Abstract
Objective
To determine the effect of erenumab, a human monoclonal antibody targeting the calcitonin gene-related peptide receptor, on health-related quality of life (HRQoL), headache impact, and disability in patients with chronic migraine (CM).
Methods
In this double-blind, placebo-controlled study, 667 adults with CM were randomized (3:2:2) to placebo or erenumab (70 or 140 mg monthly). Exploratory endpoints included migraine-specific HRQoL (Migraine-Specific Quality-of-Life Questionnaire [MSQ]), headache impact (Headache Impact Test–6 [HIT-6]), migraine-related disability (Migraine Disability Assessment [MIDAS] test), and pain interference (Patient-Reported Outcomes Measurement Information System [PROMIS] Pain Interference Scale short form 6b).
Results
Improvements were observed for all endpoints in both erenumab groups at month 3, with greater changes relative to placebo observed at month 1 for many outcomes. All 3 MSQ domains were improved from baseline with treatment differences for both doses exceeding minimally important differences established for MSQ–role function-restrictive (≥3.2) and MSQ–emotional functioning (≥7.5) and for MSQ–role function-preventive (≥4.5) for erenumab 140 mg. Changes from baseline in HIT-6 scores at month 3 were −5.6 for both doses vs −3.1 for placebo. MIDAS scores at month 3 improved by −19.4 days for 70 mg and −19.8 days for 140 mg vs −7.5 days for placebo. Individual-level minimally important difference was achieved by larger proportions of erenumab-treated participants than placebo for all MSQ domains and HIT-6. Lower proportions of erenumab-treated participants had MIDAS scores of severe (≥21) or very severe (≥41) or PROMIS scores ≥60 at month 3.
Conclusions
Erenumab-treated patients with CM experienced clinically relevant improvements across a broad range of patient-reported outcomes.
Clinicaltrials.gov identifier
Classification of evidence
This study provides Class II evidence that for patients with CM, erenumab treatment improves HRQoL, headache impact, and disability.
Migraine is a disabling neurologic disorder affecting 15% of the global population.1–5 Disability increases progressively with increasing frequency of migraine headache days; patients with chronic migraine (CM), who comprise approximately 10% of the total migraine population, are most disabled.4,6 Migraine and its associated symptoms result in decreased health-related quality of life (HRQoL), social and psychological impact, and increased disability.2,4,5,7 In addition to reducing the frequency, intensity, and duration of attacks, international treatment guidelines state that preventive treatments for migraine should restore ability to function.8 Erenumab (in the United States, erenumab-aooe) is a fully human anti-canonical calcitonin gene-related peptide (CGRP) receptor monoclonal antibody approved in the United States for migraine prevention,9 with demonstrated clinically relevant efficacy in CM.10 The clinical safety and efficacy of erenumab was assessed in a pivotal, randomized, double-blind, placebo-controlled study of patients with CM. Erenumab 70 and 140 mg showed significant reductions from baseline in monthly migraine days compared with placebo (both doses 6.6 days vs placebo 4.2 days) with a safety profile similar to placebo.10 Herein, we present a secondary analysis of these clinical trial data to examine the effect of erenumab treatment on multiple patient-reported outcomes (PROs) measuring a broad range of complementary outcomes in patients with CM following 3 months of treatment.
Methods
The objective of this analysis was to assess the efficacy of erenumab on HRQoL, headache impact, and disability in patients with CM. This study provides Class II evidence that for patients with CM, erenumab treatment improves HRQoL, headache impact, and disability.
Patients and data source
This was an exploratory analysis of PRO data from a pivotal study that evaluated safety and efficacy of erenumab in patients with CM (≥15 headache days per month, of which ≥8 were migraine days). The eligibility criteria, design, and primary results of the phase 2 study were previously published.10 The study comprised 667 participants at 69 study sites worldwide. Participants were randomized to 1 of 3 treatment arms in a 3:2:2 ratio (placebo, erenumab 70, or erenumab 140 mg monthly) stratified by region (North America vs other) and medication overuse (yes or no) for a 3-month double-blind treatment phase.
Standard protocol approvals, registrations, and patient consents
The study was approved by an independent ethics committee or local institutional review board at each participating site. Written informed consent was obtained from all enrolled participants. The study was conducted in accordance with the International Conference on Harmonisation Tripartite Guideline on Good Clinical Practice. Clinicaltrials.gov identifier: NCT02066415.
Outcome measures
Migraine-Specific Quality-of-Life Questionnaire
The Migraine-Specific Quality-of-Life Questionnaire (MSQ) is a self-administered, migraine-specific, 14-item instrument assessment of quality of life that was developed to assess the effect of migraine on daily functioning across 3 domains.11,12 The role function-restrictive (MSQ-RFR) domain measures the effect of migraine on daily social and work-related activities, the role function-preventive (MSQ-RFP) domain assesses whether migraine prevents the individual from performing these activities, and the emotional functioning (MSQ-EF) domain measures emotions associated with migraine. Items are rated on a 6-point scale (none of the time, a little bit of the time, some of the time, a good bit of the time, most of the time, and all of the time). Raw domain scores are summed and transformed to a 100-point scale with higher scores indicating better quality of life and increased scores consistent with improvement. The between-group minimally important difference (MID) for MSQ-RFR is 3.2, for MSQ-RFP is 4.6, and for MSQ-EF is 7.5.13 For within-group analyses for each participant's change with responder analyses, the MID is 5.0 for MSQ-RFR, 5.0–7.9 for MSQ-RFP, and 8.0–10.6 for MSQ-EF.13 In the clinical trial, the MSQ was completed at baseline and then every 4 weeks during the study using an eDiary.
Headache Impact Test–6
The Headache Impact Test–6 (HIT-6) is a short-form, self-administered questionnaire developed as a global measure of adverse headache impact to assess headache severity using widely measured, functionally relevant domains: pain, social and role limitations, cognitive functioning, vitality, and psychological distress.14,15 The 6 questions are scored 6, 8, 10, 11, or 13 points based on 5 response categories that assess how often headaches interfere with activities or cause distress (never, rarely, sometimes, very often, or always). Scores for all 6 items are summed to produce a total HIT-6 score (range: 36–78), interpreted as little or no impact (49 or less), some impact (50–55), substantial impact (56–59), and severe impact (60–78) due to headache with higher scores indicating greater impact and decreased scores consistent with improvement. The between-group MID for HIT-6 in CM is 2.3 points16 and the within-patient MID for HIT-6 is a score reduction of 5.0 points.17 The recall period for 3 questions is 1 month and the other 3 do not have a specific recall period. The HIT-6 was completed every 4 weeks during the study using an eDiary.
Migraine Disability Assessment test
The Migraine Disability Assessment (MIDAS) test is a 5-item self-administered questionnaire that sums the number of productive days lost over the past 3 months in the workplace and assesses disability in family, social, and leisure activities at home (e.g., How many days in the last 3 months was the patient at least 50% disabled at work, home, school, or recreational activities due to migraine?).18 In addition to a total score, there are subdomains of absenteeism (missed days, attributable to a headache, from paid work, housework, and nonwork activities) and presenteeism (days at paid work or housework in which productivity was reduced by at least half). MIDAS scores are interpreted as grade I = 0–5 (minimal or infrequent disability), grade II = 6–10 (mild or infrequent disability), grade III = 11–20 (moderate disability), grade IVa = 21–40 and higher (severe disability), grade IVb = 41 and higher (very severe disability) with higher scores indicating greater disability and decreased scores consistent with improvement. Although no MID has been established for MIDAS, a preliminary analysis based on an anchor of 25% change in monthly headache days estimated that an increase or decrease of 5 days of migraine-related disability per 3 months represents meaningful within-patient change.19 MIDAS was completed at the end of the 3-month treatment period using an eDiary.
Pain Interference Scale short form 6b
The Patient-Reported Outcomes Measurement Information System (PROMIS) Pain Interference Scale short form 6b is a 6-item instrument that measures the level of pain interference on enjoyment of life, ability to concentrate, day-to-day activities, enjoyment of recreational activities, doing activities away from home, and socializing with others.20 Each question is scored on a 5-point scale, summed, and translated into a t score or standardized score with a mean of 50 (average for the US general population) and an SD of 10. The transformed PROMIS Pain Interference 6b short form t scores range from 41 to 78.3 with higher scores indicating greater interference and decreased scores consistent with improvement. No MID has been established for the PROMIS Pain Interference Scale for patients with migraine. The recall period used was the past 7 days, and participants completed the PROMIS Pain Interference Scale short form weekly during the study using the eDiary.
Statistical analysis
Descriptive analyses were conducted reporting the change from baseline and proportion of patients achieving clinically relevant changes from baseline. Descriptive comparisons between erenumab groups and placebo are reported as differences in change from baseline or odds ratios, respectively. Changes from baseline in PRO scores were preplanned exploratory endpoints. Dichotomized endpoints were post hoc exploratory endpoints. Differences in MSQ, HIT-6, and PROMIS scores were assessed based on a generalized linear mixed model including treatment, visit, treatment by visit interaction, stratification factors (region and medication overuse), and baseline value as covariates and assuming a first-order autoregressive covariance structure. There was no imputation for missing data. Differences in MIDAS scores were assessed based on an analysis of covariance model including treatment, stratification factors (region and medication overuse), and baseline value as covariates. MSQ subdomains and HIT-6 were dichotomized based on respective MIDs (MSQ-RFR ≥5, MSQ-RFP ≥5, MSQ-EF ≥8, HIT-6 ≥5), PROMIS was dichotomized based on 1 SD worse than the average US general population (≥60), and MIDAS was dichotomized based on severe (≥21) and very severe (≥41) ratings. The randomization analysis set included all participants who were randomized in the study. The efficacy analysis set included participants who received ≥1 dose of randomized treatment and completed at least one postbaseline monthly eDiary measurement. No formal hypothesis was tested. The p values for the difference between erenumab dose groups and placebo were not adjusted for multiple comparisons.
Data availability
Qualified researchers may request data from Amgen clinical studies. Complete details are available at amgen.com/datasharing.
Results
Participants and baseline
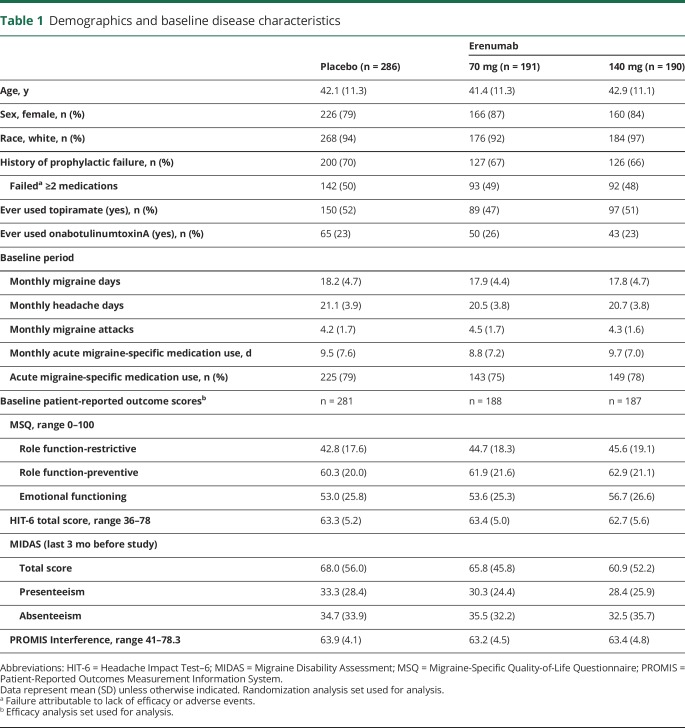
Of 667 participants randomized, 83% were female with a mean (SD) age of 42 (11) years (table 1). Mean duration since first migraine diagnosis was 22 (12) years and participants had a mean 18.0 (4.6) monthly migraine days and 20.8 (3.9) monthly headache days during the baseline period (table 1). Baseline PRO scores, which were similar among treatment groups, were reflective of the severe effect of migraine (table 1).
Table 1.
Demographics and baseline disease characteristics
Changes in PRO score over time
MSQ scores
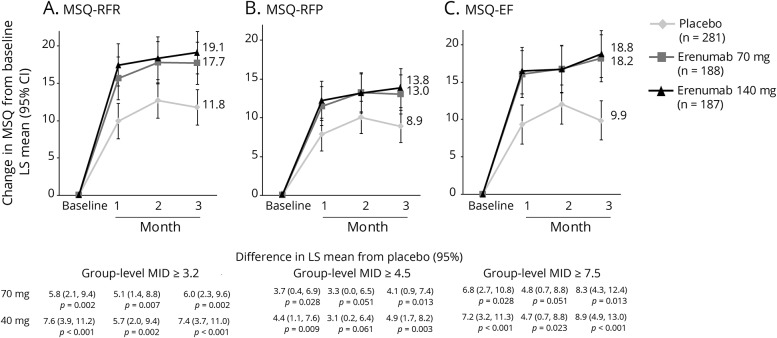
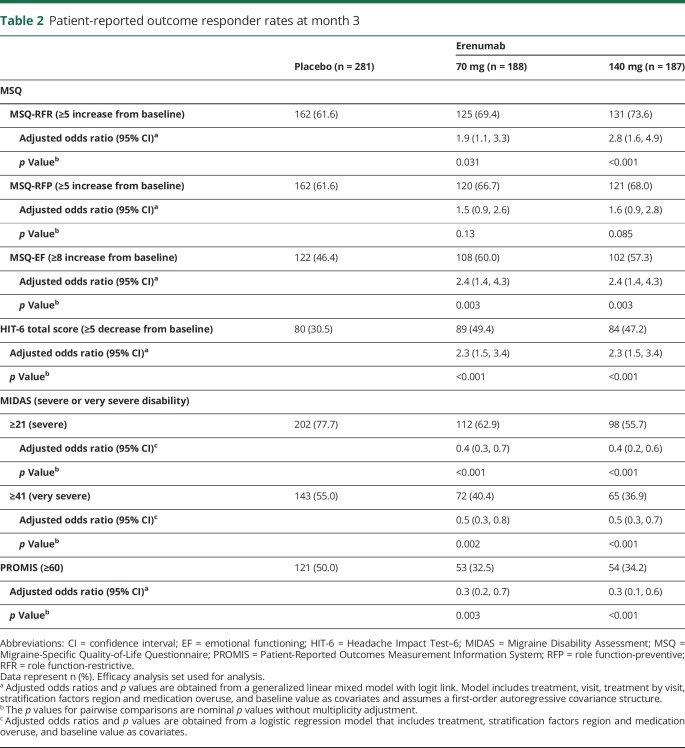
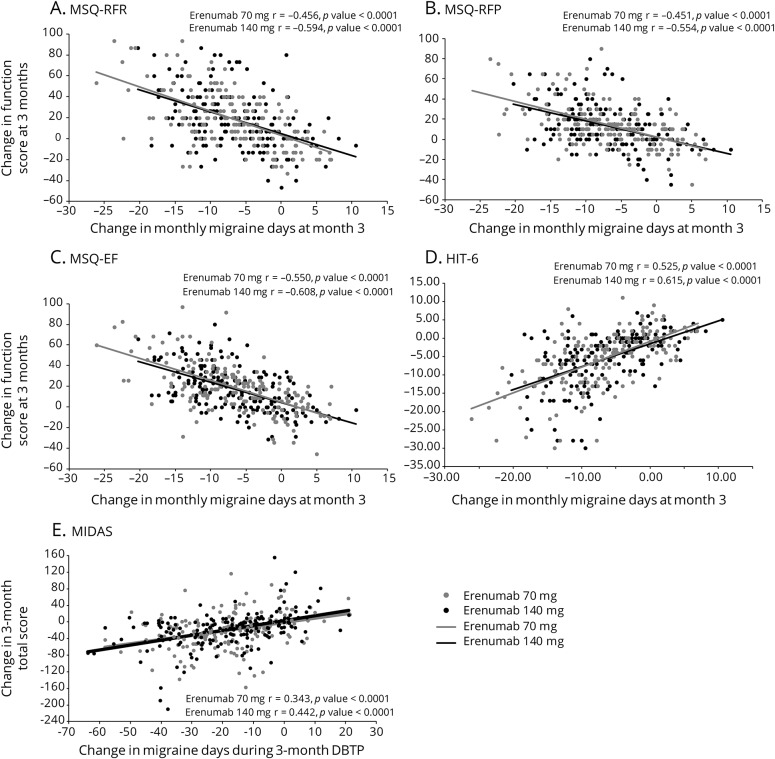
MSQ scores at baseline were similar among treatment groups and were indicative of substantial limitations to daily social and work-related activities with scores of approximately 45, 60, and 53 for MSQ-RFR, MSQ-RFP, and MSQ-EF, respectively (table 1). At month 3, increases from baseline (improvements) in all 3 MSQ domain scores were greater in the erenumab groups compared with placebo (figure 1). The least-squares mean (95% confidence interval [CI]) changes from baseline in MSQ-RFR were 17.7 (14.9, 20.6) for 70 mg and 19.1 (16.3, 22.0) for 140 mg vs 11.8 (9.4, 14.1) for placebo. Respective changes for MSQ-RFP were 13.0 (10.5, 15.6) and 13.8 (11.3, 16.4) vs 8.9 (6.8, 11.0) and for MSQ-EF were 18.2 (15.0, 21.3) and 18.8 (15.6, 21.9) vs 9.9 (7.3, 12.5). These differences from placebo were observed at the earliest time point assessed, month 1, and were observed throughout the double-blind period (figure 1). At month 3, the differences between the erenumab groups and placebo exceeded the established group-level MID for all MSQ domains for both doses except for MSQ-RFP with the erenumab 70-mg group (figure 1). At month 3, a greater proportion of participants in the erenumab group than in the placebo group had changes in MSQ scores that exceeded the within-patient MID for all 3 subdomains (table 2). There were moderately negative linear relationships between change in MSQ scores and change in monthly migraine days (MMD) (correlation coefficient r values ranged from −0.61 to −0.45 in the active treatment groups) that were significantly different from zero (p < 0.001) (figures 4, A–C).
Figure 1. Change in Migraine-Specific Quality-of-Life Questionnaire (MSQ) scores from baseline over 3 months.
Least-squares (LS) mean changes from baseline in (A) MSQ–role function-restrictive (MSQ-RFR), (B) MSQ–role function-preventive (MSQ-RFP), and (C) MSQ–emotional functioning (MSQ-EF) scores among participants with chronic migraine who were assigned to receive erenumab 70 mg, erenumab 140 mg, or placebo every month. The error bars represent 95% confidence intervals (CIs). Figure based on the efficacy analysis set. All p values ≤0.05, except for MSQ-RFP at month 2: erenumab 70 mg, p = 0.051, and erenumab 140 mg, p = 0.061. MID = minimally important difference.
Table 2.
Patient-reported outcome responder rates at month 3
Figure 4. Scatterplots of correlation between change in migraine days and change in patient-reported outcome scores.
(A) Migraine-Specific Quality-of-Life Questionnaire–role function-restrictive (MSQ-RFR), (B) Migraine-Specific Quality-of-Life Questionnaire–role function-preventive (MSQ-RFP), (C) Migraine-Specific Quality-of-Life Questionnaire–emotional functioning (MSQ-EF), (D) Headache Impact Test–6 (HIT-6), and (E) Migraine Disability Assessment (MIDAS).
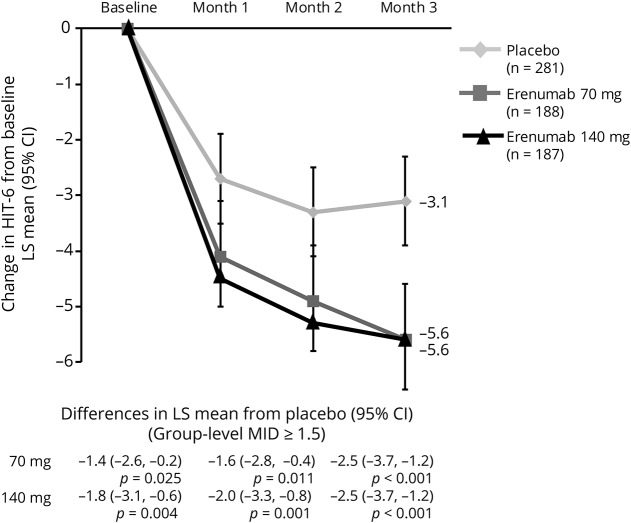
HIT-6 scores
Mean total HIT-6 score at baseline, similar among treatment groups, was approximately 63 (table 1), reflective of severe impact of headaches (>60). At month 3, mean (SE) HIT-6 scores were 57.6 (0.5) for 70 mg and 57.0 (0.6) for 140 mg vs 60.2 (0.4) for placebo and reflected greater changes from baseline. The change from baseline in total HIT-6 score was greater in the erenumab groups than in placebo as early as month 1 and was sustained throughout the double-blind period (figure 2). The least-squares mean (95% CI) changes from baseline at month 3 in HIT-6 were −5.6 (−6.5, −4.6) for both 70 mg and 140 mg vs −3.1 (−3.9, −2.3) for placebo. The between-group HIT-6 MID (≥1.5 points) was achieved at months 2 and 3 for both doses and by month 1 for 140 mg erenumab. A greater proportion of erenumab-treated participants than placebo achieved the within-patient MID for HIT-6 (score reduction of ≥5 points) at month 3, indicating improvement (table 2). There was a moderately positively linear relationship between change in HIT-6 score and change in MMD (correlation coefficient r values ranged from 0.53 to 0.62 in the active treatment groups) that were significantly away from zero (p < 0.001) (figure 4D).
Figure 2. Change in Headache Impact Test–6 (HIT-6) total score from baseline over 3 months.
Least-squares (LS) mean changes from baseline in HIT-6 scores among participants with chronic migraine who were assigned to receive erenumab 70 mg, erenumab 140 mg, or placebo every month. The error bars represent 95% confidence intervals (CIs). Figure based on the efficacy analysis set. All p values ≤0.05. MID = minimally important difference.
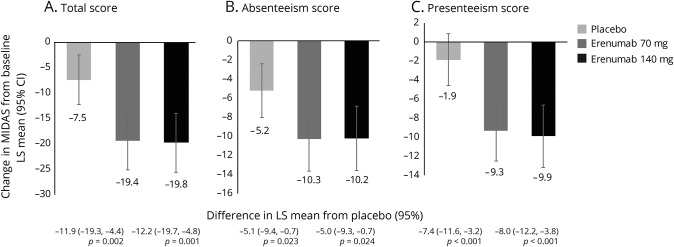
MIDAS scores
MIDAS scores at baseline indicated severe disability; at baseline, 81.9% of participants had MIDAS scores of ≥21 (severe disability) and 61.4% had scores of ≥41 (very severe disability). Reductions from baseline to month 3 in MIDAS total, absenteeism, and presenteeism scores were greater in the erenumab groups as compared with placebo, indicating better improvement (figure 3). The least-squares mean (95% CI) changes from baseline in MIDAS total scores were −19.4 (−25.2, −13.6) for 70 mg and −19.8 (−25.6, −14.0) for 140 mg vs −7.5 (−12.4, −2.7) for placebo. Respective changes for absenteeism were −10.3 (−13.6, −6.9) and −10.2 (−13.6, −6.8) vs −5.2 (−8.0, −2.4) and for presenteeism were −9.3 (−12.6, −6.1) and −9.9 (−13.2, −6.7) vs −1.9 (−4.7, 0.8). At month 3, a lower proportion of participants in the erenumab groups had MIDAS scores ≥21 compared with placebo (table 2). Likewise, the proportion of participants with MIDAS scores ≥41 was lower in the erenumab groups than in the placebo group (table 2). There was a moderately positive linear relationship between change in MIDAS score and change in total migraine days over the 12-week double-blind treatment phase (correlation coefficient r values ranged from 0.34 to 0.44 in the active treatment groups) that were significantly away from zero (p < 0.001) (figure 4E).
Figure 3. Change in Migraine Disability Assessment (MIDAS) scores from baseline to month 3.
Least-squares (LS) mean changes from baseline in (A) MIDAS total, (B) absenteeism, and (C) presenteeism scores among participants with chronic migraine who were assigned to receive erenumab 70 mg, erenumab 140 mg, or placebo every month. The error bars represent 95% confidence intervals (CIs). Figure based on the efficacy analysis set. All p values ≤0.05.
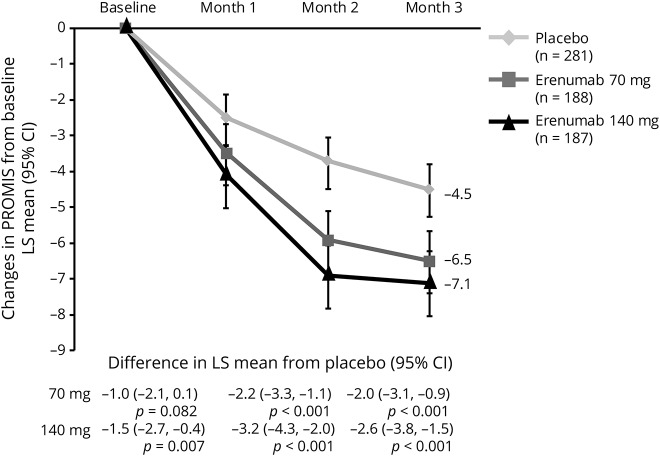
PROMIS scores
PROMIS scores at baseline (mean 63.4) suggested participants had pain interference >1 SD worse than the average US general population. At month 3, decreases in PROMIS scores were greater (better) in the erenumab groups as compared with placebo (figure 5). The least-squares mean (95% CI) changes from baseline in PROMIS scores were −6.5 (−7.4, −5.6) for 70 mg and −7.1 (−8.0, −6.2) for 140 mg vs −4.5 (−5.2, −3.8) for placebo. The response to erenumab 140-mg dosage was observed as early as month 1. At month 3, 50.0% of participants in the placebo group, compared with 32.5% and 34.2% of erenumab 70 and 140 mg, had PROMIS scores ≥60 (table 2).
Figure 5. Change in Patient-Reported Outcomes Measurement Information System (PROMIS) scores from baseline over 3 months.
Least-squares (LS) mean changes from baseline in PROMIS scores among participants with chronic migraine who were assigned to receive erenumab 70 mg, erenumab 140 mg, or placebo every month. The error bars represent 95% confidence intervals (CIs). Figure based on the efficacy analysis set. All p values ≤0.01, except for erenumab 70 mg at month 1, p = 0.082.
Discussion
CM is a severely disabling disease associated with poor HRQoL as a result of headache, associated symptoms, and comorbidities.1,4,6 Currently, there are few evidenced-based treatment options that patients can use to manage CM.
Erenumab is effective in reducing monthly migraine days in patients with CM.10 Both erenumab 70 and 140 mg reduced monthly migraine days by 6.6 days compared with 4.2 days for placebo (mean treatment difference −2.5).10 PROs are designed to capture the effect of migraine beyond simple counts of migraine days and can be used to more completely represent the patient perspective and experience. PROs provide context to more fully characterize the effect of illness and the benefits of treatment.
HIT-6 and MIDAS, the most frequently used PRO instruments in headache studies, measure headache impact and migraine-specific disability. HIT-6 assesses headache severity by determining how often headaches interfere with activities or cause distress, whereas MIDAS measures the number of productive days lost, and the MSQ assesses the effect of migraine on daily functioning. The mean total HIT-6 score of approximately 63 is reflective of the severe impact of headaches (>60), consistent with the 61% of patients with MIDAS scores ≥41 (very severe disability). Likewise, the baseline MSQ scores were reflective of substantial impairments in migraine-specific quality of life. In contrast to the headache-specific measures, PROMIS is a more generic instrument, which measures pain interference and allows for comparison with other disease states and the general population. PROMIS scores at baseline (mean 63.4) suggested that participants had pain interference >1 SD worse than the average US general population. Although each of the PRO measures different aspects of patient well-being, the convergence of the results indicated how severely CM affected these patients and similar to findings of prior CM studies.16,21
The PRO results from this pivotal study showed consistent benefits of erenumab with improvement across a broad set of PROs measuring a range of complementary outcomes. The observed treatment effect on these outcomes exceeded the established clinically meaningful between-group minimum difference for all measures with established MIDs. Moreover, with one exception (i.e., MSQ-RFP), significantly larger percentages of participants achieved clinically meaningful improvements in all measures.16 Compared with patients who reported no change, patients who reported feeling “somewhat better” had HIT-6 scores differing by 2.3 points, which was considered evidence of clinically meaningful improvement.16 This threshold was achieved at months 2 and 3 for both doses and by month 1 for 140 mg erenumab. Similarly, differences from baseline in MIDAS total score were >5 days, indicative of a clinically meaningful change.19 These treatment benefits emerged early, detected at the first 1-month posttreatment assessment (the earliest time point measured). Treatment benefits were sustained through the 3-month double-blind phase.
Although improvements in PRO scores correlated with reduction in migraine frequency with erenumab treatment, the proportion of patients achieving an MID with the PROs in this analysis was higher than the proportion of patients achieving a ≥50% reduction in MMD. The 50% threshold (e.g., for reduction in migraine or headache days) is an arbitrary way to dichotomize clinical response/nonresponse, and although widely used in practice and as a clinical trial endpoint, it has been shown that response at a 30% threshold can be clinically meaningful for patients with CM.22 Achievement of a 50% response has no direct relationship to the MIDs of PROs, which are established to identify a clinically meaningful change on specific attributes of migraine. This is illustrated by the onabotulinum toxin A data in the PREEMPT (Phase 3 Research Evaluating Migraine Prophylaxis Therapy) studies in which only 27% of treated patients had both a ≥5-point reduction in HIT-6 score and a ≥50% reduction in headache days. Furthermore, the different PROs have entirely different scales, measure different domains relevant to patients with migraine, and the MIDs are specific to a given PRO; i.e., clinical relevance for the domain(s) tested is independent of other PROs, such that for an individual patient it is not surprising that the relative change on one PRO might be different than the change on a second or third PRO, which measure different facets of relevance to patients. In addition, the within-patient differences for the PROs were estimated based on a variety of methods (e.g., anchor and distribution-based analyses). Therefore, one would not expect a direct relationship between MMD ≥50% responder rates and PRO MIDs. In a preliminary assessment of the relationship between change in PRO score and change in MMD, we found that there was a moderate correlation between change in MMD for each PRO with correlation coefficient r values of approximately +0.5 or −0.5 for all the PROs. However, between-subject variation is also observed based on the scatterplots. Any comparison of PRO “response” with percent migraine reduction in an individual patient will depend on what is most meaningful to a patient. Thus, the quantitative relationship between the level of change in migraine frequency and the level of change in PROs requires a more detailed and nuanced analysis. Overall, these data demonstrate that erenumab yields reductions in migraine frequency that are accompanied by improvements in ability to perform daily activities and reductions in feelings of hopelessness and/or frustration from the effect of CM. Further studies are required to determine the relationship between the level of change in migraine frequency and the level of change in the PROs to confirm whether the improvement in PROs is mediated by a reduction in headache days.
Clinical trials of parenteral pain treatments consistently report high placebo rates. Similar to the high placebo response seen for the primary and secondary endpoints in the main study, the placebo response rates for the PRO endpoints in this analysis were high. However, even with these large placebo responses, the between-group differences were still clinically relevant.
In previous studies of prevention treatments for CM, topiramate consistently showed significant reductions in MMD, but the effect on HRQoL has been mixed. In one study, MIDAS scores did not significantly improve. MSQ scores were initially improved, but the benefit diminished over time.23 A separate study of topiramate found no between-group differences for MSQ or HIT-6.24 The PREEMPT and PREEMPT 2 studies showed that onabotulinumtoxinA reduced headache days at week 24 by 8.4 days and migraine days by 8.2 days compared to 6.6 and 6.2 days, respectively, in the placebo group.25 These changes in headache frequency were paralleled by clinically meaningful treatment differences in total HIT-6 scores (approximately −2.5) and all 3 MSQ domains (MSQ-RFR: 17.0 vs 8.6; MSQ-RFP: 13.1 vs 6.4; MSQ-EF: 17.9 vs 9.5).25,26 Results from these different studies reveal that improvements in clinical outcomes (e.g., reductions in MMD) are not necessarily linked to improvements in patient functioning or HRQoL. Some medications may reduce MMD but produce side effects that offset the benefits measured by PROs.
This large placebo-controlled study demonstrated consistent treatment effects across multiple measures. These effects may be accounted for by the combination of efficacy and tolerability erenumab delivers. However, the results may be limited by the short duration of the study relative to the time that patients will remain on treatment in the real world. Further trials are needed to determine the long-term safety of erenumab and the durability of its effects. Heightened expectations arising from the promise of CGRP targeted therapies and the increased clinical attention in the trial, could lead to overestimation of treatment effects in comparison with real-world treatment. These results are unlikely to differentially influence response to active drug and placebo, however.
Overall, these results demonstrate that erenumab led to sustained, significant improvements that are consistent across multiple measures of HRQoL, social and psychological effect, and disability in patients with CM. A comparison of this number of PRO instruments has not been done in such detail in other migraine preventive trials.
This study provides Class II evidence that erenumab improves HRQoL, headache impact, and disability in patients with CM.
Glossary
- CGRP
calcitonin gene-related peptide
- CI
confidence interval
- CM
chronic migraine
- EF
emotional functioning
- HIT-6
Headache Impact Test–6
- HRQoL
health-related quality of life
- MID
minimally important difference
- MIDAS
Migraine Disability Assessment
- MMD
monthly migraine days
- MSQ
Migraine-Specific Quality-of-Life Questionnaire
- PREEMPT
Phase 3 Research Evaluating Migraine Prophylaxis Therapy
- PRO
patient-reported outcome
- PROMIS
Patient-Reported Outcomes Measurement Information System
- RFP
role function-preventive
- RFR
role function-restrictive
Footnotes
Class of Evidence: NPub.org/coe
CME Course: NPub.org/cmelist
Author contributions
R.B.L., S.J.T., U.R., S.S., W.F.S., D.K.L., P.D., S.C., D.D.M., R.A.L. interpreted the data and revised the manuscript for content; J.N. wrote the first draft of the manuscript based on an outline developed with all co-authors and revised the manuscript for content.
Study funding
This study was fully funded by Amgen. Erenumab is codeveloped in partnership with Amgen and Novartis.
Disclosure
R. Lipton declared consultant fees, honoraria, and/or research grants from Alder, Allergan, Inc., Amgen, Avanir, Biohaven, Dr. Reddy's Laboratories, eNeura, electroCore, Lilly, Novartis, Teva, and Trigemina. He has stock options in eNeura and Biohaven. U. Reuter declared consulting fees from Allergan, Amgen, Autonomic Technologies, Eli Lilly and Co., Novartis, CoLucid, Teva; speaking/teaching fees from Amgen, Novartis, CoLucid; Pharm Allergan, TEVA advisory board for Amgen, Autonomic Technologies, Novartis, Pharm Allergan, TEVA. S. Tepper declared research grants (no personal compensation) from Alder, Allergan, Amgen, ATI, Avanir, Dr. Reddy's, electroCore, eNeura, Scion NeuroStim, Teva, Zosano; consultant and/or advisory board for Acorda, Alder, Alexza, Allergan, Amgen, ATI, Avanir, Axsome, Charleston Laboratories, Dr. Reddy's, electroCore, eNeura, Eli Lilly, GLG, Guidepoint Global, Kimberly-Clark, Novartis, Pernix, Pfizer, Scion NeuroStim, Supernus, Teva, Zosano; stock option from ATI; salary from Dartmouth-Hitchcock Medical Center, American Headache Society; royalties from Springer. S. Silberstein declared consultant/ad board fees from Alder Biopharmaceuticals, Allergan, Inc., Amgen, Avanir Pharmaceuticals, Inc., Curelator, Inc., Dr. Reddy's Laboratories, eNeura Inc., electroCore Medical, LLC, Lilly USA, LLC, Medscape, LLC, NINDS, Supernus Pharmaceuticals, Inc., Teva Pharmaceuticals, Theranica, and Trigemina, Inc. W. Stewart declared consultant fees from Allergan and Amgen Inc. J. Nilsen is an employee and stockholder of Amgen Inc. D. Leonardi was an employee of and held stock/stock options in Amgen Inc. at the time of the study. P. Desai is an employee and stockholder of Amgen Inc. S. Cheng is an employee and stockholder of Amgen Inc. D. Mikol is an employee and stockholder of Amgen Inc. R. Lenz is an employee and stockholder of Amgen Inc. Go to Neurology.org/N for full disclosures.
References
- 1.Stovner LJ, Hagen K, Jensen R, et al. The global burden of headache: a documentation of headache prevalence and disability worldwide. Cephalalgia 2007;27:193–210. [DOI] [PubMed] [Google Scholar]
- 2.Lipton RB, Manack Adams A, Buse DC, Fanning KM, Reed ML. A comparison of the Chronic Migraine Epidemiology and Outcomes (CaMEO) Study and American Migraine Prevalence and Prevention (AMPP) Study: demographics and headache-related disability. Headache 2016;56:1280–1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bebbington P. The World Health Report 2001. Soc Psychiatry Psychiatr Epidemiol 2001;36:473–474. [DOI] [PubMed] [Google Scholar]
- 4.Buse DC, Manack AN, Fanning KM, et al. Chronic migraine prevalence, disability, and sociodemographic factors: results from the American Migraine Prevalence and Prevention Study. Headache 2012;52:1456–1470. [DOI] [PubMed] [Google Scholar]
- 5.Steiner TJ, Stovner LJ, Birbeck GL. Migraine: the seventh disabler. Cephalalgia 2013;33:289–290. [DOI] [PubMed] [Google Scholar]
- 6.Blumenfeld AM, Varon SF, Wilcox TK, et al. Disability, HRQoL and resource use among chronic and episodic migraineurs: results from the International Burden of Migraine Study (IBMS). Cephalalgia 2011;31:301–315. [DOI] [PubMed] [Google Scholar]
- 7.Serrano D, Manack AN, Reed ML, Buse DC, Varon SF, Lipton RB. Cost and predictors of lost productive time in chronic migraine and episodic migraine: results from the American Migraine Prevalence and Prevention (AMPP) Study. Value Health 2013;16:31–38. [DOI] [PubMed] [Google Scholar]
- 8.Holland S, Silberstein SD, Freitag F, Dodick DW, Argoff C, Ashman E. Evidence-based guideline update: NSAIDs and other complementary treatments for episodic migraine prevention in adults: report of the Quality Standards Subcommittee of the American Academy of Neurology and the American Headache Society. Neurology 2012;78:1346–1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aimovig (Erenumab-aooe) [US Package Insert]. Thousand Oaks: Amgen Inc.; 2018. [Google Scholar]
- 10.Tepper S, Ashina M, Reuter U, et al. Safety and efficacy of erenumab for preventive treatment of chronic migraine: a randomised, double-blind, placebo-controlled phase 2 trial. Lancet Neurol 2017;16:425–434. [DOI] [PubMed] [Google Scholar]
- 11.Jhingran P, Davis SM, LaVange LM, Miller DW, Helms RW. MSQ: Migraine-Specific Quality-of-Life Questionnaire: further investigation of the factor structure. Pharmacoeconomics 1998;13:707–717. [DOI] [PubMed] [Google Scholar]
- 12.Jhingran P, Osterhaus JT, Osterhaus JT, Lee JT, Kirchdoerfer L. Development and validation of the Migraine-Specific Quality of Life Questionnaire. Headache 1998;38:295–302. [DOI] [PubMed] [Google Scholar]
- 13.Cole JC, Lin P, Rupnow MF. Minimal important differences in the Migraine-Specific Quality of Life Questionnaire (MSQ) version. Cephalalgia 2009;29:1180–1187. [DOI] [PubMed] [Google Scholar]
- 14.Dowson AJ. Assessing the impact of migraine. Curr Med Res Opin 2001;17:298–309. [PubMed] [Google Scholar]
- 15.Kosinski M, Bayliss MS, Bjorner JB, et al. A six-item short-form survey for measuring headache impact: the HIT-6. Qual Life Res 2003;12:963–974. [DOI] [PubMed] [Google Scholar]
- 16.Coeytaux RR, Kaufman JS, Chao R, Mann JD, Devellis RF. Four methods of estimating the minimal important difference score were compared to establish a clinically significant change in Headache Impact Test. J Clin Epidemiol 2006;59:374–380. [DOI] [PubMed] [Google Scholar]
- 17.Yang M, Rendas-Baum R, Varon SF, Kosinski M. Validation of the Headache Impact Test (HIT-6™) across episodic and chronic migraine. Cephalalgia 2011;31:357–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stewart WF, Lipton RB, Dowson AJ, Sawyer J. Development and testing of the Migraine Disability Assessment (MIDAS) Questionnaire to assess headache-related disability. Neurology 2001;56:S20–S28. [DOI] [PubMed] [Google Scholar]
- 19.Lipton R, Desai P, Sapra S, Buse D, Fanning K, Reed M. How Much Change in Headache-Related Disability Is Clinically Meaningful? Estimating Minimally Important Difference (MID) or Change in MIDAS Using Data from the AMPP Study [Abstract]. Boston: American Headache Society Annual Meeting; 2017. [Google Scholar]
- 20.Amtmann D, Cook KF, Jensen MP, et al. Development of a PROMIS item bank to measure pain interference. Pain 2010;150:173–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lipton RB, Varon SF, Grosberg B, et al. OnabotulinumtoxinA improves quality of life and reduces impact of chronic migraine. Neurology 2011;77:1465–1472. [DOI] [PubMed] [Google Scholar]
- 22.Silberstein S, Tfelt-Hansen P, Dodick DW, et al. Guidelines for controlled trials of prophylactic treatment of chronic migraine in adults. Cephalalgia 2008;28:484–495. [DOI] [PubMed] [Google Scholar]
- 23.Dodick DW, Silberstein S, Saper J, et al. The impact of topiramate on health-related quality of life indicators in chronic migraine. Headache 2007;47:1398–1408. [DOI] [PubMed] [Google Scholar]
- 24.Diener HC, Bussone G, Oene JCV, Lahaye M, Schwalen S, Goadsby PJ. Topiramate reduces headache days in chronic migraine: a randomized, double-blind, placebo-controlled study. Cephalalgia 2007;27:814–823. [DOI] [PubMed] [Google Scholar]
- 25.Dodick DW, Turkel CC, DeGryse RE, et al. OnabotulinumtoxinA for treatment of chronic migraine: pooled results from the double-blind, randomized, placebo-controlled phases of the PREEMPT clinical program. Headache 2010;50:921–936. [DOI] [PubMed] [Google Scholar]
- 26.Diener HC, Dodick DW, Aurora SK, et al. OnabotulinumtoxinA for treatment of chronic migraine: results from the double-blind, randomized, placebo-controlled phase of the PREEMPT 2 trial. Cephalalgia 2010;30:804–814. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Qualified researchers may request data from Amgen clinical studies. Complete details are available at amgen.com/datasharing.