Abstract
Background
An association between increased relative abundance of specific bacterial taxa in the intestinal microbiota and bacteremia has been reported in some high-risk patient populations.
Methods
We collected weekly rectal swab samples from patients at 1 long-term acute care hospital (LTACH) in Chicago from May 2015 to May 2016. Samples positive for Klebsiella pneumoniae carbapenemase-producing Klebsiella pneumoniae (KPC-Kp) by polymerase chain reaction and culture underwent 16S rRNA gene sequence analysis; relative abundance of the operational taxonomic unit containing KPC-Kp was determined. Receiver operator characteristic (ROC) curves were constructed using results from the sample with highest relative abundance of KPC-Kp from each patient admission, excluding samples collected after KPC-Kp bacteremia. Cox regression analysis was performed to evaluate risk factors associated with time to achieve KPC-Kp relative abundance thresholds calculated by ROC curve analysis.
Results
We collected 2319 samples from 562 admissions (506 patients); KPC-Kp colonization was detected in 255 (45.4%) admissions and KPC-Kp bacteremia in 11 (4.3%). A relative abundance cutoff of 22% predicted KPC-Kp bacteremia with sensitivity 73%, specificity 72%, and relative risk 4.2 (P = .01). In a multivariable Cox regression model adjusted for age, Charlson comorbidity index, and medical devices, carbapenem receipt was associated with achieving the 22% relative abundance threshold (P = .044).
Conclusion
Carbapenem receipt was associated with increased hazard for high relative abundance of KPC-Kp in the gut microbiota. Increased relative abundance of KPC-Kp was associated with KPC-Kp bacteremia. Whether bacteremia arose directly from bacterial translocation or indirectly from skin contamination followed by bloodstream invasion remains to be determined.
Keywords: carbapenemase-producing Klebsiella pneumoniae, microbiome, intestinal domination, bloodstream infection, long-term acute care hospital
In adult long-term acute care hospital patients, carbapenem receipt was associated with increased hazard for high relative abundance of Klebsiella pneumoniae carbapenemase-producing Klebsiella pneumoniae (KPC-Kp) in the gut microbiota. Increased relative abundance of KPC-Kp was associated with KPC-Kp bacteremia.
Overgrowth of potentially pathogenic microbes in the large intestine followed by systemic invasion (bacterial translocation) has been recognized among patients with neutropenia, inflammatory bowel disease, intestinal obstruction, and liver cirrhosis and in neonates [1–4]. Recently, sequence analysis of the gene that encodes the RNA component of the small ribosomal subunit (the 16S rRNA-encoding gene) has allowed culture-independent determination of the composition of gut bacterial communities (intestinal microbiota) in these and other patients [5]. In one study of adult allogeneic hematopoietic stem cell transplant recipients, a relative abundance of 30% or more of enterococci in the microbial community (ie, enterococcal “domination”) increased the risk of vancomycin-resistant enterococcal bacteremia 9-fold, and domination by Proteobacteria increased the risk of gram-negative rod bacteremia 5-fold [6]. Similar observations have been made in preterm infants with late- onset sepsis and in children undergoing therapy for newly diagnosed acute lymphoblastic leukemia [7, 8]. While the presumed mechanism for the increased risk of blood stream infection (BSI) associated with intestinal domination in these populations is bacterial translocation, alternative mechanisms are possible. These include a cutaneous pathway in which intestinal domination increases the risk of skin colonization and contamination of central venous catheter exit sites or wounds followed by bloodstream invasion with or without local infection [9].
Various factors are known to influence the intestinal microbiota, including diet, host immunity, and receipt of medications including antibiotics [10, 11]. Long-term acute care hospital (LTACH) patients are at high risk of microbiota disruption due to waning mucosal immunity associated with advanced age, underlying comorbid medical conditions, and frequent antibiotic exposures [12]. In turn, a disrupted microbiota may place LTACH patients at risk of colonization or infection with antibiotic-resistant bacteria such as Klebsiella pneumoniae carbapenemase-producing Klebsiella pneumoniae (KPC-Kp). In Chicago, KPC-Kp is endemic in LTACHs, with colonization prevalence at some facilities as high as 50% [13, 14]. While KPC-Kp BSI has been reported in LTACH patients, an association between BSI and high relative abundance of KPC-Kp in the intestinal microbiota has not been investigated [14–16].
Here, we report results of a longitudinal study in a Chicago LTACH to examine risk factors for development of increased relative abundance of KPC-Kp in the intestinal microbiota and to determine whether increased relative abundance of KPC-Kp in the gut was associated with risk of BSI.
METHODS
Study Design and Medical Record Review
We conducted a prospective, longitudinal, observational study at a 106-bed LTACH in Chicago. We collected rectal swab samples from patients at admission and weekly from 4 May 2015 to 13 May 2016. Admissions among patients who had at least 1 swab sample positive for KPC-Kp (colonized patients) were analyzed. Clinical and microbiological data were extracted from hospital information systems. We reviewed medical records of all patients with KPC-Kp BSI to investigate the cause of bacteremia. The study was reviewed and approved by the Rush University Medical Center Institutional Review Board, to which the participating LTACH had formally ceded oversight. Informed consent was waived.
Laboratory Methods
All rectal swab samples were screened for KPC-Kp by polymerase chain reaction (PCR) [17–19]. Screen-positive samples were cultured for K. pneumoniae on sheep’s blood and MacConkey agars. Mucoid, lactose-positive, oxidase-negative colonies underwent identification to the species level and were determined to be resistant to carbapenems by the MicroScan Walkaway System (Beckman Coulter, Indianapolis, IN). Klebsiella pneumoniae isolates that were confirmed to be carbapenem resistant then underwent a second round of PCR to document the presence of the blaKPC gene [17–19].
DNA was purified directly from rectal swabs using the PowerMag Soil DNA Isolation Kit (Mo Bio Laboratories, Inc., Carlsbad, CA) using the EpMotion 5075 (Eppendorf, Hamburg, Germany). We have shown in a similar patient population that the fecal microbiota from rectal swabs and fecal samples collected from the same individual were highly similar, justifying the use of rectal swabs to survey the fecal microbiota in our study [20]. Dual-index primers specific to the V4 region were used to PCR amplify the bacterial 16S rRNA gene [21] from 1μL of the sample DNA, as described previously [22, 23]. Amplicons were prepared for sequencing and sequenced using the 500 cycle MiSeq Reagent Kit v2 (Illumina, catalog no. MS-102–2003) on a MiSeq (Illumina, San Diego, CA) by the University of Michigan Microbial Systems Molecular Biology Laboratory as described previously [22]. Sequences were processed and analyzed using mothur (v.1.39.5) [24]. The mothur-adapted SILVA SEED reference alignment (release 119) was used to align and trim sequences [25]. Uchime was used to remove chimeric sequences [26]. Samples with fewer than 3000 sequences after processing were excluded. For all microbiota analyses of KPC-Kp–positive samples (n = 892), a 97% sequence similarity was used to cluster sequences into operational taxonomic units (OTUs) using the average neighbor method. Relative abundance of OTUs in each sample was calculated. The Ribosomal Database Project training set (v10) was used to obtain taxonomic classification of OTUs [27]. Raw sequence data for the samples used in this project were deposited in BioProjects PRJNA485316 and PRJNA428477 (see Supplementary Table 1 for a list of BioSample accession numbers).
We determined that 1 OTU represented KPC-Kp. This OTU contained a single unique sequence that matched various Enterobacteriaceae family members, including 16S rRNA gene sequences from K. pneumoniae, Klebsiella variicola, and members of the genera Enterobacter and Buttiauxella. We observed several exact matches to at least 1 copy of the 16S rRNA gene sequence in genomes from K. pneumoniae strain multilocus sequence type ST258, K. pneumoniae subsp. pneumoniae ST258-K26BO, and K. pneumonia subsp. pneumoniae strain ST258_FL, confirming that the canonical KPC-Kp strains belonging to ST258 would fall into this OTU.
Statistical Analyses
Receiver operator characteristic (ROC) curves were constructed using results from analysis of the rectal sample with the highest relative abundance of KPC-Kp (reads of the OTU that contained KPC-Kp divided by the total number of reads in the sample) from each unique patient admission, excluding any samples collected after KPC-Kp bacteremia. Cox regression analysis was performed to evaluate clinical risk factors associated with the time to achieve KPC-Kp relative abundance thresholds determined by ROC curve analysis. We considered a P value of <.05 to be significant. All statistical analyses were performed using SPSS v.22.0 (IBM Corp., Armonk, NY) and R version 3.4.3 [28].
RESULTS
We collected 2319 rectal swab samples (median, 3; range, 1–18) from 562 admissions (506 patients), of whom 255 (45.4%) were colonized with KPC-Kp and 11 (4.3%) developed KPC-Kp bacteremia. Bacteremia was observed only in patients with documented KPC-Kp colonization. Mean age of KPC-Kp colonized patients was 63.2 years (standard deviation ± 15.9 years), and median length of hospital stay was 40 days (interquartile range, 27–65 days). A total of 235 (92.2%) patients received at least 1 dose of antibiotic during their LTACH stay. Other patient characteristics are summarized in Table 1.
Table 1.
Demographic and Clinical Characteristics of Klebsiella pneumoniae Carbapenemase-producing Klebsiella pneumoniae Colonized Patients
| Parameter | Value (N = 255 admissions)a |
|---|---|
| Age (years), mean ± SD | 63.2 ± 15.9 |
| Female sex, n (%) | 106 (41.6) |
| Length of hospital stay in days, median (IQR) | 40 (27–65) |
| Body mass index, mean ± SD | 27.7 ± 9.9 |
| Devices, n (%) | |
| Mechanical ventilation | 98 (38.4) |
| Central venous catheter | 130 (51.6) |
| Oral diet | 97 (38.0) |
| Gastrostomy tube | 142 (55.7) |
| Indwelling or suprapubic urinary catheter | 159 (62.4) |
| Charlson score, median (IQR) | 3 (2–5) |
| Comorbidities, n (%) | |
| Diabetes mellitus | 123 (48.2) |
| Congestive heart failure | 82 (32.2) |
| Stroke | 72 (28.2) |
| Decubitus ulcer | 193 (75.7) |
| End-stage renal disease on hemodialysis | 35 (13.8) |
| Antibiotic use, n (%) | 235 (92.2) |
| Carbapenem | 102 (40.0) |
| Beta-lactam/beta-lactamase inhibitor | 69 (27.1) |
| Vancomycin (intravenous) | 133 (52.2) |
| Metronidazole | 49 (19.2) |
Abbreviations: IQR, interquartile range; SD, standard deviation..
aNumber of unique admissions colonized with KPC-Kp.
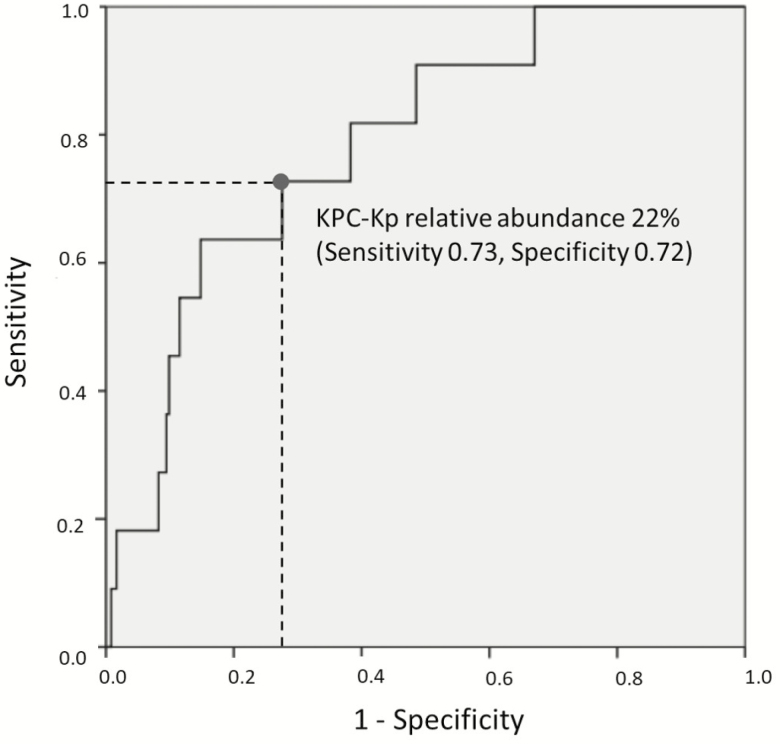
ROC curve analysis for the relative abundance of KPC-Kp in the intestinal microbiota vs KPC-Kp bacteremia showed an area under the curve of 0.78 (95% confidence interval [CI], 0.66–0.91; P = .002), indicating fair predictive ability (Figure 1). A KPC-Kp relative abundance cutoff of 22% predicted KPC-Kp bacteremia with sensitivity 73%, specificity 72%, and relative risk 4.2 (95% CI, 1.3–14.0; P = .01). A previously published cutoff for intestinal domination by a single bacterial taxon (≥30%) [6] yielded sensitivity 64%, specificity 79%, and relative risk 6.1 (95% CI, 1.8–20.0; P < .001).
Figure 1.
Receiver operating characteristic curve analysis of the relationship between relative abundance of Klebsiella pneumoniae carbapenemase-producing Klebsiella pneumoniae (KPC-Kp) and subsequent KPC-Kp bloodstream infection. Abbreviation: KPC-Kp, Klebsiella pneumoniae carbapenemase-producing Klebsiella pneumoniae.
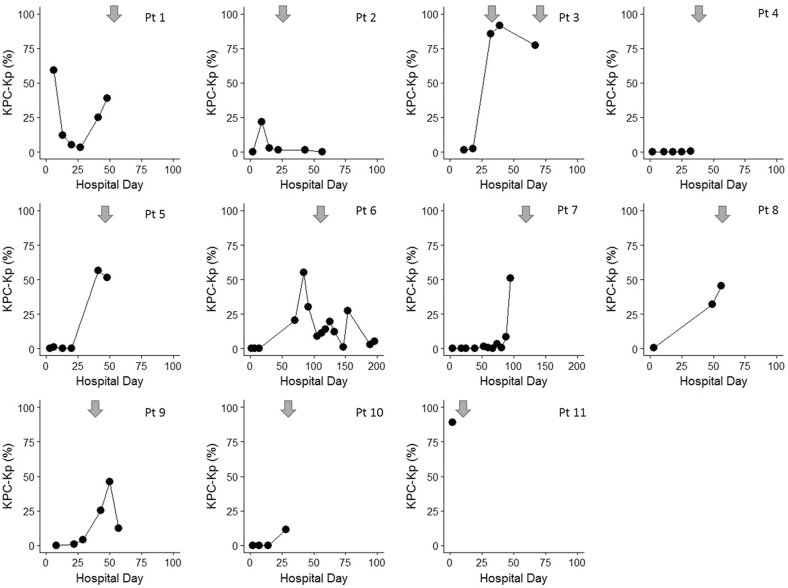
Temporal trends of KPC-Kp relative abundance for the 11 patients who developed KPC-Kp BSI are shown in Figure 2; clinical presentations and outcomes are summarized in Table 2. Six patients had a single positive blood culture reported and 3 patients (patients 3, 7, 9) had multiple blood cultures that grew KPC-Kp. At the time of KPC-Kp bacteremia, 1 patient (patient 1) had Clostridium difficile infection with abdominal distention and another patient (patient 8) had hypoxia without other clinical or radiographic evidence of pneumonia. No other bacteremic patient had organ-specific signs or symptoms of infection reported, and no patient had a microbiologically identified source of bacteremia [29]. Nine patients were reported to have a central venous catheter in place at the time of first positive blood culture. Catheters were removed from 2 patients (patients 7, 11) and catheter tips were cultured; 1 tip was positive for KPC-Kp (patient 7). No patient was neutropenic or receiving cancer chemotherapy. Four patients (patients 3, 7, 8, 9) had been receiving glucocorticoid doses equivalent to greater than 20 mg of prednisone daily for more than 2 weeks when bacteremia was identified.
Figure 2.
Chronological change of relative abundance of Klebsiella pneumoniae carbapenemase-producing Klebsiella pneumoniae (KPC-Kp) in the gut microbiota of 11 patients with KPC-Kp bacteremia. This operational taxonomic unit (OTU) contained a single unique sequence that matched various Enterobacteriaceae family members, including the 16S rRNA gene sequences from K. pneumoniae. It contained several exact matches to at least 1 copy of the 16S rRNA gene sequence in genomes from several variants of K. pneumoniae ST258, confirming that the canonical KPC-Kp strains belonging to ST258 would fall into this OTU. Each panel shows data for a single patient’s admission. Dot markers indicate the relative abundance of KPC-Kp (%) measured on the hospital day indicated. Arrows indicate date of first positive KPC-Kp blood culture. For patients with multiple positive blood cultures (patients 3, 7, 9), another arrow was added only if new infection was suspected (bacteremia episodes separated by ≥2 weeks) based on National Healthcare Safety Network surveillance definitions [29]. Abbreviations: KPC-Kp, Klebsiella pneumoniae carbapenemase-producing Klebsiella pneumoniae; Pt, patient.
Table 2.
Clinical Presentation and Outcomes for 11 Patients With Klebsiella pneumoniae Carbapenemase-producing Klebsiella pneumoniae Bloodstream Infection
| ID | Sex | Age | Reason for Hospitalization | Comorbidity | Hospital Event Preceding or Concurrent With KPC-Kp BSI | At the Time of First KPC-Kp BSI | Disposition | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Clinical Symptoms | Central Venous Catheter | Other Device | Immunosuppressants | ||||||||
| Type | Location | ||||||||||
| 1 | F | 73 | Recent pneumonia, transferred to continue care | Hypothyroidism, A fib, PVD, Pressure ulcer | Clostridium difficile infection | Abdominal distention, fever | PICC | Upper extremity | Tracheostomy tube, mechanical ventilator, gastrostomy tube, urinary catheter | … | Transfer to ACH |
| 2 | M | 64 | Recent pneumonia, cardiac arrest, transferred to continue care | COPD, pulmonary HTN | Fever followed by small bowel obstruction due to ruptured appendix | Fever | CVC | Subclavian | Tracheostomy tube, mechanical ventilator, gastrostomy tube | … | Transfer to ACH |
| 3 | F | 81 | Recent right groin infected hematoma around arteriovenous fistula, transferred to continue care | COPD, chronic myelogenous leukemia, ESRD on HD | General deterioration | None documented | Long-term HD catheter | Groin | Tracheostomy tube, mechanical ventilator | Glucocorticoids | Deceased |
| 4 | M | 62 | Recent pneumonia, transferred to continue care | COPD, DM, PVD, HTN, CHF |
Septic shock during hemodialysis | Shock | Long-term HD catheter |
Subclavian | Tracheostomy tube, mechanical ventilator, gastrostomy tube | … | Deceased |
| 5 | F | 68 | Direct admission for infected PEG tube | Ischemic stroke, MS | Persistent leak around PEG tube despite tube exchange | Fever | … | … | Tracheostomy tube, mechanical ventilator, gastrostomy tube | … | Transfer to ACH |
| 6 | F | 63 | Recent cardiac arrest, transferred to continue care | Asthma, CHF, obesity | Candida endocarditis, pyelonephritis, PEG tube infection | Fever | PICC | Unknown | Tracheostomy tube, mechanical ventilator, gastrostomy tube | … | Transfer to skilled nursing facility |
| 7 | F | 67 | Recent pneumonia, transferred to continue care | Adrenal insufficiency, COPD | Acute kidney injury requiring hemodialysis | Bradycardia | CVC, short-term HD catheter, PICC | Subclavian, internal jugular, lower extremity | Endotracheal tube, mechanical ventilator, gastrostomy tube | Glucocorticoids | Deceased |
| 8 | M | 56 | Recent ischemic stroke, transferred to continue care | HTN, CKD, dilated cardiomyopathy, A Fib | Respiratory failure, intubation | Hypoxia | … | … | Automated implantable cardioverter-defibrillator, gastrostomy tube | Glucocorticoids | Transfer to hospice |
| 9 | F | 60 | Recent left leg abscess, acalculous cholecystitis, transferred to continue care | COPD, CHF, seizure | PEG tube placement complicated by liver injury | Altered mental status, hypotension | CVC | Subclavian | Gastrostomy tube | Glucocorticoids | Transfer to ACH |
| 10 | F | 73 | Recent ischemic stroke, transferred to continue care | CKD, HTN, ESRD on HD, remote history of stomach cancer | Septic shock | Shock, hypoglycemia | Short-term HD catheter, port-catheter | Internal jugular, chest | Gastrostomy tube | … | Deceased |
| 11 | M | 48 | Recent gastrointestinal bleeding from unclear source, transferred to continue care | HTN, DM, right below knee amputation, sacral decubitus ulcer, ESRD on HD |
Deep venous thrombosis around PICC line | Fever | Short-term HD catheter, PICC | Internal jugular, upper extremity | … | … | Deceased |
Abbreviations: A fib, atrial fibrillation; ACH, acute care hospital; BSI, bloodstream infection; CHF, congestive heart failure; CKD, chronic kidney disease; COPD, chronic obstructive pulmonary disease; CVC, central venous catheter; DM, diabetes mellitus; ESRD, end-stage renal disease; F, female; HD, hemodialysis; HTN, hypertension; KPC-Kp, Klebsiella pneumoniae carbapenemase-producing Klebsiella pneumoniae; M, male; MS, multiple sclerosis; PEG, percutaneous endoscopic gastrostomy; PICC, peripherally inserted central venous catheter; PVD, peripheral vascular disease.
Results of the investigation of risk factors for increased relative abundance (≥22%) of KPC-Kp are shown in Table 3. In Cox univariate analyses, there were no significant associations between age, comorbid medical conditions, or presence of a medical device and increased relative abundance of KPC-Kp. Among antibiotics analyzed, only preceding carbapenem use was associated with a relative abundance of KPC-Kp ≥22% (hazard ratio [HR], 2.19; 95% CI, 1.06–4.55; P = .036). While no clinical variables were significantly associated with achievement of domination in univariate analysis, we included 3 variables (age, Charlson comorbidity index, and any medical device) in a multivariable model to evaluate for confounding, since these factors are frequently associated with multidrug-resistant organism colonization and acquisition. The association between carbapenem receipt and subsequent high relative abundance of KPC-Kp remained significant after adjustment (HR, 2.14; 95% CI, 1.02–4.49; P = .044).
Table 3.
Risk Factors Associated With ≥22% Relative Abundance of Klebsiella pneumoniae Carbapenemase-producing Klebsiella pneumoniae in the Gut Microbiota
| Clinical Predictor | Hazard Ratio (95% Confidence Interval) | P Value |
|---|---|---|
| Age, years | 0.99 (0.97–1.02) | .549 |
| Charlson comorbidity index | 0.90 (0.74–1.09) | .277 |
| Any medical device use | 1.05 (0.25–4.48) | .943 |
| Mechanical ventilation | 0.82 (0.39–1.71) | .588 |
| Gastrostomy tube | 0.62 (0.30–1.29) | .204 |
| Central line | 1.17 (0.55–25) | .689 |
| Hemodialysis | 0.77 (0.23–2.54) | .666 |
| Urinary catheter | 0.73 (0.34–1.55) | .409 |
| Any antibiotic exposure | 0.70 (0.24–2.07) | .519 |
| Carbapenem | 2.19 (1.06–4.55) | .036 |
| Beta-lactam/beta-lactamase inhibitor | 0.66 (0.23–1.90) | .436 |
| Vancomycin (intravenous) | 0.79 (0.38–1.66) | .537 |
| Metronidazole | 0.50 (0.12–2.12) | .351 |
DISCUSSION
In this single-center longitudinal study of LTACH patients who were colonized with KPC-Kp, high relative abundance of KPC-Kp in the intestinal microbiota was associated with increased risk of KPC-Kp bacteremia. Few patients had localizing signs or symptoms of infection at the time of bacteremia, no patient had a microbiologic source of bacteremia identified, and most patients (9 of 11) had a central venous catheter. Given the absence of evidence for an alternative primary source of bacteremia, the majority of BSI cases in our study would have been classified as central-line associated using National Healthcare Safety Network surveillance definitions [29]. While our clinical evaluation supports this as a potential source of bacteremia, the high relative abundance of KPC-Kp in patients’ intestinal microbiota suggests that gut translocation is also a possibility, despite the absence of classic immune dysfunction or medical conditions associated with intestinal mucosal barrier injury. In a similar study, an association between central venous catheter–associated bacteremia and the intestinal microbiota was investigated in infants with small bowel syndrome and long-term parenteral nutrition, but the exact mechanism of bacteremia remained undefined [30]. Regardless of the mechanism, our findings suggest that preventing an increase in relative abundance of KPC-Kp in the gut may hold promise as a means of reducing the risk (directly or indirectly) of KPC-Kp bacteremia.
Previous studies of immunocompromised children and adults with traditional risk factors for bacterial translocation in the gut used a relative abundance threshold of 30% as a marker of increased risk of Enterococcaceae, Streptococcaceae, and Proteobacteria bacteremia or other infections [6, 8]. Since we did not know if this threshold would apply to our patient population, we constructed an ROC curve to evaluate the sensitivity and specificity of different KPC-Kp relative abundance cutoffs for predicting KPC-Kp bacteremia. While application of the 30% KPC-Kp relative abundance threshold predicted KPC-Kp bacteremia with good specificity and reasonable sensitivity, we chose to apply a threshold of 22% since it yielded more balanced specificity and sensitivity predictions in our study cohort. It is important to recognize that both 30% and 22% relative abundances are much higher than the typical relative abundance of Enterobacteriaceae reported in healthy human intestinal bacterial communities, which is usually less than 1% [31]. Furthermore, measurement of relative abundance of OTUs in fecal or rectal swab samples may not reflect an absolute quantification of the entire intestinal bacterial community, nor does it provide information about in situ function. A more comprehensive analysis that combines 16S rRNA gene sequencing, proteomics, and metabolomics may provide better insight into the pathophysiology of bacteremia associated with increased relative abundance of KPC-Kp and other enteric bacteria.
We also evaluated clinical factors associated with microbiota compositional changes. Among the factors we evaluated, only carbapenem use was independently associated with high relative abundance of KPC-Kp. It is biologically plausible that the relative abundance of KPC-Kp increased after carbapenem exposure, which has activity against a majority of indigenous enteric bacteria but not against KPC-Kp. This finding supports the importance of antimicrobial stewardship efforts to reduce the adverse outcome of microbiome disruption, which can be associated with morbid clinical outcomes. A recent publication showed an association between exposure to broad-spectrum antibiotics and subsequent sepsis within 90 days [32]. The authors postulated that disruption of the microbiota was the potential cause of sepsis, which is consistent with our findings. Reversing antibiotic-associated microbiome disruption through interventions such as fecal microbiota transplantation or administration of beneficial microorganisms should also continue to be explored [5, 33].
Longitudinal specimen collection from a large sample of adult patients is a strength of our study, providing new insights into the chronological change of the gut microbiota in LTACH patients who are colonized with KPC-Kp [13, 14]. Our study also has several limitations. The number of patients with bacteremia was small, precluding a multivariable analysis of risk factors. Most patients (92%) received antibiotics at some point during their admission, so that we lacked a nonexposed comparator group for analysis of aggregate antibiotic effects. Rectal swab samples were collected weekly. Given the dynamic changes that occur in the gut microbiota of hospital patients, we may have missed high relative abundance of KPC-Kp in some patients, resulting in misclassification bias. We used an observational study design and conducted the study at a single LTACH, and our study patients were typical of an LTACH population with multiple comorbid medical conditions, extensive medical device use, and antibiotic exposure. These factors may limit the generalizability of our findings to non-LTACH patients. Still, that our results are consistent with those of prior studies of very different patient populations suggests that they may be relevant broadly to hospital patients.
In conclusion, we found that carbapenem use was associated with increased hazard for high relative abundance of KPC-Kp in the gut microbiota. High relative abundance of KPC-Kp was associated with KPC-Kp bacteremia. Future research should focus on investigating the mechanism of bacteremia risk and on mitigating this risk. In the meantime, our findings suggest a role for antimicrobial stewardship in limiting carbapenem use among patients who are colonized with KPC-Kp.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Financial support. This work was supported by the Centers for Disease Control and Prevention (cooperative agreement U54CK000481), the Michigan Institute for Clinical and Health Research Postdoctoral Translational Scholars Program of the National Institutes of Health (UL1TR0000433), the NIH/National Institute for Allergy and Infectious Diseases Cooperative Agreement (U01AI124255), and the Host Microbiome Initiative at the University of Michigan, Ann Arbor.
Potential conflicts of interest. M. K. H. reports participation in clinical trials in which participating healthcare facilities received contributed product from Sage Products Inc., Molnlycke, Clorox, Medline, or OpGen. Neither M. K. H. nor her hospital received product, funding, or payments. M. K. H. reports receipt of an investigator-initiated research grant from Clorox, outside of the submitted work. grants from Clorox outside the submitted work. M. Y. L. reports receipt of research support in the form of contributed product from Sage Products (now part of Stryker Corporation) and OpGen and receipt of an investigator-initiated grant from CareFusion Foundation (now part of BD). N. M. M. reports receipt of grants from Cepheid, Inc. and bioMerieux, Inc. outside the submitted work. R. A. W. reports participation in clinical studies where participating hospitals or nursing homes received contributed product from Sage Products Inc., Molnlycke, Clorox, Medline, or Bio-K+. Neither R. A. W. nor his hospital received product, funding, or payments. V. B. Y. reports disclosures from Vedanta Biosciences and nonfinancial support from Exarca Pharmaceuticals outside the submitted work. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. van der Velden WJ, Herbers AH, Netea MG, Blijlevens NM. Mucosal barrier injury, fever and infection in neutropenic patients with cancer: introducing the paradigm febrile mucositis. Br J Haematol 2014; 167:441–52. [DOI] [PubMed] [Google Scholar]
- 2. Balzan S, de Almeida Quadros C, de Cleva R, Zilberstein B, Cecconello I. Bacterial translocation: overview of mechanisms and clinical impact. J Gastroenterol Hepatol 2007; 22:464–71. [DOI] [PubMed] [Google Scholar]
- 3. Wiest R, Garcia-Tsao G. Bacterial translocation (BT) in cirrhosis. Hepatology 2005; 41:422–33. [DOI] [PubMed] [Google Scholar]
- 4. Sherman MP. New concepts of microbial translocation in the neonatal intestine: mechanisms and prevention. Clin Perinatol 2010; 37:565–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Young VB. The role of the microbiome in human health and disease: an introduction for clinicians. BMJ 2017; 356:j831. [DOI] [PubMed] [Google Scholar]
- 6. Taur Y, Xavier JB, Lipuma L, et al. Intestinal domination and the risk of bacteremia in patients undergoing allogeneic hematopoietic stem cell transplantation. Clin Infect Dis 2012; 55:905–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Stewart CJ, Embleton ND, Marrs ECL, et al. Longitudinal development of the gut microbiome and metabolome in preterm neonates with late onset sepsis and healthy controls. Microbiome 2017; 5:75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hakim H, Dallas R, Wolf J, et al. Gut microbiome composition predicts infection risk during chemotherapy in children with acute lymphoblastic leukemia. Clin Infect Dis. 2018; 67:541–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Weinstein RA. Intensive care unit environments and the fecal patina: a simple problem?Crit Care Med 2012; 40:1333–4. [DOI] [PubMed] [Google Scholar]
- 10. Lynch SV, Pedersen O. The human intestinal microbiome in health and disease. N Engl J Med 2016; 375:2369–79. [DOI] [PubMed] [Google Scholar]
- 11. David LA, Maurice CF, Carmody RN, et al. Diet rapidly and reproducibly alters the human gut microbiome. Nature 2014; 505:559–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Halpin AL, de Man TJ, Kraft CS, et al. Intestinal microbiome disruption in patients in a long-term acute care hospital: a case for development of microbiome disruption indices to improve infection prevention. Am J Infect Control 2016; 44:830–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lin MY, Lyles-Banks RD, Lolans K, et al. ; Centers for Disease Control and Prevention Epicenters Program. The importance of long-term acute care hospitals in the regional epidemiology of Klebsiella pneumoniae carbapenemase-producing Enterobacteriaceae. Clin Infect Dis 2013; 57:1246–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hayden MK, Lin MY, Lolans K, et al. ; Centers for Disease Control and Prevention Epicenters Program. Prevention of colonization and infection by Klebsiella pneumoniae carbapenemase-producing Enterobacteriaceae in long-term acute-care hospitals. Clin Infect Dis 2015; 60:1153–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Chitnis AS, Caruthers PS, Rao AK, et al. Outbreak of carbapenem-resistant Enterobacteriaceae at a long-term acute care hospital: sustained reductions in transmission through active surveillance and targeted interventions. Infect Control Hosp Epidemiol 2012; 33:984–92. [DOI] [PubMed] [Google Scholar]
- 16. Han JH, Goldstein EJ, Wise J, Bilker WB, Tolomeo P, Lautenbach E. Epidemiology of carbapenem-resistant Klebsiella pneumoniae in a network of long-term acute care hospitals. Clin Infect Dis 2017; 64:839–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Cole JM, Schuetz AN, Hill CE, Nolte FS. Development and evaluation of a real-time PCR assay for detection of Klebsiella pneumoniae carbapenemase genes. J Clin Microbiol 2009; 47:322–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Mangold KA, Santiano K, Broekman R, et al. Real-time detection of blaKPC in clinical samples and surveillance specimens. J Clin Microbiol 2011; 49:3338–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Rasheed JK, Kitchel B, Zhu W, et al. New Delhi metallo-β-lactamase-producing Enterobacteriaceae, United States. Emerg Infect Dis 2013; 19:870–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bassis CM, Moore NM, Lolans K, et al. ; CDC Prevention Epicenters Program. Comparison of stool versus rectal swab samples and storage conditions on bacterial community profiles. BMC Microbiol 2017; 17:78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kozich JJ, Westcott SL, Baxter NT, Highlander SK, Schloss PD. Development of a dual-index sequencing strategy and curation pipeline for analyzing amplicon sequence data on the MiSeq Illumina sequencing platform. Appl Environ Microbiol 2013; 79:5112–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Seekatz AM, Theriot CM, Molloy CT, Wozniak KL, Bergin IL, Young VB. Fecal microbiota transplantation eliminates Clostridium difficile in a murine model of relapsing disease. Infect Immun 2015; 83:3838–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Seekatz AM, Bassis CM, Fogg L, et al. ; Centers for Disease Control and Prevention Epicenters Program. Gut microbiota and clinical features distinguish colonization with Klebsiella pneumoniae carbapenemase-producing Klebsiella pneumoniae at the time of admission to a long-term acute care hospital. Open Forum Infect Dis 2018; 5:ofy190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Schloss PD, Westcott SL, Ryabin T, et al. Introducing mothur: open-source, platform-independent, community-supported software for describing and comparing microbial communities. Appl Environ Microbiol 2009; 75:7537–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Pruesse E, Quast C, Knittel K, et al. SILVA: a comprehensive online resource for quality checked and aligned ribosomal RNA sequence data compatible with ARB. Nucleic Acids Res 2007; 35:7188–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Edgar RC, Haas BJ, Clemente JC, Quince C, Knight R. UCHIME improves sensitivity and speed of chimera detection. Bioinformatics 2011; 27:2194–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wang Q, Garrity GM, Tiedje JM, Cole JR. Naive Bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Appl Environ Microbiol 2007; 73:5261–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. The R Foundation. The R project for statistical computing. Available at: https://www.r-project.org/. Accessed 7 July 2018. [Google Scholar]
- 29. National Healthcare Safety Network (NHSN). Bloodstream infection event (central line-associated bloodstream infection and non-central line associated bloodstream infection). https://www.cdc.gov/nhsn/pdfs/pscmanual/4psc_clabscurrent.pdf. Accessed 7 July 2018.
- 30. Wang P, Wang Y, Lu L, et al. Alterations in intestinal microbiota relate to intestinal failure-associated liver disease and central line infections. J Pediatr Surg 2017; 52:1318–26. [DOI] [PubMed] [Google Scholar]
- 31. Bradley PH, Pollard KS. Proteobacteria explain significant functional variability in the human gut microbiome. Microbiome 2017; 5:36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Baggs J, Jernigan JA, Halpin AL, Epstein L, Hatfield KM, McDonald LC. Risk of subsequent sepsis within 90 days after a hospital stay by type of antibiotic exposure. Clin Infect Dis 2018; 66:1004–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Pamer EG. Resurrecting the intestinal microbiota to combat antibiotic-resistant pathogens. Science 2016; 352:535–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.