Abstract
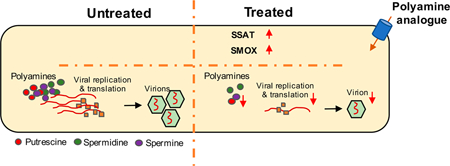
The Zika virus (ZIKV) is primarily transmitted via an infected mosquito bite, during sexual intercourse, or in utero mother to child transmission. When a fetus is infected, both neurological malformations and deficits in brain development are frequently manifested. As such, there is a need for vaccines or drugs that may be used to cure ZIKV infections. Metabolic pathways play a crucial role in cell differentiation and development. More importantly, polyamines play a key role in replication and translation of several RNA viruses, including ZIKV, Dengue virus, and Chikungunya virus. Here, we present polyamine analogues (BENSpm and PG11047) and their corresponding polymer prodrug derivatives for inhibiting ZIKV infection by intersecting with polyamine catabolism pathways. We tested the compounds against ZIKV African (MR766) and Asian (PRVABC59) strains in human kidney epithelial (Vero) and glioblastoma derived (SNB-19) cell lines. Our results demonstrate potent inhibition of ZIKV viral replication in both cell lines tested. This antiviral effect was mediated by the upregulation of two polyamine catabolic enzymes, spermine oxidase, and spermidine (SMOX)/spermine N1-acetyltransferase (SAT1) as apparent reduction of the ZIKV infection following heterologous expression of SMOX and SAT1. On the basis of these observations, we infer potential use of these polyamine analogues to treat ZIKV infections.
Keywords: Zika virus, virus replication, polyamines, bisethylnorspermine, prodrugs, polyamine metabolism, SMOX, SAT1
Graphical Abstract
INTRODUCTION
The Zika virus (ZIKV) and other related viruses, such as the Dengue virus (DENV) 1–4 serotypes, West Nile virus (WNV), and Yellow fever virus (YFV), belong to the family Flaviviridae.1–3 ZIKV is transmitted mainly through infected mosquitos, mother-to-child during pregnancy, and also during sexual intercourse.3–5 ZIKV infections have raised serious public health concerns due to the resultant neurological disease/disorders, such as microcephaly, hydrops fetalis, hydrancephaly, and Guillain–Barre syndrome.6–9 ZIKV (MR766) was first isolated in Uganda in 1947 in sentinel macaques and subsequently in 1952 in humans.10–12 Increased rates of ZIKV infections have been seen in Yap Islands (2007), French Polynesia (2013), Easter Island (2014), and Brazil (2015).3,13–16 These recent outbreaks warrant urgent development of targeted drugs and vaccines to treat the ZIKV infection.
Entry of flaviviruses into mammalian cells is facilitated by AXL, DC-SIGN, TIM-1, and Tyro-3 cell surface receptors.17–20 On the basis of our current understanding of the ZIKV-host interactions, infected mosquitoes transmit ZIKV to highly susceptible epidermal keratinocytes and dendritic cells.17,21 The envelope (E) glycoprotein of flaviviruses plays a crucial role in the virus—cell surface receptor interaction.22–24 Similar to other flaviviruses, ZIKV replication is restricted by the Type I interferon signaling pathway via upregulation of antiviral genes.25–28 Multiple interferon-inducible genes control flavivirus replication.29–31 These include spermidine/spermine N1-acetyltransferase 1 (SAT1), interferon-induced protein with tetratricopeptide repeats, interferon-induced GTP-binding protein, 2′−5′-oligoadenylate synthetase 2, and interferon-stimulated gene 15.32–35 Polyamines (putrescine, spermidine, and spermine) are essential in numerous cellular processes, such as gene expression and replication.36,37 Recent reports have highlighted the role of polyamines in viral replication and translation.34,38 Both spermine oxidase (SMOX) and SAT1 play crucial roles in the polyamine metabolic pathway.36,37 For example, SMOX is involved in the conversion of spermine to spermidine. Similarly, SAT1 converts spermine to acetylspermine and spermidine to acetyl-spermidine in two independent rate-limiting steps.36,39
Depletion of polyamines represents a promising approach for anti-Zika therapy.40,41 Synthetic polyamine analogues compete with natural polyamines for transport, biosynthesis, and catabolism.42 Previously developed polyamine analogs, such as symmetrically substituted bis(ethyl)norspermine (BENSpm), use the polyamine transport mechanism to gain entry into cells, where they upregulate the polyamine catabolic enzymes SMOX and SAT1, and downregulate polyamine biosynthesis and uptake, thereby depleting the cells of the natural polyamines.42 We have reported the development of the BENSpm-based biodegradable polymeric prodrug (DSS-BEN) to target polyamine metabolism and deliver therapeutic nucleic acids.43,44 Furthermore, the PG11047-based biodegradable polymeric prodrug (DSS-PG) was also successfully developed to target polyamine metabolism. In this study, we tested whether antiviral efficacy of the two polyamine prodrugs (DSS-BEN and DSS-PG) improves the ability of the parent compounds BENSpm and PG11047 to inhibit ZIKV replication and understand pathways they target.
MATERIALS AND METHODS
Materials.
The polymeric prodrugs DSS-BEN (Mw = 3.8 kDa, Mw/Mn = 1.1) and DSS-PG (Mw = 7.2 kDa, Mw/Mn = 1.8) were synthesized and characterized as previously described.43,45 Parent drugs (BENSpm or PG11047) accounted for 44-wt % in the prodrug (DSS-BEN and DSS-PG).
Viruses and Cell Lines.
ZIKV strains PRVABC59 (Human/2015/Puerto Rico; BEI Cat. # NR-50240) and MR766 (Rhesus/1947/Uganda; BEI Cat. # NR-50065) were obtained from the BEI Resources (https://www.beiresources.org/Organism/118/Zika-virus.aspx). Viral stocks were produced and titered in Vero cells. Vero cells and SNB-19 cells were cultured in Dulbecco’s modified eagle medium (DMEM, Gibco, Life Technologies, CA, USA) supplemented with 10% heat-inactivated fetal bovine serum (Invitrogen, CA, USA) at 37 °C in a 5% carbon dioxide humidified environment.
Cell Viability Assay.
Cytotoxicity of BENSpm, PG11047, DSS-BEN, and DSS-PG compounds in Vero cells as well as SNB-19 cells was evaluated by the CellTiter-Blue assay. The cells (3000/well) were seeded in 96-well microplates 24 h before drug exposure. Subsequently, cells were incubated for 72 h in culture medium containing polyamine analogue drugs. Following incubation, the medium was replaced with 100 μL of serum-free media and 20 μL of CellTiter-Blue reagent (CellTiter-Blue Cell Viability Assay, Promega, WI, USA). After a 2 h incubation, the fluorescence intensity [I] (560/590 nm) was measured on a SpectraMax iD3Multi-Mode Microplate Reader (Molecular Devices, CA, USA). The relative cell viability (%) was calculated as [I] treated/[I] untreated ×100%.
ZIKV Infection and Polyamine Prodrug Incubation.
Vero cells or SNB-19 cells were plated in 24-well plates. After 24 h, Vero of SNB-19 cells were exposed to different concentrations of BENSpm or PG11047 (range 0.52–13.2 μg/mL) and DSS-BEN or DSS-PG (range 1.2–30 μg/mL) for 16 h in complete medium. The cells were washed with PBS and then infected with ZIKV (MR766 and PRVABC59) at a multiplicity of infection (MOI) of 0.1 for Vero cells and 0.2 for SNB-19 cells. Cells were incubated for 1 h at 37 °C with intermittent gentle swirling. Culture media containing the same dose of compounds (BENSpm, PG11047, DSS-BEN, and DSS-PG) was added back to the cells and incubated at 37 °C. Supernatants were collected at 24 and 48 h post-infection (p.i.).
Viral RNA Extraction.
Viral RNA was extracted from cell culture supernatants of ZIKV-infected Vero or SNB-19 with a QIAamp Viral RNA kit (Qiagen, MD, USA) as per the manufacturer’s instructions. Briefly, AVL-carrier RNA buffer was prepared by dissolving 310 μg of carrier RNA in 310 μL of elution buffer. AVL-carrier RNA buffer (530 μL) was added to the culture supernatant (140 μL). After a 10 min incubation at room temperature, 530 μL of ethanol was added. The mix was centrifuged through a silica column (6000g; 1 min). The columns were washed with buffer AW1 (6000g; 1 min) and AW2 (20 000g; 3 min), respectively. Finally, RNA was eluted in 40 μL of TE buffer and stored at –80 °C until use.
Negative Strand RNA Quantification.
For negative strand quantitation, cellular RNA was extracted with an RNAeasy mini kit according to the manufacturer’s instructions (Qiagen, Germany). ZIKA negative strand RNA was detected with a two-step RT-PCR as described previously.46 The intensity of the amplified DNA was measured with ImageJ software (NIH, Bethesda, MD).
One-Step Real-Time qRT-PCR.
The PCR master mix (TaqMan RNA-to-Ct 1-Step Kit) and enzyme were procured from Applied Biosystems (CA, USA). Genomic standards (NR-50085-MR 766 and NR-50244-PRVABC59) were obtained from BEI Resources. The primers and probes used for the qRT-PCR assay include the forward primer (5′-TTGGTCATGATACTGCTGATTGC-3′), reverse primer (5′-CCTTCCACAAAGTCCCTATTGC-3′), and probe (5′-CGGCATACAGCATCAGGTGCATAGGAG-3′). Viral RNA was quantified with an one-step real-time qRT-PCR method using a CFX Real-Time PCR System, Bio-Rad (CA, USA), as published previously by our group.47 Briefly, 20 μL of the reaction mixture contains 10 μL of the 2X RT-PCR mix, forward and reverse primer in 400 nM concentration each, probe at 100 nM concentration, 0.5 μL TaqMan enzyme (40×), and nuclease-free water. qRT-PCR was performed using the one-step PCR cycle condition (reverse transcription 15 min at 48 °C, followed by 95 °C for 10 min, denaturation step for 15 s at 95 °C, and anneal/extended temperature for 1 min at 60 °C for 40 cycles). The water alone and nontemplate controls were used as negative controls.
Heterologous Expression of SAT1 and SMOX Proteins.
Vero cells in 96-well plates were transfected with 0.1 μg/well of pCMV7.1 3X FLAG/SAT1 or phCMV3 HA c-terminal-tagged SMOX in quadruplicate using the jetPRIME transfection reagent (Polyplus-Transfection Inc., NY, USA). After incubation at 37 °C for 24 h, the transfected cells were infected with African strain-MR766 or Asian strain-PRVABC59 at a MOI of 1. After 24 h of infection, cells were fixed in 4% paraformaldehyde and permeabilized with 0.5% Triton X-100. The cells were immunoassayed with rabbit polyclonal antibody against the E protein of ZIKV (Genetex Inc., Irvine, CA) and in a combination either with mouse monoclonal antibody against FLAG-tag (FG4R) or monoclonal antibody against the HA-tag (5B1D10) (Invitrogen, Rockford, IL, USA). Subsequently, cells were incubated with Alexa Fluor 488, and 594-conjugated secondary antibody (Invitrogen, Rockford, IL, USA) was used to detect mouse monoclonal antibody and rabbit polyclonal antibody, respectively. Images were captured and cells were counted under the microscope in eight fields and expressed as % reduction as compared to mock transfected virus-infected cells only.
Time-of-Compound Addition Assay.
Vero cells and SNB-19 cells seeded in 24-well plates were incubated overnight. Subsequently, BENSpm (13.2 μg/mL), PG11047 (13.2 μg/mL), DSS-BEN (30 μg/mL), and DSS-PG (30 μg/ mL) were added to the cells either 16 or 4 h prior to infection (–16 h, –4 h), during infection (0 h), or 4 h p.i. (+4 h). The cells were infected with ZIKV (MR766 and PRVABC59) at MOI 0.1 and 0.2, respectively. At 24 h p.i., supernatants were collected and layered onto a monolayer of Vero cells in a 96-well plate to calculate the number of foci present under each exposure condition using an immunofluorescence assay by the Operetta instrument (PerkinElmer, Waltham, MA, USA).
Immunostaining Assay.
Infected Vero and SNB-19 cells were visualized by a monoclonal anti-flavivirus group antigen antibody (clone D1–4G2–4-15 at 1:500 dilution; Millipore, MA, USA). Briefly, Vero cells and SNB-19 cells were infected with MR766 or PRVABC59 viruses at an MOI of 0.1 and 0.2, respectively. After 24 h, infected cells were fixed with ice-cold 4% paraformaldehyde for 30 min at room temperature (RT) and washed three times with 1X PBS. Subsequently, cells were permeabilized with 0.3% Triton-X100 and blocked in 1X PBS containing Avidin (Vector Laboratories, CA, USA). Next, the cells were washed twice with 1X PBS and incubated with a primary anti-flavivirus monoclonal antibody (1:500 dilution) in blocking solution (5% goat serum in PBS) for 1 h. The cells were washed twice with 1X PBS to remove the excess unbound antibody followed by incubation with biotinylated-conjugated goat anti-Mouse IgG (VECTASTAIN Elite ABC Kits Mouse IgG, Vector Laboratories, CA, USA) for 30 min at RT in blocking buffer at 1:1000 dilution. Cells were then washed twice with 1X PBS and treated with ABC solution (VECTASTAIN Elite ABC Kits) for 30 min, followed by DAB solution at RT for 30 min until infected cells changed to a brown color.
Quantitation of Focus Forming Units.
Using a 20× microscope objective, 10 fields in each well were randomly chosen to calculate an average number of positive cells (foci). Subsequently, the average number of foci was used to calculate relative foci forming units per well using the following formula: (average # of positive cells per field) × (fields of well). Example: 24-well tissue-culture plate. If the average number of positive cells per field = 20, FFU/field = (20 cells/field) × (314 fields/well) = 6280.
Quantitation of Expression of the Polyamine Catabolic Enzymes.
The expression of the polyamine catabolic enzymes SMOX and SAT1 in Vero and SNB-19 cells was quantified using qRT-PCR. Cells were treated with BENSpm and PG11047 (both 13.2 Mg/mL) along with DSS-BEN and DSS-PG (both 30 Mg/mL) for 24 h. Total RNA was isolated using a mirVana miRNA isolation kit (Ambion, USA) and reverse-transcribed to cDNA using a QuantiTect reverse transcription kit (Qiagen): the relative amount of mRNA was determined by RT-PCR with a Rotor-Gene Q instrument (Frederick, MD, USA). The GAPDH primer assay and QuantiFast SYBR Green PCR kit (Qiagen) were used following the manufacturer’s protocol. The following primers were used: human SMOX (forward 5′-CGCAGACT-TACTTCCCCGGC-3′, reverse, 5′-CGCTCAATTCCT-CAACCACG-3′) and human SAT1 (forward, 5′-ATC-TAAGCCAGGTTGCAATGA-3′, reverse, 5′-GCACTCCT-CACTCCTCTGTTG-3′). Relative mRNA expression levels of the enzymes were calculated from the Ct values of the target genes and the housekeeping gene GAPDH.
Analysis of Intracellular Polyamines.
Cells were incubated with BENSpm, PG11047, DSS-BEN, and DSS-PG for 24 h. Next, the cells were trypsinized and lysed; the concentrations of natural polyamines (SPM, SPD, PUT) were determined by HPLC, as described previously.48
Statistical Analyses.
Graphs were generated and statistical tests were performed using the Prism 7 (GraphPad, La Jolla, CA, USA) software. Experimental data are presented as mean ± SD. The one-way ANOVA test was used for comparison between groups, and a p-value of less than 0.05 was considered statistically significant.
RESULTS
Synthesis and Characterization of Polyamine Prodrugs.
A first-generation symmetrically bis(ethyl)-substituted polyamine analogue, BENSpm (Figure 1A), uses the poly-amine transport mechanism to gain entry into cells, where it readily upregulates the polyamine catabolic enzymes SMOX and/or SAT1, while down-regulating polyamine biosynthesis and uptake, thereby depleting the cells of the natural polyamines.42 The DSS-BEN (Figure 1C) was developed to target polyamine metabolism and deliver therapeutic nucleic acids, such as genes or miRNA.43,44 The synthesized biodegradable prodrug undergoes intracellular degradation into the parent drug BENSpm, which further depletes cellular natural polyamines. PG11047 is a second-generation conformationally restricted polyamine analogue with a cis double bond between its central carbons (Figure 1B). The spatial rigidity of PG11047 is expected to enhance the selective binding of polyamine targets.49 More recently, DSS-PG was also successfully developed to target polyamine metabolism (Figure 1D).45
Figure 1.

Chemical structures of polyamine analogs and their polymeric prodrugs. BENSpm, PG11047, DSS-BEN, and DSS-PG. Chemical structure of a first-generation polyamine analogue, BENSpm (A), a second-generation polyamine analogue PG-11047 (B), polyamine drugs DSS-BEN (C), and DSS-PG (D) derived from parental drugs, respectively.
Cytotoxicity of Polymeric Prodrugs.
We performed cell viability assays with the polyamine analogs and their polymeric prodrugs at different concentrations in the two cell lines (Vero and SNB-19) in which ZIKV is known to replicate. The polyamine analogues showed no cytotoxic effect in either the Vero cells (Figure 2A) or the SNB-19 cells (Figure 2B). Cell viability remained greater than 90% even at the highest concentration tested (200 μg/mL) and after 72 h of drug exposure. Equivalent doses of the polymeric prodrugs exerted negligible cytotoxicity at concentrations below 30 μg/mL (Figure 2A,B). At concentrations above 50 μg/mL, significant cytotoxicity was observed, mainly due to the polycationic character of the prodrugs and related adverse effects on cell membranes. These results established a safe working concentration range for the compounds. On the basis of the cytotoxicity study, we selected the highest dose of the prodrugs DSS-BEN and DSS-PG as 30 μg/mL and an equivalent parent drug dose (13.2 μg/mL) for subsequent testing of the potential antiviral effects on Zika viral strains.
Figure 2.
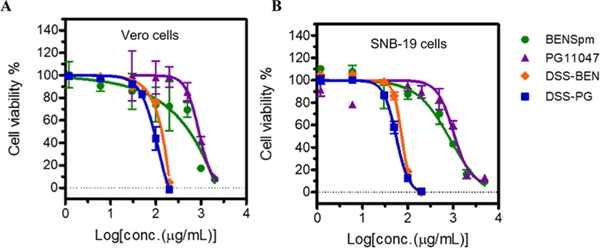
Cytotoxicity of the tested compounds in noninfected Vero cells and SNB-19 cells. In vitro cytotoxicity assay of polyamine drugs (BENSpm, PG11047, DSS-BEN, and DSS-PG) of different concentrations after incubation for 72 h was performed in Vero cell line (A) and in SNB19 cell line (B) by the CellTiter-Blue assay. The CC50 value was calculated using GraphPad Prism version 7.0 software. The results are shown as the mean percentage relative cell viability with three independent experiments in triplicate.
Inhibition of Asian and African ZIKV Replication in Vero Cells.
On the basis of the multiple sequence alignment, distant related ZIKV strains (AfTican-MR766 and Asian-PRVABC59) were chosen to test anti-ZIKV effects of the polyamine prodrugs and their parental compounds. This selection was based on multiple sequence alignments and phylogenetic tree analysis, as described previously by our group.20
The in vitro anti-ZIKV (MR766 and PRVABC59) activity of DSS-BEN and DSS-PG and their parent compounds was evaluated by the ZIKV foci quantification assay and viral RNA yield reduction assay. Vero cells were treated with BENSpm and PG11047 at 0.52, 2.6, and 13.2 μg/mL and DSS-BEN and DSS-PG at 1.2, 6, and 30 μg/mL concentrations for 16 h and then infected with an Asian strain (PRVABC59) or an African strain (MR766) for 24–48 h. The anti-ZIKV effect was further confirmed by a viral RNA yield reduction assay using qRT-PCR (Figure 3). As shown in Figure 3C, 24 h later, DSS-BEN treatment reduced PRVABC59 viral RNA by 0.78 ± 0.05, 1.22 ± 0.14, and 2.05 ± 0.27 log units and MR766 viral RNA by 0.68 ± 0.05, 1.84 ± 0.08, and 2.3 ± 0.11 log units. Furthermore, this effect on MR766 was increased after 48 h to 4.01 ± 0.15, 4.43 ± 0.04, and 4.54 ± 0.03 log units. Similarly, DSS-PG treatment (Figure 3D) reduced PRVABC59 viral RNA copies by 0.36 ± 0.08, 0.74 ± 0.18, and 1.51 ± 0.06 log units at 24 h p.i. and 0.84 ± 0.07, 1.11 ± 0.1, and 2.12 ± 0.08 log units at 48 h p.i. Similar levels of inhibition were noted for MR677 at 24 h, but higher levels of inhibition were noted at 48 h (~4 to 4.5 log units for all the concentrations). Next, BENSpm or PG11047 were tested as shown Figure 3A,B. BENSpm reduced PRVABC59 RNA levels by 1.49 ± 0.42, 2.16 ± 0.54, and 3.06 ± 0.13 log units and MR766 RNA levels by 1.56 ± 0.07, 1.82 ± 0.06, and 2.19 ± 0.07 log units compared with those of the control. Furthermore, at 48 h p.i., the same level of inhibition was observed in PRVABC59 viral RNA loads, but increased inhibition was observed in the case of MR766 RNA levels. As shown, the PG11047 treatment decreased the extracellular PRVABC59 RNA copies by approximately 0.56 ± 0.04, 0.94 ± 0.03, and 1.74 ± 0.06 log units and MR766 RNA by 1.46 ± 0.03, 1.66 ± 0.05, and 1.94 ± 0.15 log units. However, PG11047 also showed notable effects on MR766 RNA levels at 48 h p.i., (~3.7 to 4.5 log units for all the concentrations). Collectively, these data suggest that polyamine inhibition hampers viral RNA synthesis by acting differentially on the rate of the Zika viral RNA synthesis.
Figure 3.
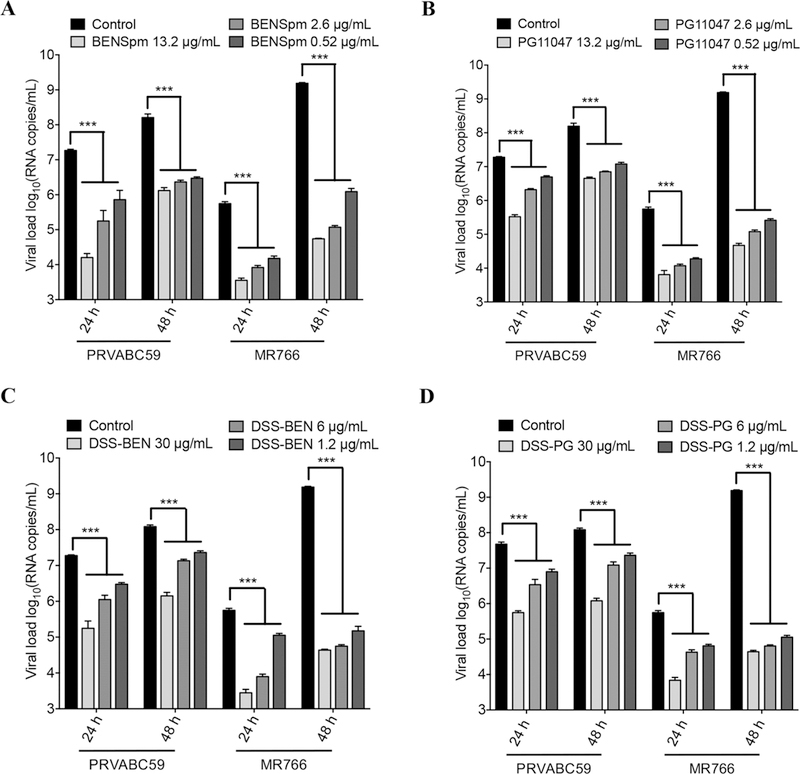
Evaluation of anti-ZIKV activity of polyamine drugs on viral RNA loads in Vero cells. The Vero cells were treated with (A) BENSpm, (B) PG11047, (C) DSS-BEN, and (D) DSS-PG at indicated concentrations. After a 16 h treatment, cells were infected with ZIKV at 0.1 MOI (PRVABC59 or MR766) for 24–48 h. Viral RNA levels were measured in cell culture supernatant from Vero cells. Control means untreated but ZIKV infected. One-way ANOVA was used, and *** indicates p ≤ 0.001. Error bars represent the standard error of the mean.
We examined the antiviral spectrum of polyamine drugs (BENSpm, DSS-BEN, PG11047, and DSS-PG) against an Asian strain (PRVABC59) and an African strain (MR766) of ZIKV using foci assay. The inhibitory effects of all four compounds showed dose-dependent patterns. Vero cells were treated with various concentrations (5-fold) of indicated polyamine drug for 16 h, infected with PRVABC59 and MR766 ZIKV at a MOI of 0.1. After 24 h, the cells were fixed and processed for foci quantification. As shown in Figure 4A,C, BENSpm inhibited PRVABC59 and MR766 with an IC50 of 0.16 and 0.21 μg/mL, respectively. Similarly, PG11047 exhibited an IC50 of 0.23 and 0.17 μg/mL, respectively. In contrast, the IC50 values of DSS-BEN were around 3.27 and 2.45 μg/mL against PRVABC59 and MR766, respectively. Also, the IC50 values of DSS-PG against PRVABC59 and MR766 were around 3.04 and 2.72 μg/mL, respectively (Figure 4B,D). These results indicate that polyamine drugs are very efficient in restricting distantly related ZIKV in Vero cells.
Figure 4.
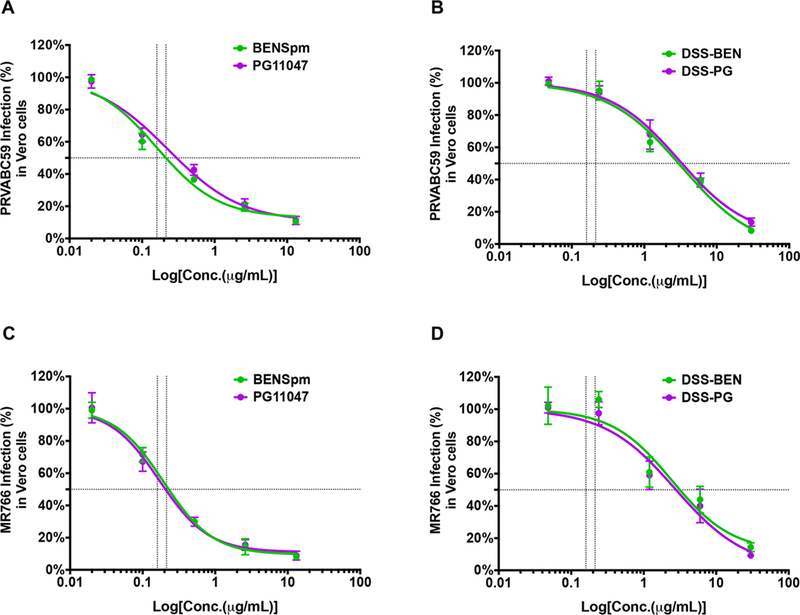
Effect of polyamine drug treatment on ZIKV foci formation in Vero cells. Vero cells were treated with serial diluted (5-fold) polyamine drug concentrations of (A and C) BENSpm and PG11047 and (B and D) DSS-BEN and DSS-PG for 16 h and infected with an Asian strain, PRVABC59 (A and B), and an African strain, MR766 (C and D), at 0.1 MOI. After 24 h, the anti-flavivirus group antigen antibody (clone D1–4G2–4-15; mouse) was used to visualize infected cells (foci) in polymeric prodrugs treated and also in untreated but infected controls. The IC50 value was calculated using GraphPad Prism version 7.0 software. Error bars represent the standard error of the mean.
Anti-ZIKV Activity in Human Glioblastoma (SNB-19) Cells.
To further characterize the polyamine prodrugs and their parent compounds ability to inhibit ZIKV infection in human glioblastoma (SNB-19) cells, we followed similar methods and infection protocols as described for Vero cells. After 48 h of infection, total RNA was isolated from infected cell culture supernatants, and viral RNA was measured using qRT-PCR (Supporting Information; Figure S1). We found that tested compounds BENSpm and PG11047 at a concentration of 13.2 μg/mL and DSS-BEN and DSS-PG at a concentration 30 μg/mL reduced ZIKV (PRVABC59 or MR766) RNA loads in SNB-19 cells compared with the nontreated infected cells. Importantly, DSS-PG exhibited the best anti-ZIKV activity in SNB-19 cells.
To quantify the antiviral effects of the polyamine drugs in SNB-19 cells, the inhibition rates of the four polyamine drugs IC50s were calculated using a foci assay. All compounds exhibited an antiviral state on both of the Zika (Asian and African) strains in treated cells, compared with control cells (Figure 5A–D). The IC50 values of BENSpm on PRVABC59 and MR766 were on 0.51 and 0.67 jWg/mL, respectively. Similarly, PG11047 exhibited a similar dose of inhibition on PRVABC59 and MR766 with the IC50 value of 0.41 and 0.91 μg/mL, respectively. The IC50s of DSS-BEN against PRVABC59 and MR766 were 2.71 and 2.68 μg/mL, respectively. Likewise, DSS-PG exhibited a similar dose of inhibition against PRVABC59 and MR766 of 3.63 and 2.41 μg/mL, respectively.
Figure 5.

Treatment effect of polyamine drugs on ZIKV foci formation in SNB-19 cells. SNB19 cells were treated with serially diluted (5-fold) polyamine drug concentrations of (A and C) BENSpm and PG11047 and (B and D) DSS-BEN and DSS-PG for 16 h and infected with an Asian strain, PRVABC59 (A and B), and an African strain, MR766 (C and D), at 0.2 MOI. After 24 h, the anti-flavivirus group antigen antibody (clone D1–4G2–4-15; mouse) was used to visualize infected cells (foci) in polymeric prodrug compounds treated and also in untreated but infected controls. The IC50 value was calculated using GraphPad Prism version 7.0 software. Error bars represent the standard error of the mean.
Taken together, all the polymeric drugs showed potential antiviral activity against Asian and African strains of ZIKVs in SNB-19 cells.
Polymeric Prodrugs Target the Cellular Metabolisms To Restrict ZIKV Replication in Both the Cell Lines.
In general, flaviviruses synthesize viral proteins in the first 1–5 h p.i., and newly formed virions are released at 12 h p.i.55 Thus, we determined optimum timing of the treatment on ZIKV replication. As shown in Figure 6A, the compounds were added to the Vero and SNB-19 cells at various time points relative to the infection (0 h), before infection (16 h preinfection, 4 h preinfection), during adsorption, and 4 h p.i. Viral titers in the culture supernatants were quantified using an immunofluorescence assay 24 h later. As shown in Figure 6B,C, for BENSpm (–16 h pretreatment), the percentage of the MR766-infected cells was reduced by 80.37 ± 1.73%, and the PRVABC59-infected cells were reduced by 88.19 ± 0.69%. However, in the case of BENSpm pretreatment, we observed a significant decrease in the percentage of the MR766-infected cells by 40.56 ± 1.92% and the PRVABC59-infected cells by 27.12 ± 0.88%. Similarly, when Vero cells were pretreated with PG11047 for 16 h, the percentage of the MR766-infected cells was reduced by 73.02 ± 0.86% and of the PRVABC59-infected cell by 88.12 ± 1.88%. However, when the PG11047 pretreatment was shortened to 4 h, the percentage of the MR766-infected cells was reduced by 42.48 ± 1.68% and the PRVABC59-infected cells by 20.16 ± 0.26%. Next, when Vero cells were pretreated with DSS-BEN for 16 h, the percentage of the MR766-infected cells was decreased by 89.95 ± 1.86% and the PRVABC59-infected cells by 92.63 ± 1.73%. When the DSS-BEN pretreatment was shortened to 4 h, the percentage of the MR766-infected cells was decreased by 28.29 ± 1.19% and the PRVABC59-infected cells by 41.85 ± 1.08%. However, DSS-BEN pretreatment for 4 h (–4 h) exhibited no effect on the PRVABC59. DSS-PG pretreatment for 4 h (–4 h) was able to reduce the percentage of MR76-infected cells by only 21.87 ± 1.57%. Furthermore, BENSpm treatment during virus adsorption (0 h), or 4 h after infection (+4 h), had no effect on the virus replication. Similar experiments were conducted in the SNB-19 cells (Figure 6D,E). BENSpm pretreatment for 16 h decreased the percentage of the MR766-infected cells by 75.67 ± 4.74% and PRVABC59-infected cells by 88.87 ± 5.68%. Likewise, PG11047 pretreatment decreased the percentage of the MR766-infected cells by 68.42 ± 0.83% and PRVABC59-infected cells by 84.96 ± 7.63%. Pretreatment with DSS-BEN decreased the percentage of MR766- and PRVABC59-infected cells by 71.34 ± 2.53 and 75.08 ± 6.43%, respectively. Pretreatment with DSS-PG decreased the percentage of the MR766-infected cells by 82.74 ± 3.07% and PRVABC59-infected cells by 87.11 ± 11.05%. However, treatment at the time of infection and 4 h before infection in SNB-19 cells failed to lower the viral titers, possibly due to differences in the intracellular pharmacokinetics of the polyamine prodrugs in the two cell lines. Collectively, our time-of-addition results suggest that polyamine prodrugs and their parent compounds could effectively control the ZIKV infections in the preattachment stage of the virus infection life cycle.
Figure 6.
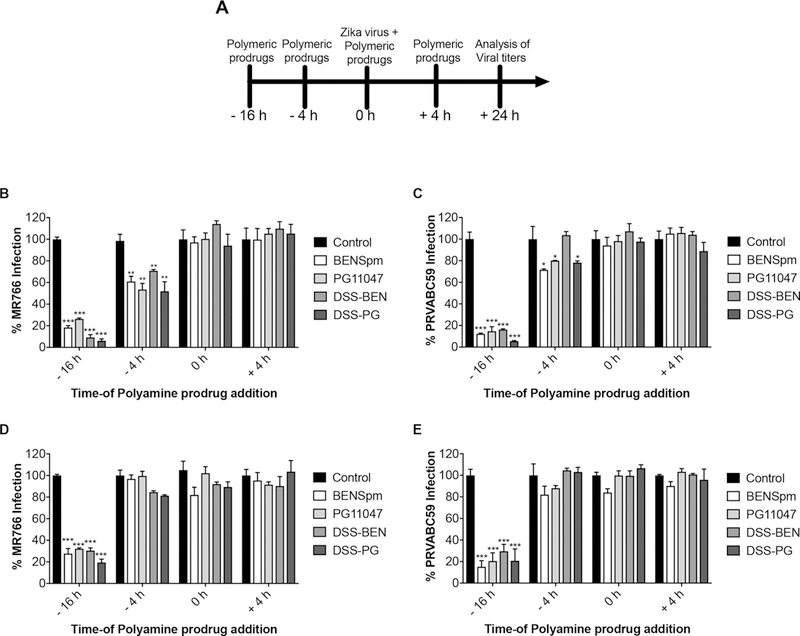
Treatment effect of polyamine drug timing on ZIKV replication in both cell lines. Cells were treated with BENSpm (13.2 μg/mL), PG11047 (13.2 μg/mL), DSS-BEN (30 μg/mL), and DSS-PG (30 μg/mL) at specified times (A). At 16 and 4 h before infection (–16 h, –4 h), during virus adsorption (0 h), and after infection (+4 h), polyamine prodrugs were added to Vero cells (B and C) or SNB-19 cells (D and E). Cells were grown in a 24-well plate and infected with ZIKV (MR766 (B and D) or PRVABC59 (C and E) at a MOI of 0.1 and 0.2, respectively. Titers were determined at 24 h p.i.; the representative results are presented. The statistical analysis was performed using GraphPad Prism Software Version 7, and two-way ANOVA was used to calculate the statistical significance (*p < 0.05, **p < 0.01, and ***p < 0.001).
Regulation of Polyamine Metabolism in Vero and SNB-19 Cells.
The capacity of the prodrugs to degrade into parent drugs and affect polyamine metabolism was evaluated by incubating the compounds with Vero cells and SNB-19 cells, followed by HPLC analyses of polyamine levels in the cell lysates. As shown in Figure 7A, DSS-BEN showed effective intracellular degradation into parent BENSpm in Vero cells, which was indicated by increased intracellular BENSpm concentrations. BENSpm was not detected in untreated cells (data not shown). The amount of BENSpm present in the cells treated with DSS-BEN was around 26% of the levels observed in cells treated with equivalent free BENSpm. Similarly, DSS-PG also showed effective intracellular degradation into parent PG11047 (32%). Moreover, DSS-BEN and DS-PG also showed effective intracellular degradation in SNB-19 cells (Figure 7D). This lower intracellular accumulation of parent drugs delivered by the prodrugs is likely a complex function of the rate and extent of cellular uptake, intracellular trafficking, rate of degradation, and extent of polyamine excretion from the cells.
Figure 7.

Mechanisms of antiviral activity of polyamine drugs. Cells were treated with BENSpm, PG11047, DSS-BEN, or DSS-PG (BENSpm and PG11047 at 13.2 μg/mL, DSS-BEN and DSS-PG at 30 μg/mL) for 24 h. Intracellular concentration of BENSpm or PG11047 was determined by HPLC (n = 3) in Vero cells (A) and SNB-19 cells (D). Relative changes in the expression of SMOX and SAT1 mRNA in Vero cells (B) and SNB-19 cells (E). mRNA levels were measured by qRT-PCR. Results are expressed as the fold induction of specific mRNA in treated cells relative to the PBS-treated group (n = 3). Intracellular polyamine concentration determined by HPLC (n = 3) in Vero cells (C) and SNB-19 cells (F). Putrescine upon BENSpm and PG11047 treatment was not detectable. ***p < 0.001 and **p < 0.01, versus PBS.
Next, we examined the effect of the compounds on changes in the expression of SMOX and SAT1 using RT-PCR. SMOX and SSAT are the two main enzymes regulating polyamine metabolism. As shown in Figure 7B, treatment with free BENSpm upregulated the mRNA levels of both SMOX and SAT1 in Vero cells. Overall, treatment with DSS-BEN resulted in a smaller up regulation of the catabolic enzymes in comparison with treatment by free BENSpm. For example, BENSpm increased the expression of SMOX mRNA 2.9-fold; whereas, the DSS-BEN increased the expression 2.1-fold. Similarly, BENSpm upregulated SAT1 mRNA expression 6.8-fold; whereas, the DSS-BEN achieved only a 1.4-fold increase. Treatment with DSS-PG induced a smaller upregulation of the mRNA of the catabolic enzymes in comparison with treatment by free PG11047. This observation is likely a consequence of the delayed release of the free drug from the prodrug due to the need to traffic into the cytoplasm, where the disulfide reduction needed for parent drug release takes place. Having confirmed the induction of polyamine catabolic enzyme expression, we evaluated the effect of the tested compounds on intracellular polyamine depletion. As shown in Figure 7C, the DSS-BEN and DSS-PG efficiently decreased the intracellular concentration of SPD and SPM. The decrease in the intracellular levels of spermidine and spermine, the substrates of SMOX and SAT1, following treatment is indicative of significant increases in enzymatically active SMOX and SAT1. It is interesting that DDS-BEN and DSS-PG failed to deplete intracellular PUT. In contrast to the prodrugs, free BENSpm and PG-11047 showed a higher ability to deplete all main intracellular polyamines (PUT, SPD, and SPM). Moreover, polyamine analysis was also performed in SNB-19 cells after treatment by the drugs (Figure 7E). Parents drugs and polymeric prodrugs all showed activity to reduce intracellular polyamine levels. the polymeric prodrugs DSS-BEN and DSS-PG also depleted the polyamines SPD and SPM to a lower extent than the parent drugs BENSpm and PG11047 in SNB-19 cells (Figure 7F).
Heterologous Expression of SAT1 and SMOX Proteins Efficiently Restrict ZIKV Replication.
Previous experiments provided evidence for anti-ZIKV activity of polyamine drugs by upregulating the expression of SAT1 and SMOX. To further examine whether the SAT1 and SMOX supplied in trans would have an inhibitory effect on ZIKV replication, Vero cells were transiently transfected with SAT1 and SMOX expressing plasmids and infected with both Asian and African ZIKV strains. To distinguish the transiently and endogenously expressed SAT1 and SMOX, the SAT1 and SMOX were tagged to FLAG and HA, respectively. The cells were superinfected with ZIKV at an MOI of 1 and incubated for 24 h. The infected cells were examined by immunofluorescence microscopy, as described in Figure 8A,B. The transiently expressed FLAG-tagged SAT1 or HA-tagged SMOX proteins are shown in green, and the ZIKV-infected cells are shown in red (Figure 8A). We observed that 52.3 ± 1.76% control-transfected cells were infected by the African strain (MR766), and 83.9 ± 5.58% were infected by the Asian strain (PRVABC59). During SAT1 overexpression, only 15.38 ± 1.76 and 9.37 ± 3.17% of cells were infected by MR766 and PRVABC59 strains, respectively. Similarly, overexpression of SMOX resulted in only 7.14 ± 3.37 and 6.25 ± 2.62% of cells getting infected by MR766 and PRVABC59 strains, respectively. On the basis of these observations, we inferred that transient overexpression of SAT1 and SMOX restricted ZIKV replication.
Figure 8.

Effects of heterologous expression of SAT1 and SMOX proteins on ZIKV replication. (A) Vero cells were mock-transfected or transfected with either FLAG-tagged SAT1 or HA-tagged SMOX expressing constructs and were infected with either MR766 or PRVABC59 at a MOI of 1. At 24 hpi, cells were processed for the immunofluorescence assay to visualize heterologous expression of proteins (green), virus infection (red), and cell nuclei (blue). Representative images are presented. (B) Stained cells were analyzed and quantified for the percent infected and cotransfected infected cells under each condition. Error bars indicate standard deviations calculated from 4 replicate wells in a 96-well plate. ***p ≤ 0.001 using a two-way ANOVA.
Polyamine Prodrugs Target the Synthesis of Negative-Sense RNA Strand during ZIKV Replication.
ZIKV generates a negative strand replicative intermediate, which is utilized as a template for producing positive strand RNA. In order to test the effect of polyamine drugs on the synthesis of the negative stand, Vero cells were treated with 13.2 μg/mL of BENSpm or PG11047 and 30 μg/mL of DSS-BEN or DSS-PG for 16 h. These cells were subsequently infected by the African or Asian ZIKV strain for 16 h. Cellular RNA from these cells was isolated for negative strand quantitation with RT-PCR. At 16 h p.i., negative strand RNA was decreased to 49.21 and 47.57% in BENSpm-and PG11047-treated cells, respectively, as compared to untreated MR766 ZIKV-infected controls. However, negative strand RNA was decreased to 42.08 and 41.95% in BENSpm-and PG11047-treated cells, respectively, as compared to untreated PRVABC59 ZIKV-infected controls. Similarly, DSS-BEN and DSS-PG reduced the negative-strand RNA of PRVABC59 to 41.29 and 43.43%, respectively, as compared to untreated infected controls. DSS-BEN and DSS-PG decreased the negative-strand RNA of MR766 by 76.71 and 77.18%, respectively, as compared to untreated ZIKV-infected controls (Supporting Information; Figure S2). These observations suggest that polyamine prodrugs utilized in our study also target the negative-strand during viral replication.
DISCUSSION
In this study, we showed that polyamine analogs and their polymeric prodrugs inhibit ZIKV replication in Vero cells and in SNB-19 cells in vitro. In addition, we demonstrated that polymeric prodrugs target pathways involving polyamine-catabolizing enzymes SMOX and SAT1. Polymeric prodrugs, used in this study, directly targeted host polyamine metabolism pathways that are required for the ZIKV replication and translation. Treatment of Vero cells and SNB-19 cells with various concentrations of the tested compounds exhibited no cytotoxicity but restricted MR766 (African) and PRVABC59 (Asian) strain replication and foci formation in a dose-dependent manner. The polymeric prodrugs upregulated the expression of polyamine catabolic enzymes, such as SMOX and SAT1, during pretreatment prior to viral infection. Upregulated SMOX and SAT1 enzymes increase the polyamine catabolism, thereby decreasing polyamine availability for viral replication and translation. Therefore, the inhibitory effect of BENSpm, PG11047, DSS-BEN, and DSS-PG is likely due to an increased rate of polyamine catabolism as a result of overexpression of polyamine catabolic enzymes, such as SMOX and SAT1.
There are several potential targets to develop unique anti-ZIKV drugs or vaccines, including E glycoprotein, proteases NS2B3 and NS3, NS3 helicase, NS5 methyl transferase, NS5 RNA-dependent RNA polymerase, and various host factors.50 For example, novobiocin and lopinavir-ritonavir exhibit anti-ZIKV activity by inhibiting NS2B–NS3 protease activity;51 NITD-448, castanospermine, celgosvir, and peptide DN59 are reported to target E glycoprotein.50,52 Similarly, ST-148, the Bowman–Birk inhibitor, and aprotinin are reported to target the DENV C protein and NS3 serine protease.50 The compounds that target NS3-helicase are halogenated benztrioles, invermectin, and suramin. NITD-618 is reported to target NS4B.53–55 Compounds that target NS5-methyltransferase are sinefungin, S-adenosyl homocysteine, ivermectin, and ribavirin.50 There are many drugs designed and targeted at an enzymatic function of NS5 RNA-dependent RNA polymerase, including N-(4 hydroxy-phenyl) retinamide, 7-deaza-2′-C methyladeno-sine, NITD 008, and balapiravir.53,55,56 In this study, we showed that all four polyamine compounds effectively inhibited the replication of both ZIKV strains. Parent polyamine analogs, BENSpm and PG11047, exhibited no cytotoxicity in both tested cell lines and showed a higher selectivity index. The timing of the treatment was crucial for the antiviral activity, with the best outcomes observed when the cells were pretreated 16 h before infection. This suggested that the compounds target cellular polyamine metabolisms to inhibit ZIKV replication. Furthermore, these polyamine prodrugs (BENSpm, PG11047, DSS-BEN, and DSS-PG) are very effective in restricting the ZIKV replication in vitro, compared to other compounds regulating polyamine metabolisms, i.e., difluoromethylornithine (DFMO).34
Polyamines play diverse roles in RNA viruses, like DENV and CHIKV, and in DNA virus, like HSV-2.38,57 These molecules are crucial in replication and translation of RNA viruses; whereas, in DNA viruses, they are known to help in facilitating DNA compaction and encapsidation.34,38 The viral replication/translation has been shown to be reduced by polyamine depletion and rescued by polyamine addition.34 This is due to polyamines playing numerous roles within the cell, such as attachment, changing the RNA conformation, altering transcription, and translational modifications, finally affecting pathways involved in signaling.58 Also, expression of viral structural proteins and nonstructural proteins are essential in the positive-strand RNA virus life cycle. Depletion of polyamines by a known SAT1 inducer, N1,N11-di-ethyl-norspermine (DENSpm), limits viral infection by upregulating the expression of the SAT1 mRNA and protein.34 Overexpression of SAT1 also exhibits a similar antiviral activity on CHIKV and ZIKV.34 Acetyltransferase activity of SAT1 is essential to acetylate spermidine and spermine pools that are available to viral translation. Similarly, DFMO also depletes putrescine, spermidine, and spermine pools but by targeting the ODC1 inhibitor.34 However, a recent report by Mounce et al. showed that the mutation in nonstructural protein 1 (nsp1) restores replicative fitness in polyamine-depleted cells.59 In an earlier study, BHK-21 cells treated with 91.1 μg/mL (500 μM) of DFMO for 4 days did not exhibit any cytotoxicity and also failed to recover any CHIKV virus in the infected cells.59 Next, Mounce et al. showed that the pretreatment of BHK-21 cells with 2.44 μg/mL (10 μM) of DENSp m for 16 h decreases the ZIKV virion production by 2-fold.34 BENSpm and PG11047 exhibit multiple functions in the regulation of intracellular polyamines. Furthermore, they can act as potent inducers for the expression of the key catabolic enzymes SMOX and SAT1. Furthermore, DSS-BEN and DSS-PG are degraded into the parent drugs BENSpm and PG11047 by cytoplasmic disulfide reduction.
Polyamine drug time-of-addition experiments were performed to identify the stage of the viral life cycle disruption due to antiviral environment created by polyamine drug addition. Overexpression of SAT1 often goes together with a compensatory induction of ODC resulting in an accelerated metabolic cycling of polyamine biosynthesis and catabolism. Our data along with several other reports suggest that a pretreatment time minimum of 12–24 h is needed to upregulate the polyamine catabolizing enzymes (SMOX and SAT1), which leads to a decrease in levels of intracellular polyamines (putrescine, spermidine, and spermine).37 However, threshold levels of polyamines are essential for viral RNA synthesis and viral protein synthesis. The polyamine drugs mediated intracellular polyamine depletion to below threshold levels needed for adequate time to establish an antiviral environment. If the cells are infected before this period, the virus enters inside the cells normally and continues to replicate normally by using available polyamines. In other words, it is not the total amount of time but the time of pretreatment before viral translation and replication occur. It is because the replication cycle of flavivirus is very short (8–12 h). The viral RNA translation occurs in the first 1–5 h p.i.; viral RNA synthesis occurs at ≥5 h p.i.; and progeny virions are released at ≥12 h p.i.60 Translation is the first step in positive-strand RNA virus replication upon entry; however, since polyamine depletion limits this important step required for the expression of viral proteins, further viral replication is restricted.
We evaluated the levels of the polyamine catabolizing enzymes (SMOX and SAT1) at 4 and 16 h pretreatment timings in Vero and SNB19 cells (data not shown). Neither of the compounds failed to increase the expression of SMOX and SAT1 mRNA level after the 4 h-treatment. At 16 h, pretreatment showed very limited expression and could be a starting point for the induction of catabolizing enzymes and aligning with viral protein translation. At 24 h pretreatment, we noticed a sharp rise in the SMOX and SAT1 levels (Figure 7). However, the 16 h time of the polyamine drug pretreatment was decided on the basis of the study described previously,40 in which induction of SAT1 was noted after the 16 h pretreatment of 10 000 U IFNβ or 10 mM DENSpm and correlated with the depletion of spermine and spermidine. The differences in the observation could be due to differences in the methods employed. Our results confirmed that prolonged time treatments were needed to induce SMOX and SAT1 expression to the levels required for effective antiviral activity. The detailed studies on the induction of polyamine catabolizing enzymes and levels of polyamine at different time points will be perused in future studies.
Next, we also demonstrated that heterologous expression of SAT1 and SMOX restricted replication of both the Asian and African strains of ZIKV with involvement of both proteins (SAT1 and SMOX). However, SMOX exhibited greater antiviral activity compared to that of SAT1 on both of the strains of ZIKV tested. This could result from SAT1-and SMOX1-mediated decrease in cellular pools of spermidine and spermine that ultimately inhibit viral replication. Our data are similar to those of previous studies, in which SAT1 overexpression in BHK-21 cells or SAT1-inducible stable cell lines was used to measure the antiviral (ZIKV and CHIKV) nature of the SAT1.34 We observed that polyamine drugs appear to block ZIKV replication at an early stage by dramatically decreasing both negative-and positive-sense RNA. A plausible mechanism for this phenomenon would be by directly inhibiting synthesis of structural and nonstructural proteins, which are required for the viral replication complex formation. Polyamine (putrescine, spermidine, and spermine) play an important role in viral replication and associated disease pathogenesis.34 Several reports suggest that the polycationic nature of polyamines is crucial in stabilizing the virion capsid by strengthening the interactions with negatively charged viral genomes (DNA or RNA).61 Recent reports further support the roles for the polyamine in viral replication. For example, overexpression or induction of polyamine catabolic enzymes, such as SAT1 or ODC, decrease the replication of ZIKV and CHIKV.34 Also, the SMOX protein was shown to be upregulated in HIV (tat)-induced neuro-toxicity.62 Our study on heterologous expression of SMOX evidently provided the support for the anti-ZIKV nature of the SMOX protein in vitro, along with the known SAT1 protein. Our data are similar to other in vitro studies that demonstrated the requirement for polyamines in the initiation translation step, and thus they strengthen the crucial role of spermidine and spermine in the viral protein translation.
In conclusion, we demonstrated that polymeric prodrugs DSS-BEN and DSS-PG and their parent compounds BENSpm and PG11047 showed antiviral activity against ZIKV infection in vitro and provided the direct evidence that overexpression of spermidine-spermine acetyltransferase and depletion of polyamines are in fact what leads to an antiviral effect. However, the detailed mechanisms of polyamine prodrug effects on ZIKV replication can be best studied using SAT1 transgenic mice, and a SAT1 adenovirus mice model of Zika infection will be assessed in the future studies. Therefore, our results suggest potent antiviral effects of polyamine drugs against ZIKV and its potential use in the treatment of ZIKV diseases.
Supplementary Material
ACKNOWLEDGMENTS
This work is supported in part by National Institutes of Health R01AI113883 and Nebraska Neuroscience Alliance Endowed Fund Award to S.N.B. We thank Dr. Dave for critical reading and Robin Taylor for editorial assistance. We thank Dr. Marton for providing PG11047. We thank Dr. Myung Hee Park (NIH/NIDCR) and Dr. Robert A. Casero, Jr., (JHSM) for kindly providing pCMV7.1 3X FLAG/SAT1 and phCMV3 HA c-terminal tagged SMOX vectors, respectively. We thank Dr. Gmeiner, Wake Forest School of Medicine, for providing SNB-19 cells.
Footnotes
ASSOCIATED CONTENT
Supporting Information
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acs.molpharmaceut.8b00068.
Antiviral effects of polyamine drug treatment on Zika viral RNA loads in SNB-19 cells and polyamine prodrug target synthesis of negative-sense RNA strand during Zika virus replication (PDF)
The authors declare the following competing financial interest(s): Invention disclosures describing the use of the reported and related compounds were filled with the University of Nebraska Medical Center.
REFERENCES
- (1).Saiz JC; Vazquez-Calvo A; Blazquez AB; Merino-Ramos T; Escribano-Romero E; Martin-Acebes MA Zika Virus: the Latest Newcomer. Front. Microbiol. 2016, 7, 496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (2).Gould EA; Solomon T Pathogenic flaviviruses. Lancet 2008, 371 (9611), 500–9. [DOI] [PubMed] [Google Scholar]
- (3).Musso D; Gubler DJ Zika Virus. Clin. Microbiol. Rev. 2016, 29 (3), 487–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (4).Musso D; Roche C; Robin E; Nhan T; Teissier A; Cao-Lormeau VM Potential sexual transmission of Zika virus. Emerging Infect. Dis. 2015, 21 (2), 359–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (5).D’Ortenzio E; Matheron S; Yazdanpanah Y; de Lamballerie X; Hubert B; Piorkowski G; Maquart M; Descamps D; Damond F; Leparc-Goffart I Evidence of Sexual Transmission of Zika Virus. N. Engl. J. Med. 2016, 374 (22), 2195–8. [DOI] [PubMed] [Google Scholar]
- (6).Blazquez AB; Saiz JC Neurological manifestations of Zika virus infection. World J. Virol 2016, 5 (4), 135–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (7).Calvet GA; Santos FB; Sequeira PC Zika virus infection: epidemiology, clinical manifestations and diagnosis. Curr. Opin. Infect. Dis. 2016, 29 (5), 459–66. [DOI] [PubMed] [Google Scholar]
- (8).Sarno M; Sacramento GA; Khouri R; do Rosario MS; Costa F; Archanjo G; Santos LA; Nery N Jr.; Vasilakis N; Ko AI; de Almeida AR Zika Virus Infection and Stillbirths: A Case of Hydrops Fetalis, Hydranencephaly and Fetal Demise. PLoS Neglected Trop. Dis. 2016, 10 (2), e0004517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (9).Krauer F; Riesen M; Reveiz L; Oladapo OT; Martinez-Vega R; Porgo TV; Haefliger A; Broutet NJ; Low N Zika Virus Infection as a Cause of Congenital Brain Abnormalities and Guillain-Barre Syndrome: Systematic Review. PLoS Med. 2017, 14 (1), e1002203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (10).Wikan N; Smith D R Zika virus: history of a newly emerging arbovirus. Lancet Infect. Dis. 2016, 16 (7), e119–26. [DOI] [PubMed] [Google Scholar]
- (11).Macnamara FN Zika virus a report on three cases of human infection during an epidemic of jaundice in Nigeria. Trans. R. Soc. Trop. Med. Hyg. 1954, 48 (2), 139–45. [DOI] [PubMed] [Google Scholar]
- (12).Bearcroft WG Zika virus infection experimentally induced in a human volunteer. Trans. R. Soc. Trop. Med. Hyg. 1956, 50 (5), 438. [PubMed] [Google Scholar]
- (13).Slavov SN; Otaguiri KK; Kashima S; Covas DT Overview of Zika virus (ZIKV) infection in regards to the Brazilian epidemic. Braz. J. Med. Biol. Res. 2016, 49 (5), e5420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (14).Cao-Lormeau VM; Blake A; Mons S; Lastere S; Roche C; Vanhomwegen J; Dub T; Baudouin L; Teissier A; Larre P; Vial AL; Decam C; Choumet V; Halstead SK; Willison HJ; Musset L; Manuguerra JC; Despres P; Fournier E; Mallet HP; Musso D; Fontanet A; Neil J; Ghawche F Guillain-Barre Syndrome outbreak associated with Zika virus infection in French Polynesia: a case-control study. Lancet 2016, 387 (10027), 1531–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (15).Duffy MR; Chen TH; Hancock WT; Powers AM; Kool JL; Lanciotti RS; Pretrick M; Marfel M; Holzbauer S; Dubray C; Guillaumot L; Griggs A; Bel M; Lambert AJ; Laven J; Kosoy O; Panella A; Biggerstaff BJ; Fischer M; Hayes EB Zika virus outbreak on Yap Island, Federated States of Micronesia. N. Engl. J. Med. 2009, 360 (24), 2536–43. [DOI] [PubMed] [Google Scholar]
- (16).Miner JJ; Daniels BP; Shrestha B; Proenca-Modena JL; Lew ED; Lazear HM; Gorman MJ; Lemke G; Klein RS; Diamond MS The TAM receptor Mertk protects against neuroinvasive viral infection by maintaining blood-brain barrier integrity. Nat. Med. 2015, 21 (12), 1464–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (17).Hamel R; Dejarnac O; Wichit S; Ekchariyawat P; Neyret A; Luplertlop N; Perera-Lecoin M; Surasombatpattana P; Talignani L; Thomas F; Cao-Lormeau VM; Choumet V; Briant L; Despres P; Amara A; Yssel H; Misse D Biology of Zika Virus Infection in Human Skin Cells. J. Virol. 2015, 89 (17), 8880–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (18).Meertens L; Carnec X; Lecoin MP; Ramdasi R; Guivel-Benhassine F; Lew E; Lemke G; Schwartz O; Amara A The TIM and TAM families of phosphatidylserine receptors mediate dengue virus entry. Cell Host Microbe 2012, 12 (4), 544–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (19).Davis CW; Nguyen HY; Hanna SL; Sanchez MD; Doms RW; Pierson TC West Nile virus discriminates between DC-SIGN and DC-SIGNR for cellular attachment and infection. J. Virol 2006, 80 (3), 1290–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (20).Routhu NK; Byrareddy SN Host-Virus Interaction of ZIKA Virus in Modulating Disease Pathogenesis. J. Neuroimmune Pharmacol 2017, 12 (2), 219–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (21).Tiwari SK; Dang J; Qin Y; Lichinchi G; Bansal V; Rana TM Zika virus infection reprograms global transcription of host cells to allow sustained infection. Emerging Microbes Infect. 2017, 6 (4), e24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (22).Dai L; Song J; Lu X; Deng YQ; Musyoki AM; Cheng H; Zhang Y; Yuan Y; Song H; Haywood J; Xiao H; Yan J; Shi Y; Qin CF; Qi J; Gao GF Structures of the Zika Virus Envelope Protein and Its Complex with a Flavivirus Broadly Protective Antibody. Cell Host Microbe 2016, 19 (5), 696–704. [DOI] [PubMed] [Google Scholar]
- (23).Zhang Y; Zhang W; Ogata S; Clements D; Strauss JH; Baker TS; Kuhn RJ; Rossmann MG Conformational changes of the flavivirus E glycoprotein. Structure 2004, 12 (9), 1607–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (24).Modis Y; Ogata S; Clements D; Harrison SC Structure of the dengue virus envelope protein after membrane fusion. Nature 2004, 427 (6972), 313–9. [DOI] [PubMed] [Google Scholar]
- (25).Morrison TE; Diamond MS Animal Models of Zika Virus Infection, Pathogenesis, and Immunity. J. Virol. 2017, 91 (8), e00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (26).Bowen JR; Quicke KM; Maddur MS; O’Neal JT; McDonald CE; Fedorova NB; Puri V; Shabman RS; Pulendran B; Suthar MS Zika Virus Antagonizes Type I Interferon Responses during Infection of Human Dendritic Cells. PLoS Pathog. 2017, 13 (2), e1006164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (27).Wu Y; Liu Q; Zhou J; Xie W; Chen C; Wang Z; Yang H; Cui J Zika virus evades interferon-mediated antiviral response through the co-operation of multiple nonstructural proteins in vitro. Cell Discov 2017, 3, 17006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (28).Kumar A; Hou S; Airo AM; Limonta D; Mancinelli V; Branton W; Power C; Hobman TC Zika virus inhibits type-I interferon production and downstream signaling. EMBO Rep. 2016, 17 (12), 1766–1775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (29).Sadler AJ; Williams BR Interferon-inducible antiviral effectors. Nat. Rev. Immunol. 2008, 8 (7), 559–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (30).Munoz-Jordan JL; Fredericksen BL How flaviviruses activate and suppress the interferon response. Viruses 2010, 2 (2), 676–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (31).Szretter KJ; Brien JD; Thackray LB; Virgin HW; Cresswell P; Diamond MS The interferon-inducible gene viperin restricts West Nile virus pathogenesis. J. Virol 2011, 85 (22), 11557–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (32).Katze MG; Fornek JL; Palermo RE; Walters KA; Korth MJ Innate immune modulation by RNA viruses: emerging insights from functional genomics. Nat. Rev. Immunol. 2008, 8 (8), 644–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (33).Huang YL; Taylor MW Induction of spermidine/spermine N1-acetyltransferase by interferon type I in cells of hematopoietic origin. J. Interferon Cytokine Res. 1998, 18 (5), 337–44. [DOI] [PubMed] [Google Scholar]
- (34).Mounce BC; Poirier EZ; Passoni G; Simon-Loriere E; Cesaro T; Prot M; Stapleford KA; Moratorio G; Sakuntabhai A; Levraud JP; Vignuzzi M Interferon-Induced Spermidine-Spermine Acetyltransferase and Polyamine Depletion Restrict Zika and Chikungunya Viruses. Cell Host Microbe 2016, 20 (2), 167–77. [DOI] [PubMed] [Google Scholar]
- (35).Hsu YL; Shi SF; Wu WL; Ho LJ; Lai JH Protective roles of interferon-induced protein with tetratricopeptide repeats 3 (IFIT3) in dengue virus infection of human lung epithelial cells. PLoS One 2013, 8 (11), e79518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (36).Pegg AE Spermidine/spermine-N(1)-acetyltransferase: a key metabolic regulator. Am. J. Physiol Endocrinol Metab 2008, 294 (6), E995–1010. [DOI] [PubMed] [Google Scholar]
- (37).Mandal S; Mandal A; Johansson HE; Orjalo AV; Park MH Depletion of cellular polyamines, spermidine and spermine, causes a total arrest in translation and growth in mammalian cells. Proc. Natl. Acad. Sci. U. S. A. 2013, 110 (6), 2169–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (38).Mounce BC; Cesaro T; Moratorio G; Hooikaas PJ; Yakovleva A; Werneke SW; Smith EC; Poirier EZ; Simon-Loriere E; Prot M; Tamietti C; Vitry S; Volle R; Khou C; Frenkiel MP; Sakuntabhai A; Delpeyroux F; Pardigon N; Flamand M; Barba-Spaeth G; Lafon M; Denison MR; Albert ML; Vignuzzi M Inhibition of Polyamine Biosynthesis Is a Broad-Spectrum Strategy against RNA Viruses. J. Virol. 2016, 90 (21), 9683–9692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (39).Casero RA; Pegg AE Polyamine catabolism and disease. Biochem. J. 2009, 421 (3), 323–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (40).Mounce BC; Poirier EZ; Passoni G; Simon-Loriere E; Cesaro T; Prot M; Stapleford KA; Moratorio G; Sakuntabhai A; Levraud J-P; Vignuzzi M Interferon-induced spermidine-spermine acetyltransferase and polyamine depletion restrict Zika and chikungunya viruses. Cell Host Microbe 2016, 20 (2), 167–177. [DOI] [PubMed] [Google Scholar]
- (41).Mounce BC; Cesaro T; Moratorio G; Hooikaas PJ; Yakovleva A; Werneke SW; Smith EC; Poirier EZ; Simon-Loriere E; Prot M Inhibition of polyamine biosynthesis is a broad-spectrum strategy against RNA viruses. J Virol 2016, 90 (21), 9683–9692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (42).Casero RA Jr; Marton LJ Targeting polyamine metabolism and function in cancer and other hyperproliferative diseases. Nat. Rev. Drug Discovery 2007, 6 (5), 373. [DOI] [PubMed] [Google Scholar]
- (43).Zhu Y; Li J; Kanvinde S; Lin Z; Hazeldine S; Singh RK; Oupický D Self-immolative polycations as gene delivery vectors and prodrugs targeting polyamine metabolism in cancer. Mol. Pharmaceutics 2015, 12 (2), 332–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (44).Xie Y; Murray-Stewart T; Wang Y; Yu F; Li J; Marton LJ; Casero RA; Oupický D Self-immolative nanoparticles for simultaneous delivery of microRNA and targeting of polyamine metabolism in combination cancer therapy. J. Controlled Release 2017, 246, 110–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (45).Murray-Stewart T; Ferrari E; Xie Y; Yu F; Marton LJ; Oupicky D; Casero RA Jr Biochemical evaluation of the anticancer potential of the polyamine-based nanocarrier Nano11047. PLoS One 2017, 12 (4), e0175917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (46).Yi G; Xu X; Abraham S; Petersen S; Guo H; Ortega N; Shankar P; Manjunath N A DNA Vaccine Protects Human Immune Cells against Zika Virus Infection in Humanized Mice. EBioMedicine 2017, 25, 87–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (47).Silveira ELV; Rogers KA; Gumber S; Amancha P; Xiao P; Woollard SM; Byrareddy SN; Teixeira MM; Villinger F Immune Cell Dynamics in Rhesus Macaques Infected with a Brazilian Strain of Zika Virus. J. Immunol. 2017, 199 (3), 1003–1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (48).Kabra PM; Lee HK; Lubich WP; Marton LJ Solidphase extraction and determination of dansyl derivatives of unconjugated and acetylated polyamines by reversed-phase liquid chromatography: improved separation systems for polyamines in cerebrospinal fluid, urine and tissue. J. Chromatogr., Biomed. Appl 1986, 380, 19–32. [DOI] [PubMed] [Google Scholar]
- (49).Reddy VK; Valasinas A; Sarkar A; Basu HS; Marton LJ; Frydman B Conformationally restricted analogues of 1 N, 12 N-bisethylspermine: synthesis and growth inhibitory effects on human tumor cell lines. J. Med. Chem. 1998, 41 (24), 4723–4732. [DOI] [PubMed] [Google Scholar]
- (50).Ekins S; Mietchen D; Coffee M; Stratton TP; Freundlich JS; Freitas-Junior L; Muratov E; Siqueira-Neto J; Williams AJ; Andrade C Open drug discovery for the Zika virus. F1000Research 2016, 5, 150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (51).Yuan S; Chan JF; den-Haan H; Chik KK; Zhang AJ; Chan CC; Poon VK; Yip CC; Mak WW; Zhu Z; Zou Z; Tee KM; Cai JP; Chan KH; de la Pena J; Perez-Sanchez H; Ceron-Carrasco JP; Yuen KY Structure-based discovery of clinically approved drugs as Zika virus NS2B-NS3 protease inhibitors that potently inhibit Zika virus infection in vitro and in vivo. Antiviral Res. 2017, 145, 33–43. [DOI] [PubMed] [Google Scholar]
- (52).Deng YQ; Zhang NN; Li CF; Tian M; Hao JN; Xie XP; Shi PY; Qin CF Adenosine Analog NITD008 Is a Potent Inhibitor of Zika Virus. Open Forum Infect Dis 2016, 3 (4), ofw175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (53).Garcia LL; Padilla L; Castano JC Inhibitors compounds of the flavivirus replication process. Virol. J. 2017, 14 (1), 95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (54).Lim SP; Wang QY; Noble CG; Chen YL; Dong H; Zou B; Yokokawa F; Nilar S; Smith P; Beer D; Lescar J; Shi PY Ten years of dengue drug discovery: progress and prospects. Antiviral Res. 2013, 100 (2), 500–19. [DOI] [PubMed] [Google Scholar]
- (55).Patkar CG; Kuhn RJ Development of novel antivirals against flaviviruses. Novartis Found Symp. 2008, 277, 41–52 discussion 52–6, 71–3, 251–3.. [PubMed] [Google Scholar]
- (56).Zmurko J; Marques RE; Schols D; Verbeken E; Kaptein SJ; Neyts J The Viral Polymerase Inhibitor 7-Deaza-2’-C-Methyladenosine Is a Potent Inhibitor of In Vitro Zika Virus Replication and Delays Disease Progression in a Robust Mouse Infection Model. PLoS Neglected Trop. Dis. 2016, 10 (5), e0004695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (57).Gibson W; Roizman B Compartmentalization of spermine and spermidine in the herpes simplex virion. Proc. Natl. Acad. Sci. U. S. A. 1971, 68 (11), 2818–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (58).Miller-Fleming L; Olin-Sandoval V; Campbell K; Ralser M Remaining Mysteries of Molecular Biology: The Role of Polyamines in the Cell. J. Mol. Biol. 2015, 427 (21), 3389–406. [DOI] [PubMed] [Google Scholar]
- 59.() Mounce BC; Cesaro T; Vlajnic L; Vidina A; Vallet T; Weger-Lucarelli J; Passoni G; Stapleford KA; Levraud JP; Vignuzzi M Chikungunya Virus Overcomes Polyamine Depletion by Mutation of nsP1 and the Opal Stop Codon To Confer Enhanced Replication and Fitness. J. Virol. 2017, 91, (15).e00344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- (60).Wang QY; Kondreddi RR; Xie X; Rao R; Nilar S; Xu HY; Qing M; Chang D; Dong H; Yokokawa F; Lakshminarayana SB; Goh A; Schul W; Kramer L; Keller TH; Shi PY A translation inhibitor that suppresses dengue virus in vitro and in vivo. Antimicrob. Agents Chemother. 2011, 55 (9), 4072–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (61).Roos WH; Ivanovska IL; Evilevitch A; Wuite GJ Viral capsids: mechanical characteristics, genome packaging and delivery mechanisms. Cell. Mol. Life Sci. 2007, 64 (12), 1484–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (62).Capone C; Cervelli M; Angelucci E; Colasanti M; Macone A; Mariottini P; Persichini T A role for spermine oxidase as a mediator of reactive oxygen species production in HIV-Tat-induced neuronal toxicity. Free Radical Biol. Med. 2013, 63, 99–107. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


