Abstract
Head and neck cancer is the sixth most common cancer with over 500000 annually reported incident cases worldwide. Besides major risk factors tobacco and alcohol, oropharyngeal squamous cell carcinomas (OSCC) show increased association with human papillomavirus (HPV). HPV-associated and HPV-negative OSCC are 2 different entities regarding biological characteristics, therapeutic response, and patient prognosis. In HPV OSCC, viral oncoprotein activity, as well as genetic (mutations and chromosomal aberrations) and epigenetic alterations plays a key role during carcinogenesis. Based on improved treatment response, the introduction of therapy de-intensification and targeted therapy is discussed for patients with HPV OSCC. A promising targeted therapy concept is immunotherapy. The use of checkpoint inhibitors (e.g. anti-PD1) is currently investigated. By means of liquid biopsies, biomarkers such as viral DNA or tumor mutations in the will soon be available for disease monitoring, as well as detection of treatment failure. By now, primary prophylaxis of HPV OSCC can be achieved by vaccination of girls and boys.
Key words: Head and neck cancer, Oropharyngeal squamous cell carcinoma, Carcinogenesis, Human Papillomavirus, Immunotherapy
1. Introduction and Summary
Oropharyngeal squamous cell carcinoma (OSCC) is the only head and neck tumor entity with clearly increasing incidence. Infections with oncogenic high-risk (HR) human papillomaviruses (HPV) are responsible for this development as they are increasingly found in OSCC. The transmission pathways and persistence of HPV in the oropharynx are still unknown. However, there are numerous hints that the transmission of HR HPV occurs through sexual contact. The carcinogenesis of HPV-positive OSCC (HPV OSCC) is mainly promoted by viral oncoproteins. However, genetic modifications also play a key role and often additional risk factors of classic carcinogenesis are observed (tobacco). Up to now, genetic examinations do not show a clear picture of HPV OSCC-specific mutations. Investigations of epigenetic modifications (DNA methylation, microRNA, tumor metabolism, immune escape, gene expression) identified HPV-specific aberrations that reveal approaches to future targeted therapies. Patients with HPV OSCC are often rather young, relatively healthy, and have accumulated less lifestyle risks; in comparison to HPV-negative OSCC, the overall survival (OS) of those patients is significantly better. The better OS and less additional risk factors make these patients suitable to benefit from de-intensification of the treatment or targeted therapy options. Since January 2017, revised TNM classifications and staging are applied for HPV OSCC. As test procedure, the p16INK4a (p16) test is suggested internationally. However, testing of HPV OSCC should be performed by means of dual detection of HPV DNA and p16 expression if possible. HPV OSCC will then, in contrast to former times, be classified into lower UICC stage groups. After therapy, patients with HPV OSCC have about 30% better 5-year OS rates in all therapeutic modalities. HPV is no predictor for surgery or radiotherapy (RT) so that surgical tumor resection still has a high significance. Currently, numerous studies are conducted with less intensive therapy; however, up to now results have not been published. Other trials focus on the significance of new immunotherapies for HPV OSCC. Surgical therapy options for distant metastasis are noteworthy; there are still possibilities of curative therapy in cases of distant failure. Beside the assessment of functional impairment, this is relevant for the follow-up of our patients. In the future, it is very probable that specific as well as de-intensified therapies are available for patients with HPV OSCC. Regarding the assignment to specific therapies, risk models are currently developed and discussed. Possibly, the viral carcinogenesis provides a valuable option for molecular early detection and follow-up by means of blood samples (so-called liquid biopsy). Finally, ENT-specialists should promote HPV vaccination for girls and boys because probably nearly all cases of HPV OSCC might hereby be avoided.
2. Epidemiology
2.1 Update on increased incidence of oropharyngeal cancer
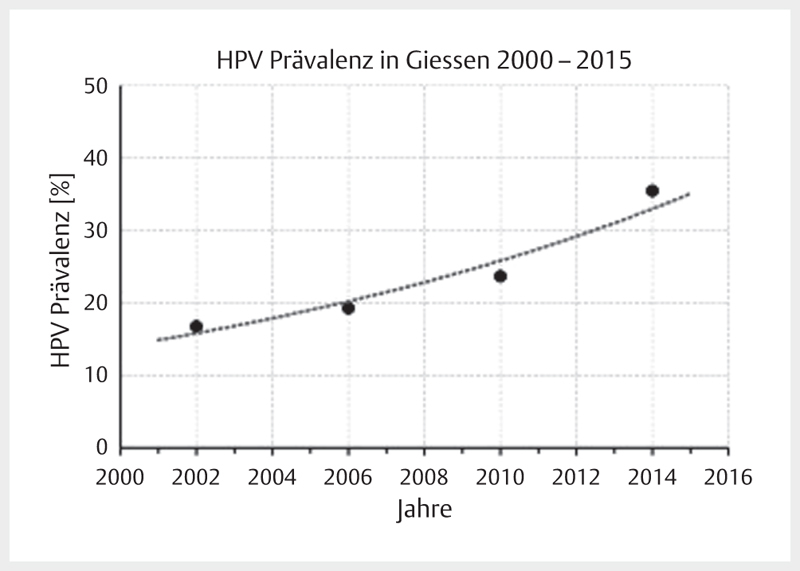
Increasing incidence rates are described for HPV-associated head and neck tumors whereas the incidence of all other head and neck carcinomas decreases in developed countries. A comparative analysis of data of US American registries from 1973–2012 and 2000–2012 revealed a doubling for OSCC (frequently HPV-associated) with simultaneous decrease of the incidence for cancer of the oral cavity (rarely HPV-associated) 1 . Canadian registries currently also report a decrease of the general incidence of head and neck cancer with simultaneous increase of OSCC 2 . This epidemiological trend is explained by the increasing prevalence of oncogenic HPV in OSCC, based on nearly all published original papers 3 . Depending on the study design and detection procedures, the prevalence of oncogenic HPV in OSCC reaches up to 85% in recently published series from Scandinavia 4 . It may at least be assumed that the increased prevalence described is already overestimated because of methodical flaws. With regard to the design, for example older specimens were compared with newer ones, this might explain a systematic incorrectness. In German-speaking countries, a HPV prevalence for OSCC is currently assumed with 20–40% 5 6 7 . For tonsillar carcinomas, oncogenic HPV was detected in more than 50% of the cases already 15 years ago 8 , here the percentage of HPV-associated OSCC can be expected to be much higher. A comparative investigation of 599 patients of our own patient population ( Fig. 1 ) with OSCC showed an increase of the HPV prevalence of about 20% of the early patients to currently over 50% 7 . A comparative analysis of the HPV prevalence in cervical CUP syndrome could reveal a clear increase of currently nearly 75% HPV-positivity rate at our department. In summary, the published data show a continuous increase of OSCC incidence rates and correspondingly, the increased incidence rates are due to the HPV epidemic.
Fig. 1.
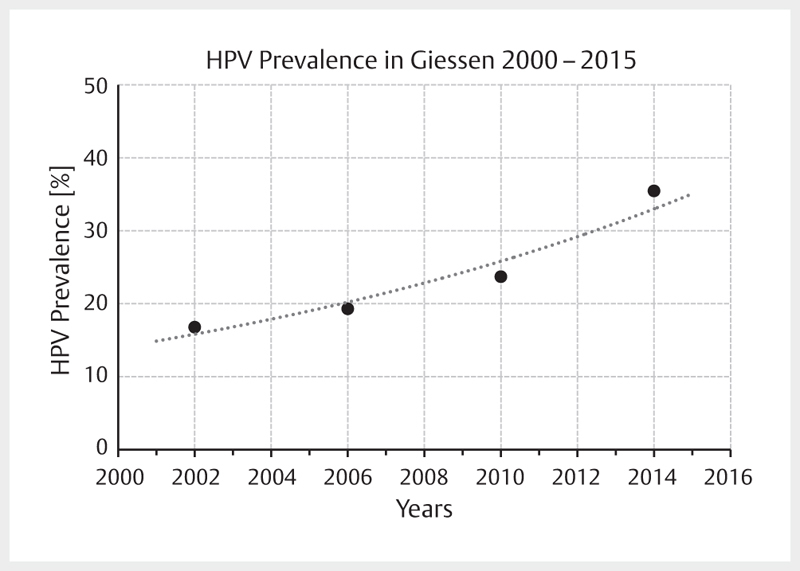
Prevalence of oncogenic HPV in OSCC patients who were treated in Giessen is increasing. Currently the prevalence amounts to more than 50%. The data points represent the mean value of 4 years each.
2.2 Significance of HPV detection outside the oropharynx
From a health economic point of view, the percentage of HPV-associated head and neck tumors in other anatomical locations than the oropharynx is of high interest, too. For example, those cases might be avoided by HPV vaccination. Furthermore, patients could also benefit from de-intensified therapy and reduced side effects. The first question that is relevant in this context is, if the detection of HPV in tissues outside the oropharynx reveals true HPV-associated carcinogenesis or if it is an incidentally detected infection without further relevance. The second question in this context is, if the detection of HPV in non-OSCC is associated with an improved prognosis of the patients.
A recent US American publication from 2015 could reveal a high rate of HPV-positive DNA test results (70.1% in the oropharynx, 32.0% in the oral cavity, and 20.9% in the larynx) also outside the oropharynx 9 . However, considering all publications and meta-analyses on HPV positivity outside the oropharynx, the results are inconsistent 10 11 12 . A data set of a meta-analysis of 12,263 patients showed HPV association in the oral cavity (24.2%) and the larynx (22.1%) based on DNA test. Outside the oropharynx, however, very few data sets with dual test results (HPV DNA and p16 test) are published 13 . Currently, an extensive investigation from Spain was presented with results from 3,680 patients with head and neck tumors after combined testing for DNA, RNA, and p16. Hereby, the HPV prevalence for oral cavity cancer amounted to 4.4% and for laryngeal cancer to 3.5%; in cases of positivity of all 3 tests, the results were even much lower. This relativizes significantly the mentioned, sometimes very high rates of HPV-associated head and neck tumors outside the oropharynx 14 . A high percentage of positive HPV test results outside the oropharynx probably does not show HPV-associated carcinogenesis but acute infections or false-positive test results.
Prospective investigations on the relevance of HPV detection outside the oropharynx with regard to prognosis of the patients are not available. However, based on retrospective data of patients who underwent radiotherapy (RT) or combined radiochemotherapy (RCT) in the context of clinical studies, it can be assumed that a positive p16 test outside the oropharynx has low prognostic significance. The DAHANCA consortium in Denmark treated 1,294 patients with advanced head and neck cancer by means of RT or RCT; and in head and neck cancer outside the oropharynx no prognostic significance could be elaborated 15 . In addition, p16 positive non-OSCC patients were evaluated after treatment in 3 RTOG studies. In comparison to p16 positive OSCC patients, non-OSCC patients had a mortality risk increase of 50% 16 . For patients with laryngeal cancer and positive p16 test, even poorer survival rates have been published 17 . Serological examinations also contradict to a correlation between the risk of head and neck tumor disease (apart from oropharynx) and HR HPV infection. In an analysis of HPV 16 specific antibodies, the odds ratios for the risk to develop OSCC amounted to 14.6 compared to 3.6 (oral cavity) and 2.4 (larynx) 18 . A more recent investigation (ARCAGE study) evaluated 1,496 head and neck cancer patients. Positivity for HPV16 L1 and E6 antibodies increased the risk for the development of OSCC by factor 8.6 and 132.0, respectively. In contrast, marginal values of 1.54 and 4.18, respectively, were described for laryngeal cancer 19 .
In summary, the prevalence of HPV-induced tumors outside the oropharynx is clearly lower than assumed and roughly estimated to be less than 5%. There is no reliable evidence that the prognosis of those patients is better in comparison to OSCC patients.
2.3 Epidemiology of carcinogenic HPV infections
Since nearly all adults in Germany have contact to oncogenic HPV during adolescence, it is important to understand why HPV OSCC increases during the last decades and develops mainly in male patients. The most common manifestation of HPV infection are warts and genital condylomas. In more than 90% of cases, these diseases are caused by non-oncogenic HPV types 6 and 11. The infection can already be transmitted at birth and presents to ENT specialists in particular as respiratory papillomatosis. True neoplastic lesions of the cervix are sometimes caused by type 6 and type 11, too. However, in the majority cervical lesions typical oncogenic HPV types 16, 18, 31, and 45 are found.
Regarding the prevalence of oral infection with HPV in the general population, cross-sectional studies are available, but only few data are published on the temporal dynamics. A review of 18 trials with 4,581 healthy adults described an estimated incidence of oral HR HPV infection with 1.3% 20 . The age distribution of oral HPV infection shows a bimodal distribution. The first peak could be found between 30 and 34 years of age and the second peak between 60 and 64 years. The infection occurred significantly more frequently in males 21 . Generally, the data situation is not evident because in another investigation, females had genital oncogenic HPV infections with the same frequency than males 22 , only the duration to “clearance” was (slightly) different to the disadvantage of the men. Incidence and type of sexual contact (oral sex, deep kisses, promiscuity) as well as age at first sexual intercourse, marihuana consumption, cigarette consumption, and genital HPV infections could be identified as risk factors 23 . The average duration of an oral HPV infection was assessed in 1,626 male persons and amounted to about 7 months; the follow-up, however, was only 13 months 24 . The majority of oral HPV infections heal within several months without further consequences. Reinfections occur only rarely. It is worth mentioning that even partners of HPV OSCC patients only have an infection rate slightly above 1% 25 . In addition, immunodeficiency (HIV infection), cigarette consumption, and high age are reported as risk factors for persisting oral HPV infection 26 . For better understanding the increased incidence of oral HPV infections in males, other data describe a higher number of sexual partners, younger age at the first sexual contact, and numerous oral sexual contacts 27 . Another hint to the susceptibility of males for HPV type 16 (HPV16)-caused OSCC is that genital HPV infections in men are mainly due to HPV 16 and not type 18 28 .
Reliable data why mostly men develop HPV 16-induced OSCC are not available, but numerous hints are found for an accumulation of risks (kinetics of the infection, nicotine, sexual risks, see chapter 4). Overall, this may explain why currently an estimated percentage of 75% of the patients with HPV OSCC are male. In contrast to the data for an increased incidence of OSCC, no data are available that confirm an increase of oral HR HPV infections.
2.4 Development in regions with consequent primary prophylaxis
Primary prophylaxis against carcinogenic HPV is available as HPV vaccination. The Sanofi Pasteur MSD Company produced the quadrivalent vaccine Gardasil that was approved in the USA and Europe in 2006. One year later, the bivalent vaccine Cervarix was approved. Both vaccines contain the recombinant capsid protein L1 of the HPV types 16 and 18, and 6, 11, and 18, respectively. Since April 2016, the 9-valent vaccine Gardasil 9 is available and additionally protects against the HR HPV strains 31, 33, 45, 52, and 58. The advantages are an extended protection given by vaccination and a 2 dose scheme (in intervals of 5-13 months). The HPV vaccines are approved as of the age of 9 years and vaccination should be performed before the first sexual contact. The approval applies for girls and boys, however, currently the German Standing Committee on Immunization (Ständige Impfkommission, STIKO) currently recommends only vaccination of girls, which is also paid by the health insurers.
In Germany, the HPV vaccine is currently not widely administered. According to an analysis of health insurance data of the AOK of Baden-Württemberg, only 37% of young women born in 1996 had complete protection provided by vaccination. In comparison, the vaccination rate against mumps and rubella is about 92% according to the Robert Koch Institute. Comparable data with vaccination rates of <40% in girls were published by the ministry of health in 2014 29 . The data clearly indicate vaccination of boys. However, the registration trials was naturally conducted based on precancerous lesions of the cervix and accordingly, the cost-benefit analyses refer to the diseases of the uterine cervix 30 .
In 2015, 34% of the countries worldwide had a HPV immunization program. However, population-related only less than 5% of all nations (in countries with high incidences often no program was established) benefited from vaccination in this time. From countries with a high coverage, numerous data are available that report an effect on HPV-associated diseases even apart from cervix cancer. For example, a review of the literature from 2015 could reveal that HR HPV infections were reduced by 68% and anogenital warts decreased by 60%, in countries with an immunization rate of more than 50% 31 . The highest decrease of HR HPV-related new diseases apart from cervix cancer were consistently reported from countries with vaccination programs and so-called catch-up vaccination of older, non-vaccinated people (Australia, Canada, Denmark, and New Zealand). The programs were implemented nearly always accompanying school education.
Convincing data on respiratory papillomatosis (RRP) are available from Australia 32 . Between 2011 and 2015, pediatricians and otolaryngologists collected data of newly diagnosed cases of juvenile RRP and published them in a meeting report. Only 13 cases had been registered (7 in 2012, 3 in 2013, 2 in 2014, and 1 case in 2015). None of the mothers of those cases had received vaccination. Two strategies are additionally discussed regarding the prophylaxis of RRP in children: first, vaccination of newborns if the mother had condylomas, and second, vaccination of pregnant women with confirmed HPV 6 or 11 infection in order to protect the child against infection by transmission of antibodies. In cases of vaccinated mothers, a similar antibody titer could be measured in newborns 33 .
Oropharyngeal cancer mostly occurs in male patients, in RRP the gender distribution is nearly the same. Numerous other diseases with high stress for the affected patients are related to carcinogenic and non-carcinogenic HPV. What is the benefit that can be expected for other diseases apart from cervix cancer? If consequent vaccination prophylaxis is performed, a dramatic effect might be expected for the incidence of HPV OSCC. Hence, many publications also recommend vaccination of boys, which is absolutely supported by the authors.
3. Carcinogenesis
Carcinogenesis is a process consisting of several steps where genetic and epigenetic modifications in cancer-associated signaling pathways accumulate over time. This results in the typical phenotype of malignant cells characterized by: unlimited replication potential, independence of growth factors, suppressed ability of apoptosis, invasive growth, and metastatic potential as well as increased angiogenesis 34 35 . The individual risk to develop cancer disease depends on extremely diverse and sometimes interdepending factors and is therefore difficult to be determined. The most important risk factor groups include: environmental influences (UV and other natural radiation, anthropogenic substances/radiation), noxae (tobacco/alcohol consumption, HPV infection), genetic predisposition (e. g., BRCA1/2 mutations in hereditary breast and ovarian cancer), immune factors (vaccination, immunosuppression), and age.
The majority of head and neck cancers are squamous cell carcinomas that are mainly associated with the risk factors of tobacco and alcohol consumption or oncogenic HPV. The carcinogenesis of HPV-associated and HPV-negative head and neck cancer is associated with other specific risk factors (see chapter 2). A separate risk to develop one of those two cancer diseases is difficult to estimate because none of the risk factors appears isolated and overlapping of risks is not the exception but the rule.
3.1 Leukoplakia – premalignant alterations
Regarding HPV-negative head and neck cancer, premalignant alterations have been known for several decades, especially in the oral cavity 36 37 . Depending on different risk factors (gender, extent of the lesion, and WHO stage of dysplasia), a transformation rate of 1-2% is estimated. Genetic changes seem to be most probably responsible for malignant transformation while HPV could only be found in 1% of the leukoplakia 38 39 . Generally, the aberration probability of premalignancies cannot be safely predicted and precancerous stages in HPV OSCC could not be reliably identified (see below).
3.2 Field cancerization
Leukoplakias are visible changes that are preceded by macroscopically invisible premalignant lesions. Those invisible lesions may possibly explain the tendency to develop locoregional recurrences after treatment. The correlation of locoregional recurrences with the occurrence of dysplastic changes in neighboring regions coined the term of field cancerization in 1953 40 . Meanwhile this term could be defined with molecular biological and genetic methods. A multistep development model consisting of morphological and genetic modifications was already suggested in 1996 including typical genetic alterations of dysplasia (loss of heterozygosity [LOH] on the chromosomes 3p, 9p, and 17p) and carcinomas (LOH on chromosomes 11q, 4q, and 8) 41 . After some time, it could be shown that at least 35% of oral and oropharyngeal tumors had genetic mutations in mucosal cells in the environment of the carcinomas whereas the epithelium in this area appeared to be normal. This allows the assumption that the carcinogenesis comprises a range of different precancerous stages that are macroscopically invisible and go beyond the resection margins developing locoregional recurrences. Furthermore, focal areas with immunohistological p53 positivity were identified in the neighborhood of carcinomas that characterized “clonal units” and originate from a common precancerous lesion 42 . Mutations in TP53 lead to the expression of an (inactive) tumor suppressor protein p53 and are considered as the earliest oncogenic modification. Together with field cancerization, the multistep development represents the current model of carcinogenesis of HPV-negative head and neck cancer 43 .
3.3 HPV
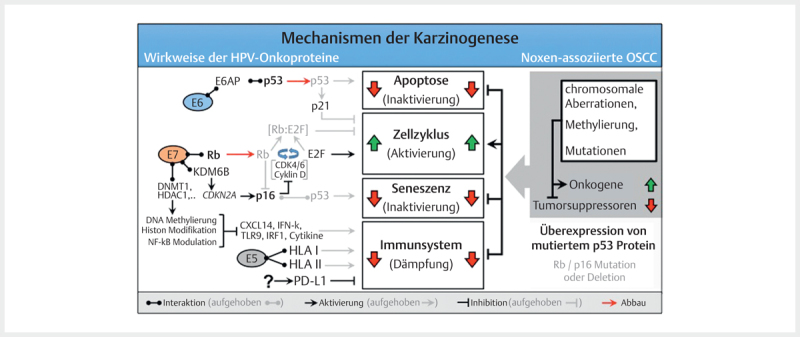
The classic assumption is that viral DNA is integrated into the host cell´s genome during a latently persisting infection with oncogenic HPV. This integration requires linearization of the viral DNA that often occurs as break within the E2 reading frame. The viral E2 protein controls the activity of the viral oncoproteins E6 and E7 and the disruption of the E2 reading frame leads to its enhanced expression. In the natural epidermal life cycle of HPV, E6 and E7 inhibit apoptosis and promote the cell cycle, which leads to proliferation of the epithelial cells, and the infections persists ( Fig. 2 ). As a consequence, infected cells are moved into higher skin layers where the activity of E6 and E7 decreases and envelope proteins of the viral capsids are produced. During HPV-associated carcinogenesis, p53 is marked for proteolytic degradation by E6 activity and thus inactivated. E7 binds to the retinoblastoma protein (RB) that triggers the cell cycle and releases the transcription factor E2F. This increases the transcription of genes that are relevant for cell proliferation.
Fig. 2.
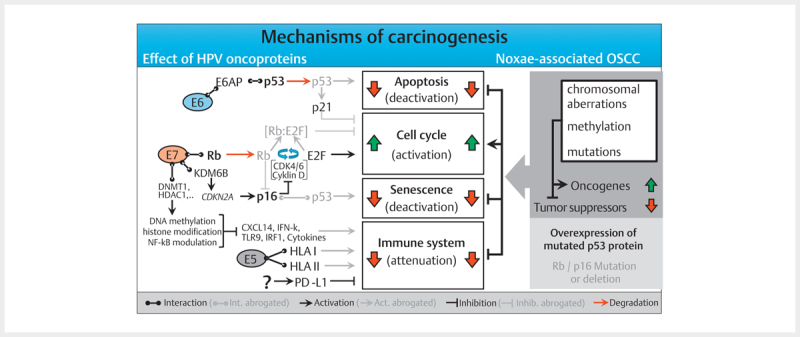
The molecular mechanisms of carcinogenesis of HPV- and noxae-associated OSCC (simplified). Dysfunction of the same cellular programs (apoptosis, cell cycle, senescence, and immune system) leads to carcinogenesis in both groups. Multiple genetic mutations that may affect numerous components of the signaling pathways, lead to activation of oncogenes in noxae-associated OSCC and inactivation of tumor suppressors. In contrast, the HPV oncoproteins E5, E6, and E7 lead to interventions in the signaling pathways which dysregulate the same cellular programs. Characteristic for noxae-associated OSCC are mutations of TP53 resulting in inactive p53 overexpression, as well as mutations in the genes coding for Rb and p16INK4A (p16) so that both proteins are reduced. Generally, those mutations are not found in HPV-associated OSCC, and due to the activity of E7, p16 is overexpressed.
In contrast to the stepwise accumulation of genetic modifications in HPV-negative head and neck cancer, these two significant steps occur due to the activity of viral oncoproteins in HPV OSCC. Mutations in TP53 (and the associated overexpression of p53) and HPV induced fields of carcinogenesis are unknown in HPV-associated head and neck cancer. This could be confirmed experimentally by the absence of viral E6 transcription at the resection margins of HPV-associated head and neck cancer 44 . In contrast to cervix carcinoma, where premalignant stages can be detected by staining with acetic acid, premalignancies of HPV-associated OSCC are unknown.
3.4 Genetic modifications
3.4.1 Mutations
In solid tumors, TP53 is the gene most frequently affected by mutations. In a comparative study, whole exome analyses were performed in 15 types of solid tumors, 11 of them revealed TP53 as the most frequently mutated gene, in the other entities it ranked second twice and third once (preceded by KRAS or BRAF and NRAF , respectively) 45 . In HNSCC, the mutation rate of TP53 is in the upper third of solid tumors with about 40%. Interestingly, the cervix carcinoma shows a particularly low percentage of only 6% TP53 mutations, which depends on the very high rate of HPV-associated carcinomas 46 . Mutations appear in many locations of TP53 ; 12 hotspots are known with more than 1% of all mutations each. Nine of those hotspots concern amino acids that contribute directly to the specific DNA binding domain of TP53 or those that are responsible for correct folding of the DNA binding domain. Other mutations are located in introns and influence an alternative splicing of TP53 which has an effect on TP53 isoforms.
Besides TP53 , mutations of CDKN2a and RB1 (RB, retinoblastoma-associated protein) are often observed in HPV-negative head and neck cancer, however, they are missing in HPV-associated OSCC. RB1 encodes RB and such as in p53, the activity of this signaling pathway is dysregulated in HPV-associated head and neck cancer by viral oncoproteins, which may explain the low mutation rate. CDKN2a (cyclin-dependent kinase inhibitor 2 A) encodes the tumor suppressor protein p16; its effect in HPV-associated head and neck cancer is eliminated by downstream inactivation of RB. Activating mutations of catalytic subunits of PI3K (phosphoinsitid-3-kinases), in particular in PIK3CA , have been described in several studies, mainly for HPV OSCC 47 48 . In contrast, inactivating mutations in the PIK3CA inhibitor PTEN were often found in HPV-negative HNSCC 49 . PI3K is a multiprotein complex that is involved in the regulation of important functions such as cell growth/proliferation, cell adhesion/migration, differentiation, and survival and that is important for HPV-negative and HPV-associated HNSCC to the same extent.
In several studies, other activating mutations were detected in FGFR3 and FBXW7 in HPV OSCC 47 48 50 51 . The membrane-bound fibroblast growth factor receptor 3 (FGFR3) is an activator of the PI3K signaling pathway and FBXW7 is involved in the inactivation of cyclin E, c-Jun, c-Myc, and Notch 1. Mutations in KRAS were also described for HPV OSCC 47 48 , however, this could not be confirmed by own studies 49 . Currently, mutations in HLA and β2 microglobulin genes seem to be relevant that are more often found in HPV OSCC 50 . This could be confirmed by immunohistochemical examinations 52 and might become relevant with regard to immune checkpoint therapies.
3.4.2 Genetic aberrations (copy number variation, CNV)
Because of their extent reaching up to the loss of entire chromosomes or their arms, chromosomal aberrations were the first genetic mutations that could be confirmed in malignant cells. Complex karyotypes with comprehensive numeric and structural chromosomal aberrations are characteristic for head and neck cancer 53 . According to the model of field cancerization, distinct chromosomal modifications with progression of a dysplasia up to invasive carcinoma could be correlated in CGH (comparative genomic hybridization) analyses. The transition from light to moderate dysplasia was characterized by gains on chromosomes 3q26-qter, 5p15, 8q11-21, and 8q24.1-qter and losses on 18q22-qter. Gains on 11q13, 14q, 17q11-22, and 20q and losses on 9p, however, were typical for the transition from moderate to severe dysplasia. Invasive growth correlated with losses occurring together on chromosomes 3p14-21 and 5q12-22 and lymphogenic metastasis with loss on 4p 54 . For the latter ones, also gains on chromosomes 10p11-12 and 11p as well as losses on 4q22-31, 9p13-24, and 14q were described that were not present in respective primary tumors 55 . Interestingly, in the mentioned areas, genes are found that are involved in cell adhesion as well as factors of the MAP (mitogen-activated protein) kinase and PI3K (phosphoinositide-3-kinase) signaling pathway that are also frequently affected by mutations.
Amplifications that are often described for head and neck cancer, are found on the chromosomes 3q-, 8q-, and 20p, independent from the HPV status 47 48 50 56 . Important genes in this region are for example PIK3CA, TP63, SOX2 as well as the oncogene MYC that probably has enhanced activity due to gene amplification. However, 3q amplification was described in the context of the integration of the HPV genome in cervix cancer 57 . In addition, deletions of 13q in HPV-associated and HPV-negative HNSCC have been described, but more rarely in HPV-associated ones which could also be confirmed by whole genome NGS (next generation sequencing) analysis 50 . The chromosomal segment 11q codes genes such as RB1 and CCNA1 (cyclin A) that are involved in the regulation of the cell cycle and that seem to be dysregulated in HPV OSCC by viral oncoproteins.
Generally, an increased chromosomal instability in head and neck cancer seems to be associated with inferior prognosis, which could also be demonstrated in HPV OSCC 58 . Even if nearly the same, but differently dysregulated signaling pathways may be crucial for carcinogenesis of HPV OSCC and HPV-negative head and neck cancer, a series of specific genetic aberrations can be defined for both subgroups. For example, amplifications of 5p, 7p, 8p, 11q, 17q, and 18q could not be verified for HPV OSCC. Clearly more rarely, also losses of 3p, 4q, 5q, 18, and 9p are found. On the last one, for example p16 is encoded which allows the interpretation why the p16 expression works as marker in HPV-associated carcinomas 8 59 60 61 62 .
HPV-specific aberrations are losses on chromosome 16q that are associated with a better prognosis of the patients 50 56 63 . Interestingly, the tumor suppressor gene WWOX is located on 16q. WWOX spans one of 3 most frequent “common chromosomal fragile sites” (FRA16D). Aberrations of FRA16D with dysregulated WWOX expression are known for different tumor types and are associated with a poor prognosis of the patients 64 . Data of the cancer genome project (TCGA, The Cancer Genome Atlas) identified a HPV-specific amplification on chromosome 20q11 ( E2F1 gene) and a deletion on chromosome 14q32.32 ( TRAF3 gene, TNF receptor associated factor 3) by means of NGS in 279 head and neck carcinomas 50 . An overexpression of E2F caused by amplification of 20q11 might develop synergistic effects in the context of viral oncoproteins (E6&E7 that also activate E2F). TRAF3 loss interferes with the NFκB signaling pathway and thus plays a role in inflammatory reactions as well as the innate and adaptive immune response against viruses 65 .
3.4.3 HPV integration
Although linearization in the E2 reading frame of the HPV genome is understood as first step in the classic model of HPV-induced carcinogenesis, the expression of the oncogene E6 and E7 is independent from the number of copies or the integration of viral DNA, and in more than 60% of HPV OSCC only episomal virus DNA was detected by means of PCR 66 67 . Data of current sequence analyses show that all 3 possible stages of the HPV genome (only episomal or integrated, or a mixture of both) occur with nearly the same frequency and probably several mechanisms lead to dysregulated expression of the viral oncoproteins 68 , including methylation of E2 in the regulator region of E6 and E7 (see below).
It could be shown in HPV transfected keratinocytes that viral DNA integration occurs at many positions within the cellular genome, and also in or near important regulator genes of cell proliferation 69 . In one case of malignant transformation of juvenile (HPV type 6 associated) RRP, HPV DNA integration into the human AKR1C3 gene was described. AKR1C3 encodes an enzyme (aldo-keto reductase family 1 member C3) of the androgen and estrogen metabolism and is described for prostate cancer in the context of PSA production, however, it is mostly undescribed in HNSCC 70 . The role of the virus DNA integration in HPV-associated carcinogenesis is not finally clarified. There seems to be a correlation between chromosomal instability and tumor progression. In contrast, in the same study, HPV DNA integration was associated with a better prognosis of patients with tonsillar carcinomas 58 .
3.5 Epigenetic modifications
3.5.1 Epigenetic modifications of nucleic acids
Epigenetic modifications describe modifications of the hereditary information, whereby the “gene activity” but not the sequence of the nucleic acid is changed. The modification of the nucleic acid influences the phenotype and can be transferred to daughter cells. The most important types are methylation of the DNA and modification of histones. Methylation of DNA (such as the modification of histones) is reversible and its function is to use static information of the nucleic acid sequence in a variable manner. By methylation of transcription factor binding site, the activity of single genes, groups of genes, or entire chromosomes can be controlled, for example in the context of gender-specific inactivation of the X chromosome or genomic imprinting in dependence of the parents’ origin of certain alleles.
Different methylation patterns were described in the context of tumor viruses including HPV 71 72 . The most important example of epigenetic gene regulation with regard to HPV is CDKN2A that is located on the chromosome 9p and encodes the tumor suppressor gene p16. p16 inhibits the cell cycle and its expression is often inhibited in head and neck cancer by gene promotor methylation, mutation, or homozygous deletion of the gene 50 73 . In contrast, a strong overexpression of p16 is observed in HPV OSCC that is understood as surrogate marker for this entity. Contrary to earlier assumptions, this overexpression is not due to the E7-related transcriptional activation of p16 by releasing E2F. Moreover, a direct activation of the cellular senescence by expression of E7 was detected. In this way, the histone H3K27-specific lysine demethylase 6B ( KDM6B ) and its downstream target gene CDKN2A is activated 74 . In HPV-associated tumor cells, Rb is inhibited by E7. Thus, the overexpression of p16 does not lead to an inhibitory effect on tumor cells ( Fig. 2 ). Moreover, the activity of the cyclin-depending kinases 4 and 6 (CDK4/6) seems to be intolerable in the context of Rb inhibition of tumor cells, which causes dependence from the expression of CDK4/6 inhibitor protein p16 and its overexpression promotes carcinogenesis in contrast to HPV-negative tumors 75 .
Besides p16, alternative splicing of CDKN2A produces another gene product, p14ARF. The protein sequence of p14ARF develops through reading an alternative reading frame ( ARF ) of CDKN2A and differs fundamentally from p16. p14ARF inhibits ubiquitin ligase MDM2, whereby p53 is stabilized and the cell cycle regulator p21 is expressed. p21 interacts with and inhibits cyclin CDK complexes, which stops the cell cycle between G2 and the metaphase. The regulation of p14ARF expression occurs by modification of CpG loci downstream the transcription start of p14ARF and p16. Correlation was found regarding their methylation in OSCC with positive HPV status and increased expression of p14ARF but not p16 76 . A relationship of the increasing methylation degree of CDKN2A with increasing grade of dysplasia was observed in the cervix, which in fact does not concern the according promotor region 77 . Also in patients with head and neck cancer, a correlation exists between the methylation pattern and the clinical course. For example, the therapy success could be successfully predicted in head and neck cancer patients based on promotor methylation of only 5 genes ( ALDH1A2, OSR2, GRIA4, IRX4 , and GATA4 ) 71 78 .
The classic explanation model of HPV-associated carcinogenesis is based on an integration of viral DNA into the human genome, which leads to an interruption of the E2 reading frame and an elimination of the inhibition of viral oncoproteins E6 and E7. Frequently, further episomal HPV copies are present beside integrated HPV DNA; and in about one third of HPV-associated OSCC, exclusively episomal virus DNA is detected. Here, the classic explanation model is apparently not satisfactory and a methylation of the E2 binding site in the regulation region for E6 and E7 in the HPV genome was identified as further integration-independent regulatory mechanism for the expression of E6 and E7 79 80 .
3.5.2 microRNA expression
microRNAs (miRNA) develop from hairpin bend-like precursor transcripts of 60-70 nucleotides that are shortened to a length of about 22 nucleotides. Together with the proteins DICER1 and Argonaute (AGO) they are integrated in the miRNA-induced silencing complex (miRISC) and guide it, based on their sequence, to corresponding target sequences of the mRNA that is subsequently cleaved enzymatically and thereby inactivated. This relatively simple regulatory mechanism of gene expression is clearly more complex in reality because miRNAs – depending on the conservation grade of their target sequence – may bind to different mRNAs and mRNA may dispose of binding sites for more than one miRNA.
Despite methodical progress during the last years, only few comparative studies have been conducted on the differential expression of miRNAs with regard to the HPV status in head and neck cancer; only a “handful” of miRNAs have been mentioned in more than one study 81 . In one of the most recent trials, 1,719 miRNA sequences were evaluated in 15 HPV-negative and 11 HPV-associated OSCC by means of microarrays. A total of 25 differentially expressed miRNAs could be identified, their functions were elaborated in silico in the context of the PI3K and Wnt signaling pathways, the regulation of the cytoskeleton, and the focal adhesion 82 . The mostly known miRNAs include Has-miR-363 that is upregulated in HPV-associated HNSCC in contrast to HPV-negative ones 83 84 85 . Target sequences of Has-miR-363 are found for example in CDKN1A (cyclin-dependent kinase inhibitor 1), CASP3 (Caspase-3), and CD274 (programmed cell death 1 ligand 1, PD-L1) and they indicate regulatory functions in apoptosis, cell cycle, transcription, and immunology. Another example is miRNA203; its expression is downregulated by the HPV oncoprotein E7 during cellular differentiation. A target gene of miRNA203 is the transcription factor p63; and the expression of p63 as well as its downstream target genes like CARM-1, p21 , and Bax , are increased by the inhibition of miRNA203 by E7 86 . Hereby, epithelial cells remain proliferative and in an undifferentiated stage which is required for the natural lifecycle of HPV. In the HPV E6/E7 induced tumor model in human keratinocytes, p63 enhances the invasiveness by modulation of the Src-FAK (focal adhesion kinase) signaling pathway by dissolving focal cell contacts (cell adhesion) and restructuring the extracellular matrix (ECM) 87 .
Beside cellular miRNAs, miRNAs were detected encoded in the HPV genome, which could be confirmed experimentally. Potential target sequences of those miRNAs are found in the HPV genome but also in the human genome 88 . Interestingly, target sequences of two less frequent human miRNAs were also identified in the HPV- E6 gene (miR-875 and miR-3144). In HPV16-positive cell cultures, both inhibit growth and induce apoptosis 89 , which demonstrates the complex regulatory possibilities by means of miRNAs.
3.6 Dysregulation of tumor metabolism
Tumor hypoxia was described as being important for the survival and therapy response of head and neck cancer 90 91 92 . It is well-known that patients with tumor hypoxia respond poorly to irradiation because of the reduced presence of reactive oxygen species (ROS). During the tumor growth, also a tumor-specific metabolism develops in order to assure the supply and the proliferation of the cells. A specific feature of this metabolism is the increased decomposition of glucose to lactate which was first described under aerobic conditions as “Warburg effect” in 1924. The decomposition of glucose to lactate, however, only provides 2 Mol ATP per Mol of glucose, which is compensated by an increased glucose rate 93 94 95 . Beside energy, this adapted glucose metabolism of the tumor serves for providing important basic building blocks (e. g., nucleic acids, amino acids, and lipids) 96 .
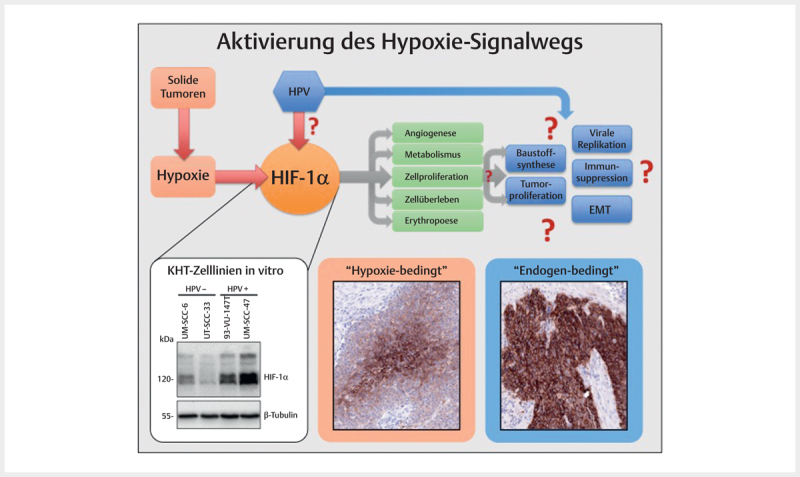
Hypoxia occurs frequently in many solid tumors and arises because tumor cells proliferate rapidly,exceeding a critical mass which leads to obstruction and compression of the blood vessels in the direct neighborhood of the tumor. This finally results in poor oxygen supply of the tumor centers so that the tumor cells adapt to this oxygen deprivation and several signaling pathways are switched on in order to secure cell survival and to change the glucose metabolism from efficient oxidative phosphorylation to inefficient glycolytic metabolism 97 . Hereby, the group of HIF (hypoxia-inducing factor) transcription factors, in particular HIF-1 (HIF-1α & HIF-1β), play a key role for the cellular adaptation to hypoxic conditions. HIF-1 activates a series of target genes that secure cell survival, serve for the modification of the metabolism, and promote invasion, cell proliferation, metastasis, erythropoiesis, and angiogenesis 97 98 99 . Beside real tumor hypoxia, it could be demonstrated in cell lines that HPV oncoproteins contribute to hypoxia pathway dysregulation by stabilizing HIF-1α ( Fig. 3 ) 100 101 102 .
Fig. 3.
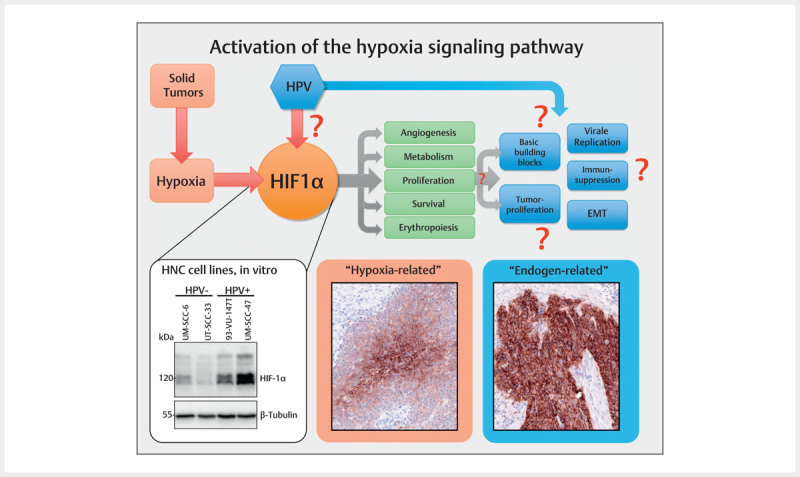
Frequently, in solid tumors an activation of the hypoxia signaling pathway is found that is obvious due to the central expression of respective marker proteins (here: Glut I) in tumor nests and that can be confirmed by immunohistochemistry (hypoxia-related). Some tumors, however, show a consistently high expression of the same marker that suggest other activation mechanisms of the signaling pathway (endogen-related). The central regulator protein of the hypoxia signaling pathway HIF-1α is present in HPV associated tumor cells in an overexpressed way compared to HPV-negative tumor cells (Western-blot, bottom left). In analogy to fig. 2 , the viral activity leads to activation of the hypoxia signaling pathways and thus to processes that are favorable for carcinogenesis.
Thus, oncogenic viruses are able to influence the tumor metabolism by direct and indirect interaction with cellular regulators such as HIF-1α, in order to adapt cellular pathways for viral replication and synthesis, which also promotes carcinogenesis and progression. This metabolic phenotype allows tumor cells to proliferate despite adverse circumstances like oxygen deprivation 103 . Hence, the signaling pathways that are used for modification of the metabolism and their regulators such as e. g., HIF-1 represent potential targets for an inhibition, in particular for those tumors that largely depend on glucose and aerobic glycolysis.
3.7 Tumor environment/immune escape mechanisms
During the development of invasive, HPV-associated squamous cell carcinoma, several lines of defense mechanisms have to be overcome. Viral infection is the first step, for which the physical barrier of skin/mucosa plays a crucial role. After absorption of viral particles, those have to traverse the cell and reach the nucleus. In the following persisting infection, the HPV oncoproteins E5, E6, and E7 have important functions to remain undetected by the immune system as long as possible and to maintain the production of new viruses in the epithelial cells. In the microenvironment of HPV-infected cells, increasingly cells of the innate immune response are found such as dendritic cells (DC) Langerhans cells (LC), natural killer cells (NK), and natural killer T cells (NKT) 104 .
To a high percentage, HPV infections heal by themselves, and only in a small part, cancer develops. In such cases, further modifications have to take place that enable infected cells to overcome the physical barrier of the basement membrane and to be resistant against the continuous attacks of the immune system. For example, higher rates of HPV infections and HPV-associated carcinomas are known in patients with different NK cell dysfunction 105 . In the context of viral reproduction and evolution, this last step of carcinogenesis has a dead end, because due to the missing differentiation of the epithelial cells, virus particles can neither be produced nor transmitted to the outside. HPV-associated tumors, as well as HPV-negative tumors, are in a steady-state with the immune system and when the disease is diagnosed, this equilibrium has already been shifted to the benefit of the tumor, and growth is observed that cannot be controlled by the immune system. The understanding of the immune escape mechanisms can be used to restore the equilibrium or to shift it to the benefit of the immune system.
A physical immune escape mechanism of HPV consists in operating its complete lifecycle within the epithelial cells and not releasing virus particles into blood or tissue. Thus, HPV antigens are barely exposed to the immune system and antibody titers are not high enough during natural HPV infection to have a protective effect 106 . Nonetheless, apparently T cell response is required for regression of an infection because it correlates with the presence of granzyme B positive cytotoxic T cells in the context of cervix premalignancies 107 .
The oncoproteins E5, E6, and E7 have an effect on many cellular mechanisms, among others they suppress signaling pathways that are necessary for the recognition of virus-infected cells by the immune system. For example, the surface protein CXCL14 works as chemokine and attracts different cells of the immune system such as DC, LC, NK, and NKT cells. E7 interacts with the cellular DNA methyltransferase DNMT1; and an E7 dependent promotor methylation and thus repression of CXCL14 could be shown 108 . Furthermore, E7 modulates the methylation and acetylation of histones, which lead (among others) to the reduction of the TLR9 (toll-like receptor 9) expression and transcriptional activity of IRF1. TLR9 is able to recognize viral DNA and to activate the innate immune system 109 . IRF1 response elements are found in promotors of a series of genes such as TAP1 (transporter associated with antigen processing 1), which plays a role in antigen charging of HLA-I in the endoplasmatic reticulum 110 . In addition, E7 interacts with NFκB and inhibits its translocation in the nucleus. Hereby, for example activation of IFN-α, IL-6, and TNF-α is stopped, which leads to attenuation of the inflammatory reaction 111 .
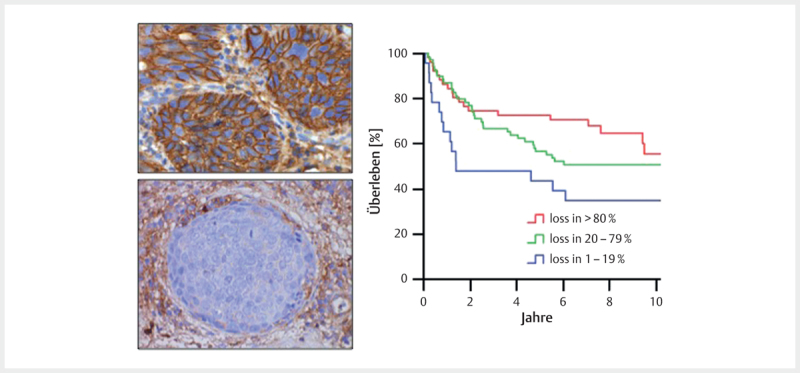
Inflammation inhibiting functions could be revealed for E6 in the context of the pro-inflammatory cytokine IL-1β. Depending on E6AP, E6 causes the ubiquitination of the precursor of IL-1β (pro-IL-1β), which is followed by its proteasomal degradation 112 . For the E5 protein, an interaction with the heavy chain of HLA-A and -B could be detected, which leads to retention of the HLA-I complex in the Golgi apparatus and in the endoplasmatic reticulum 113 114 . HLA-C and HLA-E seem to be downregulated by other mechanisms. The loss of HLA-I on the cell surface correlates with a reduced response of CD8+ T cells in E5 expressing cells. However, loss of HLA-I leads to attraction and activation of NK cells, which was already described for HPV-associated OSCC and correlates with an improved overall survival of the patients ( Fig. 4 ) 115 . Beside HLA-I, the functional surface location of HLA-II as well as CD1d is also inhibited by E5 116 117 . The viral capsid protein L2 seems to block the maturation and antigen presentation of DC and LC by disturbing the intracellular transportation and processing of virus particles after integration of DC and LC 118 .
Fig. 4.
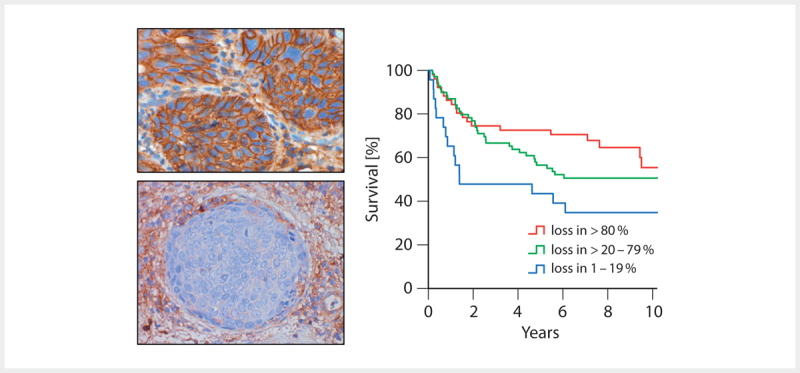
Immunohistochemical proof of the expression of β2 microglobulin (β2M) as marker of functional membrane-based HLA I expression (top left). The loss of the expression of β2M on tumor cells (bottom left) correlates with a better overall survival of patients with OSCC (right).
3.8 Molecular subtypes and gene expression profiles
Whole genome gene expression analysis are generally based on a comparative hybridization (microarrays) or sequencing of mRNA. The capacity of microarrays as well as the sequencing techniques have continuously improved over time, which leads to an increasing coverage of the genome, but also to limited comparability of former and current data.
In one of the first gene expression studies of head and neck cancer, 60 differentially expressed genes were identified from 1,187 examined tumor-associated genes on a cDNA microarray. They correlated with the radioresistance or the response to radiotherapy 119 . Already 3 years later, 60 head and neck tumors were examined on a cDNA microarray with probes against 12,814 human genes. In this study, 4 subtypes could be identified based on the gene expression. Signatures were found with a focus in the EGFR signaling pathway, a mesenchymal subtype, a subtype with expression pattern of normal epithelium, and a subtype with enhanced antioxydase enzymes 120 . However, in all early studies, no attention was paid to the HPV status of the samples. Similar gene expression profile groups, called basal, mesenchymal, atypical, and classic types, were identified in another study by means of Agilent 44 K microarrays. An enhancement of HPV associated specimens was observed in the group of atypical gene expression (e. g., with increased expression of CDKN2A ) 121 . By means of another platform (Illumina Expression BeadChips) also 4 subtypes were identified. However, only the classic expression type was confirmed as being comparable to the above-mentioned study 122 , which is probably due to technical differences or the heterogeneity of the specimens.
In 2015, a clinically relatively homogenous cohort of 134 head and neck tumors with a percentage of 44% HPV-association, was examined with an Agilent 4×44Kv2 expression array. Afterwards, the data were summarized with already published data to a cohort of more than 900 patients. In this trial, 5 subtypes were identified that included 2 groups of HPV-associated and 3 groups of HPV-negative head and neck tumors. One HPV-associated and one HPV-negative subgroup showed an immune/mesenchymal expression pattern as well as the expression type that was described above as “classic” 123 124 125 . The remaining HPV-negative group showed a basal expression pattern with overrepresentation of hypoxia-associated genes (e. g., HIF1A, CA9 , and VEGF ), epithelial markers (P-cadherin, cytokeratin KRT1 and KRT9 ), and components of the neuregulin signaling pathway. In contrast to this basal expression group, both HPV-associated groups did not reveal modification regarding the number of copies or the expression of EGFR/HER ligands 126 .
The knowledge gained from genome-wide expression analyses could not be implemented translationally until now. This is due to missing technical standards, which limits comparability of the results. On the other hand, the total number of analyzed samples is relatively low, so that for example heterogeneity because of patient characteristics cannot be subtracted. In the future, this might be different due to technical advances analyzing retrospective, formalin-fixed paraffin embedded (FFPE) archive specimens. In a pilot study, 4 tumor samples of HPV-associated and 2 HPV-negative OSCC were analyzed by means of a NanoString gene expression assay and Ion Torrent AmpliSeq cancer panel tNGS. From 230 tumor-associated genes, several ones were correlated with a positive HPV status (e. g., WNT1, PDGFA , and OGG1 ). By hierarchic classification, 6 groups of differentially expressed genes were identified 127 . Thus, the use of FFPE materials that are currently not broadly analyzed, might increase the significance and reliability of data from expression analyses in future.
4. Clinical Particularities
4.1 Is HPV-associated OSCC a sexually transmitted disease?
The transmission of HPV occurs mainly via skin contact or contaminated objects. Afterwards, infection of epithelial cells may develop with extremely high host specificity. HPV infects undifferentiated cells directly above the basement membrane through microwounds or in very thin epithelia. While the infected cells remain close to the basement membrane, the viral DNA replication is reduced. This is due to the fact that the viral development processes are coupled with the differentiation processes of the infected cells while they move up to the epithelial surface. Whereas the regulatory “early” proteins (E) are produced in the early HPV cycle, the “late” proteins L1 and L2 that represent the capsular structure of the viral particles are processed later in the life cycle. Together with viral DNA they build infectious virus particles that are released to the environment together with the external epithelial cells.
Typically, for example after visiting swimming pools children develop plantar warts because of infections with the low risk HPV types 1, 2, and 4. Another transmission pathway is the perivaginal transmission during birth which may induce the development of laryngeal papillomatosis in infants and toddlers 128 . For the HPV-associated OSCC, the sexual transmission pathway with the high-risk papillomaviruses 16 and 18 is in the focus of discussion. The severely increasing incidence in the last decades is mainly explained by changed sexual behavior, younger age at first sexual contact as well as the increased practice of oral sex 129 . Even if the genital-genital transmission of HPV infection seems to be predominant, also other transmission pathways such as anal-genital, oral-genital, manual-genital contact, the use of sex toys as well as autoinoculation are possible 130 . In 2 cohorts in the USA, it could be shown that patients with HPV OSCC had a higher rate of promiscuity (vaginal, anal, oral) in comparison to patients with HPV-negative tumor. Furthermore, (oral) sex with frequently changing partners, casual sex as well as rare use of condoms were reported. Light-skinned patients, singles as well as divorced patients mentioned a higher number of sex partners. Regarding the income, no difference could be found concerning the number of sex partners, while patients with a higher educational status reported a higher number of sex partners. After performing gender stratification, the changed sexual behavior could be confirmed mainly in men 131 132 .
For new life partners, there seems to be the risk of transmission. But the data up to now do not allow valid conclusions. Since the intermittent or missing use of condoms is associated with an increased risk of oral HPV infection or HPV-associated OSCC, the use of condoms probably protects against transmission of oncogenic HPV 21 129 . For nicotine and alcohol, no association with HPV OSCC could be detected. However, the consumption of marihuana was strongly associated with HPV-associated tumors. Patients with more than 10 pack-years of tobacco consumption had a higher number of sex partners than patients without or only low nicotine abuse. There was no evidence for multiplicative effects for HPV OSCC between nicotine and alcohol, marihuana and nicotine, or marihuana and alcohol 131 132 .
In summary, the reason for the increased occurrence of HPV OSCC is seen in the changed sexual behavior. However, it must be questioned in which way and actually if it has really changed over the years. The causal reason for the increase of HPV associated carcinomas in the oropharynx still cannot be answered with certainty.
4.2 Clinical particularities of HPV-associated OSCC
In some countries, patients with HPV-associated OSCC are often rather young 131 133 , but regional differences are observed. In our own cohort of 396 patients who were treated in Giessen between 2000 and 2009, no significant age difference could be detected in OSCC patients depending on the HPV status ( Table 1 ). Often a higher socio-demographic as well as socio-economic status (higher education level, higher profession position as well as income) is found in comparison to patients with HPV-negative OSCC 134 . Especially in the USA, males are generally more frequently affected (ratio male/female: 1.5), while the quotient for Asia and some European countries is only 0.7 135 . It is assumed that this is due to a higher transmission rate of HPV infections during orogenital sex 130 , and the higher nicotine abuse of males predisposes them for infection 21 .
Table 1 Clinical differences in HPV OSCC, n=396.
| Non-HPV OSCC | HPV OSCC | |||||||
|---|---|---|---|---|---|---|---|---|
| N | N | % | N | % | p value | |||
| 308 | 80.6 | 74 | 19.4 | |||||
| Gender | Male | 306 | 238 | 80.7 | 57 | 19.3 | 0.964 | |
| Female | 90 | 70 | 80.5 | 17 | 19.5 | |||
| Comorbidity | ECOG | |||||||
| Healthy | 0 | 257 | 87 | 76.0 | 59 | 24.0 | 0.002 | |
| 1–2 | ||||||||
| Sick | 3–4 | 134 | 118 | 89.4 | 14 | 10.6 | ||
| ≥5 | ||||||||
| Age | Young | (<60 years) | 210 | 162 | 80.6 | 39 | 19.4 | 0.987 |
| Old | (≥60 years) | 186 | 146 | 80.7 | 35 | 19.3 | ||
| Alcohol | >2 standard glasses | 161 | 144 | 92.3 | 12 | 7.7 | 0.000 | |
| <2 standard glasses | 123 | 69 | 59.0 | 48 | 41.0 | |||
| Nicotin | >10 py | 319 | 270 | 87.7 | 38 | 12.3 | 0.000 | |
| No | 60 | 29 | 50.0 | 29 | 50.0 | |||
First symptoms that occur in patients with OSCC include sore throat, odynophagia, or globus sensation. In the further course, dysphagia or cervical swelling may be observed. Frequently, the cervical swelling is the first and only symptom of HPV OSCC that lets patients seek for medical advice. This is mainly due to the already advanced N stage with low T stage. In the context of HPV association, the primary tumor is often located at the tonsil or the base of the tongue 133 136 whereas other locations of the oropharynx are rarely affected.
While smoking and alcohol abuse are the classic risk factors for head and neck cancer, there are important geographic differences with regard to the incidence of nicotine abuse. A significant decrease could be observed between 1980 and 2012 in Northern Europe as well as North America 137 . While HPV 16 and nicotine abuse were considered as independent risk factor till recently 131 , a patient cohort in the USA revealed a higher risk of HPV OSCC after nicotine abuse 138 . In cases of HPV OSCC, nicotine abuse seems to have a negative impact on the survival whereas alcohol seems to play only a secondary role 139 140 . In total, the mortality risk of patients with HPV-associated tumors, however, seems to be reduced by more than 50% in comparison to patients with HPV-negative OSCC. This improved therapy outcome is most likely associated with the improved locoregional control, among others by increased radiation sensitivity (see chapter 6).
Second primaries are significantly more rarely observed in patients with HPV OSCC. If this is possibly due to missing risk factors such as nicotine or alcohol abuse is unclear because more recent studies report about increased nicotine abuse also in patients with HPV-associated OSCC. The good prognosis of those patients increases the number of patients in follow-up examinations and the duration of follow-up is longer so that therapy-associated long-term complications such as dysphagia, xerostomia, or dysgeusia are in the focus. In future, therapy de-escalation plays a key role in order to improve the patients’ quality of life. Furthermore, the implementation of a sufficient tertiary prophylaxis in those patients with long-term survival might be important in order to early detect recurrences or distant metastases even in the long-term follow-up (see chapter 7).
5. Diagnostics and Staging
HPV-negative and HPV-associated OSCC vary significantly regarding their clinical course and biology. It is worth mentioning that a clear and valid procedure for the diagnosis of HPV-induced head and neck carcinoma does not exist. In single cases, even after performing extensive laboratory examinations, it is not evident if a tumor is HPV induced or not. Probably, it can be assumed that the triggering factors of carcinogenesis coincide in many cases of OSCC. The established methods include immunohistochemical p16 staining (p16 test), the detection of HPV-specific nucleic acids (HPV DNA test), and in situ hybridization (HPV ISH) in tissue section.
5.1 Test procedures for the diagnosis of HPV-associated oropharyngeal cancer
For clear definition of the HPV status in head and neck cancer, the presence of HPV as well as the detection of oncogenic activity in tissue specimens is required. The test results are then applicable as prognostic markers for patient counseling and also for planning future therapies. Testing of both preconditions, however, may also provide false-positive or false-negative results because of technical and biological reasons. So the misinterpretation of a test may have substantial consequences for the patients. Up to now, prospective studies are not available that justify concrete adaption of the therapy based on the HPV status, although a recent study from the USA reveals that already more than half of the physicians choose treatment strategies based on HPV tests 141 .
The laboratory diagnosis of the HPV status generally consists of the detection of viral DNA in biopsies and is performed mainly by sensitive PCR-based test procedures or the less sensitive ISH 142 . The high sensitivity of PCR-based procedures bears the disadvantage of contamination, for example due to parallel HPV infections. Biologically inactive HPV DNA in the tumor tissue show signals that cannot be differentiated. In contrast, the signal distribution in HPV ISH may provide hints regarding the HPV association, which, however, requires more efforts and does not differentiate biologically inactive HPV DNA as well. As “gold standard” for oncogenic activity, the detection of viral mRNA transcripts of the oncogenes E6 and E7 by means of RT-PCR is acknowledged. The natural instability of mRNA leads to a high specificity because free mRNA can practically be excluded as basis of contamination, but hereby also the sensitivity is lower. Furthermore, the examination of specimens for mRNA is more complex, unfixed tissue is needed in most cases and the detection of mRNA transcripts does not necessarily correlate with a protein expression of viral oncoproteins or their biological activity.
The relevant characteristic of HPV-associated carcinogenesis is the virus-oncoprotein-caused dysregulation of the cell cycle via the Rb signaling pathway and the inhibition of apoptosis by inactivation of p53 (see chapter 3). Also in HPV-negative tumors, inactivation of p53 occurs, however, generally due to mutations in TP53 , which may become obvious immunohistologically by detection of overexpressed but inactivated p53. In HPV-associated carcinomas, p53 is missing and the tumor suppressor protein p16 is overexpressed due to viral oncoprotein activity ( Fig. 5 ). The overexpression of p16 in tumor cells is rare, however, it is observed in different cancer entities and in about 5% of oropharyngeal carcinomas even independent from HPV 59 . Because of a moderate specificity, the p16 test alone is only partially sufficient for determination of the HPV status. In combination with a detection of viral nucleic acids, the sensitivity and specificity can be increased significantly ( Fig. 6 ). The combination of p16 test with HPV DNA tests is acknowledged to be the most practicable test combination for the clinical use 142 .
Fig. 5.
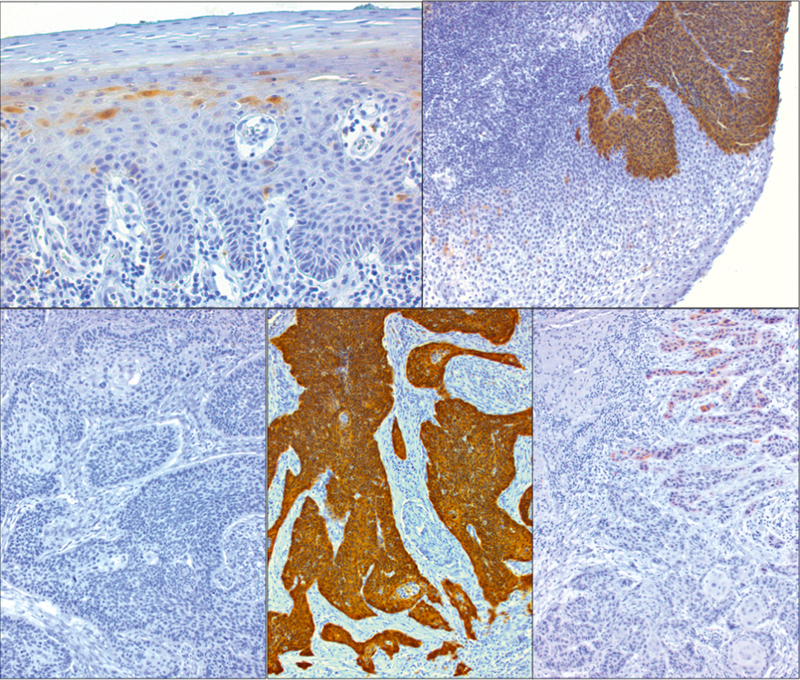
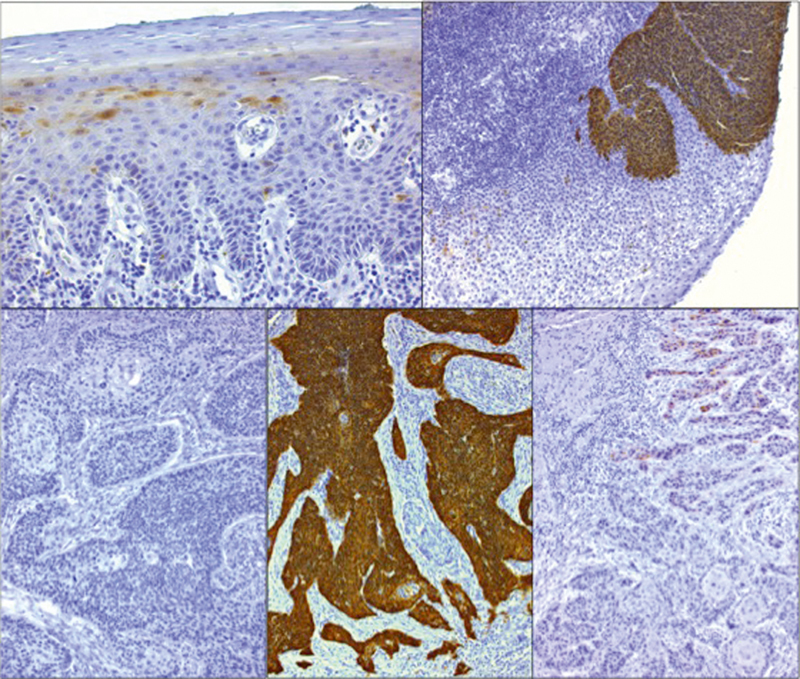
Immunohistochemical proof of p16INK4A protein expression in single cells of healthy squamous epithelium (top left). Generally, p16INK4A is missing in HPV-negative squamous cell carcinomas of the oropharynx (bottom left). However, strongly overexpressed p16INK4A is present in HPV-associated OSCC (bottom, in the middle) and dysplasia (top right). Single OSCC sometimes show a weak expression of p16INK4A (bottom right) that cannot be considered as positive in the context of HPV-diagnostics.
Fig. 6.
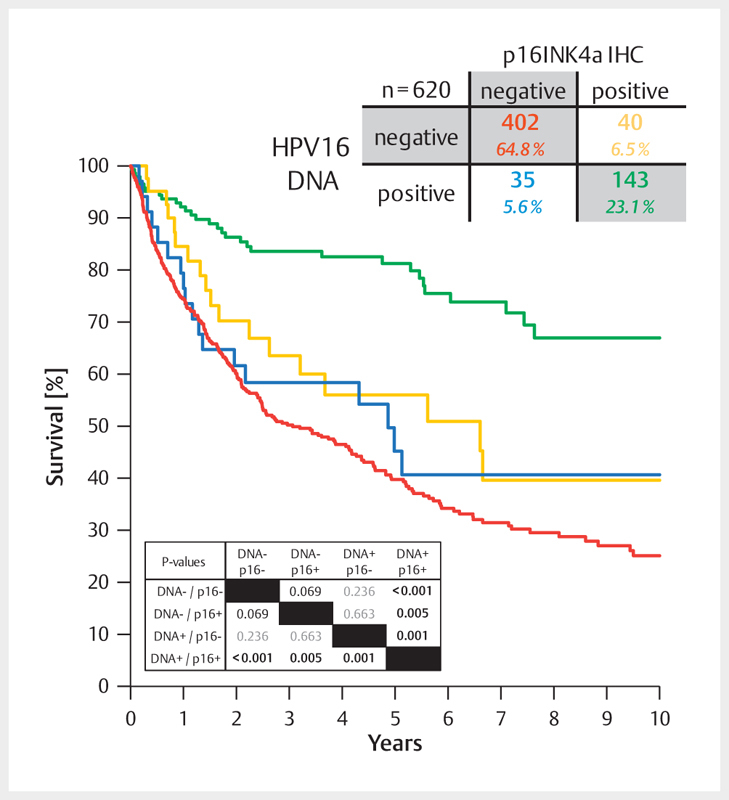
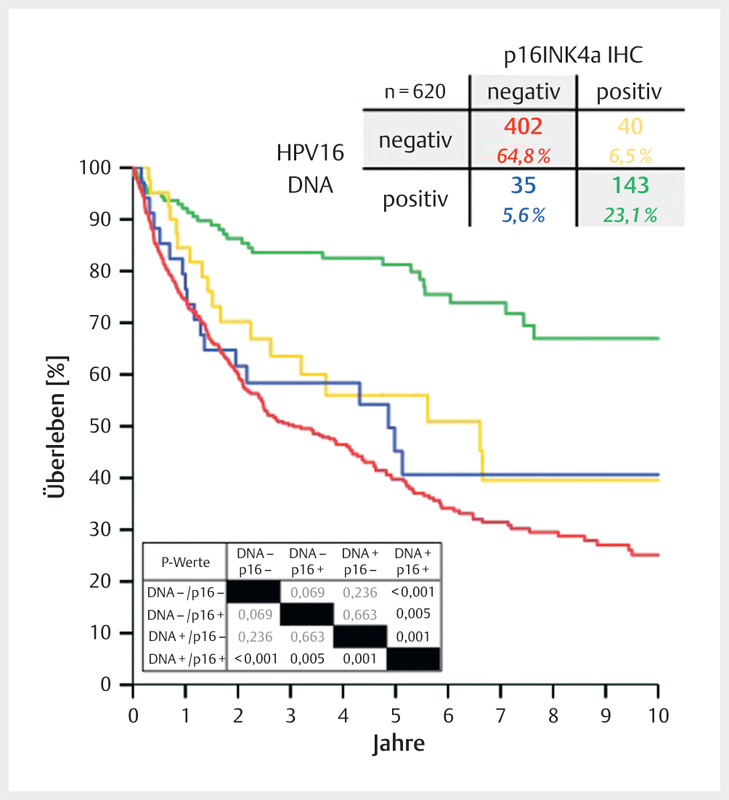
Between 2000 and 2015, the average prevalence of HPV-associated OSCC (HR-HPV DNA and p16INK4A-positive samples) amounted to 23% in Giessen. About 6% of all cases revealed discordant results of HPV DNA and p16INK4A tests, each. The survival of those patients (blue and yellow lines) was significantly inferior compared to patients with HPV-associated OSCC. However, it hardly differed from the survival of patients with HPV-negative OSCC (red line).
The examination of saliva was also evaluated regarding HPV association. This method is easy, inexpensive, and might be applicable for prophylaxis, therapy monitoring, and follow-up. First articles on this topic were already published more than 20 years ago; a good correlation of PCR test results from saliva (oral rinses) and tumor biopsies of 190 patients could be elaborated 143 . However, a really convincing specificity and sensitivity (between 50 and 70%) could not even be reported in recent investigations 144 . In the context of local tumor recurrences, it could be shown exemplarily that the detection of HPV material is possible 145 . The results, however, are naturally falsified by frequent oral HPV infections. Also the detection of oncogenic active HPV infections could not be successfully performed.
In most patients with HPV-associated OSCC, HPV-specific antibodies are found in the blood already several years before diagnosis 146 147 . The antibodies directed against the oncoproteins of HPV probably do not develop during infection but only years later during malignant transformation. This could be demonstrated in a cohort of young men with HPV infections who had no seropositivity against HPV 16 E6 protein 148 . The detection of antibodies against HR-HPV E6 and E7 correlates well with the prognosis of patients, comparable to the tissue HPV test 149 . Based on annual blood tests, a recently published investigation of about 1000 control patients calculated the risk to develop HPV OSCC with more than 5% (more than 100 times higher than with negative test) when E6 antibodies could be found at the testing time 150 . A positive antibody test cannot be assigned to a certain lesion, neither under a time nor a spatial aspect, so the diagnostic benefit for the determination of the HPV status is rather low. However, excellent applications are possible for early detection. As a limitation, however, it must be mentioned that the test procedures are not generally available.
5.2 Significance of tumor endoscopy
Tumor endoscopy is mainly used for painless histology gaining as well as estimation of the tumor size in order to determine the resectability of the tumor and possible reconstructive procedures. Furthermore, in the context of tumor endoscopy the presence of a secondary carcinoma shall be excluded, this mainly applies for patients with noxae abuse. However, the performance of tumor endoscopy or panendoscopy or triple endoscopy is critically discussed for all head and neck tumors. So there is nearly no international consensus regarding the significance and technique. Based on the further development of imaging procedures, the risk of rigid endoscopy, and unclear incidence of second primaries, it is increasingly negatively discussed 151 . The significance of tumor endoscopy in the context of HPV OSCC can be questioned most critically because those patients often do not have a positive history of noxae abuse that might lead to secondary carcinoma 152 153 . So the value of endoscopy is rather low regarding the question of secondary carcinoma in those cases. In Germany, the performance of tumor endoscopy with rigid instruments is still widespread 154 . As long as there is no reliable evidence, endoscopy may be performed in the current standardized way, however, for HPV OSCC also system oriented biopsy under general or local anesthesia can be performed without any concern.
5.3 Imaging
Imaging diagnostics of HPV OSCC correspond to the standardized imaging of head and neck cancer. For example, ultrasound of the neck is performed for imaging of regional tumor disease. Also computed tomography (CT) and magnet resonance imaging (MRI) are routinely applied. Those procedures are used for morphological description of head and neck tumors. In comparison, positron emission tomography (PET) in combination with CT scan is a hybrid procedure that shows a functional image of the metabolic situation in the affected tissue. Hereby the radioactive isotope 18 F of fluorine is the nuclide that is mostly used in PET and can be combined with several pharmaceutics. The combination that is most frequently applied, is the metabolic radiotracer 18 F-2-fluoro-2-deoxy-glucose (FDG), possible alternatives are hypoxic radiotracers such as 18 F-fluoromisonidazol (FMISO) or the next generation 18 F-fluoroazomycin arabinoside (FAZA) 155 .
Because of the distinct tumor metabolism of HPV associated OSCC in comparison to HPV-negative OSCC (see chapter 3.6) ( Fig. 7 ), differences in the functional imaging may be expected 156 . So HPV-specific tumor characteristics possibly reflect in 18 F-FDG PET-CT. For example, it could be shown that HPV-associated OSCC in the context of epithelial mesenchymal transition (EMT) has clearly more homogenous FDG and FAZA tracer uptake 155 157 , and own data (in press)]. In concordance, a significant increase of the PET parameters of HPV-negative OSCC is observed with increased size of the primary tumor 155 . In comparison, HPV OSCC provide a clearly more homogenous image of tracer uptake in different tumor stages.
Fig. 7.
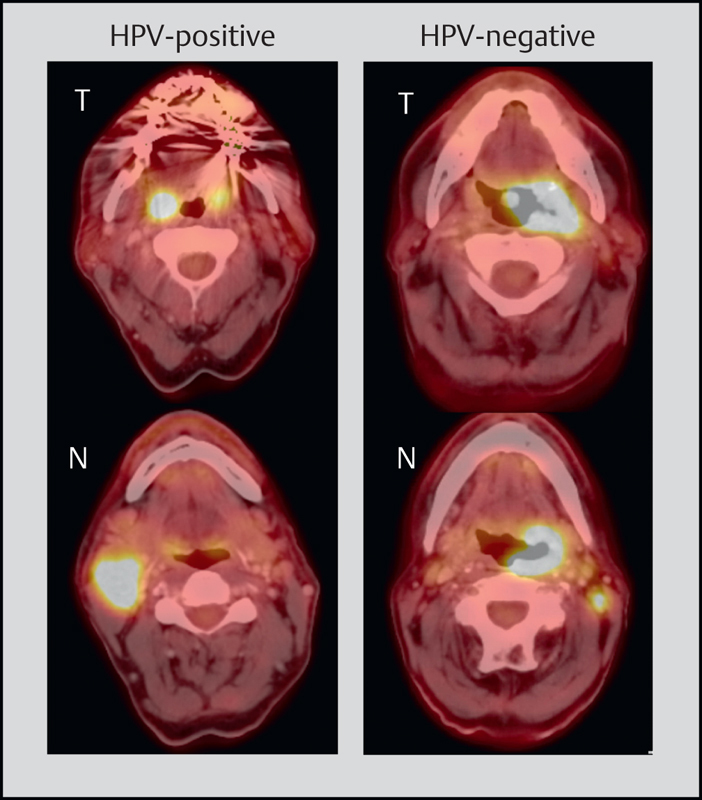
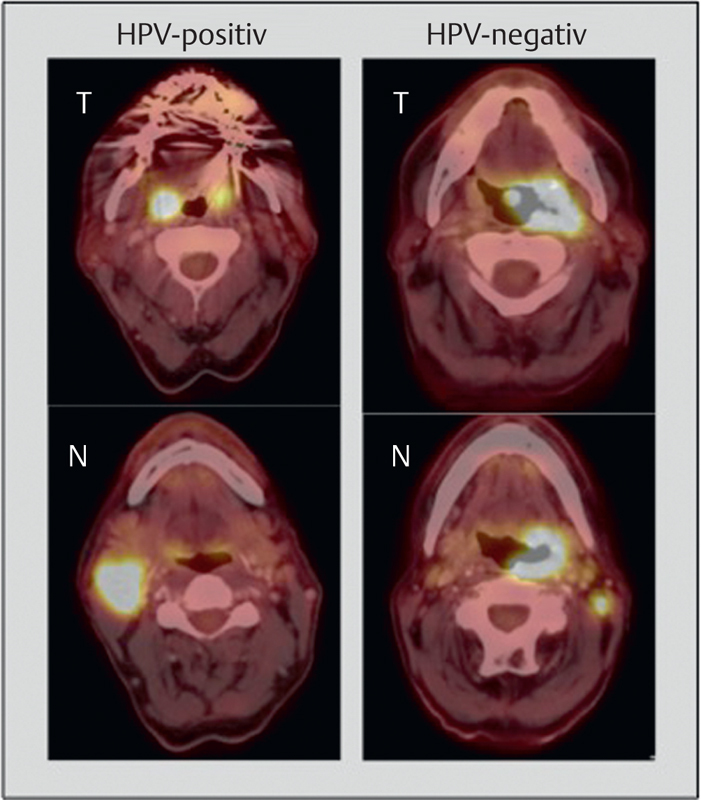
The current staging for HPV-positive and HPV-negative OSCC was changed significantly. On the left, metastatic HPV OSCC (ipsilateral, <6 cm) is displayed, this means tumor stage I; according to the former edition, the tumor would have to be classified as stage IVa (T1, N2b). On the right, a T3N1 OSCC, HPV-negative, is displayed, to be classified as stage III. Thus, the tumor stage of the patient on the left is lower in comparison to the patient on the right.
But functional imaging is not only applied in the context of staging procedures, it serves also as therapy monitoring. Currently, the controversially discussed therapy de-escalation for HPV OSCC is in the focus. In a prospective study (DAHANCA 24), it was recently possible to demonstrate that performing FAZA-PET/CT in the context of primary radiotherapy may be promising as monitoring for positive therapy response 158 . In another pilot study, it could be shown for HPV-positive OSCC patients that FMISO PET before and during therapy reflects the tumor charge. It might also be possible to reduce the irradiation dose in cases of positive treatment response 159 . Furthermore, the functional imaging has become essential for follow-up. In a prospective multicenter trial, a high significance of 18 F-FDG PET CT could be revealed for the follow-up of OSCC primarily treated with RCT. Hereby it became obvious that 18 F-FDG PET-CT as diagnostic tool for detection of regional residues was not inferior to a standard arm with post-therapeutic salvage neck dissection, which is due to the high sensitivity of this test procedure. In addition, complications and expenses could be reduced by imaging 160 .
A new option of imaging are radiomics procedures. Hereby image features are quantified by computer assistance, clusters are created and then compared with imaging databases in order to draw conclusions regarding tissue properties, diagnosis, and courses of the disease. For example, such a computer-assisted prediction of the HPV status is relatively reliable based on a CT dataset 161 . Radiomics signatures were applied successfully as prognosticators for example in breast cancer patients, but also in lung and head and neck cancer 162 163 . By combining the radiomics signature and the p16 test, the prognostic selectivity between 2 groups of head and neck cancer patients could be improved 164 . In the future, radiomics datasets might be included in prognostic models.
5.4 Revised TNM classification and staging rules
The TNM classification of malignant tumors mainly serves as prognosticator. The increasing incidence, different biology of the disease, and the clearly improved prognosis after therapy justify the necessity to consider HPV OSCC as independent tumor entity. The main reason is the fact that the established staging rules only insufficiently reflect the prognosis of the patients. In particular regarding the nodal status, it was demonstrated several times that there is no significant influence on the prognosis of the patients based on former TNM rules 165 166 . Only with regard to advanced T stages, a selectivity for the prognosis based on former TNM rules was reported 167 168 . With the recent publication of the 8 th edition of the TNM classification of malignant tumors, the HPV status of OSCC is considered. Also the TNM rules for HPV-negative OSCC were modified, the factor extracapsular spread (ECS) or extranodal extension (ENE) is now included. HPV-negative OSCC are classified as hypopharyngeal carcinomas and described in an own chapter of the cancer staging manual. Since January 1, 2017, the TNM rules are modified with regard to the nodal status of HPV OSCC – this relevantly influences the tumor stage according to the UICC (Union internationale contre le cancer).
Regarding HPV OSCC, the new edition follows study results of the ICON-S group (International Collaboration on Oropharyngeal Cancer Network for Staging) in Canada, USA, Denmark, and the Netherlands. In this multicenter cohort study, 2,603 patients with known HPV status were included. Nearly all patients received primary RCT (98% of the patients) and more than 70% of the examined patients were HPV-positive 169 . For both groups, the overall survival was analyzed according to previous recursive partition analysis with deduction of a revised staging system for the group of HPV-associated OSCC and HPV-negative OSCC. The proposals of the authors were implemented unchanged in the 8 th edition for patients treated without surgery. Since the applicability is not confirmed for patients who underwent tumor surgery, modified criteria were suggested for those patients. For this purpose, retrospectively assessed results of a surgically treated cohort of 220 American patients were included for whom the presence of 5 or more lymph node metastases was associated with a high risk of tumor recurrence 170 . All patients were p16 positive and underwent transoral surgery; 80% had ECS-positive lymph nodes, this factor was not relevant for prognosis.
Up to now, ECS was considered as indicator for poor prognosis and had a decisive impact on the therapy 171 172 . So the extranodal growth is an indication for adjuvant platinum application during postoperative RT 173 . The exclusion of ECS in the new staging system for HPV OSCC is based – in analogy to the procedure described above – on results of other publications. Retrospective investigations could confirm that the factor ECS is probably not relevant for the outcome of HPV OSCC 174 175 . In addition, the factor ECS is assessed with high interobserver variance 176 . Based on these results, the value of RCT in adjuvant settings of ECS positive HPV OSCC can be doubted 177 . The prospective verification of this assumption is urgently needed because this question is often discussed in tumor boards. Currently 3 prospective studies are conducted that deal with therapy de-escalation including ECS positive HPV OSCC to avoid acute and late toxicity (ECOG 3311, ADEPT, PATHOS, see Table 2 ). Only as a consequence, it will be possible to state if therapy de-escalation is justified despite the presence of ECS in HPV OSCC.
Table 2 Adaptive de-escalation treatment in HPV-positive OSCC.
| Beginning of the study | NCT code | Short name | Phase | HPV diagnosis | Strategy for patients with HPV OSCC | Primary aim of the study | Study title | Recruiting | Ongoing | Completed |
|---|---|---|---|---|---|---|---|---|---|---|
| 2009 | NCT01088802 | 2 | HPV DNA and/or p16INK4A | De-intensification of radiation dose | Comparable therapeutic outcome with lower long-term toxicity | Treatment de-intensification for squamous cell carcinoma of the oropharynx | X | |||
| 2010 | NCT01084083 | 2 | HPV ISH and/or p16INK4A | Induction chemotherapy, reduction of radiation dose, followed by Cetuximab | Comparable therapy outcome | Induction chemotherapy followed by Cetuximab and radiation in HPV-associated resectable stage III/IV oropharyngeal cancer | X | |||
| 2011 | NCT01302834 | RTOG-1016 | 3 | p16INK4A | Substitution of Cisplatin by Cetuximab in RCT | Comparable therapy outcome | Radiation therapy with Cisplatin or Cetuximab in treating patients with oropharyngeal cancer | X | ||
| NCT01530997 | 2 | HPV DNA and/or p16INK4A | Reduction of chemotherapy and radiation dose, limited neck dissection | Comparable therapy outcome with lower toxicity | Phase-II-study of de-intensification of radiation and chemotherapy for low-risk HPV-related oropharyngeal squamous cell carcinoma | X | ||||
| 2012 | NCT01530997 | 2 | HPV DNA and/or p16INK4A | Reduction of chemotherapy and radiation dose | Comparable therapy outcome with lower toxicity | De-intensification of radiation and chemotherapy for low-risk human papillomavirus-related oropharyngeal squamous cell carcinoma | X | |||
| NCT01687413 | ADEPT | 3 | p16INK4A | Postoperative radiotherapy with and without Cisplatin (in R0 and N>0) | Comparable therapy outcome and toxicity | Post-operative adjuvant therapy de-intensification trial for human papilloavirus-related, p16+ oropharynx cancer | X | |||
| NCT01716195 | 2 | p16INK4A | Induction chemotherapy (Carboplatin/ Paclitaxel), reduction of radiation dose and chemotherapy (Paclitaxel) | Comparable therapy outcome with lower toxicity | Induction chemotherapy followed by chemoradiotherapy for head and neck cancer | X | ||||
| 2012 | NCT01706939 | Quarterback Trial | 3 | HPV DNA and p16INK4A | Reduction of the radiation dose (56 Gy) with weekly Carboplatin vs. 70 Gy radiation dose and weekly Carboplatin | Comparable therapy outcome with reduced radiation dose | The Quarterback Trial: A randomized phase III clinical trial comparing reduced and standard radiation therapy doses for locally advanced HPV-positive oropharynx cancer | X | ||
| NCT01663259 | HPV DNA and/or p16INK4A | Substitution of Cisplatin by Cetuximab in radiochemotherapy | Comparable therapy outcome with lower toxicity | Reduced intensity therapy for oropharyngeal cancer in non-smoking HPV 16 positive patients | X | |||||
| 2013 | NCT01898494 | ECOG 3311 | 2 | p16INK4A | Reduction of the radiation dose after transoral tumor resection (advanced OSCC) | Comparable therapy outcome | Transoral surgery followed by low-dose or standard-dose radiation therapy with or without chemotherapy in treating patients with HPV positive stage III-IVA oropharyngeal cancer | X | ||
| NCT01874171 | De-ESCALaTE-HPV | 3 | p16INK4A | Substitution of Cisplatin by Cetuximab in radiochemotherapy | Improved quality of life and reduced toxicity | Determination of Cetuximab versus Cisplatin early and late toxicity events in HPV+ OPSCC | X | |||
| 2013 | NCT01891695 | 1 | p16INK4A | Reduction of the radiation dose for cervical lymph nodes (39.6 Gy) with clinical N0 | Comparable therapy outcome with lower toxicity | A pilot single arm study of intensity modulated radiation therapy elective nodal dose de-escalation for HPV-associated squamous cell carcinoma or the oropharynx | X | |||
| NCT01855451 | TROG12.01 | 3 | p16INK4A | Substitution of Cisplatin by Cetuximab in radiochemotherapy | Comparable quality of life and toxicity | Weekly Cetuximab/RT versus weekly Cisplatin/RT in HPV-associated oropharyngeal squamous cell carcinoma (HPV Oropharynx) | X | X | ||
| 2014 | NCT02281955 | 2 | HPV DNA and/or p16INK4A | Reduction of chemotherapy and radiation dose (follow-up study of NCT01530997) | Comparable therapy outcome with lower toxicity | De-intensification of radiation and chemotherapy for low-risk HPV-related oropharyngeal SCC: Follow-up study | X | X | ||
| NCT02072148 | SIRS TRIAL | 2 | HPV DNA and p16INK4A | Surgery alone for low-risk patients | Comparable therapy outcome | The Sinai Robotic Surgery Trial in HPV-positive oropharyngeal squamous cell carcioma (SCCA) | X | X | ||
| 2014 | NCT02254278 | 2 | p16INK4A | Reduction of radiation dose with or without Cisplatin | Comparable therapy outcome with lower toxicity | A randomized phase II trial for patients with p16 positive, non-smoking associated, locoregionally advanced oropharyngeal cancer | X | |||
| NCT02215265 | PATHOS | 2+3 | HPV (no further data) | Reduction of the adjuvant therapy after transoral resection | Improvement of swallowing | Post-operative adjuvant treatment for HPV-positive tumours | X | |||
| 2016 | NCT02784288 | 2 | p16INK4A | Treatment stratification after pathology of neck dissection | Improvement of the quality of life | Phase II treatment stratification trial using neck dissection-driven selection to improve quality of life for low-risk patients with HPV+ oropharyngeal squamous cell cancer | ||||
| 2017 | NCT03210103 | ORATOR2 | HPV DNA or p16INK4A | Primary de-intensified radiotherapy vs. transoral surgery with neck dissection (+/-) adjuvant radiotherapy | Comparable therapy outcome | A randomized trial of treatment de-escalation for HPV-associated oropharyngeal squamous cell carcinoma: radiotherapy vs. trans-oral surgery | ||||
| 2017 | NCT03215719 | 2 | p16INK4A | Reduction of radiation dose in responders during standard radiotherapy | Comparable therapy outcome | Adaptive treatment de-escalation in favorable risk HPV-positive oropharyngeal carcinoma | X | X |
In the Cancer Staging Manual, the p16 test is suggested as surrogate marker for HPV infection and the headline of the chapter is not “HPV-positive OSCC” but “p16-positive OSCC”. The group of authors decided for this classification because the detection of HPV association is based on a combination of test procedures that are complex and not always clear. In contrast, the p16 test is simple, inexpensive, and widespread. Numerous studies could further reveal the significance of the p16 test for the prognosis of OSCC patients 8 178 179 . The naturally existing problems of the test procedure (high subjectivity regarding the evaluation, biology-related variable p16 expression in numerous cases) often do not lead to clear test results. According to the authors, patients with negative p16 test should be classified in the same way as HPV-negative and hypopharyngeal carcinomas in the Cancer Staging Manual. In our own cohort, we could analyze the prognosis of the patients in that way that a positive result could be shown only for patients with double positive testing ( Fig. 6 ). So the new staging system bears the risk that up to 10% of the patients are falsely classified according to the rules for p16-positive OSCC. It is recommended to secure the HPV status by bimodal procedure if possible and to additionally examine HPV DNA or mRNA for immunohistochemical evidence of p16 (see above).
5.4.1 HPV-associated OSCC
T category: In p16-positive and p16-negative OSCC, the clinical (c) T category corresponds to the pathological (p) T category. Differences of this category only exist regarding T4. HPV-negative OSCC are classified as T4a and T4b, depending on the tumor extension. In HPV OSCC, however, no further classification of the T4 category is performed ( Table 3 ).
Table 3 TNM classifications of OSCC in the 7 th and 8 th edition.
| TNM, 7 th edition | TNM, 8 th edition | ||||
|---|---|---|---|---|---|
| p16-negative | p16-positive | ||||
| T | T | T | |||
| c/p T1 | ≤2 cm | c/p T1 | ≤2 cm | c/p T1 | ≤2 cm |
| c/p T2 | >2 cm, ≤4 cm | c/p T2 | >2 cm, ≤4 cm | c/p T2 | >2 cm, ≤4 cm |
| c/p T3 | >4 cm without extension to the lingual epiglottis | c/p T3 | >4 cm without extension to the lingual epiglottis | c/p T3 | >4cm without extension to the lingual epiglottis |
| c/p T4a | Infiltration of the larynx, outer tongue muscles, hard palate, mandible | c/p T4a | Infiltration of the larynx, outer tongue muscles, hard palate, mandible, lamina med. pterygoid process | c/p T4 | Infiltration of the larynx, outer tongue muscles, lamina med./lat. pterygoid process, hard palate, mandible, lateral pterygoid muscle, skull base, ACI, lateral nasopharynx |
| c/p T4b | Infiltration of the lateral pterygoid muscle, skull base, ACI | c/p T4b | Infiltration of the lateral pterygoid muscle, skull base, ACI | ||
| N | cN | cN | |||
| c/p N0 | No regional lymph node metastases | cN0 | No regional lymph node metastases | cN0 | No regional lymph node metastases |
| c/p N1 | Ipsilateral, solitary ≤3 cm | cN1 | Ipsilateral, solitary ≤3 cm | cN1 | Ipsilateral, solitary or multiple, ≤6 cm |
| c/p N2a | Ipsilateral, solitary, >3-6 cm | cN2a | Ipsilateral, multiple, >3-6 cm | cN2 | Contralateral or bilateral, ≤6 cm |
| c/p N2b | Ipsilateral, multiple, ≤6 cm | cN2b | Ipsilateral, multiple, ≤6 cm | ||
| c/p N2c | Bilateral, contralateral ≤6 cm | cN2c | Bilateral, contralateral ≤6 cm | ||
| c/p N3 | Metastases >6 cm | cN3a | Metastases >6 cm | cN3 | Metastases >6 cm |
| cN3b | ECS | ||||
| pN | pN | ||||
| pN0 | No regional lymph node metastases | pN0 | No regional lymph node metastases | ||
| pN1 | Ipsilateral solitary ≤3 cm | pN1 | ≤4 affected lymph nodes | ||
| pN2a | Ipsilateral solitary, ≤3 with ECS or ≤6 cm without ECS | pN2 | ≥5 affected lymph nodes | ||
| pN2b | Ipsilateral multiple ≤6 cm, without ECS | ||||
| pN2c | Bilateral, contralateral ≤6 cm without ECS | ||||
| pN3a | Metastases >6 cm, no ECS | ||||
| pN3b | Metastases >3 cm with ECS or contra-/bilateral with ECS | ||||
Note.
The subcategories of T4a and T4b are eliminated for HPV OSCC.
N category: With the 8 th edition, the most important actualization is introduced with regard to the nodal status of p16-positive OSCC. The differences between the c category and the p category of affected cervical nodes must be considered. The clinical classification of the nodal status of p16-positive OSCC (cN) is now significantly simplified. A unilateral affection is called cN1, bilateral or contralateral affection is classified as cN2. cN3 remains unchanged with metastases >6 cm. After surgery of the cervical lymph nodes (pN) only the categories of pN1 and pN2 are included. The limit value is the affection of 4 cervical lymph nodes. If 5 or more cervical lymph node metastases are found, a pN2 status is classified. Neither the size nor the presence of ECS are considered in the classification.
Note.
The N category of HPV OSCC differentiates cN and pN. cN: ipsilateral -> cN1 | bilateral ->cN2 |> 6 cm -> cN3. pN: 4 lymph nodes -> pN1 | ≥ 5 lymph nodes -> pN2.
5.4.2 HPV-negative OSCC
In the new edition, the T category for HPV-negative OSCC remains unchanged. For the N category, the difference is made between clinical and pathological status and the factor of ECS is considered as an upgrading into the next higher category ( Table 3 ). ECS is defined as skin invasion, infiltration of the muscles, nerves, or bones in cN and should only then be applied. The same TNM rules are applicable for squamous cell carcinomas of the hypopharynx.
Note.
The N category for HPV-negative OSCC differentiates cN and pN.In clinical staging, ECS becomes the new category of cN3b.In the context of pathology-based staging, the factor of ECS leads to upgrading.
5.4.3 UICC staging
The differences of the TNM classification are also reflected in the UICC staging. A difference is also made regarding p16-positive and p16-negative OSCC. The rules for classification into tumor stages has not been modified for HPV-negative OSCC, in the context of p16-positive OSCC, the UICC staging is performed based on clinically or pathologically verified TNM categories. It is particular that now advanced lymphogenic metastasis is categorized as N1 (e. g., 4 positive lymph node metastases with ECS) and classified in the tumor stage 1 ( Fig. 7 , Table 4 ). Only distant metastasis justifies the tumor stage IV. In an own evaluation of the new TNM rules and UICC staging groups, we could show that the new TNM edition reaches a significant UICC down-staging of HPV OSCC 7 . In a patient cohort of 150 HPV OSCC patients, it became obvious that the new UICC staging increases the number of patients in the stages I and II and the number of patients in stage 4 is significantly reduced ( Fig. 8 ). Currently, the revised TNM rules have already been verified in several cohorts and described as valuable 180 or improvements were suggested 181 .
Table 4 Groups of tumor stages in oropharyngeal cancer, 8 th edition.
| p16 negative | p16 positive | ||||||
|---|---|---|---|---|---|---|---|
| Stage | Stage | Clinical | |||||
| 0 | Tis | N0 | M0 | 0 | Tis | N0 | M0 |
| I | T1 | N0 | M0 | I | T1, T2 | N0, N1 | M0 |
| II | T2 | N0 | M0 | II | T1, T2 | N2 | M0 |
| III | T3 | N0 | M0 | T3 | N0, N1, N2 | M0 | |
| T1, T2, T3 | N1 | M0 | III | T1, T2, T3, T4 | N3 | M0 | |
| IVA | T4a | N0, N1 | M0 | T4 | Each N | M0 | |
| T1, T2,T3 | N2 | M0 | IV | Each T | Each N | M1 | |
| IVB | Each T | N3 | M0 | ||||
| T4b | Each N | M0 | |||||
| IVC | Each T | Each N | M1 | Stage | pathological | ||
| 0 | Tis | N0 | M0 | ||||
| I | T1, T2 | N0, N1 | M0 | ||||
| II | T1, T2 | N2 | M0 | ||||
| T3 | N0, N1 | M0 | |||||
| III | T3, T4 | N2 | M0 | ||||
| IV | Each T | Each N | M1 | ||||
Fig. 8.
Because of the new staging rules for HPV OSCC, stage IV tumors are meanwhile rare, so that there is now a relevant percentage of stage I and II tumors.
In summary, the development of the staging system for HPV OSCC corresponds to the high significance of this disease and leads to an improved selectivity of prognostic groups. However, in the future, probably further revisions of the current edition will be required. It is critical that the p16 testing alone leads to false estimations in up to 10%. Furthermore, the downstaging of HPV-positive OSCC should not lead to uncritical de-escalation of therapy regimen. Molecular signatures and properties (comorbidity) or habits (nicotine abuse) of the patients may play a more important role for the estimation of the prognosis and will probably influence future TNM classifications.
6. Decision-Making Tools for Therapy
Based on the significantly improved prognosis of HPV-associated OSCC regarding recurrence-free survival as well as overall survival, the question must be asked if consequences for therapy strategies can be expected. Hereby, two different approaches are considered. Since multimodal therapy strategies applied for HPV OSCC are apparently much more effective, the question arises whether parts of these multimodal therapies for HPV OSCC might be de-escalated and whether less intensive therapy may lead to the same outcome. Second, the question is asked if this applies for all patients or if de-intensification can only be performed in certain subgroups of patients without jeopardizing the outcome. With this background, the implementation of different prognostic models also from retrospective cohorts is extremely important for estimation of the value of predictors and also with regard to different treatment strategies (see chapter 8). Beside the approach of de-escalation, the question arises if HPV is a predictive marker of a specific therapy. Based on retrospective cohorts, there is primarily no hint in this regard because the prognosis of HPV-associated tumors is better after primary radiotherapy as well as after surgical therapy. In the context of this comparative evaluation, the problem is that around 80% of the patients who underwent primary surgery, have also been irradiated.
6.1 Radiotherapy
Cell culture models indicate that the radiosensitivity of HPV-associated OSCC is higher compared to HPV-negative OSCC 182 . Own investigations on cell lines could reveal a significantly reduced clonogene survival of HPV-positive tumor cell lines after radiotherapy. Comparable results were also described by several research groups 183 . According to a meta-analysis of 30 clinical trials, the improved survival of HPV OSCC patients after radiotherapy alone is confirmed also in the clinical context 184 . However, HPV evidence alone is not predictive for primary radiotherapeutic treatment. It may be interpreted as a hint that when patients with HPV OSCC are only treated with radiotherapy, the locoregional tumor control of p16-positive tumors amounts to 58% after 5 years and the overall survival is only 62% 185 . Comparative investigations on RCT or primary surgical therapy are not available. The prognosis of HPV-associated OSCC after radiotherapy alone can be estimated as being 10-15% poorer compared to patients who underwent multimodal therapy (e. g., radiochemotherapy) 61 139 169 .
This allows drawing the conclusion that de-escalation is probably not suitable for all HPV-associated OSCC based on RT alone. Furthermore, patients with very advanced inoperable OSCC have a poor prognosis of less than 40% for progression-free survival even after intensified definitive RCT 186 . Possibly, there are subgroups of HPV-associated OSCC, e. g., with small primary tumors and only low-grade cervical lymph node metastasis that might be adequately treated with RT alone. A retrospective study of 900 patients provides hints in this regard. The patients have been selected primarily based on the situation if they had phenotypically a HPV-associated tumor, HPV test result were not available 187 . Currently clinical de-escalation trials check which additional predictors are suitable for such therapeutic de-escalation beside HPV ( Table 2 ). In addition to the problem that advanced HPV OSCC might not be treated adequately with RT alone, tumors with advanced cervical metastasis (N2c according to TNM classification of the 7 th edition) also reveal a relatively high rate of distant metastases (estimated 30% of the patients) after RT alone 188 . So patients with advanced tumor disease of HPV OSCC have to receive also chemotherapy in addition to irradiation. Nonetheless, the fact that de-escalation has already started in RT, can be seen in publications of several retrospective series. In 261 patients the tumor bed was left out in the adjuvant situation without reduction of the local control 189 . Control rates of more than 97% have been described in a meta-analysis when radiotherapy of the contralateral lymphatic drainage was omitted in HPV OCSS 190 .
6.2 The role of surgery
Beside de-escalation by performing RT alone, there are also approaches to reduce adjuvant therapy by means of surgical interventions in the primary therapy ( Table 2 ). The basic principle is to elaborate histologically confirmed predictors by upfront surgery that allow de-escalation of the adjuvant therapy. Furthermore, those studies may check if risk factors that have led to the application of simultaneous chemotherapy in the adjuvant situation are justified in HPV-positive tumors (see chapter 5.4). It is rather complicated to elaborate the value of surgery for the survival or function after therapy of HPV OSCC because around 80% of all patients who underwent primary surgery rely on adjuvant therapy. Currently, clinical studies with surgery are conducted with the following questions: reduction of the adjuvant radiotherapy dose after postoperative determination of risk factors (ECOG 3311); omission of chemotherapy in cases of postoperative radiation (ADEPT); comparison of no adjuvant radiotherapy vs. adjuvant radiation with 50 Gy, 60 Gy, or 60 Gy plus platinum, depending on the risk factors (PATHOS).
In this context, 2 studies are funded by the German Cancer Aid (Deutsche Krebshilfe) that compare prospectively primary surgery of oropharyngeal cancer with primary RT. Not only HPV-associated tumors are included in those trials but the HPV status is determined and subgroup analyses are possible. The EORTC study “Best of-1420” conducted in Europe and in Germany focuses on early stages of oropharyngeal cancer with functional endpoints. In addition, the TopROC study compares different therapy strategies for advanced OSCC and the endpoint is the survival.
The decision to perform primary surgery followed by risk-adapted adjuvant therapy or primary non-surgical intervention depends rather on the local or regional particularities and guidelines. Recently, a review article about a histopathological marker was published. Hereby, in particular an advanced T stage could be identified as risk factor for failed tumor control 191 . For smokers, a current retrospective analysis describes that the prognosis is more similar to HPV-negative OSCC patients. For primarily surgically treated cohorts it will be important in the future to identify risk factors in a prospective setting that are relevant for the patients’ prognosis because in Germany currently 75% of the OSCC patients undergo primary surgery. As an example, the cystic degenerated metastasis is mentioned, hereby poor local control rates after therapy without surgery are described 192 . In our own cohort, especially a young age could be identified as additional predictor for a good outcome of HPV OSCC patients 193 .
6.3 Chemotherapy and antibody therapy
Patients with HPV OSCC have a better outcome after RCT than patients with HPV-negative tumors. This fact was first described in the convincing Ang study 139 . This survival benefit was found also in the adjuvant setting with RCT 194 , and after induction chemotherapy the response is better as well 195 . However, compared to the descriptions above, we actually do not know if HPV positivity is a predictor for chemotherapy application, i. e., if HPV OSCC should undergo preferably chemotherapy. For the combined treatment with Nimorazole and radiotherapy, it could be shown for example that in the context of HPV OSCC neither patients with hypoxic nor less hypoxic tumors have a better survival whereas HPV-negative hypoxic tumors had an advantage 196 . In another clinical study with a target for hypoxic tumor cells, a subgroup analysis revealed an even poorer response for patients with HPV OSCC 197 . Experiments indicate a particularly good response of HPV-positive cell lines to chemotherapy; in an own investigation, we could demonstrate a better chemosensitivity of HPV-positive cell lines in comparison with a platinum derivate combined with radiation 198 .
Studies on de-intensification of chemotherapy are pursued for HPV OSCC by means of replacing a platinum-based chemotherapy by antibody therapy with Cetuximab. An analysis of HPV OSCC patients from a cohort of the so-called Bonner trial revealed no specific benefit for patients with HPV OSCC 199 and a RTOG study that included Cetuximab in platinum-based radiochemotherapy did not show a benefit for patients with HPV OSCC as well 200 . So clinical studies do not provide any direct indication that HPV is predictive for antibody therapy with Cetuximab. In several clinical studies, however, it is currently verified in a randomized way if Cisplatin may be replaced by Cetuximab. Endpoints of the study are a lower toxicity of the treatment in analogy to the possible benefit for tumor control. Studies on the de-intensification of the radiotherapeutic dose for definite combined platinum containing RCT are currently conducted with the objective to reduce the dose in the area of the primary tumor or in the neck. Inclusion criteria for these de-escalation studies are additional favorable risk profiles of the patients (non-smokers or <10 pack-years, <T3 etc.) 201 . In another clinical trial (NCT02254278), patients with HPV OSCC are randomized for a platinum-based radiochemotherapy or radiotherapy alone.
Retrospective studies on adjuvant RCT for HPV OSCC are important. The indications are acknowledged, i. e., the incomplete excision (R1) and extracapsular spread (ECS). For HPV OSCC, retrospective data have been published repeatedly that allow the conclusion that platinum-based RCT in the adjuvant setting does not add important benefit for tumor control of patients with HPV OSCC. In a cohort of 29 patients with HPV OSCC (>90% of the patients did not have tumor-free margins!), no difference in the tumor control was described, independently from the fact if the patients underwent postoperative irradiation with or without chemotherapy 202 . Regarding the question of indications of adjuvant RCT in ECS-positive lymph node metastasis, data of several studies are available. They all state that combined adjuvant therapy of HPV OSCC is not associated with a benefit for tumor control 17 175 203 . However, the published patient populations are small, selected, and retrospectively evaluated so that based on the published articles, no reliable recommendation can be given for the omission of chemotherapy with postoperative radiation and existing risk factors, for example in the case of tumor-infiltrated margins.
Regarding induction chemotherapy, only limited data on HPV OSCC are available. In an ECOG phase-II study of resectable head and neck carcinomas, 2 cycles of carboplatin and paclitaxel were applied followed by radiation with 70 Gy. In 62 patients with OSCC (38/61% HPV OSCC) a better response to induction (82 vs. 55%; P=0.01) was reported for HPV-induced tumors and a better survival after 2 years (95 vs. 62%; P=0.005) 204 .
For HPV-positive patients from the TAX 324 study (n=56), after induction (cisplatin 100 mg/5-FU 1000 mg/± docetaxel 75 mg) followed by RCT, a better tumor control could be revealed, however, the rate of distant metastases was not significantly lower 195 . In a German study with TPF induction followed by surgery and adjuvant therapy, the HPV status was not predictive for a good response to chemotherapy 205 . In prospective clinical trials, it is currently verified for HPV-associated OSCC if induction may serve as switch function for de-intensified radiotherapy or radiochemotherapy (Quarterback, ECOG 1308). In both studies, HPV-positive patients showed high complete remission rates (about 80%) after induction chemotherapy.
6.4 Immunotherapy
It is unknown if the HPV status may be a predictive marker for new immuno-oncological therapy approaches. The programmed death receptor 1 (PD1) belongs to the T cell receptor family and is expressed on the surface of immune cells. The ligand of PD1 is the so-called programmed death receptor ligand 1 (PD-L1) and is often expressed on the surface of tumor cells. In this way, a cytotoxic T cell response is suppressed, which actually serves to protect against autoimmune diseases. The effectiveness of a pharmaceutical blockade of this interaction was confirmed in melanoma, bronchial carcinoma, renal cell carcinoma, and other tumor entities 206 . For HPV OSCC, we could show in an own investigation (submitted for publication) an increased expression of the PD-L1 receptor. In analogy, also an increased PD-L1 expression was revealed for tonsillar carcinomas 207 . However, only recently efforts are undertaken to standardize the PD-L1 immunohistology 208 209 so that different methods (antibody, cut-off etc.) may explain different results. Not only the results of HPV status and PD-L1 expression are inconsistent, it is in particular unclear if an immunohistochemically visible PD-L1 expression alone is predictive for antibody therapy targeted against the PD1/PD-L1 axis 210 .
For HPV OSCC, differential immune infiltrates in tumors have been described several times. CD8 positive T cell infiltrates 211 , NK cell infiltrates 115 , and PD1 positive T cell infiltrates were described with an improved outcome for HPV OSCC 212 . In a current investigation, it was shown for HPV OSCC by means of the immune score (CD8, PD-L1, and CD68) that cases with dense CD8+ T cell infiltrates in the stroma and low PD-L1 level in the tumor had the best prognosis 213 . Currently, it is not clarified for HPV OSCC which value PD-L1 expression may have as biomarker. Probably, combinations of immunological markers will reliably predict the response to an immune therapy especially for HPV OSCC because the carcinogenesis of HPV OSCC is triggered by viral immunomodulation in a particular extent. The combination of immunotherapeutic medication based on strengthening the antitumor microenvironment by T lymphocyte co-stimulating agents (e. g., CD27 agonist), chemokine receptor blockade (CXCR2, CSF1R, and CCR4 blockade) and direct antitumor medication (EGFR, STAT3) are probably very promising in the treatment of HPV OSCC.
Two checkpoint inhibitors, Pembrolizumab (Keytruda) and Nivolumab (Opdivo) were verified in patients with platinum resistance. In the KEYNOTE-012 study (Pembrolizumab) a response of 32% was evaluated in HPV-positive head and neck tumors compared to 18% in HPV-negative tumors 214 . In the CheckMate-141 study (Nivolumab), p16 test results were available in 178 of 361 patients. Regardless of the p16 status, the survival in the therapy arm with Nivolumab was significantly longer. HPV-positive tumors had a longer tumor control (overall survival of 9.1 months vs. 7.5 months in HPV-negative head and neck tumors) 215 . The results of both large therapy studies do not allow the conclusion in the palliative setting that the HPV-induced carcinogenesis is predictive for therapy with antibodies that are only targeted against the PD1/PD-L1 axis 216 .
Current clinical trials focusing on viral carcinogenesis include combinations of RT, immunotherapy, chemotherapy, and vaccination ( Table 5 ). Therapeutic vaccination is directed for example against E6/E7 oncoproteins or p16 protein 217 218 . Another approach is the infection of tumor-specific T cells in HPV OSCC patients. In contrast to prophylactic HPV vaccines, therapeutic vaccines allow fighting against an existing infection of antigen-positive tumor cells. One example is the attenuated bacterial strain of ADXS11-011 that secretes the E7 oncoprotein and is infused in 30 study patients before transoral surgery (NCT02002182). Protein-based vaccines (ProCervix, TA-CIN) for HPV OSCC are currently not included in clinical trials. Vaccines based on DNA (VGX-3100, INO-3112) were successfully applied in CIN-III lesions of the cervix and are currently tested for HPV OSCC (NCT02163057) whereby the transfer of DNA vaccine is problematic (gene gun, electroporation).
Table 5 Selection of checkpoint inhibitors with which clinical studies in HPV OSCC will be conducted now or in the future.
| Medication | Status | Study title | Inclusion criteria |
|---|---|---|---|
| Pembrolizumab | Not yet recruiting | Pembrolizumab combined with chemoradiotherapy in squamous cell carcinoma of the head and neck | Head and neck cancer, stratified for HPV |
| Recruiting | E7 TCR T cells with or without PD-1 blockade for human papillomavirus-associated cancers | HPV-induced carcinomas, including OSCC | |
| Nivolumab | Active, not recruiting | Nivolumab and HPV 16 vaccination in patients with HPV 16 positive incurable solid tumors | HPV-induced carcinomas, including OSCC |
| Recruiting | HPV 16/18 E6/E7-specific T lymphocytes, relapsed HPV-associated cancers, HESTIA | HPV-induced carcinomas, including OSCC | |
| Recruiting | Oropharyngeal tumor induction chemotherapy and response-stratified locoregional therapy trial in order to minimized long-term adverse events | HPV OSCC | |
| Recruiting | An investigational immuno-therapy study to investigate the safety and effectiveness of Nivolumab, and Nivolumab combination therapy in virus-associated tumors | HPV-induced carcinomas, including OSCC | |
| Durvalumab | Not yet recruiting | Safety and efficacy of MEDIO457 and Durvalumab in patients with HPV associated recurrent/metastatic head and neck cancer | HPV OSCC, recurrences |
| Recruiting | Phase 1-2 study of ADXS11-001 or MEDI4736 alone or combo in cervical or HPV+ head & neck cancer | HPV OSCC, cervix cancer | |
| Recruiting | Durvalumab before surgery in treating patients with oral cavity or oropharynx cancer | OSCC and oral cavity | |
| Avelumab | Not yet recruiting | Phase Ib/II or TG4001 and Avelumab in HPV16 positive R/M cancers and expansion cohort to oropharyngeal SCCHN | HPV-induced carcinomas, including OSCC |
| Ipilimumab | Recruiting | An investigational immuno-therapy study to investigate the safety and effectiveness of Nivolumab, and Nibolumab combination therapy in virus-associated tumors | HPV-induced carcinomas, including OSCC |
In summary, no fundamentally modified therapeutic concepts can be concluded based on the definition of the HPV status of oropharyngeal cancer. Hence also for HPV-associated OSCC, primary surgery should be discussed individually. The advantage of primary surgery of HPV-associated oropharyngeal cancer is based on the following reflections:
In cases of R0 resection of the primary tumor and neck dissection, mostly adjuvant RCT is not needed.
In cases of N0 situation after histological examination, adjuvant therapy is probably not necessary.
The applied radiation dose and the radiation fields can possibly be reduced. One precondition is the reliable R0 resection.
So the indication for surgery of HPV-associated OSCC must be made carefully under the aspect if R0 resection was successful. It can then lead to an optimized therapy regimen of the individual patient.
6.5 Options after treatment failure
Treatment failure in the context of OSCC is generally associated with a poor prognosis, so patients often receive palliative chemotherapy or no tumor-specific therapy. The average 5-year survival after first-line treatment failure is estimated to amount generally to about 25% and the median survival to 1.5 years after diagnosis of a recurrence of OSCC. However, our knowledge regarding the management in recurrent head and neck cancer cannot be easily transferred to patients with HPV OSCC; and the increased incidence is only observed within the last 2 decades. So the published series of recurrent/metastatic HPV OSCC are rather small. Also large therapy studies on palliative combination chemotherapy (EXTREME, SPECTRUM) do not allow specific treatment recommendations for HPV OSCC because the number of patients was too low and the HPV status was often not clearly defined.
In retrospective studies, significantly better survival rates were published for HPV OSCC also in cases of tumor recurrences. In a large investigation of >1,000 patients who were treated in the context of 2 RTOG studies and had recurrences, the 2-year survival amounted to >50% (n=105 HPV OSCC) vs. <30% (n=76 HPV-negative OSCC). Interestingly, a longer survival was significantly associated with salvage surgery 219 . In addition, other types of treatment failure in HPV OSCC occurred together with a higher incidence of soft tissue and distant metastases 220 . The estimated rate after evaluation of our patients and based on published cohorts amounts to more than 50% ( Fig. 9 ). Also after longer intervals of tumor control, an accumulation of hematogenic metastases is proven 221 . In particular in cases of hematogenic metastasis, a better tumor control for HPV OSCC is described. In a series, the mean survival in distant-metastatic HPV OSCC amounts to 42 months 222 and in another series to 34 months 223 . In contrast, the mean survival as of the diagnosis of distant metastases amounts to about 4 months in other head and neck tumors 224 . The mean survival in HPV OSCC therefore might be about 10 times as long.
Fig. 9.
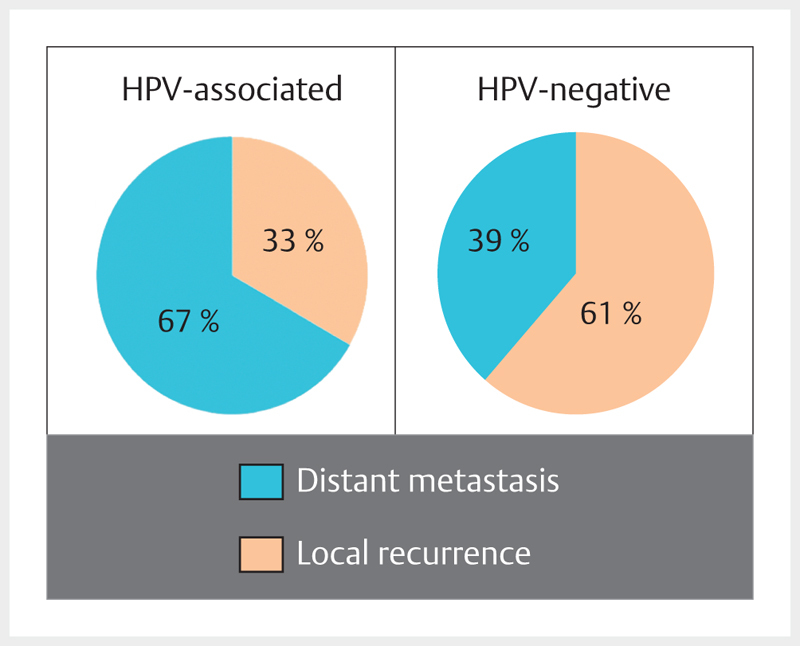
The type of treatment failure in cases of HPV-associated and HPV-negative OSCC is different: patients with HPV-induced OSCC frequently develop distant metastases, in cases of HPV-negative tumors, local and locoregional tumor recurrences prevail.
For salvage therapy with regard to HPV OSCC, it seems to be true that long-term tumor control by surgery is not successful in cases of local treatment failure. In two retrospective series comparing HPV OSCC and HPV-negative patients, no significant differences for the prognosis were published regarding local tumor recurrence or lymph node recurrence 225 226 . In another recently published series, tumor control of distant metastasis of 100% (!) was observed 3 years after surgical therapy of the distant metastasis (n=18) 227 . Also for the systemic therapy of HPV OSCC, however, long-term surviving patients are published from a very small cohort 228 .
In summary, the conclusion can be drawn from various series published up to now that for treatment failure and in particular oligo distant metastases surgical therapy of the recurrences or targeted radiotherapy even with curative intention may be considered. Most suitable candidates for such salvage therapy are patients with isolated pulmonary or bone metastases.
7. Follow-up
7.1 Assessment of therapy-related side effects
Generally, curative therapy of HPV OSCC often leads to permanent healing of the tumor disease. So the assessment of the toxicity of the treatment plays a major role. Dysphagia is a key symptom of oropharyngeal cancer because the tumor always develops near the anatomical crossover of trachea and esophagus. Directly after surgery and during RT, dysphagia and mucositis and their management are in the focus and after therapy the disturbed swallowing is the most important symptom that impairs the quality of life. Reliable evaluation of the impaired swallowing is of utmost importance for the therapy management of OSCC.
One practicable instrument to assess impairments are patient- or physician-related questionnaires. In a review article from 2014, however, more than 20 different screening instruments with the focus on patient questionnaires were described for the evaluation of swallowing disorders 229 . Which instrument is mostly accepted, simple to handle, and provides valid test results for our patients? In currently recruiting international clinical studies, also in Europe the MD Anderson Dysphagia Inventory (MDADI) is often applied. It was developed with focus on head and neck cancer, a validation of the test results is available for numerous languages. The 19 items assess emotion (6 questions), function (5 questions), body function (8 questions) and one global question.
Fiberendoscopic evaluation of swallowing (FEES) is a standard examination technique for patients suffering from dysphagia. In this context, indirect flexible laryngoscopy is performed transnasally. Larynx and oropharynx are observed at rest and during swallowing with different consistencies. Evaluation criteria are the quantification of penetration (entrance of the material into the larynx to the glottis) and aspiration (entrance of the material below the glottic level). In order to standardize the evaluation, in Germany mostly the penetration-aspiration scale according to Rosenbek is applied. The scaling was validated by means of FEES 230 . It cannot be stated if FEES is superior to radiological evaluation of aspiration by means of classic barium swallow. The results of both procedures are highly influenced by the individual and by the examiner.
Regarding toxicity and tumor therapy, reports state for HPV OSCC patients that the rate of late toxicities is lower under RCT. However, there are also data from the literature that the treatment side effects of HPV OSCC patients are perceived as particularly severe 231 . It is especially known from quality of life evaluations that patients with HPV OSCC experience the acute phase of therapy with severe impairment 232 . For the daily routine in tumor follow-up, the use of so-called ICS core sets was suggested for standardized assessment of treatment side effects in German speaking countries 233 . In an own investigation, we found that questionnaires were useful but they are associated with high efforts in the follow-up.
7.2 Early detection of treatment failure
With the increasing incidence of HPV OSCC, the risk of developing secondary carcinoma decreases after therapy of OSCC 234 . Re-occurring of a tumor is mostly discovered by the patient because of increased local pains, development of new nodules in the neck, or weight loss and swallowing disorders. In order to detect treatment failure before the re-appearance of new symptoms, intensive research is performed on methods to early detect recurrences and/or metastases. A promising method is the so-called liquid biopsy, which is a non-invasive biopsy-taking of human liquids like blood, urine, or saliva. Those samples can be examined with regard to tumor markers that may encompass cell-free circulating tumor DNA, circulating tumor cells, or – as in the case of HPV – viral DNA ( Fig. 10 ) 235 236 .
Fig. 10.
Diagram of the principle of non-invasive liquid biopsy. The circulating tumor cells (CTC), tumor DNA, tumor DNA with integrated HPV DNA as well as episomal HPV DNA are displayed.
Circulating tumor cells (CTC) were the first tumor markers that were examined in liquid biopsies. They get from solid tumors into the blood cycle, however, only in very low concentrations (1 tumor cell per 1 million healthy cells). Accordingly, either a high quantity of blood has to be examined or an extremely sensitive method must be applied.
Cell-free circulating DNA develops by apoptosis or necrosis of healthy as well as tumor cells. Apoptosis results in fragments of a length of 180 base pairs or a multiple of them, while necrosis leads to fragments of irregular length of more than 1,000 base pairs 237 . The percentage of tumor DNA in the entire circulating cell-free DNA may amount to 0.01% up to 50%. It could already be shown for different entities that tumor patients had a higher total content of cell-free DNA in the blood compared to healthy individuals. Interestingly, the concentration correlates with the presence of cervical metastases and indicates a poor prognosis 238 . Methodically, liquid biopsies are often examined by means of quantitative or digital PCR, sequencing methods (Sanger sequencing, pyrosequencing, NGS, whole genome/exome sequencing, CAPP-Seq [cancer personalized profiling by deep sequencing]) or BEAMing (beads, emulsion, amplification, and magnetics) 239 . By next generation sequencing, certain gene segments or entire genes may be sequenced and alterations may be detected in comparison to a reference genome. In cases of targeted sequencing (targeted NGS) the gene segments to be examined are selected by the user. Regions are thus examined that have already been described in the literature. The advantage of this technology is that a higher coverage of the target region and thus detection of rare variants is achieved. By means of PCR, BEAMing, and targeted NGS, known (point) mutations can be detected while whole genome or whole exome sequencing may identify unknown mutations, chromosomal aberrations, and altered numbers of copies as well as viral DNA sequences and their integration sites in the human genome 240 . Disadvantages of NGS technology for application in this routine are still the high costs, the precondition of high-quality DNA as well as the abundance of generated data that have to be analyzed and interpreted.
In cases of viral infections, the blood can be additionally examined with regard to viral DNA. Nasopharyngeal carcinomas (NPC) revealed an increased virus load in tumor patients with Epstein Barr virus infection and after therapy, the charge decreased 241 . Furthermore, it could be shown in areas with high incidence of NPC that detection of EBV-DNA in the blood plasma can be used as screening for NPC 242 . In this study, sera of more than 20,000 cases were examined. NPC could be detected earlier so that the prognosis of the patients was significantly improved. These investigations demonstrate how the detection of virus DNA in virus-induced malignomas can be used as marker. HPV DNA could be found in plasma and saliva of head and neck tumor patients. In the same study it could be shown that tumor DNA was detected before the diagnosis of recurrence, but not in recurrence-free patients. In 2 patients, tumor DNA was detected after treatment of the primary tumor and 9-15 months before clinical diagnosis of the recurrence 235 . In an own investigation, we could demonstrate successful tumor control as well as treatment failure in single patients by means of tumor DNA in the blood ( Fig. 11 ). In one study that examined HPV DNA in the serum of OSCC patients, a reduction of the DNA could be observed under RCT. Four patients developed recurrences (1 locoregional recurrence, 3 distant metastases). For 3 patients with distant metastases, again HPV DNA could be detected at the time of relapse, however, not for the patient with the locoregional recurrence 243 .
Fig. 11.
Correlation between the detection of HPV DNA in blood under therapy as well as during follow-up.
Beside viral DNA, tumor-specific mutations can also be detected in cell-free tumor DNA. This became possible due to the high sensitivity of NGS technologies. In this way, patient-specific tumor mutation patterns can be established in the future and examined in the plasma and the saliva. Furthermore, current research deals with the question if sequencing of tumor cell clones may assess additionally acquired resistances and if adaptation of therapy is possible. For example, resistances against Gefitinib and Erlotinib could be identified for non-small-cell lung cancer (NSCLC) in the EGFR gene 244 .
7.3 Consequences for tumor follow-up
For follow-up examinations of head and neck cancer patients with low risk, the German Cancer Society suggests 3 months intervals in the first year, every 4-6 months in the second year, 6 months in the 3 rd and 4 th years, and annual intervals as of the 5 th year after the end of therapy. For tumor patients with high risk of recurrence, an examination is recommended every 6 weeks during the first year, every 3 months in the second year and every 6 months in the third and fourth years, and once a year as of the 5 th year ( www.krebsgesellschaft.de/onko-internetportal/basis-informationen-krebs/krebsarten/andere-krebsarten/kopf-hals-tumoren/kopf-hals-tumoren-nachsorge-und-reh.html ; status: August 1, 2017).
Patients with HPV OSCC may remain in the follow-up program for more than 5 years because long-term survival is typical and the assessment of late toxicity is important. A particular circumstance is the incidence of hematogenic tumor dissemination. After therapy of HPV OSCC it is extraordinarily high with about 50%. Therefore and also because of the possibility of good tumor control even in cases of oligo-metastasis, imaging in narrow intervals for HPV OSCC patients is recommended ( Fig. 12 ). Possibly, the detection of viral DNA will be significant in the follow-up of HPV OSCC patients in the future.
Fig. 12.
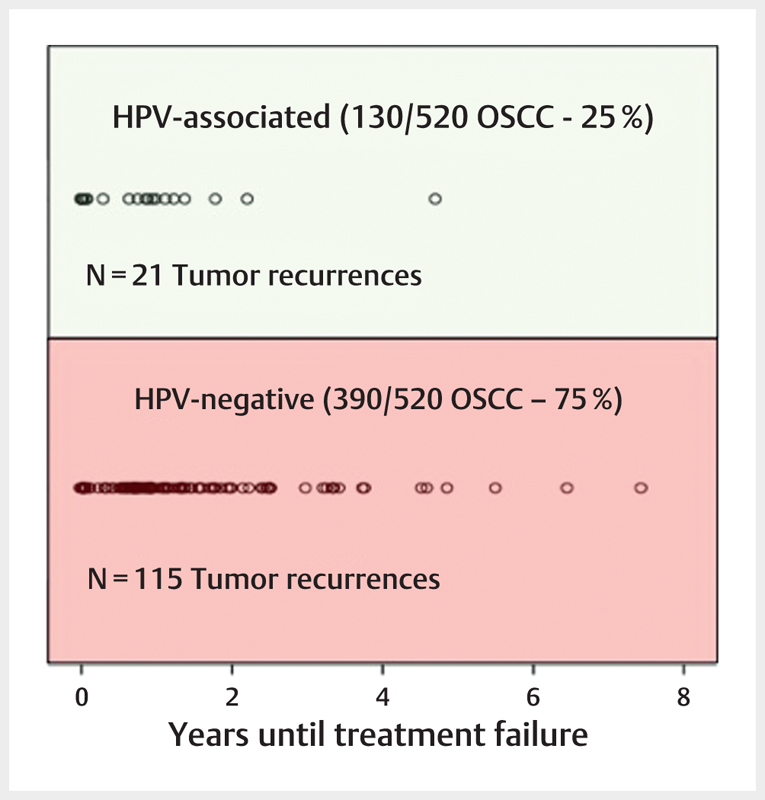
Treatment failure in 520 patients with HPV-associated and HPV-negative OSCC. When tumor control fails in HPV-induced tumors, it occurs frequently within the first year after the end of therapy.
8. Outlook
The epidemiological development of head and neck cancer cannot be compared to other entities. Head and neck carcinomas encompass 2 clearly differentiated subentities with regard to clinical and biological aspects. The prevalence of HPV-negative head and neck tumors decreased due to the success of anti-smoking campaigns and reduced tobacco consumption, but the HPV-associated head and neck tumors continuously increase in many countries. It remains open if the percentage of HPV-negative head and neck cancer will be replaced by HPV-associated tumors or if even the current total incidence of head and neck cancer increases. Plausible and verifiable reasons for the increased HPV prevalence in head and neck cancer, as for example a relevant change of the sexual behavior in the last decades, are unknown. Further, specific measures for reduction of the HPV prevalence in head and neck cancer are missing so that based on current data only a further increase of the HPV prevalence of head and neck tumors can be expected.
The value of prophylaxis by vaccination is acknowledged for many infectious diseases. Recent studies from Australia confirm the impressive success of vaccination against genital HPV infections. In a timeframe of about 10 years after introduction of the national quadrivalent HPV vaccination program in young women, the incidence of genital warts decreased of more than 90%, the one of high-grade cervical lesions of more than 50% in the investigated population. Meanwhile, vaccination in Australia was extended also to boys. The most important virus types (HPV 16 and 18) are identical in cervix cancer and head and neck cancer and on a molecular level many aspects of carcinogenesis correspond in both entities. Since there are no counterarguments, it must be assumed that primary prophylaxis by vaccination against the most important virus types may protect against HPV-associated head and neck cancer.
In order to describe the effectiveness of vaccination for HPV-associated head and neck cancer, there are 2 fundamental problems. First, this is the extremely long latency from the time of infection to the development of HPV-associated head and neck carcinoma, and second, a head and neck cancer pre-malignancy is missing so that its reduction cannot serve as endpoint of a vaccination study. The current vaccination rates in Germany amount to 42.5% of young women which is clearly below the one in Australia and for herd protection a percentage of around 85% would be needed. So there is only an individual protection and a measurable protection of the population can only be identified after several years. In addition, vaccination is recommended mainly for women in the context of cervix cancer prevention. Head and neck cancer and HPV-associated head and neck carcinomas, however, mostly occur in males. Since the vaccines are also approved for boys, HPV vaccination should also be recommended for boys. This also corresponds to the recommendation that all boys as of the age of 9 should be vaccinated against HPV as early as possible issued by the respective S3 guideline. However, currently HPV vaccination for boys is not yet recommended by the Standing Vaccination Committee (Ständige Impfkommission, STIKO). Nonetheless, health insurers sporadically pay for this vaccination on application. Fortunately, meanwhile more and more health insurers reimburse the expenses of vaccination also for women older than 18 years and hopefully, the vaccination rates increase in the long run.
In several studies, models have been developed to investigate the significance of risk factors for the prognosis of head and neck cancer. In summary they show that there are many different risk groups characterized by clinical and lifestyle factors. The most important factor hereby seems to be HPV, followed by tumor-specific properties such as T and N status, tobacco and alcohol consumption as well as the physical condition of the patient. The weighting of the factors in those models seems to be important for the treatment strategy because there are differences in the models depending on whether they were established based on patient populations that underwent primary surgery or radiotherapy. Regardless of the model, there are patients with low and those with high risks. The first ones are possibly overtreated with conventional treatment procedures which is associated with unnecessary impairment of the quality of life. For the latter ones, therapy has to be improved. In several current studies, de-escalation of the treatment is tested. The selection of the patients is performed based on the HPV status of the tumors, however, it must be questioned if this is the only factor of importance regarding possible de-escalation. Even in low-risk groups of patients there might be differences which may lead to treatment failure after deescalated therapy. For those cases, other options have to be developed and made available. The further development of prognostic models and inclusion of further factors may help to identify suitable patients for de-escalation or more specific therapies.
New treatment strategies currently emerge in particular in the context of modulation of immune checkpoints. The activation of the immune system seems to be enormously important for the treatment success because many studies show that tumors influence immune cells and “hide” so that they are not recognized by the immune system. Treatment with immunotherapeutics in combination with conventional methods such as surgery and irradiation is promising and current studies will show which patient groups may benefit most.
Beside new therapy approaches, the further development of diagnostic methods and the definition of suitable tumor markers has to be fostered in order to improve the security of stratification of patients for different therapy arms and to recognize treatment failure as early as possible. Progress in sequencing techniques allows the determination of the genetic background of a tumor. In this way, affected signaling pathways may be identified that are the target structures for molecular treatment approaches. But also individual markers might be detected that can be used to monitor the therapy course during follow-up by means of liquid biopsies and to early detect tumor recurrence. Possibly, individual genetic markers may also be used in the future to develop patient-specific therapy strategies with optimal effectiveness and minimal side effects or long-term damage. However, the clonal selection and genetic development of tumor cells must also be taken into account and a better knowledge of molecular processes during carcinogenesis as well as clinical studies are essential to implement scientific knowledge into clinical practice.
Footnotes
Interessenkonflikt Die Autoren geben an, dass kein Interessenkonflikt besteht.
Literatur
- 1.Javadi P et al. Evolving disparities in the epidemiology of oral cavity and oropharyngeal cancers. Cancer Causes Control. 2017;28:635–645. doi: 10.1007/s10552-017-0889-8. [DOI] [PubMed] [Google Scholar]
- 2.Mifsud M et al. Evolving trends in head and neck cancer epidemiology: Ontario, Canada 1993–2010. Head Neck. 2017;39:1770–1778. doi: 10.1002/hed.24829. [DOI] [PubMed] [Google Scholar]
- 3.Chaturvedi A K et al. Human papillomavirus and rising oropharyngeal cancer incidence in the United States. J Clin Oncol. 2011;29:4294–4301. doi: 10.1200/JCO.2011.36.4596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nasman A et al. Incidence of human papillomavirus (HPV) positive tonsillar carcinoma in Stockholm, Sweden: an epidemic of viral-induced carcinoma? Int J Cancer. 2009;125:362–366. doi: 10.1002/ijc.24339. [DOI] [PubMed] [Google Scholar]
- 5.Wittekindt C et al. Expression of p16 protein is associated with human papillomavirus status in tonsillar carcinomas and has implications on survival. Adv Otorhinolaryngol. 2005;62:72–80. doi: 10.1159/000082474. [DOI] [PubMed] [Google Scholar]
- 6.Quabius E S et al. Geographical and anatomical influences on human papillomavirus prevalence diversity in head and neck squamous cell carcinoma in Germany. Int J Oncol. 2015;46:414–422. doi: 10.3892/ijo.2014.2697. [DOI] [PubMed] [Google Scholar]
- 7.Wurdemann N et al. Prognostic Impact of AJCC/UICC 8th Edition new staging rules in oropharyngeal squamous cell carcinoma. Front Oncol. 2017;7:129. doi: 10.3389/fonc.2017.00129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Klussmann J P et al. Expression of p16 protein identifies a distinct entity of tonsillar carcinomas associated with human papillomavirus. Am J Pathol. 2003;162:747–753. doi: 10.1016/S0002-9440(10)63871-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Saraiya M et al. US assessment of HPV types in cancers: implications for current and 9-valent HPV vaccines. J Natl Cancer Inst. 2015;107:djv086. doi: 10.1093/jnci/djv086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mehanna H et al. Prevalence of human papillomavirus in oropharyngeal and nonoropharyngeal head and neck cancer--systematic review and meta-analysis of trends by time and region. Head Neck. 2013;35:747–755. doi: 10.1002/hed.22015. [DOI] [PubMed] [Google Scholar]
- 11.Abogunrin S et al. Prevalence of human papillomavirus in head and neck cancers in European populations: a meta-analysis. BMC Cancer. 2014;14:968. doi: 10.1186/1471-2407-14-968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Isayeva T et al. Human papillomavirus in non-oropharyngeal head and neck cancers: a systematic literature review. Head Neck Pathol. 2012;6 01:S104–S120. doi: 10.1007/s12105-012-0368-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ndiaye C et al. HPV DNA, E6/E7 mRNA, and p16INK4a detection in head and neck cancers: a systematic review and meta-analysis. Lancet Oncol. 2014;15:1319–1331. doi: 10.1016/S1470-2045(14)70471-1. [DOI] [PubMed] [Google Scholar]
- 14.Castellsague X et al. HPV involvement in head and neck cancers: comprehensive assessment of biomarkers in 3680 Patients. J Natl Cancer Inst. 2016;108:djv403. doi: 10.1093/jnci/djv403. [DOI] [PubMed] [Google Scholar]
- 15.Lassen P et al. Impact of HPV-associated p16-expression on radiotherapy outcome in advanced oropharynx and non-oropharynx cancer. Radiother Oncol. 2014;113:310–316. doi: 10.1016/j.radonc.2014.11.032. [DOI] [PubMed] [Google Scholar]
- 16.Chung C H et al. p16 protein expression and human papillomavirus status as prognostic biomarkers of nonoropharyngeal head and neck squamous cell carcinoma. J Clin Oncol. 2014;32:3930–3938. doi: 10.1200/JCO.2013.54.5228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Scheel A et al. Classification of TP53 mutations and HPV predict survival in advanced larynx cancer. Laryngoscope. 2016;126:E292–E299. doi: 10.1002/lary.25915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mork J et al. Human papillomavirus infection as a risk factor for squamous-cell carcinoma of the head and neck. N Engl J Med. 2001;344:1125–1131. doi: 10.1056/NEJM200104123441503. [DOI] [PubMed] [Google Scholar]
- 19.Anantharaman D et al. Human papillomavirus infections and upper aero-digestive tract cancers: the ARCAGE study. J Natl Cancer Inst. 2013;105:536–545. doi: 10.1093/jnci/djt053. [DOI] [PubMed] [Google Scholar]
- 20.Kreimer A R et al. Oral human papillomavirus in healthy individuals: a systematic review of the literature. Sex Transm Dis. 2010;37:386–391. doi: 10.1097/OLQ.0b013e3181c94a3b. [DOI] [PubMed] [Google Scholar]
- 21.Gillison M L et al. Prevalence of oral HPV infection in the United States, 2009-2010. JAMA. 2012;307:693–703. doi: 10.1001/jama.2012.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Giuliano A R et al. Incidence, prevalence, and clearance of type-specific human papillomavirus infections: The Young Women's Health Study. J Infect Dis. 2002;186:462–469. doi: 10.1086/341782. [DOI] [PubMed] [Google Scholar]
- 23.Pickard R K et al. The prevalence and incidence of oral human papillomavirus infection among young men and women, aged 18-30 years. Sex Transm Dis. 2012;39:559–566. doi: 10.1097/OLQ.0b013e31824f1c65. [DOI] [PubMed] [Google Scholar]
- 24.Kreimer A R et al. Incidence and clearance of oral human papillomavirus infection in men: the HIM cohort study. Lancet. 2013;382:877–887. doi: 10.1016/S0140-6736(13)60809-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.D'Souza G et al. Oral human papillomavirus (HPV) infection in HPV-positive patients with oropharyngeal cancer and their partners. J Clin Oncol. 2014;32:2408–2415. doi: 10.1200/JCO.2014.55.1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.D'Souza G et al. Six-month natural history of oral versus cervical human papillomavirus infection. Int J Cancer. 2007;121:143–150. doi: 10.1002/ijc.22667. [DOI] [PubMed] [Google Scholar]
- 27.D'Souza G et al. Differences in oral sexual behaviors by gender, age, and race explain observed differences in prevalence of oral human papillomavirus infection. PLoS One. 2014;9:e86023. doi: 10.1371/journal.pone.0086023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Partridge J M et al. Genital human papillomavirus infection in men: incidence and risk factors in a cohort of university students. J Infect Dis. 2007;196:1128–1136. doi: 10.1086/521192. [DOI] [PubMed] [Google Scholar]
- 29.Poethko-Muller C, Buttmann-Schweiger N, Ki G.G.S.S.G. [HPV vaccination coverage in German girls: results of the KiGGS study: first follow-up (KiGGS Wave 1)] Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz. 2014;57:869–877. doi: 10.1007/s00103-014-1987-3. [DOI] [PubMed] [Google Scholar]
- 30.Armstrong E P. Prophylaxis of cervical cancer and related cervical disease: a review of the cost-effectiveness of vaccination against oncogenic HPV types. J Manag Care Pharm. 2010;16:217–230. doi: 10.18553/jmcp.2010.16.3.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Drolet M et al. Population-level impact and herd effects following human papillomavirus vaccination programmes: a systematic review and meta-analysis. Lancet Infect Dis. 2015;15:565–580. doi: 10.1016/S1473-3099(14)71073-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Novakovic D et al. Juvenile recurrent respiratory papillomatosis: 10-year audit and Australian prevalence estimates. Laryngoscope. 2016;126:2827–2832. doi: 10.1002/lary.26005. [DOI] [PubMed] [Google Scholar]
- 33.Matys K et al. Mother-infant transfer of anti-human papillomavirus (HPV) antibodies following vaccination with the quadrivalent HPV (type 6/11/16/18) virus-like particle vaccine. Clin Vaccine Immunol. 2012;19:881–885. doi: 10.1128/CVI.00002-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hanahan D, Weinberg R A. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 35.Hanahan D, Weinberg R A. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 36.Napier S S, Speight P M. Natural history of potentially malignant oral lesions and conditions: an overview of the literature. J Oral Pathol Med. 2008;37:1–10. doi: 10.1111/j.1600-0714.2007.00579.x. [DOI] [PubMed] [Google Scholar]
- 37.van der Waal I. Potentially malignant disorders of the oral and oropharyngeal mucosa; terminology, classification and present concepts of management. Oral Oncol. 2009;45:317–323. doi: 10.1016/j.oraloncology.2008.05.016. [DOI] [PubMed] [Google Scholar]
- 38.Zhang L et al. Loss of heterozygosity (LOH) profiles--validated risk predictors for progression to oral cancer. Cancer Prev Res (Phila) 2012;5:1081–1089. doi: 10.1158/1940-6207.CAPR-12-0173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ha P K, Califano J A. The role of human papillomavirus in oral carcinogenesis. Crit Rev Oral Biol Med. 2004;15:188–196. doi: 10.1177/154411130401500402. [DOI] [PubMed] [Google Scholar]
- 40.Slaughter D P, Southwick H W, Smejkal W. Field cancerization in oral stratified squamous epithelium; clinical implications of multicentric origin. Cancer. 1953;6:963–968. doi: 10.1002/1097-0142(195309)6:5<963::aid-cncr2820060515>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 41.Califano J et al. Genetic progression model for head and neck cancer: implications for field cancerization. Cancer Res. 1996;56:2488–2492. [PubMed] [Google Scholar]
- 42.van Houten V M et al. Mutated p53 as a molecular marker for the diagnosis of head and neck cancer. J Pathol. 2002;198:476–486. doi: 10.1002/path.1242. [DOI] [PubMed] [Google Scholar]
- 43.Leemans C R, Braakhuis B J, Brakenhoff R H. The molecular biology of head and neck cancer. Nat Rev Cancer. 2011;11:9–22. doi: 10.1038/nrc2982. [DOI] [PubMed] [Google Scholar]
- 44.Rietbergen M M et al. No evidence for active human papillomavirus (HPV) in fields surrounding HPV-positive oropharyngeal tumors. J Oral Pathol Med. 2014;43:137–142. doi: 10.1111/jop.12123. [DOI] [PubMed] [Google Scholar]
- 45.Watson I R et al. Emerging patterns of somatic mutations in cancer. Nat Rev Genet. 2013;14:703–718. doi: 10.1038/nrg3539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Olivier M, Hollstein M, Hainaut P. TP53 mutations in human cancers: origins, consequences, and clinical use. Cold Spring Harb Perspect Biol. 2010;2:a001008. doi: 10.1101/cshperspect.a001008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Seiwert T Y et al. Integrative and comparative genomic analysis of HPV-positive and HPV-negative head and neck squamous cell carcinomas. Clin Cancer Res. 2015;21:632–641. doi: 10.1158/1078-0432.CCR-13-3310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lechner M et al. Targeted next-generation sequencing of head and neck squamous cell carcinoma identifies novel genetic alterations in HPV+ and HPV- tumors. Genome Med. 2013;5:49. doi: 10.1186/gm453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wagner S et al. [HPV-associated head and neck cancer: mutational signature and genomic aberrations] HNO. 2015;63:758–767. doi: 10.1007/s00106-015-0074-x. [DOI] [PubMed] [Google Scholar]
- 50.Cancer Genome Atlas, N . Comprehensive genomic characterization of head and neck squamous cell carcinomas. Nature. 2015;517:576–582. doi: 10.1038/nature14129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gaykalova D A et al. Novel insight into mutational landscape of head and neck squamous cell carcinoma. PLoS One. 2014;9:e93102. doi: 10.1371/journal.pone.0093102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Keating P J et al. Frequency of down-regulation of individual HLA-A and -B alleles in cervical carcinomas in relation to TAP-1 expression. Br J Cancer. 1995;72:405–411. doi: 10.1038/bjc.1995.346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gollin S M. Cytogenetic alterations and their molecular genetic correlates in head and neck squamous cell carcinoma: a next generation window to the biology of disease. Genes Chromosomes Cancer. 2014;53:972–990. doi: 10.1002/gcc.22214. [DOI] [PubMed] [Google Scholar]
- 54.Noutomi Y et al. Comparative genomic hybridization reveals genetic progression of oral squamous cell carcinoma from dysplasia via two different tumourigenic pathways. J Pathol. 2006;210:67–74. doi: 10.1002/path.2015. [DOI] [PubMed] [Google Scholar]
- 55.Wreesmann V B et al. Genetic abnormalities associated with nodal metastasis in head and neck cancer. Head Neck. 2004;26:10–15. doi: 10.1002/hed.10344. [DOI] [PubMed] [Google Scholar]
- 56.Klussmann J P et al. Genetic signatures of HPV-related and unrelated oropharyngeal carcinoma and their prognostic implications. Clin Cancer Res. 2009;15:1779–1786. doi: 10.1158/1078-0432.CCR-08-1463. [DOI] [PubMed] [Google Scholar]
- 57.Hopman A H et al. Genomic integration of oncogenic HPV and gain of the human telomerase gene TERC at 3q26 are strongly associated events in the progression of uterine cervical dysplasia to invasive cancer. J Pathol. 2006;210:412–419. doi: 10.1002/path.2070. [DOI] [PubMed] [Google Scholar]
- 58.Mooren J J et al. Chromosome stability in tonsillar squamous cell carcinoma is associated with HPV16 integration and indicates a favorable prognosis. Int J Cancer. 2013;132:1781–1789. doi: 10.1002/ijc.27846. [DOI] [PubMed] [Google Scholar]
- 59.Prigge E S et al. p16(INK4a) /Ki-67 co-expression specifically identifies transformed cells in the head and neck region. Int J Cancer. 2015;136:1589–1599. doi: 10.1002/ijc.29130. [DOI] [PubMed] [Google Scholar]
- 60.Mooren J J et al. P16(INK4A) immunostaining is a strong indicator for high-risk-HPV-associated oropharyngeal carcinomas and dysplasias, but is unreliable to predict low-risk-HPV-infection in head and neck papillomas and laryngeal dysplasias. Int J Cancer. 2014;134:2108–2117. doi: 10.1002/ijc.28534. [DOI] [PubMed] [Google Scholar]
- 61.Reimers N et al. Combined analysis of HPV-DNA, p16 and EGFR expression to predict prognosis in oropharyngeal cancer. Int J Cancer. 2007;120:1731–1738. doi: 10.1002/ijc.22355. [DOI] [PubMed] [Google Scholar]
- 62.Vent J et al. p16 expression in carcinoma of unknown primary: diagnostic indicator and prognostic marker. Head Neck. 2013;35:1521–1526. doi: 10.1002/hed.23190. [DOI] [PubMed] [Google Scholar]
- 63.Jung A C et al. Biological and clinical relevance of transcriptionally active human papillomavirus (HPV) infection in oropharynx squamous cell carcinoma. Int J Cancer. 2010;126:1882–1894. doi: 10.1002/ijc.24911. [DOI] [PubMed] [Google Scholar]
- 64.Gao G, Smith D I. Very large common fragile site genes and their potential role in cancer development. Cell Mol Life Sci. 2014;71:4601–4615. doi: 10.1007/s00018-014-1753-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Karim R et al. Human papillomavirus (HPV) upregulates the cellular deubiquitinase UCHL1 to suppress the keratinocyte's innate immune response. PLoS Pathog. 2013;9:e1003384. doi: 10.1371/journal.ppat.1003384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Olthof N C et al. Comprehensive analysis of HPV16 integration in OSCC reveals no significant impact of physical status on viral oncogene and virally disrupted human gene expression. PLoS One. 2014;9:e88718. doi: 10.1371/journal.pone.0088718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Olthof N C et al. Viral load, gene expression and mapping of viral integration sites in HPV16-associated HNSCC cell lines. Int J Cancer. 2015;136:E207–E218. doi: 10.1002/ijc.29112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Nulton T J et al. Analysis of The Cancer Genome Atlas sequencing data reveals novel properties of the human papillomavirus 16 genome in head and neck squamous cell carcinoma. Oncotarget. 2017;8:17684–17699. doi: 10.18632/oncotarget.15179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lace M J et al. Human papillomavirus type 16 (HPV-16) genomes integrated in head and neck cancers and in HPV-16-immortalized human keratinocyte clones express chimeric virus-cell mRNAs similar to those found in cervical cancers. J Virol. 2011;85:1645–1654. doi: 10.1128/JVI.02093-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.
- 71.Kostareli E et al. HPV-related methylation signature predicts survival in oropharyngeal squamous cell carcinomas. J Clin Invest. 2013;123:2488–2501. doi: 10.1172/JCI67010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Minarovits J et al. Epigenetic dysregulation in virus-associated neoplasms. Adv Exp Med Biol. 2016;879:71–90. doi: 10.1007/978-3-319-24738-0_4. [DOI] [PubMed] [Google Scholar]
- 73.Reed A L et al. High frequency of p16 (CDKN2/MTS-1/INK4A) inactivation in head and neck squamous cell carcinoma. Cancer Res. 1996;56:3630–3633. [PubMed] [Google Scholar]
- 74.McLaughlin-Drubin M E, Park D, Munger K. Tumor suppressor p16INK4A is necessary for survival of cervical carcinoma cell lines. Proc Natl Acad Sci U S A. 2013;110:16175–16180. doi: 10.1073/pnas.1310432110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Munger K, Gwin T K, McLaughlin-Drubin M E. p16 in HPV-associated cancers. Oncotarget. 2013;4:1864–1865. doi: 10.18632/oncotarget.1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Schlecht N F et al. Epigenetic changes in the CDKN2A locus are associated with differential expression of P16INK4A and P14ARF in HPV-positive oropharyngeal squamous cell carcinoma. Cancer Med. 2015;4:342–353. doi: 10.1002/cam4.374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wijetunga N A et al. Novel epigenetic changes in CDKN2A are associated with progression of cervical intraepithelial neoplasia. Gynecol Oncol. 2016;142:566–573. doi: 10.1016/j.ygyno.2016.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kostareli E et al. Gene promoter methylation signature predicts survival of head and neck squamous cell carcinoma patients. Epigenetics. 2016;11:61–73. doi: 10.1080/15592294.2015.1137414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Reuschenbach M et al. Methylation status of HPV16 E2-binding sites classifies subtypes of HPV-associated oropharyngeal cancers. Cancer. 2015;121:1966–1976. doi: 10.1002/cncr.29315. [DOI] [PubMed] [Google Scholar]
- 80.Chaiwongkot A et al. Differential methylation of E2 binding sites in episomal and integrated HPV 16 genomes in preinvasive and invasive cervical lesions. Int J Cancer. 2013;132:2087–2094. doi: 10.1002/ijc.27906. [DOI] [PubMed] [Google Scholar]
- 81.Wagner S et al. Human papillomavirus-related head and neck cancer. Oncol Res Treat. 2017;40:334–340. doi: 10.1159/000477252. [DOI] [PubMed] [Google Scholar]
- 82.Mirghani H et al. Comparative analysis of micro-RNAs in human papillomavirus-positive versus -negative oropharyngeal cancers. Head Neck. 2016;38:1634–1642. doi: 10.1002/hed.24487. [DOI] [PubMed] [Google Scholar]
- 83.Lajer C B et al. Different miRNA signatures of oral and pharyngeal squamous cell carcinomas: a prospective translational study. Br J Cancer. 2011;104:830–840. doi: 10.1038/bjc.2011.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lajer C B et al. The role of miRNAs in human papilloma virus (HPV)-associated cancers: bridging between HPV-related head and neck cancer and cervical cancer. Br J Cancer. 2012;106:1526–1534. doi: 10.1038/bjc.2012.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wald A I et al. Alteration of microRNA profiles in squamous cell carcinoma of the head and neck cell lines by human papillomavirus. Head Neck. 2011;33:504–512. doi: 10.1002/hed.21475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Melar-New M, Laimins L A. Human papillomaviruses modulate expression of microRNA 203 upon epithelial differentiation to control levels of p63 proteins. J Virol. 2010;84:5212–5221. doi: 10.1128/JVI.00078-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Srivastava K et al. p63 drives invasion in keratinocytes expressing HPV16 E6/E7 genes through regulation of Src-FAK signalling. Oncotarget. 2017;8:16202–16219. doi: 10.18632/oncotarget.3892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Qian K et al. Identification and validation of human papillomavirus encoded microRNAs. PLoS One. 2013;8:e70202. doi: 10.1371/journal.pone.0070202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Lin L et al. Two less common human microRNAs miR-875 and miR-3144 target a conserved site of E6 oncogene in most high-risk human papillomavirus subtypes. Protein Cell. 2015;6:575–588. doi: 10.1007/s13238-015-0142-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Janssen H L et al. Hypoxia in head and neck cancer: how much, how important? Head Neck. 2005;27:622–638. doi: 10.1002/hed.20223. [DOI] [PubMed] [Google Scholar]
- 91.Lindel K et al. Human papillomavirus positive squamous cell carcinoma of the oropharynx: a radiosensitive subgroup of head and neck carcinoma. Cancer. 2001;92:805–813. doi: 10.1002/1097-0142(20010815)92:4<805::aid-cncr1386>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 92.Aebersold D M et al. Expression of hypoxia-inducible factor-1alpha: a novel predictive and prognostic parameter in the radiotherapy of oropharyngeal cancer. Cancer Res. 2001;61:2911–2916. [PubMed] [Google Scholar]
- 93.Gatenby R A, Gillies R J. Why do cancers have high aerobic glycolysis? Nat Rev Cancer. 2004;4:891–899. doi: 10.1038/nrc1478. [DOI] [PubMed] [Google Scholar]
- 94.Denko N C. Hypoxia, HIF1 and glucose metabolism in the solid tumour. Nat Rev Cancer. 2008;8:705–713. doi: 10.1038/nrc2468. [DOI] [PubMed] [Google Scholar]
- 95.Mazurek S. Pyruvate kinase type M2: a key regulator of the metabolic budget system in tumor cells. Int J Biochem Cell Biol. 2011;43:969–980. doi: 10.1016/j.biocel.2010.02.005. [DOI] [PubMed] [Google Scholar]
- 96.Vander Heiden M G, Cantley L C, Thompson C B. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science. 2009;324:1029–1033. doi: 10.1126/science.1160809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Masoud G N, Li W. HIF-1alpha pathway: role, regulation and intervention for cancer therapy. Acta Pharm Sin B. 2015;5:378–389. doi: 10.1016/j.apsb.2015.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Bardos J I, Ashcroft M. Negative and positive regulation of HIF-1: a complex network. Biochim Biophys Acta. 2005;1755:107–120. doi: 10.1016/j.bbcan.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 99.Hoppe-Seyler K et al. Induction of dormancy in hypoxic human papillomavirus-positive cancer cells. Proc Natl Acad Sci U S A. 2017;114:E990–E998. doi: 10.1073/pnas.1615758114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Nakamura M et al. Hypoxia-specific stabilization of HIF-1alpha by human papillomaviruses. Virology. 2009;387:442–448. doi: 10.1016/j.virol.2009.02.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Bodily J M, Mehta K P, Laimins L A. Human papillomavirus E7 enhances hypoxia-inducible factor 1-mediated transcription by inhibiting binding of histone deacetylases. Cancer Res. 2011;71:1187–1195. doi: 10.1158/0008-5472.CAN-10-2626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Cuninghame S, Jackson R, Zehbe I. Hypoxia-inducible factor 1 and its role in viral carcinogenesis. Virology. 2014;456-457:370–383. doi: 10.1016/j.virol.2014.02.027. [DOI] [PubMed] [Google Scholar]
- 103.Noch E, Khalili K. Oncogenic viruses and tumor glucose metabolism: like kids in a candy store. Mol Cancer Ther. 2012;11:14–23. doi: 10.1158/1535-7163.MCT-11-0517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Amador-Molina A et al. Role of innate immunity against human papillomavirus (HPV) infections and effect of adjuvants in promoting specific immune response. Viruses. 2013;5:2624–2642. doi: 10.3390/v5112624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Orange J S.Natural killer cell deficiency J Allergy Clin Immunol 2013132515–525.quiz 526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Viscidi R P et al. Seroreactivity to human papillomavirus (HPV) types 16, 18, or 31 and risk of subsequent HPV infection: results from a population-based study in Costa Rica. Cancer Epidemiol Biomarkers Prev. 2004;13:324–327. doi: 10.1158/1055-9965.epi-03-0166. [DOI] [PubMed] [Google Scholar]
- 107.Woo Y Let al. Characterising the local immune responses in cervical intraepithelial neoplasia: a cross-sectional and longitudinal analysis BJOG 20081151616–1621.discussion 1621–1622 [DOI] [PubMed] [Google Scholar]
- 108.
- 109.Pacini L et al. Downregulation of Toll-like receptor 9 expression by beta human papillomavirus 38 and implications for cell cycle control. J Virol. 2015;89:11396–11405. doi: 10.1128/JVI.02151-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Eggensperger S, Tampe R. The transporter associated with antigen processing: a key player in adaptive immunity. Biol Chem. 2015;396:1059–1072. doi: 10.1515/hsz-2014-0320. [DOI] [PubMed] [Google Scholar]
- 111.Richards K H et al. The human papillomavirus (HPV) E7 protein antagonises an Imiquimod-induced inflammatory pathway in primary human keratinocytes. Sci Rep. 2015;5:12922. doi: 10.1038/srep12922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Niebler M et al. Post-translational control of IL-1beta via the human papillomavirus type 16 E6 oncoprotein: a novel mechanism of innate immune escape mediated by the E3-ubiquitin ligase E6-AP and p53. PLoS Pathog. 2013;9:e1003536. doi: 10.1371/journal.ppat.1003536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Ashrafi G H et al. E5 protein of human papillomavirus type 16 selectively downregulates surface HLA class I. Int J Cancer. 2005;113:276–283. doi: 10.1002/ijc.20558. [DOI] [PubMed] [Google Scholar]
- 114.Ashrafi G H et al. E5 protein of human papillomavirus 16 downregulates HLA class I and interacts with the heavy chain via its first hydrophobic domain. Int J Cancer. 2006;119:2105–2112. doi: 10.1002/ijc.22089. [DOI] [PubMed] [Google Scholar]
- 115.Wagner S et al. CD56-positive lymphocyte infiltration in relation to human papillomavirus association and prognostic significance in oropharyngeal squamous cell carcinoma. Int J Cancer. 2016;138:2263–2273. doi: 10.1002/ijc.29962. [DOI] [PubMed] [Google Scholar]
- 116.Miura S et al. CD1d, a sentinel molecule bridging innate and adaptive immunity, is downregulated by the human papillomavirus (HPV) E5 protein: a possible mechanism for immune evasion by HPV. J Virol. 2010;84:11614–11623. doi: 10.1128/JVI.01053-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Zhang B et al. The E5 protein of human papillomavirus type 16 perturbs MHC class II antigen maturation in human foreskin keratinocytes treated with interferon-gamma. Virology. 2003;310:100–108. doi: 10.1016/s0042-6822(03)00103-x. [DOI] [PubMed] [Google Scholar]
- 118.Fahey L M et al. A major role for the minor capsid protein of human papillomavirus type 16 in immune escape. J Immunol. 2009;183:6151–6156. doi: 10.4049/jimmunol.0902145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Hanna E et al. A novel alternative approach for prediction of radiation response of squamous cell carcinoma of head and neck. Cancer Res. 2001;61:2376–2380. [PubMed] [Google Scholar]
- 120.Chung C H et al. Molecular classification of head and neck squamous cell carcinomas using patterns of gene expression. Cancer Cell. 2004;5:489–500. doi: 10.1016/s1535-6108(04)00112-6. [DOI] [PubMed] [Google Scholar]
- 121.Walter V et al. Molecular subtypes in head and neck cancer exhibit distinct patterns of chromosomal gain and loss of canonical cancer genes. PLoS One. 2013;8:e56823. doi: 10.1371/journal.pone.0056823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Wichmann G et al. The role of HPV RNA transcription, immune response-related gene expression and disruptive TP53 mutations in diagnostic and prognostic profiling of head and neck cancer. Int J Cancer. 2015;137:2846–2857. doi: 10.1002/ijc.29649. [DOI] [PubMed] [Google Scholar]
- 123.Verhaak R G et al. Integrated genomic analysis identifies clinically relevant subtypes of glioblastoma characterized by abnormalities in PDGFRA, IDH1, EGFR, and NF1. Cancer Cell. 2010;17:98–110. doi: 10.1016/j.ccr.2009.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Wilkerson M D et al. Lung squamous cell carcinoma mRNA expression subtypes are reproducible, clinically important, and correspond to normal cell types. Clin Cancer Res. 2010;16:4864–4875. doi: 10.1158/1078-0432.CCR-10-0199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Sorlie T et al. Repeated observation of breast tumor subtypes in independent gene expression data sets. Proc Natl Acad Sci U S A. 2003;100:8418–8423. doi: 10.1073/pnas.0932692100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Keck M K et al. Integrative analysis of head and neck cancer identifies two biologically distinct HPV and three non-HPV subtypes. Clin Cancer Res. 2015;21:870–881. doi: 10.1158/1078-0432.CCR-14-2481. [DOI] [PubMed] [Google Scholar]
- 127.Saba N F et al. Mutation and transcriptional profiling of formalin-fixed paraffin embedded specimens as companion methods to immunohistochemistry for determining therapeutic targets in Oropharyngeal Squamous Cell Carcinoma (OPSCC): A Pilot of Proof of Principle. Head Neck Pathol. 2015;9:223–235. doi: 10.1007/s12105-014-0566-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Alberico S et al. [Maternal-fetal transmission of human papillomavirus] Minerva Ginecol. 1996;48:199–204. [PubMed] [Google Scholar]
- 129.D'Souza G et al. Case-control study of human papillomavirus and oropharyngeal cancer. N Engl J Med. 2007;356:1944–1956. doi: 10.1056/NEJMoa065497. [DOI] [PubMed] [Google Scholar]
- 130.Hernandez B Y et al. Transmission of human papillomavirus in heterosexual couples. Emerg Infect Dis. 2008;14:888–894. doi: 10.3201/eid1406.070616.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Gillison M L et al. Distinct risk factor profiles for human papillomavirus type 16-positive and human papillomavirus type 16-negative head and neck cancers. J Natl Cancer Inst. 2008;100:407–420. doi: 10.1093/jnci/djn025. [DOI] [PubMed] [Google Scholar]
- 132.Dahlstrom K R et al. Socioeconomic characteristics of patients with oropharyngeal carcinoma according to tumor HPV status, patient smoking status, and sexual behavior. Oral Oncol. 2015;51:832–838. doi: 10.1016/j.oraloncology.2015.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Stenmark M H et al. Influence of human papillomavirus on the clinical presentation of oropharyngeal carcinoma in the United States. Laryngoscope. 2017;127:2270–2278. doi: 10.1002/lary.26566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.D'Souza G et al. Moderate predictive value of demographic and behavioral characteristics for a diagnosis of HPV16-positive and HPV16-negative head and neck cancer. Oral Oncol. 2010;46:100–410. doi: 10.1016/j.oraloncology.2009.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Combes J D, Chen A A, Franceschi S. Prevalence of human papillomavirus in cancer of the oropharynx by gender. Cancer Epidemiol Biomarkers Prev. 2014;23:2954–2958. doi: 10.1158/1055-9965.EPI-14-0580. [DOI] [PubMed] [Google Scholar]
- 136.Klussmann J P et al. Prevalence, distribution, and viral load of human papillomavirus 16 DNA in tonsillar carcinomas. Cancer. 2001;92:2875–2884. doi: 10.1002/1097-0142(20011201)92:11<2875::aid-cncr10130>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 137.Ng M et al. Smoking prevalence and cigarette consumption in 187 countries, 1980-2012. JAMA. 2014;311:183–192. doi: 10.1001/jama.2013.284692. [DOI] [PubMed] [Google Scholar]
- 138.Chaturvedi A K et al. Burden of HPV-positive oropharynx cancers among ever and never smokers in the U.S. population. Oral Oncol. 2016;60:61–67. doi: 10.1016/j.oraloncology.2016.06.006. [DOI] [PubMed] [Google Scholar]
- 139.Ang K K et al. Human papillomavirus and survival of patients with oropharyngeal cancer. N Engl J Med. 2010;363:24–35. doi: 10.1056/NEJMoa0912217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Gillison M L et al. Tobacco smoking and increased risk of death and progression for patients with p16-positive and p16-negative oropharyngeal cancer. J Clin Oncol. 2012;30:2102–2111. doi: 10.1200/JCO.2011.38.4099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Maniakas A et al. North-American survey on HPV-DNA and p16 testing for head and neck squamous cell carcinoma. Oral Oncol. 2014;50:942–946. doi: 10.1016/j.oraloncology.2014.07.004. [DOI] [PubMed] [Google Scholar]
- 142.Prigge E S et al. Diagnostic accuracy of p16INK4a immunohistochemistry in oropharyngeal squamous cell carcinomas: A systematic review and meta-analysis. Int J Cancer. 2017;140:1186–1198. doi: 10.1002/ijc.30516. [DOI] [PubMed] [Google Scholar]
- 143.Smith E M et al. Human papillomavirus in oral exfoliated cells and risk of head and neck cancer. J Natl Cancer Inst. 2004;96:449–455. doi: 10.1093/jnci/djh074. [DOI] [PubMed] [Google Scholar]
- 144.Nordfors C et al. Human papillomavirus prevalence is high in oral samples of patients with tonsillar and base of tongue cancer. Oral Oncol. 2014;50:491–497. doi: 10.1016/j.oraloncology.2014.02.012. [DOI] [PubMed] [Google Scholar]
- 145.Chuang A Y et al. Presence of HPV DNA in convalescent salivary rinses is an adverse prognostic marker in head and neck squamous cell carcinoma. Oral Oncol. 2008;44:915–919. doi: 10.1016/j.oraloncology.2008.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Rotnaglova E et al. HPV involvement in tonsillar cancer: prognostic significance and clinically relevant markers. Int J Cancer. 2011;129:101–110. doi: 10.1002/ijc.25889. [DOI] [PubMed] [Google Scholar]
- 147.Kreimer A R et al. Evaluation of human papillomavirus antibodies and risk of subsequent head and neck cancer. J Clin Oncol. 2013;31:2708–2715. doi: 10.1200/JCO.2012.47.2738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Beachler D C et al. HPV16 E6 seropositivity among cancer-free men with oral, anal or genital HPV16 infection. Papillomavirus Res. 2016;2:141–144. doi: 10.1016/j.pvr.2016.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.
- 150.Kreimer A R et al. Kinetics of the human papillomavirus type 16 e6 antibody response prior to oropharyngeal cancer. J Natl Cancer Inst. 2017;109 doi: 10.1093/jnci/djx005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Guardiola E et al. Is there still a role for triple endoscopy as part of staging for head and neck cancer? Curr Opin Otolaryngol Head Neck Surg. 2006;14:85–88. doi: 10.1097/01.moo.0000193177.62074.fd. [DOI] [PubMed] [Google Scholar]
- 152.Martel M et al. The role of HPV on the risk of second primary neoplasia in patients with oropharyngeal carcinoma. Oral Oncol. 2017;64:37–43. doi: 10.1016/j.oraloncology.2016.11.011. [DOI] [PubMed] [Google Scholar]
- 153.Jain K S et al. Synchronous cancers in patients with head and neck cancer: risks in the era of human papillomavirus-associated oropharyngeal cancer. Cancer. 2013;119:1832–1837. doi: 10.1002/cncr.27988. [DOI] [PubMed] [Google Scholar]
- 154.Sharma S J et al. [Current practice of tumour endoscopy in German ENT-clinics] Laryngorhinootologie. 2013;92:166–169. doi: 10.1055/s-0032-1331759. [DOI] [PubMed] [Google Scholar]
- 155.Graves E E et al. Quantitative and qualitative analysis of [(18)F]FDG and [(18)F]FAZA positron emission tomography of head and neck cancers and associations with HPV status and treatment outcome. Eur J Nucl Med Mol Imaging. 2016;43:617–625. doi: 10.1007/s00259-015-3247-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Hanns E et al. Human Papillomavirus-related tumours of the oropharynx display a lower tumour hypoxia signature. Oral Oncol. 2015;51:848–856. doi: 10.1016/j.oraloncology.2015.06.003. [DOI] [PubMed] [Google Scholar]
- 157.Schouten C S et al. Interaction of quantitative (18)F-FDG-PET-CT imaging parameters and human papillomavirus status in oropharyngeal squamous cell carcinoma. Head Neck. 2016;38:529–535. doi: 10.1002/hed.23920. [DOI] [PubMed] [Google Scholar]
- 158.Thorwarth D et al. Combined uptake of [18 F]FDG and [18 F]FMISO correlates with radiation therapy outcome in head-and-neck cancer patients. Radiother Oncol. 2006;80:151–156. doi: 10.1016/j.radonc.2006.07.033. [DOI] [PubMed] [Google Scholar]
- 159.Mortensen L S et al. FAZA PET/CT hypoxia imaging in patients with squamous cell carcinoma of the head and neck treated with radiotherapy: results from the DAHANCA 24 trial. Radiother Oncol. 2012;105:14–20. doi: 10.1016/j.radonc.2012.09.015. [DOI] [PubMed] [Google Scholar]
- 160.Mehanna H et al. PET-NECK: a multicentre randomised Phase III non-inferiority trial comparing a positron emission tomography-computerised tomography-guided watch-and-wait policy with planned neck dissection in the management of locally advanced (N2/N3) nodal metastases in patients with squamous cell head and neck cancer. Health Technol Assess. 2017;21:1–122. doi: 10.3310/hta21170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.
- 162.Parmar C et al. Radiomic machine-learning classifiers for prognostic biomarkers of head and neck cancer. Front Oncol. 2015;5:272. doi: 10.3389/fonc.2015.00272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Aerts H J et al. Decoding tumour phenotype by noninvasive imaging using a quantitative radiomics approach. Nat Commun. 2014;5:4006. doi: 10.1038/ncomms5006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Ou D et al. Predictive and prognostic value of CT based radiomics signature in locally advanced head and neck cancers patients treated with concurrent chemoradiotherapy or bioradiotherapy and its added value to Human Papillomavirus status. Oral Oncol. 2017;71:150–155. doi: 10.1016/j.oraloncology.2017.06.015. [DOI] [PubMed] [Google Scholar]
- 165.Klozar J et al. Nodal status is not a prognostic factor in patients with HPV-positive oral/oropharyngeal tumors. J Surg Oncol. 2013;107:625–633. doi: 10.1002/jso.23292. [DOI] [PubMed] [Google Scholar]
- 166.Straetmans J M et al. Human papillomavirus reduces the prognostic value of nodal involvement in tonsillar squamous cell carcinomas. Laryngoscope. 2009;119:1951–1957. doi: 10.1002/lary.20593. [DOI] [PubMed] [Google Scholar]
- 167.Sedaghat A R et al. Prognostic significance of human papillomavirus in oropharyngeal squamous cell carcinomas. Laryngoscope. 2009;119:1542–1549. doi: 10.1002/lary.20533. [DOI] [PubMed] [Google Scholar]
- 168.Wittekindt C, Klussmann J P. Tumor staging and HPV-related oropharyngeal cancer. Recent Results Cancer Res. 2017;206:123–133. doi: 10.1007/978-3-319-43580-0_9. [DOI] [PubMed] [Google Scholar]
- 169.O'Sullivan B et al. Development and validation of a staging system for HPV-related oropharyngeal cancer by the International Collaboration on Oropharyngeal cancer Network for Staging (ICON-S): a multicentre cohort study. Lancet Oncol. 2016;17:440–451. doi: 10.1016/S1470-2045(15)00560-4. [DOI] [PubMed] [Google Scholar]
- 170.Sinha P et al. High metastatic node number, not extracapsular spread or N-classification is a node-related prognosticator in transorally-resected, neck-dissected p16-positive oropharynx cancer. Oral Oncol. 2015;51:514–520. doi: 10.1016/j.oraloncology.2015.02.098. [DOI] [PubMed] [Google Scholar]
- 171.Coatesworth A P, MacLennan K. Squamous cell carcinoma of the upper aerodigestive tract: the prevalence of microscopic extracapsular spread and soft tissue deposits in the clinically N0 neck. Head Neck. 2002;24:258–261. doi: 10.1002/hed.10020. [DOI] [PubMed] [Google Scholar]
- 172.Greenberg J S et al. Extent of extracapsular spread: a critical prognosticator in oral tongue cancer. Cancer. 2003;97:1464–1470. doi: 10.1002/cncr.11202. [DOI] [PubMed] [Google Scholar]
- 173.Bernier J et al. Defining risk levels in locally advanced head and neck cancers: a comparative analysis of concurrent postoperative radiation plus chemotherapy trials of the EORTC (#22931) and RTOG (# 9501) Head Neck. 2005;27:843–850. doi: 10.1002/hed.20279. [DOI] [PubMed] [Google Scholar]
- 174.Sinha P et al. Extracapsular spread and adjuvant therapy in human papillomavirus-related, p16-positive oropharyngeal carcinoma. Cancer. 2012;118:3519–3530. doi: 10.1002/cncr.26671. [DOI] [PubMed] [Google Scholar]
- 175.Maxwell J H et al. Extracapsular spread in head and neck carcinoma: impact of site and human papillomavirus status. Cancer. 2013;119:3302–3308. doi: 10.1002/cncr.28169. [DOI] [PubMed] [Google Scholar]
- 176.van den Brekel M W et al. Observer variation in the histopathologic assessment of extranodal tumor spread in lymph node metastases in the neck. Head Neck. 2012;34:840–845. doi: 10.1002/hed.21823. [DOI] [PubMed] [Google Scholar]
- 177.Lewis J S, Jr et al. Extracapsular extension is a poor predictor of disease recurrence in surgically treated oropharyngeal squamous cell carcinoma. Mod Pathol. 2011;24:1413–1420. doi: 10.1038/modpathol.2011.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Wittekindt C et al. Basics of tumor development and importance of human papilloma virus (HPV) for head and neck cancer. GMS Curr Top Otorhinolaryngol Head Neck Surg. 2012;11:Doc09. doi: 10.3205/cto000091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.El-Naggar A K, Westra W H. p16 expression as a surrogate marker for HPV-related oropharyngeal carcinoma: a guide for interpretative relevance and consistency. Head Neck. 2012;34:459–461. doi: 10.1002/hed.21974. [DOI] [PubMed] [Google Scholar]
- 180.Malm I J et al. Evaluation of proposed staging systems for human papillomavirus-related oropharyngeal squamous cell carcinoma. Cancer. 2017;123:1768–1777. doi: 10.1002/cncr.30512. [DOI] [PubMed] [Google Scholar]
- 181.Husain Z A et al. A comparison of prognostic ability of staging systems for human papillomavirus-related oropharyngeal squamous cell carcinoma. JAMA Oncol. 2017;3:358–365. doi: 10.1001/jamaoncol.2016.4581. [DOI] [PubMed] [Google Scholar]
- 182.Arenz A et al. Increased radiosensitivity of HPV-positive head and neck cancer cell lines due to cell cycle dysregulation and induction of apoptosis. Strahlenther Onkol. 2014;190:839–846. doi: 10.1007/s00066-014-0605-5. [DOI] [PubMed] [Google Scholar]
- 183.Rieckmann T et al. HNSCC cell lines positive for HPV and p16 possess higher cellular radiosensitivity due to an impaired DSB repair capacity. Radiother Oncol. 2013;107:242–246. doi: 10.1016/j.radonc.2013.03.013. [DOI] [PubMed] [Google Scholar]
- 184.Petrelli F, Sarti E, Barni S. Predictive value of human papillomavirus in oropharyngeal carcinoma treated with radiotherapy: An updated systematic review and meta-analysis of 30 trials. Head Neck. 2014;36:750–759. doi: 10.1002/hed.23351. [DOI] [PubMed] [Google Scholar]
- 185.Lassen P et al. Effect of HPV-associated p16INK4A expression on response to radiotherapy and survival in squamous cell carcinoma of the head and neck. J Clin Oncol. 2009;27:1992–1998. doi: 10.1200/JCO.2008.20.2853. [DOI] [PubMed] [Google Scholar]
- 186.Semrau R et al. Prognostic impact of human papillomavirus status, survivin, and epidermal growth factor receptor expression on survival in patients treated with radiochemotherapy for very advanced nonresectable oropharyngeal cancer. Head Neck. 2013;35:1339–1344. doi: 10.1002/hed.23126. [DOI] [PubMed] [Google Scholar]
- 187.Garden A S et al. Radiation therapy (with or without neck surgery) for phenotypic human papillomavirus-associated oropharyngeal cancer. Cancer. 2016;122:1702–1707. doi: 10.1002/cncr.29965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.O'Sullivan B et al. Deintensification candidate subgroups in human papillomavirus-related oropharyngeal cancer according to minimal risk of distant metastasis. J Clin Oncol. 2013;31:543–550. doi: 10.1200/JCO.2012.44.0164. [DOI] [PubMed] [Google Scholar]
- 189.Sinha P et al. Does elimination of planned postoperative radiation to the primary bed in p16-positive, transorally-resected oropharyngeal carcinoma associate with poorer outcomes? Oral Oncol. 2016;61:127–134. doi: 10.1016/j.oraloncology.2016.08.013. [DOI] [PubMed] [Google Scholar]
- 190.Al-Mamgani A, Verheij M, van den Brekel MW M. Elective unilateral nodal irradiation in head and neck squamous cell carcinoma: A paradigm shift. Eur J Cancer. 2017;82:1–5. doi: 10.1016/j.ejca.2017.05.035. [DOI] [PubMed] [Google Scholar]
- 191.Tassone P et al. Pathologic markers in surgically treated hpv-associated oropharyngeal cancer: retrospective study, systematic review, and meta-analysis. Ann Otol Rhinol Laryngol. 2017;126:365–374. doi: 10.1177/0003489417693014. [DOI] [PubMed] [Google Scholar]
- 192.Huang Y H et al. Cystic nodal metastasis in patients with oropharyngeal squamous cell carcinoma receiving chemoradiotherapy: Relationship with human papillomavirus status and failure patterns. PLoS One. 2017;12:e0180779. doi: 10.1371/journal.pone.0180779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Wuerdemann N et al. Risk factors for overall survival outcome in surgically treated human papillomavirus-negative and positive patients with oropharyngeal cancer. Oncol Res Treat. 2017;40:320–327. doi: 10.1159/000477097. [DOI] [PubMed] [Google Scholar]
- 194.Lohaus F et al. HPV16 DNA status is a strong prognosticator of loco-regional control after postoperative radiochemotherapy of locally advanced oropharyngeal carcinoma: results from a multicentre explorative study of the German Cancer Consortium Radiation Oncology Group (DKTK-ROG) Radiother Oncol. 2014;113:317–323. doi: 10.1016/j.radonc.2014.11.011. [DOI] [PubMed] [Google Scholar]
- 195.Posner M R et al. Survival and human papillomavirus in oropharynx cancer in TAX 324: a subset analysis from an international phase III trial. Ann Oncol. 2011;22:1071–1077. doi: 10.1093/annonc/mdr006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Toustrup K et al. Gene expression classifier predicts for hypoxic modification of radiotherapy with nimorazole in squamous cell carcinomas of the head and neck. Radiother Oncol. 2012;102:122–129. doi: 10.1016/j.radonc.2011.09.010. [DOI] [PubMed] [Google Scholar]
- 197.Rischin D et al. Prognostic significance of p16INK4A and human papillomavirus in patients with oropharyngeal cancer treated on TROG 02.02 phase III trial. J Clin Oncol. 2010;28:4142–4148. doi: 10.1200/JCO.2010.29.2904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Ziemann F et al. Increased sensitivity of HPV-positive head and neck cancer cell lines to x-irradiation +/- Cisplatin due to decreased expression of E6 and E7 oncoproteins and enhanced apoptosis. Am J Cancer Res. 2015;5:1017–1031. [PMC free article] [PubMed] [Google Scholar]
- 199.Rosenthal D I et al. Association of human papillomavirus and p16 status with outcomes in the IMCL-9815 phase iii registration trial for patients with locoregionally advanced oropharyngeal squamous cell carcinoma of the head and neck treated with radiotherapy With or Without Cetuximab. J Clin Oncol. 2016;34:1300–1308. doi: 10.1200/JCO.2015.62.5970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Ang K K et al. Randomized phase III trial of concurrent accelerated radiation plus cisplatin with or without cetuximab for stage III to IV head and neck carcinoma: RTOG 0522. J Clin Oncol. 2014;32:2940–2950. doi: 10.1200/JCO.2013.53.5633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Chera B S et al. Phase 2 trial of de-intensified chemoradiation therapy for favorable-risk human papillomavirus-associated oropharyngeal squamous cell carcinoma. Int J Radiat Oncol Biol Phys. 2015;93:976–985. doi: 10.1016/j.ijrobp.2015.08.033. [DOI] [PubMed] [Google Scholar]
- 202.Molony P et al. Impact of positive margins on outcomes of oropharyngeal squamous cell carcinoma according to p16 status. Head Neck. 2017;39:1680–1688. doi: 10.1002/hed.24824. [DOI] [PubMed] [Google Scholar]
- 203.Kharytaniuk N et al. Association of extracapsular spread with survival according to human papillomavirus status in oropharynx squamous cell carcinoma and carcinoma of unknown primary site. JAMA Otolaryngol Head Neck Surg. 2016;142:683–690. doi: 10.1001/jamaoto.2016.0882. [DOI] [PubMed] [Google Scholar]
- 204.Fakhry C et al. Improved survival of patients with human papillomavirus-positive head and neck squamous cell carcinoma in a prospective clinical trial. J Natl Cancer Inst. 2008;100:261–269. doi: 10.1093/jnci/djn011. [DOI] [PubMed] [Google Scholar]
- 205.Inhestern J et al. A two-arm multicenter phase II trial of one cycle chemoselection split-dose docetaxel, cisplatin and 5-fluorouracil (TPF) induction chemotherapy before two cycles of split TPF followed by curative surgery combined with postoperative radiotherapy in patients with locally advanced oral and oropharyngeal squamous cell cancer (TISOC-1) Ann Oncol. 2017;28:1917–1922. doi: 10.1093/annonc/mdx202. [DOI] [PubMed] [Google Scholar]
- 206.Topalian S L et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366:2443–2454. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Hong A M et al. PD-L1 expression in tonsillar cancer is associated with human papillomavirus positivity and improved survival: implications for anti-PD1 clinical trials. Oncotarget. 2016;7:77010–77020. doi: 10.18632/oncotarget.12776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Scheel A Het al. Interlaboratory-concordance of PD-L1 immunohistochemistry for non-small cell lung cancer Histopathology 2017 10.1111/his.13375[Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 209.Scheel A H et al. [Predictive PD-L1 immunohistochemistry for non-small cell lung cancer: Current state of the art and experiences of the first German harmonization study] Pathologe. 2016;37:557–567. doi: 10.1007/s00292-016-0189-1. [DOI] [PubMed] [Google Scholar]
- 210.Gandini S, Massi D, Mandala M. PD-L1 expression in cancer patients receiving anti PD-1/PD-L1 antibodies: A systematic review and meta-analysis. Crit Rev Oncol Hematol. 2016;100:88–98. doi: 10.1016/j.critrevonc.2016.02.001. [DOI] [PubMed] [Google Scholar]
- 211.Oguejiofor K et al. Stromal infiltration of CD8 T cells is associated with improved clinical outcome in HPV-positive oropharyngeal squamous carcinoma. Br J Cancer. 2015;113:886–893. doi: 10.1038/bjc.2015.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Badoual C et al. PD-1-expressing tumor-infiltrating T cells are a favorable prognostic biomarker in HPV-associated head and neck cancer. Cancer Res. 2013;73:128–138. doi: 10.1158/0008-5472.CAN-12-2606. [DOI] [PubMed] [Google Scholar]
- 213.Oguejiofor K et al. Distinct patterns of infiltrating CD8+ T cells in HPV+ and CD68 macrophages in HPV- oropharyngeal squamous cell carcinomas are associated with better clinical outcome but PD-L1 expression is not prognostic. Oncotarget. 2017;8:14416–14427. doi: 10.18632/oncotarget.14796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Seiwert T Y et al. Safety and clinical activity of pembrolizumab for treatment of recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE-012): an open-label, multicentre, phase 1b trial. Lancet Oncol. 2016;17:956–965. doi: 10.1016/S1470-2045(16)30066-3. [DOI] [PubMed] [Google Scholar]
- 215.Ferris R L et al. Nivolumab for recurrent squamous-cell carcinoma of the head and neck. N Engl J Med. 2016;375:1856–1867. doi: 10.1056/NEJMoa1602252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Addeo R, Caraglia M, Iuliano G. Pembrolizumab: the value of PDL1 biomarker in head and neck cancer. Expert Opin Biol Ther. 2016;16:1075–1078. doi: 10.1080/14712598.2016.1211635. [DOI] [PubMed] [Google Scholar]
- 217.Kenter G G et al. Vaccination against HPV-16 oncoproteins for vulvar intraepithelial neoplasia. N Engl J Med. 2009;361:1838–1847. doi: 10.1056/NEJMoa0810097. [DOI] [PubMed] [Google Scholar]
- 218.Reuschenbach M et al. A phase 1/2a study to test the safety and immunogenicity of a p16(INK4a) peptide vaccine in patients with advanced human papillomavirus-associated cancers. Cancer. 2016;122:1425–1433. doi: 10.1002/cncr.29925. [DOI] [PubMed] [Google Scholar]
- 219.Fakhry C et al. Human papillomavirus and overall survival after progression of oropharyngeal squamous cell carcinoma. J Clin Oncol. 2014;32:3365–3373. doi: 10.1200/JCO.2014.55.1937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Huang S H et al. Natural course of distant metastases following radiotherapy or chemoradiotherapy in HPV-related oropharyngeal cancer. Oral Oncol. 2013;49:79–85. doi: 10.1016/j.oraloncology.2012.07.015. [DOI] [PubMed] [Google Scholar]
- 221.Trosman S J et al. Effect of human papillomavirus on patterns of distant metastatic failure in oropharyngeal squamous cell carcinoma treated with chemoradiotherapy. JAMA Otolaryngol Head Neck Surg. 2015;141:457–462. doi: 10.1001/jamaoto.2015.136. [DOI] [PubMed] [Google Scholar]
- 222.Dave E et al. The prognostic impact of human papillomavirus status following treatment failure in oropharyngeal cancer. PLoS One. 2017;12:e0181108. doi: 10.1371/journal.pone.0181108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Sinha P et al. Distant metastasis in p16-positive oropharyngeal squamous cell carcinoma: a critical analysis of patterns and outcomes. Oral Oncol. 2014;50:45–51. doi: 10.1016/j.oraloncology.2013.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Duprez F et al. Distant metastases in head and neck cancer. Head Neck. 2017;39:1733–1743. doi: 10.1002/hed.24687. [DOI] [PubMed] [Google Scholar]
- 225.Sweeny L et al. Outcomes after surgical salvage for recurrent oropharyngeal squamous cell carcinoma. Oral Oncol. 2016;60:118–124. doi: 10.1016/j.oraloncology.2016.07.006. [DOI] [PubMed] [Google Scholar]
- 226.Patel S N et al. Salvage surgery for locally recurrent oropharyngeal cancer. Head Neck. 2016;38 01:E658–E664. doi: 10.1002/hed.24065. [DOI] [PubMed] [Google Scholar]
- 227.Sims J R et al. Management of recurrent and metastatic hpv-positive oropharyngeal squamous cell carcinoma after transoral robotic surgery. Otolaryngol Head Neck Surg. 2017;157:69–76. doi: 10.1177/0194599817696304. [DOI] [PubMed] [Google Scholar]
- 228.Dang R P et al. Clinical outcomes in patients with recurrent or metastatic human papilloma virus-positive head and neck cancer. Anticancer Res. 2016;36:1703–1709. [PubMed] [Google Scholar]
- 229.Etges C L et al. Screening tools for dysphagia: a systematic review. Codas. 2014;26:343–349. doi: 10.1590/2317-1782/20142014057. [DOI] [PubMed] [Google Scholar]
- 230.Colodny N. Interjudge and intrajudge reliabilities in fiberoptic endoscopic evaluation of swallowing (fees) using the penetration-aspiration scale: a replication study. Dysphagia. 2002;17:308–315. doi: 10.1007/s00455-002-0073-4. [DOI] [PubMed] [Google Scholar]
- 231.Frakes J M et al. Determining optimal follow-up in the management of human papillomavirus-positive oropharyngeal cancer. Cancer. 2016;122:634–641. doi: 10.1002/cncr.29782. [DOI] [PubMed] [Google Scholar]
- 232.Sharma A et al. Human papillomavirus-positive oral cavity and oropharyngeal cancer patients do not have better quality-of-life trajectories. Otolaryngol Head Neck Surg. 2012;146:739–745. doi: 10.1177/0194599811434707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Stier-Jarmer M et al. Assessment of functional outcomes in head and neck cancer. Eur Arch Otorhinolaryngol. 2014;271:2021–2044. doi: 10.1007/s00405-013-2744-1. [DOI] [PubMed] [Google Scholar]
- 234.Morris L G et al. Second primary cancers after an index head and neck cancer: subsite-specific trends in the era of human papillomavirus-associated oropharyngeal cancer. J Clin Oncol. 2011;29:739–746. doi: 10.1200/JCO.2010.31.8311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Wang Y et al. Detection of somatic mutations and HPV in the saliva and plasma of patients with head and neck squamous cell carcinomas. Sci Transl Med. 2015;7:293ra104. doi: 10.1126/scitranslmed.aaa8507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Cheng F, Su L, Qian C. Circulating tumor DNA: a promising biomarker in the liquid biopsy of cancer. Oncotarget. 2016;7:48832–48841. doi: 10.18632/oncotarget.9453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Jahr S et al. DNA fragments in the blood plasma of cancer patients: quantitations and evidence for their origin from apoptotic and necrotic cells. Cancer Res. 2001;61:1659–1665. [PubMed] [Google Scholar]
- 238.Bettegowda C et al. Detection of circulating tumor DNA in early- and late-stage human malignancies. Sci Transl Med. 2014;6:224ra24. doi: 10.1126/scitranslmed.3007094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Haber D A, Velculescu V E. Blood-based analyses of cancer: circulating tumor cells and circulating tumor DNA. Cancer Discov. 2014;4:650–661. doi: 10.1158/2159-8290.CD-13-1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Amirian E S et al. Presence of viral DNA in whole-genome sequencing of brain tumor tissues from the cancer genome atlas. J Virol. 2014;88:774. doi: 10.1128/JVI.02725-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Lo Y M et al. Quantitative analysis of cell-free Epstein-Barr virus DNA in plasma of patients with nasopharyngeal carcinoma. Cancer Res. 1999;59:1188–1191. [PubMed] [Google Scholar]
- 242.Chan KC A et al. Analysis of plasma epstein-barr virus dna to screen for nasopharyngeal cancer. N Engl J Med. 2017;377:513–522. doi: 10.1056/NEJMoa1701717. [DOI] [PubMed] [Google Scholar]
- 243.Cao H et al. Quantitation of human papillomavirus DNA in plasma of oropharyngeal carcinoma patients. Int J Radiat Oncol Biol Phys. 2012;82:e351–e358. doi: 10.1016/j.ijrobp.2011.05.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Yun C H et al. The T790M mutation in EGFR kinase causes drug resistance by increasing the affinity for ATP. Proc Natl Acad Sci U S A. 2008;105:2070–2075. doi: 10.1073/pnas.0709662105. [DOI] [PMC free article] [PubMed] [Google Scholar]