Abstract
Background:
Re-expression of the imprinted tumor suppressor gene DIRAS3 (ARHI) induces autophagy and tumor dormancy in ovarian cancer xenografts, but drives autophagic cancer cell death in cell culture. This study explores the tumor and host factors required to prevent autophagic cancer cell death in xenografts and use of antibodies against those factors or their receptors to eliminate dormant autophagic ovarian cancer cells.
Methods:
Survival factors (IGF-1, VEGF and IL8) were detected with growth factor arrays and measured by ELISA analysis. Phosphorylation of AKT, phosphorylation of ERK, nuclear localization of TFEB or FOXo3a and expression of MAPLC3B (LC3B) were examined using western blot analysis. The effect of treatment with antibodies against survival factors or their receptors were studied with DIRAS3-induced dormant xenograft models.
Results:
Ovarian cancer cells grown subcutaneously in nude mice exhibited higher levels of pERK/pAKT activity and lower levels of nuclear TFEB/FOXo3a, MAPLC3B and autophagy than cells grown in culture. Induction of autophagy and dormancy with DIRAS3 was associated with decreased ERK/AKT signaling. Addition of VEGF, IGF-1 and IL-8 weakened the inhibitory effect of DIRAS3 on ERK/AKT activity and reduced DIRAS3-mediated TFEB or FOXo3a nuclear localization and MAPLC3B expression in ovarian cancer cells. Treatment with antibodies against VEGF, IL-8 and IGFR inhibited the growth of dormant xenografts prolonging survival from 99 to >220 days (P<0.05) and curing a fraction of mice.
Conclusion:
Treatment with a combination of anti-VEGF, anti-IL8 and anti-IGFR antibodies prevented outgrowth of dormant cells and prolonged survival in a preclinical model.
Keywords: Ovarian Cancer, Autophagy, tumor dormancy, VEGF, IL8 and IGF-R
Translational Relevance:
Over the last 3 decades, despite improvement in 5-year survival for patients with ovarian cancer, the overall cure rate of approximately 35% has not changed due to late diagnosis and persistence of dormant, chemotherapy-resistant cancer cells. In more than 80% of cases, dormant ovarian cancer cells found at “second look” surgery express DIRAS3 and are undergoing autophagy. Selective elimination of dormant autophagic ovarian cancer cells could improve patient outcomes. In this study, we have found that neutralization of survival factors with monoclonal antibodies against VEGF, IL-8 and IGFR can drive dormant xenografts that express DIRAS3 to autophagic cancer cell death, curing the majority of mice. These studies could be translated to patients with minimal residual disease who might benefit from bevacizumab therapy, alone or in combination with monoclonal antibodies against IL-8 and/or IGFR. This could provide a novel approach to eliminating dormant, drug resistant ovarian cancer cells.
Introduction
Ovarian cancer develops in more than 22,440 women each year in the United States.1 Over the past four decades, the 5-year survival has increased from 37% to ~50% with optimal cytoreductive surgery and combination chemotherapy using platinum- and taxane-based regimens, but cure rates have not changed and remain at ~35% for all stages, due, in large part, to the persistence of dormant, drug-resistant ovarian cancer cells.2 Second-look surgery has documented small deposits of residual disease on the peritoneal surface in nearly half of patients following apparently successful chemotherapy.3 Approximately half of advanced-stage patients will relapse within 12 months, but some patients will relapse only after 40 months reflecting the persistence of dormant ovarian cancer cells.3–5 Factors controlling dormancy are not well understood.
Our group has studied an imprinted tumor suppressor gene DIRAS3 (ARHI) that plays an important role in inducing dormancy in ovarian cancers. DIRAS3 expression is downregulated in 60% of ovarian cancers by multiple mechanisms6–8, associated with shortened progression-free survival9. The re-expression of DIRAS3 in ovarian cancer cells inhibits proliferation, motility and xenograft growth, triggers autophagy, and induces tumor dormancy.8–19 OVCAR8-DIRAS3 and SKOv3-DIRAS3 ovarian cancer cell sublines have been developed with doxycycline-inducible expression of DIRAS3. After doxycycline-induced re-expression of DIRAS3 in SKOv3-DIRAS3 xenografts, cancer cells remain dormant and angiogenesis is inhibited. When doxycycline is withdrawn and DIRAS3 levels are reduced, xenografts acquire a blood supply and grow promptly to kill their host. The clinical relevance of this model is supported by the observation that more than 80% of patients with ovarian cancer who have completed initial chemotherapy, exhibit nodules on the peritoneal surface which contain cancer cells that are undergoing autophagy and that express DIRAS3, whereas only 23% of their primary cancers exhibit autophagy and express DIRAS3.20
Re-expression of DIRAS3 inhibits signaling through the Ras/MEK/ERK and PI3K/AKT signaling pathways. Activation of these signaling pathways plays a major role in oncogenesis.36 The PI3K signaling pathway is dysregulated in a majority of ovarian cancers due to the amplification, genetic mutation of PI3K gene and the components of the PI3K pathway including AKT.37, 38 Stimulation of the PI3K/Akt/mTOR and Ras/Raf/MEK/ERK pathways enhances growth, survival, and metabolism of cancer cells.37, 39
Autophagy is a catabolic process that results in lysosomal degradation of cytoplasmic contents ranging from abnormal proteins to damaged cell organelles.21, 22 The amino acids and fatty acids produced by proteolysis and lipolysis are further catabolized producing cellular energy. Autophagy is activated under diverse conditions. The rapid induction of autophagy, e.g. in response to starvation, is mediated by post-translational protein modification and protein-protein interactions, whereas transcriptional mechanisms are required for a sustained autophagic response.23, 24 Conserved transcriptional factors, particularly the helix-loop-helix transcriptional factor TFEB and forkhead transcriptional factor FOXO, control the expression of autophagy-related genes including MAPLC3B and are important for long-term extension of autophagy induction.24–26 Both TFEB and FOXO are negatively regulated by several growth-promoting signaling molecules.24, 27 Nuclear localization and activity of TFEB are regulated by serine phosphorylation mediated by extracellular signal-regulated kinase (ERK), belonging to the MAPK pathway27–29, whose activity can be regulated by the level of survival factors in the tumor microenvironment. The transcription factor FOXo3a has been reported to contribute to the induction of autophagy in muscle cells. FOXo3a is negatively regulated by several growth-promoting signaling molecules, including AKT phosphorylation of FOXo3a at Ser318 by AKT which induces rapid proteasomal degradation in the cytoplasm.30–33
Tumor dormancy is characterized by active equilibrium between proliferation and cell death including apoptosis or autophagic cell death. Many factors could contribute to cancer cells becoming dormant, including exiting the cell cycle, failing to establish effective angiogenesis and/or evoking a partially effective immune response.40, 41 Under certain circumstances such as the addition of growth factors and cytokines, dormant cells can exit the equilibrium state resulting in tumor growth and proliferation. Growth factors and cytokines are an important class of signaling molecules which bind to cell surface receptors and initiate intracellular signaling cascades resulting in the activation of oncogenes or inhibition of tumor suppressor genes.42–44
DIRAS3-induced autophagy in dormant xenografts appears to be important for survival of cancer cells in a poorly-vascularized, nutrient-poor microenvironment. When mice with dormant SKOv3-DIRAS3 xenografts are treated with chloroquine, a functional inhibitor of autophagy, subsequent outgrowth of tumors is significantly delayed when DIRAS3 levels are reduced15, providing a rationale for clinical trials with inhibitors of autophagy. The impact of autophagy on cancer cell survival is, however, contextual. While low levels of autophagy may facilitate survival of dormant cancer cells in a nutrient poor environment, more intense and persistent autophagy can produce cancer cell death. In contrast to observations in xenografts, re-expression of DIRAS3 in culture, kills 90% of autophagic ovarian cancer cells within 3 days.15 VEGF, IL-8 and IGF-1 are found in the xenograft microenvironment and can rescue ovarian cancer cells from DIRAS3-induced autophagic death in culture, consistent with the possibility that survival factors in the tumor microenvironment prevent autophagic death in dormant cancer cells.15 Blocking these factors could provide an alternative strategy for eliminating dormant ovarian cancer xenografts.
In this study, we have examined the effect of survival factors on cultured and xenograft-derived SKOv3-DIRAS3 and OVCAR8-ARHI ovarian cancer cells in the presence and absence of DIRAS3 expression to identify mechanisms by which VEGF, IL-8 and IGF prevent autophagic cancer cell death. Antibodies against these survival factors or their receptors have also been tested for the ability to eliminate dormant SKOv3-DIRAS3 ovarian cancer xenografts.
Materials and Methods
Antibodies and reagents.
Antibodies against EGFR (#4267), IGF-1R (#9750), IR (#3025), VEGF2 (#2479), p-AKT (#4060), p-ERK (#4370), AKT (#9272), ERK (#4695), TFEB (#4240), FOX3a (#2497 and #12829), EPCAM (#2929), actin (#4970) and LC3B (#3868 and #2775) were purchased from Cell Signaling Technology (Danvers, MA). Anti-PARP was purchased from BD Biosciences (San Jose, CA). Anti-tubulin was purchased from Sigma (St. Louis, MO). Anti-AKT (sc-5298) and anti-ERK (sc-514302) were purchased from Santa Cruz (Dallas, TX). Anti-VEGFR1 (32152) was purchased from Abcam (Cambridge, MA). CXCR1 (55450–1-AP) was purchased from Proteintech (Rosemont, IL). CXCR2 (PA1–31217) was purchased from Fisher (Waltham, MA). An anti-DIRAS3 murine monoclonal antibody (ID8) was generated in our laboratory. Doxycycline hyclate (DOX, D9891), Vascular Endothelial Growth factor (VEGF, V-7259), Interleukin-8 human (IL8, I1645), Insulin-Like Growth Factor-I human (IGF-1, I3769) and AKT 1/2 kinase inhibitor (A6730) were purchased from Sigma-Aldrich (St. Louis, MO). For MAPK inhibitors, U0126 (#9903), SB203500 (#5633) were purchased from Cell Signaling Technology and JNK inhibitor II was purchased from Calbiochem (Burlington, MA)
Cell culture and lentiviral infection.
Tet-on inducible SKOv3-DIRAS3 ovarian cancer cells were grown in McCoy’s and, tet-on inducible OVCAR8-DIRAS3 ovarian cancer cells were cultured in RPMI-1640 media and tet-on inducible Hey-DIRAS3 ovarian cancer cells were cultured in RPMI-1640 media. FOXo3a, TFEB overexpression were achieved by transducing SKOv3-DIRAS3 ovarian cancer cells with adenovirus encoding FOXo3a, TFEB and control vector. FOXo3a (1026), TFEB (ADV-225358) and control vector (1060) adenoviruses were purchased from Vector BioLABS (Philadelphia, PA).
ELISA.
VEGF ELISA (DVE00, R&D Systems, Minneapolis, MN), IL8 ELISA (D8000C, R&D System, Minneapolis, MN) and IGF-1 ELISA (ELH-IGF-1, Ray Bio, Norcross, GA) assays were performed according to manufacturer instructions. In brief, SKOv3-DIRAS3 and OVCAR8-DIRAS3 cell lysates, as well as tumor lysates from frozen tissues of isolated xenografts, were collected and triplicate aliquots (100 microliter per well) of lysates with multiple dilutions were loaded onto ELISA plates.. The plates were incubated for 1–2 hrs at RT. After washing, streptavidin-conjugated HRP reagent was added to the wells, and plates were incubated for 30 min at RT. Color development was performed by incubation with HRP substrate and the optical density (O.D.) at 450 nm was determined using a microplate reader.
Antibody array analysis.
Semi-quantitative sandwich-based antibody arrays (RayBio® Human Angiogenesis Array C-Series1000) were used to analyze tumor lysates according to the manufacturer’s instructions.. Signals were measured with a chemiluminescence imaging system. Averages of the signal intensities for duplicate spots relative to positive controls were calculated.
Clonogenic assays.
Cells were seeded in 6-well plates at a density of 2,000 cells per well and cultured overnight at 37°C. Cells were then incubated for 24 hrs with or without DOX and with or without growth factor plus specific antibodies as indicated. Media was then changed every 48 hrs with and without treatment of growth factors as indicated for the first 6 days. After treatment, cells were grown for an additional 7–8 days. Colonies were stained with Coomassie blue and counted.
Preparation of total RNA and qRT-PCR analysis of mRNA expression.
48 hrs prior to lysate collection, DOX was added to cell culture media. After 32 hrs, cells were serum starved with or without DOX for 16 hrs. In addition, at 1.5 hrs prior to lysate collection, cells were treated with or without an AKT inhibitor or a MAPK inhibitor and then stimulated with or without growth factors for the last 15 min. Cells were harvested and total RNA was extracted using RNA easy kits (Qiagen). cDNA was synthesized from 1 μg of RNA using the Superscript II First Strand Synthesis Kit (Invitrogen). qRT-PCR was performed using CFX Connect Real-time System (Bio-Rad, Hercules, CA).. LC3B primers were 5’-GAATTCTCCCACACCAAGTG-3’ (forward primer) and 5’-AAATAGTGAACCCCATGCAA-3’ (Reverse primer) (VHPS-5518, RealtimePrimers.com, Elkins, PA). Experiments were run in triplicate.
Immunoblot and nucleus / cytoplasmic protein extraction.
48 hrs prior to lysate collection, DOX was added to cell culture media After 32 hrs, cells were serum starved with or without DOX for 16 hrs. In addition, at 1.5 hrs prior to lysate collection, cells were treated with or without an AKT inhibitor or an ERK inhibitor and then stimulated with or without growth factors for the last 15 min. Cells were incubated in lysis buffer for 30 min on ice, and then centrifuged at 17,000 × g for 30 min at 4°C. Nuclear and cytoplasmic fractions were isolated using a NE-PER Nuclear and Cytoplasmic Extraction Kit (Thermo Scientific, Rockford, IL). Whole cell Lysates or cytoplasmic/nuclear fractions were separated by SDS-PAGE and then transferred to Polyvinylidene difluoride (PVDF) membranes. Immunoblot analysis was performed with the indicated antibodies and visualized with an ECL enhanced chemiluminescence detection kit (GE Healthcare, Pittsburgh, PA).
Immunofluorescent staining.
Cells were grown on cover slips. DOX was added to cell culture media for 32 hrs, and then cells were serum starved with or without DOX for 16 hrs. In addition, at 1.5 hrs prior to fixation of cells, cells were treated with or without an AKT inhibitor or an ERK inhibitor and then stimulated with or without growth factors for last 15 min. Cells were then fixed in 4% formaldehyde in PBS for 10 min, and then permeabilized. Cells on the cover slips were blocked with 5% BSA in PBS for 1 hr at room temperature followed by incubation with primary antibodies. Cells were incubated with secondary antibodies diluted in 1.5% BSA for 1 hr at room temperature. Slips were then examined using fluorescence microscopy (Olympus 1×71, Center Valley, PA).
Transmission electron microscopy (TEM).
For TEM examination of autophagosomes and autolysosomes, SKOv3-DIRAS3, OVCAR8-DIRAS3 and Hey-DIRAS3 cells were treated with or without DOX for 48 hrs and with or without growth factors for the last 24 hrs as indicated. Cells were washed in PBS and fixed with 2.5% glutaraldehyde in 0.1 M PBS buffer and further fixed with 1% osmium tetroxide in 0.1 M cacodylate buffer. Specimens were stained with aqueous uranyl acetate and lead citrate before observed with a Jeol-100 CX II (JEOL, Peabody, MA) TEM at 80 kV.
Immunohistochemistry.
Formalin-fixed, paraffin embedded SKOv3 and cell pellets as well as isolated xenografts were collected. After slides were rehydrated, antigen retrieval was performed in 6.5 mM sodium citrate buffer (pH 6.0) for 10 min. 3% bovine serum albumin in 0.1 M Tris-buffered saline was used for blocking. Sections were incubated at 4°C overnight with each of the primary antibodies including anti-EPCAM (1:100), anti-p-AKT (1:50), anti-AKT (1:500), anti-p-ERK (1:400) and anti-ERK (1:1000). And then secondary antibodies were applied for 1 hr at room temperature followed by washing 3 times in PBS for 10 min. DAB chromagen was added for 1 min to each slide. H&E staining was performed by the MD Anderson Cancer Center Research Histopathology Facility.
Murine xenografts.
Six-week-old athymic nu/nu mice were purchased from MD Anderson Cancer Center Department of Veterinary Medicine and Surgery. SKOv3-DIRAS3 cells (5 × 106) were injected subcutaneously into the flank of each mouse. DOX (2 mg/ml) in 5% sucrose or sucrose alone was added to the drinking water on the day of injection. On the following day, mice were injected i.p. with 1 μg/g control IgG (Amgen) or AMG79 (Amgen) or ABX-IL-8 (Amgen) or Avastin (Genentech) alone or combination with two antibodies or three antibodies as indicated. Antibodies were injected once each week for 6 weeks. DOX was withdrawn from the drinking water after 6 weeks. Tumors were measured once each week and animal survival were evaluated from the day of cancer cell injection until the ethical end point. To examine the activity of AKT and ERK signaling in vivo, cells were injected subcutaneously into the flank of each mouse with or without DOX and with or without 3 antibodies. After 10-day inoculation, DOX was added to the drinking water for 4 consecutive days. On the following day of DOX treatment, mice were injected i.p. with 3 μg/g control IgG or 3 antibodies for 3 consecutive days and then on the next day, xenografts were dissected after euthanasia. All procedures were carried out according to the animal protocol approved by the Institutional Animal Care and Use Committee of the M.D. Anderson Cancer Center at the University of Texas.
Statistical analysis.
All experiments were repeated independently at least twice and the data expressed as mean ± SE. Statistical analysis was performed using Student’s t test (two-sample assuming unequal variances) or one-way ANOVA. Differences were considered statistically significant at p<0.05 (two-sided).
Results
Levels of VEGF, IL-8, IGF1, phospho-AKT and phospho-ERK are elevated in ovarian cancer xenografts.
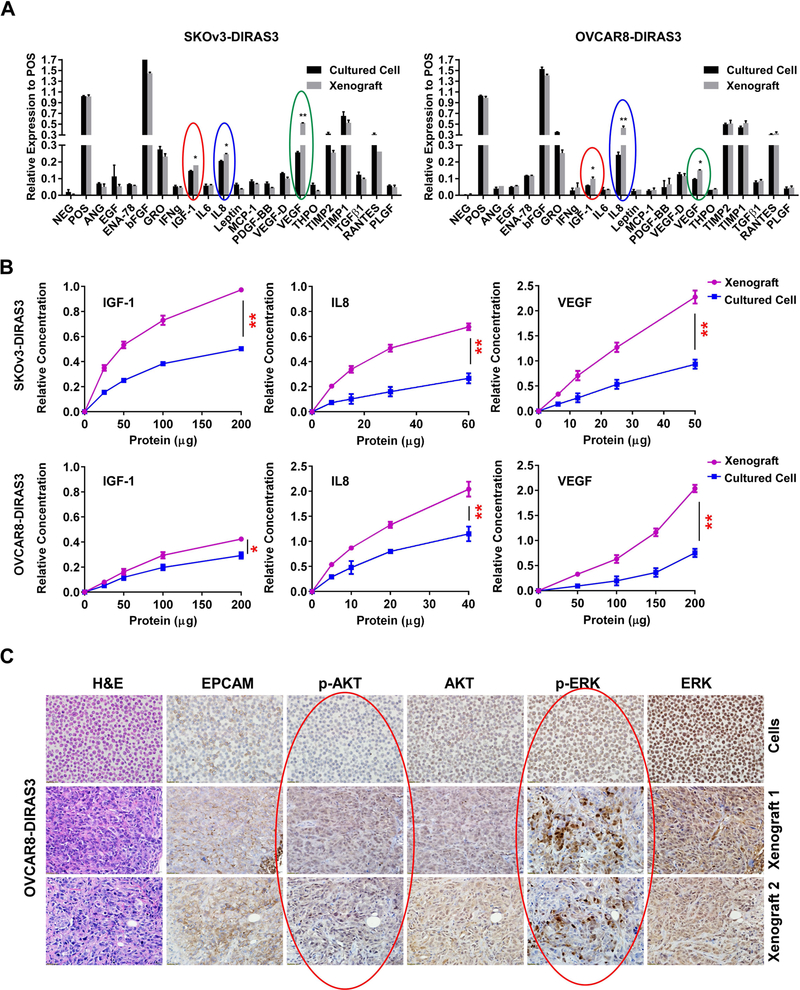
In previous studies, we reported that the re-expression of DIRAS3 (ARHI) in ovarian cancer cells induces autophagic cancer cell death in culture.15, 45 When the same cell lines are grown as xenografts, re-expression of DIRAS3 also induces autophagy, but cancer cells remain dormant and grow promptly when DIRAS3 levels are subsequently reduced.15 We hypothesized that dormant cancer cells require the presence of survival factors in their microenvironment to prevent autophagic cell death. To test this hypothesis, we examined the presence of potential survival factors and the activation of AKT and ERK signaling. First, we measured the survival factors in both cells grown subcutaneously in nude mice and grown in cell culture for two ovarian cancer cell lines (SKOv3-DIRAS3 and OVCAR8-DIRAS3) using a commercial antibody array and found that VEGF, IGF-1 and IL-8 are elevated in xenograft tumor cells when compared to their levels in media from cultured tumor cells (Figure 1A and S1). Importantly, these results were confirmed with a quantitative ELISA (Figure 1B). When signaling was evaluated, we observed that SKOv3-DIRAS3 and OVCAR8-DIRAS3 cells grown subcutaneously in nude mice exhibited higher levels of phospho-AKT and particularly phospho-ERK by immunohistochemical staining, indicating increased kinase activity, compared to cells grown in cell culture, while total AKT and ERK expression were similar (Figure 1C and S2). Together, these results suggest that VEGF, IL-8 and IGF-1 found in tumor environment could activate the AKT and/or ERK signaling pathways in ovarian cancer.
Figure 1. Levels of VEGF, IL-8 and IGF1 are elevated and signaling through PI3K/AKT and MAPK/ERK is activated in ovarian cancer xenografts.
(A) Growth factor/cytokine antibody arrays were performed using cultured cell lysates and xenograft lysates from SKOv3-DIRAS3 and OVCAR8-DIRAS3 ovarian cancer cell lines. Levels of IGF-1 (red circles), IL-8 (blue circles) and VEGF (green circles) were significantly elevated in xenografts when compared with cultured cells (NEG: negative control, POS: positive control). (B) ELISAs were performed to confirm observations in (* p<0.05, ** p<0.01). (A). (C) Phospho-AKT and phospho-ERK levels were increased in xenografts compared with cultured cells. Sections from formalin-fixed, paraffin embedded OVCAR8-DIRAS3 cell pellets and xenografts were examined by immunohistochemistry using antibodies against EpCAM, p-AKT, total AKT, p-ERK and total ERK as indicated.
Treatment of ovarian cancer cells with VEGF, IL-8 and IGF-1 impairs DIRAS3-mediated induction of autophagy and enhances signaling through PI3K/AKT and MAPK/ERK.
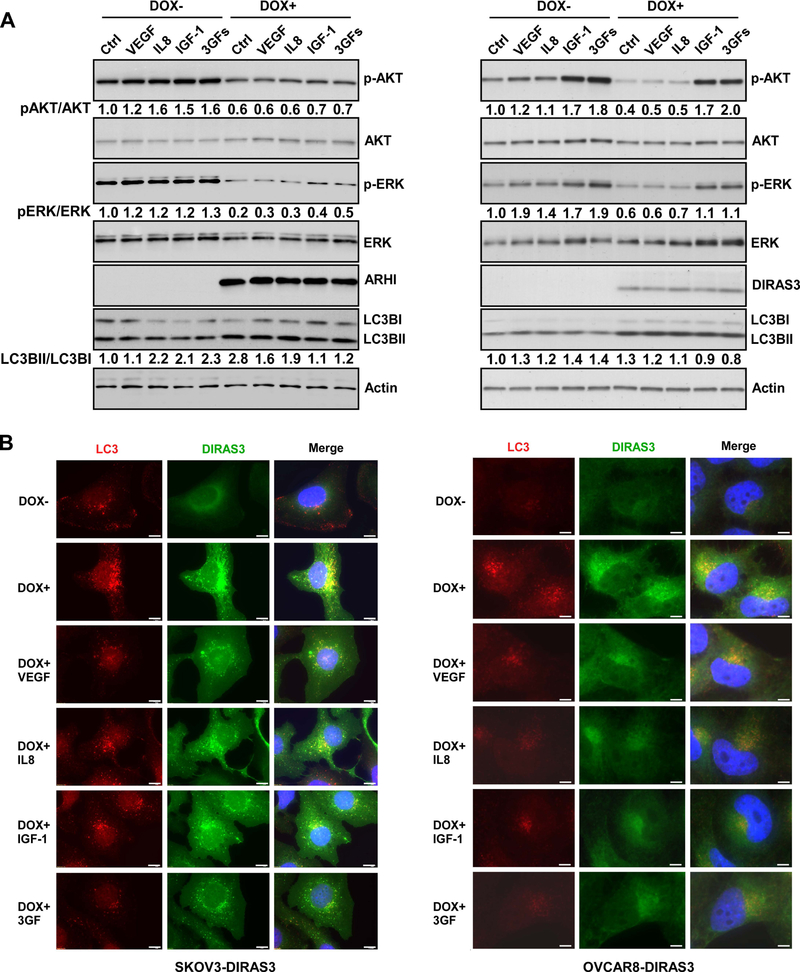
To determine whether elevated levels of VEGF, IGF and IL-8 could activate AKT and ERK signaling, we first verified that there are detectable levels of receptors for VEGF, IGF-1 and IL-8 in SKOv3-DIRAS3 and OVCAR8-DIRAS3 ovarian cancer cells (Figure S3). Doxycycline was added to the culture media for 48 hrs to induce expression of DIRAS3 and receptor expression was measured by western blot analysis. Re-expression of DIRAS3 did not affect levels of VEGF receptor (VEGFR1 and VEGFR2) or IL-8 receptor (CXCR1 and CXCR2) (Figure S3), but significantly decreased IGF1R expression in SKOv3-DIRAS3 cells. IGF-1 is a hormone similar in structure to insulin and can stimulate intracellular signaling by activation of the insulin-like growth factor receptor (IGF1R) and also to a lesser extent the insulin receptor (IR).46 Induction of DIRAS3 expression only slightly decreased IR level (Figure S3). To determine whether survival factors play a critical role in activating the AKT and/or ERK signaling pathways, rescuing the ovarian cancer cells from autophagic cell death in vitro, we treated SKOv3-DIRAS3 and OVCAR8-DIRAS3 inducible cells with DOX for 30 hrs (inducing DIRAS3 expression) before switching to serum free media in the presence or absence of additional survival factors (VEGF, IL8, IGF-1). As demonstrated by western blot analysis, supplementing the culture media with survival factors increased p-AKT and p-ERK signaling in the absence of DIRAS3. In the presence of DIRAS3, all three survival factors together increased p-AKT and p-ERK signaling, more robustly in OVCAR8 than in SKOv3, whereas the expression of DIRAS3 decreased signaling through both pathways (Figure 2A). Using conversion of LC3B I to LC3B II to measure autophagic flux, we found that the expression of DIRAS3 significantly enhanced conversion of LC3B I to LC3B II, consistent with the induction of autophagy. Addition of survival factors (VEGF, IL-6, IGF) decreased conversion of LC3B I to LC3B II (Figure 2A). In the absence of DIRAS3 (DOX-), serum starvation and the addition of the survival factors to the culture media resulted in decreased LC3B I expression (Figure 2A). Enhancement and inhibition of autophagy were confirmed using fluorescence microscopy where staining of LC3B (red) and DIRAS3 (green) document increased LC3 punctae and co-localization with DIRAS3 upon DOX induction and re-expression of DIRAS3 in SKOv3 and OVCAR8 ovarian cancer cells. The number of LC3 punctae decreased in the presence of VEGF, IL8, IGF-1 or a combination of all three survival factors (Figure 2B and S4), suggesting that activation of the AKT and MAPK signaling pathways inhibits the induction of DIRAS3-mediated autophagy. Transmission electron microscopy (TEM) also documented similar effects on autophagy, where re-expression of DIRAS3 induced the accumulation of double-membrane autophagosomes which could be reversed by the addition of survival factors to the culture media (Figure S6). The same was true for Hey-DIRAS3 inducible cells (Figure S5–6). To further document that survival factors can modulate DIRAS3-induced autophagy, we examined the phosphorylation of AKT and ERK in serum free medium and found that expression of DIRAS3 further inhibited p-AKT and p-ERK (Figure 3A), increasing LC3B I to LC3B II conversion (Figure 3A) and enhancing expression of LC3B (Figure 3B) in both SKOv3-DIRAS3 and OVCAR8-DIRAS3 cells. Taken together, these data and our previously published findings15, suggest that DIRAS3 induces autophagy by inhibiting the PI3K/AKT and MAPK/ERK signaling pathways, while survival factors found in the tumor microenvironment can mitigate this inhibition, preventing DIRAS3-mediated autophagy in SKOv3-DIRAS3 and OVCAR8-DIRAS3 inducible ovarian cancer cells.
Figure 2. Treatment of ovarian cancer cells with VEGF, IL-8 and IGF-1 inhibits DIRAS3-mediated induction of autophagy and enhances signaling through PI3K/AKT and MAPK/ERK measured by Western blot analysis and immunofluorescence staining.
(A) VEGF, IL8 and IGF-1 increased phosphorylation of AKT and ERK and deceased autophagy in SKOv3-DIRAS3 and OVCAR8-DIRAS3 ovarian cancer cells. Cells were collected and lysed for Western blot analysis after treatment with or without DOX for a total 48 hrs with or without serum starvation for the last 16 hrs. In addition, 15 min prior to lysate collection, cells were treated with or without growth factors as indicated. Final concentrations of VEGF, IL8 and IGF-1 were 20 ng/ml, 20 ng/ml and 40 ng/ml respectively. The numbers below the bands indicate band intensity compared to control. (B) VEGF, IL8 and IGF-1 deceased the formation of autophagy in SKOv3-DIRAS3 and OVCAR8-DIRAS3 cells by immunofluorescence staining. Autophagy in cells treated with or without DOX (to induce expression of DIRAS3) and with or without growth factors were determined by LC3B punctae using immunofluorescence. Scale bar: 20 μM. The autophagic vesicle counts are plotted in Figure S4.
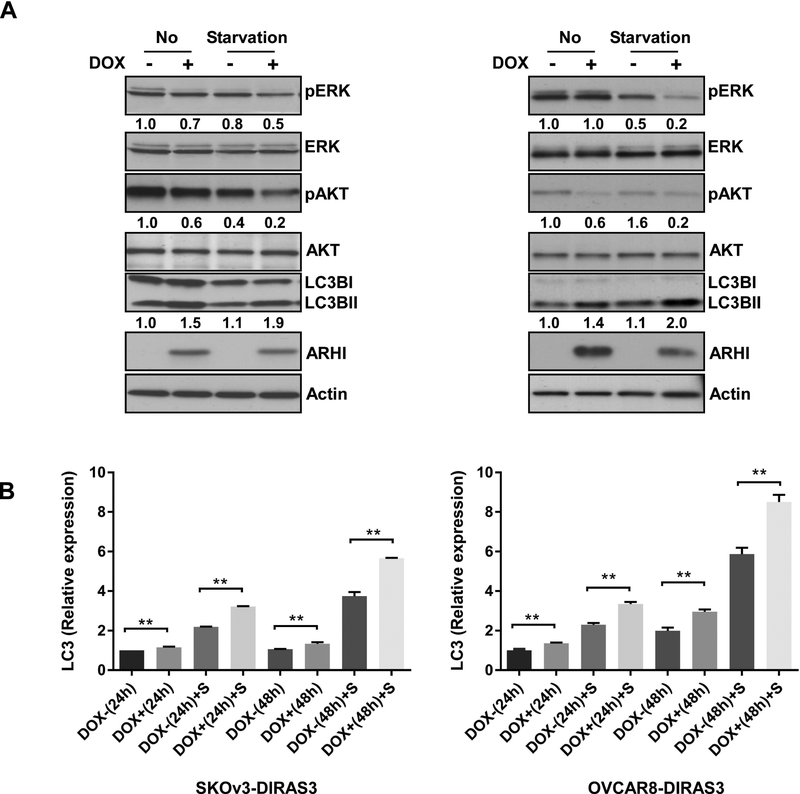
Figure 3. Serum starvation decreases the activity of AKT and ERK and enhances DIRAS3-induced autophagy in ovarian cancer cells.
(A) Serum starvation decreases phosphorylation of AKT and ERK and increases LC3B II / LC3B I conversion in SKOv3-DIRAS3 and OVCAR8-DIRAS3 cells. Cells were treated with or without DOX for 48 hrs and treated with or without serum free media for the final 24 hrs. Cell lysates were collected for Western blot analysis. Numbers below the bands indicate the band intensity compared to control. (B) Serum starvation (S) enhances messenger RNA (mRNA) expression of LCB3 in SKOv3-DIRAS3 and OVCAR8-DIRAS3 cells. Cells were treated with or without DOX for 48 hrs and treated with or without serum free media for the final 24 hrs. RT-PCR was performed to measure mRNA expression of LC3B. The columns indicate the mean and the bars indicate the S.E. (** p<0.01).
Survival factor-mediated activation of MAPK/ERK and PI3K/AKT signaling blocks DIRAS3-induced TFEB and FOXo3a nuclear localization and down-regulated the transcription of LC3B.
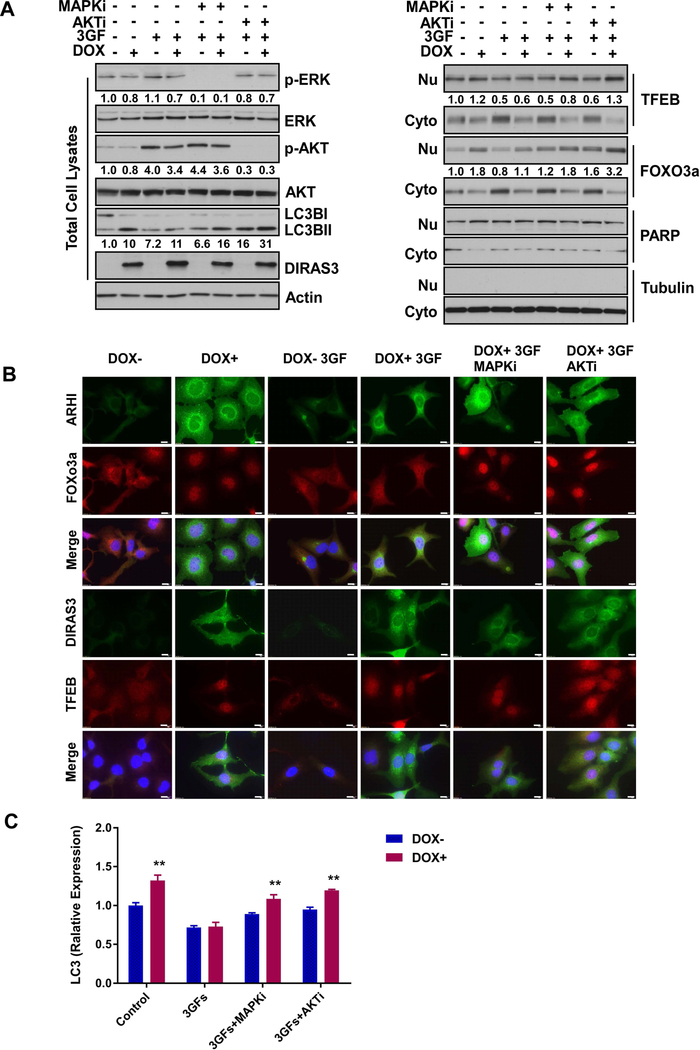
To determine whether survival factors can affect the ability of DIRAS3 to activate transcriptional programs that upregulate proteins that participate in autophagy, we evaluated the nuclear localization of TFEB and FOXo3a. Re-expression of DIRAS3 resulted in increased nuclear localization of TFEB and FOXo3a (Figure S7A), but that the addition of survival factors to the tissue culture media inhibited DIRAS3-mediated nuclear localization of both transcription factors (Figure S7B). To further analyze the relationship between MAPK/ERK and PI3K/AKT signaling as well as the downstream target regulated by survival factors in the tumor microenvironment, we used selective inhibitors of MAPK and AKT and observed their effect on survival factor-mediated inhibition of DIRAS3-induced autophagy and nuclear localization of TFEB and FOXo3a. A MAPK inhibitor (MAPKi) or an AKT inhibitor (AKTi) selectively reduced MAPK/ERK and PI3K/AKT signaling, respectively (Figure 4A and S8A) as well as blocked the survival factor-mediated decrease in the nuclear localization of TFEB and FOXo3a (Figure 4B and S8B). Notably, AKT inhibition also enhanced TFEB nuclear localization and MAPK inhibition enhanced FOXo3a nuclear localization, consistent with the overlapping signaling cascades that regulate these transcriptional programs. In addition, inhibition of MAPK or AKT restored the DIRAS3-mediated nuclear localization of TFEB and FOXo3a as well as the conversion of LC3B I to LC3B II which were suppressed by the addition of the three survival factors (Figure 4A–B and Figure S8A–B). Finally, we documented a significant decrease in expression of the TFEB and FOXo3a target gene, LC3B, by quantitative real time polymerase chain reaction (qRT-PCR) when ovarian cancer cells were treated with three growth factors compared to control treatment (Figure 4C and S8C).
Figure 4. Survival factor-mediated activation of MAPK/ERK and PI3K/AKT signaling blocks DIRAS3-induced TFEB and FOXo3a nuclear localization and down-regulates transcription of MAPLC3B.
(A) VEGF, IL8 and IGF-1 increased the levels of AKT and ERK phosphorylation and decreased nuclear localization of TFEB and FOXo3a resulting in inhibition of autophagy in SKOv3-DIRAS3 ovarian cancer cells. Cells were collected for nuclear and cytoplasmic extraction after treatment with or without DOX for a total of 48 hrs with or without serum starvation for the final 16 hrs. In addition, at 1.5 hrs prior to harvest, cells were treated with or without an AKT1/2 inhibitor (0.5 μM) or a MAPK inhibitor U0126 (5μM; SB253080, 5μM; JNK II, 5μM) and then stimulated with or without growth factors for the last 15 min. The concentrations of growth factors used the experiment are the same as in Figure 2A. Whole cell lysates or cytoplasmic/nuclear fractions were analyzed by Western blot. Numbers below the bands indicate band intensity compared to controls. (B) VEGF, IL8 and IGF-1 decreased nuclear localization of TFEB and FOXo3a. SKOv3-DIRAS3 cells were treated as described in Figure 5A. Scale bar: 20 μM. (C) VEGF, IL8 and IGF-1 decreased mRNA expression of LC3B in SKOv3-DIRAS3, which was partially rescued by AKT or MAPK inhibitor. The treatment of cells is the same as in Figure 5A. The columns indicate the mean, and the bars indicate the S.E. (** p<0.01).
To further document the contribution of ERK and AKT signaling to the regulation of TFEB and FOXo3a, SKOv3-DIRAS3 cells were transduced with lentiviral vectors expressing either the TFEB or FOXo3a genes. Either in the presence or absence of IGF-1, VEGF and IL-8, overexpression of TFEB or FOXo3a increased mRNA of LC3B, suggesting that overexpression of these two transcriptional factors may attenuate survival factor mediated down-regulation of autophagy (Figure S9). These results are consistent with the observation that inhibition of ERK or AKT phosphorylation using MAPK or AKT inhibitors increased DIRAS3-TFEB/FOX3a-mediated expression of LC3B.
Taken together, these data indicate that survival factors found in the tumor microenvironment can inhibit DIRAS3-mediated autophagy by increasing MAPK/ERK and PI3K/AKT signaling and decreasing transcriptional upregulation of the MAPLC3B gene by decreasing TFEB and FOXo3a nuclear localization, facilitating cancer cell survival.
Treatment with a combination of anti-VEGF, anti-IL8 and anti-IGF-1 antibodies prevents outgrowth of human ovarian cancer cells in culture and in dormant xenografts.
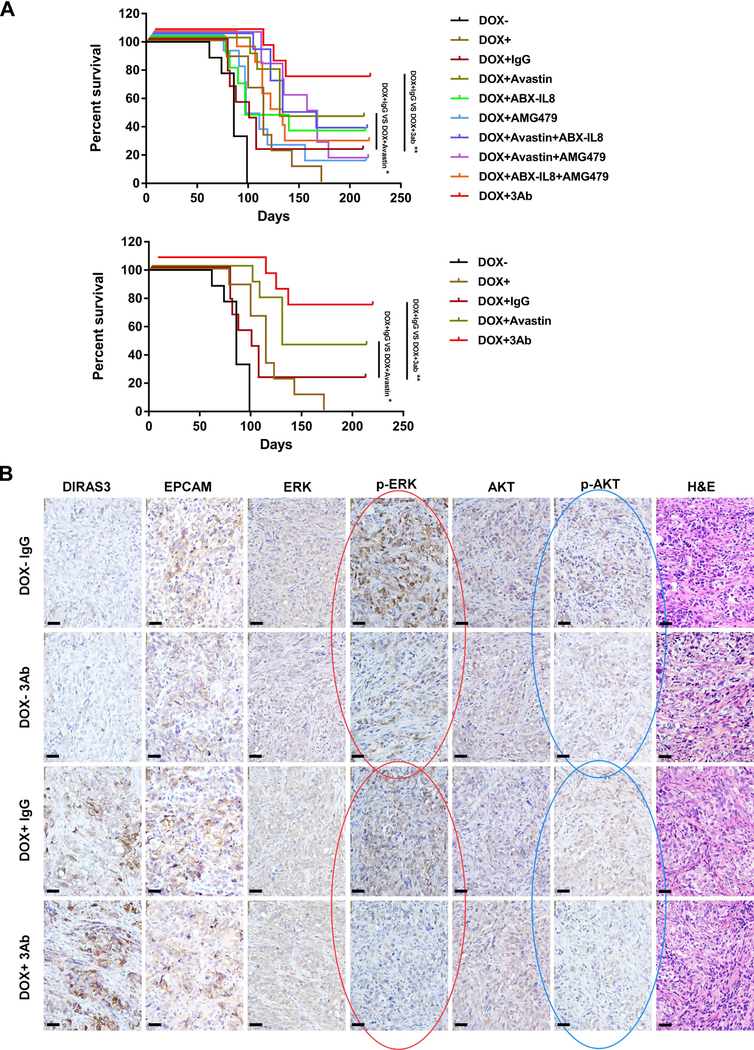
Using clonogenic assays in cell culture, we confirmed our previous observation15, 45 that re-expression of DIRAS3 in human ovarian cancer cells resulted in decreased colony formation (Figure S10). When survival factors (VEGF, IL-8, IGF-1) were added to the tissue culture media, colony counts increased compared to controls, demonstrating that DIRAS3-induced autophagic cell death could be rescued by the addition of survival factors to the cell culture media (Figure S10). Addition of antibodies against VEGF, IL8 and IGF-1 to the tissue culture media blocked growth factor increased colony formation (Figure S10). To evaluate the therapeutic potential of these observations, we tested the same antibodies for their effect on outgrowth of dormant SKOv3-DIRAS3 ovarian cancer xenografts (Figure 5A and Figure S11). Expression of DIRAS3 (DOX+) significantly inhibited the growth of xenografts. Xenografts that did not express DIRAS3 (DOX-) grew progressively. In groups where doxycycline was withdrawn after 6 weeks, xenografts were initially inhibited and then grew promptly after downregulation of DIRAS3. Treatment with control IgG did not affect xenograft growth. Treatment of nu/nu mice bearing dormant autophagic xenografts with anti-VEGF, anti-IL-8 and anti-IGFR prolonged median survival from 86 to 125 days (P<0.001) and cured 1 of 5 mice with survival >300 days (Figure S11). Importantly, the repeat experiment documented a similar effect on xenograft growth and prolonged survival in the nu/nu mice, where treatment of dormant xenografts with individual antibody delayed their outgrowth, but greater delay was observed with a combination of the three antibodies against VEGF, IL-8 and the IGFR. Treatment of nu/nu mice bearing dormant autophagic xenografts with anti-VEGF, anti-IL-8 and anti-IGFR prolonged median survival from 99 to >220 days (P<0.05) and cured 6 of 9 mice (red line, >220 days) (Figure 5A). Bevacizumab provided the greatest contribution of the three antibodies, producing long term survival in 4 of 9 mice (dark green line, >220 days) (Figure 5A). Furthermore, antibodies against these survival factors (VEGF and IL8) and their receptors (IGF-1) decreased AKT and ERK signaling in vivo (Figure 5B). Thus, combined treatment with three antibodies, which block AKT and ERK signaling produced long term survival in a majority of mice.
Figure 5. Treatment of dormant xenografts with anti-VEGF, anti-IL8 and anti-IGFR-1 antibodies prevents outgrowth of dormant cells in vivo.
(A) The effect of antibodies on outgrowth of dormant SKOv3-DIRAS3 ovarian cancer xenografts was measured. SKOv3-DIRAS3 cells (5 × 106) were injected subcutaneously into the flank of each mouse. DOX (2 mg/ml) in 5% sucrose or sucrose alone was added to the drinking water on the day of injection for 6 continuous weeks. On the day following cell inoculation, mice were injected i.p. with 1 ug/g control IgG (Amgen) or AMG79 (Amgen) or ABX-IL-8 (Amgen) or Avastin (Genentech) alone or combination with two antibodies or three antibodies as indicated for total six weeks. Animal survival was evaluated from the day of cancer cell injection until the ethical end point. Survival curves were generated by GraphPad Prism 6. Top figure presents all the experimental groups. Bottom figure presents only DOX-, DOX+, DOX+IgG, DOX+Avastin and DOX+3Ab groups.(B) Antibodies against these survival factors (VEGF and IL8) or their receptors (IGF-1) decreased AKT and ERK signaling in vivo. Xenograft tumors were examined by immunohistochemistry with anti-EPCAM, anti-DIRAS3, anti-p-AKT, anti-AKT anti-p-ERK and anti-ERK antibodies as indicated. (*p<0.05; ** p<0.01).
Discussion
In this report, we have confirmed that re-expression of DIRAS3 induces high levels of autophagy in ovarian cancer cells by inhibiting the PI3K/AKT and MAPK/ERK signaling pathways and enhancing the expression of MAPLC3B through increased transcription mediated by the nuclear localization of TFEB and FOXo3a. DIRAS3-induces both autophagy and dormancy in ovarian cancer xenografts, but induces autophagic cancer cell death in cultured cells. VEGF, IL-8 and IGF-1, are growth factors and cytokines that are elevated in the microenvironment of xenografts. Addition of these three survival factors can rescue ovarian cancer cells in culture from DIRAS3-induced autophagic cell death. Treatment with the three survival factors can also increase PI3K/AKT and MAPK/ERK signaling, decrease nuclear localization of TFEP and FOXo3a and decrease DIRAS3-induced autophagy. Antibodies against VEGF, IL-8 and IGFR-1 can eliminate dormant ovarian cancer xenografts by enhancing autophagic cell death. While re-expression of DIRAS3 decreases levels of pAKT and pERK in cancer cells in vivo, treatment with the three antibodies further decreases levels of both signaling molecules in xenografts.
We have demonstrated that DIRAS3 induces autophagy by inhibiting the PI3K/AKT and MAPK/ERK signaling pathways and enhancing TFEB and FOXo3a-mdiated the expression of LC3B in ovarian cancer cells resulting in the induction of autophagy and autophagic cell death in cell culture. Autophagy could be a mechanism of tumor suppression, but it also can confer stress tolerance that enables tumor cell to survive under adverse conditions. Previously we reported that the re-expression of DIRAS3 induces autophagy and growth arrest in SKOv3 ovarian cancer cells both in culture and in athymic nu/nu mouse xenografts. In xenografts, cancer cells remain dormant, but grow promptly when DIRAS3 levels are reduced. If mice are treated with chloroquine, a functional inhibitor of autophagy, while ovarian cancer cells are dormant, outgrowth of tumors is significantly delayed after DIRAS3 levels are reduced.15 In culture, 90% of autophagic cancer cells die within 3 days, but can be rescued by survival factors found in the xenograft microenvironment, including VEGF, IL-8 and IGF-1. These observations are consistent with a model where dormant ovarian cancer cells require low levels of autophagy to generate ATP in order to survive in an avascular, nutrient-poor environment.
Overexpression of growth factors is commonly observed in malignancies including ovarian cancer, resulting in further activation of associated pathways. Ovarian cancer cells grown as xenografts exhibited higher levels of VEGF, IL8 and IGF1, as well as higher levels of phospho-AKT and phospho-ERK than the same cells grown in culture, suggesting that survival factors found in the tumor microenvironment play an important role in activating the AKT and/or ERK signaling pathways. Thus overexpression of survival factors in the tumor microenvironment may be one mechanism that dormant cells ovarian cancer cells survive from DIRAS3-mediated autophagic cell death.
The clinical relevance of our xenograft model is supported by the observation that DIRAS3 and punctate LC3B is expressed by <20% of ovarian cancers at primary surgery, but in 80% of cancers detected at second-look operations where small clusters of ovarian cancer cells persist in scars on the surface of the peritoneal cavity.20 Our preclinical and patient-derived data support the hypothesis that autophagy favors survival of dormant drug resistant ovarian cancer cells and that inhibition of autophagy delays outgrowth and prolongs overall survival of nude mice with dormant human ovarian cancer xenografts.15, 20 Conversely, excessive autophagy can eliminate dormant ovarian cancer cells. Observations with our xenograft model suggest that dormant, autophagic cancer cells that have not yet established a vascular supply can be eliminated with bevacizumab that removes the VEGF required to protect dormant cancer cells from excessive autophagy. Our study raises the possibility that this is an important effect of bevacizumab, independent of its anti-vascular activity. Thus, patients with minimal residual disease might receive benefit from bevacizumab therapy, alone or in combination with monoclonal antibodies against IL-8 and/or IGFR.
Supplementary Material
Acknowledgements
This work was supported by NCI R01 CA135354, the Cancer Prevention and Research Institute of Texas RP110595-P1, the MD Anderson SPOREs in Ovarian Cancer NCI P50 CA83639 and NCI P50 CA217685 and the Shared Resources of the MD Anderson CCSG grant NCI P30 CA16672, The National Foundation for Cancer Research, the philanthropic support from generous donations from Stuart and Gaye-Lynn Zarrow, the Mossy Foundation and the Roberson endowment.
Footnotes
The authors declare no potential conflicts of interest
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2017. CA: A Cancer Journal for Clinicians. 2017;67: 7–30. [DOI] [PubMed] [Google Scholar]
- 2.Marchetti C, Pisano C, Facchini G, et al. First-line treatment of advanced ovarian cancer: current research and perspectives. Expert review of anticancer therapy. 2010;10: 47–60. [DOI] [PubMed] [Google Scholar]
- 3.Damak T, Chargui R, Ben Hassouna J, Hechiche M, Rahal K. Results of second-look laparotomy in advanced ovarian cancer: one single center experience. ISRN Obstet Gynecol. 2012;2012: 849518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bookman MA. Dose-dense chemotherapy in advanced ovarian cancer. Lancet. 2009;374: 1303–1305. [DOI] [PubMed] [Google Scholar]
- 5.Ushijima K Treatment for recurrent ovarian cancer-at first relapse. J Oncol. 2010;2010: 497429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Feng W, Lu Z, Luo RZ, et al. Multiple histone deacetylases repress tumor suppressor gene ARHI in breast cancer. International Journal of Cancer. 2007;120: 1664–1668. [DOI] [PubMed] [Google Scholar]
- 7.Lu H, Li G, Liu L, Feng L, Wang X, Jin H. Regulation and function of mitophagy in development and cancer. Autophagy. 2013;9: 1720–1736. [DOI] [PubMed] [Google Scholar]
- 8.Yu Y, Fujii S, Yuan J, et al. Epigenetic regulation of ARHI in breast and ovarian cancer cells. Annals of the New York Academy of Sciences. 2003;983: 268–277. [DOI] [PubMed] [Google Scholar]
- 9.Rosen DG, Wang L, Jain AN, et al. Expression of the tumor suppressor gene ARHI in epithelial ovarian cancer is associated with increased expression of p21WAF1/CIP1 and prolonged progression-free survival. Clinical Cancer Research. 2004;10: 6559–6566. [DOI] [PubMed] [Google Scholar]
- 10.Badgwell DB, Lu Z, Le K, et al. The tumor-suppressor gene ARHI (DIRAS3) suppresses ovarian cancer cell migration through inhibition of the Stat3 and FAK/Rho signaling pathways. Oncogene. 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bao JJ, Le XF, Wang RY, et al. Reexpression of the tumor suppressor gene ARHI induces apoptosis in ovarian and breast cancer cells through a caspase-independent calpain-dependent pathway. Cancer Research. 2002;62: 7264–7272. [PubMed] [Google Scholar]
- 12.Chen MY, Liao WS, Lu Z, et al. Decitabine and suberoylanilide hydroxamic acid (SAHA) inhibit growth of ovarian cancer cell lines and xenografts while inducing expression of imprinted tumor suppressor genes, apoptosis, G2/M arrest, and autophagy. Cancer. 2011;117: 4424–4438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Feng W, Marquez RT, Lu Z, et al. Imprinted tumor suppressor genes ARHI and PEG3 are the most frequently down-regulated in human ovarian cancers by loss of heterozygosity and promoter methylation. Cancer. 2008;112: 1489–1502. [DOI] [PubMed] [Google Scholar]
- 14.Li J, Cui G, Sun L, et al. ARHI overexpression induces epithelial ovarian cancer cell apoptosis and excessive autophagy. Int J Gynecol Cancer. 2014;24: 437–443. [DOI] [PubMed] [Google Scholar]
- 15.Lu Z, Luo RZ, Lu Y, et al. The tumor suppressor gene ARHI regulates autophagy and tumor dormancy in human ovarian cancer cells. Journal of Clinical Investigation. 2008;118: 3917–3929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lu Z, Luo RZ, Peng H, et al. Transcriptional and posttranscriptional down-regulation of the imprinted tumor suppressor gene ARHI (DRAS3) in ovarian cancer. Clinical Cancer Research. 2006;12: 2404–2413. [DOI] [PubMed] [Google Scholar]
- 17.Lu Z, Yang H, Sutton MN, et al. ARHI (DIRAS3) induces autophagy in ovarian cancer cells by downregulating the epidermal growth factor receptor, inhibiting PI3K and Ras/MAP signaling and activating the FOXo3a-mediated induction of Rab7. Cell Death and Differentiation. 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nishimoto A, Yu Y, Lu Z, et al. A Ras homologue member I directly inhibits signal transducers and activators of transcription 3 translocation and activity in human breast and ovarian cancer cells. Cancer Research. 2005;65: 6701–6710. [DOI] [PubMed] [Google Scholar]
- 19.Yu Y, Xu F, Peng H, et al. NOEY2 (ARHI), an imprinted putative tumor suppressor gene in ovarian and breast carcinomas. Proceedings of the National Academy of Sciences of the United States of America. 1999;96: 214–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lu Z, Baquero MT, Yang H, et al. DIRAS3 regulates the autophagosome initiation complex in dormant ovarian cancer cells. Autophagy. 2014;10: 1071–1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Strzyz P Autophagy: Membrane contacts lend a hand. Nat Rev Mol Cell Biol. 2017;18: 404–405. [DOI] [PubMed] [Google Scholar]
- 22.Kaur J, Debnath J. Autophagy at the crossroads of catabolism and anabolism. Nat Rev Mol Cell Biol. 2015;16: 461–472. [DOI] [PubMed] [Google Scholar]
- 23.Fuchs Y, Steller H. Live to die another way: modes of programmed cell death and the signals emanating from dying cells. Nat Rev Mol Cell Biol. 2015;16: 329–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Warr MR, Binnewies M, Flach J, et al. FOXO3A directs a protective autophagy program in haematopoietic stem cells. Nature. 2013;494: 323–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fullgrabe J, Heldring N, Hermanson O, Joseph B. Cracking the survival code: autophagy-related histone modifications. Autophagy. 2014;10: 556–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fullgrabe J, Klionsky DJ, Joseph B. The return of the nucleus: transcriptional and epigenetic control of autophagy. Nat Rev Mol Cell Biol. 2014;15: 65–74. [DOI] [PubMed] [Google Scholar]
- 27.Settembre C, Di Malta C, Polito VA, et al. TFEB links autophagy to lysosomal biogenesis. Science. 2011;332: 1429–1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yonekawa T, Gamez G, Kim J, et al. RIP1 negatively regulates basal autophagic flux through TFEB to control sensitivity to apoptosis. EMBO Rep. 2015;16: 700–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Settembre C, Ballabio A. TFEB regulates autophagy: an integrated coordination of cellular degradation and recycling processes. Autophagy. 2011;7: 1379–1381. [DOI] [PubMed] [Google Scholar]
- 30.Chaanine AH, Kohlbrenner E, Gamb SI, et al. FOXO3a regulates BNIP3 and modulates mitochondrial calcium, dynamics, and function in cardiac stress. Am J Physiol Heart Circ Physiol. 2016;311: H1540–H1559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jain N, Mishra R, Ganesh S. FoxO3a-mediated autophagy is down-regulated in the laforin deficient mice, an animal model for Lafora progressive myoclonus epilepsy. Biochemical and Biophysical Research Communications. 2016;474: 321–327. [DOI] [PubMed] [Google Scholar]
- 32.Shin HJ, Kim H, Oh S, et al. AMPK-SKP2-CARM1 signalling cascade in transcriptional regulation of autophagy. Nature. 2016;534: 553–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang R, Yang Q, Wang X, et al. FoxO3alpha-mediated autophagy contributes to apoptosis in cardiac microvascular endothelial cells under hypoxia. Microvascular Research. 2016;104: 23–31. [DOI] [PubMed] [Google Scholar]
- 34.Gao N, Zhang Z, Jiang BH, Shi X. Role of PI3K/AKT/mTOR signaling in the cell cycle progression of human prostate cancer. Biochemical and Biophysical Research Communications. 2003;310: 1124–1132. [DOI] [PubMed] [Google Scholar]
- 35.Engelman JA. Targeting PI3K signalling in cancer: opportunities, challenges and limitations. Nat Rev Cancer. 2009;9: 550–562. [DOI] [PubMed] [Google Scholar]
- 36.Chang F, Lee JT, Navolanic PM, et al. Involvement of PI3K/Akt pathway in cell cycle progression, apoptosis, and neoplastic transformation: a target for cancer chemotherapy. Leukemia. 2003;17: 590–603. [DOI] [PubMed] [Google Scholar]
- 37.Asati V, Mahapatra DK, Bharti SK. PI3K/Akt/mTOR and Ras/Raf/MEK/ERK signaling pathways inhibitors as anticancer agents: Structural and pharmacological perspectives. European journal of medicinal chemistry. 2016;109: 314–341. [DOI] [PubMed] [Google Scholar]
- 38.Arcaro A, Guerreiro AS. The phosphoinositide 3-kinase pathway in human cancer: genetic alterations and therapeutic implications. Curr Genomics. 2007;8: 271–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Roberts PJ, Der CJ. Targeting the Raf-MEK-ERK mitogen-activated protein kinase cascade for the treatment of cancer. Oncogene. 2007;26: 3291–3310. [DOI] [PubMed] [Google Scholar]
- 40.Sosa MS, Bragado P, Aguirre-Ghiso JA. Mechanisms of disseminated cancer cell dormancy: an awakening field. Nat Rev Cancer. 2014;14: 611–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Aguirre-Ghiso JA. Models, mechanisms and clinical evidence for cancer dormancy. Nat Rev Cancer. 2007;7: 834–846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Senft D, Ronai ZE. Adaptive Stress Responses During Tumor Metastasis and Dormancy. Trends Cancer. 2016;2: 429–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang K, Jiang W, Cheng C, Li Y, Tu M. Cell dormancy and tumor refractory. Anti-cancer agents in medicinal chemistry. 2013;13: 1312–1316. [PubMed] [Google Scholar]
- 44.Wang SH, Lin SY. Tumor dormancy: potential therapeutic target in tumor recurrence and metastasis prevention. Exp Hematol Oncol. 2013;2: 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Washington MN, Suh G, Orozco AF, et al. ARHI (DIRAS3)-mediated autophagy-associated cell death enhances chemosensitivity to cisplatin in ovarian cancer cell lines and xenografts. Cell Death Dis. 2015;6: e1836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Buck E, Gokhale PC, Koujak S, et al. Compensatory insulin receptor (IR) activation on inhibition of insulin-like growth factor-1 receptor (IGF-1R): rationale for cotargeting IGF-1R and IR in cancer. Molecular cancer therapeutics. 2010;9: 2652–2664. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.