Abstract
Background:
The frequent occurrence of bacteria-associated diarrhea together with increased antimicrobial resistance poses a significant public health challenge worldwide.
Objectives:
The aim of this study was to assess the prevalence, antimicrobial susceptibility pattern, and associated factors of Salmonella and Shigella among patients with diarrhea at public health facilities in Adama, Ethiopia.
Methods:
A cross-sectional study was conducted among 232 patients with diarrhea at public health facilities in Adama, Ethiopia, from February 2017 to March 2017. Data were collected using a structured questionnaire. Stool samples were examined for Salmonella and Shigella species using the culture and serological methods. Descriptive statistics were used to summarize the findings. Logistic regression models were used to assess the association of independent variables with the outcome. A p-value ⩽ 0.05 was considered to be statistically significant.
Results:
The prevalence of Salmonella and Shigella-associated acute diarrhea was 18.1%. The most common isolates were Shigella dysenteriae (23.8%) and Salmonella typhi (21.4%). S. dysenteriae was 80% resistant to both chloramphenicol and tetracycline. S. typhi was 66.7% resistant to ampicillin, ciprofloxacin, and tetracycline. Those patients aged 11–20 years (adjusted odds ratio: 4.61, 95% confidence interval: 2.48, 7.34), who feed raw vegetables (adjusted odds ratio: 3.67, 95% confidence interval: 1.32, 8.59), and who did not wash hands with soap before a meal (adjusted odds ratio: 2.68, 95% confidence interval: 1.96, 7.48) and after using the toilet (adjusted odds ratio: 3.25, 95% confidence interval: 1.43, 7.36) had higher odds of acute bacterial diarrhea.
Conclusion:
S. dysenteriae and S. typhi were the major causes of acute diarrhea. Most of the isolates showed resistance to ampicillin, ciprofloxacin, and tetracycline. Patients aged 11–20 years, who feed raw vegetables, and who did not wash hands with soap before the meal and after using the toilet had higher odds of acute bacterial diarrhea. Continuous surveillance and the implementation of infection prevention strategies are needed to mitigate acute bacterial diarrhea.
Keywords: Salmonellosis, shigellosis, gastroenteritis, risk factors, drug resistance
Introduction
Diarrheal diseases are one of the leading causes of deaths worldwide, with an estimated 1.4 million deaths in 2010.1 The morbidity and mortality are high in developing countries where living standards, access to safe and adequate clean water supply, and proper sewage disposal system are often limited.2,3 Diarrhea is defined as the passage of three or more loose or watery stools in the 24-h period within the 2-week period.4 Its epidemiology is aggravated by the lack of access to clean and safe drinking water, poor sanitary disposal of human waste, lack of washing hands, poor housing conditions, cohabitation with domestic animals, and lack of access to adequate and affordable health care.5,6
Potential enteric bacterial pathogens that cause life-threatening diarrheal diseases across the world include Salmonella species, Shigella spp., Campylobacter spp., Vibrio cholerae, Escherichia coli, Yersinia enterocolitica, and to less extent Aeromonas spp.7,8 Of these, Salmonella and Shigella spp. continue to be the major cause of acute diarrhea in resource-limited countries,2,7 thus presenting a serious challenge to health authorities.
Salmonella affects only human and causes typhoidal salmonellosis (caused by Salmonella typhi and salmonella paratyphi) and non-typhoidal salmonellosis (all Salmonella serovars). Salmonellosis is characterized by diarrhea, fever, vomiting, and abdominal cramps after 12–72 h of infection.9 The infection can be more serious for the sick, infants, and elders.10,11 Shigella spp. (S. flexneri, S. dysenteriae, S. sonnei, and S. boydii) cause an infection known as shigellosis or bacillary dysentery. Approximately 10–100 Shigella organisms are enough to initiate the infection.12 S. dysenteriae serotype 1 that releases a shiga toxin and AB exotoxin is particularly virulent, causing endemic and epidemic bacterial dysentery with high death rates.9
The increase in drug resistance in Salmonella and Shigella spp. is a global challenge.12–14 The problem is exacerbated in developing countries, where the use of an antimicrobial in humans and animals is largely unrestricted,15,16 and the treatment is solely based on clinical findings due to the lack of laboratory facilities.17 Most resistance to commonly used antimicrobial drugs is associated with self-replicating R plasmid.18 The resistant genes can be transferred from resistant to sensitive bacteria. A transfer occurs in the intestine of persons treated with oral antimicrobials due to selection pressure provided by the drug.15 The relative incidence of resistance and the serotypes in which it occurs differs from country to country.15,19 A study conducted in Butajira, Ethiopia, revealed higher resistance of Salmonella spp. to ampicillin and tetracycline while Shigella spp. to tetracycline and co-trimoxazole.20
There are pocket studies on diarrhea from different regions of Ethiopia. However, most of them were restricted either to clinical data, specific age group, or common bacterial strains with or without associated factors and antimicrobial sensitivity tests.6,20–22 This study investigated the prevalence, antimicrobial susceptibility pattern, and associated factors of Salmonella and Shigella among patients with diarrhea at public health facilities in Adama, Ethiopia.
Methods
Study settings, design, and period
The facility-based cross-sectional study was conducted at public health facilities in Adama, Ethiopia, from February 2017 to March 2017. Adama, which is found at 99 km southeast of Addis Ababa, Ethiopia, has 10 public health facilities. Approximately 5000 patients with diarrhea visited these health facilities every year.23
Study population and exclusion criteria
Patients who reported three or more diarrhea episodes within the last 24 h were enrolled in the study. Newborn, inpatients and those who had persistence diarrhea and taken antimicrobial treatment 2 weeks prior to and at the time of data collection were excluded from the study.
Sample size and sampling technique
The sample size was calculated using a single population proportion formula considering the prevalence of 17.4% culture-confirmed bacteria-associated diarrhea,20 95% confidence interval (CI), and a 5% margin of error. After adding 10% non-response rate, the final sample size was 243. Out of the total (10) public health facilities, six (Adama Hospital Medical College (AHMC), Adama Health Center (AHC), Biftu Health Center (BHC), Denbela Health Center (DHC), Hawas Health Center (HHC), and San Francisco Health Center (SHC)) were selected using a simple random sampling technique (lottery method). Proportional allocation of the sample size was made for each health facility based on their average size of patients with diarrhea (Figure 1). The study participants were enrolled consecutively until the intended sample size fulfilled.
Figure 1.
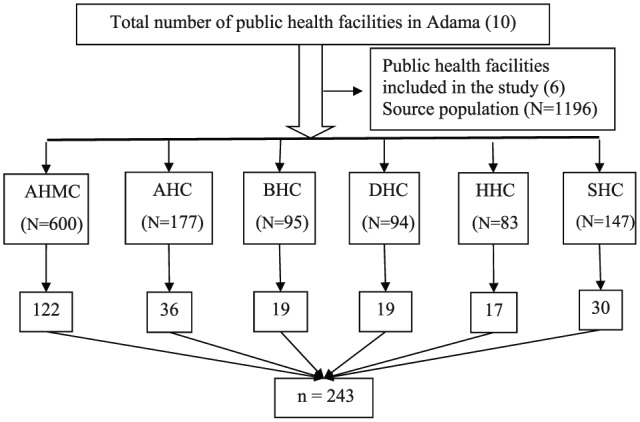
Schematic presentation of sample size allocation.
N: total number of patients with diarrhea at selected public health facilities; n: sample size.
Data collection and laboratory procedures
Data were collected using a pre-tested structured questionnaire adapted from the World Health Organization (WHO) core questions and different kinds of literature that were designed to explore factors related to diarrhea.6,19,24,25 The adapted questionnaire was contextualized to the local situation and to the study objectives. The questionnaire comprised sociodemographic characteristics (such as sex, age, residence, level of education, and occupational status) and associated risk factors (feeding of raw/uncooked food, washing hands before the meal and after using the toilet, the frequency of washing hands, contact with domestic animals among other related factors). Participants were instructed to collect ~1 g of fresh stool (or 1 mL if loose) in a sterile screw-capped tube containing 9 mL buffered peptone water (Oxoid, Basingstoke, England). The stool specimens were transported in cold box to the Oromia Public Health Research, Capacity Building and Quality Assurance Laboratory, Adama, Ethiopia for further processing and microbiological analysis.
In the laboratory, the isolation and characterization of Salmonella and Shigella spp. were done using differential and selective culture media (Oxoid, Basingstoke, England) as described by Cheesbrough.9 After overnight incubation in peptone water at 37°C, 1 mL of the sample was transferred into 9 mL Selenite F broth (Oxoid, Ltd, UK) and incubated at 37°C for 24 h. A loopful of culture from Selenite F broth showing growth was subcultured onto MacConkey agar (MAC), deoxycholate citrate agar (DCA), xylose lysine deoxycholate agar (XLD) and incubated at 37°C for 24 h. The growth of Salmonella and Shigella spp. was detected by their characteristic appearance on MAC (Salmonella and Shigella: colorless and transparent), XLD (Salmonella red with a black center, Shigella: red colonies), and DCA (Salmonella black center pale colonies, Shigella: pale colonies). Presumptive colonies were further investigated biochemically using Gram’s reactions, lactose, mannitol, lysine decarboxylase, indole, urea, triple sugar iron agar, oxidase, β-galactosidase, Simmons citrate agar, and motility test.9,26 Confirmation of Salmonella and Shigella spp. was done by a slide agglutination test using polyvalent O antigen grouping sera, followed in some cases by testing with monovalent antisera for specific serotyping (Denka Seiken Co., Ltd., Tokyo, Japan) as described.27
Antimicrobial susceptibility testing
Each isolated strain was tested in vitro for antimicrobial susceptibility according to a modified Kirby Bauer disk diffusion method as described by the Clinical and Laboratory Standards Institute (CLSI).28 The commonly prescribed antimicrobials such as ampicillin (10 µg), amoxicillin/clavulanate (20/10 µg), cefoxitin (30 µg), ciprofloxacin (5 µg), chloramphenicol (30 µg), tetracycline (30 µg), and nalidixic acid (30 µg) (Oxoid, Ltd., UK) were used to screen the susceptibility of the isolates. Three to five pure colonies of bacteria were picked and suspended in sterile normal saline (0.85% NaCl) until the suspension became equivalent to 0.5 McFarland turbidity standard. A bacterial suspension was placed at the center of Mueller Hinton agar plate (Oxoid, Ltd., UK) supplemented with 5% sheep blood and evenly spread using a sterile cotton-tipped applicator. After drying for 3–5 min, antimicrobial disks were placed and incubated aerobically at 37°C. After overnight incubation, the diameter of the zone of inhibition was measured using a digital caliper and interpreted as sensitive (S), intermediate (I), or resistance (R) based on the CLSI interpretive criteria.28
Operational definitions
Acute bacterial diarrhea is defined as the passage of three or more loose or watery stools in a 24-h period before the study and the isolation of at least one Salmonella or Shigella spp.
Antimicrobial resistance is the complete insensitivity of the isolates for which they are sensitive before.28
Data quality control
The questionnaire was initially prepared in English and translated into local languages (Afaan Oromo and Amharic) by language experts and back to English by other language experts to check its consistency. The questionnaire was pre-tested on 5% of the patients with diarrhea at Bishoftu Referral Hospital, Ethiopia, to check its practicability and applicability. Some questions were modified based on the feedback. Data collectors were trained for 5 days on the method of data collection, culture, and characterization of the isolates. Standard operating procedures and manufacturer’s instructions were strictly followed. Close supervision was undertaken by the professional nurse and medical microbiologist. Hand washing facility was facilitated during sample collection to prevent cross-contamination. Each sample was safely stored and transported at recommended conditions. The sterility of newly opened culture media was checked before use by incubating at 37°C for 24 h. American Type Culture Collection (ATCC) reference strains such as E. coli (ATCC® 25922), S. flexneri (ATCC® 25931), Staphylococcus aureus (ATCC® 25923), and S. typhimurium (ATCC® 13311) were used to test the performance of each culture medium and antimicrobial disks before use.
Data analysis
Data were checked for completeness, coded, and entered into the EpiData software (version 3.1, EpiData Association, Odense, Denmark), cleaned, and exported to the Statistical Package for Social Sciences software version 25.0 (SPSS, IL, USA) for analysis. Data were summarized using descriptive statistics (frequency, percentage, mean, and standard deviation). Bivariate and multivariate logistic regression analyses were performed to identify factors associated with the outcome variable. Variables with a p-value of <0.25 in the bivariate analysis were considered in the multivariate logistic regression model. A variable with a p-value ⩽ 0.05 at 95% CI in multivariate logistic regression was considered to be statistically significant.
Results
Participant characteristics
Of the total (243), 232 patients with diarrhea were enrolled in this study, making a response rate of 95.5%. The majority of them were females (57.3%). The age of the study participants ranges from 1 to 70 years with a mean age of 22.3 years (±16.5 standard deviation). Most of the study participants were urban dweller (89.2%) and had a primary level of education (43.1%) (Table 1).
Table 1.
Sociodemographic characteristics of patients with diarrhea at public health facilities in Adama, Ethiopia, 2017.
| Sociodemographic characteristics | n (%) |
|---|---|
| Sex | |
| Male | 99 (42.7) |
| Female | 133 (57.3) |
| Age (in years) | |
| <11 | 75 (32.3) |
| 11–20 | 40 (17.2) |
| 21–30 | 45 (19.4) |
| 31–40 | 41 (17.7) |
| >40 | 31 (13.4) |
| Place of residence | |
| Urban | 207 (89.2) |
| Rural | 25 (10.8) |
| Level of education | |
| Not read and write | 72 (31) |
| Read and write | 14 (6) |
| Primary cycle (Grades 1–8) | 100 (43.1) |
| Secondary cycle (Grades 9–12) and above | 46 (19.8) |
| Occupational status | |
| Government employee | 21 (9.1) |
| Merchant | 37 (15.9) |
| Farmer, housewife, and daily labor | 67 (28.9) |
| Students | 71 (30.6) |
| Children and elders | 36 (15.5) |
Prevalence of acute bacterial diarrhea
Out of the 232 stool specimens investigated, 42 Salmonella and Shigella spp. were recovered, making a prevalence of 18.1% (95% CI: 15.9, 21.4). Shigella (9.5%) was most frequently isolated followed by Salmonella spp. (8.6%). The predominant bacteria were S. dysenteriae (23.8%), S. typhi (21.4%) and S. flexneri (19.1%) (Table 2).
Table 2.
Antimicrobial susceptibility pattern of Salmonella and Shigella spp. isolated from stool of patients with diarrhea at public health facilities in Adama, Ethiopia, 2017.
| Bacterial isolates | Total, n (%) | Pattern | Antimicrobial susceptibility,
n (%) |
||||||
|---|---|---|---|---|---|---|---|---|---|
| AM | AMC | FOX | CIP | C | TE | NAL | |||
| S. dysenteriae | 10 (23.8) | S | 3 (30) | 7 (70) | 7 (70) | 4 (40) | 2 (20) | 2 (20) | 8 (80) |
| I | 0 (0) | 1 (10) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | ||
| R | 7 (70) | 2 (20) | 3 (30) | 6 (60) | 8 (80) | 8 (80) | 2 (20) | ||
| S. typhi | 9 (21.4) | S | 3 (33.3) | 7 (77.8) | 6 (66.7) | 2 (22.2) | 5 (55.6) | 3 (33.3) | 5 (55.6) |
| I | 0 (0) | 0 (0) | 0 (0) | 1 (11.1) | 0 (0) | 0 (0) | 0 (0) | ||
| R | 6 (66.7) | 2 (22.2) | 3 (33.3) | 6 (66.7) | 4 (44.4) | 6 (66.7) | 4 (44.4) | ||
| S. flexneri | 8 (19.1) | S | 1 (12.5) | 7 (87.5) | 6 (75) | 3 (37.5) | 6 (75) | 2 (25) | 7 (87.5) |
| R | 7 (87.5) | 1 (12.5) | 2 (25) | 5 (62.5) | 2 (25) | 6 (75) | 1 (12.5) | ||
| Non-typhoidal Salmonella spp. | 6 (14.3) | S | 1 (16.7) | 5 (83.3) | 4 (66.7) | 1 (16.7) | 4 (66.7) | 2 (33.3) | 4 (66.7) |
| R | 5 (83.3) | 1 (16.7) | 2 (33.3) | 5 (83.3) | 2 (33.3) | 4 (66.7) | 2 (33.3) | ||
| S. paratyphi | 5 (11.9) | S | 2 (40) | 3 (60) | 3 (60) | 2 (40) | 2 (40) | 2 (40) | 4 (80) |
| I | 1 (20) | 0 (0) | 0 (0) | 1 (20) | 0 (0) | 0 (0) | 1 (20) | ||
| R | 2 (40) | 2 (40) | 2 (40) | 2 (40) | 3 (60) | 3 (60) | 0 (0) | ||
| S. boydii | 4 (9.5) | S | 2 (50) | 2 (50) | 2 (50) | 1 (25) | 2 (150) | 1 (25) | 2 (50) |
| I | 0 (0) | 0 (0) | 1 (25) | 1 (25) | 0 (0) | 1 (25) | 0 (0) | ||
| R | 2 (50) | 2 (50) | 1 (25) | 2 (50) | 2 (50) | 2 (50) | 2 (50) | ||
| Total | 42 (100) | S | 12 (28.6) | 31 (73.8) | 28 (66.7) | 13 (30.9) | 21 (50) | 12 (28.6) | 30 (71.4) |
| I | 1 (2.4) | 1 (2.4) | 1 (2.4) | 3 (7.2) | 0 (0) | 1 (2.4) | 1 (2.4) | ||
| R | 29 (69) | 10 (23.8) | 13 (30.9) | 26 (61.9) | 21 (50) | 29 (69) | 12 (26.2) | ||
AM: ampicillin; AMC: amoxicillin–clavulanate; FOX: cefoxitin; CIP: ciprofloxacin; C: chloramphenicol; TE: tetracycline; NAL: nalidixic acid; S: sensitive; I: intermediate; R: resistance.
Antimicrobial susceptibility pattern of the isolates
High rates of resistance against multiple antimicrobials were observed in most of the isolates. The isolates showed 69% resistance to each of ampicillin and tetracycline, and 61.9% to ciprofloxacin. The most resistant isolates from Shigella spp. were S. flexneri, which showed 87.5% resistance to ampicillin, 75% to tetracycline, and 62.5% to ciprofloxacin. S. dysenteriae was the second most resistant bacteria, which showed 80% resistance to chloramphenicol and tetracycline, 70% to ampicillin, and 60% to ciprofloxacin. S. typhi showed 66.7% resistance to ampicillin, ciprofloxacin, and tetracycline (Table 2).
Factors associated with acute diarrhea
Patients in the age range of 11–20 years had almost five times higher odds of getting acute bacterial diarrhea than those in the age of less than 11 years (adjusted odds ratio (AOR): 4.61, 95% CI: 2.48, 7.34). The odds of having acute bacterial diarrhea were about fourfold higher among patients who feed raw vegetables compared with their counterpart (AOR: 3.67, 95% CI: 1.32, 8.59). The odds of being infected with acute bacterial diarrhea were almost three times higher among patients who did not wash their hands before a meal with soap compared to those who did (AOR: 2.68, 95% CI: 1.96, 7.48). The odds of acute bacterial diarrhea were about threefold among patients who did not wash their hands after using the toilet compared to those who did (AOR: 3.25, 95% CI: 1.43, 7.36) (Table 3).
Table 3.
Factors associated with Salmonella and Shigella-associated acute diarrhea at public health facilities in Adama, Ethiopia, 2017.
| Characteristics | Acute bacterial diarrhea |
Crude OR (95% CI) | Adjusted OR (95% CI) | |
|---|---|---|---|---|
| Yes, n (%) | No, n (%) | |||
| Sex | ||||
| Male | 16 (16.2) | 83 (83.8) | 1 | |
| Female | 26 (19.5) | 107 (80.5) | 1.26 (0.64, 2.50) | |
| Age (in years) | ||||
| <11 | 9 (11.7) | 68 (88.3) | 1 | 1 |
| 11–20 | 11 (26.8) | 30 (73.2) | 2.77 (1.04, 7.38)* | 4.61 (2.48, 7.340)** |
| 21–30 | 8 (17.8) | 37 (82.2) | 1.63 (0.58, 4.59) | 2.40 (0.73, 7.91) |
| 31–40 | 7 (18.4) | 31 (81.6) | 1.71 (0.58, 5.00) | 2.76 (0.85, 9.11) |
| >40 | 7 (22.6) | 24 (77.4) | 2.20 (0.74, 6.57) | 3.26 (0.99, 8.59) |
| Educational status | ||||
| Read and write | 31 (17.4) | 147 (82.6) | 1 | |
| Not read and write | 11 (20.4) | 43 (79.6) | 1.21 (0.56, 2.61) | |
| Occupation | ||||
| Employee | 5 (23.8) | 16 (76.2) | 1 | |
| Non-employee | 37 (17.5) | 174 (82.5) | 0.68 (0.24, 1.97) | |
| Feeding of raw meat | ||||
| No | 36 (17.9) | 165 (82.1) | 1 | |
| Yes | 6 (19.4) | 25 (80.6) | 0.91 (0.35, 2.38) | |
| Feeding of raw milk | ||||
| No | 29 (17.7) | 135 (82.3) | 1 | |
| Yes | 13 (19.1) | 55 (80.9) | 1.10 (0.53, 2.27) | |
| Feeding of raw vegetables | ||||
| No | 29 (16) | 152 (84) | 1 | 1 |
| Yes | 13 (25.5) | 38 (74.5) | 1.79 (0.85, 3.78)* | 3.67 (1.32, 8.59)** |
| Hand washing before meal | ||||
| Soap and water | 8 (12.7) | 55 (87.3) | 1 | 1 |
| Water only | 34 (20.1) | 135 (79.9) | 1.73 (0.75, 3.98)* | 2.68 (1.96, 7.48)** |
| Frequency of hand washing habit before meal | ||||
| Always | 17 (17) | 83 (83) | 1 | |
| Occasionally | 25 (18.9) | 107 (81.1) | 1.14 (0.58, 2.25) | |
| Hand washing with detergents before meal | ||||
| No | 18 (18) | 82 (82) | 1 | |
| Yes | 24 (18.2) | 108 (81.8) | 1.01 (0.51, 1.99) | |
| Hand wash after using the toilet | ||||
| Yes | 26 (14.7) | 151 (85.3) | 1 | 1 |
| No | 15 (31.2) | 33 (68.8) | 2.64 (1.26, 5.23)* | 3.25 (1.43, 7.36)** |
| Hand washing facility around the latrine | ||||
| Yes | 34 (17.9) | 156 (82.1) | 1 | |
| No | 8 (19) | 34 (81) | 1.08 (0.46, 2.54) | |
| Contact with domestic animals (cat, dog, and hen) | ||||
| No | 24 (16.1) | 125 (83.9) | 1 | |
| Yes | 18 (21.7) | 65 (78.3) | 1.44 (0.73, 2.85) | |
OR: odds ratio; CI: confidence interval.
Statistically significant association at a p-value < 0.25.
Statistically significant association at a p-value ⩽ 0.05.
Discussion
Bacteria-associated diarrheal diseases are a major public health problem in developing countries where illiteracy, poverty, overcrowding, poor sanitation, and unsafe drinking water supply are common.1–3 In this study, the prevalence of Salmonella and Shigella-associated acute diarrhea was 18.1% (95% CI: 15.9, 21.4). This was comparable with studies conducted in other parts of Ethiopia such as Harar (18.4%),29 and Gondar (17.9%),30 but it was higher than another study conducted in Gondar (5.7%).19 The variation among the above-mentioned findings might be largely due to substandard environmental and personal hygiene, ignorance of health promotion, and methodological difference (the type of study participants, sample size, study design, study period, and diagnostic techniques).
Acute diarrhea caused by Salmonella is often a mild and self-limiting disease, occasionally they may cause life-threatening diseases.3,10 The prevalence of Salmonella spp. (8.6%) in this study was relatively comparable to the study conducted in Harar, Ethiopia (11.5%),29 Ebonyi, Nigeria, (10.7%),31 and Butajira, Ethiopia (10.5%).20 The variation among the studies might be attributed to the presence of basic social facilities such as access to safe drinking water, personal hygiene, housing condition, sewage disposal, and ecological (animal reservoirs) and/or geographical condition.24
The ecological repartition of the four pathogenic Shigella spp. is dynamic. S. flexneri is endemic in many developing countries and causes a higher rate of mortality than other species.11,12 However, the most prevalent Shigella strains that cause the majority of acute diarrheal diseases in this study belonged to S. dysenteriae (23.8%). The finding was comparable with reports from Borno, Nigeria (37.5%),24 Gondar, Ethiopia (10%),32 and Accra, Ghana (16.7%).14 The prevalence of S. flexneri (19.1%) was found to be lower than the study conducted in Mwanza, Tanzania (90%),13 Rosario, Argentina (74%),12 Gondar, Ethiopia (64.7%),19 Wuhan, China (60%),7 and Addis Ababa, Ethiopia (54%).33 The decrease of the more virulent S. flexneri in the present study might be attributed to the difference in environmental conditions.
Antimicrobial treatment can reduce the symptoms of a disease, decrease the number of carriers, and prevent the spread of the infection. However, in resource-limited countries, clinicians are enforced to clinically diagnose and prescribe broad-spectrum antimicrobials empirically that led drug-resistant bacterial strains to emerge.17 Infections caused by these bacteria could lead to a high cost of treatment, prolonged hospital stays, and an increase in mortality with its concomitant loss in manpower and societies.24,31 In this study, Salmonella and Shigella strains showed higher resistance to ampicillin, ciprofloxacin, and tetracycline. These antimicrobials are no longer recommended for empirical treatment. The observed resistance could be due to their wide use as first-line drugs with and without prescription in Ethiopia for a long time, easy availability, and misuse.17 The finding was in agreement with most studies conducted in Ethiopia.19,20,32 S. dysenteriae was found to be resistant to chloramphenicol, tetracycline, and ampicillin. This was in agreement with the reports from Gondar, Ethiopia,32 and Mwanza, Tanzania.13 The resistance observed by S. typhi to ampicillin, ciprofloxacin, and tetracycline was also in agreement with the studies reported in many developing countries.13,34,35 The higher resistance to commonly used antimicrobials in this study is of critical concern to modern medicine, and clinicians may face the looming of encountering untreatable diarrhea caused by Salmonella and Shigella spp. in the near future. Sensitivity patterns can also be changed rapidly and they need to be monitored closely because of their implications for public health and as an indicator of drug misuse in a particular area. The appropriate choice, rational use, and considering the benefit–risk ratio of administering antimicrobials for the treatment of Salmonella and Shigella-associated diarrhea at different levels of health facilities would help to mitigate the evolution of antimicrobial resistance.
In this study, patients aged 11–20 years had higher odds of getting acute bacterial diarrhea. This may be due to their substandard personal hygiene and higher exposure to unavailability of safe drinking water and lack of washing hands. The higher odds of acute bacterial diarrhea among patients who did not wash their hands with soap before a meal and after using the toilet were in accordance with the findings of the study conducted in Mekelle, Ethiopia.6 Another factor that was found to increase the odds of acquiring acute bacterial diarrhea was the feeding of raw vegetables. This was supported by a large variety of evidence that demonstrated as the feeding of contaminated vegetables predisposes consumers to infectious diarrhea.36,37 The occurrence of acute diarrhea could be decreased by interventions aimed to increase awareness in the identified associated factors.
This study has some limitations. The likelihood of underestimation of the prevalence of Salmonella and Shigella-associated acute diarrhea was high since the study was based on the small sample size. The resistance observed to antimicrobials tested may not necessarily reflect in vivo resistance as the study was an in vitro one. Moreover, it is difficult to declare the cause–effect relationship between the identified associated factors and the outcome as the temporality between exposure and outcome cannot be ascertained with precision in the cross-sectional study. Thus, further studies using a large sample size and another study design are required to elucidate the relationship between associated factors, antimicrobial resistance, and acute bacterial diarrhea. Regardless of these limitations, the data described in this study could provide valuable information to clinicians, health authorities and researchers regarding Salmonella and Shigella-associated diarrhea, its associated factors, and antimicrobial susceptibility pattern.
Conclusion
In the present study, S. dysenteriae and S. typhi were the common causes of acute diarrhea. The higher resistance observed to tetracycline, ampicillin, and ciprofloxacin is of major concern. Treatments need to be based on species identification and antimicrobial susceptibility testing results rather than the currently practiced empirical treatment. Patients aged 11–20 years, who feed raw vegetables, and who did not wash hands with soap before the meal and after using the toilet had higher odds of acute bacterial diarrhea. Preventive measures focusing on these factors are needed to mitigate the spread of diarrhea. Moreover, further studies on large population are highly recommended to validate this study and to design cost-effective and cost-efficient infection control programs.
Supplemental Material
Supplemental material, Questionnaire for Salmonella and Shigella among patients with diarrhea at public health facilities in Adama, Ethiopia: Prevalence, antimicrobial susceptibility pattern, and associated factors by Bedada Teshome, Zelalem Teklemariam, Desalegn Admassu Ayana, Dadi Marami and Nega Asaminew in SAGE Open Medicine
Acknowledgments
The authors would like to thank Haramaya University for providing an ethical clearance, Oromia Public Health Research Capacity Building and Quality Assurance Laboratory for material support, and the study participants for their valuable information.
Footnotes
Author contributions: B.T. conceived the study and participated in data collection and laboratory analysis. B.T., Z.T., D.A.A., and D.M. have participated in study design, proposal development, interpretation, and an initial and final write-up of the manuscript. N.A. participated in proposal development, sample analysis, and manuscript write-up. All authors read and approved the final manuscript.
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethical approval: Ethical clearance was obtained from the Institutional Health Research Ethics Review Committee of the College of Health and Medical Sciences, Haramaya University. Permission to conduct the study was also secured from respective health facilities. Ethical approval for this study was obtained from the Institutional Health Research Ethics Review Committee of the College of Health and Medical Sciences, Haramaya University (approval number: IHRERC 065/2017).
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was materially supported by the Oromia Public Health Research, Capacity Building and Quality Assurance Laboratory, Adama, Ethiopia. The supporter had no role in study design, data collection, and analysis, decision to publish, or preparation of the manuscript.
Informed consent: Data were collected after informed, voluntary, written, and signed consent was obtained from the study participants aged ⩾18 years and assent for those <18 years of age from the child’s parent or legally authorized representative guardian before the commencement of the study. The confidentiality of the information was strictly maintained.
Supplemental material: The data used to support the findings of this study are available from the corresponding author upon request.
ORCID iDs: Zelalem Teklemariam  https://orcid.org/0000-0001-7290-8913
https://orcid.org/0000-0001-7290-8913
Dadi Marami  https://orcid.org/0000-0002-4793-3575
https://orcid.org/0000-0002-4793-3575
References
- 1. Lozano R, Naghavi M, Foreman K, et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease. Lancet 2013; 380: 2095–2128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bartram J, Cronk R, Montgomery M, et al. Lack of toilets and safe water in health-care facilities. Bull World Health Organ 2015; 93(4): 210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Pruss-Ustun A, Bartram J, Clasen T, et al. Burden of disease from inadequate water, sanitation and hygiene in low- and middle-income settings: a retrospective analysis of data from 145 countries. Trop Med Int Health 2014; 19(8): 894–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. World Health Organization (WHO). The treatment of diarrhea, whqlibdoc.who.int/publications/2005/9241593180.pdf (2005, accessed 12 September 2016). [Google Scholar]
- 5. Mama M, Alemu G. Prevalence, antimicrobial susceptibility patterns and associated risk factors of Shigella and Salmonella among food handlers in Arba Minch University, South Ethiopia. BMC Infect Dis 2016; 16(1): 686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gebrekidan A, Dejene TA, Kahsay G, et al. Prevalence and antimicrobial susceptibility patterns of Shigella among acute diarrheal outpatients in Mekelle hospital, Northern Ethiopia. BMC Res Notes 2015; 8: 611–617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Zhu X, Tian L, Cheng Z, et al. Viral and bacterial etiology of acute diarrhea among children under 5 years of age in Wuhan, China. Chin Med J 2016; 129(16): 1939–1944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Vargas M, Gascon J, Casals C, et al. Etiology of diarrhea in children less than five years of age in Ifakara, Tanzania. Am J Trop Med Hyg 2004; 70(5): 536–539. [PubMed] [Google Scholar]
- 9. Cheesbrough M. District laboratory practice in tropical countries. 2nd ed. New York: Cambridge University Press, 2006. [Google Scholar]
- 10. Coburn B, Grassl G, Finlay B. Salmonella, the host and disease: a brief review. Immunol Cell Biol 2007; 85(2): 112–118. [DOI] [PubMed] [Google Scholar]
- 11. Tsen H, Hu H, Lin J, et al. Analysis of the Salmonella typhimurium isolates from food-poisoning cases by molecular subtyping methods. Food Microbiol 2000; 17: 143–152. [Google Scholar]
- 12. Casabonne C, Gonzalez A, Aquili V, et al. Prevalence and virulence genes of Shigella spp. Jpn J Infect Dis 2016; 69(6): 477–481. [DOI] [PubMed] [Google Scholar]
- 13. Temu M, Kaatano G, Miyaye N, et al. Antimicrobial susceptibility of Shigella flexneri and S. dysenteriae isolated from stool specimens of patients with bloody diarrhoea in Mwanza, Tanzania. Tanzan Health Res Bull 2007; 9: 186–189. [PubMed] [Google Scholar]
- 14. Opintan JA, Newman MJ. Distribution of serogroups and serotypes of multiple drug resistant Shigella isolates. Ghana Med J 2007; 41(1): 8–29. [PMC free article] [PubMed] [Google Scholar]
- 15. Magiorakos AP, Srinivasan A, Carey RB, et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clin Microbiol Infect 2012; 18(3): 268–281. [DOI] [PubMed] [Google Scholar]
- 16. Okeke I, Aboderin O, Byarugaba D, et al. Growing problem of multidrug-resistant enteric pathogens in Africa. Emerg Infect Dis 2007; 13(11): 1640–1646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Food, Medicine and Health Care Administration and Control Authority of Ethiopia (FMHACA). Standard treatment guidelines for general hospitals. 3rd ed. Addis Ababa, Ethiopia: Food, Medicine and Health Care Administration and Control Authority of Ethiopia, 2014. [Google Scholar]
- 18. Buckner M, Ciusa M, Piddock L. Strategies to combat antimicrobial resistance: anti-plasmid and plasmid curing. FEMS Microbiol Rev 2018; 42(6): 781–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Demissie AT, Wubie TM, Yehuala FM, et al. Prevalence and antimicrobial susceptibility patterns of Shigella and Salmonella species among patients with diarrhea attending Gondar town health institutions, Northwest Ethiopia. Sci J Publ Heal 2014; 2: 469–475. [Google Scholar]
- 20. Mengistu G, Mulugeta G, Lema T, et al. Prevalence and antimicrobial susceptibility patterns of Salmonella serovars and Shigella species. J Microb Biochem Technol 2014; 2: 1–7. [Google Scholar]
- 21. Lamboro T, Ketema T, Bacha K. Prevalence and antimicrobial resistance in Salmonella and Shigella species isolated from outpatients, Jimma University Specialized Hospital, Southwest Ethiopia. Can J Infect Dis Med Microbiol 2016; 2016: 4210760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Yeshwondm M, Metaferia G, Birhanu A, et al. Isolation and antibiotic susceptibility patterns of Shigella and Salmonella among under 5 children with acute diarrhoea: a cross-sectional study at selected public health facilities in Addis Ababa, Ethiopia. Clin Microbiol 2015; 4: 186. [Google Scholar]
- 23. Adama City Administration Health Office. Heath and health related annual report, http://www.aarc.gov.et/index.php/component/content/article/8-yt-sample-data/85-adama-city-health-office-page (2015, accessed 12 August 2016).
- 24. Ngoshe I, Denue B, Bello H, et al. Prevalence and antimicrobial susceptibility of Shigella species isolates from diarrheal stool of patients in a tertiary health facility in northeastern Nigeria. Sub-Saharan African J Med 2017; 4: 96–101. [Google Scholar]
- 25. World Health Organization (WHO). Core questions on drinking-water and sanitation for household surveys, https://www.who.int/water_sanitation_health/monitoring/oms_brochure_core_questionsfinal24608.pdf (2006, accessed 12 September 2016).
- 26. Humphries RM, Linscott J. Laboratory diagnosis of bacterial gastroenteritis. Clin Microbiol Rev 2015; 28(1): 3–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Schrader KN, Fernandez-Castro A, Cheung WK, et al. Evaluation of commercial antisera for Salmonella serotyping. J Clin Microbiol 2008; 46(2): 685–688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Clinical and Laboratory Standards Institute (CLSI). Performance standards for antimicrobial disk susceptibility tests. 27th ed. Wayne, PA: Clinical and Laboratory Standards Institute, 2017. [Google Scholar]
- 29. Ayalu AR, Seyoum B, Yimam J, et al. Antibiotic susceptibility patterns of Salmonella and Shigella isolates in Harar, Eastern Ethiopia. J Infect Dis Immun 2011; 3: 134–139. [Google Scholar]
- 30. Huruy K, Kassu A, Mulu A, et al. High level of antimicrobial resistance in Shigella species isolated from diarrhoeal patients in University of Gondar Teaching Hospital, Gondar, Ethiopia. Pharmacologyonline 2008; 2: 328–340. [Google Scholar]
- 31. Olugbue V, Nwaugo V, Okata M, et al. Antimicrobial susceptibility profiles of Pseudomonas aeruginosa isolates from patients attending health care facilities, Ebonyi State, Nigeria. Asian J Res Med Pharm Sci 2018; 3: 1–8. [Google Scholar]
- 32. Tiruneh M. Serodiversity and antimicrobial resistance pattern of Shigella isolates at Gondar University Teaching Hospital, Northwest Ethiopia. Jpn J Infect Dis 2009; 62(2): 93–97. [PubMed] [Google Scholar]
- 33. Asrat D. Shigella and Salmonella serogroups and their antibiotic susceptibility patterns in Ethiopia. East Mediterr Health J 2008; 14(4): 760–767. [PubMed] [Google Scholar]
- 34. Mathew B, Ugboko H, De N. Prevalence of multidrug resistant Salmonella enterica serovar Typhi in Kaduna Metropolis, Kaduna, Nigeria. Int J Curr Microbiol Appl Sci 2015; 4: 323–335. [Google Scholar]
- 35. Rahman MA. Antimicrobial resistance patterns of Salmonella typhi isolated from stool culture. Chattagram Maa-O-Shishu Hosp Med Coll J 2015; 14: 26–30. [Google Scholar]
- 36. Saeed HA, Hamid HH. Bacteriological and parasitological assessment of food handlers in the Omdurman area of Sudan. J Microbiol Immunol Infect 2010; 43(1): 70–73. [DOI] [PubMed] [Google Scholar]
- 37. Taulo S, Wetlesen A, Abrahamsen R, et al. Microbiological hazard identification and exposure assessment of food prepared and served in rural households of Lungwena, Malawi. Int J Food Microbiol 2008; 125(2): 111–116. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, Questionnaire for Salmonella and Shigella among patients with diarrhea at public health facilities in Adama, Ethiopia: Prevalence, antimicrobial susceptibility pattern, and associated factors by Bedada Teshome, Zelalem Teklemariam, Desalegn Admassu Ayana, Dadi Marami and Nega Asaminew in SAGE Open Medicine


