Abstract
The Nrf2 pathway protects against oxidative stress and induces regeneration of various tissues. Here, we investigated whether Nrf2 protects from sclerosing cholangitis and biliary fibrosis and simultaneously induces liver regeneration. Diet containing 3,5-diethoxycarbonyl-1,4-dihydrocollidine (DDC) was fed to Nrf2-KO mice (Nrf2−/−), mice with liver-specific hyperactivated Nrf2 (HKeap1−/−) and wild-type (WT) littermates to induce cholangitis, liver fibrosis, and oval cell expansion. HKeap1−/−-mice were protected from almost all DDC-induced injury compared with WT and Nrf2−/−. Liver injury in Nrf2−/− and WT mice was mostly similar, albeit Nrf2−/− suffered more from DDC diet as seen for several parameters. Nrf2 activity was especially important for the expression of the hepatic efflux transporters Abcg2 and Abcc2-4, which are involved in hepatic toxin elimination. Surprisingly, cell proliferation was more enhanced in Nrf2−/−- and HKeap1−/−-mice compared with WT. Interestingly, Nrf2−/−-mice failed to sufficiently activate oval cell expansion after DDC treatment and showed almost no resident oval cell population under control conditions. The resident oval cell population of untreated HKeap1−/−-mice was increased and DDC treatment resulted in a stronger oval cell expansion compared with WT. We provide evidence that Nrf2 activation protects from DDC-induced sclerosing cholangitis and biliary fibrosis. Moreover, our data establish a possible role of Nrf2 in oval cell expansion.
Keywords: Nrf2, porphyrin clearance, liver fibrosis, cholangitis, oval cell expansion
Liver remodeling and fibrosis progression are often the result of an insufficient response of hepatocytes and biliary epithelial cells to chronic liver injury. A complex network of different liver and immune cells activates cytokines and growth factors that trigger fibrosis. Feeding mice 3,5-diethoxycarbonyl-1,4-dihydrocollidine (DDC) is a well-established model to study such complex interactions in a highly relevant model of chronic cholestatic liver injury, which lead to injury of both hepatocytes and biliary epithelial cells with rapid onset of hepatocellular liver injury involving necrosis and apoptosis and the induction of ductular proliferation (Fickert et al., 2007). This process triggers transformation of hepatic stellate cells into myofibroblasts and contributes to the progression of liver fibrosis. The animals also develop rapid and sustained jaundice. Therefore, DDC feeding resembles certain metabolic, toxic, and cholestatic liver diseases (Wakabayashi et al., 2010a).
Moreover, the liver has high potential to regenerate by hepatocyte proliferation in response to various types of injuries. When this regenerative ability is impaired, the hepatic stem cells called progenitor or oval cells proliferate and differentiate in hepatocytes and cholangiocytes. Feeding a DDC-containing diet is one of the most efficient rodent models for chemical-induced oval cell proliferation (Jakubowski et al., 2005).
The transcription factor Nrf2 (nuclear factor [erythroid-derived 2]-like 2 or NFE2L2) is well established as the major regulator of oxidative stress defense. In common with other stress-responsive transcription factors, Nrf2 is constitutively and ubiquitously expressed with its turnover largely controlled at the level of protein stability. Under unstressed conditions, Nrf2 is rapidly degraded by the proteasome via interaction with its adaptor protein Keap1. However, potentially reactive molecules such as reactive oxygen species (ROS) or xenobiotics modify Keap1 to impede degradation and enhance accumulation of Nrf2 leading to elevated signaling. Nrf2 binds to the “antioxidant response element” (ARE) and induces the expression of target genes coding for antioxidative enzymes and proteins. In addition, Nrf2 regulates the expression of genes that influence tissue regeneration such as Notch, amphiregulin, VEGF, and IL-6 (Kobayashi et al., 2016; Reiss et al., 2014; Serhan and Savill, 2005; Wakabayashi et al., 2010a; Wruck et al., 2011). Inducers of Nrf2 signaling such as isothiocyanates, flavonoids, or kavalactones are shown to be protective against various diseases (Brandenburg et al., 2010; Fragoulis et al., 2012, 2017; Wruck et al., 2007, 2008). Recently, Nrf2-activating pharmaceuticals found the way in the clinical practice (Al-Sawaf et al., 2015; O'Connell and Hayes, 2015). Moreover, Nrf2 is also activated by various cytokines and growth factors and is able to upregulate the expression of cytokines and growth factors, suggesting a function of Nrf2 beyond oxidative stress defense (Tebay et al., 2015; Wakabayashi et al., 2010b). In case of injury, Nrf2 activation is required for stem cell proliferation. Kim et al. showed that Nrf2 activation is required to induce hematopoietic stem cell proliferation after potentially lethal doses of myelosuppressive ionizing radiation (Kim et al., 2014). Moreover, Paul et al. showed the same role for Nrf2 in a model of lung injury where Nrf2 activation is required to induce airway basal stem cell proliferation (Paul et al., 2014). We et al., have shown that Nrf2 is necessary to induce satellite cell proliferation to regenerate the muscle after injury (Al-Sawaf et al., 2014a,b; Narasimhan et al., 2014). Because of the possible links between Nrf2, oxidative stress, cholestasis, and oval cell activation, we tested whether Nrf2 protects against DDC-induced liver injury and whether Nrf2 plays a role during oval cell expansion.
MATERIALS AND METHODS
Mouse Strains
Conditional AlbCre::Keap1loxP/loxP knockout mice (referred to as HKeap1−/−) were generated by crossing Keap1loxP/loxP with mice expressing Cre recombinase under the control of the albumin (Alb) promoter (Okawa et al., 2006). In these mice, Keap1 deletion is limited to hepatocytes, oval cells, and biliary epithelial cells (Ishikawa et al., 2012). To visualize Cre expression, we crossbred the Alb-Cre::Keap1loxP/loxP mice with B6.Cg-Gt(ROSA)26Sortm9(CAG-tdTomato)Hze/J: (JAX labs stock No: 007909, also called Ai9 or Ai9[RCL-tdT]) mice to obtain AlbCre::Keap1loxP/loxP::Ai9 mice (Supplementary Figure 1). Ai9 mice harbor a targeted mutation of the Gt(ROSA)26Sor locus with a loxP-flanked STOP cassette preventing transcription of a CAG promoter-driven red fluorescent protein variant (tdTomato). Nrf2−/− mice were generated as described previously (Chan et al., 1996). Wild-type (WT) littermates of Nrf2−/− mice (Nrf2+/+) were used for WT group. The Keap1/Nrf2 double-knockout (designated as HKeap1−/−::Nrf2−/−) strain was generated by crossbreeding of HKeap1−/− with Nrf2−/− mice. Transgenic C57BL6/J ARE-luciferase reporter gene mice (ARE-luc) were purchased from Cgene (Oslo, Norway) (Dohlen et al., 2008). These mice express the firefly luciferase under the control of an ARE and were used to quantify Nrf2 activity.
All mice used in this study were generated on a C57BL/6J background (Chan et al., 1996; Dohlen et al., 2008; Okawa et al., 2006).
DDC Treatment
To determine the impact of DDC feeding on Nrf2 activity, ARE-luc mice were fed with standard chow diet supplemented with 0.1% (w/w) DDC (Sigma-Aldrich, Steinheim, Germany) for 5 consecutive days.
To investigate the course of disease induced by DDC treatment, mice were fed with standard chow containing 0.1% DDC and normal drinking water for 4 weeks. Untreated control mice were fed with standard rodent chow diet.
At the start of the study, all mice were 8–10 weeks old and maintained in our animal facilities under specific pathogen-free conditions. Mice were housed in 12 h light/dark cycles with free access to food and water.
All experiments were conducted in accordance with German legislation governing animal studies. The Principles of Laboratory Animal Care were followed and experiments were approved by the “Landesamt fuer Natur, Umwelt und Verbraucherschutz Nordrhein-Westfalen.”
Sample Isolation
After euthanasia, blood was taken for analysis of liver transaminases and bilirubin concentrations in the serum. Liver samples were shock-frozen for isolation of RNA and proteins or embedded in both paraffin and Tissue-Tek O.C.T. Compound (cat. no.: 4583, Sakura Finetek, Staufen, Germany) for histochemical and immunofluorescent analyses.
Histology and Immunofluorescence
Hematoxylin and eosin staining
Liver samples were fixed in 4% formaldehyde, embedded in paraffin, sectioned (3 µm) and stained with hematoxylin and eosin (H&E). Pictures of each group were taken using a Keyence BZ-9000 microscope.
Sirius-red staining
For Sirius-red staining paraffin-embedded liver sections (3 µm) were incubated with 0.1% Sirius-red-staining solution (cat. no.: 365548, Sigma Aldrich, Frankfurt, Germany) for 1 h after dewaxing and hydration of the sections. Thereafter the slides were incubated in 0.1 M HCl for 5 min, treated with an ascending ethanol series and finally incubated in Roti-Histol (Roth, Karlsruhe, Germany) and covered with Roti-Histokit (Roth, Karlsruhe, Germany). The sections were analyzed under polarized light.
Immunofluorescence
Cryopreserved liver tissue sections (5 µm) were fixed with 4% PFA (Roth, Karlsruhe, Germany) or ice-cold acetone, washed in PBS containing 0.02% sodium-azide (Roth, Karlsruhe, Germany) and blocked using 2% BSA in PBS or 1:5 dilution of blocking reagent (Promega, Mannheim, Germany) before antibody incubation. For paraffin-embedded tissue, we conducted a standard dewax protocol using a descending alcohol series to water. Antigen retrieval was achieved by boiling in sodium citrate solution (pH 6.0) for 20 min using a microwave. Sections were blocked with 5% goat serum in PBS. Targets were detected via Alexa488 or Alexa555 labeled secondary antibody (Molecular Probes/Invitrogen, Karlsruhe). Detailed information regarding applied reagents and antibodies are depicted in Supplementary Table 1.
TUNEL staining
Apoptotic cells were visualized and quantified on paraffin-embedded liver sections (3 µm) by TUNEL staining (In Situ Cell death Detection Fluorescein Kit, cat. no.: 11684795910, Roche Diagnostics GmbH, Mannheim, Germany) as recommended by the manufacturer.
Porphyrin Quantification
Livers were homogenized in 9 volumes of homogenization buffer (0.25 M sucrose containing 10 µM MG132, 10 µM Na3VO3, 100 µM NaF, and cOmplete EDTA-free inhibitor cocktail). The protein content was measured by BCA assay (Thermo Scientific, Pierce Protein Biology Products; cat. no.: 23227; Rockford, Illinois) and the samples were adjusted to 5 mg/ml. Samples (technical duplicates) of all groups (n = 6) were transferred to a black 96-well micro titer plate with glass bottom. Porphyrin content was visualized by red fluorescence imaging using Cy5.5 excitation/emission filters in an IVIS Lumina system (PerkinElmer, Waltham, Massachusetts) using the following settings: binning = 2, FOV = 12.5 cm, f/stop = 2, integration time = 15 s. Because the Cy5.5 filter setting of the IVIS system is not optimal for porphyrin fluorescence, porphyrins were additionally quantified in the infinite M200 microplate reader (Tecan Group, Maennedorf, Switzerland). Experimental properties were set as follows: excitation at 405 nm and emission at 635 nm, integration time at 20 µs with 5 flashes.
Measurement of Serum Transaminases and Bilirubin
Serum was extracted from blood of each animal. ALT and AST activities were determined by an automated enzyme assay (Roche, Mannheim, Germany) as described before (Wruck et al., 2011). For the measurement of bilirubin content, serum samples were processed according to the standard procedures of the Central Laboratory Facility of the Uniklinik RWTH Aachen.
RNA Isolation, cDNA Synthesis, and qPCR
The qRT-PCR study was conducted in compliance with the MIQE guidelines (Bustin et al., 2009). Total RNA was isolated from 30 mg of liver tissue using the NucleoSpin RNA kit as recommended by the manufacturer (Macherey Nagel, Dueren, Germany). Nucleic acid quantity and purity was determined by spectrophotometry (A260/A280 and A260/A230) using the NanoDrop ND-1000 device (Thermo Scientific, Waltham, Massachusetts). Good RNA integrity was ensured by MOPS-buffered denaturizing RNA gel electrophoresis (28S/18S rRNA ratio of at least 1.8). Reverse transcription was performed according to the supplier’s instructions using the Maxima Reverse Transcriptase (Thermo Fisher Scientific, Waltham, Massachusetts) with mixed priming (oligo-[dT]18: random hexamer, 3:1, v/v) and 2 µg total RNA/per reaction. Real-time PCR was conducted on an ABI StepOne Plus system using PowerSYBR Green PCR Master Mix (Thermo Fisher Scientific, Waltham, Massachusetts). PCR was performed with primer-specific pre-evaluated annealing temperatures and a standard 2-step protocol. Primer specificity was determined by melt curve analysis and TAE-buffered DNA agarose gel electrophoresis of the PCR products. Amplification efficiency was calculated with LinRegPCR 2016.0 software (Heart Failure Research Center, Amsterdam, The Netherlands) as described before (Ramakers et al., 2003). A reference gene evaluation including 10 representative samples and 10 potential reference targets was conducted with geNorm calculations (part of the Biogazelle qbase+ software) prior to the actual study to establish a sufficient reference gene index for data normalization. This evaluation recommended the combination of succinate dehydrogenase complex subunit A (SDHA) with glyceraldehyde 3-phosphate dehydrogenase (GAPDH) as the most suitable normalization strategy for this experimental setup. Inter-Run-Variation was corrected by the use of inter-run calibrators in each PCR run. The relative fold change in gene expression was calculated with qbase+ according to the recommended efficiency corrected ΔΔCq method as reported by Pfaffl in 2001 (Pfaffl, 2001). All applied qPCR primers are listed in Supplementary Table 2.
OxyBlot
Protein oxidation was shown by determining levels of protein carbonylation. By this method, carbonyls are derivatized with 2,4-dinitrophenylhydrazine (DNPH) that can be detected with a specific anti-DNP antibody on immunoblots (Colombo et al., 2015). Samples (5 µl containing 10 µg total protein) were initially denaturized by adding 5 µl of 12% SDS and boiling at 95°C for 15 min. Derivatization was achieved subsequently by the addition of 5 µl 10 mM DNPH (in 2 N HCl) solution and incubation at room temperature (RT) for 20 min. Samples were subsequently neutralized with 7.5 µl of neutralization solution (2 M Tris in 30% glycerol) and loaded on 12% polyacrylamide gels for electrophoretic separation. For controls, the same samples were treated as described except that a control solution (2 N HCl without DNPH) was used instead of the DNPH-containing derivatization solution. Proteins were blotted to a PVDF membrane by semidry electroblotting (Trans-Blot Turbo, Bio-Rad, Hercules, California). Washing steps were done with TBS solution containing 0.04% (v/v) Tween 20 (WB). Membranes were blocked at RT for 1 h with 3% bovine serum albumin dissolved in WB before incubation with the rabbit polyclonal anti-DNP primary antibody (1:100; cat. no.: D9656; Sigma Aldrich, Frankfurt, Germany) at RT for 2 h under constant rocking. The HRP-conjugated anti-rabbit immunoglobulin G (1:20000; cat. no. 7074; CST, Danvers, Massachusetts) was used as secondary antibody. Immunoreactivity was visualized using the chemiluminescence reagent Immobilon Western (Millipore, Darmstadt, Germany) as recommended by the manufacturer. Luminescence signals were detected on photosensitive Hyperfilm ECL (GE Healthcare Life Science, Chalfont St. Giles; United Kingdom) in a dark room under exclusion of light. For densitometry, the blots were captured using a flatbed scanner and the intensity of each lane was calculated with Quantity One Software 4.6.9 (Biorad; Hercules, California).
Luminex Multi-Plex Assay
Cytokine expression in liver tissue was measured by Luminex Multi-Plex technology. The Bio-Plex Pro Mouse Cytokine 23-plex assay (cat. no.: M60009RDPD, Bio-Rad laboratories, Munich, Germany) was used as recommended by the manufacturer. The assay was analyzed on a Luminex 200 system. Tissue homogenization was achieved using the Bio-Plex Cell Lysis Kit (Bio-Rad laboratories; cat. no.: 171-304011; Munich, Germany) as recommended for tissue samples. Therefore, 30 mg of snap frozen liver tissue was transferred to 2 ml Precellys tubes filled with 1.4 mm ceramic beads and 500 µl Cell Lysis Buffer. Homogenization was performed with the Precellys 24 system at 5000 rpm for 20 s. The protein content was determined by BCA assay (Thermo Scientific, Pierce Protein Biology Products; cat. no.: 23227; Rockford, Illinois) and the samples were adjusted to 2 mg/ml in a reaction volume of 50 µl. Cytokine concentrations are depicted as pg/ml.
ARE Reporter Gene Assay
ARE-luciferase reporter gene mice were fed with DDC for 5 consecutive days. Untreated control animals were fed with standard chow diet. Subsequently, the animals were euthanatized, livers removed, and immediately snap-frozen in liquid nitrogen. For luciferase activity measurements, liver tissue was lysed in passive lysis buffer from Promega Corporation (Madison, Wisconsin) and photon emission was determined using a 96-well plate luminometer (Glomax 96 microplate) with the Luciferase Assay Reagent I (LAR I) as recommended by the manufacturer. The signal was normalized to the total amount of liver tissue weight.
Statistics
Variance homogeneity was checked with the Bartlett test. The Shapiro–Wilk test was used to test normal distribution. BoxCox-Y transformation was conducted to achieve homoscedasticity if necessary. One-way ANOVA followed by Tukey post hoc test was performed to analyze parametric data. Nonparametric data were analyzed using the Kruskal–Wallis test followed by Dunn’s post hoc testing. Data are shown as mean + SEM. Statistical analyses were performed with GraphPad Prism 5.03 (GraphPad, La Jolla) and JMP 10 (Böblingen, Germany).
RESULTS
Nrf2 Protects Against DDC-Induced Liver Injury
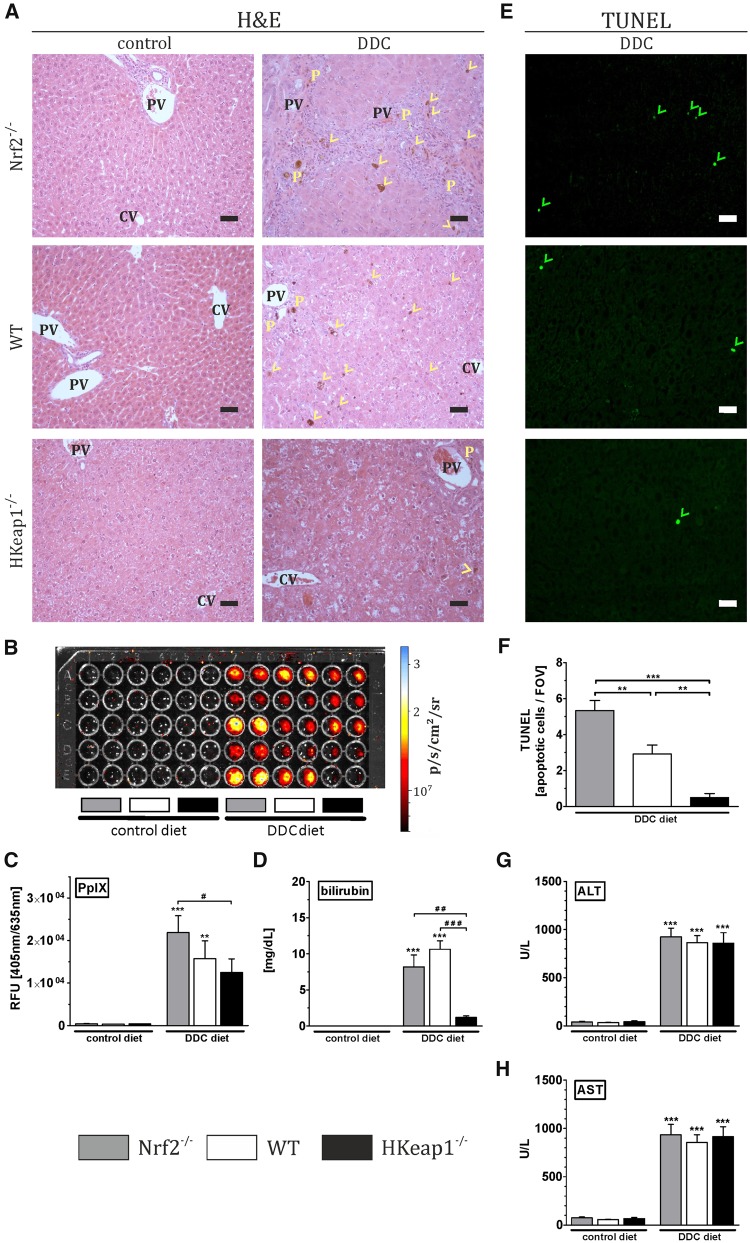
DDC-induced liver injury was investigated by the determination of bilirubinostasis, apoptosis, and the increase of ALT and AST in the serum. H&E staining revealed several ductular porphyrin plugs as well as porphyrin incorporation in the parenchyma/bile canaliculi of Nrf2−/− and WT livers whereas HKeap1−/− livers were almost unaffected (Figure 1A, yellow P, ductular porphyrin plugs; yellow arrow heads, porphyrin accumulation in the parenchyma/bile canaliculi). Porphyrin detection and quantification showed that porphyrin fluorescence was equal in all genotypes under control diet (Nrf2−/−: 0.04 × 104 RFU, WT: 0.03 × 104 RFU, and HKeap1−/−: 0.30 × 104 RFU). DDC treatment increased porphyrin-associated fluorescence in all DDC-treated mice with the most intense signal in Nrf2−/− livers (2.19 × 104 RFU). Porphyrin content of DDC-treated WT livers did not differ significantly from Nrf2−/− livers (1.57 × 104 RFU). However, porphyrin accumulation was significantly lower in HKeap−/− mice compared with Nrf2−/− (1.24 × 104 RFU) (Figs. 1B and 1C) after DDC treatment. Serum bilirubin was significantly elevated in Nrf2−/− and WT mice after DDC treatment, further confirming impaired bilirubin excretion in these livers. In contrast to that, HKeap1−/− mice were protected against DDC-induced bilirubinostasis (Figure 1D). WT showed slightly reduced bilirubin incorporation compared with Nrf2−/− in the histological analysis but that was not paralleled by serum bilirubin levels (Figs. 1A and 1D). TUNEL staining was conducted to investigate apoptosis under DDC treatment. As shown in Figure 1E, DDC induced apoptosis in all genotypes but to a variable extent. Quantification of TUNEL-positive cells per field of view (FOV) revealed a clear Nrf2-dependency (Figure 1F). Apoptosis was most severe in Nrf2−/− (5.33 ± 0.55) whereas WT mice showed significantly reduced and moderate amount of apoptotic cells per FOV (2.91 ± 0.49). Almost no apoptotic cells (0.50 ± 0.22) were detected in DDC-treated HKeap1−/− livers. ALT (Figure 1G) and AST (Figure 1H) levels were increased after 4 weeks of DDC treatment in all experimental groups. However, the amount of transaminases in all genotypes was equal, although otherwise expected.
Figure 1.
Nrf2 prevents DDC-mediated porphyrin accumulation and apoptosis. Nrf2−/−, WT, and HKeap1−/− were fed for 4 weeks with normal chow (control) or 0.1% DDC-containing diet (DDC). (A) H&E staining for general histological evaluation of liver damage, such as bile duct proliferation, bilirubin/porphyrin accumulation in the bile canaliculi (yellow arrow heads), or ductular bilirubin/porphyrin plugs (yellow P). CV, central vein; PV, portal vein; representative pictures out of n = 6; scale = 50 µm. (B) Porphyrin detection in liver homogenates using a Cy5.5 filter set in the IVIS Lumina system. Samples (technical duplicates) of each group (n = 6) were measured. Fluorescence signals (p/s/cm2/sr) are visualized with the help of a false-color scale. (C) Porphyrin content in liver homogenates were quantified in microplate reader using 405 nm excitation and 635 nm emission setting. n = 6. (D) Bilirubin content in the sera of the animals was determined and quantified, n ≥ 8. (E) TUNEL staining was conducted to examine apoptosis after DDC treatment. Representative images are shown, TUNEL-positive apoptotic cells are marked by green arrowheads; scale = 50 µm. (F) Quantification of TUNEL-positive nuclei per field of view (FOV). (G and H) The amount of liver-specific transaminases (ALT and AST) in the serum was quantified with an automated enzyme assay. n ≥ 8; data = mean + SEM; **p < .01, ***p < .001 versus control diet; #p < .05, ##p < .01, ###p < .001 as indicated.
Nrf2 Mitigates Porphyrin/Bilirubin Accumulation by Inducing the Expression of Essential Cellular Toxin Transporters
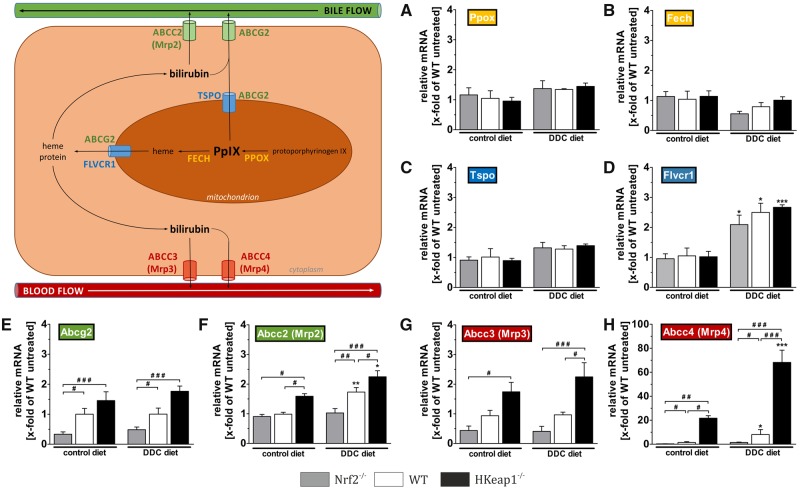
To investigate the mechanism responsible for the observed decrease in porphyrin accumulation in HKeap1−/− livers, we analyzed gene expression levels of essential enzymes involved in porphyrin processing and transport by qRT-PCR (Figure 2). DDC-mediated hepatotoxicity is mainly based on the inhibition of the final enzyme involved in heme synthesis (Fech: ferrochelatase) resulting in protoporphyrin IX accumulation that leads to tissue damage. Our analyzes showed that neither the expression of the protoporphyrin IX (PpIX) producing enzyme (Figure 2A, Ppox: protoporphyrinogen IX oxidase) nor the expression of the final enzyme of heme synthesis (Figure 2B, Fech: ferrochelatase) were altered in between Nrf2−/−, WT, and HKeap1−/− mice. Moreover, DDC treatment did not affect the expression of both enzymes.
Figure 2.
Porphyrin/bilirubin export but not production is regulated by Nrf2. Heme biosynthesis pathway and export mechanisms were investigated by qRT-PCR as depicted in the schematic overview. Gene expression of (A) protoporphyrin IX (PpIX)-producing protoporphyrinogen oxidases (Ppox) and (B) PpIX-facilitating and heme-synthetizing ferrochelatase (Fech) are shown. Gene expression analyses of the mitochondrial efflux transporters (C) translocator protein (Tspo) and (D) feline leukemia virus subgroup C cellular receptor 1 (Flvcr1) are depicted. The hepatic efflux transporters ATP-binding cassette subfamily G/C member 2 (Abcg2 and Abcc2) toward bile ducts are shown in (E and F). Results for the hepatic efflux transporters toward sinusoidal blood flow (Abcc3 and Abcc4) are shown in (G and H). n = 6; data = mean + SEM; *p < .05, **p < .01, and ***p < .001 versus control diet; #p < .05, ##p < .01, and ###p < .001 as indicated.
As porphyrin accumulation could also be altered due to varying expression levels of transporter that are involved in the removal of intracellular toxic intermediates, we expanded these experiments by expression studies of intracellular transporters. These analyses included Tspo (translocator protein), Flvcr1 (feline leukemia virus subgroup C cellular receptor 1), and Abcg2 (ATP-binding cassette subfamily G member 2) as responsible proteins for mitochondrial efflux of PpIX and heme, respectively. As shown in Figure 2C, expression levels of Tspo were affected neither by genotype nor by the treatment. The expression of Flvcr1 was significantly induced under DDC diet but without differences between the genotypes (Nrf2−/−: 2.09-fold, WT: 2.50-fold, and HKeap1−/−: 2.67-fold; Figure 2D). The only Nrf2-dependent difference in this transporter group was found for Abcg2 expression. This transporter is responsible for mitochondrial export of both PpIX as well as heme. As can be seen in Figure 2E, under both conditions we found significant differences between Nrf2−/− and WT (control diet: 0.33-fold vs. 1.00-fold, DDC diet: 0.48-fold vs. 1.00-fold) as well as significant differences between Nrf2−/− and HKeap1−/− mice (control diet: 0.33-fold vs. 1.46-fold, DDC diet: 0.48-fold vs. 1.77-fold). However, the expression of Abcg2 was not altered due to DDC treatment.
Besides, from the mitochondrial efflux, Abcg2 can also be involved in the cellular efflux toward bile canaliculi through the apical cell membrane. Another important transporter for this kind of efflux is Abcc2 (ATP-binding cassette subfamily C member 2) also known as Mrp2 (multidrug resistance-associated protein 2). Under control conditions, Abcc2 was significantly higher expressed in HKeap1−/− livers (1.59-fold) compared with WT (1.00-fold) and Nrf2−/− (0.91-fold), although its expression was not significantly reduced in Nrf2−/− livers compared with WT. However, although Abcc2 mRNA expression was increased by DDC treatment in the livers of WT (1.73-fold) and even more pronounced in livers of HKeap1−/− mice (2.31-fold), DDC-mediated induction of Abcc2 mRNA expression failed in Nrf2−/− livers (1.03-fold) (Figure 2F).
If this way of toxic removal is impaired in a hepatocyte, the cell is able to excrete toxic intermediates toward the sinusoids. Thereby, downstream hepatocytes can take them up and guide them to own ABCG2/ABCC2-mediated biliary efflux. The transporters responsible therefore are among others ABCC3 and ABCC4 (ATP-binding cassette subfamily C member 3 and 4) also known as Mrp3 and Mrp4 (multidrug resistance-associated protein 3 and 4). qRT-PCR analysis revealed that Abcc3 gene expression was not different between control and DDC diet-treated animals. Nevertheless, we observed different expression levels between the genotypes for this transporter. Under control conditions, expression levels showed an Nrf2-dependet trend with less Abcc3 expression in Nrf2−/− (0.44-fold) and highest expression in HKeap1−/− (1.75-fold). Nevertheless, only the difference between Nrf2−/− and HKeap1−/− mice was statistically significant. After DDC treatment this trend was similar, though expression levels of HKeap−/− and WT livers were significantly different as well (2.25-fold HKeap−/− vs. 0.96-fold WT). The most intense effect of Nrf2 as well as DDC was observed for Abcc4 gene expression. Even under control conditions, Abcc4 gene expression was Nrf2 dependent with the lowest expression in Nrf2−/− (0.33-fold) and the highest expression in HKeap1−/− mice (21.73-fold). DDC increased Abcc4 gene expression slightly in WT (up to 8.09-fold) and extensively in HKeap1−/− mice (up to 68.21-fold) but not in Nrf2−/− livers (1.44-fold), remaining significant differences between these genotypes (Figure 2H).
Nrf2 Protects Against Fibrosis
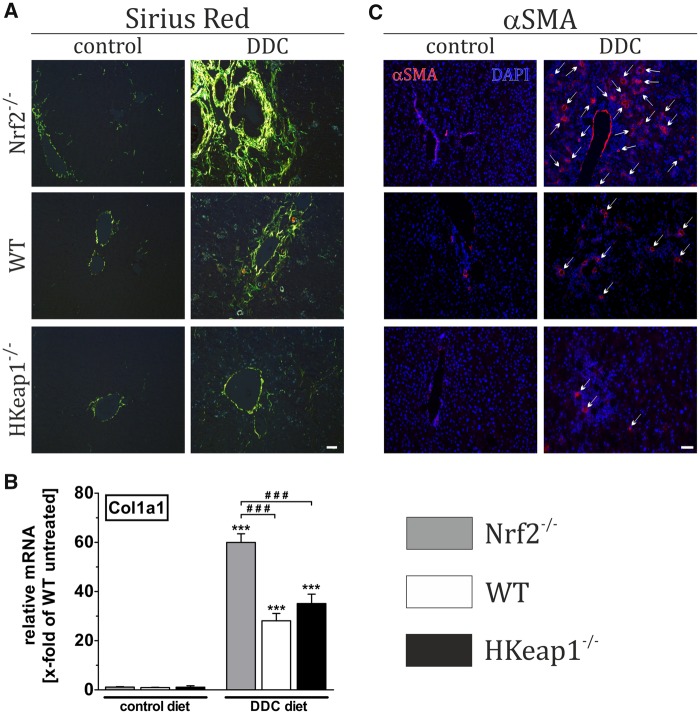
To study the role of Nrf2 for liver remodeling and fibrosis, DDC was applied to Nrf2−/−, WT, and HKeap1−/− mice. To estimate the degree of liver fibrosis, we conducted Sirius-red staining, mRNA expression analysis of Col1a1, and immunofluorescent detection of α-SMA positive cells (alpha smooth muscle actin: marker of activated stellate cells). Under control conditions, there were no differences between all three genotypes with respect to fibrosis as shown in Figure 3A (Sirius red), Figure 3B (qRT-PCR Col1a1), and Figure 3C (α-SMA, arrows). However, DDC treatment led to increased collagen fiber formation, increased expression of Col1a1 mRNA, and α-SMA immunoreactivity in WT. These effects were even intensified in Nrf2−/−-livers as collagen fiber formation, Col1a1 mRNA expression as well as the amount of activated stellate cells were significantly increased compared with WT livers. Compared with that, the livers of HKeap1−/− mice exhibited a very slight fibrosis with almost no collagen fiber formation and very few activated stellate cells (Figs. 2A and 2C, right columns). Nevertheless, the expression level of Col1a1 mRNA was comparable to DDC-treated WT mice (Figure 3B).
Figure 3.
Nrf2 prevents liver fibrosis and stellate cell activation. Nrf2−/−, WT, and HKeap1−/− were fed for 4 weeks with normal chow (control) or 0.1% DDC-containing diet (DDC). Afterward, liver tissue was collected and prepared as described in the Materials and Methods section. (A) Sirius-red staining to visualize fibrotic changes. (B) mRNA expression level of Col1a1 as a fibrotic marker using qRT-PCR. (C) Immunofluorescent staining against αSMA for the detection of activated stellate cells. A and C show representative pictures out of n = 6; data = mean + SEM; n = 6; ***p < .001 versus control diet; ###p < .001 as indicated.
DDC Treatment Induces Nrf2 Signaling to Counteract Liver Injury
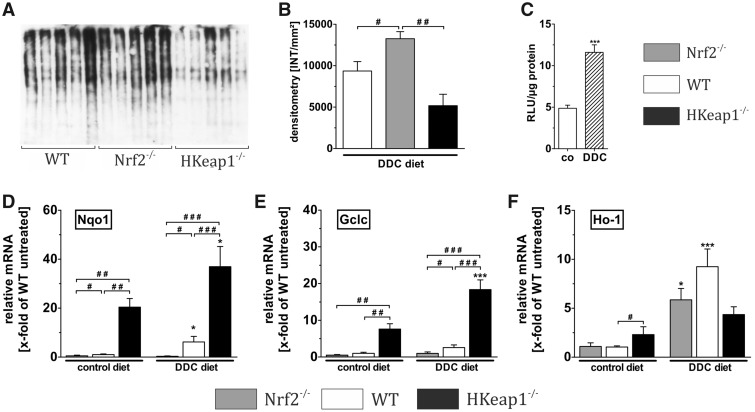
DDC treatment has been frequently described to induce oxidative stress and associated tissue damage of the liver. Protein carbonylation—a harmful oxidative modification of proteins—is considered to be mainly driven by the radical 4-hydroxy-nonenal (4-HNE) which itself is the product of oxidative modification of lipids called lipid peroxidation. This makes the measurement of protein carbonylation a suitable tool to investigate oxidative-stress-related damage to liver proteins (Singla et al., 2012). In our study, protein carbonylation was detected and quantified by OxyBlot technique. As expected, the livers of Nrf2−/− mice showed significantly more protein carbonylation compared with WT and HKeap1−/− mice (Figs. 4A and 4B). Strikingly, livers of HKeap1−/− mice were almost totally protected against DDC-induced oxidative damage indicating an enhanced oxidative-stress defense in the liver of these mice. To estimate the reason for increased susceptibility of Nrf2−/− livers to DDC-associated pathogenesis, we focused on Nrf2 activation upon DDC feeding. For this purpose, we analyzed the potency of DDC feeding to induce the Nrf2/ARE system. We fed ARE-luc mice with DDC chow for 5 days and analyzed the Nrf2-dependent luminescence signals of liver homogenate in a luminometer assay. This experiment showed that DDC-treatment-induced Nrf2 signaling 2.50-fold compared with untreated mice (Figure 4C).
Figure 4.
Nrf2 regulates cytoprotective proteins. To figure out the underlying mechanisms of Nrf2-mediated protection against DDC diet, we analyzed oxidative stress-associated protein carbonylation via OxyBlot technique, which is depicted in (A) with the resulting densitometry results shown in (B). DDC-induced Nrf2 activation was confirmed by ARE-luciferase reporter gene assays with control and DDC-treated ARE-luciferase mice as depicted in (C). The mRNA expression of essential cytoprotective targets was analyzed. These analyses included the expression of important Nrf2 target genes that are involved in the oxidative stress defense such as (D) Nqo1: NADP(H) dehydrogenase quinone 1, (E) Gclc: glutamate-cysteine ligase catalytic subunit, and (F) Ho-1: heme oxygenase-1. DDC treatment regiments: A, B, D–H treatment for 4 weeks; C treatment for 5 days. Data = mean + SEM, OxyBlot, n = 6, reporter gene assay, n = 5, qRT-PCR, n = 6; *p < .05 and ***p < .001 versus control diet; #p < .05, ##p < .01, and ###p < .001 as indicated.
We then analyzed the mRNA expression of Nrf2 target genes to understand how Nrf2 might promote liver protection against DDC-associated injury. We included the representative Nrf2 target genes NAD(P)H quinone dehydrogenase 1 (Nqo1), γ-glutamylcysteine synthetase (γGcs or Gclc), and heme oxygenase-1 (Ho-1) that are involved in the oxidative stress response and thereby important for cytoprotection.
Under control conditions, Nqo1, Gclc, and Ho-1 were significantly higher expressed in livers from HKeap1−/− (Nqo1: 20.41-, Gclc: 7.61-, and Ho-1: 2.31-fold) when compared with WT mice (Figs. 4D–F, black vs. white column, control diet). Although Nrf2 knockout resulted in significantly reduced expression of Nqo1 (0.50-fold), Gclc (0.52-fold) and Ho-1 expression were not significantly reduced due to Nrf2 depletion (1.09-fold) (Figs. 4D–F, gray column, control diet). Although DDC treatment further increased the gene expression of Nqo1 (up to 36.94-fold) and Gclc (up to 18.37-fold) in HKeap1−/− and of Nqo1 (up to 6.17-fold) in WT livers, the induction of these important protective targets failed in Nrf2−/− (Nqo1: 0.32-fold, Gclc: 0.98-fold). Ho-1 mRNA expression was induced by DDC feeding in Nrf2−/− and WT but not HKeap1−/−, albeit the induction showed no conclusive Nrf2 dependency. Both Nrf2−/− and WT livers showed a significantly increased Ho-1 expression after DDC treatment, whereas to a higher amount in WT livers (Nrf2−/−: 5.86-fold, WT: 9.24-fold). Nevertheless, after DDC treatment, the Ho-1 mRNA expression in livers of HKeap1−/−-mice was not further increased and was similar to those of Nrf2−/− mice and thus not further increased (4.36-fold) (Figure 4F).
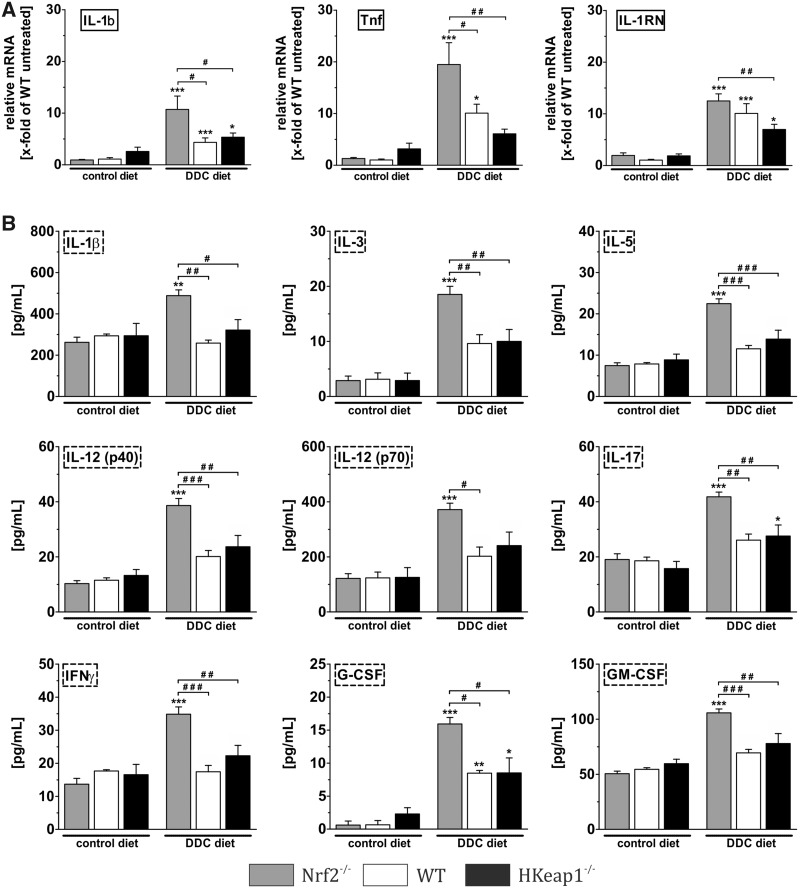
Nrf2 Ameliorates DDC-Associated Inflammation
DDC diet for 4 weeks slightly induced inflammation. In our study, we used quantitative PCR and multiplex Luminex analyses to quantify inflammation-related mediators. Our results demonstrate that DDC treatment only resulted in a moderate inflammatory reaction. Even though all 3 genotypes reacted with increased mRNA expression of IL-1β, TNF, and IL-1RN, the induction of these factors was significantly higher in livers of Nrf2−/− mice compared with WT and HKeap1−/− mice (Figure 5A). On protein level, only 2 inflammatory markers (IL-17 and G-CSF) showed a significant induction by DDC in WT and HKeap1−/−. In contrast, Nrf2−/−-mice revealed a highly increased expression of all investigated inflammatory mediators in the liver in response to DDC (Figure 5B). This indicates a crucial role for Nrf2 on inflammation in this model.
Figure 5.
Nrf2 inhibits intrahepatic inflammation. The inflammatory response after DDC treatment was investigated. (A) qRT-PCR data for the pro-inflammatory cytokines IL-1β and TNF as well as the anti-inflammatory cytokine IL-1RN, n = 6. (B) Luminex multiplex assay using liver homogenates to determine the protein concentration of the pro-inflammatory mediators: IL-1β, IL-12, GM-CSF, IL-17, IFNγ, IL-5, IL-3, and G-CSF, n = 5. Data = mean + SEM; *p < .05, **p < .01, and ***p < .001 versus control diet; #p < .05, ##p < .01, and ###p < .001 as indicated.
Nrf2 Is Not Essential for Cholangiocyte and Hepatocyte Proliferation
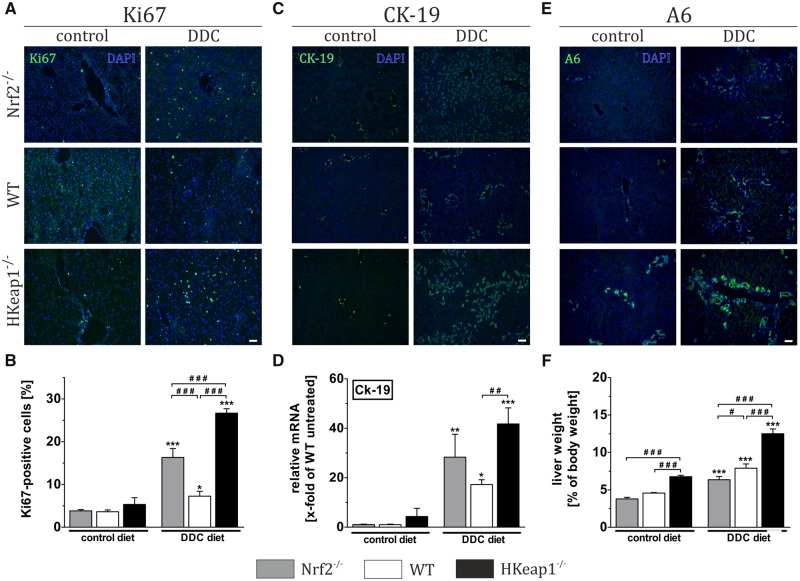
Directly after scarification, the livers were examined and weighted. The livers of mice that received the control diet did not alter macroscopically in between the genotypes with respect to size and color (data not shown). However, liver-to-body weight assessment revealed that the liver weights significantly differed in an Nrf2-dependent manner. Livers of Nrf2−/−- and WT-mice were comparable without significant differences (4.00 and 4.58%; Figure 6F, gray and white column, control diet), whereas liver weights of HKeap1−/− mice were significantly increased under control conditions (6.77% of total body weight, Figure 6F, black column, control diet). All DDC-fed mice developed a hepatomegaly due to DDC treatment. The liver weight of Nrf2−/− animals increased from 4.00% to 6.35%, of WT animals from 4.58% to 7.90% and of HKeap1−/− from 6.77% to 12.50% (Figure 6F, columns under DDC diet). In other words, the relative increase of liver weight due to DDC treatment corresponds to a fold-change of 1.59 in Nrf2−/−, 1.72 in WT, and 1.85 in HKeap1−/− mice. To understand the underlying mechanisms of the observed hepatomegaly, we performed further analyses to examine proliferation (Figs. 6A and 6B, Ki67 IF and cell counting), ductular reaction (Figs. 6C and 6D, CK-19 IF and mRNA expression) as well as oval cell activation/expansion (Figs. 6E, A6 IF). For Ki67 analysis, Ki67-positive were counted and related to the total amount of cells. As in a healthy liver, only very few hepatocytes proliferate actively, the Ki67 immunoreactivity was low and particularly similar between all genotypes under control conditions as expected (Figs. 6A and 6B). DDC diet indeed increased proliferation, but interestingly the slightest effect was found in WT livers (3.61% to 7.26%), whereas livers of Nrf2−/− showed a moderate (3.83% to 16.32%) and livers of HKeap1−/− mice the most intensive increase of proliferating cells (5.33% to 26.67%). Although the increased proliferation in the livers of Nrf2−/− livers can be explained by the immense tissue injury and the requirement for regeneration, this cannot be the explanation for the HKeap1−/− livers, because DDC-mediated injury was almost absent in these mice.
Figure 6.
Nrf2 enhances liver regeneration due to increased oval cell activation. Examination of liver regeneration was conducted by Ki67 staining (A) and quantification of Ki67-positive cells (B). Cholangiocytes/cholangitis were visualized by immunofluorescent labeling of CK-19 protein (C) and further quantified by mRNA expression analysis of Ck-19 (D). Examination of oval cell activation was conducted by immunofluorescent detection using an antibody against A6 (E). Relative liver weights are depicted in (F) as calculated, n ≥ 8. Representative images out of n = 6 are shown (scale = 100 µm). Data = mean + SEM, *p < .05, **p < .01, and ***p < .001 versus control diet; ###p < .001 as indicated.
Increased amounts of cholangiocytes can be taken as a measure for ductular reaction or cholangitis respectively. To do so, we used CK-19 immunofluorescence staining and conducted CK-19 qRT-PCR experiments. Under control conditions, all 3 genotypes showed comparable numbers of CK-19-positive cells as well as Ck-19 mRNA expression (Figs. 6C and 6D, control diet). DDC enhanced the number of cholangiocytes in all treated mice. Nrf2−/−-mice showed a greater increase of cholangiocytes compared with WT mice. Surprisingly, also the DDC-treated HKeap1−/−-mice showed a greater number of cholangiocytes (Figure 6C, DDC diet). This finding was further confirmed by qRT-PCR analysis of CK-19 mRNA expression showing increased CK-19 expression in all DDC-treated animals but with the highest expression in HKeap1−/− mice (Figure 6D, DDC diet). This might imply that the hepatic knockout of Keap1 makes the liver more accessible to developing a cholangitis. Because our analyses on inflammatory mediators (Figure 5) did not support this assumption, we continued with further investigations. Although CK-19 is a prominent and often used marker for cholangiocytes, it is not exclusively expressed by these cells. Oval cells, the liver-specific progenitor cells, which can be activated and expanded in the course of liver regeneration, express CK-19 as well. That means that the CK-19-positive cells in the livers might be at least partly oval cells rather than cholangiocytes. The detection of oval cells was achieved by immunofluorescence staining with A6 antibody. The immunoreactivity of A6 antibody correlated well with the graded Nrf2 activity in the examined mice. Under control conditions, Nrf2−/−-mice showed less and HKeap1−/−-mice showed an even greater oval cell population when compared with WT mice (Figure 6E, control diet). DDC feeding induced oval cell expansion in all tested mice. Here as well, the intensity of A6 immunoreactivity (oval cells) correlated with the Nrf2 activity of the treated genotypes. Under DDC-treatment, Nrf2−/−-mice showed less and HKeap1−/−-mice showed significantly more oval cell expansion when compared with WT mice (Figure 6E, DDC diet).
To make sure that these observed effects are Nrf2 dependent and not an effect of Keap1-deletion, we crossed HKeap1−/− mice with Nrf2−/−-mice (HKeap1−/−::Nrf2−/−). This double knockout resulted in almost complete loss of the oval cell population under control conditions similar to that seen in Nrf2−/−-mice (Supplementary Figure 3).
DISCUSSION
The goal of this study was to define the role of the Nrf2/Keap1 pathway in severe chronic liver injury and subsequent regeneration by utilizing mice with inactivated and hyper-activated Nrf2. As a model for chronic liver injury, we used DDC feeding because it reflects many aspects of certain human chronic liver diseases such as primary sclerosing cholangitis, primary biliary fibrosis, and drug-induced bile duct damage (Fickert et al., 2007). In addition, DDC diet is one of the most efficient models for chemical-induced oval cell expansion (Jakubowski et al., 2005; Wakabayashi et al., 2010a).
Our data strongly support previous publications describing the protective role of Nrf2 (O'Connell and Hayes, 2015). We and other showed that Nrf2 signaling is induced by DDC feeding, most likely by increased PpIX-mediated oxidative stress and the inhibition of Nrf2 proteasomal degradation (Singla et al., 2012; Taguchi et al., 2019). That indicates the necessity of Nrf2 activation to upregulate the expression of liver-protective proteins, including detoxifying enzymes but also essential transporter proteins to eliminate upcoming toxic intermediates. Unexpectedly, after 4 weeks of DDC treatment, the liver injury marker ALT and AST were upregulated in all genotypes to the same amount. In line with that, Taguchi et al. described similar findings after 4 weeks DDC treatment. Interestingly, they showed that ALT values after short-term DDC feeding (1 week) were significantly lower in Keap1 knockout compared with WT mice and that ALT concentrations decreased after 4 weeks DDC feeding to adapt to Keap1-KO levels (Taguchi et al., 2019). Therefore, with respect to this parameter, it can be concluded that during acute injury, Nrf2 activity partly ameliorates ALT increase. However, after chronic exposure to DDC, transaminases decrease to a common level in all genotypes, so that Nrf2 activity alone might not be sufficient to prevent the whole injury. Nevertheless, most of the other investigated DDC-triggered symptoms (apoptosis, porphyrin accumulation, fibrosis, oxidative damage, and inflammation) were highly exacerbated in DDC-fed Nrf2 knockout (Nrf2−/−) mice. However, also livers of WT mice were damaged from DDC feeding indicating that a natural Nrf2 level might not be sufficient or that Nrf2 induction occurs too late to entirely protect against DDC-induced stress. This situation was different in mice with liver-specific Keap1 deletion (HKeap1−/−) expressing constitutively upregulated Nrf2 target genes. This Nrf2 hyper-activation widely protected these mice against DDC toxicity including strikingly lower levels of bilirubin/porphyrin accumulation and jaundice, fibrosis, oxidative stress damage, and inflammation.
DDC is a potent inhibitor of ferrochelatase (Cole and Marks, 1984), the final enzyme in heme synthesis (De Matteis et al., 1973), and leads to an accumulation of protoporphyrin IX (PpIX) in hepatocytes, bile canaliculi and bile ducts, which in turn results in severe fibrosis and tissue damage. Therefore, efficient removal of emerging PpIX and bilirubin is essential for liver protection against DDC toxicity (Sachar et al., 2016). The observed reduction of porphyrin accumulation in the tissue and bilirubin concentration in the sera of HKeap1−/− mice indicates a beneficial role of Nrf2 in these processes. Gene expression analyses of protoporphyrinogen IX oxidase (Ppox) and ferrochelatase (Fech) ensured that PpIX production itself is not altered due to Nrf2 activity, because this could be a reason for reduced PpIX accumulation. Furthermore, the primarily involved mitochondrial exporter the translocator protein (Tspo) and the feline leukemia virus subgroup C cellular receptor 1 (Flvcr1) were not regulated by Nrf2. However, Flvcr1 gene expression was slightly upregulated after DDC treatment most likely as a compensatory mechanism due to DDC-mediated lack of cellular heme. Interestingly, gene expression of ATP-binding cassette subfamily G member 2 (Abcg2)—another mitochondrial exporter for PpIX as well as heme and a confirmed Nrf2 target (Adachi et al., 2007)—was at least significantly reduced in Nrf2−/− mice, suggesting an impaired mitochondrial efflux in these animals although its expression was not regulated by DDC treatment. Besides mitochondrial efflux, Abcg2 is also involved in cellular removal of toxins toward the bile flow and thus Nrf2−/− mice seem to have a reduced capacity of PpIX removal that in turn increases intercellular toxicity and consequential damages. Further members of ABC transporters confirmed our assumption, that Nrf2 might be a crucial factor for efficient PpIX and bilirubin excretion. ATP-binding cassette subfamily C member 2 (ABCC2/Mrp2) which is also found at the apical ductular membrane was significantly upregulated in HKeap1−/− mice under control condition and the expression was even increased after DDC treatment. This further improves PpIX export in this mouse strain. Although already in 2006, Abcc2/Mrp2 was identified as direct transcriptional target of Nrf2 (Vollrath et al., 2006), Abcc2 gene expression was not reduced in Nrf2−/− compared with WT livers, suggesting an alternative regulatory pathway for its expression in these mice. However, in contrast to WT mice, the induction of Abcc2 gene expression upon DDC treatment failed in Nrf2−/−, thereby possibly decreasing PpIX excretion via the bile ducts. Hepatic clearance of toxins is further ensured by the membrane efflux transporters Abcc3 and Abcc4 that allow hepatocytes to release toxic intermediates into sinusoidal blood flow for re-uptake by downstream hepatocytes, which is thought to be a mechanism to counteract a local saturation of biliary detoxification (Iusuf et al., 2012). In 2008, Aleksunes et al. showed that both Abcc3 and Abcc4 were induced under acetaminophen (APAP) treatment in an Nrf2-dependet manner (Aleksunes et al., 2008) and just recently, promoter analyses of the human Abcc3 gene confirmed Nrf2 as transcriptional regulator (Canet et al., 2015). In our model, gene expression of both transporters exhibited an Nrf2 dependency, confirming the findings of the APAP study. However, Abcc3 gene expression was indeed upregulated in HKeap1−/− livers, but not significantly reduced in Nrf2−/− livers. This again suggest other transcriptional regulators that might regulate Abcc3 transcription in Nrf2−/− mice. Analyses of Abcc4 gene expression revealed the most prominent effect in our model. We showed that Abcc4 was significantly downregulated in Nrf2−/− and upregulated in HKeap1−/− mice, respectively even under control conditions. After DDC diet, Abcc4 expression was induced in WT and further increased in HKeap1−/− mice, but not in Nrf2−/− mice. Our finding strongly suggests that Nrf2 is important for toxin excretion and enhances the cellular capacity for this process, even though only Abcc4 gene expression exhibited a clear Nrf2 dependency. Actually, in 2009 Aleksunes and et al. applied multivariate statistical procedures to identify transcription factors that correlate with Abcc2, 3, and 4 transcription in adult human livers and gave evidence that expression levels of all 3 transporters significantly correlate with Nrf2 activity. However, they also showed that other transcription factors such CAR, HNF1 alpha. and PPAR alpha exhibit greater correlation with them, explaining the observed inconsistencies (Aleksunes et al., 2009).
Our study revealed that despite similar porphyrin/bilirubin accumulation and transaminases as WT mice, Nrf2−/− mice suffered most from DDC-associated fibrosis. DDC-induced collagen deposition as well as activation of hepatic stellate cells were clearly less intense in HKeap1−/− mice compared with both WT and Nrf2−/−, suggesting effective protection against fibrosis. However, Col1a1 mRNA expression levels were comparable to that of DDC-treated WT mice and did not conclusively confirm that. Anyway, there are several possible reasons for an increased Col1a1 mRNA expression with simultaneous absence of collagen deposition. Just recently, Ju et al. showed that ECM accumulation by hepatic stellate cells was significantly reduced in an in vitro approach by promoting MMP-9- and MMP-13-mediated COL1 protein degradation instead of inhibiting Col1a1 mRNA expression (Fu and Ohnishi, 2018). Moreover, Endo et al. already showed a positive transcriptional regulation of MMP-9 by Nrf2 (Endo et al., 2018). Whether this is the case in our model has to be elucidated in future studies.
Besides fibrosis, Nrf2 protected the livers against oxidative-stress-associated tissue damage by upregulating the cytoprotective target genes NAD(P)H quinone dehydrogenase 1 (Nqo1), γ-glutamylcysteine synthetase (γGcs or Gclc), and heme oxygenase-1 (Ho-1). Excessive oxidative stress induces inflammation.
We showed that even if gene expression of inflammatory mediators was induced after DDC treatment, the induction negatively correlated with Nrf2 activity. Furthermore, multiplex analyses on protein level revealed that besides from IL-17 and G-CSF, which were elevated in WT and/or HKeap1−/− livers as well, all other inflammatory mediators were exclusively increased in Nrf2−/− livers by DDC.
We and others have shown that Keap1 deletion in the liver resulted in hepatomegaly even under physiological conditions (Figure 6F) (Shirasaki et al., 2014; Taguchi et al., 2014). DDC treatment intensifies this process. However, with respect to hepatomegaly, our analyses on proliferation (Ki67), ductular reaction (CK-19), and oval cell expansion (A6) did not correlate with the observed changes of liver weights. Although each staining would explain the highest liver weight in HKeap1−/− under both control as well as DDC diet, this is not as obvious for WT and Nrf2−/− mice. Certainly, enhanced proliferation did not exclusively account for these enlarged livers because proliferation rates under control conditions are not significantly elevated in consequence of Keap1 deletion (Figure 6B) (Shirasaki et al., 2014). Moreover, DDC-induced additional proliferation probably to compensate for occurring cell death due to apoptosis as shown by TUNEL staining (Figure 1E) (Haybaeck et al., 2012) resulted in further enlarged livers. Interestingly, although proliferation rates were higher in both Nrf2−/− and HKeap1−/− mice compared with WT, livers of DDC-treated Nrf2−/− mice were significantly smaller than these of treated WT mice. This might be explained as a result of graver defects in Nrf2−/−. Obviously, this enhanced proliferation still failed to completely compensate tissue damage. Livers of HKeap1−/− showed the highest proliferation rate and thereby the highest liver weight of all DDC-treated mice. This was not to be expected considering that DDC treatment caused only minimal liver damage in this genotype and, therefore, livers of these mice had rather less need to compensate. Wakabayashi et al. were able to demonstrate a functional bidirectional interaction between the Keap1-Nrf2-ARE and the Notch1 signaling cascade in the liver (Wakabayashi et al., 2010a, 2014). Notch1 signaling has already been described to be essential for the amplification of several somatic stem cells including the hepatic stem cells (oval cells) (Jensen et al., 2004) triggering liver regeneration (Kohler et al., 2004; Wakabayashi et al., 2010a). Thus, DDC treatment might activate Nrf2-dependent and independent pathways that induce cell proliferation that is important to be elucidated in future studies.
However, our analysis regarding DDC-mediated cholangitis was initially not absolutely congruent. The immunohistochemical staining against CK-19, a commonly used cholangiocyte marker, showed that both Nrf2−/− and HKeap1−/− mice exhibited increased CK-19 immunoreactivity after DDC treatment. Unexpectedly, the cholangitis seemed to be more pronounced in HKeap−/− mice although these mice were protected against most of the other DDC-induced liver damages. In addition, it made us wonder that the inflammatory parameters were not notably elevated in HKeap−/− mice, which is not in line with advancing cholangitis.
We therefore looked for an alternative explanation for this CK-19-positive staining in HKeap−/− mice. In addition to cholangiocytes, CK-19 is also expressed in the progenitor cell population located at the portal field called oval cells (Chiu et al., 2007; de Lima et al., 2008). To distinguish cholangiocytes from oval cells, we used the selective oval cell marker A6. Interestingly, DDC treatment increased the population of A6-positive cells extensively in HKeap1−/− but only faintly in Nrf2−/− livers. We therefore believe that the observed increase of CK-19-positive cells and the increased inflammatory parameters in Nrf2−/− mice after DDC treatment are due to a fulminant cholangitis. In HKeap1−/− livers, CK-19- and A6-positive cells might rather be proliferating oval cells. This observation would be in line with other studies investigating the role of Nrf2 in homeostatic mechanisms that maintain the stem cell pool. It has already been shown that Nrf2 maintains the stem cell pool of the intestine (Hochmuth et al., 2011), lung (Paul et al., 2014), bone marrow (Tsai et al., 2013), and skeletal muscle (Al-Sawaf et al., 2014b; Narasimhan et al., 2014) and enables these stem cells to respond to injury and to proliferate for repair. In a similar context, we have shown that Nrf2 activation starts and prolongs the proliferation phase of satellite cells by upregulating the pro-proliferative factor MyoD and inhibiting the differentiation factor myogenin (Al-Sawaf et al., 2014a,b). Therefore, it might be reasonably assumed that Nrf2 might play an analogous role in the liver progenitor cell proliferation and increased A6 immunoreactivity in livers of HKeap1−/− implies an enhanced stem cell pool (oval cells) in these mice. This in combination with slightly increased proliferation might explain increased liver weights nicely.
These findings indicate that Nrf2 activation is both protective against liver injuries caused by chronic liver diseases and might induce progenitor cell proliferation to initiate liver regeneration.
CONCLUSION
In conclusion, these results provide new evidence for an important role of Nrf2 in the coordinated stress response against DDC-induced chronic liver injury by increasing hepatocyte-mediated porphyrin excretion. Another important finding was that Nrf2 might be important for the maintenance of the oval cell pool and the regulation of regenerative responses through oval cell proliferation.
DECLARATION OF CONFLICTING INTERESTS
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
AUTHOR CONTRIBUTION
A.F., K.L.S., and C.J.W. designed the study. T.W.K. provided the HKeap1−/− mouse strain. A.F., J.S., M.H., and T.S. conducted the experiments. A.F., J.S., M.H., and T.S. performed data collection. A.F., H.J., T.P., C.T., K.L.S., and C.J.W. conducted data analysis and interpretation. A.F., K.L.S., and C.J.W. drafted the manuscript. A.F., J.S., M.H., T.S., H.J., T.P., C.T., T.W.K., K.L.S., and C.J.W. revised the manuscript content. All authors read and approved the final manuscript.
SUPPLEMENTARY DATA
Supplementary data are available at Toxicological Sciences online.
Supplementary Material
ACKNOWLEDGMENTS
We thank Nina Koch, Angela Rüben, and Stefanie Erschfeld for their excellent technical assistance.
FUNDING
This research project was partly supported by the START program of the medical faculty of the RWTH Aachen University. T.W.K. received National Institutes of Health funding (R35 CA197222).
REFERENCES
- Adachi T., Nakagawa H., Chung I., Hagiya Y., Hoshijima K., Noguchi N., Kuo M. T., Ishikawa T. (2007). Nrf2-dependent and -independent induction of ABC transporters ABCC1, ABCC2, and ABCG2 in HepG2 cells under oxidative stress. J. Exp. Ther. Oncol. 6, 335–348. [PubMed] [Google Scholar]
- Al-Sawaf O., Clarner T., Fragoulis A., Kan Y. W., Pufe T., Streetz K., Wruck C. J. (2015). Nrf2 in health and disease: current and future clinical implications. Clin. Sci. (Lond.) 129, 989–999. [DOI] [PubMed] [Google Scholar]
- Al-Sawaf O., Fragoulis A., Rosen C., Kan Y. W., Sonmez T. T., Pufe T., Wruck C. J. (2014a). Nrf2 protects against TWEAK-mediated skeletal muscle wasting. Sci. Rep. 4, 3625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Sawaf O., Fragoulis A., Rosen C., Keimes N., Liehn E. A., Holzle F., Kan Y. W., Pufe T., Sonmez T. T., Wruck C. J. (2014b). Nrf2 augments skeletal muscle regeneration after ischemia-reperfusion injury. J. Pathol. 234, 538–547. [DOI] [PubMed] [Google Scholar]
- Aleksunes L. M., Slitt A. L., Maher J. M., Augustine L. M., Goedken M. J., Chan J. Y., Cherrington N. J., Klaassen C. D., Manautou J. E. (2008). Induction of Mrp3 and Mrp4 transporters during acetaminophen hepatotoxicity is dependent on Nrf2. Toxicol. Appl. Pharmacol. 226, 74–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aleksunes L. M., Yeager R. L., Klaassen C. D. (2009). Application of multivariate statistical procedures to identify transcription factors that correlate with MRP2, 3, and 4 mRNA in adult human livers. Xenobiotica 39, 514–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandenburg L. O., Kipp M., Lucius R., Pufe T., Wruck C. J. (2010). Sulforaphane suppresses LPS-induced inflammation in primary rat microglia. Inflamm. Res. 59, 443–450. [DOI] [PubMed] [Google Scholar]
- Bustin S. A., Benes V., Garson J. A., Hellemans J., Huggett J., Kubista M., Mueller R., Nolan T., Pfaffl M. W., Shipley G. L., et al. (2009). The MIQE guidelines: minimum information for publication of quantitative real-time PCR experiments. Clin. Chem. 55, 611–622. [DOI] [PubMed] [Google Scholar]
- Canet M. J., Merrell M. D., Harder B. G., Maher J. M., Wu T., Lickteig A. J., Jackson J. P., Zhang D. D., Yamamoto M., Cherrington N. J. (2015). Identification of a functional antioxidant response element within the eighth intron of the human ABCC3 gene. Drug Metab. Dispos. 43, 93–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan K., Lu R., Chang J. C., Kan Y. W. (1996). NRF2, a member of the NFE2 family of transcription factors, is not essential for murine erythropoiesis, growth, and development. Proc. Natl. Acad. Sci. U.S.A. 93, 13943–13948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu C. C., Huang G. T., Chou S. H., Chien C. T., Chiou L. L., Chang M. H., Lee H. S., Chen D. S. (2007). Characterization of cytokeratin 19-positive hepatocyte foci in the regenerating rat liver after 2-AAF/CCl4 injury. Histochem. Cell Biol. 128, 217–226. [DOI] [PubMed] [Google Scholar]
- Cole S. P., Marks G. S. (1984). Ferrochelatase and N-alkylated porphyrins. Mol. Cell. Biochem. 64, 127–137. [DOI] [PubMed] [Google Scholar]
- Colombo G., Clerici M., Garavaglia M. E., Giustarini D., Rossi R., Milzani A., Dalle-Donne I. (2015). A step-by-step protocol for assaying protein carbonylation in biological samples. J. Chromatogr. B. Analyt. Technol. Biomed. Life Sci. 1019, 178–190. [DOI] [PubMed] [Google Scholar]
- de Lima V. M., Oliveira C. P., Alves V. A., Chammas M. C., Oliveira E. P., Stefano J. T., de Mello E. S., Cerri G. G., Carrilho F. J., Caldwell S. H. (2008). A rodent model of NASH with cirrhosis, oval cell proliferation and hepatocellular carcinoma. J. Hepatol. 49, 1055–1061. [DOI] [PubMed] [Google Scholar]
- De Matteis F., Abbritti G., Gibbs A. H. (1973). Decreased liver activity of porphyrin-metal chelatase in hepatic porphyria caused by 3,5-diethoxycarbonyl-1,4-dihydrocollidine. Studies in rats and mice. Biochem. J. 134, 717–727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dohlen G., Odland H. H., Carlsen H., Blomhoff R., Thaulow E., Saugstad O. D. (2008). Antioxidant activity in the newborn brain: a luciferase mouse model. Neonatology 93, 125–131. [DOI] [PubMed] [Google Scholar]
- Endo H., Owada S., Inagaki Y., Shida Y., Tatemichi M. (2018). Glucose starvation induces LKB1-AMPK-mediated MMP-9 expression in cancer cells. Sci. Rep. 8, 10122.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fickert P., Stoger U., Fuchsbichler A., Moustafa T., Marschall H. U., Weiglein A. H., Tsybrovskyy O., Jaeschke H., Zatloukal K., Denk H., et al. (2007). A new xenobiotic-induced mouse model of sclerosing cholangitis and biliary fibrosis. Am. J. Pathol. 171, 525–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fragoulis A., Laufs J., Muller S., Soppa U., Siegl S., Reiss L. K., Tohidnezhad M., Rosen C., Tenbrock K., Varoga D., et al. (2012). Sulforaphane has opposing effects on TNF-alpha stimulated and unstimulated synoviocytes. Arthritis Res. Ther. 14, R220.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fragoulis, A., Siegl, S., Fendt, M., Jansen, S., Soppa, U., Brandenburg, L. O., Pufe, T., Weis, J., and Wruck, C. J. (2017). Oral administration of methysticin improves cognitive deficits in a mouse model of Alzheimer's disease. Redox Biol 12, 843–853. [DOI] [PMC free article] [PubMed]
- Fu Q., Ohnishi S.. 2018. Conditioned medium from human amnion-derived mesenchymal stem cells regulates activation of primary hepatic stellate cells. Stem Cells Int 2018: 4898152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haybaeck J., Stumptner C., Thueringer A., Kolbe T., Magin T. M., Hesse M., Fickert P., Tsybrovskyy O., Muller H., Trauner M., et al. (2012). Genetic background effects of keratin 8 and 18 in a DDC-induced hepatotoxicity and Mallory-Denk body formation mouse model. Lab. Invest. 92, 857–867. [DOI] [PubMed] [Google Scholar]
- Hochmuth C. E., Biteau B., Bohmann D., Jasper H. (2011). Redox regulation by Keap1 and Nrf2 controls intestinal stem cell proliferation in Drosophila. Cell Stem Cell 8, 188–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishikawa T., Factor V. M., Marquardt J. U., Raggi C., Seo D., Kitade M., Conner E. A., Thorgeirsson S. S. (2012). Hepatocyte growth factor/c-met signaling is required for stem-cell-mediated liver regeneration in mice. Hepatology 55, 1215–1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iusuf D., van de Steeg E., Schinkel A. H. (2012). Hepatocyte hopping of OATP1B substrates contributes to efficient hepatic detoxification. Clin. Pharmacol. Ther. 92, 559–562. [DOI] [PubMed] [Google Scholar]
- Jakubowski A., Ambrose C., Parr M., Lincecum J. M., Wang M. Z., Zheng T. S., Browning B., Michaelson J. S., Baetscher M., Wang B., et al. (2005). TWEAK induces liver progenitor cell proliferation. J. Clin. Invest. 115, 2330–2340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen C. H., Jauho E. I., Santoni-Rugiu E., Holmskov U., Teisner B., Tygstrup N., Bisgaard H. C. (2004). Transit-amplifying ductular (oval) cells and their hepatocytic progeny are characterized by a novel and distinctive expression of delta-like protein/preadipocyte factor 1/fetal antigen 1. Am. J. Pathol. 164, 1347–1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J. H., Thimmulappa R. K., Kumar V., Cui W., Kumar S., Kombairaju P., Zhang H., Margolick J., Matsui W., Macvittie T., et al. (2014). NRF2-mediated Notch pathway activation enhances hematopoietic reconstitution following myelosuppressive radiation. J. Clin. Invest. 124, 730–741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi E. H., Suzuki T., Funayama R., Nagashima T., Hayashi M., Sekine H., Tanaka N., Moriguchi T., Motohashi H., Nakayama K., et al. (2016). Nrf2 suppresses macrophage inflammatory response by blocking proinflammatory cytokine transcription. Nat. Commun. 7, 11624.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohler C., Bell A. W., Bowen W. C., Monga S. P., Fleig W., Michalopoulos G. K. (2004). Expression of Notch-1 and its ligand Jagged-1 in rat liver during liver regeneration. Hepatology 39, 1056–1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narasimhan M., Hong J., Atieno N., Muthusamy V. R., Davidson C. J., Abu-Rmaileh N., Richardson R. S., Gomes A. V., Hoidal J. R., Rajasekaran N. S. (2014). Nrf2 deficiency promotes apoptosis and impairs PAX7/MyoD expression in aging skeletal muscle cells. Free Radic. Biol. Med. 71, 402–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Connell M. A., Hayes J. D. (2015). The Keap1/Nrf2 pathway in health and disease: from the bench to the clinic. Biochem. Soc. Trans. 43, 687–689. [DOI] [PubMed] [Google Scholar]
- Okawa H., Motohashi H., Kobayashi A., Aburatani H., Kensler T. W., Yamamoto M. (2006). Hepatocyte-specific deletion of the keap1 gene activates Nrf2 and confers potent resistance against acute drug toxicity. Biochem. Biophys. Res. Commun. 339, 79–88. [DOI] [PubMed] [Google Scholar]
- Paul M. K., Bisht B., Darmawan D. O., Chiou R., Ha V. L., Wallace W. D., Chon A. T., Hegab A. E., Grogan T., Elashoff D. A., et al. (2014). Dynamic changes in intracellular ROS levels regulate airway basal stem cell homeostasis through Nrf2-dependent Notch signaling. Cell Stem Cell 15, 199–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfaffl M. W. (2001). A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 29, e45.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramakers C., Ruijter J. M., Deprez R. H., Moorman A. F. (2003). Assumption-free analysis of quantitative real-time polymerase chain reaction (PCR) data. Neurosci. Lett. 339, 62–66. [DOI] [PubMed] [Google Scholar]
- Reiss L. K., Fragoulis A., Siegl S., Platen C., Kan Y. W., Nautiyal J., Parker M., Pufe T., Uhlig U., Martin C., et al. (2014). Interplay between Nrf2 and amphiregulin during mechanical ventilation. Am. J. Respir. Cell Mol. Biol. 51, 668–677. [DOI] [PubMed] [Google Scholar]
- Sachar M., Anderson K. E., Ma X. (2016). Protoporphyrin IX: the good, the bad, and the ugly. J. Pharmacol. Exp. Ther. 356, 267–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serhan C. N., Savill J. (2005). Resolution of inflammation: the beginning programs the end. Nat. Immunol. 6, 1191–1197. [DOI] [PubMed] [Google Scholar]
- Shirasaki K., Taguchi K., Unno M., Motohashi H., Yamamoto M. (2014). Nrf2 promotes compensatory liver hypertrophy after portal vein branch ligation in mice. Hepatology59, 2371–2382. [DOI] [PubMed] [Google Scholar]
- Singla A., Moons D. S., Snider N. T., Wagenmaker E. R., Jayasundera V. B., Omary M. B. (2012). Oxidative stress, Nrf2 and keratin up-regulation associate with Mallory-Denk body formation in mouse erythropoietic protoporphyria. Hepatology 56, 322–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taguchi K., Hirano I., Itoh T., Tanaka M., Miyajima A., Suzuki A., Motohashi H., Yamamoto M. (2014). Nrf2 enhances cholangiocyte expansion in Pten-deficient livers. Mol. Cell. Biol. 34, 900–913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taguchi K., Masui S., Itoh T., Miyajima A., Yamamoto M. (2019). Nrf2 activation ameliorates hepatotoxicity induced by a heme synthesis inhibitor. Toxicol. Sci. 167, 227–238. [DOI] [PubMed] [Google Scholar]
- Tebay L. E., Robertson H., Durant S. T., Vitale S. R., Penning T. M., Dinkova-Kostova A. T., Hayes J. D. (2015). Mechanisms of activation of the transcription factor Nrf2 by redox stressors, nutrient cues, and energy status and the pathways through which it attenuates degenerative disease. Free Radic Biol. Med. 88, 108–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai J. J., Dudakov J. A., Takahashi K., Shieh J. H., Velardi E., Holland A. M., Singer N. V., West M. L., Smith O. M., Young L. F., et al. (2013). Nrf2 regulates haematopoietic stem cell function. Nat. Cell Biol. 15, 309–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vollrath V., Wielandt A. M., Iruretagoyena M., Chianale J. (2006). Role of Nrf2 in the regulation of the Mrp2 (ABCC2) gene. Biochem. J. 395, 599–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakabayashi N., Shin S., Slocum S. L., Agoston E. S., Wakabayashi J., Kwak M. K., Misra V., Biswal S., Yamamoto M., Kensler T. W. (2010). Regulation of notch1 signaling by nrf2: implications for tissue regeneration. Sci. Signal. 3, ra52.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakabayashi N., Skoko J. J., Chartoumpekis D. V., Kimura S., Slocum S. L., Noda K., Palliyaguru D. L., Fujimuro M., Boley P. A., Tanaka Y., et al. (2014). Notch-Nrf2 axis: regulation of Nrf2 gene expression and cytoprotection by notch signaling. Mol. Cell. Biol. 34, 653–663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakabayashi N., Slocum S. L., Skoko J. J., Shin S., Kensler T. W. (2010). When NRF2 talks, who's listening? Antioxid. Redox Signal. 13, 1649–1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wruck C. J., Claussen M., Fuhrmann G., Romer L., Schulz A., Pufe T., Waetzig V., Peipp M., Herdegen T., Gotz M. E. (2007). Luteolin protects rat PC12 and C6 cells against MPP+ induced toxicity via an ERK dependent Keap1-Nrf2-ARE pathway. J. Neural Transm Suppl. 72, 57–67. [DOI] [PubMed] [Google Scholar]
- Wruck C. J., Gotz M. E., Herdegen T., Varoga D., Brandenburg L. O., Pufe T. (2008). Kavalactones protect neural cells against amyloid beta peptide-induced neurotoxicity via extracellular signal-regulated kinase 1/2-dependent nuclear factor erythroid 2-related factor 2 activation. Mol. Pharmacol. 73, 1785–1795. [DOI] [PubMed] [Google Scholar]
- Wruck C. J., Streetz K., Pavic G., Gotz M. E., Tohidnezhad M., Brandenburg L. O., Varoga D., Eickelberg O., Herdegen T., Trautwein C., et al. (2011). Nrf2 induces interleukin-6 (IL-6) expression via an antioxidant response element within the IL-6 promoter. J. Biol. Chem. 286, 4493–4499. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.