Summary
A short form of cellular FLICE-inhibitory protein encoded by CFLARs promotes necroptosis. Although necroptosis is involved in various pathological conditions, the detailed mechanisms are not fully understood. Here we generated transgenic mice wherein CFLARs was integrated onto the X chromosome. All male CFLARs Tg mice died perinatally due to severe ileitis. Although necroptosis was observed in various tissues of CFLARs Tg mice, large numbers of intestinal epithelial cells (IECs) died by apoptosis. Deletion of Ripk3 or Mlkl, essential genes of necroptosis, prevented both necroptosis and apoptosis, and rescued lethality of CFLARs Tg mice. Type 3 innate lymphoid cells (ILC3s) were activated and recruited to the small intestine along with upregulation of interleukin-22 (Il22) in CFLARs Tg mice. Deletion of ILC3s or Il22 rescued lethality of CFLARs Tg mice by preventing apoptosis, but not necroptosis of IECs. Together, necroptosis-dependent activation of ILC3s induces lethal ileitis in an IL-22-dependent manner.
Subject Areas: Immunology, Immune Response, Cell Biology, Functional Aspects of Cell Biology
Graphical Abstract
Highlights
-
•
CFLARs Tg mice develop severe ileitis in utero
-
•
Intestinal epithelial cells die by apoptosis and necroptosis in CFLARs Tg mice
-
•
Blockade of necroptosis rescues lethality of CFLARs Tg mice
-
•
Necroptosis activates type 3 innate lymphoid cells, resulting in severe ileitis
Immunology; Immune Response; Cell Biology; Functional Aspects of Cell Biology
Introduction
Apoptosis is the prototype of programmed cell death or regulated cell death and is executed by sequential activation of cysteine proteases, named caspases (Riedl and Salvesen, 2007, Yuan, 2006). Recent studies have reported another form of regulated cell death, which is also referred to as necroptosis (Christofferson and Yuan, 2010). Activation of death receptors induced by cognate death ligands including tumor necrosis factor (TNF), Fas, and TRAIL triggers the formation of death-inducing signaling complex, termed complex IIb, that is composed of Fas-associated protein with death domain (FADD), receptor-interacting protein kinase (RIPK)1, RIPK3, and caspase 8 (Pasparakis and Vandenabeele, 2015). Once caspase 8 is activated, it subsequently activates downstream caspases 3, 6, and 7, resulting in the execution of apoptosis. Activation of caspase 8 normally suppresses the execution of necroptosis by inactivating RIPK1 and CYLD (Chan et al., 2003, O'Donnell et al., 2011). In sharp contrast, in the presence of either caspase inhibitors, or deletion of Fadd or Caspase 8, the complex IIb evolves into the necrosome that is composed of RIPK1, RIPK3, and mixed lineage kinase domain-like (MLKL). Sequential phosphorylation of RIPK1, RIPK3, and MLKL results in oligomerization and subsequent plasma membrane translocation of MLKL, resulting in membrane permeabilization and necroptosis (Pasparakis and Vandenabeele, 2015). Necroptosis is induced by death ligands, polyinosinic:polycytidylic acid, and viral infection and is involved in various pathological conditions including drug-induced pancreatitis, ischemic reperfusion injury, and elimination of some types of viruses (Weinlich et al., 2017). Taken that germline deletion of Fadd and Caspase 8 results in embryonic lethality due to an increase in necroptosis (Kaiser et al., 2011, Oberst et al., 2011, Zhang et al., 2011), the FADD/caspase 8-dependent apoptotic pathway normally suppresses the necroptotic pathway during normal development. However, an interplay between apoptosis and necroptosis in vivo is not fully understood.
Cellular FLICE-inhibitory protein (cFLIP) is a catalytically inactive homolog of the initiator caspase, caspase 8, and blocks cell death induced by death ligands (Budd et al., 2006, Nakano et al., 2017). We and others have generated conditional Cflar-deficient mice and reported that cFLIP plays a crucial role in preventing cells from apoptosis and necroptosis (Dillon et al., 2012, Panayotova-Dimitrova et al., 2013, Piao et al., 2012, Piao et al., 2018, Schattenberg et al., 2011, Zhang and He, 2005). CFLAR gene encodes two proteins, designated as long form (cFLIPL) and short form (cFLIPs) due to alternative splicing. Intriguingly, recent studies have shown that cFLIPL blocks both apoptosis and necroptosis, whereas cFLIPs blocks apoptosis but promotes necroptosis (Feoktistova et al., 2011, Oberst et al., 2011). However, it is unclear whether the expression of cFLIPs promotes necroptosis in vivo, and the consequences of cFLIPs-dependent necroptosis are largely unknown.
Tissue homeostasis of the intestine is regulated by epithelial cells and various types of immune cells, including dendritic cells, macrophages, B and T cells, and innate lymphoid cells (Honda and Littman, 2016, Maloy and Powrie, 2011). Among them, TH17 cells and type 3 innate lymphoid cells (ILC3s) play a crucial role in preventing infection of the intestine from pathogenic bacteria (Ohnmacht, 2016, Vivier et al., 2018). The development of TH17 cells and ILC3s totally depends on the Rorc gene that encodes RAR-related orphan receptor gamma t (RORγt) protein. Under normal conditions, various stimuli such as colonization of commensal bacteria, food-derived metabolites, and cytokines activate macrophages or dendritic cells, resulting in the production of interleukin (IL)-23 and IL-1β (Manta et al., 2013, Mortha et al., 2014). IL-23 and IL-1β subsequently activate TH17 cells and ILC3s. IL-22 produced by activated ILC3s plays a dominant role in maintaining intestinal homeostasis and controls a set of genes showing antimicrobial activities, such as Regenerating islet-derived protein (Reg)3b and Reg3g (Eidenschenk et al., 2014, Parks et al., 2015). In sharp contrast, aberrantly activated ILC3s produce excessive amounts of IL-22, resulting in intestinal tissue injury under certain conditions including injection of anti-CD40 antibody, immaturity of acquired immunity, absence of regulatory T (Treg) cells, or transgenic expression of Il23 (Bauche et al., 2018, Buonocore et al., 2010, Chen et al., 2015). However, the mechanism underlying aberrant activation of ILC3s and ILC3-dependent tissue injury are not fully understood.
X chromosome inactivation is a process in which one of the two X chromosomes is randomly inactivated in female mammalian cells (Lyon, 1971). Hence integration of gene A onto one allele of two X chromosomes results in a mosaic pattern expression of gene A due to random inactivation of X chromosome. During generation of a promoter trap library, we obtained one ES line, designated B210, where a trap vector was integrated into the Diap2 locus on the X chromosome (Taniwaki et al., 2005). Using B210 ES line, we previously reported that mice harboring human SPINK1 gene in the Diap2 locus expressed human SPINK1 in a mosaic pattern (Sakata et al., 2016). This strategy might be useful to express cell death-promoting gene in mice by preventing potentially embryonic lethal phenotype.
To further understand the consequences of necroptosis and an interplay between apoptosis and necroptosis in vivo, we generated CFLARs Tg mice wherein the CFLARs gene was specifically integrated onto the X chromosome. Male and female CFLARs Tg mice were referred to as XCFY and XCFX mice, respectively. All XCFY mice died in utero due to severe ileitis. Immunohistochemistry (IHC) with anti-phosphorylated RIPK3 (pRIPK3) antibody and transmission electron microscopy (TEM) revealed that a number of intestinal epithelial cells (IECs) died by necroptosis. Unexpectedly, large numbers of IECs died by apoptosis in the SI of CFLARs Tg mice. Surprisingly, deletion of Ripk3 or Mlkl rescued embryonic lethality of CFLARs Tg mice by preventing not only necroptosis but also apoptosis of IECs. Moreover, deletion of Rorc or Il22 prevented lethal ileitis in CFLARs Tg mice by preventing apoptosis, but not necroptosis of IECs. Together, necroptosis of IECs activated ILC3s, which further induced apoptosis of IECs in an IL-22-dependent manner.
Results
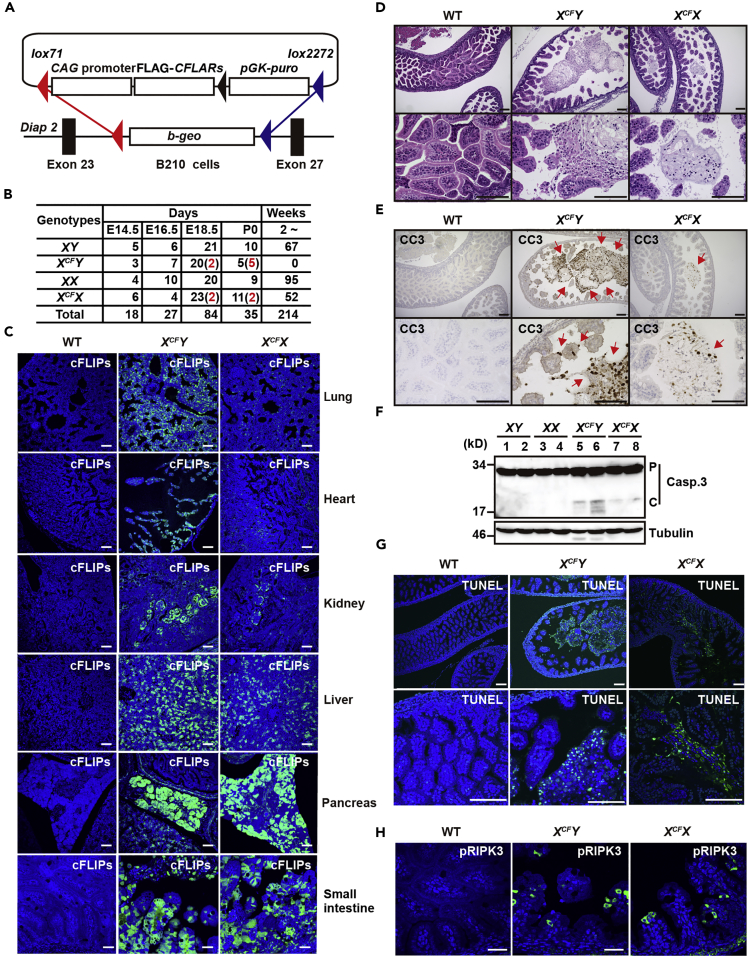
CFLARs Transgenic Mice Die Perinatally
To circumvent embryonic lethality potentially induced by overexpression of cFLIPs in mice, we generated CFLARs Tg mice by utilizing X chromosome inactivation (Figure 1A). As we assumed that XCFY mice might be embryonic lethal, we performed timed mating. XCFY mice developed normally until embryonic day embryonic day (E) 16.5 but began to die at E17.5 to E18.5, and the rest of XCFY mice died at birth (Figure 1B). Only 10% of XCFX mice died perinatally, but the other XCFX mice survived until adulthood. Although cFLIPs were expressed in various tissues (Figure 1C), an apparent histological abnormality was restricted to the small intestine (SI) (Figure S1A). The intestinal lumen was mostly occupied with villous structure in the SI of wild-type embryos, whereas the lumen was dilated and the length of villi was severely shortened in XCFY mice, and to a lesser extent, in XCFX mice at E18.5 (Figure 1D). IECs were detached from villi and accumulated in the lumen of the SI of XCFY mice. Surprisingly, IECs in the lumen and a few IECs in villi were positive for cleaved caspase (CC) 3 staining, suggesting that these IECs died by apoptosis (Figure 1E). Consistently, CC3 was also detected in tissue extracts of the SI of CFLARs Tg mice at E18.5 (Figure 1F). We also found that large numbers of cells were positive for TUNEL staining in the lumen of the SI of XCFY mice, and to a lesser extent, in XCFX mice at E18.5 (Figure 1G).
Figure 1.
CFLARs Tg Mice Die Perinatally
(A) Diagram of a vector for CFLARs Tg mice and genomic organization of the Diap2 locus of B210 cells.
(B) XCFY mice die perinatally. After timed mating, mice were sacrificed at the indicated days after coitus, and the genotypes of embryos were determined by PCR. Numbers in parentheses written in red characters indicate dead pups at the sacrifice. The genotypes of 2- to 4-week-old mice were determined by PCR.
(C) Tissue sections from mice of the indicated genotypes at E18.5 were stained with anti-cFLIP antibody (n = 3–5 mice per each genotype). The tyramid signal amplification (TSA) method was used to enhance cFLIPs-positive signals. Scale bars, 50 μm. Notably, anti-cFLIP antibody recognized exogenously expressed human cFLIPs, but endogenous murine cFLIP at least under our experimental conditions.
(D) H&E-stained small intestinal sections of mice of the indicated genotypes at E18.5 (n = 10 mice per each genotype). Scale bars, 100 μm.
(E, G, and H) Small intestinal tissue sections of mice of the indicated genotypes at E18.5 were stained with anti-cleaved caspase 3 (CC3) (E) or pRIPK3 (H) antibodies, or subjected to TUNEL staining (G) (n = 3–4 mice per each genotype). Scale bars, 100 μm. Red arrows indicate CC3+ IECs.
(F) Tissue extracts of the SI of mice of the indicated genotypes at E18.5 were immunoblotted with the indicated antibodies (n = 2 per genotype). Each number indicates an individual mouse. P and C indicate the proform and cleaved form caspase 3, respectively. Results are representative of two independent experiments. See also Figures S1 and S2.
We next tested whether IECs of CFLARs Tg mice died by necroptosis. As phosphorylation of RIPK3 (pRIPK3) is a hallmark of cells dying by necroptosis, antibodies that recognize pRIPK3 have been used to detect necroptotic cells by IHC (Webster et al., 2018). We found that small numbers of pRIPK3-positive cells were detected in the SI and other tissues of CFLARs Tg mice (Figures 1H and S1B), suggesting these cells died by necroptosis. In contrast, apoptotic cells were not detected in tissues other than SI (Figure S1C). Together, these results suggest that IECs mainly died by apoptosis rather than necroptosis in CFLARs Tg mice at E18.5.
We established two lines of CFLARs Tg mice, designated C9 and C28, and verified the expression of cFLIPs in various tissues of adult XCFX mice by western blot (Figure S1D). We found that a few CC3-positive cells were still observed in the SI, but not in the colon of adult XCFX mice (Figures S1E–S1G). As the phenotypes of C9 and C28 mice were identical, we mainly analyzed a C28 line for further experiments.
To exclude the possibility that the phenotype of CFLARs Tg mice might come from inactivation of Diap2 gene, we also generated another Tg mice line, in which Cre-ERT2 was integrated into the same locus on the X chromosome, designated Cre-ERT2 Tg mice (Figure S2A). Cre-ERT2 Tg mice were born at the expected Mendelian ratios (Figure S2B) and did not show any abnormality of the SI and colon (Figures S2C–S2E). These results indicate that the phenotype of CFLARs Tg mice is not caused by inactivation of Diap2 gene, but the expression of CFLARs gene.
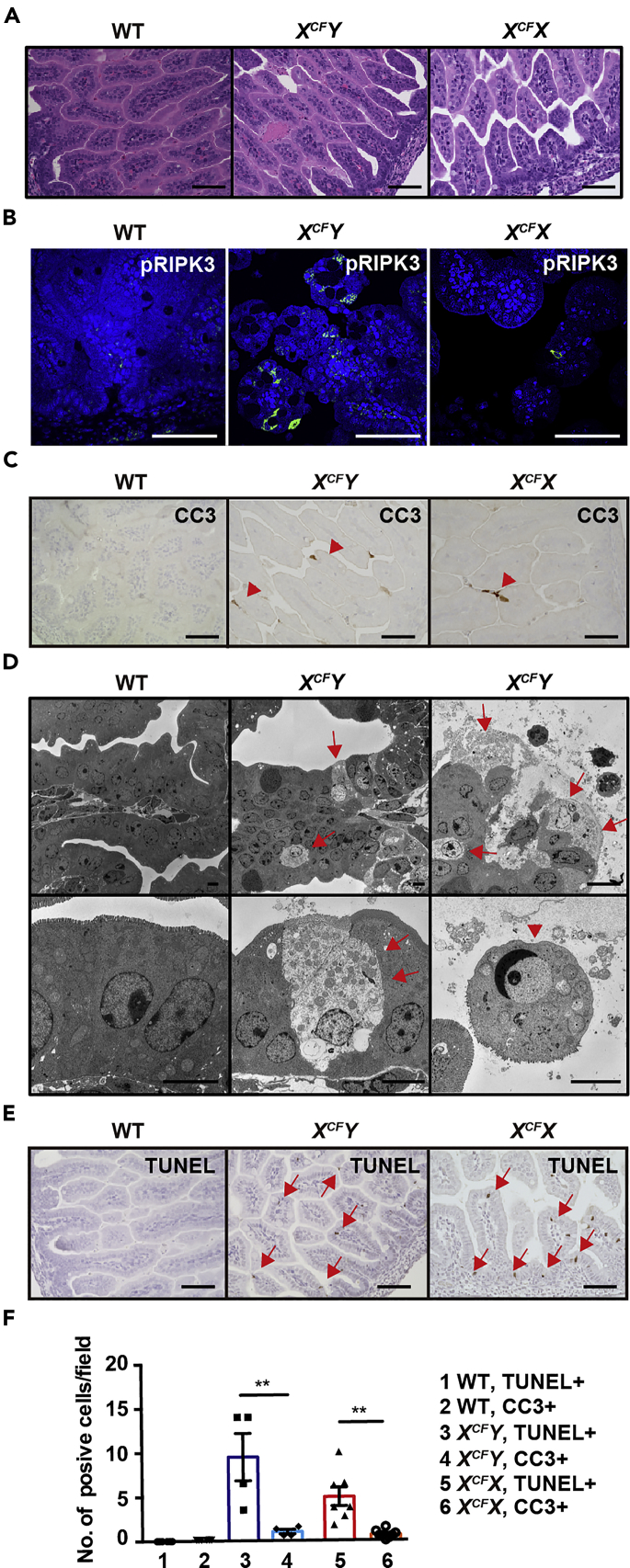
A Few IECs Already Undergo Necroptosis in the SI of CFLARs Tg Mice at E17.5
To investigate an interplay between apoptosis and necroptosis, we analyzed histology of IECs at an earlier time point E17.5. IECs of CFLARs Tg mice appeared to be normal at E17.5 compared with those at E18.5 (Figure 2A). Notably, pRIPK3-positive cells were detected in the SI of CFLARs Tg mice similarly to E18.5 (Figure 2B), whereas very few IECs were positive for CC3 staining (Figure 2C). TEM analysis revealed that some IECs exhibited a drastic decrease in electron densities of the cytoplasm with the dilatation of the endoplasmic reticulum and mitochondria in CFLARs Tg mice, suggesting that these IECs died by necroptosis (Figure 2D). In sharp contrast, a few IECs were detached from villi and exhibited chromatin condensation, a hallmark of apoptosis (Figure 2D). To quantify the relative populations of necroptotic and apoptotic cells, we calculated TUNEL+ and CC3+ cells, respectively. TUNEL staining recognizes both necroptotic and apoptotic cells. Numbers of TUNEL+ cells were higher than those of CC3+ cells in the SI of CFLARs Tg mice (Figures 2C, 2E, and 2F), thus IECs already started to die by necroptosis, and to a lesser extent, by apoptosis at E17.5.
Figure 2.
A Few IECs Already Undergo Necroptosis in the SI of CFLARs Tg Mice at E17.5
(A–C and E) Small intestinal sections of mice of the indicated genotypes at E17.5 were stained with H&E (A), anti-pRIPK3 (B), or anti-CC3 (C) antibodies, or subjected to TUNEL staining (E). Red arrowheads and arrows indicate CC3+ (C) and TUNEL+ cells (E), respectively. Results are representative of four independent experiments (n = 4 mice per each genotype). Scale bars, 100 μm.
(D) Tissue sections described as in (A) were analyzed by TEM (n = 4 mice per each genotype). Red arrows and arrowheads indicate cells showing necroptotic and apoptotic morphology, respectively. Scale bars, 5 μm.
(F) Numbers of CC3-positive and TUNEL-positive cells were counted in randomly selected fields and are expressed as numbers of positive cells per field. Results are mean ± SEM (n = 4–7 mice per genotype). Statistical significance was determined by two-tailed unpaired Student's t test. **p < 0.01; ns, not significant.
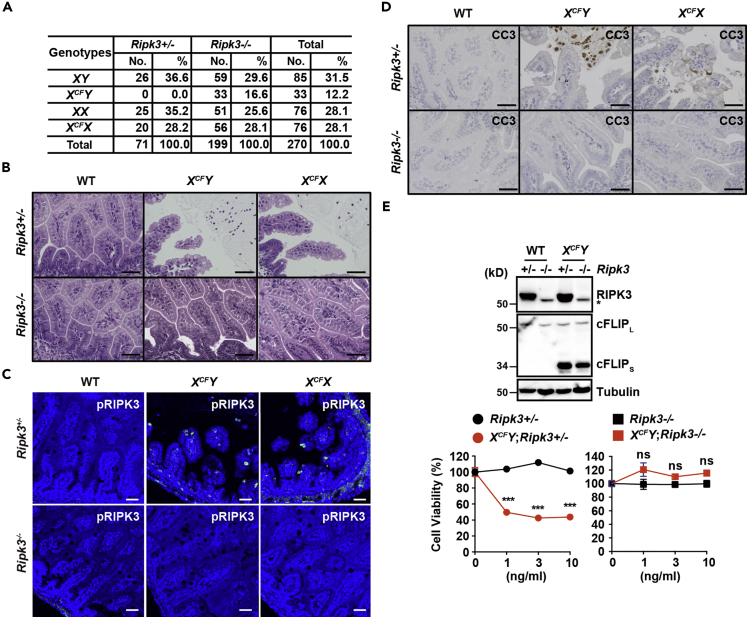
Deletion of Ripk3 Gene Partially Rescues Embryonic Lethality of CFLARs Tg Mice
To investigate the causal relationship between necroptosis and apoptosis, we crossed CFLARs Tg mice with Ripk3−/− mice (Newton et al., 2004). Deletion of Ripk3 partially rescued embryonic lethality of XCFY mice and blocked the destruction of the villous structure in the SI of both XCFY and XCFX mice (Figures 3A and 3B). As expected, pRIPK3-positive IECs disappeared in the SI of XCFY;Ripk3−/− and XCFX;Ripk3−/− mice at E18.5 (Figure 3C). More importantly, CC3-positive IECs also disappeared in the SI of XCFY;Ripk3−/− and XCFX;Ripk3−/− mice at E18.5 (Figure 3D). TEM analysis confirmed that apoptosis and necroptosis of IECs disappeared in the SI of XCFY;Ripk3−/− mice (Figure S3A). Survived XCFY;Ripk3−/− mice appeared to be healthy (Figures S3B–S3D). cFLIPs protein was ubiquitously expressed in various tissues of surviving XCFY;Ripk3−/− mice, and their expression levels were higher than those of XCFX mice on a Ripk3−/− or Ripk3+/− background (Figure S3E). Necroptosis of IECs occurs in IEC-specific Fadd- or Caspase 8-deficient mice (Gunther et al., 2011, Welz et al., 2011), suggesting that necroptosis independently occurs in the absence of apoptosis. However, it is unclear whether necroptosis promotes or suppresses apoptosis in vivo. Taken that deletion of Ripk3 blocked necroptosis and apoptosis, necroptosis might promote apoptosis of IECs of CFLARs Tg mice in vivo.
Figure 3.
Deletion of Ripk3 Partially Rescues Embryonic Lethality of CFLARs Tg Mice
(A) The progeny of crossing male Ripk3−/− mice with XCFX;Ripk3−/− or XCFX;Ripk3+/− mice. The genotypes of 3- to 4-week-old mice were determined by PCR.
(B) Small intestinal sections of mice of the indicated genotypes at E18.5 were stained with H&E (n = 4 mice per each genotype). Scale bars, 100 μm.
(C and D) Intestinal sections of mice of the indicated genotypes at E18.5 were stained with anti-pRIPK3 (C) or anti-CC3 (D) antibodies (n = 3–4 mice per each genotype). Scale bars, 100 μm.
(E) Primary MEFs were prepared from mice of the indicated genotypes at E14.5 and the expression of each protein was verified by western blot with the indicated antibodies. MEFs were stimulated with the indicated concentrations of TNF and zVAD-fmk (20 μM) for 7 h. Cell viability was determined by water soluble tetrazolium monosodium salt (WST) assay. Results are mean ± SD of triplicate samples and representative of three independent experiments. Statistical significance was determined by the two-tailed unpaired Student's t test. ***p < 0.001; ns, not significant. See also Figure S3.
Consistent with these results, TNF and zVAD-fmk (TNF/zVAD)-induced necroptosis were enhanced in murine embryonic fibroblasts (MEFs) derived from XCFY;Ripk3+/− mice compared with Ripk3+/− mice, and TNF/zVAD-induced necroptosis was abolished in MEFs from XCFY mice on a Ripk3-deficient background (Figure 3E).
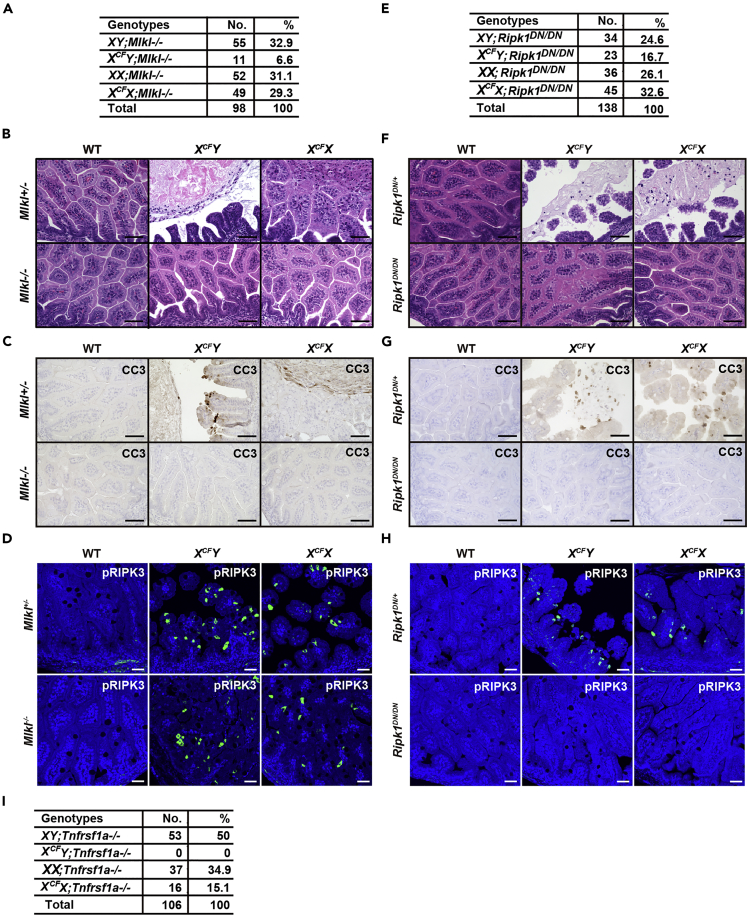
Deletion of Mlkl or Inactivation of RIPK1 Kinase Activity Partially Rescue Embryonic Lethality of CFLARs Tg Mice
Although RIPK3 is essential for necroptosis, several studies have shown that RIPK3 promotes apoptosis and also inflammation under certain conditions (Mandal et al., 2014, Newton et al., 2014). Taken that MLKL solely promotes necroptosis (Murphy et al., 2013, Wu et al., 2013), we crossed CFLARs Tg mice with Mlkl−/− mice (Dannappel et al., 2014). Consistent with deletion of Ripk3, deletion of Mlkl partially rescued the lethal phenotype of XCFY mice and blocked the disruption of the villous structure of the SI of XCFY mice (Figures 4A and 4B). Moreover, CC3-positive, but not pRIPK3-positive, IECs disappeared in the SI of XCFY;Mlkl−/− mice (Figures 4C and 4D). These results suggest that MLKL-dependent necroptosis subsequently triggers apoptosis of IECs in vivo.
Figure 4.
Deletion of Mlkl or Inactivation of RIPK1 Kinase Activity Partially Rescues Embryonic Lethality of CFLARs Tg Mice
(A, E, and I) The progeny of crossing wild-type male mice with XCFX mice on an Mlkl−/− (A), Ripk1DN/DN (E), or Tnfrsf1a−/− (I) background. The genotypes of 3- to 4-week-old mice were determined by PCR.
(B and F) Small intestinal sections of mice of the indicated genotypes at E18.5 were stained with H&E (n = 3–4 mice per each group).
(C, D, G, and H) Small intestinal sections of mice of the indicated genotypes at E18.5 were stained with anti-CC3 (C and G) or anti-pRIPK3 (D and H) antibodies (n = 3–4 mice per each genotype).
Scale bars, 100 μm.
RIPK1 kinase activity is required for formation of the complex IIb that is composed of FADD, caspase 8, RIPK1, and RIPK3 and induces apoptosis or necroptosis in a context-dependent manner (Feoktistova et al., 2011, Tenev et al., 2011). Crossing of CFLARs Tg mice with mice expressing a kinase-inactive mutant of Ripk1 (Ripk1DN/DN) (Polykratis et al., 2014) more efficiently rescued lethal phenotype of CFLARs Tg mice compared with Mlkl−/− mice by preventing both necroptosis and apoptosis (Figures 4E–4H). Unexpectedly, Tnfrsf1a deficiency (Pfeffer et al., 1993) could not rescue lethal phenotype of XCFY mice (Figure 4I), suggesting that the cFLIPs promote the RIPK3-MLKL-dependent necroptotic pathway in IECs of CFLARs Tg mice in a TNFR1-independent manner.
The Expressions of Reg3β and Reg3γ Are Elevated in the SI of CFLARs Tg Mice
To further investigate the consequences of cFLIPs-dependent cell death in vivo, we performed genome-wide transcriptome analysis of the SI of wild-type and CFLARs Tg mice at E18.5. We found that genes associated with cytokine responses, inflammatory responses, innate immune responses, and host defense responses were enriched in the SI of CFLARs Tg mice compared with wild-type mice (Figures S4A–S4C; Table S1). We focused on Regenerating gene (Reg)3b and Reg3g. Reg3β and Reg3γ are produced by IECs and act as antimicrobial proteins (Vaishnava et al., 2011). Notably, the expressions of Reg3b and Reg3g are tightly regulated at the developmental stages: both Reg3b and Reg3g are not expressed in the SI of wild-type fetus, but their expression is gradually increased in the SI after birth possibly due to colonization with commensal bacteria (Matsumoto et al., 2012). We confirmed that the expression of Reg3b and Reg3g was undetectable in the SI of wild-type mice, but elevated in the SI of CFLARs Tg mice at E18.5 by qPCR, IHC, and western blot (Figures S5A–S5C; Table S2).
We next investigated the mechanism underlying the elevation of Reg3b and Reg3g in the SI of adult mice. The expression of Reg3b and Reg3g in the SI of adult mice was abolished in Il22−/− (Zheng et al., 2008) or Rorc-gfp/gfp (Eberl et al., 2004), but not in Rag2−/− (Hao and Rajewsky, 2001) mice (Figure S5D). However, Rorc is essential for the development of ILC3s and TH17 cells, and the expression of Il22 was abolished in the SI of Rorc-gfp/gfp, but not in Rag2−/− mice (Figure S5E). These results suggest that IL-22 produced by RORγt+ ILC3s, but not TH17 cells, is essential for the expression of Reg3b and Reg3g in the SI of adult mice. Thus we surmised that RORγt+ ILC3s might be activated in response to cell death of IECs of CFLARs Tg mice at the prenatal stage.
ILC3s Accumulate in the SI of CFLARs Tg Mice
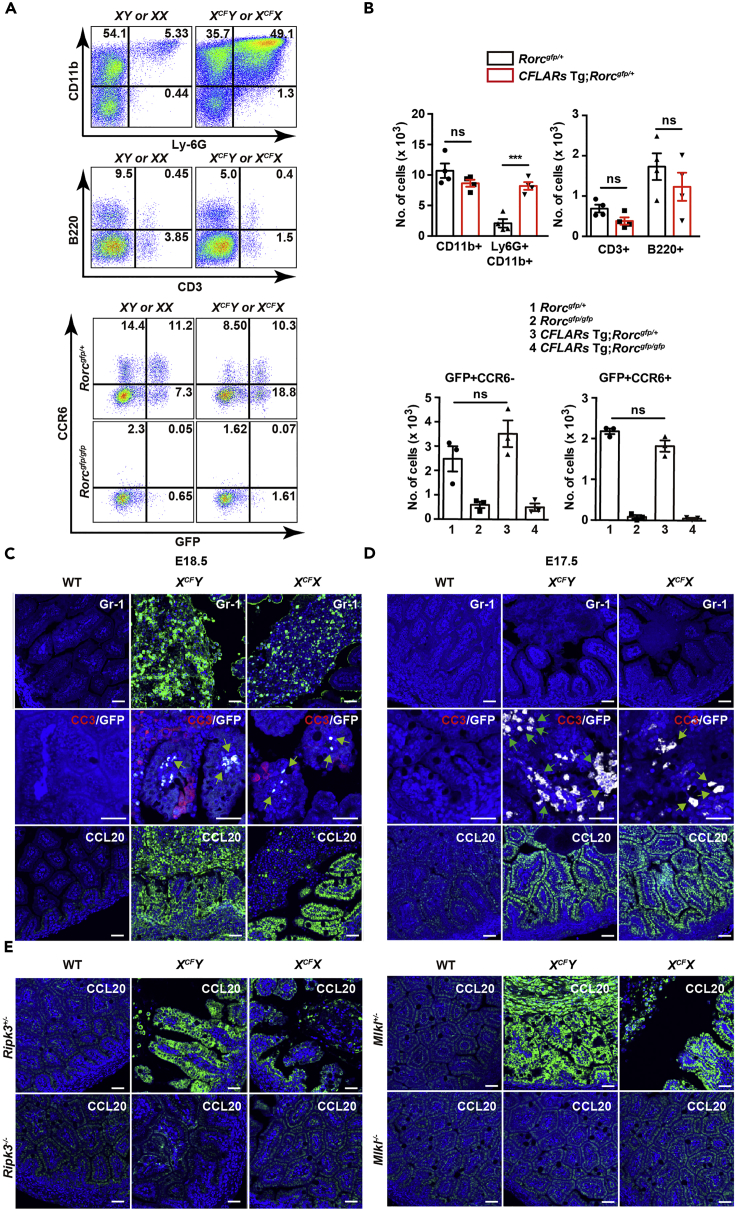
Although ILC3s play a crucial role in maintaining tissue homeostasis of the intestine (Ouyang and Valdez, 2008, Parks et al., 2015), activation of ILC3s promotes intestinal tissue injury under certain conditions (Bauche et al., 2018, Eken et al., 2014). ILC3s are aberrantly activated in the SI of mice lacking CD4+ T cells or young mice wherein acquired immunity is not fully maturated (Mao et al., 2018). As expected, the expression of Foxp3, a hallmark of Treg cells, was very low in the SI of either wild-type or CFLARs Tg mice at E18.5 compared with that of adult mice (Figure S6A). To test whether ILC3s contribute to the development of ileitis at preterm mice, we first crossed Rorc-gfp reporter mice with CFLARs Tg mice. As the expression of the green fluorescent protein (GFP) is under the control of endogenous promoter of Rorc in Rorc-gfp reporter mice, RORγt+ cells that contain both TH17 cells and ILC3s are recognized as GFP+ cells by flow cytometry or IHC. We found that large numbers of CD11b+Ly-6G+ neutrophils infiltrated in the SI of CFLARs Tg mice at E18.5 (Figures 5A and 5B). CCR6+ ILC3s are recruited to the SI via its ligand CCL20 that is produced by IECs (Esplugues et al., 2011). Although total numbers of GFP (RORγt)+ ILC3s were not increased in CFLARs Tg mice compared with wild-type mice (Figures 5A and 5B), RORγt+ cells were recruited to the SI along with an increase in the expression of CCL20 and an appearance of apoptotic cells (Figure 5C).
Figure 5.
ILC3s Accumulate in the SI of CFLARs Tg Mice
(A) Percentages of neutrophils, but not T cells, B cells, or RORγt+ cells are increased in the SI of CFLARs Tg mice at E18.5 compared with WT mice. Cells were prepared from the SI of WT or CFLARs Tg mice at E18.5, and pooled samples (approximately 3–4 fetal SI per each genotype) were stained with the indicated antibodies and analyzed by flow cytometry. Results are representative of four independent experiments.
(B) Cells were prepared as in (A); absolute cell numbers of the indicated populations were calculated and are expressed as mean ± SEM of four independent experiments. Statistical significance was determined by the two-tailed unpaired Student's t test. ***p < 0.001; ns, not significant.
(C and D) Small intestinal sections of mice of WT, XCFY;Rorc-gfp/+, and XCFX;Rorc-gfp/+ mice at E18.5 (C) and E17.5 (D) were stained with anti-Gr-1, combination of anti-GFP (to detect RORγt+ cells) (white) and anti-CC3 (red), or anti-CCL20 antibodies (n = 3 mice per each genotype). Green arrows indicate RORγt+ cells. Scale bars, 100 μm.
(E) Small intestinal sections of mice of the indicated genotypes were stained with anti-CCL20 (n = 3 mice per each genotype). Scale bars, 100 μm.
See also Figures S4–S6, and Table S1.
To determine whether apoptotic cells were responsible for accumulation of RORγt+ cells, we tested whether RORγt+ cells accumulated in the SI at E17.5. Although we hardly detected or only detected very few apoptotic cells in the SI of CFLARs Tg mice (Figures 2C and 5D), a number of RORγt+ cells were already recruited to the SI along with an increase in the expression of CCL20 (Figure 5D). As we hardly detected CD3+ T cells in the SI of CFLARs Tg mice at E17.5 and E18.5 by IHC (data not shown), accumulated RORγt+ cells were ILC3s, but not TH17 cells. Accumulation of neutrophils appeared to delay compared with that of ILC3s (Figures 5C and 5D), suggesting that neutrophils might not be primarily responsible for apoptosis induction, but accumulated in response to apoptotic cells. The expression of CCL20 was elevated in the SI of CFLARs Tg mice along with an appearance of necroptotic cells, whereas its expression was abrogated in CFLARs Tg mice on a Ripk3−/− or Mlkl−/− background (Figure 5E). Notably, although only small numbers of IECs underwent necroptosis (Figure 1H), CCL20 was ubiquitously expressed in all IECs (Figure 5C). Inflammatory cytokines such as TNF and IL-1β have been shown to induce CCL20 production by IECs (Kwon et al., 2002), and macrophages and dendritic cells might produce these cytokines in response to danger-associated molecular pattern (DAMP)s released from necroptotic IECs. Taken that the expression of Il1b was elevated in the SI of CFLARs Tg mice (Figure S6C), IL-1β might be responsible for the production of CCL20 by IECs. Consistently, the expression of both Il1b and Ccl20, but not Il22, was significantly elevated in the SI of CFLARs Tg mice at E17.5 (Figure S6D), suggesting an intimate cross talk between the expression of Il1b and Ccl20. Moreover, IL-1β and IL-23 have been shown to activate ILC3s (Manta et al., 2013, Mortha et al., 2014). Together, IL-1β might be responsible for CCL20 production by IECs and activation of ILC3s.
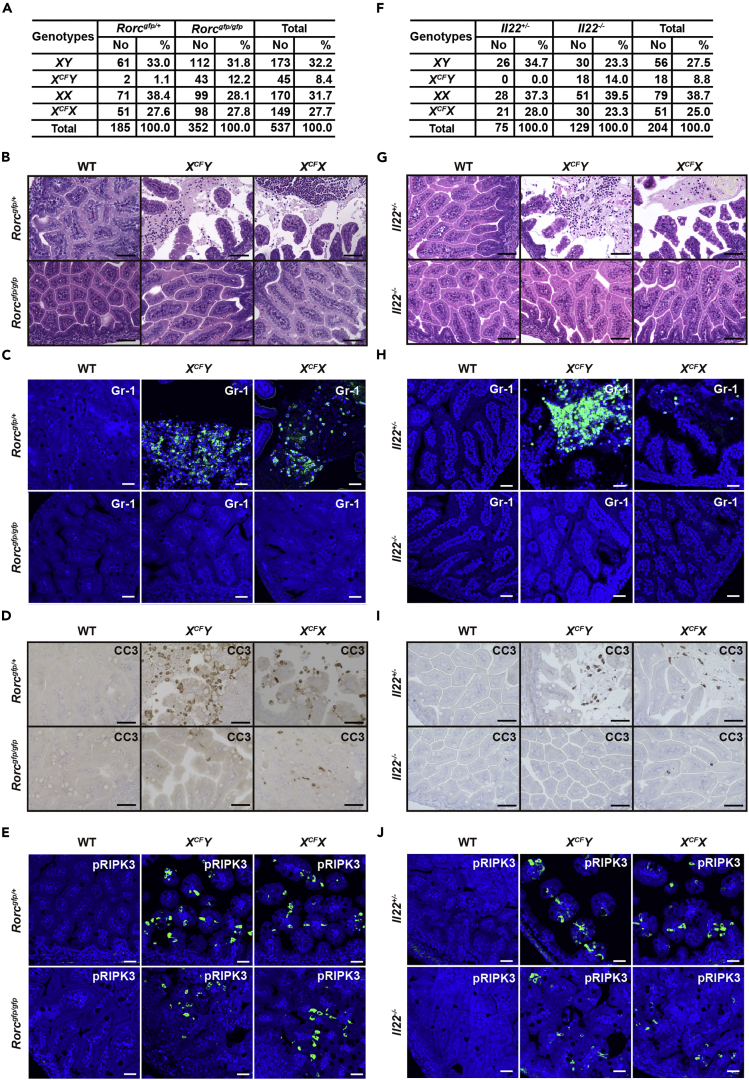
Deletion of Rorc or Il22 Partially Rescues Embryonic Lethality of CFLARs Tg Mice
We next tested whether ILC3s might contribute to the development of ileitis in CFLARs Tg mice. Deletion of Rorc partially rescued embryonic lethality of XCFY mice and blocked the destruction of villi structure of the SI of CFLARs Tg mice (Figures 6A and 6B). A large numbers of neutrophils and CC3-positive IECs were detected in the SI of XCFY;Rorc-gfp/+ mice, whereas infiltration of these cells almost completely disappeared in the SI of XCFY;Rorc-gfp/gfp mice (Figures 6C and 6D). CXCL2 is a chemokine that recruits neutrophils (Kobayashi, 2008). Consistently, the expressions of Reg3b, Reg3g, Il22, Cxcl2, and Ccl20 were downregulated in the SI of CFLARs Tg mice on a Rorc-gfp/gfp background (Figure S7A). pRIPK3-positive IECs were still detected in the SI of CFLARs Tg mice on a Rorc-gfp/gfp background (Figure 6E), further substantiating that activation of RORγt+ ILC3s is a downstream event of necroptosis of IECs of CFLARs Tg mice.
Figure 6.
Deletion of Rorc or Il22 Partially Rescues Embryonic Lethality of CFLARs Tg Mice
(A and F) The progeny of crossing male Rorc-gfp/gfp (A) or Il22−/− (F) mice with XCFX mice. The genotypes of 3- to 4-week-old mice were determined by PCR.
(B–E and G–J) Intestinal sections of mice of the indicated genotypes at E18.5 were stained with H&E (B and G), anti-Gr-1 (C and H), anti-CC3 (D and I), or anti-pRIPK3 (E and J) antibodies (n = 3–4 mice per group).
Scale bars, 100 μm. See also Figure S7.
Previous studies have reported that IL-22 has anti-colitogenic or colitogenic functions in a context-dependent manner (Eken et al., 2014, Ouyang and Valdez, 2008). To determine the contribution of IL-22 to the development of ileitis in CFLARs Tg mice, we finally crossed CFLARs Tg mice with Il22−/− mice. Deletion of Il22 partially rescued embryonic lethality and prevented the development of ileitis in CFLARs Tg mice (Figures 6F–6H). Notably, CC3-positive cells completely disappeared in the SI of CFLARs Tg;Il22−/− mice (Figure 6I), but pRIPK3-positive cells were still detected (Figure 6J). Moreover, the expression of Reg3b, Reg3g, Cxcl2, and Ccl20 was downregulated in the SI of CFLARs Tg;Il22−/− mice (Figure S7A). Thus IL-22 contributes, at least in part, to intestinal injury at perinatal stages. Moreover, deletion of Ripk3, Mlkl, or kinase activity of RIPK1 downregulated the expression of these genes (Figure S7A), suggesting that the execution of necroptosis finally converged on recruitment and activation of ILC3s and IL-22 production.
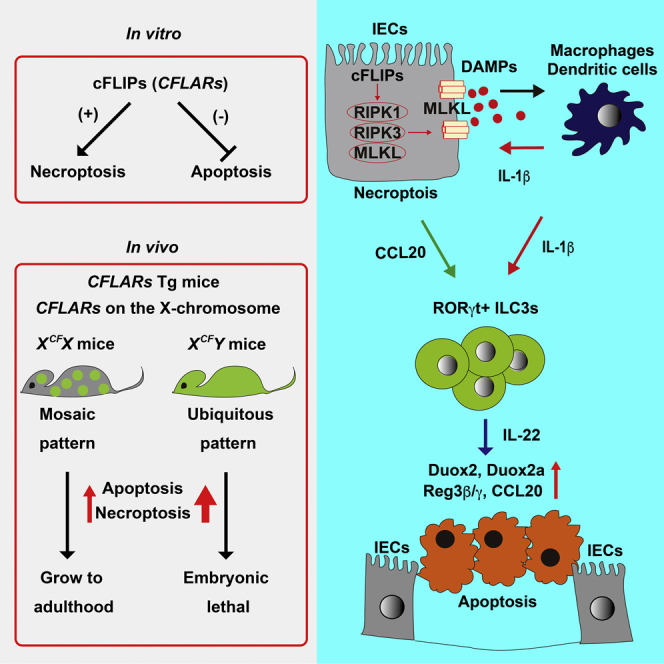
We finally investigated the mechanism underlying IL-22-dependent tissue injury. We found that the expression of reactive oxygen species (ROS)-producing enzyme, Duox2 and its regulatory subunit, Duoxa2, were significantly elevated in the SI of CFLARs Tg mice (Figure S7B). DUOX2 is upregulated in patients with inflammatory bowel disease (IBD) before the onset of inflammation and is a marker of perturbed mucosal homeostasis in patients with early-stage IBD (Grasberger et al., 2015). Taken that IL-22 induces the expression of Duox2 of IECs (Grasberger et al., 2015), it is reasonable to speculate that ILC3s might induce ROS-dependent apoptosis of IECs through the IL-22-Duox2 pathway. Together, these results indicate that a positive feedforward loop between cFLIPs-dependent necroptosis (Figure 7A) and ILC3-dependent apoptosis might critically contribute to the development of lethal ileitis in neonatal mice (Figure 7B).
Figure 7.
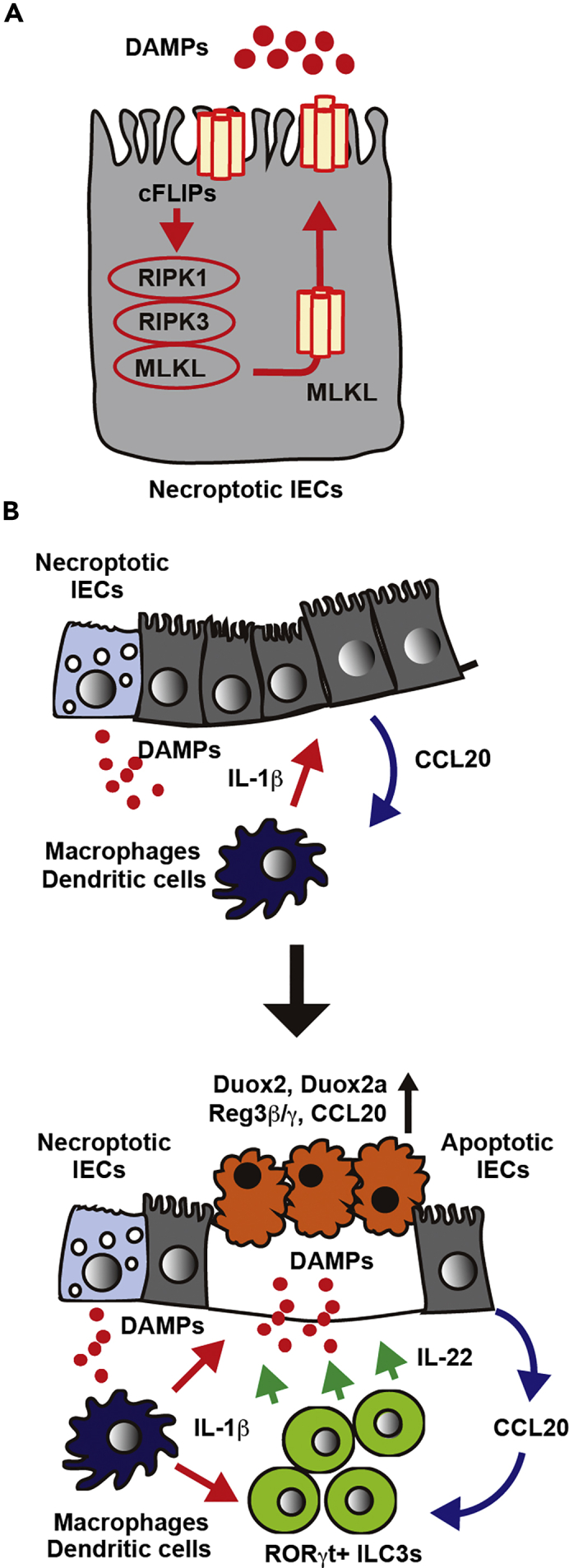
A Model for cFLIPs-Dependent Lethal Ileitis
(A) The complex IIb spontaneously forms in IECs overexpressing cFLIPs. As cFLIPs blocks activation of caspase 8, the complex IIb evolves into the necrosome, resulting in oligomerization of MLKL and subsequent membrane permeabilization.
(B) Necroptotic cells of IECs release DAMPs that subsequently activate nearby macrophages or dendritic cells, resulting in IL-1β production. In addition to the release of DAMPs, IECs in response to IL-1β produce CCL20 that recruits CCR6-positive ILC3s into IECs. Recruited IEC3s are activated by IL-1β and release IL-22 that acts on IECs, resulting in upregulation of Reg3b, Reg3g, Duox2, and Duoxa2. Importantly, aberrantly activated ILC3s induce apoptosis of IECs possibly through upregulation of several ROS-producing enzymes. Apoptotic IECs might enhance inflammation and further activate ILC3s. Thus blockade of the necroptotic pathway and depletion of ILC3s or Il22 suppress lethal ileitis.
Discussion
In the present study, we generated transgenic mice expressing human CFLARs on the X chromosome and investigated cellular responses triggered by necroptosis in vivo. As expected, all male CFLARs Tg mice died in utero, but female CFLARs Tg mice survived due to X chromosome inactivation. Thus the expression of cell-death-promoting gene on the X chromosome might be one of the strategies to evaluate cellular responses triggered by cell death in vivo. Although cFLIPs blocks caspase-dependent apoptosis but promotes necroptosis in vitro (Feoktistova et al., 2011, Oberst et al., 2011), IECs died by necroptosis and apoptosis in CFLARs Tg mice. Deletion of Ripk3 or Mlkl, or inhibition of kinase activity of RIPK1, partially prevented embryonic lethality of CFLARs Tg mice by suppressing both apoptosis and necroptosis of IECs. We finally showed that ILC3s induced apoptosis of IECs in an IL-22-dependent manner, culminating in the development of lethal ileitis in CFLARs Tg mice.
In sharp contrast to in vitro studies including ours (Feoktistova et al., 2011, Oberst et al., 2011, Shindo et al., 2013), overexpression of cFLIPs in vivo resulted in both apoptosis and necroptosis of IECs in mice. Taken that cFLIPs inhibits death receptor-induced apoptosis in vitro (Feoktistova et al., 2011, Oberst et al., 2011), and deletion of Tnfrsf1a did not attenuate embryonic lethality of CFLARs Tg mice, death receptor-induced caspase 8-dependent pathway is not primarily responsible for the execution of apoptosis of IECs of CFLARs Tg mice. Notably, the expression of Duox2 and its regulatory subunit, Duoxa2, were elevated in the SI of CFLARs Tg mice compared with control mice. Taken that oxidative stress has been shown to induce apoptosis under certain conditions (Circu and Aw, 2010), ROS-dependent activation of effector caspases might promote apoptosis of IECs in CFLARs Tg mice.
Histological analysis revealed that severe tissue injury was restricted to the SI of CFLARs Tg mice. We found that ILC3s were only detected in the SI and the liver, but not other tissues. Intriguingly, ILC3s were detected even in the liver of wild-type mice, suggesting that these cells were resident ILC3s, but not infiltrated ILC3s in response to certain stimuli observed in the SI of CFLARs Tg mice. These results suggest that cFLIPs-dependent tissue injury is correlated with infiltration of ILC3s in response to necroptotic cells. Although male CFLARs Tg;Ripk3−/− mice did not show any abnormality of the SI at E18.5, deletion of Ripk3 did not completely rescue embryonic lethality of CFLARs Tg mice. This suggests that the cause of lethality of CFLARs Tg;Ripk3−/− mice was not ileitis. A recent study showed that white blood cells are markedly increased in the peripheral blood of Ripk1−/− mice and Ripk1−/− hematopoietic cells fail to engraft efficiently in lethally irradiated wild-type (WT) mice (Peltzer et al., 2018, Rickard et al., 2014). Thus bone marrow (BM) failure might induce embryonic lethality of CFLARs Tg;Ripk3−/− mice when intestinal tissue injury was attenuated. Moreover, percentages of survived XCFY mice on an Mlkl−/− background were lower than those on Ripk3−/− or Ripk1DN/DN background. Notably, RIPK1 kinase activity and RIPK3 are involved in inflammation and apoptosis under certain conditions (Pasparakis and Vandenabeele, 2015), suggesting that the MLKL-independent pathways also contribute to embryonic lethality of CFLARs Tg mice. Further study will be required to address these issues.
Although approximately 10% of XCFX mice died perinatally, other XCFX mice survived until adulthood. One might surmise that IECs susceptible to cFLIPs-induced cell death have been largely eliminated during the development in utero or soon after birth. Therefore few IECs might be positive for CC3 staining. On the other hand, Treg cells are very few in the SI during embryonic stages and then gradually expand along with colonization of commensal bacteria (Honda and Littman, 2016). We found that the expression of Foxp3 in the SI of either wild-type or CFLARs Tg mice at E18.5 was very low compared with that of adult mice. Intriguingly, LAG3+ regulatory T cells restrain IL-23- and IL-1β-producing macrophages, thereby suppressing activation of ILC3s and the development of intestinal injury (Bauche et al., 2018). Aberrant activation of ILC3 by IL-23 has been shown to drive IL-22-dependent intestinal inflammation (Buonocore et al., 2010). Moreover, Il23 transgenic mice spontaneously develop severe intestinal inflammation along with accumulation of ILC3s and neutrophils in the SI at neonatal stages (Chen et al., 2015). The development of intestinal inflammation of Il23 Tg mice is blocked when ILC3s are depleted, or mice are treated with antibiotics, suggesting that commensal bacteria-dependent production of IL-23 might contribute to the development of severe intestinal inflammation at the neonatal stage. Given that numbers of maturated Treg cells were very few in CFLARs Tg at E18.5, Treg cells did not attenuate ILC3s-dependent ileitis in neonatal CFLARs Tg mice. In contrast, ileitis might be attenuated by Treg cells in adult XCFX mice. Together, these studies have revealed the critical contribution of ILC3s to the development of severe ileitis in mice at prenatal stages.
Previous studies have shown that germline deletion of Caspase 8 or Fadd in mice promotes necroptosis in utero, resulting in embryonic lethality due to a defect in formation of yolk sac vasculature at E10.5 (Kaiser et al., 2011, Oberst et al., 2011, Varfolomeev et al., 1998, Zhang et al., 2011). As CFLARs Tg mice did survive at least until E16.5, the phenotype of CFLARs Tg mice is completely different from that of Caspase 8−/− or Fadd−/− mice. This might come from the timing of the expression of cFLIPs driven by the CAG promoter during development. In contrast, IEC-specific deletion of Caspase 8 or Fadd (Caspase 8IEC-KO or FaddIEC-KO) results in severe intestinal inflammation in mice after birth, suggesting that the phenotype of CFLARs Tg mice might be more severe than that of these murine models (Gunther et al., 2011, Welz et al., 2011). There are several differences among CFLARs Tg mice and these murine models. First, the development of ileitis in CFLARs Tg mice occurs earlier than other murine models, where the colitis usually starts after birth. Second, CFLARs Tg mice only developed ileitis, but Caspase 8IEC-KO or FaddIEC-KO mice develop both ileitis and colitis. Third, deletion of Tnfsfr1a did not rescue embryonic lethality of CFLARs Tg mice, whereas deletion of Tnf or Tnfsfr1a attenuates intestinal tissue injury in Caspase 8IEC-KO or FaddIEC-KO mice. Finally, large numbers of apoptotic cells were detected in the intestinal lumen, but very few apoptotic cells were detected in villi of CFLARs Tg mice. Although necroptosis was detected in various tissues, necroptotic cells per se were not sufficient to induce severe tissue damage in CFLARs Tg mice. Severe tissue damage of the SI of CFLARs Tg mice is tightly correlated with infiltration of ILC3s. Intriguingly, a recent study has reported that IL-22 enhances proliferation of IECs, but inhibits the expansion of intestinal stem cell (ISC)s, resulting in a decrease in organoid survival (Zwarycz et al., 2019). Thus it might be plausible that aberrantly produced IL-22 increases the turnover of IECs and suppresses the expansion of ISCs, resulting in anoikis-dependent apoptosis of IECs in the SI of CFLARs Tg mice. Further study will be required to address this issue.
Approximately 10% population of extremely preterm infants spontaneously develops severe necrotizing enterocolitis (Lim et al., 2015, Tanner et al., 2015). The histology of necrotizing enterocolitis in human is characterized by the dilatation of the intestine, destruction of the villi structures, and intestinal bleeding. Intriguingly, these futures are reminiscent of the histological features of the SI of CFLARs Tg mice. Immaturity of host defense, type of infant feeding, ischemia, anatomical anomaly, and bacterial infection are considered to be responsible for the development of necrotizing enterocolitis. However, the detailed mechanisms remain unclear. As overexpression of cFLIPs induced ILC3s-dependent lethal ileitis, it would be interesting to test whether the expression of cFLIPs was increased in such patients. Given that IECs produce CCL20 in response to inflammatory cytokines such as TNF or IL-1β (Kwon et al., 2002), IECs might further recruit ILC3s, culminating in the development of lethal ileitis. Therefore it would be also intriguing to test whether ILC3s accumulate in the lesions of necrotizing enterocolitis in preterm infants.
Limitations of the Study
Our results demonstrate that blockade of necroptosis completely suppresses apoptosis of IECs, but only partially rescues embryonic lethality of CFLARs Tg mice. We do not currently know which pathway(s) other than necroptosis induces embryonic lethality of CFLARs Tg mice. Moreover, we cannot show genetic evidence that IL-22-dependent ROS production induces apoptosis of IECs of CFLARs Tg mice. Another limitation of our current study is the inability to answer whether the RORγt+ cells/IL-22 axis operates and contributes to the exacerbation of intestinal diseases in human patients. Further investigation will be required to address these issues.
Methods
All methods can be found in the accompanying Transparent Methods supplemental file.
Acknowledgments
We thank M. Pasparakis for Mlkl−/− and Ripk1DN/DN mice, K. Honda for Rorc-gfp/gfp mice, V. Dixit and K. Newton for Ripk3−/− mice, Genentech, Inc. for Il22−/− mice, and T.W. Mak for Tnfrsf1a−/− mice. We thank H. Ohno and N. Sato for technical advice. R.S. was supported by a Research Fellowship from Japan Society for the Promotion of Science (JSPS), Japan. This work was supported in part by Grants-in-Aid from Scientific Research (B) 17H04069 (to H.N.) and Challenging Exploratory Research 17K19533 (to H.N.) from Japan Society for the Promotion of Science (JSPS) and Scientific Research on Innovative areas 26110003 (to H.N.), the Japan Agency for Medical Research and Development (AMED) through AMED-CREST with a grant number JP18gm1210002 (to H.N.), and Private University Research Branding project (to H.N.) from MEXT (Ministry of Education, Culture, Sports, Science and Technology), Japan.
Author Contributions
Conceptualization, R.S., M.O., and H.N.; Investigation, R.S., S.K-S., S.M., Y.D., S.Y., T.N., S.K., and K.M.; Resources, M.O., T.Y., H. Konishi, H. Kiyama, T.M., and K.A.; Writing-Original Draft, R.S. and H.N.; Writing-Review & Editing, R.S., S.Y., K.M., and H.N.; Supervision, Y.U.
Declaration of Interests
The authors declare that they do not have competing financial interests. Data and Software Availability The accession number for the microarray data reported in this paper is NCBI GEO: GSE120982.
Published: May 31, 2019
Footnotes
Supplemental Information can be found online at https://doi.org/10.1016/j.isci.2019.05.011.
Supplemental Information
References
- Bauche D., Joyce-Shaikh B., Jain R., Grein J., Ku K.S., Blumenschein W.M., Ganal-Vonarburg S.C., Wilson D.C., McClanahan T.K., Malefyt R.W. LAG3(+) regulatory T cells restrain interleukin-23-producing CX3CR1(+) gut-resident macrophages during group 3 innate lymphoid cell-driven colitis. Immunity. 2018;49:342–352.e5. doi: 10.1016/j.immuni.2018.07.007. [DOI] [PubMed] [Google Scholar]; Bauche, D., Joyce-Shaikh, B., Jain, R., Grein, J., Ku, K.S., Blumenschein, W.M., Ganal-Vonarburg, S.C., Wilson, D.C., McClanahan, T.K., Malefyt, R.W., et al. (2018). LAG3(+) regulatory T cells restrain interleukin-23-producing CX3CR1(+) gut-resident macrophages during group 3 innate lymphoid cell-driven colitis. Immunity 49, 342-352.e5. [DOI] [PubMed]
- Budd R.C., Yeh W.C., Tschopp J. cFLIP regulation of lymphocyte activation and development. Nat. Rev. Immunol. 2006;6:196–204. doi: 10.1038/nri1787. [DOI] [PubMed] [Google Scholar]; Budd, R.C., Yeh, W.C., and Tschopp, J. (2006). cFLIP regulation of lymphocyte activation and development. Nat. Rev. Immunol. 6, 196-204. [DOI] [PubMed]
- Buonocore S., Ahern P.P., Uhlig H.H., Ivanov I.I., Littman D.R., Maloy K.J., Powrie F. Innate lymphoid cells drive interleukin-23-dependent innate intestinal pathology. Nature. 2010;464:1371–1375. doi: 10.1038/nature08949. [DOI] [PMC free article] [PubMed] [Google Scholar]; Buonocore, S., Ahern, P.P., Uhlig, H.H., Ivanov, II, Littman, D.R., Maloy, K.J., and Powrie, F. (2010). Innate lymphoid cells drive interleukin-23-dependent innate intestinal pathology. Nature 464, 1371-1375. [DOI] [PMC free article] [PubMed]
- Chan F.K., Shisler J., Bixby J.G., Felices M., Zheng L., Appel M., Orenstein J., Moss B., Lenardo M.J. A role for tumor necrosis factor receptor-2 and receptor-interacting protein in programmed necrosis and antiviral responses. J. Biol. Chem. 2003;278:51613–51621. doi: 10.1074/jbc.M305633200. [DOI] [PubMed] [Google Scholar]; Chan, F.K., Shisler, J., Bixby, J.G., Felices, M., Zheng, L., Appel, M., Orenstein, J., Moss, B., and Lenardo, M.J. (2003). A role for tumor necrosis factor receptor-2 and receptor-interacting protein in programmed necrosis and antiviral responses. J. Biol. Chem. 278, 51613-51621. [DOI] [PubMed]
- Chen L., He Z., Slinger E., Bongers G., Lapenda T.L., Pacer M.E., Jiao J., Beltrao M.F., Soto A.J., Harpaz N. IL-23 activates innate lymphoid cells to promote neonatal intestinal pathology. Mucosal Immunol. 2015;8:390–402. doi: 10.1038/mi.2014.77. [DOI] [PMC free article] [PubMed] [Google Scholar]; Chen, L., He, Z., Slinger, E., Bongers, G., Lapenda, T.L., Pacer, M.E., Jiao, J., Beltrao, M.F., Soto, A.J., Harpaz, N., et al. (2015). IL-23 activates innate lymphoid cells to promote neonatal intestinal pathology. Mucosal Immunol. 8, 390-402. [DOI] [PMC free article] [PubMed]
- Christofferson D.E., Yuan J. Necroptosis as an alternative form of programmed cell death. Curr. Opin. Cell Biol. 2010;22:263–268. doi: 10.1016/j.ceb.2009.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]; Christofferson, D.E., and Yuan, J. (2010). Necroptosis as an alternative form of programmed cell death. Curr. Opin. Cell Biol. 22, 263-268. [DOI] [PMC free article] [PubMed]
- Circu M.L., Aw T.Y. Reactive oxygen species, cellular redox systems, and apoptosis. Free Radic. Biol. Med. 2010;48:749–762. doi: 10.1016/j.freeradbiomed.2009.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]; Circu, M.L., and Aw, T.Y. (2010). Reactive oxygen species, cellular redox systems, and apoptosis. Free Radic. Biol. Med. 48, 749-762. [DOI] [PMC free article] [PubMed]
- Dannappel M., Vlantis K., Kumari S., Polykratis A., Kim C., Wachsmuth L., Eftychi C., Lin J., Corona T., Hermance N. RIPK1 maintains epithelial homeostasis by inhibiting apoptosis and necroptosis. Nature. 2014;513:90–94. doi: 10.1038/nature13608. [DOI] [PMC free article] [PubMed] [Google Scholar]; Dannappel M., Vlantis K., Kumari S., Polykratis A., Kim C., Wachsmuth L., Eftychi C., Lin J., Corona T., Hermance N., et al., (2014). RIPK1 maintains epithelial homeostasis by inhibiting apoptosis and necroptosis, Nature 513, 90-94. [DOI] [PMC free article] [PubMed]
- Dillon C.P., Oberst A., Weinlich R., Janke L.J., Kang T.B., Ben-Moshe T., Mak T.W., Wallach D., Green D.R. Survival function of the FADD-CASPASE-8-cFLIP(L) complex. Cell Rep. 2012;1:401–407. doi: 10.1016/j.celrep.2012.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]; Dillon, C.P., Oberst, A., Weinlich, R., Janke, L.J., Kang, T.B., Ben-Moshe, T., Mak, T.W., Wallach, D., and Green, D.R. (2012). Survival function of the FADD-CASPASE-8-cFLIP(L) complex. Cell Rep. 1, 401-407. [DOI] [PMC free article] [PubMed]
- Eberl G., Marmon S., Sunshine M.J., Rennert P.D., Choi Y., Littman D.R. An essential function for the nuclear receptor RORgamma(t) in the generation of fetal lymphoid tissue inducer cells. Nat. Immunol. 2004;5:64–73. doi: 10.1038/ni1022. [DOI] [PubMed] [Google Scholar]; Eberl G., Marmon S., Sunshine M.J., Rennert P.D., Choi Y. and Littman D.R. (2004). An essential function for the nuclear receptor RORgamma(t) in the generation of fetal lymphoid tissue inducer cells, Nat. Immunol. 5, 64-73. [DOI] [PubMed]
- Eidenschenk C., Rutz S., Liesenfeld O., Ouyang W. Role of IL-22 in microbial host defense. Curr. Top. Microbiol. Immunol. 2014;380:213–236. doi: 10.1007/978-3-662-43492-5_10. [DOI] [PubMed] [Google Scholar]; Eidenschenk, C., Rutz, S., Liesenfeld, O., and Ouyang, W. (2014). Role of IL-22 in microbial host defense. Curr. Top. Microbiol. Immunol. 380, 213-236. [DOI] [PubMed]
- Eken A., Singh A.K., Treuting P.M., Oukka M. IL-23R+ innate lymphoid cells induce colitis via interleukin-22-dependent mechanism. Mucosal Immunol. 2014;7:143–154. doi: 10.1038/mi.2013.33. [DOI] [PMC free article] [PubMed] [Google Scholar]; Eken, A., Singh, A.K., Treuting, P.M., and Oukka, M. (2014). IL-23R+ innate lymphoid cells induce colitis via interleukin-22-dependent mechanism. Mucosal Immunol. 7, 143-154. [DOI] [PMC free article] [PubMed]
- Esplugues E., Huber S., Gagliani N., Hauser A.E., Town T., Wan Y.Y., O'Connor W., Jr., Rongvaux A., Van Rooijen N., Haberman A.M. Control of TH17 cells occurs in the small intestine. Nature. 2011;475:514–518. doi: 10.1038/nature10228. [DOI] [PMC free article] [PubMed] [Google Scholar]; Esplugues, E., Huber, S., Gagliani, N., Hauser, A.E., Town, T., Wan, Y.Y., O'Connor, W., Jr., Rongvaux, A., Van Rooijen, N., Haberman, A.M., et al. (2011). Control of TH17 cells occurs in the small intestine. Nature 475, 514-518. [DOI] [PMC free article] [PubMed]
- Feoktistova M., Geserick P., Kellert B., Dimitrova D.P., Langlais C., Hupe M., Cain K., MacFarlane M., Hacker G., Leverkus M. cIAPs block ripoptosome formation, a RIP1/caspase-8 containing intracellular cell death complex differentially regulated by cFLIP isoforms. Mol. Cell. 2011;43:449–463. doi: 10.1016/j.molcel.2011.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]; Feoktistova, M., Geserick, P., Kellert, B., Dimitrova, D.P., Langlais, C., Hupe, M., Cain, K., MacFarlane, M., Hacker, G., and Leverkus, M. (2011). cIAPs block ripoptosome formation, a RIP1/caspase-8 containing intracellular cell death complex differentially regulated by cFLIP isoforms. Mol. Cell 43, 449-463. [DOI] [PMC free article] [PubMed]
- Grasberger H., Gao J., Nagao-Kitamoto H., Kitamoto S., Zhang M., Kamada N., Eaton K.A., El-Zaatari M., Shreiner A.B., Merchant J.L. Increased expression of DUOX2 is an epithelial response to mucosal dysbiosis required for immune homeostasis in mouse intestine. Gastroenterology. 2015;149:1849–1859. doi: 10.1053/j.gastro.2015.07.062. [DOI] [PMC free article] [PubMed] [Google Scholar]; Grasberger, H., Gao, J., Nagao-Kitamoto, H., Kitamoto, S., Zhang, M., Kamada, N., Eaton, K.A., El-Zaatari, M., Shreiner, A.B., Merchant, J.L., et al. (2015). Increased expression of DUOX2 is an epithelial response to mucosal dysbiosis required for immune homeostasis in mouse intestine. Gastroenterology 149, 1849-1859. [DOI] [PMC free article] [PubMed]
- Gunther C., Martini E., Wittkopf N., Amann K., Weigmann B., Neumann H., Waldner M.J., Hedrick S.M., Tenzer S., Neurath M.F. Caspase-8 regulates TNF-alpha-induced epithelial necroptosis and terminal ileitis. Nature. 2011;477:335–339. doi: 10.1038/nature10400. [DOI] [PMC free article] [PubMed] [Google Scholar]; Gunther, C., Martini, E., Wittkopf, N., Amann, K., Weigmann, B., Neumann, H., Waldner, M.J., Hedrick, S.M., Tenzer, S., Neurath, M.F., et al. (2011). Caspase-8 regulates TNF-alpha-induced epithelial necroptosis and terminal ileitis. Nature 477, 335-339. [DOI] [PMC free article] [PubMed]
- Hao Z., Rajewsky K. Homeostasis of peripheral B cells in the absence of B cell influx from the bone marrow. J. Exp. Med. 2001;194:1151–1164. doi: 10.1084/jem.194.8.1151. [DOI] [PMC free article] [PubMed] [Google Scholar]; Hao Z. and Rajewsky K. (2001). Homeostasis of peripheral B cells in the absence of B cell influx from the bone marrow. J. Exp. Med. 194, 1151-1164. [DOI] [PMC free article] [PubMed]
- Honda K., Littman D.R. The microbiota in adaptive immune homeostasis and disease. Nature. 2016;535:75–84. doi: 10.1038/nature18848. [DOI] [PubMed] [Google Scholar]; Honda, K., and Littman, D.R. (2016). The microbiota in adaptive immune homeostasis and disease. Nature 535, 75-84. [DOI] [PubMed]
- Kaiser W.J., Upton J.W., Long A.B., Livingston-Rosanoff D., Daley-Bauer L.P., Hakem R., Caspary T., Mocarski E.S. RIP3 mediates the embryonic lethality of caspase-8-deficient mice. Nature. 2011;471:368–372. doi: 10.1038/nature09857. [DOI] [PMC free article] [PubMed] [Google Scholar]; Kaiser, W.J., Upton, J.W., Long, A.B., Livingston-Rosanoff, D., Daley-Bauer, L.P., Hakem, R., Caspary, T., and Mocarski, E.S. (2011). RIP3 mediates the embryonic lethality of caspase-8-deficient mice. Nature 471, 368-372. [DOI] [PMC free article] [PubMed]
- Kobayashi Y. The role of chemokines in neutrophil biology. Front. Biosci. 2008;13:2400–2407. doi: 10.2741/2853. [DOI] [PubMed] [Google Scholar]; Kobayashi, Y. (2008). The role of chemokines in neutrophil biology. Front. Biosci. 13, 2400-2407. [DOI] [PubMed]
- Kwon J.H., Keates S., Bassani L., Mayer L.F., Keates A.C. Colonic epithelial cells are a major site of macrophage inflammatory protein 3alpha (MIP-3alpha) production in normal colon and inflammatory bowel disease. Gut. 2002;51:818–826. doi: 10.1136/gut.51.6.818. [DOI] [PMC free article] [PubMed] [Google Scholar]; Kwon, J.H., Keates, S., Bassani, L., Mayer, L.F., and Keates, A.C. (2002). Colonic epithelial cells are a major site of macrophage inflammatory protein 3alpha (MIP-3alpha) production in normal colon and inflammatory bowel disease. Gut 51, 818-826. [DOI] [PMC free article] [PubMed]
- Lim J.C., Golden J.M., Ford H.R. Pathogenesis of neonatal necrotizing enterocolitis. Pediatr. Surg. Int. 2015;31:509–518. doi: 10.1007/s00383-015-3697-9. [DOI] [PubMed] [Google Scholar]; Lim, J.C., Golden, J.M., and Ford, H.R. (2015). Pathogenesis of neonatal necrotizing enterocolitis. Pediatr. Surg. Int. 31, 509-518. [DOI] [PubMed]
- Lyon M.F. Possible mechanisms of X chromosome inactivation. Nature New Biol. 1971;232:229–232. doi: 10.1038/newbio232229a0. [DOI] [PubMed] [Google Scholar]; Lyon, M.F. (1971). Possible mechanisms of X chromosome inactivation. Nature New Biol. 232, 229-232. [DOI] [PubMed]
- Maloy K.J., Powrie F. Intestinal homeostasis and its breakdown in inflammatory bowel disease. Nature. 2011;474:298–306. doi: 10.1038/nature10208. [DOI] [PubMed] [Google Scholar]; Maloy, K.J., and Powrie, F. (2011). Intestinal homeostasis and its breakdown in inflammatory bowel disease. Nature 474, 298-306. [DOI] [PubMed]
- Mandal P., Berger S.B., Pillay S., Moriwaki K., Huang C., Guo H., Lich J.D., Finger J., Kasparcova V., Votta B. RIP3 induces apoptosis independent of pronecrotic kinase activity. Mol. Cell. 2014;56:481–495. doi: 10.1016/j.molcel.2014.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]; Mandal, P., Berger, S.B., Pillay, S., Moriwaki, K., Huang, C., Guo, H., Lich, J.D., Finger, J., Kasparcova, V., Votta, B., et al. (2014). RIP3 induces apoptosis independent of pronecrotic kinase activity. Mol. Cell 56, 481-495. [DOI] [PMC free article] [PubMed]
- Manta C., Heupel E., Radulovic K., Rossini V., Garbi N., Riedel C.U., Niess J.H. CX(3)CR1(+) macrophages support IL-22 production by innate lymphoid cells during infection with Citrobacter rodentium. Mucosal Immunol. 2013;6:177–188. doi: 10.1038/mi.2012.61. [DOI] [PMC free article] [PubMed] [Google Scholar]; Manta, C., Heupel, E., Radulovic, K., Rossini, V., Garbi, N., Riedel, C.U., and Niess, J.H. (2013). CX(3)CR1(+) macrophages support IL-22 production by innate lymphoid cells during infection with Citrobacter rodentium. Mucosal Immunol. 6, 177-188. [DOI] [PMC free article] [PubMed]
- Mao K., Baptista A.P., Tamoutounour S., Zhuang L., Bouladoux N., Martins A.J., Huang Y., Gerner M.Y., Belkaid Y., Germain R.N. Innate and adaptive lymphocytes sequentially shape the gut microbiota and lipid metabolism. Nature. 2018;554:255–259. doi: 10.1038/nature25437. [DOI] [PubMed] [Google Scholar]; Mao, K., Baptista, A.P., Tamoutounour, S., Zhuang, L., Bouladoux, N., Martins, A.J., Huang, Y., Gerner, M.Y., Belkaid, Y., and Germain, R.N. (2018). Innate and adaptive lymphocytes sequentially shape the gut microbiota and lipid metabolism. Nature 554, 255-259. [DOI] [PubMed]
- Matsumoto S., Konishi H., Maeda R., Kiryu-Seo S., Kiyama H. Expression analysis of the regenerating gene (Reg) family members Reg-IIIbeta and Reg-IIIgamma in the mouse during development. J. Comp. Neurol. 2012;520:479–494. doi: 10.1002/cne.22705. [DOI] [PubMed] [Google Scholar]; Matsumoto, S., Konishi, H., Maeda, R., Kiryu-Seo, S., and Kiyama, H. (2012). Expression analysis of the regenerating gene (Reg) family members Reg-IIIbeta and Reg-IIIgamma in the mouse during development. J. Comp. Neurol. 520, 479-494. [DOI] [PubMed]
- Mortha A., Chudnovskiy A., Hashimoto D., Bogunovic M., Spencer S.P., Belkaid Y., Merad M. Microbiota-dependent crosstalk between macrophages and ILC3 promotes intestinal homeostasis. Science. 2014;343:1249288. doi: 10.1126/science.1249288. [DOI] [PMC free article] [PubMed] [Google Scholar]; Mortha, A., Chudnovskiy, A., Hashimoto, D., Bogunovic, M., Spencer, S.P., Belkaid, Y., and Merad, M. (2014). Microbiota-dependent crosstalk between macrophages and ILC3 promotes intestinal homeostasis. Science 343, 1249288. [DOI] [PMC free article] [PubMed]
- Murphy J.M., Czabotar P.E., Hildebrand J.M., Lucet I.S., Zhang J.G., Alvarez-Diaz S., Lewis R., Lalaoui N., Metcalf D., Webb A.I. The pseudokinase MLKL mediates necroptosis via a molecular switch mechanism. Immunity. 2013;39:443–453. doi: 10.1016/j.immuni.2013.06.018. [DOI] [PubMed] [Google Scholar]; Murphy, J.M., Czabotar, P.E., Hildebrand, J.M., Lucet, I.S., Zhang, J.G., Alvarez-Diaz, S., Lewis, R., Lalaoui, N., Metcalf, D., Webb, A.I., et al. (2013). The pseudokinase MLKL mediates necroptosis via a molecular switch mechanism. Immunity 39, 443-453. [DOI] [PubMed]
- Nakano H., Piao X., Shindo R., Komazawa-Sakon S. Cellular FLICE-inhibitory protein regulates tissue homeostasis. Curr. Top. Microbiol. Immunol. 2017;403:119–141. doi: 10.1007/82_2015_448. [DOI] [PubMed] [Google Scholar]; Nakano, H., Piao, X., Shindo, R., and Komazawa-Sakon, S. (2017). Cellular FLICE-inhibitory protein regulates tissue homeostasis. Curr. Top. Microbiol. Immunol. 403, 119-141. [DOI] [PubMed]
- Newton K., Sun X., Dixit V.M. Kinase RIP3 is dispensable for normal NF-kappa Bs, signaling by the B-cell and T-cell receptors, tumor necrosis factor receptor 1, and Toll-like receptors 2 and 4. Mol. Cell. Biol. 2004;24:1464–1469. doi: 10.1128/MCB.24.4.1464-1469.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]; Newton K., Sun X. and Dixit V.M., (2004). Kinase RIP3 is dispensable for normal NF-kappa Bs, signaling by the B-cell and T-cell receptors, tumor necrosis factor receptor 1, and Toll-like receptors 2 and 4, Mol. Cell. Biol. 24, 1464-1469. [DOI] [PMC free article] [PubMed]
- Newton K., Dugger D.L., Wickliffe K.E., Kapoor N., de Almagro M.C., Vucic D., Komuves L., Ferrando R.E., French D.M., Webster J. Activity of protein kinase RIPK3 determines whether cells die by necroptosis or apoptosis. Science. 2014;343:1357–1360. doi: 10.1126/science.1249361. [DOI] [PubMed] [Google Scholar]; Newton, K., Dugger, D.L., Wickliffe, K.E., Kapoor, N., de Almagro, M.C., Vucic, D., Komuves, L., Ferrando, R.E., French, D.M., Webster, J., et al. (2014). Activity of protein kinase RIPK3 determines whether cells die by necroptosis or apoptosis. Science 343, 1357-1360. [DOI] [PubMed]
- O'Donnell M.A., Perez-Jimenez E., Oberst A., Ng A., Massoumi R., Xavier R., Green D.R., Ting A.T. Caspase 8 inhibits programmed necrosis by processing CYLD. Nat. Cell Biol. 2011;13:1437–1442. doi: 10.1038/ncb2362. [DOI] [PMC free article] [PubMed] [Google Scholar]; O'Donnell, M.A., Perez-Jimenez, E., Oberst, A., Ng, A., Massoumi, R., Xavier, R., Green, D.R., and Ting, A.T. (2011). Caspase 8 inhibits programmed necrosis by processing CYLD. Nat. Cell Biol. 13, 1437-1442. [DOI] [PMC free article] [PubMed]
- Oberst A., Dillon C.P., Weinlich R., McCormick L.L., Fitzgerald P., Pop C., Hakem R., Salvesen G.S., Green D.R. Catalytic activity of the caspase-8-FLIP(L) complex inhibits RIPK3-dependent necrosis. Nature. 2011;471:363–367. doi: 10.1038/nature09852. [DOI] [PMC free article] [PubMed] [Google Scholar]; Oberst, A., Dillon, C.P., Weinlich, R., McCormick, L.L., Fitzgerald, P., Pop, C., Hakem, R., Salvesen, G.S., and Green, D.R. (2011). Catalytic activity of the caspase-8-FLIP(L) complex inhibits RIPK3-dependent necrosis. Nature 471, 363-367. [DOI] [PMC free article] [PubMed]
- Ohnmacht C. Tolerance to the intestinal microbiota mediated by ROR(gammat)(+) cells. Trends Immunol. 2016;37:477–486. doi: 10.1016/j.it.2016.05.002. [DOI] [PubMed] [Google Scholar]; Ohnmacht, C. (2016). Tolerance to the intestinal microbiota mediated by ROR(gammat)(+) cells. Trends Immunol. 37, 477-486. [DOI] [PubMed]
- Ouyang W., Valdez P. IL-22 in mucosal immunity. Mucosal Immunol. 2008;1:335–338. doi: 10.1038/mi.2008.26. [DOI] [PubMed] [Google Scholar]; Ouyang, W., and Valdez, P. (2008). IL-22 in mucosal immunity. Mucosal Immunol. 1, 335-338. [DOI] [PubMed]
- Panayotova-Dimitrova D., Feoktistova M., Ploesser M., Kellert B., Hupe M., Horn S., Makarov R., Jensen F., Porubsky S., Schmieder A. cFLIP regulates skin homeostasis and protects against TNF-induced keratinocyte apoptosis. Cell Rep. 2013;5:397–408. doi: 10.1016/j.celrep.2013.09.035. [DOI] [PubMed] [Google Scholar]; Panayotova-Dimitrova, D., Feoktistova, M., Ploesser, M., Kellert, B., Hupe, M., Horn, S., Makarov, R., Jensen, F., Porubsky, S., Schmieder, A., et al. (2013). cFLIP regulates skin homeostasis and protects against TNF-induced keratinocyte apoptosis. Cell Rep. 5, 397-408. [DOI] [PubMed]
- Parks O.B., Pociask D.A., Hodzic Z., Kolls J.K., Good M. Interleukin-22 signaling in the regulation of intestinal health and disease. Front. Cell Dev. Biol. 2015;3:85. doi: 10.3389/fcell.2015.00085. [DOI] [PMC free article] [PubMed] [Google Scholar]; Parks, O.B., Pociask, D.A., Hodzic, Z., Kolls, J.K., and Good, M. (2015). Interleukin-22 signaling in the regulation of intestinal health and disease. Front. Cell Dev. Biol. 3, 85. [DOI] [PMC free article] [PubMed]
- Pasparakis M., Vandenabeele P. Necroptosis and its role in inflammation. Nature. 2015;517:311–320. doi: 10.1038/nature14191. [DOI] [PubMed] [Google Scholar]; Pasparakis, M., and Vandenabeele, P. (2015). Necroptosis and its role in inflammation. Nature 517, 311-320. [DOI] [PubMed]
- Peltzer N., Darding M., Montinaro A., Draber P., Draberova H., Kupka S., Rieser E., Fisher A., Hutchinson C., Taraborrelli L. LUBAC is essential for embryogenesis by preventing cell death and enabling haematopoiesis. Nature. 2018;557:112–117. doi: 10.1038/s41586-018-0064-8. [DOI] [PMC free article] [PubMed] [Google Scholar]; Peltzer, N., Darding, M., Montinaro, A., Draber, P., Draberova, H., Kupka, S., Rieser, E., Fisher, A., Hutchinson, C., Taraborrelli, L., et al. (2018). LUBAC is essential for embryogenesis by preventing cell death and enabling haematopoiesis. Nature 557, 112-117. [DOI] [PMC free article] [PubMed]
- Pfeffer K., Matsuyama T., Kundig T.M., Wakeham A., Kishihara K., Shahinian A., Wiegmann K., Ohashi P.S., Kronke M., Mak T.W. Mice deficient for the 55 kd tumor necrosis factor receptor are resistant to endotoxic shock, yet succumb to L. monocytogenes infection. Cell. 1993;73:45467. doi: 10.1016/0092-8674(93)90134-c. [DOI] [PubMed] [Google Scholar]; Pfeffer K., Matsuyama T., Kundig T.M., Wakeham A., Kishihara K., Shahinian A., Wiegmann K., Ohashi P.S., Kronke M. and Mak T.W. (1993). Mice deficient for the 55 kd tumor necrosis factor receptor are resistant to endotoxic shock, yet succumb to L. monocytogenes infection, Cell 73, 45467. [DOI] [PubMed]
- Piao X., Komazawa-Sakon S., Nishina T., Koike M., Piao J.H., Ehlken H., Kurihara H., Hara M., Van Rooijen N., Schutz G. c-FLIP maintains tissue homeostasis by preventing apoptosis and programmed necrosis. Sci. Signal. 2012;5:ra93. doi: 10.1126/scisignal.2003558. [DOI] [PMC free article] [PubMed] [Google Scholar]; Piao, X., Komazawa-Sakon, S., Nishina, T., Koike, M., Piao, J.H., Ehlken, H., Kurihara, H., Hara, M., Van Rooijen, N., Schutz, G., et al. (2012). c-FLIP maintains tissue homeostasis by preventing apoptosis and programmed necrosis. Sci. Signal. 5, ra93. [DOI] [PMC free article] [PubMed]
- Piao X., Miura R., Miyake S., Komazawa-Sakon S., Koike M., Shindo R., Takeda J., Hasegawa A., Abe R., Nishiyama C. Blockade of TNF receptor superfamily 1 (TNFR1)-dependent and TNFR1-independent cell death is crucial for normal epidermal differentiation. J. Allergy Clin. Immunol. 2018;143:213–228.e10. doi: 10.1016/j.jaci.2018.02.043. [DOI] [PubMed] [Google Scholar]; Piao, X., Miura, R., Miyake, S., Komazawa-Sakon, S., Koike, M., Shindo, R., Takeda, J., Hasegawa, A., Abe, R., Nishiyama, C., et al. (2018). Blockade of TNF receptor superfamily 1 (TNFR1)-dependent and TNFR1-independent cell death is crucial for normal epidermal differentiation. J. Allergy Clin. Immunol. in press.143(1):213-228.e10 [DOI] [PubMed]
- Polykratis A., Hermance N., Zelic M., Roderick J., Kim C., Van T.M., Lee T.H., Chan F.K.M., Pasparakis M., Kelliher M.A. Cutting edge: RIPK1 Kinase inactive mice are viable and protected from TNF-induced necroptosis in vivo. J. Immunol. 2014;193:1539–1543. doi: 10.4049/jimmunol.1400590. [DOI] [PMC free article] [PubMed] [Google Scholar]; Polykratis A., Hermance N., Zelic M., Roderick J., Kim C., Van T.M., Lee T.H., Chan F.K.M., Pasparakis M. and Kelliher M.A. (2014). Cutting edge: RIPK1 Kinase inactive mice are viable and protected from TNF-induced necroptosis in vivo, J. Immunol. 193, 1539-1543. [DOI] [PMC free article] [PubMed]
- Rickard J.A., O'Donnell J.A., Evans J.M., Lalaoui N., Poh A.R., Rogers T., Vince J.E., Lawlor K.E., Ninnis R.L., Anderton H. RIPK1 regulates RIPK3-MLKL-driven systemic inflammation and emergency hematopoiesis. Cell. 2014;157:1175–1188. doi: 10.1016/j.cell.2014.04.019. [DOI] [PubMed] [Google Scholar]; Rickard, J.A., O'Donnell, J.A., Evans, J.M., Lalaoui, N., Poh, A.R., Rogers, T., Vince, J.E., Lawlor, K.E., Ninnis, R.L., Anderton, H., et al. (2014). RIPK1 regulates RIPK3-MLKL-driven systemic inflammation and emergency hematopoiesis. Cell 157, 1175-1188. [DOI] [PubMed]
- Riedl S.J., Salvesen G.S. The apoptosome: signalling platform of cell death. Nat. Rev. Mol. Cell Biol. 2007;8:405–413. doi: 10.1038/nrm2153. [DOI] [PubMed] [Google Scholar]; Riedl, S.J., and Salvesen, G.S. (2007). The apoptosome: signalling platform of cell death. Nat. Rev. Mol. Cell Biol. 8, 405-413. [DOI] [PubMed]
- Sakata K., Araki K., Nakano H., Nishina T., Komazawa-Sakon S., Murai S., Lee G.E., Hashimoto D., Suzuki C., Uchiyama Y. Novel method to rescue a lethal phenotype through integration of target gene onto the X-chromosome. Sci. Rep. 2016;6:37200. doi: 10.1038/srep37200. [DOI] [PMC free article] [PubMed] [Google Scholar]; Sakata, K., Araki, K., Nakano, H., Nishina, T., Komazawa-Sakon, S., Murai, S., Lee, G.E., Hashimoto, D., Suzuki, C., Uchiyama, Y., et al. (2016). Novel method to rescue a lethal phenotype through integration of target gene onto the X-chromosome. Sci. Rep. 6, 37200. [DOI] [PMC free article] [PubMed]
- Schattenberg J.M., Zimmermann T., Worns M., Sprinzl M.F., Kreft A., Kohl T., Nagel M., Siebler J., Bergkamen H.S., He Y.W. Ablation of c-FLIP in hepatocytes enhances death-receptor mediated apoptosis and toxic liver injury in vivo. J. Hepatol. 2011;55:1272–1280. doi: 10.1016/j.jhep.2011.03.008. [DOI] [PubMed] [Google Scholar]; Schattenberg, J.M., Zimmermann, T., Worns, M., Sprinzl, M.F., Kreft, A., Kohl, T., Nagel, M., Siebler, J., Bergkamen, H.S., He, Y.W., et al. (2011). Ablation of c-FLIP in hepatocytes enhances death-receptor mediated apoptosis and toxic liver injury in vivo. J. Hepatol. 55, 1272-1280. [DOI] [PubMed]
- Shindo R., Kakehashi H., Okumura K., Kumagai Y., Nakano H. Critical contribution of oxidative stress to TNFalpha-induced necroptosis downstream of RIPK1 activation. Biochem. Biophys. Res. Commun. 2013;436:212–216. doi: 10.1016/j.bbrc.2013.05.075. [DOI] [PubMed] [Google Scholar]; Shindo, R., Kakehashi, H., Okumura, K., Kumagai, Y., and Nakano, H. (2013). Critical contribution of oxidative stress to TNFalpha-induced necroptosis downstream of RIPK1 activation. Biochem. Biophys. Res. Commun. 436, 212-216. [DOI] [PubMed]
- Taniwaki T., Haruna K., Nakamura H., Sekimoto T., Oike Y., Imaizumi T., Saito F., Muta M., Soejima Y., Utoh A. Characterization of an exchangeable gene trap using pU-17 carrying a stop codon-beta geo cassette. Dev. Growth Differ. 2005;47:163–172. doi: 10.1111/j.1440-169X.2005.00792.x. [DOI] [PubMed] [Google Scholar]; Taniwaki, T., Haruna, K., Nakamura, H., Sekimoto, T., Oike, Y., Imaizumi, T., Saito, F., Muta, M., Soejima, Y., Utoh, A., et al. (2005). Characterization of an exchangeable gene trap using pU-17 carrying a stop codon-beta geo cassette. Dev. Growth Differ. 47, 163-172. [DOI] [PubMed]
- Tanner S.M., Berryhill T.F., Ellenburg J.L., Jilling T., Cleveland D.S., Lorenz R.G., Martin C.A. Pathogenesis of necrotizing enterocolitis: modeling the innate immune response. Am. J. Pathol. 2015;185:4–16. doi: 10.1016/j.ajpath.2014.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]; Tanner, S.M., Berryhill, T.F., Ellenburg, J.L., Jilling, T., Cleveland, D.S., Lorenz, R.G., and Martin, C.A. (2015). Pathogenesis of necrotizing enterocolitis: modeling the innate immune response. Am. J. Pathol. 185, 4-16. [DOI] [PMC free article] [PubMed]
- Tenev T., Bianchi K., Darding M., Broemer M., Langlais C., Wallberg F., Zachariou A., Lopez J., MacFarlane M., Cain K. The Ripoptosome, a signaling platform that assembles in response to genotoxic stress and loss of IAPs. Mol. Cell. 2011;43:432–448. doi: 10.1016/j.molcel.2011.06.006. [DOI] [PubMed] [Google Scholar]; Tenev, T., Bianchi, K., Darding, M., Broemer, M., Langlais, C., Wallberg, F., Zachariou, A., Lopez, J., MacFarlane, M., Cain, K., et al. (2011). The Ripoptosome, a signaling platform that assembles in response to genotoxic stress and loss of IAPs. Mol. Cell 43, 432-448. [DOI] [PubMed]
- Vaishnava S., Yamamoto M., Severson K.M., Ruhn K.A., Yu X., Koren O., Ley R., Wakeland E.K., Hooper L.V. The antibacterial lectin RegIIIgamma promotes the spatial segregation of microbiota and host in the intestine. Science. 2011;334:255–258. doi: 10.1126/science.1209791. [DOI] [PMC free article] [PubMed] [Google Scholar]; Vaishnava, S., Yamamoto, M., Severson, K.M., Ruhn, K.A., Yu, X., Koren, O., Ley, R., Wakeland, E.K., and Hooper, L.V. (2011). The antibacterial lectin RegIIIgamma promotes the spatial segregation of microbiota and host in the intestine. Science 334, 255-258. [DOI] [PMC free article] [PubMed]
- Varfolomeev E.E., Schuchmann M., Luria V., Chiannilkulchai N., Beckmann J.S., Mett I.L., Rebrikov D., Brodianski V.M., Kemper O.C., Kollet O. Targeted disruption of the mouse Caspase 8 gene ablates cell death induction by the TNF receptors, Fas/Apo1, and DR3 and is lethal prenatally. Immunity. 1998;9:267–276. doi: 10.1016/s1074-7613(00)80609-3. [DOI] [PubMed] [Google Scholar]; Varfolomeev, E.E., Schuchmann, M., Luria, V., Chiannilkulchai, N., Beckmann, J.S., Mett, I.L., Rebrikov, D., Brodianski, V.M., Kemper, O.C., Kollet, O., et al. (1998). Targeted disruption of the mouse Caspase 8 gene ablates cell death induction by the TNF receptors, Fas/Apo1, and DR3 and is lethal prenatally. Immunity 9, 267-276. [DOI] [PubMed]
- Vivier E., Artis D., Colonna M., Diefenbach A., Di Santo J.P., Eberl G., Koyasu S., Locksley R.M., McKenzie A.N.J., Mebius R.E. Innate lymphoid cells: 10 years on. Cell. 2018;174:1054–1066. doi: 10.1016/j.cell.2018.07.017. [DOI] [PubMed] [Google Scholar]; Vivier, E., Artis, D., Colonna, M., Diefenbach, A., Di Santo, J.P., Eberl, G., Koyasu, S., Locksley, R.M., McKenzie, A.N.J., Mebius, R.E., et al. (2018). Innate lymphoid cells: 10 years on. Cell 174, 1054-1066. [DOI] [PubMed]
- Webster J.D., Solon M., Haller S., Newton K. Detection of necroptosis by phospho-RIPK3 immunohistochemical labeling. Methods Mol. Biol. 2018;1857:153–160. doi: 10.1007/978-1-4939-8754-2_15. [DOI] [PubMed] [Google Scholar]; Webster, J.D., Solon, M., Haller, S., and Newton, K. (2018). Detection of necroptosis by phospho-RIPK3 immunohistochemical labeling. Methods Mol. Biol. 1857, 153-160. [DOI] [PubMed]
- Weinlich R., Oberst A., Beere H.M., Green D.R. Necroptosis in development, inflammation and disease. Nat. Rev. Mol. Cell Biol. 2017;18:127–136. doi: 10.1038/nrm.2016.149. [DOI] [PubMed] [Google Scholar]; Weinlich, R., Oberst, A., Beere, H.M., and Green, D.R. (2017). Necroptosis in development, inflammation and disease. Nat. Rev. Mol. Cell Biol. 18, 127-136. [DOI] [PubMed]
- Welz P.S., Wullaert A., Vlantis K., Kondylis V., Fernandez-Majada V., Ermolaeva M., Kirsch P., Sterner-Kock A., van Loo G., Pasparakis M. FADD prevents RIP3-mediated epithelial cell necrosis and chronic intestinal inflammation. Nature. 2011;477:330–334. doi: 10.1038/nature10273. [DOI] [PubMed] [Google Scholar]; Welz, P.S., Wullaert, A., Vlantis, K., Kondylis, V., Fernandez-Majada, V., Ermolaeva, M., Kirsch, P., Sterner-Kock, A., van Loo, G., and Pasparakis, M. (2011). FADD prevents RIP3-mediated epithelial cell necrosis and chronic intestinal inflammation. Nature 477, 330-334. [DOI] [PubMed]
- Wu J., Huang Z., Ren J., Zhang Z., He P., Li Y., Ma J., Chen W., Zhang Y., Zhou X. Mlkl knockout mice demonstrate the indispensable role of Mlkl in necroptosis. Cell Res. 2013;23:994–1006. doi: 10.1038/cr.2013.91. [DOI] [PMC free article] [PubMed] [Google Scholar]; Wu, J., Huang, Z., Ren, J., Zhang, Z., He, P., Li, Y., Ma, J., Chen, W., Zhang, Y., Zhou, X., et al. (2013). Mlkl knockout mice demonstrate the indispensable role of Mlkl in necroptosis. Cell Res. 23, 994-1006. [DOI] [PMC free article] [PubMed]
- Yuan J. Divergence from a dedicated cellular suicide mechanism: exploring the evolution of cell death. Mol. Cell. 2006;23:1–12. doi: 10.1016/j.molcel.2006.06.008. [DOI] [PubMed] [Google Scholar]; Yuan, J. (2006). Divergence from a dedicated cellular suicide mechanism: exploring the evolution of cell death. Mol. Cell 23, 1-12. [DOI] [PubMed]
- Zhang H., Zhou X., McQuade T., Li J., Chan F.K., Zhang J. Functional complementation between FADD and RIP1 in embryos and lymphocytes. Nature. 2011;471:373–376. doi: 10.1038/nature09878. [DOI] [PMC free article] [PubMed] [Google Scholar]; Zhang, H., Zhou, X., McQuade, T., Li, J., Chan, F.K., and Zhang, J. (2011). Functional complementation between FADD and RIP1 in embryos and lymphocytes. Nature 471, 373-376. [DOI] [PMC free article] [PubMed]
- Zhang N., He Y.W. An essential role for c-FLIP in the efficient development of mature T lymphocytes. J. Exp. Med. 2005;202:395–404. doi: 10.1084/jem.20050117. [DOI] [PMC free article] [PubMed] [Google Scholar]; Zhang, N., and He, Y.W. (2005). An essential role for c-FLIP in the efficient development of mature T lymphocytes. J. Exp. Med. 202, 395-404. [DOI] [PMC free article] [PubMed]
- Zheng Y., Valdez P.A., Danilenko D.M., Hu Y., Sa S.M., Gong Q., Abbas A.R., Modrusan Z., Ghilardi N., de Sauvage F.J. Interleukin-22 mediates early host defense against attaching and effacing bacterial pathogens. Nat. Med. 2008;14:282–289. doi: 10.1038/nm1720. [DOI] [PubMed] [Google Scholar]; Zheng Y., Valdez P.A., Danilenko D.M., Hu Y., Sa S.M., Gong Q., Abbas A.R., Modrusan Z., Ghilardi N., de Sauvage F.J., et al., (2008). Interleukin-22 mediates early host defense against attaching and effacing bacterial pathogens, Nat. Med. 14, 282-289. [DOI] [PubMed]
- Zwarycz B., Gracz A.D., Rivera K.R., Williamson I.A., Samsa L.A., Starmer J., Daniele M.A., Salter-Cid L., Zhao Q., Magness S.T. IL22 inhibits epithelial stem cell expansion in an ileal organoid model. Cell. Mol. Gastroenterol. Hepatol. 2019;7:1–17. doi: 10.1016/j.jcmgh.2018.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]; Zwarycz, B., Gracz, A.D., Rivera, K.R., Williamson, I.A., Samsa, L.A., Starmer, J., Daniele, M.A., Salter-Cid, L., Zhao, Q., and Magness, S.T. (2019). IL22 inhibits epithelial stem cell expansion in an ileal organoid model. Cell. Mol. Gastroenterol. Hepatol. 7, 1-17. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.