Abstract
Roundup is a popular herbicide containing glyphosate as an active ingredient. The formulation of Roundup is speculated to have critical toxic effects, one among which is chronic inflammation. The present study analyzed adverse inflammatory effects in the liver and adipose tissue of rats after a subacute exposure of Roundup. Adult male rats were exposed to various doses of Roundup (0, 5, 10, 25, 50, 100 and 250 mg/kg bodyweight [bw] glyphosate) orally, everyday for 14 days. On day 15, liver and adipose tissues from dosed rats were analyzed for inflammation markers. C-reactive protein in liver, cytokines IL-1β, TNF-α, IL-6, and inflammatory response marker, and prostaglandin–endoperoxide synthase were upregulated in liver and adipose of rats exposed to higher (100 and 250 mg/kg bw/d) doses of Roundup. Cumulatively, our data suggest development of inflammation in lipid and hepatic organs upon exposure to Roundup. Furthermore, liver histological studies showed formation of vacuoles, fibroid tissue, and glycogen depletion in the groups treated with doses of higher Roundup. These observations suggest progression of fatty liver disease in Roundup-treated adult rats. In summary, our data suggest progression of multiorgan inflammation, liver scarring, and dysfunction post short-term exposure of Roundup in adult male rats.
Keywords: glyphosate, Roundup, liver, adipose tissue, inflammation, non-alcoholic fatty liver disease
Introduction
The exposure to xenobiotics is increasing every day,1 leading to several health complications, including chronic inflammation.2-4 Inflammation may in turn lead to metabolic disorders5,6 such as fatty liver,7-9 irregular adipose tissue development,10,11 and obesity.12 Systematic as well as hepatic inflammation is involved in the pathogenesis of nonalcoholic fatty liver disease and steatohepatitis.13 Nonalcoholic fatty liver disease includes liver dysfunction ranging from the simple accumulation of fat known as fatty liver to an advanced stage of cirrhosis, non-alcoholic steatohepatitis (NASH), and liver fibrosis.14 The systemic inflammation is also associated with various types of cancer.15,16
During inflammatory reactions, blood flow and permeability of capillaries increases, and recruitment of neutrophils occurs in the target tissues. The migrated macrophages secrete proinflammatory cytokines, interleukin-1β (IL-1β), tumor necrosis factor-α (TNF-α), and interleukin-6 (IL-6), which initiate inflammatory reactions in the target tissue such as the increased production of cytokines, alteration in endothelial gene expression, chemotaxis, leukocyte adherence, and activation of fibroblasts.17,18 C-reactive protein (CRP) is an acute inflammatory protein secreted by the target tissue as a result of inflammatory processes such as IL-6 and TNF-α secretion and in host–pathogen responses.19 Inflammation also leads to the production of prostaglandin at the reaction site by mast cells which is regulated by cyclooxygenase 2 or prostaglandin–endoperoxide synthase (PTGS) gene involved mainly in vasodilation20-22 and results in the activation of nuclear factor κB in target tissues.23
Glyphosate or N-(phosphonomethyl) glycine-based herbicide, Roundup, is the most commonly used, nonselective, broad-spectrum weed control chemical formulation.24 Roundup is used in agricultural fields, gardens, playgrounds, and roadsides. According to the USDA’s Agricultural Research Service Glyphosate fact sheet 1999, the half-life of glyphosate varies from 2 to 174 days in soil and up to 90 days in water. Glyphosate and its metabolite aminomethyl-phosphonic acid have been detected in water and crops.25 Notably, the commercial formulation Roundup has been reported to have more adverse effect than the active ingredient glyphosate alone.26,27 Study with various cell lines suggested that the major adjuvant polyethoxylated tallowamine was more toxic compared to other components of Roundup.28 Therefore, toxicology studies are recommended to be designed with the complete formulation of Roundup.
There are very few in vivo studies describing the inflammatory effect of Roundup. One study reported systemic inflammation and its damaging effects on brain and renal tissue of rats post herbicide exposure.29,30 In another report, lung inflammation was studied leading to signs of asthma in a murine model.31 Recent reports on long-term exposure of Roundup in liver dysfunction in female rats30,32 have been discussed in relation to aging-related complexity. There are no reports presenting adverse inflammatory effects for a short-term Roundup exposure in the liver and adipose tissue. The liver and adipose tissue are major metabolic organs involved in energy homeostasis. Health problems resulting from abrupt energy homeostasis such as obesity and diabetes are of much concern. It warrants study on plausible effects of xenobiotics to metabolic organs. To our knowledge, the current study is the only one that aims to analyze inflammatory response toward subacute exposure of Roundup in metabolic tissues such as liver and adipose.
Materials and Methods
Reagents
Herbicide Roundup (41% w/w glyphosate) was procured from Monsanto India Ltd (Mumbai, India). TRIzol, custom-made primers, oligo(dT), dNTPs, Trichrome Stain (Masson) kit, Schiff reagent, and Periodic acid–Schiff were purchased from Sigma-Aldrich Co (Bangalore, India). DyNAmo ColorFlash SYBR Green quantitative polymerase chain reaction (qPCR) Kit was purchased from ThermoFisher Scientific (Waltham, Massachusetts). Reverse transcriptase (EasyScript) was obtained from Applied Biological Materials Inc (Richmond, Canada). DNase 1 (RNase-free) was from New England Biolabs Inc (Ipswich, Massachusetts). Picro Sirius Red Stain Kit was from Abcam (Cambridge, United Kingdom). All other chemicals, unless otherwise noted, were purchased from Sigma-Aldrich Co (Bangalore, India) or sourced locally.
Animal Experiments
All experimental procedure and housing management of rats were approved by the Institutional Animal Ethical Committee, Indian Institute of Science (Bangalore, India). Adult male rats (2 to 2.5 months age) were housed in a temperature-controlled environment of 25° to 26°C with a photoperiod cycle of 12-hour light, 12-hour darkness. Rats had ad libitum access to food and water. One rat was housed per cage. For this study, rats were randomly divided into 7 groups with 5 or more rats per group. Rats were administered with 5, 10, 25, 50, 100, and 250 mg/kg bodyweight (bw)/d of glyphosate, and the unexposed group (ie, 0 mg/kg bw/d) received 300 μL of deionized water. The treatments were administered orally and once daily (08:00-09:00 hours) for 14 days. Each component of Roundup formulation in the highest dose tested was set well below lethal dose 50 (LD50; 4900 mg/kg bw) of glyphosate for rodents. As short-term exposure effects could also be due to components other than glyphosate (eg, surfactants) present in Roundup formulation, which are not disclosed by the manufacturer, the experiment readouts for each group were designated with formulation trade name Roundup and the doses calculated based on its active ingredient glyphosate. On day 15 of treatment, rats were anesthetized with 50 mg/kg bw pentobarbitone sodium (Sigma Chemical Co, St Louis, Missouri). The rats were killed by cervical dislocation, and liver and gonadal adipose tissues were dissected out. Tissues were transferred to neutral-buffered formaldehyde (NBF) solution or snap frozen in liquid nitrogen and stored in −70° freezer until analyzed.
Liver Index Calculation
On day 15 of the experiment, body weight was determined, and liver tissue weight was measured post dissection. Tissue index for liver was calculated using following formula for each rat33:
Quantitative Polymerase Chain Reaction Analysis
Total RNA was isolated from tissues by TRIzol reagent according to the manufacturer’s protocol. The quality and quantity of RNA samples were examined spectrophotometrically using ND-1000 NanoDrop, Thermo Scientific (Delaware). The analysis of RNA spectrophotometrically at optical density A260: A280 consistently provided a ratio >1.8. The quality and quantity of RNA were verified by electrophoresis on a 1% (w/v) formaldehyde agarose gel along with RNA samples of good quality and known concentration. RNA was stored in 0.1% diethyl pyrocarbonate (DEPC)-treated water at −70°C until further use. Total RNA of 1 μg was converted into complementary DNA (cDNA) by EasyScript Reverse Transcriptase (Transgene biotech., China) with oligo(dT), post DNase I treatment. Real-time PCR was carried out using gene-specific primers, and details are provided in Table 1. The gene-specific primers were designed using exon junction sequence of gene transcripts retrieved from the National Centre for Biotechnology Information, and primer parameters were tested with primer express software v2.0, Applied Biosystems (California). The qPCR was set up using 10 ng of cDNA, Power DyNAmo ColorFlash SYBR Green qPCR Kit (Applied Biosystems, CA), and 12.5 pM/μL primer on an Applied Biosystems 7500 Real-time PCR machine following standard protocol. The reaction mixtures were incubated at 95° for 5 minutes, followed by 40 cycles of 95° for 30 seconds, and 60° for 30 seconds. Samples were always assayed in duplicates. Cycle threshold (Ct) values were obtained, and dCt values were generated by subtracting reference (REF) gene Ct value from gene of interest Ct value. The housekeeping gene, rpl19 is REF gene. The dCt (dCT) values were plotted for each group utilizing ABI PRISM 7500 software (California). The lowered slope of the graph represents upregulation in the gene expression.
Table 1.
List of Primers used in the Expression Analysis.a
| Gene | NCBI Gene ID | Sequence | Annealing Temperature, °C | Amplicon, bp |
|---|---|---|---|---|
| RPL 19 | 81767 | F: CTGTAACCAGGTCGAATGTCAC R: GGGTAGTTCGGGTTCACCC |
62 | 214 |
| IL-1β | 24494 | F: AACATAAGCCAACAAGTGGTA R: TTGGGATCCACACTCTCC |
60 | 162 |
| TNF-α | 24835 | F: CCAGACCCTCACACTCAGA R: GGTACCACCAGTTGGTTG |
60 | 165 |
| IL-6 | 24498 | F: CTTCCAGCCAGTTGCCTTC R: TGGTCTGTTGTGGGTGGTAT |
60 | 109 |
| CRP | 25419 | F: TGGTCATGAAGACATGTCTAA R: ACAGTGAAGGCTTCCAGTG |
60 | 108 |
| PTGS | 29527 | F: TGACTGTACCCGGACTGGATTC R: GTGATCTGGACGTCAACACG |
60 | 197 |
Abbreviations: CRP, C-reactive protein; F, forward primer; IL, interleukin; PTGS, prostaglandin–endoperoxide synthase; R, reverse primer; TNF, tumor necrosis factor.
aProvided are NCBI gene ID for each of the genes, primer sequences, annealing temperature and product size.
Histopathology
The liver tissues isolated from the Roundup and vehicle-treated animal groups were fixed in 4% NBF (Fine-chem, Mumbai, India) until further use. The tissues were dehydrated in ethanol solutions of decreasing concentration, cleared in xylene, and embedded in paraffin (Sigma-Aldrich, Bangalore, India). Five-micron thick sections were cut and deparaffinized by xylene (Fine-chem, Mumbai, India). Series of increased concentration of ethanol solutions were used to rehydrate the tissue section. Sections were washed with distilled sterile water before staining.
For hematoxylin and eosin (H&E) staining, sections were stained with hematoxylin and counterstained with eosin (Nice chemicals, Kerala, India) and mounted in DPX [AW; Please provide the expansion for DPX.] (Sigma-Aldrich, Bangalore, India) as described elsewhere.34 Images were captured under Olympus IX81inverted light microscope (Olympus, Tokyo, Japan).
Periodic acid–Schiff staining of tissues was conducted as per protocol reported elsewhere.35 In short, sections were hydrated and oxidized in acid, washed under running water followed by addition of Shiff reagent to the slides. The slides were washed with running water and counterstained with Mayer haematoxylin, dehydrated and mounted in DPX (Sigma-Aldrich). Sections were visualized under a light microscope (Olympus IX81inverted light microscope).
Masson trichrome staining was conducted as reported previously.36 In brief, rehydrated and washed liver sections were stained with Biebrich scarlet-acid fuchsin solution which was differentiated in phosphomolybdic–phosphotungstic acid solution. Sections were further stained with aniline blue solution, differentiated in 1% acetic acid solution, and counterstained with Weigert iron hematoxylin. The slides were dehydrated through ascending concentration grades of ethanol and cleared in xylene and mounted in DPX (Sigma-Aldrich). Sections were visualized under Olympus IX81inverted light microscope.
The Sirius red staining was performed as per the manufacturer’s protocol. In brief, deparaffinized sections were hydrated in distilled water. Sections were submerged into Picro-Sirius Red solution and incubated for 60 minutes. Slides were differentiated in acetic acid solution and dehydrated before mounting in DPX (Sigma-Aldrich). Sections were visualized under crossed polars utilizing polarized light microscope from Olympus, BX51 (Tokyo, Japan).
Statistical Analysis
Data values are expressed as mean ± standard error of mean. The data were graphed and analyzed using Prism Graph pad software, version 2 (California). A value of P < .05 was considered statistically significant. Analysis between groups was done by Kruskal-Wallis test.
Results
Effect of Roundup Treatment on Liver Index
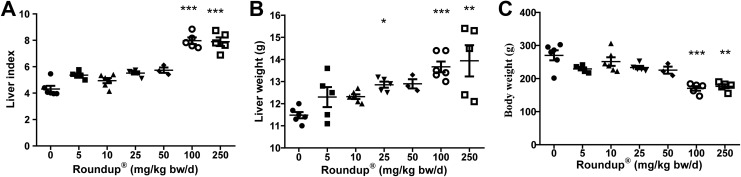
Tissue index is calculated as a primary step33 toward assessing the effects of xenobiotics in any tissue. There was a significant difference (P < .001) observed in the tissue index from rats dosed with 5 mg/kg bw/d of Roundup when compared to vehicle-treated rats (Figure 1A). At higher doses, that is, 100 mg/kg bw/d of Roundup, body weight was significantly lower compared to vehicle-treated group which indicates systemic effects of Roundup (Figure 1C). Also, a trend of weight increase in liver tissue was observed in a dose-dependent manner (Figure 1B).
Figure 1.
Liver index (A), liver weight (B), and body weight (C) in the different treatment groups. Rats were provided with ad libitum access to food during experiments. Vehicle group received milli-Q water and other groups administered with various doses of Roundup for 14 days daily. On day 15, body and liver weights were measured, and index was calculated with formula provided in the Material and Methods section. Values are mean ± SEM (n = 5). Statistical significance from vehicle group was determined by one-way ANOVA followed by Kruskall-Wallis test. *P < .05, **P < .01, ***P < .001. SEM indicates standard error of mean; ANOVA, analysis of variance.
Effect of Roundup on Cytokine Expression in Liver and Adipose Tissue
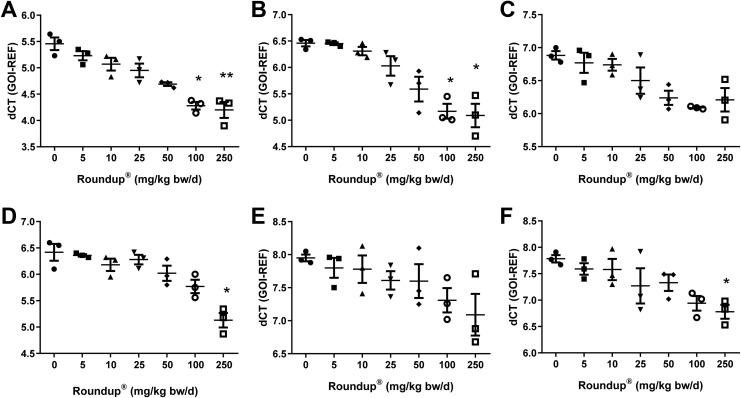
Cytokine expression was elevated in liver tissue of all adult male rats exposed to various doses of Roundup. There was a dose-dependent increase observed in IL-1β and TNF-α which was significantly high at doses 100 and 250 mg/kg bw/d of Roundup when compared to the vehicle group (P < .05; Figure 2A and B). On the other hand, IL-6 expression in liver tissue increased from 25 mg/kg bw/d dose of Roundup exposure when compared to vehicle-treated rats (Figure 2C).
Figure 2.
Expression analysis of cytokines in rat liver and adipose tissue with various doses of Roundup treatment. Total RNA was isolated from liver and adipose tissue post-Roundup treatment and converted to cDNA. The cDNA was utilized to perform qPCR to examine (A and D) IL-1β, (B and E) TNF-α, and (C and F) IL-6 cytokines levels in liver and adipose tissue viz. Values are mean ± SEM (n = 3). Statistical significance of gene expression when compared to vehicle group was determined by Kruskal-Wallis test. *, **, significantly different by P < .05 and .01 viz. SEM indicates standard error of mean; cDNA, complementary DNA; IL, interleukin; TNF-α, tumor necrosis factor α; qPCR, quantitative polymerase chain reaction.
In adipose tissues, IL-1β expression decreased and was significantly lower for dose 250 mg/kg bw/d and higher when compared to vehicle-treated rats (P < .05; Figure 2D). The TNF-α had a nonsignificant trend of upregulation with a dose of 5 to 50 mg/kg bw/d of Roundup in a dose-dependent fashion (Figure 2E). Although, IL-6 had high adipose tissue expression from dose of 25 mg/kg bw/d of Roundup, there was significant increase in 250 mg/kg bw/d when compared to vehicle-treated rats (P < .05; Figure 2F).
Effect of Roundup on PTGS in Liver
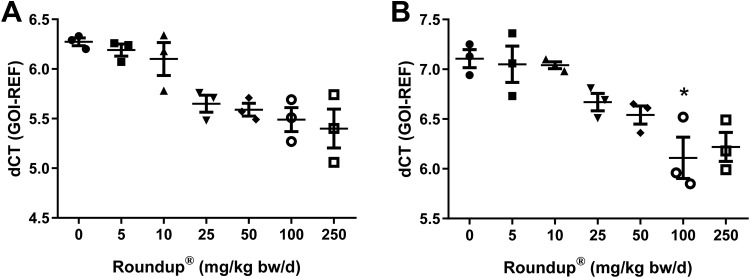
Prostaglandin–endoperoxide synthase or cyclooxygenase 2 is an enzyme that is a key regulator of prostaglandin formation. Prostaglandins are involved in vasodilation in target tissue during inflammatory response that improves blood flow and macrophage recruitment to the inflamed tissue. Expression of PTGS was analyzed in the liver tissues of rats exposed to different doses of Roundup. There was an increased level of PTGS expression from dose 50 mg/kg bw/d dose when compared to vehicle-treated group (Figure 3A). In adipose tissue, however, PTGS expression was found to be unchanged upto dose 10 mg/kg bw/d of Roundup when compared to vehicle-treated group (Figure 3B). Levels of PTGS show a significant increase at the dose 100 mg/kg bw/d of Roundup (P < .05; Figure 3B).
Figure 3.
Effects of Roundup on PTGS expression in liver and adipose tissue. The liver and adipose tissue total RNA was extracted from rats administered with various doses of Roundup and vehicle. cDNA was prepared and qPCR was performed to determine PTGS levels. Values are presented as mean ± SEM (n = 3). Statistical significance of gene expression as compared to the vehicle-treated group was calculated by Kruskall-Wallis test. *significantly different by P < .05. SEM indicates standard error of mean; cDNA, complementary DNA; qPCR, quantitative polymerase chain reaction; PTGS, prostaglandin-endoperoxide synthase.
Effect of Roundup on Liver CRP
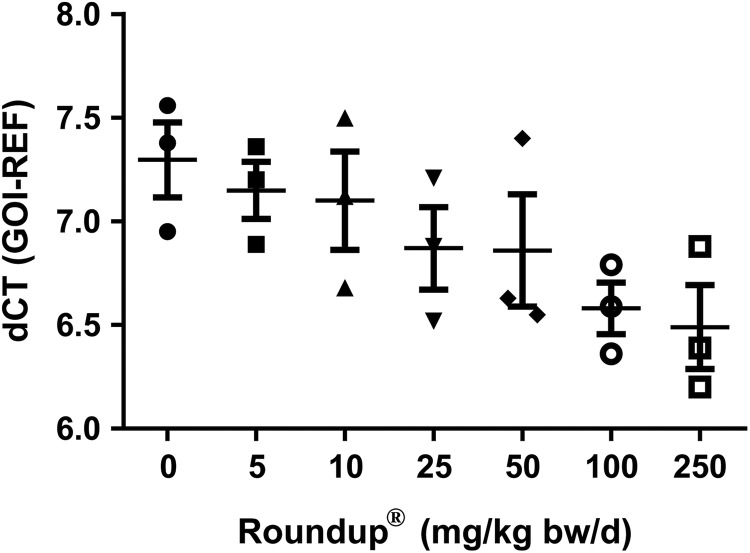
The CRP is secreted by liver tissue in response to cytokines and adipokines released from macrophages and adipose tissue during inflammation. It binds to phosphatidylcholine which is expressed on the surface of dead cells to activate the complement system to improve phagocyte action. The liver had an increasing trend of CRP expression post-Roundup treatment when compared to vehicle-treated group (Figure 4).
Figure 4.
Effects of Roundup on CRP expression in liver. The liver tissue was collected at the end of various doses exposure of Roundup from adult male rats. Total RNA was isolated, converted into cDNA and qPCR was performed to determine CRP levels. Values are represented as mean ± SEM (n = 3). Statistical significance between vehicle and treatment groups was determined by Kruskall-Wallis test. SEM indicates standard error of mean; cDNA, complementary DNA; qPCR, quantitative polymerase chain reaction; CRP, C-reactive protein.
Effect of Roundup on Histopathological Changes and Glycogen Depletion
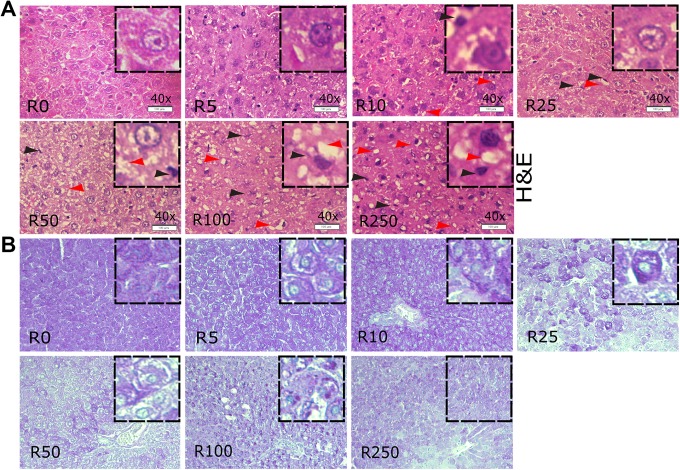
The histopathological changes in the liver were examined with H&E staining. There were a higher number of macrophages at dose 50 mg/kg bw/d of Roundup and higher when compared to the vehicle-treated group (Figure 5A). A moderate microvesicular steatosis, ballooning degeneration was observed at dose 50 mg/kg bw/d when compared to vehicle-treated group (Figure 5A). At higher doses such as 100 and 250 mg/kg bw/d of Roundup, rat liver cells contain severe micro-vesicular and macro-vesicular steatosis, vacuolation as well as higher number of macrophages compared to vehicle group rats (Figure 5A).
Figure 5.
Roundup exposure, histopathological changes and glycogen content in rat liver. (A) hematoxylin and eosin (H&E) stained and (B) Periodic acid–Schiff (PAS) stained a transverse section of liver from vehicle-treated and different doses of Roundup-administered rats. Tissues were collected and fixed post completion of various doses of Roundup treatment. Tissue sections stained with H&E as well as with PAS and images are presented at ×200 magnifications. Hepatocyte nuclei are round and located inside the cells, while macrophages nuclei are present in interstitial spaces and indicated with a black arrow head in the H&E-stained tissue images. White patches around the nuclei depict lipid vesicles present inside the hepatocytes, indicated with red arrowheads (A). In the PAS-stained sections, glycogen stains with purple–magenta color, while nuclei were counterstained to deep blue–purple color with hematoxylin (B). Images are representative of 3 runs, and each group consists of 3 rats per treatment of Roundup.
To analyze liver glycogen accumulation due to herbicide exposure, PAS staining was performed. It was observed that from dose 25 mg/kg bw/d of Roundup onward uniform glycogen storage was absent, and sparely distributed glycogen aggregates were noted. A depletion of glycogen content was spotted in the liver of adult rats exposed to dose 250 mg/ kg w/d as compared to the vehicle-treated group. Overall, liver sections presented a sign of steatohepatitis characterized by the presence of inflammation and cell injury, that is, hepatocyte ballooning, chronic inflammatory cell infiltrates with multiple vesicle formations with liver dysfunction in the form of uneven, and absent glycogen storage.
Effect of Roundup on Fibroid Formation at Higher Doses
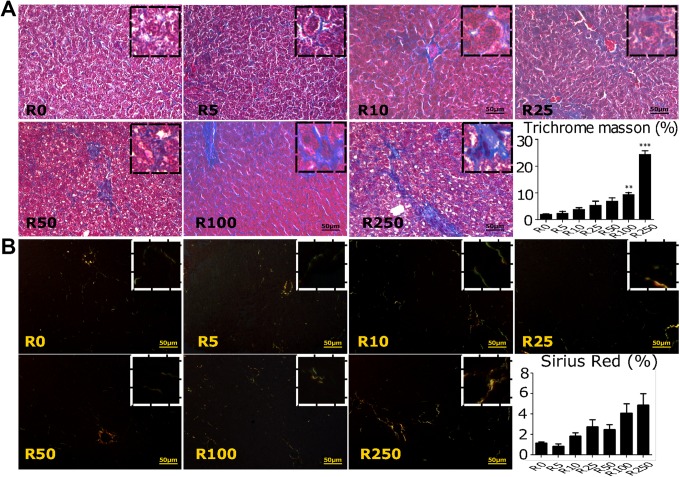
Liver tissue samples were also analyzed for fibroid formation in Roundup-treated male rats. The MTS staining revealed a significant increase (P < .01) in fibrosis at higher doses of 100 and 250 mg/kg bw/d of Roundup when compared to vehicle-treated group (Figure 6A). The rat group treated with 5 and 10 mg/kg bw/d of Roundup presented mild perisinusoidal fibrosis. Groups exposed to 25 and 50 mg/kg bw/d of Roundup had moderate signs of fibrosis mainly perisinusoidal, periportal, and centrilobular. The higher doses of Roundup, that is, 100 and 250 mg/kg bw/d Roundup had severe bridging fibrosis. Sirius red stain for examining collagen accumulation in the liver sample from Roundup-treated groups had similar findings as from MTS (Figure 6B). Collagen accumulation was higher in a high dose of Roundup treatment group such as 250 mg/kg bw/d of Roundup compared to the vehicle-treated group. Therefore, it was found that the high doses, that is, 100 and 250 mg/kg bw/d of Roundup caused scarring or fibrosis in liver tissue due to a progression of hepatosteatosis.
Figure 6.
Effect of Roundup on liver fibrosis. Transverse section of liver obtained from male rats exposed to various doses of Roundup was stained with (A) Masson trichrome stain (TMS) and (B) Sirius red. Tissues were collected and fixed in formalin post-Roundup treatments. Tissue sections are stained with TMS and Sirius red with standard staining methods, and images are presented at ×200. Images are representative of 3 runs. In the TMS-stained sections, connective tissue stains blue, nuclei are stained dark red/purple, and cytoplasm is stained red/pink (A). Sirius red–stained sections, under a polarized light, present the collagen fibers as yellow–orange with finest fibers (eg, reticular fibers) as green (B). ImageJ software was utilized to calculate stained area, and values are presented as graph via GraphPad software, arranged after each staining panels. Statistical significance between groups was calculated by Kruskall-Wallis test. **, and ***significantly different by P < .01 and .001.
Discussion
The current study describes for the first time the effects of a subacute exposure of Roundup in developing multi-organ inflammation and non-alcoholic fatty liver (NAFL) condition. After a 2-week exposure of herbicide doses well below LD50, increased expression levels of inflammatory markers were seen in adipose and liver tissues of treated rats. Liver tissue showed signs of developing glycogen storage imbalance and fibrosis at higher doses. Taken together, the report presents dose-dependent adverse inflammatory effects of short-term exposure of Roundup on the liver and adipose tissues of treated rats.
The liver index was altered in Roundup-treated rats with doses as low as 5 mg/kg bw/d indicative of preliminary harmful effects of Roundup exposure. Further, liver histology revealed severe damages to the tissue due to Roundup exposure. Microvesicle formation was distinctly observed at doses 50 mg/kg bw/d Roundup and higher, indicating signs of liver steatosis or NAFL. Non-alcoholic fatty liver is the most common form of chronic liver disease and is indicative of systemic metabolic syndromes.37 When fat accumulates in the liver, it can cause liver inflammation, functional impairment, fibrosis, and hepatocellular carcinoma. The condition is known as NASH.14 Steatosis in NAFL usually has macro-vesicular steatosis (large droplet steatosis) in which large vacuoles of fat fills up the hepatocyte and displaces the nucleus to the periphery. Often macro-vesicular steatosis is related to progression to fibrosis or cirrhosis of the liver. Micro-vesicular steatosis is characterized by distended hepatocytes with foamy-appearing cytoplasm and small lipid vesicles (less than 1 µm in diameter) may or may not be discernible. The nucleus is typically centrally located unlike in macro-vesicular steatosis, where the nucleus is displaced peripherally. Micro-vesicular steatosis corelates with liver failure due to severe mitochondrial β-oxidation defects caused by exposure to toxins.38,39 In our study, the formation of micro- and macro-vesicules was observed in higher doses of 100 and 250 mg/kg bw/d of Roundup. Fibrosis, one of the end stages of various liver diseases, developed in a dose-dependent fashion. Adult male rats exposed to doses higher than 10 mg/kg bw/d of Roundup had increased fibrosis with dramatic increase at 100 and 250 mg/kg bw/d. Glycogen depletion was also observed in the liver of male rats exposed to 25 mg/ kg bw/d and higher doses of Roundup and is an indicator of late stages of fibrosis or cirrhosis.40 Taken together, our data indicate a gradual decrease in the functional efficiency of the liver post short-term treatment of the common herbicide, Roundup.
Liver fibrosis occurs in response to inflammation with increased cytokine production leading to activation of many pathways that further raise the expression of TNF-α and IL-6.41 Thus, we wanted to look at the inflammatory response to short-term exposure to Roundup in adult male rats. We found that, inflammatory cytokines levels were upregulated in liver tissues of male rats treated with 50 mg/kg w/d and higher doses of Roundup. C-reactive protein is an acute-phase reactant protein, mainly produced by the liver during inflammation. There is strong evidence to suggest a correlation between increased CRP levels and NAFL. Targher et al found high CRP levels in patients with fatty liver,42 while Nigham et al observed a statistically significant association between different grades of fatty liver and CRP levels found in serum.43 Similar correlations between inflamed liver and CRP levels were observed in other studies, too.44-46 We also found liver CRP expression to be increased in a dose-dependent fashion in Roundup-treated rats. In summary, the overall liver data of the present study suggest liver tissue to be structurally deformed, inflamed, and fibroidal following short-term exposure to Roundup.
The mechanism of NAFL development is not fully understood, but it is suggested that adipose tissue dysfunction is associated with the development of fatty liver.47 White adipose tissue along with being the storage depot of energy in the form of fat is also an important endocrine organ that synthesizes and secretes various adipokines or cytokines such as TNF-α, IL-6, and so on.48 Adipose tissue inflammation is reported to be an important link that explains the relationship between obesity and hepatic steatosis.49-51 Studies have shown that the recruitment of macrophages to adipose tissue subsequent to inflammation contributes to hepatic lipid accumulation.52-54 In the present study, adipose tissues of male rats exposed to varying doses of the popular herbicide Roundup and expressed increased levels of cytokines TNF-α, IL-6, and IL-1β postexposure in a dose-dependent fashion. It was observed that cytokine levels in adipose tissue increased at higher doses, starting at 100 mg/kg bw/d of Roundup, coinciding with a clear indication of fatty liver development in those rats. A study with the active ingredient glyphosate has also presented elevation in liver cytokines in rats.55 Prostaglandin–endoperoxide synthase is crucial in inflammatory responses, as it mediates inflammation which is in turn related to increases in tissue mass and fatty liver.56 Prostaglandin–endoperoxide synthase has been the main target of other external chemicals that initiate an inflammatory response.57 We found an upregulation of PTGS gene expression in adipose tissues of treated rats starting at a low dose of 25 mg/kg bw/d of Roundup, indicating an early initiation of inflammation in the adipose tissue. The mechanism behind adipose tissue inflammation causing the development of NAFL is yet to be examined post herbicide exposure.
The liver is a vital organ involved in the metabolism and biotransformation of xenobiotics.58 Eventually, due to inflammation and fibrosis, steatosis or NAFL degenerates into NASH and liver function declines. Thus, increase in the incidence of NAFL is of great concern.59,60 Metabolomics and proteomic studies reported signs of liver dysfunction and hepatic lipid accumulation in adult61 and in pregnant rats.62 Another group also suggested a fatty liver condition development in female rats with a long-term exposure of Roundup,32 based on a multi-omics study. The present study adds to the current understanding of the adverse effects of herbicides on liver and adipose tissues by reporting an increase in inflammation and fibrosis of liver with a subacute exposure of Roundup. Thus, studies such as the present one are relevant and essential addition to the human health-care system.
A report presented that xenobiotics can activate the hypothalamic–pituitary–adrenal axis in rats and increases plasma levels of the glucocorticoid corticosterone.63 Glucocorticoids are principal molecules involved in the control of inflammation. Corticosterone levels are reported to be higher in the case of chronic inflammations64,65 in rodents. A previous publication from the current group reported an increase in glucocorticoid level per g body weight (data not presented) in the serum of male rats exposed to dose 100 and 250 mg/ kg bw/d of Roundup.34 Our present study reiterates this finding as we found that when rats were exposed to 100 and 250 mg/kg bw/d of Roundup for 2 weeks, it elevated glucocorticoids, apart from causing metabolic organ inflammation and liver dysfunction.
In conclusion, the present study suggests that short-term exposure of Roundup causes multi-organ inflammation and has adverse effects on metabolic tissues such as liver and adipose. In future, a detailed analysis would be required to understand the mechanism behind the triggering of inflammation in liver, fat, and other tissues, and its role in the progression of NAFL upon herbicide exposure.
The presence of Roundup has been reported in various natural resources that make toxicological studies,66 such as the current one imperative to understand the short-term effects of these herbicides on human health. Roundup has effects on various organ systems altering physiology such as endocrine,34 reproduction,67,68 urinary,69 and neuronal70 system. The current study discussed inflammatory effects of acute exposure to Roundup. Chronic inflammation is linked to insulin resistance, type 2 diabetes,71 and various cancer types.72 Therefore, studies such as the one presented provides information urging cautious usage of xenobiotics and are highly relevant to health care.
Acknowledgments
Authors are thankful to Dr Ruchir Patel from the Department of Pathology, Dr D.Y. Patil Medical College, Hospital & Research Centre, Pimpri, Pune, India for the expert comments on liver histology slides. Tissue sectioning and staining procedure were performed by Sapthagiri central laboratory, Bangalore, India. Macro utilized for image quantitation was provided by Dr Damir Krunic, DKFZ, Heidelberg, Germany. Authors acknowledge Prof. Rudraiah Medhamurthy from the Department of Molecular Reproduction, Development and Genetics of Indian Institute of Science, India for providing laboratory facility to conduct the research work as a part of AP’s graduation program. The authors would like to thank Dr Sujatha P. Koduvayur, Research Project Manager, Penn State Hershey Medical Center, for her valuable comments and edits to the manuscript. AP was supported by a senior research fellowship from the Council of Scientific and Industrial Research, New Delhi, India.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Aparamita Pandey  https://orcid.org/0000-0003-1510-9562
https://orcid.org/0000-0003-1510-9562
Reference
- 1. Stapleton PA, Minarchick VC, McCawley M, Knuckles TL, Nurkiewicz TR. Xenobiotic particle exposure and microvascular endpoints: a call to arms. Microcirculation. 2012;19(2):126–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Peuschel H, Sydlik U, Grether-Beck S, et al. Carbon nanoparticles induce ceramide- and lipid raft-dependent signalling in lung epithelial cells: a target for a preventive strategy against environmentally-induced lung inflammation. Part Fibre Toxicol. 2012;9:48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Waring RH, Harris RM, Hunter JO, Mitchell SC. Xenobiotic sulphation and its variability during inflammation: a factor in adverse drug reactions? Curr Drug Metab. 2013;14(3):361–365. [DOI] [PubMed] [Google Scholar]
- 4. Fedeli D, Montani M, Carloni M, Nasuti C, Amici A, Gabbianelli R. Leukocyte Nurr1 as peripheral biomarker of early-life environmental exposure to permethrin insecticide. Biomarkers. 2012;17(7):604–609. [DOI] [PubMed] [Google Scholar]
- 5. Zhou SS, Zhou YM, Li D, Lun YZ. Dietary methyl-consuming compounds and metabolic syndrome. Hypertens Res. 2011;34(12):1239–1245. [DOI] [PubMed] [Google Scholar]
- 6. Lee DH, Steffes MW, Jacobs DR., Jr Can persistent organic pollutants explain the association between serum gamma-glutamyltransferase and type 2 diabetes? Diabetologia. 2008;51(3):402–407. [DOI] [PubMed] [Google Scholar]
- 7. Woolbright BL, Jaeschke H. Xenobiotic and endobiotic mediated interactions between the cytochrome P450 system and the inflammatory response in the liver. Adv Pharmacol. 2015;74:131–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Zhang Y, Zhang Z, Zhao Y, Cheng S, Ren H. Identifying health effects of exposure to trichloroacetamide using transcriptomics and metabonomics in mice (Mus musculus). Environ Sci Technol. 2013;47(6):2918–2924. [DOI] [PubMed] [Google Scholar]
- 9. Osterreicher CH, Trauner M. Xenobiotic-induced liver injury and fibrosis. Expert Opin Drug Metab Toxicol. 2012;8(5):571–580. [DOI] [PubMed] [Google Scholar]
- 10. Garcia-Mayor RV, Larranaga Vidal A, Docet Caamano MF, Lafuente Gimenez A. Endocrine disruptors and obesity: obesogens [in Spanish]. Endocrinol Nutr. 2012;59(4):261–267. [DOI] [PubMed] [Google Scholar]
- 11. Barouki R. Can xenobiotics accumulated in adipose tissue contribute to a carcinogenic risk? [in French]. Ann Endocrinol. 2013;74(2):154–155. [DOI] [PubMed] [Google Scholar]
- 12. Thayer KA, Heindel JJ, Bucher JR, Gallo MA. Role of environmental chemicals in diabetes and obesity: a National Toxicology Program workshop review. Environ Health Perspect. 2012;120(6):779–789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gao B, Tsukamoto H. Inflammation in alcoholic and nonalcoholic fatty liver disease: friend or foe? Gastroenterology. 2016;150(8):1704–1709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Sergio C, González-Díaz V, Martínez-Bonilla G, et al. Non-alcoholic fatty steatohepatitis an inflammatory disorder beyond the liver. J Clin Cel Immunol. 2013;4(159):2155–9899. [Google Scholar]
- 15. Irigaray P, Newby JA, Lacomme S, Belpomme D. Overweight/obesity and cancer genesis: more than a biological link. Biomedicine & pharmacotherapy = Biomedecine & pharmacotherapie. 2007;61(10):665–678. [DOI] [PubMed] [Google Scholar]
- 16. Naik A, Belic A, Zanger UM, Rozman D. Molecular interactions between NAFLD and xenobiotic metabolism. Front Genet. 2013;4:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Arango Duque G, Descoteaux A. Macrophage cytokines: involvement in immunity and infectious diseases. Front Immunol. 2014;5:491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Slaats J, Ten Oever J, van de Veerdonk FL, Netea MG. IL-1beta/IL-6/CRP and IL-18/ferritin: Distinct Inflammatory Programs in Infections. PLoS Pathog. 2016;12(12):e1005973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sproston NR, Ashworth JJ. Role of C-reactive protein at sites of inflammation and infection. Front Immunol. 2018;9:754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ilangumaran S, Ferbeyre G. Editorial: cytokines in inflammation, aging, cancer and obesity. Cytokine. 2016;82:1–3. [DOI] [PubMed] [Google Scholar]
- 21. Harris SG, Padilla J, Koumas L, Ray D, Phipps RP. Prostaglandins as modulators of immunity. Trends Immunol. 2002;23(3):144–150. [DOI] [PubMed] [Google Scholar]
- 22. Theoharides TC, Alysandratos KD, Angelidou A, et al. Mast cells and inflammation. Biochimt Biophys Acta. 2012;1822(1):21–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Jiang CY, Wang W, Tang JX, Yuan ZR. The adipocytokine resistin stimulates the production of proinflammatory cytokines TNF-alpha and IL-6 in pancreatic acinar cells via NF-kappaB activation. J Endocrinol Invest. 2013;36(11):986–992. [DOI] [PubMed] [Google Scholar]
- 24. Heap I, Duke SO. Overview of glyphosate-resistant weeds worldwide. Pest Manag Sci. 2018;74(5):1040–1049. [DOI] [PubMed] [Google Scholar]
- 25. Cox C, Surgan M. Unidentified inert ingredients in pesticides: implications for human and environmental health. Environ Health Perspect. 2006;114(12):1803–1806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Benachour N, Seralini GE. Glyphosate formulations induce apoptosis and necrosis in human umbilical, embryonic, and placental cells. Chem Res Toxicol. 2009;22(1):97–105. [DOI] [PubMed] [Google Scholar]
- 27. Mesnage R, Defarge N, Spiroux de Vendomois J, Seralini GE. Potential toxic effects of glyphosate and its commercial formulations below regulatory limits. Food Chem Toxicol. 2015;84:133–153. [DOI] [PubMed] [Google Scholar]
- 28. Mesnage R, Bernay B, Seralini GE. Ethoxylated adjuvants of glyphosate-based herbicides are active principles of human cell toxicity. Toxicology. 2013;313(2-3):122–128. [DOI] [PubMed] [Google Scholar]
- 29. Astiz M, de Alaniz MJ, Marra CA. The oxidative damage and inflammation caused by pesticides are reverted by lipoic acid in rat brain. Neurochem Int. 2012;61(7):1231–1241. [DOI] [PubMed] [Google Scholar]
- 30. Mesnage R, Arno M, Costanzo M, Malatesta M, Seralini GE, Antoniou MN. Transcriptome profile analysis reflects rat liver and kidney damage following chronic ultra-low dose Roundup exposure. Environ Health. 2015;14:70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kumar S, Khodoun M, Kettleson EM, et al. Glyphosate-rich air samples induce IL-33, TSLP and generate IL-13 dependent airway inflammation. Toxicology. 2014;325:42–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Mesnage R, Renney G, Seralini GE, Ward M, Antoniou MN. Multiomics reveal non-alcoholic fatty liver disease in rats following chronic exposure to an ultra-low dose of Roundup herbicide. Sci Rep. 2017;7:39328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Bailey SA, Zidell RH, Perry RW. Relationships between organ weight and body/brain weight in the rat: what is the best analytical endpoint? Toxicol Pathol. 2004;32(4):448–466. [DOI] [PubMed] [Google Scholar]
- 34. Pandey A, Rudraiah M. Analysis of endocrine disruption effect of Roundup((R)) in adrenal gland of male rats. Toxicol Rep. 2015;2:1075–1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Qu D, Teckman JH, Perlmutter DH. Review: alpha 1-antitrypsin deficiency associated liver disease. J Gastroenterol Hepatol. 1997;12(5):404–416. [DOI] [PubMed] [Google Scholar]
- 36. Xia JL, Dai C, Michalopoulos GK, Liu Y. Hepatocyte growth factor attenuates liver fibrosis induced by bile duct ligation. Am J Pathol. 2006;168(5):1500–1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. McNear S, Harrison SA. Current status of therapy in nonalcoholic fatty liver disease. Therap Adv Gastroenterol. 2009;2(1):29–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Fromenty B, Berson A, Pessayre D. Microvesicular steatosis and steatohepatitis: role of mitochondrial dysfunction and lipid peroxidation. J Hepatol. 1997;26(Suppl 1):13–22. [DOI] [PubMed] [Google Scholar]
- 39. Galli SJ, Weintraub HP, Proppe KH. Malignant fibrous histiocytoma and pleomorphic sarcoma in association with medullary bone infarcts. Cancer. 1978;41(2):607–619. [DOI] [PubMed] [Google Scholar]
- 40. Krahenbuhl L, Lang C, Ludes S, et al. Reduced hepatic glycogen stores in patients with liver cirrhosis. Liver Int. 2003;23(2):101–109. [DOI] [PubMed] [Google Scholar]
- 41. Riquelme A, Arrese M, Soza A, et al. Non-alcoholic fatty liver disease and its association with obesity, insulin resistance and increased serum levels of C-reactive protein in Hispanics. Liver Int. 2009;29(1):82–88. [DOI] [PubMed] [Google Scholar]
- 42. Targher G, Bertolini L, Rodella S, et al. NASH predicts plasma inflammatory biomarkers independently of visceral fat in men. Obesity. 2008;16(6):1394–1399. [DOI] [PubMed] [Google Scholar]
- 43. Nigam P, Bhatt SP, Misra A, Vaidya M, Dasgupta J, Chadha DS. Non-alcoholic fatty liver disease is closely associated with sub-clinical inflammation: a case-control study on Asian Indians in North India. PloS One. 2013;8(1):e49286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Oruc N, Ozutemiz O, Yuce G, et al. Serum procalcitonin and CRP levels in non-alcoholic fatty liver disease: a case control study. BMC Gastroenterol. 2009;9:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Uchihara M, Izumi N. High-sensitivity C-reactive protein (hs-CRP): a promising biomarker for the screening of non-alcoholic steatohepatitis (NASH) [in Japanese]. Nihon Rinsho. Jpn J Clin Med. 2006;64(6):1133–1138. [PubMed] [Google Scholar]
- 46. Foroughi M, Maghsoudi Z, Khayyatzadeh S, Ghiasvand R, Askari G, Iraj B. Relationship between non-alcoholic fatty liver disease and inflammation in patients with non-alcoholic fatty liver. Adv Biomed Res. 2016;5:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Duval C, Thissen U, Keshtkar S, et al. Adipose tissue dysfunction signals progression of hepatic steatosis towards nonalcoholic steatohepatitis in C57BL/6 mice. Diabetes. 2010;59(12):3181–3191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Ouchi N, Parker JL, Lugus JJ, Walsh K. Adipokines in inflammation and metabolic disease. Nat Rev Immunol. 2011;11(2):85–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Olefsky JM, Glass CK. Macrophages, inflammation, and insulin resistance. Annu Rev Physiol. 2010;72:219–246. [DOI] [PubMed] [Google Scholar]
- 50. Kolak M, Westerbacka J, Velagapudi VR, et al. Adipose tissue inflammation and increased ceramide content characterize subjects with high liver fat content independent of obesity. Diabetes. 2007;56(8):1960–1968. [DOI] [PubMed] [Google Scholar]
- 51. Richard P. The role of adipose tissue in fatty liver disease. Liver Res. 2018;2(1):35–42. [Google Scholar]
- 52. Strissel KJ, Stancheva Z, Miyoshi H, et al. Adipocyte death, adipose tissue remodeling, and obesity complications. Diabetes. 2007;56(12):2910–2918. [DOI] [PubMed] [Google Scholar]
- 53. Murano I, Barbatelli G, Parisani V, et al. Dead adipocytes, detected as crown-like structures, are prevalent in visceral fat depots of genetically obese mice. J Lipid Res. 2008;49(7):1562–1568. [DOI] [PubMed] [Google Scholar]
- 54. Cinti S, Mitchell G, Barbatelli G, et al. Adipocyte death defines macrophage localization and function in adipose tissue of obese mice and humans. J Lipid Res. 2005;46(11):2347–2355. [DOI] [PubMed] [Google Scholar]
- 55. Tang J, Hu P, Li Y, Win-Shwe TT, Li C. Ion imbalance is involved in the mechanisms of liver oxidative damage in rats exposed to glyphosate. Front Physiol. 2017;8:1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Hsieh PS, Jin JS, Chiang CF, Chan PC, Chen CH, Shih KC. COX-2-mediated inflammation in fat is crucial for obesity-linked insulin resistance and fatty liver. Obesity. 2009;17(6):1150–1157. [DOI] [PubMed] [Google Scholar]
- 57. Thompson PA, Khatami M, Baglole CJ, et al. Environmental immune disruptors, inflammation and cancer risk. Carcinogenesis. 2015;36(Suppl 1):S232–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Strohmeyer G, Weik C. Liver damage caused by drugs [in German]. Z Gastroenterol. 1999;37(5):367–378. [PubMed] [Google Scholar]
- 59. Perumpail BJ, Khan MA, Yoo ER, Cholankeril G, Kim D, Ahmed A. Clinical epidemiology and disease burden of nonalcoholic fatty liver disease. World J Gastroenterol. 2017;23(47):8263–8276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Allen AM, Therneau TM, Larson JJ, Coward A, Somers VK, Kamath PS. Nonalcoholic fatty liver disease incidence and impact on metabolic burden and death: a 20 year-community study. Hepatology. 2018;67(5):1726–1736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Ford B, Bateman LA, Gutierrez-Palominos L, Park R, Nomura DK. Mapping proteome-wide targets of glyphosate in mice. Cell Chem Biol. 2017;24(2):133–140. [DOI] [PubMed] [Google Scholar]
- 62. Bonvallot N, Canlet C, Blas YEF, et al. Metabolome disruption of pregnant rats and their offspring resulting from repeated exposure to a pesticide mixture representative of environmental contamination in Brittany. PloS One. 2018;13(6):e0198448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Thomson EM, Pal S, Guenette J, et al. Ozone Inhalation provokes glucocorticoid-dependent and -independent effects on inflammatory and metabolic pathways. Toxicol Sci. 2016;152(1):17–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Wolff C, Krinner K, Schroeder JA, Straub RH. Inadequate corticosterone levels relative to arthritic inflammation are accompanied by altered mitochondria/cholesterol breakdown in adrenal cortex: a steroid-inhibiting role of IL-1beta in rats. Ann Rheum Dis. 2015;74(10):1890–1897. [DOI] [PubMed] [Google Scholar]
- 65. Landgraf MA, Silva RC, Correa-Costa M, et al. Leptin downregulates LPS-induced lung injury: role of corticosterone and insulin. Cell Physiol Biochem. 2014;33(3):835–846. [DOI] [PubMed] [Google Scholar]
- 66. Acquavella JF, Alexander BH, Mandel JS, et al. Glyphosate biomonitoring for farmers and their families: results from the Farm Family Exposure Study. Environ Health Perspect. 2004;112(3):321–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Owagboriaye FO, Dedeke GA, Ademolu KO, Olujimi OO, Ashidi JS, Adeyinka AA. Reproductive toxicity of Roundup herbicide exposure in male albino rat. Exp Toxicol Pathol. 2017;69(7):461–468. [DOI] [PubMed] [Google Scholar]
- 68. Ren X, Li R, Liu J, et al. Effects of glyphosate on the ovarian function of pregnant mice, the secretion of hormones and the sex ratio of their fetuses. Environ Pollut. 2018;243(Pt B):833–841. [DOI] [PubMed] [Google Scholar]
- 69. Dedeke GA, Owagboriaye FO, Ademolu KO, Olujimi OO, Aladesida AA. Comparative assessment on mechanism underlying renal toxicity of commercial formulation of roundup herbicide and glyphosate alone in male albino rat. Int J Toxicol. 2018;37(4):285–295. [DOI] [PubMed] [Google Scholar]
- 70. Martinez MA, Ares I, Rodriguez JL, Martinez M, Martinez-Larranaga MR, Anadon A. Neurotransmitter changes in rat brain regions following glyphosate exposure. Environ Res. 2018;161:212–219. [DOI] [PubMed] [Google Scholar]
- 71. Rehman K, Akash MS. Mechanisms of inflammatory responses and development of insulin resistance: how are they interlinked? J Biomed Sci. 2016;23(1):87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420(6917):860–867. [DOI] [PMC free article] [PubMed] [Google Scholar]