Abstract
Background
Fistulas occur in about 25% of patients with Crohn’s disease (CD) and can be difficult to treat. The aim of this consensus was to provide guidance for the management of patients with perianal fistulizing CD.
Methods
A systematic literature search identified studies on the management of fistulizing CD. The quality of evidence and strength of recommendations were rated according to the Grading of Recommendation Assessment, Development and Evaluation (GRADE) approach. Statements were developed through an iterative online platform using a modified Delphi process, then finalized, and voted on by a group of specialists.
Results
The quality of evidence for treatment of fistulizing CD was generally of very low quality, and because of the scarcity of good randomized controlled trials (RCTs), these consensus statements generally provide conditional suggestions (5 of 7 statements). Imaging and surgical consultations were recommended in the initial assessment of patients with active fistulizing CD, particularly those with complicated disease. Antibiotic therapy is useful for initial symptom control. Antitumor necrosis factor (anti-TNF) therapy was recommended to induce symptomatic response, and continued use was suggested to achieve and maintain complete remission. The use of concomitant immunosuppressant therapies may be useful to optimize pharmacokinetic parameters when initiating anti-TNF therapy. When there has been an inadequate symptomatic response to medical management strategies, surgical therapy may provide effective fistula healing for some patients.
Conclusions
Optimal management of perianal fistulizing CD requires a collaborative effort between gastroenterologists and surgeons and may include the evidence-based use of existing therapies, as well as surgical assessments and interventions when needed.
Keywords: antibiotic, antitumor necrosis factor, endoscopic ultrasound, magnetic resonance imaging, recommendations
INTRODUCTION
The development of perianal abscesses and fistulas are recognized complications of Crohn’s disease (CD), which can lead to significant morbidity and reduced quality of life. About 25% of patients develop perianal fistulas during long-term follow-up, with the cumulative risk being 21% after 10 years and 26% after 20 years.1 Fistulizing perianal CD is a risk factor for poor outcomes,2–4 with about 70% of patients requiring surgical treatment during long-term follow-up.1 About 34% of patients have been reported to have recurrent fistulas,1 but data suggest that healing, if achieved, is maintained long-term for many patients.5,6
At the time the literature searches were conducted for this consensus (April 2016), the most recent clinical practice guidelines on the treatment of perianal fistulizing CD were the ‘Guidelines for the Multidisciplinary Management of Crohn’s Perianal Fistulas: Summary statement’ published in 2015,7 and the ‘Global Consensus on the Classification, Diagnosis and Multidisciplinary Treatment of Perianal Fistulizing Crohn’s Disease’ published in 2014.8 The current guidelines differ from these previously published documents in a number of areas. These include the evidence searches (literature searches through 2016 vs 2010), the use of the formal GRADE process for assessing the evidence, the vetting process (large faculty and availability for full Canadian Association of Gastroenterology [CAG] membership review before submission), and the content of the conclusions and recommendations (eg, timing of use of antibiotics, value of repeat imaging to ensure healing). These differences should make this guideline a valuable additional resource for clinicians.
Unfortunately, the quality of evidence for the treatment of fistulizing CD is generally very low, and as a result of the lack of randomized controlled trials (RCTs), these consensus statements primarily provide conditional suggestions (5 of 7 statements). In addition, the consensus group recognized that perianal fistulizing CD has a substantial impact on patient quality of life (QoL), and the patient’s perspective should be considered when making treatment decisions.
The purpose of these consensus statements is to review the literature relating to the medical management of perianal fistulizing CD and to develop specific recommendations for a multidisciplinary approach. These statements do not apply to patients with fistulizing disease outside of the immediate perianal region (eg, enterovesical or enterocutaneous fistulizing disease). In addition, it is important to recognize that the evaluation and treatment of associated luminal CD (CAG consensus guidelines published separately), particularly rectal disease, is a critical factor in the management of fistulizing CD that can influence outcomes. While a comparative assessment of surgical procedures was not conducted, the consensus group included a colorectal surgeon and recognized the need for an ongoing collaborative approach between gastroenterologists and surgeons.
METHODS
Scope and Purpose
These consensus statements focused on specific questions, as identified and discussed by the participants, regarding the management of perianal fistulizing CD. Statements on the management of luminal CD and postoperative patients were also developed and will be presented in separate publications. The development of this clinical practice guideline began in September 2015, with the full consensus group participating in a face-to-face meeting in September 2016.
Sources and Searches
The editorial office of the Cochrane Upper Gastrointestinal and Pancreatic Diseases Group at McMaster University performed a systematic literature search of MEDLINE (from 1946 onward), EMBASE (from 1980 onward), and CENTRAL (Cochrane Central Register of Controlled Trials) for trials published through February to April 2016. Key search terms included Crohn, fistula, and fistulizing in trials published through February to April 2016. Only human studies published in English were considered (see Appendix 1 of supplemental content, which provides details of the search strategy used for preparing the initial consensus statements). Additional focused but nonsystematic searches were also performed up to the September 2016 consensus meeting.
Review and Grading of Evidence
Two nonvoting methodologists (GL, PM) used the GRADE (Grading of Recommendation Assessment, Development and Evaluation) approach9 to assess the risk of bias (of individual studies and overall across studies), indirectness, inconsistency, imprecision, and other considerations (including publication bias) to determine the overall quality of evidence for each statement. The quality of evidence for each statement was graded as high, moderate, low, or very low, as described in GRADE (Table 1)9, 10 and used in prior Canadian Association of Gastroenterology (CAG) consensus documents.11–14 GRADE assessments were reviewed and agreed upon by voting members of the consensus group at the meeting.
Table 1.
Quality of Evidence and Definitions
| High quality | Further research is very unlikely to change our confidence in the estimate of effect |
| Moderate quality | Further research is likely to have an important impact on our confidence in the estimate effect and may change the estimate |
| Low quality | Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate |
| Very low quality | Any estimate of effect is very uncertain |
*Adapted from reference 9
Approved product labeling from government regulatory agencies varies from country to country, and though not ignored, recommendations are based on evidence from the literature and consensus discussion and may not fully reflect the product labeling for a given country.
Consensus Process
The consensus group comprised 21 voting participants with expertise in the management of CD, including the chairs (RP, AHS), academic and community gastroenterologists, a nurse practitioner, and a colorectal surgeon. Nonvoting participants included a patient representative, nonvoting observers, the GRADE experts (GL, PM), and a moderator (JM).
The CAG used a web-based platform (ECD solutions, Atlanta, Georgia, USA) to aid in the consensus process before the 2-day, face-to-face consensus meeting held in Toronto, Ontario, Canada, in September 2016. The steering committee (RP, AHS, BB, RK, JKM, and LT) and one of the nonvoting methodologists (GL) developed the initial statements. Using the consensus web-based platform, the steering committee reviewed the results of initial literature searches and identified relevant references that were then ‘tagged’ (ie, selected and linked) to each statement. All participants then used the web-based platform and a modified Delphi process15, 16 to vote anonymously on their level of agreement with the statements, suggest revisions, and provide comments. The statements were revised through 2 separate iterations and finalized at the consensus meeting. All participants had access to all abstracts and electronic copies of the individual tagged references. The GRADE evaluations of the evidence for each statement were provided at the meeting.
At the consensus conference, participants presented data, reviewed GRADE evaluations of the evidence for the individual statements, and discussed the phrasing of specific statements before their subsequent finalization. Participants then indicated their level of agreement for each statement by voting. A statement was accepted if >75% of participants voted 4 (agree) or 5 (strongly agree) on a scale of 1 to 5 (with 1, 2, and 3 indicating disagree strongly, disagree, and uncertain, respectively). Following acceptance of a statement, participants voted on the “strength” of the recommendation, with a vote of ≥75% of participants needed to classify a statement as “strong” (recommended); if this threshold was not met, the statement defaulted to “conditional” (suggested). The strength of the recommendation considered risk-benefit balance, patients’ values and preferences, cost and resource allocation, and the quality of the evidence. Therefore, it was possible for a recommendation to be classified as strong despite having low-quality evidence or conditional despite the existence of high-quality evidence.17 As per the GRADE method, a strong recommendation is indicative of a more broadly applicable statement, whereas a conditional recommendation suggests that that different choices will be appropriate for different patients (Table 2).17 In light of the low- or very low-quality evidence in fistulizing disease, the majority of the statements in this area were designated as suggestions.
Table 2.
Implications of Strength of Recommendation
| Strong recommendation | Weak recommendation | |
|---|---|---|
| Patients | Most people in your situation would want the recommended course of action and only a small proportion would not; request discussion if the intervention is not offered | Most people in your situation would want the recommended course of action but many would not |
| Clinicians | Most patients should receive the recommended course of action | You should recognize that different choices will be appropriate for different patients and that you must help each patient to arrive at a management decision consistent with her or his values and preferences |
| Policy makers | The recommendation can be adopted as a policy in most situations | Policy making will require substantial debate and involvement of many stakeholders |
*From reference 17
The manuscript was drafted, reviewed, and approved by the cochairs (AHS, RP), the steering committee members, and the remaining members of the consensus group. As per Canadian Association of Gastroenterology (CAG) policy for all clinical practice guidelines, the manuscript was made available to all CAG members for comments before submission for publication. Members were notified that the manuscript was available on the members-only section of the CAG website and open for comment for a 2-week period.
In accordance with CAG policy, written disclosures of any potential conflicts of interest for the 24 months before the consensus meeting were provided by all participants and made available to all group members.
Role of the Funding Sources
Funding for the consensus meeting was provided by unrestricted, arms-length grants to the CAG by AbbVie Corporation, Janssen Inc., Pfizer Canada Inc., and Takeda Canada Inc. The CAG administered all aspects of the meeting, and the funding sources had no involvement in the process at any point nor were they made aware of any part of the process—from development of search strings and statements to drafting and approval of these guidelines.
PERIANAL FISTULIZING CROHN’S DISEASE DEFINITIONS
Before finalizing the individual statements for the management of perianal fistulizing CD, the consensus group first discussed and agreed on definitions of terminology for fistulizing disease that were then used throughout the consensus process. Definitions were presented by a member of the steering committee (JM), discussed, revised, and then agreed upon by the group.
Definition and Classification of Perianal Fistulas
A perianal fistula is an abnormal communication between the rectum or anal canal and the external perianal or ischioanal skin. Perianal fistulas generally arise from perianal abscesses and are classified by the Parks classification as intersphincteric, transphincteric, supraphincteric and extrasphincteric. Over 90% of perianal fistulas are intersphincteric and transphincteric, and therefore, these fistulas are the focus of this guideline.8, 18
Factors Associated With High Risk of Relapse, Surgery, or Complicated Course
Symptoms of perianal fistulizing disease often include drainage, bleeding, pain, and swelling from one or more external openings, as well as diminished, disease-related quality of life. In addition to symptoms, severity assessments should consider the overall risk profile and the impact of the disease on the patient. In patients with CD, factors that have been associated with a higher risk of clinical relapse or a more aggressive or complicated disease course include clinical factors (eg, younger age, smoking, longer disease duration, early need for corticosteroids, and fistulizing perianal CD1–4), laboratory markers (eg, low hemoglobin, low albumin, high CRP, and high fecal calprotectin levels19–23), endoscopic appearance (eg, the presence of deep ulcers), and the total disease burden and location of associated luminal disease. Patients lacking these factors would generally be classified as low-risk. It is important to note that fistulizing perianal CD itself is a risk factor for poor outcomes.1–4
Outcomes in Perianal, Fistulizing Crohn’s Disease
Terminology and definitions regarding fistulizing CD used in this guideline are shown in Table 3. While complete remission, defined as both symptomatic and radiographic remission, is the preferred outcome, it was recognized that radiography to document healing is not routinely performed in this patient group, and standardized radiographic definitions of healing have not been developed. The Van Assche score has been proposed to measure fistula activity. The severity of fistulas is rated based on the number of fistula tracts, fistula location, fistula extension, hyperintensity on T2-weighted images, collections or abscesses, and rectal wall involvement.24 This system is not widely used in clinical practice and includes anatomic variables that may not improve despite effective medical therapy (eg, a tract may still be visible radiologically after a fistula has “closed”).
Table 3.
Defining Remission and Response in Patients With Perianal, Fistulizing CD
| Complete remission | Symptomatic and radiographic remission (defined below) |
|---|---|
| Symptomatic remission | Absence of both pain and drainage* from the fistula tract |
| Symptomatic response | Meaningful improvement in symptoms of pain and drainage as judged by both the patient and physician in the absence of remission. Response should not be considered a desirable final outcome, but is useful to assess early response to treatments |
| Radiographic remission | Absence of inflammation in any fistula tract and the absence of any abscess |
*Absence of drainage is considered to be no drainage from the fistula tract with the application of gentle pressure
Some data suggest that the use of magnetic resonance imaging (MRI) of the pelvis or transperineal and endoscopic ultrasound (EUS) to guide therapy in patients with CD perianal fistulas is associated with improved short- and long-term symptomatic response rates.5, 25, 26 However, there are currently insufficient data to make a recommendation for or against serial routine imaging to monitor, or increase, response to treatment.
If radiography is not used to document fistula closure, symptomatic remission without the occurrence of any complications (eg, anal stenosis, perianal abscess, systemic sepsis, fecal incontinence) or the need for fecal diversion or proctectomy is an acceptable outcome. Symptomatic response should not be the goal of therapy but may be useful to assess early improvement with therapy. In addition, symptomatic response may be an acceptable outcome in some cases when symptoms are only intermittent and not associated with the development of the previously mentioned complications. This acknowledges a patient preference for not escalating or changing therapy if there is only scant intermittent drainage from an uncomplicated fistula that does not confer a significant impact on the patient’s QoL. It is extremely important to discuss treatment goals with the patient to ensure that these goals align with those outlined in these consensus statements.
Statements in this guideline regarding fistulizing disease are limited to patients with uncomplicated or complicated perianal fistulizing disease. For the purpose of this guideline, complicated fistulizing CD was defined as multiple and/or branching fistula tracts, rectovaginal fistula and/or fistulas associated with active rectal disease or anal stenosis. As previously noted, these statements do not apply to patients with any fistulizing disease outside of the immediate perianal region (eg, enterovesical, enterocutaneous fistulizing disease).
RECOMMENDATION STATEMENTS FOR PERIANAL FISTULIZING CROHN’S DISEASE
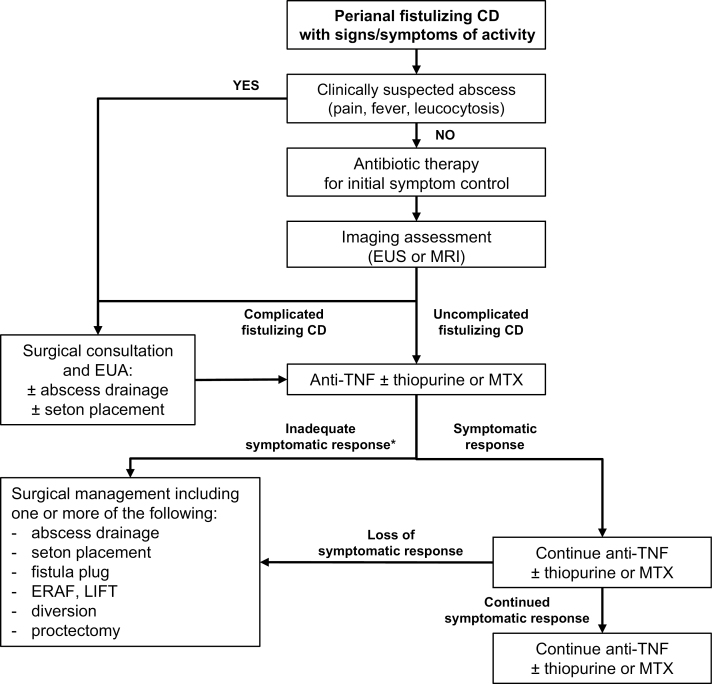
The individual recommendation statements are provided and include the “GRADE” of supporting evidence and the voting results, after which a discussion of the evidence considered for the specific statement is presented. A summary of the recommendation statements is provided in Table 4, and an algorithmic, consensus-guided approach is shown in Fig. 1.
Statement 1: In patients with Crohn’s disease and signs and/or symptoms of active fistulizing disease, we recommend imaging (EUS or MRI, based on availability and local expertise) be obtained to delineate the anatomy of the fistula tract(s).
GRADE: Strong recommendation, very low-quality evidence. Vote: strongly agree, 62%; agree, 38%.
Table 4.
Summary of Consensus Recommendations for the Management of Perianal Fistulizing Crohn’s Disease*
| 1: In patients with Crohn’s disease and signs and/or symptoms of active fistulizing disease, we recommend imaging (EUS or MRI, based on availability and local expertise) be obtained to delineate the anatomy of the fistula tract(s). GRADE: Strong recommendation, very low-quality evidence. |
| 2: In patients with Crohn’s disease and evidence of complicated fistulizing disease, we suggest surgical consultation. GRADE: Conditional recommendation, very low-quality evidence. |
| 3: In patients with Crohn’s disease and evidence of fistulizing disease, we suggest the use of antibiotic therapy for initial management to achieve symptomatic response. GRADE: Conditional recommendation, very low-quality evidence |
| 4: In patients with Crohn’s disease and evidence of fistulizing disease, we recommend the use of anti-TNF therapy, to induce symptomatic response. GRADE: Strong recommendation, very low-quality evidence. |
| 5: In patients with Crohn’s disease and evidence of fistulizing disease who have achieved symptomatic response on anti-TNF therapy, we suggest the use of continued therapy, to achieve and maintain complete remission. GRADE: Conditional recommendation, low-quality evidence. |
| 6: In patients with Crohn’s disease and evidence of fistulizing disease, when starting anti-TNF therapy, we suggest it be combined with a thiopurine or methotrexate over monotherapy to optimize pharmacokinetic parameters. GRADE: Conditional recommendation, low-quality evidence for infliximab, very low-quality evidence for adalimumab. |
| 7: In patients with Crohn’s disease and evidence of fistulizing disease, we suggest referral for surgical management when there has been an inadequate symptomatic response to medical management strategies. GRADE: Conditional recommendation, very low-quality evidence. |
EUS, endoscopic ultrasound; MRI, magnetic resonance imaging; TNF, tumor necrosis factor; *The strength of each recommendation was assigned by the consensus group, per the GRADE system, as strong (“we recommend . . .”) or conditional (“we suggest . . .”). A recommendation could be classified as strong despite low quality evidence to support it or conditional despite the existence of high quality evidence due to the 4 components considered in each recommendation (risk:benefit balance, patients’ values and preferences, cost and resource allocation, and quality of evidence).
Figure 1.
Consensus guided algorithm for the management of patients with active perianal fistulizing CD. MTX, methotrexate; MAF, mucosal advancement flap.
*Medical therapy should be optimized, informed by therapeutic drug monitoring (if needed) before patients are considered refractory to treatment.
Key evidence
The evidence for the utility of EUS or MRI is from observational studies and small RCTs. In a prospective, blinded comparison, EUS, MRI, and exam under anesthesia (EUA) were shown to have >85% accuracy for the classification of fistulas.27 Accuracy reached 100% when a combination of any 2 of the tests was used. In small RCTs, the use of EUS to guide medical and surgical therapy was associated with a higher rate of cessation of fistula drainage,28 earlier escalation of dosing of medical therapy, and a faster rate of fistula resolution.29 Observational studies show that MRI correctly classified 90% of fistulas30 and provided useful information related to healing of fistulas in patients receiving antitumor necrosis factor (anti-TNF) therapy.5, 26
In a prospective study, healing demonstrated by MRI was maintained during long-term follow-up in patients continuing or stopping anti-TNF therapy.5 However in one study, improvement seen on MRI correlated with clinical and endoscopic responses to anti-TNF therapy in only about 50% of patients.26
Discussion
Imaging modalities are recommended for diagnostic purposes in patients with suspected fistulizing CD.31 Data show good diagnostic accuracy with these techniques for classifying fistulas according to Parks criteria.27 Imaging can also detect associated abscesses or collections that may merit surgical drainage with or without placement of setons. Data also suggest imaging may be useful to evaluate treatment response,5, 26, 28, 29 allowing for earlier escalation of therapy in patients who do not demonstrate fistula healing29 or discontinuation of therapy in patients who do.5 However, when used to monitor responses to therapy, expertise in evaluating serial radiological studies is necessary.
Computed tomography (CT) is generally not recommended because it lacks the sensitivity and specificity of MRI32 and because of concerns around the exposure to radiation.8 When other imaging techniques are not available, CT can be useful to detect fistulas, although in one case series, CT had a 50% false-negative rate.32 Computed tomography is generally reserved for cases of severe CD with clinical suspicion of sepsis and has been shown to accurately detect abscesses.32, 33
The consensus group concluded that EUS and MRI are accurate for the detection and classification of fistulas and recommended imaging be performed in patients with suspicion of active fistulizing disease to delineate the anatomy of the fistula. However, there were insufficient data to comment on the use of serial imaging to monitor response to treatment.
Statement 2: In patients with Crohn’s disease and evidence of complicated fistulizing disease, we suggest surgical consultation.
GRADE: Conditional recommendation, very low-quality evidence. Vote: strongly agree, 67%; agree, 29%; uncertain, 5%.
Key evidence
Data suggest that combining surgery with medical therapy may have additional beneficial effects on perianal fistula healing, compared with surgery or medical therapy alone. In a systematic review of 8 cohort studies, the rate of fistula healing was 43% with medical therapy alone and 53% with combination surgical and medical therapy.34
Discussion
In a natural history study, about 25% of patients developed 1 or more perianal fistulas during long-term follow-up, of which approximately 70% required surgical treatment. The majority of patients underwent minor procedures (eg, drainage of abscesses, fistulotomy/fistulectomy, and seton placement), but approximately one third required bowel resection.1 Surgeons play a pivotal role in defining the anatomy during EUA, draining pelvic and perianal sepsis, and placing setons. They also play an important role in a collaborative care model when more advanced surgery is needed.
Referral for surgical management has also been suggested for patients with uncomplicated, single, superficial fistulas that do not involve a significant amount of anal sphincter muscle. In such patients, surgeons may consider a fistulotomy and may obtain good symptomatic results without the need for medical therapy. However, these types of superficial fistulas are very uncommon in patients with CD, and as a result, this type of primary surgical approach would be used only in highly selected patients.
Based on the high rate of surgical treatment of fistulizing disease, and the suggestion that combined surgical and medical therapy may be more effective than medical therapy alone, the consensus group conditionally suggested that patients undergo a surgical consultation, preferably with a colorectal surgeon if available.
Statement 3: In patients with Crohn’s disease and evidence of fistulizing disease, we suggest the use of antibiotic therapy for initial management to achieve symptomatic response.
GRADE: Conditional recommendation, very low-quality evidence. Vote: strongly agree, 48%; agree, 48%; uncertain, 5%.
Key evidence
A systematic review and meta-analysis of 3 RCTs in patients with perianal CD fistula (n = 123) showed that treatment with antibiotic therapy (ciprofloxacin or metronidazole) given for between 4 and 12 weeks was associated with a significant improvement in the outcome of failure to reduce fistula drainage (relative risk [RR], 0.80; 95% CI, 0.66–0.98).35 In the ADAFI RCT, in patients with active perianal fistulizing CD, which was published after the meta-analysis, the combination of ciprofloxacin and adalimumab resulted in significantly greater symptomatic remission (closure of all draining fistulas) at week 12 (65 vs 33%; P = 0.009), compared with adalimumab alone.36
In the meta-analysis, results were not significant for each of the individual antibiotics tested (ciprofloxacin and metronidazole).35 A more recent meta-analysis including data on ciprofloxacin from 3 RCTs, showed a significantly higher rate of response (>50% reduction in the number of fistulas) compared with placebo (RR, 1.64; 95% CI, 1.16–2.32; P = 0.005).37
The quality of evidence was downgraded for imprecision because of the small number of patients and the different types of antibiotics used.
Discussion
The evidence suggests that, while antibiotic treatment may reduce fistula drainage, it does not appear to have long-term benefits after the antibiotic is discontinued. In the 2 trials, where ciprofloxacin was added to anti-TNF therapy, there were no significant differences in outcomes at long-term follow-up (18 and 24 weeks).36, 38 However, in the larger trial, results were significant at week 12.36 These data are limited to ciprofloxacin and metronidazole, but they suggest that further research and assessment of other antibiotics may be warranted.
Based on the sparse evidence, but also the suggestion of short-term benefits, the consensus group conditionally suggested that antibiotics may be useful as an initial management strategy to decrease drainage, to prevent abscess formation, and to act a bridge to a more definitive treatment strategy.
Statement 4: In patients with Crohn’s disease and evidence of fistulizing disease, we recommend the use of anti-TNF therapy to induce symptomatic response.
GRADE: Strong recommendation, very low-quality evidence. Vote: strongly agree, 33%; agree, 67%.
Key evidence
Evidence for anti-TNF therapies in fistulizing disease comes from 1 positive RCT with infliximab that was conducted specifically in this patient group.39 But the majority of evidence is from subgroup analyses of RCTs with limited statistical power. Among 3 trials with adalimumab, 1 was positive,40 and 2 were negative.41, 42 One RCT of certolizumab pegol induction therapy (PRECISE-1) had negative results among the subgroup of patients with fistulizing disease.43 A systematic review of 6 trials reported no significant effect of anti-TNF therapy for the outcome of failure to heal fistulizing disease (RR, 0.88; 95% CI, 0.73–1.05).44 When we removed the certolizumab pegol studies from the meta-analysis, the results remained nonsignificant (RR, 0.88; 95% CI, 0.66–1.11). In another meta-analysis, the 4 RCTs with adalimumab or infliximab found no significant benefit for anti-TNF therapy for the outcomes of complete fistula closure or partial fistula closure.45
Although data did not show a significant benefit overall, there was significant heterogeneity between studies, and the populations were not restricted to patients with perianal fistulas. However, the trial from Present et al designed with healing of fistulas as the primary outcome,39 in which over 90% of the population had perianal fistulas, showed a clear benefit of infliximab over placebo (RR, 0.62; 95% CI, 0.48–0.81).44 In addition, the effect was significant in studies in which patients were treated for more than 4 weeks (RR, 0.80; 95% CI, 0.65–0.98).44
Discussion
Although the quality of evidence is very low, the trial specifically designed to assess anti-TNF therapy in patients with fistulizing disease did show a significant benefit for healing of fistulas.39 In addition, data suggest that a longer course of therapy is needed before therapy should be considered ineffective.44
Based on the positive data, the consensus group recommended anti-TNF therapy with adalimumab or infliximab for fistulizing disease. Although RCTs with fistula-specific primary endpoints have only been performed with infliximab, there are fistula outcome data from subgroup analyses of RCTs of adalimumab that support its efficacy in this indication. Therefore, the consensus group concluded that there was not sufficient data to recommend one anti-TNF over the other. In light of the fact that there were no positive studies with certolizumab pegol, this agent was not recommended. Patients with uncomplicated perianal fistulas and mild luminal disease may not require anti-TNF therapy.
The timing of outcome assessment of symptomatic response to therapy in the studies has been in the range of 12 to 14 weeks. Although this seems to be a reasonable time for initial assessment of response, the full extent of response may not be realized until after that time.44
Among patients with fistulizing disease, an outcome of symptomatic response rather than complete remission may be acceptable when imaging to demonstrate healing is not available. However, the consensus group suggested that imaging be performed if discontinuation of treatment is being considered. In addition, some participants argued that treatment of fistulizing disease should strive for complete remission because fistulas are a risk factor for poor long-term outcomes,1 and there is evidence that healing, if achieved, can be maintained long-term.5, 6
Statement 5: In patients with Crohn’s disease and evidence of fistulizing disease who have achieved symptomatic response on anti-TNF therapy, we suggest the use of continued therapy to achieve and maintain complete remission.
GRADE: Conditional recommendation, low-quality evidence. Vote: strongly agree, 52%; agree, 38%; uncertain, 5%; disagree, 5%.
Key evidence
Evidence for maintenance anti-TNF therapies in fistulizing disease comes from 1 positive RCT with infliximab that was conducted specifically in this patient group (ACCENT II)46 and a subgroup analysis of a RCT with adalimumab (CHARM).6, 40 In ACCENT II, 195 patients with fistulas (90% perianal) who had responded to infliximab induction therapy were randomized to infliximab or placebo maintenance therapy. At week 54, 42% of patients treated with infliximab had lost response compared with 62% with placebo (P < 0.001). Complete absence of draining fistulas was seen in 36% of patients with infliximab and 19% with placebo (P = 0.009).46 The CHARM RCT included 117 patients with fistulas at baseline (97% perianal), and fistula closure rates at week 56 were 33% with adalimumab therapy and 13% with placebo (P = 0.043). Among patients who had fistula closure at week 26, 100% maintained healing at week 56 in both treatment groups.6
Subgroup analysis of patients with draining fistulas (95% perianal) at baseline who had responded to certolizumab pegol induction therapy in the PRECISE-2 RCT showed no significant improvement in the rate of fistula closure with continued certolizumab (54%) compared with switching to placebo (43%; P = 0.069).47, 48
Discussion
In the ACCENT II trial, the median length of time during which fistulas remained closed after cessation of infliximab therapy was 14 weeks, with over 60% of patients experiencing loss of response through 54 weeks of follow-up.46, 49 During longer follow-up of patients treated with adalimumab, fistula healing rates were maintained for up to 4 years.40, 50
Based on the positive data, the consensus group recommended anti-TNF therapy with adalimumab or infliximab for maintenance therapy in patients with fistulizing disease who have responded to induction therapy. Based on the fact that the maintenance data for certolizumab pegol were negative, this agent was not recommended.
Statement 6: In patients with Crohn’s disease and evidence of fistulizing disease, when starting anti-TNF therapy, we suggest it be combined with a thiopurine or methotrexate over monotherapy to optimize pharmacokinetic parameters.
GRADE: Conditional recommendation, low-quality evidence for infliximab; very low-quality evidence for adalimumab. Vote: strongly agree, 5%; agree, 81%; uncertain, 5%; disagree, 10%.
Key evidence
Few data are available on the role of combination immunosuppressant therapy in patients initiating anti-TNF therapy. Several cohort studies have demonstrated healing of perianal fistulas with the combination of infliximab induction therapy, followed by immunosuppressant maintenance therapy.51–54 In one cohort study in which azathioprine was started at initiation of infliximab or adalimumab therapy, the response rates were 66% and 43%, respectively, at 3 years.55 However, none of these studies included an anti-TNF monotherapy group. The placebo controlled trial of infliximab induction therapy found no difference between fistula response in patients receiving (49%) and those not receiving concomitant immunosuppressants (54%).39 However, patients on immunosuppressants had been receiving those drugs before initiation of infliximab and presumably had not responded to immunosuppressant monotherapy and were therefore less likely to obtain benefit from combination therapy. Similarly, in the open label induction phase of the ACCENT II study, the rate of fistula response at week 14 was identical in patients receiving concomitant immunosuppressants and those receiving only infliximab monotherapy.46 In an uncontrolled, open-label case series, combination therapy with infliximab and an immunosuppressant was associated with a greater likelihood of first perianal fistula closure compared with infliximab alone, but this was not statistically significant (HR 1.44; 95% CI, 0.79–2.66; P = 0.23).56 However, among patients who were naïve to immunosuppressants, the combination was significantly more likely to result in fistula closure (HR 2.58; 95% CI, 1.16–5.6; P = 0.02).
Discussion
The consensus group determined that, although the quality of the evidence was very low, the data on the impact of immunosuppressant therapy on anti-TNF drug levels, the correlation between levels and fistula outcomes, and thr extrapolation of higher quality evidence in luminal CD provided sufficient support for a conditional statement for the use of concomitant, antimetabolite, immunosuppressant therapy with anti-TNF therapy.
Although there was a lack of controlled trial evidence to demonstrate a clinical benefit of combination immunosuppressant and anti-TNF therapy over anti-TNF monotherapy in fistulizing disease, the combination has demonstrated good long-term response rates in IBD overall. In addition, one placebo-controlled, cross-over trial of 6-mercaptopurine in CD did assess fistula outcomes.57 However, this study was considered to be of low quality, and although it did report an effect of the drug on fistula response, the consensus group concluded that it was not sufficient on its own to make a statement regarding immunosuppressant monotherapy.
In the SONIC trial, in patients with luminal CD, combination therapy was more effective than either infliximab or azathioprine monotherapies in inducing symptomatic remission and mucosal healing.58 In addition, patients who received combination therapy were less likely to develop anti-TNF antibodies and had higher median serum anti-TNF trough levels.
Cohort studies have suggested that higher anti-TNF drug levels may be needed to promote fistula resolution.59, 60 In one report, patients with fistula response had significantly higher infliximab trough drug levels (weeks 2, 6, and 14) compared with those who did not respond.59 The presence of antidrug antibodies at week 14 was negatively associated with fistula response in the univariate analysis but not the multivariate analysis. This was confirmed in a larger study, in which patients with fistula healing had significantly higher infliximab drug levels (P < 0.0001) and healing rates increased incrementally with increasing drug levels.60 This study did find that patients with antidrug antibodies had a significantly lower chance of fistula resolution. In both trials, concomitant immunosuppressant therapy was not significantly more prevalent among responders compared with nonresponders, but comparisons of response rates among patients on combination therapy vs those not receiving immunosuppressants were not preformed.59, 60
The consensus group made their decision largely based on extrapolating data from patients in luminal CD and the fact that higher anti-TNF levels have been associated with improved rates of fistula healing. But given the scarce data on the use of combination therapy in patients with fistulizing disease, the consensus group made a conditional suggestion in favor of initiating azathioprine or methotrexate when starting anti-TNF therapy in this patient group.
Statement 7: In patients with Crohn’s disease and evidence of fistulizing disease, we suggest referral for surgical management when there has been an inadequate symptomatic response to medical management strategies.
GRADE: Conditional recommendation, very low-quality evidence. Vote: strongly agree, 57%; agree, 43%.
Key evidence
Very low-quality evidence from retrospective and prospective surgical case series suggest successful fistula closure in about 50% to 60% of patients at 6 months or more of follow-up.61–65 Surgical strategies included seton placement,61, 63, 65 use of fibrin glue,61 fistula plug insertion,64, 65 fistulotomy,62, 63 endorectal advancement flap (ERAF),66 ligation of the intersphincteric fistula tract (LIFT),67 and fecal diversion with or without proctectomy.68 Repeat procedures were common.62, 63
A systematic review of cohort studies reported fistula closure in 58% of patients with use of an anal fistula plug.64 However, a subsequent open-label, RCT found no significant difference between the rate of fistula closure at 3 months, with an anal fistula plug compared with seton removal alone (31.5% vs 23.1%; P = 0.19).65
A meta-analysis of cohort studies of fecal diversion reported that 63.8% (95% CI, 54.1–72.5) of patients had early response after treatment for refractory perianal CD. Restoration of bowel continuity was attempted in 34.5% of patients and was successful in only 16.6%. Overall, 41.6% of patients required proctectomy after failure of temporary fecal diversion.68 One cohort study comparing diversion vs no diversion found no differences in outcomes between the two groups, but these were patients with rectovaginal fistulas, and patients receiving diversion had more complicated disease.69
Discussion
Medically refractory disease implies that the disease has remained active despite optimal medical therapy. As discussed in statement 6, low anti-TNF drug levels or the presence of antidrug antibodies may affect fistula response rates.59, 60 Therefore, before surgical referral, dose optimization should be attempted, and for patients who lose response to anti-TNF therapy, therapeutic drug monitoring may be useful to help guide medical treatment decisions and dose optimization. As discussed in statement 2, a natural history study found that about 70% of patients with perianal fistulas required surgical treatment, including bowel resection in roughly one third of patients.1 Furthermore, data suggest that combining surgery with medical therapy may have additional beneficial effects on perianal fistula healing, compared with surgery or medical therapy alone.34
An ongoing collaborative approach between the gastroenterologist and the surgeon is important to optimize outcomes. In patients who are not responding to medical therapy, assessment or reassessment by a surgeon and EUA may reveal areas of undrained sepsis or the need to add or reposition setons. In addition, in very severe, medically refractory disease, proctectomy or fecal diversion with an ileostomy or colostomy may be required.1, 70
Based on the high rate of surgical treatment of fistulizing disease and cohort studies suggesting that surgical therapy may provide effective fistula healing for some patients, the consensus group conditionally suggested that patients be referred for surgical management when there has been an inadequate response to medical therapies.
FUTURE DIRECTIONS
The management of patients with perianal fistulizing disease has been inadequately studied, with most outcome data coming from subgroup analyses of RCTs for which perianal fistula outcomes were secondary. While additional RCTs specifically enrolling a perianal fistulizing patient population are needed for most treatments, there are some areas that particularly warrant study.
Although some of the anti-TNF agents have demonstrated efficacy for fistulizing disease, the optimal use of these therapies has not yet been defined. Further studies are needed to determine if drug dose or serum drug levels affect response and remission rates. If serum drug levels are determinants of response, then further studies are needed to define the optimal levels and to assess the impact of therapeutic drug monitoring on clinical and radiologic outcomes.
More robust data are needed on the newer non-anti-TNF biologics, vedolizumab and ustekinumab, in this patient population. In the subgroup of patients with perianal fistulas in the GEMINI-2 RCT, vedolizumab was associated with closure of fistulas in 23% of patients treated with vedolizumab every 8 weeks and 41% of patients treated with vedolizumab every 4 weeks compared with 11% of patients who received placebo (P = 0.32 and P = 0.03).71 Several small, open-label case series in CD patients with perianal disease have reported a symptomatic response rate of 60%–70% with ustekinumab.72, 73
Cohort studies74, 75 and one small RCT76 have suggested that some patients with fistulas may benefit from treatment with oral tacrolimus. In the RCT, fistula response was seen in significantly more patients receiving tacrolimus compared with placebo (48% vs 8%, P = 0.004), but closure rates were very low and similar between groups (10% vs 8%, P = 0.86).76 One other small RCT reported no benefit of tacrolimus ointment in patients with fistulas.77
Data suggest that stem cells may be a beneficial treatment particularly for patients with complicated, treatment-refractory fistulas. A large RCT demonstrated the efficacy of expanded, allogeneic-derived, mesenchymal stem cells (ASC) in inducing fistula remission in patients with complex perianal fistulas. Rates of complete remission were significantly higher with ASC compared with placebo at week 24 (50% vs 34%; P = 0.024),78 and the 1-year relapse rates among these patients were significantly lower (25% vs 44%).79 The high placebo remission rates in this trial may be related to the conditioning procedures received by all patients (curettage of fistula tracts, drainage of abscesses, and insertion of setons where necessary), which may have provided short-term fistula response for some patients without the need for additional medical therapy. A number of other surgical approaches to the management of perianal fistulizing disease have been described, but unlike ASC therapy, these have not been studied in RCTs. RCTs of surgical approaches would help to better define the role and optimal timing of surgical management of these patients.
Open-label case series suggest that hyperbaric oxygen therapy (HBOT) may promote healing in patients with severe or refractory perianal disease, but this strategy has not been systematically evaluated.80–82 In a recent case series, the rate of perianal fistula healing was 65%.82 The median number of 2-hour sessions per patient was 20 (range, 10–86).
Further data are needed on the role of imaging such as MRI and EUS to monitor patient response to treatment and to guide therapy, including de-escalation of medical therapy. In addition, further studies are needed to determine if effective surgical therapy for patients with medically refractory disease can obviate the need for further medical therapy. Finally, there is a need for a more definitive demonstration of whether the addition of immunosuppressive therapy (eg, methotrexate or a thiopurine) during initiation of biologic therapy provides any real efficacy benefits.
SUMMARY
These guidelines present recommendations for the patient with active perianal fistulizing CD. Consensus was reached on 7 statements pertaining to assessments, management with antibiotics, immunosuppressants, anti-TNF therapies, the role of surgical consultations, and interventions (Table 4). An algorithm summarizing the consensus-guided approach to the management of active perianal fistulizing CD is shown in Fig. 1. The evaluation and treatment of associated luminal CD (CAG consensus guidelines published separately), particularly rectal disease, is an important part of the management of fistulizing CD.
While the preferred goal of therapy is complete remission, the target outcomes in the recommendation statements also include symptomatic response in some cases, especially when assessing early results of therapy. A CD patient-advocate, who participated in this consensus, stressed the importance of a collaborative approach between patient and physician.
These guidelines should help to optimize the use and proper positioning of existing medical therapies and the role of surgical consultations and thus improve outcomes in patients with perianal fistulizing CD. Imaging (eg, MRI and EUS) to monitor patient response to treatment and investigative treatments, such as the non-anti-TNF biologics (vedolizumab and ustekinumab), tacrolimus, stem cells, and hyperbaric oxygen therapy, may provide additional options for patients with fistulizing CD and will be considered in future iterations of these guidelines.
Optimal management of perianal fistulizing CD requires a collaborative effort between gastroenterologists and surgeons and may include the evidence-based use of existing therapies and surgical assessments and interventions when needed.
Supplementary Data
Supplementary data is available at Inflammatory Bowel Diseases online.
Conflicts of Interest
AB has served on advisory boards for AbbVie, Allergan, Ferring, Janssen, Merck, Pfizer, Shire, and Takeda, has received educational support from AbbVie, Allergan, Janssen, and Takeda, has received research grants or clinical trial funding from AbbVie, and has participated in speaker’s bureaus for AbbVie, Shire, and Takeda. AHS has served on advisory boards for AbbVie, Actavis, Ferring, Janssen, Merck, Pendopharm, Pfizer, Shire, Takeda, has consulted for Pfizer, has received educational support from Janssen, has received research grants or clinical trial funding from AbbVie, Amgen, Celgene, Genentech, Millennium, and Redhill Biopharmn, and has participated in speaker’s bureaus for AbbVie, Ferring, Hospira, Janssen, Shire, and Takeda. BB has served on advisory boards for AbbVie, Actavis, Celltrion, Ferring, Genentech, Pendopharm, Shire, and Takeda, has received research grants or clinical trial funding from AbbVie, Alvine, Amgen, Boehringer Ingelheim, Bristol-Myers Squibb, Celegene, Genentech, Glaxo-Smith Kline, Janssen, Merck, Qu Biologic, RedHill Biopharm, has participated in speaker’s bureaus for AbbVie, Actavis, Ferring, Shire, and Takeda, and has stock options in Qu Biologic. BH has served on advisory boards for AbbVie and Janssen and has participated in speaker’s bureaus for AbbVie, Janssen, Pendopharm, and Shire. CNB has served on advisory boards for AbbVie, Cubist, Shire, and Takeda, has consulted for Theradig, and has participated in speaker’s bureaus for AbbVie, Janssen, Shire, and Takeda. CHS has served on advisory boards for AbbVie, Actavis, Janssen, Shire, and Takeda. Chadwick Williams has served on advisory boards for AbbVie, Ferring, and Shire and has participated in speaker’s bureaus for Janssen and Takeda. DS has consulted for AbbVie, Janssen, Takeda, Tigenix, and UCB and has received research grants or clinical trial funding from AbbVie and UCB. EVL has consulted for AbbVie, Amgen, Bristol-Myers Squibb, Celgene, CVS Caremark, Eli Lilly, Genentech, Janssen, Mesoblast, Pfizer, Salix, Seres Therapeutics, Sun Pharmaceuticals, Takeda, Theradig, and UCB, and has received research grants or clinical trial funding from AbbVie, Amgen, Celgene, Genentech, Gilead, Janssen, MedImmune, Pfizer, Receptos, Robarts Clinical Trials, Seres Therapeutics, Takeda, and UCB. GR has served on advisory boards for AbbVie, Janssen, Pendopharm, Shire, and Takeda, has consulted for AbbVie, has received educational support from Janssen, and has participated in speaker’s bureaus for AbbVie, Janssen, Shire, and Takeda. JJ has served on advisory boards for Takeda, has consulted for AbbVie, Janssen, and Takeda, and has participated in speaker’s bureaus for Ferring, Janssen, and Shire. John K. Marshall has consulted for AbbVie, Actavis, AstraZeneca, Celltrion, Cubist, Ferring, Hospira, Janssen, Shire, and Takeda, and has participated in speaker’s bureaus for AbbVie, Actavis, Ferring, Hospira, Janssen, Shire, and Takeda. Laura Targownik has served on advisory boards for AbbVie, Janssen, and Takeda, has received educational support from Pfizer, Shire, and Takeda, and has received research grants or clinical trial funding from AbbVie and Pfizer. MB has served on advisory boards for AbbVie and Shire and has participated in speaker’s bureaus for AbbVie, Allergan/Forest, Janssen, Pendopharm, Shire, and Takeda. RK has served on advisory boards for AbbVie, Janssen, and Takeda and has participated in speaker’s bureaus for Abbvie, Janssen, and Takeda. RP has served on advisory boards for AbbVie, Abbott, Amgen, Aptalis, AstraZeneca, Baxter, Bristol-Meyers Squibb, Celgene, Centocor, Cubist, Eisai, Elan, Ferring, Genentech, Glaxo-Smith Kline, Janssen, Merck, Pfizer, Salix, Schering-Plough, Shire, Takeda, UCB, and Warner Chilcott, has consulted for AbbVie, Abbott, Amgen, Aptalis, AstraZeneca, Baxter, Bristol-Meyers Squibb, Celgene, Centocor, Cubist, Eisai, Elan, Ferring, Glaxo-Smith Kline, Janssen, Merck, Pfizer, Schering-Plough, Shire, Takeda, UCB, and Warner Chilcott, has received educational support from AbbVie, Abbott, Bristol-Meyers Squibb, Centocor, Elan, Ferring, Janssen, Millennium, Proctor and Gamble, and Schering-Plough, has received research grants or clinical trial funding from AbbVie, Abbott, Bristol-Meyers Squibb, Centocor, Elan, Ferring, Janssen, Millennium, Proctor and Gamble, and Schering-Plough, and has participated in speaker’s bureaus for AbbVie, Abbott, AstraZeneca, Centocor, Elan, Janssen, Prometheus, Schering-Plough, Shire, Takeda, and Warner Chilcott. SM has served on advisory boards for AbbVie, Shire, and Takeda and has participated in speaker’s bureaus for AbbVie and Takeda. Sophie Plamondon has served on advisory boards for AbbVie, Janssen, and Takeda, has received educational support from AbbVie, and has participated in speaker’s bureaus for AbbVie, Janssen, and Shire. UC has served on advisory boards for AbbVie, Janssen, and Takeda, has received educational support from AbbVie and Aptalis, and has participated in speaker’s bureaus for AbbVie, Aptalis, and Janssen. WA has served on advisory boards for AbbVie, Ferring, Janssen, Shire, and Takeda, has received research grants or clinical trial funding from Prometheus, and has participated in speaker’s bureaus for AbbVie, Janssen, and Takeda. E, GIL, JM, and PM have no industry or government relationships to report.
Supported by: This guideline was supported through unrestricted grants to the Canadian Association of Gastroenterology by AbbVie Canada, Janssen, Pfizer, and Takeda Canada, who had no involvement in any aspect of the guideline development.
Canadian Association of Gastroenterology Statement
This clinical practice guideline (CPG) on the management of fistulizing perianal Crohn’s disease was developed under the direction of Drs. A. Hillary Steinhart and Remo Panaccione, in accordance with the policies and procedures of the Canadian Association of Gastroenterology (CAG) and under the direction of CAG Clinical Affairs. It has been reviewed by the CAG Practice Affairs and Clinical Affairs Committees and the CAG Board of Directors. The CPG was developed following a thorough consideration of medical literature and the best available evidence and clinical experience. It represents the consensus of a Canadian and international panel comprised of experts on this topic. The CPG aims to provide a reasonable and practical approach to care for specialists, and allied health professionals are charged with the duty of providing optimal care to patients and families. The CPG can be subject to change as scientific knowledge and technology advance and as practice patterns evolve. The CPG is not intended to be a substitute for physicians using their individual judgment in managing clinical care in consultation with the patient, with appropriate regard to all the individual circumstances of the patient, diagnostic, and treatment options available, in addition to available resources. Adherence to these recommendations will not necessarily produce successful outcomes in every case.
Acknowledgements
The CAG would like to thank AbbVie Corp., Janssen Inc., Pfizer Canada Inc., and Takeda Canada Inc. for their generous support of the guideline process. The consensus group would like to thank the following people for their contributions: Paul Sinclair, Lesley Marshall (CAG representatives, administrative and technical support, and logistics assistance), Pauline Lavigne, and Steven Portelance (unaffiliated, editorial assistance). Finally, we would like to thank our patient advocate, Jenna Rines, for invaluable insights.
Abbreviations
- ASC
allogenic-derived stem cell
- CAG
Canadian Association of Gastroenterology
- CI
confidence interval
- CPG
clinical practice guideline
- CT
computed tomography
- EUA
exam under anesthesia
- EUS
endoscopic ultrasound
- GRADE
Grading of Recommendation Assessment, Development and Evaluation
- HBOT
hyperbaric oxygen therapy
- MRI
magnetic resonance imaging
- MTX
methotrexate
- QoL
quality of life
- RCT
randomized controlled trial
- RR
relative risk
- TNF
tumor necrosis factor
References
- 1. Schwartz DA, Loftus EV Jr, Tremaine WJ, et al. . The natural history of fistulizing Crohn’s disease in Olmsted county, Minnesota. Gastroenterology. 2002;122:875–880. [DOI] [PubMed] [Google Scholar]
- 2. Beaugerie L, Seksik P, Nion-Larmurier I, et al. . Predictors of Crohn’s disease. Gastroenterology. 2006;130:650–656. [DOI] [PubMed] [Google Scholar]
- 3. Loly C, Belaiche J, Louis E. Predictors of severe Crohn’s disease. Scand J Gastroenterol. 2008;43:948–954. [DOI] [PubMed] [Google Scholar]
- 4. Beaugerie L, Sokol H. Clinical, serological and genetic predictors of inflammatory bowel disease course. World J Gastroenterol. 2012;18:3806–3813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ng SC, Plamondon S, Gupta A, et al. . Prospective evaluation of anti-tumor necrosis factor therapy guided by magnetic resonance imaging for Crohn’s perineal fistulas. Am J Gastroenterol. 2009;104:2973–2986. [DOI] [PubMed] [Google Scholar]
- 6. Colombel JF, Sandborn WJ, Rutgeerts P, et al. . Adalimumab for maintenance of clinical response and remission in patients with Crohn’s disease: the CHARM trial. Gastroenterology. 2007;132:52–65. [DOI] [PubMed] [Google Scholar]
- 7. Schwartz DA, Ghazi LJ, Regueiro M, et al. ; Crohn’s & Colitis Foundation of America, Inc Guidelines for the multidisciplinary management of Crohn’s perianal fistulas: summary statement. Inflamm Bowel Dis. 2015;21:723–730. [DOI] [PubMed] [Google Scholar]
- 8. Gecse KB, Bemelman W, Kamm MA, et al. ; World Gastroenterology Organization, International Organisation for Inflammatory Bowel Diseases IOIBD, European Society of Coloproctology and Robarts Clinical Trials; World Gastroenterology Organization International Organisation for Inflammatory Bowel Diseases IOIBD European Society of Coloproctology and Robarts Clinical Trials A global consensus on the classification, diagnosis and multidisciplinary treatment of perianal fistulising Crohn’s disease. Gut. 2014;63:1381–1392. [DOI] [PubMed] [Google Scholar]
- 9. Guyatt GH, Oxman AD, Vist GE, et al. ; GRADE Working Group GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. Bmj. 2008;336:924–926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sultan S, Falck-Ytter Y, Inadomi JM. The AGA institute process for developing clinical practice guidelines part one: grading the evidence. Clin Gastroenterol Hepatol. 2013;11:329–332. [DOI] [PubMed] [Google Scholar]
- 11. Bressler B, Marshall JK, Bernstein CN, et al. ; Toronto Ulcerative Colitis Consensus Group Clinical practice guidelines for the medical management of nonhospitalized ulcerative colitis: the toronto consensus. Gastroenterology. 2015;148:1035–1058.e3. [DOI] [PubMed] [Google Scholar]
- 12. Nguyen GC, Bernstein CN, Bitton A, et al. . Consensus statements on the risk, prevention, and treatment of venous thromboembolism in inflammatory bowel disease: Canadian Association of Gastroenterology. Gastroenterology. 2014;146:835–848.e6. [DOI] [PubMed] [Google Scholar]
- 13. Fallone CA, Chiba N, van Zanten SV, et al. . The Toronto consensus for the treatment of Helicobacter pylori infection in adults. Gastroenterology. 2016;151:51–69.e14. [DOI] [PubMed] [Google Scholar]
- 14. Nguyen GC, Seow CH, Maxwell C, et al. ; IBD in Pregnancy Consensus Group; Canadian Association of Gastroenterology The Toronto consensus statements for the management of inflammatory bowel disease in pregnancy. Gastroenterology. 2016;150:734–757.e1. [DOI] [PubMed] [Google Scholar]
- 15. Dalkey N. An experimental study of group opinion: the Delphi method. Futures. 1969;1:408–426. [Google Scholar]
- 16. Cook DJ, Greengold NL, Ellrodt AG, et al. . The relation between systematic reviews and practice guidelines. Ann Intern Med. 1997;127:210–216. [DOI] [PubMed] [Google Scholar]
- 17. Guyatt GH, Oxman AD, Kunz R, et al. ; GRADE Working Group Going from evidence to recommendations. Bmj. 2008;336:1049–1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Parks AG, Gordon PH, Hardcastle JD. A classification of fistula-in-ano. Br J Surg. 1976;63:1–12. [DOI] [PubMed] [Google Scholar]
- 19. Gisbert JP, Marín AC, Chaparro M. Systematic review: factors associated with relapse of inflammatory bowel disease after discontinuation of anti-TNF therapy. Aliment Pharmacol Ther. 2015;42:391–405. [DOI] [PubMed] [Google Scholar]
- 20. Kiss LS, Papp M, Lovasz BD, et al. . High-sensitivity C-reactive protein for identification of disease phenotype, active disease, and clinical relapses in Crohn’s disease: a marker for patient classification?Inflamm Bowel Dis. 2012;18:1647–1654. [DOI] [PubMed] [Google Scholar]
- 21. Mao R, Xiao YL, Gao X, et al. . Fecal calprotectin in predicting relapse of inflammatory bowel diseases: a meta-analysis of prospective studies. Inflamm Bowel Dis. 2012;18:1894–1899. [DOI] [PubMed] [Google Scholar]
- 22. Ghaly S, Murray K, Baird A, et al. . High vitamin D-binding protein concentration, low albumin, and mode of remission predict relapse in Crohn’s disease. Inflamm Bowel Dis. 2016;22:2456–2464. [DOI] [PubMed] [Google Scholar]
- 23. Qin G, Tu J, Liu L, et al. . Serum albumin and C-reactive protein/albumin ratio are useful biomarkers of Crohn’s disease activity. Med Sci Monit. 2016;22:4393–4400. [DOI] [PubMed] [Google Scholar]
- 24. Van Assche G, Vanbeckevoort D, Bielen D, et al. . Magnetic resonance imaging of the effects of infliximab on perianal fistulizing Crohn’s disease. Am J Gastroenterol. 2003;98:332–339. [DOI] [PubMed] [Google Scholar]
- 25. Schwartz DA, White CM, Wise PE, et al. . Use of endoscopic ultrasound to guide combination medical and surgical therapy for patients with Crohn’s perianal fistulas. Inflamm Bowel Dis. 2005;11:727–732. [DOI] [PubMed] [Google Scholar]
- 26. Karmiris K, Bielen D, Vanbeckevoort D, et al. . Long-term monitoring of infliximab therapy for perianal fistulizing Crohn’s disease by using magnetic resonance imaging. Clin Gastroenterol Hepatol. 2011;9:130–136. [DOI] [PubMed] [Google Scholar]
- 27. Schwartz DA, Wiersema MJ, Dudiak KM, et al. . A comparison of endoscopic ultrasound, magnetic resonance imaging, and exam under anesthesia for evaluation of Crohn’s perianal fistulas. Gastroenterology. 2001;121:1064–1072. [DOI] [PubMed] [Google Scholar]
- 28. Spradlin NM, Wise PE, Herline AJ, et al. . A randomized prospective trial of endoscopic ultrasound to guide combination medical and surgical treatment for Crohn’s perianal fistulas. Am J Gastroenterol. 2008;103:2527–2535. [DOI] [PubMed] [Google Scholar]
- 29. Wiese DM, Beaulieu D, Slaughter JC, et al. . Use of endoscopic ultrasound to guide adalimumab treatment in perianal Crohn’s disease results in faster fistula healing. Inflamm Bowel Dis. 2015;21:1594–1599. [DOI] [PubMed] [Google Scholar]
- 30. Villa C, Pompili G, Franceschelli G, et al. . Role of magnetic resonance imaging in evaluation of the activity of perianal Crohn’s disease. Eur J Radiol. 2012;81:616–622. [DOI] [PubMed] [Google Scholar]
- 31. Gionchetti P, Dignass A, Danese S, et al. ; ECCO 3rd european evidence-based consensus on the diagnosis and management of Crohn’s disease 2016: part 2: surgical management and special situations. J Crohns Colitis. 2017;11:135–149. [DOI] [PubMed] [Google Scholar]
- 32. Seastedt KP, Trencheva K, Michelassi F, et al. . Accuracy of CT enterography and magnetic resonance enterography imaging to detect lesions preoperatively in patients undergoing surgery for Crohn’s disease. Dis Colon Rectum. 2014;57:1364–1370. [DOI] [PubMed] [Google Scholar]
- 33. Maconi G, Sampietro GM, Parente F, et al. . Contrast radiology, computed tomography and ultrasonography in detecting internal fistulas and intra-abdominal abscesses in Crohn’s disease: a prospective comparative study. Am J Gastroenterol. 2003;98:1545–1555. [DOI] [PubMed] [Google Scholar]
- 34. Yassin NA, Askari A, Warusavitarne J, et al. . Systematic review: the combined surgical and medical treatment of fistulising perianal Crohn’s disease. Aliment Pharmacol Ther. 2014;40:741–749. [DOI] [PubMed] [Google Scholar]
- 35. Khan KJ, Ullman TA, Ford AC, et al. . Antibiotic therapy in inflammatory bowel disease: a systematic review and meta-analysis. Am J Gastroenterol. 2011;106:661–673. [DOI] [PubMed] [Google Scholar]
- 36. Dewint P, Hansen BE, Verhey E, et al. . Adalimumab combined with ciprofloxacin is superior to adalimumab monotherapy in perianal fistula closure in Crohn’s disease: a randomised, double-blind, placebo controlled trial (ADAFI). Gut. 2014;63:292–299. [DOI] [PubMed] [Google Scholar]
- 37. Su JW, Ma JJ, Zhang HJ. Use of antibiotics in patients with Crohn’s disease: a systematic review and meta-analysis. J Dig Dis. 2015;16:58–66. [DOI] [PubMed] [Google Scholar]
- 38. West RL, van der Woude CJ, Hansen BE, et al. . Clinical and endosonographic effect of ciprofloxacin on the treatment of perianal fistulae in Crohn’s disease with infliximab: a double-blind placebo-controlled study. Aliment Pharmacol Ther. 2004;20:1329–1336. [DOI] [PubMed] [Google Scholar]
- 39. Present DH, Rutgeerts P, Targan S, et al. . Infliximab for the treatment of fistulas in patients with Crohn’s disease. N Engl J Med. 1999;340:1398–1405. [DOI] [PubMed] [Google Scholar]
- 40. Colombel JF, Schwartz DA, Sandborn WJ, et al. . Adalimumab for the treatment of fistulas in patients with Crohn’s disease. Gut. 2009;58:940–948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Hanauer SB, Sandborn WJ, Rutgeerts P, et al. . Human antitumor necrosis factor monoclonal antibody (adalimumab) in Crohn’s disease: the CLASSIC-I trial. Gastroenterology. 2006;130:323–333; quiz 591. [DOI] [PubMed] [Google Scholar]
- 42. Sandborn WJ, Rutgeerts P, Enns R, et al. . Adalimumab induction therapy for crohn disease previously treated with infliximab: a randomized trial. Ann Intern Med. 2007;146:829–838. [DOI] [PubMed] [Google Scholar]
- 43. Sandborn WJ, Feagan BG, Stoinov S, et al. ; PRECISE 1 Study Investigators Certolizumab pegol for the treatment of Crohn’s disease. N Engl J Med. 2007;357:228–238. [DOI] [PubMed] [Google Scholar]
- 44. Ford AC, Sandborn WJ, Khan KJ, et al. . Efficacy of biological therapies in inflammatory bowel disease: systematic review and meta-analysis. Am J Gastroenterol. 2011;106:644–659, quiz 660 [DOI] [PubMed] [Google Scholar]
- 45. de Groof EJ, Sahami S, Lucas C, et al. . Treatment of perianal fistula in Crohn’s disease: a systematic review and meta-analysis comparing seton drainage and anti-tumour necrosis factor treatment. Colorectal Dis. 2016;18:667–675. [DOI] [PubMed] [Google Scholar]
- 46. Sands BE, Anderson FH, Bernstein CN, et al. . Infliximab maintenance therapy for fistulizing Crohn’s disease. N Engl J Med. 2004;350:876–885. [DOI] [PubMed] [Google Scholar]
- 47. Schreiber S, Khaliq-Kareemi M, Lawrance IC, et al. ; PRECISE 2 Study Investigators Maintenance therapy with certolizumab pegol for Crohn’s disease. N Engl J Med. 2007;357:239–250. [DOI] [PubMed] [Google Scholar]
- 48. Schreiber S, Lawrance IC, Thomsen OØ, et al. . Randomised clinical trial: certolizumab pegol for fistulas in Crohn’s disease - subgroup results from a placebo-controlled study. Aliment Pharmacol Ther. 2011;33:185–193. [DOI] [PubMed] [Google Scholar]
- 49. Lichtenstein GR, Yan S, Bala M, et al. . Infliximab maintenance treatment reduces hospitalizations, surgeries, and procedures in fistulizing Crohn’s disease. Gastroenterology. 2005;128:862–869. [DOI] [PubMed] [Google Scholar]
- 50. Panaccione R, Colombel JF, Sandborn WJ, et al. . Adalimumab maintains remission of Crohn’s disease after up to 4 years of treatment: data from CHARM and ADHERE. Aliment Pharmacol Ther. 2013;38:1236–1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Roumeguère P, Bouchard D, Pigot F, et al. . Combined approach with infliximab, surgery, and methotrexate in severe fistulizing anoperineal Crohn’s disease: results from a prospective study. Inflamm Bowel Dis. 2011;17:69–76. [DOI] [PubMed] [Google Scholar]
- 52. Topstad DR, Panaccione R, Heine JA, et al. . Combined seton placement, infliximab infusion, and maintenance immunosuppressives improve healing rate in fistulizing anorectal Crohn’s disease: a single center experience. Dis Colon Rectum. 2003;46:577–583. [DOI] [PubMed] [Google Scholar]
- 53. Schröder O, Blumenstein I, Schulte-Bockholt A, et al. . Combining infliximab and methotrexate in fistulizing Crohn’s disease resistant or intolerant to azathioprine. Aliment Pharmacol Ther. 2004;19:295–301. [DOI] [PubMed] [Google Scholar]
- 54. Ochsenkühn T, Göke B, Sackmann M. Combining infliximab with 6-mercaptopurine/azathioprine for fistula therapy in Crohn’s disease. Am J Gastroenterol. 2002;97:2022–2025. [DOI] [PubMed] [Google Scholar]
- 55. Tozer P, Ng SC, Siddiqui MR, et al. . Long-term MRI-guided combined anti-TNF-α and thiopurine therapy for Crohn’s perianal fistulas. Inflamm Bowel Dis. 2012;18:1825–1834. [DOI] [PubMed] [Google Scholar]
- 56. Bouguen G, Siproudhis L, Gizard E, et al. . Long-term outcome of perianal fistulizing Crohn’s disease treated with infliximab. Clin Gastroenterol Hepatol. 2013;11:975–81.e1. [DOI] [PubMed] [Google Scholar]
- 57. Present DH, Korelitz BI, Wisch N, et al. . Treatment of Crohn’s disease with 6-mercaptopurine. A long-term, randomized, double-blind study. N Engl J Med. 1980;302:981–987. [DOI] [PubMed] [Google Scholar]
- 58. Colombel JF, Sandborn WJ, Reinisch W, et al. ; SONIC Study Group Infliximab, azathioprine, or combination therapy for Crohn’s disease. N Engl J Med. 2010;362:1383–1395. [DOI] [PubMed] [Google Scholar]
- 59. Davidov Y, Ungar B, Bar-Yoseph H, et al. . Association of induction infliximab levels with clinical response in perianal Crohn’s disease. J Crohns Colitis. 2017;11:549–555. [DOI] [PubMed] [Google Scholar]
- 60. Yarur AJ, Kanagala V, Stein DJ, et al. . Higher infliximab trough levels are associated with perianal fistula healing in patients with Crohn’s disease. Aliment Pharmacol Ther. 2017;45:933–940. [DOI] [PubMed] [Google Scholar]
- 61. de Parades V, Far HS, Etienney I, et al. . Seton drainage and fibrin glue injection for complex anal fistulas. Colorectal Dis. 2010;12:459–463. [DOI] [PubMed] [Google Scholar]
- 62. Hyman N, O’Brien S, Osler T. Outcomes after fistulotomy: results of a prospective, multicenter regional study. Dis Colon Rectum. 2009;52:2022–2027. [DOI] [PubMed] [Google Scholar]
- 63. Graf W, Andersson M, Åkerlund JE, et al. ; Swedish Organization for Studies of Inflammatory Bowel Disease Long-term outcome after surgery for Crohn’s anal fistula. Colorectal Dis. 2016;18:80–85. [DOI] [PubMed] [Google Scholar]
- 64. Nasseri Y, Cassella L, Berns M, et al. . The anal fistula plug in Crohn’s disease patients with fistula-in-ano: a systematic review. Colorectal Dis. 2016;18:351–356. [DOI] [PubMed] [Google Scholar]
- 65. Senéjoux A, Siproudhis L, Abramowitz L, et al. ; Groupe d’Etude Thérapeutique des Affections Inflammatoires du tube Digestif [GETAID] Fistula plug in fistulising ano-perineal Crohn’s disease: a randomised controlled trial. J Crohns Colitis. 2016;10:141–148. [DOI] [PubMed] [Google Scholar]
- 66. Soltani A, Kaiser AM. Endorectal advancement flap for cryptoglandular or Crohn’s fistula-in-ano. Dis Colon Rectum. 2010;53:486–495. [DOI] [PubMed] [Google Scholar]
- 67. Gingold DS, Murrell ZA, Fleshner PR. A prospective evaluation of the ligation of the intersphincteric tract procedure for complex anal fistula in patients with Crohn’s disease. Ann Surg. 2014;260:1057–1061. [DOI] [PubMed] [Google Scholar]
- 68. Singh S, Ding NS, Mathis KL, et al. . Systematic review with meta-analysis: faecal diversion for management of perianal Crohn’s disease. Aliment Pharmacol Ther. 2015;42:783–792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Lambertz A, Lüken B, Ulmer TF, et al. . Influence of diversion stoma on surgical outcome and recurrence rates in patients with rectovaginal fistula - A retrospective cohort study. Int J Surg. 2016;25:114–117. [DOI] [PubMed] [Google Scholar]
- 70. Bell SJ, Williams AB, Wiesel P, et al. . The clinical course of fistulating Crohn’s disease. Aliment Pharmacol Ther. 2003;17:1145–1151. [DOI] [PubMed] [Google Scholar]
- 71. Sandborn WJ, Feagan BG, Rutgeerts P, et al. ; GEMINI 2 Study Group Vedolizumab as induction and maintenance therapy for Crohn’s disease. N Engl J Med. 2013;369:711–721. [DOI] [PubMed] [Google Scholar]
- 72. Khorrami S, Ginard D, Marín-Jiménez I, et al. . Ustekinumab for the treatment of refractory Crohn’s disease: the spanish experience in a large multicentre open-label cohort. Inflamm Bowel Dis. 2016;22:1662–1669. [DOI] [PubMed] [Google Scholar]
- 73. Kopylov U, Afif W, Cohen A, et al. . Subcutaneous ustekinumab for the treatment of anti-TNF resistant Crohn’s disease–the mcgill experience. J Crohns Colitis. 2014;8:1516–1522. [DOI] [PubMed] [Google Scholar]
- 74. Lowry PW, Weaver AL, Tremaine WJ, et al. . Combination therapy with oral tacrolimus (FK506) and azathioprine or 6-mercaptopurine for treatment-refractory Crohn’s disease perianal fistulae. Inflamm Bowel Dis. 1999;5:239–245. [DOI] [PubMed] [Google Scholar]
- 75. González-Lama Y, Abreu L, Vera MI, et al. . Long-term oral tacrolimus therapy in refractory to infliximab fistulizing Crohn’s disease: a pilot study. Inflamm Bowel Dis. 2005;11:8–15. [DOI] [PubMed] [Google Scholar]
- 76. Sandborn WJ, Present DH, Isaacs KL, et al. . Tacrolimus for the treatment of fistulas in patients with Crohn’s disease: a randomized, placebo-controlled trial. Gastroenterology. 2003;125:380–388. [DOI] [PubMed] [Google Scholar]
- 77. Hart AL, Plamondon S, Kamm MA. Topical tacrolimus in the treatment of perianal Crohn’s disease: exploratory randomized controlled trial. Inflamm Bowel Dis. 2007;13:245–253. [DOI] [PubMed] [Google Scholar]
- 78. Panés J, García-Olmo D, Van Assche G, et al. ; ADMIRE CD Study Group Collaborators Expanded allogeneic adipose-derived mesenchymal stem cells (cx601) for complex perianal fistulas in Crohn’s disease: a phase 3 randomised, double-blind controlled trial. Lancet. 2016;388:1281–1290. [DOI] [PubMed] [Google Scholar]
- 79. Panes J, García-Olmo D, Van Assche G, et al. . Long-term efficacy and safety of Cx601, allogeneic expanded adipose-derived mesenchymal stem cells, for complex perianal fistulas in Crohn’s disease: 52-week results of a phase III randomised controlled trial [Abstract OP009]. J Crohn’s Colitis. 2017;11:S5. [Google Scholar]
- 80. Noyer CM, Brandt LJ. Hyperbaric oxygen therapy for perineal Crohn’s disease. Am J Gastroenterol. 1999;94:318–321. [DOI] [PubMed] [Google Scholar]
- 81. Iezzi LE, Feitosa MR, Medeiros BA, et al. . Crohn’s disease and hyperbaric oxygen therapy. Acta Cir Bras. 2011;26(Suppl 2):129–132. [DOI] [PubMed] [Google Scholar]
- 82. Feitosa MR, Féres Filho O, Tamaki CM, et al. . Adjunctive hyperbaric oxygen therapy promotes successful healing in patients with refractory Crohn’s disease. Acta Cir Bras. 2016;31(Suppl 1):19–23. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.