Abstract
Starting two decades ago with the discoveries of genetic links between alpha-synuclein and Parkinson’s disease risk and the identification of aggregated alpha-synuclein as the main protein constituent of Lewy pathology, alpha-synuclein has emerged as the major therapeutic target in Parkinson’s disease and related synucleinopathies. Following the suggestion that alpha-synuclein pathology gradually spreads through the nervous system following a stereotypic pattern and the discovery that aggregated forms of alpha-synuclein can propagate pathology from one cell to another, and thereby probably aggravate existing deficits as well as generate additional symptoms, the idea that alpha-synuclein is a viable therapeutic target gained further support. In this review we describe current challenges and possibilities with alpha-synuclein as a therapeutic target. We briefly highlight gaps in the knowledge of the role of alpha-synuclein in disease, and propose that a deeper understanding of the pathobiology of alpha-synuclein can lead to improved therapeutic strategies. We describe several treatment approaches that are currently being tested in advanced animal experiments or already are in clinical trials. We have divided them into approaches that reduce alpha-synuclein production; inhibit alpha-synuclein aggregation inside cells; promote its degradation either inside or outside cells; and reduce its uptake by neighbouring cells following release from already affected neurons. Finally, we briefly discuss challenges related to the clinical testing of alpha-synuclein therapies, for example diffculties in monitoring target engagement and the need for relatively large trials of long duration. We conclude that alpha-synuclein remains one of the most compelling therapeutic targets for Parkinson’s disease, and related synucleinopathies, and that the multitude of approaches being tested provides hope for the future.
Keywords: Alpha-synuclein, Parkinson’s disease, Dementia with Lewy bodies, Multiple system atrophy, Therapy, Immunotherapy, Biomarker, Clinical Trial, Protein aggregation
1. Introduction
Undoubtedly, accumulation of misfolded alpha-synuclein (a-syn) into aggregates has a central place in the pathogenesis of Parkinson’s disease (PD) and other related synucleinopathies, such as Dementia with Lewy Bodies (DLB) and multiple system atrophy (MSA). In all three of these disorders, the neuropathology is characterised by a-syn accumulation and aggregation, albeit with different cellular predilections and neuroanatomical distributions (Halliday et al., 2011; McCann et al., 2014). The normal function of a-syn in neurons is not well understood, and it is believed that it plays a role in synaptic transmission, specifically in the recycling of synaptic vesicles (Burré, 2015; Burré et al., 2017). The protein is also highly abundant in red blood cells, but its function there is also unknown (Barbour et al., 2008). Under normal conditions a-syn is bound to the membranes of synaptic vesicles (Logan et al., 2017), and is present in the cytosol as a soluble and natively unfolded monomer (Bendor et al., 2013; Burré, 2015; Burré et al., 2017), and possibly also as a tetramer (Bartels et al., 2011), although the existence of a tetrameric form is debated (Fauvet et al., 2012). During the pathogenic process, a-syn misfolds and forms insoluble protein amyloid fibrils, that are the pathological hallmarks of PD, DLB and MSA (Burré et al., 2017; Lashuel et al., 2013).
As has been detailed in other review articles, genetic evidence supports the notion that a-syn can play a causative role in PD and related disorders. In brief, point mutations and multiplications of the SNCA gene lead to PD or neurological disorders with parkinsonian features (Lashuel et al., 2013; Nalls et al., 2014; Wales et al., 2013). Normal aging is also associated with increased cytoplasmic levels of soluble a-syn and decreased levels of markers coupled to dopaminergic function in substantia nigra neuronal cell bodies (Chu and Kordower, 2007). Furthermore, single nucleotide polymorphisms close to the SNCA locus are also associated with significantly altered PD risk (Nalls et al., 2014). The fact that increased levels of a-syn (due to gene multiplications, nucleotide polymorphisms or normal aging) are associated with neuropathology and disease, suggests that just decreasing the cellular levels of the protein is a possible approach to therapy, and further below we will discuss this as one possible therapeutic strategy.
While there is little doubt that a-syn plays some role in the pathogenesis of PD and related conditions, the precise role is not fully elucidated. It is beyond the scope of this review to discuss in detail which molecular species of a-syn that are pathogenic and how these initially develop (Burré et al., 2017; Dehay et al., 2015; Lashuel et al., 2013; Oueslati, 2016; Wales et al., 2013). Although we will touch upon this topic briefly in a later section, in this review we instead focus on describing a series of therapeutic approaches that all target a-syn in one way or another. We also want to clarify that this review mostly exemplifies treatments directed at PD, but several of the therapeutic approaches are likely also relevant to other synucleinopathies.
Before describing the different approaches to therapy, we provide a short list of outstanding questions regarding the role of a-syn pathology in neurological diseases, together with relevant references to review articles. This list can be used as a backdrop to the discussion of different therapeutic approaches because the questions are relevant to our understanding of how a-syn pathology might be targeted therapeutically. The seven listed issues regarding the role of a-syn in disease pathogenesis are frequently discussed, and therefore worthy of special mention.
First, it remains controversial how upstream in the pathogenic cascade a-syn aggregation is, and whether other molecular events (e.g. mitochondrial failure, oxidative stress, decline of lysosomal function and inflammation) are required triggers (Burré et al., 2017; Dehay et al., 2015; Lashuel et al., 2013; Oueslati, 2016; Wales et al., 2013; Wong and Krainc, 2017). Can a-syn aggregation develop in in otherwise healthy cells, or is failure in energy metabolism, protein homeostasis, antioxidant defences etc. necessary for disease to occur? In some patients (especially those with known mutations in proteins involved in mitochondrial health), is the misfolding of a-syn simply a marker of cell stress and not the root cause of the disease? Considering that aging is the greatest risk factor for PD, even normal cellular aging might be considered the “most upstream” event in the pathogenesis. Aged neurons might represent fertile ground for the seeds of a-syn misfolding to take hold, whereas misfolded a-syn can be effectively cleared by well-functioning, proteostatic mechanisms in young, but otherwise equivalent, neurons (Chu and Kordower, 2007; Jin et al., 2016; Wong and Krainc, 2017). Indeed, such failure of proteostatic mechanisms might explain, at least in part, the selective vulnerability of certain neurons that is seen in the diseased brain (George and Brundin, 2017; Kim et al., 2016a, b). This has implications on therapies that target a-syn, because approaches that counteract cellular aging in the brain might therefore be helpful in reducing PD risk.
Second, it is debated whether the a-syn aggregates that are viewed in the microscope as Lewy pathology, either in neuronal cell bodies (Lewy bodies) or neurites (Lewy neurites), or, in the case of MSA inside oligodendroglia, are the primary cause of cell death. Indeed, it has even been suggested that these large a-syn aggregates actually sequester the more harmful conformers of the protein and might be neuroprotective. Therefore, alternatively, smaller oligomeric species have been suggested to be neurotoxic, and it has even been speculated that monomeric a-syn at supraphysiological levels might be a trigger of lethal molecular cascades. Understanding the role of a-syn oligomers versus fibrils is important when considering what form(s) of a-syn the therapies (e.g. small molecules, antibodies) should target (Melki, 2015; Peng et al., 2017; Wang et al., 2016).
Third, recent discoveries in laboratory models have shown that aggregation-prone a-syn species can be released into the extracellular space and be taken up by neighbouring neurons (Brundin et al., 2016; Goedert et al., 2017; Guo and Lee, 2014; Lee et al., 2014). Once inside the new neuron, the a-syn can seed aggregation of endogenous a-syn. Since a-syn aggregates can be transported along the axon between interconnected brain regions, this “prion-like” mechanism can explain, at least in part, why the disease progresses with both additional symptoms and more widespread Lewy pathology appearing in the brain over time. The implications for this discovery are wide-reaching in a therapy context. If extracellular a-syn plays an important role in the propagation of pathology from one brain region to another in patients, it might be possible to therapeutically minimize the release of misfolded a-syn or reduce the uptake of the protein by neighbouring neurons. Indeed, if a-syn accumulation begins in the periphery (outside the brain), then preventing its propagation to the central nervous system (CNS) could theoretically prevent many of the neurological manifestations of the synucleinopathies.
Fourth, it has been suggested that there exist different molecular species of a-syn aggregates, akin to the “strains” of prion protein that cause different clinical forms of prion disease (Bousset et al., 2013; Melki, 2015; Peng et al., 2017). The putative existence of such a-syn strains could, at least partly, explain why some patients develop PD and others DLB, or MSA. This idea assumes that the biophysical characteristics of the different strains make them more prone to preferentially invade certain cell types. As we will discuss briefly below, this in turn would suggest that a therapy that is effective in one of the synucleinopathies by targeting the prevalent strains of a-syn in that particular disease, might not work in other synucleinopathies. It is even conceivable that the a-syn strains differ between individuals with the same clinical diagnosis, or potentially between different brain regions in the same patient. This would require individualised therapies, or even multiple therapies that selectively target different types of strains in the same patient.
Fifth, while it is widely recognised that Lewy pathology does not develop simultaneously in all parts of the nervous system, there is not full agreement on which brain regions are first vulnerable. Evidence suggests that the olfactory bulb, with closely associated olfactory pathways, and the dorsal motor nucleus of the vagus are two areas that among the first to exhibit Lewy pathology (Braak and Del Tredici, 2017; Del Tredici and Braak, 2016; Rey et al., 2016b; Beach et al., 2009). The a-syn pathology that occurs in sites outside the substantia nigra is likely one of the causes of the signs and symptoms that characterise “prodromal” PD, and therefore this pathology is particularly important when considering early therapeutic intervention. This concept means that monitoring non-motor features of prodromal PD (e.g. anosmia, constipation, sleep disorders, depression), could be excellent measures of therapeutic success; although it should be noted that these features in isolation are not specific for PD (Mahlknecht et al., 2015; Poewe et al., 2017; Schrag et al., 2015). Furthermore, reports that at post-mortem examination 10–30% of normal aged individuals exhibit Lewy pathology in the brain without any associated symptoms (the so called Incidental Lewy Body Disease, which could be a forerunner of the clinical disorders) (Iacono et al., 2015), also suggests that therapies which target a-syn will become increasingly important as the average life-span of populations continue to increase worldwide.
Sixth, it is debated what the true relationship is between accumulation of a-syn, neuronal death and neurological deficits. Some argue that the correlation between Lewy bodies and neuronal death in the afficted brain regions is poor (Surmeier et al., 2017). It has also been suggested that the accumulation of a-syn in small synaptic aggregates (Lewy neurites) as opposed to the larger Lewy bodies in the soma, are the drivers of neuronal dysfunction and, e.g., cognitive decline (Kramer and Schulz-Schaeffer, 2007). While this is an important consideration, it does not necessarily question the validity of a-syn as a therapeutic target.
Seventh, the role of post-translational modifications of a-syn is not fully understood. In Lewy pathology, some of the a-syn has been cleaved at the C-terminus by calpains (Dufty et al., 2007) while an estimated 90% of the a-syn in the aggregates is phosphorylated at serine residue 129, which can be taken to suggest that this modified form of asyn is particularly detrimental (Lashuel et al., 2013; Oueslati, 2016). However, experimental studies involving modified forms of a-syn, that cannot undergo this phosphorylation, have yielded conflicting results and do not unequivocally support a key role for this post-translational modification in PD pathogenesis (Febbraro et al., 2013). Therapies that target post-translational modifications of a-syn have to viewed in the light that we do not really understand the significance of the modifications in the pathogenesis of the disease.
Eighth, the interaction between a-syn misfolding and neuroin-flammation is complex. Studies have shown that genes encoding immune system molecules affect PD risk (S. G. Coetzee et al., 2016; Nalls et al., 2014; Pierce and Coetzee, 2017). Several experiments also indicate that misfolded a-syn can trigger microglial activation (Béraud et al., 2013; Reynolds et al., 2009), and it is also suggested that an activated neuroimmune response can promote post-translational modifications (e.g. oxidation and nitrosylation) of a-syn which triggers misfolding of the protein (Gao et al., 2008). Recent work has even suggested that peptides derived from a-syn can trigger an autoimmune component to PD (Sulzer et al., 2017). Taken together, a complex vicious circle of immune activation and a-syn misfolding can be envisaged. These complex interactions are particularly important when considering therapies that have immune modulatory effects or utilise microglia as a means to attain the goal (e.g. immunotherapies) (George and Brundin, 2015).
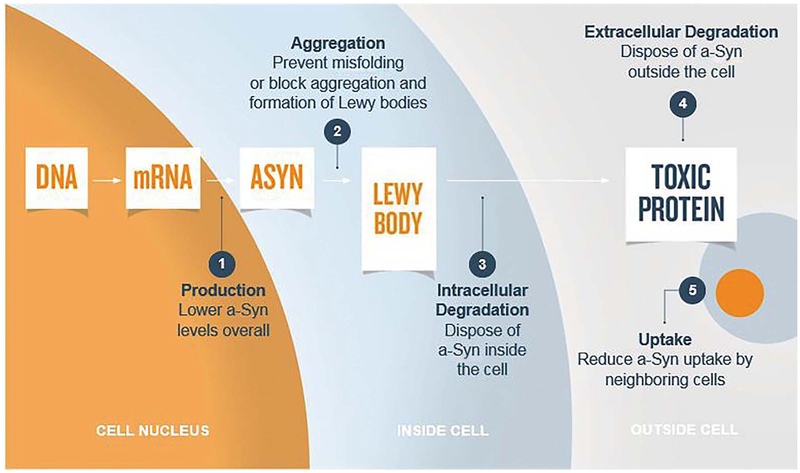
Notwithstanding the uncertainties regarding the pathobiology of asyn mentioned above - some of which are going to very challenging to resolve in disease models in the laboratory - it remains highly warranted to test therapies that target a-syn pathology. Below, we systematically divide different strategies to target a-syn according to the steps in the molecular pathogenesis that are in focus and the cellular compartments that are relevant (Fig. 1). Thus, in separate sections we describe approaches to inhibit a-syn production; prevent a-syn aggregation; promote intracellular a-syn degradation; enhance extra-cellular a-syn degradation and reduce uptake of extracellular a-syn.
Fig. 1.
Schematic view of a neuron and adjacent extracellular space, illustrating 5 principally different ways in which a-syn can be targeted therapeutically, when trying to reduce neurodegeneration due to misfolded a-syn. These 5 strategies are 1) reduce a-syn production; 2) inhibit a-syn aggregation inside cells; 3) promote a-syn degradation inside cells; 4) promote a-syn degradation outside cells; 5) reduce a-syn uptake by neighbouring cells from the extracellular space following release from affected neurons.
2. Reducing alpha-synuclein production
Especially since a-syn gene duplication and triplication causes PD, a logical therapeutic approach is to reduce a-syn production. Aging is the number one risk factor for the development of PD and as humans age there is an accumulation of non-aggregated, i.e. soluble, a-syn that is associated with decreased dopamine levels in the striatum, but not death of substantia nigra neurons (Chu and Kordower, 2007; McCormack et al., 2012). Reducing the cytoplasmic levels of a-syn also mitigates the risk that the protein will oligomerize and adopt an abnormal conformation. Thus, decreasing a-syn production in PD at a time prior to its aggregation might prevent its aggregation and rescue the function and viability of neuronal populations that are vulnerable in PD.
One way to reduce production is with RNA interference (RNAi). Initial investigations tested RNA molecules targeting a-syn via RNAi in neuron-like cell cultures as well as in rodent models in vivo. In the latter, short hairpin (sh) a-syn RNA delivered via a lentiviral vector silenced ectopic expression of human a-syn in the rat striatum, and small interfering RNA (siRNA) directed against a-syn reduced the expression of endogenous a-syn after a two-week infusion into the mouse hippocampus (Lewis et al., 2008; Sapru et al., 2006). No signs of toxi-city were reported as a consequence of these treatments. As a follow up, the Michael J. Fox Foundation for Parkinson’s Research (MJFF) and Alnylam Pharmaceuticals sponsored studies in nonhuman primates in preparation for possible clinical trials. Di Monte and coworkers tested the ability of chronic siRNA infusions directed against a-syn to reduce asyn levels in squirrel monkeys (McCormack et al., 2010). Following a unilateral infusion, a-syn levels were reduced monkeys by 40–50% relative to the untreated side. This approach, however, became problematic as the sponsor for this program withdrew their support and this program has not been picked up by other funding sources. In a different project, Burton and coworkers reduced a-syn by 35% in rats receiving shRNA’s (Zharikov et al., 2015). They found no toxicity in rats and prevented the structural and functional changes seen in rotenone treated rats. The fact that a-syn can be presumed to normally have important functions in the CNS suggests that one must be cautious if designing a therapy aimed at reducing its levels dramatically. Indeed, in other experiments where extensive loss of endogenous a-syn was achieved, significant neurotoxicity was observed. Specifically, Manfredsson and coworkers reported that when using viral vectors to achieve marked (> 90%) reductions of a-syn in the substantia nigra of rats and nonhumans primates, the nigrostriatal system degenerates (Collier et al., 2016; Gorbatyuk et al., 2010; Kanaan and Manfredsson, 2012). Furthermore, unless targeted specifically to the brain, therapies that reduce a-syn levels in the CNS could have similar effects in peripheral tissues. Indeed, therapies administered systemically might even gain greater access to peripheral tissues, since they are not behind the blood brain barrier. Under normal conditions a-syn is present in e.g. peripheral nerves and red blood cells, and the consequences of reducing a-syn in these tissues are not well understood. Thus, if this approach of reducing a-syn production is to go forward to clinical trials, a safe degree of knockdown will have to be established pre-clinically.
In addition to targeting mRNA translation process, another approach to reduce protein expression could be to modify transcription of a-syn gene. A recent publication from Scherzer and colleagues (Mittal et al., 2017) conducted a high-throughput screen of an FDA drug library and natural products. They identified beta-2-adrenoreceptor agonism as a mechanism to reduce a-syn gene expression. For example, beta-2AR agonists (currently approved for asthma), such as clenbuterol, lowered a-syn expression by over 35%, in dose-dependent manner, in a neuroblastoma cell line and in rat cortical neurons. Furthermore, in neuronal precursors derived from iPSCs from a patient with a SNCA-triplication clenbuterol reduced a-syn levels, mitigated mitochondrial stress, and improved cell survival. Clenbuterol, which readily passes the blood-brain barrier, also reduced a-syn expression in substantia nigra of wild-type mice and protected TH-neurons from MPTP toxicity in mice. Mechanistically, beta-2AR ligands were shown to modulate transcription by histone 3 lysine 27 acetylation of its promoters and enhancers within the gene locus. These preclinical results were supported by findings in two epidemiological studies in Norway demonstrating that treatment with beta-2AR agonist salbutamol was associated with lower risk of PD and that, conversely, beta-2AR antagonist propanolol was associated with an increased PD risk (Mittal et al., 2017). This report suggests that beta-2AR agonists should be tested as potential disease-modifying agents in PD, and provides support for exploring and pursuing other druggable targets that may modify protein expression via transcription or translation processes.
3. Inhibiting alpha-synuclein aggregation
Clearly, if aggregation of a-syn can be prevented then its normal function can be sustained and the toxicity that follows misfolding can be prevented. Heat shock proteins (HSP), especially small HSPs are molecular chaperones that prevent protein aggregation. An interesting question is how aggregation-prone molecules, e.g. a-syn, evade the effects of small HSPs when they form pathological aggregates. A variety of HSPs have been effective in reducing a-syn aggregation in vitro and in vivo (Gorenberg and Chandra, 2017; Klucken et al., 2004; McLean et al., 2002). The use of small HSPs may be particularly useful for a-syn, because small HSPs are most effective when the formation of the aggregates is slow; as it appears to be in degenerative diseases. To date, however, there are no clinical trials utilising strategies to modulate levels of small HSPs in PD.
One approach to reducing the risk of a-syn aggregation inside cells that is gaining attention is the use of intrabodies which can bind a-syn monomers and prevent them from oligomerizing. Intrabodies are small antibody fragments, 140–250 amino acids in length, that target antigens intracellularly. The Fv variable regions responsible for antibody speci-ficity can be expressed separately from the full-length immunoglobulin, retaining many advantages of conventional antibodies, including high specificity and affnity for target epitopes. Intrabodies that can bind asyn come in many different forms and several different antibodies have shown promise (Bhatt et al., 2013). in vitro as molecules that specifically bind different forms (monomeric, oligomeric, fibrillar) of a-syn and target specific regions (Non-Amyloid Component, i.e. NAC; C-terminal region) of a-syn. The different binding sites of various intrabodies/nanobodies were recently reviewed (Bhatt et al., 2013). Emerging data suggest that intrabodies may be neuroprotective by attenuating/neutralizing/modulating aggregated a-syn, possibly by interfering with the aggregation prone region (NAC). For example, na-nobody VH14*PEST has target specificity for the NAC of α-syn which is known to be aggregation prone and nanobody NbSyn87 has target specificity for both the C-terminal of a-syn as well as fibrillar forms (De Genst et al., 2010). Viral vector-mediated gene delivery of VH14*PEST and NbSyn87 can protect against nigrostriatal degeneration in rats injected intranigrally with a viral vector overexpressing a-syn. Indeed, both of the two intrabodies completely eliminated aggregated a-syn, restored striatal dopamine, and improved motor function as assessed on the stepping test. These data suggest that intrabodies may be effective at lowering levels of soluble a-syn and thereby preventing a-syn aggregation. A challenge for the clinical application of intrabodies for therapeutic intervention is going to be how to achieve high levels in the CNS for prolonged periods. Unlike immunotherapies, intrabodies will require direct CNS delivery using viral vectors. New vectors that can deliver payloads throughout the CNS following systemic injection are being characterized (Chan et al., 2017) and could prove a powerful way to dispense intrabodies.
Two industry programs that also aim to inhibit a-syn aggregation are currently in clinical testing phase. First, Neuropore therapies is advancing their therapeutic small-molecule lead NPT200–11, in partnership with UCB Pharma. NPT200–11 has been reported to prevent the formation of toxic oligomeric aggregates and improve behavioral, neuropathological and biochemical outcome measures in a-syn over-expressing transgenic mouse model (Koike et al., 2014). Second, Pro-clara Bioscience’s clinical candidate NPT088 is a fusion protein combining human immunoglobulin backbone as well as a General Amyloid Interaction Motif (or GAIM) and is designed to simultaneously target multiple misfolded proteins. NPT088 has been reported to bind a-syn aggregates, reduce the accumulation of proteinase K-resistant protein and have a positive effect on tyrosine hydroxylase levels in an a-syn overexpressing transgenic mouse model (Krishnan et al., 2014).
As evidenced by the examples mentioned above, there are multiple approaches aiming to prevent a-syn aggregation, which can be considered to interfere at a very upstream point in the pathogenic cascade mediated by a-syn misfolding.
4. Promoting degradation of intracellular alpha-synuclein aggregates
Autophagy is believed to play a major role in degradation of intracellular a-syn aggregates (Decressac and Björklund, 2013; Decressac et al., 2013; Spencer et al., 2009b). The idea is that targeting enhancement of autophagic processes would lead to increased clearance of pathological a-syn. Rapamycin and its analogues, act via the mammalian target of rapamycin (mTOR) to increase macroautophagy function. This class of drugs has been shown in numerous studies to reduce a-syn aggregation and resulting toxicity in various over-expression-based cellular and animal models (Moors et al., 2017). However, lack of specificity (effects on other essential pathways) and side-effects (such as immunosuppression) have limited the potential use of such drugs for PD where long term treatment would be needed.
Given rapamycin’s therapeutic limitations, other autophagy enhancers have been tested in models of PD. For example, trehalose works through an mTOR-independent pathway (Sarkar et al., 2007). Treha-lose is a natural sugar molecule found in many organisms and has been reported to increase autophagy function by targeting lysosomal bio-genesis and resulting in an increased clearance of protein aggregates (Ghavami et al., 2014). While a recent study reported that trehalose did not protect cultured cortical neurons against toxicity in a model involving exposure to a-syn preformed fibrils (Redmann et al., 2017), in light of earlier studies and a good safety profile more work in animal models are needed to determine if trehalose still might be effcacious in other contexts relevant to PD.
Another strategy to achieve mTOR inhibition works by reducing pyruvate transport into mitochondria using a modulator of the mitochondrial pyruvate carrier (MPC) called MSDC-0160. This drug has immediate (within minutes) beneficial effects on mitochondrial oxygen consumption in stressed cells, e.g. midbrain neurons exposed to an inhibitor of the electron transport chain such as MPP+ (Ghosh et al., 2016). Following a delay of over 24 h, the changes in mitochondrial metabolism induced by MPC inhibition result in mTOR inhibition and upregulation of autophagy in neurons. As a result, MSDC-0160 protects midbrain neurons protects from MPP+ induced death (Ghosh et al., 2016). Furthermore, MSDC-0160 enhances autophagy and protects nigral dopamine neurons in a “chronic” genetic mouse model of PD (the Engrailed1 heterozygous knock out mouse). Perhaps most important in this context, inhibiting MPC with MSDC-0160 also enhances autophagy in a model of a-syn-induced toxicity in c elegans, and using genetic tools it has been possible to demonstrate that the rescue effect is mediated via mTOR inhibition. Taken together, the findings with MSDC-0160 strongly suggest that inhibition of MPC can be used as a strategy to reduce a-syn aggregation (Ghosh et al., 2016), but so far there are no published reports on this strategy in mammalian models of a-syn aggregation.
Recent studies in experimental and clinical PD which have gained media attention and generated debate in the scientific community regarding the underlying mechanisms of action have used a drug re-purposed drug from oncology. Nilotinib is approved for chronic myelogenous leukemia, a cancer of the white blood cells. It works by inhibiting protein target c-abl, which is an Abelson murine leukemia viral oncogene found in cells. c-Abl has been implicated in regulation of many physiological processes including cell growth, differentiation, proliferation, survival and phosphorylation of downstream proteins (Brahmachari et al., 2017; Lindholm et al., 2016). Mutations in c-abl produce an increase in kinase activity which in turn leads to increased cell proliferation. Thus, many specific c-abl inhibitors have been developed as effective anti-tumor agents. c-Abl inhibition has also been validated as a rational approach for therapeutic development in PD, based on both human and experimental target validation data (Lindholm et al., 2016). Several studies have showed that c-abl activity is enhanced in brain tissue of PD patients and that this increased c-abl activity leads to a downstream increase in phosphorylation and aggregation of a-syn. Increased c-abl activity also reduced function of Parkin, a key protein involved in mitochondrial biogenesis (Lonskaya et al., 2013). Additionally, pharmacological and genetic manipulations to reduce c-abl activity resulted in neuroprotective effects in animal models of PD. For example, nilotinib was shown to attenuate a-syn levels in A53T transgenic mice and protect substantia nigra dopamine neurons from toxicity in a with model viral-vector mediated a-syn overexpression (Hebron et al., 2013). Despite these positive results in PD, c-abl inhibitors have been limited in their therapeutic potential for CNS disorders because of pharmacokinetics (poor absorption across the blood brain barrier) and small therapeutic window for chronic usage such as what would be required in PD. A recent small, open-label study (lacking a placebo control) in patients suffering from PD and dementia or DLB reported that nilotinib was safe and tolerable at doses much lower than those used against cancer (Pagan et al., 2016). Results from future systematic, randomized, double-blind, placebo-controlled and adequately powered trials are needed to understand the safety implications of long term use, whether the drug gets into the brain in optimal concentrations, whether it modulates a-syn levels and whether it produces clinical effcacy (Wyse et al., 2016).
An exciting approach to promote a-syn degradation through autophagy-lysosomal pathway is to increase the expression, stability or delivery of the lysosomal enzyme β-glucocerebrosidase (GCase). Mutations in the GBA1 gene, which encodes for GCase, have been identified as causative for Gaucher disease, a rare lysosomal storage disorder and represent the most common genetic risk factor for PD (Sidransky et al., 2009). The proportion of PD patients that carry GBA1 mutations is estimated to be between 7 and 10%, and that these mutations confer a 20- to 30- fold increased risk of developing PD with penetrance estimated up to 30% at 80 years of age (Migdalska-Richards and Schapira, 2016). These mutations have been reported to reduce GCase activity in PD brain tissue (Murphy et al., 2014) and cerebrospinal fluid (Parnetti et al., 2017). Emerging experimental evidence in cell-free systems, cell lines, animal models and patient samples suggests a correlation between this decreased activity and accumulation of a-syn (for extensive review on GBA1 and synucleinopathy, see Sardi et al., 2015). These strong genetic data and the mechanistic link to asyn have made GCase an attractive target for PD drug development. Recently, Genzyme/Sanofi recently announced a phase 2 trial (named MOVES-PD) testing the effcacy of the compound GZ/SAR402671 in GBA1-mutation carriers. This drug works upstream of GCase in that it inhibits the production of glycosphingolipids (substrate for GCase) (Sanofi Genzyme Company Website, 2017). Another approach to target this pathway has been to use small-molecule chaperones that would drive correct folding of mutant GCase molecules in the ER, resulting in effcient transport to the lysosomes and increased GCase activity. Ambroxol, an FDA-approved mucolytic, acts as a chaperone and improves lysosomal function in cells with GBA mutations in vitro (McNeill et al., 2014) and increases GCase activity in non-human primates in vivo (Migdalska-Richards et al., 2017). There are currently two separate phase 2 trials ongoing that are testing safety, tolerability and effcacy of Ambroxol in PD (ClinicalTrials.gov Identifier: NCT02941822; NCT02914366). The feasibility and success of other modalities such as direct activators of GCase or gene-delivery would need to be tested in the near future to warrant further development.
5. Increase extracellular alpha-synuclein degradation
Active and passive immunotherapies are possibly among some of the most exciting therapeutic approaches for PD and in both cases, it is considered that the antibodies, which are unable to enter cells, target extracellular a-syn. Active immunization stimulates the immune system to produce antibodies against target proteins, while for passive immunization, antibodies are directly administered that confer temporary protection against the disease. In animal models, both passive and active immunotherapy reduce a-syn aggregation in transgenic mice overexpressing a-syn and prevent the associated behavioural deficits (George and Brundin, 2015). Immunotherapy has also been shown to induce a physiological microglial activation and reduce the production of pro-inflammatory cytokines, thus exerting an anti-inflammatory effect in neurodegenerative disorders. However, immunotherapy has the potential to trigger off-target responses and non-specific inflammatory reactions. Furthermore, the need for repetitive administration, lack of response due to senescence of the innate immune system (in the case of active immunisation therapy), and limited penetration of antibodies into the CNS all represent challenges (George and Brundin, 2015; Lindström et al., 2014). Finally, it is unclear how immunotherapies that are effcacious in animal models of PD will work in the specific form of inflammation and glial activation that is present in advanced PD patients (George and Brundin, 2015).
Despite of all these potential caveats aside, several immunotherapy programs targeting a-syn are currently in clinical trials and additional programs are probably on the verge of starting clinical trials. We only highlight three that have presented results in the public domain. The biotech company Affris recently completed a Phase I clinical trial with an active immunotherapy vaccine called AFFITOPE PD03A. AFFITOPE® PD03A is a synthetically produced vaccine containing an a-syn-mimicking peptide formulated with adjuvant. The trial is a randomized, placebo-controlled, parallel group, patient-blinded, bi-center study, assessing tolerability and safety of repeated subcutaneous administration of two doses of AFFITOPE® PD03A to patients with early PD. In study AFFiRiS011, 36 patients were randomized to either AFFITOPE® PD03A high dose or low dose or to the placebo group treated with the adjuvant (Affris Company Website, 2017). Both doses were well tolerated and no drug-related serious adverse events or reactions occurred. The majority of adverse events were mild and local reactions. It was also reported that AFFITOPE® PD03A exhibited a dose-dependent immune response against the peptide itself and crossreactivity against asyn targeted epitope over time, and that the antibody titer went up (Affris Company Website, 2017).
The Prothena a-syn passive immunotherapy approach uses a humanized monoclonal antibody against a-syn (PRX002). It has been tested in Phase 1a and 1b clinical trials. In the Phase 1a trial, free serum a-syn levels were reduced up to 96.5% (Schenk et al., 2017). In the Phase 1b trial, performed in PD patients, the reduction of free serum asyn was replicated (Prothena Company Website, 2017a). Patients were randomized into six escalating dose cohorts to receive PRX002 or placebo. In this six-month study, patients received three monthly doses (intravenous infusion once every 28 days) of PRX002 or placebo and were followed for an additional observational period of three months. No serious adverse events or dose-limiting toxicities were observed, and PRX002 demonstrated acceptable pharmacokinetics and penetration into the CNS (i.e. a dose-dependent increase in PRX002 levels in cerebrospinal fluid, CSF). These results have enabled Prothena to move forward to a Phase 2 trial in patients with early PD (Prothena Company Website, 2017b).
Biogen is also investigating passive immunotherapy using the antibody BIIB-054 which is directed against a-syn. As presented at an international meeting (Brys et al., 2017), they report that this antibody was well tolerated in healthy volunteers, up to the highest tested dose, had a favorable pharmacokinetic profile, and was detectable within the CSF in a range expected for monoclonal antibodies. These data provided support for the continuation of the Biogen immunotherapy program directed for a-syn (Alzforum, 2017).
Additional clinical immunotherapy programs targeting a-syn in PD are on the horizon. In 2016, BioArctic Neuroscience announced that they would work with AbbVie to commercialize their portfolio of antibodies against a-syn (BioArctic Company Website, 2016). BAN0805 is their clinical-phase antibody which is a development of antibodies that target oligomeric forms of a-syn that are thought to be pathogenic (Fagerqvist et al., 2013). In 2017, AstraZeneca and Takeda Pharmaceutical Company Limited declared that they have entered an agreement to jointly develop the a-syn antibody MEDI1341 for PD (AstraZeneca Company Website, 2017). In the press material they state that MED11341 has high affnity for the target and reduced effector function, meaning a lower interaction with the immune system, and therefore has the potential to more effcacious and safe.
Taken together, there are several immunotherapy programs aimed at degrading extracellular a-syn already in early stage clinical trials, with additional programs about to enter the clinical arena. In addition to these advanced immunotherapy programs, other approaches to reducing extracellular a-syn have been tested in experimental animals, but are further from clinical application. For example, overexpressing the serine protease Kalekrein 6 (also known as KLK6 or neurosin) has been suggested to degrade extracellular a-syn in a models over-expressing a-syn (Spencer et al., 2009a; Pampalakis et al., 2017) by triggering a proteolytic cascade involving unidentified metallopro-teases. While this latter concept is currently not close to a clinical application, it illustrates that novel therapeutic approaches to reducing extracellular a-syn might emerge in the future.
6. Reducing uptake of extracellular alpha-synuclein
Until recently, there was little information available regarding the molecules involved with the secretion/expulsion of a-syn from neurons or glial cells into the extracellular space where it can then be taken up by other cells, cause permissive templating, and propagate pathology to distant neural circuits. One pioneering study suggested that amyloid fibrils, including those composed of a-syn, bind to heparan sulfate proteoglycans on the cell surface and are then taken up by endocytosis (Holmes et al., 2013). Therefore limiting endocytosis of extracellular asyn might be a viable strategy to slow propagation of Lewy pathology. Although it is fully defined how a-syn that has undergone endocytosis can escape the endosome and access the cytoplasm in order to seed further aggregation as part of the pathology propagation cycle, it has been suggested that a-syn penetrates the membrane of endosomes, in manner akin to certain viral peptides (Freeman et al., 2013). In their initial study, Diamond and collaborators showed that addition of heparin or chloral hydrate, which both interfere with heparan sulfate proteoglycans, reduced the endocytic uptake of a-syn in vitro (Holmes et al., 2013). In remains to be seen whether more specific inhibitors of heparan sulfate proteoglycans - that do not interfere with vital cellular processes - can be developed and used to slow propagation of a-syn pathology.
Another strategy has been to search for a potential receptor that is required for uptake of oligomeric or fibrillar a-syn. Through a comprehensive series of experiments, the Dawson team recently demonstrated that a-syn preformed fibrils, but not the a-syn monomer, binds to lymphocyte-activation-gene 3 (LAG3) protein on the cell surface with high affnity (Mao et al., 2016). They described that binding initiated the endocytosis of pathological a-syn into neurons, its transport and structural and functional toxicity. Antibodies to LAG3 blocked these effects indicating that that decreasing or eliminating LAG3 binding site might be a therapeutic target to prevent a-syn propagation (Mao et al., 2016). These are still early days for this biology and it is not clear how important quantitatively LAG3-mediated uptake of pathogenic a-syn species is in disease. There are likely other proteins involved in a-syn uptake (Shrivastava et al., 2015), even some of which remain to be discovered and characterised, but this recent paper opened an avenue for investigation into a new way of inhibiting propagation of a-syn pathology.
7. Challenges facing clinical trials with alpha-synuclein therapies
As of today, at least five a-syn targeting programs (all mentioned above) have now entered phase 1 or phase 2 clinical testing (with many more expected over the next few years). The prevailing a-syn hypothesis for PD is analogous to the amyloid hypothesis of Alzheimer’s disease in that toxic gain-of-function results in protein aggregation and neurotoxicity. Thus, the recent AD clinical trials testing this hypothesis can be informative for PD. Unfortunately, so far the large AD trials have failed to show meaningful effcacy due to various challenges including lack of target engagement, sub-optimal patient selection/stratification and lack of sensitivity in clinical outcome measures (Vellas et al., 2013). Lack of objective biomarkers that track with the progression of the disease have led to longer, larger and costlier trials, and ultimately leading to inconclusive testing of the hypothesis and uninformed results. In the following six sections, we discuss different challenges that face clinical trials with therapies that target a-syn.
7.1. What is the molecular species of alpha-synuclein that is the best target?
Unfortunately, it remains unclear what molecular species of a-syn is the best target and which is the “toxic” species for this protein. The current dogma (for review, see Ingelsson, 2016)) is that a-syn is normally a natively unfolded, monomeric protein and that specific events such as toxin exposure, gene mutations, cellular stress and post-translational modifications drives a conformational shift to the larger, mis-folded multimeric states. More specifically, unfolded monomers can aggregate first into smaller and soluble oligomeric species followed by β-sheet-like larger oligomers/protofibrils and finally into higher molecular weight insoluble a-syn fibrils found to aggregate into Lewy bodies (Wang et al., 2016). However, due to the nature of a-syn to be an intrinsically disordered protein with extreme conformational diversity, it has been diffcult to associate a particular structural species to neuronal toxicity. For example, large number of studies have investigated effects of various oligomeric and fibrillar species in non-cellular, cellular and animal models (For review, see Cookson, 2009). While most of these studies have shown that recombinant oligomers (as characterized by molecular weight) can be toxic, it is not clear whether how many monomeric molecules constitute that “toxic” oligomer (a 26-mer, a 113-mer or a 212-mer molecule, etc.), whether toxicity results from a particular post-translational modification or truncated species, or whether aggregation is even necessary for toxicity. The key challenge for the field currently is to first identify the most prevalent species in human disease samples and then work back to isolate and overexpress that species in different models of the disease to assess its toxicity. The MJFF is addressing this challenge by bringing together a consortium of key opinion leaders and researchers and funding a large discovery initiative focused on using different technologies/methodologies (mass-spectrometry, sequential extraction through size-exclusion chromatography, etc.) to identify, replicate and validate specific molecular conformations of a-syn in human CSF and brain samples. Identification of such specific a-syn conformations would allow for more specific targeting of the protein and development of biomarker assays for measuring target engagement and pharmacodynamic response.
7.2. Pathogenic alpha-synuclein conformers might differ between patients
A confounding challenge in trying to identify a “toxic” species in PD pathology is the possibility that the type (strain) and amount of multimeric species might vary from patient to patient (Melki, 2015; Peng et al., 2017). The hypothesis is that a-syn may adopt different conformations depending on its environment. A seminal paper in support of this hypothesis from Dr. Melki and colleagues (Bousset et al., 2013), demonstrated that two different a-syn fibril strains (with distinct morphologies) produced different higher-order oligomeric structures, levels of toxicity, prion-amplification, cell-to-cell propagation and pathology. This was further supported by studies where extracts directly obtained from either human PD or MSA brains were injected into animals and these precipitated PD-like (Recasens et al., 2014) or MSA-like pathology (Watts et al., 2013), respectively. These different strains could be generated due to differential post-translational modifications (Ma et al., 2016) or exposure to exogenous pathogens (Kim et al., 2016a, b), have the ability to cross the blood-brain barrier and distribute to the CNS (Peelaerts et al., 2015), and produce differential seeding and cross-seeding with other aggregation-prone proteins in the brain (Guo et al., 2013).
7.3. The need for alpha-synuclein biomarkers
A key challenge for any a-syn clinical trial is going to be lack of imaging, bio-fluid or non-invasive a-syn related biomarkers that can used for patient selection/stratification, assessment of target engagement, testing proof-of-mechanism, and determination of effcacy (proof-of-concept). Since a-syn pathology is a pathological hallmark of PD and that the load and distribution of Lewy pathology can only be assessed at autopsy, the ability to image a-syn pathology in the brain would be a game-changing achievement for the PD field. This is considered a research priority and significant resources have been deployed to develop a positron emission tomography (PET) tracer for asyn (for review, see Eberling et al., 2013). As discussed at length in the review, significant challenges exist in developing such a tracer including the ability of ligands to cross the blood-brain barrier and the need for these ligands to possess high affnity and demonstrate selectivity for the aggregate form of a-syn.
In the absence of being able to directly visualize aggregate load in the brain, bio-fluid assays have been developed to measure in more easily accessible fluids such as CSF, blood and saliva. For example, immunoassays exist which measure total levels of a-syn and studies in large cohorts have showed that total a-syn in CSF is significantly (20–25%) lower in de novo PD patients compared to healthy controls (Kang et al., 2016; Mollenhauer et al., 2013). A user’s guide was recently published providing guidelines and recommendations for considering pre-analytical variables and analytical confounders for biomarker studies measuring total a-syn (Mollenhauer et al., 2017). Assays to measure the putative pathological forms such as phosphorylated (pS129) and oligomeric a-syn species have also been reported recently (Majbour et al., 2016; Stewart et al., 2015), however, such assays need to be replicated in independent laboratories with blinded samples from larger cohorts.
In addition to immunoassays, other novel assays to measure a-syn in biofluids are either being generated or undergoing validation. These include mass spectrometry methods to measure various post-translationally modified or truncated a-syn species (Schmid et al., 2013) and “prion-type” amplification assays to detect small levels of oligomeric a-syn and its ability to seed and nucleate further aggregation (Fairfoul et al., 2016; Shahnawaz et al., 2017). Finally, efforts are also underway to further validate (or invalidate) reports of synucleinopathy observed in peripheral biopsies from submandibular gland, skin and colon tissues (Lee et al., 2017, Visanji et al., 2017).
7.4. Can short term symptomatic benefit be expected with therapies targeting alpha-synuclein?
There is substantial evidence that indicates that a-syn accumulation and aggregation causes a phenotypic down-regulation in dopaminergic markers. In PD, ageing and animal models of PD, nigral cells with a-syn inclusions exhibit reductions in tyrosine hydroxylase and/or Nurr1; a transcription factor involved dopamine synthesis (Chu and Kordower, 2007; Decressac et al., 2012). One might suspect, that if a-syn accumulation reduces nigrostriatal tone (Lundblad et al., 2012), then reduction in a-syn through therapeutic interventions could increase dopaminergic tone. Thus, in addition to potentially slowing disease progression, approach that reduce a-syn levels have the potential to induce acute symptomatic benefit by enhancing nigrostriatal function.
7.5. The challenge of monitoring effects in a protracted disease
A major challenge with targeting a-syn therapeutically is that, most likely, changes in disease progression are going to have to be the primary outcome. Considering that PD progresses relatively slowly and that there is great variation in the rate of progression between patients (Espay et al., 2016, 2017) this poses great challenges when it comes to the design and financing of trials that target a-syn. The duration of the trial will have to be long (one year or more) or the patient cohorts will need to be so large that cost can become prohibitive. The trials will also need to demonstrate effcacy in settings where other anti-parkinsonian, symptomatic treatments can constitute significant confounding factors, and consequently novel and innovative study designs are currently being considered (Thibault et al., 2017). These are issues that are not exclusive to therapies that target a-syn, but they also apply to other novel therapies aiming to achieve disease-modification in PD.
7.6. The possibility of targeting prodromal Parkinson’s disease
The realisation that the underlying disease process in PD begins several years, or even one to two decades, before the development of motor symptoms has opened up for ideas about starting therapies even before the formal diagnosis of PD, i.e. during the phase that has recently been defined as prodromal PD (Mahlknecht et al., 2015; Postuma et al., 2012; Postuma and Berg, 2016). During this phase, one or more of several non-motor symptoms can appear, such as hyposmia, constipation, sleep disorder and depression. It has been suggested that some of the very first events in PD are a-syn misfolding in enteric nerves and the olfactory bulb, which could explain why constipation and hyposmia are prevalent in the prodromal phase (Rey et al., 2016b; Stokholm et al., 2016). Therefore, targeting a-syn, possibly even locally at these anatomical sites which both are relatively easy to access, during prodromal PD might slow the disease process and even prevent the occurrence of motor symptoms during the lifetime of a patient. Furthermore, targeting a-syn pathology in the enteric nervous system and olfactory system means that it might be possible to use outcome measures related to gastrointestinal function and olfaction. A recent study in mice reported that it is possible to mimic the progressive development a-syn pathology in the olfactory system, concomitant with the emergence of olfactory deficits, suggesting that this novel model could be used to evaluate novel a-syn therapies aimed at slowing progression in the prodromal phase (Rey et al., 2016a).
8. Concluding remarks
We hope we have presented a convincing argument that a-syn is the most relevant therapeutic target in PD and related diseases where a-syn aggregation is a pivotal part of the pathogenesis. In this regard, evidence from genetics, neuropathology, and preclinical experimental models paints a compelling picture suggesting that a-syn misfolding is a key player in disease pathogenesis. Based upon this concept, there is increased interest in a-syn targeted therapy. The realisation that a-syn is present in the extracellular space, as aggregates likely propagate and template from cell-to-cell, is important because it makes the putative target more accessible to systemically administered agents; although there is also interest in developing intracellular antibody (intrabody) approaches. The emergence of a wide range of diverse strategies, ranging from immunotherapy to small molecules, is encouraging because alone or in combination they all show promise. Indications that a-syn misfolding occurs very early in the disease process support the concept that targeting a-syn in the disease prodrome, before extensive neuro-degeneration has occurred, might be effective. This will require the development of effective and sensitive biomarkers that allow diagnosis with a high degree of accuracy, even in the absence of motor symptoms. It will also be important to develop biomarkers that reflect target engagement of any novel therapy with a-syn. In that regard, an imaging marker that can be used to detect misfolded a-syn in brain tissue would be ideal because it would allow for non-invasive monitoring of the effectiveness of novel treatments. The fact that misfolded a-syn is also present in peripheral nerves and organs is important to consider because it opens up for the possibility to track therapeutic effcacy in readily accessible tissue samples.
While we believe that there is reason for optimism regarding a-syn as a therapeutic target, several fundamental challenges still remain. Lessons learnt from Alzheimer’s disease clinical trials (Vellas et al., 2013) suggest that it likely will be both costly and take a long time to develop a new therapy that slows PD progression by targeting a-syn. Admittedly there is even still an incomplete understanding of the precise role of a-syn in disease development. For example, it is unclear which conformation of a-syn should be targeted. The existence of multiple fibrillar conformations of a-syn further complicates the picture. There is some concern that an excessive therapy-induced down-regulation of the levels of a-syn monomer could be detrimental and could itself cause toxicity. Assuming that the ongoing therapy programs targeting a-syn provide a proof-of-principle that it is a valid target, future research into basic a-syn pathobiology will undoubtedly provide new insights that can guide the development of refined therapeutic strategies.
Acknowledgements
PB reports relevant grants from National Institutes of Health (R01DC016519-01 and 5R21NS093993-02), Department of Defense (W81XWH-17-1-0534), The Michael J. Fox Foundation for Parkinson’s Research and Cure Parkinson’s Trust. JHK is supported in part by grants from the Parkinson’s disease Foundation and National Institutes of Health (NS070577 and R01NS094460–01). We would also like to acknowledge Maggie McGuire Kuhl from The Michael J. Fox Foundation and her team for their help with the figure showing different modes of targeting a-syn.
Abbreviations:
- a-syn
Alpha-synuclein
- PD
Parkinson’s disease
- DLB
Dementia with Lewy bodies
- MSA
Multiple system atrophy
- CNS
central nervous system
- MJFF
Michael J Fox Foundation for Parkinson’s Research
- HSP
Heat shock proteins
- mTOR
mammalian target of rapamycin
- NAC
Non-Amyloid Component
- CSF
cerebrospinal fluid
- GAIM
General Amyloid Interaction Motif
- MPC
mitochondrial pyruvate carrier
- LAG3
lymphocyte-activation-gene 3
- PET
positron emission tomography
Footnotes
Conflicts of interest
PB has received commercial support as a consultant from Renovo Neural, Inc., Roche, Teva, Lundbeck, AbbVie, NeuroDerm, Cellular Dynamics International Inc and Axial Biotherapeutics. Additionally, he has received commercial support for grants/research from Renovo, Teva and Lundbeck. PB has ownership interests in Acousort AB and Parkcell AB. JHK has received commercial support as a consultant and/or a research grant from Cellular Dynamics International Inc.; BrainEver, NeuroDerm, NsGene, and AbbVie.
References
- Affris Company Website, 2017. AFFiRiS Announces Top Line Results of First-in-Human Clinical Study Using AFFITOPE® PD03A, confirming immunogenicity and safety profile in Parkinson’s disease patients www.affris.comnewsaffris-announces-top-line-results-of-first-in-human-clinical-study-using-afftope.
- Alzforum, 2017. α-Synuclein antibodies enter phase 2, sans biomarker http://www.alzforum.org/news/conference-coverage/synuclein-antibodies-enter-phase-2-sansbiomarker.
- Barbour R, Kling K, Anderson JP, Banducci K, Cole T, Diep L, Fox M, Goldstein JM, Soriano F, Seubert P, Chilcote TJ, 2008. Red blood cells are the major source of alpha-synuclein in blood. Neurodegener Dis 5, 55–59. 10.1159/000112832. [DOI] [PubMed] [Google Scholar]
- Bartels T, Choi JG, Selkoe DJ, 2011. α-Synuclein occurs physiologically as a helically folded tetramer that resists aggregation. Nature 477, 107–110. 10.1038/nature10324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beach TG, White CL, Hladik CL, Sabbagh MN, Connor DJ, Shill HA, Sue LI, Sasse J, Bachalakuri J, Henry-Watson J, Akiyama H, Adler CH, Arizona Parkinson’s Disease Consortium, 2009. Olfactory bulb alpha-synucleinopathy has high specificity and sensitivity for Lewy body disorders. Acta Neuropathol 117, 169–174. 10.1007/s00401-008-0450-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bendor JT, Logan TP, Edwards RH, 2013. The function of α-synuclein. Neuron 79, 1044–1066. 10.1016/j.neuron.2013.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Béraud D, Hathaway HA, Trecki J, Chasovskikh S, Johnson DA, Johnson JA, Federoff HJ, Shimoji M, Mhyre TR, Maguire-Zeiss KA, 2013. Microglial Activation and Antioxidant Responses Induced by the Parkinson’s Disease Protein α-synuclein. J Neuroimmune Pharmacol 8, 94–117. 10.1007/s11481-012-9401-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhatt MA, Messer A, Kordower JH, 2013. Can intrabodies serve as neuroprotective therapies for Parkinson’s disease? Beginning thoughts. J Parkinsons Dis 3, 581–591. 10.3233/JPD-130252. [DOI] [PubMed] [Google Scholar]
- BioArctic Company Website, 2016. BioArctic enters into collaboration with AbbVie for Parkinson’s disease research https://www.bioarctic.se/en/bioarctic-enters-into-collaboration-with-abbvie-for-parkinsons-disease-research-2815/.
- Bousset L, Pieri L, Ruiz-Arlandis G, Gath J, Jensen PH, Habenstein B, Madiona K, Olieric V, Böckmann A, Meier BH, Melki R, 2013. Structural and functional characterization of two alpha-synuclein strains. Nature Communications 4, 2575 10.1038/ncomms3575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braak H, Del Tredici K, 2017. Neuropathological Staging of Brain Pathology in Sporadic Parkinson’s disease: Separating the Wheat from the Chaff. J Parkinsons Dis 7, S71–S85. 10.3233/JPD-179001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brahmachari S, Karuppagounder SS, Ge P, Lee S, Dawson VL, Dawson TM, Ko HS, 2017. c-Abl and Parkinson’s disease: mechanisms and therapeutic potential. J Parkinsons Dis [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brundin P, Ma J, Kordower JH, 2016. How strong is the evidence that Parkinson’s disease is a prion disorder? Curr. Opin. Neurol 29, 459–466. 10.1097/WCO.0000000000000349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brys M, Hung S, Fanning L, Penner N, Yang M, David E, Fox T, Makh S, Graham D, Cedarbaum JM, 2017. Randomized, double-blind, placebo-controlled, single ascending dose study of anti-alpha-synuclein antibody BIIB054 in healthy volunteers
- Burré J, 2015. The Synaptic Function of α-synuclein. J Parkinsons Dis 5, 699–713. 10.3233/JPD-150642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burré J, Sharma M, Südhof TC, 2017. Cell Biology and Pathophysiology of α-synuclein. Cold Spring Harb Perspect Med a024091–29. 10.1101/cshperspect.a024091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan KY, Jang MJ, Yoo BB, Greenbaum A, Ravi N, Wu W-L, Sánchez-Guardado L, Lois C, Mazmanian SK, Deverman BE, Gradinaru V, 2017. Engineered AAVs for effcient noninvasive gene delivery to the central and peripheral nervous systems. Nat Neurosci 20, 1172–1179. 10.1038/nn.4593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu Y, Kordower JH, 2007. Age-associated increases of alpha-synuclein in monkeys and humans are associated with nigrostriatal dopamine depletion: Is this the target for Parkinson’s disease? Neurobiol Dis 25, 134–149. 10.1016/j.nbd.2006.08.021. [DOI] [PubMed] [Google Scholar]
- ClinicalTrials.gov Identifier: NCT02914366. Ambroxol as a treatment for Parkinson’s disease dementia https://clinicaltrials.gov/ct2/show/NCT02914366?cond=Ambroxol&draw=1&rank=3
- ClinicalTrials.gov Identifier: NCT02941822. Ambroxol in Disease Modification in Parkinson Disease (AiM-PD) https://clinicaltrials.gov/ct2/show/NCT02941822?cond=Ambroxol&draw=1&rank=6
- Coetzee SG, Pierce S, Brundin P, Brundin L, Hazelett DJ, Coetzee GA, 2016. Enrichment of risk SNPs in regulatory regions implicate diverse tissues in Parkinson’s disease etiology. Sci. Rep 6 srep30509 10.1038/srep30509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collier TJ, Redmond DE, Steece-Collier K, Lipton JW, Manfredsson FP, 2016. Is alpha-synuclein loss-of-function a contributor to Parkinsonian pathology? Evidence from non-human primates. Front Neurosci 10 239–7. 10.3389/fnins.2016.00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cookson MR, 2009. alpha-Synuclein and neuronal cell death. Mol. Neurodegener 4, 9 10.1186/1750-1326-4-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Genst EJ, Guilliams T, Wellens J, O’Day EM, Waudby CA, Meehan S, Dumoulin M, Hsu S-TD, Cremades N, Verschueren KHG, Pardon E, Wyns L, Steyaert J, Christodoulou J, Dobson CM, 2010. Structure and properties of a complex of α-synuclein and a single-domain camelid antibody. J Mol Biol 402, 326–343. 10.1016/j.jmb.2010.07.001. [DOI] [PubMed] [Google Scholar]
- Decressac M, Björklund A, 2013. TFEB: Pathogenic role and therapeutic target in Parkinson disease. Autophagy 9, 1244–1246. 10.4161/auto.25044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decressac M, Kadkhodaei B, Mattsson B, Laguna A, Perlmann T, Björklund A, 2012. α-Synuclein-Induced Down-Regulation of Nurr1 Disrupts GDNF Signaling in Nigral Dopamine Neurons. Sci. Transl. Med 4 163ra156 10.1126/scitranslmed.3004676. [DOI] [PubMed] [Google Scholar]
- Decressac M, Mattsson B, Weikop P, Lundblad M, Jakobsson J, Björklund A, 2013. TFEB-mediated autophagy rescues midbrain dopamine neurons from α-synuclein toxicity. Proc Natl Acad Sci USA 110, E1817–26. 10.1073/pnas.1305623110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dehay B, Bourdenx M, Gorry P, Przedborski S, Vila M, Hunot S, Singleton A, Olanow CW, Merchant KM, Bezard E, Petsko GA, Meissner WG, 2015. Targeting α-synuclein for treatment of Parkinson’s disease: mechanistic and therapeutic considerations. Lancet Neurol 14, 855–866. 10.1016/S1474-4422(15)00006-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Tredici K, Braak H, 2016. Review: Sporadic Parkinson’s disease: development and distribution of α-synuclein pathology. Neuropathol Appl Neurobiol 42, 33–50. 10.1111/nan.12298. [DOI] [PubMed] [Google Scholar]
- Dufty BM, Warner LR, Hou ST, Jiang SX, Gómez-Isla T, Leenhouts KM, Oxford JT, Feany MB, Masliah E, Rohn TT, 2007. Calpain-cleavage of alpha-synuclein: connecting proteolytic processing to disease-linked aggregation. Am J Pathol 170, 1725–1738. 10.2353/ajpath.2007.061232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eberling JL, Dave KD, Frasier MA, 2013. α-Synuclein imaging: a critical need for Parkinson’s disease research. J Parkinsons Dis 3, 565–567. 10.3233/JPD-130247. [DOI] [PubMed] [Google Scholar]
- Espay AJ, Brundin P, Lang AE, 2016. Precision medicine for disease modification in Parkinson disease. Nat. Rev. Neurol [DOI] [PubMed] [Google Scholar]
- Espay AJ, Schwarzschild MA, Tanner CM, Fernandez HH, Simon DK, Leverenz JB, Merola A, Chen-Plotkin A, Brundin P, Kauffman MA, Erro R, Kieburtz K, Woo D, Macklin EA, Standaert DG, Lang AE, 2017. Biomarker-driven pheno-typing in Parkinson’s disease: A translational missing link in disease-modifying clinical trials. Mov Disord 32, 319–324. 10.1002/mds.26913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagerqvist T, Lindström V, Nordström E, Lord A, Tucker SME, Su X, Sahlin C, Kasrayan A, Andersson J, Welander H, Näsström T, Holmquist M, Schell H, Kahle PJ, Kalimo H, Möller C, Gellerfors P, Lannfelt L, Bergström J, Ingelsson M, 2013. Monoclonal antibodies selective for α-synuclein oligomers/protofibrils recognize brain pathology in Lewy body disorders and α-synuclein transgenic mice with the disease-causing A30P mutation. J Neurochem 126, 131–144. 10.1111/jnc.12175. [DOI] [PubMed] [Google Scholar]
- Fairfoul G, McGuire LI, Pal S, Ironside JW, Neumann J, Christie S, Joachim C, Esiri M, Evetts SG, Rolinski M, Baig F, Ruffmann C, Wade-Martins R, Hu MTM, Parkkinen L, Green AJE, 2016. Alpha-synuclein RT-QuIC in the CSF of patients with alpha-synucleinopathies. Ann Clin Transl Neurol 1–7. 10.1002/acn3.338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fauvet B, Mbefo MK, Fares M-B, Desobry C, Michael S, Ardah MT, Tsika E, Coune P, Prudent M, Lion N, Eliezer D, Moore DJ, Schneider B, Aebischer P, El-Agnaf OM, Masliah E, Lashuel HA, 2012. α-Synuclein in central nervous system and from erythrocytes, mammalian cells, and Escherichia coli exists predominantly as disordered monomer. J Biol Chem 287, 15345–15364. 10.1074/jbc.M111.318949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Febbraro F, Sahin G, Farran A, Soares S, Jensen PH, Kirik D, Romero-Ramos M, 2013. Ser129D mutant alpha-synuclein induces earlier motor dysfunction while S129A results in distinctive pathology in a rat model of Parkinson’s disease. Neurobiol Dis 56, 47–58. [DOI] [PubMed] [Google Scholar]
- Freeman D, Cedillos R, Choyke S, Lukic Z, McGuire K, Marvin S, Burrage AM, Sudholt S, Rana A, O’Connor C, Wiethoff CM, Campbell EM, 2013. Alpha-synuclein induces lysosomal rupture and cathepsin dependent reactive oxygen species following endocytosis. PLoS ONE 8, e62143 10.1371/journal.pone.0062143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao H-M, Kotzbauer PT, Uryu K, Leight S, Trojanowski JQ, Lee VM-Y, 2008. Neuroinflammation and oxidation/nitration of alpha-synuclein linked to dopaminergic neurodegeneration. J Neurosci 28, 7687–7698. 10.1523/JNEUROSCI.0143-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George S, Brundin P, 2015. Immunotherapy in Parkinson’s Disease: Micromanaging Alpha-synuclein Aggregation. J Parkinsons Dis 5, 413–424. 10.3233/JPD-150630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George S, Brundin P, 2017. Solving the conundrum of insoluble protein aggregates. Lancet Neurol 16, 258–259. 10.1016/S1474-4422(17)30045-5. [DOI] [PubMed] [Google Scholar]
- Ghavami S, Shojaei S, Yeganeh B, Ande SR, Jangamreddy JR, Mehrpour M, Christoffersson J, Chaabane W, Moghadam AR, Kashani HH, Hashemi M, Owji AA, Łos MJ, 2014. Autophagy and apoptosis dysfunction in neurodegenerative disorders. Prog. Neurobiol 112, 24–49. 10.1016/j.pneurobio.2013.10.004. [DOI] [PubMed] [Google Scholar]
- Ghosh A, Tyson T, George S, Hildebrandt EN, Steiner JA, Madaj Z, Schulz E, Machiela E, McDonald WG, Escobar Galvis ML, Kordower JH, Van Raamsdonk JM, Colca JR, Brundin P, 2016. Mitochondrial pyruvate carrier regulates autophagy, inflammation, and neurodegeneration in experimental models of Parkinson’s disease. Science Translational Medicine 8, 368ra174–368ra174. doi: 10.1126/scitranslmed.aag2210 [DOI] [PubMed] [Google Scholar]
- Goedert M, Masuda-Suzukake M, Falcon B, 2017. Like prions: the propagation of aggregated tau and α-synuclein in neurodegeneration. Brain 140, 266–278. 10.1093/brain/aww230. [DOI] [PubMed] [Google Scholar]
- Gorbatyuk OS, Li S, Nash K, Gorbatyuk M, Lewin AS, Sullivan LF, Mandel RJ, Chen W, Meyers C, Manfredsson FP, Muzyczka N, 2010. In Vivo RNAi-Mediated α-synuclein Silencing Induces Nigrostriatal Degeneration. Mol Ther 18, 1450–1457. 10.1038/mt.2010.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorenberg EL, Chandra SS, 2017. The Role of Co-chaperones in Synaptic proteostasis and Neurodegenerative Disease. Front Neurosci 11, 248 10.3389/fnins.2017.00248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo JL, Lee VM-Y, 2014. Cell-to-cell transmission of pathogenic proteins in neuro-degenerative diseases. Nature Medicine 20, 130–138. 10.1038/nm.3457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo JL, Covell DJ, Daniels JP, Iba M, Stieber A, Zhang B, Riddle DM, Kwong LK, Xu Y, Trojanowski JQ, Lee VM-Y, 2013. Distinct α-synuclein Strains Differentially Promote Tau Inclusions in Neurons. Cell 154, 103–117. 10.1016/j.cell.2013.05.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halliday GM, Holton JL, Revesz T, Dickson DW, 2011. Neuropathology underlying clinical variability in patients with synucleinopathies. Acta Neuropathol 122, 187–204. 10.1007/s00401-011-0852-9. [DOI] [PubMed] [Google Scholar]
- Hebron ML, Lonskaya I, Moussa CEH, 2013. Nilotinib reverses loss of dopamine neurons and improves motor behavior via autophagic degradation of -synuclein in Parkinson’s disease models. Hum Mol Genet 22, 3315–3328. 10.1093/hmg/ddt192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes BB, Devos SL, Kfoury N, Li M, Jacks R, Yanamandra K, Ouidja MO, Brodsky FM, Marasa J, Bagchi DP, Kotzbauer PT, Miller TM, Papy-Garcia D, Diamond MI, 2013. Heparan sulfate proteoglycans mediate internalization and propagation of specific proteopathic seeds. Proc Natl Acad Sci USA 110, E3138–47. 10.1073/pnas.1301440110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iacono D, Geraci-Erck M, Rabin ML, Adler CH, Serrano G, Beach TG, Kurlan R, 2015. Parkinson disease and incidental Lewy body disease: Just a question of time? Neurology 85, 1670–1679. 10.1212/WNL.0000000000002102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingelsson M, 2016. Alpha-synuclein Oligomers-Neurotoxic Molecules in Parkinson’s Disease and Other Lewy Body Disorders. Front Neurosci 10, 408 10.3389/fnins.2016.00408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin S-G, Zhang Z-M, Dunwell TL, Harter MR, Wu X, Johnson J, Li Z, Liu J, Szabó PE, Lu Q, Xu G-L, Song J, Pfeifer GP, 2016. Tet3 Reads 5-carboxylcytosine through Its CXXC Domain and Is a Potential guardian against Neurodegeneration. Cell Reports 14, 493–505. 10.1016/j.celrep.2015.12.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanaan NM, Manfredsson FP, 2012. Loss of functional alpha-synuclein: a toxic event in Parkinson’s disease? J Parkinsons Dis 2, 249–267. 10.3233/JPD-012138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang J-H, Mollenhauer B, Coffey CS, Toledo JB, Weintraub D, Galasko DR, Irwin DJ, Van Deerlin V, Chen-Plotkin AS, Caspell-Garcia C, Waligórska T, Taylor P, Shah N, Pan S, Zero P, Frasier M, Marek K, Kieburtz K, Jennings D, Tanner CM, Simuni T, Singleton A, Toga AW, Chowdhury S, Trojanowski JQ, Shaw LM, Parkinson’s Progression Marker Initiative, 2016. CSF biomarkers associated with disease heterogeneity in early Parkinson’s disease: the Parkinson’s Progression Markers Initiative study. Acta Neuropathol 131, 935–949. 10.1007/s00401-016-1552-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim D-K, Lim H-S, Kawasaki I, Shim Y-H, Vaikath NN, El-Agnaf OMA, Lee H-J, Lee S-J, 2016a. Anti-aging treatments slow propagation of synucleinopathy by restoring lysosomal function. Autophagy 12, 1849–1863. 10.1080/15548627.2016.1207014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim C, Lv G, Lee JS, Jung BC, Masuda-Suzukake M, Hong C-S, Valera E, Lee H-J, Paik SR, Hasegawa M, Masliah E, Eliezer D, Lee S-J, 2016b. Exposure to bacterial endotoxin generates a distinct strain of α-synuclein fibril. Sci. Rep 6, 30891 10.1038/srep30891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klucken J, Shin Y, Masliah E, Hyman BT, McLean PJ, 2004. Hsp70 Reduces alpha-synuclein Aggregation and Toxicity. J Biol Chem 279, 25497–25502. 10.1074/jbc.M400255200. [DOI] [PubMed] [Google Scholar]
- Koike MA, Price DL, White BM, Rockenstein E, Wrasidlo W, Tsigelny I, Meier D, Masliah E, Bonhaus DW, 2014. The novel alpha-synuclein stabilizer NPT200–11 improves behavior, neuropathology, and Biochemistry in the murine thy1-ASYN transgenic model of Parkinson’s disease. Soc. Neurosci [Google Scholar]
- Kramer ML, Schulz-Schaeffer WJ, 2007. Presynaptic alpha-synuclein aggregates, not Lewy bodies, cause neurodegeneration in dementia with Lewy bodies. J Neurosci 27, 1405–1410. 10.1523/JNEUROSCI.4564-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnan R, Tsubery H, Proschitsky MY, Asp E, Lulu M, Gilead S, Gartner M, Waltho JP, Davis PJ, Hounslow AM, Kirschner DA, Inouye H, Myszka DG, Wright J, Solomon B, Fisher RA, 2014. A bacteriophage capsid protein provides a general amyloid interaction motif (GAIM) that binds and remodels misfolded protein assemblies. J Mol Biol 426, 2500–2519. [DOI] [PubMed] [Google Scholar]
- Lashuel HA, Overk CR, Oueslati A, Masliah E, 2013. The many faces of alpha-synuclein: from structure and toxicity to therapeutic target. Nat Rev Neurosci 14, 38–48. 10.1038/nrn3406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H-J, Bae E-J, Lee S-J, 2014. Extracellular alpha-synuclein-a novel and crucial factor in Lewy body diseases. Nat Rev Neurol 10, 92–98. 10.1038/nrneurol.2013.275. [DOI] [PubMed] [Google Scholar]
- Lee JM, Derkinderen P, Kordower JH, Freeman R, Munoz DG, Kremer T, Zago W, Hutten SJ, Adler CH, Serrano GE, Beach TG, 2017. The Search for a Peripheral Biopsy Indicator of α-synuclein Pathology for Parkinson Disease. J. Neuropathol. Exp. Neurol 76, 2–15. 10.1093/jnen/nlw103. [DOI] [PubMed] [Google Scholar]
- Lewis J, Melrose H, Bumcrot D, Hope A, Zehr C, Lincoln S, Braithwaite A, He Z, Ogholikhan S, Hinkle K, Kent C, Toudjarska I, Charisse K, Braich R, Pandey RK, Heckman M, Maraganore DM, Crook J, Farrer MJ, 2008. In vivo silencing of alpha-synuclein using naked siRNA. Mol Neurodegener 3, 19 10.1186/1750-1326-3-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindholm D, Pham DD, Cascone A, Eriksson O, Wennerberg K, Saarma M, 2016. c-Abl Inhibitors Enable Insights into the Pathophysiology and Neuroprotection in Parkinson’s Disease. Front Aging Neurosci 8, 254 10.3389/fnagi.2016.00254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindström V, Ihse E, Fagerqvist T, Bergström J, Nordström E, Möller C, Lannfelt L, Ingelsson M, 2014. Immunotherapy targeting α-synuclein, with relevance for future treatment of Parkinson’s disease and other Lewy body disorders. Immunotherapy 6, 141–153. 10.2217/imt.13.162. [DOI] [PubMed] [Google Scholar]
- Logan T, Bendor J, Toupin C, Thorn K, Edwards RH, 2017. α-Synuclein promotes dilation of the exocytotic fusion pore. Nat Neurosci 20, 681–689. 10.1038/nn.4529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lonskaya I, Hebron ML, Desforges NM, Schachter JB, Moussa CEH, 2013. Nilotinib-induced autophagic changes increase endogenous parkin level and ubiquitination, leading to amyloid clearance. J Mol Med 92, 373–386. 10.1007/s00109-013-1112-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundblad M, Decressac M, Mattsson B, Björklund A, 2012. Impaired neuro-transmission caused by overexpression of α-synuclein in nigral dopamine neurons. Proc Natl Acad Sci USA 109, 3213–3219. 10.1073/pnas.1200575109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma M-R, Hu Z-W, Zhao Y-F, Chen Y-X, Li Y-M, 2016. Phosphorylation induces distinct alpha-synuclein strain formation. Sci. Rep 6, 37130 10.1038/srep37130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahlknecht P, Seppi K, Poewe W, 2015. The Concept of Prodromal Parkinson’s Disease. J Parkinsons Dis 5, 681–697. 10.3233/JPD-150685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majbour NK, Vaikath NN, van Dijk KD, Ardah MT, Varghese S, Vesterager LB, Montezinho LP, Poole S, Safieh-Garabedian B, Tokuda T, Teunissen CE, Berendse HW, van de Berg WDJ, El-Agnaf OMA, 2016. Oligomeric and phosphorylated alpha-synuclein as potential CSF biomarkers for Parkinson’s disease. Mol Neurodegener 11, 7 10.1186/s13024-016-0072-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao X, Ou MT, Karuppagounder SS, Kam TI, Yin X, Xiong Y, Ge P, Umanah GE, Brahmachari S, Shin JH, Kang HC, Zhang J, Xu J, Chen R, Park H, Andrabi SA, Kang SU, Goncalves RA, Liang Y, Zhang S, Qi C, Lam S, Keiler JA, Tyson J, Kim D, Panicker N, Yun SP, Workman CJ, Vignali DAA, Dawson VL, Ko HS, Dawson TM, 2016. Pathological alpha-synuclein transmission initiated by binding lymphocyte-activation gene 3. Science (New York, N.Y.) 353 aah3374–aah3374. 10.1126/science.aah3374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCann H, Stevens CH, Cartwright H, Halliday GM, 2014. α-Synucleinopathy phenotypes. Parkinsonism Relat D 20 (Suppl. 1) S62–7. 10.1016/S1353-8020(13)70017-8. [DOI] [PubMed] [Google Scholar]
- McCormack AL, Mak SK, Henderson JM, Bumcrot D, Farrer MJ, Di Monte DA, 2010. Alpha-synuclein suppression by targeted small interfering RNA in the primate substantia Nigra. PLoS ONE 5, e12122 10.1371/journal.pone.0012122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormack AL, Mak SK, Di Monte DA, 2012. Increased α-synuclein phosphorylation and nitration in the aging primate substantia nigra. Cell Death Dis 3, e315 10.1038/cddis.2012.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLean PJ, Kawamata H, Shariff S, Hewett J, Sharma N, Uéda K, Breakefield XO, Hyman BT, 2002. TorsinA and heat shock proteins act as molecular chaperones: suppression of α-synuclein aggregation. J Neurochem 83, 846–854. 10.1046/j.1471-4159.2002.01190.x. [DOI] [PubMed] [Google Scholar]
- McNeill A, Magalhaes J, Shen C, Chau KY, Hughes D, Mehta A, Foltynie T, Cooper JM, Abramov AY, Gegg M, Schapira AHV, 2014. Ambroxol improves lysosomal biochemistry in glucocerebrosidase mutation-linked Parkinson disease cells. Brain 137, 1481–1495. 10.1093/brain/awu020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melki R, 2015. Role of Different Alpha-synuclein Strains in synucleinopathies, Similarities with other Neurodegenerative Diseases. J Parkinsons Dis 5, 217–227. 10.3233/JPD-150543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Migdalska-Richards A, Schapira AHV, 2016. The relationship between glucocerebrosidase mutations and Parkinson disease. J Neurochem 139 (Suppl. 1), 77–90. 10.1111/jnc.13385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Migdalska-Richards A, Ko WKD, Li Q, Bezard E, Schapira AHV, 2017. Oral ambroxol increases brain glucocerebrosidase activity in a nonhuman primate. Synapse 71, e21967 10.1002/syn.21967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittal S, Bjørnevik K, Im DS, Flierl A, Dong X, Locascio JJ, Abo KM, Long E, Jin M, Xu B, Xiang YK, Rochet J-C, Engeland A, Rizzu P, Heutink P, Bartels T, Selkoe DJ, Caldarone BJ, Glicksman MA, Khurana V, Schüle B, Park DS, Riise T, Scherzer CR, 2017. β2-Adrenoreceptor is a regulator of the α-synuclein gene driving risk of Parkinson’s disease. Science (New York, NY) 357, 891–898. 10.1126/science.aaf3934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mollenhauer B, Trautmann E, Taylor P, Manninger P, Sixel-Döring F, Ebentheuer J, Trenkwalder C, Schlossmacher MG, 2013. Total CSF α-synuclein is lower in de novo Parkinson patients than in healthy subjects. Neuroscience Letters 532, 44–48. 10.1016/j.neulet.2012.11.004. [DOI] [PubMed] [Google Scholar]
- Mollenhauer B, Batrla R, El-Agnaf O, Galasko DR, Lashuel HA, Merchant KM, Shaw LM, Selkoe DJ, Umek R, Vanderstichele H, Zetterberg H, Zhang J, Caspell-Garcia C, Coffey C, Hutten SJ, Frasier M, Taylor P, Investigating Synuclein Consortium of the Michael J. Fox Foundation for Parkinson’s Research, 2017. A user’s guide for α-synuclein biomarker studies in biological fluids: perianalytical considerations. Mov Disord 32, 1117–1130. 10.1002/mds.27090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moors TE, Hoozemans JJM, Ingrassia A, Beccari T, Parnetti L, Chartier-Harlin M-C, van de Berg WDJ, 2017. Therapeutic potential of autophagy-enhancing agents in Parkinson’s disease. Mol Neurodegener 1–18. 10.1186/s13024-017-0154-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy KE, Gysbers AM, Abbott SK, Tayebi N, Kim WS, Sidransky E, Cooper A, Garner B, Halliday GM, 2014. Reduced glucocerebrosidase is associated with increased α-synuclein in sporadic Parkinson’s disease. Brain 137, 834–848. 10.1093/brain/awt367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nalls MA, Pankratz N, Lill CM, Do CB, Hernandez DG, Saad M, DeStefano AL, Kara E, Bras J, Sharma M, Schulte C, Keller MF, Arepalli S, Letson C, Edsall C, Stefansson H, Liu X, Pliner H, Lee JH, Cheng R, International Parkinson’s Disease Genomics Consortium (IPDGC), Parkinson’s Study Group (PSG) Parkinson’s Research: The Organized GENetics Initiative (PROGENI), 23andMe, GenePD, NeuroGenetics Research Consortium (NGRC), Hussman Institute of Human Genomics (HIHG), Ashkenazi Jewish Dataset Investigator, Cohorts for Health and Aging Research in Genetic Epidemiology (CHARGE), North American Brain Expression Consortium (NABEC), United Kingdom Brain Expression Consortium (UKBEC), Greek Parkinson’s Disease Consortium, Alzheimer Genetic Analysis Group, Ikram MA, Ioannidis JPA, Hadjigeorgiou GM, Bis JC, Martinez M, Perlmutter JS, Goate A, Marder K, Fiske B, Sutherland M, Xiromerisiou G, Myers RH, Clark LN, Stefansson K, Hardy JA, Heutink P, Chen H, Wood NW, Houlden H, Payami H, Brice A, Scott WK, Gasser T, Bertram L, Eriksson N, Foroud T, Singleton AB, 2014. Large-scale meta-analysis of genome-wide association data identifies six new risk loci for Parkinson’s disease. Nat. Genet 46, 989–993. 10.1038/ng.3043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oueslati A, 2016. Implication of Alpha-synuclein Phosphorylation at S129 in synucleinopathies: What Have We Learned in the Last Decade? J Parkinsons Dis 6, 39–51. 10.3233/JPD-160779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagan F, Hebron M, Valadez EH, Torres-Yaghi Y, Huang X, Mills RR, Wilmarth BM, Howard H, Dunn C, Carlson A, Lawler A, Rogers SL, Falconer RA, Ahn J, Li Z, Moussa C, 2016. Nilotinib Effects in Parkinson’s disease and Dementia with Lewy bodies. J Parkinsons Dis 6, 503–517. 10.3233/JPD-160867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pampalakis G, Sykioti V-S, Ximerakis M, Stefanakou-Kalakou I, Melki R, Vekrellis K, Sotiropoulou G, 2017. KLK6 proteolysis is implicated in the turnover and uptake of extracellular alpha-synuclein species. Oncotarget 8, 14502–14515. 10.18632/oncotarget.13264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parnetti L, Paciotti S, Eusebi P, Dardis A, Zampieri S, Chiasserini D, Tasegian A, Tambasco N, Bembi B, Calabresi P, Beccari T, 2017. Cerebrospinal fluid β-glucocerebrosidase activity is reduced in Parkinson’s disease patients. Mov Disord 35, 385 10.1002/mds.27136. [DOI] [PubMed] [Google Scholar]
- Peelaerts W, Bousset L, Van der Perren A, Moskalyuk A, Pulizzi R, Giugliano M, Van den Haute C, Melki R, Baekelandt V, 2015. α-Synuclein strains cause distinct synucleinopathies after local and systemic administration. Nature 522, 340–344. 10.1038/nature14547. [DOI] [PubMed] [Google Scholar]
- Peng C, Gathagan RJ, Lee VM-Y, 2017. Distinct α-synuclein strains and implications for heterogeneity among α-synucleinopathies. Neurobiol Dis 10.1016/j.nbd.2017.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce S, Coetzee GA, 2017. Parkinson’s disease-associated genetic variation is linked to quantitative expression of inflammatory genes. PLoS ONE 12 e0175882–11. 10.1371/journal.pone.0175882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poewe W, Seppi K, Tanner CM, Halliday GM, Brundin P, Volkmann J, Schrag A-E, Lang AE, 2017. Parkinson disease. Nat Rev Dis Primers 3, 17013 10.1038/nrdp.2017.13. [DOI] [PubMed] [Google Scholar]
- Postuma RB, Berg D, 2016. Advances in markers of prodromal Parkinson disease. Nat Rev Neurol 12, 622–634. 10.1038/nrneurol.2016.152. [DOI] [PubMed] [Google Scholar]
- Postuma RB, Aarsland D, Barone P, Burn DJ, Hawkes CH, Oertel W, Ziemssen T, 2012. Identifying prodromal Parkinson’s disease: Pre-Motor disorders in Parkinson’s disease. Mov Disord 27, 617–626. 10.1002/mds.24996. [DOI] [PubMed] [Google Scholar]
- Prothena Company Website, 2017a. Clinical results presented from Prothena’s phase 1b study of PRX002/RG7935 demonstrating robust antibody CNS penetration and significant reduction of free serum alpha-synuclein in patients with Parkinson’s disease http://ir.prothena.com/releasedetail.cfm?ReleaseID=1019672.
- Prothena Company Website, 2017b. Prothena announces initiation of phase 2 PASADENA study of PRX002/RG7935 in patients with early Parkinson’s disease http://ir.prothena.com/releasedetail.cfm?ReleaseID=1032188.
- Recasens A, Dehay B, Bové J, Carballo-Carbajal I, Dovero S, Pérez-Villalba A, Fernagut P-O, Blesa J, Parent A, Perier C, Fariñas I, Obeso JA, Bezard E, Vila M, 2014. Lewy body extracts from Parkinson disease brains trigger α-synuclein pathology and neurodegeneration in mice and monkeys. Ann Neurol 75, 351–362. 10.1002/ana.24066. [DOI] [PubMed] [Google Scholar]
- Redmann M, Wani WY, Volpicelli-Daley L, Darley-Usmar V, Zhang J, 2017. Trehalose does not improve neuronal survival on exposure to alpha-synuclein pre-formed fibrils. Redox Biology 11, 429–437. 10.1016/j.redox.2016.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rey NL, Steiner JA, Maroof N, Luk KC, Madaj Z, Trojanowski JQ, Lee VM-Y, Brundin P, 2016a. Widespread transneuronal propagation of α-synucleinopathy triggered in olfactory bulb mimics prodromal Parkinson’s disease. J. Exp. Med 213, 1759–1778. 10.1084/jem.20160368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rey NL, Wesson DW, Brundin P, 2016b. The olfactory bulb as the entry site for prion-like propagation in neurodegenerative diseases. Neurobiol. Dis 10.1016/j.nbd.2016.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds AD, Stone DK, Mosley RL, Gendelman HE, 2009. Nitrated α-synuclein-induced alterations in microglial immunity are regulated by CD4+ T cell subsets. J. Immunol 182, 4137–4149. 10.4049/jimmunol.0803982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanofi Genzyme Company Website, 2017. Sanofi initiates phase 2 clinical trial to evaluate therapy for genetic form of Parkinson’s disease http://news.genzyme.com/press-release/sanofi-initiates-phase-2-clinical-trial-evaluate-therapy-genetic-form-parkinsons-disea.
- Sapru MK, Yates JW, Hogan S, Jiang L, Halter J, Bohn MC, 2006. Silencing of human alpha-synuclein in vitro and in rat brain using lentiviral-mediated RNAi. Exp. Neurol 198, 382–390. 10.1016/j.expneurol.2005.12.024. [DOI] [PubMed] [Google Scholar]
- Sardi SP, Cheng SH, Shihabuddin LS, 2015. Gaucher-related synucleinopathies: the examination of sporadic neurodegeneration from a rare (disease) angle. Prog. Neurobiol 125, 47–62. 10.1016/j.pneurobio.2014.12.001. [DOI] [PubMed] [Google Scholar]
- Sarkar S, Davies JE, Huang Z, Tunnacliffe A, Rubinsztein DC, 2007. Trehalose, a novel mTOR-independent autophagy enhancer, accelerates the clearance of mutant huntingtin and alpha-synuclein. J. Biol. Chem 282, 5641–5652. 10.1074/jbc.M609532200. [DOI] [PubMed] [Google Scholar]
- Schenk DB, Koller M, Ness DK, Griffth SG, Grundman M, Zago W, Soto J, Atiee G, Ostrowitzki S, Kinney GG, 2017. First-in-human assessment of PRX002, an anti-α-synuclein monoclonal antibody, in healthy volunteers. Mov. Disord 32, 211–218. 10.1002/mds.26878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid AW, Fauvet B, Moniatte M, Lashuel HA, 2013. Alpha-synuclein post-translational modifications as potential biomarkers for Parkinson disease and other synucleinopathies. Mol. Cell. Proteomics 12, 3543–3558. 10.1074/mcp.R113.032730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrag A, Horsfall L, Walters K, Noyce A, Petersen I, 2015. Prediagnostic presentations of Parkinson’s disease in primary care: a case-control study. Lancet Neurol 14, 57–64. 10.1016/S1474-4422(14)70287-X. [DOI] [PubMed] [Google Scholar]
- Shahnawaz M, Tokuda T, Waragai M, Mendez N, Ishii R, Trenkwalder C, Mollenhauer B, Soto C, 2017. Development of a biochemical diagnosis of Parkinson disease by detection of α-synuclein misfolded aggregates in cerebrospinal fluid. JAMA Neurol 74, 163–172. 10.1001/jamaneurol.2016.4547. [DOI] [PubMed] [Google Scholar]
- Shrivastava AN, Redeker V, Fritz N, Pieri L, Almeida LG, Spolidoro M, Liebmann T, Bousset L, Renner M, Léna C, Aperia A, Melki R, Triller A, 2015. α-Synuclein assemblies sequester neuronal α3-Na+/K+-ATPase and impair Na+ gradient. EMBO J 34, e201591397–2423. 10.15252/embj.201591397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sidransky E, Nalls MA, Aasly JO, Aharon-Peretz J, Annesi G, Barbosa ER, Bar-Shira A, Berg D, Bras J, Brice A, Chen C-M, Clark LN, Condroyer C, De Marco EV, Durr A, Eblan MJ, Fahn S, Farrer MJ, Fung H-C, Gan-Or Z, Gasser T, Gershoni-Baruch R, Giladi N, Griffth A, Gurevich T, Januario C, Kropp P, Lang AE, Lee-Chen G-J, Lesage S, Marder K, Mata IF, Mirelman A, Mitsui J, Mizuta I, Nicoletti G, Oliveira C, Ottman R, Orr-Urtreger A, Pereira LV, Quattrone A, Rogaeva E, Rolfs A, Rosenbaum H, Rozenberg R, Samii A, Samaddar T, Schulte C, Sharma M, Singleton A, Spitz M, Tan E-K, Tayebi N, Toda T, Troiano AR, Tsuji S, Wittstock M, Wolfsberg TG, Wu Y-R, Zabetian CP, Zhao Y, Ziegler SG, 2009. Multicenter analysis of glucocerebrosidase mutations in Parkinson’s disease. N. Engl. J. Med 361, 1651–1661. 10.1056/NEJMoa0901281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer B, Michael S, Shen J, Kosberg K, Rockenstein E, Patrick C, Adame A, Masliah E, 2009a. Lentivirus mediated delivery of neurosin promotes clearance of wild-type. Mol. Ther 1–11. 10.1038/mt.2012.66. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Spencer B, Potkar R, Trejo M, Rockenstein E, Patrick C, Gindi R, Adame A, Wyss-Coray T, Masliah E, 2009b. Beclin 1 gene transfer activates autophagy and ameliorates the neurodegenerative pathology in alpha-synuclein models of Parkinson’s and Lewy body diseases. J. Neurosci 29, 13578–13588. 10.1523/JNEUROSCI.4390-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart T, Sossi V, Aasly JO, Wszolek ZK, Uitti RJ, Hasegawa K, Yokoyama T, Zabetian CP, Leverenz JB, Stoessl AJ, Wang Y, Ginghina C, Liu C, Cain KC, Auinger P, Kang UJ, Jensen PH, Shi M, Zhang J, 2015. Phosphorylated α-synuclein in Parkinson’s disease: correlation depends on disease severity. Acta Neuropathologica Communications 3, 7 10.1186/s40478-015-0185-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stokholm MG, Danielsen EH, Hamilton-Dutoit SJ, Borghammer P, 2016. Pathological α-synuclein in gastrointestinal tissues from prodromal Parkinson disease patients. Ann. Neurol 79, 940–949. 10.1002/ana.24648. [DOI] [PubMed] [Google Scholar]
- Sulzer D, Alcalay RN, Garretti F, Cote L, Kanter E, Agin-Liebes J, Liong C, McMurtrey C, Hildebrand WH, Mao X, Dawson VL, Dawson TM, Oseroff C, Pham J, Sidney J, Dillon MB, Carpenter C, Weiskopf D, Phillips E, Mallal S, Peters B, Frazier A, Arlehamn CSL, Sette A, 2017. T cells from patients with Parkinson’s disease recognize α-synuclein peptides. Nature 546, 656–661. 10.1038/nature22815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surmeier DJ, Obeso JA, Halliday GM, 2017. Selective neuronal vulnerability in Parkinson disease. Nat. Rev. Neurosci 18, 101–113. 10.1038/nrn.2016.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thibault L, Rascol O, Corvol J-C, Ferreira J, Defebvre L, Deplanque D, Bordet R, Moreau C, Devos D, 2017. New perspectives on study designs for evaluating neuroprotection in Parkinson’s disease. Mov. Disord 10.1002/mds.27055. [DOI] [PubMed] [Google Scholar]
- Vellas B, Carrillo MC, Sampaio C, Brashear HR, Siemers E, Hampel H, Schneider LS, Weiner M, Doody R, Khachaturian Z, Cedarbaum J, Grundman M, Broich K, Giacobini E, Dubois B, Sperling R, Wilcock GK, Fox N, Scheltens P, Touchon J, Hendrix S, Andrieu S, Aisen P, Task Force Members, E.U/.U.S/.C.T.A.D., 2013. Designing drug trials for Alzheimer’s disease: what we have learned from the release of the phase III antibody trials: a report from the EU/US/CTAD Task Force. In: Presented at the Alzheimer’s & Dementia: the Journal of the Alzheimer’s Association, pp. 438–444. 10.1016/j.jalz.2013.03.007. [DOI] [PubMed] [Google Scholar]
- Visanji NP, Mollenhauer B, Beach TG, Adler CH, Coffey CS, Kopil CM, Dave KD, Foroud T, Chahine L, Jennings D, Systemic Synuclein Sampling Study (S4), 2017. The systemic synuclein sampling study: toward a biomarker for Parkinson’s disease. Biomark. Med 11, 359–368. 10.2217/bmm-2016-0366. [DOI] [PubMed] [Google Scholar]
- Wales P, Pinho R, Lázaro DF, Outeiro TF, 2013. Limelight on alpha-synuclein: pathological and mechanistic implications in neurodegeneration. J Parkinsons Dis 3, 415–459. 10.3233/JPD-130216. [DOI] [PubMed] [Google Scholar]
- Wang C, Zhao C, Li D, Tian Z, Lai Y, Diao J, Liu C, 2016. Versatile structures of α-synuclein. Front. Mol. Neurosci 9, 48 10.3389/fnmol.2016.00048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watts JC, Giles K, Oehler A, Middleton L, Dexter DT, Gentleman SM, Dearmond SJ, Prusiner SB, 2013. Transmission of multiple system atrophy prions to transgenic mice. Proc. Natl. Acad. Sci. U. S. A 110, 19555–19560. 10.1073/pnas.1318268110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong YC, Krainc D, 2017. Alpha-synuclein toxicity in neurodegeneration: mechanism and therapeutic strategies. Nat. Publ. Group 23, 151–163. 10.1038/nm.4269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyse RK, Brundin P, Sherer TB, 2016. Nilotinib – differentiating the hope from the hype. J Parkinsons Dis 6, 519–522. 10.3233/JPD-160904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zharikov AD, Cannon JR, Tapias V, Bai Q, 2015. shRNA targeting α-synuclein prevents neurodegeneration in a Parkinson’s disease model. J. Clin. Invest 10.1172/JCI64502. [DOI] [PMC free article] [PubMed] [Google Scholar]