This study evaluates the feasibility of visualizing nasal cartilage using high resolution micro–computed tomography compared with the criterion standard of pathologic findings and its accuracy compared with various clinical computed tomography scanners.
Key Points
Question
Can nasal cartilage be accurately visualized on various computed tomography acquisitions?
Findings
In this anatomic cadaveric study, upper lateral cartilage, lower lateral cartilage, and septal cartilage were accurately visualized on micro–computed tomography, an imaging technology with more than 100-fold resolution compared with clinical computed tomography scanners. With review of clinical protocols, the septal cartilage was the only substructure that had no overlap in the variance between cartilage and boundary density.
Meaning
Study results reported the feasibility of visualizing nasal cartilage using micro–computed tomography with high accuracy compared with pathologic findings and suggest that a gap persists in the capability of current clinical computed tomography imaging to accurately model nasal cartilage.
Abstract
Importance
There is no imaging standard to model nasal cartilage for the planning of rhinoplasty procedures. Preoperative visualization of cartilage may improve objective evaluation of nasal deformities, surgical planning, and surgical reconstruction.
Objectives
To evaluate the feasibility of visualizing nasal cartilage using high resolution micro–computed tomography (CT) compared with the criterion standard of pathologic findings in a cadaveric specimen and to evaluate its accuracy compared with various clinical CT protocols.
Design, Setting, and Participants
Anatomic study at the University of Washington using single human cadaveric nasal specimens performed from July 10, 2017, to March 30, 2018.
Interventions
A micro-CT acquisition with 60-micron resolution was obtained of a nasal specimen. The specimen was then scanned with 5 different clinical CT protocols to span both clinical care and machine limits. The specimen was then sectioned in 5-mm axial slices for pathologic analysis.
Main Outcomes and Measures
Micro-CT images were registered to pathologic specimen cross-sections using a graphite fiducial system. Cartilage substructures were manually segmented and analyzed. A library of matched images across the micro-CT and various clinical CT protocols was then developed. Region of interest analysis was performed for each of the cartilage structures and their boundaries on clinical CT protocols and micro-CT, with the outcome of mean (SD) density using Hounsfield units.
Results
A single human cadaveric nasal specimen was used to obtain the following results. Lower lateral cartilage, upper lateral cartilage, and septal cartilage were accurately delineated on the micro-CT images compared with pathologic findings. The mean absolute deviation from pathologic findings was 0.30 mm for septal cartilage thickness, 0.98 mm for maximal upper lateral cartilage length, and 1.40 mm for maximal lower lateral cartilage length. On clinical CT protocols, only septal cartilage was well discriminated from boundary. Higher radiation dose resulted in more accurate density measurements of cartilage, but it did not ultimately improve ability to discriminate cartilage.
Conclusions and Relevance
The results of this anatomic study may represent a notable step toward advancing knowledge of the capabilities and pitfalls of nasal cartilage visualization on CT. Nasal cartilage visualization was feasible on the micro-CT compared with pathologic findings. Future research may further examine the barriers to accurately visualizing upper lateral cartilage and lower lateral cartilage, a prerequisite for clinical application.
Level of Evidence
NA.
Introduction
Rhinoplasty is one of the most technically challenging surgical procedures in facial plastic surgery because of the combination of soft tissue, bone, and cartilage structures; aesthetic and reconstructive goals; and the persistence of postoperative edema for up to a year. Multiple diverse subsets of patients undergo surgical procedures each year, including those with congenital deformities, bulbous nose, twisted nose, nasal valve compromise, and obstructive sleep apnea. Revision rates are high.1 In a study of more than 175 000 patients who underwent rhinoplasty, revision rates were 3.3% in primary cases and 11.0% in secondary rhinoplasty cases.1 In a review of 146 consecutive revision rhinoplasty cases, East et al2 identified the nasal dorsum as the most frequent key area of concern (77% of cases), followed by the nasal tip (50%) and septum (39%). The costs and consequences of these revisions includes long-term antibiotics, hospitalization, lost revenue, and psychologic association.3
A fundamental challenge in preoperative rhinoplasty evaluation is the inability to identify cartilage position and morphologic features. As a result, objective planning and evaluation is limited, and there is difficulty correlating structure with functional outcomes.4,5 Unlike facial trauma reconstruction, in which the surgeon can use navigation with 3-dimensional (3-D) bone reconstructions to enhance surgical planning, there is a gap in our ability to model cartilage. The surgeon may not accurately identify the deformities until the cartilage is directly visualized. However, intraoperative visualization alters the location of the cartilage from its native position in association with the skin and bone.
Current modalities of preoperative imaging of the nose reveal limitations in cartilage analysis. Surface topography and photogrammetry require surgeons to generate a 3-D mental model based on 2-dimensional measurements and analyses.6 Preoperative visualization via speculum examination is limited to the anterior nasal cavity.4 Endoscopic visualization is effective for septal evaluation but does not provide perspective of the lower lateral cartilage or the upper lateral cartilage anatomic features. Magnetic resonance imaging and CT are both used in orthopedics for preoperative evaluation of cartilage; however, these structures are surrounded by synovial fluid instead of soft tissue, and the articular cartilage is larger than the nasal cartilage.7,8 Ultrasonography has been used to visualize tracheal and thyroid cartilage.9 In addition, 3-D ultrasonography has been used to detect absent nasal bones in fetuses but are not designed for evaluation of adult nasal anatomic features because of probe size and practical difficulties in attaining planes on the nose.10
Recent work in visualizing cartilage in 3-D nasal reconstructions has revealed the potential of modeling nasal cartilage. Graviero et al11 used surface rendering on existing clinical CT scans to identify morphologic features of the nasal cartilages. Preoperative diagnoses based on the models of nasal cartilage found improved diagnosis of nasal pathologic findings compared with anterior rhinoscopy or nasal endoscopy. A critical limitation is that the researchers did not provide validation to verify that the identified tissues were cartilage rather than mucoperichondrium, mucosa, or skin. Visscher et al12 used magnetic resonance imaging to visualize nasal cartilage and found criterion validity associated with a high-resolution micro-CT.
Preoperative visualization offers an opportunity to improve objective evaluation of nasal deformities, surgical planning, and surgical reconstruction. However, there is limited evidence that nasal cartilage can be accurately identified with current imaging modalities. Given the gap in the literature and the importance of objective rhinoplasty evaluation, we sought to better understand the capabilities and limitations of current nasal cartilage imaging with CT technology. Current micro-CT scanners can achieve 60-micron resolution. In addition, newer clinical CT scanners have expanded from single- to dual-energy capability with improvements in tissue characterization.13
The objectives of this study were to (1) conduct a feasibility study of nasal cartilage visualization on a high-resolution micro-CT compared with the criterion standard of pathologic findings in a cadaveric sample and (2) evaluate the accuracy and discrimination of cartilage from boundary on various clinical CT protocols compared with micro-CT.
Methods
A single cadaveric nasal specimen was preserved in a 2:1 water to 100% 2-propanol mixture (Fisherbrand or Histoprep; Fisher Scientific). Radiopaque 0.7-mm graphite was selected for a fiducial system because of its accuracy, low cost, and wide availability.14 Each graphite rod was inserted using an 18-gauge needle in the coronal plane beginning at the columella and moving superiorly in 5-mm intervals. The needle was inserted through the specimen, the graphite was inserted within the needle, and the needle was withdrawn leaving the graphite in its place. Thus, the graphite rod extended from the entry point in the skin, through soft tissue and cartilage, and exited again through the skin without fracturing. The graphite was visualized to verify placement. Contrast material was also considered; however, cartilage has limited blood volume and would be unlikely to be better resolved with contrast material. The University of Washington institutional review board approved this study.
A micro-CT scan of the nasal specimen was obtained using an industrial CT scanner (NSI X5000; North Star Imaging) with 60-micron resolution. Multiple acquisitions were obtained on the single-energy and dual-energy CT scans (Siemens SOMATOM Force CT scanner; Siemens Healthcare).15 The sample was dissected in the axial plane for pathologic evaluation with photographic documentation. Cartilage was identified grossly for pathologic analysis.
Fiducial markers facilitated registration between clinical and pathologic modalities. Three axial micro-CT images were registered to corresponding pathologic cross-sections using image J/Fiji software (National Institutes of Health). A surgeon rater (R.C.S.) then manually segmented the distinct nasal cartilages from 3 sections of the high-resolution CT scan. Four outcomes (the maximal length of the lower lateral cartilage and the upper lateral cartilage, the thickness of the cartilaginous septum at midpoint, and the surface area of total cartilage after manual segmentation) were each measured 3 times and compared with each other to verify accuracy. These outcomes were selected because of high reproducibility in repeat measurements, use of landmarks, and review of measurements described in anatomic cadaveric studies.16,17
The clinical protocols were selected to span clinical care, maximize CT machine capabilities, and compare visualization to the micro-CT scan. The “Combined Applications to Reduce Exposure (CARE) on”18 setting provided protection against excessive radiation and was always in place for scans when used with patients, and the “CARE off” setting represented maximal resolution capabilities of the clinical scanner. Acquisitions included the maximal dose CARE off protocols on the clinical scanners, the clinical dose CARE on protocols, and additional standard CT scans that might be expected for rhinoplasty planning. We also used both single-energy and dual-energy CT acquisitions.
A library of matched images across the micro-CT and various clinical protocols was developed. Manual segmentation of the lower lateral cartilage, upper lateral cartilage, and septal cartilage was performed on 3 sections of each protocol in 3D Slicer,19 with 3 repeat measurements. Region of interest analysis was performed. The clinical protocols were evaluated based on accuracy and discrimination ability. Accuracy reflected how close the density value of cartilage was to that measured with micro-CT. High discrimination ability indicated little to no signal overlap between the density of cartilage and its surrounding soft-tissue boundary.
Results
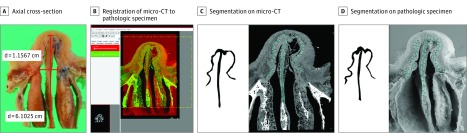
From a single human cadaveric nasal specimen, measures for the clinical CT and micro-CT acquisitions are listed in Table 1. Micro-CT had high accuracy, with a mean absolute deviation from pathologic findings of 0.30 mm for septal cartilage thickness, 0.98 mm for maximal upper lateral cartilage length, and 1.40 mm for maximal lower lateral cartilage length (Table 2). The surface area of total cartilage on the axial section closely matched that of pathologic findings with a mean absolute deviation of 10%. The masks generated from segmenting cartilage (Figure 1) further showed that the cartilage morphologic features generated from the micro-CT closely resembled those of the registered pathologic slices.
Table 1. Acquisition Techniques for the Clinical and Micro-CT System.
| Type of Scanner | Tube Type | Exposure Time, s | Tube Current Time, mAsa | Section Thickness, mm | |
|---|---|---|---|---|---|
| Voltage, kVp | Current, mA | ||||
| Siemens SOMATOM Force CT scannerb | |||||
| A, inner ear ultrahigh resolution | 70 | 525 | 1.00 | 525 | 0.400 |
| B, head | 80 | 11 | 0.91 | 10 | 0.625 |
| C, inner ear ultrahigh resolution | 100 | 98 | 0.5 | 49 | 0.600 |
| D, inner ear ultrahigh resolution | 150 | 58 | 0.5 | 29 | 0.600 |
| E, inner ear ultrahigh resolution | 100 | 98 | 0.5 | 49 | 0.600 |
| North Star Imaging micro-CT scanner | |||||
| NSI X5000 | 130 | 0.581 | 1806 | 1050 | 0.060 |
Abbreviations: CT, computed tomography; kVp, kilovoltage peak; mAs, milliampere-seconds.
The Combined Applications to Reduce Exposure (CARE) off setting represented maximal resolution capabilities of the clinical scanner and a radiation dose report cannot be generated.
Protocol B was a single-energy acquisition; protocols A, C, D, and E were dual-energy acquisitions.
Table 2. Nasal Cartilage Visualization on Axial Micro-CT Compared With Pathologic Finding.
| Nasal Cartilage Measurement | Measurement, Mean (SD), mm | Mean Absolute Deviation of Micro-CT From Pathologic Findings | ||
|---|---|---|---|---|
| Pathologic Finding | Micro-CT | Mean, mm | Mean, % | |
| Maximal axial | ||||
| Lower lateral cartilage length | 10.60 (0.59) | 11.35 (0.98) | 1.40 | 13 |
| Upper lateral cartilage length | 23.18 (2.14) | 22.40 (3.26) | 0.98 | 4 |
| Axial septal cartilage thickness at anterior-posterior midpoint | 1.70 (0.89) | 1.84 (0.94) | 0.30 | 18 |
| Axial surface area of total cartilage, mm2 | 103.11 (17.34) | 109.89 (22.07) | 9.89 | 10 |
Abbreviation: CT, computed tomography.
Figure 1. Nasal Cartilage Visualization on Micro-CT Compared With Pathologic Findings.
The masks show an axial cross-section of a pathologic specimen (A) and registration of micro–computed tomography (CT) to the pathologic specimen (B). The total surface area of cartilage was calculated from nasal cartilage segmentation on micro-CT (C) and on axial pathology (D). d indicates distance.
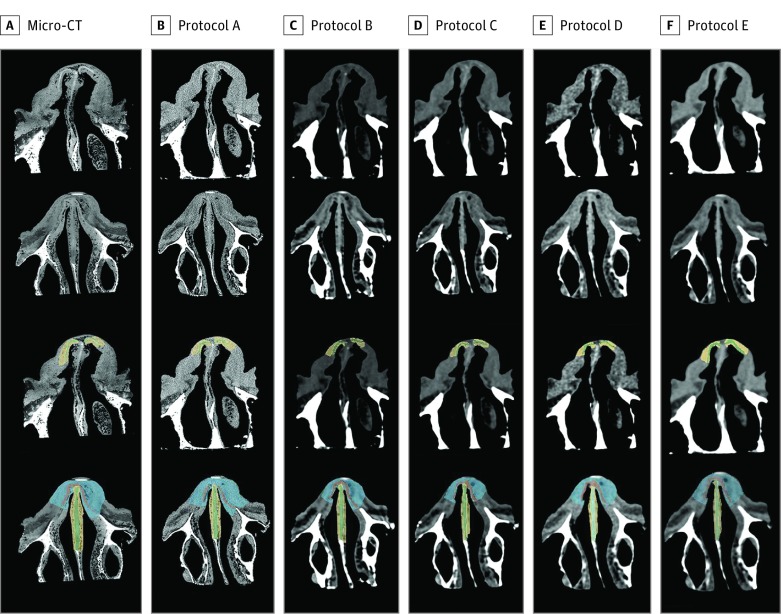
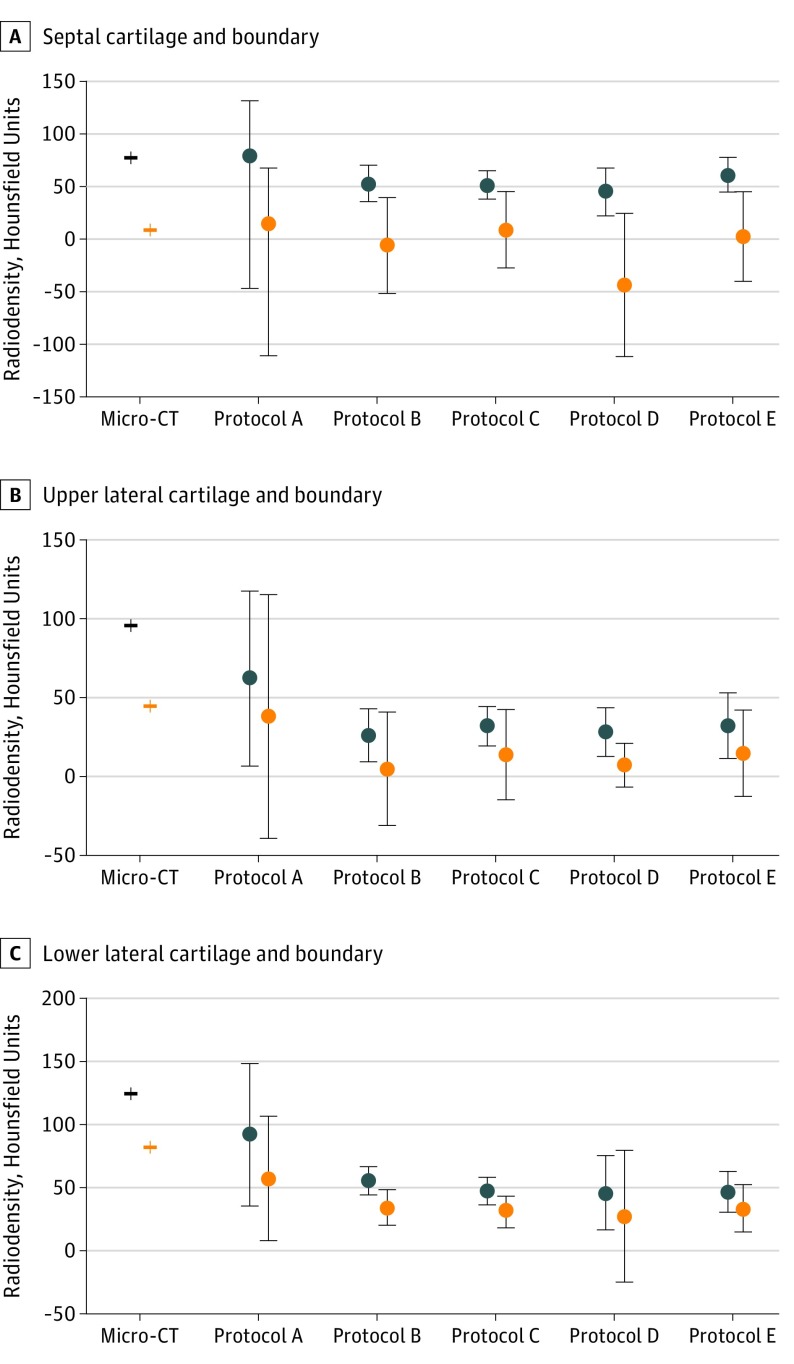
A library of registered images was developed (Figure 2), and the regions of interest for each cartilage substructure and boundary were defined on clinical CT–acquired images and the micro-CT. Figure 3 depicts the mean density (SD) for each cartilage substructure across micro-CT and clinical CT protocols. Protocol A, the highest radiation dose on the clinical scanner, had the most accurate density measurements (septal cartilage boundary mean [SD], 15.1 [125.6] Hounsfield units), but this did not translate into improved signal differentiation between cartilage and boundary. Septal cartilage was well discriminated from boundary across all clinical protocols; upper lateral cartilage and lower lateral cartilage were poorly discriminated across all clinical protocols (eTable in the Supplement).
Figure 2. Library Data Set of Micro-CT and Clinical Images.
The micro–computed tomography (CT) and clinical protocols are seen on axial section in columns A through F (images arranged from highest to lowest radiation dose). The first 2 rows show nasal cartilage visualization on the micro-CT. The third and fourth rows show the corresponding nasal cartilage visualization after manual segmentation.
Figure 3. Region of Interest Analysis for Cartilage vs Boundary Across Micro-CT and Clinical CT Protocols.
The mean (SD) of cartilage substructures and boundary was calculated in 3D Slicer for septal cartilage (A), upper lateral cartilage (B), and lower lateral cartilage (C) across the micro-computed tomography (CT) and clinical CT protocols (arranged from highest to lowest radiation dose). Less overlap in density variance between the cartilage and boundary indicates better discrimination of cartilage from boundary.
Discussion
No imaging standard for visualizing cartilage exists in otolaryngology. Our study is the first to systematically investigate the signal characteristics of nasal cartilage using micro-CT and clinical CT protocols. We showed that upper lateral cartilage, lower lateral cartilage, and septal cartilage may be accurately delineated on the micro-CT images compared with the criterion standard of pathologic findings. After validating cartilage visualization on the micro-CT, we varied the radiation dose within in vivo (CARE on) and ex vivo (CARE off) ranges to visualize nasal cartilage across a range of clinical CT acquisitions.
By comparing density, as measured by Hounsfield units, within the segmented cartilage structures to soft-tissue boundary, we developed an objective measure of how well cartilage could be discriminated from the surrounding tissue. There may have been the potential for bias from the surgeon rater in tracing where the cartilage should be based on his clinical knowledge. However, a strength of our methods was this objective evaluation in association with the micro-CT. The density for septal cartilage and boundary was most similar to the micro-CT with clinical protocol A. This result was expected given that this protocol represented maximal radiation with CARE off on the clinical dual-energy scanner. However, protocol A still retained substantial overlap in signal intensity as can be seen on the deviation plots of the cartilage and boundary and thus remained limited in delineating cartilage from boundary (Figure 3). In contrast, protocols D and E, the lowest radiation protocols, had less variance overlap, aiding in boundary delineation.
Discrimination of the upper and lower lateral cartilages from boundary was limited in all clinical CT protocols. Protocol A had the most accurate density measurements for upper and lower lateral cartilages compared with other clinical protocols. However, protocol A had the largest variance of both cartilage and boundary regions, supporting poor discrimination ability. Clinical protocols with CARE on demonstrated lower variance in some comparisons; however, little confirmation could be seen visually on the raw images. These findings suggested that maximal radiation on the clinical scanner did not improve discrimination of nasal cartilage. This was a novel finding, and the 2 protocols with the lowest radiation doses best discriminated septal cartilage from boundary. This result suggested that optimizing other measures in low radiation clinical protocols may represent a good step to improving nasal cartilage visualization on clinical CT acquisitions. Although there may be possible energy-level differences or specific reconstruction of single-energy levels that may assist in cartilage visualization, we did not see any marked difference between protocol B on the clinical single-energy scanner and protocols C, D, and E on the clinical dual-energy scanner.
Discrimination of cartilage from boundary is fundamental for segmentation. Of note, a clinically relevant level of accuracy for rhinoplasty will likely be based on identification of clinical defects and contours rather than precise cartilage measurements. The micro-CT demonstrated high resolution and more importantly, distinct density differences between each cartilage substructure and boundary that could be seen visually. Given that only septal cartilage was well discriminated from boundary on the clinical CT, these findings also reveal the limitations of current clinical CTs in visualizing nasal cartilage. It should be stressed that the type of x-ray sources and detectors are different between micro-CT and clinical CT scanners. With major differences in the type of x-ray tubes and lack of standards for micro-CT devices, it remains difficult to compare radiation doses between these systems. Table 1 reveals that the accumulated x-ray flux for the micro-CT system (tube current time) was higher (1050 milliampere-seconds [mAs] vs <525 mAs) than that of the clinical CT systems, enabling a low-noise image at the higher spatial resolution.20
Studies have shown the utility of nasal cartilage imaging and 3-D modeling. Kleinheinz and Joos21 described nasal cartilage visualization using magnetic resonance imaging, but these findings were based on clinical impression without validation. Visscher at al12 compared manually segmented magnetic resonance images with micro-CT and printed alar cartilage 3-D constructs. We used a similar method to compare clinical CT imaging with micro-CT imaging. However, the previous authors did not specifically validate micro-CT. Visscher at al12 described limitations of magnetic resonance imaging because the medial crus of the alar cartilage was consistently missed by observers compared with the micro-CT. The lateral crus had an overestimation of 2 mm in some parts. Our work had a similar finding for CT imaging in that the lower lateral cartilage was not well discriminated from boundary on all tested clinical CT protocols. Analysis of the lower lateral cartilage on micro-CT also had the largest deviation compared with all other cartilage structures, suggesting that the lower lateral cartilage may be more difficult to visualize compared with upper lateral and septal cartilages.
Several lessons may be applied from this research to clinical practice. First, we visualized nasal cartilage on micro-CT with objective accuracy compared with pathologic findings. We opted to study visualization on CT because patients who have had facial trauma often have CT scans performed before a referral; no additional imaging or insurance authorization would be required to model cartilage in this subgroup of candidates for rhinoplasty. The CT scans are also often obtained for preoperative planning of other soft-tissue or cartilage-based surgical procedures such as laryngotracheal reconstruction and microtia reconstruction.22,23 The imaging findings are more translatable to clinical application. Establishing micro-CT as a criterion standard is an important advancement, because future investigators can use this imaging modality to compare nasal cartilage visualization on CT, magnetic resonance imaging, and ultrasonography.
We found that the upper and lower lateral cartilages were poorly discriminated on multiple clinical CT protocols in this study compared with micro-CT. Multiple studies in the literature11,24 describe benefits of 3-D CT volume renderings in preoperative rhinoplasty evaluation. For example, Graviero et al11 demonstrated that 3-D volume renderings of CT improved preoperative evaluation of nasal cartilage deformities compared with anterior rhinoscopy and endoscopic evaluation. Bared at al24 used 3dMDvultus software (3dMDvultus Inc) to analyze nasal tip volume changes and lower lateral cartilage positioning after rhinoplasty. One of the inherent limitations in these clinical studies is the lack of validation. Our results may indicate that there remains a gap in the capability of current clinical CT imaging to accurately delineate lower and upper lateral cartilage. We recommend exercising caution in assuming that 3-D CT models of nasal cartilage are accurately delineating all cartilage substructures.
In contrast to the upper and lower lateral cartilages, septal cartilage was well delineated on protocols despite having a marginally higher density on micro-CT. This finding may have important clinical ramifications because septal cartilage is often used for grafting in rhinoplasty procedures. Preoperative quantification of available septal cartilage and how much cartilage has been depleted in previous procedures may be valuable in surgical planning. The ability to discriminate nasal cartilage from boundary across all clinical protocol may be because of the septal cartilage being bordered by mucoperichondrium and then air of the nasal cavity. This interface may be easier to detect radiographically compared with that of the upper and lower lateral cartilages. We also noted that there were more areas of lower density in the septal mucosa, which indirectly assists in delineating the septal cartilage boundary. Alternatively, the greater density difference may be an artifact in cadavers because of absence of blood flow, which would make septal cartilage more difficult to visualize in a clinical setting.
This study provides valuable information toward the systematic evaluation of nasal cartilage visualization on CT. Modeling nasal cartilage preoperatively is not well established. Further research is required to identify the role and benefits of this type of imaging. We developed CT protocols to evaluate cartilage accuracy and discrimination ability with multiple clinical CT protocols and micro-CT. This pilot study addressed the fundamental aspects of cartilage visualization, which has broad importance in multiple areas of otolaryngology, including planning for rhinoplasty procedures, airway surgical procedures, and microtia. The study findings showed that nasal cartilage may be accurately delineated on the micro-CT scanner, and we described a novel method of comparing multiple clinical protocols for nasal cartilage visualization with a criterion standard.
Preoperative rhinoplasty planning is challenging especially given the interplay of skin, soft tissue, cartilage, and bone. Modeling nasal cartilage may preoperatively offer the potential for improving diagnosis and surgical planning. The innovation in our work was the creation of a data set of pathologic findings, micro-CT, and clinical CT images that may establish ground truth cartilage for comparison.
Limitations
There are multiple limitations of our study. First, this was a cadaveric study, and the absence of septal blood flow, for example, may alter imaging of the septal cartilage and make it easier to distinguish in the cadaver. Preservation of the cadaveric specimen also results in shrinkage, particularly of the skin, but we would not anticipate this to have a sizable influence on cartilage analysis. We were able to perform exploratory clinical CT protocols and micro-CT in a cadaveric specimen, which would not have been possible in vivo. In addition, we focused on varying the imaging protocols with 1 cadaveric specimen. Future studies will include additional cadaveric specimens and fresh tissue samples.
We sought to reduce potential sources of error and provide optimal comparison of imaging and pathologic findings. Pathologic examination was performed by a technician along with surgeon assistance. In addition to deformation, sectioning bone and soft tissue invariably results in nonuniformity in axial planes. To address this, the first author (R.C.S.) and a radiologist on our team (J.O.) examined each of the axial pathologic specimens and selected the 3 specimens in which the outer contours and pattern of graphite fiducial markers matched the imaging most closely. A radiologist also compared the imaging with the pathologic finding as a second check for accuracy. The contours provided rapid visual confirmation that the pathologic finding and imaging were of the same part of the nose. The graphite markers facilitated the registration and postprocessing to limit deformation. The 3-D analysis of nasal contour may have allowed for enhancement alignment and registration in future investigations.
Conclusions
Our findings suggest that nasal cartilage can be accurately delineated on micro-CT. We also described a method of comparing multiple clinical protocols with micro-CT for nasal cartilage visualization. Lower radiation clinical protocols were better at discriminating septal cartilage than maximal radiation clinical protocols. We did not identify a clinical protocol that could accurately discriminate lower or upper lateral cartilage from boundary. Our findings appear to advance knowledge of the capabilities and pitfalls of nasal cartilage visualization on clinical CT.
eTable. Region of Interest Analysis for Cartilage vs Boundary Across Micro-CT and Clinical CT Protocols
References
- 1.Spataro E, Piccirillo JF, Kallogjeri D, Branham GH, Desai SC. Revision rates and risk factors of 175 842 patients undergoing septorhinoplasty. JAMA Facial Plast Surg. 2016;18(3):212-219. doi: 10.1001/jamafacial.2015.2194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.East C, Kwame I, Hannan SA. Revision rhinoplasty: what can we learn from error patterns? an analysis of revision surgery. Facial Plast Surg. 2016;32(4):409-415. doi: 10.1055/s-0036-1586176 [DOI] [PubMed] [Google Scholar]
- 3.Ishii LE, Tollefson TT, Basura GJ, et al. Clinical practice guideline: improving nasal form and function after rhinoplasty. Otolaryngol Head Neck Surg. 2017;156(2, suppl):S1-S30. doi: 10.1177/0194599816683153 [DOI] [PubMed] [Google Scholar]
- 4.Pawar SS, Garcia GJ, Kimbell JS, Rhee JS. Objective measures in aesthetic and functional nasal surgery: perspectives on nasal form and function. Facial Plast Surg. 2010;26(4):320-327. doi: 10.1055/s-0030-1262314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sharp HR, Rowe-Jones JM. Assessing outcome in aesthetic rhinoplasty. Clin Otolaryngol Allied Sci. 2003;28(5):430-435. doi: 10.1046/j.1365-2273.2003.00739.x [DOI] [PubMed] [Google Scholar]
- 6.Lekakis G, Claes P, Hamilton GS III, Hellings PW. Three-dimensional surface imaging and the continuous evolution of preoperative and postoperative assessment in rhinoplasty. Facial Plast Surg. 2016;32(1):88-94. doi: 10.1055/s-0035-1570122 [DOI] [PubMed] [Google Scholar]
- 7.Schnier M, Eckstein F, Priebsch J, et al. Three-dimensional thickness and volume measurements of the knee joint cartilage using MRI: validation in an anatomical specimen by CT arthrography [in German]. Rofo. 1997;167(5):521-526. doi: 10.1055/s-2007-1015574 [DOI] [PubMed] [Google Scholar]
- 8.Lefevre N, Naouri JF, Herman S, Gerometta A, Klouche S, Bohu Y. A current review of the meniscus imaging: proposition of a useful tool for its radiologic analysis. Radiol Res Pract. 2016;2016:8329296. doi: 10.1155/2016/8329296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kundra P, Mishra SK, Ramesh A. Ultrasound of the airway. Indian J Anaesth. 2011;55(5):456-462. doi: 10.4103/0019-5049.89868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rembouskos G, Cicero S, Longo D, Vandecruys H, Nicolaides KH. Assessment of the fetal nasal bone at 11-14 weeks of gestation by three-dimensional ultrasound. Ultrasound Obstet Gynecol. 2004;23(3):232-236. doi: 10.1002/uog.952 [DOI] [PubMed] [Google Scholar]
- 11.Graviero G, Guastini L, Mora R, Salzano G, Salzano FA. The role of three-dimensional CT in the evaluation of nasal structures and anomalies. Eur Arch Otorhinolaryngol. 2011;268(8):1163-1167. doi: 10.1007/s00405-011-1575-1 [DOI] [PubMed] [Google Scholar]
- 12.Visscher DO, van Eijnatten M, Liberton NPTJ, et al. MRI and additive manufacturing of nasal alar constructs for patient-specific reconstruction. Sci Rep. 2017;7(1):10021. doi: 10.1038/s41598-017-10602-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Postma AA, Das M, Stadler AA, Wildberger JE .. Dual-energy CT: what the neuroradiologist should know. Curr Radiol Rep. 2015;3(5):16. doi: 10.1007/s40134-015-0097-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Javadrashid R, Fouladi DF, Golamian M, et al. Visibility of different foreign bodies in the maxillofacial region using plain radiography, CT, MRI and ultrasonography: an in vitro study. Dentomaxillofac Radiol. 2015;44(4):20140229. doi: 10.1259/dmfr.20140229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Siemens SOMATOM Force CT scanner. https://www.healthcare.siemens.com/computed-tomography/dual-source-ct/somatom-force. Accessed December 29, 2018.
- 16.de Pochat VD, Alonso N, Ribeiro EB, da Rocha EA, Tenório EG, Meneses JV. Anatomical variations of the upper lateral cartilages and their implications in rhinoplasty. Aesthetic Plast Surg. 2012;36(2):285-289. doi: 10.1007/s00266-011-9824-7 [DOI] [PubMed] [Google Scholar]
- 17.Slupchynskyj O, Cranford J. Quantitative measurements of the bulbous tip in ethnic rhinoplasty. Ann Plast Surg. 2017;78(5):569-575. doi: 10.1097/SAP.0000000000000925 [DOI] [PubMed] [Google Scholar]
- 18.Combined Applications to Reduce Exposure (CARE) https://www.healthcare.siemens.com/computed-tomography/technologies-innovations/care-right/right-dose-technology. Accessed December 29, 2018.
- 19.3D Slicer. https://www.slicer.org/. Accessed December 29, 2018.
- 20.Meganck JA, Liu B. Dosimetry in micro-computed tomography: a review of the measurement methods, impacts, and characterization of the Quantum GX imaging system. Mol Imaging Biol. 2017;19(4):499-511. doi: 10.1007/s11307-016-1026-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kleinheinz J, Joos U. Imaging of cartilage and mimic muscles with MRI: anatomic study in healthy volunteers and patients with unilateral cleft lip and palate. Cleft Palate Craniofac J. 2001;38(4):291-298. doi: 10.1597/1545-1569_2001_038_0291_iocamm_2.0.co_2 [DOI] [PubMed] [Google Scholar]
- 22.Lee KS, Ashiku SK, Ernst A, et al. Comparison of expiratory CT airway abnormalities before and after tracheoplasty surgery for tracheobronchomalacia. J Thorac Imaging. 2008;23(2):121-126. doi: 10.1097/RTI.0b013e3181653c41 [DOI] [PubMed] [Google Scholar]
- 23.Qin FH, Zhang TY, Dai P, Yang L. Anatomic variants on computed tomography in congenital aural atresia and stenosis. Clin Exp Otorhinolaryngol. 2015;8(4):320-328. doi: 10.3342/ceo.2015.8.4.320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bared A, Rashan A, Caughlin BP, Toriumi DM. Lower lateral cartilage repositioning: objective analysis using 3-dimensional imaging. JAMA Facial Plast Surg. 2014;16(4):261-267. doi: 10.1001/jamafacial.2013.2552 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable. Region of Interest Analysis for Cartilage vs Boundary Across Micro-CT and Clinical CT Protocols