Abstract
Various mosquito control methods use factory raised males to suppress vector densities. But the efficiency of these methods is currently insufficient to prevent epidemics of arbovirus diseases such as dengue, chikungunya or Zika. Suggestions that the sterile insect technique (SIT) could be “boosted” by applying biopesticides to sterile males remain unquantified. Here, we assess mathematically the gains to SIT for Aedes control of either: boosting with the pupicide pyriproxifen (BSIT); or, contaminating mosquitoes at auto-dissemination stations. Thresholds in sterile male release rate and competitiveness are identified, above which mosquitoes are eliminated asymptotically. Boosting reduces these thresholds and aids population destabilisation, even at sub-threshold release rates. No equivalent bifurcation exists in the auto-dissemination sub-model. Analysis suggests that BSIT can reduce by over 95% the total release required to circumvent dengue epidemics compared to SIT. We conclude, BSIT provides a powerful new tool for the integrated management of mosquito borne diseases.
Subject terms: Ecological epidemiology, Computational models
David Pleydell and Jeremy Bouyer use mathematical modelling to examine the gains from adding biopesticides to the sterile insect technique for controlling mosquito populations. They find that boosting sterile males with pupicides drastically reduces the total release required to control dengue epidemics.
Introduction
The international spread of mosquitoes Aedes aegypti and Ae. albopictus has triggered numerous epidemics of dengue, Zika, chikungunya and yellow-fever1–4. Without effective vaccines5–7, mosquito abatement remains key to controlling most of these diseases. Mosquito borne pathogens account for one-sixth of infection-associated disability adjusted life years8, highlighting the difficulty of area-wide mosquito control9. The World Health Organisation has called for new vector control technologies10. Here, we explore the potential benefits of combining two prominent Aedes control techniques.
The auto-dissemination technique (ADT) uses mosquitoes to deposit biopesticides at larval sites—providing efficient treatment of the small, hidden and disseminated water bodies Aedes use as larval habitat11. The most common biopesticide used is pyriproxifen—a juvenile hormone analogue inhibiting metamorphosis to adult. Mosquitoes become contaminated with pyriproxifen at dissemination stations12. Field trials with pyriproxyfen have demonstrated elevated pupal mortality (emergence inhibition) of 40–70% in Ae. albopictus populations12–15, and 95–100% density reductions in Ae. aegypti populations16 and mixed Ae. aegypti/Ae. albopictus populations17,18. Whilst the scale of successful field trials has increased17,18, the required high numbers of dissemination stations19 impose large maintenance costs and the long-term efficacy of ADT has yet to be demonstrated.
The sterile insect technique (SIT) reduces female reproductive success through sexual competition between wild-type and released males sterilized with ionizing radiation (formerly with chemosterilants)20,21. Related methods include the Wolbachia-based incompatible insect technique22,23, or gene modification systems such as the release of transgenic mosquitoes carrying a dominant lethal24,25. Successful SIT programmes have eradicated screwworm and medfly from North and Central America26,27, and tsetse from Zanzibar28. Mosquito SIT is less developed—while trials have suppressed Ae. albopictus populations in Italy29, elimination requires maintaining high sterile to wild male ratios which proves prohibitively costly8. One proposed solution is to couple SIT and ADT by treating sterile males with biopesticides before release30,31. But the efficacy gain from this “boosted” sterile insect technique (BSIT) remains unquantified. Using mathematical modelling, we analyse the efficacy of SIT, BSIT and ADT for controlling Aedes vectors and Aedes borne diseases.
The dynamics of an Aedes population, under BSIT and ADT, were modelled using ordinary differential equations (Eqs. (1)–(8)). The model characterises sexual competition between sterile and wild males32, pyriproxifen transfer at dissemination stations12, during coupling30 and oviposition11, and concentration dependent emergence inhibition of juveniles33 (Supplementary Fig. 1). Sexual competition depends on the competitiveness (h) and relative frequency of sterile males. When pyriproxifen transfer is blocked, the model describes dynamics under standard SIT. Parameterisation (Supplementary Table 1) reflects possible dynamics under fixed favourable climatic conditions across a 1 ha area with 200 larval sites, each of 250 mL and a carrying capacity of 25 larvae. We assume regular maintenance and constant efficacy of dissemination stations, and neglect dispersion34, landscape effects35, risk-mitigating oviposition site selection36, sterile male induced larval site contamination37, substrate effects on pyriproxifen efficacy38 and reduced female survival due to sexual harassment39.
We present results indicating that sexual competition between sterile and wild males creates a threshold sterile male release rate, above which a population density of zero is the only stable equilibrium. Boosting with pyriproxifen generates large reductions in the elimination threshold, the sub-threshold stable equilibrium, the total number of sterile males required for elimination, and the time to elimination. An equivalent elimination threshold does not exist for the auto-dissemination technique, which is most efficient at large densities. Epidemiological analyses suggest if using SIT, without pyriproxifen and with near elimination threshold release rates, the equilibrium density of female mosquitoes can be greater than the density of females required to bring the basic reproductive number of dengue below one. This suggests that vector elimination may be required to prevent dengue epidemics—something that has yet to be achieved with mosquito SIT (or related techniques). Boosting with pyriproxifen lowers both the elimination threshold and the stable equilibrium, providing greater protection against dengue, possibly even if elimination is not achieved. We conclude that ADT and SIT are complimentary techniques and that BSIT can provide a powerful new approach for protecting populations against diseases such as dengue, chikungunya and Zika.
Results
Boosting reduces the thresholds and time for elimination
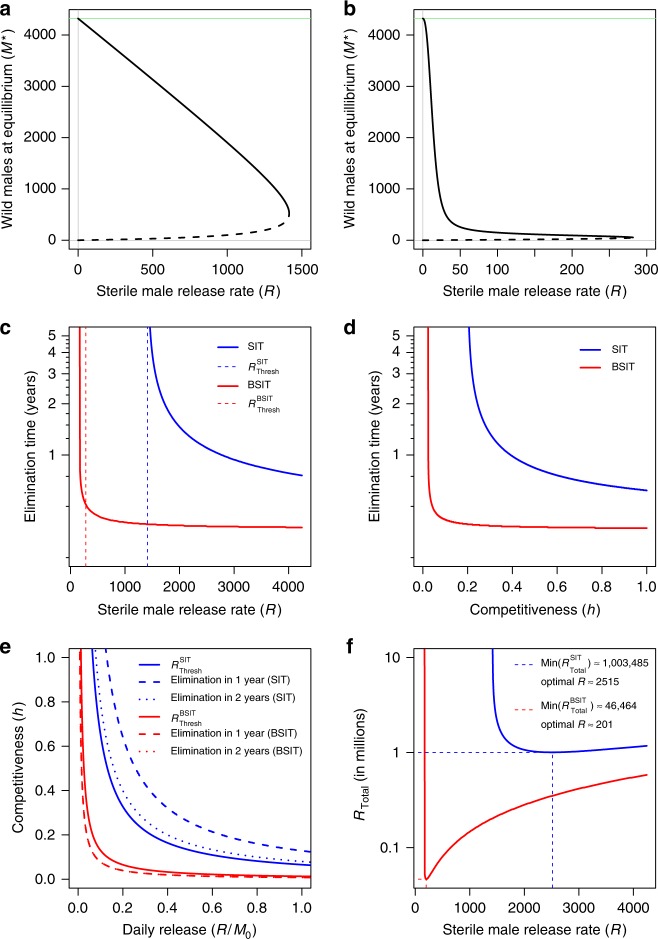
With SIT, augmenting the daily release rate (R) decreases the asymptotic population density (stable equilibrium) (Fig. 1a, Supplementary Fig. 2A). A bifurcation, where stable and unstable equilibria converge, gives a threshold release rate . Maintaining ensures eventual elimination, whilst ensures convergence to a new stable equilibrium (Supplementary Figs. 2A, B). Elimination times rise asymptotically at (Fig. 1c) and quasi-zero gradients near can trap trajectories for many years (Supplementary Fig. 2B). Thus, Aedes elimination with SIT requires and sustaining such high release rates entails non-trivial logistic difficulties29,40.
Fig. 1.
Equilibria, thresholds and optima of the BSIT model. Density of males at stable (solid lines) and unstable (dashed lines) equilibria given release rate (R) under SIT (a) and BSIT (b). A bifurcation, where stable and unstable equilibria converge, provides an elimination threshold for SIT . Pyriproxifen reduces the threshold and the distance between stable and unstable equilibria (b). With SIT, elimination time grows asymptotically at (blue dashed), whereas boosting can shift this asymptote below (red dashed) (c). With R fixed (R = 1414), elimination time responds asymptotically to competitiveness (d), and boosting shifts the threshold (hThresh) towards zero. Thresholds for the eventual elimination of any initial population (solid), and for eliminating from carrying capacity in one (dashed) or two (dotted) years respond non-linearly to release rate (R) and competitiveness (h) (e). Two years (dotted) and 1 year (dashed) elimination thresholds for BSIT (red) are indistinguishable. The total release required for elimination (RTotal) is minimised at and for SIT and BSIT, respectively (f, dashed lines). All simulations were initialised at carrying capacity, with M0 the initial density of males
Boosting reduces the bifurcation point (by 80%) and the distances between stable and unstable equilibria (Fig. 1b). Unlike SIT, for BSIT the elimination time asymptote shifts to some (Fig. 1c). The size of this shift depends on initial population densities—large populations generate high pyriproxifen concentration peaks (Supplementary Fig. 2C). When these peaks push populations beneath the unstable equilibrium elimination becomes easy, otherwise transient oscillations and population recovery lead to a new stable equilibrium (Supplementary Fig. 2D).
Elimination time responds asymptotically to sterile male competitiveness h at a threshold hThresh. With R = 1414, boosting reduces hThresh by 80% (Fig. 1d). Sensitivity analyses suggest boosting induced reductions in hThresh would be greatest for low R—but even with daily release rates as high as the adult male carrying capacity (M0) boosting could reduce hThresh by as much as one order of magnitude (Supplementary Fig. 3A). Thresholds hThresh and RThresh are highly sensitive to variation in near-threshold values of R and h, respectively (Supplementary Figs. 3A, B). Elimination with sub-threshold values of R (or h) requires sufficient pyriproxifen accumulation to prevent population recovery to a new stable equilibrium (Supplementary Figs. 3C, D). With R fixed at 500, an upper bound on hThresh is sensitive to egg viability q, whereas the expected value of hThresh shows greatest sensitivity to the quantity of pyriproxifen deposited at oviposition (p) and its longevity in the environment (1/d) (Supplementary Fig. 4A). Similar patterns are observed with RThresh (Supplementary Fig. 4B) and (with R = 1500) elimination time and RTotal (Supplementary Fig. 4C, D). The elimination thresholds RThresh and hThresh are interdependent and boosting permits rapid elimination under many R ~ h combinations that would only suppress mosquitoes under SIT (Fig. 1e).
Control practitioners need to identify release rates that can eliminate vectors with a minimum of sterile males. Numerical integration indicates that, to eradicate a population initialised at carrying capacity, SIT requires at least 1,003,485 sterile males released over 399 days, while BSIT requires just 46,464 sterile males released over 231 days (Fig. 1f)—an efficiency gain of over 95%. These results suggest that BSIT may achieve elimination in many scenarios where it is impractical with SIT.
Boosting shrinks the basic reproductive number of dengue
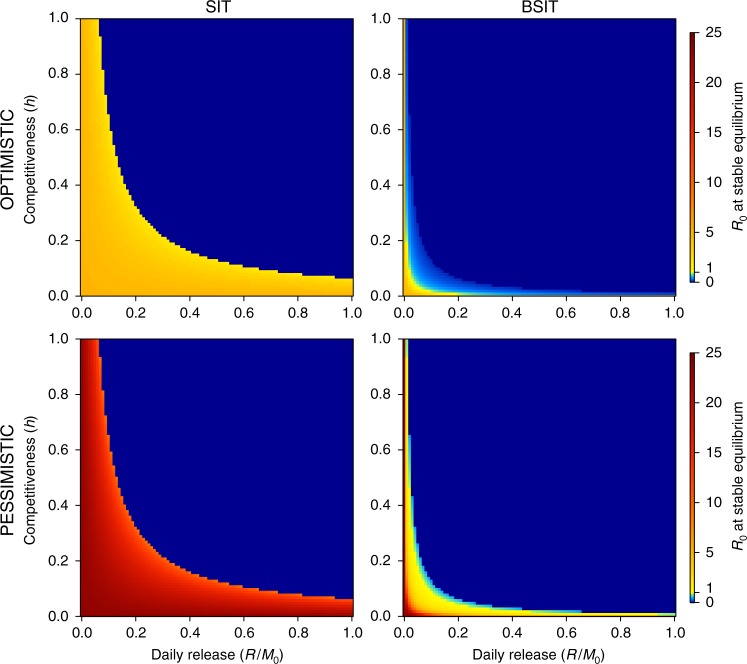
To assess the epidemiological implications of boosting, the BSIT model was coupled with a dengue transmission model where transmission occurs between susceptible, exposed, infectious or recovered humans and susceptible, exposed or infectious female mosquitoes41 (Supplementary Fig. 5, Eqs. (19)–(28)). For simplicity, we neglect spatial dynamics42,43, temperature driven parameter fluctuations41,44, inapparent infections45,46, non-linearity in bite rates47–49, multiple serotypes50–52 and multi-annual cyclicity50,53. Parameters (Supplementary Table 2) reflect transmission within 1 ha accommodating 50 susceptible humans. This small spatial scale was adopted to minimise bias from assuming homogeneous mixing and to characterise transmission at localised hot-spots with high vector-host ratios54,55. The asymptotic stable equilibrium of the system was used to calculate the basic reproductive number (R0)—recall, epidemic spread in a susceptible population requires R0 > 1. R0 calculation used two parameter sets, labelled “optimistic” and “pessimistic”, with different bite rates, transmission probabilities and extrinsic incubation periods (Supplementary Table 2). The notation and indicates the R0 associated with each parameter set. Boosting reduced R0 for many combinations of R and h and expanded the region of parameter space over which R0 < 1 (Fig. 2). For SIT, the relation between R, h and the R0 unity threshold (Fig. 2) matched the elimination thresholds (Fig. 1e). For BSIT, some R ~ h combinations lead to and (light blue) without vector elimination (dark blue). More R ~ h combinations were associated with than with . Thus, BSIT (but not SIT) might provide lasting protection against dengue without the need for elimination, particularly in situations where a more optimistic parameterization of R0 is justifiable.
Fig. 2.
Basic reproductive number (R0) of dengue transmission. Shown as a function of sterile male competitiveness (h) and release rate (R) for SIT (left) and BSIT (right). Alternative “optimistic” (top row) and “pessimistic” (bottom row) parameter sets are used (Supplementary Table 2). M0 is the control-free stable equilibrium of males
Auto-dissemination is most effective at high densities
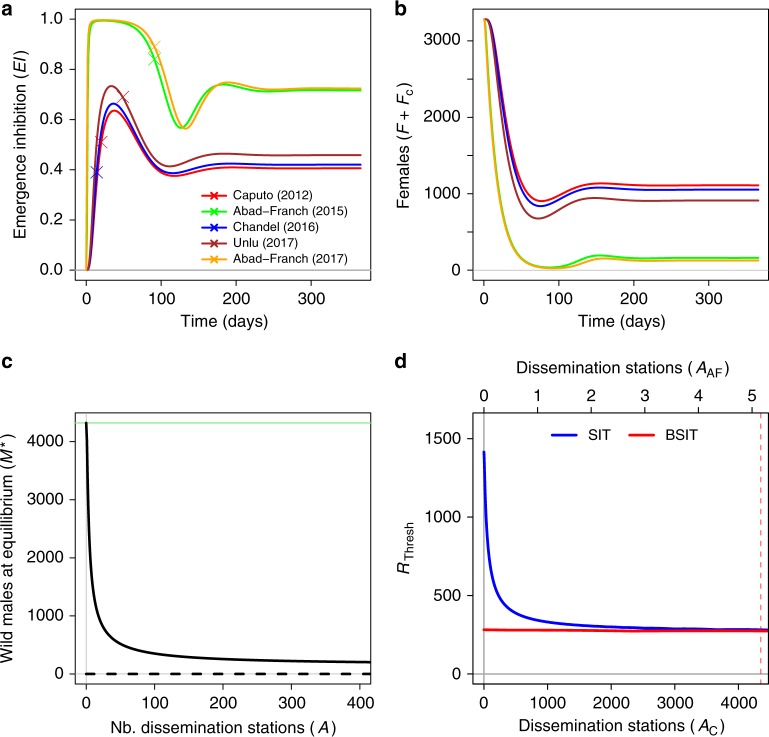
To assess whether ADT could augment SIT and BSIT efficacy, we estimated the contamination rate at dissemination stations (α) using emergence inhibition (EI) data from five field trials (Supplementary Table 3). Estimates of α were greatest for trials targeting mixed Ae. albopictus/Ae. aegypi populations (Supplementary Table 3). Some authors have suggested ADT is more efficient when Ae. aegypi is present17, and our analyses are consistent with that hypothesis. All EI trajectories peaked rapidly and then oscillated to convergence at a stable equilibrium (Fig. 3a). An inverse pattern was observed in female density, where an initial crash was followed by recovery to a stable equilibrium (Fig. 3b). The stable and unstable equilibria of the ADT sub-model do not converge when dissemination station density (A) is increased (Fig. 3c). Without a bifurcation, zero remains an unstable equilibrium, suggesting it would be highly unlikely to eliminate Aedes using ADT alone. Even if the total contamination rate (α × A) was high, low mosquito numbers would not sustain sufficient EI to prevent recovery.
Fig. 3.
Trajectories, equilibria and thresholds with ADT. Trajectories of emergence inhibition under ADT, and calibration points taken from five field trials (a). Corresponding trajectories of total female density (b). Stable (solid) and unstable (dashed) equilibria of the ADT model as a funciton of dissemination station density (c). The response of the elimination thresholds (blue) and (red) to dissemination station density (d), where AC indicates dissemination station density when contamination rate α is estimated from Caputo et al.12, and where AAF indicates dissemination station density when α is estimated from Abad-Franch et al.18 (Supplementary Table 3). The vertical pink dashed line indicates the dissemination station density required for to equal with A = 0 (intercept of red line)
Auto-dissemination improves SIT and BSIT efficacy
The elimination threshold of BSIT is reduced by ADT—but the reduction is extremely small (Fig. 3d, red line). For SIT, the effect of ADT on is greater (Fig. 3d, blue line). The size of this reduction depends on the total contamination rate α × A —we call this rate the ADT “intensity” for brevity. With α based on Caputo et al.12 data (α = 0.0035), it would require A = 4350 dissemination station per hectare for to match without ADT (Fig. 3d, pink dashed). This number drops three orders of magnitude using α estimated from Abad-Franch et al.18 (Fig. 3d, top axis).
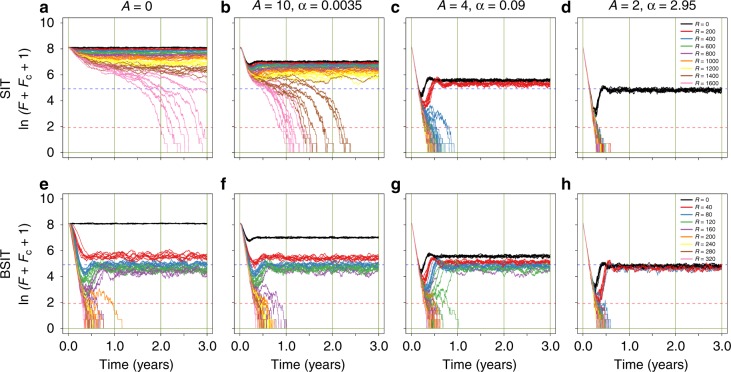
To account for small-population effects, we complimented the deterministic analyses above with stochastic simulation56,57. Mosquitoes (initialised at carrying capacity) were subjected to SIT or BSIT with ADT applied at four different intensity levels. With BSIT, trajectories either displayed transitory oscillations followed by convergence to a (stochastic) stable equilibrium, or destabilisation followed by elimination (Fig. 4). With R = 0, ADT displayed similar transitory dynamics, but with a higher stable equilibrium and no elimination (black lines). For SIT and A = 0, elimination was only achieved when (Fig. 4a)—the minimum time to elimination was over 2 years, reflecting that was too small for rapid elimination. Increasing ADT intensity lowered the stable equilibria and increased the probability and rate of elimination. At highest ADT intensity, SIT achieved elimination with R as low as 200, and trajectories resembled those of BSIT with R close to (Fig. 4d, e). When A = 0, the (non-zero) stable equilibria were lower for BSIT than for SIT. Increasing ADT intensity reduced this difference. For BSIT, only trajectories leading to elimination sustained , but several trajectories converged below the unity threshold. For SIT, only trajectories leading to elimination sustained . Without ADT, boosting reduced by one order of magnitude the release rates at which the probability of elimination became non-negligible, and elimination was faster with boosting. Using ADT alone (R = 0), even the highest intensity ADT scheme did not suppress mosquito densities sufficiently to sustain . These results suggest boosting can provide a greater level of protection against dengue than would be possible with SIT or ADT alone. Moreover, an ADT-SIT combination could only provide the same level of protection as BSIT with either highly efficient (α) or highly numerous (A) dissemination stations.
Fig. 4.
Stochastic simulation of Aedes control with SIT, BSIT and/or ADT. Mosquito populations were initiated as Poisson random variables with expectancies set to the control-free stable equilibrium. For SIT (top row) and BSIT (bottom row), nine different release rates were evaluated (see colour legends). For ADT, four different intensities (α × A) were evaluated. Values of α in columns two to four were estimated from different field trials (Supplementary Table 3), the associated values of A were adjusted to provide a more even spread of intensities. For each R ~ A combination, ten simulations are shown. Total female density (plus one) is shown on the natural log scale. Thresholds in female density corresponding to and are indicated as blue and pink dashed lines, respectively. In all simulations, an immigration rate of zero was assumed
Discussion
At present, insect control primarily depends on insecticides, with major impacts on human/animal health and food safety58. Moreover, negative effects of chemicals on predator populations, and the evolution of insecticide resistance, can trigger outbreaks of target (or secondary pest) populations and control failure59. Various mosquito release schemes (SIT, incompatible insect technique, transgenic mosquitoes) are being tested in the hope of establishing more efficient control without the undesirable impacts of insecticides. Yet despite the sophistication of modern methods, we remain incapable of preventing large-scale epidemics of mosquito borne diseases. Our analyses highlight a tight association between release rate and competitiveness thresholds which provide minimum conditions for elimination with SIT. Boosting with pyriproxifen shifts these thresholds, and could reduce by over 95% (Fig. 1f) the number of sterile males required for Aedes elimination.
Auto-dissemination field trials have reported impressive levels of suppression17,18. However, our analyses suggest several potential problems with ADT: a lack of bifurcation makes elimination difficult; ADT works well at high, but not low, mosquito densities; some degree of population recovery is expected once pyriproxifen levels fall; very high EI has yet to be demonstrated over prolonged periods, or in the absence of Ae. aegypti. Coupling ADT with SIT or BSIT therefore makes sense. These methods work best at low densities and introduce a bifurcation that renders zero a stable equilibrium. Thus, so long as release rates greater than RThresh are maintained, population recovery can be kept in check.
Our model is relatively simple and relies on laboratory data for the dose–response curve33 and venereal pyriproxifen transfer11. Using data from alternative emergence-inhibition studies had little impact on the difference between and . Although our estimate of female induced pyriproxifen transfer (p) relies on one key study11, by neglecting direct male induced contamination of larval sites we likely underestimate pyriproxifen accumulation37—particularly at low female densities. The magnitude of pupicidal action in our model corresponds well with field-cage experiments and field trials that measured the impacts of pyriproxifen transfer from (non-sterilised) males to females and larval sites37. Moreover, whilst control trials with transgenic mosquito OX513A in Brazil and the Cayman Islands have demonstrated suppression of Ae. aegypti populations, in both cases the release area was reduced mid-trial to augment R locally40,60, and elimination with transgenic mosquitoes has never been demonstrated8. Similarly, field trials with the incompatible insect technique have only demonstrated suppression, not elimination, resulting in the up-scaling of insect production61. Our analyses are consistent with these observations, and explain why (Fig. 1e) sustaining sufficient release rates for Ae. aegypti elimination appears unrealistic given the competitiveness (h < 0.0640) of OX513A. Boosting offers a powerful solution to the pragmatic and economic difficulties of Aedes elimination with released male methods.
Historically, Ae. aegypti has been the vector primarily associated with dengue. Some authors have questioned whether confounding factors, such as historical geographical distribution, have led us to underestimate the vector competence of Ae. albopictus62. The traditional view was that epidemics associated with Ae. albopictus were small—such as those in Tokyo55, Guangdong (before 2014)63 and Arunachal Pradesh64. However, this view has been challenged by large outbreaks on Reunion Island (over 8500 indigenous cases in 2018–201965) and in Guangdong (over 45,000 indigenous cases in 201466). The auto-dissemination technique has been shown to provide EI > 90% in the presence of Ae. aegypti, apparently with sufficient suppression to achieve R0 < 118. However, the same level of suppression has yet to be demonstrated where Ae. albopictus is the sole vector. Our results suggest that BSIT can provide greater protection from dengue than is possible using ADT or SIT alone.
Whether or not mosquito abatement achieves R0 < 1 depends upon numerous factors. We calculated R0 using two different parameters sets reflecting variation in the literature of some key parameters. Whether or not those parameters are appropriate in a given control scenario will depend upon the specifics of the local ecology. We neglect several sources of complexity such as temperature effects67,68, variation in the availability of alternative hosts69,70, dispersion34 and landscape effects35. We do this for simplicity and emphasise that care is required when extrapolating our results to real systems. Further simulation studies accounting for seasonality and spatial heterogeneity in mosquito ecology would be beneficial for generalising our results to real control scenarios.
One highly variable R0 parameter is the vector-host ratio—but estimating the density of an insect that is a master of stealth is difficult. Vector densities can be highly aggregated in space71 and localised hot-spots play an important epidemiological role55. Without detailed knowledge of local vector densities, R0 studies rely on assumptions such as simply assuming the ratio is two49, or that trap density and true density are equivalent18. Sophisticated density estimation studies have used mark-release-recapture with BG-Sentinel traps and modern spatial statistics72,73—although these studies often report much lower densities than those given by human landing collections. In Forli district, Italy, human landing collections in August averaged 5.73 females (s.d. = 4.48) in 15 min54. Fitting a negative binomial distribution to that data suggests 5% of sampling sites provided over 14 females per human-trap in 15 min—numbers well within the range of findings from a park in Tokyo55. Assuming the majority of biting females within a 7 m radius are sampled within 15 min, extrapolating over 1 ha and using the bite rate used in that study (0.258), gives a mean of females/ha and a 95th percentile of females/ha. This later estimate is similar to the carrying capacity of our study (F = 3283.9)—thus our ecological assumptions are coherent with known hot-spots in Italy. On Reunion Island, densities of over 5800 males/ha have been reported using mark-release-recapture with mice-baited BG-Sentinel traps74—our modelling predicts that, if using SIT alone, it would be very difficult to bring R0 below one with such high mosquito densities.
The current study has focused on control within 1 ha. Given that Aedes densities display spatial auto-correlation over just some hundreds of metres71, 1 ha would be a suitable pixel size for an R0 mapping study75,76 in an urban area. Although we have not modelled spatial effects, it is important to remember spatial processes when interpreting our results. Bringing R0 below one locally may have near-zero impact on the epidemiology across a large city77. Also, whilst BSIT might be able to achieve elimination with , this phenomenon relies on high mosquito densities generating a large pyriproxifen peak. Subsequent immigration would facilitate population recovery unless R was greater than , or some higher threshold if using a ADT-SIT combination without boosting. Thus an area-wide vector management strategy is recommended, and spatial simulation models, extending the current model by including crucial sources of ecological variation, are expected to provide valuable information for planning mosquito control.
Whilst extreme weather events can reduce pyriproxifen efficacy78, large ADT trials in towns of the Amazonian rain-forest suggest regular rainfall does not prevent population suppression17,18. In our model, we neglect this potential source of variation. We also do not include variation in container size, thus heterogeneity in pyriproxifen concentrations is neglected. Whilst water tanks and tires are known to provide good larval habitat for Aedes, large bodies of water, such as stagnant swimming pools, appear to be less important—particularly when they receive little shade79. Moreover, large non-cryptic habitats are relatively easy to identify and treat manually (with pyriproxifen granules, for example ref. 80). The greatest difficulty faced by traditional methods of Aedes control comes from the high aptitude of these mosquitoes to utilise small cryptic habitats that are protected from insecticide spraying. It is here that both SIT and ADT excel, both techniques utilise mosquito behaviour to bypass the limits of conventional spraying methods. Moreover, where bigger pools are attractive to Aedes, they will attract more pyriproxifen carrying mosquitoes, thus increasing pyriproxifen accumulation. Further research is required to fully understand the effects of container size and climate in ADT and BSIT field trials.
Pyriproxifen is highly toxic for all water invertebrates, thus care should be taken regarding undesirable ecological impacts of its use. However, with BSIT or ADT, pyriproxifen contaminated females are expected to specifically contaminate their larval habitats. In urban areas, 95% of Ae. albopictus breeding habitats are domestic containers and 99% are of artificial type81—factors which should limit the risks for non-target fauna. Thus, the environmental risks are expected to be much lower than those of the widely used technique of ultra-low volume spraying78. However, it is important to monitor the impacts of ADT and BSIT on non-target organisms when testing in field conditions—a factor that has been overlooked in many field trials to date.
In light of the large effects predicted here—and the coherence between model results and available field trial data—we urge mosquito control practitioners/developers to include BSIT in their field trials to further quantify its potential. Although we have concentrated on pyriproxifen, alternative biopesticides could be used. Densoviruses, for example, may advantageously provide: greater species specificity; replication at larval sites, ensuring efficacy even with low transfer rates; and an additional tool for resistance management31. Given the aptitude of Aedes mosquitoes for range expansion, the high burden of associated epidemics, and the resilience of R0 to modest declines in vector density, the results of such trials would be of great importance for global health management.
Methods
Boosted sterile insect technique model
The dynamics of an Aedes population in response to the sterile insect technique (SIT), boosted sterile insect technique (BSIT) and/or auto-dissemination technique (ADT) were modelled using the following system of ordinary differential equations.
| 1 |
| 2 |
| 3 |
| 4 |
| 5 |
| 6 |
| 7 |
| 8 |
Compartments in this system include eggs (E), larvae (L), pupae (P), adult females (F), adult males (M), adult sterile males (S), pyriproxifen carrying (contaminated) adult females (Fc) and the quantity of pyriproxifen at larval sites (C). Parameters (Supplementary Table 1) include daily release rate (R); dissemination station density (A); gonotrophic cycle rate (g); female fecundity per gonotrophic cycle (f ); maturation rates of juveniles (mE, mL, mP); mortality rates of eggs (μE), pupae (μP), females (μF), contaminated females (μc), males (μM) and sterile males (μS), larval mortality—a linear function of larval density rising from μ0 at L = 0 to μK at L = K (carrying capacity); the proportion of females among emerging adults (ρ); the number of larval sites (N); the volume of water at larval sites, V = V1 × N, where V1 is the mean volume per site; the carrying capacity at larval sites, K = K1 × N, where K1 is the mean carrying capacity per site; the contamination level (in parts per billion, ppb) generating 50% emergence inhibition (EI50); the slope of the dose–response curve modelling emergence inhibition among maturing juveniles (σ); the mating rate of wild males (r); competitiveness of sterile males (h); the mating rate of sterile males (rh); viability of eggs from contaminated females (q); oviposition rate (γ); expected number of ovipositions per gonotrophic cycle (κ); number of ovipositions required to clear contamination (κc) and the expected quantity of pyriproxifen deposited by contaminated females at oviposition (p).
The term is a classic representation of sexual competition32, providing the proportion of couplings involving wild-type males (Eq. (1)). Sterilisation is assumed absolute. The competitiveness of sterile males, h, is the ratio of (per capita) sterile male to wild male coupling rates. The strength of sexual competition depends on h and the relative frequency of M and S—represented as two red dashed arrows in Supplementary Fig. 1. Based on Gaugler et al.11, we assume adult females become contaminated when they couple with males carrying pyriproxifen. These events occur at rate per female (Supplementary Fig. 1, red dashed arrows). These females deposit p µg of pyriproxifen at larval sites according to oviposition rate (γ) and lose their pyriproxifen after an expected κc ovipositions—we assume κc = 1 throughout. The term provides the emergence success according to a logit(EI) ~ ln(C/V) dose–response curve (Supplementary Fig. 1, red dashed arrows). Swapping the logit function for probit, and/or the natural logarithm for log10, made little difference to the dose–response curve—hence, we adopted the algebraically and computationally more convenient form. Parameter σ gives the slope of a straight line on the transformed scales. The model assumes pyriproxifen degrades in the environment at constant rate d.
Equilibria analysis
Differential Eqs. (1)–(8) return gradients of zero at (respectively)
| 9 |
| 10 |
| 11 |
| 12 |
| 13 |
| 14 |
| 15 |
| 16 |
where * indicates the values at which the respective ordinary differential equations have zero gradient and
A trivial equilibrium of the system exists at and S* = R/μS. Non-trivial equilibria of the system are found (for a given R) at the intersections of the following two curves describing M* as a function of F. With R and F fixed, S*, and C* are obtained from Eqs. (14)–(16). Then, assuming F′ = F′c = M′ = 0, a process of substitution using Eqs. (4) and (7) gives
| 17 |
Secondly, assuming E′ = L′ = P′ = M′ = S′ = F′c = C′ = 0, we obtain
| 18 |
where
When R < RThresh, the curves (17) and (18) intersect at two points, giving one stable equilibrium and one unstable equilibrium. When R = RThresh, the curves meet at a single point. When R > RThresh, the curves no longer intersect and the population will eventually be eliminated—irrespective of the initial population density. The equilibria can be found using standard root finding algorithms.
Parameterisation of BSIT model
Parameters were set to values obtained from the literature (Supplementary Table 1). Shape parameter σ of the dose–response curve was estimated from published EI50 and EI95 data33 as the slope of the straight line linking these two data points on transformed (logit(EI) ~ ln(C/V)) scales. The quantity of pyriproxifen deposited by a female at oviposition (p) was estimated by using the dose–response curve to predict the concentration of pyriproxifen in the water of the venereal transfer experiments of Gaugler et al.11 based on their reported emergence inhibition. The quantity deposited per oviposition (p) was obtained assuming contaminated females lose their pyriproxifen in a single oviposition. The obtained value of p was then divided by five to account for Gaugler et al. using five males to one female in their venereal transfer experiment. Using alternative emergence inhibition data to generate the dose–response curve had relatively little impact on our modelling results— was estimated as 286.1, 253.9, 299.0, 174.9 and 328.2 using emergence inhibition data from refs. 33,82–84 and ref. 85 (Rockefeller strain), respectively. The relative viability of eggs from contaminated females (q) was assessed experimentally86. Two to five-day-old fertile males were sprayed with a dry powder containing 20% pyriproxifen and mated with 5-day-old virgin females. Egg papers were dried for 24 h and emergence was monitored for 8 days. The expected value of q and bootstrap 95% confidence intervals are shown in Supplementary Table 1.
In our model, each female mosquito is contaminated at ADT dissemination stations with rate α × A—for brevity we call this rate the ADT “intensity”. Field trial data (Supplementary Table 3) provided five different estimates of α. For each trial we identified the α that minimised the absolute error between reported and fitted EI at a given point in time. We assumed EI at larval sites matched EI in ovitraps. Minimisation was performed over a finite sequence of 100 evenly spaced values spanning two orders of magnitude. The appropriateness of the bounds of this finite set were checked visually by plotting the absolute error for each potential α. Since our model is deliberately simple, we did not expect it to characterise the full range of EI variation observed in the field. Therefore, we did not explore more complicated methods (such as Bayesian methods87,88) for fitting mechanistic models. Regarding uncertainty in α, we note that the estimates are highly variable between studies, and that an estimate from any one study might not transfer well to other ecological contexts.
Time to elimination
The time required to bring the total population size below one, when initialised at carrying capacity (the control-free stable equilibrium), was evaluated using R function ode89 and is called “elimination time” throughout the paper. All such simulations used a constant sterile male daily release rate R. The quantity RTotal was defined as the product of elimination time and R.
Sensitivity analyses
Eight sensitivity analyses with the BSIT/SIT model were performed (Supplementary Figs. 3 and 4). Parameters were sampled uniformly over the plotted ranges, all other parameters were set to default values (Supplementary Table 1). Each experiment consisted of 105 randomisations of the selected parameter set. Trends in the generated data clouds were explored using the R function loess90.
Dengue transmission model
The epidemiological model of dengue transmission was adapted from ref. 41 by splitting compartment F into FS, FE and FI and compartment Fc into , and . The model uses Eqs. (1)–(3), (5)–(6) and (8) (where F and Fc are the sum of their respective sub-compartments) and the following sub-system:
| 19 |
| 20 |
| 21 |
| 22 |
| 23 |
| 24 |
| 25 |
| 26 |
| 27 |
| 28 |
where is the total population density of adult females, is the total population density of humans, b is the bite rate of a single female, βF is the probability that a susceptible female mosquito becomes infected having bitten an infectious human, βH is the probability that a susceptible human becomes infected following a bite from an infectious mosquito, θF and θH are (respectively) the extrinsic and intrinsic incubation rates and αH is the recovery rate in humans.
The basic reproductive number of dengue transmission
The basic reproductive number (R0) was calculated using the next generation matrix approach91,92. Assuming the mortality rate of females carrying pyriproxifen (μc) equals that of females without pyriproxifen (μF) permits R0 to be written
| 29 |
Throughout, we assume people/ha, a population density typical of many European cities (such as Montpellier or Seville). Two alternative parameterizations were used (Supplementary Table 3), reflecting variation in the literature and providing “optimistic” and “pessimistic” estimates of R0.
Stochastic simulation of population dynamics under control
To incorporate the effects of demographic stochasticity in small populations, and the non-equilibrium dynamics in the first months of control, stochastic simulation (of integer events) was performed using a modification of Gillespie’s direct algorithm56. To reduce computation time we incorporated modifications presented in ref. 57 and adopted the following two approximations: eggs were generated in batches per oviposition event with batch size set as either a draw from a Poisson distribution (when E < 5000) or the expected number of new eggs (at higher densities); egg maturation and mortality was simulated using either the tau-leap method (when E < 5000)93 or by using the solution to the linear ordinary differential equations (at higher densities). The algorithm was coded in Nimble94, which automatically compiles code with R-like syntax to C++. Scripts for all analyses are available. For SIT and BSIT, nine values of R were evenly spaced in the intervals [0, 1600] and [0, 320], respectively. ADT was applied at four levels of intensity corresponding to either no ADT, or ADT with intensity equivalent to Caputo et al.12, Abad-Franch et al.17 with A reduced from 14 to 4 or Abad-Franch et al.18 with A increased from 1.54 to 2. The values of α and A used in each scenario are shown in Fig. 4. Ten simulations were performed for each R ~ A combination. In each simulation, mosquitoes were initialised by drawing Poisson random numbers with expectancies given by the control-free stable equilibrium, and control parameters were held constant for 3 years.
Reporting summary
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
Supplementary information
Acknowledgements
The authors would like to thank Mark Vreysen, Kostas Bourtzis, Andrew Parker, Nicole Culbert, Thierry Baldet and Haoues Alout for comments on early versions of this paper and Lyse Primault, Geoffrey Gimonneau and Fabrice Chandre for providing egg viability data. We also thank Randy Gaugler and Devi Shankar Suman for discussions regarding their venereal transfer experiment. Financial support was provided by the Institute Carnot Santé Animale (IVEMA project) and the REVOLINC (Revolutionising Insect Control) ERC Consolidator Grant. This project has received funding from the European Research Council (ERC) under the European Union’s Horizon 2020 research and innovation programme (grant agreement No 682387).
Author contributions
J.B. (lead) and D.R.J.P. (supporting) conceived the study and acquired funding. D.R.J.P. (lead) and J.B. (supporting) created and analysed the models and wrote the paper.
Data availability
Data sharing not applicable to this article as no datasets were generated or analysed during the current study.
Code availability
All code used in the current study is available at the following Bitbucket repository https://DRJP@bitbucket.org/DRJP/pleydell-bouyer-2019.git.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
David R. J. Pleydell, Email: david.pleydell@inra.fr
Jérémy Bouyer, Email: j.bouyer@iaea.org.
Supplementary information
Supplementary information accompanies this paper at 10.1038/s42003-019-0451-1.
References
- 1.Bhatt S, et al. The global distribution and burden of dengue. Nature. 2013;496:504–507. doi: 10.1038/nature12060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Leparc-Goffart I, Nougairede A, Cassadou S, Prat C, De Lamballerie X. Chikungunya in the Americas. Lancet. 2014;383:514. doi: 10.1016/S0140-6736(14)60185-9. [DOI] [PubMed] [Google Scholar]
- 3.Lessler J., Chaisson L. H., Kucirka L. M., Bi Q., Grantz K., Salje H., Carcelen A. C., Ott C. T., Sheffield J. S., Ferguson N. M., Cummings D. A. T., Metcalf C. J. E., Rodriguez-Barraquer I. Assessing the global threat from Zika virus. Science. 2016;353(6300):aaf8160–aaf8160. doi: 10.1126/science.aaf8160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Paules CI, Fauci AS. Yellow fever—once again on the radar screen in the Americas. New. Engl. J. Med. 2017;376:1397–1399. doi: 10.1056/NEJMp1702172. [DOI] [PubMed] [Google Scholar]
- 5.Poland GA, et al. Development of vaccines against Zika virus. Lancet Infect. Dis. 2018;18:e211–e219. doi: 10.1016/S1473-3099(18)30063-X. [DOI] [PubMed] [Google Scholar]
- 6.Powers AM. Vaccine and therapeutic options to control Chikungunya virus. Clin. Microbiol. Rev. 2018;31:e00104–16. doi: 10.1128/CMR.00104-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Flipse J, Smit JM. The complexity of a dengue vaccine: a review of the human antibody response. PLoS Negl. Trop. D. 2015;9:e0003749. doi: 10.1371/journal.pntd.0003749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Flores HA, O’Neill SL. Controlling vector-borne diseases by releasing modified mosquitoes. Nat. Rev. Microbiol. 2018;16:508–518. doi: 10.1038/s41579-018-0025-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Baldacchino F, et al. Control methods against invasive Aedes mosquitoes in Europe: a review. Pest Manag. Sci. 2015;71:1471–1485. doi: 10.1002/ps.4044. [DOI] [PubMed] [Google Scholar]
- 10.World Health Organization, UNICEF et al. Global Vector Control Response 2017–2030 (World Health Organization, 2017).
- 11.Gaugler R, Suman D, Wang Y. An autodissemination station for the transfer of an insect growth regulator to mosquito oviposition sites. Med. Vet. Entomol. 2012;26:37–45. doi: 10.1111/j.1365-2915.2011.00970.x. [DOI] [PubMed] [Google Scholar]
- 12.Caputo B, et al. The auto-dissemination approach: a novel concept to fight Aedes albopictus in urban areas. PLoS Negl. Trop. D. 2012;6:e1793. doi: 10.1371/journal.pntd.0001793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chandel K, et al. Targeting a hidden enemy: pyriproxyfen autodissemination strategy for the control of the container mosquito Aedes albopictus in cryptic habitats. PLoS Negl. Trop. D. 2016;10:e0005235. doi: 10.1371/journal.pntd.0005235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Unlu I, et al. Effectiveness of autodissemination stations containing pyriproxyfen in reducing immature Aedes albopictus populations. Parasit. Vectors. 2017;10:139. doi: 10.1186/s13071-017-2034-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Suman DS, et al. Seasonal field efficacy of pyriproxyfen autodissemination stations against container-inhabiting mosquito Aedes albopictus under different habitat conditions. Pest Manag. Sci. 2017;74:885–895. doi: 10.1002/ps.4780. [DOI] [PubMed] [Google Scholar]
- 16.Devine GJ, et al. Using adult mosquitoes to transfer insecticides to Aedes aegypti larval habitats. Proc. Natl Acad. Sci. USA. 2009;106:11530–11534. doi: 10.1073/pnas.0901369106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Abad-Franch F, Zamora-Perea E, Ferraz G, Padilla-Torres SD, Luz SLB. Mosquito-disseminated pyriproxyfen yields high breeding-site coverage and boosts juvenile mosquito mortality at the neighborhood scale. PLoS Negl. Trop. D. 2015;9:e0003702. doi: 10.1371/journal.pntd.0003702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Abad-Franch F, Zamora-Perea E, Luz SL. Mosquito-disseminated insecticide for citywide vector control and its potential to block arbovirus epidemics: entomological observations and modeling results from Amazonian Brazil. PLoS Med. 2017;14:e1002213. doi: 10.1371/journal.pmed.1002213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kartzinel MA, Alto BW, Deblasio MW, Burkett-Cadena ND. Testing of visual and chemical attractants in correlation with the development and field evaluation of an autodissemination station for the suppression of Aedes aegypti and Aedes albopictus in Florida. J. Am. Mosq. Contr. 2016;32:194–202. doi: 10.2987/16-6555.1. [DOI] [PubMed] [Google Scholar]
- 20.Knipling EF. Sterile-male method of population control: successful with some insects, the method may also be effective when applied to other noxious animals. Science. 1959;130:902–904. doi: 10.1126/science.130.3380.902. [DOI] [PubMed] [Google Scholar]
- 21.Bourtzis K, Lees RS, Hendrichs J, Vreysen MJB. More than one rabbit out of the hat: radiation, transgenic and symbiont-based approaches for sustainable management of mosquito and tsetse fly populations. Acta Trop. 2016;157:115–130. doi: 10.1016/j.actatropica.2016.01.009. [DOI] [PubMed] [Google Scholar]
- 22.Zhang DJ, Zheng XY, Xi ZY, Bourtzis K, Gilles JRL. Combining the sterile insect technique with the incompatible insect technique: I-impact of Wolbachia infection on the fitness of triple-and double-infected strains of Aedes albopictus. PLoS ONE. 2015;10:e0121126. doi: 10.1371/journal.pone.0121126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mains JW, Brelsfoard CL, Rose RI, Dobson SL. Female adult Aedes albopictus suppression by Wolbachia-infected male mosquitoes. Sci. Rep. 2016;6:33846. doi: 10.1038/srep33846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Alphey L. Genetic control of mosquitoes. Annu. Rev. Entomol. 2014;59:205–224. doi: 10.1146/annurev-ento-011613-162002. [DOI] [PubMed] [Google Scholar]
- 25.Kyrou K, et al. A CRISPR–Cas9 gene drive targeting doublesex causes complete population suppression in caged Anopheles gambiae mosquitoes. Nat. Biotechnol. 2018;36:1062–1066. doi: 10.1038/nbt.4245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wyss JH. Screwworm eradication in the Americas. Ann. N. Y. Acad. Sci. 2000;916:186–193. doi: 10.1111/j.1749-6632.2000.tb05289.x. [DOI] [PubMed] [Google Scholar]
- 27.Enkerlin WR, et al. The Moscamed regional programme: review of a success story of area-wide sterile insect technique application. Entomol. Exp. Appl. 2017;164:188–203. doi: 10.1111/eea.12611. [DOI] [Google Scholar]
- 28.Vreysen MJB, et al. Sterile insects to enhance agricultural development: the case of sustainable tsetse eradication on Unguja Island, Zanzibar, using an area-wide integrated pest management approach. PLoS Negl. Trop. D. 2014;8:e2857. doi: 10.1371/journal.pntd.0002857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bellini R, Medici A, Puggioli A, Balestrino F, Carrieri M. Pilot field trials with Aedes albopictus irradiated sterile males in Italian urban areas. J. Med. Entomol. 2013;50:317–325. doi: 10.1603/ME12048. [DOI] [PubMed] [Google Scholar]
- 30.Bouyer J, Lefrançois T. Boosting the sterile insect technique to control mosquitoes. Trends Parasitol. 2014;30:271–273. doi: 10.1016/j.pt.2014.04.002. [DOI] [PubMed] [Google Scholar]
- 31.Bouyer J, Chandre F, Gilles J, Baldet T. Alternative vector control methods to manage the Zika virus outbreak: more haste, less speed. Lancet Glob. Health. 2016;4:e364. doi: 10.1016/S2214-109X(16)00082-6. [DOI] [PubMed] [Google Scholar]
- 32.Fried M. Determination of sterile-insect competitiveness. J. Econ. Entomol. 1971;64:869–872. doi: 10.1093/jee/64.4.869. [DOI] [Google Scholar]
- 33.Dell Chism B, Apperson CS. Horizontal transfer of the insect growth regulator pyriproxyfen to larval microcosms by gravid Aedes albopictus and Ochlerotatus triseriatus mosquitoes in the laboratory. Med. Vet. Entomol. 2003;17:211–220. doi: 10.1046/j.1365-2915.2003.00433.x. [DOI] [PubMed] [Google Scholar]
- 34.Winskill P, et al. Dispersal of engineered male Aedes aegypti mosquitoes. PLoS Negl. Trop. D. 2015;9:e0004156. doi: 10.1371/journal.pntd.0004156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Weterings R, Umponstira C, Buckley HL. Landscape variation influences trophic cascades in dengue vector food webs. Sci. Adv. 2018;4:eaap9534. doi: 10.1126/sciadv.aap9534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fonseca DM, Kaplan LR, Heiry RA, Strickman D. Density-dependent oviposition by female Aedes albopictus (Diptera: Culicidae) spreads eggs among containers during the summer but accumulates them in the fall. J. Med. Entomol. 2015;1:8. doi: 10.1093/jme/tjv060. [DOI] [PubMed] [Google Scholar]
- 37.Mains JW, Brelsfoard CL, Dobson SL. Male mosquitoes as vehicles for insecticide. PLoS Negl. Trop. D. 2015;9:e0003406–e0003406. doi: 10.1371/journal.pntd.0003406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Suman DS, Wang Y, Dong L, Gaugler R. Effects of larval habitat substrate on pyriproxyfen efficacy against Aedes albopictus (Diptera: Culicidae) J. Med. Entomol. 2013;50:1261–1266. doi: 10.1603/ME13068. [DOI] [PubMed] [Google Scholar]
- 39.Clutton-Brock T, Langley P. Persistent courtship reduces male and female longevity in captive tsetse flies Glossina morsitans morsitans Westwood (Diptera: Glossinidae) Behav. Ecol. 1997;8:392–395. doi: 10.1093/beheco/8.4.392. [DOI] [Google Scholar]
- 40.Carvalho DO, et al. Suppression of a field population of Aedes aegypti in Brazil by sustained release of transgenic male mosquitoes. PLoS Negl. Trop. D. 2015;9:e0003864. doi: 10.1371/journal.pntd.0003864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.de Pinho STR, et al. Modelling the dynamics of dengue real epidemics. Philos. T. R. Soc. A. 2010;368:5679–5693. doi: 10.1098/rsta.2010.0278. [DOI] [PubMed] [Google Scholar]
- 42.Wesolowski A, et al. Impact of human mobility on the emergence of dengue epidemics in Pakistan. Proc. Natl Acad. Sci. USA. 2015;112:11887–11892. doi: 10.1073/pnas.1504964112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhu D, Ren J, Zhu H. Spatial-temporal basic reproduction number and dynamics for a dengue disease diffusion model. Math. Methods Appl. Sci. 2018;41:5388–5403. doi: 10.1002/mma.5085. [DOI] [Google Scholar]
- 44.Lambrechts L, et al. Impact of daily temperature fluctuations on dengue virus transmission by Aedes aegypti. Proc. Natl Acad. Sci. USA. 2011;108:7460–7465. doi: 10.1073/pnas.1101377108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Duong V, et al. Asymptomatic humans transmit dengue virus to mosquitoes. Proc. Natl Acad. Sci. USA. 2015;112:14688–14693. doi: 10.1073/pnas.1508114112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang T, et al. Evaluation of inapparent dengue infections during an outbreak in Southern China. PLoS Negl. Trop. D. 2015;9:e0003677. doi: 10.1371/journal.pntd.0003677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wonham MJ, Lewis MA, Rencławowicz J, Van den Driessche P. Transmission assumptions generate conflicting predictions in host–vector disease models: a case study in West Nile virus. Ecol. Lett. 2006;9:706–725. doi: 10.1111/j.1461-0248.2006.00912.x. [DOI] [PubMed] [Google Scholar]
- 48.Chitnis N, Cushing J, Hyman J. Bifurcation analysis of a mathematical model for malaria transmission. SIAM J. Appl. Math. 2006;67:24–45. doi: 10.1137/050638941. [DOI] [Google Scholar]
- 49.Manore CA, Hickmann KS, Xu S, Wearing HJ, Hyman JM. Comparing dengue and Chikungunya emergence and endemic transmission in Ae. aegypti and Ae. albopictus. J. Theor. Biol. 2014;356:174–191. doi: 10.1016/j.jtbi.2014.04.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wearing HJ, Rohani P. Ecological and immunological determinants of dengue epidemics. Proc. Natl Acad. Sci. USA. 2006;103:11802–11807. doi: 10.1073/pnas.0602960103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Recker M, et al. Immunological serotype interactions and their effect on the epidemiological pattern of dengue. Proc. R. Soc. Lond., B, Biol. Sci. 2009;276:2541–2548. doi: 10.1098/rspb.2009.0331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Joanne S, et al. Vector competence of Malaysian Aedes albopictus with and without Wolbachia to four dengue virus serotypes. Trop. Med. Int. Health. 2017;22:1154–1165. doi: 10.1111/tmi.12918. [DOI] [PubMed] [Google Scholar]
- 53.Amaku M, et al. Magnitude and frequency variations of vector-borne infection outbreaks using the Ross–Macdonald model: explaining and predicting outbreaks of dengue fever. Epidemiol. Infect. 2016;144:3435–3450. doi: 10.1017/S0950268816001448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Carrieri M, Angelini P, Venturelli C, Maccagnani B, Bellini R. Aedes albopictus (Diptera: Culicidae) population size survey in the 2007 Chikungunya outbreak area in Italy. II: Estimating epidemic thresholds. J. Med. Entomol. 2012;49:388–399. doi: 10.1603/ME10259. [DOI] [PubMed] [Google Scholar]
- 55.Tsuda Y, et al. Biting density and distribution of Aedes albopictus during the September 2014 outbreak of dengue fever in Yoyogi Park and the vicinity in Tokyo Metropolis, Japan. Jpn. J. Infect. Dis. 2015;69:1–5. doi: 10.7883/yoken.JJID.2014.576. [DOI] [PubMed] [Google Scholar]
- 56.Gillespie DT. A general method for numerically simulating the stochastic time evolution of coupled chemical reactions. J. Comput. Phys. 1976;22:403–434. doi: 10.1016/0021-9991(76)90041-3. [DOI] [Google Scholar]
- 57.Gibson MA, Bruck J. Efficient exact stochastic simulation of chemical systems with many species and many channels. J. Phys. Chem. A. 2000;104:1876–1889. doi: 10.1021/jp993732q. [DOI] [Google Scholar]
- 58.World Health Organization et al. Public Health Impact of Pesticides Used in Agriculture (WHO/UNEP, 1990).
- 59.Lu YH, Wu KM, Jiang YY, Guo YY, Desneux N. Widespread adoption of Bt cotton and insecticide decrease promotes biocontrol services. Nature. 2012;487:362. doi: 10.1038/nature11153. [DOI] [PubMed] [Google Scholar]
- 60.Harris AF, et al. Successful suppression of a field mosquito population by sustained release of engineered male mosquitoes. Nat. Biotechnol. 2012;30:828. doi: 10.1038/nbt.2350. [DOI] [PubMed] [Google Scholar]
- 61.Gilbert JA, Melton L. Verily project releases millions of factory-reared mosquitoes. Nat. Biotechnol. 2018;36:781–782. doi: 10.1038/nbt0918-781a. [DOI] [PubMed] [Google Scholar]
- 62.Rezza G. Aedes albopictus and the reemergence of Dengue. BMC Public Health. 2012;12:72. doi: 10.1186/1471-2458-12-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Peng HJ, et al. A local outbreak of dengue caused by an imported case in Dongguan China. BMC Public Health. 2012;12:83. doi: 10.1186/1471-2458-12-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Khan SA, Dutta P, Topno R, Soni M, Mahanta J. Dengue outbreak in a hilly state of Arunachal Pradesh in Northeast India. Sci. World J. 2014;2014:1–6. doi: 10.1155/2014/584093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Agences Régionales de Santé, Océan Indien. Epidémie de dengue à La Réunion: la circulation du virus s’accélère. https://www.ocean-indien.ars.sante.fr/system/files/2019-03/2019-03-12_CP_ARSOI_Epidemie%20Dengue%202019_1.pdf. Date accessed: 12 March 2019.
- 66.Xiao JP, et al. Characterizing a large outbreak of dengue fever in Guangdong Province, China. Infect. Dis. Poverty. 2016;5:44. doi: 10.1186/s40249-016-0131-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Carrington LB, Armijos MV, Lambrechts L, Scott TW. Fluctuations at a low mean temperature accelerate dengue virus transmission by Aedes aegypti. PLoS Negl. Trop. D. 2013;7:e2190. doi: 10.1371/journal.pntd.0002190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Carrington LB, Seifert SN, Armijos MV, Lambrechts L, Scott TW. Reduction of Aedes aegypti vector competence for dengue virus under large temperature fluctuations. Am. J. Trop. Med. 2013;88:689–697. doi: 10.4269/ajtmh.12-0488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sawabe K, et al. Host-feeding habits of Culex pipiens and Aedes albopictus (Diptera: Culicidae) collected at the urban and suburban residential areas of Japan. J. Med. Entomol. 2010;47:442–450. doi: 10.1093/jmedent/47.3.442. [DOI] [PubMed] [Google Scholar]
- 70.Barrera R, et al. Vertebrate hosts of Aedes aegypti and Aedes mediovittatus (Diptera: Culicidae) in rural Puerto Rico. J. Med. Entomol. 2012;49:917–921. doi: 10.1603/ME12046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Tantowijoyo W, et al. Spatial and temporal variation in Aedes aegypti and Aedes albopictus (Diptera: Culicidae) numbers in the Yogyakarta Area of Java, Indonesia, with implications for Wolbachia releases. J. Med. Entomol. 2015;53:188–198. doi: 10.1093/jme/tjv180. [DOI] [PubMed] [Google Scholar]
- 72.Johnson P, Spitzauer V, Ritchie S. Field sampling rate of BG-Sentinel traps for Aedes aegypti (Diptera: Culicidae) in suburban Cairns, Australia. J. Med. Entomol. 2012;49:29–34. doi: 10.1603/ME11116. [DOI] [PubMed] [Google Scholar]
- 73.Villela DA, et al. Bayesian hierarchical model for estimation of abundance and spatial density of Aedes aegypti. PLoS ONE. 2015;10:e0123794. doi: 10.1371/journal.pone.0123794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Le Goff G, et al. Field evaluation of seasonal trends in relative population sizes and dispersal pattern of Aedes albopictus males in support of the design of a sterile male release strategy. Parasit. Vectors. 2019;12:81. doi: 10.1186/s13071-019-3329-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hartemink N, et al. Mapping the basic reproduction number (R0) for vector-borne diseases: a case study on bluetongue virus. Epidemics. 2009;1:153–161. doi: 10.1016/j.epidem.2009.05.004. [DOI] [PubMed] [Google Scholar]
- 76.Guis H, et al. Modelling the effects of past and future climate on the risk of bluetongue emergence in Europe. J. R. Soc. Interface. 2011;9:339–350. doi: 10.1098/rsif.2011.0255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Greenhalgh D, et al. Modeling the effect of a novel auto-dissemination trap on the spread of dengue in high-rise condominia, Malaysia. J. Biol. Syst. 2018;26:553–578. doi: 10.1142/S0218339018500250. [DOI] [Google Scholar]
- 78.Suman DS, et al. Point-source and area-wide field studies of pyriproxyfen autodissemination against urban container-inhabiting mosquitoes. Acta Trop. 2014;135:96–103. doi: 10.1016/j.actatropica.2014.03.026. [DOI] [PubMed] [Google Scholar]
- 79.Carrieri M, Bacchi M, Bellini R, Maini S. On the competition occurring between Aedes albopictus and Culex pipiens (Diptera: Culicidae) in Italy. Environ. Entomol. 2003;32:1313–1321. doi: 10.1603/0046-225X-32.6.1313. [DOI] [Google Scholar]
- 80.Ritchie SA, Paton C, Buhagiar T, Webb GA, Jovic V. Residual treatment of Aedes aegypti (Diptera: Culicidae) in containers using pyriproxyfen slow-release granules (Sumilarv 0.5G) J. Med. Entomol. 2013;50:1169–1172. doi: 10.1603/ME13043. [DOI] [PubMed] [Google Scholar]
- 81.Chan K, Ho B, Chan Y. Aedes aegypti (L.) and Aedes albopictus (Skuse) in Singapore City: 2. Larval habitats. Bull. World Health Organ. 1971;44:629. [PMC free article] [PubMed] [Google Scholar]
- 82.Ali A, Nayar JK, Xue RD. Comparative toxicity of selected larvicides and insect growth regulators to a Florida laboratory population of Aedes albopictus. J. Am. Mosq. Contr. 1995;11:72–76. [PubMed] [Google Scholar]
- 83.Darriet F, Corbel V. Laboratory evaluation of pyriproxyfen and spinosad, alone and in combination, against Aedes aegypti larvae. J. Med. Entomol. 2006;43:1190–1194. doi: 10.1603/0022-2585(2006)43[1190:LEOPAS]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 84.Sihuincha M, et al. Potential use of pyriproxyfen for control of Aedes aegypti (Diptera: Culicidae) in Iquitos, Peru. J. Med. Entomol. 2005;42:620–630. doi: 10.1603/0022-2585(2005)042[0620:PUOPFC]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 85.Andrighetti MTM, Cerone F, Rigueti M, Galvani KC, da Graça Macoris MdL. Effect of pyriproxyfen in Aedes aegypti populations with different levels of susceptibility to the organophosphate temephos. Dengue. 2008;32:186. [Google Scholar]
- 86.Primault, L. Comment booster la technique de l’insecte stérile? Transfert de pyriproxyfène par les mâles aux femelles et impact sur leur reproduction (Master’s thesis, Université de Montpellier, 2015).
- 87.Pleydell DRJ, et al. Estimation of the dispersal distances of an aphid-borne virus in a patchy landscape. PLOS Comput. Biol. 2018;14:e1006085. doi: 10.1371/journal.pcbi.1006085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Peccoud J, Pleydell DRJ, Sauvion N. A framework for estimating the effects of sequential reproductive barriers: implementation using Bayesian models with field data from cryptic species. Evolution. 2018;72:2503–2512. doi: 10.1111/evo.13595. [DOI] [PubMed] [Google Scholar]
- 89.Soetaert K, Petzoldt T, Setzer RW. Solving differential equations in R: Package deSolve. J. Stat. Softw. 2010;33:1–25. [Google Scholar]
- 90.R Core Team. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna, Austria, https://www.R-project.org/ (2018).
- 91.Heffernan JM, Smith RJ, Wahl LM. Perspectives on the basic reproductive ratio. J. R. Soc. Interface. 2005;2:281–293. doi: 10.1098/rsif.2005.0042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Diekmann O, Heesterbeek J, Roberts MG. The construction of next-generation matrices for compartmental epidemic models. J. R. Soc. Interface. 2009;7:873–885. doi: 10.1098/rsif.2009.0386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Gillespie DT. Approximate accelerated stochastic simulation of chemically reacting systems. J. Chem. Phys. 2001;115:1716–1733. doi: 10.1063/1.1378322. [DOI] [Google Scholar]
- 94.de Valpine P, et al. Programming with models: writing statistical algorithms for general model structures with NIMBLE. J. Comput. Graph. Stat. 2017;26:403–417. doi: 10.1080/10618600.2016.1172487. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data sharing not applicable to this article as no datasets were generated or analysed during the current study.
All code used in the current study is available at the following Bitbucket repository https://DRJP@bitbucket.org/DRJP/pleydell-bouyer-2019.git.