Abstract
Brown adipose tissue (BAT) may potentially be used in strategies for preventing lifestyle-related diseases. We examine evidence that near-infrared time-resolved spectroscopy (NIRTRS) is capable of estimating human BAT density (BAT-d). The parameters examined in this study are total hemoglobin [total-Hb]sup, oxygenated Hb [oxy-Hb]sup, deoxygenated Hb [deoxy-Hb]sup, Hb O2 saturation (StO2sup), and the reduced scattering coefficient in the supraclavicular region (μs’sup), where BAT deposits can be located; corresponding parameters in the control deltoid region are obtained as controls. Among the NIRTRS parameters, [total-Hb]sup and [oxy-Hb]sup show region-specific increases in winter, compared to summer. Further, [total-Hb]sup and [oxy-Hb]sup are correlated with cold-induced thermogenesis in the supraclavicular region. We conclude that NIRTRS-determined [total-Hb]sup and [oxy-Hb]sup are useful parameters for evaluating BAT-d in a simple, rapid, non-invasive manner.
Keywords: 18F-fluorodeoxyglucose-positron emission tomography, noninvasive, brown adipose tissue (BAT), seasonal temperature fluctuations, cold-induced thermogenesis, thermogenic functional ingredients
1. Introduction
Human brown adipose tissue (BAT) functions as a tissue for non-shivering thermogenesis in response to cold exposure, and it has been shown to be present in larger amounts in winter [1,2]. It is reported that human BAT is related to lower body weight [1,2] and enhanced glucose tolerance [3]. Daily cold exposure has been shown to increase BAT activity, not only in healthy subjects [1,4,5] but also in obese individuals [6] and patients with type 2 diabetes [7]. Thus, BAT is expected to be utilized in strategies for preventing or treating obesity and lifestyle-related diseases such as diabetes.
It is generally accepted that cold-induced activation of human BAT can be detected by 18F-fluorodeoxyglucose (FDG)-positron emission tomography (PET), used in conjunction with computed tomography (CT) (18FDG-PET/CT) [4,5,6,7]. However, 18FDG–PET/CT has several limitations, including enormous instrumentation costs, ionizing radiation exposure, and acute cold exposure [8]. These make repeated 18FDG–PET/CT measurements difficult, and hinder human studies, specifically longitudinal ones. Thus, a noninvasive, simple method, that does not require exposure to cold and/or ionizing radiation, is desirable.
Near-infrared time-resolved spectroscopy (NIRTRS) is considered to be a noninvasive alternative method for evaluating BAT activity. Uniquely among commercially-available NIR techniques, NIRTRS can evaluate optical properties, such as the absorption coefficient (μa) and reduced scattering coefficient (μs’), and therefore it can be used to noninvasively quantify tissue oxygenated hemoglobin [oxy-Hb], deoxygenated Hb [deoxy-Hb], and total Hb [total-Hb] concentrations. Among these NIRTRS parameters, [total-Hb] and μs’ have been investigated for evaluating BAT, as potential indices of blood volume (or tissue vasculature) and mitochondrial concentration [9], respectively. Hence, we postulated that, as BAT has abundant capillaries and mitochondria, NIRTRS should allow assessment of vascular or mitochondrial density in BAT (BAT-d) in the supraclavicular region by measurement of [total-Hb]sup and µs′sup. It was reported that [total-Hb]sup and µs′sup, as determined by NIRTRS under both thermoneutral and cold conditions, is positively correlated with cold-induced 18FDG–PET/CT parameters in the supraclavicular region, which potentially contains BAT deposits, but not in the deltoid muscle region control site [10]. Further, a longitudinal study reported that [total-Hb]sup increases with the FDG-PET/CT parameter during repeated thermogenic capsinoid intake [11], which is known to increase BAT activity and mass. NIRTRS parameters, in particular [total-Hb]sup, can therefore evaluate BAT density under thermoneutral conditions.
However, there is a lack of reliable data on the variation of NIRTRS parameters (not only [total-Hb]sup and µs′sup, but also other parameters) with seasonal changes and during acute cold-induced thermogenesis (CIT), both of which correlate with increased BAT activity. Thus, the purpose of this study is to examine the ability of NIRTRS measurement in the supraclavicular region to evaluate BAT-d in humans. The evidence we present comprises differences in NIRTRS parameters measured in summer and winter, as well as correlations between the NIRTRS parameters and CIT.
2. Results
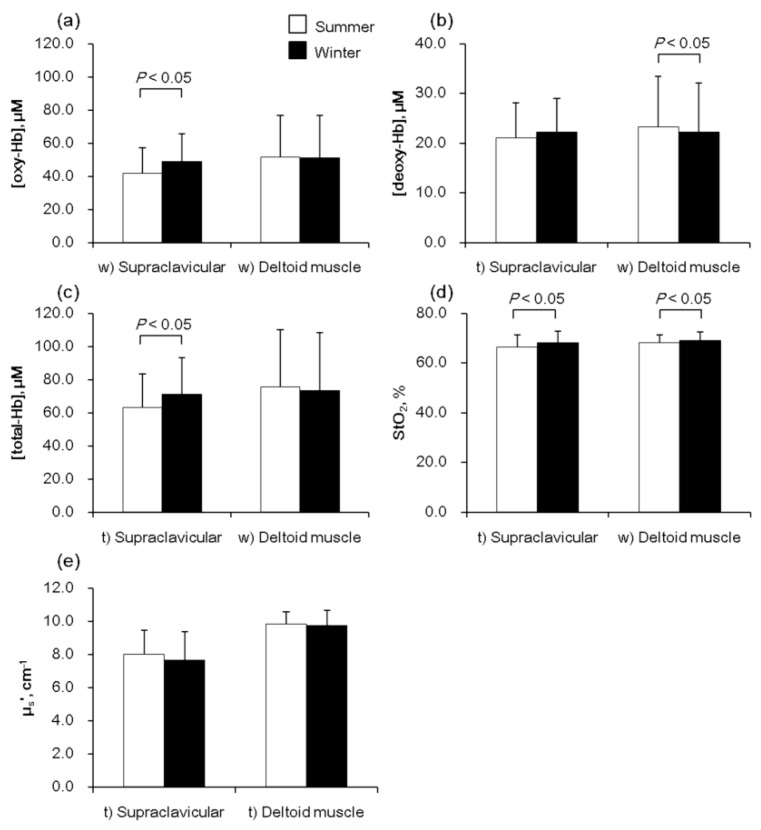
We found that [oxy-Hb]sup (42.0 ± 14.9 vs. 48.9 ± 16.7 μM, p < 0.01) and [total-Hb]sup (63.1 ± 20.6 vs. 71.2 ± 22.1 μM, p < 0.01) increased significantly, by 16.5% and 12.8%, respectively, from summer to winter, while there were no significant changes to the corresponding parameters for the deltoid muscle region ([oxy-Hb]del and [total-Hb]del) that served as a control site. The winter measurement of [deoxy-Hb]del was slightly but significantly greater, by only 4.5%, than the value in summer. StO2sup and StO2del were found to be higher in winter, compared to summer, in both the supraclavicular and deltoid muscle regions (p < 0.05) (Figure 1). The values of [total-Hb]sup and [oxy-Hb]sup showed very high correlations in both summer (r = 0.97, p < 0.01) and winter (r = 0.98, p < 0.01). The BAT-positive rate, defined as a [total-Hb]sup value greater than 74.0 µM [10], was found to be 48.3% in winter, which is significantly higher than the value obtained from the measurement in summer (27.6%) (p < 0.05).
Figure 1.
Near-infrared time-resolved spectroscopy results for 58 subjects measured during two seasons. (a) Oxygenated hemoglobin concentration [oxy-Hb], (b) deoxygenated Hb concentration [deoxy-Hb], (c) total Hb concentration [total-Hb], (d) Hb O2 saturation (StO2), and (e) reduced scattering coefficient (µs’) of 760 nm in the supraclavicular region, and as a reference, in the deltoid region. Results are presented as mean values and the error bars indicate standard deviations. t) Paired t-tests or w) Wilcoxon signed-rank tests were performed to determine the significance of seasonal differences.
Table 1 reveals the relationships between NIRTRS parameters at 19 and 27 °C and CIT in winter. We found a significant correlation between [total-Hb]sup (r = 0.48–0.64, p < 0.01) or [oxy-Hb]sup (r = 0.49–0.62, p < 0.05) and CIT, both at 27 and 19 °C. However, the correlation between [deoxy-Hb]sup and CIT is significant only at 27 °C. StO2sup and μs’sup are not significantly correlated with CIT at 19 or 27 °C. None of the parameters for the deltoid region are significantly correlated with CIT.
Table 1.
Relationship between near-infrared time-resolved spectroscopy (NIRTRS) parameters at 27 °C or 19 °C and cold-induced thermogenesis (CIT) a.
| Supraclavicular Region | Deltoid Muscle Region | ||
|---|---|---|---|
| [total-Hb] | 27 °C | 0.64 ※ | 0.24 |
| 19 °C | 0.48 ※ | 0.21 | |
| [oxy-Hb] | 27 °C | 0.62 ※ | 0.25 |
| 19 °C | 0.49 ※ | 0.15 | |
| [deoxy-Hb] | 27 °C | 0.70 ※ | 0.20 |
| 19 °C | 0.40 | 0.30 | |
| StO2 | 27 °C | −0.38 | 0.20 |
| 19 °C | −0.11 | −0.24 | |
| μs’ | 27 °C | −0.02 | 0.30 |
| 19 °C | 0.08 | 0.39 |
Determined as averages of near-infrared time-resolved spectroscopy and cold-induced thermogenesis (CIT), results for 18 young lean men in an air-conditioned room at 27 or 19 °C. ※ p < 0.05.
3. Discussion
We found a significant increase only in values for [oxy-Hb]sup and [total-Hb]sup without any changes in the corresponding parameters for the control region, indicating that [oxy-Hb]sup, but not [deoxy-Hb]sup, has an accuracy similar to that of [total-Hb]sup for detecting seasonal variations in BAT-d. Furthermore, although the reason is unclear, the decrease in [deoxy-Hb] in the deltoid region in winter indicates either that muscle metabolism decreased in the same period or that [deoxy-Hb] is a less stable index than [oxy-Hb] or [total-Hb]. We also found a significant correlation between CIT and each of the parameters [total-Hb]sup, [oxy-Hb]sup, and [deoxy-Hb]sup at 27 °C, but not between CIT and any other parameters. Taken together, among the indicators determined by NIRTRS, [total-Hb]sup, and [oxy-Hb]sup provide a reliable, sensitive, and specific means of evaluating BAT-d in both cross-sectional and longitudinal studies.
Recently a study aimed at investigating the association between near-infrared continuous-wave spectroscopy (NIRCWS) parameters (StO2, total-Hb, oxy-Hb, and deoxy-Hb), and BAT volume and activity estimated by 18FDG–PET/CT, has been reported [12]. The experiments were conducted by following the current cold exposure recommendations, and the measurements were obtained in the supraclavicular and forearm regions in young healthy women. No association between the NIRCWS parameters and BAT volume and activity under warm conditions was observed in this study. Similarly, cold-induced changes in the NIRCWS parameters were not found to be associated with BAT volume and activity. NIRCWS, therefore, does not seem to be a valid technique for indirectly assessing BAT in young healthy women. Moreover, another previous study examined the relationship between NIRCWS and 18FDG-PET/CT parameters in subjects with low and high levels of BAT during cold exposure [13]. The adjusted supraclavicular StO2 (adjStO2) parameter, which is relative to the value in a control area (deltoid region), measured by NIRCWS, was significantly correlated with oxygen uptake by BAT in the high BAT group.
The most common, commercially-available, near-infrared spectroscopy (NIRS) modality is NIRCWS. However, NIRCWS only provides relative values of tissue oxygenation. Further, the depth of light penetration is approximately 15 mm for a 30-mm optode separation in NIRCWS [14]. Thus, the main reason for the inability of the method to provide quantitative data, is that the path of NIR light through biological tissues is undetermined [14,15,16]. In contrast, NIRTRS is a method employing picosecond light pulse emissions from the surface of the skin to measure the time distribution of photons scattered and/or absorbed in tissue that is several centimeters distant from the point of light emission. It noninvasively quantifies a range of tissue optical properties, including μa, μs′, and light path length, allowing calculation of tissue [oxy-Hb], [deoxy-Hb], [total-Hb], and StO2 [16,17,18]. It can also provide absolute values for tissue hemodynamics. Furthermore, according to a recent study [19], the mean depth of light penetration should be greater (approximately 20 mm at the same 30-mm optode separation), and more homogeneous in NIRTRS. Thus, it is clear that instrumentation differences between NIRCWS and NIRTRS influence the accuracy of these methods with respect to BAT detection.
As BAT is activated by acute cold exposure, it is believed that it increases in winter, and this was confirmed by the majority of the 18FDG–PET/CT studies [2,20,21], with the sole exception being a paper showing that BAT activity was higher in early winter than in late winter or early spring [22]. In one cross-sectional study, [total-Hb]sup was reported to be significantly higher in winter than in summer [23]. Here, we confirm that [total-Hb]sup, [oxy-Hb]sup, and StO2sup increase in winter, which is consistent with the increase in BAT activity determined via the 18FDG-PET/CT studies [2,20,21,22]. To examine how BAT-d could be evaluated from data collected in summer, we correlated winter and summer data. There was a significant correlation between the summer and winter BAT indicators measured in the supraclavicular region, including [total-Hb]sup (r = 0.78, p < 0.01), [oxy-Hb]sup (r = 0.77, p < 0.01), and µs’sup (r = 0.52, p < 0.01). Thus, it could be possible to use these parameters determined in summer as an alternative to the winter parameters, which data might be unavailable, albeit acknowledging the lower accuracy of the summer data. Although we cannot identify the reason why [deoxy-Hb]del decreased and StO2del increased in winter, muscle vascularity and metabolism might be expected to show seasonal changes, and this should be further elucidated in a future study.
It is well known that whole-body oxygen consumption increases under non-shivering cold conditions, and that the mechanism involved comprises CIT brought about by the upregulation of uncoupling protein-1 in brown adipocytes. Those who have significantly greater BAT activity exhibit larger CIT than those with lower BAT activity. In addition, there is a correlation between BAT activity and CIT [1,24]. We have previously observed a strong correlation between [total-Hb]sup or µs′sup and mean standardized uptake value (SUVmean) assessed by 18FDG–PET/CT [10]. We have demonstrated in this study that [total-Hb]sup and [oxy-Hb]sup might be reliable markers for evaluating BAT activity. However, we also showed that StO2sup and µs′sup are less sensitive as markers, which is in contrast with the results of previous studies that showed a significant correlation between adjStO2 or µs′ and 18FDG–PET/CT parameters [10,12].
For in vivo imaging, the short-wavelength infrared region (SWIR; 1000-2000 nm) provides several advantages over the visible and near-infrared regions: In blood and tissue, there is a general lack of autofluorescence, low light absorption, and reduced scattering upon irradiation with these wavelengths. A recent animal study using SWIR with contrast agents has demonstrated visualization of BAT characteristics [25]. However, this imaging protocol is not suitable for human subjects because of the necessity of injecting contrast agents. Further, light absorption by water limits photon penetration through biological tissue when wavelengths above 900 or 1000 nm are used for emission or detection [17], as was reported for the aforementioned BAT visualization experiment. To allow better penetration, almost all human NIRS studies, in common with our work, have utilized wavelengths in the region of 650–850 nm [10,11,12,13,14,15,16,17,18,19].
Our study has several limitations that we now discuss. First, the supraclavicular region is heterogeneous, and hence any NIRTRS index results might be influenced by multiple tissue types other than BAT (e.g., white adipose tissue, muscle tissue, etc.). However, our previous work revealed a significant relationship between [total-Hb] and the 18FDG-PET/CT index in the supraclavicular region (r = 0.74), but not in the deltoid muscle region. Second, NIRTRS cannot differentiate Hb from myoglobin (Mb), and it has been reported that Mb is present in BAT [26]. In order to investigate the proportion of Hb in human BAT, 1proton (1H)-magnetic resonance spectroscopy measurements are needed. Third, NIRTRS indices could be interpreted as measurements of BAT density rather than BAT activity. Therefore, we believe that NIRTRS does not detect the oxidative metabolism of BAT in either winter or summer. Fourth, the supraclavicular region is one of the representative locations for BAT deposits. We examined this location, because NIRS can only be applied with a mean light depth of 20 mm at a 3-cm optode separation [19]; unfortunately, we were not able to evaluate other deeper regions in which BAT is deposited.
4. Materials and Methods
4.1. Subjects and Study Design
We tested whether near-infrared time-resolved spectroscopy (NIRTRS) parameters differ between seasons, taking measurements in summer [minimum temperature, 23.2 (22.0, 25.8) °C; maximum temperature, 32.3 (28.4, 33.7) °C, median (the first quartile, the third quartile)] and winter [minimum temperature, 3.2 (0.5, 5.4) °C; maximum temperature, 12.2 (9.6, 16.1) °C]. We recruited 58 subjects [men/women, 35/23; age, 40.5 (25.8, 47.0) year; BMI, 21.8 (20.4, 23.8) kg/m2, median (the first quartile, the third quartile)] to investigate changes in NIRTRS measurements conducted in the range of 23 to 27 °C.
We also investigated whether or not NIRTRS parameters were correlated with cold-induced thermogenesis (CIT), in 18 young men [age, 20.0 (19.0, 21.0) year; BMI, 24.2 (21.6, 25.7) kg/m2]. After fasting for 6–12 h, the subjects rested in an air-conditioned room at 27 °C, wearing light clothing (usually a T-shirt with underwear) for 20 min. This was followed by 5 min of NIRTRS measurements. Then, the subjects entered an air-conditioned room at 19 °C and rested for 2 h; they also intermittently placed their feet on an ice block wrapped in cloth, usually for 4 min every 5 min [1,2]. The NIRTRS parameters and energy expenditure were evaluated before and during the 2-h cold exposure.
All subjects gave informed consent for their participation in the study before the experiments were carried out. The study was conducted in accordance with the Declaration of Helsinki, and the protocol was approved by the Ethics Committees of Ritsumeikan University (IRB 2014-022, 8 December, 2014) and Tokyo Medical University (IRB 2017-199, 26 December, 2017).
4.2. NIRTRS Parameters
The NIRTRS parameters were measured by using a commercial NIRTRS device (TRS-20; Hamamatsu Photonics K.K., Hamamatsu, Japan). Each measurement procedure required 1 min. The probes were placed on the skin of the supraclavicular region that potentially contained brown adipose tissue (BAT), and on the deltoid muscle region as a control site. The participants were required to remain in a sitting position during the measurements, as previously described [10,11,21]. Compared to visible light wavelengths, NIR wavelengths (700 to 3000 nm) are scattered less and, consequently, they show better penetration in biological tissue. However, light absorption by water limits tissue penetration above a limiting wavelength of about 900 nm; thus, the 650–850-nm range is suitable for measurements [27]. Accordingly, we used NIR wavelengths of 760, 800, and 830 nm to evaluate [oxy-Hb], [deoxy-Hb], and [total-Hb], respectively. With the 3 cm probe used in this study, light can penetrate to a mean depth of 2 cm [19], where BAT is potentially located. For the sake of accuracy, it should be noted that NIRTRS cannot distinguish Hb from myoglobin (Mb). Hence, many studies have been reported in which results are presented as oxy-Hb/oxy-Mb, deoxy-Hb/deoxy-Mb, and total-Hb/total-Mb concentrations [17]. However, for simplicity, in this work we present the absorption measurements as [oxy-Hb], [deoxy-Hb], and [total-Hb].
Tissue was illuminated via a 200-μm-core-diameter optical fiber by a tunable picosecond pulsed light source (100-ps full width at half-maximum; 5-MHz repetition rate; 80-μW average power for each wavelength). The emitted photons penetrated the tissue, and were reflected into a 3-mm-diameter optical bundle fiber, through which they were sent to a photomultiplier tube for single-photon detection, using a signal processing circuit for time-resolved measurements. The nonlinear least-squares method was used to fit the digitized temporal profile measurement data with a theoretical temporal profile; the latter was derived from the analytical solution of the photon diffusion theory with a semi-infinite homogeneous reflectance model. After convolution with the instrumental response function to compensate for the time response of the instrument itself, the absorption and scattering coefficients, µa and μs′, at 760, 800, and 830 nm were obtained using the least-squares fitting method. Then, absolute [total-Hb], [oxy-Hb], [deoxy-Hb], and StO2 values were calculated [17,28].
4.3. Cold Induced Thermogenesis (CIT)
Whole-body energy expenditure was estimated in winter using an automatic respiratory gas analyzer (AE300S, Minato Medical Science Co., Ltd., Tokyo, Japan) before (at 27 °C) and during 2 h of cold exposure (at 19 °C), during which the participants wore light clothing. Stable values for the 10-min period at the end of the 2-h duration of cold exposure were used as the measured values of energy expenditure. Thus, CIT values were calculated as differences between energy expenditure at 27 °C and at the end of the period of cold exposure at 19 °C [1,24].
4.4. Statistical Analyses
Data are expressed as median (first quartile, third quartile) or mean ± standard deviation (SD). If a normal distribution was detected by the Shapiro-Wilk test, we used a paired t-test to test for seasonal differences in the NIRTRS parameters; otherwise, a Wilcoxon signed-rank test was conducted. The Pearson product-moment correlation coefficient was evaluated to analyze the relationship between CIT and NIRTRS parameters at 19 and 27 °C. BAT-positive rates were compared in winter and summer using chi-squared tests. All analyses were performed using SPSS software (IBM SPSS Statistics 25, IBM Japan, Tokyo, Japan) and p < 0.05 values were considered statistically significant.
5. Conclusions
We observed that seasonal temperature fluctuations influenced [total-Hb]sup and [oxy-Hb]sup and that there were significant correlations between CIT and each of the parameters [total-Hb]sup, [oxy-Hb]sup, and [deoxy-Hb]sup (only at 27 °C) in winter. These relationships were seen specifically in measurements of the supraclavicular region, which potentially contains BAT deposits. Thus, among the indicators determined by NIRTRS, [total-Hb]sup and [oxy-Hb]sup provide a reliable and sensitive means for evaluating BAT-d in both cross-sectional and longitudinal studies. We conclude that NIRTRS is a new approach for evaluating BAT-d, and could prove to be a useful tool for simple, non-invasive measurements in the clinic. However, a further validation study is definitely needed to confirm our results via side-by-side NIRTRS and conventional gold-standard 18FDG–PET/CT measurements.
Abbreviations
| adjStO2 | adjusted supraclavicular hemoglobin oxygen saturation |
| BAT | brown adipose tissue |
| BAT-d | vascular or mitochondrial density in brown adipose tissue |
| CIT | cold-induced thermogenesis |
| CT | computed tomography |
| deoxy-Hb | deoxygenated hemoglobin |
| FDG | 18F-fluorodeoxyglucose |
| Hb | hemoglobin |
| NIRS | near-infrared spectroscopy |
| NIRCWS | near-infrared continuous-wave spectroscopy |
| NIRTRS | near-infrared time-resolved spectroscopy |
| oxy-Hb | oxygenated hemoglobin |
| PET | positron emission tomography |
| StO2 | hemoglobin oxygen saturation |
| SUVmean | mean standardized uptake value |
| total-Hb | total hemoglobin |
| µa | absorption coefficient |
| µs’ | scattering coefficient |
Author Contributions
Conceptualization, S.N. and T.H.; formal analysis, S.N. and S.F.; investigation, S.N., S.F., S.A., T.H., R.K., M.K., T.E. and Y.K.; data curation, S.N. and S.F.; writing—original draft preparation, S.N., S.F.; writing—review and editing, N.S., M.M., Y.K., M.S., and T.H.; funding acquisition, T.H.
Funding
This work was supported by a Grant-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science, and Technology of Japan (15H03100).
Conflicts of Interest
The authors declare that there are no conflicts of interest.
References
- 1.Yoneshiro T., Aita S., Matsushita M., Kayahara T., Kameya T., Kawai Y., Iwanaga T., Saito M. Recruited brown adipose tissue as an antiobesity agent in humans. J. Clin. Investig. 2013;123:3404–3408. doi: 10.1172/JCI67803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Saito M., Okamatsu-Ogura Y., Matsushita M., Watanabe K., Yoneshiro T., Nio-Kobayashi J., Iwanaga T., Miyagawa M., Kameya T., Nakada K., et al. High incidence of metabolically active brown adipose tissue in healthy adult humans: Effects of cold exposure and adiposity. Diabetes. 2009;58:1526–1531. doi: 10.2337/db09-0530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Matsushita M., Yoneshiro T., Aita S., Kameya T., Sugie H., Saito M. Impact of brown adipose tissue on body fatness and glucose metabolism in healthy humans. Int. J. Obes. (Lond.) 2014;38:812–817. doi: 10.1038/ijo.2013.206. [DOI] [PubMed] [Google Scholar]
- 4.Blondin D.P., Labbe S.M., Tingelstad H.C., Noll C., Kunach M., Phoenix S., Guerin B., Turcotte E.E., Carpentier A.C., Richard D., et al. Increased brown adipose tissue oxidative capacity in cold-acclimated humans. J. Clin. Endocrinol. Metab. 2014;99:E438–E446. doi: 10.1210/jc.2013-3901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.van der Lans A.A., Hoeks J., Brans B., Vijgen G.H., Visser M.G., Vosselman M.J., Hansen J., Jorgensen J.A., Wu J., Mottaghy F.M., et al. Cold acclimation recruits human brown fat and increases nonshivering thermogenesis. J. Clin. Investig. 2013;123:3395–3403. doi: 10.1172/JCI68993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hanssen M.J., van der Lans A.A., Brans B., Hoeks J., Jardon K.M., Schaart G., Mottaghy F.M., Schrauwen P., van Marken Lichtenbelt W.D. Short-term Cold Acclimation Recruits Brown Adipose Tissue in Obese Humans. Diabetes. 2016;65:1179–1189. doi: 10.2337/db15-1372. [DOI] [PubMed] [Google Scholar]
- 7.Hanssen M.J., Hoeks J., Brans B., van der Lans A.A., Schaart G., van den Driessche J.J., Jorgensen J.A., Boekschoten M.V., Hesselink M.K., Havekes B., et al. Short-term cold acclimation improves insulin sensitivity in patients with type 2 diabetes mellitus. Nat. Med. 2015;21:863–865. doi: 10.1038/nm.3891. [DOI] [PubMed] [Google Scholar]
- 8.Borga M., Virtanen K.A., Romu T., Leinhard O.D., Persson A., Nuutila P., Enerback S. Brown adipose tissue in humans: Detection and functional analysis using PET (positron emission tomography), MRI (magnetic resonance imaging), and DECT (dual energy computed tomography) Methods Enzym. 2014;537:141–159. doi: 10.1016/B978-0-12-411619-1.00008-2. [DOI] [PubMed] [Google Scholar]
- 9.Beauvoit B., Chance B. Time-resolved spectroscopy of mitochondria, cells and tissues under normal and pathological conditions. Mol. Cell Biochem. 1998;184:445–455. doi: 10.1023/A:1006855716742. [DOI] [PubMed] [Google Scholar]
- 10.Nirengi S., Yoneshiro T., Sugie H., Saito M., Hamaoka T. Human brown adipose tissue assessed by simple, noninvasive near-infrared time-resolved spectroscopy. Obesity (Silver Spring) 2015;23:973–980. doi: 10.1002/oby.21012. [DOI] [PubMed] [Google Scholar]
- 11.Nirengi S., Homma T., Inoue N., Sato H., Yoneshiro T., Matsushita M., Kameya T., Sugie H., Tsuzaki K., Saito M., et al. Assessment of human brown adipose tissue density during daily ingestion of thermogenic capsinoids using near-infrared time-resolved spectroscopy. J. Biomed. Opt. 2016;21:091305. doi: 10.1117/1.JBO.21.9.091305. [DOI] [PubMed] [Google Scholar]
- 12.Acosta F.M., Berchem J., Martinez-Tellez B., Sanchez-Delgado G., Alcantara J.M.A., Ortiz-Alvarez L., Hamaoka T., Ruiz J.R. Near-Infrared Spatially Resolved Spectroscopy as an Indirect Technique to Assess Brown Adipose Tissue in Young Women. Mol. Imaging Biol. 2018 doi: 10.1007/s11307-018-1244-5. [DOI] [PubMed] [Google Scholar]
- 13.Muzik O., Mangner T.J., Leonard W.R., Kumar A., Janisse J., Granneman J.G. 15O PET measurement of blood flow and oxygen consumption in cold-activated human brown fat. J. Nucl. Med. 2013;54:523–531. doi: 10.2967/jnumed.112.111336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chance B., Dait M.T., Zhang C., Hamaoka T., Hagerman F. Recovery from exercise-induced desaturation in the quadriceps muscles of elite competitive rowers. Am. J. Physiol. 1992;262:C766–C775. doi: 10.1152/ajpcell.1992.262.3.C766. [DOI] [PubMed] [Google Scholar]
- 15.Ferrari M., Mottola L., Quaresima V. Principles, techniques, and limitations of near infrared spectroscopy. Can. J. Appl. Physiol. 2004;29:463–487. doi: 10.1139/h04-031. [DOI] [PubMed] [Google Scholar]
- 16.Chance B., Nioka S., Kent J., McCully K., Fountain M., Greenfeld R., Holtom G. Time-resolved spectroscopy of hemoglobin and myoglobin in resting and ischemic muscle. Anal. Biochem. 1988;174:698–707. doi: 10.1016/0003-2697(88)90076-0. [DOI] [PubMed] [Google Scholar]
- 17.Hamaoka T., McCully K.K., Quaresima V., Yamamoto K., Chance B. Near-infrared spectroscopy/imaging for monitoring muscle oxygenation and oxidative metabolism in healthy and diseased humans. J. Biomed. Opt. 2007;12:062105. doi: 10.1117/1.2805437. [DOI] [PubMed] [Google Scholar]
- 18.Hamaoka T., Katsumura T., Murase N., Nishio S., Osada T., Sako T., Higuchi H., Kurosawa Y., Shimomitsu T., Miwa M., et al. Quantification of ischemic muscle deoxygenation by near infrared time-resolved spectroscopy. J. Biomed. Opt. 2000;5:102–105. doi: 10.1117/1.429975. [DOI] [PubMed] [Google Scholar]
- 19.Gunadi S., Leung T.S., Elwell C.E., Tachtsidis I. Spatial sensitivity and penetration depth of three cerebral oxygenation monitors. Biomed. Opt. Express. 2014;5:2896–2912. doi: 10.1364/BOE.5.002896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cohade C., Mourtzikos K.A., Wahl R.L. “USA-Fat”: Prevalence is related to ambient outdoor temperature-evaluation with 18F-FDG PET/CT. J. Nucl. Med. 2003;44:1267–1270. [PubMed] [Google Scholar]
- 21.Au-Yong I.T., Thorn N., Ganatra R., Perkins A.C., Symonds M.E. Brown adipose tissue and seasonal variation in humans. Diabetes. 2009;58:2583–2587. doi: 10.2337/db09-0833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim S., Krynyckyi B.R., Machac J., Kim C.K. Temporal relation between temperature change and FDG uptake in brown adipose tissue. Eur. J. Nucl. Med. Mol. Imaging. 2008;35:984–989. doi: 10.1007/s00259-007-0670-4. [DOI] [PubMed] [Google Scholar]
- 23.Nirengi S., Sakane N., Amagasa S., Wakui S., Homma T., Kurosawa Y., Hamaoka T. Seasonal differences in brown adipose tissue density and pulse rate variability in a thermoneutral environment. J. Physiol. Anthropol. 2018;37:6. doi: 10.1186/s40101-018-0166-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yoneshiro T., Aita S., Matsushita M., Kameya T., Nakada K., Kawai Y., Saito M. Brown adipose tissue, whole-body energy expenditure, and thermogenesis in healthy adult men. Obesity (Silver Spring) 2011;19:13–16. doi: 10.1038/oby.2010.105. [DOI] [PubMed] [Google Scholar]
- 25.Bruns O.T., Bischof T.S., Harris D.K., Franke D., Shi Y., Riedemann L., Bartelt A., Jaworski F.B., Carr J.A., Rowlands C.J., et al. Next-generation in vivo optical imaging with short-wave infrared quantum dots. Nat. Biomed. Eng. 2017;1:pii: 0056. doi: 10.1038/s41551-017-0056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Watanabe M., Yamamoto T., Kakuhata R., Okada N., Kajimoto K., Yamazaki N., Kataoka M., Baba Y., Tamaki T., Shinohara Y. Synchronized changes in transcript levels of genes activating cold exposure-induced thermogenesis in brown adipose tissue of experimental animals. Biochim. Biophys. Acta. 2008;1777:104–112. doi: 10.1016/j.bbabio.2007.10.014. [DOI] [PubMed] [Google Scholar]
- 27.Boushel R., Saltin B. Ex vivo measures of muscle mitochondrial capacity reveal quantitative limits of oxygen delivery by the circulation during exercise. Int. J. Biochem. Cell Biol. 2013;45:68–75. doi: 10.1016/j.biocel.2012.09.024. [DOI] [PubMed] [Google Scholar]
- 28.Delpy D.T., Cope M. Quantification in tissue near-infrared spectroscopy. Philos. Trans. R. Soc. Lond. B. 1997;352:649–659. doi: 10.1098/rstb.1997.0046. [DOI] [Google Scholar]