Abstract
With the progressive epidemics of obesity, non-alcoholic fatty liver disease (NAFLD) has become the most common cause of chronic liver disease in adults and children. The increasing prevalence and incidence of NAFLD with advanced fibrosis is concerning because patients appear to experience higher non-liver-related morbidity and mortality than the general population. Recent clinical evidence suggests that NAFLD is directly associated with an increased risk of cardio-metabolic disorders. This mini review describes briefly the current understanding of the pathogenesis of NAFLD, summarizing the link between NAFLD and cardio-metabolic complications, focusing mainly upon ischemic stroke, type 2 diabetes mellitus (DM), hypertension, chronic kidney disease (CKD) and cardiac arrhythmias. In addition, it describes briefly the current understanding of the pathogenesis of NAFLD.
Keywords: non-alcoholic fatty liver disease, cardio-metabolic disorders, hypertension, diabetes, dyslipidemia, chronic kidney disease, cardiac arrhythmia, ischemic stroke
1. Introduction
Non-alcoholic fatty liver disease (NAFLD) is the most common form of liver disease and a leading cause of morbidity and mortality in both developed and developing countries [1]. A large body of literature currently suggests that NAFLD is not only confined to the liver but might rather represent a major part of a multisystemic disease. As early as 1995 it was first suggested that NAFLD was a systemic condition with a specific cardio-metabolic involvement [2], a notion which is now universally accepted. As is well known, NAFLD patients usually die of extra-hepatic causes, frequently for cardiovascular diseases (CVD), which sustains the importance of an early diagnosis and a prompt treatment of CVD risk factors. There is abundant evidence of a direct link between NAFLD and multiple cardio-metabolic disorders including ischemic stroke, insulin resistance, hypertension, chronic kidney disease (CKD) and cardiac arrhythmias [3]. The current mini review will briefly highlight the current understanding of the pathogenesis of NAFLD, describing the association between NAFLD and cardio-metabolic disorders and discussing the underlying pathogenic mechanisms.
2. NAFLD: Definition, Epidemiology and Pathogenesis
In addition, NAFLD is a condition characterized by different hepatic abnormalities, ranging from simple liver steatosis to cirrhosis, with an increased risk for the development of hepatocellular carcinoma (HCC). The diffusion of the disease has reached epidemic levels in the last few decades, with an increased prevalence overlapping the spread of obesity and metabolic syndrome worldwide. As a consequence, NAFLD actually represents the most common chronic liver disorder, with a global prevalence of about 24%. Current data estimate that NAFLD affects 30% of the United States, 30% of the South American, 27% of the Asian, 24% of the European, 32% of the Middle East and 13% of the African population [1,4,5]. Today, this condition poses a relevant problem for all health systems because of the high prevalence of cardio-metabolic comorbidities and high liver-related mortality observed in these patients.
Non-alcoholic fatty liver (NAFL) or simple steatosis (a condition characterized by ≥5% hepatic steatosis without evidence of hepatocellular injury) is the starting point of NAFLD, and the majority of patients show this pattern [6]. Liver steatosis is usually considered as a benign condition, but a significant percentage of these patients’ conditions will evolve to liver fibrosis through via non-alcoholic steatohepatitis (NASH) [7], which is defined by the presence of histological abnormalities such as hepatocyte ballooning and lobular necro-inflammation, which may progress to irreversible damage [8]. Remarkably, the progression from NASH to fibrosis is associated with some predisposing factors, such as arterial hypertension, obesity and type 2 diabetes mellitus (DM) and, as a unique in liver pathology, HCC in these patients may develop in NASH also in the absence of liver cirrhosis. The risk of neoplastic degeneration in NAFLD patients is different according to the different population study [9]. Cumulative incidence ranges from 0.25% to 7.6% at 5 years in subjects with advanced fibrosis or established cirrhosis [10]. Several risk factors have been associated with an increased risk for neoplastic progression. Patatin-like phospholipase domain-containing protein-3 (PNPLA3) polymorphisms, elderly status, metabolic abnormalities and drugs may modulate the risk of developing HCC.
According to the current guidelines, the diagnosis of NAFLD may be performed with ultrasound, although it has limited sensitivity for people with a low degree of steatosis (<20%) and for individuals with a high body mass index (BMI) (>40 kg/m2). To this regard, liver biopsy is the gold standard for NAFLD diagnosis but it is impractical as a diagnostic tool as it is invasive and expensive. Currently, liver biopsy is implied in the histological definition and assessment of fibrosis in NASH patients [11]. Proton magnetic resonance spectroscopy represents the best method for an accurate quantification of liver fat accumulation, but it is applied only in the context of clinical trials and experimental purposes. In clinical practice, abnormal levels of hepatic transaminases are used for the diagnosis despite their elevation being non-specific and not correlating with the severity of fibrosis.
The pathophysiology of NAFLD is complex and multifactorial, involving different risk factors, both genetic (in particular, polymorphisms of the PNPLA3 gene) and environmental factors (Western diet, low physical activity), which probably act in a different manner along with the different phases of the disease, leading to both liver-specific and extra-hepatic manifestations. To this respect, a growing interest has generated the observations that NAFLD seems independently associated with an increased risk of CVDs, and cardiovascular complications are probably the main cause of death in these patients [3].
The strong epidemiological association with obesity and type 2 DM has led to considering NAFLD as an additional manifestation in the context of insulin resistance, despite the underlying mechanisms remaining not completely understood. The severity of obesity seems positively correlated with the degree of the histological pattern of NAFLD, as it can be observed by the high rates of NASH and advanced fibrosis up to cirrhosis in morbidly obese patients. In fact, while in non-obese subjects the prevalence of NAFLD is 16%, about two-thirds of patients with liver steatosis are overweight or obese [12], and in this population weight loss has been shown to be the most effective strategy for NAFLD improvement. Bariatric surgery seems the most effective approach for weight loss, although NAFLD is not an indication to surgery despite its high prevalence in severe obesity (BMI>40), ranging from 84% for NAFL to about 62% for NASH [13].
Considering the independent burden associated with the disease, recent recommendations suggest performing a screening for NAFLD in all subjects with metabolic syndrome and/or obesity to prevent possible complications, as the majority of patients with liver steatosis and even NASH are asymptomatic [14]. Moreover, a growing body of evidence emphasizes the importance of a broader patient assessment and demonstrates that NAFLD is a disease with systemic pathophysiologic consequences. Therefore, in patients with NAFLD a clinical evaluation with the assessment of blood pressure, waist circumference, BMI, plasma cholesterol, triglyceride levels, plasma glucose or glycosylated haemoglobin estimated that glomerular filtration rate (eGFR) and albuminuria is fundamental to recognize the presence of concomitant cardio-metabolic complications [15].
The hypothesis that was proposed to explain the interplay between NAFLD and metabolic disease has been modeled considering the effect of a hypercaloric diet (and also reduced physical activity) on fat mass. In obesity and metabolic syndrome, the excessive calorie intake promotes an expansion of adipose tissue with dysfunctional adipocytes and an increased triglycerides hydrolysis and a consequent increase of circulating free fatty acids (FFA). The initial phase in the development of NAFLD results from the increased uptake of FFA by the hepatocytes, with consequent liver steatosis [16].
The role of visceral adiposity as a better predictor of NAFLD rather than body weight and BMI [16,17] is progressively increasing. Several studies have suggested a high correlation between visceral adiposity and the development of fatty liver [18,19]. An increase in visceral fat results in increased triglycerides hydrolysis and consequent FFA delivery to the liver, which in turn is closely related to the development of insulin resistance.
In recent years, it has become increasingly obsolescent to regard the so called NAFLD “two hit hypothesis”, considering the fact that insulin resistance as the “first hit” induces lipid accumulation within the hepatocytes, thus increasing the vulnerability of the liver to further insults—referred to as the “second hit” that in turn promoted hepatic injury, inflammation and fibrosis. Today, a “multiple parallel hits hypothesis” seems to provide a more accurate explanation of NAFLD pathogenesis [20].
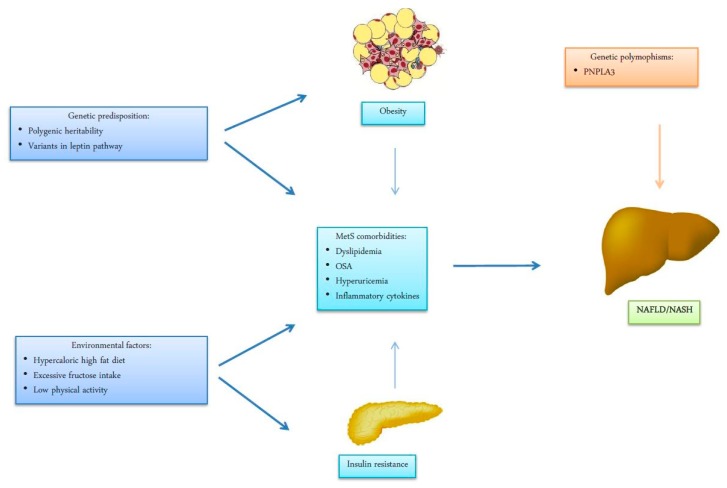
In the model of a “multiple hit process”, adipocyte dysfunction in genetically predisposed subjects is associated with a concomitant reduction of insulin sensitivity which is responsible for a reduced liver fatty acid oxidation and intra-hepatic accumulation of triglycerides (IHTG). The contribution to insulin resistance is due to the abnormal production of adipose-derived cytokines and hormones, such as tumor necrosis factor alpha (TNF-α), interleukin (IL)-6, C-reactive protein (CRP), leptin, adiponectin and resist in, with the induction of an inflammatory pathway which amplifies liver lipotoxic damage. In fact, from adipocyte-derived inflammation the condition evolves to an intrahepatic inflammation with hepatocyte injury, which is responsible for the progression of liver disease to NASH and cirrhosis (Figure 1). Increased levels of FFA, IHTG and the increased production of diacylglicerol and other lipotoxins activate protein kinase C-delta (PKC-δ) and nuclear factor kinase-B (NF-κB), leading to liver inflammation and insulin resistance. These hits also contribute multiple nutritional factors and gut microbiota [20,21].
Figure 1.
Environmental factors, life style and genetic susceptibility have shown to be implicated in the pathogenesis of visceral obesity and insulin resistance. The metabolic and respiratory complications associated with obesity and insulin resistance as dyslipidemia, OSA (obstructive sleep apnea) Hyperuricemia, hormonal imbalance and systemic low-grade inflammation are established factors in the pathogenesis of non-alcoholic fatty liver disease (NAFLD) and progression towards non-alcoholic steatohepatitis (NASH). Remarkably, genetic variation in the patatin-like phospholipase domain-containing protein-3 (PNPLA3) gene is considered a prominent risk factor for progression of liver injury from steatosis to steatohepatitis, cirrhosis and hepatocellular carcinoma (HCC).
In addition, other obesity-associated comorbidities, such as obstructive sleep apnea [22], hyperuricemia and even hypo-testosteronemia in men and polycystic ovary syndrome in women [23] have been associated with the development of NAFLD, suggesting a more complex interaction between obesity and liver function (Figure 1).
3. Association between NAFLD and Cardio-Metabolic Disorders
3.1. Ischemic Stroke in NAFLD
Ischemic stroke is one of the leading causes of mortality and long-term disability. The main initiating event consists in an impairment of blood flow in a portion of the brain, mainly due to occlusion of an intracranial or neck blood vessel. Emerging evidence suggests a relationship between ischemic stroke and biomarkers of NAFLD. In a cross-sectional study including 103 patients with acute ischemic stroke diagnosed by magnetic resonance tomography (MRT) and 200 healthy controls, elevations of alanine aminotransferase (ALT) or aspartate aminotransferase (AST) have been shown to be independently associated with ischemic stroke [24]. In a case-control study performed considering ongoing cohort studies in three European countries (United Kingdom, Netherlands and Finland), the incidence of ischemic stroke has been shown to be strongly associated with increased gamma-glutamyl transferase (GGT) levels [25]. GGT is known to be one of the main contributors in atherosclerosis and subsequent cerebrovascular diseases, mainly by inducing production of pro-inflammatory mediators and increasing the release of reactive oxygen species (ROS) [26]. In a recent study, NAFLD has been shown to be associated with an increased prevalence of lacunar infarcts in non-obese subjects [27]. A recent meta-analysis evaluating nine case-control studies showed that NAFLD is related to a higher risk of ischemic stroke even after adjustment for cardiovascular risk factors such as obesity, dyslipidemia and type 2 DM [28]. In a two-year prospective study including 200 patients with acute ischemic stroke, NAFLD (based on a combination of ultrasound findings and elevated aminotransferase levels) was found in 42.5% of the study population. In addition, patients with NAFLD have been shown to be associated with a worse clinical outcome at the moment of hospital discharge. However, these verity of stroke in the study population was not adjusted for the main cardiovascular risk factors such as obesity, dyslipidemia and type 2 DM [29]. Similarly, it has been shown that NAFLD is associated with an increased prevalence of brainstem infarctions. In this retrospective study, NAFLD was an independent determinant of the severity and progression of acute brainstem infarctions after adjustment of other risk factors, such as age, gender, DM and inflammatory markers [30].
3.2. Arterial Hypertension in NAFLD
Arterial hypertension is considered a major cardiovascular risk factor and represents a leading contributor to stroke and ischemic heart disease. In a large prospective cohort of Korean men, baseline NAFLD, assessed by ultrasonography, was independently associated with an increased incidence rate of hypertension. In addition, this rate was potentially associated with different degrees of NAFLD [31].
In another five-year prospective study, the incidence of NAFLD was associated with increased odd ratios for incident hypertension even after adjustment of potential risk factors for hypertension. In this study, individuals improving fatty liver during the follow-up period presented the same risk of incident hypertension compared with subjects without NAFLD, whereas the resolution of fatty liver during the same period was not associated with a significant increase in incident hypertension, suggesting that the change in fatty liver status overtime could influence the incidence of hypertension [32].
In another observational study including participants randomly selected from the general population, NAFLD was independently associated with prevalent hypertension and high-normal systolic blood pressure [33].
Moreover, a large study comparing the prevalence of NAFLD in lean and overweight-obese individuals using ultrasonography and its association with other cardio-metabolic disorders reported a higher incidence of hypertension in lean individuals with NAFLD. These results suggested that NAFLD seems to be related to a higher cardiovascular risk in normal weight patients [34].
In actuality, it is believed that the relationship between NAFLD and hypertension is bidirectional. This was first reported in two large cohort prospective studies that investigated the causality between incident NAFLD and metabolic syndrome components. In these studies, arterial hypertension was found to be an independent predictor for ultrasound-diagnosed NAFLD [35,36].
In another large cohort study on Brazilian individuals, hypertension was independently associated with an increase in the odds of prevalent NAFLD diagnosed by ultrasound. In addition, using the noninvasive fibrosis risk score (FIB-4), it was reported that the optimal control of blood pressure protects against moderate-to-severe hepatic fibrosis risk [37].In another cross-sectional study among hypertensive patients with NAFLD proven by biopsy, the use of renin-angiotensin-aldosterone system (RAAS)inhibitors combined with either an angiotensin converting enzyme inhibitor (ACE-I) or an angiotensin receptor blocker (ARB) was associated with less advanced hepatic fibrosis compared with the control group [38].
Recently, this bi-directional relationship was observed also in the prospective cohort study of the Framingham Heart Study over a six-year of follow-up in a group of middle-aged to older adults.
NAFLD at baseline, assessed by multiple-detector computed tomography, was associated with increased odds of incident hypertension. Paralleling this, arterial hypertension was associated with an increased risk of developing NAFLD [39].
3.3. Diabetes in NAFLD
Diabetes is one of the most common metabolic disorders in the world. The link between NAFLD and type 2 DM is complex, since both disorders share multiple common pro-inflammatory and pro-fibrotic pathways [40,41]. Several extensive studies have indicated that more than 70% of patients with type 2 DM develop NAFLD [42,43].
Two large meta-analyses reported that NAFLD is associated with an increased risk of incident DM. In both meta-analyses, most included observational studies relying on abnormal liver enzymes to identify patients with NAFLD [44,45].Similar findings have been reported using non-invasive imaging techniques to diagnose NAFLD, such as ultrasonography or computed tomography (CT) [46,47].
Besides type 2 DM, NAFLD has been shown to also be associated with impaired fasting glucose [48]. In a large cross-sectional study, NAFLD diagnosed by ultrasound scan was shown to be an independent risk factor for incident diabetes under conditions of impaired insulin secretion [49]. In a five-year prospective study, the degree of hepatic steatosis was shown to be positively correlated with the future development of type 2 DM, even after adjustment for risk factors [50].
Increasing evidence from studies with biopsy-diagnosed NAFLD suggest that the coexistence of type 2 DM represents an independent predictor of histological severity and progression in advanced fibrosis of NAFLD [51,52,53]. Further investigations based on liver histology reported that patients with type 2 DM have more severe NAFLD than those without type 2 DM, with a higher prevalence of NASH (80.2% vs 64.4%, respectively) and advanced fibrosis (40.3% vs 17.0%, respectively) [54]. Despite the debate concerning the causality between DM and NAFLD, increasing epidemiological evidence suggests that there is a bidirectional relationship between NAFLD and type 2 diabetes and that NAFLD may precede the development of type 2 DM [40,41,55]. Therefore type 2 DM screening should be suggested and implemented in patients with NAFLD and/or advanced liver fibrosis.
Since insulin resistance is considered as a main pathogenetic factor for the development of NAFLD, pharmacological strategies to improve insulin sensitivity have been investigated as potential treatment options for NAFLD, but the studies have yielded conflicting results [56].
Metformin, the most widely used insulin-sensitizing agent, improves the biochemical and metabolic features of NAFLD. However, it showed a scarce positive impact on histological markers associated with NAFLD [57].
Pioglitazone is another family of insulin-sensitizing agents that have been associated with improvements in histological findings in NAFLD [58]. However, concerns about the side effects and long-term safety of these molecules have limited their widespread use in NAFLD and NASH treatment.
Glucagon-like peptide-1 (GLP-1) agonists represent a novel class of antidiabetic drugs. They have been shown to be effective in improving liver histology and reducing aminotransferase levels in patients with NASH [59]. However, the evidence of an actual effectiveness of GLP-1 receptor agonist in the treatment of NAFLD is still lacking.
The most recent drugs introduced in the treatment of diabetes are the sodium glucose cotransporter type-2 (SGLT2) inhibitors, a class of glucose-lowering agents improving glucose control by excreting glucose with the urine. These agents also exert important cardiovascular protection in type 2DM patients with established cardiovascular disease. Recent studies have shown that the treatment of diabetic patients with SGLT2 inhibitors reduces fatty liver content, as assessed by different imaging techniques, also improving biological markers of NAFLD [60,61].
3.4. Dyslipidemia in NAFLD
As previously mentioned, abnormal lipid metabolism is a cause and a consequence of NAFLD, and the development of dyslipidemia may further account for the CV risk observed in these patients [62].
Excessive intra-hepatic fat deposition characterizing hepatic steatosis is promoted by the increased circulating levels of FFA (resulting from an enhanced lipolysis within adipose tissue), finally resulting in very low-density lipoprotein (VLDL) overproduction. Hepatic FFA uptake is the key point for increased triglycerides synthesis, resulting from the balance between circulating FFA and hepatocytes uptake capacity deriving from the activity of membrane transporters (fatty acid transport proteins). Patients with NAFLD show an increased FFA uptake, leading to an abnormal “de novo lipogenesis” and higher triglyceride levels. Reduced mitochondrial oxidation of FFA is the second step of this process, responsible for the overall reduced clearance of several lipoproteins.
Lipid accumulation within hepatocytes is responsible for the initial hepatic damage, and then lipotoxicity, oxidative stress, mitochondrial dysfunction and the induction of inflammatory cascade with the expression of TNF-α, IL-1 and IL-6 promote the progression to the advanced stages of the disease. To this respect, it is interesting to note that the severity of serum lipid profile seems related to the degree of hepatic injury, with higher disturbances in NASH [63].
Lipid profile in NAFLD is characterized by decreased levels of high-density lipoprotein (HDL)- and increased levels of low-density lipoprotein (LDL)-cholesterol and triglycerides, and even by a high apolipoprotein B to apolipoprotein A-1 ratio. In particular, NAFLD has been independently associated with small dense LDL particles, resulting in a more atherogenic profile [64]. This condition reflects the qualitative derangements of lipid profile observed in NAFLD—hypertriglyceridemia activates the cholesteryl-ester-transfer protein, enhancing the synthesis of these triacylglycerol-rich LDL particles.
NAFLD also seems to be associated with lower levels of HDL, which are usually considered to have a high anti-atherogenic potential [65].
3.5. Chronic Kidney Disease in NAFLD
CKD is increasingly regarded as a health problem worldwide and is considered an independent cardiovascular risk factor leading to decreased quality of life and premature mortality [66]. It is defined by the presence of renal dysfunction, as measured by the estimated eGFR (derived from serum creatinine using standard estimating equations) and abnormal albuminuria or overt proteinuria [66]. Evidence accumulated in the last few years suggests a direct relationship between NAFLD and CKD. It has been estimated that the prevalence of CKD in NAFLD patients, diagnosed either by imaging or histology, ranges approximately from 20% to 55%, compared with5% to30% in patients without NAFLD [67].
In a recent meta-analysis including twenty studies (11 cross-sectional and 9 longitudinal), NAFLD patients were associated with an increased risk and severity of prevalent and incident CKD, even after adjustment for significant risk factors. Interestingly, NASH and advanced fibrosis were also associated with higher odd ratios for proteinuria and with a lower eGFR than simple hepatic steatosis and non-advanced fibrosis, respectively [68].
These findings were further confirmed by several case-control studies that utilized liver biopsy to diagnose NAFLD/NASH [69,70,71,72,73]. These studies have shown that patients with histologically defined NAFLD/NASH have lower eGFRs and a higher frequency of CKD or abnormal albuminuria than matched controls. The severity of NAFLD or NASH was positively correlated with that of CKD regardless of other components of the metabolic syndrome.
3.6. Cardiac Arrhythmias in NAFLD
Recent clinical investigations revealed a significant increase of cardiac arrhythmias among patients with NAFLD, suggesting that NAFLD could represent a risk factor for various types of arrhythmias including atrial fibrillation, cardiac conduction defects and ventricular arrhythmias.
Two large population-based cohort studies described the association between mildly elevated liver enzymes (ALT and AST), as markers of NAFLD, and atrial fibrillation (AF). Furthermore, it has been reported that elevated AST levels were independently associated with increased long-term risk of incident AF [74,75].
The diagnosis of NAFLD by ultrasonography has also been associated with an increased prevalence and incidence of AF in a hospital-based sample of patients with type 2 DM, independently from several clinical risk factors for atrial fibrillation such asage, sex, arterial hypertension and electrocardiographic features [76,77].These findings were later confirmed by another prospective study showing that NAFLD was independently associated with an increased risk of incident AF over a mean follow-up of 16 years [78].
In a recent meta-analysis of five observational cohort studies (two cross-sectional and three longitudinal studies) with 238,129 participants, NAFLD assessed by ultrasonography was associated with a nearly two-fold increase in the prevalence and incidence of atrial fibrillation [79].
Abnormal prolongation of the electrocardiographic corrected QT interval (QTc) predisposes to malignant ventricular arrhythmias and sudden cardiac death. In a recent cross-sectional study considering nearly 31,000 patients, the presence and severity of NAFLD was associated with an increased risk of QTc interval prolongation in patients with and without type 2 DM [80].
A retrospective study reported that patients with NAFLD, when compared with patients without NAFLD, had a nearly three-fold increased risk of ventricular arrhythmia demonstrated by 24-hour Holter monitoring (defined as more than 30 premature ventricular contractions per hour, non-sustained ventricular tachycardia or both). This association also remained consistent after adjusting for different cardiovascular risk factors, comorbidities and the use of various medications [81].
In the same context, patients with ultrasound-diagnosed NAFLD showed a three-fold increase risk of persistent heart block (defined as at least one block among first-degree a trio-ventricular block, second-degree block, third-degree block, left bundle branch block, right bundle branch block, left anterior hemi-block or left posterior hemi-block) compared with those without NAFLD. Notably, this risk was positively correlated with the severity of liver disease even after adjustment for age, gender, hypertension and prior ischemic heart disease, among other potentially confounding factors [82].
4. Mechanisms Linking Cardio-Metabolic Disorders with NAFLD
The mechanisms underlying the possible independent association between NAFLD and cardio-metabolic disorders have not been fully elucidated yet. In the last decade the link between these two conditions has been a hot topic in basic and clinical research.
Low-grade inflammation characterizes metabolic disorders, such as NAFLD and visceral obesity which is mainly determined by the expansion of ectopic abdominal fat, which leads to an increased release of pro-inflammatory cytokines that could crucially contribute to the development of insulin resistance and other NAFLD-related extrahepatic complications [83]. Insulin resistance, a characteristic feature in NAFLD, has been suggested to promote the progression of CKD by worsening renal haemodynamics, increasing sodium retention and the activation of the sympathetic nervous system [84].
As mentioned in the previous section, the inflammatory process within the expanding white adipose tissue has been suggested to activate the pro-inflammatory NF-κB pathway in the liver and increase the transcription of a variety of pro-inflammatory genes that can amplify systemic chronic inflammation, thus leading to extrahepatic cellular injury [85].
Another feature in NAFLD is atherogenic dyslipidemia, which comprises a triad of elevated levels of triglycerides and small-dense low-density lipoprotein and low levels of high-density lipoprotein cholesterol. This represents a potential risk factor of endothelial dysfunction and reno-vascular damage [86]. NAFLD, especially in its necro-inflammatory form, is often associated with an increased release of pro-coagulant, pro-oxidant and pro-fibrogenic factors mediating additional organ dysfunction [87].It has been suggested that pro-inflammatory cytokines and different pro-thrombotic factors are associated with increased arrhythmogenic complications, possibly by cardiac structural and electrical remodeling [88].
NAFLD is associated with an increase in components of the renin–angiotensin system, such as angiotensin II, that can contribute to vascular damage via increases in oxidative stress and subsequently blocking the insulin-signaling pathways and accelerating atherosclerosis [89]. In addition, the impairment of insulin-signaling in the endothelium leads to vasoconstriction, thus promoting arterial hypertension [90]. Angiotensin II also accelerates the progression of NAFLD to NASH and then to fibrosis by stimulating fibroblasts and inducing the release of pro-inflammatory cytokines [91].
Recent data support a role for adiponectin in mediating the cardio-metabolic complications in patients with NAFLD. Adiponectin is an adipocyte-derived anti-inflammatory and anti-atherogenic mediator. Patients with NAFLD have shown reduced circulating adiponectin concentrations independent of other metabolic confounding factors [92]. The regulation of adiponectin levels have been shown to be mediated by fetuin-A, a liver-secreted protein that is strongly correlated with fatty liver, impaired glucose tolerance and insulin resistance [93]. Decreased plasma adiponectin levels lead to a suppression of 5’ adenosine monophosphate-activated protein kinase (AMPK)-activation, thereby stimulating pro-inflammatory and pro-fibrogenic cascades in hepatocytes and podocytes, possibly causing liver and kidney damage [94].
There is growing evidence that the imbalance of the intestinal microbiota, termed dysbiosis, is involved in the pathogenesis and severity of hepatic steatosis [95]. This is usually associated with increased intestinal permeability by disrupting intracellular tight junctions leading to the release of lipopolysaccharide (LPS), cytokines and gut microbiota DNA into the circulation and to the liver, promoting hepatic and systemic inflammation. Dysbiosis also promotes the increased production of secondary bile acids that, in turn, have been linked to toxic effects on mitochondrial and cell membranes [96].
Different molecules generated by intestinal microbiota (such as trimethylamine, p-cresol and indole) have been described after further metabolism within the liver to promote atherosclerosis, probably through the upregulation of multiple macrophage scavenger receptors [79].
Additionally, an increase of systemic levels of trimethylamine-N-oxide (TMAO) has shown to be associated with poor clinical outcomes, probably by promoting tubule-interstitial fibrosis in the kidney [97].
5. Conclusions
There is now strong evidence that NAFLD is a systemic disease that contributes to the development and progression of extra-hepatic diseases such as type 2 DM, CKD, atherosclerosis, arterial hypertension, cardiovascular diseases and cardiac arrhythmias. Therefore, these data imply that patients with diagnosed NAFLD should be screened at an earlier stage for associated cardio-metabolic disorders and a multi-disciplinary-team-based approach should be involved in the management and treatment of these patients.
Currently, the histopathological examination of liver biopsy represents the gold standard for diagnosis of NAFLD. As a result, the non-invasive assessment of hepatic steatosis using serological tests and radiological imaging can be associated with substantial false positive and false negative rates. Therefore, the lack of standardized diagnostic methods in NAFLD makes the determination of the relationships between NAFLD and cardiometabolic disorders even more challenging.
The causal factors linking NAFLD to cardiometabolic complications remain to be definitively established. Therefore, more research is required to elucidate the pathophysiological links between NAFLD and these systemic complications. This will possibly lead to the development of new therapeutic approaches for NAFLD, thus leading also to a decrease of the global burden of related cardio-metabolic diseases.
Furthermore, more prospective and intervention studies of well-characterized patients with NAFLD are needed to definitively prove whether the improvement or resolution of NAFLD can prevent the development of cardio-metabolic risks.
Abbreviations
| ACE-I | angiotensin converting enzyme inhibitor |
| AF | atrial fibrillation |
| ALT | alanine aminotransferase |
| AMP | adenosine monophosphate |
| AMPK 5′ | AMP-activated protein kinase |
| ARB | angiotensin receptor blocker |
| AST | aspartate aminotransferase |
| BMI | body mass index |
| CKD | chronic kidney disease |
| CRP | C-reactive protein |
| CT | computed tomography |
| CVD | cardiovascular diseases |
| DM | diabetes mellitus |
| eGFR | estimated glomerular filtration rate |
| FFA | free fatty acids |
| GGT | gamma-glutamyl transferase |
| HCC | hepatocellular carcinoma |
| HDL | high-density lipoprotein |
| IHTG | intra-hepatic accumulation of triglycerides |
| IL | interleukin |
| LPS | Lipopolysaccharide |
| LDL | low-density lipoprotein |
| MRT | magnetic resonance tomography |
| NAFL | non-alcoholic fatty liver |
| NAFLD | non-alcoholic fatty liver disease |
| NASH | non-alcoholic steatohepatitis |
| NF-κB | protein kinase C-delta (PKC-δ) and nuclear factor kinase-B |
| OSA | obstructive sleep apnea |
| PNPLA3 | patatin-like phospholipase domain-containing protein-3 |
| QTc | corrected QT interval |
| RAAS | renin-angiotensin-aldosterone system |
| ROS | reactive oxygen species |
| SGLT2 | sodium glucose cotrasporter type-2 |
| TMAO | trimethylamine-N-oxide |
| TNF-α | tumor necrosis factor alpha |
| VLDL | very low-density lipoprotein |
Author Contributions
H.E.H. and A.D.V. wrote the manuscript. M.R. proofread the manuscript and provided guidance on the overall direction of the manuscript. All authors critically appraised the final version of the paper.
Funding
Project n. DOR 1848773/18 from the University of Padova.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Younossi Z., Anstee Q.M., Marietti M., Hardy T., Henry L., Eslam M., George J., Bugianesi E. Global burden of NAFLD and NASH: Trends, predictions, risk factors and prevention. Nat. Rev. Gastroenterol. Hepatol. 2018;15:11–20. doi: 10.1038/nrgastro.2017.109. [DOI] [PubMed] [Google Scholar]
- 2.Lonardo A., Bellini M., Tondelli E., Frazzoni M., Grisendi A., Pulvirenti M., Della Casa G. Nonalcoholic steatohepatitis and the “bright liver syndrome”: Should a recently expanded clinical entity be further expanded? Am. J. Gastroenterol. 1995;90:2072–2074. [PubMed] [Google Scholar]
- 3.Targher G., Byrne C.D., Lonardo A., Zoppini G., Barbui C. Non-alcoholic fatty liver disease and risk of incident cardiovascular disease: A meta-analysis. J. Hepatol. 2016;65:589–600. doi: 10.1016/j.jhep.2016.05.013. [DOI] [PubMed] [Google Scholar]
- 4.Vernon G., Baranova A., Younossi Z.M. Systematic review: The epidemiology and natural history of non-alcoholic fatty liver disease and non-alcoholic steatohepatitis in adults. Aliment. Pharmacol. Ther. 2011;34:274–285. doi: 10.1111/j.1365-2036.2011.04724.x. [DOI] [PubMed] [Google Scholar]
- 5.Petersen K.F., Dufour S., Feng J., Befroy D., Dziura J., Dalla Man C., Cobelli C., Shulman G.I. Increased prevalence of insulin resistance and nonalcoholic fatty liver disease in asian-indian men. Proc. Natl. Acad. Sci. USA. 2006;103:18273–18277. doi: 10.1073/pnas.0608537103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Petäjä E.M., Yki-Järvinen H. Definitions of Normal Liver Fat and the Association of Insulin Sensitivity with Acquired and Genetic NAFLD-A Systematic Review. Int. J. Mol. Sci. 2016;17:633. doi: 10.3390/ijms17050633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pais R., Charlotte F., Fedchuk L., Bedossa P., Lebray P., Poynard T., Ratziu V., LIDO Study Group A systematic review of follow-up biopsies reveals disease progression in patients with non-alcoholic fatty liver. J. Hepatol. 2013;59:550–556. doi: 10.1016/j.jhep.2013.04.027. [DOI] [PubMed] [Google Scholar]
- 8.Italian Association for the Study of the Liver (AISF) AISF position paper on nonalcoholic fatty liver disease (NAFLD): Updates and future directions. Dig. Liver Dis. 2017;49:471–483. doi: 10.1016/j.dld.2017.01.147. [DOI] [PubMed] [Google Scholar]
- 9.Mittal S., El-Serag H.B., Sada Y.H., Kanwal F., Duan Z., Temple S., May S.B., Kramer J.R., Richardson P.A., Davila J.A. Hepatocellular Carcinoma in the Absence of Cirrhosis in United States Veterans is Associated with Nonalcoholic Fatty Liver Disease. Clin. Gastroenterol. Hepatol. 2016;14:124–131. doi: 10.1016/j.cgh.2015.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.European Association for the Study of the Liver, European Association for the Study of Diabetes, European Association for the Study of Obesity EASL-EASD- EASO Clinical Practice Guidelines for the management of non-alcoholic fatty liver disease. J. Hepatol. 2016;64:1388–1402. doi: 10.1016/j.jhep.2015.11.004. [DOI] [PubMed] [Google Scholar]
- 11.Ekstedt M., Hagstrom H., Nasr P., Fredrikson M., Stal P., Kechagias S., Hultcrantz R. Fibrosis stage is the strongest predictor for disease-specific mortality in NAFLD after up to 33 years of follow-up. Hepatology. 2015;61:1547–1554. doi: 10.1002/hep.27368. [DOI] [PubMed] [Google Scholar]
- 12.Lazo M., Clark J.M. The epidemiology of nonalcoholic fatty liver disease: A global perspective. Semin. Liver Dis. 2008;28:339–350. doi: 10.1055/s-0028-1091978. [DOI] [PubMed] [Google Scholar]
- 13.Morita S., NetoDde S., Morita F.H., Morita N.K., Lobo S.M. Prevalence of Non-alcoholic Fatty Liver Disease and Steatohepatitis Risk Factors in Patients Undergoing Bariatric Surgery. Obes. Surg. 2015;25:2335–2343. doi: 10.1007/s11695-015-1696-5. [DOI] [PubMed] [Google Scholar]
- 14.Corey K.E., Klebanoff M.J., Tramontano A.C., Chung R.T., Hur C. Screening for Nonalcoholic Steatohepatitis in Individuals with Type 2 Diabetes: A Cost-Effectiveness Analysis. Dig. Dis. Sci. 2016;61:2108–2117. doi: 10.1007/s10620-016-4044-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Patil R., Sood G.K. Non-alcoholic fatty liver disease and cardiovascular risk. World J. Gastrointest. Pathophysiol. 2017;8:51–58. doi: 10.4291/wjgp.v8.i2.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ha Y., Seo N., Shim J.H., Kim S.Y., Park J.A., Han S., Kim K.W., Yu E., Kim K.M., Lim Y.-S., et al. Intimate association of visceral obesity with non-alcoholic fatty liver disease in healthy Asians: A case-control study. J. Gastroenterol. Hepatol. 2015;30:1666–1672. doi: 10.1111/jgh.12996. [DOI] [PubMed] [Google Scholar]
- 17.Lee J.Y., Kim K.M., Lee S.G., Yu E., Lim Y.S., Lee H.C., Chung Y.-H., Lee Y.S., Suh D.-J. Prevalence and risk factors of non-alcoholic fatty liver disease in potential living liver donors in Korea: A review of 589 consecutive liver biopsies in a single center. J. Hepatol. 2007;47:239–244. doi: 10.1016/j.jhep.2007.02.007. [DOI] [PubMed] [Google Scholar]
- 18.Ko Y.H., Wong T.C., Hsu Y.Y., Kuo K.L., Yang S.H. The Correlation Between Body Fat, Visceral Fat, and Nonalcoholic Fatty Liver Disease. Metab. Syndr. Relat. Disord. 2017;15:304–311. doi: 10.1089/met.2017.0001. [DOI] [PubMed] [Google Scholar]
- 19.Omagari K., Kadokawa Y., Masuda J., Egawa I., Sawa T., Hazama H., Ohba K., Isomoto H., Mizuta Y., Hayashida K., et al. Fatty liver in non-alcoholic non-overweight Japanese adults: Incidence and clinical characteristics. J. Gastroenterol. Hepatol. 2002;17:1098–1105. doi: 10.1046/j.1440-1746.2002.02846.x. [DOI] [PubMed] [Google Scholar]
- 20.Buzzetti E., Pinzani M., Tsochatzis E.A. The multiple-hit pathogenesis of non-alcoholic fatty liver disease (NAFLD) Metabolism. 2016;65:1038–1048. doi: 10.1016/j.metabol.2015.12.012. [DOI] [PubMed] [Google Scholar]
- 21.Fabbrini E., Sullivan S., Klein S. Obesity and nonalcoholic fatty liver disease: Biochemical, metabolic, and clinical implications. Hepatology. 2010;51:679–689. doi: 10.1002/hep.23280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Musso G., Cassader M., Olivetti C., Rosina F., Carbone G., Gambino R. Association of obstructive sleep apnoea with the presence and severity of non-alcoholic fatty liver disease. A systematic review and meta-analysis. Obes. Rev. 2013;14:417–431. doi: 10.1111/obr.12020. [DOI] [PubMed] [Google Scholar]
- 23.Yim J.Y., Kim J., Kim D., Ahmed A. Serum testosterone and non-alcoholic fatty liver disease in men and women in the, U.S. Liver Int. 2018;38:2051–2059. doi: 10.1111/liv.13735. [DOI] [PubMed] [Google Scholar]
- 24.Ying I., Saposnik G., Vermeulen M.J., Leung A., Ray J.G. Nonalcoholic fatty liver disease and acute ischemic stroke. Epidemiology. 2011;22:129–130. doi: 10.1097/EDE.0b013e3181feb50a. [DOI] [PubMed] [Google Scholar]
- 25.Bots M.L., Salonen J.T., Elwood P.C., Nikitin Y., Freire de Concalves A., Inzitari D., Sivenius J., Trichopoulou A., Tuomilehto J., Koudstaal P.J. Gamma-glutamyltransferase and risk of stroke: The EUROSTROKE project. J. Epidemiol. Community Health. 2002;56(Suppl. 1):i25–i29. doi: 10.1136/jech.56.suppl_1.i25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ndrepepa G., Colleran R., Kastrati A. Gamma-glutamyl transferase and the risk of atherosclerosis and coronary heart disease. Clin. Chim. Acta. 2018;476:130–138. doi: 10.1016/j.cca.2017.11.026. [DOI] [PubMed] [Google Scholar]
- 27.Kwak M.S., Kim K.W., Seo H., Chung G.E., Yim J.Y., Kim D. Non-obese fatty liver disease is associated with lacunar infarct. Liver Int. 2018;38:1292–1299. doi: 10.1111/liv.13663. [DOI] [PubMed] [Google Scholar]
- 28.Hu J., Xu Y., He Z., Zhang H., Lian X., Zhu T., Liang C., Li J. Increased risk of cerebrovascular accident related to non-alcoholic fatty liver disease: A meta-analysis. Oncotarget. 2017;9:2752–2760. doi: 10.18632/oncotarget.22755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Abdeldyem S.M., Goda T., Khodeir S.A., AbouSaif S., Abd-Elsalam S. Nonalcoholic fatty liver disease in patients with acute ischemic stroke is associated with more severe stroke and worse outcome. J. Clin. Lipidol. 2017;11:915–919. doi: 10.1016/j.jacl.2017.04.115. [DOI] [PubMed] [Google Scholar]
- 30.Li H., Hu B., Wei L., Zhou L., Zhang L., Lin Y., Qin B., Dai Y., Lu Z. Non-alcoholic fatty liver disease is associated with stroke severity and progression of brainstem infarctions. Eur. J. Neurol. 2018;25:577-e34. doi: 10.1111/ene.13556. [DOI] [PubMed] [Google Scholar]
- 31.Ryoo J.H., Suh Y.J., Shin H.C., Cho Y.K., Choi J.M., Park S.K. Clinical association between non-alcoholic fatty liver disease and the development of hypertension. J. Gastroenterol. Hepatol. 2014;29:1926–1931. doi: 10.1111/jgh.12643. [DOI] [PubMed] [Google Scholar]
- 32.Sung K.C., Wild S.H., Byrne C.D. Development of new fatty liver, or resolution of existing fatty liver, over five years of follow-up, and risk of incident hypertension. J. Hepatol. 2014;60:1040–1045. doi: 10.1016/j.jhep.2014.01.009. [DOI] [PubMed] [Google Scholar]
- 33.López-Suárez A., Guerrero J.M., Elvira-González J., Beltrán-Robles M., Cañas-Hormigo F., Bascuñana-Quirell A. Nonalcoholic fatty liver disease is associated with blood pressure in hypertensive and nonhypertensive individuals from the general population with normal levels of alanine aminotransferase. Eur. J. Gastroenterol. Hepatol. 2011;23:1011–1017. doi: 10.1097/MEG.0b013e32834b8d52. [DOI] [PubMed] [Google Scholar]
- 34.Feng R.N., Du S.S., Wang C., Li Y.C., Liu L.Y., Guo F.C., Sun C.H. Lean-nonalcoholic fatty liver disease increases risk for metabolic disorders in a normal weight Chinese population. World J. Gastroenterol. 2014;20:17932–17940. doi: 10.3748/wjg.v20.i47.17932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang T., Zhang C., Zhang Y., Tang F., Li H., Zhang Q., Lin H., Wu S., Liu Y., Xue F. Prediction of metabolic syndrome by non-alcoholic fatty liver disease in northern urban Han Chinese population: A prospective cohort study. PLoS ONE. 2014;9:e96651. doi: 10.1371/journal.pone.0096651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang T., Zhang C., Zhang Y., Tang F., Li H., Zhang Q., Lin H., Wu S., Liu Y., Xue F. Metabolic syndrome and its components as predictors of nonalcoholic fatty liver disease in a northern urban Han Chinese population: A prospective cohort study. Atherosclerosis. 2015;240:144–148. doi: 10.1016/j.atherosclerosis.2015.02.049. [DOI] [PubMed] [Google Scholar]
- 37.Aneni E.C., Oni E.T., Martin S.S., Blaha M.J., Agatston A.S., Feldman T., Veledar E., Conçeicao R.D., Carvalho J.A., Santos R.D., et al. Blood pressure is associated with the presence and severity of nonalcoholic fatty liver disease across the spectrum of cardiometabolic risk. J. Hypertens. 2015;33:1207–1214. doi: 10.1097/HJH.0000000000000532. [DOI] [PubMed] [Google Scholar]
- 38.Goh G.B., Pagadala M.R., Dasarathy J., Unalp-Arida A., Sargent R., Hawkins C., Sourianarayanane A., Khiyami A., Yerian L., Pai R., et al. Renin-angiotensin system and fibrosis in non-alcoholic fatty liver disease. Liver Int. 2015;35:979–985. doi: 10.1111/liv.12611. [DOI] [PubMed] [Google Scholar]
- 39.Ma J., Hwang S.J., Pedley A., Massaro J.M., Hoffmann U., Chung R.T., Benjamin E.J., Levy D., Fox C.S., Long M.T. Bi-directional analysis between fatty liver and cardiovascular disease risk factors. J. Hepatol. 2017;66:390–397. doi: 10.1016/j.jhep.2016.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Adams L.A., Anstee Q.M., Tilg H., Targher G. Non-alcoholic fatty liver disease and its relationship with cardiovascular disease and other extrahepatic diseases. Gut. 2017;66:1138–1153. doi: 10.1136/gutjnl-2017-313884. [DOI] [PubMed] [Google Scholar]
- 41.Targher G., Marchesini G., Byrne C.D. Risk of type 2 diabetes in patients with non-alcoholic fatty liver disease: Causal association or epiphenomenon? Diabetes Metab. 2016;42:142–156. doi: 10.1016/j.diabet.2016.04.002. [DOI] [PubMed] [Google Scholar]
- 42.Loomba R., Abraham M., Unalp A., Wilson L., Lavine J., Doo E., Nathan M. Bass the Nonalcoholic Steatohepatitis Clinical Research Network. Association between diabetes, family history of diabetes, and risk of nonalcoholic steatohepatitis and fibrosis. Hepatology. 2012;56:943–951. doi: 10.1002/hep.25772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Williams C.D., Stengel J., Asike M.I., Torres D.M., Shaw J., Contreras M., Landt C.L., Harrison S.A. Prevalence of nonalcoholic fatty liver disease and nonalcoholic steatohepatitis among a largely middle-aged population utilizing ultrasound and liver biopsy: A prospective study. Gastroenterology. 2011;140:124–131. doi: 10.1053/j.gastro.2010.09.038. [DOI] [PubMed] [Google Scholar]
- 44.Musso G., Gambino R., Cassader M., Pagano G. Meta-analysis: Natural history of non-alcoholic fatty liver disease (NAFLD) and diagnostic accuracy of non-invasive tests for liver disease severity. Ann. Med. 2011;43:617–649. doi: 10.3109/07853890.2010.518623. [DOI] [PubMed] [Google Scholar]
- 45.Ballestri S., Zona S., Targher G., Romagnoli D., Baldelli E., Nascimbeni F., Roverato A., Guaraldi G., Lonardo A. Nonalcoholic fatty liver disease is associated with an almost twofold increased risk of incident type 2 diabetes and metabolic syndrome. Evidence from a systematic review and meta-analysis. J. Gastroenterol. Hepatol. 2016;31:936–944. doi: 10.1111/jgh.13264. [DOI] [PubMed] [Google Scholar]
- 46.Lonardo A., Bellentani S., Argo C.K., Ballestri S., Byrne C.D., Caldwell S.H., Cortez-Pinto H., Grieco A., Machado M.V., Miele L., et al. Epidemiological modifiers of non-alcoholic fatty liver disease: Focus on high-risk groups. Dig. Liver Dis. 2015;47:997–1006. doi: 10.1016/j.dld.2015.08.004. [DOI] [PubMed] [Google Scholar]
- 47.Machado M.V., Cortez-Pinto H. Non-invasive diagnosis of non-alcoholic fatty liver disease. A critical appraisal. J. Hepatol. 2013;58:1007–1019. doi: 10.1016/j.jhep.2012.11.021. [DOI] [PubMed] [Google Scholar]
- 48.Yamada T., Fukatsu M., Suzuki S., Wada T., Yoshida T., Joh T. Fatty liver predicts impaired fasting glucose and type 2 diabetes mellitus in Japanese undergoing a health checkup. J. Gastroenterol. Hepatol. 2010;25:352–356. doi: 10.1111/j.1440-1746.2009.05998.x. [DOI] [PubMed] [Google Scholar]
- 49.Bae J.C., Cho Y.K., Lee W.Y., Seo H.I., Rhee E.J., Park S.E., Park C.Y., Oh K.W., Sung K.C., Kim B.I. Impact of nonalcoholic fatty liver disease on insulin resistance in relation to HbA1c levels in nondiabetic subjects. Am. J. Gastroenterol. 2010;105:2389–2395. doi: 10.1038/ajg.2010.275. [DOI] [PubMed] [Google Scholar]
- 50.Park S.K., Seo M.H., Shin H.C., Ryoo J.H. Clinical availability of nonalcoholic fatty liver disease as an early predictor of type 2 diabetes mellitus in Korean men: 5-year prospective cohort study. Hepatology. 2013;57:1378–1383. doi: 10.1002/hep.26183. [DOI] [PubMed] [Google Scholar]
- 51.McPherson S., Hardy T., Henderson E., Burt A.D., Day C.P., Anstee Q.M. Evidence of NAFLD progression from steatosis to fibrosing-steatohepatitis using paired biopsies: Implications for prognosis and clinical management. J. Hepatol. 2015;62:1148–1155. doi: 10.1016/j.jhep.2014.11.034. [DOI] [PubMed] [Google Scholar]
- 52.Hossain N., Afendy A., Stepanova M., Nader F., Srishord M., Rafiq N., Goodman Z., Younossi Z. Independent predictors of fibrosis in patients with nonalcoholic fatty liver disease. Clin. Gastroenterol. Hepatol. 2009;7:1224–1229. doi: 10.1016/j.cgh.2009.06.007. [DOI] [PubMed] [Google Scholar]
- 53.Fracanzani A.L., Valenti L., Bugianesi E., Andreoletti M., Colli A., Vanni E., Bertelli C., Fatta E., Bignamini D., Marchesini G., et al. Risk of severe liver disease in nonalcoholic fatty liver disease with normal aminotransferase levels: A role for insulin resistance and diabetes. Hepatology. 2008;48:792–798. doi: 10.1002/hep.22429. [DOI] [PubMed] [Google Scholar]
- 54.Goh G.B., Pagadala M.R., Dasarathy J., Unalp-Arida A., Sargent R., Hawkins C., Sourianarayanane A., Khiyami A., Yerian L., Pai R.K., et al. Clinical spectrum of non-alcoholic fatty liver disease in diabetic and non-diabetic patients. BBA Clin. 2014;3:141–145. doi: 10.1016/j.bbacli.2014.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bril F., Cusi K. Management of nonalcoholic fatty liver disease in patients with type 2 diabetes: A call to action. Diabetes Care. 2017;40:419–430. doi: 10.2337/dc16-1787. [DOI] [PubMed] [Google Scholar]
- 56.Mills E.P., Brown K.P.D., Smith J.D., Vang P.W., Trotta K. Treating nonalcoholic fatty liver disease in patients with type 2 diabetes mellitus: A review of efficacy and safety. Ther. Adv. Endocrinol. Metab. 2018;9:15–28. doi: 10.1177/2042018817741852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Li Y., Liu L., Wang B., Wang J., Chen D. Metformin in non-alcoholic fatty liver disease: A systematic review and meta-analysis. Biomed Rep. 2013;1:57–64. doi: 10.3892/br.2012.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cusi K., Orsak B., Bril F., Lomonaco R., Hecht J., Ortiz-Lopez C., Tio F., Hardies J., Darland C., Musi N., et al. Long-Term Pioglitazone Treatment for Patients with Nonalcoholic Steatohepatitis and Prediabetes or Type 2 Diabetes Mellitus: A Randomized Trial. Ann. Intern Med. 2016;165:305–315. doi: 10.7326/M15-1774. [DOI] [PubMed] [Google Scholar]
- 59.Chalasani N., Younossi Z., Lavine J.E., Charlton M., Cusi K., Rinella M., Harrison S.A., Brunt E.M., Sanyal A.J. The diagnosis and management of nonalcoholic fatty liver disease: Practice guidance from the American Association for the Study of Liver Diseases. Hepatology. 2018;67:328–357. doi: 10.1002/hep.29367. [DOI] [PubMed] [Google Scholar]
- 60.Portillo-Sanchez P., Cusi K. Treatment of Nonalcoholic Fatty Liver Disease (NAFLD) in patients with Type 2 Diabetes Mellitus. Clin. Diabetes Endocrinol. 2016;2:9. doi: 10.1186/s40842-016-0027-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Aso Y., Kato K., Sakurai S., Kishi H., Shimizu M., Jojima T., Iijima T., Maejima Y., Shimomura K., Usui I. Impact of dapagliflozin, an SGLT2 inhibitor, on serum levels of soluble dipeptidyl peptidase-4 in patients with type 2 diabetes and non-alcoholic fatty liver disease. Int. J. Clin. Pract. 2019;27:e13335. doi: 10.1111/ijcp.13335. [DOI] [PubMed] [Google Scholar]
- 62.Chatrath H., Vuppalanchi R., Chalasani N. Dyslipidemia in patients with nonalcoholic fatty liver disease. Semin. Liver Dis. 2012;32:22–29. doi: 10.1055/s-0032-1306423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Alkhouri N., Tamimi T.A., Yerian L., Lopez R., Zein N.N., Feldstein A.E. The inflamed liver and atherosclerosis: A link between histologic severity of nonalcoholic fatty liver disease and increased cardiovascular risk. J. Gastroenterol. Hepatol. 2002;17:1098–1105. doi: 10.1007/s10620-009-1075-y. [DOI] [PubMed] [Google Scholar]
- 64.Sonmez A., Nikolic D., Dogru T., Ercin C.N., Genc H., Cesur M., Tapan S., Karslioğlu Y., Montalto G., Banach M., et al. Low- and high-density lipoprotein subclasses in subjects with nonalcoholic fatty liver disease. J. Clin. Lipidol. 2015;9:576–582. doi: 10.1016/j.jacl.2015.03.010. [DOI] [PubMed] [Google Scholar]
- 65.Kantartzis K., Rittig K., Cegan A., Machann J., Schick F., Balletshofer B., Fritsche A., Schleicher E., Häring H.U., Stefan N. Fatty liver is independently associated with alterations in circulating HDL2 and HDL3 subfractions. Diabetes Care. 2008;31:366–368. doi: 10.2337/dc07-1558. [DOI] [PubMed] [Google Scholar]
- 66.Hill N.R., Fatoba S.T., Oke J.L., Hirst J.A., O’Callaghan C.A., Lasserson D.S., Hobbs F.D. Global Prevalence of Chronic Kidney Disease—A Systematic Review and Meta-Analysis. PLoS ONE. 2016;11:e0158765. doi: 10.1371/journal.pone.0158765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Targher G., Byrne C.D. Non-alcoholic fatty liver disease: An emerging driving force in chronic kidney disease. Nat. Rev. Nephrol. 2017;13:297–310. doi: 10.1038/nrneph.2017.16. [DOI] [PubMed] [Google Scholar]
- 68.Musso G., Gambino R., Tabibian J.H., Ekstedt M., Kechagias S., Hamaguchi M., Hultcrantz R., Hagström H., Yoon S.K., Charatcharoenwitthaya P., et al. Association of non-alcoholic fatty liver disease with chronic kidney disease: A systematic review and meta-analysis. PLoS Med. 2014;11:e1001680. doi: 10.1371/journal.pmed.1001680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Targher G., Bertolini L., Rodella S., Lippi G., Zoppini G., Chonchol M. Relationship between kidney function and liver histology in subjects with nonalcoholic steatohepatitis. Clin. J. Am. Soc. Nephrol. 2010;5:2166–2171. doi: 10.2215/CJN.05050610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yilmaz Y., Alahdab Y.O., Yonal O., Kurt R., Kedrah A.E., Celikel C.A., Ozdogan O., Duman D., Imeryuz N., Avsar E., et al. Microalbuminuria in nondiabetic patients with nonalcoholic fatty liver disease: Association with liver fibrosis. Metabolism. 2010;59:1327–1330. doi: 10.1016/j.metabol.2009.12.012. [DOI] [PubMed] [Google Scholar]
- 71.Yasui K., Sumida Y., Mori Y., Mitsuyoshi H., Minami M., Itoh Y., Kanemasa K., Matsubara H., Okanoue T., Yoshikawa T. Nonalcoholic steatohepatitis and increased risk of chronic kidney disease. Metabolism. 2011;60:735–739. doi: 10.1016/j.metabol.2010.07.022. [DOI] [PubMed] [Google Scholar]
- 72.Park C.W., Tsai N.T., Wong L.L. Implications of worse renal dysfunction and medical comorbidities in patients with NASH undergoing liver transplant evaluation: Impact on MELD and more. Clin. Transplant. 2011;25:E606–E611. doi: 10.1111/j.1399-0012.2011.01497.x. [DOI] [PubMed] [Google Scholar]
- 73.Machado M.V., Gonçalves S., Carepa F., Coutinho J., Costa A., Cortez-Pinto H. Impaired renal function in morbid obese patients with nonalcoholic fatty liver disease. Liver Int. 2012;32:241–248. doi: 10.1111/j.1478-3231.2011.02623.x. [DOI] [PubMed] [Google Scholar]
- 74.Sinner M.F., Wang N., Fox C.S., Fontes J.D., Rienstra M., Magnani J.W., Vasan R.S., Calderwood A.H., Pencina M., Sullivan L.M., et al. Relation of circulating liver transaminase concentrations to risk of new-onset atrial fibrillation. Am. J. Cardiol. 2013;111:219–224. doi: 10.1016/j.amjcard.2012.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Alonso A., Misialek J.R., Amiin M.A., Hoogeveen R.C., Chen L.Y., Agarwal S.K., Loehr L.R., Soliman E.Z., Selvin E. Circulating levels of liver enzymes and incidence of atrial fibrillation: The Atherosclerosis Risk in Communities cohort. Heart. 2014;100:1511–1516. doi: 10.1136/heartjnl-2014-305756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Targher G., Mantovani A., Pichiri I., Rigolon R., Dauriz M., Zoppini G., Morani G., Vassanelli C., Bonora E. Non-alcoholic fatty liver disease is associated with an increased prevalence of atrial fibrillation in hospitalized patients with type 2 diabetes. Clin. Sci. (Lond) 2013;125:301–309. doi: 10.1042/CS20130036. [DOI] [PubMed] [Google Scholar]
- 77.Targher G., Valbusa F., Bonapace S., Bertolini L., Zenari L., Rodella S., Zoppini G., Mantovani W., Barbieri E., Byrne C.D. Non-alcoholic fatty liver disease is associated with an increased incidence of atrial fibrillation in patients with type 2 diabetes. PLoS ONE. 2013;8:e57183. doi: 10.1371/journal.pone.0057183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Käräjämäki A.J., Pätsi O.P., Savolainen M., Kesäniemi Y.A., Huikuri H., Ukkola O. Non-Alcoholic Fatty Liver Disease as a Predictor of Atrial Fibrillation in Middle-Aged Population (OPERA Study) PLoS ONE. 2015;10:e0142937. doi: 10.1371/journal.pone.0142937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wijarnpreecha K., Boonpheng B., Thongprayoon C., Jaruvongvanich V., Ungprasert P. The association between non-alcoholic fatty liver disease and atrial fibrillation: A meta-analysis. Clin. Res. Hepatol. Gastroenterol. 2017;41:525–532. doi: 10.1016/j.clinre.2017.08.001. [DOI] [PubMed] [Google Scholar]
- 80.Hung C.S., Tseng P.H., Tu C.H., Chen C.C., Liao W.C., Lee Y.C., Chiu H.M., Lin H.J., Ho Y.L., Yang W.S., et al. Nonalcoholic Fatty Liver Disease Is Associated with QT Prolongation in the General Population. J. Am. Heart Assoc. 2015;4:e001820. doi: 10.1161/JAHA.115.001820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Mantovani A., Rigamonti A., Bonapace S., Bolzan B., Pernigo M., Morani G., Franceschini L., Bergamini C., Bertolini L., Valbusa F., et al. Nonalcoholic Fatty Liver Disease Is Associated with Ventricular Arrhythmias in Patients with Type 2 Diabetes Referred for Clinically Indicated 24-Hour Holter Monitoring. Diabetes Care. 2016;39:1416–1423. doi: 10.2337/dc16-0091. [DOI] [PubMed] [Google Scholar]
- 82.Mantovani A., Rigolon R., Pichiri I., Bonapace S., Morani G., Zoppini G., Bonora E., Targher G. Nonalcoholic fatty liver disease is associated with an increased risk of heart block in hospitalized patients with type 2 diabetes mellitus. PLoS ONE. 2017;12:e0185459. doi: 10.1371/journal.pone.0185459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Saltiel A.R., Olefsky J.M. Inflammatory mechanisms linking obesity and metabolic disease. J. Clin. Investig. 2017;127:1–4. doi: 10.1172/JCI92035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Spoto B., Pisano A., Zoccali C. Insulin resistance in chronic kidney disease: A systematic review. Am. J. Physiol. Renal Physiol. 2016;311:F1087–F1108. doi: 10.1152/ajprenal.00340.2016. [DOI] [PubMed] [Google Scholar]
- 85.Sharma M., Mitnala S., Vishnubhotla R.K., Mukherjee R., Reddy D.N., Rao P.N. The Riddle of Nonalcoholic Fatty Liver Disease: Progression from Nonalcoholic Fatty Liver to Nonalcoholic Steatohepatitis. J. Clin. Exp. Hepatol. 2015;5:147–158. doi: 10.1016/j.jceh.2015.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Katsiki N., Mikhailidis D.P., Mantzoros C.S. Non-alcoholic fatty liver disease and dyslipidemia: An update. Metabolism. 2016;65:1109–1123. doi: 10.1016/j.metabol.2016.05.003. [DOI] [PubMed] [Google Scholar]
- 87.Targher G., Chonchol M., Miele L., Zoppini G., Pichiri I., Muggeo M. Nonalcoholic fatty liver disease as a contributor to hypercoagulation and thrombophilia in the metabolic syndrome. Semin. Thromb. Hemost. 2009;35:277–287. doi: 10.1055/s-0029-1222606. [DOI] [PubMed] [Google Scholar]
- 88.Harada M., Van Wagoner D.R., Nattel S. Role of inflammation in atrial fibrillation pathophysiology and management. Circ. J. 2015;79:495–502. doi: 10.1253/circj.CJ-15-0138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Cabandugama P.K., Gardner M.J., Sowers J.R. The Renin Angiotensin Aldosterone System in Obesity and Hypertension: Roles in the Cardiorenal Metabolic Syndrome. Med. Clin. N. Am. 2017;101:129–137. doi: 10.1016/j.mcna.2016.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Artunc F., Schleicher E., Weigert C., Fritsche A., Stefan N., Häring H.U. The impact of insulin resistance on the kidney and vasculature. Nat. Rev. Nephrol. 2016;12:721–737. doi: 10.1038/nrneph.2016.145. [DOI] [PubMed] [Google Scholar]
- 91.Warner F.J., Lubel J.S., McCaughan G.W., Angus P.W. Liver fibrosis: A balance of ACEs? Clin. Sci. (Lond) 2007;113:109–118. doi: 10.1042/CS20070026. [DOI] [PubMed] [Google Scholar]
- 92.Feldman A., Eder S.K., Felder T.K., Kedenko L., Paulweber B., Stadlmayr A., Huber-Schönauer U., Niederseer D., Stickel F., Auer S., Haschke-Becher E., et al. Clinical and Metabolic Characterization of Lean Caucasian Subjects with Non-alcoholic Fatty Liver. Am. J. Gastroenterol. 2017;112:102–110. doi: 10.1038/ajg.2016.318. [DOI] [PubMed] [Google Scholar]
- 93.Ju H., Zhou Z., Sun M., Chen H. Association of fetuin-A to adiponectin ratio with metabolic syndrome: A cross-sectional study. Endocrine. 2017;58:190–193. doi: 10.1007/s12020-017-1383-5. [DOI] [PubMed] [Google Scholar]
- 94.Ix J.H., Sharma K. Mechanisms linking obesity, chronic kidney disease, and fatty liver disease: The roles of fetuin-A, adiponectin, and AMPK. J. Am. Soc. Nephrol. 2010;21:406–412. doi: 10.1681/ASN.2009080820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Saltzman E.T., Palacios T., Thomsen M., Vitetta L. Intestinal Microbiome Shifts, Dysbiosis, Inflammation, and Non-alcoholic Fatty Liver Disease. Front. Microbiol. 2018;9:61. doi: 10.3389/fmicb.2018.00061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Wang Z., Klipfell E., Bennett B.J., Koeth R., Levison B.S., Dugar B., Feldstein A.E., Britt E.B., Fu X., Chung Y.M., et al. Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature. 2011;472:57–63. doi: 10.1038/nature09922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Tang W.H., Wang Z., Kennedy D.J., Wu Y., Buffa J.A., Agatisa-Boyle B., Li X.S., Levison B.S., Hazen S.L. Gut microbiota-dependent trimethylamine N-oxide (TMAO) pathway contributes to both development of renal insufficiency and mortality risk in chronic kidney disease. Circ. Res. 2015;116:448–455. doi: 10.1161/CIRCRESAHA.116.305360. [DOI] [PMC free article] [PubMed] [Google Scholar]