Abstract
A better understanding of the mechanisms of beige and brown adipogenesis is needed for developing strategies to prevent and treat obesity and associated metabolic disorders. Phytanic acid (PA) exists in a wide range of foods, especially in milk fat and marine foods, but its effects on obesity and beige adipogenesis remain poorly defined. The objective is to investigate the effects and regulatory mechanisms of PA in the beige adipogenesis. In 3T3-L1 preadipocytes, PA elevated the expression of brown adipogenic markers, suggesting that PA promotes beige adipogenic differentiation in committed adipogenic cells. In uncommitted C3H10T1/2 cells, while PA increased PGC1α expression, it did not increase brown adipogenic regulators PRDM16 or UCP1 expression, suggesting that PA had no significant effects on brown adipocyte commitment. PA also enhanced mitochondrial biogenesis and oxygen consumption. Promotion of both mitochondriogenesis and beige adipogenic differentiation were blocked by using PPARα antagonist or with Pparα knockdown, showing that PA-mediated beige/brown adipogenic differentiation is dependent on PPARα. Additionally, the PA-regulated effect is independent on β3-adrenergic receptor. Taken together, PA promotes beige adipogenic differentiation, but not the commitment of progenitor cells to the brown adipocyte lineage. PPARα is a key mediator during PA-induced beige/brown adipogenic differentiation.
Keywords: Beige adipogenesis, Brown adipogenic differentiation, Phytanic acid, PPARα
1. Introduction
Based on morphological and functional differences, adipose tissue can be classified as white adipose tissue (WAT) and brown adipose tissue (BAT). WAT is mainly responsible for storage of energy in the form of triglycerides droplets [1]. Excessive and abnormal WAT deposition may lead to metabolic diseases, such as obesity, type 2 diabetes and cardiovascular disease [1, 2]. In contrast, BAT dissipates energy in the form of heat due to the presence of numerous mitochondria and abundant uncoupling protein 1 (UCP1) [3]. Recently, brown-like adipocytes (termed beige adipocytes) have been discovered in WAT [3, 4]. Similar to classic brown adipocytes, beige adipocytes dissipate calories as heat, and importantly, they are highly inducible [4]. Therefore, promoting beige adipogenesis and the browning of WAT through nutritional and pharmacological interventions are attractive strategies for preventing and treating obesity-linked dysfunctions [5].
Beige adipocytes are derived from PDGFRα+ cells within WAT [4, 6]. Both brown and beige adipogenesis is regulated by transcriptional regulators including PR domain zinc-finger protein 16 (PRDM16), peroxisome proliferator-activated receptor (PPAR) γ and PPARγ coactivator 1α (PGC-1α) [7-9]. In addition, fibroblast growth factor 21 (FGF21) and bone morphogenetic protein 7 (BMP7) play important roles in brown adipogenesis [10, 11]. Micro RNA (i.e. miR-196a) could also have positive effects on the browning of WAT [12]. In addition, pharmacological and nutritional agents, such as resveratrol [13], retinoic acid [14], curcumin [15], berberine [16], cryptotanshinone [17], sulforaphane [18] and phytol [19], enhance WAT browning by activating brown adipocytes-related signaling pathways.
Phytanic acid (PA) is a branched-chain fatty acid that is produced by the degradation of phytol, a constituent of chlorophyll [20]. As a result, PA is abundant in a variety of foods especially in marine foods, beef and dairy products [20]. The daily consumption per person is estimated at 50 to 100 mg [21]. PA acts via (PPARs) to regulate glucose metabolism in rat primary hepatocytes [22]. PA also induced white adipocyte differentiation [23]. Besides, PA has been found to activate Ucp1 gene transcription and brown adipocyte differentiation in HIB-1B cells and mouse primary brown preadipocytes [24-26]. A phytol-enriched diet may boost PA levels in the liver of mouse, thereby leading to activation of PPARα [26]. To date, effects of PA on the formation of brown and beige adipocytes have only been sparsely explored and the underlying mechanism is unknown.
PPARα regulates fatty acid oxidation in many organs [27]. It has been showed that PPARα agonist fenofibrate promoted the expression of brown adipocyte marker genes in subcutaneous WAT and ultimately resulted in beige adipocyte formation [28, 29]. PPARα could also cooperate with SIRT1 to increase metabolic activity and promote browning of WAT [30]. PA is considered as a ligand of mouse PPARα [31], but whether PPARα is involved in PA-mediated brown or beige adipogenesis remains undefined. The objective of this work is to explore the effects of PA on beige adipogenesis. Excitingly, we found that PA promotes beige adipogenic differentiation of preadipocytes but not uncommitted progenitor cells, and PPARα is a key mediator of PA-induced beige adipogenic differentiation.
2. Materials and Methods
2.1. Antibodies and chemicals
Antibodies against β-actin (#4967), AMPKα (#5832), and phospho-AMPKα at Thr172 (#2535) were purchased from Cell Signaling Technology (Danvers, MA, USA). Antibodies against FABP4 (ab92501), PPARγ (ab41928), PGC1α (ab54481), PRDM16 (ab106410), UCP1 (ab10983), and PPARα (ab3484) were purchased from Abcam (Cambridge, UK). Goat anti-rabbit IgG HRP (A0208) and goat anti-mouse IgG HRP (A0216) secondary antibodies were bought from Beyotime Institute of Biotechnology (Haimen, Jiangsu, China).
Insulin (91077C), indomethacin (I7378), dexamethasone (D4902), PA (P4060), 3-isobutyl-1-methylxanthine (I5878), triiodothyronine (T3) (I2877), DMSO (D2650), Polybrene (H9268) and Oil-Red O (O0625) were purchased from Sigma (St Louis, MO, USA). DMEM (11960–044) and Pierce™ ECL Western Blotting Substrate (#32109) were purchased from Thermo Fisher Scientific (Waltham, MA, USA). GW6471 (4618) and SR59230A (1511) were purchased from Tocris Bioscience (Avonmouth, Bristol, UK). Mito Stress Test Kit (103015–100) was purchased from Agilent Technologyies (Wilmington, USA).
2.2. Cell culture and induction of adipogenesis
3T3-L1 and C3H10T1/2 cell lines were purchased from China Infrastructure of Cell Line Resource (Beijing, China). The cell lines were maintained in DMEM supplemented with 10% fetal bovine serum (FBS) and 1% penicillin-streptomycin solution (called basic medium) in a humidified atmosphere containing 5% CO2 at 37 °C. For inducing beige/brown adipogenesis, confluent cells were cultured in the basic medium containing 1 μM dexamethasone, 0.5 mM 3-isobutyl-1-methylxanthine, 5 μg/mL insulin, 50 nM T3 and 125 μM indomethacin (called differentiation medium) with or without PA for 2 days and then switched to the basic medium containing 5 μg/mL insulin and 50 nM T3 with or without PA for 6 days. The medium was changed every other day. To induce adipogenic commitment, C3H10T1/2 cells were pretreated with 50 ng/mL BMP7 [10, 11] or PA until the cells became confluent. The confluent cells were cultured in the differentiation medium for 2 days and then switched to the basic medium including 5 μg/mL insulin and 50 nM T3 for 6 days. The medium was changed every other day. Unless otherwise specified, PA was used at 50 μM to treat cells. To inhibit PPARα, 10 µM GW6471, a PPARα inhibitor, was used. To inhibit β3-AR, 10 µM β3-AR antagonist SR59230A was used.
2.3. Isolation and differentiation of primary brown adipocytes
Isolation of primary brown preadipocytes were performed as described previously [32]. Briefly, the interscapular brown adipose tissue was dissected from mice, minced, and digested for 20 min at 37°C in the digestion medium (1.5 U/mL Collaginase D, 2.4 U/mL Dispase II and 10 mM CaCl2). Digested tissue was filtered through a 70 μm cell strainer and the cell suspension was then centrifuged for 10 min at 700 g to pellet cells. Primary brown preadipocytes were resuspended in complete culture medium containing DMEM/F12, Pen/Strep and 10% FBS, and then plated onto culture dishes. The differentiation of primary brown adipocytes were performed as mentioned above.
2.4. Oil-Red O staining
Oil-Red O staining was conducted as previously described [33, 34]. Briefly, differentiated cells were fixed with 4% paraformaldehyde (PFA) and stained with 0.15% Oil-Red O dye. The stained Oil-Red O was then extracted with isopropanol and the absorbance was spectrophotometrically measured at 530 nm.
2.5. Quantitative real-time PCR
Total RNA was extracted from cells using RNAprep pure Cell Kit (Tiangen biotech, Beijing, China) according to the manufacturer’s protocol and cDNA was synthesized from 1–2 μg of total RNA using M-MLV Reverse Transcriptase (Promega, Fitchburg, WI, USA). The cDNAs were used for quantitative real-time PCR analysis with SYBR™ Select Master Mix (Applied Biosystems™, Waltham, MA, USA). The relative expression level was calculated after normalization to β-actin reference using 2−ΔΔCT method. Primer sequences are shown in Table 1.
Table 1.
The primer sequences
| Gene | Forward primer sequence (5’ to 3’) | Reverse primer sequence (5’ to 3’) |
|---|---|---|
| β-actin | GATCTGGCACCACACCTTCT | GGGGTGTTGAAGGTCTCAAA |
| PGC1α | ATGTGTCGCCTTCTTGCTCTTCC | GGACCTTGATCTTGACCTGGAATATGG |
| Prdm16 | CAGCAACCTCCAGCGTCACATC | GCGAAGGTCTTGCCACAGTCAG |
| Ucp1 | ACTCAGGATTGGCCTCTACGACTC | GCATTCTGACCTTCACGACCTCTG |
| Pparα | CTTCACGATGCTGTCCTCCTTGATG | GATGTCACAGAACGGCTTCCTCAG |
| Cidea | GCCGTGTTAAGGAATCTGCTGAGG | GGATGGCTGCTCTTCTGTATCGC |
| Tbx1 | GGCAGACGAATGTTCCCCAC | CATCCACTGTGCGCCCTTAG |
| Cd137 | TGGTCATTGTGCTGCTGCTAGTG | CAGTTCGGCTGTCCACCTATGC |
| Ffg21 | CTGCTGCTGGCTGTCTTCCTG | GTGTCTTGGTCGTCATCTGTGTAGAG |
| Resistin | TCCTGTGGCTCTGCCTGTGG | GCTGCTGTCCAGTCTATCCTTGC |
| Retnla | ACTTCTTGCCAATCCAGCTAACTATCC | GTCCAGTCAACGAGTAAGCACAGG |
| Psat1 | CTTCCCCTGCTGTCGCCTTA | AGCACACTGATGCCGAGTCC |
| Zfp423 | GAGAGTGCTGAGGACCTGGAGAG | GGTTGGCGACGTGGATCTGAATC |
| Tfam | CCATGCAAGGCTTTTCCTCAG | TGGGATTTGCCAGCTCAAAGT |
| Cox7a | CGTGTGGCAGAGAAGCAGAA | GCCCAGCCCAAGCAGTATAA |
| Sox9 | GAGTGTCCCTTAGCCTTCCT | CCAAGAGCCTGGGGAATTAATCA |
| MyoD [40] | CGCCACTCCGGGACATAG | GAAGTCGTCTGCTGTCTCAAAGG |
| Col1a1 [17] | GCATGGCCAAGAAGACATCC | CCTCGGGTTTCCACGTCTC |
| Col2a1 [17] | CCACACCAAATTCCTGTTCA | ACTGGTAAGTGGGGCAAGAC |
| Nrf1 [16] | AGCACGGAGTGACCCAAAC | TGTACGTGGCTACATGGACCT |
| Nrf2 [35] | TGAAGTTCGCATTTTGATGGC | CTTTGGTCCTGGCATCTCTAC |
| Tfb1m [35] | ATAGAGCCCAAGATCAAGCAG | TGTAACAGCCTTCCAGTGC |
| Uqcrc1 [35] | ATCAAGGCACTGTCCAAGG | TCATTTTCCTGCATCTCCCG |
| Sdhb [35] | ACCCCTTCTCTGTCTACCG | AATGCTCGCTTCTCCTTGTAG |
| ATP5a1 [35] | CATTGGTGATGGTATTGCGC | TCCCAAACACGACAACTCC |
| COX2 [35] | ATAACCGAGTCGTTCTGCCAAT | TTTCAGAGCATTGGCCATAGAA |
| β-globin [36] | GAAGCGATTCTAGGGAGCAG | GGAGCAGCGATTCTGAGTAGA |
2.6. Western blotting
Cells were lysed in the cell lysis buffer (Beyotime, Haimen, Jiangsu, China) containing 1 mM phenylmethylsulfonyl fluoride (PMSF) on ice for 20 min and the supernatant was collected after centrifugation at 10000 g for 15 min. The protein concentration was measured using the BCA Protein Assay Kit (Beyotime, Haimen, Jiangsu, China). The samples were boiled at 100 ℃ for 10 min to denature the protein. The proteins were subjected to SDS-polyacrylamide gel and transferred to PVDF membranes (Millipore, Billerica, MA, USA). These membranes were in 5% bovine serum albumin for blocking and incubated overnight at 4 ℃ with primary antibodies. Secondary antibodies were then incubated for 1 h at room temperature. Protein bands were detected by SuperSignal West Dura Extended Duration Substrate (Thermo Scientific, Waltham, MA, USA) and images were captured by Amersham Imager 600 imaging system (GE Healthcare Life Sciences, Pittsburgh, PA, USA). Gray analysis was performed using image J software and the protein band density was normalized to the β-actin expression.
2.7. Mitochondrial DNA (mtDNA) Measurement
Mt DNA measurement was conducted as previously described [35, 36]. Genomic DNA was isolated with TIANamp Genomic DNA Kit (Tiangen biotech, Beijing, China) according to the manufacturer’s protocol and then used for quantitative real-time PCR analysis with SYBR™ Select Master Mix (Applied Biosystems™, Waltham, MA, USA). The mtDNA was amplified using primers specific for the mitochondrial COX2 gene and normalized to genomic DNA by amplification of the β-globin nuclear gene. Primer sequences are shown in Table 1.
2.8. Oxygen consumption rate measurements
3T3-L1 cells were plated in an XF24-well microplate (Seahorse Bioscience) and differentiated into beige adipocytes. Oxygen consumption rate (OCR) was measured using an XF24 analyser (Seahorse Bioscience) according to the manufacturer’s instructions. 1 µM oligomycin, 1 µM FCCP and 0.5 µM rotenone/antimycin were injected to detect ATP production, maximal respiration and non-mitochondrial respiration, respectively.
2.9. PPARα gene silencing in 3T3-L1 cells and C3H10T1/2 cells
To knockdown PPARα, shRNA and control scramble lentivirus were designed and synthesized by Genomeditech Company. For lentivirus production, 293T cells were transfected with the vector using Lipofectamine 3000 (Invitrogen, Waltham, MA, USA)) and the conditioned medium was centrifuged at 4,500g for 20 min 2 days later. The supernatant was used to infect 3T3-L1 and C3H10T1/2 cells in the presence of 8μg/mL polybrene. Infected cells were selected 2 days later using 2μg/mL puromycin (Sigma, St Louis, MO, USA). The sequences used for shRNA-mediated experiments were: sh Pparα No.1, 5’- GGAAGCCGTTCTGTGACATCA −3’; sh Pparα No.2, 5’-GCTAAAGTACGGTGTGTATGA-3’.
2.10. Statistical Analyses
The data were generated from three independent experiments and three parallels were used in each experiment. All data were reported as means ± standard deviations (SD). Differences between groups were calculated using the Student’s t test or one-way ANOVA (for multiple comparison). P < 0.05 was considered to be statistically significant.
3. Results
3.1. PA promotes beige adipogenesis of 3T3-L1 preadipocytes, but not in C3H10T1/2 progenitor cells.
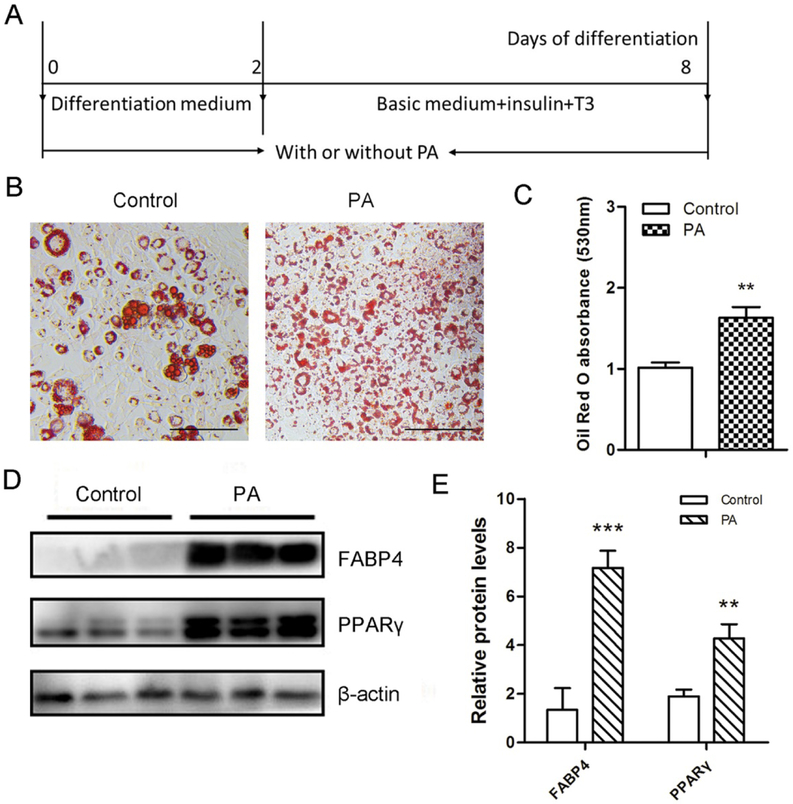
To determine the optimal concentration of PA in inducing beige adipogenesis, 3T3-L1 cells were treated with PA at various concentrations including 10 μM, 50 μM and 100 μM. 100 μM PA caused toxicity to cells and 50 μM PA was used for subsequent experiments for its strong effect on beige adipogenesis based on Oil-Red O staining. PA treatment (Fig. 1A) increased the density of multilocular lipid droplets during 3T3-L1 beige adipogenesis (Fig. 1B and 1C). Consistently, PA promoted protein contents of adipogenic markers PPARγ and FABP4 in 3T3-L1 cells (Fig. 1D and 1E). To explore the effect of PA on the commitment of C3H10T1/2, PA was used to treat cells for 2 days before the differentiation of progenitor cells into brown adipocytes (Fig. S1A). BMP7 profoundly stimulates brown adipogenesis [10]. Here, we compared the effects of PA with BMP7, and we found that BMP7 promoted brown adipogenic differentiation as shown by increased lipid droplets, while PA did not affect the lipid accumulation (Fig.S1B and S1C). We also tested the effect of PA on the differentiation of C3H10T1/2 into brown adipocytes (Fig. S1D). We found that PA did not enhance the lipid accumulation in this process either (Fig. S1E and S1F). These findings demonstrated that PA had no obvious effects on lipid accumulation in C3H10T1/2 mesenchymal progenitor cells under brown adipogenic induction. Taken together, these data suggest that PA stimulates the beige adipogenesis in committed preadipocytes but not in uncommitted progenitor cells.
Figure 1. Effects of PA on the lipid accumulation and the expression of adipogenic markers in differentiated 3T3-L1 cells.
3T3-L1 preadipocytes were induced into beige adipocytes through the treatment of PA for 8 days. (A) Confluent 3T3-L1 cells were cultured in the differentiation medium with or without PA for 2 days and then switched to the basic medium including insulin and T3 with or without PA for 6 days. (B) Oil-Red O staining of differentiated 3T3-L1 white adipocytes. Bar, 100 μm. (C) The absorbance of the stained Oil-Red O at 530 nm. (D) Western blotting analysis of adipogenic markers FABP4 and PPARγ in the differentiated 3T3-L1 white adipocytes. β-actin was used as the control. (E) Relative quantitative analysis of protein bands. Data are shown as means ± SD of three independent experiments. **p≤0.01 versus control; ***p≤0.001 versus control.
3.2. PA promotes formation of beige adipocytes.
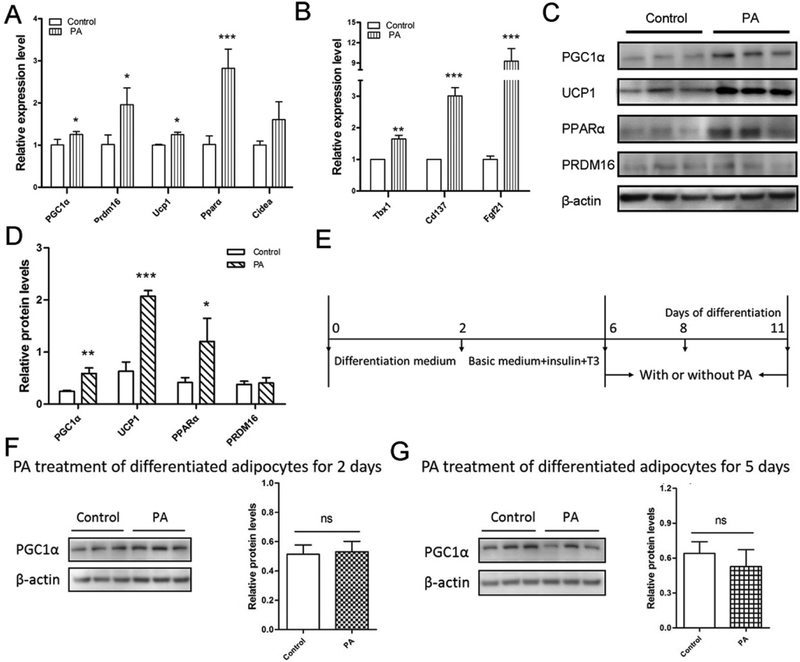
Furthermore, in 3T3-L1 cells, PA elevated the mRNA expression of brown adipocyte marker genes including PGC1α, Prdm16, Ucp1 and Pparα (Fig. 2A), and also markedly increased beige adipocyte markers such as Fgf21, Cd137 and Tbx1 (Fig. 2B). Consistently, PGC1α, UCP1 and PPARα protein contents in PA-treated cells were 2-fold higher than those of control cells (Fig. 2c and 2d). However, the protein content of PRDM16, a key brown adipogenic regulator [7], remained unchanged (Fig. 2C and 2D). These data demonstrate that PA has positive effects on promotion of beige adipogenic differentiation of committed preadipocytes. To test whether PA treatment could promote white differentiated adipocytes beiging, PA was used to culture differentiated adipocytes (Fig. 2E). Followed by PA treatment for 2 and 5 days respectively, PGC1α protein expression was examined. The PGC1α content was not significantly changed in the PA presence (Fig. 2F and 2G), indicating that PA hardly plays a role in the differentiated white adipocytes beiging, and it mainly functions in beige adipogenesis using committed but undifferentiated 3T3-L1 as the starting point.
Figure 2. PA promotes beige adipocyte biogenesis.
3T3-L1 preadipocytes were induced into beige adipocytes through the treatment of PA for 8 days. (A and B) Quantitative real-time PCR analysis of brown (A) and beige (B) adipocyte marker genes in beige adipocytes from 3T3-L1 white adipocytes. Data are shown as means ± SD of three independent experiments. (C) Western blotting analysis of brown adipogenic markers in beige adipocytes. β-actin was used as the control. (D) Relative quantitative analysis of protein bands. (E) Confluent 3T3-L1 cells were cultured in the differentiation medium for 2 days, switched to the basic medium including insulin and T3 for 4 days and then changed to the medium (DMEM and 10% FBS) with or without PA for 2 days and 5 days, respectively. (F and G) Western blotting analysis of PGC1α content and relative quantitative analysis of protein bands. Data are shown as means ± SD of three independent experiments. *p≤0.05 versus control; **p≤0.01 versus control; ***p≤0.001 versus control; ns indicates no significant differences between PA treatment and the control group.
Next, we determined whether PA could stimulate the browning of C3H10T1/2 progenitor cells. PA notably increased PGC1α expression, but not other brown fat markers such as Ucp1 and Prdm16 or beige adipocyte markers Fgf21, Cd137 and Tbx1 (Fig. S2A and S2B). Similarly, PGC1α protein content was elevated in PA-treated group while PRDM16 and UCP1 contents were not significantly changed (Fig. S2C and S2D). These data indicate that PA did not trigger the differentiation of progenitor cells to brown adipocyte lineage while it promoted the expression of PGC1α that is a master regulator of mitochondrial biogenesis [37].
Because C3H10T1/2 cells are multipotent, to further examine the effect of PA on lineage differentiation of C3H10T1/2 cells, we tested marker gene expression of other lineages. Both 10 μM and 50 μM PA treatment did not alter white adipocyte markers including Resitin, Psat1, Zfp423, Retnla (Fig. S2E), nor chondrogenesis markers Sox9, Col1a1, Col2a1 and myogenesis marker MyoD (Fig. S2F). Taken together, these results demonstrate that PA had no noticeable effects on other lineage differentiation of C3H10T1/2 cells to white adipocytes, myocytes and chondrocytes.
3.3. PA increases mitochondrial biogenesis and oxygen consumption.
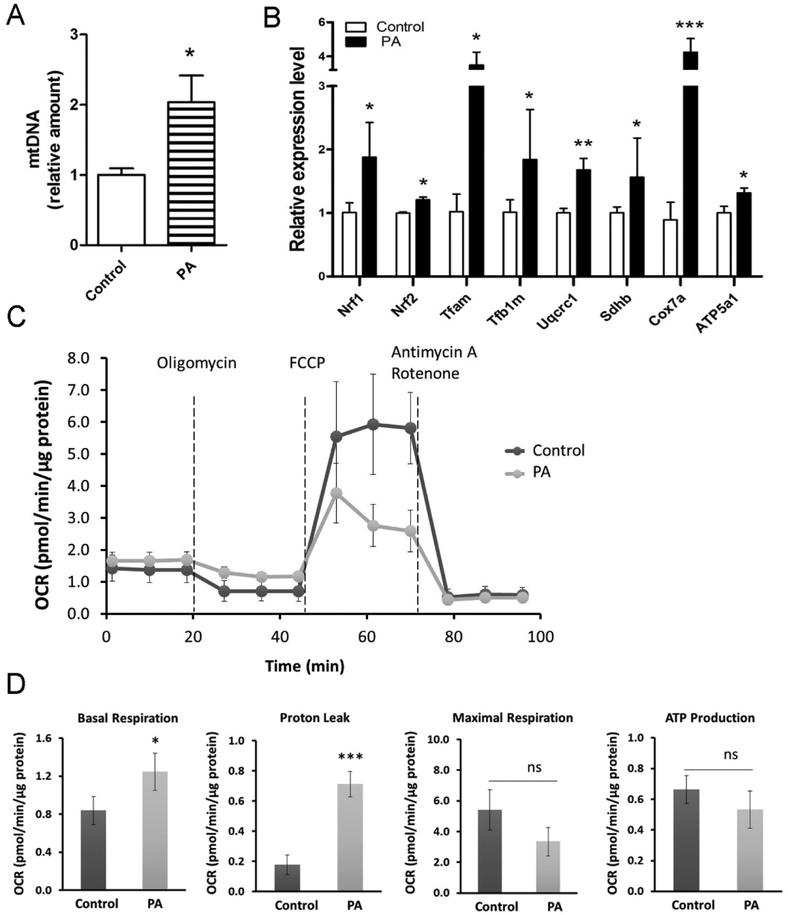
Mitochondrial biogenesis is key for brown fat thermogenesis and browning of WAT [1]. Treatment with PA increased mtDNA content in both 3T3-L1 (Fig. 3A) and C3H10T1/2 (Fig. S3A) during brown adipogenic induction, suggesting that PA increased mitochondrial biogenesis. As a master regulator of mitochondrial biogenesis, PGC1α was substantially elevated by PA treatment at both mRNA and protein levels (Fig. 2A, 2C, S2A and S2C). In agreement, mitochondrial transcription factors (i.e. Tfam and Tfb2m), nuclear respiratory factors (i.e. Nrf1 and Nrf2) and components of the mitochondrial electron transport chain (i.e. Uqcrc1, Sdhb, Cox7a and ATP5a1) were also elevated in the PA treatment group (Fig. 3B and S3B). Additionally, using a Seahorse XF-24 extracellular flux analyzer, we observed that basal mitochondrial respiration and proton leak significantly increased in PA-treated beige adipocytes (Fig. 3C and 3D), indicating that PA could promote the oxygen consumption and uncoupled respiration during beige adipogenesis.
Figure 3. PA stimulates mitochondrial biogenesis.
3T3-L1 preadipocytes were induced into beige adipocytes through the treatment of PA for 8 days. (A) Mitochondrial DNA content analyzed by quantitative real-time PCR in differentiated 3T3-L1 white adipocytes treated with PA. Relative expression values were normalize to control cells. (B) Quantitative real-time PCR analysis of mitochondrial marker genes in beige adipocytes. Data are shown as means ± SD of three independent experiments. (C and D) The oxygen consumption rate (OCR) in differentiated beige adipocytes from 3T3-L1 cells (n=3). The OCR was measured before and after injection of 1 µM oligomycin (the ATP synthase inhibitor), 1 µM FCCP (the uncoupling agent) and 0.5 µM Antimycin A/rotenone (the inhibitor of complex III and complex I). Basal respiration was measured before addition of oligomycine. Proton leak (uncoupled respiration) and ATP production were measured after addition of oligomycine. Maximal respiration was measured after addition of FCCP. *p≤0.05 versus control; ***p≤0.001 versus control; ns indicates no significant differences.
Taken together, these data demonstrated that PA promoted mitochondrial biogenesis and oxygen consumption during beige adipogenesis.
3.4. PA stimulates the phosphorylation of AMPK.
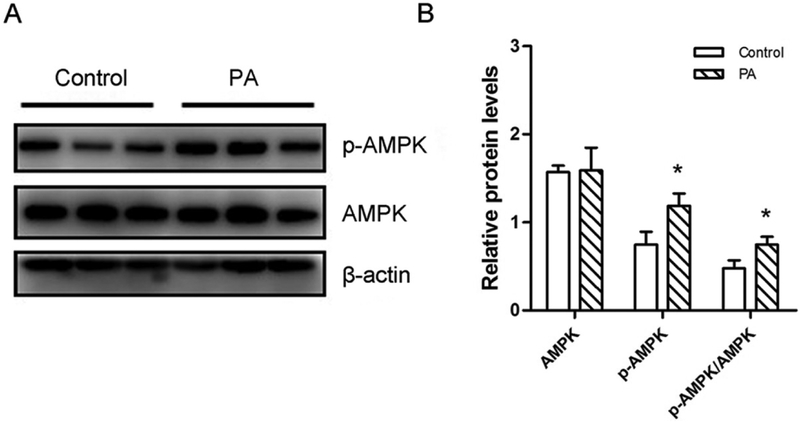
AMPK, an energy sensor of cells, is crucially involved in energy metabolism and is highly expressed during browning [13, 35, 38]. To understand whether AMPK was involved in the PA-mediated browning process, we tested the effect of PA on the phosphorylation of AMPKα (p-AMPK). PA increased the AMPKα phosphorylation in differentiated 3T3-L1 and C3H10T1/2 cells without obvious effects on total AMPKα content (Fig. 4 and S4). Moreover, the ratio of p-AMPK to AMPK was upregulated in the PA-treated group (Fig. 4 and S4). These data suggested that the AMPKα signaling pathway was activated in response to PA.
Figure 4. PA has a positive effect on the phosphorylation of AMPK.
3T3-L1 preadipocytes were induced into beige adipocytes through the treatment of PA for 8 days. (A) Western blotting analysis of p-AMPK and AMPK in beige adipocytes. β-actin was used as the control. (B) Relative quantitative analysis of protein bands. Data are shown as means ± SD of three independent experiments. *p≤0.05 versus control.
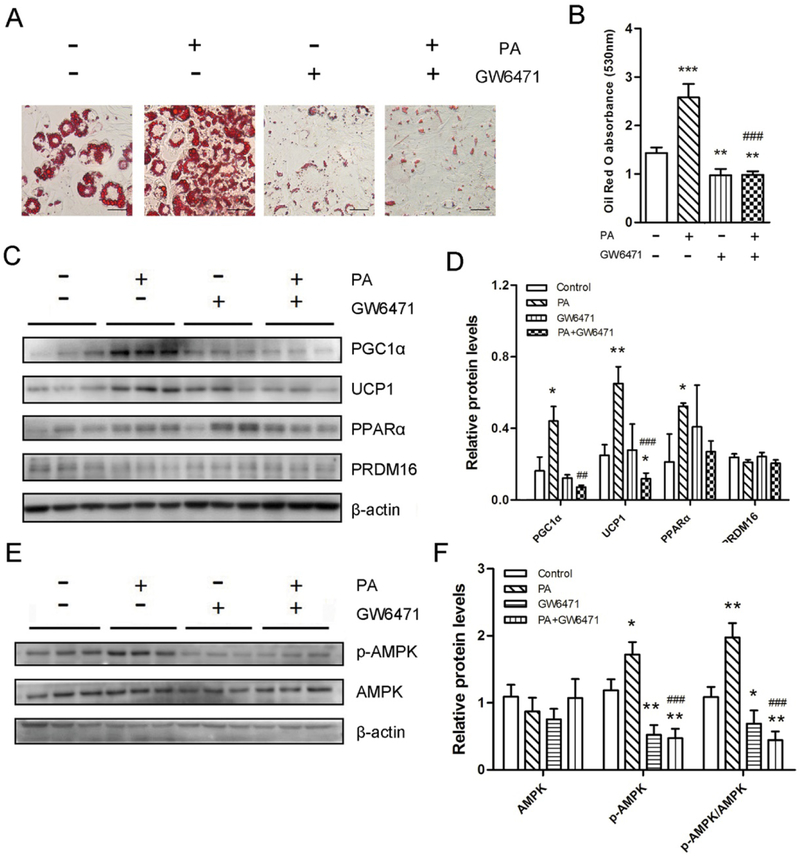
3.5. PPARa antagonist eliminates browning effects of PA.
Given the previous findings that PPARα has a positive role in the browning process [28, 29] and PA was a natural PPARα ligand [31, 39], we hypothesized that PPARα was required for PA-mediated beige adipocyte formation. PPARα antagonist GW6471 was used to inhibit PPARα. Remarkably, GW6471 abolished the lipid accumulation in both 3T3-L1 (Fig. 5A and 5B) and C3H10T1/2 cells (Fig. S5A and S5B), suggesting that GW6471 inhibited beige and brown adipocyte differentiation. Besides, compared with PA-treated group, brown adipocytes and mitochondrial biogenesis marker proteins (UCP1 and PGC1α) were down-regulated in the presence of GW6471 (Fig. 5C, 5D, S5C and S5D). The master brown adipogenic regulator PRDM16 remained unchanged (Fig. 5C, 5D, S5C and S5D). Taken together, these results indicate that PPARα inhibition eliminates PA-mediated browning effects in both committed preadipocytes and uncommitted progenitor cells.
Figure 5. PPARα antagonism inhibits PA-stimulated browning of white adipocytes.
3T3-L1 preadipocytes were induced into beige adipocytes through the treatment of PA or GW6471 for 8 days. (A) The PPARα antagonist GW6471 inhibits PA- mediated browning effects. Oil-Red O staining of differentiated 3T3-L1 white adipocytes. Bar, 100 μm. (B) The absorbance of the stained Oil-Red O at 530 nm. (C and E) Western blotting analysis of PGC1α, UCP1, PPARα, PRDM16 (C) and p-AMPK/AMPK (E) in beige adipocytes. β-actin was used as the control. (D and F) Relative quantitative analysis of protein bands. Data are shown as means ± SD of three independent experiments. *p≤0.05 versus control; **p≤0.01 versus control; ## p ≤0.01 versus PA treatment; ### p ≤0.001 versus PA treatment.
The elevated phosphorylation of AMPKα was also reduced while total AMPK was not changed by GW6471 (Fig. 5E, 5F, S5E and S5F), suggesting that PA enhances the phosphorylation of AMPKα during the browning process, at least in part, via PPARα.
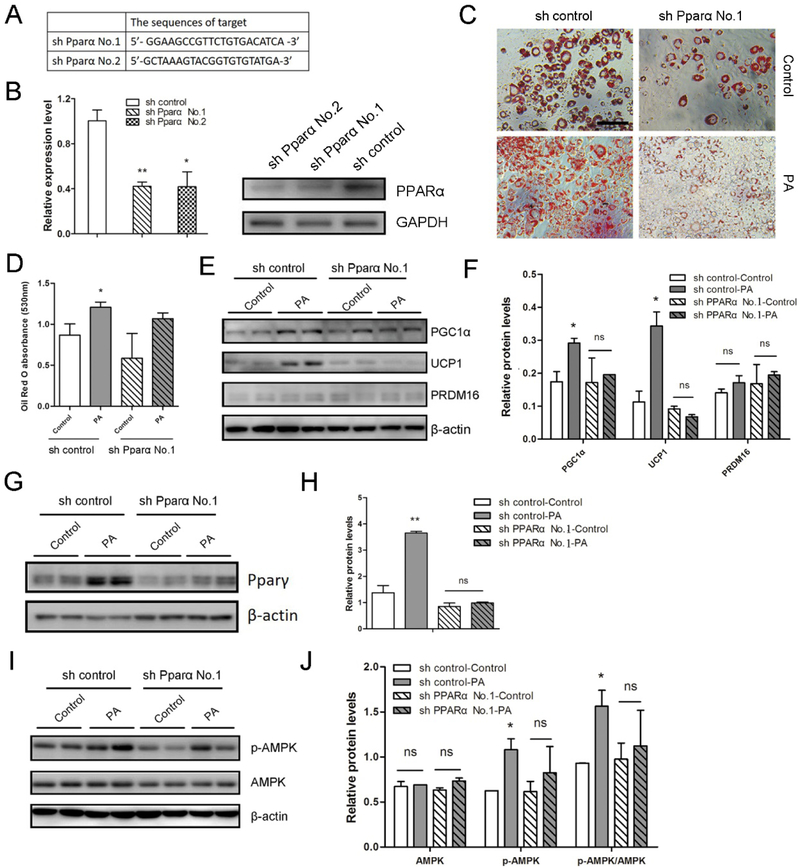
3.6. Knockdown of PPARα abolishes PA- mediated brown and beige adipogenesis.
To exclude the possible nonspecific effects of inhibitor, we further knocked down Pparα gene to confirm the roles of PPARα in PA-mediated beige adipogenesis. We introduced a short hairpin (sh) RNA for PPARα into 3T3-L1 cells, which effectively knocked down PPARα protein levels (Fig. 6A and 6B). Following Pparα knockdown in 3T3-L1 cells, the enhanced lipid accumulation by PA treatment was largely abolished (Fig. 6C and 6D). The upregulation of UCP1 and PGC1α protein contents in PA-treated group were profoundly decreased (Fig. 6E, 6F, S6A and S6B), while the effect of Pparα knockdown on the PRDM16 protein expression was not significant (Fig. 6E and 6F). PPARγ content was also decreased in the PA presence (Fig. 6G, 6H, S6C and S6D). These data indicate that PPARα is required for brown-adipocyte specific marker expression in the PA-mediated browning process. Additionally, the elevated phosphorylation of AMPKα was eliminated while total AMPK was not significantly changed (Fig. 6I and 6J), indicating that PA enhances the phosphorylation of AMPKα through activation of PPARα during beige adipogenesis.
Figure 6. PPARα is necessary for the effect of PA on beige adipocyte biogenesis.
3T3-L1 preadipocytes with knockdown of PPARα were induced into beige adipocytes through the treatment of PA for 8 days. (A) The target sequences used for shRNA-mediated experiments. (B) Quantitative real-time PCR analysis of Pparα (left) and western blot analysis of PPARα (right) in shRNA control and shRNA PPARα 3T3-L1 cells. (C) Oil-Red O staining of differentiated 3T3-L1 cells. Bar, 100 μm. (D) The absorbance of the stained Oil-Red O at 530 nm. (E, G and I) Western blotting analysis of brown adipogenic markers (E), PPARγ (G), AMPK and p-AMPK (I) contents in the indicated cells. β-actin was used as the control. (F, H and J) Relative quantitative analysis of protein bands. *p≤0.05 versus control; **p≤0.01 versus control; ns indicates no significant differences between PA treatment and the control group.
To further test PPARα effects on PA-mediated brown adipocyte differentiation, we also introduced shRNA for PPARα into C3H10T1/2 cell line (Fig. S6E). In knockdown cells, lipid accumulation was not significantly changed (Fig. S6F and S6G), while elevation of PGC1α by PA treatment was decreased (Fig. S6H and S6I). The data suggest that PPARα is necessary for PGC1α expression in the PA-mediated mitochondriogenesis from C3H10T1/2 progenitor cells. Taken together, PPARα is a key factor during PA-mediated beige adipogenesis, primarily through enhancing PGC1α expression and mitocondriogenesis.
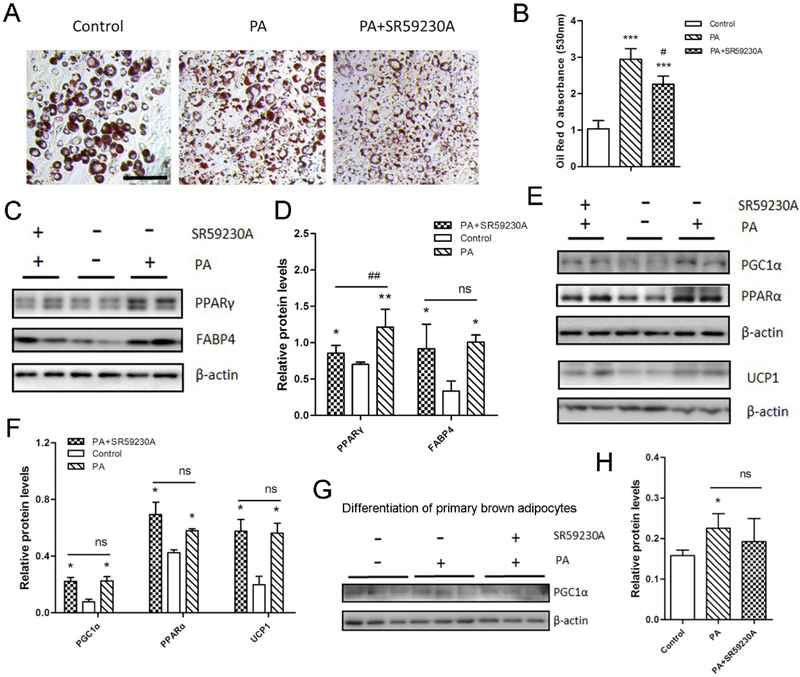
3.7. PA-mediated beige adipogenesis is independent on β3-adrenergic receptor.
To explore whether PA-mediated beiging is through β3-adrenergic receptor (β3-AR) independent mechanism, the β3-AR antagonist SR59230A was used for PA-stimulated beige adipocyte differentiation. Through Oil-Red O staining, we found that with the addition of SR59230A, lipid droplets were slightly reduced compared with that in PA-treated cells. However, the lipid droplets were still significantly higher compared with the control group (Fig.7A and 7B). Additionally, in the PA treatment group, adipocyte markers PPARγ and FABP4 protein contents were both higher regardless of the presence of SR59230A or not (Fig.7C and 7D). The upregulation of UCP1, PGC1α and PPARα protein contents in PA-treated group were not downregulated with the addition of SR59230A (Fig.7E and 7F). Similarly, PA-mediated PGC1α upregulation in the differentiation of primary brown adipocytes did not significantly change in the presence of SR59230A (Fig.7G and 7H).
Figure 7. PA-stimulated browning of white adipocytes is independent on β3-AR.
3T3-L1 preadipocytes were induced into beige adipocytes through the treatment of PA and SR59230A for 8 days. (A) Oil-Red O staining of differentiated 3T3-L1 white adipocytes. Bar, 100 μm. (B) The absorbance of the stained Oil-Red O at 530 nm. (C and E) Western blotting analysis of adipocyte markers (C) and brown adipocyte markers (E) in beige adipocytes. β-actin was used as the control. (D and F) Relative quantitative analysis of protein bands. Data are shown as means ± SD of three independent experiments. (G and H) Western blotting analysis of PGC1α during differentiation of primary brown adipocytes (G) and relative quantitative analysis of protein bands (H). Data are shown as means ± SD of three independent experiments.*p≤0.05 versus control; **p≤0.01 versus control; ***p≤0.001 versus control; # p ≤0.05 versus PA treatment; ## p ≤0.01 versus PA treatment; ns indicates no significant differences.
Taken together, β3-AR antagonist SR59230A did not abolish the PA-promoted browning of 3T3-L1 cells and primary brown adipocytes. PA-stimulated beige adipogenesis is independent on β3-AR.
4. Discussion
Due to their ability to effectively burn lipids for thermogenesis, enhancing brown and beige adipogenesis is a promising method to treat obesity and associated metabolic diseases [40, 41]. PA is a bioactive component of milk and dairy products, marine foods and meat from ruminant animals [20]. It has been reported to increase brown adipogenesis in HIB-1B cells and primary brown preadipocytes, but mechanisms remain undefined [24, 25]. Brown/beige adipogenesis can be separated into the commitment of progenitor cells into preadipocytes, and the further differentiation of preadipocytes into mature brown/beige adipocytes. The major difference between white and beige adipocytes is the expression of UCP1. New beige adipocytes are induced from PDGFRa+ progenitor cells through a two-step process, adipogenic commitment and differentiation [42]. Beige adipogenesis is initiated by PRDM16, which induces the expression of brown/beige-specific genes, including UCP1, while white adipocytes is initiated by the expression of Zinc Finger protein (ZFP) 423 [4, 6]. To our knowledge, the effect of PA on beige adipogenic commitment and differentiation has not been systemically explored. In this study, we found that PA promoted multilocular lipid accumulation and gene expression of beige (i.e. Fgf21, Tbx1 and Cd137) and brown (i.e. Ucp1, PGC1α and Pparα) fat cell markers. In addition, protein expression levels of PGC1α, UCP1 and PPARα in beige adipocytes, which are known as the brown adipocytes-selective markers, were elevated by PA treatment. These findings strongly support that PA promotes the formation of beige adipocytes.
Interestingly, the master brown adipocyte regulator PRDM16 were not increased in the protein level following PA stimulation, suggesting that PA might not have significant effects on the early stage of brown adipogenesis. Consistently, we found that, in uncommitted C3H10T1/2 progenitor cells, PA did not increase lipid accumulation and UCP1 and PRDM16 protein expression levels, nor the expression of other brown adipocyte-selective markers (i.e. Ucp1, PGC1α and Prdm16), white adipocyte markers (i.e. Resitin, Psat1, Zfp423 and Retnla) and markers of chondrogenesis and myogenesis (i.e. Sox9, Col1a1, Col2a1 and MyoD). In combination, these data show that PA positively affects the beige adipogenic differentiation but not the commitment of progenitor cells.
Given our observation that PA increased the mRNA and protein levels of PGC1α, which is a key regulator of mitochondrial biogenesis regulator, we further analyzed mitochondriogenesis which was promoted by PA treatment. Consistently, AMPK was also activated, which is in agreement with the effect of phytol in the browning process [19]. This phenomenon suggests that phytol and its metabolite PA may share signal pathways to promote beige adipocyte differentiation.
It has been reported that PA was a natural ligand of PPARα in the human diet [31, 39]. Thus, the mediatory role of PPARα was further tested by using antagonist and knockdown. Inhibition of PPARα decreased the promotion effects of PA in the browning process, demonstrating that PA requires PPARα to promote beige adipocyte differentiation. Consistently, in the mouse model, a phytol-enriched diet elevated PA levels and activated PPARα in the liver and BAT, which increase thermogenesis [26]. Our results were consistent with these findings, and we further discovered that PA promotes beige adipocyte differentiation but not commitment. In addition, we established the mediatory role of PPARα through both using antagonist and also gene knockdown.
Our study also indicated that as a PPARα ligand, PA promoted the phosphorylation of AMPKα possibly via PPARα, which is similar to the previous reports that PPARα activators such as fenofibrate and WY14643 could activate AMPK signaling pathway [43, 44]. AMPK plays an important role in regulating energy homeostasis in cells [27]. AMPK activation impedes hepatic lipogenesis and adipocyte differentiation and promotes glucose uptake in muscle [45, 46]. Therefore, PPARα agonists including PA may activate AMPK to exert beneficial effects on the prevention of metabolic syndrome.
Activation of β3-AR has been shown to have profound effects on browning [47, 48]. However, we found that PA maintained the ability to stimulate the browning of 3T3-L1 cells and primary brown adipocytes in the presence of β3-AR antagonist, SR59230A, demonstrating that PA-mediated browning is independent on β3-AR.
In conclusion, our data revealed that PA mainly boosts beige adipogenesis via stimulation of PPARα pathway, and it functions primarily to enhance beige/brown adipogenic differentiation not the commitment. Moreover, the effects of PA on beige adipogenesis is independent on β3-AR. Compared to these chemosynthesis drugs [49], PA, a natural agent, has low toxicity and is safer for the promotion of beige adipogenesis. In our study, we used 50 μM PA for testing, which represents the high end of physiological PA level and is consistent the previous report [5]. The concentration of PA in the plasma of healthy human varies from 0.04–9.88 μM [3], whereas, in Refsum disease, patient levels have been reported to be 240–1400 μM [4]. PA is readily available through food (i.e. milk and dairy products, beef and fatty fish), which is in contrast to synthetic agonists of PPARα such as fibrates (i.e. clofibrate, fenofibrate and bezafibrate), available only as dietary supplements [20, 39]. Because of the wide availability of PA in diets, increasing PA intake provides an effective method for enhancing beige adipocyte differentiation, thus preventing or reducing obesity and its associated metabolic dysfunction.
Supplementary Material
Acknowledgements
This work was supported by the National Natural Science Foundation of China (Grant No. 31371753), and the Beijing Dairy Industry Innovation Team (BAIC06–2018) to XM, and NIH R01HD067449 to MD.
Abbreviations:
- AMPK
AMP-activated protein kinase
- BAT
Brown adipose tissue
- PA
Phytanic acid
- PPARα
Peroxisome proliferator-activated receptor α
- PGC-1α
Peroxisome proliferator-activated receptor γ coactivator 1α
- UCP1
Uncoupling protein 1
- WAT
White adipose tissue
Footnotes
Conflict of Interest
The authors declare no conflict of interest.
References
- [1].Rosen ED, Spiegelman BM. What We Talk About When We Talk About Fat. Cell 2014; 156:20–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Van Gaal LF, Mertens IL, De Block CE. Mechanisms linking obesity with cardiovascular disease. Nature 2006; 444:875–880. [DOI] [PubMed] [Google Scholar]
- [3].Inagaki T, Sakai J, Kajimura S. Transcriptional and epigenetic control of brown and beige adipose cell fate and function. Nature Reviews Molecular Cell Biology 2016; 17:480–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Wu J, Bostrom P, Sparks LM, Ye L, Choi JH, Giang AH et al. Beige adipocytes are a distinct type of thermogenic fat cell in mouse and human. Cell 2012; 150:366–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Villarroya F, Vidal-Puig A. Beyond the sympathetic tone: the new brown fat activators. Cell Metab 2013; 17:638–643. [DOI] [PubMed] [Google Scholar]
- [6].Bostrom P, Wu J, Jedrychowski MP, Korde A, Ye L, Lo JC et al. A PGC1-alpha-dependent myokine that drives brown-fat-like development of white fat and thermogenesis. Nature 2012; 481:463–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Seale P, Bjork B, Yang W, Kajimura S, Chin S, Kuang S et al. PRDM16 controls a brown fat/skeletal muscle switch. Nature 2008; 454:961–967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Ohno H, Shinoda K, Spiegelman BM, Kajimura S. PPARgamma agonists induce a white-to-brown fat conversion through stabilization of PRDM16 protein. Cell Metab 2012; 15:395–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Puigserver P, Wu Z, Park CW, Graves R, Wright M, Spiegelman BM. A cold-inducible coactivator of nuclear receptors linked to adaptive thermogenesis. Cell 1998; 92:829–839. [DOI] [PubMed] [Google Scholar]
- [10].Tseng YH, Kokkotou E, Schulz TJ, Huang TL, Winnay JN, Taniguchi CM et al. New role of bone morphogenetic protein 7 in brown adipogenesis and energy expenditure. Nature 2008; 454:1000–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Fisher FM, Kleiner S, Douris N, Fox EC, Mepani RJ, Verdeguer F et al. FGF21 regulates PGC-1alpha and browning of white adipose tissues in adaptive thermogenesis. Genes Dev 2012; 26:271–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Mori M, Nakagami H, Rodriguez-Araujo G, Nimura K, Kaneda Y. Essential role for miR-196a in brown adipogenesis of white fat progenitor cells. PLoS Biol 2012; 10:e1001314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Wang S, Liang X, Yang Q, Fu X, Rogers CJ, Zhu M et al. Resveratrol induces brown-like adipocyte formation in white fat through activation of AMP-activated protein kinase (AMPK) alpha 1. Int J Obesity 2015; 39:967–976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Wang B, Fu X, Liang X, Deavila JM, Wang Z, Zhao L et al. Retinoic acid induces white adipose tissue browning by increasing adipose vascularity and inducing beige adipogenesis of PDGFRalpha(+) adipose progenitors. Cell Discov 2017; 3:17036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Lone J, Choi JH, Kim SW, Yun JW. Curcumin induces brown fat-like phenotype in 3T3-L1 and primary white adipocytes. J Nutr Biochem 2016; 27:193–202. [DOI] [PubMed] [Google Scholar]
- [16].Zhang Z, Zhang H, Li B, Meng X, Wang J, Zhang Y et al. Berberine activates thermogenesis in white and brown adipose tissue. Nat Commun 2014; 5:5493. [DOI] [PubMed] [Google Scholar]
- [17].Imran KM, Rahman N, Yoon D, Jeon M, Lee BT, Kim YS. Cryptotanshinone promotes commitment to the brown adipocyte lineage and mitochondrial biogenesis in C3H10T1/2 mesenchymal stem cells via AMPK and p38-MAPK signaling. Biochimica Et Biophysica Acta-Molecular and Cell Biology of Lipids 2017; 1862:1110–1120. [DOI] [PubMed] [Google Scholar]
- [18].Zhang HQ, Chen SY, Wang AS, Yao AJ, Fu JF, Zhao JS et al. Sulforaphane induces adipocyte browning and promotes glucose and lipid utilization. Mol Nutr Food Res 2016; 60:2185–2197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Zhang F, Ai W, Hu X, Meng Y, Yuan C, Su H et al. Phytol stimulates the browning of white adipocytes through the activation of AMP-activated protein kinase (AMPK) alpha in mice fed high-fat diet. Food Funct 2018; 9:2043–2050. [DOI] [PubMed] [Google Scholar]
- [20].Roca-Saavedra P, Marino-Lorenzo P, Miranda JM, Porto-Arias JJ, Lamas A, Vazquez BI et al. Phytanic acid consumption and human health, risks, benefits and future trends: A review. Food Chem 2017; 221:237–247. [DOI] [PubMed] [Google Scholar]
- [21].Allen NE, Grace PB, Ginn A, Travis RC, Roddam AW, Appleby PN et al. Phytanic acid: measurement of plasma concentrations by gas-liquid chromatography-mass spectrometry analysis and associations with diet and other plasma fatty acids. The British journal of nutrition 2008; 99:653–659. [DOI] [PubMed] [Google Scholar]
- [22].Heim M, Johnson J, Boess F, Bendik I, Weber P, Hunziker W et al. Phytanic acid, a natural peroxisome proliferator-activated receptor (PPAR) agonist, regulates glucose metabolism in rat primary hepatocytes. FASEB J 2002; 16:718–720. [DOI] [PubMed] [Google Scholar]
- [23].Schluter A, Yubero P, Iglesias R, Giralt M, Villarroya F. The chlorophyll-derived metabolite phytanic acid induces white adipocyte differentiation. Int J Obes Relat Metab Disord 2002; 26:1277–1280. [DOI] [PubMed] [Google Scholar]
- [24].Schluter A, Barbera MJ, Iglesias R, Giralt M, Villarroya F. Phytanic acid, a novel activator of uncoupling protein-1 gene transcription and brown adipocyte differentiation. Biochem J 2002; 362:61–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Schluter A, Giralt M, Iglesias R, Villarroya F. Phytanic acid, but not pristanic acid, mediates the positive effects of phytol derivatives on brown adipocyte differentiation. FEBS Lett 2002; 517:83–86. [DOI] [PubMed] [Google Scholar]
- [26].An JY, Jheng HF, Nagai H, Sanada K, Takahashi H, Iwase M et al. A Phytol-Enriched Diet Activates PPAR-alpha in the Liver and Brown Adipose Tissue to Ameliorate Obesity-Induced Metabolic Abnormalities. Mol Nutr Food Res 2018; 62:e1700688. [DOI] [PubMed] [Google Scholar]
- [27].Lee WH, Kim SG. AMPK-Dependent Metabolic Regulation by PPAR Agonists. PPAR Res 2010; 2010: [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Rachid TL, Penna-de-Carvalho A, Bringhenti I, Aguila MB, Mandarim-de-Lacerda CA, Souza-Mello V. Fenofibrate (PPARalpha agonist) induces beige cell formation in subcutaneous white adipose tissue from diet-induced male obese mice. Mol Cell Endocrinol 2015; 402:86–94. [DOI] [PubMed] [Google Scholar]
- [29].Carmona MC, Louche K, Nibbelink M, Prunet B, Bross A, Desbazeille M et al. Fenofibrate prevents Rosiglitazone-induced body weight gain in ob/ob mice. Int J Obes (Lond) 2005; 29:864–871. [DOI] [PubMed] [Google Scholar]
- [30].Wang L, Teng R, Di L, Rogers H, Wu H, Kopp JB et al. PPARalpha and Sirt1 mediate erythropoietin action in increasing metabolic activity and browning of white adipocytes to protect against obesity and metabolic disorders. Diabetes 2013; 62:4122–4131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Ellinghaus P, Wolfrum C, Assmann G, Spener F, Seedorf U. Phytanic acid activates the peroxisome proliferator-activated receptor alpha (PPARalpha) in sterol carrier protein 2-/ sterol carrier protein x-deficient mice. J Biol Chem 1999; 274:2766–2772. [DOI] [PubMed] [Google Scholar]
- [32].Aune UL, Ruiz L, Kajimura S. Isolation and differentiation of stromal vascular cells to beige/brite cells. J Vis Exp 2013; [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Yang Q, Liang X, Sun X, Zhang L, Fu X, Rogers CJ et al. AMPK/alpha-Ketoglutarate Axis Dynamically Mediates DNA Demethylation in the Prdm16 Promoter and Brown Adipogenesis. Cell Metab 2016; 24:542–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Wang H, Xiang J, Zhang W, Li J, Wei Q, Zhong L et al. Induction of Germ Cell-like Cells from Porcine Induced Pluripotent Stem Cells. Sci Rep 2016; 6:27256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Price NL, Gomes AP, Ling AJY, Duarte FV, Martin-Montalvo A, North BJ et al. SIRT1 Is Required for AMPK Activation and the Beneficial Effects of Resveratrol on Mitochondrial Function. Cell Metab 2012; 15:675–690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Morita M, Gravel SP, Chenard V, Sikstrom K, Zheng L, Alain T et al. mTORC1 Controls Mitochondrial Activity and Biogenesis through 4E-BP-Dependent Translational Regulation. Cell Metab 2013; 18:698–711. [DOI] [PubMed] [Google Scholar]
- [37].Ventura-Clapier R, Garnier A, Veksler V. Transcriptional control of mitochondrial biogenesis: the central role of PGC-1alpha. Cardiovasc Res 2008; 79:208–217. [DOI] [PubMed] [Google Scholar]
- [38].van Dam AD, Kooijman S, Schilperoort M, Rensen PC, Boon MR. Regulation of brown fat by AMP-activated protein kinase. Trends Mol Med 2015; 21:571–579. [DOI] [PubMed] [Google Scholar]
- [39].Grygiel-Gorniak B Peroxisome proliferator-activated receptors and their ligands: nutritional and clinical implications - a review. Nutr J 2014; 13: [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Nie BM, Nie T, Hui XY, Gu P, Mao LF, Li K et al. Brown Adipogenic Reprogramming Induced by a Small Molecule. Cell Reports 2017; 18:624–635. [DOI] [PubMed] [Google Scholar]
- [41].Harms M, Seale P. Brown and beige fat: development, function and therapeutic potential. Nat Med 2013; 19:1252–1263. [DOI] [PubMed] [Google Scholar]
- [42].Lee YH, Petkova AP, Mottillo EP, Granneman JG. In vivo identification of bipotential adipocyte progenitors recruited by beta3-adrenoceptor activation and high-fat feeding. Cell Metab 2012; 15:480–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Chanda D, Lee CH, Kim YH, Noh JR, Kim DK, Park JH et al. Fenofibrate differentially regulates plasminogen activator inhibitor-1 gene expression via adenosine monophosphate-activated protein kinase-dependent induction of orphan nuclear receptor small heterodimer partner. Hepatology 2009; 50:880–892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Liangpunsakul S, Wou SE, Wineinger KD, Zeng Y, Cyganek I, Jayaram HN et al. Effects of WY-14,643 on the phosphorylation and activation of AMP-dependent protein kinase. Arch Biochem Biophys 2009; 485:10–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Towler MC, Hardie DG. AMP-activated protein kinase in metabolic control and insulin signaling. Circ Res 2007; 100:328–341. [DOI] [PubMed] [Google Scholar]
- [46].Lage R, Dieguez C, Vidal-Puig A, Lopez M. AMPK: a metabolic gauge regulating whole-body energy homeostasis. Trends Mol Med 2008; 14:539–549. [DOI] [PubMed] [Google Scholar]
- [47].Cypess AM, Weiner LS, Roberts-Toler C, Franquet Elia E, Kessler SH, Kahn PA et al. Activation of human brown adipose tissue by a beta3-adrenergic receptor agonist. Cell Metab 2015; 21:33–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Ghorbani M, Claus TH, Himms-Hagen J. Hypertrophy of brown adipocytes in brown and white adipose tissues and reversal of diet-induced obesity in rats treated with a beta3-adrenoceptor agonist. Biochem Pharmacol 1997; 54:121–131. [DOI] [PubMed] [Google Scholar]
- [49].Kang JG, Park CY. Anti-Obesity Drugs: A Review about Their Effects and Safety. Diabetes Metab J 2012; 36:13–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.