Abstract
Cardiovascular and respiratory parameters change during sleep and wakefulness. This observation underscores an important, albeit incompletely understood, role for the central nervous system in the differential regulation of autonomic functions. Understanding sleep/wake-dependent sympathetic modulations provides insights into diseases involving autonomic dysfunction. The purpose of this review was to define the central nervous system nuclei regulating sleep and cardiovascular function and to identify reciprocal networks that may underlie autonomic symptoms of disorders such as insomnia, sleep apnea, restless leg syndrome, rapid eye movement sleep behavior disorder, and narcolepsy/cataplexy. In this review, we examine the functional and anatomical significance of hypothalamic, pontine, and medullary networks on sleep, cardiovascular function, and breathing.
Keywords: Autonomic nervous system, Breathing, Sleep, Sympathetic, Parasympathetic
Introduction
Seventeenth-century philosophers were fascinated with sleep [1, 2]. Descartes grappled with an uncertain human existence during unconsciousness, concluding that the sleeping brain continues to think [1]. Locke separated the awake and sleeping minds, suggesting the latter state to be inactive and—by extension—with limited purpose [ 2]. By the 1950s, however, discoveries about paradoxical sleep (a sleep stage with rapid movements of the eyes and cortical activity resembling wakefulness) challenged the notion that the sleeping brain was inactive [3]. Modern definitions acknowledge that many biologic processes are active during sleep, particularly networks regulating the autonomic nervous system (ANS).
Understanding the neural mechanisms regulating sleep and autonomic function is important for defining the relationship between sleep disorders and cardiovascular (CV) morbidity. The purpose of this review was to examine neural networks differentially controlling the ANS during wakefulness versus sleep. The review also summarizes knowledge about the following clinical disorders: insomnia, sleep apnea, restless leg syndrome (RLS), rapid eye movement (REM), sleep behavior disorder, and narcolepsy/cataplexy.
Neural networks
Knowledge about central nervous system (CNS) networks is important for determining relationships between the CNS sleep/wake control circuitry and the pathways regulating autonomic function. Evidence from preclinical and clinical studies indicates that the modulation of ANS reflexes is dependent on behavioral state (i.e., sleep vs. wake). The key CNS nuclei associated with these functions are summarized in Table 1.
Table 1.
Summary of the major central nervous system mechanisms regulating autonomic and sleep/wake functions
| CNS region | CNS nuclei | Important anatomical connections | Functional significance |
|---|---|---|---|
| Hypothalamus | Preoptic area (VLPO) | Provides GABAergic projections to hypothalamic monoamerinergic systems [30] | Inhibits wakefulness [30] |
| Provides a GABAergic projection to the paraventricular nucleus [24, 25] | Inhibits vasopressin synthesis, possibly leading to blood pressure reduction [26] | ||
| Subparaventricular zone | Receives efferents from the suprachiasmatic nucleus [32] | Integrates signals regulating body temperature, locomotion, and level of alertness [30] | |
| SCN | Provides polysynaptic GABAergic input to pineal gland and receives light signals from retinal ganglion cells [32] | Regulates melatonin secretion from the pineal gland; contributes to regulation of circadian rhythms [32] | |
| Midbrain and pons | Parabrachial nucleus | Provides inhibitory projections to the NTS [17] | Suggests a role in baroreflex modulation. |
| PPT | Provides cholinergic projections to the thalamus [12] | Promotes/modulates sensory input to the cortex (alertness) and contributes to the EEG activity of REM sleep [12] | |
| Provides cholinergic projections to RVLM [37, 40] | Suggests a role in sympathetic/CV regulation | ||
| PAG | Provides GABAergic projections to pontine REM-regulating neurons [12] | Regulates the timing of REM sleep [12, 32] | |
| Provides input to the parabrachial nucleus [13] | Suggests a role in baroreflex modulation | ||
| Medulla | NTS | Receives afferent signals from peripheral baroreceptors, chemoreceptors, cardiac sympathetic nerves, and vagus nerve [5] | Regulates sympathetic outflow to the heart |
| Bötzinger complex | Receives projections from NTS [11] | Innervates the respiratory control centers [11] | |
| Caudal ventrolateral medulla | Provides GABAergic projections to RVLM [7] | Regulates sympathetic outflow to the kidney [7] | |
| RVLM | Pre-motor neurons project to the intermediolateral column of the spinal cord, which synapse with renal post-ganglionic neurons [8] | Regulates renal blood flow, sodium and water reabsorption and excretion, and renin–angiotensin system [7, 9] |
CNS Central nervous system, VLPO ventrolateral preoptic nucleus, SCN suprachiasmatic nucleus/nuclei, PPT pedunculopontine tegmentum, PAG periaqueductal grey, NTS nucleus of the solitary tract, RVLM rostral ventrolateral medula, GABA gamma-aminobutyric acid, REM rapid eye movement, EEG electroencephalogram
Cardiorespiratory regulation and CNS activities during wakefulness
During wakefulness, particularly during periods of physical activity, there are significant fluctuations in cardiac output and respiratory flow. The brainstem reflexively responds to afferent signals communicating these changes from peripheral baroreceptors, chemoreceptors, and the cardiac sympathetic nerves. The nucleus of the solitary tract (NTS) and the rostral ventrolateral medulla (RVLM) are the primary regulators of CV adjustments [4]. Baroreceptors in the aortic arch sense blood pressure (BP) changes and relay this information to the NTS via the vagus nerve [5]. When the BP is elevated, NTS efferents decrease sympathetic outflow to the heart while simultaneously increasing vagal tone [6]. NTS neurons project to gamma-aminobutyric acid (GABA)-ergic neurons in the caudal ventrolateral medulla, which provides inhibitory inputs to the RVLM, thereby reducing sympathetic outflow to the kidney [7]. Pre-motor neurons in the RVLM project to the intermediolateral column of the spinal cord, which synapse with renal post-ganglionic neurons [8]. Via this neural network, RVLM neurons regulate renal blood flow, sodium and water reabsorption, and excretion, as well as the activity of the renin–angiotensin system [9].
Baroreceptor activation also reduces respiratory rate, a response attributed to the activation of neurons in the Bötzinger complex, a cluster of neurons in the RVLM and ventral respiratory column. The Bötzinger complex receives projections from the NTS and innervates respiratory control centers [10, 11]. In the RVLM, sympathetic neurons are intermingled with respiratory-modulating neurons [7]. Chemoreceptors in the carotid body provide another afferent signal to the NTS in response to hypoxia. Low levels of arterial oxygen increase afferent discharge via the glossopharyngeal nerve; this chemosensory information is relayed to the NTS, and the NTS neurons excite central respiratory centers, including the retrotrapezoid nucleus/parafacial respiratory group and Bötzinger complex [11]. The chemoreflex enhances respiratory drive and sympathoexcitation.
In circuits originating from the midbrain and pons, the fast neurotransmitters glutamate and GABA play critical roles in maintaining alertness [12]; these midbrain/pontine networks also influence cardiorespiratory activities, possibly via efferents to the NTS or RVLM. In addition, several modulatory CNS circuits exist to regulate wakefulness. A cholinergic pathway (originating from the laterodorsal tegmentum and pedunculopontine tegmentum [PPT]) promotes arousal. The cholinergic projections innervate the thalamus, promoting the transmission of sensory input to the cortex [12]. Monoaminergic neurons (originating from the parabrachial nucleus, periaqueductal grey [PAG], locus coeruleus, and raphe nuclei) project to the cortex, lateral hypothalamus, and basal forebrain and also modulate alertness [13]. Orexinergic neurons in the lateral hypothalamus represent another system promoting wakefulness; these neurons provide excitatory projections that activate the ascending monoaminergic system and brainstem cholinergic systems [14]. Collectively, these pathways activate the cortex and contribute to the low-voltage, high-frequency electroencephalogram (EEG) pattern occurring during wakefulness. Glutamatergic neurons in the parabrachial nucleus of the rostral pons are preferentially active during wakefulness [15, 16]; projections from the parabrachial nucleus to the NTS may represent a mechanism for adjusting BP between wakefulness and sleep. Increased parabrachial nucleus activity may inhibit the baroreflex [17–19], representing a mechanism for maintaining higher BP during wakefulness compared with sleep.
Networks regulating sympathetic/respiratory activities during sleep
During non-REM sleep, parasympathetic drive increases, with an associated reduction in cardiac sympathetic activity [20]. The decline in sympathetic drive accounts for the dipping phenomenon, which is an approximately 10% decrease in mean arterial pressure compared with that during wakefulness. BP dipping represents a healthy cardiovascular response; non-dipping, rising, or extreme-dipping is associated with elevated CV disease risk [21]. Non-REM sleep is also accompanied by decreased muscle tone and reduced respiratory rate [22].
Hypothalamic nuclei, specifically the ventrolateral preoptic nucleus (VLPO) and median preoptic nucleus, promote non-REM sleep via descending GABAergic projections to the arousal systems of the hypothalamus and brainstem. These sleep-promoting pathways are regulated by neuromodulators, such as adenosine, that accumulate during wakefulness to increase the physiologic pressure to sleep [23]. Activity of the preoptic nuclei may also influence ANS functions. For example, anatomical evidence suggests that non-REM sleep-promoting neurons in the VLPO inhibit the hypothalamic structures with pressor and sympathoexcitatory functions. For example, Uschakov and colleagues demonstrated the presence of an inhibitory projection from the VLPO to the paraventricular nucleus of the hypothalamus in rats [24, 25]. Inhibition of the paraventricular nucleus may account for a reduction in sympathetic drive, taking into account the possible impact on vasopressin synthesis/secretion. Vasopressin, synthesized by the paraventricular nucleus, is an important neurotransmitter/hormone for maintaining BP. As a potent vasoconstrictor, vasopressin contributes to elevated BP, in addition to its role in increasing fluid reabsorption from the filtrate in the nephron [26].
The occurrence of non-REM sleep requires inhibition of the wake-promoting networks, which is dependent on neurotransmission involving GABA and galanin [12]. Inhibition of the networks exciting the cortex results in a slower-frequency, higher-voltage EEG pattern during non-REM sleep, although slow waves are not uniformly distributed across the cortical surface [27]. The VLPO of the anterior hypothalamus plays an important role in promoting the transition from wake to sleep [28]. VLPO neurons fire rapidly during sleep; via GABAergic and galaninergic efferents, the VLPO inhibits the discharge of wake-promoting monoaminergic cells [28, 29]. Data also support a possible role for the preoptic area in promoting REM sleep, as evidenced by firing rates of the VLPO and the median preoptic nucleus increasing during both non-REM and REM sleep in rats [30]. REM sleep is a paradoxical stage consisting of a high-frequency and low-amplitude EEG pattern (similar to the EEG pattern observed during wakefulness) with muscle atonia.
Unlike non-REM sleep, REM sleep involves an increase in cholinergic activity in the pons, particularly in the PPT and laterodorsal tegmentum [31]. The EEG features of REM sleep and the rapid eye movements are controlled by neurons in the sublaterodorsal region of the pons [12]. Skeletal muscle paralysis, a key feature of REM sleep, is associated with increased glutamatergic neuron activity in the dorsal pons [29]. Typically, REM sleep bouts are preceded by longer periods of non-REM sleep. A network of CNS nuclei regulates the timing of REM sleep. REM-promoting neurons in the pons are inhibited by GABAergic projections from the PAG and VLPO, as well as by the wake-promoting structures of the lateral hypothalamus [12, 32]. Inhibition of REM sleep may also involve serotonergic input from the raphe nuclei, albeit this connection is incompletely understood [33].
Autonomic instability with fluctuations in BP and respiratory rate have been reported during REM sleep [34–36]; these events may be related to the activities of pontine nuclei, such as the PPT, that have connections with sympathetic/respiratory control centers (NTS and RVLM) [37, 38]. Functional and anatomical data from rat studies support a role for the PPT in regulating sympathetic nerve activity. For example, studies in anesthetized rats demonstrated the ability to increase renal and splanchnic sympathetic nerve activity by stimulating neurons in the PPT [39, 40], which also evoked respiratory dysrhythmia [39]. Sympathetic activity in different vascular beds as modulated by the PPT, however, has yet to be examined during non-REM and REM sleep. The irregular breathing patterns associated with REM sleep may be attributed to chemoreflex and baroreflex control [41]. During non-REM sleep, chemoreceptors promptly recognize small fluctuations in oxygen and carbon dioxide, which results in minor adjustments in breathing rate and depth via NTS pathways [11]. Non-REM sleep is therefore associated with stability in respiration and BP, but chemo- and baroreflex response time may be altered during REM sleep. For example, transitions from non-REM to REM sleep evoke increases in mean arterial pressure in humans [42] and in rats [43, 44], and these BP fluctuations may lead to baroreflex instability. These observations suggest that an increase in the loop gain of these feedback systems may accompany REM sleep. Loop gain measures the propensity for a feedback system to become unstable [45, 46]. Although REM sleep is associated with skeletal muscle atonia, results from several studies have challenged the notion that upper airway muscles lose tone during REM sleep. For example, Fraigne and Orme demonstrated that genioglossus muscle activity increased in rats during REM sleep compared with non-REM sleep, suggesting the possibly that REM-specific muscle recruitment could contribute to changes in respiration [47].
Features of the cortical EEG relevant to understanding state‑dependent cardiorespiratory patterns
Figures 1 and 2 illustrate examples from our laboratory of polysomnography in freely moving rats. Our procedures conformed to the American Physiological Society’s Guiding Principles for the Care and Use of Vertebrate Animals and were approved by the University of Illinois at Chicago Institutional Animal Care and Use Committee. In Fig. 1, non-REM sleep is associated with a high-amplitude/low-frequency cortical EEG pattern (Fig. 1a), and a transition from non-REM sleep to wakefulness shifts the EEG to a lower amplitude/higher frequency pattern accompanied by adjustments in BP (Fig. 1b). With the transition from non-REM sleep to REM sleep, the EEG shifts to a high-frequency/low-amplitude pattern (similar to wakefulness), while the electromyogram (EMG) signal provides evidence of skeletal muscle paralysis, a defining characteristic of REM sleep (Fig. 1c). Figure 2 illustrates a non-REM to REM sleep transition in a rat. An increase in the EEG frequency with low EMG tone signifies the transition to REM sleep, a sleep stage in which respiratory rate can be irregular (Fig. 2 a, c). Both rodent and human sleep demonstrate alternating patterns of non-REM and REM sleep, which can be accompanied by fluctuations in BP, heart rate, ventilation, and arterial concentrations of oxygen and carbon dioxide.
Fig. 1.
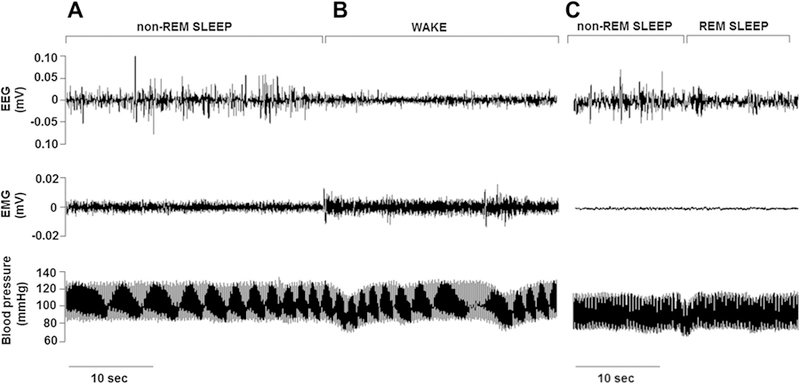
Cardiovascular and electroencephalogram/electromyogram (EEG/EMG) characteristics of non-rapid eye movement (REM) sleep, wakefulness, and REM sleep in the rat. EEG, EMG, and blood pressure (BP) patterns during sleep and wakefulness in an adult male Wistar-Kyoto rat are shown. During non-REM sleep (a), BP and heart rate were stable. The non-REM sleep EEG demonstrated a lower frequency, higher amplitude pattern compared with wakefulness. When the rat awoke (b), BP became less stable, and the EEG pattern shifted to a lower amplitude/increased frequency waveform; the increased EMG indicated skeletal muscle activity. c Illustration of a transition occurring 2 h later in the same rat; from non-REM sleep, the rat transitioned to REM sleep, which is demonstrated by a lower amplitude/higher frequency EEG with muscle atonia. The latter is indicated by the very low EMG tone. Data were acquired using the Data Sciences International (Minneapolis, MN) implantable telemetry system (transmitter models 4ET/HDS-11; 500 Hz sampling rate). Data were graphed using Igor Pro version 6.3 (WaveMetrics Inc.)
Fig. 2.
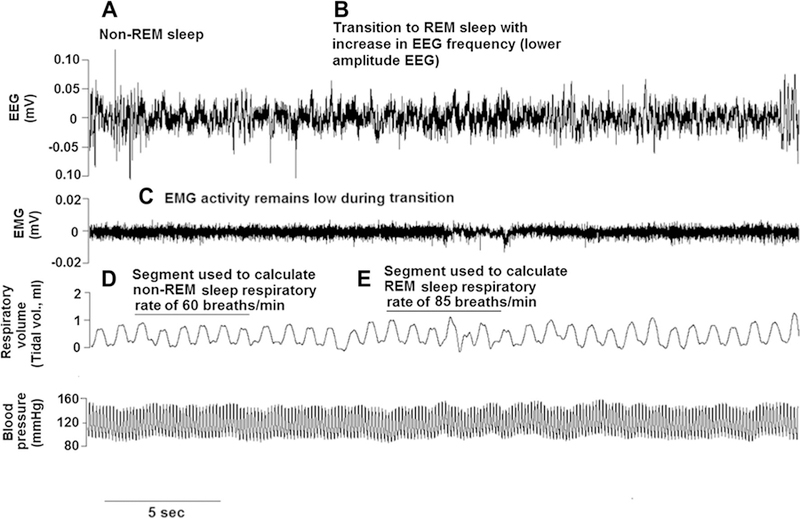
Cortical EEG, respiratory, and BP activities during a sleep transition in the rat. Patterns in EEG, EMG, breathing, and BP in an adult male Sprague–Dawley rat are shown. a–c A transition from non-REM to REM sleep (a, b) is illustrated by increasing frequency in the EEG while EMG tone remains unchanged (c). Non-REM sleep is typically associated with stability in respiratory and CV parameters (d). Compared with non-REM sleep, REM sleep can evoke more variability in breathing and/or BP. In this example, the respiratory rate increased [from approx. 60 breaths/min to 85 breaths/min during the transition (e)]. BP remained relatively stable in this rat. Data were acquired using implantable telemetry system and analyzed using Neuroscore software (Data Sciences International, Minneapolis, MN)
Pathophysiology of sleep disorders associated with ANS dysfunction
Insomnia
Insomnia is accompanied by hyperarousal, elevated BP, and reduced heart rate variability [48]. To understand ANS dysfunction with insomnia, autonomic parameters have been examined in individuals with objective short sleep duration (< 6 h) and in those with genetic disorders causing severe sleep loss and hyperarousal (e.g., fatal familial insomnia [FFI]). Jarrin and colleagues demonstrated that, compared with controls with normal sleep duration (≥ 6 h), short sleep duration was associated with elevated mean heart rate and with heart rate variability metrics indicating reduced parasympathetic activity and sympathovagal imbalance [49]. In FFI, a progressive, inherited (autosomal-dominant) neurodegenerative disease, the inability to sleep leads to unbalanced autonomic control, coma, and death [50]. No single CNS anomaly is linked with FFI, but dysfunction of the sleep- and autonomic-regulating systems of the thalamus, hypothalamus, parabrachial nucleus, PAG, and NTS have been investigated. FFI is characterized by severe sympathetic over-activity (tachycardia, hypertension, and hyperthermia), which is hypothesized to involve impaired inhibition of the baroreflex, possibly resulting from over-excitation of the RVLM [51].
Sleep apnea and airway resistance syndromes
Sleep apnea involves a complex group of disorders causing adverse CV and metabolic consequences in addition to excessive daytime sleepiness. Sleep-disordered breathing can cause intermittent hypoxia and chronic activation of the sympathetic nervous system—two mechanisms hypothesized to link sleep apnea with an elevated risk for CV diseases, such as hypertension and stroke [52]. Patients experience frequent arousals from sleep, resulting from increased respiratory effort in response to hypoxia or hypercapnia [53]. Respiratory events can be apneas (defined as the absence of inspiratory flow for ≥ 10 s) or hypoapneas (shallow, slow respiration lasting ≥ 10 s), leading to EEG arousals and varying degrees of arterial oxygen desaturation [52]. The etiology of these respiratory events may be obstructive or central. Obstructive sleep apnea (OSA) involves repetitive airway blockage caused by the surrounding soft tissue and can be correlated with obesity; central sleep apneas result from reduced neural output of the brainstem neurons innervating the upper airway and thoracic inspiratory muscles [52]. Many patients, however, experience both obstructive and central events [54]. Interestingly, treating airway obstruction with positive airway pressure or tracheostomy results in central apnea, suggesting that underlying CNS neural mechanisms are implicated in both types of apnea and contribute to complex sleep apnea phenotypes [55, 56].
Sleep apnea disrupts sleep architecture, evoking frequent arousals and modifying sleep stage-dependent interactions between sympathetic and parasympathetic tone [57]. In OSA, hypoxia is a powerful stimulus for increasing sympathetic drive via the chemoreflex [58–60]. In humans, obstructive sleep apnea has been associated with an impaired nocturnal dipping pattern in BP. Mokhlesi and colleagues demonstrated a dose–response risk for developing systolic and diastolic non-dipping BP with increasing severity of sleep apnea [61]. Experiments in rats have demonstrated how repetitive apneas have an additive effect on widening pulse pressure and increasing BP; these responses activate CNS ascending arousal systems, increase respiratory muscle effort, cause changes in intrathoracic blood volume, and activate sympathetic activity [59, 60, 62]. Data from rat models of chronic intermittent hypoxia have also provided evidence for renal mechanisms in the development of chronically elevated BP. Rats with renal sympathetic denervation did not demonstrate hypertension in response to chronic intermittent hypoxia, but sham-operated rats exhibited a 10 ± 3 mmHg increase in mean arterial pressure after 5 weeks of hypoxia exposure. Plasma renin activity increased fourfold in the latter group but remained at baseline levels in denervated rats [63]. It remains to be determined whether afferents from state-regulating nuclei, such as the PPT, that have projections to the RVLM exert a pathophysiologic influence on renal function or sympathetic nerve activity. Other candidates for understanding CV morbidity with sleep apnea include the pontine and medullary respiratory control systems, which receive projections from the PAG. As explained above, in addition to regulating wakefulness/arousal, the PPT modulates respiratory patterns [64], and PAG neuronal activity is synchronized with respiratory cycles [65]. Therefore, dysfunction of these nuclei could contribute to central sleep apneas and sympathetic consequences.
Investigators have examined airflow restriction and oxygen desaturation to determine how autonomic responses depend on the degree of airway obstruction, sleep disruption, or oxygen desaturation. Normally, parasympathetic activity increases as a patient transitions from wakefulness to non-REM sleep, but this response may be overactive or reduced depending on the type of sleep-related breathing disorder. For example, Lin and colleagues studied beat-to-beat heart rate (RR intervals) and finger photoplethysmography in patients with upper airway resistance syndrome (UARS); these patients had reduced airway diameter during sleep, but airway restriction had a very limited effect on oxygen saturation. During non-REM sleep, patients with UARS demonstrated parasympathetic nervous system hyperactivation with inspiratory flow limitation/increased respiratory effort. Chronic vagal stimulation during sleep may, therefore, evoke irregularities in BP control with UARS [66]. Patients with sleep-related alveolar hypoventilation (SRAH), however, have oxygen desaturation without significant limitations in airflow. Palma and colleagues found SRAH to be associated with two autonomic abnormalities: (1) an increase in the low-frequency component of the heart rate variability spectrum (0.04–0.15 Hz) and (2) a decrease in the high-frequency component (0.15–0.40 Hz). These findings suggest a reduction in parasympathetic activity, and the researchers found that this abnormality was particularly evident during REM sleep [67]. These studies emphasize the importance of considering that different autonomic profiles, with different underlying mechanisms, are associated with sleep-related breathing disorders, depending on how the disease involves airflow limitation and changes in oxygenation.
Patients with postural tachycardia syndrome (POTS) often undergo evaluation for sleep-disordered breathing. POTS is defined by orthostatic intolerance; when patients move from the recumbent sleeping position, they demonstrate syncope and excessively elevated heart rate [68]. Patients with POTS have markedly elevated sympathetic outflow in response to hypotensive challenges and enhanced activation of the parasympathetic nervous system [69]. The presence of sleep-disordered breathing and changes in sleep architecture are controversial with POTS, considering that several studies reported no polysomnographic changes compared with controls [69, 70]. When mild airflow limitations accompany POTS, they may not cause significant oxygen desaturation but still appear to contribute to significant autonomic impairment. This may be of greatest concern when POTS is diagnosed in patients with disorders affecting connective tissue flexibility, such as Ehlers–Danlos syndrome, which contribute to airway collapse during sleep [71].
Restless leg syndrome
Restless movements of the legs are monitored during polysomnography and have provided insights into how these pathological movements correlate with heart rate variability metrics. The pathophysiology may be complex because some studies demonstrate no impact of leg movements on heart rate variability [72], while others indicate the presence of elevated sympathetic activity during non-REM sleep [73]. Inconsistencies among study findings may result from the observation that not all leg movements cause arousals from sleep. For example, patients with RLS are kept awake by their leg movements. In contrast to RLS, patients with periodic limb movement disorder exhibit involuntary movement during sleep. Barone and colleagues demonstrated that periodic limb movement disorder is associated with sympathetic over-activity during non-REM sleep, a finding not associated with the autonomic profile of RLS [73]. These observations underscore the importance of measuring sleep—and arousals from sleep—to characterize the etiology of autonomic dysfunction with restless leg movements.
REM sleep behavior disorder
Skeletal muscle paralysis normally accompanies REM sleep. With REM sleep behavior disorder, however, muscle paralysis is incomplete or absent, resulting in forceful movements during REM sleep. Dreams may also be intense and vivid. REM sleep behavior disorder has been associated with CNS neurodegenerative diseases, and the presence of comorbid neurodegeneration and REM sleep behavior disorder is associated with a greater degree of autonomic impairment [74]. The neurobiology of REM sleep behavior disorder remains incompletely defined, but the role of CNS REM sleep circuitry has been investigated. For example, degeneration of the sublaterodorsal region of the pons and the associated projections to spinal interneurons have been proposed to contribute to abnormal REM sleep and motor regulation [74]. Pontine nuclei involved in REM sleep regulation, such as the PPT, are also implicated in regulating balance, locomotion, and gain [64]. Although the findings of some investigations are equivocal, studies of patients with REM sleep behavior disorders have revealed reduced variability in heart rate in addition to abnormal heart rate responses to arousals from sleep [75].
Narcolepsy/cataplexy
Narcolepsy is a rare disease involving excessive daytime sleepiness, uncontrollable bouts of sleep, the presence of REM sleep immediately upon sleep onset, and/or episodes of muscle weakness (cataplexy). The condition results from the partial or complete destruction of orexinergic neurons in the hypothalamus, which project to the CNS nuclei regulating arousal and wake–sleep switching [14, 76]. Data from mouse models indicate that, during wakefulness, orexinergic neurons exhibit slow tonic firing, and these neurons are not active during non-REM sleep [77]. There is evidence suggesting that narcolepsy affects the ability to modulate BP during sleep. For example, Grimaldi and colleagues demonstrated different 24-h BP patterns in patients with narcolepsy compared with controls. Narcolepsy was associated with a nighttime non-dipping pattern, although it remains unknown whether this pattern contributes to CV disease development in this population [78]. Sieminski and colleagues also observed a non-dipping profile with narcolepsy; BP, however, did not correlate with cerebrospinal fluid orexin levels or sleep characteristics [79]. Findings from other studies indicated reduced sympathetic drive during wakefulness with narcolepsy [80, 81]. For example, Fronczek and colleagues found evidence of reduced sympathetic tone in a small group of men with narcolepsy [81]. Collectively, these findings suggest that the loss of orexinergic neurons affects BP in a manner that differs according to sleep–wake states.
Conclusions and future directions
Networks of CNS nuclei play critical roles in differentially regulating autonomic CV function during wakefulness, non-REM sleep, and REM sleep. Dysfunction of these networks underlies sleep and autonomic disorders. The etiologies are complex and can involve neurodegeneration of CNS nuclei, genetic factors, and plasticity of the nervous system. Arousals, hypoxia, and other stimuli may contribute to chronic changes in neuronal signaling, promoting an imbalance in sympathetic and parasympathetic drive. Conditions that arouse a patient from sleep (e.g., insomnia, OSA) evoke hyperarousal of the CNS networks promoting wakefulness and sympathetic parameters (e.g., thalamus, hypothalamus, parabrachial nucleus, PAG, and NTS). Short sleep duration contributes to elevated heart rate and sympathovagal imbalance, and in severe forms of the disease (e.g., fatal familial insomnia), severe dysfunction of CV reflexes contributes to mortality. Diseases that disrupt sleep architecture (e.g., OSA, UARS, RLS) also alter the sleep stage-dependent interactions of the sympathetic and parasympathetic nervous systems. The existing literature indicates that it is important to differentiate patients with different phenotypes of a sleep disorder (e.g., OSA vs. UARS, RLS vs. periodic limb movement disorder) because their autonomic profiles may differ depending on the disease-specific neural mechanisms. There is a critical need to devise animal models and obtain clinical data to understand disorders associated with progressive neuron destruction (e.g., REM sleep behavior disorder, narcolepsy/cataplexy) given that there is evidence linking these conditions with widespread/progressive neurodegeneration, chronic autonomic impairment, and impaired quality of life.
Acknowledgements
The first author is supported by the National Institute for Nursing Research (R00NR014369). The authors acknowledge Kevin Grandfield, Publication Manager, for editorial assistance.
Abbreviations
- ANS
Autonomic nervous system
- BP
Blood pressure
- CNS
Central nervous system
- CV
Cardiovascular
- EEG
Electroencephalogram
- EMG
Electromyogram
- FFI
Fatal familial insomnia
- GABA
Gamma-aminobutyric acid
- NTS
Nucleus of the solitary tract
- OSA
Obstructive sleep apnea
- PAG
Periaqueductal grey
- POTS
Postural orthostatic tachycardia syndrome
- PPT
Pedunculopontine tegmentum
- REM
Rapid eye movement
- RLS
Restless leg syndrome
- RVLM
Rostral ventrolateral medulla
- SRAH
Sleep-related alveolar hypoventilation
- UARS
Upper airway resistance syndrome
- VLPO
Ventrolateral preoptic nucleus
Footnotes
Compliance of ethical standards
Conflict of interest The first author serves on the Customer Advisory Board for Data Sciences International.
References
- 1.Descartes R, Cress D (1993) Meditations on first philosophy Hackett, Indianapolis [Google Scholar]
- 2.Locke J, Nidditch P (1975) An essay concerning human understanding Clarendon Press, Oxford [Google Scholar]
- 3.Aserinsky E, Kleitman N (1953) Regularly occurring periods of eye motility, and concomitant phenomena, during sleep. Science 118:273–274 [DOI] [PubMed] [Google Scholar]
- 4.Chen WW, Xiong XQ, Chen Q, Li YH, Kang YM, Zhu GQ (2015) Cardiac sympathetic afferent reflex and its implications for sympathetic activation in chronic heart failure and hypertension. Acta Physiol (Oxf) 213:778–794 [DOI] [PubMed] [Google Scholar]
- 5.Andresen MC, Doyle MW, Jin YH, Bailey TW (2001) Cellular mechanisms of baroreceptor integration at the nucleus tractus solitarius. Ann New York Acad Sci 940:132–141 [DOI] [PubMed] [Google Scholar]
- 6.Michelini LC (2007) The NTS and integration of cardiovascular control during exercise in normotensive and hypertensive individuals. Curr Hypertens Rep 9:214–221 [DOI] [PubMed] [Google Scholar]
- 7.Zoccal DB, Furuya WI, Bassi M, Colombari DSA, Colombari E (2014) The nucleus of the solitary tract and the coordination of respiratory and sympathetic activities. Front Physiol 5:238 10.3389/fphys.2014.00238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nishi EE, Bergamaschi CT, Campos RR (2015) The crosstalk between the kidney and the central nervous system: the role of renal nerves in blood pressure regulation. Exp Physiol 100:479–484 [DOI] [PubMed] [Google Scholar]
- 9.DiBona GF, Kopp UC (1997) Neural control of renal function. Physiol Rev 77:75–197 [DOI] [PubMed] [Google Scholar]
- 10.Baekey DM, Molkov YI, Paton JF, Rybak IA, Dick TE (2010) Effect of baroreceptor stimulation on the respiratory pattern: insights into respiratory–sympathetic interactions. Respir Physiol Neurobiol 174:135–145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Spyer KM, Gourine AV (2009) Chemosensory pathways in the brainstem controlling cardiorespiratory activity. Philos Trans R Soc Lond B Biol Sci 364:2603–2610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Saper CB, Fuller PM (2017) Wake–sleep circuitry: an overview. Curr Opin Neurobiol 44:186–192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Harris CD (2005) Neurophysiology of sleep and wakefulness. Respir Care Clin N Am 11:567–586 [DOI] [PubMed] [Google Scholar]
- 14.Gvilia I (2010) Underlying brain mechanisms that regulate sleep– wakefulness cycles. Int Rev Neurobiol 93:1–21 [DOI] [PubMed] [Google Scholar]
- 15.Saper CB, Fuller PM, Pedersen NP, Lu J, Scammell TE (2010) Sleep state switching. Neuron 68:1023–1042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.de Zambotti M, Trinder J, Silvani A, Colrain IM, Baker FC (2018) Dynamic coupling between the central and autonomic nervous systems during sleep: a review. Neurosci Biobehav Rev 90:84–103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Krukoff TL, Harris KH, Jhamandas JH (1993) Efferent projections from the parabrachial nucleus demonstrated with the anterograde tracer Phaseolus vulgaris leucoagglutinin. Brain Res Bull 30:163–172 [DOI] [PubMed] [Google Scholar]
- 18.Felder RB, Mifflin SW (1988) Modulation of carotid sinus afferent input to nucleus tractus solitarius by parabrachial nucleus stimulation. Circ Res 63:35–49 [DOI] [PubMed] [Google Scholar]
- 19.Hayward LF (2007) Midbrain modulation of the cardiac baroreflex involves excitation of lateral parabrachial neurons in the rat. Brain Res 1145:117–127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jafari B (2017) Sleep architecture and blood pressure. Sleep Med Clin 12:161–166 [DOI] [PubMed] [Google Scholar]
- 21.Silvani A, Dampney RA (2013) Central control of cardiovascular function during sleep. Am J Physiol Heart Circ Physiol 305:H1683–H1692 [DOI] [PubMed] [Google Scholar]
- 22.Sowho M, Amatoury J, Kirkness JP, Patil SP (2014) Sleep and respiratory physiology in adults. Clin Chest Med 35:469–481 [DOI] [PubMed] [Google Scholar]
- 23.Donlea JM, Alam MN, Szymusiak R (2017) Neuronal substrates of sleep homeostasis; lessons from flies, rats and mice. Curr Opin Neurobiol 44:228–235 [DOI] [PubMed] [Google Scholar]
- 24.Uschakov A, Gong H, McGinty D, Szymusiak R (2006) Sleep-active neurons in the preoptic area project to the hypothalamic paraventricular nucleus and perifornical lateral hypothalamus. Eur J Neurosci 23:3284–3296 [DOI] [PubMed] [Google Scholar]
- 25.Uschakov A, Gong H, McGinty D, Szymusiak R (2007) Efferent projections from the median preoptic nucleus to sleep- and arousal-regulatory nuclei in the rat brain. Neuroscience 150:104–120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lozić M, Šarenac O, Murphy D, Japundžić-Žigon N (2018) Vasopressin, central autonomic control and blood pressure regulation. Curr Hypertens Rep 20:11. [DOI] [PubMed] [Google Scholar]
- 27.Siclari F, Tononi G (2017) Local aspects of sleep and wakefulness. Curr Opin Neurobiol 44:222–227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schwartz JRL, Roth T (2008) Neurophysiology of sleep and wakefulness: basic science and clinical implications. Curr Neuropharmacol 6:367–378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Saper CB (2013) The neurobiology of sleep. Continuum (Minneap Minn) 19:19–31 [DOI] [PubMed] [Google Scholar]
- 30.Suntsova N, Szymusiak R, Alam MN, Guzman-Marin R, McGinty D (2002) Sleep–waking discharge patterns of median preoptic nucleus neurons in rats. J Physiol 543:665–677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Van Dort CJ, Zachs DP, Kenny JD, Zheng S, Goldblum RR, Gelwan NA, Ramos DM, Nolan MA, Wang K, Weng FJ, Lin Y, Wilson MA, Brown EN (2015) Optogenetic activation of cholinergic neurons in the PPT or LDT induces REM sleep. Proc Natl Acad Sci USA 112:584–589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Saper CB, Scammell TE, Lu J (2005) Hypothalamic regulation of sleep and circadian rhythms. Nature 437:1257–1263 [DOI] [PubMed] [Google Scholar]
- 33.Monti JM (2010) The structure of the dorsal raphe nucleus and its relevance to the regulation of sleep and wakefulness. Sleep Med Rev 14:307–317 [DOI] [PubMed] [Google Scholar]
- 34.Gould GA, Gugger M, Molloy J, Tsara V, Shapiro CM, Douglas NJ (1988) Breathing pattern and eye movement density during REM sleep in humans. Am Rev Respir Dis 138:874–877 [DOI] [PubMed] [Google Scholar]
- 35.Sei H (2012) Blood pressure surges in REM sleep: a mini review. Pathophysiology 19:233–241 [DOI] [PubMed] [Google Scholar]
- 36.Cabiddu R, Cerutti S, Viardot G, Werner S, Bianchi AM (2012) Modulation of the sympatho-vagal balance during sleep: frequency domain study of heart rate variability and respiration. Front Physiol 3:45. doi: 10.3389/fphys.2012.00045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yasui Y, Cechetto DF, Saper CB (1990) Evidence for a cholinergic projection from the pedunculopontine tegmental nucleus to the rostral ventrolateral medulla in the rat. Brain Res 517:19–24 [DOI] [PubMed] [Google Scholar]
- 38.Steininger TL, Rye DB, Wainer BH (1992) Afferent projections to the cholinergic pedunculopontine tegmental nucleus and adjacent midbrain extrapyramidal area in the albino rat. I. Retrograde tracing studies. J Comp Neurol 321:515–543 [DOI] [PubMed] [Google Scholar]
- 39.Fink AM, Dean C, Piano MR, Carley DW (2017) The pedunculopontine tegmentum controls renal sympathetic nerve activity and cardiorespiratory activities in nembutal-anesthetized rats. PLoS One 12:e0187956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Padley JR, Kumar NN, Li Q, Nguyen TB, Pilowsky PM, Good-child AK (2007) Central command regulation of circulatory function mediated by descending pontine cholinergic inputs to sympathoexcitatory rostral ventrolateral medulla neurons. Circ Res 100:284–291 [DOI] [PubMed] [Google Scholar]
- 41.Bourke SC, Gibson GJ (2002) Sleep and breathing in neuromuscular disease. Eur Respir J 19:1194–1201 [DOI] [PubMed] [Google Scholar]
- 42.Somers VK, Dyken ME, Mark AL, Abboud FM (1993) Sympathetic-nerve activity during sleep in normal subjects. N Engl J Med 328:303–307 [DOI] [PubMed] [Google Scholar]
- 43.Nagura S, Sakagami T, Kakiichi A, Yoshimoto M, Miki K (2004) Acute shifts in baroreflex control of renal sympathetic nerve activity induced by REM sleep and grooming in rats. J Physiol 558:975–983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Miki K, Kato M, Kajii S (2003) Relationship between renal sympathetic nerve activity and arterial pressure during REM sleep in rats. Am J Physiol Regul Integr Comp Physiol 284:R467–R473 [DOI] [PubMed] [Google Scholar]
- 45.Naughton MT (2010) Loop gain in apnea: gaining control or controlling the gain? Am J Respir Crit Care Med 181:103–105 [DOI] [PubMed] [Google Scholar]
- 46.Burgess KR (2012) New insights from the measurement of loop gain in obstructive sleep apnoea. J Physiol 590:1781–1782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fraigne JJ, Orem JM (2011) Phasic motor activity of respiratory and non-respiratory muscles in REM sleep. Sleep 34:425–434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Vgontzas AN, Fernandez-Mendoza J, Liao D, Bixler EO (2013) Insomnia with objective short sleep duration: the most biologically severe phenotype of the disorder. Sleep Med Rev 17:241–254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jarrin DC, Ivers H, Lamy M, Chen IY, Harvey AG, Morin CM (2018) Cardiovascular autonomic dysfunction in insomnia patients with objective short sleep duration. J Sleep Res 27:e12663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tabaee Damavandi P, Dove MT, Pickersgill RW (2017) A review of drug therapy for sporadic fatal insomnia. Prion 11:293–299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Benarroch EE, Stotz-Potter EH (1998) Dysautonomia in fatal familial insomnia as an indicator of the potential role of the thalamus in autonomic control. Brain Pathol 8:527–530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Javaheri S, Barbe F, Campos-Rodriguez F, Dempsey JA, Khayat R, Javaheri S, Malhotra A, Martinez-Garcia MA, Mehra R, Pack AI, Polotsky VY, Redline S, Somers VK (2017) Sleep apnea: types, mechanisms, and clinical cardiovascular consequences. J Am Coll Cardiol 69:841–858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rosenzweig I, Williams SC, Morrell MJ (2014) The impact of sleep and hypoxia on the brain: potential mechanisms for the effects of obstructive sleep apnea. Curr Opin Pulm Med 20:565–571 [DOI] [PubMed] [Google Scholar]
- 54.Salloum A, Rowley JA, Mateika JH, Chowdhuri S, Omran Q, Badr MS (2010) Increased propensity for central apnea in patients with obstructive sleep apnea: effect of nasal continuous positive airway pressure. Am J Respir Crit Care Med 181:189–193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gilmartin GS, Daly RW, Thomas RJ (2005) Recognition and management of complex sleep-disordered breathing. Curr Opin Pulm Med 11:485–493 [DOI] [PubMed] [Google Scholar]
- 56.Onal E, Lopata M (1982) Periodic breathing and the pathogenesis of occlusive sleep apneas. Am Rev Respir Dis 126:676–680 [DOI] [PubMed] [Google Scholar]
- 57.Guilleminault C, Poyares D, Rosa A, Huang YS (2005) Heart rate variability, sympathetic and vagal balance and EEG arousals in upper airway resistance and mild obstructive sleep apnea syndromes. Sleep Med 6:451–457 [DOI] [PubMed] [Google Scholar]
- 58.Fletcher EC (2000) Cardiovascular consequences of obstructive sleep apnea: experimental hypoxia and sympathetic activity. Sleep 23[Suppl 4]:S127–S131 [PubMed] [Google Scholar]
- 59.Ferreira CB, Cravo SL, Stocker SD (2018) Airway obstruction produces widespread sympathoexcitation: role of hypoxia, carotid chemoreceptors, and NTS neurotransmission. Physiol Rep 6:3 10.14814/phy2.13536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Prabhakar NR, Kumar GK (2010) Mechanisms of sympathetic activation and blood pressure elevation by intermittent hypoxia. Respir Physiol Neurobiol 174:156–161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mokhlesi B, Hagen EW, Finn LA, Hla KM, Carter JR, Peppard PE (2015) Obstructive sleep apnoea during REM sleep and incident non-dipping of nocturnal blood pressure: a longitudinal analysis of the Wisconsin Sleep Cohort. Thorax 70:1062–1069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Costa-Silva JH, Zoccal DB, Machado BH (2012) Chronic intermittent hypoxia alters glutamatergic control of sympathetic and respiratory activities in the commissural NTS of rats. Am J Physiol Regul Integr Comp Physiol 302:R785–R793 [DOI] [PubMed] [Google Scholar]
- 63.Fletcher EC, Bao G, Li R (1999) Renin activity and blood pressure in response to chronic episodic hypoxia. Hypertension 34:309–314 [DOI] [PubMed] [Google Scholar]
- 64.Saponjic J, Radulovacki M, Carley DW (2003) Respiratory pattern modulation by the pedunculopontine tegmental nucleus. Respir Physiol Neurobiol 138:223–237 [DOI] [PubMed] [Google Scholar]
- 65.Ni HF, Zhang JX, Harper RM (1990) Cardiovascular-related discharge of periaqueductal gray neurons during sleep–waking states. Brain Res 532:242–248 [DOI] [PubMed] [Google Scholar]
- 66.Lin C, Lo M-T, Guilleminault C (2017) Exploring the abnormal modulation of the autonomic systems during nasal flow limitation in upper airway resistance syndrome by Hilbert–Huang transform. Front Med 4:161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Palma JA, Urrestarazu E, Lopez-Azcarate J, Alegre M, Fernandez S, Artieda J, Iriarte J (2013) Increased sympathetic and decreased parasympathetic cardiac tone in patients with sleep related alveolar hypoventilation. Sleep 36:933–940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Goodman BP (2018) Evaluation of postural tachycardia syndrome (POTS). Auton Neurosci 10.1016/j.autneu.2018.04.004 [DOI] [PubMed] [Google Scholar]
- 69.Pengo MF, Higgins S, Drakatos P, Martin K, Gall N, Rossi GP, Leschziner G (2015) Characterisation of sleep disturbances in postural orthostatic tachycardia syndrome: a polysomnography-based study. Sleep Med 16:1457–1461 [DOI] [PubMed] [Google Scholar]
- 70.Bagai K, Peltier A, Malow B, Diedrich A, Shibao C, Black B, Paranjape S, Orozco C, Biaggioni I, Robertson D, Raj S (2016) Objective sleep assessments in patients with postural tachycardia syndrome using overnight polysomnograms. J Clin Sleep Med 12:727–733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Guilleminault C, Primeau M, Chiu HY, Yuen KM, Leger D, Metlaine A (2013) Sleep-disordered breathing in Ehlers–Danlos syndrome: a genetic model of OSA. Chest 144:1503–1511 [DOI] [PubMed] [Google Scholar]
- 72.Manconi M, Ferri R, Zucconi M, Clemens S, Rundo F, Oldani A, Ferini-Strambi L (2011) Effects of acute dopamine-agonist treatment in restless legs syndrome on heart rate variability during sleep. Sleep Med 12:47–55 [DOI] [PubMed] [Google Scholar]
- 73.Barone DA, Ebben MR, DeGrazia M, Mortara D, Krieger AC (2017) Heart rate variability in restless legs syndrome and periodic limb movements of sleep. Sleep Sci 10:80–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Chiaro G, Calandra-Buonaura G, Cecere A, Mignani F, Sambati L, Loddo G, Cortelli P, Provini F (2017) REM sleep behavior disorder, autonomic dysfunction and synuclein-related neurodegeneration: where do we stand? Clin Auton Res doi: 10.1007/s10286-017-0460-4 [DOI] [PubMed] [Google Scholar]
- 75.Sorensen GL, Kempfner J, Zoetmulder M, Sorensen HB, Jennum P (2012) Attenuated heart rate response in REM sleep behavior disorder and Parkinson’s disease. Mov Disord 27(7):888–894 [DOI] [PubMed] [Google Scholar]
- 76.Berteotti C, Silvani A (2017) The link between narcolepsy and autonomic cardiovascular dysfunction: a translational perspective. Clin Auton Res 10.1007/s10286-017-0473-z [DOI] [PubMed] [Google Scholar]
- 77.Bastianini S, Silvani A, Berteotti C, Elghozi JL, Franzini C, Lenzi P, Lo Martire V, Zoccoli G (2011) Sleep related changes in blood pressure in hypocretin-deficient narcoleptic mice. Sleep 34:213–218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Takahashi K, Lin JS, Sakai K (2008) Neuronal activity of orexin and non-orexin waking-active neurons during wake–sleep states in the mouse. Neuroscience 153:860–870 [DOI] [PubMed] [Google Scholar]
- 79.Grimaldi D, Calandra-Buonaura G, Provini F, Agati P, Pierangeli G, Franceschini C, Barletta G, Plazzi G, Montagna P, Cortelli P (2012) Abnormal sleep-cardiovascular system interaction in narcolepsy with cataplexy: effects of hypocretin deficiency in humans. Sleep 35:519–528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Sieminski M, Chwojnicki K, Sarkanen T, Partinen M (2017) The relationship between orexin levels and blood pressure changes in patients with narcolepsy. PLoS One 12:e0185975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Fronczek R, Overeem S, Reijntjes R, Lammers GJ, van Dijk JG, Pijl H (2008) Increased heart rate variability but normal resting metabolic rate in hypocretin/orexin-deficient human narcolepsy. J Clin Sleep Med 4:248–254 [PMC free article] [PubMed] [Google Scholar]


