lignoceric | 0.0068 ± 0.0003 | 0.0095 ± 0.0015 | ||
| (Z)-15-Tetracosenoic acid (C24:1 n-9) | Nervonic | 0.0228 ± 0.0009 | 0.0616 ± 0.0018 | ||
| (Z,Z,Z,Z,Z)-7,10,13,16,19-Docosapentaenoic acid (C22:5 n-3) | DPA | 0.014 ± 0.001 | 0.0202 ± 0.0007 | ||
| (Z,Z,Z,Z,Z,Z)-4,7,10,13,16,19-Docosahexaenoic acid (C22:6 n-3) | DHA | 0.1701 ± 0.0077 | |||
Certified values are in italic type; reference/indicative values are in normal type. See Certificate of Analysis for NIST SRMs at http://www.nist.gov/srm/index.cfm and for BCR-163 at https://crm.jrc.ec.europa.eu
Certified values based on GC-MS and GC-FID measurements at NIST; reference values for SRM 1849a are based only on results from interlaboratory exercise
Values include positional and geometrical (i.e., cis/trans) isomers, as appropriate; Mass fraction values reported as grams of FAME per 100 g of FAME (fatty acid methyl esters)
Since 2007 NIST has developed 10 additional CRMs related to dietary supplement matrices that have values assigned for fatty acids including saw palmetto [68], botanical oils [69], and fish oils [69]. These CRMs are summarized in Table 8 with information on the number of values assigned for fatty acids. The specific nutritional interest in omega-3 and omega-6 fatty acids, often denoted as essential fatty acids, was the motivation for the development of SRM 3274 botanical oils containing omega-3 and omega-6 fatty acids [69]. SRM 3274 consists of four botanical oils (borage, evening primrose, flax, and perilla), which have different profiles of the content of various fatty acids, and in particular, differing levels of α-linolenic acid (an omega-3) and linoleic and γ-linolenic acids (both omega-6) as shown in Fig. 5 (and summarized in ESM, Table S7).
Table 8.
Dietary supplement-matrix CRMs for fattv acid and tocopherol content
| SRM | Name | Issue date | No. of fatty acid values assigneda | Additional constituents with values assigned | Total values assigneda |
|---|---|---|---|---|---|
| 3250 | Serenoa repens (Fruit)–Saw Palmetto | 2009 | 14 (4) | Phytosterols (3); free fatty acids (16) | 37 |
| 3251 | Serenoa repens Extract–Saw Palmetto | 2009 | 17 (3) | Phytosterols (3); free fatty acids (17); tocopherols (2), carotenoids (3), cycloartenol | 45 |
| 3274 | Botanical oils containing Omega-3 and Omega-6 fatty acids | 2009 | 35 (33) | 68 | |
| 3274-1 | Borage (Borago officinalis) | 2009 | 9 (8) | 17 | |
| 3274-2 | Evening Primrose (Oenothera biennis) | 2009 | 10 (8) | 18 | |
| 3274-3 | Flax (Linium usitatissimum) | 2009 | 9 (7) | 16 | |
| 3274-4 | Perilla (Perilla frutescens) | 2009 | 7 (10) | 17 | |
| 3275 | Omega-3 and Omega-6 fatty acids in fish oil | 2010 | 54 | ||
| 3275-1 | Concentrate high in DHA | 2010 | 9 (7) | 16 | |
| 3275-2 | Anchovy oil (high in DHA and EPA) | 2010 | 11 (7) | 18 | |
| 3275-3 | Concentrate containing 60% long chain omega-3 fatty acids | 2010 | 11 (9) | 20 | |
| 3277 | Krill oil | 2019b | Fatty acids | ||
| 3278 | Tocopherols in Edible oils | 2009 | Tocopherols (4) | 4 |
See Certificate of Analysis for SRMs at http://www.nist.gov/srm/index.cfm
Number of certified, (reference/indicative), and [information] values
In progress; anticipated completion in 2019
Fig. 5.
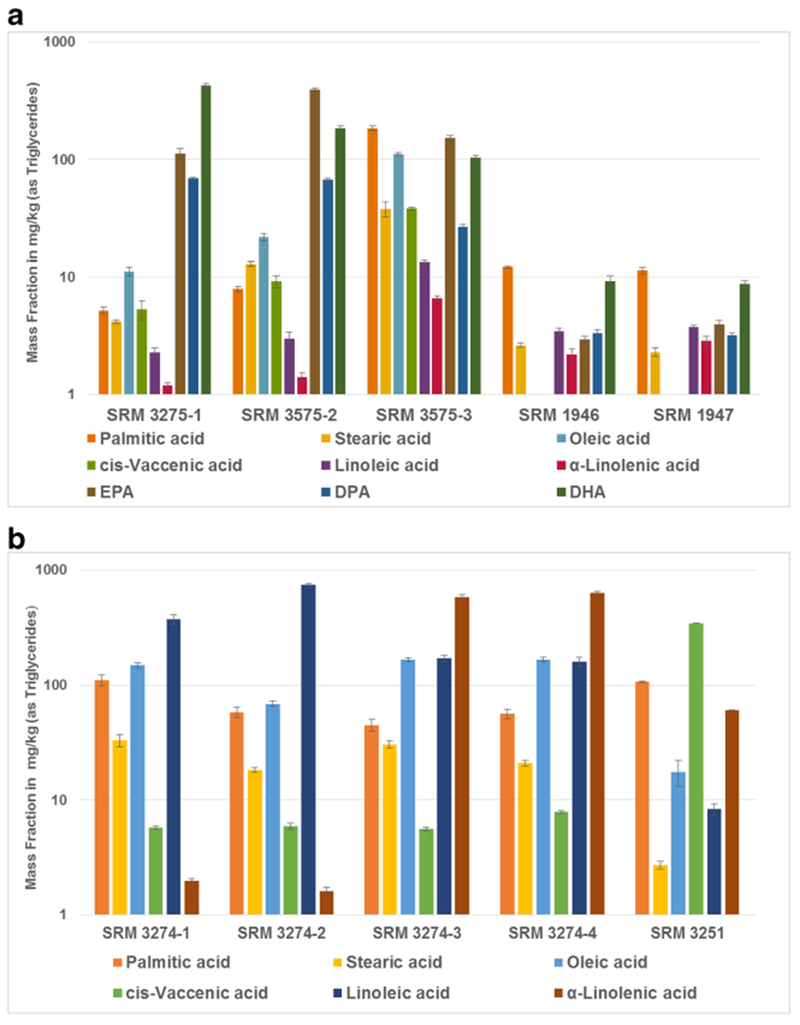
Bar graphs of the distribution of mass fractions of selected fatty acids (as triglycerides) in fish-matrix CRMs (a) and plant-matrix CRMs (b). Note the logarithmic scale for mass fractions. Error bars are the expanded uncertainties of the certified values with 95% confidence
Fish oils are high in long-chain polyunsatuarated fatty acids such as eicosapenaenoic acid (EPA, C20), docosapentaenoic acid (DPA, C22), and docosahexaenoic acid (DHA, C22). SRM 3275 omega-3 and omega-6 fatty acids in fish oil was developed specifically to provide values for fatty acid content with particular emphasis on DPA, DHA, and EPA. SRM 3275 consists of three individual fish oils: 3275-1 is a fish oil concentrate high in DHA, 3275-2 is an anchovy oil that is high in DHA and EPA, and 32750-3 is a fish oil concentrate with nominally 60% long-chain omega-3 fatty acids [57]. The levels of selected fatty acids including EPA, DPA, and DHA in SRM 3275 are shown in Fig. 5 and summarized in Table S8 in ESM. As described above, two frozen fish tissue SRMs [39] also have values assigned for fatty acids and are also included in Table S8 (see ESM). A krill oil CRM is under development (SRM 3277) that will have values assigned for fatty acids which are present predominantly as phospholipids rather than as triglycerides in fish oil. Krill oil is high in omega-3 fatty acid content including EPA and DHA.
Evolution of fatty acid value assignment
SRM 1544 fatty acids and cholesterol in a frozen diet composite, issued in 1996, was the first NIST CRM with values assigned for individual fatty acids based on the combination of ID GC-MS measurements at NIST and results from three contract laboratories. For the remaining first-generation NIST food-matrix CRMs, fatty acid values were assigned using a several approaches. Reference/indicative values for fatty acids were assigned in the infant formula (SRM 1846) and baby food (SRM 2383) materials based on data from collaborating laboratories and interlaboratory exercises only. Certified and reference/indicative values were assigned for fatty acids in the meat homogenate (SRM 1546), baking chocolate (SRM 2384), peanut butter (SRM 2387), and fish tissue (SRMs 1946 and 1947) materials using data from interlaboratory exercises in combination with NIST GC-MS or GC-FID results.
In 2009 SRM 1849 infant/adult nutritional formula was the first NIST food-matrix material issued with certified values for fatty acids based on results from two NIST methods, i.e., GC-FID and GC-MS using three isotopically labeled fatty acids as internal standards (i.e., not true ID analyses since labeled standards were not used for each analyte), in combination with results from the manufacturer and an interlaboratory exercise. For the food-matrix CRMs listed in Tables 7 and S6 (see ESM), all certified values for fatty acids were assigned based on NIST GC-FID and GC-MS measurements in combination with the results of an interlaboratory exercise or with confirmation only by the interlaboratory exercise results (SRM 1845a). As shown in Tables 7 and S6, the relative expanded uncertainties of the certified values in these recent food-matrix CRMs typically range from 5 to 11%, with the egg powder uncertainties lower at 3 to 4%, which is perhaps an indication that the use of the interlaboratory results in conjunction with NIST results generally increases the uncertainty associated with the certified value. However, for BCR-163 the fatty acid methyl esters values were based on results from 10 to 12 laboratories using GC-FID resulting in uncertainties as low as 1% (95% confidence interval, see Certificate of Analysis for details) for the three major fatty acids indicating the maturity of GC-FID measurements for fatty acids and the excellent capabilities of the laboratories involved.
Value assignment of cholesterol content at NIST has remained consistent since the ID GC-MS method was developed in the 1980s for determination of serum cholesterol [56]. This method was applied to the first NIST food-matrix CRMs, i.e., coconut oil (SRM 1563) at 638 mg/kg ± 8 mg/kg, 1.3% and egg powder (SRM 1845) (18.64 g/kg ± 0.39 g/kg, 2.0%), and then SRM 1544 fatty acids and cholesterol in diet composite (148.3 mg/kg ± 9.4 mg/kg, 6.3%). For the five recent NIST food-matrix CRMs with cholesterol values assigned, the relative uncertainties of the certified values are 1.1% (protein drink mix) to 3.1% (meat homogenate) for four of the materials with only the milk powder higher at 7.2%. For BCR-163 the value for cholesterol was based on results from nine laboratories using GC-FID resulting in a relative uncertainty of only 3.7% (95% confidence interval).
Recent food-matrix CRMs from JRC-Geel
The only recent food-matrix CRM for organic nutrients from JRC-Geel is ERM-BD600 Whole Milk Powder, which was produced as the replacement for BCR-421 (milk powder) (see Table 2). The European Reference Material (ERM) concept was launched in 2004 as a partnership of the three major European CRM producers (JRC-Geel, Federal Institute for Materials Research and Testing (BAM), and LGC) to provide a European-branded CRM with added value based on stringent quality criteria throughout the production, homogeneity and stability testing, and chemical characterization. Issued in 2011, ERM-BD600 Whole Milk Powder was the first food-matrix ERM produced by JRC-Geel for vitamins since the original BCR materials described above. ERM BD600 was prepared from 4000 L of bovine whole milk, which was fortified with a vitamin mixture, pasteurized, homogenized, concentrated, and spray dried at a food-grade pilot plant to produce 389 kg of material [70]. ERM-BD600 has certified values assigned for seven FSVs and WSVs and indicative and informational values assigned for five additional constituents (see ESM, Tables S1 and S2). The certified values in ERM-BD600 were assigned based on the same approach as for the previous BCR materials, namely a collaborative study among expert laboratories that have provided sufficient evidence of demonstrated capabilities (e.g., participation in proficiency testing, use of validated methods, and accreditation to ISO 17025 for the relevant measurements). From 6 to 18 laboratories provided measurements for the determination of the vitamins in ERM-BD600 based on normal-phase LC (vitamin A) and combinations of LC-MS, LC-FL, and LC-UV for the remaining vitamins. The indicative and informational values also included measurements by microbiological assays.
Recent food- and dietary supplement-matrix CRMs from other NMIs
Most NMIs producing matrix CRMs (e.g., IAEA, NMIJ, LGC, and NMIA) have food-matrix CRMs for trace element content or food safety concerns (e.g., allergens, aflatoxins, additives), but not for organic nutrient content. In addition to NIST and JRC-Geel, only KRISS and NIMC have produced food or dietary supplement CRMs for the determination of vitamins. As shown in Table 9, both KRISS and NIMC have produced infant formula and multivitamin (powder or tablets) CRMs with values assigned for selected vitamins.
Table 9.
Mass fractions of vitamins in infant formula and multivitamin CRMs from KRISS, NIMC, and NIST
| Infant formula Mass fraction (mg/kg, dry-mass basis)a |
Multivitamin tablet/powder Mass fraction (mg/kg, dry-mass basis) |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Vitamins |
KRISS 108-02-003 | NIMC GBW(E) 100227b | NIMC GBW 10037b | NIST SRM 1849a | KRISS 108-10-019 (Powder) | NIMC GBW(E) 100,273 (Powder) | NIMC GBW(E) 100,228 (Tablets) | NIST SRM 3280 (Tablets) | |
| Issue date | 2015 | 2011c | 2007 | 2012 | 2016 | 2013 | 2011c | 2009 | |
| Thiamine | B1 | 5.54 ± 0.97 | 6.60 ± 0.46 | 12.57 ± 0.98 | 23,380 ± 310 | 1010 ± 80 | 2760 ± 110 | 1060 ± 120d | |
| Riboflavin | B2 | 18.49 ± 0.18 | 20.2 ± 2.4 | 20.37 ± 0.52 | 22,800 ± 1700 | 1240 ± 100 | 4280 ± 170 | 1320 ± 170 | |
| Niacin | B3 | 60.6 ± 1.3c | 39.8 ± 1.2e | 65.3 ± 3.7f | 108 ± 10 | 15,190 ± 440 | 9890 ± 180 | 14,100 ± 230 | |
| Pantothenic acid | B5 | 48.36 ± 0.84 | 68.2 ± 1.9 | 25,090 ± 400 | 4062 ± 262g | 7300 ± 960 | |||
| Pyridoxine | B6 | 5.57 ± 0.14 | 4.71 ± 0.31 | 13.46 ± 0.93 | 21,620 ± 730 | 1800 ± 130 | 300 ± 20 | 1810 ± 170 | |
| Folic acid | B9 | 1.210 ± 0.042 | 2.293 ± 0.062 | 109.9 ± 6.0 | 394 ± 22 | ||||
| Biotin | B7 | 0.291 ± 0.060 | 24.8 ± 1.1 | 23.4 ± 3.2 | |||||
| Cyanocobalamin | B12 | 0.0415 ± 0.0027 | |||||||
| Ascorbic acid | C | 670 ± 27 | 784 ± 65 | 39,400 ± 5600 | 42,200 ± 3700 | ||||
| Retinol | A | 6.21 ± 0.23 | 7.68 ± 0.23 | 14.3 ± 2.6 | 444 ± 46 | ||||
| α-Tocopherol | E | 82.8 ± 4.9 | 158 ± 18 | 14.3 ± 3.4 | 21,400 ± 3500 | ||||
| Cholecalciferol | D3 | 0.095 ± 0.007 | 0.111 ± 0.017 | 0.125 ± 0.003 | – | ||||
| Phylloquinone | K1 | 0.515 ± 0.026 | 1.06 ± 0.17 | 16.7 ± 3.0 | 22.8 ± 2.2 | ||||
Certified values in italic type; reference/indicative values in normal type. Information regarding NIMC CRMs available at www.en.nim.ac.cn/service/168; Information regarding KRISS CRMs available at www.kriss.re.kr/eng. Certificate of Analysis for each NIST SRM is available at http://www.nist.gov/srm
Mass fraction for SRM 1849a is on an as-received basis (not dry-mass)
Certificate of Analysis for this CRM provides the uncertainty as a relative percent of the certified value; uncertainties have been converted to mg/kg
CRM issued in 2011 and values updated in 2015
Thiamine reported as thiamine hydrochloride
Value reported as nicotinic acid
Value reported as nicotinamide
Certificate of Analysis for this CRM provides the value as calcium pantothenate (8830 mg/kg ± 570 mg/kg); value has been converted to pantothenic acid for comparison
NIMC has produced two infant formula CRMs, one with a value assigned for only niacin (GBW 10037), and the second with values assigned for four B vitamins and 10 trace elements (GBW 100227). Huang et al. [71] reported the development of the infant formula CRM GBW10037, with a certified value for niacin based on combining results from ID LC-MS and LC-UV, which were in excellent agreement. The GBW(E)100227 infant formula has values assigned for four B vitamins based on results from ID LC-MS/MS (B1, B3, and B6) or LC-MS/MS (B2) and LC-UV. NIMC used SRM 1849 as a control sample during the analyses [71]. For the multivitamin powder [GBW(E)100273], the certified values were based on results from four collaborating laboratories in China and from NIMC; NIMC used an LC-UV method and analyzed SRM 3280 for method validation. The NIMC multivitamin tablet CRM, GBW(E)100228, has certified values for four WSVs, which are based on combining LC-UV and ID LC-MS/MS (B1, B3, B6) or LC-MS/MS (B2) results from NIMC, as well as certified values for 10 element nutrients. SRM 3280 was used for validation of both methods.
The infant formula and multivitamin powder CRMs from KRISS shown in Table 9 are both the second issue of these matrices. KRISS first issued CRM 108-02-001 infant formula and CRM 108-10-010 multivitamin powder in 2014 with certified values for three and five WSVs, respectively, as shown in Table S9 (see ESM). KRISS assigned the certified values to these first food and supplement materials based on measurements using ID LC-MS methods developed specifically for the determination of each vitamin individually. Shin et al. [72] developed an ID LC-MS method for niacin, validated the method using SRM 1849, and applied the method for the certification of infant formula CRM 108-02-001. In this same investigation, they confirmed the niacin value in BCR-431 (43 mg/kg ± 3 mg/kg) by their ID LC-MS method as 41.2 mg/kg ± 2.1 mg/kg. Lee et al. [73] developed an ID LC-MS method for determination of tocopherols (α, β, and γ) in CRM 108-02-001 infant formula using a standard addition approach. When Lee et al. [73] used the traditional ID approach to LC-MS analysis using deuterium-labeled internal standards for the three tocopherols, they observed high biases compared to a standard addition (SA) ID LC-MS approach for their analysis of CRM 108-02-001 and NIST SRM 1849; they attributed these biases to hydrogen/deuterium exchanges during the sample preparation saponification. However, the ID LC-MS results of Lee et al. [73] are in better agreement with the certified values in SRM 1849 than the SA-ID-LC-MS results except for α-tocopherol. The NIST certified values for the three tocopherols were determined using results from LC-UV and LC-FL measurements at NIST combined with results from collaborating laboratories. Lim et al. [74] developed an ID LC-MS method for vitamin A (retinol) in infant formula and used SRM 1846 infant formula, which had only a reference/indicative value, as part of the method validation. Lim et al. [74] obtained a value of 5.58 mg/kg ± 0.14 mg/kg (expressed as retinol equivalents) using the ID LC-MS method compared to the NIST value of 5.84 mg/kg ± 0.68 mg/kg, which was assigned based on a LC-UV method, and later (2004) removed from the Certificate of Analysis due to increasing difficulty in extraction recovery as the material aged.
KRISS expanded the list of nutrients with assigned values for both the infant formula and the multivitamin CRMs when they issued CRM 108-02-003 as the replacement for CRM 108-02-001 and CRM 108-10-019 as the replacement for 108-10-010, both in 2016 (see Table 9). In addition to vitamin B2, B3, and B9, which were certified in the original CRM 108-02-001 infant formula (see ESM, Table S9), values for other WSVs were added (i.e., C, B1, B5, B6, B7, and B12). The utility of the new infant formula CRM was greatly increased with values assigned for FSVs [vitamin A, E (α-, β-, and γ-tocopherols), D3, and K1]; cholesterol; fatty acids (8); amino acids (10), and proximates. As with their original infant formula CRM (108-02-001), KRISS developed higher order methods for value assignment of the new infant formula CRM based on ID LC-MS and ID LC-MS/MS for riboflavin (B2) and K1, respectively. Lee et al. [75] described the ID LC-MS method for riboflavin using 13C4,15N2-riboflavin as the internal standard and validating the method using SRM 1849 and SRM 3280. For determination of vitamin K1, Lee et al. [76] used an ID LC-MS/MS method, with a C30 column and deuterium-labeled vitamin K1 as the internal standard, to assign values for both trans-vitamin K1 (0.434 mg/kg ± 0.025 mg/kg) and cis-vitamin K1 (0.080 mg/kg ± 0.005 mg/kg). Using this ID LC-MS/MS method, Lee et al. [76] analyzed SRM 1849a, which has a certified value for total vitamin K1 (1.06 mg/kg ± 0.17 mg/kg), and reported values for trans- and cis-vitamin K1 of 0.958 mg/kg ± 0.055 mg/kg and 0.082 mg/kg ± 0.002 mg/kg, which when combined (1.039 mg/kg ± 0.055 mg/kg) agree with the NIST certified value for total vitamin K1. For value assignment of α-, β-, and γ-tocopherols and retinol in CRM 108-02-003, the methods of Lee et al. [73] and Lim et al. [74] were used.
The number of vitamins with assigned values was also significantly increased for the new multivitamin CRM (108-10-019) compared with the original material (108-10-010), i.e., 12 vs. 5 values. In addition to the five B vitamins (B2, B3, B5, B6, and B9) certified in CRM 108-10-010 (see ESM, Table S9), the new multivitamin CRM has certified values assigned for vitamins B1 and B7 and information values assigned for vitamins C, A, E, D3, and K1 (see Table 9). In addition to the increased number of vitamins with values assigned in the new infant formula and multivitamin CRMs, the relative expanded uncertainties associated with the certified values have decreased. For example, for the infant formula, the relative uncertainties on the original material for B2, B3, and B9 were 1.5%, 4.0%, and 5.7%, respectively, compared to 1%, 2.1%, and 3.5% for the new material. Similarly, for the multivitamin CRM, relative expanded uncertainties on the certified values for B3, B5, B6, and B9 were 7.0%, 4.0%, 4.3%, and 6.8%, respectively, compared with 2.9%, 1.6%, 3.4%, and 5.5%. Only the relative expanded uncertainty for B2 increased (4.4% vs 7.4%). In addition, the new KRISS infant formula and multivitamin CRMs also have generally improved on the relative expanded uncertainties compared to similar NIST CRMs issued several years earlier (see Table 4 and Table 9) demonstrating the rapid improvements among CRM producers for these measurements.
The new infant formula and multivitamin powder CRMs from KRISS with certified values for 15 and 12 vitamins/organic nutrients, respectively, are significant additions to the international inventory of food and dietary supplement CRMs. Although not distributed significantly outside Asia, the CRMs from NIMC and KRISS play a critical role in ensuring the quality of measurements for vitamins in food and dietary supplements within Korea and China.
At KRISS the infant formula and multivitamin CRMs, and the methods used to value assign these CRMs, have been developed through a substantial program in measurement standards for organic nutrients [B. Kim, KRISS, personal communication]. As part of this continuing effort, KRISS plans to develop food-matrix CRMs for organic nutrients based on rice (108-01-007), wheat flour (108-01-005), spinach (108-05-010), cabbage (108-05-011), apple (108-05-012), beef (108-03-007), and tuna (108-04-005), which when completed will significantly expand and complement the current inventory of food-matrix CRMs available.
Nutrients in fortified vs. non-fortified food matrices
Many foods are fortified (nutrients are added) to supplement the intake of nutrients in the diet; however, the majority of food matrices are not fortified. Of the first generation of food-matrix CRMs listed in Tables 1 and 2, all but one (SRM 1846 infant formula) were non-fortified food matrices, which also contributed to the limited number of organic nutrient values assigned to these materials. Significant measurement challenges are associated with development of CRMs for non-fortified foods including: (1) the content of vitamins and nutrients is often significantly lower in non-fortified foods, and (2) multiple natural forms of a vitamin may be present in a non-fortified food, whereas a single form of a vitamin is used in fortification. While the levels may be higher for fortified food matrices, the extractability and the stability of the fortified nutrients may change over time, as has been observed for encapsulated FSVs in SRM 1846 and SRM 3280 [61]. The ability to measure all forms of each vitamin using techniques such as ID LC-MS/MS, even at low endogenous levels, contributes to greater information about the vitamin content of foods and thus the impact of food intake on nutrient status. In some cases, even in foods with high levels of a fortified vitamin, small quantities of other vitamin forms may be measurable. For example, the milk base used for many infant formulas may contribute small quantities of endogenous vitamins, and for regulatory purposes these vitamins must be considered in the label claims as they contribute to the total nutritional impact of the food product when consumed.
Of the recent food and dietary supplement CRMs from NIST shown in Table 3, only six (three infant/adult nutritional formulas, multivitamin tablets, breakfast cereal, protein drink powder, and dry cat food) are fortified foods or supplements. All recent food-matrix CRMs from JRC-Geel (Table 2, ERM-BD600), KRISS, and NIMC (Table 9) are fortified materials. As shown in Tables 5 and 6, the four fortified NIST food-matrix CRMs have mass fractions of most vitamins ranging from 10 to 50 times higher than the non-fortified matrices. The report on the production of ERM BD600 states that it was fortified with a vitamin mix during preparation. A comparison of levels of WSVs in ERM-BD600 Milk Powder (Table S1, see ESM) and SRM 1549a Whole Milk Powder (Table 6), which was not fortified, indicates that the difference is small (less than a factor of 2) in this instance. The multivitamin supplements, as expected, have levels significantly higher than even the fortified foods (20 to 100 times higher). The proposed new food matrices planned at KRISS will significantly expand the available non-fortified food-matrix CRMs for organic nutrient analysis.
Vitamin metabolites in food-matrix CRMs
Folates
Folate is the general term for various forms of vitamin B9. Endogenous folates in foods exist in various forms, with 5-methyltetrahydrofolate (5-MTHF) as the predominant form. Folic acid is the synthetic form used in supplements and fortification of foods, particularly enriched flour and cereals. While most fortified food-matrix CRMs have values assigned for folic acid, relatively few food-matrix CRMs have values assigned for 5-MTHF. The first CRMs with values assigned for 5-MTHF were BCR-421 milk powder, BCR-485 mixed vegetables, BCR-121 wholemeal flour, and BCR-487 pig liver, all issued in 1998; the values were listed as indicative or informational values (see ESM, Table S1) and were based on results from three laboratories using a common sample preparation procedure followed by analysis using an LC-FL detection method. In a study by Koontz et al. [77] comparing total folate content in foods determined by a number of commercial laboratories using microbiological assays, they measured 5-MTHF in BCR-485 using an LC method and obtained a value of 2.532 mg/kg ± 0.115 mg/kg (compared to the indicative value of 2.15 mg/kg ± 0.42 mg/kg). For all BCR food CRMs issued in the late 1990s (see ESM, Table S1), the values for total folate were assigned using results from multiple laboratories using microbiological assays. The most recent food-matrix CRM from JRC-Geel, ERM-BD600 whole milk powder, still has a total folate value based only on microbiological assay results.
Camara et al. [65] developed an ID LC-MS/MS method for the determination of folic acid and 5-MTHF in both fortified and non-fortified food-matrix CRMs. The ID LC-MS/MS method was used to analyze two fortified food-matrix materials, SRM 1849a infant/adult nutritional formula and SRM 3233 fortified breakfast cereal, and two non-fortified materials, SRM 1549a whole milk powder and SRM 1845a whole egg powder. ID LC-MS/MS results for folic acid in the fortified CRMs were combined with results from collaborating laboratories to assign certified values (Table 5); a reference/indicative value for 5-MTHF was assigned in the infant/adult nutritional formula, and results for 5-MTHF were reported for the milk powder and egg powder CRMs [65] (Table 6) but are not listed on the Certificate of Analysis.
Vitamin D and metabolites
Increasing interest in the content of vitamin D in food has led recently to proposals in the U.S. to update the Nutrition Labels to include vitamin D [78]. There are two major forms of vitamin D, i.e., vitamin D3 (cholecalciferol), which is produced in the skin from exposure to sunlight and obtained from food sources, and vitamin D2 (ergocalciferol), which is obtained primarily from plant food sources and supplementation. Both forms of vitamin D are metabolized by animals to 25-hydroxyvitamin D [25(OH)D] and other metabolites. Consequently, animal-derived foods may contain 25(OH)D as well as unmetabolized vitamin D. Recent work has indicated that vitamin D intake and status may be attributed to both vitamin D and 25(OH)D, and intake of 25(OH)D present in foods such as meat, poultry, and eggs could significantly contribute to vitamin D health status [79] since 25(OH)D is reported to be up to five times more potent than the unmetabolized vitamin D3 in raising serum 25(OH)D levels. Even if the reported levels of 25(OH)D are lower than the vitamin D content, the enhanced bioavailability of 25(OH)D could mean that these foods would have a significant impact on estimates of vitamin D intake.
This emerging interest in vitamin D metabolites in food suggests the need for CRMs to validate the potential growth and importance of the measurement of 25(OH)D in food matrices. To address the impact of 25(OH)D in food, the U.S. Department of Agriculture (USDA) recently conducted an interlaboratory comparison study for the determination of vitamin D and 25(OH)D in several different food and dietary supplement matrices to assess the state-of-the-art of current measurements and to identify suitable materials for use as reference materials [80]. Study samples included a vitamin D supplement, cooked chicken liver and ground beef, and three NIST food-matrix CRMs, i.e., bovine liver (SRM 1577c), meat homogenate (SRM 1546a) and whole egg powder (SRM 1845a). The six food and supplement materials were analyzed for the determination of vitamin D3, vitamin D2, 25(OH)D3 and 25(OH)D2 by five participating laboratories using LC-MS/MS-based methods. The results of the study indicated that existing methodology for vitamin D and 25(OH)D measurements in food provided consistent results and the existing bovine liver, meat homogenate, and egg powder CRMs were suitable matrices for vitamin D and 25(OH)D value assignment.
NIST recently developed an ID LC-MS/MS method for the determination of vitamin D3 and 25(OH)D3 in non-fortified foods [81]. Briefly, the method is based on addition of vitamin D2-13C5 and 25(OH)D3-13C5 as internal standards, enzymatic digestion to hydrolyze the fat and release the vitamin D3 and 25(OH)D3, extraction with hexane, derivatization using 4-phenyl-1,2,4-triazoline-3,5-dione (PTAD), followed by analysis of the derivatized extract using LC-MS/MS with a pentafluorophenyl stationary phase column. The derivatization with PTAD was necessary to provide the selectivity and sensitivity necessary to measure the low levels of vitamin D3 and 25(OH)D3 in non-fortified food matrices. Using this method, vitamin D3 and 25(OH)D3 were determined in four non-fortified food matrix SRMs (whole egg powder, meat homogenate, whole milk powder, and bovine liver). The results of these analyses were combined with results from the USDA interlaboratory study to assign certified and/or reference/indicative values for levels of vitamin D3 and 25(OH)D3 in four NIST CRMs as summarized in Table 10. These four materials are the first non-fortified food-matrix CRMs with values assigned for vitamin D3 and 25(OH)D3. Four additional NIST CRMs, all fortified materials, are available with values assigned for vitamin D3 or vitamin D2. The infant/adult nutritional formula (SRM 1849a), and its predecessor SRM 1849 [61], were the first NIST food-matrix CRMs with values assigned for vitamin D3. SRM 3280 multivitamin/multielement tablets was the first dietary supplement CRM with a certified value for vitamin D, which was manufactured with the addition of vitamin D2 [44, 58]. A recent dietary supplement material, SRM 3532 calcium-containing solid oral dosage form, was also certified for vitamin D3 content using the ID LC-MS/MS method [82]. The ID LC-MS/MS method was also used to assign values for vitamin D2 in a soy milk CRM as shown in Table 10.
Table 10.
Values assigned for vitamin D2, vitamin D3, and 25-hydroxyvitamin D3 [25-(OH)D3] in food and dietary supplement CRMs
| SRM | Vitamin D3 Mass fraction (ng/g) |
25(OH)D3 |
|---|---|---|
| Non-fortified food matrices | ||
| SRM 1845a whole egg powder | 48.8 ± 4.7a,c | 12.2 ± 1.5a,c |
| SRM 1546a meat homogenate | 2.56 ± 0.53a,d | 0.90 ± 0.12a,c |
| SRM 1549a whole milk powder | 1.88 ± 0.35a,d | 0.53 ± 0.05a,d |
| SRM 1577c bovine liver | 13.7 ± 3.1b,c | |
| Fortified food and dietary supplement matrices | ||
| SRM 1849a infant/adult nutritional formula | 111 ± 1.7a,e | ND |
| SRM 3532 calcium-containing solid oral dosage form | 1310±33b,d | ND |
| Vitamin D2 Mass fraction (ng/g) |
||
| SRM 3235 soy milk | 12.0 ± 2.4a,f | ND |
| SRM 3280 multivitamin/multielement tablets | 8600±2600b,g | ND |
Number of certified, (reference/indicative), and [information] values. See Certificate of Analysis for SRMs at http://www.nist.gov/srm/index.cfm
Mass fraction reported on as-received basis
Mass fraction reported on dry-mass basis
Value assigned based on combination of results from NIST ID LC-MS/MS (with derivatization) and USDA interlaboratory study [80]
Value based on only NIST ID LC-MS/MS (with derivatization) results
Value based on results from NIST ID LC-MS, collaborating laboratories, and the manufacturer [57]
Value based on results from NIST ID LC-MS/MS (with derivatization) and from collaborating laboratories
Value based on NIST LC-MS results [44]
ND = not determined
Certified values in italic type; reference/indicative values in normal type
For current food-matrix CRMs available from JRC-Geel, only BCR-122 margarine has a certified value for vitamin D3 (0.125 mg/kg ± 0.007 mg/kg) and ERM-BD600 has an indicative value as a range (0.057 mg/kg–0.269 mg/kg). The recent infant formula CRM from KRISS has a certified value for vitamin D3. Thus, significant progress has been made recently in addressing this growing need. The characterization of vitamin D in appropriate existing food-matrix CRMs and/or the development of new food-matrix CRMs specifically for vitamin D measurements would be beneficial to the food nutritional assessment community to meet potential new labeling regulations.
Vitamin K1 in food and dietary supplement CRMs
Vitamin K1 (phylloquinone) has been a relatively neglected FSV as far as values assigned in food and dietary supplement CRMs. The first certified values assigned for vitamin K1 were in SRM 1849 infant/adult nutritional formula and SRM 3280 multivitamin/multielement tablets (see Table 4). Values were also assigned for vitamin K1 in SRM 1849a. In addition to a value for total vitamin K1, the recent KRISS infant formula CRM has values for trans- and cis-isomers of vitamin K1. NIST recently issued SRM 3232 Kelp Powder (Thallus laminariae) [83], which has certified and reference values assigned for 20 trace elements including total arsenic, arsenic species, and arsenosugars. Since kelp is a good source of vitamin K1, reference values were also assigned for total vitamin K1 (0.431 mg/kg ± 0.081 mg/kg), cis-vitamin K1 (0.0353 mg/kg ± 0.0067 mg/kg) and trans-vitamin K1 (0.396 mg/kg ± 0.075 mg/kg) using an ID LC-MS/MS method [83]. The vitamin K1 levels in the kelp are similar to the level in the KRISS infant formula and about 40% of the level in the NIST infant formula, which are both fortified materials. The kelp CRM and KRISS infant formula CRM are the only CRMs with values assigned for trans-vitamin K1 and cis-vitamin K1 isomers.
Carnitine and choline in food-matrix CRMs
Although not considered vitamins, choline and carnitine are important water-soluble nutrients that can be synthesized in the body or consumed through the diet. Carnitine is important for brain and heart health, and choline is critical in the synthesis of constructional components in cell membranes. Until recently no food-matrix CRMs had been characterized for these nutrients. The first CRM with values assigned for carnitine and choline was SRM 1849 infant/adult nutritional formula, in which a reference/indicative value for choline (882 mg/kg ± 88 mg/kg) was assigned based on a combination of results from the manufacturer and an interlaboratory comparison. An information value of 85 mg/kg was provided for carnitine in SRM 1849 based on only the manufacturer’s measurements. Recently, the AOAC International Stakeholder Panel on Infant Formula and Adult Nutritionals (SPIFAN) declared both choline and carnitine to be priority nutrients in infant formulas and analytical methods were updated [84, 85]. Phillips and Sander [86] reported the development of a method for the simultaneous determination of carnitine and choline in food matrices based on microwave-assisted extraction and ID LC-MS analysis. Using this method, they reported the choline and carnitine content of four NIST food-matrix CRMs including infant/adult nutritional formula, whole milk powder, whole egg powder, and soy flour. Certified values for choline and carnitine were assigned in SRM 1849a based on the ID LC-MS results combined with the manufacturer’s measurements and the results from an interlaboratory study. A certified value was also assigned for choline in SRM 3234 soy flour based on the ID LC-MS results combined with the results from an interlaboratory study. Reference/indicative values were assigned for choline and carnitine in the milk and egg powder CRMs and for carnitine in the soy flour CRM based on the ID LC-MS measurements. The values assigned for carnitine and choline in six NIST CRMs are shown in Tables 5 and 6. Values for choline span the range from 536 mg/kg (meat homogenate) to 16,400 mg/kg (egg powder) and values for carnitine range from approximately 5 mg/kg (egg powder and protein drink mix) to 173 mg/kg (infant formula).
Storage of food-matrix CRMs
Since many of the early food-matrix CRMs were intended for trace element analysis, long-term stability of the certified properties was not a significant issue. However, as more food-matrix CRMs are developed for the determination of vitamins and other organic nutrients, the physical form of the matrix (i.e., dry, freeze-dried, fresh, or wet) and the storage temperature are critical to the long-term stability of the certified constituents. From the standpoint of production of a CRM, a dry, homogeneous powder matrix that can be stored at room temperature would be ideal for long-term maintenance and for ease of distribution worldwide. Unfortunately, most food matrices that are analyzed routinely are not dry, homogeneous powders but are fresh (wet) or cooked inhomogeneous mixtures.
The current NIST CRMs listed in Table 3 are stored under several conditions including room temperature (20 to 25 °C), refrigeration (4 °C), −20 °C, and −80 °C [63]. The JRC-Geel CRMs listed in Table 2 are all stored at −40 °C [87]. The most characterized NIST food-matrix CRM, SRM 1849a, is stored at −80 °C to maintain the stability of the vitamins. The original infant formula, SRM 1846, which was packaged in a similar manner, was stored at room temperature, and before the inventory was depleted after 12 years, difficulties were encountered in extracting some vitamins from the matrix [44, 61]. For some recent NIST food-matrix CRMs (milk powder and egg powder), the samples have been sealed in aluminized pouches (typically 10-g samples) and are stored at 4 °C specifically to maintain stability of the vitamins and organic nutrients. Instructions for use for these materials state: “For elemental analyses, the packet can be resealed, stored under refrigeration, and test portions removed and analyzed until the material reaches its expiration date. For organic analyses, the packet can be resealed, stored under refrigeration, and test portions removed and analyzed for three weeks after the packet was initially opened [88].”
The soy flour SRM, a dry powder, which was originally intended primarily for isoflavones, was stored in bottles at room temperature. Values were added for nutritional elements, WSVs, and fatty acids to expand the use of the material for nutritional measurements. Unfortunately, after 5 years, presumably due to the room temperature storage, the values for all the WSVs, except B2 and B5, and the fatty acids were removed from the Certificate of Analysis [89] because of instability. Based on the experiences of the past 20 years, CRMs intended for organic nutrients should be stored at or below 4 °C to maintain long-term stability.
As part of the design in developing SRM 3280 multivitamin/multielement tablets, the decision was made not to grind the individual tablets to create a powder (the ideal homogeneous form for a CRM) to avoid potential decreased stability for some vitamins after grinding based on the manufacturer’s recommendations. Therefore, SRM 3280 is packaged in tablet form and specific instructions are provided on grinding a minimum of 15 tablets to obtain suitable test portions for valid comparison to the certified values. This potential stability issue for ground tablets was confirmed during the development of another tablet-matrix CRM, SRM 3532 calcium-containing solid oral dosage form. Since the primary intended use was for calcium determinations, the decision was made to provide a homogeneous powder from ground tablets. However, with the high interest in assigning vitamin D values to food- and supplement-matrix CRMs, SRM 3532 was evaluated for cholecalciferol content. The content of cholecalciferol in the ground tablet material (SRM 3532) was significantly lower (−63%) when compared to freshly ground material [82]. Fortunately, after the initial decrease in cholecalciferol content with grinding, the powdered material is stable even when stored at room temperature, and a reference/indicative value for cholecalciferol was assigned to SRM 3532 (see Table 10).
Current status of food and dietary supplement CRMs
The currently available food-matrix CRMs for organic nutrients are shown in the AOAC Food Triangle in Fig. 1. The NIST and KRISS CRMs have values assigned for proximates, which were used to position the CRMs in the triangle. For the JRC-Geel and NIMC CRMs, the approximate position in the triangle was determined using protein, fat, and carbohydrate values from food composition databases [90]. The CRMs shown in Fig. 1 are color-coded in four categories: (1) NIST SRMs with organic nutrients only, (2) NIST SRMs with both organic and trace element nutrients, (3) NIST SRMs with trace element content only, and (4) non-NIST CRMs. Most of the recent NIST food-matrix CRMs for organic nutrients also have values assigned for nutritional elements.
As shown in Fig. 1, Tables 1, 2, and 3, and Tables 9 and 10, a wide range of food- and dietary supplement-matrix CRMs are available for the determination of organic nutrients. In the cases where more than one similar matrix CRM exists, e.g. milk powder, infant formula, and multivitamin tablets/powder, the materials provide a reasonable range of mass fraction levels. For example, for the four infant formula CRMs (see Tables 3 and 9), levels of vitamin B3 are conveniently at nominal levels of 40 mg/kg, 60 mg/kg, and 110 mg/kg. All four CRMs have nominally 20 mg/kg of vitamin B2. With the exception of vitamin B2, the mass fractions of the WSVs and FSVs are all higher in the NIST infant/adult nutritional formula than in the other three infant formula CRMs by factors of 1.5 to 3. This difference is probably because SRM 1849a is not a commercial product but a hybrid formula prepared specifically as an internal control material for the manufacturer and used as the CRM. For the multivitamin CRMs, mass fractions generally differ by factors of 2 to 20 for the vitamins reported in multiple materials. Note also that the NIST multivitamin/multielement SRM is in tablet form while the NIM China and KRISS materials are both powders. The powder materials may be more homogeneous than the powder produced from grinding the tablets following the suggested protocol, which does add this step into the uncertainty; however, the vitamins in the powder materials may be less stable. The third similar matrix among the CRM producers is the milk powder (SRM 1549a and ERM-BD600), and the mass fractions for the WSVs are a factor of 2 to 3 higher in ERM-BD600, which was fortified in the preparation process.
For the fortified food-matrices in Table 5, most of the B vitamins cover good ranges of mass fractions, e.g., B1 mg/kg to 60 mg/kg; B2 20 mg/kg to 76 mg/kg (nominally 20 mg/kg, 30 mg/kg, 40 mg/kg, and 80 mg/kg); total B3 109 mg/kg to 822 mg/kg (nominally 100 mg/kg, 200 mg/kg, 300 mg/kg, and 800 mg/kg); B5 68 mg/kg to 540 mg/kg; B6 mg/kg to 82 mg/kg; B7 1.4 mg/kg to 4.4 mg/kg; and B9 2.2 mg/kg to 15 mg/kg. The non-fortified food-matrices in Tables S1 and S3 (see ESM) and in Table 6 cover good ranges of mass fractions for the WSVs. For vitamin B1 and vitamin B6, the mass fractions range nominally over an order of magnitude, 0.6 mg/kg to 9 mg/kg for B1 and 0.2 mg/kg to 1.8 mg/kg for B6. For vitamin B3 and B5, the mass fractions cover a range of about a factor of 10 and 20, respectively. Vitamin B2 mass fractions cover the greatest range of nearly three orders of magnitude (0.18 mg/kg in peanut butter to 106 mg/kg in pig liver). The lowest mass fractions are assigned for vitamin B12 ranging from 0.0055 mg/kg in the meat homogenate to 1.12 mg/kg in the pig liver.
There are only a limited number of food-matrix CRMs with values assigned for the FSVs and/or carotenoids. For FSVs the three infant formulas (CRM 108-02-003, SRM 1849a, and SRM 1869) have 5, 7, and 16 values, respectively (Table 9 and Table S5, ESM); the milk powder (ERM-BD600) and margarine (BCR-122) CRMs have values assigned for four and two FSVs, respectively (Table S2, ESM). The remaining NIST materials have limited values assigned for FSVs (Tables S5 and S6, ESM). SRM 3233 fortified breakfast cereal has a certified value for α-tocopherol; SRM 1845a whole egg powder has reference/indicative values for retinol, α-tocopherol, and γ-tocopherol; and SRM 3252 protein drink mix and SRM 3290 dry cat food both have reference/indicative values for vitamin A and α-tocopherol (Table S5, ESM). The most characterized CRMs for carotenoids are BCR-485 mixed vegetables with nine values assigned (Table S2, ESM), and SRM 2383a baby food composite with six values assigned (Table S6, ESM). Two of the NIST first-generation CRMs that are still available have values for several FSVs or carotenoids (Table S6, ESM). The peanut butter (SRM 2387) has values assigned for three tocopherols and SRM 2385 slurried spinach has the only values for carotenoids (trans-β-carotene and lutein) (Table S5, ESM). Three of the dietary supplement CRMs listed in Table 8 have values assigned for tocopherols. More food-matrix CRMs with values assigned for FSVs (other than tocopherols) are needed.
The mass fractions of individual fatty acids and cholesterol in selected NIST and BCR CRMs are summarized in Table 7 and Table S4, ESM. These CRMs have cholesterol content ranging over more than two orders of magnitude from 0.1374 mg/g for infant/adult nutritional formula to 17.67 mg/g for the whole egg powder. The three BCR butter fat CRMs (Table 2) have cholesterol levels at 300 mg/g (data not shown). The NIST materials have fat content ranging from 19% for the meat homogenate to 43% for the egg powder. Of the four BCR materials, only BCR-163 beef-pork fat blend has C14 to C18 fatty acids for comparison with the NIST CRM levels of C8 to C24 fatty acids, and BCR-163 has a significantly higher fatty acid content than the NIST CRMs (3 to 10 times higher for C16 fatty acids and 6 to 20 times higher for C18 fatty acids). With the dietary supplement CRMs shown in Table 8 (all but one material are oils) and the two fish tissues (Table S8, ESM), a good variety of CRMs with certified values assigned for fatty acid content are available, and they exhibit a wide range of mass fraction levels and fatty acid profiles as shown in Fig. 5.
Customer demand and use of food- and supplement-matrix CRMs
The evolution of and the increasing number of food-matrix CRMs over the past 30 years (see Fig. 2 and Fig. S2, ESM) have been paralleled by increasing use of these materials within the analytical food testing community. The distribution of the infant formula CRMs from NIST over the past 20 years, as shown in Fig. 6, demonstrates the increasing usage as the materials have evolved. When issued in 1996, the original infant formula (SRM 1846) started with a distribution rate of about 50 units per year. The distribution of SRM 1846 steadily increased over its 12-year lifetime to about 250 units per year. The distribution of the replacement material, SRM 1849, initially doubled, and the inventory was depleted in less than 3 years. SRM 1849a, issued in 2011, continued the upward trend with sales now above 700 per year. The increasing usage of the three NIST infant formula CRMs can be attributed to several factors including (1) increasing regulations, (2) increasing number of constituents with values assigned in each succeeding CRM reissue (see Fig. 6), and (3) the involvement of the manufacturers as part of the AOAC stakeholder process to develop analytical methods for nutrients in infant formula [91] and the recognition of the need to include CRMs in this process [92]. SRM 1546 meat homogenate had a similar distribution pattern with sales of less than 50 units/year when issued in 1999 increasing to a plateau of approximately 180 units/year after a decade. The replacement material, SRM 1546a, issued in 2014, has continued the increasing trend. The infant/adult nutritional formula and the meat homogenate are the two most widely distributed NIST food-matrix CRMs with organic nutrient values assigned. Other popularNIST food-matrix CRMs with sales of approximately 40 units to 80 units per year include the peanut butter, milk powder, and breakfast cereal. The remaining NIST food-matrix CRMs listed in Table 3 have average sales of 10 units to 30 units per year. For the dietary supplement-matrix CRMs, the multivitamin/multielement tablets is the most popular with distribution of about 65 units/year (multivitamin/mineral supplements are the most widely used in the U.S. with sales of $5.6 billion in 2016) [93]. The fish oil and botanical oil CRMs, shown in Table 8, are distributed currently at rates of about 25 units/year, and the remaining dietary supplement materials average sales of about 10 units per year.
Fig. 6.
Distribution/sales of three different NIST infant formula CRMs over two decades
The JRC-Geel food-matrix CRMs (Table 2) have similar distribution rates when compared to comparable NIST CRMs [94]. For example, the JRC-Geel milk powder (ERM-BD600), mixed vegetables (BCR-485), and pig liver (BCR-487), which are the three materials with the largest number of values assigned for FSVs and WSVs, have sales rates of 35 units to 50 units each per year. For the JRC-Geel CRMs with values assigned for cholesterol and fatty acids, the soyamaize oil blend is the most popular with sales of over 50 units per year, and the beef-pork fat blend (BCR-163) and anhydrous butter fat have average sales of about 40 units per year. The remaining JRC-Geel food-matrix materials in Table 2 are distributed at a rate of between 15 units to 20 units per year. The food-matrix CRMs from NIST and JRC-Geel are distributed worldwide. However, the CRMs from NIMC and KRISS are distributed primarily within China and Korea, respectively.
In the context of numbers of units of CRMs distributed, it should be stressed that every single unit of a CRM has a multiplicative effect when used appropriately in a measurement laboratory. One unit of a CRM used for method validation/trueness assessment or used for control charts normally translate into hundreds or even thousands of accurate measurements in routine samples, year in and year out.
As uncertainties associated with values assigned for content of vitamins in food-matrix CRMs become smaller, a frequently asked question regarding the CRMs is how to compare a laboratory measurement result on a CRM with the certified value provided on the CRM Certificate of Analysis. JRC-Geel published a brief Application Note [95] to address this question focusing on the calculation and comparison of uncertainties for both the customer’s measurements and the certified value. Sharpless et al. [96] prepared a practical guide on the use of CRMs in the analysis of food and dietary supplements. This guide covers the information in the JRC-Geel Application Note and provides practical guidance on selection of an appropriate CRM and use to establish metrological traceability and trueness of results, to assess precision, to characterize in-house control materials, and to assign values to secondary reference materials. All of the examples in this guide are based on food and dietary supplement CRMs.
Future CRM needs for organic nutrients
As shown in the AOAC Food Triangle in Fig. 1, the majority of the food-matrix CRMs are in sectors 5, 6, and 7; however, as shown by Wolf and Andrews [27] the majority of commonly consumed foods also appear in these sectors. Additional CRMs in the less-populated sectors should be a priority for future CRM development. Food-matrix CRMs with high fat content but not oils (sectors 2, 3, and 4) are still limited; the recent renewals of whole egg powder (SRM 1845a) and meat homogenate (SRM 1546a) in sector 4 helped to reduce this deficiency. An avocado material (SRM 2386) is in progress as a high-fat, powdered matrix located in sector 2. Two soy-related SRMs (SRM 3236 soy protein isolate and SRM 3237 soy protein concentrate) [46], which were developed for isoflavone measurements, reside in sector 9 (not shown), and they could be useful protein-rich matrices, if additional characterization for nutrients is performed. Existing food-matrix CRMs for trace elements only (e.g., wheat and rice flour, bovine and porcine liver, spinach, etc.) could be characterized for organic nutrients by the CRM producer, or by users and reported in the literature, to provide added value for these materials. However, stability of the vitamins could be an issue depending on the current storage conditions for these CRMs. Two human milk materials, SRM 1953 and SRM 1954, have been issued with certified values for mass fractions of selected organic contaminants and selected inorganic nutrients [97]. Both CRMs were prepared from the same pool of human milk with one portion spiked with a mixture of over 170 contaminants. Either of these human milk CRMs, which would reside in sector 6, could also be analyzed to determine organic nutrients. A reference value for iodine was added recently to SRM 1953 to address this important inorganic nutrient in human milk [98] and to complement the other nutritional elements listed. Yerba mate (SRM 3253), shown in sector 5 of Fig. 1, has values assigned for polycyclic aromatic hydrocarbons but has been characterized for proximate content.
The current food-matrix CRMs in Tables 2, 3, and 10 are primarily characterized for WSVs. With the recent additions of the whole egg, protein drink mix, and dry cat food, more materials are now available that have been characterized for a limited number of FSVs. Only the BCR-485 mixed-vegetables and NIST SRM 2383a baby food composite and SRM 2385 slurried spinach have been characterized for carotenoids, and since BCR-485 was characterized over 20 years ago, an update of the Certificate of Analysis with contemporary measurements would be useful to the community. A great need exists for additional CRMs with values assigned for a wider variety of FSVs. Even with the recent addition of vitamin D values to several CRMs (see Table 10), the increasing interest in vitamin D will necessitate the development of additional food-matrix CRMs with values assigned for vitamin D and its metabolites. Vitamin K is another FSV with limited availability in existing CRMs. Similarly, there is a need for more food-matrix CRMs characterized for folate vitamers.
Conclusions
Even though CRMs with a limited number of values assigned for organic nutrients have existed for 30 years, only in the past decade have food and dietary supplement CRMs with extensive characterization of the organic nutrient content, particularly vitamins, become available. There has been a significant evolution of food-matrix CRMs during the past three decades. Food-matrix CRMs were originally intended only as a biological matrix for elemental analysis, all trace elements and not just nutritional elements, and the matrices were not specifically intended as foods. The first food-matrix CRMs for vitamins were limited in the quantity and quality of values assigned for vitamins because of the lack of suitable multiple analytical methods. Values assigned for vitamins in first-generation CRMs were often based on microbiological assays with ill-defined measurands. As LC-MS and LC-MS/MS methods have become available, particularly in conjunction with ID approaches for quantification, food-matrix CRMs have appeared with significant numbers of values assigned for WSVs and FSVs. Many of the recent food-matrix CRMs have values assigned for 35 to 75 organic nutrients with additional values for nutritional elements. Multiple, improved analytical methods for the determination of vitamins, carotenoids, and fatty acids have also produced lower relative uncertainties associated with the assigned certified values (typically 4 to 10%). The form of the food-matrix CRMs has also evolved from originally only dry powders stored at room temperature to numerous fresh, frozen, cooked, or processed food matrices stored refrigerated (4 °C) or frozen at temperatures as low as −80 °C. Early food-matrix CRMs were either certified for elements or organic nutrients but rarely for both. Many of the recent food-matrix CRMs have values assigned for both organic and element nutrients; however, this often restricts the form of the matrix and/or the storage conditions required to maintain stability for the organic nutrients.
What will the next decade in the development of food and dietary supplement CRMs bring? With the emergence of ID LC-MS and ID LC-MS/MS methods for the determination of vitamins and other organic nutrients, the capabilities exist to provide food-matrix CRMs, particularly non-fortified materials, with more accurate assigned values and with lower expanded uncertainties, potentially < 3% depending on material homogeneity. From a metrological standpoint, the values assigned for all vitamins in food- and dietary supplement-matrix CRMs should ultimately be determined using MS-based methods with ID quantification, if feasible, rather than microbiological assays. However, confirmation by another analytical method and often multiple laboratories as in an intercomparison exercise provides additional assurance that the true value is reported. Based on the recent accomplishments and emphasis of KRISS to produce more food-matrix materials, we would expect to see a broader scope of food matrices available and from more CRM producers during the next decade.
Supplementary Material
Acknowledgements
The authors acknowledge Zhang Qinghe of NIMC and Byungjoo Kim of KRISS for providing valuable information regarding their CRMs and Thomas Linsinger, Hakan Emteborg, and Marta Dabrio (JRC) for useful input regarding the JRC food-matrix CRMs. Katherine E. Sharpless (NIST) and Lane C. Sander (NIST) are acknowledged for their contributions in the coordination of the development of many of the NIST food- and dietary supplement-matrix SRMs. Joseph M. Betz, Director of the Analytical Methods and Reference Materials (AMRM) Program at the Office of Dietary Supplements, National Institutes of Health, is acknowledged for his guidance and leadership in the development of dietary supplement SRMs. The contributions of the numerous analysts at NIST, JRC, NIMC, and KRISS who have provided measurements used in the certification of organic nutrients in the CRMs discussed in this review are also acknowledged.
Footnotes
Electronic supplementary material The online version of this article (https://doi.org/10.1007/s00216-018-1473-0) contains supplementary material, which is available to authorized users.
Conflict of interest The authors declare that they have no conflict of interest.
References
- 1.Bowen HJM. Comparative elemental analyses of a standard plant material. Analyst. 1967;92(1091):124 10.1039/an9679200124. [DOI] [Google Scholar]
- 2.Bowen HJM (1985) Kale as a reference material In: Biological reference materials—availability, uses, and need for validation of nutrient measurement. John Wiley & Sons, New York [Google Scholar]
- 3.Katz SA. Bowen’s kale: a brief review dedicated to the late Professor Humphry John Moule Bowen 1929–2001. J Radioanal Nucl Chem. 2002;251(1):3–5. 10.1023/a:1015021823497. [DOI] [Google Scholar]
- 4.Epstein MS. The independent method concept for certifying chemical-composition reference materials. Spectroc Acta Pt B-Atom Spectr. 1991;46(12):1583–91. 10.1016/0584-8547(91)80162-v. [DOI] [Google Scholar]
- 5.May W, Parris R, Beck C II, Fassett J, Greenberg R, Guenther F, et al. Definition of terms and modes used at NIST for value-assignment of reference materials for chemical measurements NIST Special Publication, vol 260–136 Gaithersburg: National Institute of Standards and Technology (NIST); 2000. [Google Scholar]
- 6.Belliardo JJ, Wagstaffe PJ. BCR reference materials for food and agricultural analysis—an overview. Fresenius Z Anal Chem. 1988;332(6):533–8. 10.1007/bf00472637. [DOI] [Google Scholar]
- 7.Hollman PCH, Boenke A, Wagstaffe PJ (1993) The certification of major components and major elements in five food reference mterials. Fresenius J Anal Chem 345 (2–4):174–179. doi: 10.1007/bf00322583 [DOI] [Google Scholar]
- 8.Wagstaffe PJ, Belliardo JJ. The BCR program and measurements for food and agriculture. Fresenius J Anal Chem. 1990;338(4):469–72. 10.1007/bf00322519. [DOI] [Google Scholar]
- 9.Parr RM, Fajgelj A, Dekner R, Ruiz HV, Carvalho FP, Povinec PP (1998) IAEA analytical quality assurance programmes to meet the present and future needs of developing countries. Fresenius J Anal Chem 360 (3–4):287–290. doi: 10.1007/s002160050694 [DOI] [Google Scholar]
- 10.Berman SS, Sturgeon RE. A new approach to the preparation of biological reference materials for trace-metals. Fresenius Z Anal Chem. 1988;332(6):546–8. 10.1007/bf00472639. [DOI] [Google Scholar]
- 11.Ihnat M, Dabeka RW, Wolynetz MS (1993) Summary of preparation and homogeneity characterization of 10 agricultural food reference materials for elemental composition. Fresenius J Anal Chem 345 (2–4):221–223. doi: 10.1007/bf00322594 [DOI] [Google Scholar]
- 12.Ihnat M, Dabeka RW, Wolynetz MS. Preparation and homogeneity characterization of 10 agricultural food reference materials for elemental composition. Fresenius J Anal Chem. 1994;348(7):445–51. 10.1007/bf00325310. [DOI] [Google Scholar]
- 13.Ihnat M (2001) Twenty five years of reference material activity at Agriculture and Agri-Food Canada. Fresenius J Anal Chem 370 (2–3):279–285. doi: 10.1007/s002160100803 [DOI] [PubMed] [Google Scholar]
- 14.National Research Council Canada, Certified Reference Materials, www.nrc-cnrc.gc.ca/eng/solutions/advisory/crm_index.html, accessed 4/12/2018.
- 15.Wolf WR, Iyengar GV, Tanner JT. Mixed diet reference materials for nutrient analysis of foods—preparation of SRM 1548 total diet. Fresenius J Anal Chem. 1990;338(4):473–5. 10.1007/bf00322520. [DOI] [Google Scholar]
- 16.U.S. Food And Drug Administration Total Diet Study. (2014) U.S. Department of Health and Human Resources, www.fda.gov/food/FoodScienceResearch/TotalDietStudy/default.htm, accessed 07-03-2018.
- 17.U.S. Infant Formula Act (1980). Public Law 96–359 [H.R. 6940]; September 26, 1980, www.gpo.gov//fdsys/pkg/Statute-94/pdf/statute-94-Pg1190.pdf, accessed 07-03-2018.
- 18.U.S. Food and Drug Administration (1990) Nutrition Labeling and Education Act. Public Law 101–535 [H.R. 3562], November 8 1990, www.gpo.gov/fdsys/pkg/statute-104/statute-104-Pg2353.pdf, accessed 07–03-2018.
- 19.U.S. Department Health and Human Services, Food and Drug Administration, Food Safety Moderization Act (FSMA) (2011), www.fda.gov/food/guidanceRegulation/FSMA, accessed 2/17/2018.
- 20.U.S. Dietary Supplement Health and Education Act (DSHEA) of 1994 Public Law 103–417, 103rd Congress, accessed 2/17/2018.
- 21.U.S. Department Health and Human Services, Food and Drug Administration, Current Good Manufacturing Practices (CGMPs) for Dietary Supplements (2007), www.fda.gov/food/guidanceRegulation/CGMP, accessed 2/17/2018.
- 22.European Commission Directive, Food (2014), www.ec.europa.eu/food/safety/labeling_nutrition/labelling_legislation_en, accessed 2/18/2018.
- 23.National Food Safety Standard - Infant Formula (2009). The Ministry of Health of the People’s Republic of China, https://gain.fas.usda.gov/Recent%20GAIN%20Publications/National%20Dairy%20Standard%20-%20Infant%20Formulas_Beijing_China%20-%20Peoples%20Republic%20of_12-1-2009.pdf, accessed on 2/18/2018. [Google Scholar]
- 24.International Standards Organization (ISO) (2015) Reference materials—selected terms and definitions ISO Guide 30:2015, 3rd Ed. [Google Scholar]
- 25.Ikins W, DeVries J, Wolf WR, Oles P, Carpenter D, Fraley N, et al. A food matrix organizational system applied to collaborative studies. The Referee AOAC International. 1993;17:1–7. [Google Scholar]
- 26.Wolf WR. Reference materials In: Methods of analysis for nutrition labeling. Arlington: AOAC International; 1993. [Google Scholar]
- 27.Wolf WR, Andrews KW (1995) A system for defining reference materials applicable to all food matrices. Fresenius J Anal Chem 352 (1–2):73–76. doi: 10.1007/bf00322300 [DOI] [Google Scholar]
- 28.Phillips MM, Sharpless KE, Wise SA. Standard reference materials for food analysis. Anal Bioanal Chem. 2013;405(13):4325–35. 10.1007/s00216-013-6890-5. [DOI] [PubMed] [Google Scholar]
- 29.Sharpless KE, Colbert JC, Greenberg RR, Schantz MM, Welch MJ (2001) Recent developments in food-matrix reference materials at NIST. Fresenius J Anal Chem 370 (2–3):275–278. doi: 10.1007/s002160100826 [DOI] [PubMed] [Google Scholar]
- 30.Sharpless KE, Greenberg RR, Schantz MM, Welch MJ, Wise SA, Ihnat M. Filling the AOAC triangle with food-matrix standard reference materials. Anal Bioanal Chem. 2004;378(5):1161–7. 10.1007/s00216-003-2384-1. [DOI] [PubMed] [Google Scholar]
- 31.Sharpless KE, Thomas JB, Christopher SJ, Greenberg RR, Sander LC, Schantz MM, et al. Standard reference materials for foods and dietary supplements. Anal Bioanal Chem. 2007;389(1):171–8. 10.1007/s00216-007-1315-y. [DOI] [PubMed] [Google Scholar]
- 32.Sharpless KE, Welch MJ, Greenberg RR, Iyengar GV, Colbert JC (1998) Recent SRMs for organic and inorganic nutrients in food matrices. Fresenius J Anal Chem 360 (3–4):456–458. doi: 10.1007/s002160050738 [DOI] [Google Scholar]
- 33.Wise SA, Sharpless KE, Sander LC, May WE (2004) Standard reference materials to support US regulations for nutrients and contaminants in food and dietary supplements. Accredit Qual Assur 9 (9):543–550. doi: 10.1007/s00769-004-0820-3 [DOI] [Google Scholar]
- 34.Brown-Thomas JM, Moustafa AA, Wise, May WE. Determination of fat-soluble vitamins in oil matrices by multidimensional high-performance liquid chromatography. Anal Chem. 1988;60(18):1929–33. 10.1021/ac00169a018. [DOI] [PubMed] [Google Scholar]
- 35.Sharpless KE, Schiller SB, Margolis SA, Thomas JB, Iyengar GV, Colbert JC, Gills TE, Wise SA (1997) Certification of nutrients in standard reference material 1846: infant formula. J AOAC Int 80 (3):611–621. [PubMed] [Google Scholar]
- 36.Sharpless KE, Arce-Osuna M, Thomas JB, Gill LM (1999) Value assignment of retinol, retinyl palmitate, tocopherol, and carotenoid concentrations in standard reference material 2383 (baby food composite). J AOAC Int 82 (2):288–296. [PubMed] [Google Scholar]
- 37.Sharpless KE, Gill LM, Margolis SA, Wise SA, Elkins E (1999) Preparation of standard reference material 2383 (baby food composite) and use of an interlaboratory comparison exercise for value assignment of its nutrient concentrations. J AOAC Int 82 (2):276–287. [PubMed] [Google Scholar]
- 38.Sharpless KE, Thomas JB, Nelson BC, Phinney CS, Sieber JR, Wood LJ, Yen JH, Howell DW (2002) Value assignment of nutrient concentrations in standard reference material 2384 baking chocolate. J Agric Food Chem 50 (24):7069–7075. doi: 10.1021/fj020610d [DOI] [PubMed] [Google Scholar]
- 39.Poster DL, Kucklick JR, Schantz MM, Porter BJ, Leigh SD, Wise SA. Determination of polychlorinated biphenyl congeners and chlorinated pesticides in a fish tissue standard reference material. Anal Bioanal Chem. 2003;375(2):223–41. 10.1007/s00216-002-1680-5. [DOI] [PubMed] [Google Scholar]
- 40.Vandendriessche S, Pauwels J. Certification of reference materials by European Commission services. Fresenius J Anal Chem. 1995;352(1–2):7–10. 10.1007/bf00322285. [DOI] [Google Scholar]
- 41.Finglas PM, Scott KJ, Witthoft H, van den Berg H, de Froidmont-Goertz I. The certification of the mass fractions of vitamins in four reference materials: wholemeal flour (CRM 121), milk powder (CRM 421), lyophilized mixed vegetabbles (CRM 485) and lyophilized pigs liver (CRM 487). Luxembourg: Office for Official Publications of the European Communities; 1999. [Google Scholar]
- 42.Finglas PM, van den Berg H, de Froidmont-Goertz I. The certification of the mass fractions of vitamins in three reference materials: margarine (CRM 121), milk powder (CRM 421), and lyophilized brussels sprout powder (CRM 431). Luxembourg: Office for Official Publications of the European Communities; 1997. [Google Scholar]
- 43.Zeleny R, Bernreuther T, Linsinger T, Schimmel H, Sejeroe-Olsen B, Kramer GN, et al. The method-specific certification of thee cholesterol and triglyceride contents ofa pure and an adulterated butter fat reference material BCR-632-A and BCR-632B. Geel: European Commission Joint Research Centre; 2005. [Google Scholar]
- 44.Sander LC, Sharpless KE, Wise SA, Nelson BC, Phinney KW, Porter BJ, et al. Certification of vitamins and carotenoids in SRM 3280 multivitamin/multielement tablets. Anal Chem. 2011;83(1): 99–108. 10.1021/ac101953u. [DOI] [PubMed] [Google Scholar]
- 45.Phillips MM, Case RJ, Rimmer CA, Sander LC, Sharpless KE, Wise SA, et al. Determination of organic acids in Vaccinium berry standard reference materials. Anal Bioanal Chem. 2010;398(1): 425–34. 10.1007/s00216-010-3916-0. [DOI] [PubMed] [Google Scholar]
- 46.Phillips MM, Bedner M, Reitz M, Burdette CQ, Nelson MA, Yen JH, et al. Liquid chromatography with absorbance detection and with isotope-dilution mass spectrometry for determination of isoflavones in soy standard reference materials. Anal Bioanal Chem. 2017;409(4):949–60. 10.1007/s00216-016-9997-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rimmer CA, Sharpless KE, Wise SA, Betz JM, Coates PM. Standard reference materials for dietary supplement analysis. Anal Bioanal Chem. 2013;405(13):4337–44. 10.1007/s00216-013-6942-x. [DOI] [PubMed] [Google Scholar]
- 48.Sander LC, Sharpless KE, Satterfield MB, Ihara T, Phinney KW, Yen JH, et al. Determination of ephedrine alkaloids in dietary supplement standard reference materials. Anal Chem. 2005;77(10): 3101–12. 10.1021/ac0484530. [DOI] [PubMed] [Google Scholar]
- 49.Sharpless KE, Anderson DL, Betz JM, Butler TA, Capar SG, Cheng J, et al. Preparation and characterization of a suite of ephedra-containing standard reference materials. J AOAC Int. 2006;89(6):1483–95. [PubMed] [Google Scholar]
- 50.International Standards Organization (ISO) (2016) General requirements for the competence of reference material producers ISO Guide 17034, 1st Ed. [Google Scholar]
- 51.Ihnat M A synopsis of different approaches to the certification of reference materials. Fresenius J Anal Chem. 1998;360(3–4):308–11. 10.1007/s002160050699. [DOI] [Google Scholar]
- 52.Wise SA, Poster DL, Kucklick JR, Keller JM, VanderPol SS, Sander LC, et al. Standard reference materials (SRMs) for determination of organic contaminants in environmental samples. Anal Bioanal Chem. 2006;386(4):1153–90. 10.1007/s00216-006-0719-4. [DOI] [PubMed] [Google Scholar]
- 53.Sharpless KE, Gill LM. Value assignment of nutrient concentrations in five standard reference materials and six reference materials. J AOAC Int. 2000;83(2):413–23. [PubMed] [Google Scholar]
- 54.Sharpless KE, Margolis S, Thomas JB. Determination of vitamins in food-matrix standard reference materials. J Chromatogr A. 2000;881(1–2):171–81. 10.1016/s0021-9673(00)00260-0. [DOI] [PubMed] [Google Scholar]
- 55.Sharpless KE, Phinney CS, Wood LJ, Yen JH, Howell DW. Value assignment of nutrient and aflatoxin concentrations in standard reference material 2387 peanut butter. J Agric Food Chem. 2003;51(23):6745–51. 10.1021/jf034637o. [DOI] [PubMed] [Google Scholar]
- 56.Cohen A, Hertz HS, Mandel J, Paule RC, Schaffer R, Sniegoski LT, Sun T, Welch MJ, White E (1980) Total serum-cholesterol by isotope dilution-mass spectrometry—a candidate definitive method. Clin Chem 26 (7):854–860. [PubMed] [Google Scholar]
- 57.Phinney KW, Rimmer CA, Thomas JB, Sander LC, Sharpless KE, Wise SA. Isotope dilution liquid chromatography-mass spectrometry methods for fat- and water-soluble vitamins in nutritional formulations. Anal Chem. 2011;83(1):92–8. 10.1021/ac101950r. [DOI] [PubMed] [Google Scholar]
- 58.Turk GC, Sharpless KE, Cleveland D, Jongsma C, Mackey EA, Marlow AF, et al. Certification of elements in and use of standard reference material 3280 multivitamin/multielement tablets. J AOAC Int. 2013;96(6):1281–7. 10.5740/jaoacint.13-135. [DOI] [PubMed] [Google Scholar]
- 59.Nelson BC, Sharpless KE, Sander LC. Quantitative determination of folic acid in multivitamin/multielement tablets using liquid chromatography/tandem mass spectrometry. J Chromatogr A. 2006;1135(2):203–11. 10.1016/j.chroma.2006.09.040. [DOI] [PubMed] [Google Scholar]
- 60.Nelson BC, Sharpless KE, Sander LC. Improved liquid chromatography methods for the separation and quantification of biotin in NIST standard reference material 3280: multivitamin/multielement tablets. J Agric Food Chem. 2006;54(23):8710–6. 10.1021/jf062000+. [DOI] [PubMed] [Google Scholar]
- 61.Sharpless KE, Lindstrom RM, Nelson BC, Phinney KW, Rimmer CA, Sander LC, Schantz MM, Spatz RO, Thomas JB, Turk GC, Wise SA, Wood LJ, Yen JH (2010) Preparation and characterization of standard reference material 1849 infant/adult nutritional formula. J AOAC Int 93 (4):1262–1274. [PubMed] [Google Scholar]
- 62.Thomas JB, Yen JH, Sharpless KE. Characterization of NIST food-matrix standard reference materials for their vitamin C content. Anal Bioanal Chem. 2013;405(13):4539–48. 10.1007/s00216-013-6891-4. [DOI] [PubMed] [Google Scholar]
- 63.Certificate of Analysis, Standard Reference Materials (SRMs), National Institute of Standards and Technology (NIST) www.nist.gov/srm [Google Scholar]
- 64.Phillips MM. Liquid chromatography with isotope-dilution mass spectrometry for determination ofwater-soluble vitamins in foods. Anal Bioanal Chem. 2015;407(11):2965–74. 10.1007/s00216-014-8354-y. [DOI] [PubMed] [Google Scholar]
- 65.Camara JE, Lowenthal MS, Phinney KW. Determination of fortified and endogenous folates in food-based standard reference materials by liquid chromatography-tandem mass spectrometry. Anal Bioanal Chem. 2013;405(13):4561–8. 10.1007/s00216-013-6733-4. [DOI] [PubMed] [Google Scholar]
- 66.Raju CSK, Yu LL, Schiel JE, Long SE. A simple and sensitive LC-ICP-MS method for the accurate determination of vitamin B-12 in fortified breakfast cereals and multivitamin tablets. J Anal At Spectrom. 2013;28(6):901–7. 10.1039/c3ja30383g. [DOI] [Google Scholar]
- 67.Plockington WD, Pearse J, Lognay G, Wagstaffe PJ, Boenke A, Schurer B. The certification of the fatty acid profile and the cholesterol mass fraction of two edible oil and fat materials - soya-maise oil blend CRM 162, beef-pork fat blend CRM 163. Luxembourg; 1993. [Google Scholar]
- 68.Schantz MM, Bedner M, Long SE, Molloy JL, Murphy KE, Porter BJ, et al. Development of saw palmetto (Serenoa repens) fruit and extract standard reference materials. Anal Bioanal Chem. 2008;392(3):427–38. 10.1007/s00216-008-2297-0. [DOI] [PubMed] [Google Scholar]
- 69.Schantz MM, Sander LC, Sharpless KE, Wise SA, Yen JH, NguyenPho A, et al. Development ofbotanical and fish oil standard reference materials for fatty acids. Anal Bioanal Chem. 2013;405(13):4531–8. 10.1007/s00216-013-6747-y. [DOI] [PubMed] [Google Scholar]
- 70.Dabrio Ramos M, Schimmel H, Emons H. Certification of the mass fractions of vitamins in whole milk powder. Geel: Joint Research Centre, European Commission; 2011. [Google Scholar]
- 71.Huang T, Zhang W, Liu J, Tian Y, Yang GX, Quan C. A new certified reference material (GBW10037) of vitamin B-3 in infant formula. J Food Compos Anal. 2010;23(4):367–72. 10.1016/j.jfca.2009.12.013. [DOI] [Google Scholar]
- 72.Shin H, Kim B, Lee J. Investigation of isotope dilution mass spectrometric (ID-MS) method to determine niacin in infant formula, breakfast cereals and multivitamins. Food Chem. 2013;138(2–3): 1109–15. 10.1016/j.foodchem.2012.11.046. [DOI] [PubMed] [Google Scholar]
- 73.Lee J, Jang ES, Kim B. Development of isotope dilution-liquid chromatography/mass spectrometry combined with standard addition techniques for the accurate determination of tocopherols in infant formula. Anal Chim Acta. 2013;787:132–9. 10.1016/j.aca.2013.05.034. [DOI] [PubMed] [Google Scholar]
- 74.Lim YO, Kim B, Ahn S, Kim J. Improvement of accuracy for the determination of vitamin A in infant formula by isotope dilution-liquid chromatography/tandem mass spectrometry. Food Chem. 2011;126(3):1393–8. 10.1016/j.foodchem.2010.11.118. [DOI] [Google Scholar]
- 75.Lee J, Song YS, Sim HJ, Kim B. Isotope dilution-liquid chromatography/mass spectrometric method for the determination of riboflavin content in multivitamin tablets and infant formula. J Food Compos Anal. 2016;50:49–54. 10.1016/j.jfca.2016.05.008. [DOI] [Google Scholar]
- 76.Lee H, Lee J, Choi K, Kim B. Development of isotope dilution-liquid chromatography/tandem mass spectrometry for the accurate determination of trans- and cis-vitamin K-1 isomers in infant formula. Food Chem. 2017;221:729–36. 10.1016/j.foodchem.2016.11.112. [DOI] [PubMed] [Google Scholar]
- 77.Koontz JL, Phillips KM, Wunderiach KM, Exler J, Holden JM, Gebhardt SE, et al. Comparison of total folate concentrations in foods determined by microbiological assay at several experienced US commercial laboratories. J AOAC Int. 2005;88(3):805–13. [PubMed] [Google Scholar]
- 78.U.S. Food and Drug Administration Proposed Rule 79 FR 11879 3-3-2014 (2014). www.federalregister.gov/documents/2014/0303/2014-04387/food-labeling-revision-of-the-nutrition-and-supplement-fact-labels, accessed 07-03-2018.
- 79.Taylor CL, Patterson KY, Roseland JM, Wise SA, Merkel JM, Pehrsson PR, et al. Including food 25-hydroxyvitamin D in intake estimates may reduce the discrepancy between dietary and serum measures of vitamin D status. J Nutr. 2014;144(5):654–9. 10.3945/jn.113.189811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Roseland JM, Patterson KY, Andrews KW, Phillips KM, Phillips MM, Pehrsson PR, et al. Interlaboratory trial for measurement of vitamin D and 25-hydroxyvitamin D 25(OH)D in foods and a dietary supplement using liquid chromatography-mass spectrometry. J Agric FoodChem. 2016;64(16):3167–75. 10.1021/acs.jafc.5b05016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Burdette CQ National Institute of Standards and Technology, Gaithersburg, MD, USA, personal communication. [Google Scholar]
- 82.Burdette CQ. Determination of cholecalciferol (vitamin D3) in standard reference material 3532 calcium-containing solid oral dosage form. J AOAC Intl. 2017;100(5):1304–7. 10.5740/jaoacint.17-0083. [DOI] [PubMed] [Google Scholar]
- 83.Yu LL, Browning JF, Burdette CQ, Caceres KD, Davis WC, Ellisor MB, et al. Development of a kelp powder (Thallus laminariae) standard reference material. Anal Bioanal Chem. 2018;410(4): 1265–78. 10.1007/s0021-6-017-0766-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Jing W, Thompson JJ, Jacobs WA, Salvati LM. Determination of free and total carnitine and choline in infant formulas and adult nutritional products by UPLC/MS/MS: single-laboratory validation, First Action 2014.04. J AOAC Intl. 2015;98(5):1395–406. 10.5740/jaoacint.12-137. [DOI] [PubMed] [Google Scholar]
- 85.Ellingson DJ, Shippar JJ, Gilmore JM. Determination of free and total choline and carntine in infant formula and adult/pediatric nutritional formula by liquid chromatography/tandem mass spectrometry (LC/MS/MS): Single-laboratory validation, First Action 2015.10. J. AOAC Intl. 2016;99(1):204–9. 10.5740/jaoacint.15102. [DOI] [PubMed] [Google Scholar]
- 86.Phillips MM, Sander LC. Microwave-assisted extraction and quantitative LC/ID-MS measurement of total choline and free carnitine in food standard reference materials. J AOAC Int. 2012;95(5): 1479–86. 10.5740/jaoacint.12-137. [DOI] [PubMed] [Google Scholar]
- 87.European Commission, Joint Research Centre (JRC), Certified Reference Material Catalogue, https://crm.jrc.ec.europa.eu, assessed 10-25-2018. [Google Scholar]
- 88.Certificate of Analysis, Standard Reference Material 1845a Whole Egg Powder, National Institute of Standards and Technology (NIST) (2018). [Google Scholar]
- 89.Certificate of Analysis, Standard Reference Material (SRM) 3234 Soy Flour, National Institute of Standards and Technology (NIST) (2017). [Google Scholar]
- 90.U.S. Department of Agriculture, Agricultural Research Service, USDA Food Composition Databases. https://ndb.nal.usda.gov, accessed 10-25-2018. [Google Scholar]
- 91.Sullivan D Modernization of AOAC Nutrient methods by stakeholder panel on infant formula and adult nutritionals. J AOAC Int. 2016;99(1):3–6. 10.5740/jaoacint.15-0248. [DOI] [PubMed] [Google Scholar]
- 92.Wargo WF. Reference materials: critical importance to the infant formula industry. J AOAC Int. 2017;100(5):1376–8. 10.5740/jaoacint.17-0231. [DOI] [PubMed] [Google Scholar]
- 93.Top 100 dietary supplements by U.S. sales 2012–2016 (2017). Nutrition Business Journal, www.nutritionbusinessjournal.com [Google Scholar]
- 94.Emteborg H, Linsinger T, European Commission Joint Research Centre, Geel, Belgium, personal communication. [Google Scholar]
- 95.Linsinger T Application Note 1 Comparison of a measurement result with the certified value. Geel: European Commission - Joint Research Centre, Institute for Reference Materials and Measurements (IRMM); 2014. [Google Scholar]
- 96.Sharpless KE, Lippa KA, Duewer DL, Rukhin AL. Standard reference materials in the analysis of foods and dietary supplements: a practical guide. Gaithersburg: NIST Special Publication 260–181, National Institute of Standards and Technology (NIST); 2014. [Google Scholar]
- 97.Schantz MM, Eppe G, Focant JF, Hamilton C, Heckert NA, Heltsley RM, et al. Milk and serum standard reference materials for monitoring organic contaminants in human samples. Anal Bioanal Chem. 2013;405(4):1203–11. 10.1007/s00216-012-6524-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Long SE, Catron BL, Boggs ASP, Tai SS-C, Wise SA. Development of standard reference materials to support assessment of iodine status for nutritional and public health purposes. Am J Clin Nutr. 2016;104(Supplement):902S–6S. 10.3945/ajcn.115.110361. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


