Abstract
Eukaryotic cells have evolved a myriad of ion channels, transporters, and pumps to maintain and regulate transmembrane ion gradients. As intracellular parasites, viruses also have evolved ion channel proteins, called viroporins, which disrupt normal ionic homeostasis to promote viral replication and pathogenesis. The first viral ion channel (influenza M2 protein) was confirmed only 23 years ago, and since then studies on M2 and many other viroporins have shown they serve critical functions in virus entry, replication, morphogenesis, and immune evasion. As new candidate viroporins and viroporin-mediated functions are being discovered, we review the experimental criteria for viroporin identification and characterization to facilitate consistency within this field of research. Then we review recent studies on how the few Ca2+-conducting viroporins exploit host signaling pathways, including store-operated Ca2+ entry, autophagy, and inflammasome activation. These viroporin-induced aberrant Ca2+ signals cause pathophysiological changes resulting in diarrhea, vomiting, and proinflammatory diseases, making both the viroporin and host Ca2+ signaling pathways potential therapeutic targets for antiviral drugs.
Keywords: viroporin, calcium homeostasis, store-operated calcium entry, autophagy, inflammasome
HISTORY OF VIROPORINS
Ion channels are transmembrane pore-forming proteins that gate the flow of ions across the membrane through distinct open and closed states and thereby regulate a wide range of critical cellular functions, such as establishing the resting membrane potential, controlling the flow of ions through epithelial cells to regulate fluid absorption and secretion, and shaping action potentials for muscle contraction and nerve transmission. Ion channel proteins are found in all kingdoms of life, and therefore it is no surprise that viruses have also evolved their own ion channels to subvert the host cell’s normal ion gradients to facilitate virus replication and pathogenesis.
The concept of virus-encoded pore-forming proteins was first introduced after recognition that membrane leakiness can develop after virus infection, and the idea was proposed that viral proteins can create holes in cellular membranes through which ions and low-molecular-weight compounds can freely pass (1). Initially, these were thought to be relatively nonspecific pores formed by a viral protein and capable of passing compounds as large as 750 Da, therefore potentially functioning during late stages of viral replication to induce cell lysis. Subsequently, the term viroporin was proposed to describe virus-encoded pore-forming proteins, primarily the alphavirus 6K and poliovirus 2B and 3A proteins (2). Initially, viroporins were mainly identified using Escherichia coli permeability assays (reviewed in detail below), wherein inducible expression of viroporins allows release of small molecules (e.g., preloaded radiolabeled nucleotides) or entry of antibiotics (e.g., hygromycin B) that are normally excluded (3, 4). At about the same time, the influenza A virus (IAV) M2 protein was predicted to be an ion channel due to its sensitivity to amantadine, a drug that was shown to block both the entry and assembly/release stages of IAV infection (5). Voltage-clamp electrophysiology experiments using M2 expression in Xenopus oocytes showed that M2 carries an electrical current across the plasma membrane (PM), confirming that M2 is a bona fide ion channel (5, 6). The discrete ion channel activity of M2 compared with the broader permeability induced by other viroporins suggested they were functionally different classes of viral proteins, but when tested in the E. coli permeability assay, M2 showed the same permeability-inducing activity as the previously characterized viroporins (7). Subsequent electrophysiology studies on additional viroporins [e.g., alphavirus 6K and human immunodeficiency virus type 1 (HIV-1) Vpu] showed that these proteins function as bona fide ion channels with distinct open and closed conductance states rather than simply causing perturbation of membrane integrity or forming unregulated pores (8, 9). Thus, from distinct roots in both electrophysiology and biochemistry, the viroporin field has converged to study the structure, channel activity, and biological functions of these unconventional ion channels.
The viroporin field is still in a relatively early stage, with most of the currently characterized viroporins having been identified in the past 10 years and from a diverse range of viruses (Table 1) (10, 11). Currently characterized viroporins are primarily found in mammalian viruses, with only a few examples from plant viruses (chlorella virus Kcv) and from arboviruses that infect insects as well as mammalian hosts (togavirus 6K, orbivirus NS3, ephemerovirus α−1) (12). No other viroporins are reported from plant viruses, and none have been identified in protozoa viruses, archaeal phages, or bacteriophages, likely due to a lack of searching rather than an absence of viroporins in these virus families. Further, the majority of characterized viroporins are specific proton (H+) channels (e.g., IAV M2) or nonspecific monovalent cation (Na+ or K+) channels (e.g., alphavirus 6K, HIV-1 Vpu); only a few viroporins show divalent cation (Ca2+) flux ( e.g., rotavirus NSP4), and there have been no reports of anion selectivity over cations (see Table 1) (10, 11). Because ion homeostasis is a fundamental aspect of cell physiology, we anticipate the viroporin field will continue to grow as more viroporins are discovered, which will be facilitated by establishing the basic tenets of viroporin biology and continuing to characterize the diversity of viroporin structure and function.
Table 1.
List of currently recognized viroporins
| Virus family | Virus(es) | Viroporin | Class | Ion conductance | Reference(s) |
|---|---|---|---|---|---|
| Orthomyxoviridae | Influenza A virus | M2 | IA | H+, K+ | 5 |
| PB1-F2 | ND | MVC, Ca2+ | 25 | ||
| Influenza B virus | BM2 | IA | H+, K+ | 111 | |
| Picornaviridae | Poliovirus, coxsackievirus 3B, human rhinovirus, encephalomyocarditis virus, enterovirus 71 | 2B/2BC | IIB | Ca2+ | 17, 34, 38, 46 |
| Poliovirus | 3A | IB | MVC | 4 | |
| Togaviridae | Sindbis virus, Semliki Forest virus, Ross River virus | 6K | IIB | MVC, Ca2+ | 8,94,96 |
| Retroviridae | Human immunodeficiency virus type 1 | Vpr | ND | MVC | 112 |
| Vpu | IA | MVC | 9, 113 | ||
| Human T lymphotropic virus 1 | P13II | IA | K+ | 114 | |
| Flaviviridae | Hepatitis C virus, bovine viral diarrhea virus, classical swine fever virus | p7 | IIA | H+, MVC | 116, 117 |
| Coronaviridae | Severe acute respiratory syndrome coronavirus, human coronavirus 229E, mouse hepatitis virus, infectious bronchitis virus, feline infectious peritonitis virus | Envelope (E) | IA | MVC | 118, 119 |
| Severe acute respiratory syndrome coronavirus | ORF3a | IIIA | K+, Ca2+ | 19 | |
| Human coronavirus 229E | ORF4a | IIIA | MVC | 120 | |
| Reoviridae | Rotavirus (serogroup A) | NSP4 | IIIA | MVC, Ca2+ | 18, 35 |
| Avian reovirus | p10 | IA | ND | 121 | |
| Polyomaviridae | JC virus | Agnoprotein | ND | Ca2+ | 36 |
| Simian virus 40 | VP2 | ND | ND | 122 | |
| VP3 | ND | ND | 122 | ||
| VP4 | ND | ND | 123 | ||
| Papillomaviridae | Human papillomavirus | E5 | IIIB | H+ | 124 |
| Rhabdoviridae | Bovine ephemeral fever virus | α1 | IA | ND | 125 |
| Phycodnaviridae | Paramecium bursaria chlorella virus, Acanthocystis turfacae chlorella virus | Kcv | IIB | K+ | 126 |
Abbreviations: MVC, monovalent cation; ND, not determined.
The functional roles of only a few viroporins have been characterized in detail; the proton channel activity of IAV M2 has been the most extensively studied, making M2 the best characterized viroporin (13, 14). As more viroporins are identified and characterized, new viroporin-mediated functions are being elucidated, and currently, four categories of viroporin functions are known: (a) virus entry, (b) virus morphogenesis and release, (c) modulation of apoptosis, and (d) disruption in Ca2+ homeostasis. In general, viroporins within categories a–c are proton channels or nonspecific monovalent cation channels, and several other review articles have detailed these functional properties of viroporins (see 3, 10–12, 15). In contrast, only a few viroporins have been shown to directly disrupt Ca2+ homeostasis, and although this group of viroporins has been studied less extensively, recent work has begun providing insight into the functions and consequences of this viroporin subgroup (16, 17). This article is targeted to the novice viroporin hunter as a practical guide for viroporin identification and functional characterization, as well as to the seasoned viroporin researcher as a review of newly identified biological functions of Ca2+-conducting viroporins and their role in viral pathogenesis.
IDENTIFICATION AND FUNCTIONAL CHARACTERIZATION OF CANDIDATE VIROPORINS
Identification of Candidate Viroporins
A major challenge to identifying viroporins stems from a lack of both nucleotide and protein sequence homology among viroporins from different viruses; however, viroporins do share some common structural characteristics and signature motifs that have become the basis for viroporin identification. First, viroporins are small integral membrane proteins, typically around 100 aa long but occasionally smaller [e.g., severe acute respiratory syndrome coronavirus ( SARS-CoV ) ORF8, 39 aa] or larger [e.g., rotavirus nonstructural protein 4 (NSP4), 175 aa; SARS-CoV ORF3, 274 aa] (18–20). Second, viroporins generally are hydrophobic and are predicted to be largely α-helical proteins that readily oligomerize. Third, viroporins contain two signature motifs: (a) an amphipathic α-helix that forms the aqueous channel and (b) an adjacent cluster of positively charged residues (i.e., lysine or arginine) that anchor the viroporin in the membrane by interacting with the negatively charged phosphate head groups of surrounding lipids. Because these motifs are necessary for proper assembly of a stable transmembrane pore through which ions can pass, the amphipathic α-helix and clustered basic residues are essential for viroporin function; typically, deletion or mutation of these residues abolishes viroporin activity (3).
As more viroporins are identified, efforts are being made to classify them based on structural and/or predicted functional activities. A recently established classification system groups viroporins based on the number an dorientation of the transmembrane segments(10).Aviroporinclass is defined by the number of transmembrane segments, and a subclass is defined by whether the N terminus is in the endoplasmic reticulum (ER) lumen (Subclass A) or cytoplasm (Subclass B). For example,IAVM2 is a Class 1A viroporin, because it has one transmembrane segment and the N terminus is in the ER, but picornavirus 2B is a Class IIB viroporin, because it is has two transmembrane segments and the N terminus is in the cytoplasm (Table 1, column 4) (10). Thus, the signature viroporin motifs and the viroporin classification system can be used as initial tools for the identification of a putative viroporin domain that can then be tested for viroporin function in a biological assay.
Characterization of Viroporin Activity
Once a candidate viroporin is identified, a wide variety of functional assays can be used to test for viroporin activity. The most commonly used assays measure the viroporin’s ability to induce PM permeability upon recombinant expression in either E. coli or mammalian cells (reviewed in 2, 21).
Viroporin permeability assays.
One hallmark of viroporins is their ability to induce general membrane permeability when overexpressed in either E. coli or eukaryotic cells, or when the purified viroporin is incorporated in to a synthetic liposome membrane. Researchers have capitalizedon this property to develop a range of permeability assays that are used to assess viroporin functions, test mutations that are expected to disrupt viroporin functions, and screen for viroporin-blocking small molecules that can be developed into antiviral drugs.
Cell permeability assays.
Generally, viroporin cell permeability assays employ inducible expression in either E. coli or mammalian cells. For E. coli assays, the viroporin is produced from an inducible expression vector [e.g., an isopropyl β-D-1-thiogalactopyranoside ( IPTG)-inducible expression vector], and upon induction of viroporin expression, the PM becomes permeable to small molecules that cannot typically cross, which can then be assayed in a variety of ways. Two examples of E. coli permeability assays are as follows: (a) Cells can be loaded with 3H-uridine prior to being induced, and leak of 3H-uridine into the media is then measured. (b) Upon induction of viroporin expression, cells can be treated with hygromycin B (a translation inhibitor) in the presence of 35S-methionine to measure the rate of protein synthesis. Increased viroporin activity allows hygromycin B to enter the cell, thereby causing decreased 35S-methionine incorporation (4, 22). Other small molecules that have been used include β-galactosidase substrates, fluorescent molecules (FITC dextran, propidium iodide), and fluorescent redox sensors (4, 23–27). Mammalian cell permeability assays are conducted in a similar fashion, such that virus infection or viroporin expression increases the cell’s permeability to small molecules. For mammalian cells, use of translation inhibitors (e.g., hygromycin B) and 35S-methionine labeling of protein synthesis or fluorescent molecules (e.g., propidium iodide) are the most commonly employed techniques (21).
These permeability assays measure nonspecific alterations in membrane integrity. Therefore, to demonstrate specificity to viroporin activity rather than cell stress or cytotoxicity from overexpression of transmembrane proteins, experiments must also be done to test for activity of the candidate viroporin bearing mutations of the amphipathic α-helix and clustered basic residues (28). These relatively simple assays serve as an important foundation for understanding viroporin structure/function relationships, as a rapid means for screening for mutations that disrupt viroporin activity, and as a basis for drug screens to identify compounds that may act as viroporin channel inhibitors (23).
Liposome permeability assays.
An alternative to cell-based assays is a synthetic liposome permeability assay. In this assay, synthetic liposomes, which are single-bilayer liposomes made from (commercially available) purified lipids, are produced in the presence of self-quenching concentrations of a fluorescent dye, such as carboxyfluorescein. When the viroporin is added to the liposome, it permeabilizes the liposome, causing increased fluorescence due to unquenching of the released dye (29). This assay has been successfully used to assess viroporin function and to screen for antiviral drugs that target viroporins (30). The primary limitation to this assay is that either the candidate viroporin has to be small enough (<100 aa) that a peptide can be synthesized or a system for recombinant expression and purification must have been established. Variations in the liposome assays have also been used to study ion-specific conductance by the IAV M2 proton channel, to show Ca2+ and anion conductance by the PB1-F2 viroporin, and to screen for K+ conductance by the respiratory syncytial virus (RSV) small hydrophobic (SH) viroporin (25, 27, 30). Despite the utility of the cell and liposome permeability assays, these do not measure biologically relevant permeability. Therefore, more sophisticated methods are needed to confirm that the candidate viroporin domain either has bona fide ion channel activity (using electrophysiology) or specifically disrupts ion homeostasis in the context of virus infection (using fluorescent ion imaging).
Viroporin functional assays.
Although permeability assays are important tools for initial viroporin studies, they measure relatively nonspecific permeability, and without more specific functional studies a candidate protein cannot be defined as a bona fide viroporin. There are several types of assays used to examine viroporin functions, primarily direct ion channel studies using electrophysiology and indirect measurement of specific ion conductance using fluorescent ion indicators.
Electrophysiology.
Electrophysiology techniques measure ion conductance by pores and channels through membranes, and this remains the gold standard for demonstrating and characterizing a protein’s ion channel activity. The two electrophysiology techniques used to study viroporins are whole-cell voltage clamp (commonly referred to as patch clamp) and planar lipid bilayer. Several viroporins have been studied extensively by electrophysiology (reviewed in 31), and in general these studies have confirmed that viroporins are true ion channels, in that they have discrete conductance patterns with defined open and closed states (Figure 1), and that some viroporins are gated by biological conditions, such as low-pH activation by IAV M2 (5).
Figure 1.
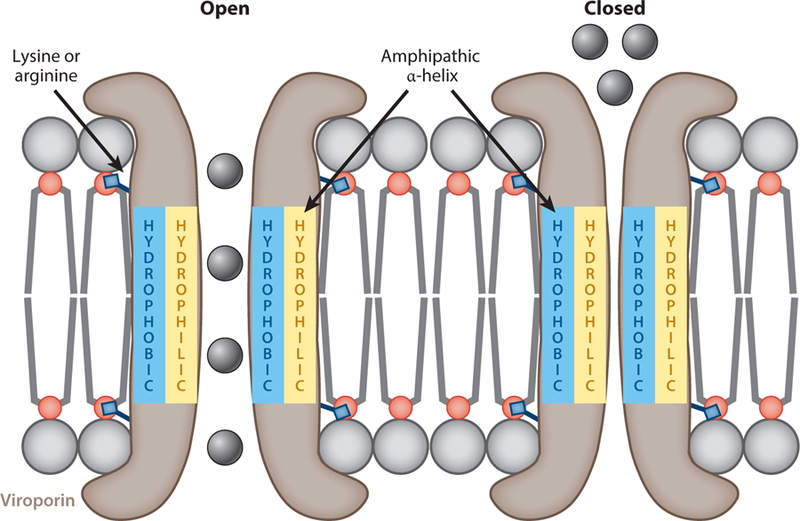
Illustration of viroporin motifs and conductance states. A viroporin (brown) is shown inserted into a lipid bilayer. Positively charged residues such as lysine or arginine anchor the viroporin into the membrane. Upon oligomerization, the viroporin’s amphipathic α-helix creates the aqueous channel for ion conductance. When the channel is in the open conformation (left), ions are conducted through the pore; however, conformational changes can cause the pore to close (right), preventing the flow of ions through the pore.
Fluorescent imaging.
Some viroporin effects are mediated through changes in cell signalling pathways, as illustrated by the putative Ca2+-conducting viroporins (Table 1). Ca2+ is a ubiquitous secondary messenger in eukaryotic cells, and the flux of Ca2+ into and out of the cytoplasm is a major mode for eukaryotic cell signal transduction (32). The importance of Ca2+ as a signal transduction molecule has resulted in the development of sensitive fluorescent indicators to detect the rapid changes in the concentration of cytoplasmic calcium ([Ca2+]c) that occur during Ca2+ release from the ER or entry into cells through PM-localized Ca2+ channels. The availability of fluorescent Ca2+ sensors has made live cell Ca2+ imaging a fundamental assay to study the putative Ca2+-conducting viroporins. Initial studies of the rotavirus NSP4 and picornavirus 2B proteins (primarily from coxsackievirus 3B and poliovirus) utilized acetoxymethyl (AM) ester Ca2+-sensitive fluorescent dyes, including Quin2, Fura-2, and Fluo-3, which must be loaded into host cells, followed by fluorescence emission measurements by a spectrofluorometer, fluorescence microscope, or flow cytometer to quantitate [Ca2+]c (33, 34). More recent studies have used genetically encoded Ca2+ indicators (GECIs) that exhibit a bioluminescent or fluorescent response to changes in [Ca2+]c (35, 36). GECIs are recombinantly engineered hybrid proteins that combine a fluorescent protein with a specific Ca2+-binding site such that the spectral properties of the fluorescent protein are modified upon Ca2+ binding. For example, a popular GECI is GCaMP6s, in which calmodulin (a Ca2+-binding protein) and M13 (a calmodulin target peptide) are inserted at opposite termini of a destabilized green fluorescent protein (GFP). GCaMP6s is expressed in the cytoplasm, and when a Ca2+ flux occurs, Ca2+ binds calmodulin, and calmodulin then binds the M13 peptide, which stabilizes the chromophore and increases the fluorescence emission. Because GECIs are proteins, they can be expressed from standard mammalian expression vectors and targeted to Ca2+ storage organelles such as the ER or Golgi using canonical host cell sorting signals. This enables specific measurement of viroporin-induced changes within these compartments [concentration of calcium in the ER ([Ca2+]ER) and concentration of calcium in the Golgi ([Ca2+]Golgi) (37,38)]. Ca2+ indicators and GECIs can be used to measure viroporin-induced changes to steady-state [Ca2+]c (e.g., infected cells have a higher mean fluorescence intensity from the indicator). Additionally, changes in the Ca2+ indictor’s fluorescence upon either (a) addition of a sarco/endoplasmic reticulum calcium ATPase (SERCA) pump inhibitor (e.g., thapsigargin) or (b) increasing the concentration of extracellular Ca2+ are used to measure Ca2+ permeability of the ER and PM (see below). In addition to Ca2+ indicators, pH-sensitive fluorescent dyes such as LysoSensor Yellow/Blue DND-160 can be used to measure Golgi alkalinization due to H+ flux out of the Golgi (39).
Experimental demonstration of viroporin function.
The assays summarized above have identified several new proteins as viroporins (Table 1), yet the field still lacks a consensus of what criteria must be met to definitively characterize a viroporin. However, two critical pieces of evidence are known to be needed to establish a protein as a viroporin. First, the viroporin activity of the candidate viroporin domain should be demonstrated, and specificity of this activity should be demonstrated by its loss following mutation of the signature viroporin motifs (amphipathic α-helix and clustered basic residues) (18). This is typically accomplished using one of the permeability assays presented above. Additionally, a small molecule inhibitor of viroporin activity (typically identified using one of the bacterial or liposome permeability assays described above) can be used to demonstrate specificity for viroporin function (23). Second, it is critical to use electrophysiology and/or fluorescent imaging to demonstrate that the viroporin domain is responsible for ion conductance or alteration of ion gradients in cells, and that this activity is prevented by the same mutations or drugs that abolish viroporin activity in the permeability assays. Fulfilling both of these criteria establishes a firm experimental basis to classify a candidate protein as a viroporin and provide a framework for further biological characterization of the viroporin’s role during the virus replication cycle. Candidate viroporins (Table 2) are proteins that have viroporin structural characteristics and satisfy some of these criteria but require additional studies to confirm viroporin activity.
Table 2.
List of candidate viroporins
| Virus family | Virus(es) | Viroporin | Class | Ion conductance | Reference(s) |
|---|---|---|---|---|---|
| Orthomyxoviridae | Influenza B virus | NB | ND | ND | 127 |
| Influenza C virus | CM2 | IA | MVC | 97 | |
| Togaviridae | Sindbis virus, chikungunya virus | TF | IA | ND | 128 |
| Retroviridae | HIV-1 | gp41 (LLP domains) | ND | ND | 129 |
| HTLV-1 | p12I | IA | Ca2+? | 105 | |
| Flaviviridae | GB virus B | p13 | IVA | ND | 130, 131 |
| Dengue virus | preM/M | IIA | MVC | 132 | |
| Japanese encephalitis virus | NS2 | ND | ND | 133 | |
| Coronaviridae | Severe acute respiratory syndrome coronavirus | ORF8a | ND | MVC | 20 |
| Reoviridae | Rotavirus (serogroups B and C) | NSP4 | ND | ND | 16, 26 |
| Bluetongue virus | NS3 | IA | ND | 134 | |
| Reovirus | μN1 | ND | ND | 135 | |
| Filoviridae | Ebola virus | δ-peptide | ND | ND | 136 |
| Adenoviridae | Adenovirus 5 | E3/19K | ND | ND | 137 |
| Poxviridae | Vaccinia virus | A38L | VA | Ca2+? | 107 |
| Herpesviridae | Human cytomegalovirus | UL37×1 | IA | Ca2+? | 108, 109 |
Abbreviations: MVC, monovalent cation; ND, not determined.
VIROPORIN-MEDIATED DISRUPTION OF CALCIUM HOMEOSTASIS
As mentioned above, viroporin functions can be divided into four broad categories: (a) virus entry, (b) virus morphogenesis and release, (c) modulation of apoptosis, and (d) disruption in Ca2+ homeostasis. Despite Ca2+ being a ubiquitous secondary messenger critical for most cellular processes,only a few Ca2+-conducting viroporins have been identified, and this group of viroporins has been studied less extensively (16, 17). The best-characterized Ca2+-conducting viroporins are rotavirus NSP4 and picornavirus 2B/2BC, and recent studies defining the mechanisms by which viroporin-mediated disruption of host Ca2+ signaling contributes to virus replication and disease are considered here.
Rotavirus was one of the first virus systems shown to specifically elevate [Ca2+]c prior to overall cell permeabilization. Shortly after this finding, the ability to elevate [Ca2+]c was evaluated by individual expression of all the rotavirus proteins in Sf9 insect cells, and this phenotype was induced by the single rotavirus protein (NSP4) (33, 40). Subsequently, the consequences of NSP4-mediated disruption in Ca2+ were characterized (41, 42), but despite nearly 15 years of speculation that NSP4 could be a viroporin, identification and validation of the NSP4 viroporin domain were only recently completed (18, 26). In contrast, poliovirus 2B/2BC was one of the first viroporins identified, by Luis Carrasco—who also coined the term viroporin—based on its ability to permeabilize E. coli and mammalian cell membranes (22, 43, 44). Soon thereafter, the rapid elevation in [Ca2+]c by poliovirus was described, and it was shown that individual expression of the 2B protein of coxsackievirus (strain 3B) and expression of the 2B/2BC protein of poliovirus result in similar phenotypes (17, 34, 45). Disruption of [Ca2+]c also has been reported for other picornavirus 2B proteins (38, 46, 47). Thus NSP4 and 2B are the prototype members of the Ca2+-disrupting viroporins, and despite a lack of sequence homology, the NSP4and 2B-induced alterations in Ca2+ homeostasis share many characteristics (summarized in Figure 2). Recent studies show that by targeting intracellular Ca2+ stores, the viroporin activity of NSP4 and 2B is adapted to exploit and/or perturb multiple host pathways, which are summarized below.
Figure 2.
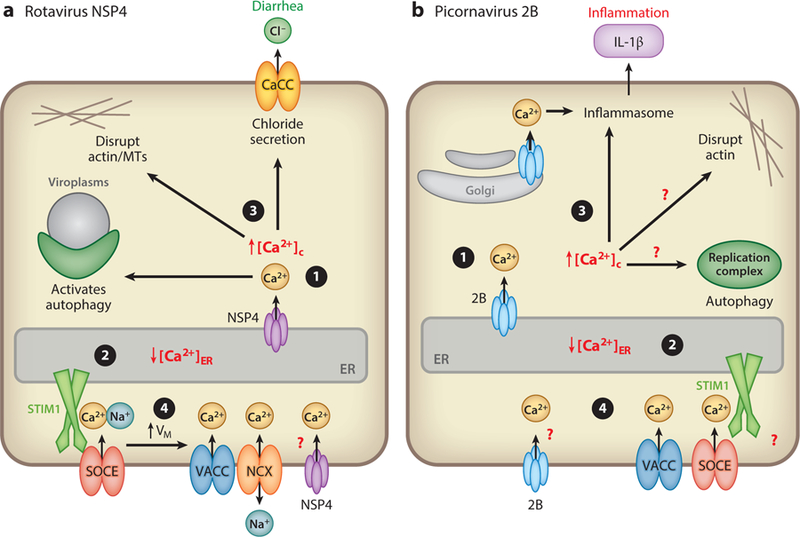
Viroporin-mediated disruption in calcium signaling by NSP4 and 2B. Changes to host cell Ca2+ homeostasis induced by (a) rotavirus NSP4 and (b) picornavirus 2B, with potential changes in Ca2+ or viroporin-induced processes indicated by red question marks. Similarities between NSP4− and 2B-induced changes include (❶) release of endoplasmic reticulum (ER) Ca2+ (NSP4) or ER/Golgi Ca2+ (2B), (❷) decreased ER calcium levels (↓[Ca2+]ER), (❸) increased cytoplasmic calcium levels (↑[Ca2+]c), and (❹) activation of plasma membrane channels. It is not known whether NSP4 or 2B directly conducts Ca2+ across the plasma membrane. Rotavirus NSP4 activates store-operated Ca2+ entry (SOCE) and the sodium-calcium exchanger (NCX), and both NSP4 and 2B activate voltage-activated Ca2+ channels (VACC) due to Ca2+ influx raising the membrane voltage (↑VM). Whether 2B-induced ↓[ Ca2+]ER activates SOCE is unknown. The cellular processes activated due to viroporin-induced ↑[ Ca2+]c include autophagy, disruption of actin and/or microtubules (MTs), induction of chloride secretion, and inflammasome activation of IL-1β secretion.
Effects of NSP4 and 2B on Calcium Homeostasis
Rotavirus NSP4.
Early during rotavirus infection (3–4 hpi), a Ca2+ influx pathway is activated, which results in an elevated steady-state [Ca2+]c concomitant with infectious particle assembly but 4–5 h prior to breakdown of PM integrity (33). The elevated [Ca2+]c is due to increased Ca2+leakage from the ER and Ca2+influx through the PM (16, 48). Expression of NSP4 alone recapitulates all of these changes in Ca2+ homeostasis, RNA interference–mediated silencing of NSP4 abrogates these phenotypes, and the ability to elevate [Ca2+]c is a conserved function of even genetically divergent NSP4 proteins from different rotavirus serogroups (16, 26, 49, 50). The mechanism for elevated [Ca2+]c was predicted to be intrinsic to NSP4 because rotavirus and NSP4 elevate[Ca2+]c in Sf9 insect cells (16) and in a wide range of mammalian cell lines, including human, monkey, and canine kidney cells (HEK293, MA104, COS7, and MDCK); intestinal epithelial cells (Caco-2 and HT-29); and human neuroendocrine enterochromaffin cells responsible for serotonin production in the gastrointestinal tract (33, 48, 51–54).
The relationship between host cell Ca2+ homeostasis and NSP4 is more complex than initially anticipated, because NSP4 has two distinct functional domains related to Ca2+ signaling: the viroporin domain (amino acids ∼47–90) and the enterotoxin domain (amino acids ∼114–135) (18, 55). Exogenous treatment of noninfected cells with NSP4 enterotoxin domain–containing peptides induces chloride secretion from enterocytes due to a phospholipase C ( PLC)-dependent transient increase in [Ca2+]c that requires cell surface receptors, including integrins α1β1 and α2β1 (56–58). In contrast, endogenous expression of full-length NSP4 (as occurs during infection) causes a PLC-independent steady-state increase in [Ca2+]c, similar to what is observed in rotavirus-infected cells (49, 52). Earlier studies on NSP4 identified a membrane-destabilizing domain that permeabilizes E. coli and mammalian cell membranes (59, 60). This NSP4 domain contains the viroporin-identifying motifs: It (a) is predicted to be α-helical, (b) oligomerizes, and (c) has a conserved cluster of five lysine residues (pentalysine domain) and a strong amphipathic α-helix (amphipathic domain) (18). Interestingly, these motifs are found in NSP4 proteins from all of the rotavirus serogroups, though their primary sequences are completely different, indicating NSP4 evolution was constrained more by biochemical function than by primary protein sequence (26). Mutation of these motifs to remove the positive charge or reduce the amphipathicity abolishes membrane permeabilization in E. coli and prevents the steady-state elevation in [Ca2+]c in mammalian cells, indicating that NSP4 viroporin activity is directly responsible for the elevation of [Ca2+]c observed in rotavirus-infected cells (18). Finally, using planar lipid bilayer and liposome patch clamp electrophysiology techniques, we have observed bona fide ion channel activity by a synthetic NSP4 viroporin domain peptide (amino acids 47–90), and the NSP4 channel is permeable to Ca2+, demonstrating NSP4 has intrinsic ion channel function ( J.M. Hyser & A.H. Delcour, unpublished data). These studies are ongoing to assess whether NSP4 is merely permeable to Ca2+ or whether it has selectivity for Ca2+ over other cations, but the ability of the NSP4 viroporin domain to form a Ca2+-permeable channel indicates that NSP4 is directly causing the observed leakiness of the ER membrane and decreased ER Ca2+ stores.
Poliovirus and coxsackievirus 2B/2BC.
Despite a complete lack of sequence similarity, the changes in Ca2+ homeostasis induced by 2B/2BC are strikingly similar to those induced by NSP4. Like rotavirus infection, poliovirus and coxsackievirus infection of HeLa cells rapidly (within 3 hpi) increases Ca2+ influx through the PM, which occurs prior to loss of PM integrity and requires viral protein synthesis (45). Within 1 hpi, there is a ∼35% decrease in ER Ca2+ levels and progressive depletion concomitant with elevation in [Ca2+]c (34). Expression of the coxsackievirus 2B protein alone elevates [Ca2+]c and depletes ER Ca2+ stores, and this is abolished by mutations that disrupt the amphipathic α-helix and polybasic viroporin motifs (34). Infectious cDNA clones of coxsackievirus bearing mutations in the 2B viroporin motifs result in poor virus replication (80–99% smaller plaques) due to deficient RNA replication; however, a direct role for Ca2+ in replication has not been explored (61). Finally, picornavirus 2B localizes to the Golgi compartment, which is also a Ca2+ storage organelle, and significantly depletes Golgi Ca2+ levels and increases the Golgi pH, presumably by release of protons (37, 62). As is the case for NSP4, elevation of [Ca2+]c and depletion of ER Ca2+ is a conserved function of 2 B from enteroviruses (poliovirus and coxsackievirus 3B), rhinoviruses (human rhinovirus 14), and cardioviruses [encephalomyocarditis virus (EMCV)], but not of 2B from a hepatovirus ( hepatitis A virus) (46). De Jong et al. (37) speculate that lack of viroporin activity from the hepatitis A virus 2B protein may be due to the use of normal Golgi-dependent secretion for virus release.
Endoplasmic Reticulum–Localized Viroporins and Store-Operated Calcium Entry
The majority of NSP4 and 2B is localized to the ER and is thought to initially target this Ca2+ store; however, smaller pools of both proteins localize to other subcellular compartments, such as the ER-Golgi intermediate compartment, Golgi, autophagosomal membranes, mitochondria, and PM (34, 37, 63–67). Despite their ER localization, both proteins rapidly induce Ca2+ influx through the PM, and researchers in both field shypothesized this could be due to activation of a cellular process initially called capacitative calcium entry (68). This process was later renamed storeoperated calcium entry (SOCE); it is a homeostatic mechanism used to maintain ER Ca2+ levels by activating Ca2+ influx through the PM subsequent to agonist-induced ER Ca2+ release (69). Despite intensive research since the 1980s, it was not until 2005–2006 that the molecular mechanism for SOCE was elucidated through the identification of the two key proteins in the pathway: stromal interaction molecule 1 (STIM1) and calcium release–activated calcium channel protein 1 (Orai1) (70). Coordination of Ca2+ release from the ER and subsequent Ca2+ influx through the PM are accomplished by STIM1 acting as an ER-localized sensor of the [Ca2+]ER via a low-affinity EF-hand Ca2+-binding site in the STIM1 N-terminal domain within the ER lumen. Decreased [Ca2+]ER causes disassociation of the Ca2+ from the EF-hand, inducing oligomerization/activation of STIM1. Active STIM1 oligomers (which remain in the ER membrane) bind to and activate Orai1 channels in the PM to allow influx of extracellular Ca2+ to be resequestered into the ER and maintain the Ca2+ store (71). Both NSP4 and 2B are poised to exploit this system by leaking ER Ca2+ through formation of Ca2+-permeable channels, thereby decreasing [Ca2+]ER to activate STIM1 and SOCE.
Using HEK293 cells stably expressing yellow fluorescent protein (YFP)-STIM1 as a biosensor of SOCE activation, we found that rotavirus infection and NSP4 expression induce STIM1 oligomerization (detected via fluorescence microscopy as formation of puncta) and that the activated STIM1 puncta localize with Orai1 at the PM (35). Activation of STIM1 and induction of Ca2+ entry through the PM are abolished in cells expressing a NSP4 viroporin–defective mutant, demonstrating SOCE is a direct consequence of NSP4 viroporin activity. Finally, RNA interference–mediated knockdown of STIM1 significantly reduces rotavirus yield, demonstrating that activation of SOCE is critical for rotavirus replication (35). Induction of SOCE and activation of Orai1 by NSP4 fit well with the prior characterization of rotavirus-induced Ca2+ entry through the PM: (a) The channels are activated at ∼3 hpi, a time when NSP4 is able to be detected in the ER; (b) the influx channels are permeable to Ca2+, Sr2+, and Ba2+ but are blocked by Ni2+, La3+, and Gd3+, which is also the case for Orai1 (48, 70). However, whereas the commonly used Orai1 blocker 2-APB inhibits NSP4-induced influx, other non-Orai1 blockers are also effective, including KB-R7943 [a sodium/calcium exchanger (NCX) reverse mode and TRPC channel blocker] and D600 [an L-type voltage-activated calcium channel ( VACC ) blocker], suggesting other channels may also be involved in Ca2+ entry into rotavirus-infected cells (48, 54). Whereas SOCE and NCX are ubiquitously expressed, VACC channel expression is more restricted to neuroendocrine cells (e.g., pancreatic β-cells), neurons, and muscle cells, suggesting rotavirus/NSP4 effects on Ca2+ entry may show cell type differences depending on channel expression and abundance. Nevertheless, because STIM1 activation and Ca2+ influx are not observed with the NSP4 viroporin–defective mutant, activation of host channels remains a consequence of viroporin activity irrespective of which channels are involved (35).
However, depletion of ER Ca2+ by NSP4 was initially controversial, because studies using 45Ca2+ loading into rotavirus-infected cells showed an increase rather than a decrease in [Ca2+]ER (49). Further, a small pool of NSP4 is detected in the PM in rotavirus-infected cells, and it has therefore been proposed that the NSP4 viroporin remains inactive in the ER but is activated upon trafficking to the PM, where it can conduct Ca2+ and Na+ into the cytoplasm (49, 66). Na+ influx would depolarize the PM, which is observed in rotavirus-infected cells (72), and this could activate further Ca2+ entry through VACC and NCX operating in reverse mode (54). The observed depletion in agonist-releasable Ca2+ stores and activation of STIM1 counter the idea that the NSP4 viroporin is inactive in the ER (35, 40). Instead, elevated loading of 45 Ca2+ in rotavirusinfected cells is possibly due to the high level of rotavirus protein expression, which includes three Ca2+-binding proteins: the capsid protein VP7, the replication complex scaffold protein NSP5, and NSP4 itself (see below) (73–75). Although a role for PM-localized NSP4 directly conducting Ca2+/Na+ cannot be ruled out, enhanced GFP (EGFP)-tagged NSP4 is not detected in the PM, and its expression recapitulates the disruption in Ca2+ homeostasis seen during a rotavirus infection, indicating the PM-associated pool is not essential for elevating [Ca2+]c (52, 63).
Expression of 2B has not yet been definitively shown to activate STIM1; however, the ability of different ion channel blockers to inhibit the 2B-induced increase in [Ca2+]c provides clues that SOCE is activated. Initial Ca2+ imaging studies of poliovirus-infected HeLa cells had reduced [Ca2+]c after treatment with verapamil, an L-type VACC blocker, and similar results are seen for coxsackievirus-infected cells (45, 76). Studies focused on ER-mitochondria Ca2+ fluxes show 2-APB significantly reduces [Ca2+]c in poliovirus-infected cells; however, 2-APB also blocks IP3R Ca2+ release, so the reduced [Ca2+]c may not be solely due to blocking Orai1 (77). Thus, further work is needed to determine whether STIM1 is activated by 2B and to define which influx pathways are induced.
During normal SOCE, refilling of the ER with Ca2+ negatively regulates STIM1 to inactivate Orai1 (71). Surprisingly, in rotavirus-infected cells, STIM1 remains active at late times postinfection, when cytoplasmic Ca2+ has reached high steady-state levels, which would normally inactive STIM1/SOCE (35). Continued activation of STIM1 could be caused by persistent depletion of ER Ca2+ levels and/or an inability of STIM1 oligomers to disassociate due to disruption in the microtubule cytoskeleton (see below), which is required for STIM1 movement to and from Orai1 in the PM. Persistent depletion of ER Ca2+ by NSP4 is supported by a significantly blunted agonist-induced ER Ca2+ release by ATP, carbachol, and thapsigargin when measured by live cell fluorescence Ca2+ imaging (40, 49). However, rotavirus morphogenesis requires high Ca2+ levels to stabilize the Ca2+-dependent assembly of the ER-localized VP7 outer capsid protein as immature virus particles bud into the ER, so persistently depleted Ca2+ could not support virus morphogenesis (78). It was postulated that rotavirus assembly may occur in an isolated ER compartment that does not respond to typical PLC-dependent agonists (78, 79). Recent data show that rotavirus induces autophagy and that VP7-containing autophagosomal membranes colocalize with viroplasms, the site of rotavirus assembly, where high Ca2+ would be needed (80). In contrast, the activated STIM1 puncta do not colocalize with the viroplasm proteins NSP2 and NSP5, supporting the concept of persistent depletion of the ER Ca2+ stores but creation of a high-Ca2+ compartment for rotavirus assembly distinct from the ER; however, direct evidence for the existence of such a compartment remains to be shown (35).
Viroporin-Mediated Exploitation of Host Calcium Signaling
Recent studies have provided new insights into the role viroporin-induced elevation in [Ca2+]c plays in subverting cellular processes, which facilitates virus replication and contributes to pathogenesis. Both NSP4 and 2B display some distinct Ca2+-evoked changes to cell physiology. For NSP4, elevated [Ca2+]c is associated with stimulation of Cl− secretion from enterocytes and serotonin secretion from enterochromaffin cells to cause diarrhea and vomiting. In contrast, 2Bmediated Ca2+ signals activate the NLRP3 inflammasome, inducing proinflammatory cytokine secretion. There are also several Ca2+-activated functions in common between NSP4 and 2 B, including activation of autophagy and disruption of the actin and tubulin cytoskeleton.
Calcium-evoked diarrhea and vomiting.
Rotavirus diarrhea is a multifactorial disease with both secretory and malabsorptive components involving the epithelia, immune system, and enteric nervous system, but one notable feature is the rapid onset of watery diarrhea driven by excess Cl− and fluid secretion (81). In contrast to most bacterial toxins that cause Cl− secretion through activation of the cystic fibrosis transmembrane regulator (CFTR) Cl− channel, rotavirus disease is not attenuated in CFTR-knockout mice but induces Cl− secretion through at least one Ca2+-activated Cl− channel, which was recently identified as anoctamin 1(Ano1, also called TMEM16A). Ca2+ signals induced by exogenous treatment of cells with the enterotoxin domain of NSP4 activate Ano1 and induce Cl− secretion (57, 82). Further, drugs and red wine extracts that inhibit Ano1 Cl− conductance prevent rotavirus diarrhea in the neonatal mouse model (83). Whether the viroporin-mediated increase in [Ca2+]c activates Ano1 independent from the enterotoxin function remains to be determined. In rotavirus-infected polarized Caco-2 monolayers, Cl− secretion (measured by Ussing chamber experiments) is abolished in Ca2+-free Ringer’s buffer or when cells are treated with Bay K8644 (an L-type VACC blocker), suggesting the importance of extracellular Ca2+ ( e.g., SOCE pathways) for Cl− secretion (84). Further studies using NSP4 mutants with attenuated viroporin and/or enterotoxin activity are needed to validate a role for NSP4 viroporin activity in rotavirus diarrhea.
In addition to diarrhea, vomiting exacerbates dehydration and limits the effectiveness of oral rehydration (81). Recently, rotavirus-induced Ca2+ signals were shown to induce serotonin secretion from primary enterochromaffin tumor cells and the GOT1 enterochromaffin cell line. In mice, rotavirus infection of the small intestine induces neuronal activity within areas of the brain associated with emesis (i.e., vomiting), and although this does not cause vomiting in mice ( mice lack an emetic reflex), it demonstrates activation of vagal efferent nerves during infection (53). Serotonin secretion was elicited from both rotavirus-infected cells (i.e., viroporin Ca2+ signal) and uninfected cells treated with NSP4 (i.e., enterotoxin Ca2+ signal) (53). Serotonin receptor antagonists are effective antiemetic drugs in children, suggesting a role for the enteric nervous system in NSP4-induced disease and a potentially promising therapy for rotavirus infections when vomiting is prominent (81).
Inflammasome activation.
Inflammasomes are multiprotein complexes that regulate innate immune responses to pathogen-associated molecular patterns to upregulate production of the proinflammatory cytokines IL-1β and IL-18 through activation of caspase 1, and Ca2+ release from intracellular stores is a stimulus for inflammasome activation, potentially as a cellular sensing mechanism for excess Ca2+ leak (85). Several recent studies have identified multiple viroporins as specific inflammasome activators, including activation of the NLRP3 inflammasome in bone marrow–derived macrophages and dendritic cells by IAV M2 and picornavirus 2B (from EMCV or enterovirus 71), and activation of the NLRP3 and/or NLRC5 inflammasomes in primary bronchial epithelial cells by human rhinovirus (HRV) 2B or RSV SH (86). Expression of EMCV, enterovirus 71, or HRV 2B protein alone is sufficient to activate the inflammasome, leading to caspase 1 cleavage and IL-1β production. Interestingly, 2B-mediated inflammasome activation requires elevation of [Ca2+]c by efflux of ER and/or Golgi Ca2+ but not influx of extracellular Ca2+ (38, 47). Although NSP4 has not been associated with inflammasome activation, rotavirus infection induces IL-1β production, suggesting a possible connection that has yet to be demonstrated (87). Because many viruses elevate [Ca2+]c, it is interesting to speculate whether Ca2+-induced inflammasome activation has evolved in part as a cellular response to combat these viral infections.
Activation of autophagy.
In rotavirus-infected cells, NSP4 traffics from the ER into a vesicular compartment that surrounds viroplasms. Expression of NSP4 alone is sufficient to induce these compartments, and they colocalize with the autophagy marker LC3 (26, 63). Induction of autophagy is linked with NSP4 viroporin activity, because treatment of cells with the Ca2+ chelator BAPTA-AM or expression of viroporin-defective NSP4 mutants prevents autophagosome formation, which in the case of BAPTA-AM treatment inhibits rotavirus replication by ∼84% (80). In contrast, exogenous elevation of [Ca2+]c induces the NSP4 viroporin mutants to form autophagosomes (18, 80). The autophagy pathway is activated by NSP4 through a viroporin-mediated Ca2+dependent signal involving calcium/calmodulin-dependent kinase kinase β (CaMKK-β) phosphorylation of the energy sensor 5 adenosine monophosphate–activated protein kinase (AMPK) (80). Chelation of cytoplasmic Ca2+ or expression of the viroporin-defective NSP4 mutant abrogates the induction of autophagy, indicating this is a viroporin-mediated autophagy pathway (88). The induced autophagosomal membranes contain rotavirus glycoproteins, NSP4 and the capsid protein VP7, and they traffic to viroplasms, facilitating the final maturation of infectious particles. Many picornaviruses also induce autophagy, and for poliovirus this is also regulated by the 2B viroporin (67). However, the mechanism by which 2B induces autophagy, and whether this mechanism is dependent on Ca2+ or viroporin function, remains unknown.
Disruption of the cytoskeleton.
Rotavirus infection or NSP4 expression substantially disrupts both the actin and microtubule cytoskeletons, and these changes occur rapidly after infection and are dependent on the elevated [Ca2+]c induced by NSP4. Infection of polarized Caco-2 cells, a human enterocyte cell line, causes loss of microvillar F-actin at 18 hpi, but treatment of cells with BAPTA-AM and use of low-Ca2+ media restore F-actin assembly (51). Subsequent studies using a HEK293 cell line with doxycycline-inducible NSP4-EGFP expression showed that NSP4 expression causes a Ca2+-dependent activation of cofilin, an actin-remodeling protein, which induces aberrant stabilization of F-actin filaments(89). In rotavirus-infected MA104 cells, an African green monkey kidney cell line commonly used in the rotavirus field, disruption in actin filaments occurs within 30 min postinfection, and buffering [Ca2+]c with BAPTA-AM or small interfering RNA (siRNA)-mediated knockdown of NSP4 abrogates these changes (90). Further, rotavirus infection or transient expression of NSP4 alone causes remodeling of the microtubule cytoskeleton, resulting in a redistribution of microtubules to the periphery of the cell. The redistribution of microtubules is inhibited by siRNA knockdown of NSP4 and can be partially blocked by buffering [Ca2+]c with BAPTA-AM (90, 91). Continued research is required to determine the ultimate benefit to virus replication that is gained by the Ca2+-mediated disruption in the host cytoskeleton, but it is known to cause breakdown in epithelial tight junctions, which could facilitate apical virus release and virus spread (51, 89).
Unlike the case of NSP4, a direct connection between 2B-mediated [Ca2+]c flux and disruption in the cytoskeleton has not been established. Poliovirus and echovirus 11 infections of intestinal cell lines (HRT-18 and Caco-2, respectively) cause similar perturbation to actin filaments, in that F-actin is aberrantly stabilized into long microfilaments (92, 93). Although this is similar to what is observed in rotavirus-infected cells, experiments have not directly attributed these alterations in actin to 2B or Ca2+ signalling (93). However, poliovirus and coxsackievirus replication induces a block in ER-to-Golgi trafficking that is due, in part, to the viroporin activity of 2B but likely requires an intact microtubule cytoskeleton (37). Because activation of the inflammasome and disruption of vesicular trafficking both require 2B viroporin activity, it is possible these two processes are mechanistically linked.
Other Calcium-Disrupting Viroporins
NSP4 and 2B are the most extensively studied Ca2+-disrupting viroporins, but altered Ca2+ homeostasis has been observed for several other previously characterized viroporins or viral proteins with structural/functional features of viroporins (i.e., candidate viroporins). Some of these proteins have been shown to directly disrupt Ca2+ signaling [e.g., human T-lymphotropic virus 1 (HTLV-1) p12I], but in most cases, the data are limited to in vitro assays with purified protein or indirect evidence that ER Ca2+ is decreased by the observed activation of SOCE and/or Ca2+-activated Cl− channels (e.g., Sindbis virus 6K). The evidence for the activity of Ca2+ disruption by these viroporins and candidate viroporins is reviewed below.
Characterized viroporins.
Most viroporins are described as monovalent cation (Na+ or K+)-conducting ion channels, primarily through electrophysiology studies. However, new studies of a subset of these viroporins have suggested the ability to conduct Ca2+ and disrupt cellular Ca2+ homeostasis.
Sindbis virus 6K.
The alphavirus 6K protein was one of the first viroporins to be identified using the E. coli cell lysis assay (94). Subsequent planar lipid bilayer electrophysiology studies demonstrated 6K is a bona fide ion channel, with relatively strong selectivity for cations over anions (16-fold) but only a slight preference for monovalent (Na+) over divalent (Ca2+) cations (3-fold) (8). Thus, purified 6K is able to conduct Ca2+, albeit less effectively than it conducts monovalent cations. Disruption of Ca2+ homeostasis by 6K has not been demonstrated in mammalian cells; however, a patch clamp study of Xenopus oocytes provided strong evidence that 6K is capable of disrupting cellular Ca2+ homeostasis. When expressed in oocytes, no 6K-specific ion channel activity is detected. However, 6K expression causes PM depolarization, with induction of strong endogenous currents, including a nonselective cation (Na+/ Ca2+) influx and Ca2+-activated Cl− efflux (95). Activation of these endogenous currents is occasionally observed in oocytes in response to the overexpression of proteins, including known viroporins (95). Undeterred, Antoine et al. (96) characterized the molecular mechanism underlying the induction of these currents by 6K. The cation influx was similar to agonist-induced SOCE in oocytes, suggesting 6K might be depleting ER Ca2+ stores, which in turn would activate Ca2+ influx and therefore Ca2+-activated Cl− efflux. Supporting this hypothesis, the authors showed that buffering cytoplasmic Ca2+ using BAPTA-AM and blocking PM Ca2+ influx reduced the Cl− efflux (96). Thus, similar to what is proposed for NSP4 and 2B, increased Ca2+ conductance (i.e., Ca2+ leak) from ER-localized 6K viroporins may prevent refilling of ER Ca2+ stores, inducing persistent STIM1 activation and Ca2+ influx through SOCE, which ultimately results in elevated [Ca2+]c. Further studies are needed to show whether this mechanism is active in alpha virus-infected cells but not mutant virus–infected cells and whether SOCE plays a role in replication and pathogenesis. In addition, the activation of these endogenous currents has been seen for other viroporins that are considered monovalent-conducting, but not divalent-conducting, viroporins, including HIV-1 Vpu and influenza C virus M2 (97–99). Though it remains possible that induction of the endogenous oocyte currents by expression of these viroporins is a nonspecific effect, it is tempting to speculate that, as with 6K, these currents are evidence of ER permeability that was previously not recognized.
Polyomavirus agnoprotein.
Agnoprotein is produced from the late coding region of some polyomaviruses, including JC virus, BK virus, and simian virus 40 (SV40), and has functional roles in virus replication, assembly, and release(36). Agnoprotein was shown to have the structural features of viroporins, such as the formation of oligomers and the presence of clustered basic residues and of an amphipathic α-helix, and expression of agnoprotein induces membrane permeabilization to hygromycin B in both E. coli and mammalian cells, which is abolished by mutation of the basic residues (36). Interestingly, agnoprotein also increases PM Ca2+ permeability, as measured by live cell Ca2+ imaging, but the agnoprotein viroporin mutant was not tested, so whether this is a specific viroporin function of agnoprotein remains unknown. Agnoprotein viroporin activity facilitates infectious virus release, and recombinant JC virus bearing the mutant agnoprotein shows a buildup of intracellular particles that are fully infectious (36). Interestingly, as occurs with poliovirus and coxsackievirus 2B proteins, agnoprotein disrupts intracellular vesicular trafficking by a direct interaction with the adaptor protein adaptor protein complex 3 (AP-3) δ subunit, enabling buildup of agnoprotein in the PM by preventing lysosomal targeting (100).
Influenza A virus PB1-F2.
Recently, the polymerase gene of IAV was found to encode a second protein called PB1-F2, which is found in many human and avian IAV strains and is a virulence factor (101). PB1-F2 primarily localizes to the mitochondria, and it induces apoptosis by depolarizing the mitochondria’s membrane potential. Early studies showed that PB1-F2 from multiple IAV strains forms nonselective cation-permeable pores in synthetic lipid bilayers; however, no canonical current fluctuations between open and closed states were observed, suggesting formation of unrestricted pores rather than ion channels (102). A recent study showed PB1-F2 can conduct Ca2+ using synthetic liposomes loaded with the Fluo-3 Ca2+-sensitive fluorescent dye. PB1-F2 addition induces Ca2+ conductance into the liposome, measured as an increase in Fluo-3 fluorescence (25). Although refined electrophysiology studies of synthetic PB1-F2 in phosphatidylcholine bilayers show canonical channel activity interspersed between bursts of highly variable conductance levels, the channels conduct cations and anions with low selectivity (25). Thus, although PB1-F2 does conduct Ca2+ and mitochondria are important Ca2+ storage organelles, it is unclear whether PB1-F2 functions are related to Ca2+ conductivity.
Severe acute respiratory syndrome coronavirus ORF3a.
SARS-CoV encodes eight unique open reading frame (ORF) products that were previously uncharacterized. ORF3a is 274aa long, making it the largest viroporin identified thus far, and its three transmembrane domains make it one of two Class III viroporins (the other is NSP4) (10, 18). Based on predicted membrane topology and the ability to form homo-oligomers, ORF3a was predicted to be an ion channel, which was confirmed by recombinant expression of ORF3a in Xenopus oocytes and patch clamp electrophysiology (19). ORF3a showed K+ conductance, and the K+ currents were insensitive to typical host K+ channel blockers but were blocked by 10 mM Ba2+ in the extracellular buffer (19). Electrophysiology of purified recombinant ORF3a inserted into synthetic lipid bilayers showed conductance of K+ as well as Ca2+ (103). The biological relevance of ORF3a Ca2+ permeability requires further study; however, ORF3a is localized to the ER, Golgi, and PM and therefore has the potential to elevate [Ca2+]c by releasing intracellular stores or enabling influx through the PM (19).
Candidate viroporins.
Disruption in Ca2+ signaling is a common theme in viruses, and it can be accomplished through mechanisms that do not involve a Ca2+-conducting viroporin (104). However,it is possible that some proteins that disrupthost Ca2+ homeostas is are indeed viroporins but that this activity has not yet been defined. Based on the common viroporin characteristics— small, α-helical, oligomeric transmembrane proteins with a polybasic domain and an amphipathic α-helix—we predict a few viral proteins may be new Ca2+-conducting viroporin candidates.
HTLV-1 is a human retrovirus that is the cause of some adult T cell leukemia and lymphoma cancers. In addition to the common retroviral genes, HTLV-1 encodes accessory genes, including the p12I protein. Studies on p12I in T cells show it elevates basal [Ca2+]c due to both depletion of ER Ca2+ and influx of extracellular Ca2+ through the PM, and this elevation activates the Ca2+dependent transcription factor nuclear factor for the activation of T cells (NFAT). Ca2+ influx and NFAT activation are blocked by the Ca2+ release–activated Ca2+ channel (CRAC) blockers 2-APB and SKF 96365, indicating that SOCE is likely involved (105). Elevated [Ca2+]c alters gene expression profiles that are implicated in cell replication, survival, and transformation (106).
Othermembrane-associated viral proteins that directly disrupt Ca2+ homeostasis and are candidate viroporins include poxvirus A38L and human cytomegalovirus(HCMV)UL37×1.Expression of the vaccinia virus A38L protein induces cell necrosis, including cell swelling, condensation of chromatin, and vacuolation of the ER, which correlate with elevated 45 Ca2+ entry, and these changes are reversed in low-Ca2+ media (107). UL37×1 is best known for localizing to the mitochondria and blocking apoptosis; however, it also localizes to the ER, where it induces release of ER Ca2+ stores, which in turn causes loss of F-actin and accumulation of cortical actin, similar to what is seen with rotavirus NSP4 (108). This occurs through a Ca2+-dependent protein kinase C pathway and also causes accumulation of large lipid vesicles, a process important for viral morphogenesis (109). While the mechanistic details of how p12I, A38L, and UL37×1 elevate [Ca2+]c remain unknown, the structural similarities to known viroporins and phenotypic similarities to NSP4 and 2B provide a good rationale for investigating their viroporin activity.
CONCLUSIONS
Among viroporins, the ability to disrupt host Ca2+ homeostasis has been definitively established only for rotavirus NSP4 and picornavirus 2B, and extensive studies on the resulting changes in Ca2+ signaling show these proteins are important for the replication and pathogenesis of their respective viruses. Electrophysiology studies have classified most viroporins as nonselective cation channels, but they primarily affect conductivity of Na+ or K+ rather than Ca2+ (10). This raises the question of whether electrophysiological studies of NSP4 and 2B will demonstrate them to be bona fide Ca2+ channels that show selectivity for divalent over monovalent cations. If they do show Ca2+ selectivity, then will structural motifs be identified that regulate Ca2+ conductance? A conserved NSP4 Ca2+-binding site in the coiled-coil domain, which is immediately downstream of the viroporin domain, may be such a motif that regulates the oligomerization state of the NSP4 (75). However, it is also possible that NSP4 and 2B are not Ca2+ selective but specifically affect Ca2+ homeostasis by capitalizing on the high electrochemical gradient present in the Ca2+ storage organelles to which they are localized. In this case, conductance of monovalent cations would be advantageous to counterflux ions into the ER or Golgi lumen, which would maintain the driving force for Ca2+ efflux by preventing charge accumulation near the membrane. Though electrophysiology studies of NSP4 are ongoing, much work is still needed to understand whether Ca2+-conducting viroporins may function differently from other viroporins. In particular, it needs to be discovered how other viroporins are regulated to remain impermeable to ER Ca2+ despite their insertion into the ER membrane during biosynthesis and in most cases substantial localization to the ER, Golgi, mitochondria, and PM, all of which have large Ca2+ gradients.
Studies on NSP4 and 2B demonstrate their ability to disrupt Ca2+ signaling and thereby produce a wide range of effects in host cells, and these signals are amplified through activation of host Ca2+ influx channels, like the SOCE Orai1 channel and voltage-activated L-type channels. The resulting Ca2+ influx reprograms cellular physiology to facilitate virus replication (e.g., autophagy) and to cause disease (e.g., Cl− secretory diarrhea). The connection between viroporin activity and induction of these cellular processes has only recently been identified, so detailed mechanisms of how perturbation of Ca2+ stores elicits these responses are still lacking. Finally, beyond the obvious role that Cl− secretion plays in rotavirus diarrhea, it is likely that viroporins also contribute to pathogenesis by inducing serotonin production and possibly by disrupting host barrier function via cytoskeleton remodeling. Additionally, it has been proposed that viroporin-triggered proinflammatory cytokine production may be linked to chronic immune-mediated diseases, such as asthma (110).
Because viroporins serve crucial functions for virus replication, identification of small molecule blockers for use as antiviral drugs is an active area of research. The M2 blocker amantadine was the first antiviroporin drug, but selection for drug-resistant mutants required only a single amino acid change, making amantadine an ineffective drug in the long term (5). In contrast, many of the cellular processes induced as a result of viroporin activity are also crucial for virus replication and pathogenesis. Therefore, drugs targeting these host processes may be an effective alternative to traditional antiviral drugs, and targeting cellular processes would be less likely to drive drug-resistant mutations. For example, the ubiquitous nature of Ca2+ signaling has led to the development of many specific Ca2+ channel blockers used to treat neurological, cardiovascular, and autoimmune diseases. These drugs possibly could be repurposed as antiviral drugs and may offer a less costly alternative to new drug development. However, continued study of viroporinmediated Ca2+ perturbations and how these Ca2+ signals exploit the host cell is needed to identify strong targets for drug development.
ACKNOWLEDGMENTS
Work on this manuscript was supported in part by NIH grants K01DK093657 and R01AI080656 and by Public Health Service grant P30DK56338, which funds the Texas Medical Center Digestive Diseases Center(TMCDDC). Additional support was provided by the Baylor College of Medicine Integrated Microscopy Core (U54HD007495 and P30CA125123), as well as by the Baylor College of Medicine Cytometry and Cell Sorting Core with funding from the NIH (AI036211, CA125123, and RR024574).
DISCLOSURE STATEMENT
J.M.H. is principal investigator and receives funding from the National Institutes of Health (NIH) through grant K01DK093657, entitled Enteric Virus Calcium Channel Inhibitors, which involves studies of rotavirus NSP4 viroporin function and cellular calcium homeostasis of rotavirusinfected cells. M.K.E. is principal investigator and receives funding from the NIH through grant R01AI080656, entitled Regulation of Rotavirus Replication, which involves studies of the function of NSP4 and other rotavirus and cellular proteins.
LITERATURE CITED
- 1.Carrasco L 1978. Membrane leakiness after viral infection and a new approach to the development of antiviral agents. Nature 272:694–99 [DOI] [PubMed] [Google Scholar]
- 2.Carrasco L, Perez L, Irurzun A, Lama J, Martinez-Abarca F, et al. 1993. Modification of membrane permeability by animal viruses. NATO Adv. Sci. Inst. Ser. A 240:283–303 [Google Scholar]
- 3.González ME, Carrasco L. 2003. Viroporins. FEBS Lett 552:28–34 [DOI] [PubMed] [Google Scholar]
- 4.Lama J, Carrasco L. 1992. Expression of poliovirus nonstructural proteins in Escherichia coli cells. Modification of membrane permeability induced by 2B and 3A. J. Biol. Chem 267:15932–37 [PubMed] [Google Scholar]
- 5.Pinto LH, Holsinger LJ, Lamb RA. 1992. Influenza virus M2 protein has ion channel activity. Cell 69:517–28 [DOI] [PubMed] [Google Scholar]
- 6.Sugrue RJ, Hay AJ. 1991. Structural characteristics of the M2 protein of influenza A viruses: evidence that it forms a tetrameric channel. Virology 180:617–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Guinea R, Carrasco L. 1994. Influenza virus M2 protein modifies membrane permeability in E. coli cells. FEBS Lett 343:242–46 [DOI] [PubMed] [Google Scholar]
- 8.Melton JV, Ewart GD, Weir RC, Board PG, Lee E, Gage PW. 2002. Alphavirus 6K proteins form ion channels. J. Biol. Chem 277:46923–31 [DOI] [PubMed] [Google Scholar]
- 9.Ewart GD, Sutherland T, Gage PW, Cox GB. 1996. The Vpu protein of human immunodeficiency virus type 1 forms cation-selective ion channels. J. Virol 70:7108–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nieva JL, Madan V, Carrasco L. 2012. Viroporins: structure and biological functions. Nat. Rev. Microbiol 10:563–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Giorda KM, Hebert DN. 2013. Viroporins customize host cells for efficient viral propagation. DNA Cell Biol 32:557–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang K, Xie S, Sun B. 2011. Viral proteins function as ion channels. Biochim. Biophys. Acta 1808:510–15 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pinto LH, Lamb RA. 2006. The M2 proton channels of influenza A and B viruses. J. Biol. Chem 281:8997–9000 [DOI] [PubMed] [Google Scholar]
- 14.Madan V, Castello A, Carrasco L. 2008. Viroporins from RNA viruses induce caspase-dependent apoptosis. Cell Microbiol 10:437–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.van Kuppeveld FJ, de Jong AS, Melchers WJ, Willems PH. 2005. Enterovirus protein 2B po(u)res out the calcium: a viral strategy to survive? Trends Microbiol 13:41–44 [DOI] [PubMed] [Google Scholar]
- 16.Tian P, Hu Y, Schilling WP, Lindsay DA, Eiden J, Estes MK. 1994. The nonstructural glycoprotein of rotavirus affects intracellular calcium levels. J. Virol 68:251–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Aldabe R, Irurzun A, Carrasco L. 1997. Poliovirus protein 2BC increases cytosolic free calcium concentrations. J. Virol 71:6214–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hyser JM, Collinson-Pautz MR, Utama B, Estes MK. 2010. Rotavirus disrupts calcium homeostasis by NSP4 viroporin activity. mBio 1:e00265–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lu W, Zheng BJ, Xu K, Schwarz W, Du L, et al. 2006. Severe acute respiratory syndrome-associated coronavirus 3a protein forms an ion channel and modulates virus release. PNAS 103:12540–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen CC, Kruger J, Sramala I, Hsu HJ, Henklein P, et al. 2011. ORF8a of SARS-CoV forms an ion channel: experiments and molecular dynamics simulations. Biochim. Biophys. Acta 1808:572–79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.González ME, Carrasco L 2005. Viral proteins that enhance membrane permeability. Protein Rev 1:79–90 [Google Scholar]
- 22.Lama J, Carrasco L. 1992. Inducible expression of a toxic poliovirus membrane protein in Escherichia coli: comparative studies using different expression systems based on T7 promoters. Biochem. Biophys. Res. Commun 188:972–81 [DOI] [PubMed] [Google Scholar]
- 23.Taube R, Alhadeff R, Assa D, Krugliak M, Arkin IT. 2014. Bacteria-based analysis of HIV-1 Vpu channel activity. PLOS ONE 9:e105387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Agirre A, Barco A, Carrasco L, Nieva JL. 2002. Viroporin-mediated membrane permeabilization: pore formation by nonstructural poliovirus 2B protein. J. Biol. Chem 277:40434–41 [DOI] [PubMed] [Google Scholar]
- 25.Henkel M, Mitzner D, Henklein P, Meyer-Almes FJ, Moroni A, et al. 2010. The proapoptotic influenza A virus protein PB1-F2 forms a nonselective ion channel. PLOS ONE 5:e11112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hyser JM, Utama B, Crawford SE, Estes MK. 2012. Genetic divergence of rotavirus nonstructural protein 4 results in distinct serogroup-specific viroporin activity and intracellular punctate structure morphologies. J. Virol 86:4921–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lin TI, Schroeder C. 2001. Definitive assignment of proton selectivity and attoampere unitary current to the M2 ion channel protein of influenza A virus. J. Virol 75:3647–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Perez M, Garcia-Barreno B, Melero JA, Carrasco L, Guinea R. 1997. Membrane permeability changes induced in Escherichia coli by the SH protein of human respiratory syncytial virus. Virology 235:342–51 [DOI] [PubMed] [Google Scholar]
- 29.Gelais C St., Tuthill TJ, Clarke DS, Rowlands DJ, Harris M, Griffin S 2007. Inhibition of hepatitis C virus p7 membrane channels in a liposome-based assay system. Antivir. Res 76:48–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li Y, To J, Verdia-Baguena C, Dossena S, Surya W, et al. 2014. Inhibition of the human respiratory syncytial virus small hydrophobic protein and structural variations in a bicelle environment. J. Virol 88:11899–914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hyser JM. 2015. Viroporins. In Electrophysiology of Unconventional Channels and Pores, ed. Delcour AH Berlin: Springer; In press [Google Scholar]
- 32.Berridge MJ, Lipp P, Bootman MD. 2000. The versatility and universality of calcium signalling. Nat. Rev. Mol. Cell Biol 1:11–21 [DOI] [PubMed] [Google Scholar]
- 33.Michelangeli F, Ruiz MC, del Castillo JR, Ludert JE, Liprandi F. 1991. Effect of rotavirus infection on intracellular calcium homeostasis in cultured cells. Virology 181:520–27 [DOI] [PubMed] [Google Scholar]
- 34.van Kuppeveld FJ, Hoenderop JG, Smeets RL, Willems PH, Dijkman HB, et al. 1997. Coxsackievirus protein 2B modifies endoplasmic reticulum membrane and plasma membrane permeability and facilitates virus release. EMBO J 16:3519–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hyser JM, Utama B, Crawford SE, Broughman JR, Estes MK. 2013. Activation of the endoplasmic reticulum calcium sensor STIM1 and store-operated calcium entry by rotavirus requires NSP4 viroporin activity. J. Virol 87:13579–88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Suzuki T, Orba Y, Okada Y, Sunden Y, Kimura T, et al. 2010. The human polyoma JC virus agnoprotein acts as a viroporin. PLOS Pathog 6:e1000801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.de Jong AS, Visch HJ, de Mattia F, Van Dommelen MM, Swarts HG, et al. 2006. The coxsackievirus 2B protein increases efflux of ions from the endoplasmic reticulum and Golgi, thereby inhibiting protein trafficking through the Golgi. J. Biol. Chem 281:14144–50 [DOI] [PubMed] [Google Scholar]
- 38.Ito M, Yanagi Y, Ichinohe T. 2012. Encephalomyocarditis virus viroporin 2B activates NLRP3 inflammasome. PLOS Pathog 8:e1002857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wozniak AL, Griffin S, Rowlands D, Harris M, Yi M, et al. 2010. Intracellular proton conductance of the hepatitis C virus p7 protein and its contribution to infectious virus production. PLOS Pathog 6:e1001087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tian P, Estes MK, Hu Y, Ball JM, Zeng CQ, Schilling WP. 1995. The rotavirus nonstructural glycoprotein NSP4 mobilizes Ca2+ from the endoplasmic reticulum. J. Virol 69:5763–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ruiz MC, Cohen J, Michelangeli F. 2000. Role of Ca2+ in the replication and pathogenesis of rotavirus and other viral infections. Cell Calcium 28:137–49 [DOI] [PubMed] [Google Scholar]
- 42.Hyser JM, Estes MK. 2009. Rotavirus vaccines and pathogenesis: 2008. Curr. Opin. Gastroenterol 25:36–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Aldabe R, Barco A, Carrasco L. 1996. Membrane permeabilization by poliovirus proteins 2B and 2 BC. J. Biol. Chem 271:23134–37 [DOI] [PubMed] [Google Scholar]
- 44.Carrasco L 1995. Modification of membrane permeability by animal viruses. Adv. Virus Res 45:171–212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Irurzun A, Arroyo J, Alvarez A, Carrasco L. 1995. Enhanced intracellular calcium concentration during poliovirus infection. J. Virol 69:5142–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.de Jong AS, de Mattia F, Van Dommelen MM, Lanke K, Melchers WJ, et al. 2008. Functional analysis of picornavirus 2B proteins: effects on calcium homeostasis and intracellular protein trafficking. J. Virol 82:3782–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Triantafilou K, Kar S, van Kuppeveld FJ, Triantafilou M. 2013. Rhinovirus-induced calcium flux triggers NLRP3 and NLRC5 activation in bronchial cells. Am. J. Respir. Cell Mol. Biol 49:923–34 [DOI] [PubMed] [Google Scholar]
- 48.Perez JF, Ruiz MC, Chemello ME, Michelangeli F. 1999. Characterization of a membrane calcium pathway induced by rotavirus infection in cultured cells. J. Virol 73:2481–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Diaz Y, Chemello ME, Pena F, Aristimuno OC, Zambrano JL, et al. 2008. Expression of nonstructural rotavirus protein NSP4 mimics Ca2+ homeostasis changes induced by rotavirus infection in cultured cells. J. Virol 82:11331–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zambrano JL, Diaz Y, Pena F, Vizzi E, Ruiz MC, et al. 2008. Silencing of rotavirus NSP4 or VP7 expression reduces alterations in Ca2+ homeostasis induced by infection of cultured cells. J. Virol 82:5815–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Brunet JP, Cotte-Laffitte J, Linxe C, Quero AM, Geniteau-Legendre M, Servin A. 2000. Rotavirus infection induces an increase in intracellular calcium concentration in human intestinal epithelial cells: role in microvillar actin alteration. J. Virol 74:2323–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Berkova Z, Morris AP, Estes MK. 2003. Cytoplasmic calcium measurement in rotavirus enterotoxin-enhanced green fluorescent protein (NSP4-EGFP) expressing cells loaded with Fura-2. Cell Calcium 34:55–68 [DOI] [PubMed] [Google Scholar]
- 53.Hagbom M, Istrate C, Engblom D, Karlsson T, Rodriguez-Diaz J, et al. 2011. Rotavirus stimulates release of serotonin (5-HT) from human enterochromaffin cells and activates brain structures involved in nausea and vomiting. PLOS Pathog 7:e1002115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Diaz Y, Pena F, Aristimuno OC, Matteo L, De Agrela M, et al. 2012. Dissecting the Ca2+ entry pathways induced by rotavirus infection and NSP4-EGFP expression in Cos-7 cells. Virus Res 167:285–96 [DOI] [PubMed] [Google Scholar]
- 55.Ball JM, Tian P, Zeng CQ, Morris AP, Estes MK. 1996. Age-dependent diarrhea induced by a rotaviral nonstructural glycoprotein. Science 272:101–4 [DOI] [PubMed] [Google Scholar]
- 56.Dong Y, Zeng CQ, Ball JM, Estes MK, Morris AP. 1997. The rotavirus enterotoxin NSP4 mobilizes intracellular calcium in human intestinal cells by stimulating phospholipase C-mediated inositol 1,4,5trisphosphate production. PNAS 94:3960–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Morris AP, Scott JK, Ball JM, Zeng CQ, O’Neal WK, Estes MK. 1999. NSP4 elicits age-dependent diarrhea and Ca2+ mediated I− influx into intestinal crypts of CF mice. Am. J. Physiol. Gastrointest. Liver Physiol 277:431–44 [DOI] [PubMed] [Google Scholar]
- 58.Seo NS, Zeng CQ, Hyser JM, Utama B, Crawford SE, et al. 2008. Integrins α1β1 and α2β1 are receptors for the rotavirus enterotoxin. PNAS 105:8811–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Newton K, Meyer JC, Bellamy AR, Taylor JA. 1997. Rotavirus nonstructural glycoprotein NSP4 alters plasma membrane permeability in mammalian cells. J. Virol 71:9458–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Browne EP, Bellamy AR, Taylor JA. 2000. Membrane-destabilizing activity of rotavirus NSP4 is mediated by a membrane-proximal amphipathic domain. J. Gen. Virol 81:1955–59 [DOI] [PubMed] [Google Scholar]
- 61.van Kuppeveld FJ, Galama JM, Zoll J, van den Hurk PJ, Melchers WJ. 1996. Coxsackie B3 virus protein 2B contains cationic amphipathic helix that is required for viral RNA replication. J. Virol 70:3876–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Campanella M, de Jong AS, Lanke KW, Melchers WJ, Willems PH, et al. 2004. The coxsackievirus 2B protein suppresses apoptotic host cell responses by manipulating intracellular Ca2+ homeostasis. J. Biol. Chem 279:18440–50 [DOI] [PubMed] [Google Scholar]
- 63.Berkova Z, Crawford SE, Trugnan G, Yoshimori T, Morris AP, Estes MK. 2006. Rotavirus NSP4 induces a novel vesicular compartment regulated by calcium and associated with viroplasms. J. Virol 80:6061–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bhowmick R, Halder UC, Chattopadhyay S, Chanda S, Nandi S, et al. 2012. Rotaviral enterotoxin nonstructural protein 4 targets mitochondria for activation of apoptosis during infection. J. Biol. Chem 287:35004–20 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 65.Bugarcic A, Taylor JA. 2006. Rotavirus nonstructural glycoprotein NSP4 is secreted from the apical surfaces of polarized epithelial cells. J. Virol 80:12343–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Storey SM, Gibbons TF, Williams CV, Parr RD, Schroeder F, Ball JM. 2007. Full-length, glycosylated NSP4 is localized to plasma membrane caveolae by a novel raft isolation technique. J. Virol 81:5472–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Taylor MP, Kirkegaard K. 2007. Modification of cellular autophagy protein LC3 by poliovirus. J. Virol 81:12543–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Putney JW Jr. 1986. A model for receptor-regulated calcium entry. Cell Calcium 7:1–12 [DOI] [PubMed] [Google Scholar]
- 69.Putney JW Jr. 2004. Store-operated calcium channels: How do we measure them, and why do we care? Sci. Signal 2004: pe37. [DOI] [PubMed] [Google Scholar]
- 70.Smyth JT, Dehaven WI, Jones BF, Mercer JC, Trebak M, et al. 2006. Emerging perspectives in storeoperated Ca2+ entry: roles of Orai, Stim and TRP. Biochim. Biophys. Acta 1763:1147–60 [DOI] [PubMed] [Google Scholar]
- 71.Soboloff J, Rothberg BS, Madesh M, Gill DL. 2012. STIM proteins: dynamic calcium signal transducers. Nat. Rev. Mol. Cell Biol 13:549–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.del Castillo JR, Ludert JE, Sanchez A, Ruiz MC, Michelangeli F, Liprandi F. 1991. Rotavirus infection alters Na+ and K+ homeostasis in MA-104 cells. J. Gen. Virol 72:541–47 [DOI] [PubMed] [Google Scholar]
- 73.Aoki ST, Settembre EC, Trask SD, Greenberg HB, Harrison SC, Dormitzer PR. 2009. Structure of rotavirus outer-layer protein VP7 bound with a neutralizing Fab. Science 324:1444–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Sen A, Sen N, Mackow ER. 2007. The formation of viroplasm-like structures by the rotavirus NSP5 protein is calcium regulated and directed by a C-terminal helical domain. J. Virol 81:11758–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sastri NP, Viskovska M, Hyser JM, Tanner MR, Horton LB, et al. 2014. Structural plasticity of the coiled-coil domain of rotavirus NSP4. J. Virol 88:13602–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Yang Y, Guo Q, Peng T, Gu Q, Zhao J, Xiong D. 1996. Effect of verapamil on Ca2+ influx and CVB3RNA replication in cultured neonatal rat heart cells infected with CVB3. Chin. Med. Sci. J 11:89–92 [PubMed] [Google Scholar]
- 77.Brisac C, Teoule F, Autret A, Pelletier I, Colbere-Garapin F, et al. 2010. Calcium flux between the endoplasmic reticulum and mitochondrion contributes to poliovirus-induced apoptosis. J. Virol 84:12226–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Michelangeli F, Liprandi F, Chemello ME, Ciarlet M, Ruiz MC. 1995. Selective depletion of stored calcium by thapsigargin blocks rotavirus maturation but not the cytopathic effect. J. Virol 69:3838–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ruiz MC, Aristimuno OC, Diaz Y, Pena F, Chemello ME, et al. 2007. Intracellular disassembly of infectious rotavirus particles by depletion of Ca2+ sequestered in the endoplasmic reticulum at the end of virus cycle. Virus Res 130:140–50 [DOI] [PubMed] [Google Scholar]
- 80.Crawford SE, Hyser JM, Utama B, Estes MK. 2012. Autophagy hijacked through viroporin-activated calcium/calmodulin-dependent kinase kinase-β signaling is required for rotavirus replication. PNAS 109:E3405–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hagbom M, Sharma S, Lundgren O, Svensson L. 2012. Towards a human rotavirus disease model. Curr. Opin. Virol 2:408–18 [DOI] [PubMed] [Google Scholar]
- 82.Ousingsawat J, Mirza M, Tian Y, Roussa E, Schreiber R, et al. 2011. Rotavirus toxin NSP4 induces diarrhea by activation of TMEM16A and inhibition of Na+ absorption. Pflüg. Arch 461:579–89 [DOI] [PubMed] [Google Scholar]
- 83.Ko EA, Jin BJ, Namkung W, Ma T, Thiagarajah JR, Verkman AS. 2014. Chloride channel inhibition by a red wine extract and a synthetic small molecule prevents rotaviral secretory diarrhoea in neonatal mice. Gut 63:1120–29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.De Marco G, Bracale I, Buccigrossi V, Bruzzese E, Canani RB, et al. 2009. Rotavirus induces a biphasic enterotoxic and cytotoxic response in human-derived intestinal enterocytes, which is inhibited by human immunoglobulins. J. Infect. Dis 200:813–19 [DOI] [PubMed] [Google Scholar]
- 85.Murakami T, Ockinger J, Yu J, Byles V, McColl A, et al. 2012. Critical role for calcium mobilization in activation of the NLRP3 inflammasome. PNAS 109:11282–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.ChenI Y,Ichinohe T 2015Response of host inflammasomes to viral infection. TrendsMicrobiol 23:55–63 [DOI] [PubMed] [Google Scholar]
- 87.Uchiyama R, Chassaing B, Zhang B, Gewirtz AT. 2015. MyD88-mediated TLR signaling protects against acute rotavirus infection while inflammasome cytokines direct Ab response. Innate Immun 21:416–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Crawford SE, Estes MK. 2013. Viroporin-mediated calcium-activated autophagy. Autophagy 9:797–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Berkova Z, Crawford SE, Blutt SE, Morris AP, Estes MK. 2007. Expression of rotavirus NSP4 alters the actin network organization through the actin remodeling protein cofilin. J. Virol 81:3545–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Zambrano JL, Sorondo O, Alcala A, Vizzi E, Diaz Y, et al. 2012. Rotavirus infection of cells in culture induces activation of RhoA and changes in the actin and tubulin cytoskeleton. PLOS ONE 7:e47612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Yang W,McCrae MA 2012The rotavirus enterotoxin (NSP4) promotes re-modeling of the intracellular microtubule network. Virus Res 163:269–74 [DOI] [PubMed] [Google Scholar]
- 92.Kang G, Desikan P, Mathan M. 2002. Cytoskeletal changes during poliovirus infection in an intestinal cell line. Indian J. Med. Res 115:37–45 [PubMed] [Google Scholar]
- 93.Sobo K, Stuart AD, Rubbia-Brandt L, Brown TD, McKee TA. 2012. Echovirus 11 infection induces dramatic changes in the actin cytoskeleton of polarized Caco-2 cells. J. Gen. Virol 93:475–87 [DOI] [PubMed] [Google Scholar]
- 94.Sanz MA, Perez L, Carrasco L. 1994. Semliki Forest virus 6K protein modifies membrane permeability after inducible expression in Escherichia coli cells. J. Biol. Chem 269:12106–10 [PubMed] [Google Scholar]
- 95.Shimbo K, Brassard DL, Lamb RA, Pinto LH. 1995. Viral and cellular small integral membrane proteins can modify ion channels endogenous to Xenopus oocytes. Biophys. J 69:1819–29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Antoine AF, Montpellier C, Cailliau K, Browaeys-Poly E, Vilain JP, Dubuisson J. 2007. The alphavirus 6K protein activates endogenous ionic conductances when expressed in Xenopus oocytes. J. Membr. Biol 215:37–48 [DOI] [PubMed] [Google Scholar]
- 97.Hongo S, Ishii K, Mori K, Takashita E, Muraki Y, et al. 2004. Detection of ion channel activity in Xenopus laevis oocytes expressing influenza C virus CM2 protein. Arch. Virol 149:35–50 [DOI] [PubMed] [Google Scholar]
- 98.Schubert U, Ferrer-Montiel AV, Oblatt-Montal M, Henklein P, Strebel K, Montal M. 1996. Identification of an ion channel activity of the Vpu transmembrane domain and its involvement in the regulation of virus release from HIV-1-infected cells. FEBS Lett 398:12–18 [DOI] [PubMed] [Google Scholar]
- 99.Coady MJ, Daniel NG, Tiganos E, Allain B, Friborg J, et al. 1998. Effects of Vpu expression on Xenopus oocyte membrane conductance. Virology 244:39–49 [DOI] [PubMed] [Google Scholar]
- 100.Suzuki T, Orba Y, Makino Y, Okada Y, Sunden Y, et al. 2013. Viroporin activity of the JC polyomavirus is regulated by interactions with the adaptor protein complex 3. PNAS 110:18668–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Gibbs JS, Malide D, Hornung F, Bennink JR, Yewdell JW. 2003. The influenza A virus PB1-F2 protein targets the inner mitochondrial membrane via a predicted basic amphipathic helix that disrupts mitochondrial function. J. Virol 77:7214–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Chanturiya AN, Basanez G, Schubert U, Henklein P, Yewdell JW, Zimmerberg J. 2004. PB1-F2, an influenza A virus-encoded proapoptotic mitochondrial protein, creates variably sized pores in planar lipid membranes. J. Virol 78:6304–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Chien TH, Chiang YL, Chen CP, Henklein P, Hanel K, et al. 2013. Assembling an ion channel: ORF 3a from SARS-CoV. Biopolymers 99:628–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Zhou Y, Frey TK, Yang JJ. 2009. Viral calciomics: interplays between Ca2+ and virus. Cell Calcium 46:1–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Ding W, Albrecht B, Kelley RE, Muthusamy N, Kim SJ, et al. 2002. Human T-cell lymphotropic virus type 1 p12I expression increases cytoplasmic calcium to enhance the activation of nuclear factor of activated T cells. J. Virol 76:10374–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Nair A, Michael B, Hiraragi H, Fernandez S, Feuer G, et al. 2005. Human T lymphotropic virus type 1 accessory protein p12I modulates calcium-mediated cellular gene expression and enhances p300 expression in T lymphocytes. AIDS Res. Hum. Retrovir 21:273–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Sanderson CM, Parkinson JE, Hollinshead M, Smith GL. 1996. Overexpression of the vaccinia virus A38L integral membrane protein promotes Ca2+ influx into infected cells. J. Virol 70:905–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Sharon-Friling R, Goodhouse J, Colberg-Poley AM, Shenk T. 2006. Human cytomegalovirus pUL37×1 induces the release of endoplasmic reticulum calcium stores. PNAS 103:19117–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Sharon-Friling R, Shenk T. 2014. Human cytomegalovirus pUL37×1-induced calcium flux activates PKCα, inducing altered cell shape and accumulation of cytoplasmic vesicles. PNAS 111:E1140–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Triantafilou K,Triantafilou M 2014Ionfluxinthelung:virus-induced inflammasome activation. Trends Microbiol 22:580–88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Mould JA, Paterson RG, Takeda M, Ohigashi Y, Venkataraman P, et al. 2003. Influenza B virus BM2 protein has ion channel activity that conducts protons across membranes. Dev. Cell 5:175–84 [DOI] [PubMed] [Google Scholar]
- 112.Piller SC, Ewart GD,Premkumar A,Cox GB,Gage PW 1996Vprprotein ofhuman immunodeficiency virus type 1 forms cation-selective channels in planar lipid bilayers. PNAS 93:111–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Gonzaleź ME, Carrasco L 1998. The human immunodeficiency virus type 1 Vpu protein enhances membrane permeability. Biochemistry 37:13710–19 [DOI] [PubMed] [Google Scholar]
- 114.Silic-Benussi M, Marin O, Biasiotto R, D’Agostino DM, Ciminale V. 2010. Effects of human T-cell leukemia virus type 1 (HTLV-1) p13 on mitochondrial K+ permeability: a new member of the viroporin family? FEBS Lett 584:2070–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Gan SW, Ng L, Lin X, Gong X, Torres J. 2008. Structure and ion channel activity of the human respiratory syncytial virus (hRSV) small hydrophobic protein transmembrane domain. Protein Sci 17:813–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Griffin SD, Beales LP, Clarke DS, Worsfold O, Evans SD, et al. 2003. The p7 protein of hepatitis C virus forms an ion channel that is blocked by the antiviral drug, amantadine. FEBS Lett 535:34–38 [DOI] [PubMed] [Google Scholar]
- 117.Griffin SD, Harvey R, Clarke DS, Barclay WS, Harris M, Rowlands DJ. 2004. A conserved basic loop in hepatitis C virus p7 protein is required for amantadine-sensitive ion channel activity in mammalian cells but is dispensable for localization to mitochondria. J. Gen. Virol 85:451–61 [DOI] [PubMed] [Google Scholar]
- 118.Wilson L, McKinlay C, Gage P, Ewart G. 2004. SARS coronavirus E protein forms cation-selective ion channels. Virology 330:322–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Liao Y,Tam JP, Liu DX 2006Viroporin activity ofSARS-CoV Eprotein. Adv. Exp. Med. Biol 581:199–202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Zhang R, Wang K, Lv W, Yu W, Xie S, et al. 2014. The ORF4a protein of human coronavirus 229 E functions as a viroporin that regulates viral production. Biochim. Biophys. Acta 1838:1088–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Bodelon G, Labrada L, Martinez-Costas J, Benavente J. 2002. Modification of late membrane permeability in avian reovirus-infected cells: viroporin activity of the S1-encoded nonstructural p10 protein. J. Biol. Chem 277:17789–96 [DOI] [PubMed] [Google Scholar]
- 122.Daniels R, Rusan NM, Wadsworth P, Hebert DN. 2006. SV40 VP2 and VP3 insertion into ER membranes is controlled by the capsid protein VP1: implications for DNA translocation out of the ER. Mol. Cell 24:955–66 [DOI] [PubMed] [Google Scholar]
- 123.Daniels R, Sadowicz D, Hebert DN. 2007. A very late viral protein triggers the lytic release of SV40. PLOS Pathog 3: e98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Wetherill LF, Holmes KK, Verow M, Muller M, Howell G, et al. 2012. High-risk human papillomavirus E5 oncoprotein displays channel-forming activity sensitive to small-molecule inhibitors. J. Virol 86:5341–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Joubert DA, Blasdell KR, Audsley MD, Trinidad L, Monaghan P, et al. 2014. Bovine ephemeral fever rhabdovirus α1 protein has viroporin-like properties and binds importin β1 and importin 7. J. Virol 88:1591–603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Kang M, Moroni A, Gazzarrini S, DiFrancesco D, Thiel G, et al. 2004. Small potassium ion channel proteins encoded by chlorella viruses. PNAS 101:5318–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Sunstrom NA, Premkumar LS, Premkumar A, Ewart G, Cox GB, Gage PW. 1996. Ion channels formed by NB, an influenza B virus protein. J. Membr. Biol 150:127–32 [DOI] [PubMed] [Google Scholar]
- 128.Snyder JE, Kulcsar KA, Schultz KL, Riley CP, Neary JT, et al. 2013. Functional characterization of the alphavirus TF protein. J. Virol 87:8511–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Costin JM, Rausch JM, Garry RF, Wimley WC. 2007. Viroporin potential of the lentivirus lytic peptide (LLP) domains of the HIV-1 gp41 protein. Virol. J 4:123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Takikawa S, Engle RE, Emerson SU, Purcell RH St., Claire M, Bukh J 2006. Functional analyses of GB virus B p13 protein: development of a recombinant GB virus B hepatitis virus with a p7 protein. PNAS 103:3345–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Premkumar A, Dong X, Haqshenas G, Gage PW, Gowans EJ. 2006. Amantadine inhibits the function of an ion channel encoded by GB virus B, but fails to inhibit virus replication. Antivir. Ther 11:289–95 [PubMed] [Google Scholar]
- 132.Premkumar A, Horan CR, Gage PW. 2005. Dengue virus M protein C-terminal peptide ( DVM-C ) forms ion channels. J. Membr. Biol 204:33–38 [DOI] [PubMed] [Google Scholar]
- 133.Chang YS, Liao CL, Tsao CH, Chen MC, Liu CI, et al. 1999. Membrane permeabilization by small hydrophobic nonstructural proteins of Japanese encephalitis virus. J. Virol 73:6257–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Han Z, Harty RN. 2004. The NS3 protein of bluetongue virus exhibits viroporin-like properties. J. Biol. Chem 279:43092–97 [DOI] [PubMed] [Google Scholar]
- 135.Ivanovic T, Agosto MA, Zhang L, Chandran K, Harrison SC, Nibert ML. 2008. Peptides released from reovirus outer capsid form membrane pores that recruit virus particles. EMBO J 27:1289–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Gallaher WR,Garry RF 2015. Modeling of the Ebola virus delta peptide reveals a potential lytic sequence motif. Viruses 7:285–305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Gros A, Martinez-Quintanilla J, Puig C, Guedan S, Mollevi DG, et al. 2008. Bioselection of a gain of function mutation that enhances adenovirus 5 release and improves its antitumoral potency. Cancer Res 68:8928–37 [DOI] [PubMed] [Google Scholar]


