Abstract
Cardiac amyloidosis, once considered untreatable, is now gaining well-deserved attention due to advances in imaging and the recent approval of targeted breakthrough therapies. In this paper, we discuss the role of radionuclide imaging in the evaluation and management of patients with the most common form of amyloidosis—cardiac transthyretin amyloidosis (ATTR). We provide a comprehensive summary of the literature interspersed with our institutional experience as appropriate, to deliver our perspective.
Keywords: Amyloid heart disease, SPECT, PET, modalities, molecular imaging
BACKGROUND
Cardiac amyloidosis, once considered untreatable, is now gaining well-deserved attention due to advances in imaging and the recent approval of targeted breakthrough therapies.1,2 In this paper, we discuss the role of radionuclide imaging in the evaluation and management of patients with the most common form of amyloidosis—cardiac transthyretin amyloidosis (ATTR). We provide a comprehensive summary of the literature interspersed with our institutional experience as appropriate, to deliver our perspective.
Cardiac amyloidosis is a disorder in which proteins mis-fold and deposit as amyloid fibrils that infiltrate the myocardial extracellular space. Transthyretin protein (ATTR) derived from the liver or immunoglobulin light chain proteins (AL), derived from a plasma cell clone, most commonly affect the heart.3 The former can be due to variant TTR from TTR gene mutations (ATTRm or hereditary amyloidosis) or wild-type TTR deposition where native TTR proteins mis-fold with aging and deposit as fibrils in the heart (ATTRwt, also previously known as senile systemic amyloidosis). With mutant TTR, although over hundred genotypic mutations have been identified in hereditary amyloidosis, variable penetrance results in varying degrees of heart involvement.4 Whereas, at least 50% of primary AL patients5 and almost all wild-type ATTR patients have cardiac involvement,6 but can be challenging to identify.
NEED FOR ACCURATE DIAGNOSIS AND TYPING OF CARDIAC AMYLOID FIBRILS
Diagnosis of cardiac amyloidosis is frequently delayed7 for a number of reasons. Clinical manifestations are varied, serum cardiac biomarker elevation is non-specific, awareness about the condition is lacking, and until recently, noninvasive techniques for specific diagnosis were not available. Appropriate therapies are delayed in a substantial proportion of the affected individuals. Now, advanced imaging, echocardiography8 and cardiac magnetic resonance (CMR),9 can detect cardiac structural and functional changes induced by amyloid deposition. However, they can neither distinguish amyloid from non-amyloid hypertrophic heart disease nor ATTR from AL (which is treated very differently using chemotherapy). This necessitates a histological diagnosis. A fat pad biopsy though simple, has low diagnostic yield in ATTR cardiac amyloidosis (sensitivity: 15% in wildtype ATTR, 45% in mutant ATTR).10 This is why historically, endomyocardial biopsy has been used to confirm cardiac amyloidosis and identify the fibril type. The good news is that data now supports the value of radionuclide imaging with bone avid radiotracers to specifically image ATTR cardiac amyloidosis—obviating the need for endomyocardial biopsy.11
Radionuclide imaging plays a critical role in the diagnosis and identification of ATTR cardiac amyloidosis. Accurate diagnosis of heart failure from cardiac amyloidosis has major implications on patient care, as well. Correct identification of hereditary ATTR cardiac amyloidosis has critical and generationally relevant implications for genetic testing and family counseling. Misidentification of the type of cardiac amyloid can be life threatening if ATTR patients are misclassified as AL and erroneously treated with chemotherapy. Conventional heart failure medications, betablockers and angiotensin-converting enzyme inhibitors, are poorly tolerated by patients with amyloidosis and frequently avoided.12 Appropriate use of bone avid radiotracer imaging can significantly increase the detection of patients with cardiac ATTR amyloidosis; these patients, will benefit from specific disease modifying therapies targeting the cause of heart failure, TTR, that improve quality of life, heart failure, and prolong life.13
BONE AVID RADIOTRACERS
Cardiac imaging with bone avid radiotracers has been used clinically for over 40 years. Early studies showed high-diagnostic accuracies leading to great excitement in the field and a profusion of studies. Curiously, some of the later studies reported lower diagnostic accuracies; one paper from 1987 concluded that “when intense uptake of 99mTc-pyrophosphate (PYP) is present amyloidosis is highly likely, but the technique is not sufficiently sensitive.”14 Astute investigators, in the early 2000s, recognized greater binding avidity of 99mTc-3,3-diphosphono-1,2-propanedicarboxylic acid (DPD) to ATTR (rather than AL). This new knowledge led to a rebirth of cardiac imaging with bone avid radiotracers for ATTR amyloidosis.
MECHANISM OF BINDING OF BONE AVID RADIOTRACERS
Pyrophosphate (PYP) and closely related bis-phosphonates (DPD, hydroxymethylene diphosphonate, HMDP) labeled with99mTc have been used for imaging cardiac ATTR amyloidosis (Figure 1). These tracers were used for myocardial infarct imaging in the 1970s and several binding sites have been identified in animal experiments, including microcalcifications, calcium deposits,15 intracellular calcium PYP,16 or intracellular macromolecules.17 In the context of amyloidosis, the fibril deposits have three major structural components, the precursor protein, heparin sulfate proteoglycan, and a calcium dependent P-component that binds the fibrils together. Pepys et al. suggest that circulating amyloid P component, a universal component of all types of amyloid, could bind to amyloid fibrils via a calcium mediated mechanism, also explaining the uptake of 99mTc-bone avid tracers.18 More recently, Stats et al.19 demonstrated that endomyocardial biopsies of ATTR hearts had greater density of small microcalcifications but fewer macrophages when compared with AL hearts. Interestingly, even though patients with ATTR cardiac amyloidosis were older than AL cardiac amyloidosis, the extent of these microcalcifications did not correlate with age in either group. It should also be recognised that in a few cases of AL cardiac amyloidosis, there were microcalcification densities comparable with ATTR hearts. This suggests a pathophysiological basis for reports of borderline positive 99mTc-PYP or 99mTc-DPD scan in AL patients.20,21 The other clinical scenario where cardiac microcalcifications might lead to positive scans are myocardial infarcts where uptake is typically regional and distinct from the diffuse uptake observed in ATTR amyloidosis.15
Figure 1.
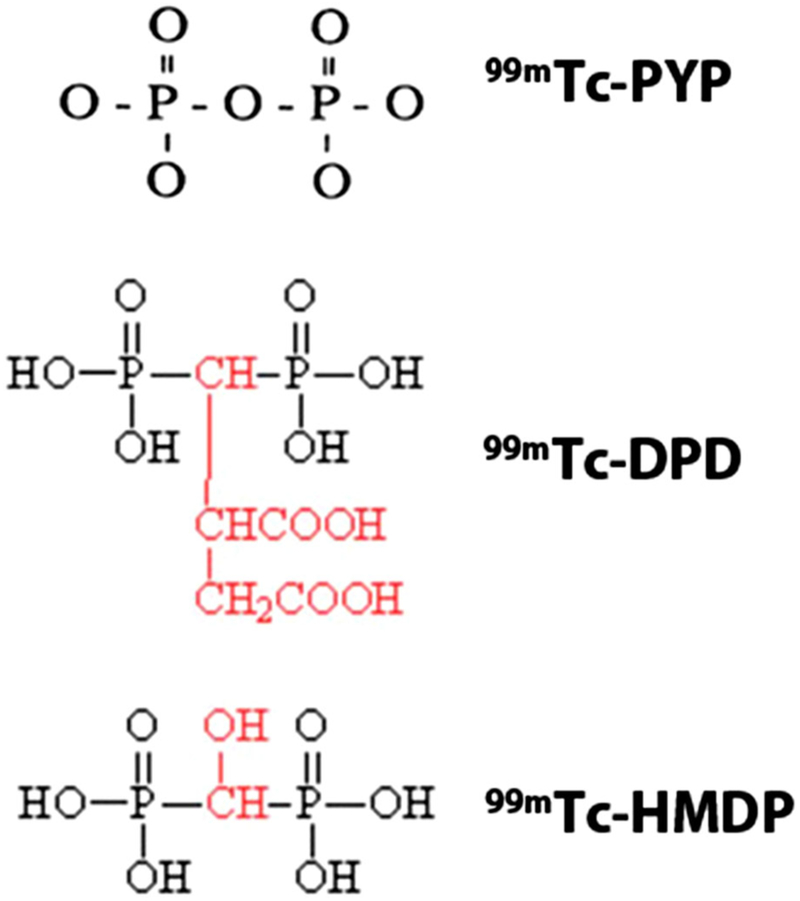
Bone avid SPECT radiotracers for imaging cardiac amyloidosis. 99mTc pyrophosphate (PYP), 3,3-diphosphono-1,2-propanedicarboxylic acid (DPD), and hydroxymethylene diphosphonate (HMDP) have been used for imaging ATTR cardiac amyloidosis. These bone avid SPECT tracers show greater uptake in ATTR cardiac amyloidosis. Images adapted from package insert of 99mTc PYP, and from Human Health Campus, IAEA.
REVIEW OF LITERATURE ON IMAGING PROTOCOLS
Radiotracers
Three tracers, 99mTc-PYP, -DPD, -HMDP, have been evaluated for imaging ATTR cardiac amyloidosis, but they are not widely available. 99mTc-PYP is available in the United States, while 99mTc-DPD, -HMDP are more widely available in Europe. For this reason, the accuracy of each tracer in relation to the others directly in the same patients could not be studied. 99mTc-MDP (methylene diphosphonate), is a widely used bone imaging tracer that has not been studied in ATTR cardiac amyloidosis; its diagnostic utility of compared to 99mTc-PYP (Figure 2) and DPD is likely suboptimal. In one study, 11 ATTR patients with positive 99mTc-DPD, all showed negative 99mTc-MDP imaging.22 On the other hand, 99mTc-HMDP and DPD imaging showed similar diagnostic utility in 6 patients with hereditary cardiac ATTR amyloidosis, but using early phase imaging (5-10 minutes after injection), not a well validated protocol.23 Direct comparison of myocardial 99mTc-PYP with 99mTc-DPD or -HMDP imaging in ATTR cardiac amyloidosis is limited.
Figure 2.
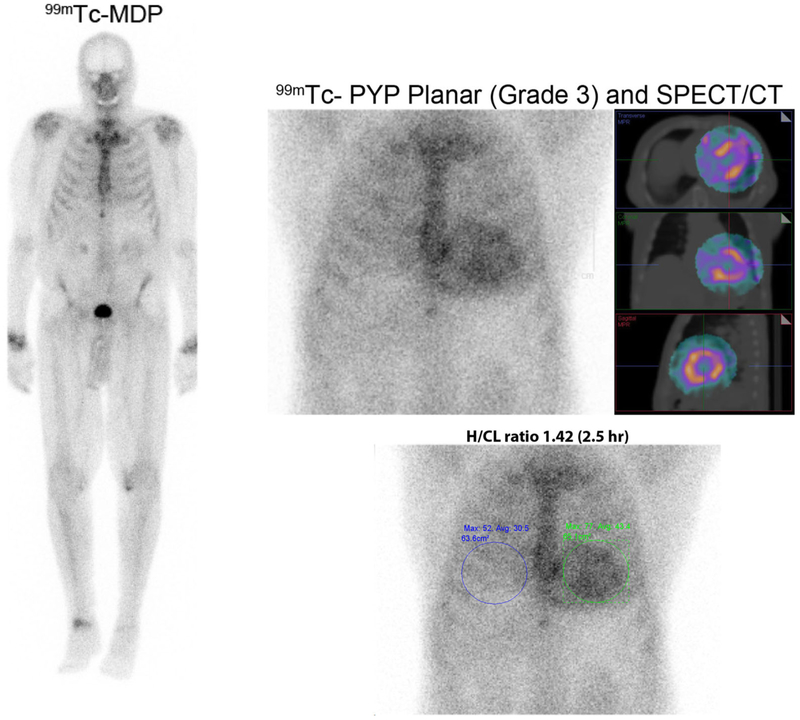
99mTc-MDP and 99mTc-PYP images in cardiac ATTR amyloidosis. Images of an 86-year-old man with prostate cancer and a 99mTc-MDP scan showing no myocardial radiotracer uptake. He presented a month later with heart failure, and echocardiography revealed classic features of cardiac amyloidosis. A clonal abnormality was excluded by serum and urine immunofixation, and serum-free light chain levels. A 99mTc-PYP scan was performed using chest planar and SPECT imaging 2.5 hours after injection of radiotracer; Grade 3 myocardial uptake on 99mTc-PYP planar, and SPECT with a heart-to-contralateral ratio of 1.42 on planar images (≥ 1.3 abnormal for late images) diagnosed cardiac ATTR amyloidosis.
Planar vs SPECT
Most experience in imaging protocols for 99mTc-PYP,-DPD, -HMDP is with planar and SPECT imaging routinely (our institutional practice)24 or planar imaging followed by SPECT if planar is positive.14,20,21 Planar imaging alone is limited as myocardial uptake cannot be discerned from blood pool uptake, overlying rib uptake may add counts to the region of the heart (Figure 3), and attenuation correction is not feasible. SPECT overcomes these challenges. Segmental evaluation, and attenuation correction offer the potential advantages of being more quantitative and a better metric of change. 99mTc-DPD studies predominantly used whole body imaging, 21,24 while 99mTc-PYP studies used chest imaging.25 In our experience, whole body imaging for 99mTc-PYP did not provide additional diagnostic valve, and we are currently imaging only the chest with planar and SPECT/CT imaging.
Figure 3.
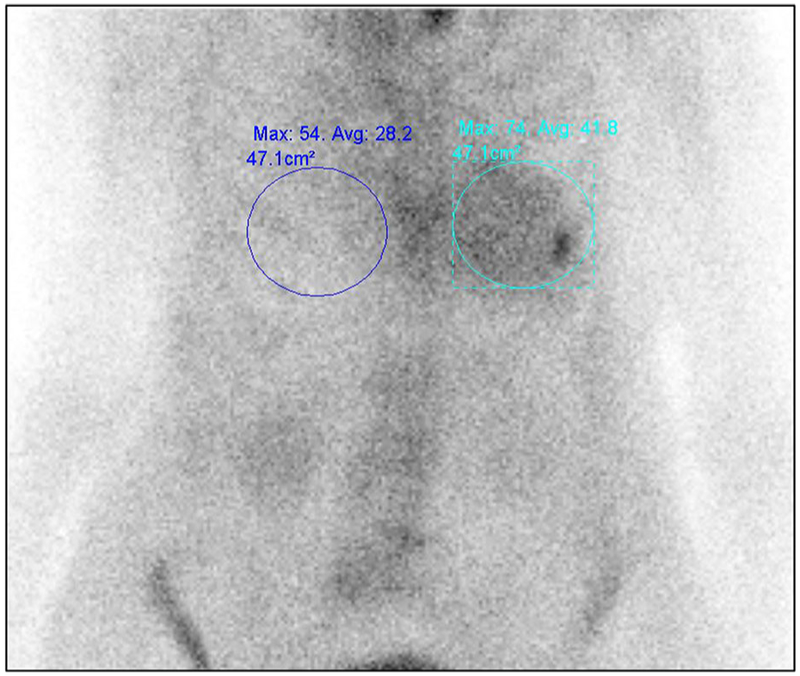
Planar 99mTc-PYP imaging limited by overlying rib uptake. When evaluating the heart-to-contralateral lung uptake ratio (H/CL), care must be taken to avoid increase the bone activity overlying the myocardial region. In this chest planar 99mTc-PYP image, focally increased radiotracer uptake in the left chest is due to a recent rib fracture.
Timing Between Injection and Scan
The timing of imaging after injection of radiotracer varies from 1 to 3 hours. Most experience with 99mTc-PYP protocols is at 1 or 3 hour11,22,24 and for 99mTc-DPD, -HMDP at 3 hours.11,22 Our institutional practice recently changed from 2.5 hours imaging to 1-hour imaging. Imaging at 1 hour offers the advantages of patient comfort, fast laboratory throughput, and high-count images allowing the use of lower radiotracer dose, but SPECT is essential to discern myocardial uptake on planar images as blood pool versus myocardial uptake (Figure 4). Data from 4 patients with multi-time point 99mTc-DPD planar images showed peak myocardial counts 60 minutes after injection of 99mTc-DPD with a gradual decline at 2 and 3 hours; bone counts increased gradually and peaked at 2-3 hours (Figure 5).24 For this reason, the bone to heart ratio (Perugini scoring, see next paragraph) may be more stable at ≥ 2 hours post injection. With 1-hour imaging, visual assessment for diagnosis is more sensitive (but less specific) than at 3 hours.25 However, the disadvantages of imaging at 1-hour include persistent radiotracer activity in the blood pool, particularly in patients with renal dysfunction, which may lower the specificity on planar images. The longer 2-3 hours uptake time allows bone tracer activity to accumulate, along with a parallel reduction in blood pool and myocardial radiotracer activity leading to an enhanced specificity at the cost of reduced sensitivity (Table 1). Following similar principles, a H/CL (heart-to-contralateral lung uptake) ratio threshold of ≥ 1.5 has been established for imaging at 1 hour and a lower cutoff of ≥ 1.3 for 3-hour imaging to distinguish ATTR from AL amyloidosis to allow for decreasing myocardial counts with time.25 In addition, studies have documented high intrareader and interreader reproducibility for visual score and H/CL ratio determination.11,26
Figure 4.
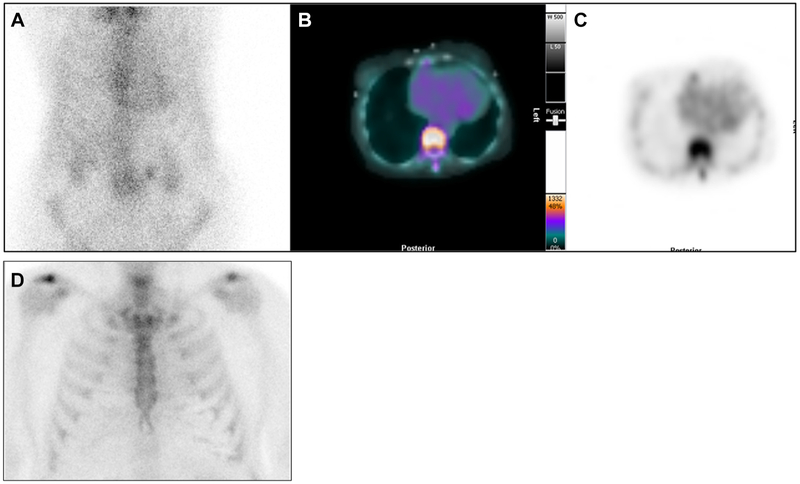
99mTc-PYP on the 1-hour planar chest images and corresponding cardiac SPECT images. 99mTc-PYP with Grade 2 cardiac uptake on the planar chest images (A), which was confirmed as blood pool activity on SPECT/CT fusion images (B), and SPECT images (C). These are 1-hour images, and low rib uptake is noted. In contrast, Panel D shows 3-hour planar chest images from another patient showing Grade 0 (no) cardiac uptake and expected rib uptake.
Figure 5.
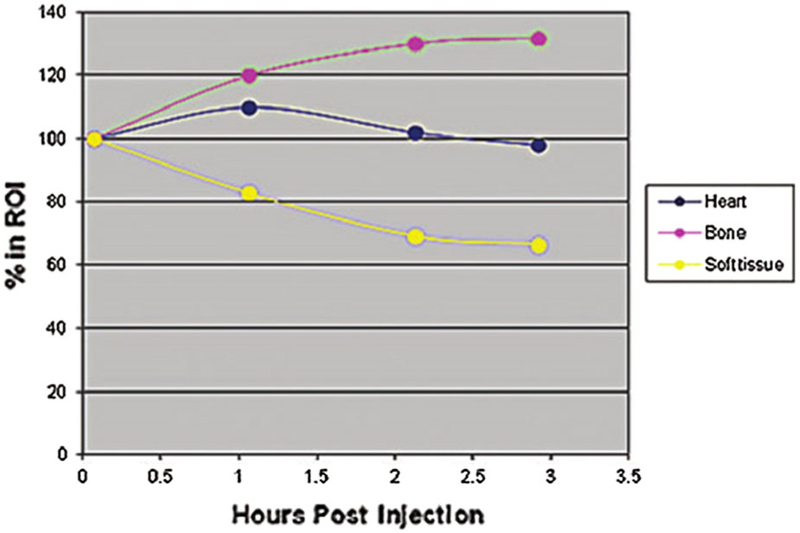
Changes in heart and bone 99mTc-DPD counts over time. Hutt et al. evaluated planar images at multiple time points after injection of 99mTc-DPD in 4 patients. As seen in this figure, myocardial counts peaked at 60 minutes, while bone counts peaked between 2 and 3 hours after injection of 99mTc-DPD.
Table 1.
Accuracy of Tc-pyrophosphate scan for detecting ATTR Cardiac amyloidosis based on semiquantitative and quantitative assessments
| Sensitivity (%) | Specificity (%) | AUC (95% CI) | |
|---|---|---|---|
| Semiquantitative visual Score | |||
| 1-hour delay positive scan ≥ 2 | 95 | 79 | 0.938 (0.873-0.984) |
| 3-hour delay positive scan ≥ 2 | 58 | 100 | 0.980 (0.932-1.000) |
| Combined analysis | 88 | 88 | 0.945 (0.901-0.977) |
| Quantitative H/CL ratio | |||
| 1-hour delay positive scan ≥ 1.5 | 92 | 97 | 0.971 (0.949-0.992) |
| 3-hour delay positive scan ≥ 1.3 | 88 | 86 | 0.935 (0.848-0.988) |
| Combined analysis | 91 | 92 | 0.960 (0.930-0.981) |
Tc-PYP, Tc pyrophosphate; ATTR, transthyretin amyloidosis; AUC, area under the curve; CI, confidence interval; H/CL, ratio = heart-to-contralateral lung ratio. Adapted from Castano et al.25
Quantitation
As 99mTc-PYP, -DPD, -HMDP are hot spot imaging agents, image interpretation typically includes a ratio of myocardial uptake to other organ uptakes (bone, whole body, lung), either visually or using semiquantitative metrics.
Visual method.
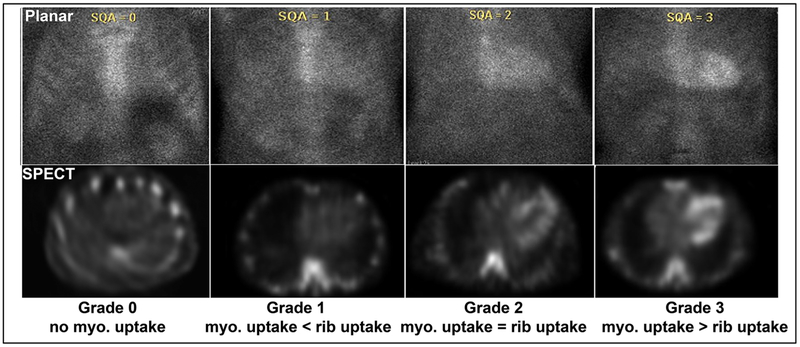
Perugini and colleagues22 described a grading scheme for 3 hours imaging where, Grade 0 = no myocardial uptake; Grade 1 = myocardial uptake < bone uptake; Grade 2 = myocardial uptake equal to bone uptake, Grade 3 = myocardial uptake > bone uptake (with attenuation of bone uptake on whole body images) (Figure 6). This is a very simple metric and includes all the limitations of 3-hour imaging listed above. The percentages of patients with the different grades of radiotracer uptake varies by center and by radiotracer used. With 99mTc-DPD/HMDP and whole-body imaging, attenuation of long bone uptake is required criterion for a Grade 3, while with 99mTc-PYP, most sites only perform chest imaging and Grade 3 is reported when myocardial tracer uptake is greater than rib uptake. Accordingly, the proportion of patients with Grade 3 uptake from major amyloidosis centers for 99mTc-PYP (46%) is higher compared to DPD/HMP (99mTc-DPD 9%; 99mTc-HMDP 9%).11 However, in this multicenter study, the proportion of ATTR positive cases (Grade 2 plus Grade 3 derived from this paper19) was 56%, 40%, and 29%, respectively with PYP, DPD, and HMDP. This may reflect differences in enrolled patient characteristics, imaging protocols, or radiotracers used.
Figure 6.
Visual grading of 99mTc PYP planar and SPECT scans. 99mTc pyrophosphate (PYP) scans are graded visually into Grades 0, 1, 2, and 3, comparing myocardial radiotracer uptake-to-rib uptake. Myo = myocardial. Grades 2 and 3 are considered positive for ATTR cardiac amyloidosis. Figure reproduced with permission.55
Semiquantitative method.
Rapezzi and colleagues21 described a heart-to-whole body retention ratio using early (5 minutes) and delayed images (3 hours). Decay-corrected counts in the late images (corrected for decay and scan speed, and subtracting the activity in the kidneys, bladder) were compared to the counts in early images to derive the heart and whole body retention.21 This method has the advantage of quantitation of radiotracer retention, but requires long time (two whole body scans, 3 hours apart), its value is not established with SPECT/CT, and novel metrics of SUV offer the potential of superior quantitation.
Bokhari and colleagues20 defined a heart-to-contralateral lung uptake ratio (H/CL ratio) on 1-hour planar imaging for distinguishing ATTR from AL amyloidosis (≥ 1.5) and validated it also for 3-hours imaging with slightly different cut off values (≥ 1.3).25 This is a simple metric, but includes all the challenges of 1-hour imaging and planar imaging outlined in the previous paragraph. As this is a ratio metric, optimally, it is used to distinguish AL from ATTR, when the scan shows cardiac uptake of radiotracer visually.
The optimal metric to use is likely a combination of above depends on the tracer used, timing of scan, and use of planar vs SPECT images. We interpret our scans using the visual score and review the H/CL in select cases. The semiquantitative metrics have demonstrated prognostic value as discussed later.
DIAGNOSIS OF CARDIAC ATTR AMYLOIDOSIS
Several single-center studies have confirmed high-diagnostic accuracy (sensitivity and specificity > 90%) of 99mTc-PYP,20 -DPD21,22,27 and -HMDP28 imaging for cardiac ATTR amyloidosis. Specificity was lower in the presence of circulating monoclonal protein and AL amyloidosis. In these studies, 25-50%11,24 of patients with AL cardiac amyloidosis demonstrated myocardial uptake of 99mTc-PYP and DPD, typically, low grade (Grade 1 or 2).
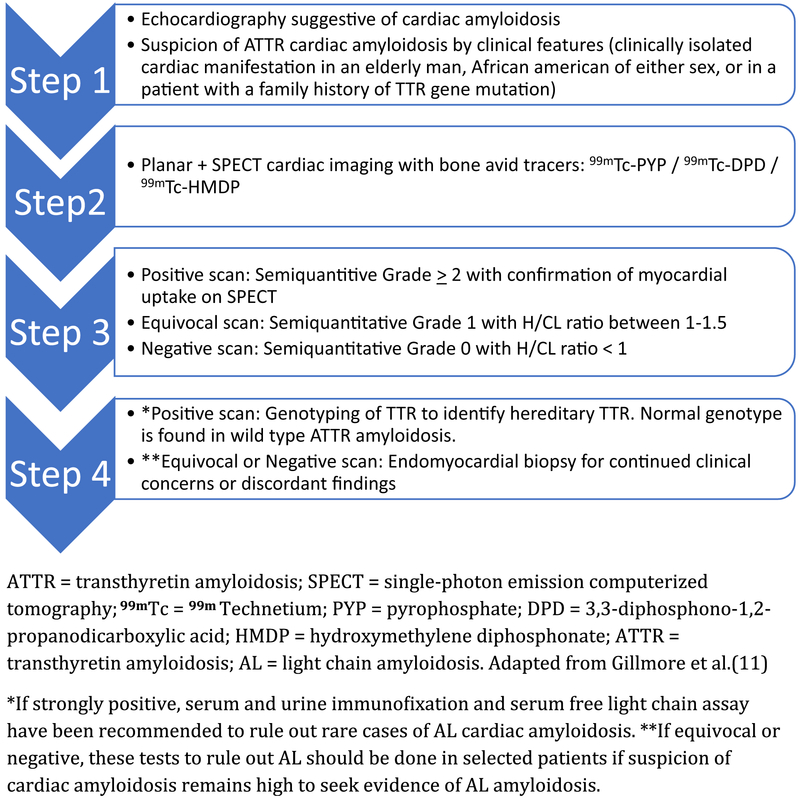
In a recent large international collaboration by Gillmore et al.,11 1217 patients (n: 99mTc-pyrophosphate, PYP = 199, 99mTc-HMDP (hydroxy methylene diphosphonate) = 141 and 99mTc-3,3-diphosphono-1,2-propanodicarboxylic acid, DPD = 877) who were referred for evaluation of suspected cardiac amyloidosis with bone compound tracers were analyzed. The authors concluded that the collective findings of ≥ Grade 2 myocardial radiotracer uptake on bone tracer cardiac scintigraphy along with absence of monoclonal gammopathy on serum and urine analysis demonstrated a specificity and positive predictive value of 100% for ATTR cardiac amyloidosis. Interestingly, up to 1/5th of patients had a monoclonal protein spike on lab evaluation, and the specificity of ≥ Grade 2 scan was only 91% in the absence of exclusion of AL cardiac amyloidosis. This highlights the absolute necessity to exclude monoclonal protein in serum and urine using immunofixation studies concurrently with bone avid tracer imaging (Figure 7). For these same parameters (≥ Grade 2 uptake and absence of monoclonal gammopathy) with a specificity of 100%, the sensitivity was 72%, 70%, and 57% for 99mTc-PYP, DPD, and HMDP respectively. As discussed previously, differences in enrolled patient features, imaging protocols, or radiotracer characteristics may account for these differences. When the clinical findings are discordant with imaging endomyocardial biopsy can be considered, in select cases, for improved detection. These data came from internationally renowned centers with expertise in clinical amyloidosis, and the diagnostic accuracy of this test when applied more broadly to centers without clinical amyloidosis expertise is yet to be determined. Integrating bone tracer cardiac scintigraphy into diagnostic algorithms could reduce healthcare costs relative to the expenses of an invasive procedure with endomyocardial biopsy (Figure 8).29
Figure 7.
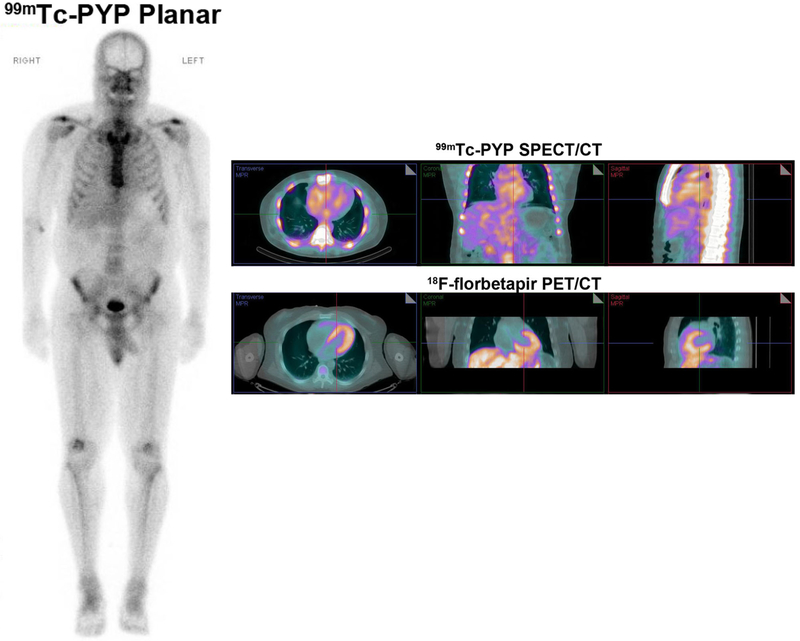
A negative 99mTc-pyrophosphate scan does not exclude cardiac amyloidosis. A 59-year-old man with Val 122 I mutation and heart failure had typical echocardiographic findings. He was referred for 99mTc-PYP imaging which was negative (Grade 0 uptake on planar whole-body images on the left; SPECT/CT fusion images in the right panel, top row show blood pool activity without myocardial uptake). Further evaluation with serum and urine immunofixation, and bone marrow biopsy confirmed AL amyloidosis. A PET/CT scan obtained as part of a research protocol using 8 mCi 18F-florbetapir with dynamic imaging, and static images reconstructed from 4 to 30 minutes, showed intense myocardial uptake (PET/CT fusion images on the right panel bottom row) confirming cardiac amyloidosis.
Figure 8.
A proposed algorithm incorporating 99mTc-PYP, DPD, and HMDP for the evaluation of suspected ATTR cardiac amyloidosis. Evaluation of patients with suspected cardiac amyloidosis typically starts with echocardiography or CMR to evaluate cardiac structure and function, and if ATTR is suspected a bone avid scintigraphy is performed. If the bone avid tracer cardiac scintigraphy is strongly positive, and monoclonal protein is excluded, transthyretin cardiac amyloidosis is diagnosed with higher specificity. In equivocal or negative cases, further evaluation may be considered including endomyocardial biopsy.
Increased 99mTc-DPD uptake in the muscle has been described in ATTR cardiac amyloidosis on planar imaging,30 and more recently in the gluteal, shoulder, chest, and abdominal walls using SPECT/CT. This pattern of increased muscle uptake of radiotracer appears to be specific to 99mTc-DPD and has not been observed with 99mTc-PYP.31 This has been our institutional experience as well.
A recent study evaluated 55 patients with biopsy proven ATTR with 99mTc-DPD scan and typing of amyloid fibril composition. In that study, 97% of patients with type A (mix of full length TTR and truncated TTR fragments) and none of the patients with type B amyloid fibrils (full length TTR only) demonstrated uptake on 99mTc-DPD scintigraphy.32 These findings of differential myocardial uptakes based on fibril type in hereditary ATTR cardiac amyloidosis have been confirmed with 11C-acetate PET,33 but not yet widely reported by other groups.
SCREENING FOR CARDIAC ATTR AMYLOIDOSIS
The prevalence of wild type ATTR amyloidosis increases with advancing age. On autopsy studies, close to 25% of individuals above the age of 80 years demonstrated cardiac ATTR amyloid deposits.34,35 In addition to older individuals, there is an increasing recognition that cardiac amyloidosis is an under-diagnosed cause of heart failure in several populations. (Table 2)99mTc-PYP and DPD imaging has been used to diagnose ATTR cardiac amyloidosis in patients with hereditary ATTR,36 heart failure with preserved ejection fraction,37,38 degenerative aortic stenosis,39 carpal tunnel syndrome,40 cardiomyopathies supposed to be idiopathic or sarcomeric (Table 2). Unexpected myocardial uptake of 99mTc-PYP, or -DPD suggesting ATTR cardiomyopathy has been reported in two large series of patients undergoing bone scintigraphy for non-cardiac reasons41,42 (Table 2). However most of these studies are limited due to the lack of a reference standard for cardiac amyloidosis in the patients with negative scans.
Table 2.
Screening for cardiac ATTR amyloidosis using 99mTc-PYP or DPD
| First Author |
Radiotracer | N | Cohort | Prevalence of ATTR |
|---|---|---|---|---|
| Gonzalez-Lopez38 | 99mTc-DPD | 120 | Heart failure with preserved EF Hospitalized patients 42% women |
13.3% |
| Castano39 | 99mTc-PYP | 151 | TAVR Age > 75 years Severe aortic stenosis Low flow low gradient AS Mean LVEF 46% |
16% |
| Haq36 | 99mTc-PYP | Hereditary ATTR No heart failure Normal echocardiogram Normal cardiac biomarkers |
83% | |
| Bennani-Smires37 | 99mTc-DPD | 49 | Age > 65 years Heart failure with preserved EF |
18% |
| Longhi S56 | 99mTc-DPD | 43 | Aortic stenosis 5 with echo red flags underwent 99mTc-DPD and all were strongly positive |
11.6% |
| Longhi S41 | 99mTc-DPD | 12400 | All bone scans performed over a 5 + year period for clinical reasons | 0.36% |
| Mohamed-Salem42 | 99mTc-DPD | 1114 | Age ≥ 75 years Bone scan for clinical reasons |
2.78% |
| Sperry40 | 99mTc-PYP | 98 | Carpal tunnel surgery Men ≥ 50 years Women ≥ 60 years 10 patients with biopsy proven amyloid from carpal tunnel procedure were evaluated by 99mTc-PYP |
10.2% |
PROPOSED CLINICAL INDICATIONS FOR 99MTC-PYP/DPD/HMDP IMAGING
Cardiac imaging with 99mTc-PYP, -DPD, and -HMDP are indicated in the below clinical scenarios (Reproduced with permission from ASNC 99mTc-Practice Points).
Individuals with heart failure and unexplained increase in left ventricular wall thickness.
Elderly African-Americans over the age of 60 years with heart failure, unexplained or with increased left ventricular wall thickness (> 12 mm).
Elderly individuals (age over 60 years) with unexplained heart failure with preserved ejection fraction.
Individuals, especially elderly males, with unexplained neuropathy, bilateral carpal tunnel syndrome or atrial fibrillation in the absence of usual risk factors, and signs/symptoms of heart failure.
Evaluation of cardiac involvement in individuals with known or suspected hereditary amyloidosis.
Diagnosis of cardiac ATTR in individuals with CMR or echocardiography consistent with cardiac amyloidosis.
Patients with suspected cardiac ATTR amyloidosis and contraindications to CMR such as renal insufficiency or an implantable cardiac device.43
An upcoming publication in JNC on multi-societal consensus recommendations on multimodality imaging in ATTR cardiac amyloidosis will provide an update on the indications for imaging.
ASSESSMENT OF RISK IN ATTR CARDIAC AMYLOIDOSIS
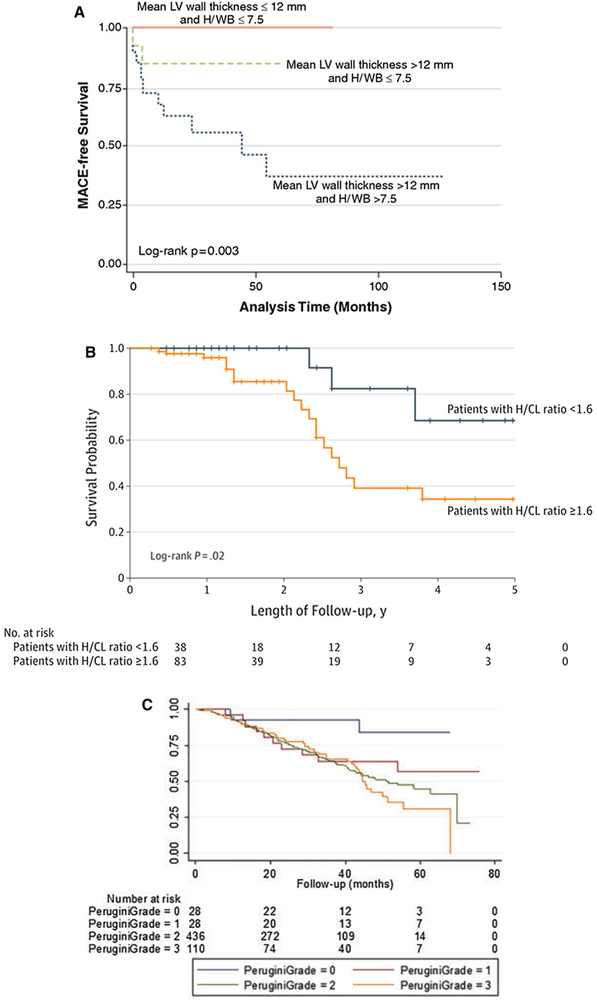
Current literature supports the ability of semiquantitative metrics of heart/whole body (H/WB) retention on 99mTc-DPD (Figure 9A),21 and H/CL ratio on 99mTc-PYP(Figure 9B)25 to identify higher risk of major adverse cardiac events (MACE) and survival, respectively. However, visual grading of 99mTc-PYP -DPD was not found to be an independent predictors of outcomes, (Figure 9C).25,44
Figure 9.
Prognostic value of 99mTc-DPD/PYP imaging in cardiac amyloidosis. Patients with mean LV wall thickness < 12 mm, heart-to-whole body retention (H/WB) ratio < 7.5 on 99mTc-DPD (A), heart/contralateral (H/CL) ratio < 1.6 on 99mTc-PYP (B), and Grade 0 uptake on 99mTc-DPD (C) showed best outcomes. Patients with Perugini Grades 1, 2, and 3 showed similar survival, and worse compared to Grades 0 (no amyloidosis). Figures reproduced with permission from refs. 21,25,44
In one study using 99mTc-DPD, Rapezzi et al.21 assessed 63 patients with ATTR (40 with and 23 without echocardiographic evidence of amyloid cardiomyopathy) and exhibited significant myocardial tracer uptake (visual score ≥ 2) in all patients, signifying that 99mTc-DPD scintigraphy has the ability to identify amyloid myocardial infiltration even before the appearance of echocardiographic abnormalities, and this has prognostic value. In addition, a combination of left ventricular wall thickness > 1.2 cm and heart/whole body retention (H/WB) > 7.5 was associated with the highest major adverse cardiac events (MACE) rate.21
In a multicenter, retrospective cohort study of 229 patients, Castano et al.25 highlighted the prognostic importance of a higher 99mTc-PYP radiotracer activity as quantified by H/CL ratio. A H/CL ratio of ≥ 1.5 was associated with a significantly worse survival (along with echocardiographic characteristics of a more advanced disease stage) over a 5-year period, whereas a visual Grade of 2 or 3 was not. This finding also emphasizes the higher sensitivity of the H/CL ratio compared to visual grading of radiotracer uptake, as quantitative assessment corrects for background, blood pool, and soft tissue activity.
Likewise, in a study of 602 patients by Hutt et al.,44 survival was significantly better in patients with 99mTc-DPD scan Grade 0 compared to Grades 1, 2, and 3. However, there was not a significant difference in survival by increasing grade of 99mTc-DPD. The only independent predictors of mortality included functional status (ECOG performance status, HR for 3 vs 0 of 9.5 [CI 1.9-47.4], P = .006), and renal function (eGFR, HR 0.98 [CI 0.96-0.99], P = .002), after adjustment for NT-proBNP levels, age, left ventricular ejection fraction, supine systolic blood pressure (< _100 mmHg vs > 100 mmHg) and 99mTc-DPD scan Grade 0 vs Grade 1/2/3.
ASSESSMENT OF DISEASE PROGRESSION AND RESPONSE TO THERAPY
Currently there are very minimal data available on the utility of serial imaging to either identify disease progression or assess response to therapy in ATTR cardiac amyloidosis. In a single-center study, 20 ATTR cardiac amyloidosis patients (wild type =10, mutant = 10) with a positive 99mTc-PYP imaging, an average of 1.5 years prior, were invited to undergo a repeat 99mTc-PYP scan. Cardiac radiotracer retention assessed by both semiquantitative (visual score) and quantitative (H/CL ratio) methods did not demonstrate any significant changes, despite clinical progression of disease as determined by worsening lab biomarkers, echocardiographic parameters, New York Heart Association (NYHA) class, progression to heart transplantation and/or death. With emerging therapies for ATTR,45–47 there is an urgent need for sensitive imaging biomarkers to monitor disease progression and response to treatment in cardiac ATTR. Quantitative imaging with echocardiography and longitudinal strain, CMR ECV, or amyloid imaging with PET tracers may play an important role.
FUTURE OF RADIONUCLIDE AMYLOID IMAGING: PET TRACERS
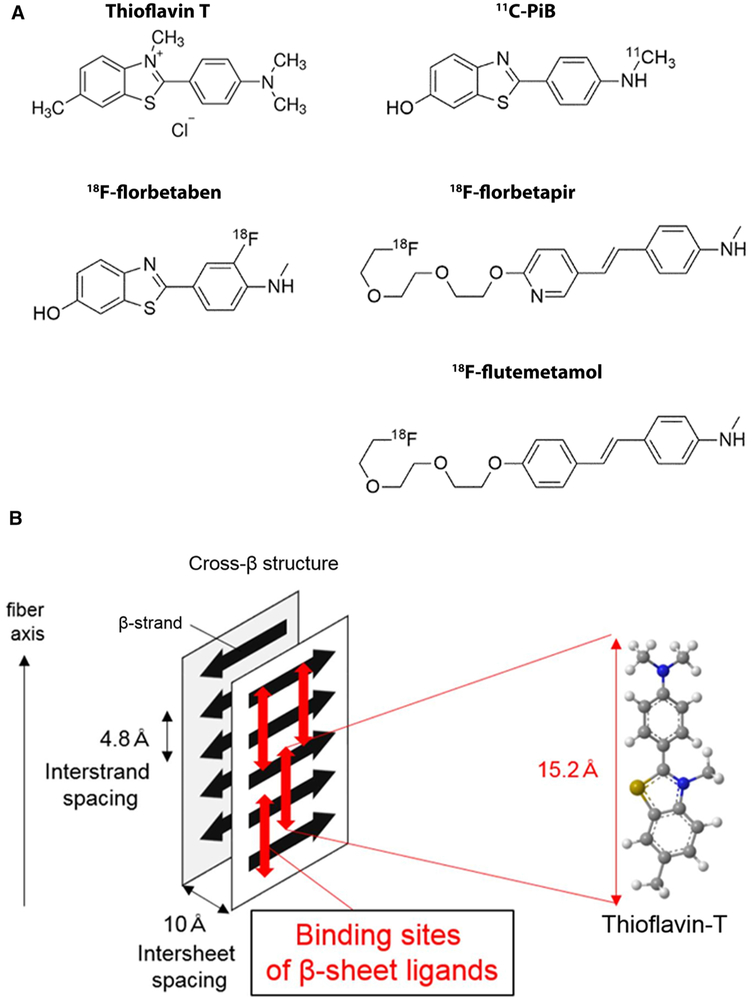
The PET tracers, originally developed for imaging β-amyloid and Alzheimer’s disease,18F-florbetapir, -florbetaben, and -flutemetamol, and 11C-Pittsburgh Compound-B (Figure 10A), have been successfully used to diagnose cardiac amyloidosis.48 These tracers are structurally similar to thioflavin-T and likely bind to the beta-pleated motif of amyloid fibril explaining their ability to image amyloid deposits independent of the precursor protein (Figure 10B). Also, compared to SPECT tracers, PET tracers are quantitative, allowing the possibility of quantifying amyloid burden and detecting a change. The 18F-tracers have the added advantage of a long half-life (109.7 minutes) and unit dose delivery to sites without a cyclotron on site. Notably, amyloid binding PET tracers are the sole clinically available radiotracers to adequately image AL amyloid burden in the heart.
Figure 10.
Amyloid PET tracers (A) and proposed mechanism of binding of amyloid PET radiotracers (B). 18F-florbetapir, 18F-florbetaben, and 18F-flutemetamol are FDA approved for β-amyloid imaging in Alzheimer’s disease. 11C-PIB is not FDA approved. Studies have reported the utility of the 11C-PIB, 18F-florbetapir, and 18F-florbetaben for imaging AL and ATTR cardiac amyloidosis, and these tracers appear to show greater uptake in AL than ATTR cardiac amyloidosis. These tracers are structurally similar to Thioflavin T and likely bind to the motif of the β pleated sheet structure. Molecular structures in Figure 10A were adapted from package inserts/product catalogue and reproduced with permission from ref. 57 Figure 10B reproduced with permission from ref. 58
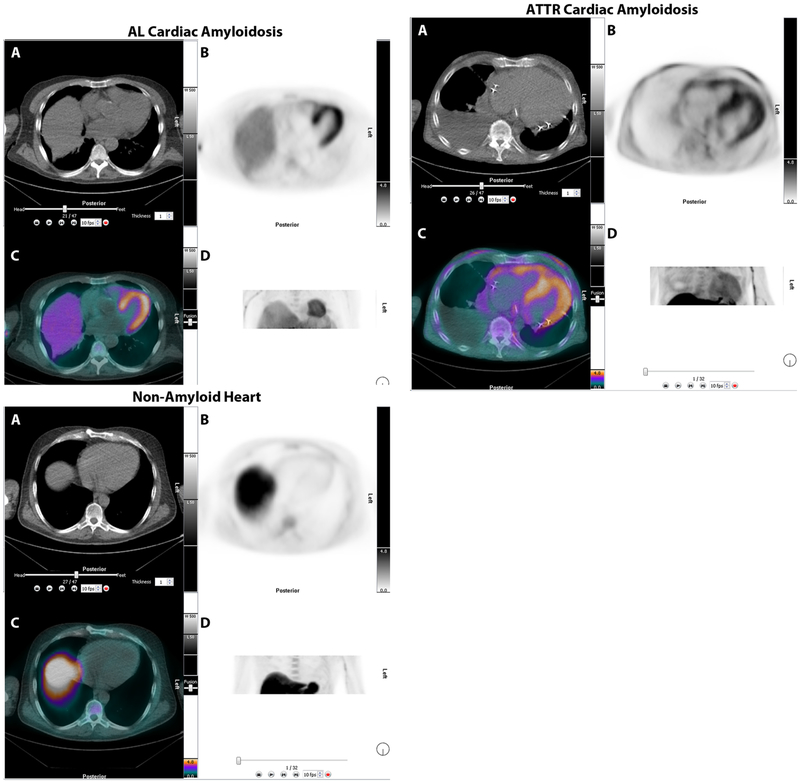
The initial publications of cardiac amyloidosis in humans were based on 11C-PiB followed by 18F-florbetapir, and 18F-florbetaben PET/CT. In these studies, all patients with AL and ATTR cardiac amyloidosis showed significant myocardial uptake while none of the control subjects showed myocardial uptake of amyloid tracer. Figure 11 shows representative images of 18F-florbetapir PET/CT in AL (A), ATTR (B) and in a healthy subject (C) from a research study.49 In that study myocardial SUV [3.84 (1.87-5.65) vs 1.35 (1.17-2.28), P < .00010]; retention index [0.043 (0.034-0.051) vs 0.023 (0.015-0.024), P < .001], were significantly higher in patients with amyloidosis compared to non-amyloid control subjects; in the ATTR cohort, myocardial retention index ranged from 0.031-0.045.49Similar results were reported by other investigators.50–52 In most of these studies, although the radiotracer signal intensity trended higher in AL compared to ATTR hearts, there was significant overlap in values making a distinction of AL from ATTR impossible.50–52 An autoradiography study evaluated 30 myocardial sections from patients with autopsy-proven ATTR (n = 10), AL (n = 10) and nonamyloid controls (n = 10) and demonstrated specific binding of 18F-florbetapir to AL and ATTR cardiac amyloidosis.53 Also, percentage myocardial 18F-florbetaben retention was an independent determinant of biventricular myocardial dysfunction as measured by longitudinal strain.54 18F-flutemetamol is an 18F structural analogue of nC-Pittsburgh compound B, however harbors the additional benefit of a longer half-life compared to 11C-PIB (t1/2 = 20 minutes) that requires an onsite cyclotron for production; this has not yet been studied in cardiac amyloidosis imaging. Access to sites without a cyclotron makes 18F-tracers more real-world surrogates for research and clinical applications compared to 11C-PIB, though they have not yet been evaluated in multicenter studies.
Figure 11.
18F-florbetapir PET/CT imaging in patients with AL, ATTR, and no-amyloidosis. Images are displayed as axial CT transmission (A), axial emission (B), fused transmission/emission (C), and a maximum intensity projection image (D). Both AL (top left), ATTR (top right) images show intense 18F-florbetapir uptake in the left ventricle. The patient without amyloidosis, volunteer, (bottom), showed no myocardial uptake of 18F-florbetapir.
CONCLUSIONS
Early diagnosis of ATTR cardiac amyloidosis is now more important than ever before. Bone avid tracer cardiac imaging has revolutionized the field’s ability to specifically diagnose ATTR cardiac amyloidosis noninvasively, obviating the need for endomyocardial biopsy. This test enables screening of specific populations at high risk for ATTR cardiac amyloidosis. Patients with heart failure can, for the first time, be diagnosed with ATTR cardiac amyloidosis and risk stratified using semiquantitative radionuclide metrics. Using bone avid tracer cardiac imaging to accurately identify ATTR cardiac amyloidosis makes possible targeted therapy with novel disease-modifying agents to substantially reduce heart failure hospitalization and improve survival.
Supplementary Material
Acknowledgments
Funding Dr. Dorbala is supported by NIH RO1 Grant (RO1 HL 130563) and the American Heart Association Grant (AHA 16 CSA 2888 0004). Dr. Falk is supported by NIH RO1 Grant (RO1 HL 130563).
Footnotes
Disclosures
Dr. Singh has no disclosures. Dr. Falk has research grants from Ionnis, Alnylam, Glaxo Smith Kline and Pfizer. He serves as a consultant for Proclara. Dr. Di Carli has research grant from Spectrum Dynamics and Gilead, and consulting fees from Sanofi and GE. Dr. Dorbala served as a consultant with Advanced Accelerator Applications, General Electric and Proclara. Dr. Rapezzi has research grants from Pfizer and consulting fees from Pfizer and Alnylam.
Electronic supplementary material The online version of this article (https://doi.org/10.1007/s12350-018-01552-4) contains supplementary material, which is available to authorized users.
References
- 1.Adams D, Gonzalez-Duarte A, O’Riordan WD, Yang CC, Ueda M, Adams AV, et al. Patisiran, an RNAi Therapeutic, for Hereditary Transthyretin Amyloidosis. N Engl J Med 2018;379:11–21. [DOI] [PubMed] [Google Scholar]
- 2.Benson MD, Waddington-Cruz M, Berk JL, Polydefkis M, Dyck PJ, Wang AK, et al. Inotersen Treatment for Patients with Hereditary Transthyretin Amyloidosis. N Engl J Med 2018;379:22–31. [DOI] [PubMed] [Google Scholar]
- 3.Falk RH. Diagnosis and management of the cardiac amyloidoses. Circulation 2005;112:2047–60. [DOI] [PubMed] [Google Scholar]
- 4.Quarta CC, Buxbaum JN, Shah AM, Falk RH, Claggett B, Kitzman DW, et al. The amyloidogenic V122I transthyretin variant in elderly black Americans. N Engl J Med 2015;372:21–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kyle RA, Gertz MA. Primary systemic amyloidosis: clinical and laboratory features in 474 cases. Semin Hematol 1995;32:45–59. [PubMed] [Google Scholar]
- 6.Ruberg FL, Berk JL. Transthyretin (TTR) cardiac amyloidosis. Circulation 2012;126:1286–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lousada I, Comenzo RL, Landau H, Guthrie S, Merlini G. Light Chain Amyloidosis: Patient Experience Survey from the Amyloidosis Research Consortium. Adv Ther 2015;32:920–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Quarta CC, Solomon SD, Uraizee I, Kruger J, Longhi S, Ferlito M, et al. Left ventricular structure and function in transthyretin-related versus light-chain cardiac amyloidosis. Circulation 2014;129:1840–9. [DOI] [PubMed] [Google Scholar]
- 9.Martinez-Naharro A, Treibel TA, Abdel-Gadir A, Bulluck H, Zumbo G, Knight DS, et al. Magnetic Resonance in Transthyretin Cardiac Amyloidosis. J Am Coll Cardiol 2017;70:466–77. [DOI] [PubMed] [Google Scholar]
- 10.Quarta CC, Gonzalez-Lopez E, Gilbertson JA, Botcher N, Rowczenio D, Petrie A, et al. Diagnostic sensitivity of abdominal fat aspiration in cardiac amyloidosis. Eur Heart J 2017;38:1905–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gillmore JD, Maurer MS, Falk RH, Merlini G, Damy T, Dispenzieri A, et al. Nonbiopsy Diagnosis of Cardiac Transthyretin Amyloidosis. Circulation 2016;133:2404–12. [DOI] [PubMed] [Google Scholar]
- 12.Falk RH, Alexander KM, Liao R, Dorbala S. AL (Light-Chain) Cardiac Amyloidosis: A Review of Diagnosis and Therapy. J Am Coll Cardiol 2016;68:1323–41. [DOI] [PubMed] [Google Scholar]
- 13.Maurer MS, Schwartz JH, Gundapaneni B, Elliott PM, Merlini G, Waddington-Cruz M, et al. Tafamidis Treatment for Patients with Transthyretin Amyloid Cardiomyopathy. N Engl J Med 2018;379:1007–16. [DOI] [PubMed] [Google Scholar]
- 14.Gertz MA, Brown ML, Hauser MF, Kyle RA. Utility of technetium Tc 99 m pyrophosphate bone scanning in cardiac amyloidosis. Arch Intern Med 1987;147:1039–44. [PubMed] [Google Scholar]
- 15.Buja LM, Parkey RW, Stokely EM, Bonte FJ, Willerson JT. Pathophysiology of technetium-99 m stannous pyrophosphate and thallium-201 scintigraphy of acute anterior myocardial infarcts in dogs. J Clin Invest 1976;57:1508–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Buja LM, Tofe AJ, Kulkarni PV, Mukherjee A, Parkey RW, Francis MD, et al. Sites and mechanisms of localization of technetium-99 m phosphorus radiopharmaceuticals in acute myocardial infarcts and other tissues. J Clin Invest 1977;60:724–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dewanjee MK, Kahn PC. Mechanism of localization of 99 mTc-labeled pyrophosphate and tetracycline in infarcted myocardium. J Nucl Med 1976;17:639–46. [PubMed] [Google Scholar]
- 18.Pepys MB, Dyck RF, de Beer FC, Skinner M, Cohen AS. Binding of serum amyloid P-component (SAP) by amyloid fibrils. Clin Exp Immunol 1979;38:284–93. [PMC free article] [PubMed] [Google Scholar]
- 19.Stats MA, Stone JR. Varying levels of small microcalcifications and macrophages in ATTR and AL cardiac amyloidosis: implications for utilizing nuclear medicine studies to subtype amyloidosis. Cardiovasc Pathol 2016;25:413–7. [DOI] [PubMed] [Google Scholar]
- 20.Bokhari S, Castano A, Pozniakoff T, Deslisle S, Latif F, Maurer MS. (99 m)Tc-pyrophosphate scintigraphy for differentiating light-chain cardiac amyloidosis from the transthyretin-related familial and senile cardiac amyloidoses. Circ Cardiovasc Imaging 2013;6:195–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rapezzi C, Quarta CC, Guidalotti PL, Pettinato C, Fanti S, Leone O, et al. Role of (99 m)Tc-DPD scintigraphy in diagnosis and prognosis of hereditary transthyretin-related cardiac amyloidosis. JACC Cardiovasc imaging 2011;4:659–70. [DOI] [PubMed] [Google Scholar]
- 22.Perugini E, Guidalotti PL, Salvi F, Cooke RM, Pettinato C, Riva L, et al. Noninvasive etiologic diagnosis of cardiac amyloidosis using 99 mTc-3,3-diphosphono-1,2-propanodicarboxylic acid scintigraphy. J Am Coll Cardiol 2005;46:1076–84. [DOI] [PubMed] [Google Scholar]
- 23.Abulizi M, Cottereau AS, Guellich A, Vandeventer S, Galat A, Van Der Gucht A et al. Early-phase myocardial uptake intensity of 99 mTc-HMDP vs 99 mTc-DPD in patients with hereditary transthyretin-related cardiac amyloidosis. J Nucl Cardiol 2016. [DOI] [PubMed] [Google Scholar]
- 24.Hutt DF, Quigley AM, Page J, Hall ML, Burniston M, Gopaul D, et al. Utility and limitations of 3,3-diphosphono-1,2-propanodicarboxylic acid scintigraphy in systemic amyloidosis. Eur Heart J Cardiovasc Imaging 2014;15:1289–98. [DOI] [PubMed] [Google Scholar]
- 25.Castano A, Haq M, Narotsky DL, Goldsmith J, Weinberg RL, Morgenstern R et al. Multicenter Study of Planar Technetium 99 m Pyrophosphate Cardiac Imaging: Predicting Survival for Patients With ATTR Cardiac Amyloidosis. JAMA Cardiol 2016. [DOI] [PubMed] [Google Scholar]
- 26.Castano A, DeLuca A, Weinberg R, Pozniakoff T, Blaner WS, Pirmohamed A, et al. Serial scanning with technetium pyrophosphate ((99m)Tc-PYP) in advanced ATTR cardiac amyloidosis. J Nucl Cardiol 2016;23:1355–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rapezzi C, Quarta CC, Guidalotti PL, Longhi S, Pettinato C, Leone O, et al. Usefulness and limitations of 99mTc-3,3-diphosphono-1,2-propanodicarboxylic acid scintigraphy in the aetiological diagnosis of amyloidotic cardiomyopathy. Eur J Nucl Med Mol Imaging 2011;38:470–8. [DOI] [PubMed] [Google Scholar]
- 28.Galat A, Rosso J, Guellich A, Van Der Gucht A, Rappeneau S, Bodez D, et al. Usefulness of (99m)Tc-HMDP scintigraphy for the etiologic diagnosis and prognosis of cardiac amyloidosis. Amyloid 2015;22:210–20. [DOI] [PubMed] [Google Scholar]
- 29.Maurer MS. Noninvasive Identification of ATTRwt Cardiac Amyloid: The Re-emergence of Nuclear Cardiology. Am J Med 2015;128:1275–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kula RW, Engel WK, Line BR. Scanning for soft-tissue amyloid. Lancet 1977;1:92–3. [DOI] [PubMed] [Google Scholar]
- 31.Sperry BW, Gonzalez MH, Brunken R, Cerqueira MD, Hanna M, Jaber WA. Non-cardiac uptake of technetium-99m pyrophosphate in transthyretin cardiac amyloidosis. J Nucl Cardiol 2018. [DOI] [PubMed] [Google Scholar]
- 32.Pilebro B, Suhr OB, Naslund U, Westermark P, Lindqvist P, Sundstrom T. (99m)Tc-DPD uptake reflects amyloid fibril composition in hereditary transthyretin amyloidosis. Ups J Med Sci 2016;121:17–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pilebro B, Arvidsson S, Lindqvist P, Sundstrom T, Westermark P, Antoni G, et al. Positron emission tomography (PET) utilizing Pittsburgh compound B (PIB) for detection of amyloid heart deposits in hereditary transthyretin amyloidosis (ATTR). J Nucl Cardiol 2018;25:240–8. [DOI] [PubMed] [Google Scholar]
- 34.Cornwell GG 3rd, Murdoch WL, Kyle RA, Westermark P, Pitkanen P. Frequency and distribution of senile cardiovascular amyloid. A clinicopathologic correlation. Am J Med 1983;75:618–23. [DOI] [PubMed] [Google Scholar]
- 35.Pomerance A. Senile cardiac amyloidosis. Br Heart J 1965;27:711–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Haq M, Pawar S, Berk JL, Miller EJ, Ruberg FL. Can 99m-Tc-Pyrophosphate Aid in Early Detection of Cardiac Involvement in Asymptomatic Variant TTR Amyloidosis? JACC Cardiovasc imaging 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bennani Smires Y, Victor G, Ribes D, Berry M, Cognet T, Mejean S, et al. Pilot study for left ventricular imaging phenotype of patients over 65 years old with heart failure and preserved ejection fraction: the high prevalence of amyloid cardiomyopathy. Int J Cardiovasc Imaging 2016;32:1403–13. [DOI] [PubMed] [Google Scholar]
- 38.Gonzalez-Lopez E, Gallego-Delgado M, Guzzo-Merello G, de Haro-Del Moral FJ, Cobo-Marcos M, Robles C, et al. Wild-type transthyretin amyloidosis as a cause of heart failure with preserved ejection fraction. Eur Heart J 2015;36:2585–94. [DOI] [PubMed] [Google Scholar]
- 39.Castano A, Narotsky DL, Hamid N, Khalique OK, Morgenstern R, DeLuca A, et al. Unveiling transthyretin cardiac amyloidosis and its predictors among elderly patients with severe aortic stenosis undergoing transcatheter aortic valve replacement. Eur Heart J 2017;38:2879–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sperry BW, Reyes BA, Ikram A, Donnelly JP, Phelan D, Jaber WA, et al. Tenosynovial and Cardiac Amyloidosis in Patients Undergoing Carpal Tunnel Release. J Am Coll Cardiol 2018;72:2040–50. [DOI] [PubMed] [Google Scholar]
- 41.Longhi S, Guidalotti PL, Quarta CC, Gagliardi C, Milandri A, Lorenzini M, et al. Identification of TTR-related subclinical amyloidosis with 99mTc-DPD scintigraphy. JACC Cardiovasc imaging 2014;7:531–2. [DOI] [PubMed] [Google Scholar]
- 42.Mohamed-Salem L, Santos-Mateo JJ, Sanchez-Serna J, Hernandez-Vicente A, Reyes-Marle R, Castellon Sanchez MI, et al. Prevalence of wild type ATTR assessed as myocardial uptake in bone scan in the elderly population. Int J Cardiol 2018;270:192–6. [DOI] [PubMed] [Google Scholar]
- 43.Falk RH, Quarta CC, Dorbala S How to image cardiac amyloidosis. Circ Cardiovasc Imaging 2014;7:552–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hutt DF, Fontana M, Burniston M, Quigley AM, Petrie A, Ross JC, et al. Prognostic utility of the Perugini grading of 99mTc-DPD scintigraphy in transthyretin (ATTR) amyloidosis and its relationship with skeletal muscle and soft tissue amyloid. Eur Heart J Cardiovasc Imaging 2017;18:1344–50. [DOI] [PubMed] [Google Scholar]
- 45.Safety and Efficacy of Tafamidis in Patients With Transthyretin Cardiomyopathy; 2014. [Google Scholar]
- 46.Phase 2 Study to Evaluate ALN-TTRSC (Revusiran) in Patients With Transthyretin (TTR) Cardiac Amyloidosis; 2014. [Google Scholar]
- 47.Tolerability and Efficacy of a Combination of Doxycycline and TUDCA in Patients With Transthyretin Amyloid Cardiomyopathy; 2013. [Google Scholar]
- 48.Vandenberghe R, Van Laere K, Ivanoiu A, Salmon E, Bastin C, Triau E, et al. 18F-flutemetamol amyloid imaging in Alzheimer disease and mild cognitive impairment: a phase 2 trial. Ann Neurol 2010;68:319–29. [DOI] [PubMed] [Google Scholar]
- 49.Dorbala S, Vangala D, Semer J, Strader C, Bruyere JR Jr, Di Carli MF, et al. Imaging cardiac amyloidosis: a pilot study using (18)F-florbetapir positron emission tomography. Eur J Nucl Med Mol Imaging 2014;41:1652–62. [DOI] [PubMed] [Google Scholar]
- 50.Law WP, Wang WY, Moore PT, Mollee PN, Ng AC. Cardiac amyloid imaging with 18F-florbetaben positron emission tomography: a pilot study. J Nucl Med 2016. [DOI] [PubMed] [Google Scholar]
- 51.Osborne DR, Acuff SN, Stuckey A, Wall J. A routine PET/CT protocol with simple calculations for assessing cardiac amyloid using 18F-Florbetapir. Front Cardiovasc Med 2015;2:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Antoni G, Lubberink M, Estrada S, Axelsson J, Carlson K, Lindsjo L, et al. In vivo visualization of amyloid deposits in the heart with 11C-PIB and PET. J Nucl Med 2013;54:213–20. [DOI] [PubMed] [Google Scholar]
- 53.Park MA, Padera RF, Belanger A, Dubey S, Hwang DH, Veeranna V et al. 18F-Florbetapir binds specifically to myocardial light chain and transthyretin amyloid deposits: Autoradiography Study. Circ Cardiovasc imaging 2015;8:e002954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Law WP, Wang W, Moore P, Mollee P, Ng A. Cardiac amyloid imaging with (18)F-florbetaben positron emission tomography: a pilot study. Amyloid 2017;24:162. [DOI] [PubMed] [Google Scholar]
- 55.Dorbala S, Bokhari S, Miller E, Bullock-Palmer R, Soman P, Thompson R. ASNC Practice Points: 99mTechnetium-Pyrophosphate Imaging for Transthyretin Cardiac Amyloidosis 2016. [Google Scholar]
- 56.Longhi S, Lorenzini M, Gagliardi C, Milandri A, Marzocchi A, Marrozzini C, et al. Coexistence of degenerative aortic stenosis and wild-type transthyretin-related cardiac amyloidosis. JACC Cardiovasc imaging 2016;9:325–7. [DOI] [PubMed] [Google Scholar]
- 57.Hayne DJ, Lim S, Donnelly PS. Metal complexes designed to bind to amyloid-beta for the diagnosis and treatment of Alzheimer’s disease. Chem Soc Rev 2014;43:6701–15. [DOI] [PubMed] [Google Scholar]
- 58.Harada R, Okamura N, Furumoto S, Yanai K. Imaging protein misfolding in the brain using beta-sheet ligands. Front Neurosci 2018;12:585. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.