Abstract
Complement-mediated damage to the neuromuscular junction (NMJ) is a key mechanism of pathology in myasthenia gravis (MG), and therapeutics inhibiting complement have shown evidence of efficacy in the treatment of MG. In this study, we describe the development of a subcutaneously administered N-acetylgalactosamine (GalNAc)-conjugated small interfering RNA (siRNA) targeting the C5 component of complement that silences C5 expression in the liver (ALN-CC5). Treatment of wild-type rodents with ALN-CC5 resulted in robust and durable suppression of liver C5 expression. Dose-dependent serum C5 suppression was observed in non-human primates, with a lowering of serum C5 of up to 97.5% and the concomitant inhibition of serum complement activity. C5 silencing was efficacious in ameliorating disease symptoms in two standard rat models of MG, demonstrating the key role of circulating C5 in pathology at the NMJ. Improvement in disease activity scores and NMJ pathology was observed at intermediate levels of complement activity inhibition, suggesting that complete ablation of complement activity may not be required for efficacy in MG. The pre-clinical studies of ALN-CC5 and efficacy of C5 silencing in rat models of MG support further clinical development of ALN-CC5 as a potential therapeutic for the treatment of MG and other complement-mediated disorders.
Keywords: myasthenia gravis, small-interfering RNA, complement pathway, siRNA therapeutic
Introduction
Myasthenia gravis (MG) is a disease caused by autoantibodies directed toward proteins of the neuromuscular junction (NMJ), primarily the acetylcholine receptor (AChR), that lead to neuromuscular transmission failure.1 Several observations support that complement activation is the primary effector mechanism in humans and in animal models of MG. C3 activation fragments, C9, and components of the membrane attack complex (MAC) are detected at motor endplates both in patients and in animals with experimental autoimmune MG (EAMG).2, 3, 4, 5, 6 Depletion of C3 by cobra venom factor protects rats against induction of EAMG.7 Complement-component-deficient rats are resistant to induction of passive transfer MG (PTMG) and EAMG.8, 9, 10 Finally, engineered knockout of intrinsic complement inhibitors enhances the severity of PTMG.11, 12, 13 Therefore, targeting complement activation is a rational approach for MG.
Strong support for complement inhibition as a therapeutic approach for MG has been demonstrated by several preclinical studies. Administration of decay accelerating factor targeted to the neuromuscular junction,14, 15 siRNA to C2,16 antibody to C6,17 soluble complement receptor 1,18 and inhibition of C5 by antibody or recombinant inhibitor19, 20 have been shown to reduce the severity of EAMG. The clinical translatability of complement inhibition in preclinical MG models has been supported by data from phase 2 and 3 trials in patients with refractory generalized MG, of eculizumab, an antibody that blocks human C5, with results demonstrating clinical benefit with limited adverse effects.21, 22 These trials led to the approval of eculizumab as a first-in-class C5 inhibitor for the treatment of generalized MG. Eculizumab’s efficacy is limited in certain individuals by genetic polymorphisms within the antibody-binding region of C5.23, 24 Additionally, a therapeutic with infrequent subcutaneous administration may be more convenient for patients than an every-2-week infusion of eculizumab.
RNAi is a naturally occurring cellular mechanism that regulates gene expression.25 Synthetic small interfering RNAs (siRNAs) can be designed to target an endogenous mRNA transcript of any gene.26 siRNAs targeted to liver-expressed genes, such as C5, can be efficiently and specifically delivered to hepatocytes using conjugation to N-acetylgalactosamine (GalNAc), which binds the asialoglycoprotein receptor (ASGPR).27 Subcutaneously administered GalNAc-siRNA conjugates have demonstrated specific, robust, and durable suppression of multiple target genes in animals. Furthermore, several investigational RNAi therapeutics have been evaluated in human trials, establishing clinical translation of GalNAc-siRNA-mediated silencing and demonstrating favorable safety results and positive efficacy, with the potential for monthly or less frequent subcutaneous (s.c.) dosing.28 In the present investigation, we describe the development of C5-targeted GalNAc-siRNAs and their impact on complement activity in wild-type animals and demonstrate their ability to ameliorate the severity of PTMG and EAMG by specifically suppressing the synthesis of liver-derived C5.
Results
Identification of GalNAc siRNA Conjugates Targeting C5
siRNA sequences that are cross-reactive to the rodent, primate, and human versions of the C5 gene were identified by using Alnylam bioinformatic tools. Chemically stabilized duplexes conjugated to a tri-antennary GalNAc on the 3′ end of the sense strand were synthesized, using solid-phase synthesis methods.27 siRNAs were tested in vitro in Hep3B cells for C5 silencing by transfection to identify the most potent duplexes. Two duplexes, siRNA-C5 and ALN-CC5, with in vitro silencing IC50s of 31 and 36 pM, respectively, were used to evaluate C5 silencing in animal studies.
Characterization of C5 Silencing in Wild-Type Rodents
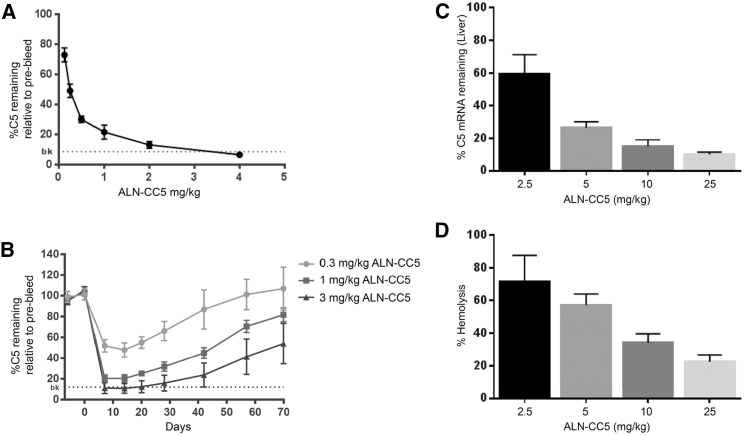
The effect of s.c. administration of ALN-CC5 on circulating C5 levels was first characterized in C57BL/6 mice. Single s.c. administration of ALN-CC5 at doses ranging from 0.1 to 4 mg/kg resulted in a dose-dependent reduction in circulating C5 levels at day 9 after injection (Figure 1A). The ALN-CC5 half-maximal effective concentration (EC50) was estimated to be 0.25 mg/kg, with >85% suppression of circulating C5 achieved in the 4 mg/kg dose group. A single s.c. administration of ALN-CC5 was highly durable, with animals treated with the 3 mg/kg dose achieving C5 silencing of greater than 85% by day 7 and 50% C5 silencing still observed by day 70 (Figure 1B).
Figure 1.
ALN-CC5 Lowers Circulating C5 Protein Levels and Complement Hemolytic Activity in Mice and Rats
(A and B) Levels of mouse serum C5 protein were measured by ELISA. (A) ALN-CC5 potency at day 9 after single-dose treatment. (B) Duration of C5 reduction after single-dose treatment. N = 5 per group. Dotted line is the assay background observed in C5-deficient DBA/2 mice. (C and D) Female rats were evaluated on day 8 following a single injection of 2.5, 5, 10, or 25 mg/kg of ALN-CC5. (C) C5 liver mRNA was quantified by qRT-PCR and normalized to untreated rats. (D) Serum hemolytic complement activity was quantified using a sensitized sheep red blood cell (RBC) lysis assay. N = 3–4 animals per group; error bars are SD. The 5 mg/kg group showed significant reduction at p < 0.05, and the 10 and 25 mg/kg groups showed significant reduction at Bonferroni-corrected p < 0.0125. Error bars are SD.
C5 silencing was further characterized in rats, where complement activity can be assessed more robustly. A single s.c. administration of ALN-CC5 at doses ranging from 2.5 to 25 mg/kg resulted in a dose-dependent reduction in rat liver C5 mRNA levels, with up to 90% reduction at the peak dose on day 8 (Figure 1C). Circulating C5 levels were correspondingly decreased (data not shown). Classical pathway hemolytic activity showed a dose-dependent reduction of up to 75% at the top dose (Figure 1D). siRNA-C5 demonstrated comparable silencing in normal rodents (data not shown).
Efficacy of ALN-CC5 in Non-human Primates
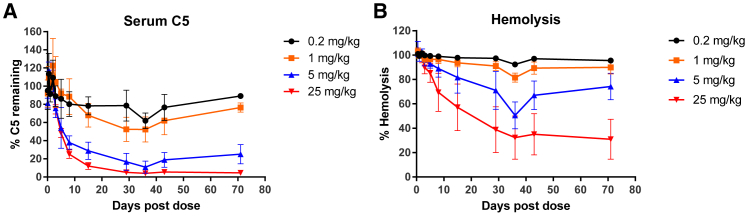
To further advance the development of ALN-CC5 as an investigational RNAi therapeutic, its pharmacodynamic activity was characterized in cynomolgus monkeys. A single s.c. administration of ALN-CC5 resulted in a dose-dependent suppression of serum C5 protein levels with a half-maximal effective dose (ED50) estimated to be ∼1 mg/kg and significant reduction in hemolytic activity at higher doses (Figure 2). Sustained reduction of circulating C5 was appreciated for 71 days (the last observation point). The nadir for serum C5 protein silencing and hemolysis suppression (65%–70%) was achieved by day 30.
Figure 2.
Potent and Durable C5 Silencing and Complement Activity Reduction in NHPs with Single-Dose ALN-CC5 Treatment
(A) C5 protein quantified by ELISA levels following a single s.c. injection of ALN-CC5. N = 6 through day 36, N = 4 on day 43, and N = 2 on day 71. For 0.2–5 mg/kg treatments, C5 levels were normalized to the average C5 levels at 2 and 15 min and 8 h after dose time points, which were expected to be equivalent to baseline levels. For the 25 mg/kg group, C5 levels are normalized to the level at 24 h after the dose, expected to be within 20% of baseline. (B) Hemolytic complement activity levels were evaluated in serum samples collected as in (A), using a sheep RBC hemolysis assay. Hemolysis values were normalized to a “maximal hemolysis” control (lysis by water). Group averages with SD are plotted for both readouts.
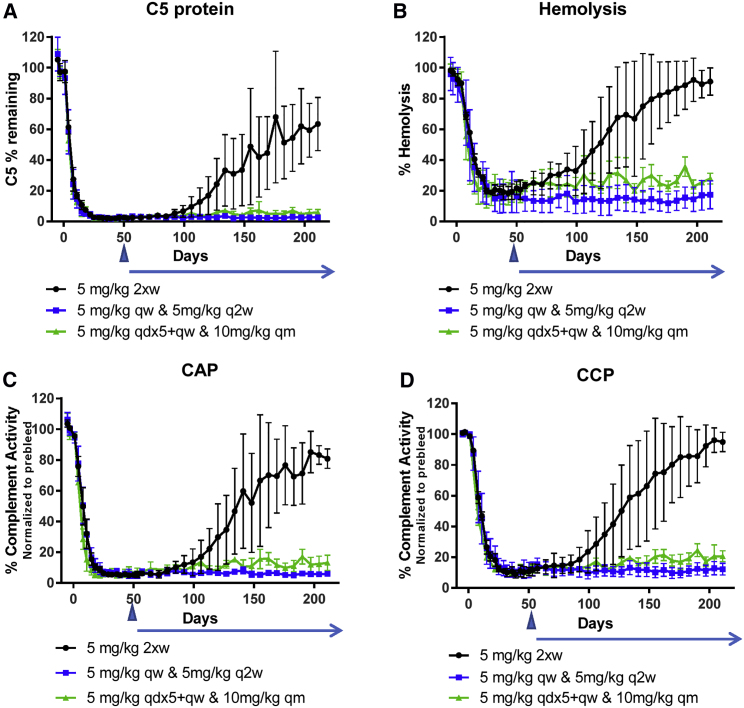
Repeat-dose pharmacology in cynomolgus monkeys was evaluated as described in the Materials and Methods to achieve maximal C5 silencing and complement inhibition. Daily, weekly, or twice-weekly s.c. administration of 5 mg/kg of CC5-GalNAc achieved equivalent decreases in serum C5 protein levels, which were maintained with continued dosing. Compared with a single 5 mg/kg dose, multiple s.c. administrations of a 5 mg/kg dose of ALN-CC5 resulted in a greater reduction in serum C5 levels: a maximal reduction of 89.1% for a single 5 mg/kg dose (Figure 2A) versus 97.5% for a weekly 5 mg/kg regimen (Figure 3A). Time to nadir was equivalent between the three induction regimens tested, suggesting that C5 lowering is limited by the protein’s half-life. At 24 weeks after the last dose for group 1, recovery of C5 levels was observed, with average levels reaching 70% of initial baseline C5 levels.
Figure 3.
Robust Reduction in C5 Protein Levels and Complement Activity in NHPs Treated with Multiple Doses of ALN-CC5
(A) Serum C5 levels were quantified by ELISA and normalized to the average of the three pre-dose samples for each animal. (B) Serum hemolytic activity was evaluated by a sensitized sheep RBC assay. Hemolysis is expressed as the percentage of “maximal hemolysis” control (lysis with water). Alternative (C) and classical (D) pathway activities were quantified by Weislab ELISA assays. CAP and CCP activities were calculated relative to the human complement standard and normalized to the average pre-dose activity. For all panels, group means (N = 3) and SDs are plotted. Initial dosing regimens were administered for 8 weeks (day 53). Arrowhead, end of initial dosing, no further dosing for the 5 mg/kg group; blue horizontal arrow, additional dosing regimens for the duration of the study; qw, weekly dosing; q2w, every-2-week dosing; 2xw, twice-a-week dosing; qdx5, daily dosing for 5 days; qm, monthly dosing.
Reduction of hemolytic activity of ≥80% was observed in animals that had a 5 mg/kg dose administered weekly or twice weekly by day 32 (Figure 3B). In group 1 hemolysis, inhibition remained stable for approximately 3 weeks after cessation of dosing, before gradually increasing, with hemolysis levels reaching within 20% of baseline by day 141. Overall, the level of hemolysis suppression achieved in cynomolgus monkeys was comparable to or greater than that observed in rats (Figure 1D). Classical complement pathway (CCP) or alternative complement pathway (CAP) activity was also evaluated by quantifying formation of the C5b-9 neo-antigen after engagement of a pathway-specific activator. Up to 89% reduction in CCP and 95% reduction in CAP were observed in ALN-CC5-treated animals (Figures 3C and 3D). All three complement activity readouts showed significant non-linear correlation with C5 level (data not shown).
C5 Silencing Eliminates PTMG-Induced Weakness
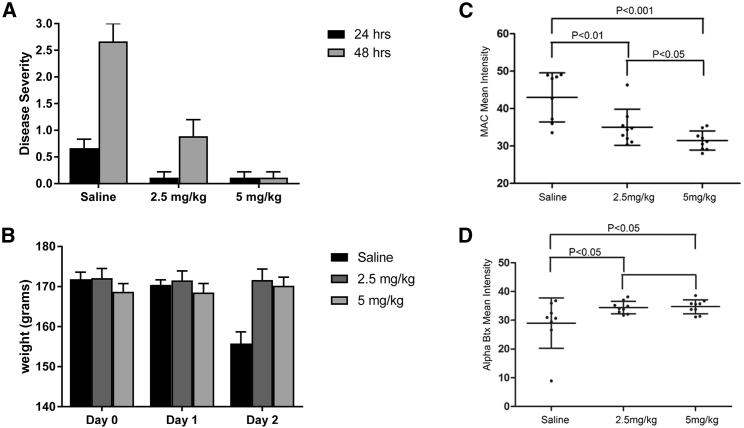
The impact of C5 silencing was then evaluated in rat MG models by using siRNA-C5, a duplex with efficacy equivalent to ALN-CC5 (data not shown). Because of the short duration of the PTMG model, animals were pre-treated with siRNA-C5 at 5 or 2.5 mg/kg to allow for maximal C5 silencing at the time of disease induction. Twenty-four hours after administration of McAb3, animals that received saline developed significant weakness, whereas siRNA-C5-treated animal had essentially no weakness (Figure 4A). At 48 hours, minimal weakness was evident in the low-dose treatment group, and no weakness was observed in the majority of animals in the high-dose group, in contrast to the severe weakness observed in the control group. Control animals had a significant 15% drop in weight on day 2 after McAb3 administration, whereas no weight loss occurred among siRNA-C5-treated animals (Figure 4B). NMJs of treated animals had significantly less intensity of MAC and greater bungarotoxin (BTX) fluorescence staining, demonstrating protection from injury (Figures 4C and 4D).
Figure 4.
C5 Silencing Reduces Weakness and Weight Loss Produced by PTMG in Rats and Protects NMJs from Complement-Mediated Destruction
Twenty-four rats were randomly assigned to three groups (n = 8 per group): siRNA-C5 (2.5 mg/mL), siRNA-C5 (5 mg/kg) or saline control. Treatment was administered 10, 7, and 3 days prior to and on the day of PTMG induction. (A) Mean disease severity of each group 24 and 48 h after PTMG induction. (B) Mean weight of animals after PTMG induction on day 0 (t test p < 0.01 for day 2 weight loss relative to both siRNA-treated groups). Error bars are SEM. Tibialis anterior muscle sections were stained with anti-membrane attack complex (MAC) antibody and detected with Alexa-488-labeled anti-rabbit IgG (C). Alexa-594-labeled bungarotoxin was used to identify the NMJ at 48 h after PTMJ induction (D). Quantitation of fluorescence intensity was performed with Zen software. Mean pixel intensity values were plotted for each group (n = 8 per group). Statistical significance was determined (ANOVA: p value is 0.00217; t test: p < 0.01 is considered significant). Error bars are SD.
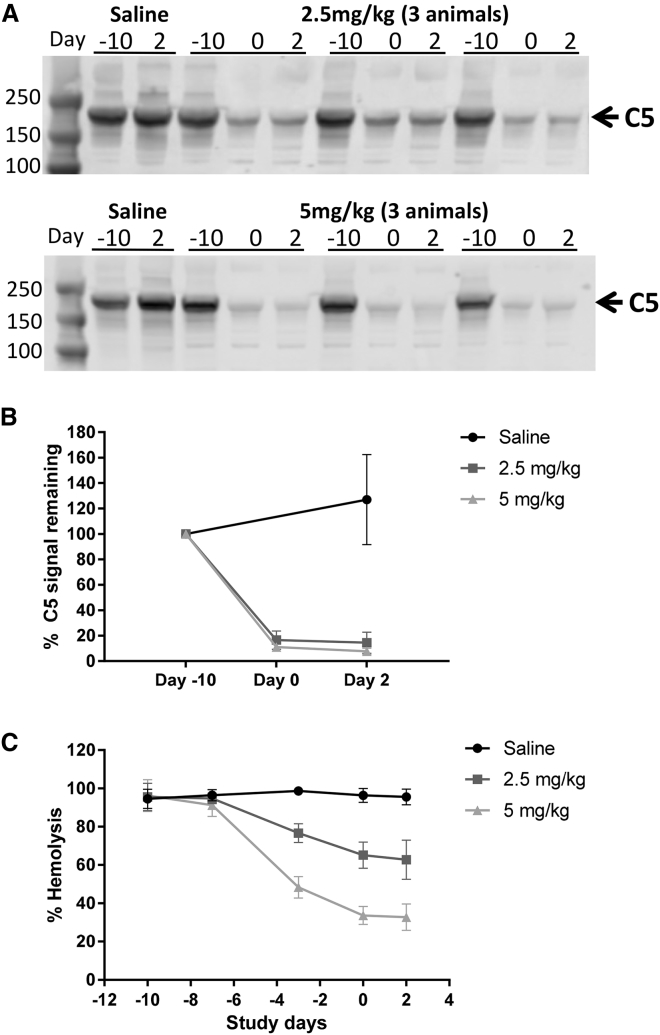
As was observed in healthy animals, liver C5 mRNA expression was reduced by approximately 10-fold, with both low- and high-dose treatments (data not shown). Serum C5 protein levels and hemolytic activity dropped after siRNA-C5 administration, reaching maximal effect at the time of PTMG induction. Serum C5 protein was reduced to less than 20% of baseline levels at day 0, with a slightly greater decrease produced by the high-dose treatment (Figures 5A and 5B). Hemolytic activity was reduced after two doses of siRNA-C5 and remained suppressed through the end of the study. High-dose siRNA-C5 group had reduced hemolytic activity by 70%, whereas the lower dose dropped hemolytic activity by 40% relative to baseline (Figure 5C).
Figure 5.
C5 siRNA Reduces C5 Protein Level and Hemolytic Activity in PTMG Rats
(A) Western blot analysis of C5 protein from rat serum prior to treatment (day −10), on the day of PTMG induction (day 0), and at the end of the experiment (day 2). Treatment with 2.5 (top) and 5 (bottom) mg/kg siRNA-C5 are shown for three representative animals in each treatment group and 1 control animal. (B) Quantification of the western blot signal; t test p < 0.01 for saline- versus siRNA-treated groups at day 2. (C) Mean hemolytic activity over the course of the experiment (n = 8 per group). The error bars are SD.
C5 Silencing Moderates EAMG Severity
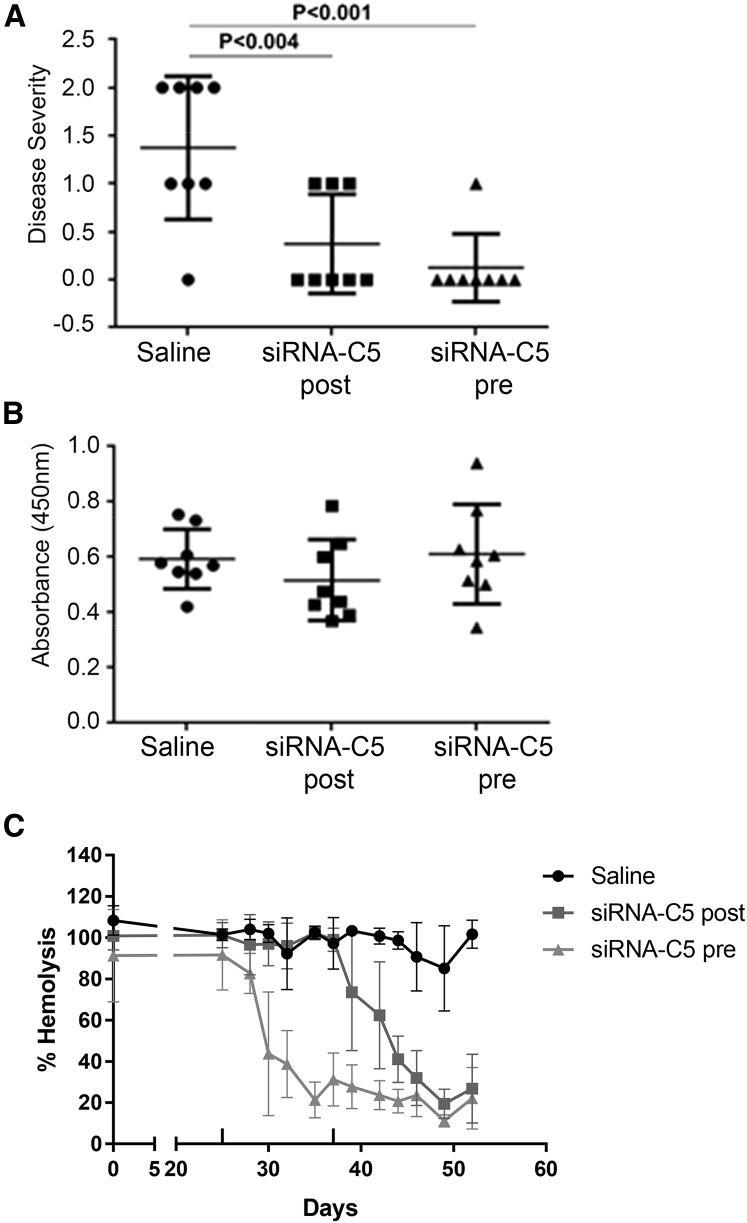
Based on its better efficacy, we used a twice-a-week 5 mg/kg treatment for an active immunization EAMG study, starting treatment either before (day 25) or after (day 37) development of weakness. At the end of the study (day 52), control rats were significantly weaker than those treated with siRNA-C5 before (p < 0.001) or after development of weakness (p < 0.004; Figure 6A). Serum AChR antibody levels were not influenced by siRNA-C5 treatment (Figure 6B). siRNA-C5 reduced serum hemolytic activity after the second dose in both the pre- and post-treatment groups, with approximately 20% hemolytic activity remaining at the end of the experiment (Figure 6C). Hemolytic activity reduction in the context of the inflammatory EAMG model (induced with complete Freund’s adjuvant) was comparable to that achieved in healthy rats (Figure 1C).
Figure 6.
C5 siRNA Treatment Ameliorates Weakness in EAMG Rats
Twenty-four rats were immunized with Torpedo acetylcholine receptor (AChR) to induce EAMG. Animals were randomized into three groups (n = 8 per group): the pre-treatment group, 5 mg/kg of siRNA-C5 twice a week before development of weakness (day 25); the post-treatment group, 5 mg/kg of siRNA-C5 twice a week after observation of weakness (day 52); and the control saline group. (A) Disease severity of EAMG rats at the end of the study. (B) ELISA of circulating AChR-specific total IgG antibodies in the serum. (C) Serum hemolytic activity (sensitized sheep RBC lysis method). Averages of eight animals per group are plotted. Statistical significance was obtained by t test, and p < 0.05 was considered significant. The error bars are SD.
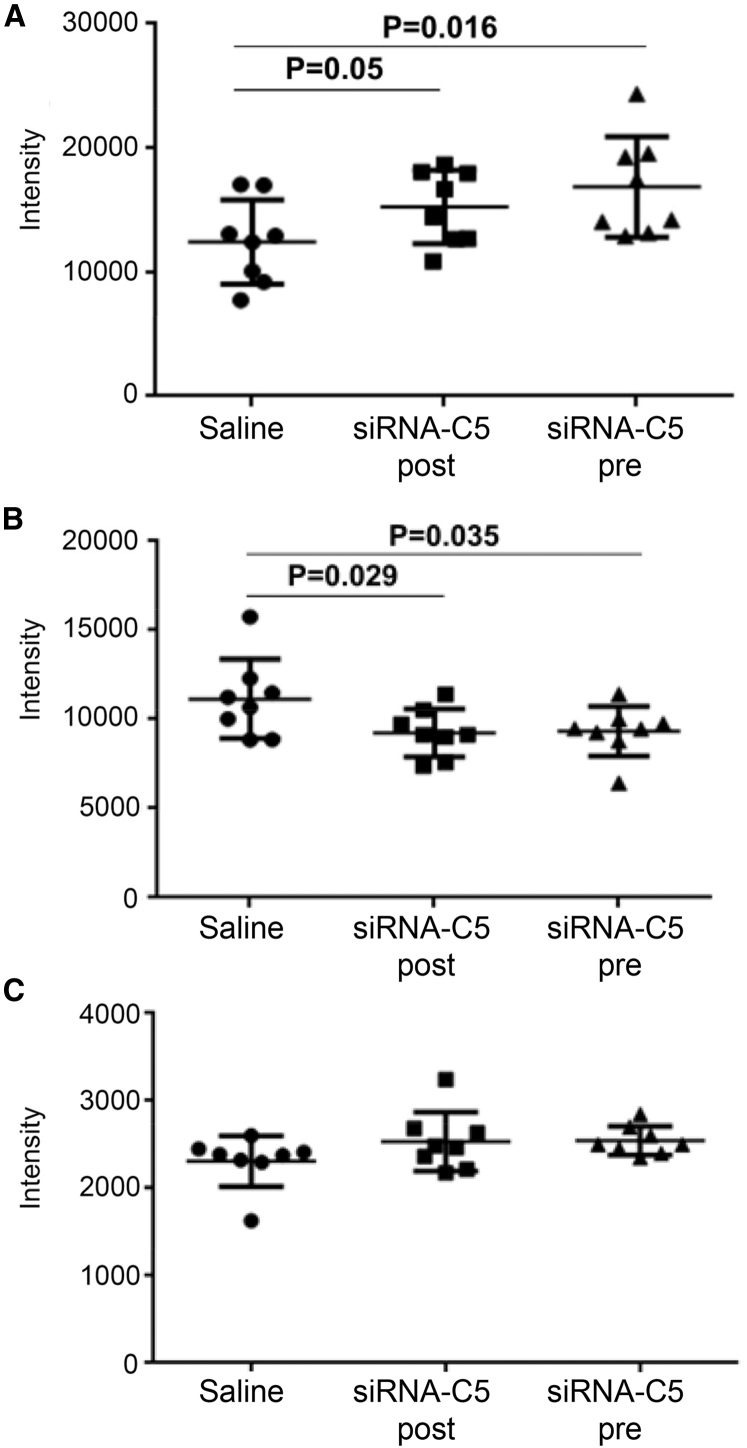
Control rats lost weight in the last 7 days (−0.93 ± 4.6 g) compared (p < 0.05) to the siRNA-C5 treatment groups (2.4 ± 2.6 g for the post-treatment group; 3.2 ± 2.1 g for the pre-treatment group; data not shown). AChR density at the NMJ was higher in both treatment groups than in the saline-treated group (Figure 7A), and MAC deposition was reduced in each treatment group compared to control, with no difference in MAC deposition between the two treatment groups (Figure 7B). C3 deposition at the NMJ was similar across all groups (Figure 7C).
Figure 7.
C5 siRNA Treatment Protects the Neuromuscular Junctions of EAMG Rats from Complement-Mediated Destruction in Both Pre- and Post-Treatment Models
Tibialis anterior muscle sections were stained with Alexa-594-labeled bungarotoxin for identification of NMJ, Alexa-488-labeled anti-rabbit IgG for detection of MAC deposition, and Alexa-488-labeled anti-mouse IgG for detection of C3. Immunofluorescence confocal microscopy was performed. Quantitation of fluorescence intensity was performed using Zen software. Mean pixel intensity values were plotted for each group (n = 8 per group). Statistical significance was obtained by t test and p < 0.05 was considered as significant. Error bars are SD. (A) Mean fluorescence intensity of bungarotoxin at the NMJ. (B) Mean fluorescence intensity of MAC deposition at the NMJ. (C) C3 deposition at the NMJ.
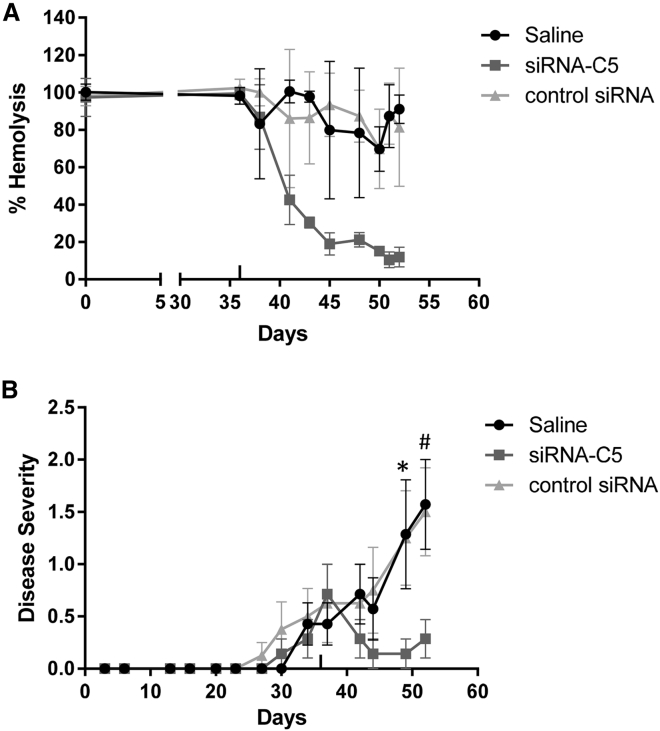
We performed a second EAMG experiment initiating treatment only after the development of weakness, including an siRNA sequence against an irrelevant transcript (luciferase) as an additional control. Serum hemolytic activity and liver C5 mRNA levels were reduced only in the siRNA-C5-treated animals (Figure 8A; data not shown). siRNA-C5 treated animals again showed an improvement in severity scores, whereas saline and irrelevant siRNA-treated controls continued to develop significant weakness (Figure 8B). siRNA-C5-treated animals showed an improvement in weakness starting on day 42 at which time 40% reduction in serum hemolytic activity was observed (Figure 8B), consistent with the disease improvement with low-dose siRNA-C5 treatment in the PTMG model.
Figure 8.
C5 siRNA Treatment Specifically Lowers Complement Activity and Reduces Symptoms in EAMG Rats as Compared to Control siRNA
In a second EAMG experiment, 24 rats were immunized with Torpedo AChR. After development of weakness, rats were randomized to three groups (n = 8 per group): 5 mg/kg siRNA-C5, 5 mg/kg control siRNA, and saline control. (A) Serum hemolytic activity (sensitized sheep RBC lysis method). Average hemolytic activity is plotted. The error bars are SD. (B) Disease severity of each group. The error bars are SEM. Statistical significance was obtained by t test, comparing saline and control siRNA to siRNA-C5 (*p < 0.05 and #p < 0.02).
Discussion
We demonstrated that GalNAc-mediated targeting of ALN-CC5 to hepatocytes enabled potent reduction of liver C5 mRNA levels and subsequent dramatic lowering of C5 protein in mice, rats, and non-human primates (NHPs). Reductions of circulating C5 of up to 97.5% were observed in NHPs, demonstrating that the vast majority of serum C5 protein is liver derived. C5 silencing robustly reduced alternative and classical complement activity in NHPs. Single-dose administration of ALN-CC5 in NHPs resulted in durable suppression of C5 and hemolytic activity for up to 71 days, suggesting that infrequent s.c. dosing of ALN-CC5 could provide efficacy in complement-mediated diseases. siRNA-C5 significantly abrogated the severity of PTMG to the point that treated animals had minimal evidence of weakness, whereas controls were nearly moribund, with significant weight loss. Similarly, in an active immunization model, EAMG severity was reduced, regardless of whether treatment was begun before or after signs of weakness were present. Importantly, control siRNA-treated animals were similar to non-treated animals, indicating the specificity of the results to C5 inhibition.
The role of complement in human MG pathology has been demonstrated in the clinic by the recent approval of the anti-C5 antibody eculizumab for the treatment of MG.21 The results presented here support that the primary mechanism of injury to the NMJ in the standard animal models of MG is through the action of serum-derived complement components. Both PTMG and EAMG experiments demonstrated a decrease in serum complement activity and reduction, but not complete elimination, of MAC deposition at the NMJ, which was associated with improved motor scores with C5-targeted siRNA treatment. Of critical importance is the observation that ablation of complement activity was not necessary to derive a therapeutic effect, and this was also observed in EAMG studies of a C5 antibody.19 This should not be surprising, since the damaged post-synaptic surface can maintain normal neuromuscular transmission despite upward of 60% loss of AChR.5
In the EAMG experiments, siRNA treatment did not affect anti-AChR antibody levels; therefore, moderation of EAMG was not mediated by reduction of antibody production. Skeletal muscle may synthesize components of complement,29 leading to the possibility that local C5 synthesis could drive NMJ injury, if only hepatic synthesis of C5 were inhibited. However, the siRNA-C5-treated animals demonstrated no significant pathology, and no C5 mRNA expression was detected in the muscle (data not shown), indicating that the C5 driving NMJ pathology is likely liver derived.
siRNA treatment after development of weakness in the EAMG model was efficacious in moderating disease severity, as evidenced by strength scores and reduction of MAC deposition, as well as retention of AChR density. Complement activity was not completely eliminated by siRNA-C5 treatment in PTMG or EAMG experiments, but normal strength was attained in many animals, indicating that a therapeutic benefit was obtained without complete ablation of complement activity. Initial improvement in weakness in the second EAMG study was observed within 1 week of the start of siRNA-C5 treatment when hemolytic activity was reduced by only 40%, in line with the improvements seen in the PTMG animals treated with a lower siRNA dose. These results suggest that a rapid improvement of complement-mediated pathology after siRNA treatment is possible with C5 silencing. Such a property would be particularly beneficial in treatment of severe MG-related weakness, such as a myasthenic crisis. The effects observed in MG models were comparable to what we have seen with C5 antibody treatment.19
ALN-CC5 produces a dose-dependent and durable reduction in C5 protein levels, with no evidence of tachyphylaxis with continued dosing for up to 7 months in NHPs. The prolonged C5 silencing observed after a single dose of ALN-CC5 could support infrequent s.c. dosing in the clinic and could be advantageous for chronic disease management. Chronic C5 inhibition has been shown to be generally safe in patients with paroxysmal nocturnal hemoglobinuria (PNH) and atypical hemolytic uremic syndrome treated with intravenous eculizumab with the potential for increased risk of meningococcal infection managed by vaccination. Early-phase clinical trials of ALN-CC5 are currently evaluating the safety and efficacy of ALN-CC5 in healthy volunteers and patients with PNH, as well as patients with IgA nephropathy (ClinicalTrials.gov: NCT02352493 and NCT03841448) The data presented here support the continued development of ALN-CC5 as a potential therapy for patients with MG and other complement-mediated disorders.
Materials and Methods
Animals (Rodents)
For PTMG and EAMG studies, female Lewis rats (100–124 g; Harlan, Indianapolis, IN, USA) were used. Animals were maintained in the George Washington University animal facility, which follows International Animal Care and Use Committee (IACUC), Association for Assessment and Accreditation of Laboratory Animal Care (AAALAC), and American Association for Laboratory Animal Science (AALAS) standards concerning appropriate housing, cage cleaning procedure, air purity, feed, temperature, humidity, and light and dark cycle. C57BL/6 mice and Sprague-Dawley rats (Charles River, Wilmington, MA, USA) were maintained at the Alnylam Pharmaceuticals animal facility, which is AALAS accredited and follows IACUC guidelines. All studies were conducted according to a protocol approved by the Alnylam IACUC. At both facilities, the animals were housed in isolator cages in a specific-pathogen-free environment, and rodent chow and water were provided ad libitum. C5 silencing was evaluated by using s.c. injection of siRNA conjugates in saline into the lower back.
Animals (NHPs)
NHP studies were conducted by Covance Laboratories in experimentally naive female Chinese cynomolgus monkeys (Macaca fascicularis) between 2 and 4 years of age and 2–4 kg in weight. Studies were conducted according to protocols developed by Covance and Alnylam and approved by the Covance IACUC. Animals were housed in accordance with the US Department of Agriculture (USDA) Animal Welfare Act (9 CFR, Parts 1, 2 and 3). Animals receiving ALN-CC5 were dosed s.c. in the scapular and mid-dorsal regions. Gross observations indicated no change in animal behavior or health throughout the study. Blood draws were collected throughout the study to evaluate the levels of C5 protein and complement activity in serum.
PTMG Model and siRNA Treatment
Twenty-four rats were randomly assigned to three groups: siRNA-C5 (2.5 mg/kg), siRNA-C5 (5 mg/kg), and saline control, by s.c. injection. Treatment was administered 10, 7, and 3 days before and on the day of PTMG induction. Blood was obtained prior to the siRNA dose and immediately prior to PTMG induction. PTMG was induced by administration of the rat anti-mouse muscle AChR monoclonal antibody (mAb) McAb3 (gift of Vanda Lennon, Mayo Clinic), similar to our previous investigations.13, 30 McAb3 is an IgG2b isotype known to activate complement. Animals underwent euthanasia 48 hours after PTMG induction. Weakness was graded on a standard 1–4 disease severity scale, with a higher score indicating more severe weakness.6 Blood, liver, diaphragm, and tibialis anterior were harvested for analysis.
EAMG Model and siRNA Treatment
Twenty-four rats received s.c. injections of 40 μg Torpedo AChR in complete Freund’s adjuvant (Sigma, St. Louis, MO, USA), given in two injections at the base of the tail. Rats were evaluated daily in a blinded fashion with a standard disease severity scale and weighed.6 Quantitative grip strength was assessed using a digital-force gauge. Animals underwent 20 test pulls prior to five measurements. On day 25 after AChR immunization, one group of eight randomly selected rats started to receive s.c. injections of siRNA-C5 (5 mg/kg) twice a week (pre-weakness treatment group). The remainder of the animals received saline injections. Once animals demonstrated weakness, saline injections were replaced by 5 mg/kg siRNA-C5 (day 37) in a second group of eight animals chosen at random (post-weakness treatment group). Animals were sacrificed 53 days after initial AChR immunization. Blood was obtained from all animals by tail vein at the time of each injection, to monitor complement activity. A second experiment was performed in an identical fashion with the following exception: treatment prior to the development of weakness was not evaluated. After development of weakness, one group of rats received siRNA-C5 (5 mg/kg) and another group received an equivalent dose of siRNA targeting the luciferase gene, which is not expressed in mammals. A third group received saline.
AChR-Specific IgG and IgG Subclass ELISA
Flat-bottomed 96-well Nunc-Immuno MicroWell plates (Sigma-Aldrich, MO, USA) were coated with purified Torpedo AChR at 10 μg/mL overnight at 4°C. Blocking buffer (PBS+0.5% [v/v] Tween 20+5% [w/v] BSA) was added. Serum was diluted to 1:1,000 for total IgG, and 1: 2,000 for IgG1 determinations; 1:64,000 and 1:128,000 dilutions were used for IgG2a, and 1:500 and 1:1,000 dilutions were used for IgG2b. Samples were assessed in duplicate. Sera were incubated for 75 minutes followed by the addition of anti-rat IgG subtypes conjugated to horseradish peroxidase (HRP; 1:1,000; Alpha Diagnostic International, TX, USA). Tetramethylbenzidine (TMB; SeraCare Lifesciences, MD, USA) was used for development and stopped with 1 M hydrochloric acid. The optical density (OD) of each plate was read at 450 nm with a Varioskan fluorescent and luminescent plate reader.
Hemolytic Assay
Serum hemolytic activity of the CCP was measured using antibody-sensitized sheep erythrocytes (EAs; Complement Technology). Sera were diluted 1:130 for rat and 1:115 for cynomolgus in gelatin-veronal-buffered saline with Ca2+ and Mg2+ (GVB2+; Complement Technology); 173 μL of diluted sample was mixed with 27 μL of EA (1.34 × 107 cells per sample). For the negative and positive controls, GVB2+ and distilled water were used, respectively. Mixed samples were incubated at 37°C with shaking at 900 rpm for 1 h and centrifuged at 1,200 rpm for 3 min, and the OD was read at 541 nm. Hemolytic activity was calculated as follows: %Hemolysis = 100 × (sample − negative control)/(positive control − negative control). The mean of each treatment group per time point was plotted, along with its SD.
C5 mRNA Quantification
C5 mRNA expression was quantified by qRT-PCR. Flash-frozen livers were pulverized by a 2000 Geno/Grinder (Spex SamplePrep), and RNA was extracted using the RNeasy Mini Kit according to the manufacturer’s protocol (QIAGEN). Reverse transcription was performed to generate cDNA according to the manufacturer’s protocol (Life Technologies). qPCR was performed on cDNA using a Roche LightCycler 480 instrument with a rat C5 Taqman FAM probe (Life Technologies) and a rat glyceraldehyde-3-phosphate dehydrogenase (GAPDH) Taqman VIC probe (Life Technologies) as the control. The C5 level was normalized to GAPDH, and the percentage of C5 mRNA remaining was calculated relative to the average of untreated or saline-treated rats.
Serum C5 Protein Assessment
Rat serum samples were analyzed by semiquantitative western blot. Sera were diluted 1:20 into 50 mM Tris (pH 7) with 1% SDS. Samples were run on 10% Bis-Tris protein gels (Life Technologies) and transferred onto a PVDF membrane (Bio-Rad). A goat anti-human C5 antibody (Complement Technology) which crosses to rat, was used at a 1:1,000 dilution with a secondary fluorophore-conjugated donkey anti-goat antibody (LI-COR) followed by imaging with the LI-COR Odyssey imaging system. Analysis and quantification of the western blot was done using LI-COR’s Image Studio software. The percent of C5 remaining in the PTMG study was calculated by normalizing rat samples from day 8 to their individual pre-bleed sample.
Mouse C5 protein levels were analyzed by ELISA. Serum was diluted 1:5,000 into 0.05 M carbonate-bicarbonate buffer (pH 9.6). The standard curve consisted of nine 2-fold dilutions beginning at 1:1,000 of wild-type C57BL/6 serum. DBA/2 serum (C5-deficient strain) was included at a 1:5,000 dilution to calculate the background of the assay. Diluted samples (100 μL) and controls were incubated at 4°C overnight in 96-well polystyrene plates (Costar). Plates were washed and blocked for 2 hours at room temperature with 1× PBS and 2% BSA. Mouse cross-reactive goat anti-human C5 antibody (Complement Technology) was added at a 1:1,000 dilution and incubated for 1 h at room temperature. HRP-conjugated anti-goat antibody (Jackson ImmunoResearch) was used at a 1:500 dilution with a TMB substrate. OD was read at 450 nm, and the relative C5 level was calculated. The percentage of C5 remaining was calculated relative to the pre-dose value, per individual mouse.
For cynomolgus serum samples, C5 protein levels were analyzed using the human C5 protein ELISA kit (ab125963; Abcam), which cross-reacts to cynomolgus C5, according to the kit’s instructions, using a purified cynomolgus C5 protein standard (Athens Biosciences) in place of the human C5 protein standard. Serum sample dilutions of 1:800 or 1:8,000 were used, depending on the expected C5 level, to ensure that signal fell within the standard curve.
Weislab ELISA Assays for Complement Activity
Plate-based complement activity assays from Svar Life Science AB (Sweden) were used to evaluate CAP or CCP in NHPs. The kits are labeled for use with human serum but cross to NHPs.31 Serum was diluted per kit instructions into plates with pathway-specific activator (IgM for CCP and lipopolysaccharide [LPS] for CAP), and the formation of the C5b-9 neoantigen was detected by an alkaline-phosphatase-conjugated antibody.
NMJ Immunohistochemistry
Ten-micron cryosections of tibialis anterior were stained for MAC deposition. Briefly, rabbit polyclonal anti-complement C5b-9 (Calbiochem), diluted 1:100, was incubated on sections for 1 h. For analysis of C3 deposition, monoclonal antibody from 12E2 clone (Enzo Life Sciences) was used at 1:100 and incubated overnight. Alexa-488-labeled anti-rabbit IgG (Molecular Probes) was used to detect MAC, Alexa-488-labeled anti-mouse IgG (cross absorbed to rat; Molecular Probes) was used to detect C3 and Alexa-594-labeled bungarotoxin (Molecular Probes) was used to identify NMJs. The sections were viewed with Leica DFC310 FX, and digital images captured with the Leica Microsystems LAS AF6000 modular systems (Wetzlar, Germany) and analyzed with Image Pro software (Media Cybernetics, Rockville, MD, USA). NMJs determined by fluorescently labeled bungarotoxin were defined as areas of interest. Pixel intensity measurements were determined, and mean values were obtained for each animal (n = 8/group).
Statistical Analysis
The data were analyzed and tested for statistical significance using paired t tests and ANOVA. Results were considered significantly different when p < 0.05 unless otherwise indicated.
Study Approval
PTMG and EAMG animal studies were conducted according to a protocol approved by the George Washington University IACUC (permit no. A247). Rats and mice were maintained at the Alnylam Pharmaceuticals animal facility, which is AALAS accredited and follows IACUC guidelines. NHP studies were performed by Covance Laboratories and conducted following protocols developed by Covance and Alnylam and approved by the Covance IACUC. Animals were housed in accordance with the USDA Animal Welfare Act (9 CFR, Parts 1, 2 and 3).
Author Contributions
L.L.K., A.B., and H.J.K. devised and performed the experiments, analyzed the data, and composed the manuscript. K.Y., A.G.S., D.D., and T.N. performed the experiments and analyzed the data. K.C., S.K., and H.J.K. designed and synthesized the siRNA molecules. K.F. designed the experiments. M.S. performed the experiments, analyzed the data, and revised the draft versions of the manuscript.
Conflicts of Interest
The work was supported by a contract from Alnylam Pharmaceuticals (with L.K.). K.Y., A.G.S., D.D., T.N., K.C., S.K., R.K., K.F., and A.B. are current or former employees of Alnylam Pharmaceuticals. A.B., K.C., S.K., K.F., and R.K. are inventors named on C5 siRNA patents.
Acknowledgments
We would like to thank members of the Alnylam small- and medium-scale synthesis teams for the synthesis, purification, and characterization of siRNAs for these studies.
References
- 1.Berrih-Aknin S., Le Panse R. [Myasthenia gravis and autoantibodies: Pathophysiology of the different subtypes] Rev. Med. Interne. 2014;35:413–420. doi: 10.1016/j.revmed.2013.09.012. [DOI] [PubMed] [Google Scholar]; Berrih-Aknin, S., and Le Panse, R. (2014). [Myasthenia gravis and autoantibodies: Pathophysiology of the different subtypes]. Rev. Med. Interne 35, 413-420. [DOI] [PubMed]
- 2.Sahashi K., Engel A.G., Linstrom J.M., Lambert E.H., Lennon V.A. Ultrastructural localization of immune complexes (IgG and C3) at the end-plate in experimental autoimmune myasthenia gravis. J. Neuropathol. Exp. Neurol. 1978;37:212–223. doi: 10.1097/00005072-197803000-00008. [DOI] [PubMed] [Google Scholar]; Sahashi, K., Engel, A.G., Linstrom, J.M., Lambert, E.H., and Lennon, V.A. (1978). Ultrastructural localization of immune complexes (IgG and C3) at the end-plate in experimental autoimmune myasthenia gravis. J. Neuropathol. Exp. Neurol. 37, 212-223. [DOI] [PubMed]
- 3.Sahashi K., Engel A.G., Lambert E.H., Howard F.M., Jr. Ultrastructural localization of the terminal and lytic ninth complement component (C9) at the motor end-plate in myasthenia gravis. J. Neuropathol. Exp. Neurol. 1980;39:160–172. doi: 10.1097/00005072-198003000-00005. [DOI] [PubMed] [Google Scholar]; Sahashi, K., Engel, A.G., Lambert, E.H., and Howard, F.M., Jr. (1980). Ultrastructural localization of the terminal and lytic ninth complement component (C9) at the motor end-plate in myasthenia gravis. J. Neuropathol. Exp. Neurol. 39, 160-172. [DOI] [PubMed]
- 4.Nakano S., Engel A.G. Myasthenia gravis: quantitative immunocytochemical analysis of inflammatory cells and detection of complement membrane attack complex at the end-plate in 30 patients. Neurology. 1993;43:1167–1172. doi: 10.1212/wnl.43.6.1167. [DOI] [PubMed] [Google Scholar]; Nakano, S., and Engel, A.G. (1993). Myasthenia gravis: quantitative immunocytochemical analysis of inflammatory cells and detection of complement membrane attack complex at the end-plate in 30 patients. Neurology 43, 1167-1172. [DOI] [PubMed]
- 5.Losen M., Martinez-Martinez P., Molenaar P.C., Lazaridis K., Tzartos S., Brenner T., Duan R.S., Luo J., Lindstrom J., Kusner L. Standardization of the experimental autoimmune myasthenia gravis (EAMG) model by immunization of rats with Torpedo californica acetylcholine receptors: Recommendations for methods and experimental designs. Exp. Neurol. 2015;270:18–28. doi: 10.1016/j.expneurol.2015.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]; Losen, M., Martinez-Martinez, P., Molenaar, P.C., Lazaridis, K., Tzartos, S., Brenner, T., Duan, R.S., Luo, J., Lindstrom, J., and Kusner, L. (2015). Standardization of the experimental autoimmune myasthenia gravis (EAMG) model by immunization of rats with Torpedo californica acetylcholine receptors: Recommendations for methods and experimental designs. Exp. Neurol. 270, 18-28. [DOI] [PMC free article] [PubMed]
- 6.Kusner L.L., Losen M., Vincent A., Lindstrom J., Tzartos S., Lazaridis K., Martinez-Martinez P. Guidelines for pre-clinical assessment of the acetylcholine receptor: specific passive transfer myasthenia gravis model. Recommendations for methods and experimental designs. Exp. Neurol. 2015;270:3–10. doi: 10.1016/j.expneurol.2015.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]; Kusner, L.L., Losen, M., Vincent, A., Lindstrom, J., Tzartos, S., Lazaridis, K., and Martinez-Martinez, P. (2015). Guidelines for pre-clinical assessment of the acetylcholine receptor: specific passive transfer myasthenia gravis model. Recommendations for methods and experimental designs. Exp. Neurol. 270, 3-10. [DOI] [PMC free article] [PubMed]
- 7.Lennon V.A., Seybold M.E., Lindstrom J.M., Cochrane C., Ulevitch R. Role of complement in the pathogenesis of experimental autoimmune myasthenia gravis. J. Exp. Med. 1978;147:973–983. doi: 10.1084/jem.147.4.973. [DOI] [PMC free article] [PubMed] [Google Scholar]; Lennon, V.A., Seybold, M.E., Lindstrom, J.M., Cochrane, C., and Ulevitch, R. (1978). Role of complement in the pathogenesis of experimental autoimmune myasthenia gravis. J. Exp. Med. 147, 973-983. [DOI] [PMC free article] [PubMed]
- 8.Christadoss P. C5 gene influences the development of murine myasthenia gravis. J. Immunol. 1988;140:2589–2592. [PubMed] [Google Scholar]; Christadoss, P. (1988). C5 gene influences the development of murine myasthenia gravis. J. Immunol. 140, 2589-2592. [PubMed]
- 9.Tüzün E., Scott B.G., Goluszko E., Higgs S., Christadoss P. Genetic evidence for involvement of classical complement pathway in induction of experimental autoimmune myasthenia gravis. J. Immunol. 2003;171:3847–3854. doi: 10.4049/jimmunol.171.7.3847. [DOI] [PubMed] [Google Scholar]; Tuzun, E., Scott, B.G., Goluszko, E., Higgs, S., and Christadoss, P. (2003). Genetic evidence for involvement of classical complement pathway in induction of experimental autoimmune myasthenia gravis. J. Immunol. 171, 3847-3854. [DOI] [PubMed]
- 10.Chamberlain-Banoub J., Neal J.W., Mizuno M., Harris C.L., Morgan B.P. Complement membrane attack is required for endplate damage and clinical disease in passive experimental myasthenia gravis in Lewis rats. Clin. Exp. Immunol. 2006;146:278–286. doi: 10.1111/j.1365-2249.2006.03198.x. [DOI] [PMC free article] [PubMed] [Google Scholar]; Chamberlain-Banoub, J., Neal, J.W., Mizuno, M., Harris, C.L., and Morgan, B.P. (2006). Complement membrane attack is required for endplate damage and clinical disease in passive experimental myasthenia gravis in Lewis rats. Clin. Exp. Immunol. 146, 278-286. [DOI] [PMC free article] [PubMed]
- 11.Lin F., Kaminski H.J., Conti-Fine B.M., Wang W., Richmonds C., Medof M.E. Markedly enhanced susceptibility to experimental autoimmune myasthenia gravis in the absence of decay-accelerating factor protection. J. Clin. Invest. 2002;110:1269–1274. doi: 10.1172/JCI16086. [DOI] [PMC free article] [PubMed] [Google Scholar]; Lin, F., Kaminski, H.J., Conti-Fine, B.M., Wang, W., Richmonds, C., and Medof, M.E. (2002). Markedly enhanced susceptibility to experimental autoimmune myasthenia gravis in the absence of decay-accelerating factor protection. J. Clin. Invest. 110, 1269-1274. [DOI] [PMC free article] [PubMed]
- 12.Kaminski H.J., Kusner L.L., Richmonds C., Medof M.E., Lin F. Deficiency of decay accelerating factor and CD59 leads to crisis in experimental myasthenia. Exp. Neurol. 2006;202:287–293. doi: 10.1016/j.expneurol.2006.06.003. [DOI] [PubMed] [Google Scholar]; Kaminski, H.J., Kusner, L.L., Richmonds, C., Medof, M.E., and Lin, F. (2006). Deficiency of decay accelerating factor and CD59 leads to crisis in experimental myasthenia. Exp. Neurol. 202, 287-293. [DOI] [PubMed]
- 13.Kusner L.L., Halperin J.A., Kaminski H.J. Cell surface complement regulators moderate experimental myasthenia gravis pathology. Muscle Nerve. 2013;47:33–40. doi: 10.1002/mus.23448. [DOI] [PMC free article] [PubMed] [Google Scholar]; Kusner, L.L., Halperin, J.A., and Kaminski, H.J. (2013). Cell surface complement regulators moderate experimental myasthenia gravis pathology. Muscle Nerve 47, 33-40. [DOI] [PMC free article] [PubMed]
- 14.Song C., Xu Z., Miao J., Xu J., Wu X., Zhang F., Lin H., Li Z., Kaminski H.J. Protective effect of scFv-DAF fusion protein on the complement attack to acetylcholine receptor: a possible option for treatment of myasthenia gravis. Muscle Nerve. 2012;45:668–675. doi: 10.1002/mus.23247. [DOI] [PMC free article] [PubMed] [Google Scholar]; Song, C., Xu, Z., Miao, J., Xu, J., Wu, X., Zhang, F., Lin, H., Li, Z., and Kaminski, H.J. (2012). Protective effect of scFv-DAF fusion protein on the complement attack to acetylcholine receptor: a possible option for treatment of myasthenia gravis. Muscle Nerve 45, 668-675. [DOI] [PMC free article] [PubMed]
- 15.Kusner L.L., Satija N., Cheng G., Kaminski H.J. Targeting therapy to the neuromuscular junction: proof of concept. Muscle Nerve. 2014;49:749–756. doi: 10.1002/mus.24057. [DOI] [PMC free article] [PubMed] [Google Scholar]; Kusner, L.L., Satija, N., Cheng, G., and Kaminski, H.J. (2014). Targeting therapy to the neuromuscular junction: proof of concept. Muscle Nerve 49, 749-756. [DOI] [PMC free article] [PubMed]
- 16.Huda R., Tüzün E., Christadoss P. Complement C2 siRNA mediated therapy of myasthenia gravis in mice. J. Autoimmun. 2013;42:94–104. doi: 10.1016/j.jaut.2013.01.003. [DOI] [PubMed] [Google Scholar]; Huda, R., Tuzun, E., and Christadoss, P. (2013). Complement C2 siRNA mediated therapy of myasthenia gravis in mice. J. Autoimmun. 42, 94-104. [DOI] [PubMed]
- 17.Biesecker G., Gomez C.M. Inhibition of acute passive transfer experimental autoimmune myasthenia gravis with Fab antibody to complement C6. J. Immunol. 1989;142:2654–2659. [PubMed] [Google Scholar]; Biesecker, G., and Gomez, C.M. (1989). Inhibition of acute passive transfer experimental autoimmune myasthenia gravis with Fab antibody to complement C6. J. Immunol. 142, 2654-2659. [PubMed]
- 18.Piddlesden S.J., Jiang S., Levin J.L., Vincent A., Morgan B.P. Soluble complement receptor 1 (sCR1) protects against experimental autoimmune myasthenia gravis. J. Neuroimmunol. 1996;71:173–177. doi: 10.1016/s0165-5728(96)00144-0. [DOI] [PubMed] [Google Scholar]; Piddlesden, S.J., Jiang, S., Levin, J.L., Vincent, A., and Morgan, B.P. (1996). Soluble complement receptor 1 (sCR1) protects against experimental autoimmune myasthenia gravis. J. Neuroimmunol. 71, 173-177. [DOI] [PubMed]
- 19.Zhou Y., Gong B., Lin F., Rother R.P., Medof M.E., Kaminski H.J. Anti-C5 antibody treatment ameliorates weakness in experimentally acquired myasthenia gravis. J. Immunol. 2007;179:8562–8567. doi: 10.4049/jimmunol.179.12.8562. [DOI] [PubMed] [Google Scholar]; Zhou, Y., Gong, B., Lin, F., Rother, R.P., Medof, M.E., and Kaminski, H.J. (2007). Anti-C5 antibody treatment ameliorates weakness in experimentally acquired myasthenia gravis. J. Immunol. 179, 8562-8567. [DOI] [PubMed]
- 20.Soltys J., Kusner L.L., Young A., Richmonds C., Hatala D., Gong B., Shanmugavel V., Kaminski H.J. Novel complement inhibitor limits severity of experimentally myasthenia gravis. Ann. Neurol. 2009;65:67–75. doi: 10.1002/ana.21536. [DOI] [PMC free article] [PubMed] [Google Scholar]; Soltys, J., Kusner, L.L., Young, A., Richmonds, C., Hatala, D., Gong, B., Shanmugavel, V., and Kaminski, H.J. (2009). Novel complement inhibitor limits severity of experimentally myasthenia gravis. Ann. Neurol. 65, 67-75. [DOI] [PMC free article] [PubMed]
- 21.Howard J.F., Jr., Utsugisawa K., Benatar M., Murai H., Barohn R.J., Illa I., Jacob S., Vissing J., Burns T.M., Kissel J.T., REGAIN Study Group Safety and efficacy of eculizumab in anti-acetylcholine receptor antibody-positive refractory generalised myasthenia gravis (REGAIN): a phase 3, randomised, double-blind, placebo-controlled, multicentre study. Lancet Neurol. 2017;16:976–986. doi: 10.1016/S1474-4422(17)30369-1. [DOI] [PubMed] [Google Scholar]; Howard, J.F., Jr., Utsugisawa, K., Benatar, M., Murai, H., Barohn, R.J., Illa, I., Jacob, S., Vissing, J., Burns, T.M., Kissel, J.T., et al.; REGAIN Study Group (2017). Safety and efficacy of eculizumab in anti-acetylcholine receptor antibody-positive refractory generalised myasthenia gravis (REGAIN): a phase 3, randomised, double-blind, placebo-controlled, multicentre study. Lancet Neurol. 16, 976-986. [DOI] [PubMed]
- 22.Howard J.F., Jr., Barohn R.J., Cutter G.R., Freimer M., Juel V.C., Mozaffar T., Mellion M.L., Benatar M.G., Farrugia M.E., Wang J.J., MG Study Group A randomized, double-blind, placebo-controlled phase II study of eculizumab in patients with refractory generalized myasthenia gravis. Muscle Nerve. 2013;48:76–84. doi: 10.1002/mus.23839. [DOI] [PubMed] [Google Scholar]; Howard, J.F., Jr., Barohn, R.J., Cutter, G.R., Freimer, M., Juel, V.C., Mozaffar, T., Mellion, M.L., Benatar, M.G., Farrugia, M.E., Wang, J.J., et al.; MG Study Group (2013). A randomized, double-blind, placebo-controlled phase II study of eculizumab in patients with refractory generalized myasthenia gravis. Muscle Nerve 48, 76-84. [DOI] [PubMed]
- 23.Razzak M. Anaemia: mutations in C5 explain eculizumab resistance. Nat. Rev. Nephrol. 2014;10:182. doi: 10.1038/nrneph.2014.30. [DOI] [PubMed] [Google Scholar]; Razzak, M. (2014). Anaemia: mutations in C5 explain eculizumab resistance. Nat. Rev. Nephrol. 10, 182. [DOI] [PubMed]
- 24.Nishimura J., Yamamoto M., Hayashi S., Ohyashiki K., Ando K., Brodsky A.L., Noji H., Kitamura K., Eto T., Takahashi T. Genetic variants in C5 and poor response to eculizumab. N. Engl. J. Med. 2014;370:632–639. doi: 10.1056/NEJMoa1311084. [DOI] [PubMed] [Google Scholar]; Nishimura, J., Yamamoto, M., Hayashi, S., Ohyashiki, K., Ando, K., Brodsky, A.L., Noji, H., Kitamura, K., Eto, T., Takahashi, T., et al. (2014). Genetic variants in C5 and poor response to eculizumab. N. Engl. J. Med. 370, 632-639. [DOI] [PubMed]
- 25.Vaishnaw A.K., Gollob J., Gamba-Vitalo C., Hutabarat R., Sah D., Meyers R., de Fougerolles T., Maraganore J. A status report on RNAi therapeutics. Silence. 2010;1:14. doi: 10.1186/1758-907X-1-14. [DOI] [PMC free article] [PubMed] [Google Scholar]; Vaishnaw, A.K., Gollob, J., Gamba-Vitalo, C., Hutabarat, R., Sah, D., Meyers, R., de Fougerolles, T., and Maraganore, J. (2010). A status report on RNAi therapeutics. Silence 1, 14. [DOI] [PMC free article] [PubMed]
- 26.Fitzgerald K., Frank-Kamenetsky M., Shulga-Morskaya S., Liebow A., Bettencourt B.R., Sutherland J.E., Hutabarat R.M., Clausen V.A., Karsten V., Cehelsky J. Effect of an RNA interference drug on the synthesis of proprotein convertase subtilisin/kexin type 9 (PCSK9) and the concentration of serum LDL cholesterol in healthy volunteers: a randomised, single-blind, placebo-controlled, phase 1 trial. Lancet. 2014;383:60–68. doi: 10.1016/S0140-6736(13)61914-5. [DOI] [PMC free article] [PubMed] [Google Scholar]; Fitzgerald, K., Frank-Kamenetsky, M., Shulga-Morskaya, S., Liebow, A., Bettencourt, B.R., Sutherland, J.E., Hutabarat, R.M., Clausen, V.A., Karsten, V., Cehelsky, J., et al. (2014). Effect of an RNA interference drug on the synthesis of proprotein convertase subtilisin/kexin type 9 (PCSK9) and the concentration of serum LDL cholesterol in healthy volunteers: a randomised, single-blind, placebo-controlled, phase 1 trial. Lancet 383, 60-68. [DOI] [PMC free article] [PubMed]
- 27.Nair J.K., Willoughby J.L., Chan A., Charisse K., Alam M.R., Wang Q., Hoekstra M., Kandasamy P., Kel’in A.V., Milstein S. Multivalent N-acetylgalactosamine-conjugated siRNA localizes in hepatocytes and elicits robust RNAi-mediated gene silencing. J. Am. Chem. Soc. 2014;136:16958–16961. doi: 10.1021/ja505986a. [DOI] [PubMed] [Google Scholar]; Nair, J.K., Willoughby, J.L., Chan, A., Charisse, K., Alam, M.R., Wang, Q., Hoekstra, M., Kandasamy, P., Kel’in, A.V., Milstein, S., et al. (2014). Multivalent N-acetylgalactosamine-conjugated siRNA localizes in hepatocytes and elicits robust RNAi-mediated gene silencing. J. Am. Chem. Soc. 136, 16958-16961. [DOI] [PubMed]
- 28.Coelho T., Adams D., Silva A., Lozeron P., Hawkins P.N., Mant T., Perez J., Chiesa J., Warrington S., Tranter E. Safety and efficacy of RNAi therapy for transthyretin amyloidosis. N. Engl. J. Med. 2013;369:819–829. doi: 10.1056/NEJMoa1208760. [DOI] [PubMed] [Google Scholar]; Coelho, T., Adams, D., Silva, A., Lozeron, P., Hawkins, P.N., Mant, T., Perez, J., Chiesa, J., Warrington, S., Tranter, E., et al. (2013). Safety and efficacy of RNAi therapy for transthyretin amyloidosis. N. Engl. J. Med. 369, 819-829. [DOI] [PubMed]
- 29.Porter J.D., Khanna S., Kaminski H.J., Rao J.S., Merriam A.P., Richmonds C.R., Leahy P., Li J., Andrade F.H. Extraocular muscle is defined by a fundamentally distinct gene expression profile. Proc. Natl. Acad. Sci. USA. 2001;98:12062–12067. doi: 10.1073/pnas.211257298. [DOI] [PMC free article] [PubMed] [Google Scholar]; Porter, J.D., Khanna, S., Kaminski, H.J., Rao, J.S., Merriam, A.P., Richmonds, C.R., Leahy, P., Li, J., and Andrade, F.H. (2001). Extraocular muscle is defined by a fundamentally distinct gene expression profile. Proc. Natl. Acad. Sci. USA 98, 12062-12067. [DOI] [PMC free article] [PubMed]
- 30.Zhou Y., Kaminski H.J., Gong B., Cheng G., Feuerman J.M., Kusner L. RNA expression analysis of passive transfer myasthenia supports extraocular muscle as a unique immunological environment. Invest. Ophthalmol. Vis. Sci. 2014;55:4348–4359. doi: 10.1167/iovs.14-14422. [DOI] [PMC free article] [PubMed] [Google Scholar]; Zhou, Y., Kaminski, H.J., Gong, B., Cheng, G., Feuerman, J.M., and Kusner, L. (2014). RNA expression analysis of passive transfer myasthenia supports extraocular muscle as a unique immunological environment. Invest. Ophthalmol. Vis. Sci. 55, 4348-4359. [DOI] [PMC free article] [PubMed]
- 31.Fridkis-Hareli M., Storek M., Mazsaroff I., Risitano A.M., Lundberg A.S., Horvath C.J., Holers V.M. Design and development of TT30, a novel C3d-targeted C3/C5 convertase inhibitor for treatment of human complement alternative pathway-mediated diseases. Blood. 2011;118:4705–4713. doi: 10.1182/blood-2011-06-359646. [DOI] [PMC free article] [PubMed] [Google Scholar]; Fridkis-Hareli, M., Storek, M., Mazsaroff, I., Risitano, A.M., Lundberg, A.S., Horvath, C.J., and Holers, V.M. (2011). Design and development of TT30, a novel C3d-targeted C3/C5 convertase inhibitor for treatment of human complement alternative pathway-mediated diseases. Blood 118, 4705-4713. [DOI] [PMC free article] [PubMed]