Abstract
Objectives
To analyse the trends of amenable mortality rates (AMRs) in children over the period 2001–2015.
Design
Time trend analysis.
Setting
Thirty-four member countries of the Organisation for Economic Co-operation and Development (OECD).
Participants
Midyear estimates of the resident population aged ≤14 years.
Primary and secondary outcome measures
Using data from the WHO Mortality Database and Nolte and McKee’s list, AMRs were calculated as the annual number of deaths over the population/100 000 inhabitants. The rates were stratified by age groups (<1, 1–4, 5–9 and 10–14 years). All data were summarised by presenting the average rates for the years 2001/2005, 2006/2010 and 2011/2015.
Results
There was a significant decline in children’s AMRs in the <1 year group in all 34 OECD countries from 2001/2005 to 2006/2010 (332.78 to 295.17/100 000; %Δ −11.30%; 95% CI −18.75% to −3.85%) and from 2006/2010 to 2011/2015 (295.17 to 240.22/100 000; %Δ −18.62%; 95% CI −26.53% to −10.70%) and a slow decline in the other age classes. The only cause of death that was significantly reduced was conditions originating in the early neonatal period for the <1 year group. The age-specific distribution of causes of death did not vary significantly over the study period.
Conclusions
The low decline in amenable mortality rates for children aged ≥1 year, the large variation in amenable mortality rates across countries and the insufficient success in reducing mortality from all causes suggest that the heath system should increase its efforts to enhance child survival. Promoting models of comanagement between primary care and subspecialty services, encouraging high-quality healthcare and knowledge, financing universal access to healthcare and adopting best practice guidelines might help reduce amenable child mortality.
Keywords: international health services, amenable mortality, child, childhooh, healthcare, oecd countries
Strengths and limitations of this study.
This is the first study to analyse trends in child mortality amenable to healthcare.
Thirty-four Organisation for Economic Co-operation and Development countries were included in the analyses to provide a thorough depiction of amenable child mortality in high-income economies.
Mortality was not disaggregated by ethnicity or socioeconomic characteristics.
Making international comparisons is difficult due to variations in birth registration laws and death certification practices.
Introduction
The health of children and adolescents is an important goal for every society, both because they are vulnerable and because diseases can affect their quality of life. Measures for protecting and improving children’s and adolescents’ health will yield economic and social benefits beyond improved health outcomes. Indeed, young people have the potential to affect the health of future populations as well as global economic development unless timely and effective strategies are put into place.1 2
For the adoption of new strategies and the planning of interventions, information on the leading causes of death is essential. Many studies have analysed the mortality rates and have identified the main causes of death of children in specific countries3 or areas.4 Studies have demonstrated that many different factors contribute to child and adolescent mortality,5 including biological and psychosocial,6 socioeconomic status,7 8 environmental and behavioural factors.9 In the landscape described, the mortality burden conditioned by the performance of the healthcare system has not been well investigated. Although there is no indicator that is able to compressively reflect the performance of the healthcare system, a suitable measurement seems to be the concept of amenable mortality.
Amenable mortality is defined as deaths that, in the light of medical knowledge and technology at the time of death, could be prevented by timely access to good quality care. The concept of mortality amenable to healthcare finds its origins in the evolution of the concept of avoidable mortality, developed by Rutstein et al 10, who created a list of conditions that were considered either treatable or preventable through healthcare services given the current medical knowledge and technology. Rutstein was the first to introduce the term amenable mortality, differentiating between causes that are responsive to medical intervention through treatment and secondary/tertiary prevention actions (eg, cervical cancer, hypertensive disease or appendicitis) and causes responsive to actions beyond healthcare services (preventable conditions such as lung cancer and liver cirrhosis).
In recent years, the concept of amenable mortality has been used as a potential indicator of the performance of healthcare systems by several countries. The amenable mortality has been chosen as indicator in the UK National Health Service Outcomes Framework for 2011–201211 and in Australian and New Zealand Atlas of Avoidable Mortality 1997–2001 12 or in European Community Atlas of ‘Avoidable Death’.13 Amenable mortality indicators have also been used to report on spatial and temporal distributions and variations in health system performance across countries,14–20 as well as across subnational entities,12 21–25 socioeconomic status, ethnic groups and sex.14 24 26 27 Most of these studies did not assess amenable mortality across ages in different populations and did not focus on child and adolescent age classes.
According to the WHO, an estimated 6.3 million children under the age of 15 years died in 2017 (117 000 in the Organisation for Economic Co-operation and Development (OECD) region). A total of 5.4 million of these children were under the age of 5 years, and 2.5 million died within the first month of life. More than half of these early child deaths were due to conditions that could be prevented or treated with access to simple, affordable interventions and were, consequently, amenable.2
The purpose of this study was to analyse the trends of amenable child mortality rates in 34 OECD countries from 2001 to 2015 and to evaluate the pattern across age classes.
Materials and methods
This descriptive study was conducted using secondary data from 34 OECD countries during the period from 2001 to 2015. Mexico and Turkey, although members of the OECD, were not included in the analysis because these countries are not listed among high-income economies in the World Development Indicators dataset (gross national income per capita ≥$12 056 for fiscal year 2019)28 and had limited data availability. The mortality and population data came from the WHO Mortality Database,29 which comprises deaths registered in national vital registration systems, with the underlying causes of death coded according to the International Classification of Diseases; no permission is required from the WHO if data are used for non-commercial purposes. If reference populations were not available in the WHO Mortality Database, the data were extracted from UN data.30 The country-level data availability is presented in online supplementary file 1.
bmjopen-2018-027909supp001.pdf (342.1KB, pdf)
The causes of death amenable to healthcare were selected by means of the list proposed by Nolte and McKee14 31 and used in a working paper by the OECD to generate estimates of amenable mortality for 31 countries.15 This list includes a selected number of conditions that are treatable based on the clinical effectiveness of existing medical interventions. The age limit for amenable deaths is set at 75 years for most conditions, such as cancer and cardiovascular diseases, but the age limit for some diseases (ie, whooping cough, measles, intestinal infections and respiratory diseases other than pneumonia/influenza) is set at 14 (see online supplementary file 2 for Nolte and McKee’s full list). The aggregation of the causes of death operated in the WHO Mortality Database prevents the use of the list of amenable deaths currently adopted by Eurostat.32
bmjopen-2018-027909supp002.pdf (130.3KB, pdf)
For each country, the amenable mortality rates were calculated as the annual number of deaths in the population aged 0–14 years per 100 000 inhabitants. The rates were stratified by the age groups adopted in the WHO Mortality Database (<1, 1–4, 5–9 and 10–14 years) and by the 33 disease categories defined by Gay et al.15 Due to the instability in the estimates of the annual amenable mortality rates, especially for small population countries, all data were summarised by presenting the average rates for the years 2001/2005, 2006/2010 and 2011/2015. The statistical significance of the percentage changes between these time periods was assessed by using the formula suggested by Hildebrandt et al.33 Data interpretation focused on the countries that showed significant percentage changes over the entire study period (ie, both between 2001/2005 and 2006/2010 and between 2006/2010 and 2011/2015). Differences in the ranking of age-specific causes of death between time periods were evaluated with the Friedman test.
All data were analysed using the Stata software package, V.15. The significance level was set at. 05.
Ethics statement
This descriptive study involved aggregate data that exist in the public domain, where it is not possible to identify individuals from the information provided. For this reason, this research did not require ethical approval.
Patient and public involvement
Patients and the public were not involved in the design or planning of the study.
Results
All-cause amenable child mortality rates
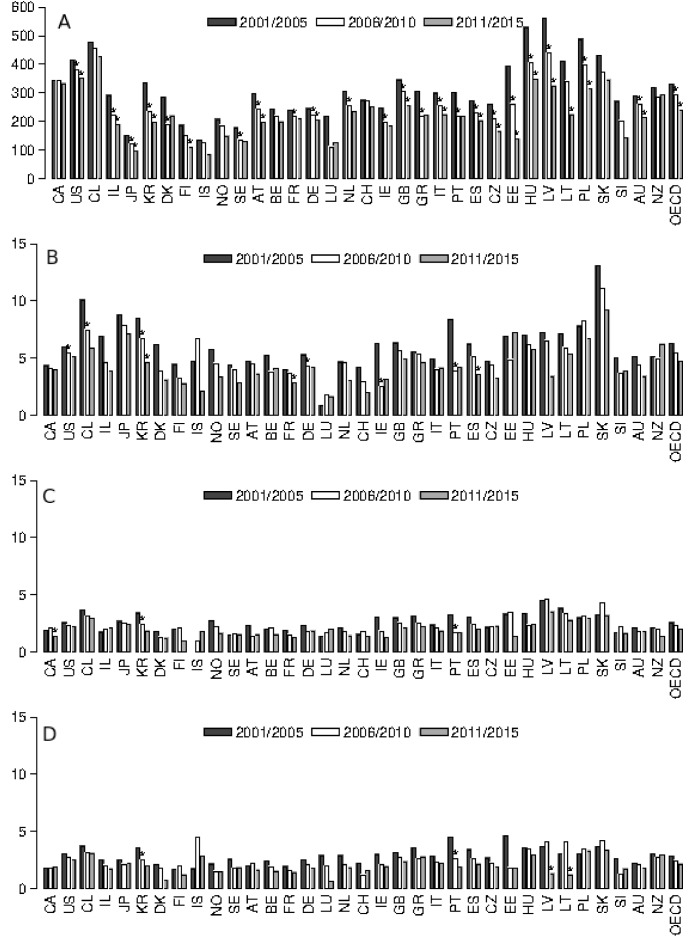
The results of the trend analyses conducted over the 5-year periods 2001/2005, 2006/2010 and 2011/2015 are presented in figure 1 and in online supplementary file 3. The amenable mortality rate of the OECD population with <1 year dropped significantly from 2001/2005 to 2006/2010 (332.78 to 295.17 per 100 000, %Δ=−11.3%) and from 2006/2010 to 2011/2015 (295.17 to 240.22 per 100 000, %Δ=−18.6%). Contrary to the OECD rate of children aged <1 year, in the population aged ≥1 year, the overall OECD rate did not decrease significantly. From 2001/2005 to 2006/2010 and from 2006/2010 to 2011/2015, the mortality rates decreased by 13.4% and 13.0%, respectively, in the 1–4 years age group; by 11.9% and 11.3% in the 5–9 years age group; and by 13.5% and 13.1% for the 10–14 years age group. In 2011/2015, the OECD rate was 4.73 per 100 000 for the 1–4 years age group, 2.02 per 100 000 for the 5–9 years age group and 2.16 per 100 000 for the 10–14 years age group.
Figure 1.
Yearly amenable mortality rates (per 100 000) for ages <1 (A), 1–4 (B), 5–9 (C) and 10–14 (D) in 34 Organisation for Economic Co-operation and Development countries, 2001/2005, 2006/2010 and 2011/2015 (see online supplementary file 1 for country-level data availability). *Percentage decrease is statistically significant (p<0.05). AT, Austria; AU, Australia; BE, Belgium; CA, Canada; CL, Chile; CZ, Czechia; CH, Switzerland; DE, Germany; DK, Denmark; EE, Estonia; ES, Spain; FI, Finland; FR, France; GR, Greece; GB, UK; HU, Hungary; IL, Israel; IS, Iceland; IE, Ireland; IT, Italy; JP, Japan; KR, Republic of Korea; LT, Lithuania; LU, Luxembourg; LV, Latvia; NL, The Netherlands; NO, Norway; NZ, New Zealand; PL, Poland; PT, Portugal; SE, Sweden; SI, Slovenia; SK, Slovakia; US, USA.
bmjopen-2018-027909supp003.pdf (258.6KB, pdf)
Cause-specific child mortality rates
When we examined the distribution of cause-specific mortality rates across multiple age groups and study periods, no statistically significant variations in the OECD population were found. The only exception was a significant decrease in deaths in the first year of life for conditions originating in the early neonatal period (2001/2005 to 2006/2010: 266.3 to 239.8 per 100 000, %Δ=−9.9%; 2006/2010 to 2011/2015: 239.8 to 193.8 per 100 000, %Δ=−19.2%) (see online supplementary file 4).
bmjopen-2018-027909supp004.pdf (212.3KB, pdf)
Globally, for the years 2001/2005, 2006/2010 and 2011/2015, there were no significant differences in the age-specific percentage distribution of causes of death (<1 year: Friedman test (p)=0.955 (0.372); 1–4 years: 0.864 (0.607); 5–9 years: 0.470 (0.740); 10–14 years: 1.773 (0.398)). As shown in table 1, the three leading causes of death by age groups over the 15 year study period were the following: conditions originating in the early neonatal period, congenital cardiovascular anomalies and pneumonia for children aged <1 year and congenital cardiovascular anomalies, leukaemia and respiratory diseases (excluding pneumonia/influenza) for all other age groups.
Table 1.
Percentage distribution of the top 10 causes of death amenable to healthcare in children in 34 OECD countries, years 2001/2005, 2006/2010 and 2011/2015
| Cause of death | 2001/2005 | 2006/2010 | 2011/2015 | |||
| Rank | % | Rank | % | Rank | % | |
| <1 year | (n=190 169.5) | (n=175 008) | (n=105 210.5) | |||
| Conditions originating in the early neonatal period | #1 | 80.02 | #1 | 81.25 | #1 | 80.69 |
| Congenital cardiovascular anomalies | #2 | 14.38 | #2 | 13.15 | #2 | 14.55 |
| Pneumonia | #3 | 1.82 | #3 | 1.53 | #3 | 1.30 |
| Septicaemia | #4 | 1.50 | #4 | 1.39 | #4 | 1.04 |
| Intestinal infections other than typhoid, diphtheria | #7 | 0.27 | #5 | 0.60 | #5 | 0.72 |
| Cerebrovascular diseases | #6 | 0.51 | #6 | 0.60 | #6 | 0.38 |
| Nephritis and nephrosis | #5 | 0.54 | #7 | 0.49 | #7 | 0.25 |
| Leukaemia | #8 | 0.21 | #8 | 0.26 | #8 | 0.24 |
| Epilepsy | #9 | 0.17 | #9 | 0.17 | #9 | 0.23 |
| Whooping cough | #11* | 0.11 | #12* | 0.10 | #10 | 0.19 |
| Other causes | – | 0.46 | – | 0.46 | – | 0.39 |
| 1–4 years | (n=14 438) | (n=12 738) | (n=8491.5) | |||
| Congenital cardiovascular anomalies | #1 | 25.67 | #1 | 23.63 | #1 | 25.21 |
| Leukaemia | #3 | 14.55 | #3 | 13.99 | #2 | 14.99 |
| All respiratory diseases, excluding pneumonia/influenza | #2 | 15.60 | #2 | 16.79 | #3 | 14.25 |
| Pneumonia | #4 | 12.35 | #4 | 12.20 | #4 | 12.24 |
| Epilepsy | #7 | 5.16 | #7 | 5.50 | #5 | 7.20 |
| Septicaemia | #5 | 7.57 | #5 | 8.18 | #6 | 6.88 |
| Conditions originating in the early neonatal period | #6 | 5.59 | #6 | 5.60 | #7 | 5.06 |
| Intestinal infections other than typhoid and diphtheria | #9 | 2.45 | #9 | 3.05 | #8 | 3.98 |
| Cerebrovascular diseases | #8 | 3.86 | #8 | 4.13 | #9 | 3.38 |
| Influenza | #10 | 2.16 | #10 | 1.92 | #10 | 2.39 |
| Other causes | – | 5.05 | – | 5.00 | – | 4.42 |
| 5–9 years | (n=7705) | (n=6706) | (n=4541) | |||
| Leukaemia | #1 | 31.72 | #1 | 28.35 | #1 | 27.31 |
| All respiratory diseases, excluding pneumonia/influenza | #3 | 12.91 | #2 | 16.28 | #2 | 13.87 |
| Congenital cardiovascular anomalies | #2 | 14.91 | #3 | 13.67 | #3 | 13.12 |
| Epilepsy | #5 | 7.81 | #5 | 7.86 | #4 | 10.37 |
| Pneumonia | #4 | 8.58 | #4 | 7.90 | #5 | 9.12 |
| Cerebrovascular diseases | #6 | 6.87 | #6 | 7.13 | #6 | 6.69 |
| Septicaemia | #7 | 5.15 | #7 | 5.67 | #7 | 5.26 |
| Influenza | #9 | 1.66 | #8 | 2.54 | #8 | 2.97 |
| Conditions originating in the early neonatal period | #8 | 2.25 | #9 | 2.48 | #9 | 2.80 |
| Intestinal infections other than typhoid and diphtheria | #13† | 0.87 | #12† | 1.25 | #10 | 2.00 |
| Other causes | – | 7.26 | – | 6.87 | – | 6.47 |
| 10–14 years | (n=9109) | (n=7579) | (n=4921) | |||
| Leukaemia | #1 | 31.30 | #1 | 30.01 | #1 | 30.24 |
| All respiratory diseases, excluding pneumonia/influenza | #3 | 13.66 | #2 | 15.28 | #2 | 13.66 |
| Congenital cardiovascular anomalies | #2 | 14.13 | #3 | 12.17 | #3 | 12.62 |
| Epilepsy | #5 | 7.73 | #5 | 7.89 | #4 | 10.36 |
| Cerebrovascular diseases | #4 | 8.12 | #4 | 8.99 | #5 | 9.08 |
| Pneumonia | #6 | 7.59 | #6 | 6.73 | #6 | 7.27 |
| Septicaemia | #7 | 4.17 | #7 | 4.92 | #7 | 4.29 |
| Diabetes mellitus | #8 | 2.49 | #8 | 2.31 | #8 | 1.97 |
| Influenza | #11‡ | 1.15 | #9 | 2.15 | #9 | 1.77 |
| Conditions originating in the early neonatal period | #10 | 1.57 | #11‡ | 1.78 | #10 | 1.52 |
| Other causes | – | 8.09 | – | 7.78 | – | 7.21 |
*Abdominal hernia was ranked #10 in 2001/2005 (0.17%) and 2006/2010 (0.13%).
†Nephritis and nephrosis were ranked #10 in 2001/2005 (1.60%) and 2006/2010 (1.69%).
‡Nephritis and nephrosis were ranked #9 in 2001/2005 (1.79%) and #10 in 2006/2010 (1.91%).
OECD, Organisation for Economic Co-operation and Development.
Country-specific amenable child mortality rates
As shown in figure 1 and online supplementary file 3, the amenable mortality rate for the population aged <1 year dropped significantly over the entire study period in 15 countries (USA, Israel, Japan, South Korea, Czechia, Estonia, Germany, Italy, Latvia, Hungary, Poland, Spain, Austria, UK and Australia). In addition to the decrease in conditions originating in the early neonatal period, other causes contributed to a significant reduction in mortality for some of these countries: septicaemia, pneumonia and nephritis/nephrosis in the USA, septicaemia in Poland and congenital cardiovascular anomalies in Japan and Spain. See online supplementary file 4 for country-specific and cause-specific mortality rates in the <1 year population.
Although the overall OECD rate did not decrease significantly in the population aged ≥1 year, some country-specific data exhibited significant trends (figure 1). However, no country showed a significant linear decline in mortality over the entire study period for all age groups (figure 1).
Discussion
In this time trend analysis, we found a significant decline in amenable child mortality rates for the <1 year age group in the 34 OECD countries between 2001/2005 and 2011/2015 and a slow decline in the other age groups. These results confirm the trend shown by previous studies conducted on six OECD countries that had been selected to provide a variety of forms of healthcare delivery between 1956 and 198021 and highlight that policies to reduce amenable mortality rates are still needed, even in settings where the quality of medical services and resources is high.
These results are driven by the fact that the only significantly reduced cause of death was conditions originating in the early neonatal period in the <1 year group. Surprisingly, the decline was not more pronounced among countries with higher mortality in 2001/2005, indicating that continued gains in child survival occurred both in low-mortality and high-mortality countries. The most representative countries are Japan, which showed an improvement in its performance despite starting from low values in 2001/2005, and Hungary and the USA, which showed high amenable mortality rates in 2001/2005.
The results concerning country-specific amenable child mortality rates showed that the reductions in the age-specific rates were not evenly distributed across OECD countries. There was not a single cause for which the age-specific mortality rates declined in the countries studied, and the success in reducing mortality rates was not consistent for all causes. These achievements corroborate another of our results, highlighting that the causal distribution of the age-specific mortality rates did not vary over the period examined and that, namely, the conditions originating in the early neonatal period, congenital cardiovascular anomalies, pneumonia, leukaemia and all respiratory diseases (excluding pneumonia/influenza) were consistently top ranked. Because these are the main causes related to chronic disorders and pathologies that require acute care delivered quickly, our results suggest that the models of care for children should be revised. Sidebotham5 has argued that child diseases might broadly be divided into chronic (eg, leukaemia) and acute diseases (eg, pneumonia) and that a single model cannot be proposed but each needs a different, although interconnected, health system solution.
Wolfe34 highlights that the presence of children with chronic disorders requires substantial changes from a hospital-centric model to a model in which primary care and secondary care providers and public health services work closely together. Models of comanagement are essential to promote ongoing communication and coordination between primary care and subspecialty services. It would be inefficient for subspecialists to provide primary care, and ineffective for primary care providers to attempt to stay abreast of the latest therapies for chronic diseases. Because the majority of chronic illness care is performed within the primary care setting and because primary care physicians spend a considerable amount of time treating chronic illness, primary care should play a central role in the overall coordination and continuity of people’s care providing greater access to specialists and more timely follow-up care after emergency room visits.35 The few studies evaluating this new approach to work showed an improvement in child survival,36–38 and thus traced a possible path to improve amenable mortality from chronic diseases.
Sidebotham and Mackenbach judge as determinants of amenable mortality in acute diseases the universal access to healthcare and the presence of professionals with appropriate training.5 18 The relevance of access may at least partially explain our results in some countries. For example, in Chile, where the mortality rate of pneumonia was consistently high for the <1 year group, the healthcare system replicates class inequalities. Studies have identified and tracked several important inequalities in the burden of infant mortality for infectious diseases by socioeconomic level in Chile, showing that this gap is discriminatory because disadvantaged households underuse healthcare services (due to social or economic exclusion).39 40 In Slovakia, where also the mortality rate of pneumonia was high for the <1 year group, social health insurance system formally covers all residents and has a benefit package that all insurance companies must provide for their insured. In theory, the insurance system is thus designed to provide everybody with the same benefit package, regardless of their health status, ability to pay or place of residence. In practice, coverage varies across the country, mainly because the supply of human resources (especially specialists and general practitioners (GPs)) is not adequate in all regions and districts, and sometimes providers are simply not available.41
The access to healthcare professionals with adequate training looks at the models of first contact between patients and clinicians and on their expertise. Although an international debate is open on the best pattern of paediatric primary care among a paediatrician-based system, a combined system or a system based on GPs/family doctors,42 some authors highlight the importance of primary care paediatricians, especially because primary care paediatricians who look after children are likely to have more professional training and competencies than GPs, who often, receiving an insufficient or not existing or non-mandatory training, do not have capabilities to diagnose and treat a child in effective times.34 43 44
Supported by the specialised literature, the availability of high-quality healthcare and knowledge and the use of best practice guidelines are also determinants of amenable mortality.5 18 These determinants provide one possible explanation for our results, in particular for the reduction in mortality by conditions originating in the early neonatal period. Several lines of evidence support the hypothesis that neonatal intensive care has resulted in decreased mortality. Marked declines in neonatal and infant mortality rates are coincident with the introduction and progressive development of neonatal intensive care45 46 and with specific neonatal intensive therapeutic improvements.47–50 The second line of evidence regarding the effect of neonatal intensive care on infant mortality is the observation that low birthweight infants born in hospitals with tertiary-level neonatal intensive care units have lower mortality rates than infants born in hospitals without such units.51 52 Finally, a third line of evidence referring to improving high-risk obstetric care has been associated with decreases in neonatal mortality.46 53
Variations in child mortality exist even in countries where high-quality healthcare is available. Part of this variation may be due to a failure in implementing treatment protocols or evidence-based best practices tailored with the local conditions. This suggestion may provide some explanation of the mortality decrease in some countries, such as Hungary and Japan, which have supported the introduction of evidence-based protocols on routine newborn care and have trained nursery staff members on the protocols.54 55
Our study has the same limitations as all studies that use secondary data. It is undeniable that international variations in birth registration laws and practices and the process of death certification have the potential to bias the international comparisons of child mortality. Additionally, international comparisons of mortality rates are confounded by the various ways in which countries classify preterm infants near the threshold of viability.56–58 Considering these issues, our study only evaluates the trend of amenable mortality rates, and a comparison analysis was not performed. Also, although much of the literature assessing the contribution of healthcare to health focuses on mortality data, data on children may be of limited value because the number of deaths is small, making interpretation difficult. However, we believe that the small numbers were mitigated by the fact that our study analysed the rates and trajectories of multiple countries over a 15-year time period. Another limitation is that our analysis did not account for mortality disparities within countries that were attributable to ethnicity, race, socioeconomic status or geographic residence, since our data sources did not include this information. Evidence from the USA, for example, showed higher levels of amenable mortality among people disadvantaged in terms of race or socioeconomic status.59 60 Considering that the perinatal mortality of the USA has a prevailing effect on premature births and that striking racial disparities persist, with African-Americans exhibiting higher rates of preterm delivery than any other major racial/ethnic group,61 potentially large variations within populations may be concealed. Lastly, our study is based on the definition of amenable death in its original sense of mortality responsive to medical intervention through treatment, although several countries, such as UK, Canada and Australia, have broadened definitions for their avoidable mortality indicators to include deaths from conditions avoidable through primary prevention. This broadened definition suggests that the models of care for children should be revised and that policies must be adopted in order to prevent specific causes of death before the point of reaching the healthcare system. These policies include population health interventions such as the obligation of physical activity during adolescence in schools or the taxation of tobacco and sugar-sweetened beverages.
Some preliminary conclusions can be drawn from this study. Over the 15-year period from 2001 to 2015, the amenable mortality rate in <1 year olds progressively declined in most OECD countries. Second, OECD countries had success in reducing mortality from conditions originating in the early neonatal period. Lastly, the low decline in amenable mortality rates for children aged ≥1 year, the high variation in amenable mortality rates across countries and the insufficient success in reducing mortality from all causes suggest that the heath system should increase its efforts to improve health outcomes for children.
Supplementary Material
Acknowledgments
Data for this study came from the WHO. All analyses, interpretations and conclusions are credited to the authors of this study, not to the WHO.
Footnotes
Contributors: MMG and GD formulated the research goals and supervised the research activity; MMG, GD and JL defined the design of the methodology; MMG wrote the article; RS, WR and MPF revised the article; MB collected the data and managed the database; JL used statistical techniques to analyse the study data. All authors have read and approved the manuscript.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Patient consent for publication: Not required.
References
- 1. BLUM R, Nelson-Mmari K. The health of young people in a global context. Journal of Adolescent Health 2004;35:402–18. 10.1016/S1054-139X(03)00537-8 [DOI] [PubMed] [Google Scholar]
- 2. WHO. Children: reducing mortality. http://www.who.int/news-room/fact-sheets/detail/children-reducing-mortality (Accessed 3 Oct 2018). [PubMed]
- 3. Fraser J, Sidebotham P, Frederick J, et al. . Learning from child death review in the USA, England, Australia, and New Zealand. The Lancet 2014;384:894–903. 10.1016/S0140-6736(13)61089-2 [DOI] [PubMed] [Google Scholar]
- 4. Kyu HH, Stein CE, Boschi Pinto C, et al. . Causes of death among children aged 5-14 years in the WHO European Region: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Child Adolesc Health 2018;2:321–37. 10.1016/S2352-4642(18)30095-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Sidebotham P, Fraser J, Covington T, et al. . Understanding why children die in high-income countries. Lancet 2014;384:915–27. 10.1016/S0140-6736(14)60581-X [DOI] [PubMed] [Google Scholar]
- 6. Alio AP, Richman AR, Clayton HB, et al. . An ecological approach to understanding black-white disparities in perinatal mortality. Matern Child Health J 2010;14:557–66. 10.1007/s10995-009-0495-9 [DOI] [PubMed] [Google Scholar]
- 7. Gakidou E, Cowling K, Lozano R, et al. . Increased educational attainment and its effect on child mortality in 175 countries between 1970 and 2009: a systematic analysis. Lancet 2010;376:959–74. 10.1016/S0140-6736(10)61257-3 [DOI] [PubMed] [Google Scholar]
- 8. Lightfoot TJ, Johnston WT, Simpson J, et al. . Survival from childhood acute lymphoblastic leukaemia: the impact of social inequality in the United Kingdom. Eur J Cancer 2012;48:263–9. 10.1016/j.ejca.2011.10.007 [DOI] [PubMed] [Google Scholar]
- 9. Gore FM, Bloem PJ, Patton GC, et al. . Global burden of disease in young people aged 10-24 years: a systematic analysis. Lancet 2011;377:2093–102. 10.1016/S0140-6736(11)60512-6 [DOI] [PubMed] [Google Scholar]
- 10. Rutstein DD, Berenberg W, Chalmers TC, et al. . Measuring the quality of medical care. A clinical method. N Engl J Med 1976;294:582–8. 10.1056/NEJM197603112941104 [DOI] [PubMed] [Google Scholar]
- 11. Department of Health. The NHS Outcomes Framework 2011/12. 2010. https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/213789/dh_123138.pdf.
- 12. Page A, Zealand N. University of Adelaide, editors. Australian and New Zealand atlas of avoidable mortality. Adelaide, S. Aust: Public Health Information Development Unit, University of Adelaide, 2006. [Google Scholar]
- 13. Holland WW. European Community atlas of “avoidable death, 1991. [Google Scholar]
- 14. Nolte E, McKee CM. Measuring the health of nations: updating an earlier analysis. Health Aff 2008;27:58–71. 10.1377/hlthaff.27.1.58 [DOI] [PubMed] [Google Scholar]
- 15. Gay JG, Paris V, Devaux M, et al. . Mortality Amenable to Health Care in 31 OECD Countries: estimates and methodological Issues. (Published Online First: 31 Jan 2011).
- 16. Treurniet HF, Boshuizen HC, Harteloh PP. Avoidable mortality in Europe (1980-1997): a comparison of trends. J Epidemiol Community Health 2004;58:290–5. 10.1136/jech.2002.006452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Nolte E, McKee M. Variations in amenable mortality--trends in 16 high-income nations. Health Policy 2011;103:47–52. 10.1016/j.healthpol.2011.08.002 [DOI] [PubMed] [Google Scholar]
- 18. Mackenbach JP, Hoffmann R, Khoshaba B, et al. . Using “amenable mortality” as indicator of healthcare effectiveness in international comparisons: results of a validation study. J Epidemiol Community Health 2013;67:139–46. 10.1136/jech-2012-201471 [DOI] [PubMed] [Google Scholar]
- 19. Mackenbach JP, Bouvier-Colle MH, Jougla E. “Avoidable” mortality and health services: a review of aggregate data studies. J Epidemiol Community Health 1990;44:106–11. 10.1136/jech.44.2.106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Gianino MM, Lenzi J, Muça A, et al. . Declining Amenable Mortality: Time Trend (2000-2013) and Geographic Area Analysis. Health Serv Res 2017;52:1908–27. 10.1111/1475-6773.12563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Charlton JR, Velez R. Some international comparisons of mortality amenable to medical intervention. Br Med J 1986;292:295–301. 10.1136/bmj.292.6516.295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. James PD, Manuel DG, Mao Y. Avoidable mortality across Canada from 1975 to 1999. BMC Public Health 2006;6:137 10.1186/1471-2458-6-137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Pampalon R. Avoidable mortality in Québec and its regions. Soc Sci Med 1993;37:823–31. 10.1016/0277-9536(93)90376-F [DOI] [PubMed] [Google Scholar]
- 24. Piers LS, Carson NJ, Brown K, et al. . Avoidable mortality in Victoria between 1979 and 2001. Aust N Z J Public Health 2007;31:5–12. 10.1111/j.1753-6405.2007.00002.x [DOI] [PubMed] [Google Scholar]
- 25. Fantini MP, Lenzi J, Franchino G, et al. . Amenable mortality as a performance indicator of Italian health-care services. BMC Health Serv Res 2012;12:310 10.1186/1472-6963-12-310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Tobias M, Jackson G. Avoidable mortality in New Zealand, 1981-97. Aust N Z J Public Health 2001;25:12–20. 10.1111/j.1467-842X.2001.tb00543.x [DOI] [PubMed] [Google Scholar]
- 27. Schoenbaum SC, Schoen C, Nicholson JL, et al. . Mortality amenable to health care in the United States: the roles of demographics and health systems performance. J Public Health Policy 2011;32:407–29. 10.1057/jphp.2011.42 [DOI] [PubMed] [Google Scholar]
- 28. World Bank. World Development Indicators | DataBank. 2018. http://databank.worldbank.org/data/reports.aspx?source=world-development-indicators (Accessed 3 Oct 2018).
- 29. World Health Organization. WHO Mortality Database. 2018. http://www.who.int/healthinfo/mortality_data/en/ (Accessed 3 Oct 2018).
- 30. United Nations. Demographic Statistics Database. 2018. http://data.un.org/Data.aspx?d=POP&f=tableCode%3A22 (Accessed 3 Oct 2018).
- 31. Nolte E, McKee M. Does health care save lives? Avoidable mortality revisited. The Nuffield Trust 2004. http://researchonline.lshtm.ac.uk/15535/ (Accessed 3 Oct 2018). [Google Scholar]
- 32. Eurostat. Amenable and preventable deaths of residents. https://ec.europa.eu/eurostat/web/products-datasets/-/hlth_cd_apr (Accessed 3 Oct 2018).
- 33. Hildebrandt M, Bender R, Gehrmann U, et al. . Calculating confidence intervals for impact numbers. BMC Med Res Methodol 2006;6:32 10.1186/1471-2288-6-32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Wolfe I, Thompson M, Gill P, et al. . Health services for children in western Europe. The Lancet 2013;381:1224–34. 10.1016/S0140-6736(12)62085-6 [DOI] [PubMed] [Google Scholar]
- 35. Expert Panel on Effective Ways of Investing in Health. Definition of a frame of reference in relation to primary care with a special emphasis on financing systems and referral systems. 2014. https://ec.europa.eu/health/expert_panel/sites/expertpanel/files/004_definitionprimarycare_en.pdf (Accessed 2 Mar 2019).
- 36. Adams JS, Woods ER. Redesign of chronic illness care in children and adolescents. Curr Opin Pediatr 2016;28:428–33. 10.1097/MOP.0000000000000368 [DOI] [PubMed] [Google Scholar]
- 37. Britto MT, Vockell AL, Munafo JK, et al. . Improving outcomes for underserved adolescents with asthma. Pediatrics 2014;133:e418–e427. 10.1542/peds.2013-0684 [DOI] [PubMed] [Google Scholar]
- 38. Mangione-Smith R, Schonlau M, Chan KS, et al. . Measuring the effectiveness of a collaborative for quality improvement in pediatric asthma care: does implementing the chronic care model improve processes and outcomes of care? Ambul Pediatr 2005;5:75–82. 10.1367/A04-106R.1 [DOI] [PubMed] [Google Scholar]
- 39. Hertel-Fernandez AW, Giusti AE, Sotelo JM. The Chilean infant mortality decline: improvement for whom? Socioeconomic and geographic inequalities in infant mortality, 1990-2005. Bull World Health Organ 2007;85:798–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Cárcamo RA, van der Veer R, Vermeer HJ, et al. . From foundling homes to day care: a historical review of childcare in Chile. Cad Saude Publica 2014;30:461–72. 10.1590/0102-311X00060613 [DOI] [PubMed] [Google Scholar]
- 41. OECD. State of Health in the EU Slovak Republic: Country Health Profile 2017 - European Observatory on Health Systems and Policies, 2017. [Google Scholar]
- 42. Katz M, Rubino A, Collier J, et al. . Demography of pediatric primary care in Europe: delivery of care and training. Pediatrics 2002;109:788–96. 10.1542/peds.109.5.788 [DOI] [PubMed] [Google Scholar]
- 43. Pearson GA, Ward-Platt M, Harnden A, et al. . Why children die: avoidable factors associated with child deaths. Arch Dis Child 2011;96:927–31. 10.1136/adc.2009.177071 [DOI] [PubMed] [Google Scholar]
- 44. van Esso D, del Torso S, Hadjipanayis A, et al. . Paediatric primary care in Europe: variation between countries. Arch Dis Child 2010;95:791–5. 10.1136/adc.2009.178459 [DOI] [PubMed] [Google Scholar]
- 45. Williams RL, Chen PM. Identifying the sources of the recent decline in perinatal mortality rates in California. N Engl J Med 1982;306:207–14. 10.1056/NEJM198201283060404 [DOI] [PubMed] [Google Scholar]
- 46. Richardson DK, Gray JE, Gortmaker SL, et al. . Declining severity adjusted mortality: evidence of improving neonatal intensive care. Pediatrics 1998;102:893–9. 10.1542/peds.102.4.893 [DOI] [PubMed] [Google Scholar]
- 47. Horbar JD, Wright LL, Soll RF, et al. . A multicenter randomized trial comparing two surfactants for the treatment of neonatal respiratory distress syndrome. National Institute of Child Health and Human Development Neonatal Research Network. J Pediatr 1993;123:757–66. [DOI] [PubMed] [Google Scholar]
- 48. Vermont-Oxford Neonatal Network. A multicenter, randomized trial comparing synthetic surfactant with modified bovine surfactant extract in the treatment of neonatal respiratory distress syndrome. Vermont-Oxford Neonatal Network. Pediatrics 1996;97:1–6. [PubMed] [Google Scholar]
- 49. Clark RH, Yoder BA, Sell MS. Prospective, randomized comparison of high-frequency oscillation and conventional ventilation in candidates for extracorporeal membrane oxygenation. J Pediatr 1994;124:447–54. 10.1016/S0022-3476(94)70374-4 [DOI] [PubMed] [Google Scholar]
- 50. Rastogi A, Akintorin SM, Bez ML, et al. . A controlled trial of dexamethasone to prevent bronchopulmonary dysplasia in surfactant-treated infants. Pediatrics 1996;98:204–10. [PubMed] [Google Scholar]
- 51. Phibbs CS, Baker LC, Caughey AB, et al. . Level and volume of neonatal intensive care and mortality in very-low-birth-weight infants. N Engl J Med 2007;356:2165–75. 10.1056/NEJMsa065029 [DOI] [PubMed] [Google Scholar]
- 52. Paneth N. Technology at birth. Am J Public Health 1990;80:791–2. 10.2105/AJPH.80.7.791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Alfirevic Z, Neilson JP. Doppler ultrasonography in high-risk pregnancies: systematic review with meta-analysis. Am J Obstet Gynecol 1995;172:1379–87. 10.1016/0002-9378(95)90466-2 [DOI] [PubMed] [Google Scholar]
- 54. Kuchna D, Hovsepyan A, Leonard S. Preventing Newborn Deaths In Romania And Hungary. Health Aff 2017;36:1160 10.1377/hlthaff.2017.0382 [DOI] [PubMed] [Google Scholar]
- 55. Maeda K. Progress of perinatal medicine in Japan. J Health Med Inform 2013:s11. [Google Scholar]
- 56. Kramer MS, Platt RW, Yang H, et al. . Registration artifacts in international comparisons of infant mortality. Paediatr Perinat Epidemiol 2002;16:16–22. 10.1046/j.1365-3016.2002.00390.x [DOI] [PubMed] [Google Scholar]
- 57. Joseph KS, Liu S, Rouleau J, et al. . Influence of definition based versus pragmatic birth registration on international comparisons of perinatal and infant mortality: population based retrospective study. BMJ 2012;344:e746 10.1136/bmj.e746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Mohangoo AD, Blondel B, Gissler M, et al. . International comparisons of fetal and neonatal mortality rates in high-income countries: should exclusion thresholds be based on birth weight or gestational age? PLoS One 2013;8:e64869 10.1371/journal.pone.0064869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Schwartz E, Kofie VY, Rivo M, et al. . Black/white comparisons of deaths preventable by medical intervention: United States and the District of Columbia 1980-1986. Int J Epidemiol 1990;19:591–8. 10.1093/ije/19.3.591 [DOI] [PubMed] [Google Scholar]
- 60. Macinko J, Elo IT. Black-white differences in avoidable mortality in the USA, 1980-2005. J Epidemiol Community Health 2009;63:715–21. 10.1136/jech.2008.081141 [DOI] [PubMed] [Google Scholar]
- 61. Riddell CA, Harper S, Kaufman JS. Trends in differences in US mortality rates between black and white infants. JAMA Pediatr 2017;171:911–3. 10.1001/jamapediatrics.2017.1365 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2018-027909supp001.pdf (342.1KB, pdf)
bmjopen-2018-027909supp002.pdf (130.3KB, pdf)
bmjopen-2018-027909supp003.pdf (258.6KB, pdf)
bmjopen-2018-027909supp004.pdf (212.3KB, pdf)