Abstract
Background
Studies about asymptomatic norovirus infections have been frequently reported. We aim to assess the global prevalence of asymptomatic infections.
Method
We identified publications that included the proportion of asymptomatic norovirus infections by searching in PubMed, Ovid, Scopus, and Web of Science and by screening references from the articles reviewed. The principal summary data were the prevalence of asymptomatic norovirus infection. Random-effect models for meta-analysis were fitted to generate estimates of overall and subgroup prevalence.
Findings
Of 81 studies included, asymptomatic norovirus prevalence was estimated at 7% (95% CI: 6%–9%). Africa, Meso America and South America had higher prevalence (15%, 14%, 11%, respectively) while the prevalence in Europe and North America was lower (4%). Prevalence was similar between community and hospital (9%). Prevalence was higher in children (8%) than adults (4%). For food handlers, prevalence was estimated at 3%. In context of outbreaks, prevalence estimated from 15 studies was as high as 18% (95% CI: 10%–30%).
Interpretation
This knowledge could have an impact on the development of transmission prevention strategies in the future. The high prevalence indicated asymptomatic individuals must not be overlooked.
Outstanding questions
The high prevalence indicated asymptomatic individuals must not be overlooked. Asymptomatic individuals may play an important role in norovirus transmission. This knowledge could have an impact on the development of transmission prevention strategies.
Keywords: Norovirus, Prevalence, Asymptomatic, Meta-analysis
Highlights
-
•
A pooled analysis of global prevalence from 36 countries was conducted.
-
•
Global prevalence of asymptomatic norovirus infection is about 7%.
-
•
Prevalence of asymptomatic norovirus infection varies among countries.
Research in context
Evidence before this study
We designed two strategies for searching records. First, we identified publications containing the proportion of asymptomatic NoV infections published before October 15, 2017 in PubMed, Ovid, Scopus and Web of Science. According to different search characters in these search engines, different search terms were used. The keywords include: “norovirus*”, “Norwalk”, “asymptom*”, “gastroenter*”, “calicivir*”, “enteric*”, “entero*”.During the stage of full-text screening, relevant references cited by those articles were reviewed for selection. Our principal summary data were the total number of asymptomatic samples together with the positive number or positive rate. The prevalence of asymptomatic NoV infections varied in different studies and ranged from 0 to more than 30%. Some factors had an impact on prevalence, for example, geographic regions.
Added value of this study
Understanding the global prevalence could be the first step for many further studies. For reference of future studies, we did a pooled analysis of global prevalence from 36 countries and assessed prevalence by different subgroups. We calculated that the pooled prevalence of asymptomatic NoV infection is about 7% and varies depending on different countries, settings and objects of study, and the pooled prevalence in context of outbreak exposures is as high as 18%.
Implications of all the available evidence
The high prevalence indicated asymptomatic individuals must not be overlooked. Asymptomatic individuals may play an important role in NoV transmission. This knowledge could have an impact on the development of transmission prevention strategies.
Alt-text: Unlabelled Box
1. Introduction
Human noroviruses (NoVs) are the most common cause of acute gastroenteritis and are responsible for substantial morbidity and mortality worldwide [1], [2]. NoV is responsible for 19–21 million illnesses, 1.7–1.9 million outpatient visits, 400,000 emergency department visits, 56,000–71,000 hospitalizations, and 570–800 deaths annually in the United States alone [3].
Studies about asymptomatic norovirus infections have been frequently reported. However, the mechanism of how NoV results in asymptomatic or symptomatic infection is unclear. The possible reasons for presence of asymptomatic infection may include long-term shedding from a previous symptomatic episode and truly asymptomatic infection due to lack of susceptible factors to symptomatic infection. However, human challenge studies [4] have showed the appearance of truly asymptomatic infection rather than long-term shedding from a previous symptomatic episode. Understanding the prevalence of asymptomatic NoV infection could be important for further studies. Less attention is usually paid to asymptomatic individuals and their environmental contaminants which may facilitate the transmission of norovirus. The understanding of asymptomatic and symptomatic infection would be useful in successfully presenting and applying public health control policy. In 2011, we conducted a study for NoV detection among asymptomatic children from kindergartens and primary schools in Changzhou City, China. The proportion of asymptomatic NoV infection was about 4% [5]. In an Australian research, NoV was not detected in 399 asymptomatic people [6]. In addition, more than 30% of samples from asymptomatic children in South Africa were determined to be NoV positive. The prevalence of asymptomatic NoV infection varied in different studies. In view of this, we aimed to summarize the overall prevalence of asymptomatic NoV infection. We assessed the prevalence by subgroup variables (study designs, geographic groups, objects and settings of study, and definition of asymptomatic infection). In addition, we also estimated prevalence of asymptomatic individuals in outbreak situations.
2. Methods
2.1. Search Strategy and Selection Criteria
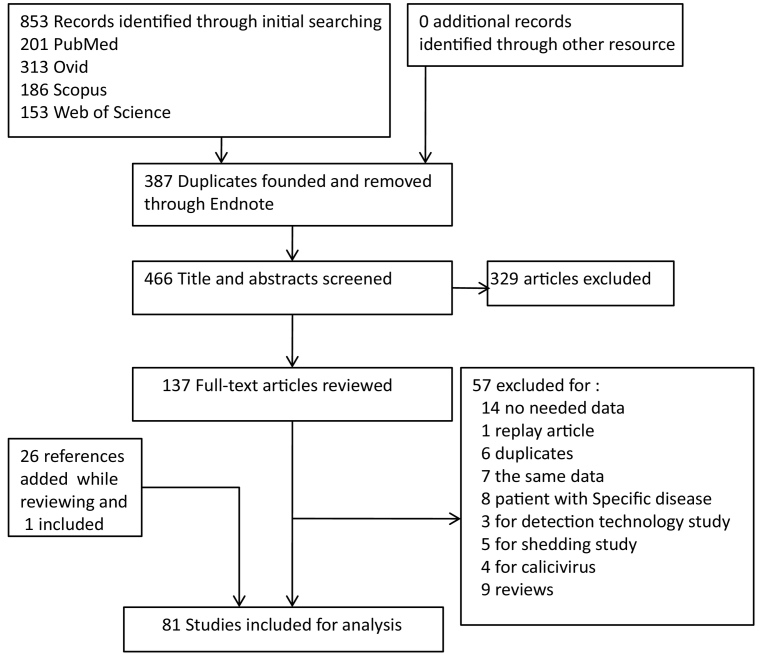
We designed two strategies for searching records. First, we identified publications containing the proportion of asymptomatic NoV infections published before October 15, 2017 in PubMed, Ovid, Scopus and Web of Science. According to different search characters in these search engines, different search terms were used. The keywords included: “norovirus*”, “Norwalk”, “asymptom*”, “gastroenter*”, “calicivir*”, “enteric*”, and “entero*”. During the stage of full-text screening, relevant references cited by those articles were reviewed for selection. Fig. 1 shows the detailed search process.
Fig. 1.
Process of study.
Two independent reviewers screened titles and abstracts for preliminary exclusion. Through preliminary screening, articles were excluded if: (1) were not written in English, (2) objects of study were not human, such as mice, non-human primates, (3) researched about special population groups, for example, people living with HIV, (4) Norovirus were not detected with PCR-based methods, and (5) volunteer challenge studies, which were artificially rather than naturally infected.
The full texts of the remaining articles were then screened in detail for eligibility. At this stage, articles were excluded if: (1) had data but did not differentiate between symptomatic and asymptomatic cases, (2) only calculated the whole proportion of calicivirus, and not the proportion of NoV individually, (3) different articles sharing the same data, (4) studies had different purposes such as aimed at duration of NoV shedding among asymptomatic individuals, and (6) review and editorial articles. Though reviews were excluded, references in those texts were screened.
2.2. Data Extraction
Our principal summary data were the total number of asymptomatic samples together with the positive number or positive rate. The following data were extracted if provided from articles: author, year of publication, country, total number, positive number, positive rate, study design, setting, object, age, specimen, definition of “asymptomatic”, study date, and method used for detection.
Asymptomatic samples were designed to be collected from healthy participants in a cross-sectional study, the control group of a case–control study, or healthy follow-up participants in a cohort study. Therefore, we stratified study designs into three groups. Settings were also stratified into three groups (community, hospital, or other). Age was not grouped because its range varied among these studies. Instead of age-stratum, we stratified a group containing children and adults by objects of studies. “Asymptomatic” is considered if a person is a carrier for a disease or infection but experiences no symptoms. There was no consensus among the studies on this definition. Some studies defined “asymptomatic” as healthy persons with no symptoms of gastroenteritis (diarrhea, vomiting, or fever, etc.). Others included people without symptoms of gastroenteritis for at least 1 week prior and more than 3 weeks after the day of stool collection. Finally, in some studies, norovirus was detected in nondiarrheal stool specimens collected from healthy persons, but it was unknown if they had vomiting or other symptoms. We grouped the studies into two categories of asymptomatic: (1) those with either a precise definition of asymptomatic or which did not fulfill a clear symptomatic definition and (2) those without diarrhea. Some studies were conducted in different settings and data were extracted individually.
2.3. Statistical Analysis
We used Q-test to provide a test of significance for heterogeneity. Meta-regression was used to examine the impact of subgroup variables on heterogeneity. R2-adjust was the percentage of heterogeneity accounted for by the addition of variables into the Meta-regression model as compared to an “empty” model. In other words, it was the percentage of the heterogeneity explained by the subgroup variables. Random-effect models were fitted to generate estimates of overall and subgroups prevalence using the inverse-variance method. To have better statistical properties, the raw proportions were first logit-transformed in order to be closer to a normal distribution and whose sampling variance can be better approximated. When the number of NoV positive samples was equal to 0, a value of 1/2 was added for calculation [7]. The test statistics of the individual coefficients in meta-regression models were based on methods of R metafor packages [7]. An omnibus test of all the model coefficients is conducted that excludes the intercept. The omnibus test is based on a chi-square distribution with m degrees of freedom (m being the number of coefficients tested). The Knapp and Hartung method [8] is an adjustment to the standard errors of the estimated coefficients, which helps to account for the uncertainty in the estimate of the amount of heterogeneity and leads to different reference distributions. Individual coefficients and confidence intervals are then based on the t-distribution with k-p degrees of freedom, while the omnibus test statistic then uses an F-distribution with m and k-p degrees of freedom (p being the total number of model coefficients). All analysis and plots were run with R software and the metafor package and rworldmap package (for world map) [7], [9].
2.4. Role of the Funding Source
The funder of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
3. Results
853 studies were identified through initial searching. Of these, 466 were then reviewed by their titles and abstracts, and 329 were excluded. We assessed the remaining 137 full-text articles for eligibility, and 57 of them were excluded because they did not fulfill inclusion criteria such as lacking pertinent information or were conducted for other aims. Twenty six references were added while reviewing those 137 full-text articles with the selection criterion being cited by articles to show prevalence of asymptomatic infection. One of these 26 references was included and the other 25 references either had been included in previous selections or did not meet the inclusion criteria. Finally, 81 studies were included for analysis (Fig. 1), and of these, 15 were under outbreaks circumstances while 71 were not. Because 5 studies reported more than one result, the number of stratum-specific studies for analysis did not sum up to the overall number.
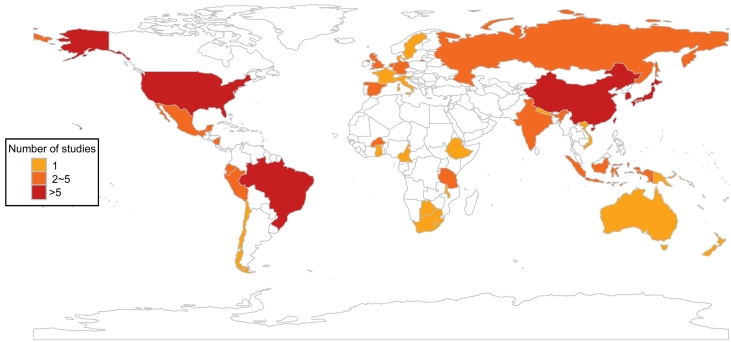
Asymptomatic individuals mainly were from: (1) cross-sectional studies, (2) case–control studies control group, and (3) cohort studies. The numbers of studies with these three designs were 33, 21, and 17, respectively. Studies were selected from 36 countries (Fig. 2). More studies were from China (9), Brazil (7), United States (6), South Korea and Japan (5) than from other countries such as Australia and New Zealand, both of which had only 1 study included. One study conducted in eight countries was not stratified data by countries due to incomplete information, and instead it was reviewed as a whole study. The number of communities (30) and hospitals (26) where studies were conducted was similar. Subjects of most studies were children (56), and only eight articles studied prevalence of adults, with five of eight focused on food handlers. Most of these studies had a definition of “asymptomatic” (or not fulfilling a defined case definition), while nineteen studies only included individuals who were without diarrhea as research objects. “Asymptomatic” was referred to but not defined in eleven studies. Among studies which had information about genotypes, about 80% (922/1157) of asymptomatic individuals were GII and 20% (235/1157) were GI.
Fig. 2.
Map of studies distribution by countries.
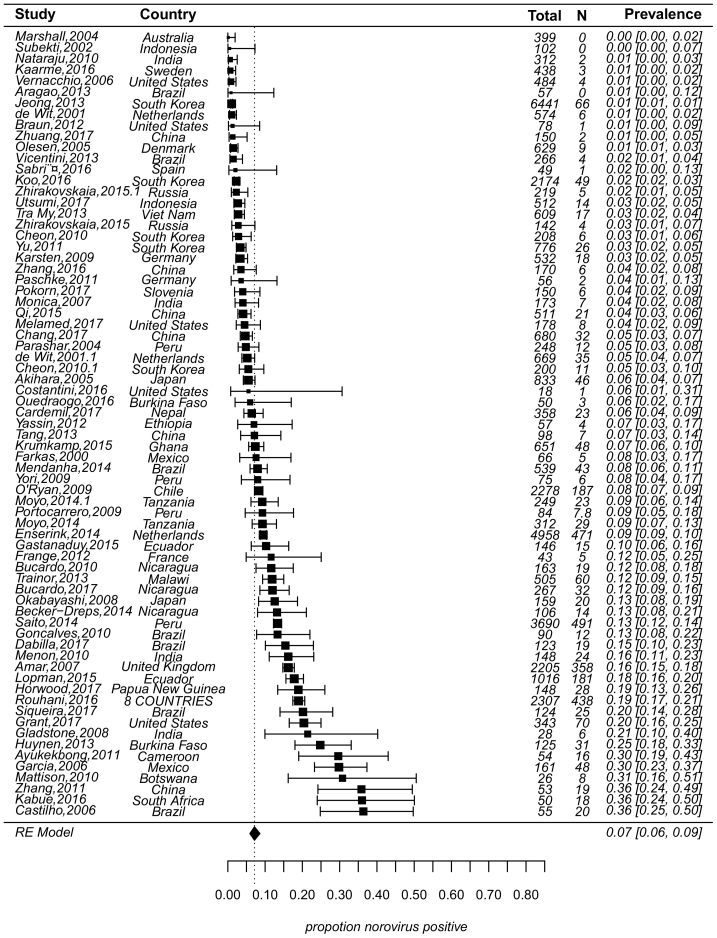
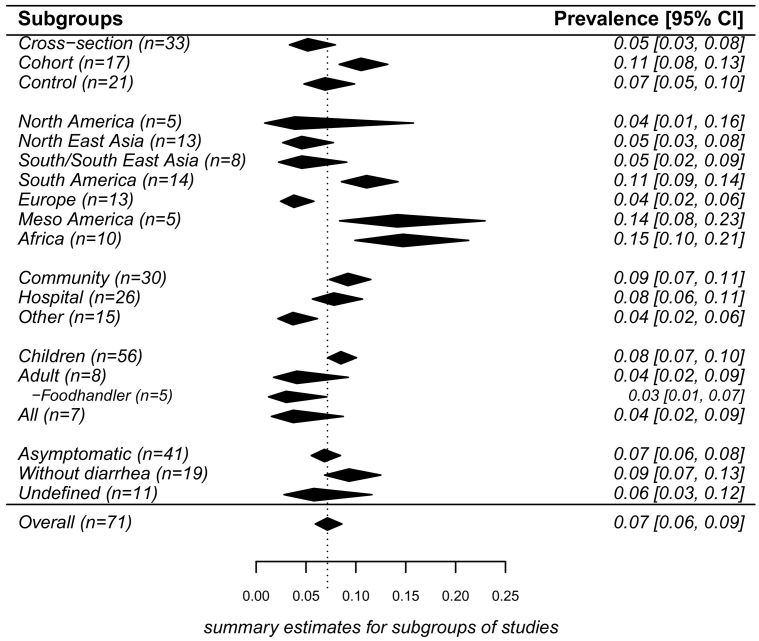
Of 71 studies, asymptomatic NoV prevalence was estimated by random-effect model at 7% (95% CI: 6%–9%, τ2 = 0.60, P < 0.01 test for heterogeneity) (Fig. 3). The sources of heterogeneity may be due to many factors such as study design, population, etc. Results of meta-regressions showed that heterogeneity was 7.53%, 8.16%, 18.10%, 17.41% and 11.13%, respectively for 5 subgroups variables (designs, environment, objects, geographic regions, and definition of “asymptomatic”). By design, prevalence from the control group (7%) in case–control studies was similar with that from cross-sectional studies (5%) (P = 0.51), but was higher (11%) from cohort studies than from cross-sectional studies (P = 0.05). By geographic regions, Africa, Meso America and South America had higher prevalence (15%, 14%, 11%, respectively) while the prevalence in Europe and North America was lower (4%). By environmental settings, prevalence was about 9% in both the community and hospital (P = 0.66). Prevalence was higher in children (8%) than adults (4%) (P = 0.07). For food handlers, prevalence was estimated at 3%, which was similar with prevalence in adults. Studies that provided a clear definition of “asymptomatic” (or not fulfilling a clear case definition) had a prevalence of 7%, which was not significantly different from prevalence of studies that only allowed individuals without diarrhea for enrollment (9%) (Results with confidence bounds were shown in Fig. 4).
Fig. 3.
Forest plot showing the results of 71 studies estimating prevalence of norovirus asymptomatic infection (τ2 = 0.60, P < 0.01 test for heterogeneity). The figure showed prevalence with 95% confidence intervals in the individual studies based on a random-effects model with studies ordered by prevalence. A line was used to represent the confidence interval of estimate. The prevalence estimated was marked with a solid black square. The size of the square represented the weight that the corresponding study exerted in the meta-analysis. The size of the square and the length of confidence interval corresponded to the size of the study and therefore the precision of the estimate. The pooled prevalence was marked with a filled polygon that had a dotted line from its upper point. Confidence intervals of pooled estimates were displayed as the width of the polygon. N was the number of NoV positive samples, value of 1/2 was added to N for calculation when N was equal to 0.
Fig. 4.
Results of pooled prevalence by subgroup. (n = number of studies in this subgroup). The figure showed pooled prevalence with 95% confidence intervals in the individual subgroups based on random-effects models. Black polygon was the estimated prevalence in the individual subgroups. The width of the polygon represented confidence interval of pooled estimate. Because some studies reported different results of strata, the number of stratum-specific studies for analysis didn't sum up to the overall number. The overall pooled estimate was added for comparison by a polygon that had a dotted line. For all subgroups, P-values of tests for heterogeneity were < 0.01. The sources of heterogeneity of prevalence studies were due to many factors, significant heterogeneities of subgroups divided respectively by each factor were still existed.
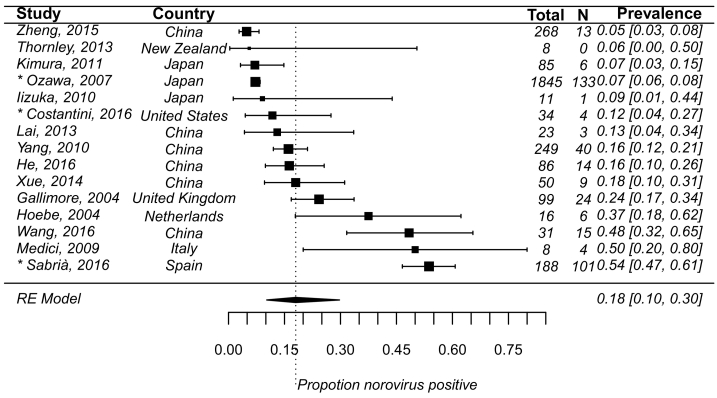
In the 15 studies where asymptomatic individuals were exposed under outbreak circumstances, pooled prevalence of infection was 18% (95% CI: 10%–30%, τ2 = 1.42, P < 0.01 test for heterogeneity) (Fig. 5). Settings where outbreaks occurred included: care facilities, catering services, hotels, schools, hospitals, and cruise ships. Transmission pathways included: person to person, foodborne, waterborne, and environment (Data not shown).
Fig. 5.
The same as in Fig. 3, but for prevalence of norovirus asymptomatic infection under outbreaks circumstance (τ2 = 1.42, P < 0.01 test for heterogeneity) from 15 studies. The length of confidence interval corresponded to the sample size of the study and therefore the precision of the estimate.
*. Studies with prevalence were calculated in N outbreaks (N > 1).
4. Discussion
Noroviruses are commonly detected in asymptomatic individuals with possible reasons including pre/post-symptomatic long-term shedding, and true asymptomatic infection due to lack of susceptible factors to symptomatic infection. In a human challenge study [4], samples from pre-challenge and post-challenge days were tested for NoV shedding. First, this experiment resulted in the appearance of asymptomatic volunteers. Second, asymptomatic and symptomatic volunteers had a similar shedding pattern (viral load, duration of shedding). Another study of a large population for NoV shedding also indicated that shedding in asymptomatic subjects was similar to symptomatic subjects [10]. Many studies reported that NoV bound to histo-blood group antigens (HBGA), which were expressed by the fucosyltransferase 2 (FUT2) gene. Individuals with a functional FUT2 gene were termed “secretors”. A systematic review [11] of association between HBGA and susceptibility showed that secretors were about 2–10 times more likely to be infected than non-secretors. HBGA had been reported to play a role as cellular receptors for NoV attachment [1]. In this view, asymptomatic infection may not only result from pre/post-symptomatic shedding. Similar norovirus shedding patterns between symptomatic and asymptomatic individuals in conjunction with lesser attention usually paid to asymptomatic individuals may facilitate the transmission of norovirus.
In practice, estimates of prevalence of asymptomatic NoV infections are affected by study design, settings, population, the distinction between symptomatic and asymptomatic individuals and other factors. Based on the 71 studies in our analysis, prevalence of asymptomatic NoV infection was 7%. The same result was obtained in another study in which prevalence was estimated from 20 studies [12], where asymptomatic individuals were only from control groups matched with cases in case–control studies. Studies with this design also met our study inclusion criteria, and our result in this design was also 7%. Beyond that, our studies included another two designs of studies. Prevalence was higher in cohort studies, and the proportion was calculated as the sum of several specimens collected from each individual divided by total number of positive individuals. Cohort studies might be a source of heterogeneity because detection of NoV in their stool may be shedding from a previously symptomatic infection. Information on the length of follow up or interval between previous reports of symptoms and sample collection was rarely given in texts. Norovirus prevalence tended to be higher in cases of acute gastroenteritis compared with asymptomatic infection. A pooled prevalence of norovirus in 187,336 patients with acute gastroenteritis from 175 articles was as high as 18% (95% CI 17–20) [12]. The high prevalence of symptomatic and asymptomatic norovirus infection showed that this pathogen brought heavy burdens and targeted control programs were needed for norovirus prevention.
We noted the same prevalence from community and hospital. Generally, prevalence of symptomatic infections in hospitals might be regarded as higher than in the community, but for asymptomatic infection, it cannot be known as the ranges of case-matched control for inclusion were not defined clearly in the studies. A few articles enrolled control individuals who were attending outpatient for routine health checks or conditions unrelated to gastroenteritis [13]. These individuals in this kind of control group had the same characteristics as those in the community. Prevalence in children was higher, suggesting that asymptomatic infection was likely associated with immunity because children have lower resistance to illness than adults [14]. One important mode of NoV infection was via contaminated food. NoV was considered in the United States the leading known foodborne agent, accounting for over 50% of foodborne illnesses annually [15]. Foodborne transmission was significant in NoV outbreaks [16]. The estimation of food handlers' asymptomatic infection from five studies was 3% with a 95% CI: 1%–7%. Due to the lower prevalence, food handlers are not suspected to be susceptible population for asymptomatic infections. In our selected studies, there was one study which was conducted in eight countries: Bangladesh, Brazil, Pakistan, Peru, South Africa, Tanzania, Nepal, and India [17]. The conclusion about asymptomatic infection in the previous study was 19%, ranging from 2.2% in Nepal to 30.4% in South Africa. Their study population was only infants aged 0–2 years old from low- and middle-income countries, which was a possible reason for the high prevalence as children are more susceptible for NoV [14] and norovirus is spread by the fecal oral route, suggesting age and hygiene status might be factors associated with norovirus infections. Due to the lack of data stratified by countries in this study, we did not include it in subgroup analysis by geographic regions. A recent study [18] generated a pooled estimate of the prevalence of asymptomatic norovirus infection from 13 articles centered in Latin America. The prevalence they estimated was 8% (95% CI: 4–13) which was similar with our result. One cause of this small variation may be because we included more articles (14 from South America and 5 from Meso America), and that their data were collected only between 1989 and 2012.
As we hypothesized, asymptomatic prevalence (18%) was much higher in the context of outbreaks than in healthy individuals not known to be in contact with other infected symptomatic people. More data with outbreak of norovirus would be needed in the future for better analysis.
Our study has some limitations. First, older adults were vulnerable to gastroenteritis [19], but there were not enough articles available for inclusion to have a stratified analysis for elderly people. Age, an important factor to NoV, could not be grouped into categories as ranges in studies were highly varied, even though we tried different ways. Other possible factors which could have an impact on prevalence were not considered, for example, seasonality. A few studies reported different prevalence of asymptomatic NoV infection by seasons [20], [21]. Many of our studies involved an extended span of time making it difficult to distinguishably estimate the pattern of seasonality. In addition, we calculated the proportion of genotypes of asymptomatic NoV cases from articles having this information. About 80% (922/1157) were GII and 20% (235/1157) were GI. From theses proportions, we could not say that GII was the major genotype associated with asymptomatic infection as determining the association between genotypes and asymptomatic cases requires minimum proportion of genotypes of symptomatic NoV cases. Although 80% of infections were GII in asymptomatic cases, symptomatic human norovirus infections were also caused mostly by GII. More evidences are needed to explore the associations between genotype and asymptomatic cases. Finally, Q test for heterogeneity in models was highly significant, suggesting that other factors that were not considered might have had remarkable effects on asymptomatic prevalence.
In conclusion, the prevalence of asymptomatic NoV infection estimated through meta-analysis is 7% and varies depending on different countries, settings and objects of study. The prevalence in context of outbreak exposure is as high as 18%. The high prevalence indicated asymptomatic individuals must not be overlooked. Asymptomatic individuals may play an important role in NoV transmission. This knowledge could have an impact on the development of transmission prevention strategies. More work will be needed to better understand and interpret the presence of asymptomatic infection and the roles it plays in NoV transmission.
Contributors
QR and HYT designed the study, did the literature search, data extraction, analyzed data and interpreted data. QR drafted the manuscript. YXJ designed the study, interpreted data, edited the manuscript and contributed to the manuscript. LJW, HHJ, SXF, QXR and LWQ helped do the literature screen and data extraction. SY, ZHM and WLJ helped update articles published in 2017. LJH and CC helped with R program.
Declaration of Interests
We declare no competing interests.
Acknowledgments
This study was supported by grants from National Natural Science Funds of China (Nos. 31570167).
Appendix A
-
1.
Yu J-H, Kim N-Y, Lee E-J, Jeon I-S. Norovirus Infections in Asymptomatic Food Handlers in Elementary Schools without Norovirus Outbreaks in Some Regions of Incheon, Korea. J Korean Med Sci 2011; 26(6): 734-9.
-
2.
Yori PPMPH, Schwab KP, Gilman RHMD, et al. Norovirus Highly Prevalent Cause Of Endemic Acute Diarrhea In Children In The Peruvian Amazon. Pediatr Infect Dis J 2009; 28(9): 844-7.
-
3.
Vicentini F, Denadai W, Gomes YM, et al. Molecular Characterization of Noroviruses and HBGA from Infected Quilombola Children in Espirito Santo State, Brazil. PLoS One 2013; 8(7).
-
4.
Tang M-B, Chen C-H, Chen S-C, Chou Y-C, Yu C-P. Epidemiological and molecular analysis of human norovirus infections in Taiwan during 2011 and 2012. BMC Infect Dis 2013; 13.
-
5.
Saito Ma, Goel-Apaza S, Espetia S, et al. Multiple Norovirus Infections in a Birth Cohort in a Peruvian Periurban Community. Clin Infect Dis 2014; 58(4): 483-91.
-
6.
Sabrià A, Pintó RM, Bosch A, et al. Norovirus shedding among food and healthcare workers exposed to the virus in outbreak settings. J Clin Virol 2016; 82: 119-25.
-
7.
Qi R, Ye C, Chen C, Yao P, Hu F, Lin Q. Norovirus prevention and the prevalence of asymptomatic norovirus infection in kindergartens and primary schools in Changzhou, China: Status of the knowledge, attitudes, behaviors, and requirements. Am J Infect Control 2015; 43(8): 833-8.
-
8.
O'Ryan ML, Lucero Y, Prado V, et al. Symptomatic and asymptomatic rotavirus and norovirus infections during infancy in a Chilean birth cohort. Pediatr Infect Dis J 2009; 28(10): 879-84.
-
9.
Okabayashi T, Yokota S-i, Ohkoshi Y, et al. Occurrence of norovirus infections unrelated to norovirus outbreaks in an asymptomatic food handler population. J Clin Microbiol 2008; 46(6): 1985-8.
-
10.
Mendanha de Oliveira DM, Souza M, Fiaccadori FS, Pereira Santos HC, de Paula Cardoso DdD. Monitoring of Calicivirus Among Day-Care Children: Evidence of Asymptomatic Viral Excretion and First Report of GI.7 Norovirus and GI.3 Sapovirus in Brazil. J Med Virol 2014; 86(9): 1569-75.
-
11.
Marshall JA, Hellard ME, Sinclair MI, et al. Failure to detect norovirus in a large group of asymptomatic individuals. Public Health 2004; 118(3): 230-3.
-
12.
Lopman BA, Trivedi T, Vicuna Y, et al. Norovirus Infection and Disease in an Ecuadorian Birth Cohort: Association of Certain Norovirus Genotypes With Host FUT2 Secretor Status. Journal of Infectious Diseases 2015; 211(11): 1813-21.
-
13.
Koo HS, Lee MO, Ku PT, Hwang SJ, Park DJ, Baik HS. Molecular epidemiology of norovirus in asymptomatic food handlers in Busan, Korea, and emergence of genotype GII.17. J Microbiol 2016; 54(10): 686-94.
-
14.
Kabue JP, Meader E, Hunter PR, Potgieter N. Norovirus prevalence and estimated viral load in symptomatic and asymptomatic children from rural communities of Vhembe district, South Africa. J Clin Virol 2016; 84: 12-8.
-
15.
Jeong AY, Jeong HS, Lee JS, et al. Occurrence of norovirus infections in asymptomatic food handlers in South Korea. J Clin Microbiol 2013; 51(2): 598-600.
-
16.
Huynen P, Mauroy A, Martin C, et al. Molecular epidemiology of norovirus infections in symptomatic and asymptomatic children from Bobo Dioulasso, Burkina Faso. J Clin Virol 2013; 58(3): 515-21.
-
17.
Goncalves Barreira DMP, Rocha Ferreira MS, Machado Fumian T, et al. Viral load and genotypes of noroviruses in symptomatic and asymptomatic children in Southeastern Brazil. J Clin Virol 2010; 47(1): 60-4.
-
18.
Gladstone BP, Iturriza-Gomara M, Ramani S, et al. Polymerase chain reaction in the detection of an 'outbreak' of asymptomatic viral infections in a community birth cohort in south India. Epidemiol Infect 2008; 136(3): 399-405.
-
19.
Garcia C, DuPont HL, Long KZ, Santos JI, Ko G. Asymptomatic norovirus infection in Mexican children. J Clin Microbiol 2006; 44(8): 2997-3000.
-
20.
Cheon D-S, Jeong HS, Jeong A, et al. Seasonal Prevalence of Asymptomatic Norovirus Infection in Korean Children. Foodborne Pathog Dis 2010; 7(11): 1427-30.
-
21.
Bucardo FP, Nordgren JP, Carlsson BM, et al. Asymptomatic Norovirus Infections in Nicaraguan Children and its Association With Viral Properties and Histo-blood Group Antigens. Pediatr Infect Dis J 2010; 29(10): 934-9.
-
22.
Menon VK, George S, Ramani S, et al. Genogroup IIb norovirus infections and association with enteric symptoms in a neonatal nursery in southern India. J Clin Microbiol 2010; 48(9): 3212-5.
-
23.
Trainor E, Lopman B, Iturriza-Gomara M, et al. Detection and molecular characterisation of noroviruses in hospitalised children in Malawi, 1997-2007. J Med Virol 2013; 85(7): 1299-306.
-
24.
Subekti D, Lesmana M, Tjaniadi P, et al. Incidence of Norwalk-like viruses, rotavirus and adenovirus infection in patients with acute gastroenteritis in Jakarta, Indonesia. FEMS Immunol Med Microbiol 2002; 33(1): 27-33.
-
25.
Moyo S, Hanevik K, Blomberg B, et al. Genetic diversity of norovirus in hospitalised diarrhoeic children and asymptomatic controls in Dar es Salaam, Tanzania. Infect Genet Evol 2014; 26: 340-7.
-
26.
Tra My PV, Thompson C, Phuc HL, et al. Endemic norovirus infections in children, Ho Chi Minh City, Vietnam, 2009-2010. Emerg Infect Dis 2013; 19(6): 977-80.
-
27.
Portocarrero DV. 262 Prevalence of Norovirus infections in children from Iquitos, Peru. J Acquir Immune Defic Syndr 2009; 51(2).
-
28.
Nataraju SM, Ganesh B, Das S, et al. Emergence of Noroviruses homologous to strains reported from Djibouti (horn of Africa), Brazil, Italy, Japan and USA among children in Kolkata, India. Eur Rev Med Pharmacol Sci 2010; 14(9): 789-94.
-
29.
Gastanaduy PAMD, Vicuna YB, Salazar FB, et al. Transmission of Norovirus Within Households in Quininde, Ecuador. Pediatr Infect Dis J 2015; 34(9): 1031-3.
-
30.
Zhang S, Chen T-H, Wang J, et al. Symptomatic and Asymptomatic Infections of Rotavirus, Norovirus, and Adenovirus Among Hospitalized Children in Xi'an, China. J Med Virol 2011; 83(8): 1476-84.
-
31.
Zhirakovskaia EV, Tikunov AY, Bodnev SA, Klemesheva VV, Netesov SV, Tikunova NV. Molecular epidemiology of noroviruses associated with sporadic gastroenteritis in children in Novosibirsk, Russia, 2003-2012. J Med Virol 2015; 87(5): 740-53.
-
32.
Akihara S, Phan TG, Nguyen TA, Hansman G, Okitsu S, Ushijima H. Existence of multiple outbreaks of viral gastroenteritis among infants in a day care center in Japan. Arch Virol 2005; 150(10): 2061-75.
-
33.
Rouhani S, Yori PP, Olortegui MP, et al. Norovirus Infection and Acquired Immunity in 8 Countries: Results From the MAL-ED Study. Clin Infect Dis 2016; 62(10): 1210-20.
-
34.
Parashar UD, Li J-F, Cama R, et al. Human Caliciviruses as a Cause of Severe Gastroenteritis in Peruvian Children. Journal of Infectious Diseases 2004; 190(6): 1088-92.
-
35.
Krumkamp R, Sarpong N, Schwarz NG, et al. Gastrointestinal infections and diarrheal disease in Ghanaian infants and children: an outpatient case-control study. PLoS Negl Trop Dis 2015; 9(3): e0003568.
-
36.
Becker-Dreps S, Bucardo F, Vilchez S, et al. Etiology of childhood diarrhea after rotavirus vaccine introduction: a prospective, population-based study in Nicaragua. Pediatr Infect Dis J 2014; 33(11): 1156-63.
-
37.
Aragao GC, Mascarenhas JD, Kaiano JH, et al. Norovirus diversity in diarrheic children from an African-descendant settlement in Belem, Northern Brazil. PLoS One 2013; 8(2): e56608.
-
38.
Yassin MA, Kirby A, Mengistu AA, et al. Unusual norovirus and rotavirus genotypes in Ethiopia. Paediatr Int Child Health 2012; 32(1): 51-5.
-
39.
Mattison K, Sebunya TK, Shukla A, Noliwe LN, Bidawid S. Molecular detection and characterization of noroviruses from children in Botswana. J Med Virol 2010; 82(2): 321-4.
-
40.
Karsten C, Baumgarte S, Friedrich AW, et al. Incidence and risk factors for community-acquired acute gastroenteritis in north-west Germany in 2004. Eur J Clin Microbiol Infect Dis 2009; 28(8): 935-43.
-
41.
Monica B, Ramani S, Banerjee I, et al. Human caliciviruses in symptomatic and asymptomatic infections in children in Vellore, South India. J Med Virol 2007; 79(5): 544-51.
-
42.
Amar CF, East CL, Gray J, Iturriza-Gomara M, Maclure EA, McLauchlin J. Detection by PCR of eight groups of enteric pathogens in 4,627 faecal samples: re-examination of the English case-control Infectious Intestinal Disease Study (1993-1996). Eur J Clin Microbiol Infect Dis 2007; 26(5): 311-23.
-
43.
Castilho JG, Munford V, Resque HR, Fagundes-Neto U, Vinje J, Racz ML. Genetic diversity of norovirus among children with gastroenteritis in Sao Paulo State, Brazil. J Clin Microbiol 2006; 44(11): 3947-53.
-
44.
Olesen B, Neimann J, Bottiger B, et al. Etiology of diarrhea in young children in Denmark: a case-control study. J Clin Microbiol 2005; 43(8): 3636-41.
-
45.
de Wit MA, Koopmans MP, Kortbeek LM, et al. Sensor, a population-based cohort study on gastroenteritis in the Netherlands: incidence and etiology. Am J Epidemiol 2001; 154(7): 666-74.
-
46.
de Wit MA, Koopmans MP, Kortbeek LM, van Leeuwen NJ, Vinje J, van Duynhoven YT. Etiology of gastroenteritis in sentinel general practices in the Netherlands. Clin Infect Dis 2001; 33(3): 280-8.
-
47.
Farkas T, Jiang X, Guerrero ML, et al. Prevalence and genetic diversity of human caliciviruses (HuCVs) in Mexican children. J Med Virol 2000; 62(2): 217-23.
-
48.
Frange P, Touzot F, Debre M, et al. Prevalence and Clinical Impact of Norovirus Fecal Shedding in Children with Inherited Immune Deficiencies. Journal of Infectious Diseases 2012; 206(8): 1269-74.
-
49.
Ayukekbong J, Lindh M, Nenonen N, Tah F, Nkuo-Akenji T, Bergstrom T. Enteric viruses in healthy children in Cameroon: viral load and genotyping of norovirus strains. J Med Virol 2011; 83(12): 2135-42.
-
50.
Braun LE, Renaud C, Fairchok MP, Kuypers J, Englund JA, Martin ET. Human Parechovirus and Other Enteric Viruses in Childcare Attendees in the Era of Rotavirus Vaccines. J Pediatric Infect Dis Soc 2012; 1(2): 136-43.
-
51.
Enserink R, Scholts R, Bruijning-Verhagen P, et al. High detection rates of enteropathogens in asymptomatic children attending day care. PLoS One 2014; 9(2): e89496.
-
52.
Kaarme J, Hickman RA, Neveus T, Blomberg J, Ohrmalm C. Reassuringly low carriage of enteropathogens among healthy Swedish children in day care centres. Public Health 2016; 140: 221-7.
-
53.
Ouedraogo N, Kaplon J, Bonkoungou IJ, et al. Prevalence and Genetic Diversity of Enteric Viruses in Children with Diarrhea in Ouagadougou, Burkina Faso. PLoS One 2016; 11(4): e0153652.
-
54.
Paschke C, Apelt N, Fleischmann E, et al. Controlled study on enteropathogens in travellers returning from the tropics with and without diarrhoea. Clin Microbiol Infect 2011; 17(8): 1194-200.
-
55.
Vernacchio L, Vezina RM, Mitchell AA, Lesko SM, Plaut AG, Acheson DWK. Diarrhea in American infants and young children in the community setting: Incidence, clinical presentation and microbiology. Pediatr Infect Dis J 2006; 25(1): 2-7.
-
56.
Zhang S-X, Zhou Y-M, Xu W, et al. Impact of co-infections with enteric pathogens on children suffering from acute diarrhea in southwest China. Infect Dis Poverty 2016; 5.
-
57.
Bucardo F, Reyes Y, Becker-Dreps S, et al. Pediatric norovirus GII.4 infections in Nicaragua, 1999-2015. Infect Genet Evol 2017; 55: 305-12.
-
58.
Cardemil CV, Sherchand JB, Shrestha L, et al. Pathogen-Specific Burden of Outpatient Diarrhea in Infants in Nepal: A Multisite Prospective Case-Control Study. J Pediatric Infect Dis Soc 2017; 6(3): e75-e85.
-
59.
Chang HMD, Zhang LMS, Ge YMDP, et al. A Hospital-based Case-control Study of Diarrhea in Children in Shanghai. Pediatr Infect Dis J 2017; 36(11): 1057-63.
-
60.
Dabilla N, Vieira Almeida TN, Reboucas Oliveira AC, et al. Norovirus in feces and nasopharyngeal swab of children with and without acute gastroenteritis symptoms: First report of GI.5 in Brazil and GI.3 in nasopharyngeal swab. J Clin Virol 2017; 87: 60-6.
-
61.
Grant LR, O'Brien KL, Weatherholtz RC, et al. Norovirus and Sapovirus Epidemiology and Strain Characteristics among Navajo and Apache Infants. PLoS One 2017; 12(1): e0169491.
-
62.
Horwood PF, Soli KW, Maure T, et al. A High Burden of Asymptomatic Gastrointestinal Infections in Traditional Communities in Papua New Guinea. Am J Trop Med Hyg 2017.
-
63.
Melamed R, Storch GA, Holtz LR, et al. Case-Control Assessment of the Roles of Noroviruses, Human Bocaviruses 2, 3, and 4, and Novel Polyomaviruses and Astroviruses in Acute Childhood Diarrhea. J Pediatric Infect Dis Soc 2017; 6(3): e49-e54.
-
64.
Pokorn MMDM, Jevsnik MP, Petrovec MMDP, et al. Respiratory and Enteric Virus Detection in Children: A Prospective Study Comparing Children With Febrile Seizures and Healthy Controls. J Child Neurol 2017; 32(1): 84-93.
-
65.
Siqueira JAM, Junior ECS, Linhares ADC, Gabbay YB. Molecular analysis of norovirus in specimens from children enrolled in a 1982-1986 study in Belem, Brazil: A community-based longitudinal study. J Med Virol 2017; 89(11): 1894-903.
-
66.
Utsumi T, Lusida MI, Dinana Z, et al. Occurrence of norovirus infection in an asymptomatic population in Indonesia. Infect Genet Evol 2017; 55: 1-7.
-
67.
Zhuang Z-L, Jin Y, Yan K-L, Cheng W-X. Study of the association between histo-blood group antigens and norovirus infection in Chinese children. Arch Virol 2017.
-
68.
Zheng QM, Zeng HT, Dai CW, et al. Epidemiological investigation of a norovirus GII.4 Sydney outbreak in a China elder care facility. Jpn J Infect Dis 2015; 68(1): 70-4.
-
69.
Xue C, Fu Y, Zhu W, et al. An outbreak of acute norovirus gastroenteritis in a boarding school in Shanghai: a retrospective cohort study. BMC Public Health 2014; 14: 1092.
-
70.
Wang X, Yong W, Shi L, et al. An outbreak of multiple norovirus strains on a cruise ship in China, 2014. Appl Microbiol 2016; 120(1): 226-33.
-
71.
Ozawa K, Oka T, Takeda N, Hansman GS. Norovirus infections in symptomatic and asymptomatic food handlers in Japan. J Clin Microbiol 2007; 45(12): 3996-4005.
-
72.
Medici MC, Morelli A, Arcangeletti MC, et al. An outbreak of norovirus infection in an Italian residential-care facility for the elderly. Clin Microbiol Infect 2009; 15(1): 97-100.
-
73.
Costantini VP, Cooper EM, Hardaker HL, et al. Epidemiologic, Virologic, and Host Genetic Factors of Norovirus Outbreaks in Long-term Care Facilities. Clin Infect Dis 2016; 62(1): 1-10.
-
74.
Yang LC, Chiang PC, Huang TH, et al. Residents had an increasing risk of norovirus gastroenteritis infection than health care workers during an outbreak in a nursing home. J Am Med Dir Assoc 2010; 11(8): 592-7.
-
75.
Thornley CN, Hewitt J, Perumal L, et al. Multiple outbreaks of a novel norovirus GII.4 linked to an infected post-symptomatic food handler. Epidemiol Infect 2013; 141(8): 1585-97.
-
76.
Lai C-C, Wang Y-H, Wu C-Y, Hung C-H, Jiang DD-S, Wu F-T. A norovirus outbreak in a nursing home: Norovirus shedding time associated with age. J Clin Virol 2013; 56(2): 96-101.
-
77.
Kimura H, Nagano K, Kimura N, et al. A norovirus outbreak associated with environmental contamination at a hotel. Epidemiol Infect 2011; 139(2): 317-25.
-
78.
Iizuka S, Oka T, Tabara K, et al. Detection of sapoviruses and noroviruses in an outbreak of gastroenteritis linked genetically to shellfish. J Med Virol 2010; 82(7): 1247-54.
-
79.
He Y, Jin M, Chen K, et al. Gastroenteritis Outbreaks Associated with the Emergence of the New GII.4 Sydney Norovirus Variant during the Epidemic of 2012/13 in Shenzhen City, China. PLoS One 2016; 11(11): e0165880.
-
80.
Gallimore CI, Cubitt D, du Plessis N, Gray JJ. Asymptomatic and symptomatic excretion of noroviruses during a hospital outbreak of gastroenteritis. J Clin Microbiol 2004; 42(5): 2271-4.
-
81.
Hoebe CJ, Vennema H, de Roda Husman AM, van Duynhoven YT. Norovirus outbreak among primary schoolchildren who had played in a recreational water fountain. J Infect Dis 2004; 189(4): 699-705.
References
- 1.Patel M.M., Widdowson M.A., Glass R.I., Akazawa K., Vinje J., Parashar U.D. Systematic literature review of role of noroviruses in sporadic gastroenteritis. Emerg Infect Dis. 2008;14(8):1224–1231. doi: 10.3201/eid1408.071114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Glass R.I., Parashar U.D., Estes M.K. Norovirus gastroenteritis. N Engl J Med. 2009;361(18):1776–1785. doi: 10.1056/NEJMra0804575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hall A.J., Lopman B.A., Payne D.C. Norovirus disease in the United States. Emerg Infect Dis. 2013;19(8):1198–1205. doi: 10.3201/eid1908.130465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Newman K.L., Moe C.L., Kirby A.E., Flanders W.D., Parkos C.A., Leon J.S. Norovirus in symptomatic and asymptomatic individuals: cytokines and viral shedding. Clin Exp Immunol. 2016;184(3):347–357. doi: 10.1111/cei.12772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Qi R., Ye C., Chen C., Yao P., Hu F., Lin Q. Norovirus prevention and the prevalence of asymptomatic norovirus infection in kindergartens and primary schools in Changzhou, China: status of the knowledge, attitudes, behaviors, and requirements. Am J Infect Control. 2015;43(8):833–838. doi: 10.1016/j.ajic.2015.04.182. [DOI] [PubMed] [Google Scholar]
- 6.Marshall J.A., Hellard M.E., Sinclair M.I. Failure to detect norovirus in a large group of asymptomatic individuals. Public Health. 2004;118(3):230–233. doi: 10.1016/j.puhe.2003.09.007. [DOI] [PubMed] [Google Scholar]
- 7.Viechtbauer W. Conducting meta-analyses in R with the metafor package. J Stat Softw. 2010;36(3):1–48. [Google Scholar]
- 8.Knapp G., Hartung J. Improved tests for a random effects meta-regression with a single covariate. Stat Med. 2003;22(17):2693–2710. doi: 10.1002/sim.1482. [DOI] [PubMed] [Google Scholar]
- 9.South A. rworldmap: a new R package for mapping global data. R J. 2011;3(1):35–43. [Google Scholar]
- 10.Teunis P.F., Sukhrie F.H., Vennema H., Bogerman J., Beersma M.F., Koopmans M.P. Shedding of norovirus in symptomatic and asymptomatic infections. Epidemiol Infect. 2015;143(8):1710–1717. doi: 10.1017/S095026881400274X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kambhampati A., Payne D.C., Costantini V., Lopman B.A. Host genetic susceptibility to enteric viruses: a systematic review and metaanalysis. Clin Infect Dis. 2016;62(1):11–18. doi: 10.1093/cid/civ873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ahmed S.M., Hall A.J., Robinson A.E. Global prevalence of norovirus in cases of gastroenteritis: a systematic review and meta-analysis. Lancet Infect Dis. 2014;14(8):725–730. doi: 10.1016/S1473-3099(14)70767-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tra My P.V., Thompson C., Phuc H.L. Endemic norovirus infections in children, Ho Chi Minh City, Vietnam, 2009–2010. Emerg Infect Dis. 2013;19(6):977–980. doi: 10.3201/eid1906.111862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.O'Brien S.J., Donaldson A.L., Iturriza-Gomara M., Tam C.C. Age-specific incidence rates for norovirus in the community and presenting to primary healthcare facilities in the United Kingdom. Emerg Infect Dis. 2016;213(Supplement(1)) doi: 10.1093/infdis/jiv411. (S15-S8) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Scallan E., Hoekstra R.M., Angulo F.J. Foodborne illness acquired in the United States—major pathogens. Emerg Infect Dis. 2011;17(1):7–15. doi: 10.3201/eid1701.P11101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moore M.D., Goulter R.M., Jaykus L.A. Human norovirus as a foodborne pathogen: challenges and developments. Annu Rev Food Sci Technol. 2015;6:411–433. doi: 10.1146/annurev-food-022814-015643. [DOI] [PubMed] [Google Scholar]
- 17.Rouhani S., Yori P.P., Olortegui M.P. Norovirus infection and acquired immunity in 8 countries: results from the MAL-ED study. Clin Infect Dis. 2016;62(10):1210–1220. doi: 10.1093/cid/ciw072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.O'Ryan M.M.D., Riera-Montes M.M.D.M., Lopman B.P. Norovirus in Latin America: systematic review and meta-analysis. Pediatr Infect Dis J. 2017;36(2):127–134. doi: 10.1097/INF.0000000000001369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kirk M.D., Veitch M.G., Hall G.V. Gastroenteritis and food-borne disease in elderly people living in long-term care. Clin Infect Dis. 2010;50(3):397–404. doi: 10.1086/649878. [DOI] [PubMed] [Google Scholar]
- 20.Jeong A.Y., Jeong H.S., Lee J.S. Occurrence of norovirus infections in asymptomatic food handlers in South Korea. J Clin Microbiol. 2013;51(2):598–600. doi: 10.1128/JCM.01856-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cheon D.-S., Jeong H.S., Jeong A. Seasonal prevalence of asymptomatic norovirus infection in Korean children. J Clin Microbiol. 2010;7(11):1427–1430. doi: 10.1089/fpd.2010.0547. [DOI] [PubMed] [Google Scholar]