Abstract
Aim
To study the relationship between obesity, insulin resistance, vitamin D deficiency and sclerostin as a bone biomarker.
Materials and methods
Cross-section study of 75 subjects grouped into 3 groups; obese (n = 31), overweight (n = 23) and normal (n = 21) subjects. Sclerostin, fasting insulin, fasting plasma glucose and 25(OH)D were measured and anthropometric measures were taken.
Results
25(OH)D was lower in obese subjects than overweight and control groups (mean ± SD 5.27 ± 5.14 vs. 12.55 ± 6.99 vs.17.65 ± 4.07 ng/L, p < 0.001). Sclerostin was significantly lower in obese subjects versus the control (mean ± SD 1.02 ± 0.45 vs 1.58 ± 0.83 ng/mL, p = 0.014).
Conclusion
These results lead us to hypothesize that the relationship between sclerostin and Vitamin D levels has an important role in the link between obesity and bone metabolism. DObesity could be an active focus of research in the coming years.
Keywords: Vitamin D, Obesity, Sclerostin, Bone metabolism, Insulin resistance
Introduction
Obesity has been considered as an epidemic with over 10% of men and 14% of women in the world being obese. In Egypt, the 2008 Egypt Demographic and Health Survey was conducted by the Ministry of Health. It showed that around 22.5% and 46.3% of males and females are obese, respectively [1].
The relationship between obesity and bone metabolism has been of major interest, controversies and debates [2]. Classically, obesity was thought to be beneficial to bone by virtue of the mechanical effect of weight upon the bones. Heavier subjects tend to have higher bone mineral content and density [2], [3]. A metanalysis has shown that lower body mass index (BMI) is associated with increased fracture risk after bone mineral density (BMD) adjustments in women but not men [4]. However, more recent studies have observed an increased fracture risk among obese subjects versus those with normal weight. The Global Longitudinal Study of Osteoporosis in Women (GLOW) has shown that obesity is not protective against fracture as it was linked with increased risk of ankle and upper leg fractures among postmenopausal women [5]. A Spanish study has shown that the association between obesity and fractures are site-specific [6]. In men, the Osteoporotic fractures in Men Study (MrOS) has shown that obesity is associated with increased risk of fractures after adjusting for BMD, although obese subjects had greater BMD than those with normal BMI [7]. It is known that fractures in obese subjects are associated with greater morbidity and mortality due to greater risk of non-union, postoperative complications, presence of comorbidities, and slower rehabilitation [8].
Sclerostin is a 190-amino acid secreted glycoprotein predominantly made by osteocytes. Sclerostin directly inhibits the Wnt pathway in osteoblasts. Therefore, it inhibits the differentiation of osteoblasts and reduces bone formation. In addition to its anti-anabolic action, sclerostin has an indirect activity in bone resorption by stimulating osteoclast differentiation in a RANKL-dependent manner [9]. Moreover, sclerostin level in serum has been evaluated in many physiological and pathological conditions. More recently, monoclonal antibodies against sclerostin were studied as a possible treatment option for postmenopausal osteoporosis where the results phase III trial have found that romosozumab, sclerostin monoclonal antibody was associated with a lower risk of vertebral fracture [10]. Serum sclerostin levels have been studied in different physiological and pathological conditions. It has been shown that sclerostin levels correlated with insulin resistance in prediabetes [11]. Body weight had different effects on sclerostin levels [12]. Moreover, there were contradictory results regarding the relationship between vitamin D status and sclerostin levels
Vitamin D role has extended beyond the classical action on calcium and phosphorus homeostasis to a possible role in obesity, insulin resistance and metabolic syndrome among others [13]. It was confirmed in earlier studies that obese subjects have lower serum levels of 25-hydroxyvitamin D [25(OH)D]. Whether this is a cause or a consequence is yet uncertain. However, interventional studies have failed to show any advantage of vitamin D supplementation in terms of weight loss [14]. In addition, vitamin D deficiency has been associated with insulin resistance [15]. Higher basal levels of 25(OH)D have been found to predict better β-cell function and lower glycaemia in subjects at risk for type 2 diabetes [16]. Possible explanations for this include the immunomodulator effects of vitamin D [17] and direct effects on insulin sensitivity through the stimulation of expression of insulin receptors on target tissues and, through the activation of PPAR-δ as well as inhibiting the renin-angiotensin-aldosterone system [18].
From previous studies, several parameters have been investigated to affect serum sclerostin levels which are obesity and insulin resistance which was shown to have a positive correlation [11], and vitamin D level which was controversial and debatable. Therefore, we aimed to study the relationship between obesity, insulin resistance, vitamin D and sclerostin as a bone biomarker.
Subjects
This was cross sectional study on 75 subjects divided into three groups matched by age and gender according to their BMI; obese (BMI ≥ 30 kg/m2), overweight (BMI ≥ 25 and <30 kg/m2) and normal BMI (BMI ≥ 18.5 and <25 kg/m2) groups (n = 31, n = 23 and n = 21 respectively) according to the WHO [19]. Obese subjects were enrolled from the obesity clinic at the Alexandria Main University Hospital. Enrolment started in June 2017 and ended in September 2017. Patients with renal, liver or cardiac disease, patients having thyroid dysfunction, diabetes mellitus, hypertension, recent fractures or prolonged immobilization together with those taking drugs affecting insulin resistance like metformin, and pioglitazone, corticosteroids and drugs affecting bone metabolism were excluded from the sample.
Materials and methods
Detailed history taking and thorough clinical examination were done for each participant including history of endocrine diseases, diabetes mellitus and diseases affecting bone metabolism and family history of obesity, metabolic syndrome and osteoporosis. Anthropometric measures were taken including weight, height, waist circumference and hip circumference, all according to the WHO protocols. BMI and waist-hip ratio (WHR) were calculated from them.
Serum calcium, phosphorus. Alkaline phosphatase and serum glucose were measured together with HDL-C, LDL-C, total cholesterol and total TG were measured. Serum levels of fasting insulin, sclerostin and 25(OH)D were measured by ELISA kits (USCN Life Science, Wuhan, China/Cloud-Clone, Houston, TX, USA). Insulin resistance was assessed using the homeostatic model assessment index for insulin resistance (HOMA-IR).
Statistical analysis
All data were tabulated and entered into the SPSS version 22 program. For the comparison of the two groups, a t-test with unequal variance was used; for the comparison of multiple groups, one-way ANOVA was used, with Dunn's multiple comparisons for post-hoc analyses. Multivariate analysis was used for group comparisons. Correlations were analysed by extracting the Pearson coefficient. Tests were conducted at 5% significance level, i.e. p < 0.05 was considered as significant.
Results
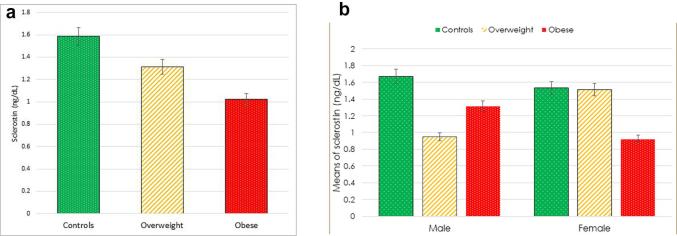
Serum concentrations of sclerostin were demonstrated to be the lowest in the obese group, followed by the overweight group and then the control group (mean ± SD 1.02 ± 0.46 vs 1.31 ± 0.82 vs 1.58 ± 0.83 ng/dL, respectively; p = 0.048) (Table 1, Fig. 1a and b).
Table 1.
Comparison between the three groups regarding: age, gender, BMI, 25(OH)D, sclerostin, HOMA-IR, fasting lipid profile and ALP.
| Normal | Overweight | Obese | Test of significance | Significance p value | |
|---|---|---|---|---|---|
| Age (years) | 28.67 ± 2.89 | 32.65 ± 5.36 | 35.06 ± 7.13 | F = 7.946 | 0.001* |
| Females (%) | 61.9% | 65.2% | 74.2% | χ2 = 0.987 | 0.611 |
| BMI (kg/m2) | 22.93 ± 1.21 | 27.56 ± 1.51 | 39.05 ± 6.50 | F = 97.49 | <0.001* |
| p1 = 0.001*, p2 < 0.001*, p3 < 0.001* | |||||
| 25(OH)D (ng/mL) | 17.65 ± 4.07 | 12.55 ± 6.99 | 5.27 ± 5.14 | F = 32.83 | <0.001* |
| p1 = 0.056, p2 < 0.001*, p3 = 0.001* | |||||
| Sclerostin (ng/dL) | 1.58 ± 0.83 | 1.31 ± 0.82 | 1.02 ± 0.45 | F = 4.12 | 0.048* |
| p1 = 0.240, p2 = 0.014*, p3 = 0.218 | |||||
| HOMA-IR | 1.15 ± 0. 06 | 2.05 ± 0.21 | 3.55 ± 0.21 | F = 32.34 | <0.001* |
| p1 < 0.001*, p2 < 0.001*, p3 < 0.001* | |||||
| TC (mg/dL) | 188.24 ± 5.40 | 196.09 ± 6.01 | 208.48 ± 12.19 | F = 33.42 | <0.001* |
| p1 = 0.015*, p2 < 0.001*, p3 < 0.001* | |||||
| TG (mg/dL) | 159.33 ± 14.37 | 174.17 ± 17.28 | 181.55 ± 19.26 | F = 10.26 | <0.001* |
| p1 = 0.018*, p2 < 0.001*, p3 = 0.385 | |||||
| HDL-C (mg/dL) | 44.76 ± 2.86 | 39.83 ± 3.50 | 35.10 ± 3.38 | F = 54.77 | <0.001* |
| p1 < 0.001*, p2 < 0.001*, p3 < 0.001* | |||||
| LDL-C (mg/dL) | 100.00 ± 10.59 | 108.09 ± 13.71 | 126.58 ± 13.68 | F = 29.42 | <0.001* |
| p1 = 0.124, p2 < 0.001*, p3 < 0.001* | |||||
| ALP | 119.86 ± 3.89 | 129.4 ± 7.34 | 132 ± 5.23 | F = 25.7 | <0.001* |
| p1 = 0.002*, p2 < 0.001*, p3 < 0.001* | |||||
| Calcium | 9.1 ± 0.4 | 8.9 ± 0.3 | 8.8 ± 0.4 | F = 2.25 | 0.113 |
| p1 = 0.258 p2 = 0.037*, p3 = 0.357 | |||||
| Phosphorus | 3.9 ± 0.6 | 4.1 ± 0.5 | 4.0 ± 0.3 | F = 2.04 | 0.138 |
| p1 = 0.142, p2=0.836, p3 = 0.836 | |||||
F,p: F and p values for ANOVA test, significance between groups was done using Post Hoc Test (LSD); 2 and p values for Chi square test for comparing between the three groups; p1: p value for comparing between controls and overweight subjects. p2: p value for comparing between controls and obese subjects. p3: p value for comparing between overweight and obese subjects. *: Statistically significant at p ≤ 0.0525(OH)D: 25-hydroxyvitamin D, ALP: alkaline phosphatase, BMI: body mass index, HOMA-IR: homeostatic model assessment of insulin resistance, HDL-C: high-density lipoprotein cholesterol, TG: total cholesterol, TG: Total triglycerides, LDL-C: low-density lipoprotein cholesterol.
Fig. 1.
(a and b). Comparison between the obese, overweight and the control groups as regards serum sclerostin concentration.
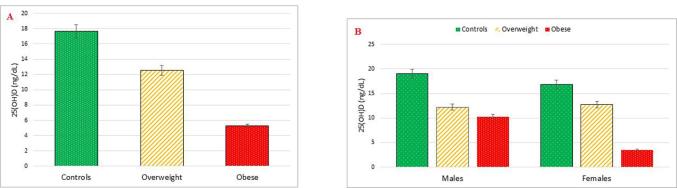
25(OH)D was measured to assess the vitamin D status. 25(OH)D serum levels were the lowest in the obese group (mean ± SD 5.27 ± 5.14 ng/mL) followed by the overweight group (mean ± SD 12.55 ± 6.99 ng/mL) and lastly the control group (mean ± SD 17.65 ± 4.07 ng/mL). The difference was statistically significant with p < 0.001. Serum levels of 25(OH)D were significantly higher in males than females (13.83 ± 7.26 vs 9.62 ± 7.34 ng/mL, respectively); with p = 0.023). This difference was mainly driven by the difference between males and females in the obese group (10.28 ± 8.30 vs 3.52 ± 1.32 ng/mL respectively); and p = 0.009 (Table 1, Fig. 2a and b).
Fig. 2.
(a and b). Comparison between the obese, overweight and the control groups regarding 25(OH)D.
HOMA-IR was calculated from fasting serum insulin concentration and fasting serum glucose concentration. It was found that there was significant difference (p < 0.001) between the three groups regarding insulin resistance, with the obese group having the highest mean followed by the overweight and then the control group (3.55 ± 0.21 vs 2.05 ± 0.21 vs 1.15 ± 0.06 respectively) (Table 1). ALP was found to be significantly the highest in obese group followed by the overweight and then the control group (132 ± 5.23, 129.4 ± 7.34, 119.86 ± 3.89 respectively, with p < 0.001) (Table 1).
Regarding lipid profile, TC, TG and LDL-C were significantly the highest in the obese group followed by the overweight and then the control group, opposite to HDL-C as shown in (Table 1).
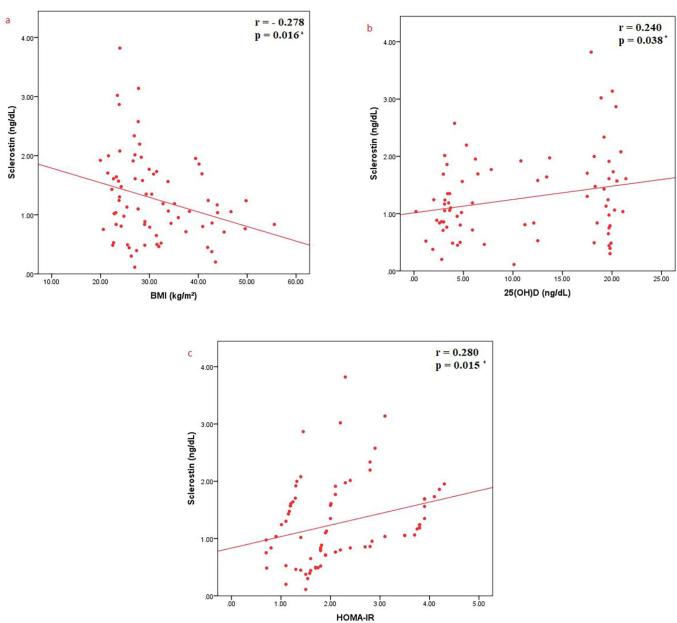
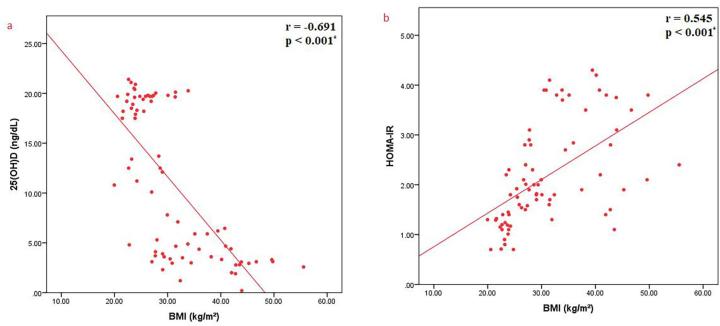
Regarding the correlations between sclerostin and various parameters, sclerostin was found to have a significant negative correlation with BMI (r = −0.278, p = 0.016), a significant positive correlation with HOMA-IR as a measure of insulin resistance (r = 0.280, p = 0.015) and a significant positive correlation between sclerostin and 25(OH)D (r = 0.240, p = 0.038) (Fig. 3a-c). BMI was shown to have a significant negative correlation with serum concentration of 25(OH)D (r = −0.691, p < 0.001) (Fig. 4a) and a significant positive correlation with HOMA-IR as a measure of insulin resistance (r = 0.545, p < 0.001) (Fig. 4b). Moreover, it has been shown that there was a significant negative correlation between 25(OH)D and HOMA-IR as a measure of insulin resistance (r = −0.473, p < 0.001) (Fig. 5).
Fig. 3.
Correlations between sclerostin and a) BMI; b) 25(OH)D; c) HOMA-IR.
Fig. 4.
Correlations between BMI and a) 25(OH)D; b) HOMA-IR.
Fig. 5.
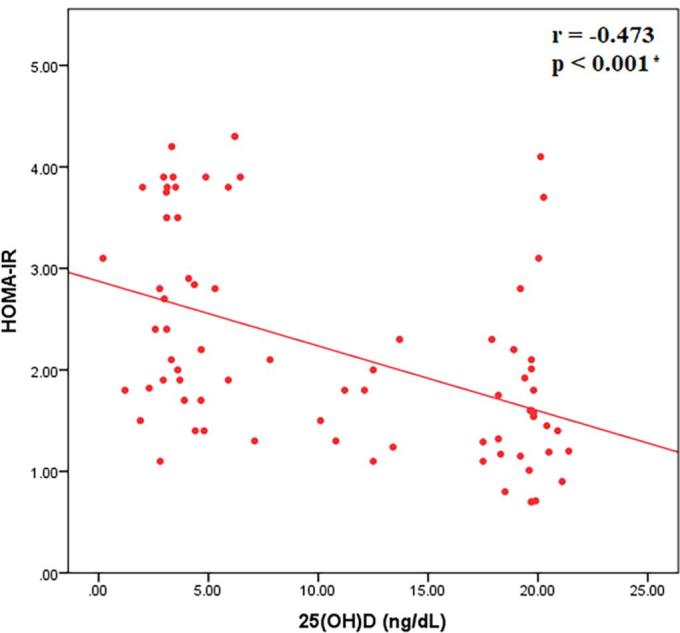
Correlation between 25(OH)D and HOMA-IR.
Discussion
The current study is a cross sectional study which attempted to investigate the relationship between obesity, insulin resistance, vitamin D and bone metabolism through the link of the recently discovered bone biomarker sclerostin.
The current study has showed that sclerostin was significantly decreased in the obese group versus both the overweight and the control groups. Previous studies comparing sclerostin in obese versus normal subjects have shown contradictory results. Grethen et al. [12] studied the difference in sclerostin level between 20 obese women before undergoing bariatric surgery and 20 control women matched for race and age. They found no significant difference between sclerostin level among the two groups. However, bariatric surgery and its associated weight loss has led to a rapid and sustained increase in serum sclerostin levels which peaked at six months (+135%; p < 0.001) and remained elevated above baseline 24 months after surgery. This resulted in BMD loss at all skeletal sites [20]. In another study, weight loss with diet alone was associated with a significant increase in sclerostin level and was associated with deterioration in hip geometry parameters. Hip is an important weight bearing skeletal site. The increase in sclerostin and the deterioration in hip geometry were attenuated by the addition of an exercise program [21]. Serum sclerostin concentration was significantly higher in osteopenic-osteoporotic obese group compared to the obese-only group [22]. Sclerostin was found to be higher in subjects with metabolic syndrome, its level increases significantly with increasing number of metabolic syndrome components. There was significant positive correlation between sclerostin level and waist circumference. However, this correlation lost its significance after correction for the whole body bone mineral content [23].
Under normal physiological circumstances, mechanical loading has been shown to play a major role in attaining bone mass, bone strength, and bone size [24]. Results from the Framingham osteoporosis study, a subset of the Framingham study cohort, showed the strong effect of weight on BMD, suggesting that this effect is due to mechanical loading on weight bearing axes [25].Obesity was thought to be protective against postmenopausal osteoporosis as obese women had higher BMD [26]. However, more recent studies have shown that increasing fat mass may not have a beneficial effect on bone mass [27]. Therefore, it may be postulated that the reason for the lower levels of serum sclerostin may be the effect of mechanical loading by the excess weight on bones, so osteocytes reduce their expression of sclerostin.
The present study shows that sclerostin positively correlated with insulin resistance (r = 0.280, p = 0.015). This agrees with a previous study where sclerostin levels were higher in subjects with impaired glucose regulation and positively correlated with HOMA-IR (r = 0.62, p < 0.001) [11]. Moreover, circulating sclerostin is increased in T2DM independently of gender and age, and it is also correlated with duration of T2DM, glycated haemoglobin, bone turnover markers, and BMD in T2DM patients [28]. Serum sclerostin was elevated in patients with both T1DM and T2DM [28], [29], [30]. It was previously mentioned that sclerostin was found to be higher in subjects with metabolic syndrome. The correlation between WC and sclerostin lost its significance after correction for BMC, suggesting that it was driven by the high BMC in obese subjects as they tend to have bigger bone [23]. On the other hand, the correlation between glycemia and TG with sclerostin persisted even after correction for BMC. This could propose a metabolic role for sclerostin apart from its role in bone physiology [23].
The pathophysiologic mechanisms relating sclerostin to glucose metabolism are still under investigations. Sclerostin has been associated with several inflammatory and metabolic conditions suggesting that sclerostin is not only a regulator of bone mass and its elevation in metabolic syndrome is not only an artefact. It is proven that TNF-α stimulated the release of sclerostin [31]. Insulin resistance is a state of low grade inflammation with slightly elevated inflammatory markers [32], [33].
Regarding the relationship between vitamin D and sclerostin, the current study has shown a significant weak positive correlation between vitamin D and sclerostin (r = 0.240, p < 0.038). There were contradictory results in the literature regarding the relationship between sclerostin and vitamin D. In one study, treatment of human primary osteoblasts, including cells differentiated to an osteocyte-like stage, with 1,25 vitamin D resulted in the dose-dependent increased expression of SOST mRNA which may in turn increase the secretion of sclerostin [34]. In another study on patients with idiopathic hypercalciuria, there was a positive correlation between sclerostin expression by osteocytes and serum 1,25D levels [35]. However; some studies reported different results. There was a decrease in serum sclerostin level after vitamin D3 treatment of vitamin D deficient young females [36]. These results were repeatedly confirmed by another study that studied both males and females [37]. The reason behind these differences may be that our study is observational without intervention and that our random small sample included subjects with vitamin D deficiency. It may be hypothesized that an interventional study trying to correct vitamin D levels may alter serum sclerostin levels in our sample.
The current study revealed that serum level of 25(OH)D correlated negatively with HOMA-IR (r = −0.692, p < 0.001); i.e. insulin resistance significantly increased as vitamin D level decreased. This agreed with the results of Liu et al. [38]. A recent study which examined standardized 25(OH)D data from the National Health and Nutrition Examination Survey (NHANES) found that low vitamin D status is a risk factor for cardiometabolic disease and insulin resistance [39]. Chiu [40] also found a positive correlation of 25(OH)D concentration with insulin sensitivity and a negative effect of hypovitaminosis D on β cell function. It was also reported that there is a significant inverse association between serum 25(OH)D and risk of T2DM [41].
Mechanisms relating vitamin D deficiency and insulin resistance are not fully elucidated. It was proven that pancreatic β-cells have vitamin D receptors on their surfaces suggesting that the active metabolite 1-α-25-dihydroxyvitamin D3 (1,25(OH)2D3) could directly influence insulin secretion [39]. Moreover, vitamin D deficiency promotes secondary hyperparathyroidism, stimulates the RAAS, which in turn increases the secretion of aldosterone [42]. Previous epidemiological studies showed plasma intact parathyroid hormone level is inversely correlated with insulin sensitivity [43]. It was also found that there is an increased risk of metabolic syndrome with elevated PTH levels in older men [44]. Elevated PTH could possibly affect insulin sensitivity by regulating the intracellular free calcium concentrations in target cells [38]. Vitamin D may play a role in insulin action by stimulating the expression of insulin receptor and thereby enhancing insulin responsiveness for glucose transport target cells [45].
To conclude, this study has highlighted the relationship between sclerostin and various metabolic parameters, confirming the direct link between bone and obesity and opening the gate for future research in this field. Sclerostin studies were introduced recently and have shown that sclerostin is a negative regulator of bone metabolism. Up to the best of our knowledge our study was the first study in medical literature to link obesity, insulin resistance and 25(OH)D to serum sclerostin in one study. Furthermore, sclerostin monoclonal antibody, romosozumab, has been recently approved by FDA in April 2019 as therapy of osteoporosis. DObesity is suggested to be a new terminology in medical literature to highlight the complex relationship between obesity and vitamin D and the cross talk between adiposity and bone metabolism via the novel bone biomarker sclerostin.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Declaration of Competing Interest
The authors declare that they have no conflicts of interest.
Acknowledgement
The authors would like to express their gratitude for the support of department of Internal medicine, Endocrinology unit, Alexandria University, Egypt.
References
- 1.Ministry of Health and Population [Egypt], El-Zanaty and Associates [Egypt], International I. Egypt Demographic and Health Survey 2014. Cairo, Egypt and Rockville, Maryland, USA: Ministry of Health and Population and ICF International; 2015.
- 2.Reid I.R., Plank L.D., Evans M.C. Fat mass is an important determinant of whole body bone density in premenopausal women but not in men. J Clin Endocrinol Metab. 1992;75(3):779–782. doi: 10.1210/jcem.75.3.1517366. [DOI] [PubMed] [Google Scholar]
- 3.Mazess R.B., Barden H.S., Drinka P.J., Bauwens S.F., Orwoll E.S., Bell N.H. Influence of age and body weight on spine and femur bone mineral density in U.S. white men. J Bone Miner Res. 1990;5(6):645–652. doi: 10.1002/jbmr.5650050614. [DOI] [PubMed] [Google Scholar]
- 4.De Laet C., Kanis J.A., Odén A., Johanson H., Johnell O., Delmas P. Body mass index as a predictor of fracture risk: a meta-analysis. Osteoporos Int. 2005;16(11):1330–1338. doi: 10.1007/s00198-005-1863-y. [DOI] [PubMed] [Google Scholar]
- 5.Compston J.E., Watts N.B., Chapurlat R., Cooper C., Boonen S., Greenspan S. Obesity is not protective against fracture in postmenopausal women: GLOW. Am J Med. 2011;124(11):1043–1050. doi: 10.1016/j.amjmed.2011.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Prieto-Alhambra D., Premaor M.O., Fina Avilés F., Hermosilla E., Martinez-Laguna D., Carbonell-Abella C. The association between fracture and obesity is site-dependent: a population-based study in postmenopausal women. J Bone Miner Res. 2012;27(2):294–300. doi: 10.1002/jbmr.1466. [DOI] [PubMed] [Google Scholar]
- 7.Nielson C.M., Marshall L.M., Adams A.L., LeBlanc E.S., Cawthon P.M., Ensrud K. BMI and fracture risk in older men: the osteoporotic fractures in men study (MrOS) J Bone Miner Res. 2011;26(3):496–502. doi: 10.1002/jbmr.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gonnelli S., Caffarelli C., Nuti R. Obesity and fracture risk. Clin Cases Miner Bone Metab. 2014;11(1):9–14. doi: 10.11138/ccmbm/2014.11.1.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hay E., Bouaziz W., Funck-Brentano T., Cohen-Solal M. Sclerostin and bone aging: a mini-review. Gerontology. 2016;62(6):618–623. doi: 10.1159/000446278. [DOI] [PubMed] [Google Scholar]
- 10.Cosman F., Crittenden D.B., Adachi J.D., Binkley N., Czerwinski E., Ferrari S. Romosozumab treatment in postmenopausal women with osteoporosis. N Engl J Med. 2016;375(16):1532–1543. doi: 10.1056/NEJMoa1607948. [DOI] [PubMed] [Google Scholar]
- 11.Daniele G., Winnier D., Mari A., Bruder J., Fourcaudot M., Pengou Z. Sclerostin and insulin resistance in prediabetes: evidence of a cross talk between bone and glucose metabolism. Diabetes Care. 2015;38(8):1509–1517. doi: 10.2337/dc14-2989. [DOI] [PubMed] [Google Scholar]
- 12.Grethen E., Hill K.M., Jones R., Cacucci B.M., Gupta C.E., Acton A. Serum leptin, parathyroid hormone, 1,25-dihydroxyvitamin D, fibroblast growth factor 23, bone alkaline phosphatase, and sclerostin relationships in obesity. J Clin Endocrinol Metab. 2012;97(5):1655–1662. doi: 10.1210/jc.2011-2280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Christakos S., Dhawan P., Verstuyf A., Verlinden L., Carmeliet G. Vitamin D: metabolism, molecular mechanism of action, and pleiotropic effects. Physiol Rev. 2016;96(1):365–408. doi: 10.1152/physrev.00014.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Landrier J.F., Karkeni E., Marcotorchino J., Bonnet L., Tourniaire F. Vitamin D modulates adipose tissue biology: possible consequences for obesity? Proc Nutr Soc. 2016;75(1):38–46. doi: 10.1017/S0029665115004164. [DOI] [PubMed] [Google Scholar]
- 15.Zhao G., Ford E.S., Li C. Associations of serum concentrations of 25-hydroxyvitamin D and parathyroid hormone with surrogate markers of insulin resistance among U.S. adults without physician-diagnosed diabetes: NHANES, 2003-2006. Diabetes Care. 2010;33(2344) doi: 10.2337/dc09-0924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kayaniyil S., Retnakaran R., Harris S.B., Vieth R., Knight J.A., Gerstein H.C. Prospective associations of vitamin D with beta-cell function and glycemia: the PROspective Metabolism and ISlet cell Evaluation (PROMISE) cohort study. Diabetes. 2011;60(11):2947–2953. doi: 10.2337/db11-0465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Garbossa S.G., Folli F. Vitamin D, sub-inflammation and insulin resistance. A window on a potential role for the interaction between bone and glucose metabolism. Rev Endocr Metab Disord. 2017;18(2):243–258. doi: 10.1007/s11154-017-9423-2. [DOI] [PubMed] [Google Scholar]
- 18.Altieri B., Grant W.B., Della Casa S., Orio F., Pontecorvi A., Colao A. Vitamin D and pancreas: the role of sunshine vitamin in the pathogenesis of diabetes mellitus and pancreatic cancer. Crit Rev Food Sci Nutr. 2017;57(16):3472–3488. doi: 10.1080/10408398.2015.1136922. [DOI] [PubMed] [Google Scholar]
- 19.WHO . World Health Organization; 2000. Obesity: preventing and managing the global epidemic. [PubMed] [Google Scholar]
- 20.Muschitz C., Kocijan R., Marterer C., Nia A.R., Muschitz G.K., Resch H. Sclerostin levels and changes in bone metabolism after bariatric surgery. J Clin Endocrinol Metab. 2015;100(3):891–901. doi: 10.1210/jc.2014-3367. [DOI] [PubMed] [Google Scholar]
- 21.Armamento-Villareal R., Sadler C., Napoli N., Shah K., Chode S., Sinacore D.R. Weight loss in obese older adults increases serum sclerostin and impairs hip geometry but both are prevented by exercise training. J Bone Miner Res. 2012;27(5):1215–1221. doi: 10.1002/jbmr.1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.JafariNasabian P., Inglis J.E., Kelly O.J., Ilich J.Z. Osteosarcopenic obesity in women: impact, prevalence, and management challenges. Int J Womens Health. 2017;9:33–42. doi: 10.2147/IJWH.S106107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Confavreux C.B., Casey R., Varennes A., Goudable J., Chapurlat R.D., Szulc P. Has sclerostin a true endocrine metabolic action complementary to osteocalcin in older men? Osteoporos Int. 2016;27(7):2301–2309. doi: 10.1007/s00198-016-3540-8. [DOI] [PubMed] [Google Scholar]
- 24.Sakhaee K., Poindexter J., Aguirre C. The effects of bariatric surgery on bone and nephrolithiasis. Bone. 2016;84:1–8. doi: 10.1016/j.bone.2015.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Felson D.T., Zhang Y., Hannan M.T., Anderson J.J. Effects of weight and body mass index on bone mineral density in men and women: the Framingham study. J Bone Miner Res. 1993;8(5):567–573. doi: 10.1002/jbmr.5650080507. [DOI] [PubMed] [Google Scholar]
- 26.Albala C., Yáñez M., Devoto E., Sostin C., Zeballos L., Santos J.L. Obesity as a protective factor for postmenopausal osteoporosis. Int J Obes Relat Metab Disord. 1996;20(11):1027–1032. [PubMed] [Google Scholar]
- 27.Zhao L.J., Liu Y.J., Liu P.Y., Hamilton J., Recker R.R., Deng H.W. Relationship of obesity with osteoporosis. J Clin Endocrinol Metab. 2007;92(5):1640–1646. doi: 10.1210/jc.2006-0572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Garcia-Martin A., Rozas-Moreno P., Reyes-Garcia R., Morales-Santana S., Garcia-Fontana B., Garcia-Salcedo J.A. Circulating levels of sclerostin are increased in patients with type 2 diabetes mellitus. J Clin Endocrinol Metab. 2012;97(1):234–241. doi: 10.1210/jc.2011-2186. [DOI] [PubMed] [Google Scholar]
- 29.Morales-Santana S., Garcia-Fontana B., Garcia-Martin A., Rozas-Moreno P., Garcia-Salcedo J.A., Reyes-Garcia R. Atherosclerotic disease in type 2 diabetes is associated with an increase in sclerostin levels. Diabetes Care. 2013;36(6):1667–1674. doi: 10.2337/dc12-1691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gennari L., Merlotti D., Valenti R., Ceccarelli E., Ruvio M., Pietrini M.G. Circulating sclerostin levels and bone turnover in type 1 and type 2 diabetes. J Clin Endocrinol Metab. 2012;97(5):1737–1744. doi: 10.1210/jc.2011-2958. [DOI] [PubMed] [Google Scholar]
- 31.Heiland G.R., Zwerina K., Baum W., Kireva T., Distler J.H., Grisanti M. Neutralisation of Dkk-1 protects from systemic bone loss during inflammation and reduces sclerostin expression. Ann Rheum Dis. 2010;69(12):2152–2159. doi: 10.1136/ard.2010.132852. [DOI] [PubMed] [Google Scholar]
- 32.Dandona P., Aljada A., Bandyopadhyay A. Inflammation: the link between insulin resistance, obesity and diabetes. Trends Immunol. 2004;25(1):4–7. doi: 10.1016/j.it.2003.10.013. [DOI] [PubMed] [Google Scholar]
- 33.Shoelson S.E., Lee J., Goldfine A.B. Inflammation and insulin resistance. J Clin Invest. 2006;116(7):1793. doi: 10.1172/JCI29069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wijenayaka A.R., Yang D., Prideaux M., Ito N., Kogawa M., Anderson P.H. 1alpha,25-dihydroxyvitamin D3 stimulates human SOST gene expression and sclerostin secretion. Mol Cell Endocrinol. 2015;413:157–167. doi: 10.1016/j.mce.2015.06.021. [DOI] [PubMed] [Google Scholar]
- 35.Menon V.B., Moyses R.M., Gomes S.A., de Carvalho A.B., Jorgetti V., Heilberg I.P. Expression of fibroblast growth factor 23, vitamin D receptor, and sclerostin in bone tissue from hypercalciuric stone formers. Clin J Am Soc Nephrol. 2014;9(7):1263–1270. doi: 10.2215/CJN.10030913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cidem M., Karacan I., Arat N.B., Zengi O., Ozkaya M., Guzel S.P. Serum sclerostin is decreased following vitamin D treatment in young vitamin D-deficient female adults. Rheumatol Int. 2015;35(10):1739–1742. doi: 10.1007/s00296-015-3294-1. [DOI] [PubMed] [Google Scholar]
- 37.Acibucu F., Dokmetas H.S., Acibucu D.O., Kilicli F., Aydemir M., Cakmak E. Effect of Vitamin D treatment on serum sclerostin level. Exp Clin Endocrinol Diabetes. 2016;125(9):634–637. doi: 10.1055/s-0035-1559790. [DOI] [PubMed] [Google Scholar]
- 38.Liu E., Meigs J.B., Pittas A.G., McKeown N.M., Economos C.D., Booth S.L. Plasma 25-hydroxyvitamin D is associated with markers of the insulin resistant phenotype in nondiabetic adults. J Nutrition. 2009;139(2):329–334. doi: 10.3945/jn.108.093831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Al-khalidi B., Kimball S.M., Rotondi M.A., Ardern C.I. Standardized serum 25-hydroxyvitamin D concentrations are inversely associated with cardiometabolic disease in U.S. adults: a cross-sectional analysis of NHANES, 2001–2010. Nutr J. 2017;16(1):16. doi: 10.1186/s12937-017-0237-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chiu K.C., Chu A., Go V.L.W., Saad M.F. Hypovitaminosis D is associated with insulin resistance and β cell dysfunction. Am J Clin Nutrition. 2004;79(5):820–825. doi: 10.1093/ajcn/79.5.820. [DOI] [PubMed] [Google Scholar]
- 41.Mattila C., Knekt P., Mannisto S., Rissanen H., Laaksonen M.A., Montonen J. Serum 25-hydroxyvitamin D concentration and subsequent risk of type 2 diabetes. Diabetes Care. 2007;30(10):2569–2570. doi: 10.2337/dc07-0292. [DOI] [PubMed] [Google Scholar]
- 42.Tomaschitz A., Ritz E., Pieske B., Fahrleitner-Pammer A., Kienreich K., Horina J.H. Aldosterone and parathyroid hormone: a precarious couple for cardiovascular disease. Cardiovasc Res. 2012;94(1):10–19. doi: 10.1093/cvr/cvs092. [DOI] [PubMed] [Google Scholar]
- 43.Chiu K.C., Chuang L.M., Lee N.P., Ryu J.M., McGullam J.L., Tsai G.P. Insulin sensitivity is inversely correlated with plasma intact parathyroid hormone level. Metabolism. 2000;49(11):1501–1505. doi: 10.1053/meta.2000.17708. [DOI] [PubMed] [Google Scholar]
- 44.Reis J.P., von Muhlen D., Kritz-Silverstein D., Wingard D.L., Barrett-Connor E. Vitamin D, parathyroid hormone levels, and the prevalence of metabolic syndrome in community-dwelling older adults. Diabetes Care. 2007;30 doi: 10.2337/dc06-2438. [DOI] [PubMed] [Google Scholar]
- 45.Maestro B., Davila N., Carranza M.C., Calle C. Identification of a Vitamin D response element in the human insulin receptor gene promoter. J Steroid Biochem Mol Biol. 2003;84(2–3):223–230. doi: 10.1016/s0960-0760(03)00032-3. [DOI] [PubMed] [Google Scholar]