Abstract
We present a case of a 64-year-old man who presented with a rapidly growing tumor in the left buttock and intergluteal cleft area, which was affected by hidradenitis suppurativa. The patient was on tumor necrosis factor-alpha inhibitors for hidradenitis suppurativa for 2 years prior to the development of the mass. Initial biopsy of the mass showed a well-differentiated squamous cell carcinoma with spindle cells and positive epithelial immunomarkers. Subsequent excisional biopsy of the tumor showed an infiltrating poorly differentiated squamous cell carcinoma composed of islands of atypical sarcomatoid spindle cells. Squamous cell carcinoma arising in hidradenitis suppurativa is a rare complication which may occur secondary to chronic inflammation and epidermal hyperproliferation in hidradenitis suppurativa–affected areas.
Keywords: Squamous cell carcinoma, hidradenitis suppurativa, adalimumab
Introduction
Squamous cell carcinoma (SCC) is a rare, yet severe complication of hidradenitis suppurativa (HS), typically presenting in the gluteal and perineal areas. Epidermal hyperproliferation in long-standing inflammatory wounds, delayed diagnosis, and local spread of malignant lesions through extensive sinus tracts networks appear to be the main factors in both development of SCC in HS and the highly aggressive nature of these tumors.1,2 Other potential contributory factors have been reported in the literature, including anti-tumor necrosis factor-alpha (TNF-α) therapy which has been reported in three cases of malignant transformation in hidradenitis-affected lesions.3–5 We hereby present the case of a 64-year-old man who presented with a rapidly progressing neoplastic mass after 2 years of adalimumab therapy for HS.
Case presentation
A 64-year-old man was first diagnosed with HS 28 years ago, following a 3-year history of painful furuncles in the axillary and gluteal regions that were unsuccessfully managed with oral antibiotics and multiple incision and drainage procedures. Comorbidities included diabetes managed with twice daily metformin, dyslipidemia, and hypertension. Adalimumab (a human monoclonal antibody against TNF-α) in 40 mg per week subcutaneous injections effectively improved symptoms and reduced the size of the nodules.
After 2 years of therapy, the patient presented with a 14 cm × 10 cm ulcerated exophytic tumor in a HS-affected area involving the left buttock and intergluteal cleft. The biopsy specimen revealed a low-grade well-differentiated SCC, and surgical excision of the lesion was performed at the community hospital. As surgical margins were incomplete, the patient was referred to our tertiary care surgical oncology service for re-excision. The tumor proved to be highly aggressive as within 2 months a new violaceous necrotic mass had rapidly emerged within the surgical wound (Figure 1). This time, an extensive surgical resection extending to the deep fascia was performed (Figure 2).
Figure 1.
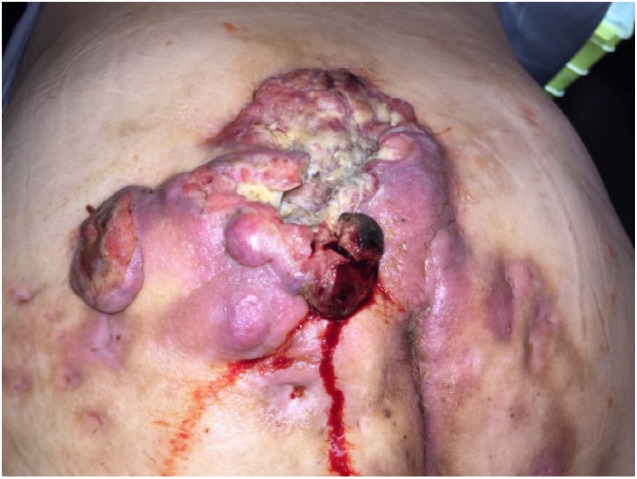
Violaceous exophytic mass arising within the surgical wound.
Figure 2.
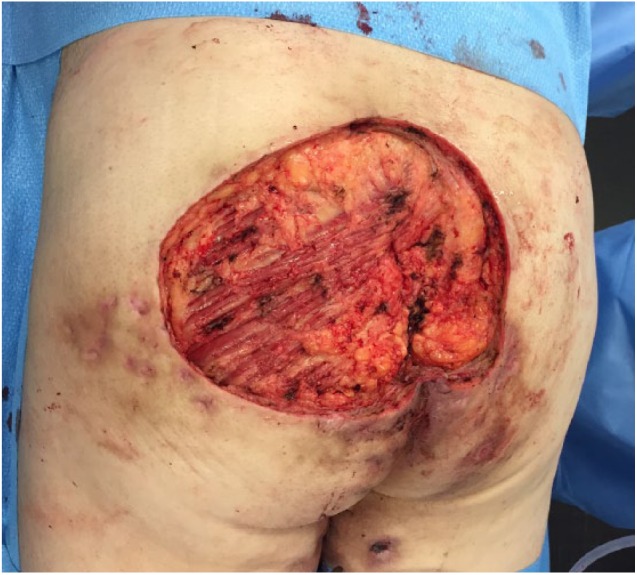
Surgical resection extending to the level of the deep fascia.
Post-operative wound care with vacuum assisted closure (VAC) therapy and pain management with oral analgesics allowed for a rapid recovery. Pathological examination of the surgical specimen revealed a markedly more aggressive and undifferentiated tumor than what was observed 2 months prior upon first resection at the community hospital. Indeed, microscopy now showed an infiltrating poorly differentiated SCC composed of islands of atypical sarcomatoid spindle cells (Figure 3), staining positive for CK5/6, CKAE1/AE3, p63, and vimentin. Numerous mitoses were noted. Melanoma was ruled out as S100, and Melan-A were negative. CD34, CD31, desmin, and p16 were also negative. There was no vascular or lymphatic invasion. Surgical margins were positive at the level of the intergluteal cleft, and a third resection was scheduled. Unfortunately, the patient died shortly after from pulmonary metastases.
Figure 3.
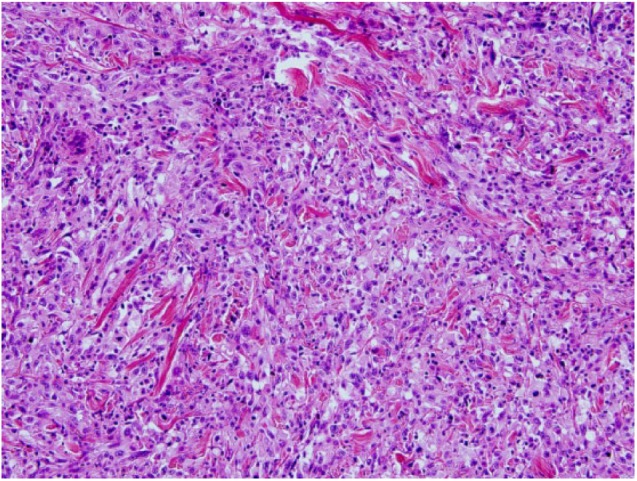
Sheets of poorly differentiated pleomorphic neoplastic cells invading the dermis, with numerous mitoses (hematoxylin and eosin, original magnification x 40).
Discussion
With an overall incidence of 1%–3.2%, SCC arising in long-standing HS is a rare entity, and its pathogenesis is not yet fully understood.6 In a recent study of 62 cases of malignant transformation in HS, Huang et al.1 reported an average latency period from diagnosis of HS to the development of SCC of approximately 27 years, as was seen in our patient, thus suggesting long-standing inflammation leading to epidermal hyperproliferation and increased rate of spontaneous mutations may play an important role in the development of SCC.
Although HS most commonly affects females, it is males with perianal hidradenitis lesions who appear to be at highest risk of developing this dreaded complication.7 Indeed, a review of 52 HS patients who developed SCC in HS-affected sites reveals a remarkable male predominance (86% of the patients).2 Importantly, these tumors tend to be both clinically and histologically aggressive, with frequent metastatic spread and high mortality rates.1 Indeed, in the aforementioned review of 52 cases of SCC lesions arising in HS, it was found that 57% of the patients died within 2 years.8 Delayed diagnosis due to the inherent challenge of identifying metastatic foci within an area of HS, which often presents with chronic abscesses and extensive scarring from previous procedures, may contribute to the poor prognosis.2 Furthermore, the presence of sinus tracts within hidradenitis-affected areas was suggested to facilitate local spread, allowing for development of extensive lesions camouflaged below the surface of chronically inflamed wounds.1 Although these appear to be the main determinants of the aggressive nature of SCCs arising within chronic inflammatory wounds, other contributory factors have been proposed, including anti-TNF-α therapy.3
In recent years, TNF-α inhibitors have been increasingly used in the treatment of refractory HS cases, as they are highly effective in reducing HS-associated morbidity.9 However, these agents have previously been linked to a theoretical increase in malignant transformation. Indeed, studies have shown that TNF-α might act as an endogenous tumor suppressor by exerting a cytotoxic effect on malignant cells through upregulation of natural killer cell and T-cell activity.10 Our literature search identified three published cases of SCC arising in long-standing HS shortly following initiation of anti-TNF-α therapy, all of which proved to be fatal.3–5 Although only rarely reported in HS cases, there is some evidence suggesting an increased risk of non-melanoma skin cancer after initiation of anti-TNF-α therapy for other chronic inflammatory conditions such as rheumatoid arthritis and inflammatory bowel disease.11 Lavogiez et al.8 suggested anti-TNF-α therapy may contribute to an increased risk of malignant transformation in HS by increasing the rate of recurrence of genital human papillomaviruses (HPV) lesions in patients following treatment initiation. Indeed, HPV, predominantly HPV-16 is a known risk factor for development of anogenital epithelial cancers.8 The authors performed polymerase chain reaction (PCR) on paraffin-embedded material from eight cases of SCC arising in HS, all of which were positive for HPV, in particular high-risk types such as HPV-16.8 Of note, immunohistochemical staining of the surgical resection specimen in our patient was unreactive for p16.
Although the precise pathogenesis of SCC in HS remains unclear, the aggressive nature of these tumors warrants the need for rapid surgical intervention.7 As for our patient, histological examination of the tumor arising within the wound 2 months after initial excision (with positive margins) showed rapid dedifferentiation of the SCC and infiltration of the surrounding soft tissue. This highlights the importance of prompt primary surgical excision for these patients, with wide surgical margins.2
To conclude, SCC is a rare complication which may occur secondary to chronic inflammation and epidermal hyperproliferation in hidradenitis-affected areas. It remains unclear if anti-TNF-α therapy such as adalimumab may have been a contributory factor for both for the development of SCC in HS and for the aggressiveness of this tumor in this patient. Further surveillance studies are required to ascertain the risks relative to this therapeutic modality in patients with HS.
Footnotes
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Informed consent: Informed consent from the next of kin was obtained for the publication of this study.
References
- 1. Huang C, Lai Z, He M, et al. Successful surgical treatment for squamous cell carcinoma arising from hidradenitis suppurativa: a case report and literature review. Medicine (Baltimore) 2017; 96(3): e5857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Yon JR, Son JD, Fredericks C, et al. Marjolin’s ulcer in chronic hidradenitis suppurativa: a rare complication of an often neglected disease. J Burn Care Res 2017; 38(2): 121–124. [DOI] [PubMed] [Google Scholar]
- 3. Scheinfeld N. A case of a patient with stage III familial hidradenitis suppurativa treated with 3 courses of infliximab and died of metastatic squamous cell carcinoma. Dermatol Online J 2014; 20(3): doj_21764. [PubMed] [Google Scholar]
- 4. Verdelli A, Antiga E, Bonciani D, et al. A fatal case of hidradenitis suppurativa associated with sepsis and squamous cell carcinoma. Int J Dermatol 2016; 55(1): e52–e53. [DOI] [PubMed] [Google Scholar]
- 5. Maalouf E, Faye O, Poli F, et al. Carcinome épidermoïde fatal compliquant une maladie de Verneuil après traitement par infliximab. Ann Dermatol Vénéréol 2006; 133(5): 473–474. [DOI] [PubMed] [Google Scholar]
- 6. Constantinou C, Widom K, Desantis J, et al. Hidradenitis suppurativa complicated by squamous cell carcinoma. The American Surgeon 2008; 74: 1177–1181. [PubMed] [Google Scholar]
- 7. Makris GM, Poulakaki N, Papanota AM, et al. Vulvar, perianal and perineal cancer after Hidradenitis suppurativa: a systematic review and pooled analysis. Dermatol Surg 2017; 43(1): 107–115. [DOI] [PubMed] [Google Scholar]
- 8. Lavogiez C, Delaporte E, Darras-Vercambre S, et al. Clinicopathological study of 13 cases of squamous cell carcinoma complicating hidradenitis suppurativa. Dermatology 2010; 220(2): 147. [DOI] [PubMed] [Google Scholar]
- 9. Bahillo Monné C, Honorato Guerra S, Schoendorff Ortega C, et al. Management of hidradenitis suppurativa with biological therapy: report of four cases and review of the literature. Dermatology 2014; 229(4): 279–287. [DOI] [PubMed] [Google Scholar]
- 10. Hessam S, Sand M, Bechara FG. When inflammation shifts to malignancy: extensive squamous cell carcinoma in a female hidradenitis suppurativa/acne inversa patient. Deustche Dermatologische Gesselschaft 2018; 15(1): 86–88. [DOI] [PubMed] [Google Scholar]
- 11. Scott FI, Mamtani R, Brensinger CM, et al. Risk of nonmelanoma skin cancer associated with the use of immunosuppressant and biologic agents in patients with a history of autoimmune disease and nonmelanoma skin cancer. JAMA Dermatol 2016; 152(2): 164–172. [DOI] [PMC free article] [PubMed] [Google Scholar]


