This cohort study of 56 adults with varying degrees of hearing impairment evaluates the clinical performance of a personal sound amplification product vs a basic hearing aid and a premium hearing aid.
Key Points
Question
For adults with mild to moderately severe hearing loss, how does the performance of a personal sound amplification product (PSAP) compare with that of a basic hearing aid (HA) and a premium HA on a series of efficacy tests and patient self-reports?
Findings
In this cohort study of 56 adults, for those with mild and moderate hearing loss, there were no differences between PSAP, basic HA, and premium HA for speech perception, sound quality, listening effort, and user preference. However, for the group with moderately severe hearing loss, the premium HA had better performance across most tests, and 70% of these participants preferred to use the premium HA.
Meaning
A PSAP may be helpful for individuals with mild to moderate hearing loss, but if the degree of hearing loss is severe, a premium HA that can provide sufficient gain across all frequencies may be needed.
Abstract
Importance
Hearing loss is a highly prevalent condition with multiple negative associated outcomes, yet few persons with hearing loss have hearing aids (HAs). Personal sound amplification products (PSAPs) could be an alternative low-cost solution to HAs, but data are lacking on the performance of PSAPs.
Objective
To evaluate the clinical efficacy of a PSAP by comparing its performance with that of a basic HA and a premium HA in participants with mild, moderate, and moderately severe hearing impairment.
Design, Setting, and Participants
A prospective, single-institution cohort study was performed with a total of 56 participants, including 19 with mild hearing loss, 20 with moderate hearing loss, and 17 with moderately severe hearing loss. All participants underwent 4 clinical hearing tests with each of the PSAP, basic HA, and premium HA, and all completed an evaluative questionnaire.
Interventions
All hearing devices (PSAP, basic HA, and premium HA) were applied by a clinician to prevent bias and order effects; participants were blinded to the device in use, and sequence of devices was randomized.
Main Outcomes and Measures
The study used the Korean version of the hearing in noise test, the speech intelligibility in noise test, listening effort measurement using a dual-task paradigm, pupillometry, and a self-rating questionnaire regarding sound quality and preference. These tests were administered under the following 4 hearing conditions: unaided hearing, use of PSAP, use of basic HA, and use of premium HA.
Results
The study included 56 participants with a mean age of 56 years (interquartile range, 48-59 years); 29 (52%) were women. In the mild and moderate hearing loss groups, there was no meaningful difference between PSAP, basic HA, and premium HA for speech perception (Cohen d = 0.06-1.05), sound quality (Cohen d = 0.06-0.71), listening effort (Cohen d = 0.10-0.92), and user preference (PSAP, 41%; basic HA, 28%; premium HA, 31%). However, for the patients with moderately severe hearing loss, the premium HA had better performance across most tests (Cohen d = 0.60-1.59), and 70% of participants preferred to use the premium HA.
Conclusions and Relevance
The results indicate that basic and premium HAs were not superior to the PSAP in patients with mild to moderate hearing impairment, which suggests that PSAPs might be used as an alternative to HAs in these patient populations. However, if hearing loss is more severe, then HAs, especially premium HAs, should be considered as an option to manage hearing loss.
Introduction
Age-related hearing loss is common and is often untreated. Elderly patients with hearing loss generally began losing their hearing in their 40s or early 50s.1 With every 10 years of age, the prevalence of hearing loss doubles.2 This is not only a personal matter for individual patients, but it is also a social and national burden; hearing loss is the fourth major contributor to worldwide disability, affecting 6% to 8% of the world population.3,4 The burden of hearing loss is increasing with increased worldwide life expectancy. In South Korea, the general prevalence of subjective hearing loss was 12.0%, and 44.7% of elderly people (>70 years old) reported hearing loss.5
Untreated hearing impairment causes communication problems and social isolation6 resulting in psychological problems such as depression.7 Other important consequences include deterioration of cognitive function,8 increased risk of hospitalization,9,10 and mortality.9 In particular, there have been reports that hearing loss, cognitive dysfunction, and dementia are very closely related.8,11,12 These problems have obvious implications for society, so persons with hearing impairment need proactive hearing rehabilitation and should be able to access hearing devices more easily. In this regard, direct-to-consumer devices, such as personal sound amplifier products (PSAPs) or over-the-counter (OTC) hearing aids (HAs), can be a good alternative to conventional HAs, which are expensive and require multiple fitting appointments. The mainstay of hearing loss management, especially age-related sensorineural hearing loss, is HAs. However, in developing countries, only 2% of the total population who were strongly encouraged to receive hearing rehabilitation were using HAs.13 In the United States, according to nationally representative estimates based on 2605 adults from 1999 through 2006, fewer than 20% of adults with hearing loss reported HA use.14 The national prevalence of regular HA use in South Korea was 12.6% among people with moderate to profound hearing loss, which is comparatively low compared with prevalence reported in other countries.15 Several reasons for the low HA adoption rate are high cost, social stigma, poor performance, and inconvenience. Among these, expensive prices (about $6000 for a pair of HAs) and inconvenience (multiple fitting and adjustment appointments) are the biggest reasons for lack of HA use.1 These problems are important barriers to HA purchase, particularly in the elderly who may be unemployed.
Personal sound amplification products have been described as the audio version of reading glasses. They do not require medical examination, fitting procedures, and more importantly, are much less expensive (ranging from $20 to $400) than conventional HAs. The United States Food and Drug Administration (FDA) defines a PSAP as a wearable customer electronic device intended for customers without hearing loss to amplify sounds in certain environments like recreational activities.16 Therefore, PSAPs have not yet been recognized as a formal hearing device and are not FDA regulated. However, with the remarkable development of wearable devices, rapid market expansion, and the familiarity of online shopping, the perception of PSAPs has changed. There has been a dramatic increase in the number of PSAPs purchasable online or in retail stores without the involvement of a licensed hearing professional.17 According to a recent study, PSAPs have a wide quality range, with a few operating electroacoustically similarly to conventional HAs.18 Other studies have shown current PSAPs’ advantages and limitations.16 There have been several research findings related to OTC HAs and PSAPs, but many studies focused only on quality of life19 or were limited to electroacoustic characteristics.20 Additionally, some results only showed differences between before and after use of a single product21 or have not reflected the results of people with various degrees of hearing loss.22 These results suggest that OTC HAs and PSAPs do not provide sufficient gain for high frequencies, so people have very low satisfaction with these devices, and those with mild to moderate hearing loss receive slight benefit. However, to our knowledge, there are no comprehensive studies on their electroacoustic characteristics, various laboratory tests, and questionnaires. There are also no studies to our knowledge that objectively evaluate listening effort using methods such as pupillometry.
It is very meaningful to compare conventional HAs and PSAPs using a comprehensive method at this point, as the market for PSAPs is growing. The present study evaluated the efficacy of a PSAP by comparing its performance with that of basic and premium HAs for individuals with mild, moderate, and moderately severe hearing loss.
Methods
Patients
As detailed in Table 1, 56 adults with hearing loss (27 men, 29 women) whose median (interquartile range [IQR]) age was 57 (48-59) years, were enrolled in this prospective single-institution study from May 2017 to September 2018. Patients who visited the outpatient clinic of Samsung Medical Center with hearing loss completed pure-tone audiometry (PTA) and speech audiometry (SA) testing. Participants were divided into 3 groups based on the 4-frequency averages (0.5, 1.0, 2.0, and 4.0 kHz): 19 with mild hearing loss (MHL: PTA, 26-40 dB of hearing loss), 20 with moderate hearing loss (MDHL: PTA, 41-55 dB of hearing loss), and 17 with moderately severe hearing loss (MSHL, PTA, 56-70 dB of hearing loss). Patients with a history of cognitive impairment, learning disabilities, and speech disorders were excluded from the study, as were patients with ophthalmic diseases based on pupillometry testing. All participants underwent the Montreal cognitive assessment (MOCA) test prior to the study. Those with a MOCA score of 22 points or higher were able to participate in the study. Of the 56 included patients, 16 were experienced HA users. No participants had ever used a PSAP. Written informed consent was obtained from all participants prior to the study. This study was approved by the institutional review board of Samsung Medical Center following the declaration of Helsinki (IRB File No. 2015-06-087).
Table 1. Clinical Characteristics of Enrolled Participants.
| Characteristic | Mild Hearing Loss (n = 19) | Moderate Hearing Loss (n = 20) | Moderately Severe Hearing Loss (n = 17) | Effect Sizea |
|---|---|---|---|---|
| Age, median (IQR), y | 57 (48-59) | 63.5 (60.5-68.0) | 48 (34-60) | 1.2 |
| Male, No. (%) | 9 (47) | 9 (45) | 9 (53) | 0.1 |
| PTA, median (IQR), dB HL | ||||
| AC, right | 31.88 (31.25-35.00) | 42.63 (42.50-51.88) | 62.50 (61.25-67.50) | 4.6 |
| AC, left | 32.50 (30.00-35.00) | 47.50 (43.75-49.38) | 63.75 (61.25-66.25) | 5.0 |
| Cognitive test | ||||
| Digit span test, median (IQR)b | 11 (10-13) | 10 (9-12) | 11 (10-13) | 0.2 |
| MOCA, median (IQR)c | 27 (27-28) | 27 (24-29) | 26 (25-29) | 0.3 |
| Previous HA experience, No. (%)d | 0 | 7 (35) | 9 (53) | 1.1 |
Abbreviations: AC, Air conduction; HA, hearing aid; HL, hearing loss; IQR, interquartile range; MOCA, Montreal cognitive assessment; PTA, pure-tone audiometry.
The effect size was calculated with Cohen d.
The digit span test is the sum of forward and backward test scores.
MOCA uses a Korean version that has been officially validated and has a total score of 30 points.
Only HA experience longer than 3 months is reported. The median duration of use was 32.6 months in the group with moderate hearing loss and 74.3 months in the group with moderately severe hearing loss.
Intervention
Two pairs of HAs and 1 pair of PSAPs were used in this study. The Opn1 (Oticon Ltd) was chosen as the premium HA, and the Ria2Pro (Oticon Ltd) was used as the basic HA. Both are receiver-in-the-canal HAs. The premium product has 64 channels with additional functions, and the basic product has 6 channels. The PSAP (Ps2500amp; Able Planet Inc) was purchased online. This PSAP has features such as noise reduction and directionality. Features and prices of each product are described in eTable 1 in the Supplement. HA fitting was performed by experienced audiologists using the NAL-NL2 formula. For the PSAP, participants selected their preferred amplification mode among 4 presets. The binaural communication function was disabled in all devices. Both HAs were set to automatic directionality. The PSAP contains a “natural directionality” feature as default, but its settings could not be modified. We did not inform the patient of product price or company information. During the testing, participants noticed that the product had changed, but they did not know which product was in use. To minimize learning effects, experiments were conducted with various speech materials. The order of the test materials was randomly assigned to avoid the adaptation effect.
Outcome Measures
Three main test batteries were administered: (1) speech intelligibility in noise test (SIN), (2) listening effort test, and (3) self-rating questionnaire. All tests were performed in unaided and aided conditions, and the order of wearing devices was randomized.
For the SIN test, the Korean version of the hearing in noise test (K-HINT) and speech intelligibility in babble noise test (SIBN) were performed. K-HINT was performed in a double-walled, sound-treated booth using a HINT Pro 7.2 Audiometric system (Bio-Logic System) with a background noise level lower than 25 dB. Recorded K-HINT sentences were presented at a 1-meter distance from the patient, and sound intensities were changed based on patient response. In quiet, the test started with the presentation level of 15 dB and adjustments were made (−4 dB for a correct response and +4 dB for and incorrect response) for the first 4 questions. From question 5 to 20, a 2-dB step size was used. In noise, target speech was presented from the front, and white noise was presented from 4 directions (45°, 135°, 225°, and 315°). Noise intensity was fixed at 65-dB sound pressure level, and the test followed the same procedures as in quiet. All sentences were randomly selected, thus reducing errors by sequence-learning effect. The reception threshold for target speech was measured for the quiet environment, and signal-to-noise ratio (SNR) was measured for the noisy environment.
For the SIBN, multiple-talker babble noise was presented from 4 directions, and target speech was presented from the front with a presentation level of 40 dBA (A-weighted decibels), after which intelligibility was evaluated. The sentence and method used are the same as in the K-HINT. Speech signal and noise were adjusted to 0-dB SNR and participants repeated words or sentences back to the tester.
We also used 2 methods to objectively measure listening effort. The first method was the SIN test with a dual-task paradigm using digital pursuit rotor tracking. This is a visual tracking test to measure listening effort. The pursuit rotor features an elliptical track with a red circle target rotating along the track. The participant moves the mouse cursor along the target. The speed adjusted for participants to obtain 80% time on target was recorded. We then created a dual-task situation; participants were told that their main task was to repeat the Korean speech audiometry sentences while tracking the moving target as best as they could.23,24,25 The noise was fixed at 65-dB sound pressure level, and the target speech was adjusted to 3-dB SNR. The correct score was calculated in the same manner as in the SIN test. Dual-task performance scores (ie, overall percentage correct speech recognition and time on target) were recorded for unaided and aided conditions. Pupillometry was performed to objectively measure listening effort using a pupillometer, REDn Scientific (SensoMotoric Instruments Inc). Pupil dilation increased when participants used greater effort to solve hard problems.26 Many studies have evaluated the degree of pupil dilation to measure cognitive load for listening effort, and it has been found that there is a significant correlation.27,28 The system uses infrared video-based tracking technology to measure pupil size. The spatial resolution of the pupillometer was 0.03 mm. The location and size of the pupil were recorded at 50 Hz automatically, and a computer connected to the pupillometer stored the data with time stamps, indicating the start of the trials and the stimuli, the prompt signal, and the response of the participant. For the first 5 seconds, we set pupil size at baseline after presenting 3 seconds of noise (multi-talker babble noise, 65-dB sound pressure level). We then asked questions with noise at 0- and 3-dB SNR, and participants answered the questions. The amount of further dilation or contraction based on the baseline pupil size, except for blinks and saccades, was measured. Peak dilated pupil diameter with respect to reference pupil diameter of each device was calculated.28 The Speech, Spatial and Qualities of Hearing Scale questionnaire was also completed. A 5-point scale was used to rate sound quality (5, very good; 4, good; 3, usual; 2, bad; and 1, very bad). Patients indicated the most preferred product.
Statistical Analysis
For descriptive statistical analysis, continuous variables were presented as medians (IQRs), and categorical variables were reported as numbers and percentages. Clinical characteristics were tested for group comparison using the Kruskal-Wallis test for continuous variables and the χ2 test for categorical variables. Differences among and between devices were analyzed using the Friedman test, followed by post hoc Wilcoxon signed-rank test. A Bonferroni correction was calculated to account for multiple testing. Effect sizes were calculated for hearing device comparisons with Cohen d. Cohen d is interpreted as 0.2, small effect size; 0.5, medium effect size; and 0.8, large effect size.29 All analyses were performed using SAS software, version 9.4 (SAS Institute Inc) and Stata/SE, version 14.
Results
The SIN Tests
The Korean Version of the Hearing in Noise Test
The K-HINT results are detailed in Table 2. In quiet, as supported by the scores reported there, the reception threshold for target speech in the aided condition was meaningfully lower than that in the unaided condition. However, the MHL group in quiet showed no significant threshold differences between the PSAP (median, 30.1 dB; IQR, 26.8-33.4 dB), the basic HA (median, 30.7 dB; IQR, 26.1-34.5 dB), and the premium HA (median, 30.7 dB; IQR, 25.9-34.8 dB). In quiet, there was a large threshold difference in the unaided (median, 50.15 dB; IQR, 43.2-58.1 dB) and aided conditions (median, 39.68 dB; IQR, 34.95-43.25 dB) of the MDHL group (Cohen d = 1.58), but there was no meaningful difference between the devices. In noise, the PSAP (median, 0.4 dB; IQR, −0.8 to 1.7 dB; Cohen d = 0.9) and the premium HA (median, −0.1 dB; IQR, −0.7 to 0.7 dB]; Cohen d = 1.05) had much lower SNRs than the basic HA (median, 1.15; IQR, −0.35 to 2.9). In quiet, for the MSHL group, the premium HA (median, 46.6 dB; IQR, 42.1-51.3 dB) showed a lower reception threshold than the PSAP (median, 60.4 dB; IQR, 49.6-66.2 dB; Cohen d = 0.99) or the basic HA (median, 54.7 dB; IQR, 46.9-60.3 dB; Cohen d = 1.18). Even in noise, the premium HA showed much better results than the other 2 devices (Table 2).
Table 2. Speech Intelligibility in Noise Test.
| Test | Group | Test Result, Median (IQR) | Effect Size (95% CI)a | |||||
|---|---|---|---|---|---|---|---|---|
| Unaided | PSAP | Basic HA | Premium HA | PSAP vs Premium HA | PSAP vs Basic HA | Premium HA vs Basic HA | ||
| K-HINT,b dB HL | MHL | 37.6 (28.2 to 38.9) | 30.1 (26.8 to 33.4) | 30.7 (26.1 to 34.5) | 30.7 (25.9 to 34.8) | 0.1 (−0.2 to 0.4) | 0.1 (−0.3 to 0.4) | 0.1 (−0.2 to 0.3) |
| MDHL | 50.15 (43.2 to 58.1) | 40.45 (35.75 to 43.05) | 39.7 (36.6 to 43.5) | 39.2 (34.75 to 42.25) | 0.8 (0.4 to 1.2) | 0.6 (0.4 to 0.9) | 0.4 (0.1 to 0.7) | |
| MSHL | 68.1 (50.3 to 71.4) | 60.4 (49.6 to 66.2) | 54.7 (46.9 to 60.3) | 46.6 (42.1 to 51.3) | 1.6 (0.9 to 2.2) | 1.2 (0.7 to 1.6) | 1.2 (0.7 to 1.8) | |
| K-HINT, SNR, dB HLc | MHL | −1.3 (−1.9 to −0.4) | −1.1 (−1.9 to −0.4) | −1.4 (−2.8 to 0.2) | −1.7 (−2.5 to −0.5) | 0.4 (−0.2 to 1.0) | 0.1 (−0.3 to 0.5) | 0.5 (0.1 to 0.9) |
| MDHL | 0.95 (−0.2 to 2.3) | 0.4 (−0.8 to 1.7) | 1.15 (−0.35 to 2.9) | −0.1 (−0.7 to 0.65) | 0.5 (0.2 to 0.8) | 0.9 (0.5 to 1.3) | 1.0 (0.6 to 1.5) | |
| MSHL | 5.5 (4.7 to 7.5) | 4.3 (2.4 to 5.0) | 4.9 (2.8 to 6.7) | 1.6 (0.8 to 3.1) | 1.0 (0.4 to 1.6) | 0.8 (0.3 to 1.2) | 1.2 (0.6 to 1.8) | |
| SIBN,d word score | MHL | 32 (27 to 38) | 35 (31 to 38) | 34 (28 to 38) | 34 (30 to 39) | 0.1 (−0.2 to 0.3) | 0.1 (−0.2 to 0.5) | 0.4 (0.0 to 0.7) |
| MDHL | 0 (0 to 15) | 16.5 (0.5 to 25.5) | 16.5 (8 to 27.5) | 20.5 (10.5 to 30) | 0.4 (−0.1 to 0.9) | 0.3 (−0.2 to 0.7) | 0.5 (0.1 to 0.8) | |
| MSHL | 0 (0 to 0) | 0 (0 to 0) | 0 (0 to 2) | 3 (0 to 7) | 1.2 (0.7 to 1.8) | 0.6 (0.2 to 1.0) | 0.9 (0.4 to 1.4) | |
| SIBN,d sentence score | MHL | 6 (3 to 9) | 8 (6 to 9) | 6 (5 to 8) | 7 (5 to 9) | 0.1 (−0.3 to 0.5) | 0.5 (0.1 to 1.0) | 0.4 (0.0 to 0.9) |
| MDHL | 0 (0 to 1) | 1.5 (0.0 to 4.0) | 2.0 (0.5 to 4.5) | 3.5 (1.5 to 5.0) | 0.4 (−0.2 to 1.0) | 0.1 (−0.3 to 0.6) | 0.4 (0.0 to 0.8) | |
| MSHL | 0 (0 to 0) | 0 (0 to 0) | 0 (0 to 0) | 0 (0 to 1) | 0.9 (0.3 to 1.5) | 0.3 (−0.1 to 0.8) | 0.6 (0.2 to 1.0) | |
Abbreviations: HA, hearing aid; HL, hearing loss; K-HINT, Korean hearing in noise test; IQR, interquartile range; MDHL, moderate hearing loss; MHL, mild hearing loss; MSHL, moderately severe hearing loss; PSAP, personal sound amplification product; SIBN, speech intelligibility in babble noise test; SNR, signal-to-noise ratio; SPL, sound pressure level.
The comparison result of each device was corrected by the Bonferroni correction method for type I error increase by multiple comparisons and effect sizes calculated with Cohen d.
In a quiet environment, the K-HINT sentence was presented from the front, and the reception threshold was measured.
Speech signal was applied from the front; white noise was presented at an intensity of 65 dB SPL in 4 directions, and SNR was measured.
The frontal speech signal was fixed at 40 dB SPL and measured at a 0-dB SNR condition.
The SIBN Test
Word and sentence SIBN tests were performed (Table 2). As with the K-HINT, in the SIBN tests, the MHL group revealed no differences in unaided and aided conditions. The MDHL group showed a large difference in score between unaided (median, 0; IQR, 0-15) and aided hearing conditions (median, 17.5; IQR, 2-28]; Cohen d = 1.42), but no difference was observed between the devices. For the MSHL group on the word tests, the score for the premium HA (median, 3; IQR, 0-7) showed a large difference compared with the PSAP (median, 0; IQR, 0-0; Cohen d = 1.25) or the basic HA (median, 0; IQR, 0-2; Cohen d = 0.93). The sentence tests showed better performance in the aided condition than in the unaided condition in both the MDHL and MSHL groups (Table 2), but no device differences were found.
Listening Effort Tests
Dual-Task Paradigm
The SIBN test was performed with the addition of forced cognitive loading using digital pursuit rotor tracking (Table 3). The SIBN scores were higher in the aided condition than in the unaided condition in all groups. In particular, the PSAP and premium HA scores were much higher in the MHL group (PSAP median, 37; IQR, 35-38; premium HA median, 38.5; IQR, 37.0-39.5; Cohen d = 0.92) and the MSHL group (PSAP median, 18; IQR, 5-20; premium HA median, 22; IQR, 14-29; Cohen d = 0.89). Time on target was measured as the percentage of time the participant was able to follow round targets. Overall, a short time on target was seen in the unaided hearing condition, but only in the MSHL group were large differences observed (unaided median, 68.2%; IQR, 59.4%-79.3% vs aided median, 76.3; IQR, 71.6-82.5) (Cohen d = 0.89).
Table 3. Listening Effort Test (Dual-Task Paradigm).
| Test | Group | Test Result, Median (IQR) | Effect Size (95% CI)a | |||||
|---|---|---|---|---|---|---|---|---|
| Unaided | PSAP | Basic HA | Premium HA | PSAP vs Premium HA | PSAP vs Basic HA | Premium HA vs Basic HA | ||
| SIBN,b word score | MHL | 40.0 (38.5-40.0) | 37 (35-38) | 38.5 (36.5-39.0) | 38.5 (37.0-39.5) | 0.9 (0.4 to 1.5) | 0.4 (−0.2 to 1.0) | 0.2 (−0.4 to 0.8) |
| MDHL | 35 (24-38) | 31.0 (28.0-34.5) | 29.5 (20.5-35.5) | 31.0 (29.5-34.0) | 0.1 (−0.2 to 0.5) | 0.5 (0.1 to 0.8) | 0.5 (0.2 to 0.8) | |
| MSHL | 7 (0-22) | 18 (5-20) | 22 (5-25) | 22 (14-29) | 0.9 (0.4 to 1.4) | 0.1 (−0.3 to 0.5) | 0.7 (0.1 to 1.2) | |
| TOT,c % | MHL | 74.85 (72.8-83.15) | 78.85 (73.85-83.8) | 81.3 (78.7-85.5) | 82.65 (77.25-87.3) | 0.8 (0.3 to 1.3) | 0.6 (0.1 to 1.2) | 0.3 (−0.1 to 0.6) |
| MDHL | 77.95 (74.55-81.10) | 80.40 (74.95-85.55) | 80.40 (74.15-84.80) | 78.35 (70.35-83.55) | 0.5 (0.1 to 0.9) | 0.1 (−0.4 to 0.5) | 0.7 (0.3 to 1.1) | |
| MSHL | 68.2 (59.4-79.3) | 78.2 (73.5-81.2) | 75.9 (69.6-82.3) | 74.9 (72.0-82.9) | 0.0 (−0.3 to 0.4) | 0.2 (−0.2 to 0.6) | 0.1 (−0.1 to 0.4) | |
Abbreviations: HA, hearing aid; IQR, interquartile range; MDHL, moderate hearing loss; MHL, mild hearing loss; MSHL, moderately severe hearing loss; PSAP, personal sound amplification product; SIBN, speech intelligibility in babble noise test; TOT, time on target.
Comparison result of each device was corrected by the Bonferroni correction method for type I error increase by multiple comparisons and effect sizes calculated with Cohen d.
In the dual-task paradigm situation, the same speech intelligibility test as in Table 1 was performed.
Calculated as the percentage of time the participant remained on the target.
Pupillometry
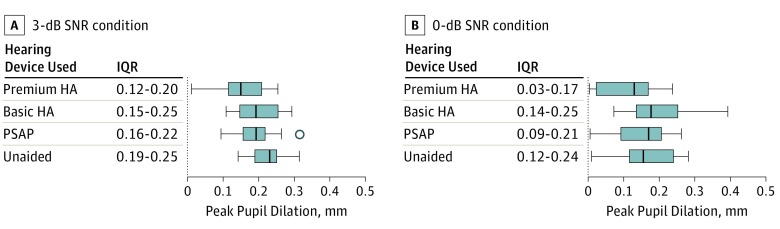
For pupillometry, we used a pupillometer to analyze the difference in how much the peak dilated pupil diameter (PPD) increased compared with the baseline pupil diameter (Figure). In the 3- and 0-dB SNR conditions, the percentage correct was not different from the SIBN results. The PPD size of the premium HA was the smallest of all the hearing conditions, analyzed by integrating the MHL, MDHL, and MSHL group results. The PPD sizes in the basic HA and PSAP groups were smaller than in the unaided 3-dB SNR condition. The 0-dB SNR condition showed no difference in PPD size between the unaided hearing, PSAP, and basic HA groups.
Figure. Pupillometry Results.
Peak pupil dilation size in millimeters was measured at 0-dB SNR (A) and 3-dB SNR (B) by adjusting the target speech value to less than 65-dB SPL babble noise. The vertical lines within the shaded bars represent the median values; shaded bars, interquartile ranges; the error whiskers, the highest and lowest points; and the circle beyond the highest point at the PSAP reading of the 3-dB SNR condition, an outlier. HA indicates hearing aid; IQR, interquartile range; SNR, signal-to-noise ratio; SPL, sound pressure level; PSAP, personal sound amplification product.
Self-rating Questionnaire
Sound Quality
There were no significant differences across all groups regarding sound quality. Generally, the worse the hearing, the lower the scores for all products. Satisfaction with basic HA showed the biggest drop for speech distortion and overall sound quality (eFigure 1 in the Supplement).
Device Preference
Device preference was investigated for all participants. There were no differences regarding personal preference in the MHL group (PSAP, 37%; basic HA, 26%; premium HA, 37%). In the MDHL group, 50% preferred the PSAP, and preferences for premium and basic HAs were equal (25%). In the MSHL group, 70% preferred the premium HA.
Discussion
A challenge in treating those with hearing impairment, which disproportionately affects the elderly population, is the high cost of HAs. Conventional HAs may be too expensive for many elderly persons to afford. Mamo et al1 compared features of direct-to-consumer hearing devices costing less than $400, but they did not mention specific performance. In contrast, Reed et al22 reported comparable SIN performance of PSAPs compared with conventional HAs, and this study is meaningful because it analyzed 5 types of PSAPs of various prices. However, it is different from the present study in that participants had only mild to moderate hearing loss; only 1 type of HA was used; and only speech comprehension was analyzed. Brody et al30 reported real-world speech recognition performance of HAs and PSAPs in 25 patients with mild to moderate hearing loss.30 The difference in sound quality found among devices was small, but well-fitted HAs showed better performance for speech recognition and listening effort than PSAPs. While this study can illuminate PSAPs’ usefulness in real life, patients were not divided based on the degree of hearing loss, and only 1 type of HA was analyzed. The present study divided the HAs into 2 types and analyzed them together with the PSAP for participants with various hearing impairments. The K-HINT, dual-task paradigm, and pupillometry tests were used as analytical tools to improve objectivity, and a comprehensive analysis was conducted (ie, satisfaction with preference and sound quality). To our knowledge, this is the first time pupillometry has been used to analyze PSAP performance. Previous studies have shown that listening effort and pupil dilation are closely related.28,31 Analysis of the peak dilation amplitude in the present study showed that although the difference was small, the premium HA had the smallest PPD value statistically for the 3-dB SNR condition (Figure, A). In the 0-dB SNR condition, all groups’ PPD values decreased. In the 0-dB SNR unaided hearing condition, the greatest decrease suggests that many participants in the unaided condition had given up listening (Figure, B).
Participants with MHL may have not felt the need for hearing enhancement. As a result, there was no difference between the PSAP and the basic and premium HAs across most tests. The MHL group also showed a similar preference for all devices, demonstrating that hearing technology was not necessary for hearing enhancement.
On the other hand, in the MDHL group, except for the objective dual-task paradigm test, the PSAP and HA groups showed a lower reception threshold (including quiet and noise conditions) and higher word and sentence scores than in unaided group. When analyzed by device, all indexes showed a good tendency in the premium HA, but large differences were shown only in the K-HINT (front noise condition). Differences between the PSAP and the basic HA were not found on most tests; that is, in the MDHL group, expensive HAs did not perform better on various laboratory analyses than the relatively inexpensive PSAP.
A total of 17 patients in the MSHL group had hearing loss greater than 56 dB of hearing loss, suggesting that they felt a greater need for hearing rehabilitation. Nine of them (52%) had previously used or were currently using HAs, but no one had ever used premium HAs. In this group, the premium HA was found to be superior on most tests, and the PSAP and basic HA did not show any clinically meaningful difference on all tests.
These results suggest that auditory enhancement using HAs or PSAPs was not meaningful for the MHL group with a PTA threshold less than 40 dB of hearing loss. However, for the MDHL group, PSAPs and HAs were associated with improved auditory comprehension and meaningfully improved speech understanding in noise. It is interesting that although the 3 devices did not show different performances in the MDHL group, 50% of patients in that group preferred PSAP over HA. In our opinion, this is probably because participants felt that there was little performance difference among the 3 devices, and the quick fitting process of the PSAP seemed more convenient than either of the HAs. In the MSHL group, which had communication difficulties without proper hearing enhancement, the premium HA showed good results and overwhelming preference. And while there was no meaningful difference between them, the basic HA obtained higher scores than the PSAP on various tests among the patients with MSHL. However, most of these participants had already been wearing HAs, indicating that HA adaptation must be considered. Additionally, since most of the patients had presbycusis, the fact that the PSAP lacked a compression function may have produced the lowered result.
Limitations
The study has several limitations. First, the degree of HA adaptation varied according to the duration of previous HA use, and we did not take this into consideration. However, no difference between the 2 HA groups was observed when we analyzed the results based on HA experience. Since only 1 product for each of the 3 device categories was compared, it is difficult to apply the results of this research to all HAs and PSAPs. Finally, the results of long-term PSAP and HA use in real life were not analyzed separately. Future field research is needed.
To our knowledge, this study is the first to compare the performance of a PSAP and HAs for various acoustic environments, especially based on the degree of hearing loss. However, careful attention should be paid when interpreting the findings because the study does not analyze different kinds of HAs or PSAPs, nor does it have long-term observation results that include individuals with different HA experiences. Nonetheless, we are confident that these results will shed light on PSAPs’ applicability. Our findings might be used as supporting evidence for future global policy decisions especially for OTC HAs and PSAPs.
Conclusions
Our results indicate that PSAPs may be helpful for individuals with mild to moderate hearing loss. For severe hearing loss, premium HAs would be more suitable. As this study cannot be applied to all HAs and PSAPs, careful interpretation of the results should be made. The lower price of PSAPs may lower the hearing enhancement barriers for patients with mild to moderate hearing loss. Further verification of safety and a comparative study on the usefulness of PSAPs and HAs in real life are needed.
eTable 1. Test device features
eFigure 1. Sound quality questionnaire survey by each hearing group
References
- 1.Mamo SK, Reed NS, Nieman CL, Oh ES, Lin FR. Personal sound amplifiers for adults with hearing loss. Am J Med. 2016;129(3):245-250. doi: 10.1016/j.amjmed.2015.09.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lin FR, Niparko JK, Ferrucci L. Hearing loss prevalence in the United States. Arch Intern Med. 2011;171(20):1851-1852. doi: 10.1001/archinternmed.2011.506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wilson BS, Tucci DL, Merson MH, O’Donoghue GM. Global hearing health care: new findings and perspectives. Lancet. 2017;390(10111):2503-2515. doi: 10.1016/S0140-6736(17)31073-5 [DOI] [PubMed] [Google Scholar]
- 4.Disease GBD, Injury I, Prevalence C; GBD 2015 Disease and Injury Incidence and Prevalence Collaborators . Global, regional, and national incidence, prevalence, and years lived with disability for 310 diseases and injuries, 1990-2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet. 2016;388(10053):1545-1602. doi: 10.1016/S0140-6736(16)31678-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cho Y-S, Choi S-H, Park KH, et al. Prevalence of otolaryngologic diseases in South Korea: data from the Korea national health and nutrition examination survey 2008. Clin Exp Otorhinolaryngol. 2010;3(4):183-193. doi: 10.3342/ceo.2010.3.4.183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Heine C, Browning CJ. Communication and psychosocial consequences of sensory loss in older adults: overview and rehabilitation directions. Disabil Rehabil. 2002;24(15):763-773. doi: 10.1080/09638280210129162 [DOI] [PubMed] [Google Scholar]
- 7.Arlinger S. Negative consequences of uncorrected hearing loss—a review. Int J Audiol. 2003;42(suppl 2):S17-S20. doi: 10.3109/14992020309074639 [DOI] [PubMed] [Google Scholar]
- 8.Lin FR, Yaffe K, Xia J, et al. ; Health ABC Study Group . Hearing loss and cognitive decline in older adults. JAMA Intern Med. 2013;173(4):293-299. doi: 10.1001/jamainternmed.2013.1868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Genther DJ, Betz J, Pratt S, et al. ; Health, Aging and Body Composition Study . Association between hearing impairment and risk of hospitalization in older adults. J Am Geriatr Soc. 2015;63(6):1146-1152. doi: 10.1111/jgs.13456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Genther DJ, Frick KD, Chen D, Betz J, Lin FR. Association of hearing loss with hospitalization and burden of disease in older adults. JAMA. 2013;309(22):2322-2324. doi: 10.1001/jama.2013.5912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lin FR. Hearing loss and cognition among older adults in the United States. J Gerontol A Biol Sci Med Sci. 2011;66(10):1131-1136. doi: 10.1093/gerona/glr115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lin FR, Ferrucci L, Metter EJ, An Y, Zonderman AB, Resnick SM. Hearing loss and cognition in the Baltimore Longitudinal Study of Aging. Neuropsychology. 2011;25(6):763-770. doi: 10.1037/a0024238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brown CS, Emmett SD, Robler SK, Tucci DL. Global hearing loss prevention. Otolaryngol Clin North Am. 2018;51(3):575-592. doi: 10.1016/j.otc.2018.01.006 [DOI] [PubMed] [Google Scholar]
- 14.Spahr AJ, Dorman MF, Litvak LM, et al. Development and validation of the AzBio sentence lists. Ear Hear. 2012;33(1):112-117. doi: 10.1097/AUD.0b013e31822c2549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moon IJ, Baek SY, Cho YS. Hearing aid use and associated factors in South Korea. Medicine (Baltimore). 2015;94(42):e1580. doi: 10.1097/MD.0000000000001580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Manchaiah V, Taylor B, Dockens AL, et al. Applications of direct-to-consumer hearing devices for adults with hearing loss: a review. Clin Interv Aging. 2017;12:859-871. doi: 10.2147/CIA.S135390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Taylor B. Hearables: the morphing of hearing aids and consumer electronic devices. Audiol Today. 2015;27(6):22-31. [Google Scholar]
- 18.Smith C, Wilber LA, Kim C. PSAPs vs hearing aids: an electroacoustic analysis of performance and fitting capabilities. Hearing Rev. 2016;23(7):18-22. [Google Scholar]
- 19.Kochkin S. MarkeTrak VIII: utilization of PSAPs and direct-mail hearing aids by people with hearing impairment. Hearing Rev. 2010;17(6):12-16. [Google Scholar]
- 20.Cheng CM, McPherson B. Over-the-counter hearing aids: electroacoustic characteristics and possible target client groups. Audiology. 2000;39(2):110-116. doi: 10.3109/00206090009073062 [DOI] [PubMed] [Google Scholar]
- 21.Sacco G, Gonfrier S, Teboul B, et al. Clinical evaluation of an over-the-counter hearing aid (TEO First®) in elderly patients suffering of mild to moderate hearing loss. BMC Geriatr. 2016;16(1):136. doi: 10.1186/s12877-016-0304-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Reed NS, Betz J, Kendig N, Korczak M, Lin FR. Personal sound amplification products vs a conventional hearing aid for speech understanding in noise. JAMA. 2017;318(1):89-90. doi: 10.1001/jama.2017.6905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Desjardins JL, Doherty KA. Age-related changes in listening effort for various types of masker noises. Ear Hear. 2013;34(3):261-272. doi: 10.1097/AUD.0b013e31826d0ba4 [DOI] [PubMed] [Google Scholar]
- 24.Downs DW. Effects of hearing and use on speech discrimination and listening effort. J Speech Hear Disord. 1982;47(2):189-193. doi: 10.1044/jshd.4702.189 [DOI] [PubMed] [Google Scholar]
- 25.Desjardins JL, Doherty KA. The effect of hearing aid noise reduction on listening effort in hearing-impaired adults. Ear Hear. 2014;35(6):600-610. doi: 10.1097/AUD.0000000000000028 [DOI] [PubMed] [Google Scholar]
- 26.Polt JM. Effect of threat of shock on pupillary response in a problem-solving situation. Percept Mot Skills. 1970;31(2):587-593. doi: 10.2466/pms.1970.31.2.587 [DOI] [PubMed] [Google Scholar]
- 27.Winn MB, Edwards JR, Litovsky RY. The impact of auditory spectral resolution on listening effort revealed by pupil dilation. Ear Hear. 2015;36(4):e153-e165. doi: 10.1097/AUD.0000000000000145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zekveld AA, Kramer SE, Festen JM. Pupil response as an indication of effortful listening: the influence of sentence intelligibility. Ear Hear. 2010;31(4):480-490. doi: 10.1097/AUD.0b013e3181d4f251 [DOI] [PubMed] [Google Scholar]
- 29.Cohen J. Statistical Power Analysis for the Behavioral Sciences. 2nd ed London, England: Routledge; 1988. [Google Scholar]
- 30.Brody L, Wu Y-H, Stangl E. A comparison of personal sound amplification products and hearing aids in ecologically relevant test environments. Am J Audiol. 2018;27(4):581-593. doi: 10.1044/2018_AJA-18-0027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Recarte MÁ, Pérez E, Conchillo A, Nunes LM. Mental workload and visual impairment: differences between pupil, blink, and subjective rating. Span J Psychol. 2008;11(2):374-385. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Test device features
eFigure 1. Sound quality questionnaire survey by each hearing group