Abstract
The extracranial carotid artery aneurysm (ECAA) is a rare pathology for which clinical treatment guidelines are lacking. In general, symptoms or growth of the aneurysm sac are thought to indicate intervention. ECAAs may present in a large variety of shapes and sizes, and conventional diameter measurements fail to indicate geometrical differences. Therefore, we propose a protocol to measure ECAA size by 3D volumetric assessment. The volumes of 40 ECAAs in computed tomography angiography (CTA) images were measured through manual segmentation, by two independent operators. Volumes of the entire internal carotid artery (ICA) and the ECAA were measured separately. Excellent inter- and intraoperator reliability was found for both ICA and ECAA volumes, with all intraclass correlation coefficients above 0.94. Bland-Altman analysis revealed normal differences for both inter- and intraoperator agreement. For all volumes, similarity of the segmentations was excellent. Outliers were explained by presence of intraluminal ECAA thrombus, which hampered identification of the aneurysm outer wall. These results implicate robustness of our protocol, which is designed as a step-up towards (semi)automatic volumetric measurements to monitor patients with ECAA. Future (semi)automatic volumetric assessments are recommended and such techniques can be developed and validated using the proposed protocol and manual reference segmentations.
Subject terms: Preclinical research, Experimental models of disease
Introduction
The extracranial carotid artery aneurysm (ECAA) is a rare vascular pathology, of which the natural clinical course, and risk factors for adverse outcome are largely unknown1,2. As a consequence, currently no evidence-based guidelines exist on treatment of ECAAs. Asymptomatic, non-growing aneurysms are generally treated conservatively3, while surgical or endovascular aneurysm exclusion may be considered in case of symptomatic or growing ECAA2. However, a standardized method to measure ECAA size or growth is currently not available.
At present, ECAA size is mostly defined by determination of maximum diameter. These diameters can be assessed by measuring the bi-directional maximum aneurysm diameter from outer to outer vessel wall, extracted from the well-established approach for abdominal aortic aneurysms (AAAs)4–6. However, ECAAs may present in a large variety of shapes and sizes. The dilatation of ECAA may be focal and saccular, or extensive and fusiform, or a combination of both, and maximum aneurysm diameters between 4 and 60 mm have been reported3,7,8. Furthermore, it is imaginable that ECAA growth might occur in other directions than just obliquely. Thus, assessment of geometrical differences by comparing maximum aneurysm diameters is inadequate as we lack the appropriate imaging tools. A standardized protocol to measure 3D ECAA volume - instead of 2D diameters - would theoretically be able to reliably determine aneurysm size and growth. These measures could then be used for follow-up of ECAA patients (and ultimately for patients with any type of aneurysm), for pre-treatment monitoring, and underpinning of treatment decisions.
Therefore, the aim of this study was to develop and evaluate a robust protocol for volume measurement of ECAAs.
Methods
Participants
Twenty patients with an ECAA located in the internal carotid artery (ICA) gave informed consent and were included from our Carotid Aneurysm Registry (www.carotidaneurysmregistry.com). Ethical approval for this study (12/020761) was provided by the Medical Research Ethics Committee of University Medical Center Utrecht, Utrecht, The Netherlands on 12 June 2012, and all research was conducted according to the principles of the Declaration of Helsinki (59th amendment, Seoul 2008) and in accordance with the Dutch Medical Research Involving Human Subjects Act (WMO). The registry protocol has been published elsewhere9. Briefly, any patient aged 18-years or older with an ECAA is included in this ongoing registry, independent of etiology or treatment strategy. Patients who, or their legal representative, are unable or unwilling to sign the informed consent were excluded. Selected patients for the current study had two computed tomography angiography (CTA) scans of the carotids made at different time points. Hence, in total 40 scans were included. The CTAs had been performed for evaluation or treatment planning of ECAAs between 2008 and 2017, in the University Medical Center Utrecht. As specified within the registry protocol9, fusiform aneurysms were defined as ≥150% diameter increase of the non-affected ICA diameter, while saccular aneurysms were defined as a distended sac of any size affecting only part of the ICA circumference.
Volume measurement protocol
The protocol for volume measurement of ECAAs was developed by an expert panel, which consisted of three vascular surgeons (G.B., J.H., C.H.) and two dedicated researchers (E.V., C.L.). Each case was measured in a twofold manner. First, the volume of the entire ICA (including ECAA) was measured, from carotid bifurcation up until the entrance of the ICA in the skull base (method 1). Hereafter, the ECAA volume was measured separately (method 2).
Entire ICA (method 1)
The starting point of the ICA was the carotid bifurcation (Fig. 1). On sagittal and coronal views, the ipsilateral common and external carotid arteries were segmented, and subtracted from the segmented proximal ICA on axial view. Distally, the ICA was segmented up until entrance in the skull base, defined as the first slice which showed at least 50% of the ICA surrounded by bone.
Figure 1.
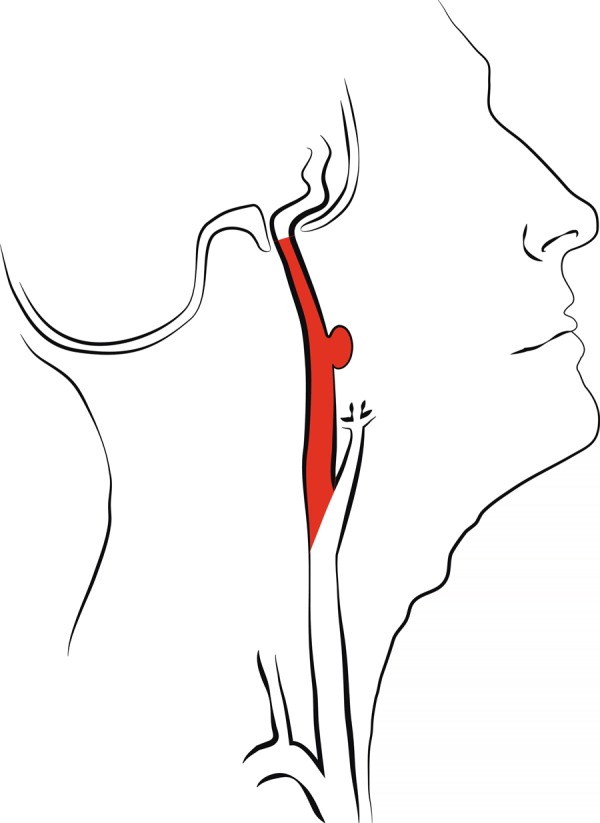
Measurement method 1: Entire ICA.
Solely the ECAA (method 2)
Since ECAAs come in different shapes, a different approach was inevitable to define the borders of saccular versus fusiform aneurysms.
Fusiform aneurysms: The aneurysm was segmented from the clear starting point of vessel dilatation onwards (immediately ≥150%; Fig. 2A). If the widening of the vessel commenced gradually, segmentation started when the vessel had widened with ≥125% compared with the healthy ipsilateral ICA.
Figure 2.
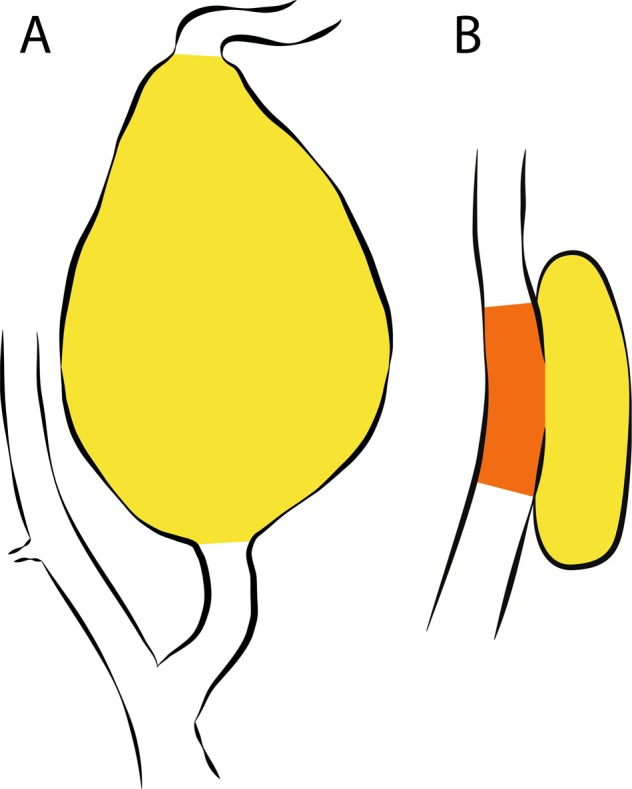
Measurement method 2: Solely the ECAA. (A) Fusiform aneurysm. (B) Saccular aneurysm: measurement of solely the aneurysm sac (yellow), and vessel adjacent to aneurysm sac (orange).
Saccular aneurysms: All saccular ECAAs were measured similarly. First, volume of just the aneurysm sac was measured. In case the outer wall of the aneurysm sac adjoined the outer wall of the ICA, volume of the aneurysm sac was additionally measured in combination with the part of the ICA adjacent to the aneurysm neck and sac (Fig. 2B). Hence, both separate segmentations were added to calculate volume of method 2 (Fig. 3).
Figure 3.
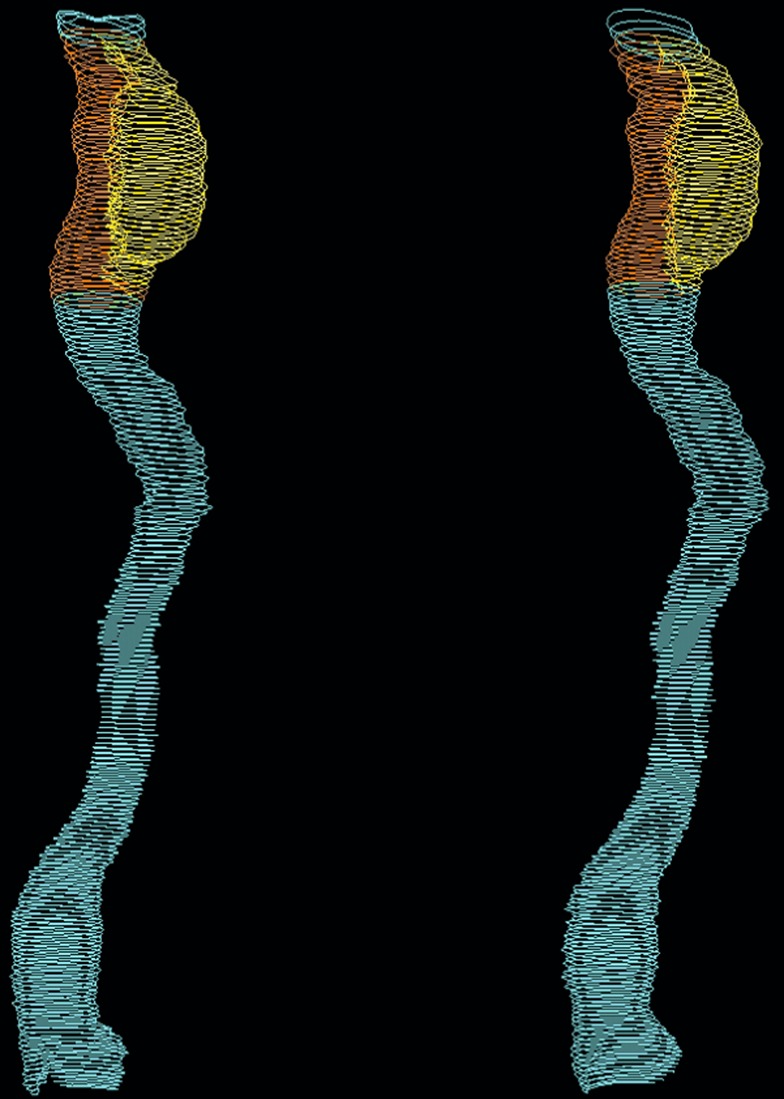
Example of segmentation of operator 1 (left) and operator 2 (right). The separate segmentations are referred to “only vessel” in blue, “aneurysm sac” in yellow, and “vessel adjacent to aneurysm sac” in orange. Total volume of method 1 (entire ICA) is calculated by adding up the volumes of all three separate segmentations. Total volume of method 2 (solely the ECAA) is calculated by adding up the volumes of “aneurysm sac” and “vessel adjacent to aneurysm sac”.
For a schematic overview of the twenty cases, we refer to Appendix A. The final segmentations as performed with MeVisLab (see next subsection) are included in Appendix B. A case example with color coding and names of separate volumes is shown in Fig. 3.
Software
In-house developed software (by H.K.) implemented in MeVisLab (MeVis Medical Solutions AG, Bremen, Germany) was used to manually trace the ICA and borders of the ECAA. This program provided a user-friendly spline drawing and editing technique, with which we were able to follow the borders of the structures closely. All volumes were manually segmented from outer to outer wall, as is standard in segmentation of abdominal aortic aneurysms4–6. All cases were segmented on axial view (sagittal and coronal views were only used to identify structure borders). Volumes (in mL) were obtained by multiplying the size of the segmented volume by the voxel size of the acquired image.
Study design
Two operators (E.V., C.L.) independently segmented each of the 40 scans according to the measurement methods 1 and 2 in the predefined protocol. Prior to measurement of the 40 scans, both operators measured three test scans in order to familiarize with the program. The results of these test segmentations were compared to overcome potential differences in protocol interpretation. Afterwards, the twenty cases were offered to the operators in randomized fashion in order to prevent a potential effect from learning. Of each case, the first and second scans were measured in succession. A random sample of five ECAAs was selected for both operators to be measured twice according to method 2.
Imaging
A 64-slice or 128-slice CT scanner (Philips Brilliance; Philips medical systems, Best, the Netherlands) was used to acquire the CTA scans. The carotid arteries were visualized from the aortic arch up until the skull base. The median slice thickness was 0.9 mm (range 0.6–3.0 mm), increment 0.33 mm, pitch 0.609 mm, and collimation 64 × 0.625 mm. Tube voltage was 100–120 kV and tube current 17–300 mA. Fields of view were set per patient. Injection of 65 milliliter intravascular contrast (ultravist 300, Schering, Berlin, Germany) was followed by a saline bolus of 40 milliliter, both at a flow rate of 6 milliliters per second.
Outcomes and statistical analysis
Crude volumes were calculated for method 1 (entire internal carotid artery ipsilateral to the ECAA) and method 2 (solely the ECAA).
The primary outcomes were measurement reliability and agreement of the protocol. Interoperator reliability of each volume was calculated by the intraclass correlation coefficient (ICC; model: two-way mixed, type: consistency). This was done for the entire ICA (method 1), as well as for the ECAA (method 2, A and B). An ICC of 1.0 equals perfect reliability, an ICC of 0.81–0.99 excellent reliability, and an ICC of 0.61–0.80 substantial reliability10. Intraoperator reliability for measurement method 2 was calculated for both operators for the random sample of five scans. Inter- and intraoperator agreement was assessed by use of Bland-Altman analysis.
In addition, Dice Similarity Coefficients (DSCs) and modified Hausdorff Distances (HDs) were calculated for measurement methods 1 and 2, to assess similarity of the segmentations. The DSC is a measure of spatial overlap of two segmentations, with perfectly overlapping segmentations resulting in a Dice score of 1, and a complete lack of overlap resulting in a Dice score of 011. The HD measures the boundary distance in mm between segmentation contours, with a HD of 0 mm meaning that the two segmentations are identical12. We used a modified HD, using the 95th percentile of the distances over the full segmentation to be less sensitive to outliers13. The amount of disagreement that was considered acceptable between segmentations was judged per case, and was mainly dependent on the size of the ECAA and the spatial resolution of the images.
All data analyses were conducted using SPSS version 25 (IBM Corp. Released 2017. IBM SPSS Statistics for Windows, Version 25.0. Armonk, NY: IBM Corp.).
Results
The median age of the subjects was 51 years (range 36–80 years) at the time of the first CTA scan. 50% of subjects was male, and aneurysms were fusiform in 35% of cases. The median time between the first and second scan was 56 months (range 3–184 months). The median aneurysm diameter reported in the patient records at the time of the first scan was 13 mm (range 9–26 mm) for fusiform aneurysms, and 17 mm (range 7–44 mm) for saccular aneurysms. The time needed to perform the manual segmentations of 40 scans was 32.3 hours, or 49 minutes per scan (as an average of both observers).
Evaluation of the protocol
Interoperator reliability and segmentation similarity
Interoperator reliability scores are summarized in Table 1 and indicate excellent consistency for measurement methods 1 and 2, with all ICCs higher than 0.94. Similarity as measured with the DSC was above 0.88 for method 1 and 2, except for the segmentation “vessel adjacent to aneurysm sac” with a DSC of 0.79. The HD ranged from median 0.801 (interquartile range [IQR] 0.430-7.006) mm for method 1 to median 1.229 (IQR 0.584-2.863) mm for segmentation of the “aneurysm sac”.
Table 1.
Reliability and similarity of both operators for measurement of the entire ICA (method 1), solely the ECAA (method 2), and the three separate segmentations “only vessel”, “aneurysm sac”, and “vessel adjacent to aneurysm sac” (also see Fig. 3).
| ICC (95% CI) | DSC (median, IQR) | HD, mm (median, IQR) | |
|---|---|---|---|
| Method 1 | 0.995 (0.991–0.998) | 0.913 (0.895–0.954) | 0.801 (0.588–1.728) |
| Method 2 | 0.996 (0.993–0.998) | 0.906 (0.860–0.957) | 1.229 (0.663–2.793) |
| Separate segmentations* | |||
| - only vessel | 0.960 (0.926–0.979) | 0.885 (0.857–0.899) | 0.947 (0.631–1.435) |
| - aneurysm sac | 0.996 (0.993–0.998) | 0.903 (0.854–0.955) | 1.229 (0.584–2.863) |
| - vessel adjacent to aneurysm sac | 0.941 (0.891–0.968) | 0.788 (0.736–0.818) | 1.208 (1.033–2.724) |
Reliability was expressed as the Intraclass Correlation Coefficient (ICC), similarity as the Dice Similarity Coefficient (DSC) and modified Hausdorff Distance (HD).
Footnotes: *See Fig. 3 for case example and description of the separate segmentations.
Abbreviations: ICC, intraclass correlation coefficient; DSC, Dice similarity coefficient; HD, modified Hausdorff distance; IQR, interquartile range; CI, confidence interval.
Intraoperator reliability and segmentation similarity
Intraoperator reliability for measurement method 2 was calculated for both operators for the random sample of five ECAAs, and was close to perfect with an average intraoperator reliability of 0.999. Similarity of segmentations as measured with the DSC was above 0.93 for both operators, and HD values were below 1 mm for both operators (data not shown).
Interoperator agreement
Bland-Altman plots were constructed to assess presence of systematic differences between both operators for method 1 and 2 (Fig. 4A,B, Appendix C). All plots reveal approximately normal differences, as 95% of all differences fell within the limits of agreement. However, proportional bias can be observed in all plots except “only vessel”, since the difference in volume increased with the mean volume.
Figure 4.
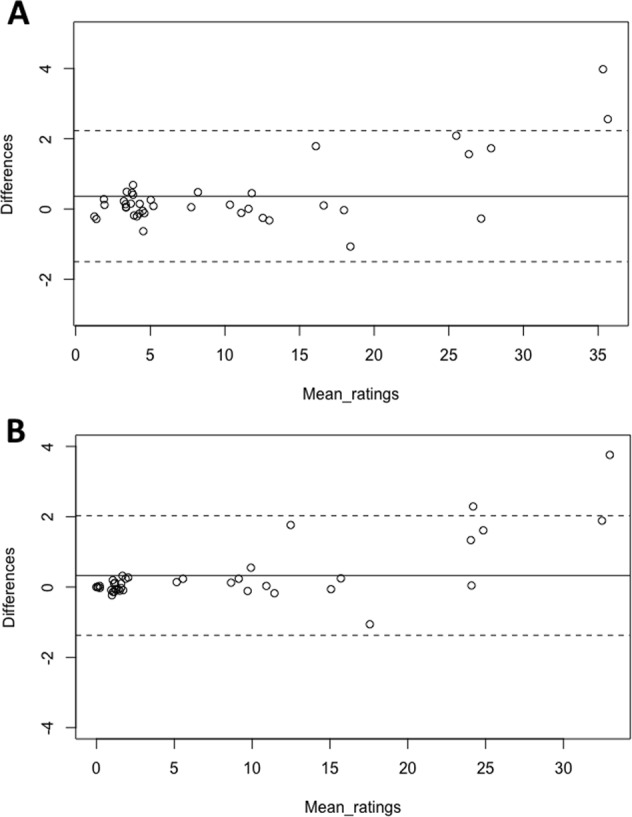
Bland-Altman plots showing agreement of two operators on the volume measurement of 40 scans according to method 1 (A) and method 2 (B). The line in the middle represents the mean difference of the volume (in mL) between the two operators, and the dashed lines represent the upper and lower limits of agreement (mean difference ±1.96 × standard deviation).
The measurements that fell outside the upper limit of agreement were cases 7a and 7b for measurement method 1, and cases 3b and 7b for measurement method 2 (Fig. 4). These cases contained aneurysm sacs with a significant amount of thrombus, which hampered identification of the aneurysm outer wall.
Discussion
In this study, we developed a new protocol for volumetric assessment of ECAAs, and demonstrated that this protocol can be applied in a reliable manner with excellent ICC, DSC and HD values.
The use of volume measurements in addition to diameter measurements for detection of aneurysm growth has been a topic of interest in multiple vascular territories. Literature on abdominal aortic aneurysms showed that volumetric measurements may be superior to conventional diameter measurements for detecting size changes as a follow-up tool after endovascular repair14,15, as well as untreated aneurysm surveillance16. In intracranial aneurysms, a moderate correlation of volume and diameter at best was only found in predominantly saccularly shaped aneurysms, and not at all in fusiform aneurysms17. However, despite abundant evidence, volume assessment of aneurysms over diameter measurements to assess size is still not standard practice in most institutions. This is largely because volume assessment is time consuming, requires dedicated software and skilled technicians, and carries significant operator variability18. Our volume measurement protocol for ECAA was applied by two operators with excellent levels of reliability and agreement, even though the current manual segmentation process was time-consuming. However, it was designed as a step-up towards development of a (semi)automatic volume measurement tool. This future application aims to minimize the time required to perform the volume assessment, and will bypass the need for skilled technicians. It is crucial to develop a robust and feasible tool to monitor the aneurysm sac, by measuring growth and geometrical alterations, and hereby aid in selection of patients who should undergo intervention to prevent future adverse events.
Our protocol comprised two measurement methods, segmentation of the entire ICA and solely the ECAA. The specific segmentation of solely the ECAA was demonstrated to be reproducible with excellent interoperator reliability. Segmentation of the entire ICA had been included in the protocol to overcome expected difficulties in identification of aneurysm starting point and terminus. However, even though in case of fusiform aneurysms the cutoff value of >125% enlargement on axial slices on CTA was pragmatic and might be considered arbitrary, this did not hamper accurate and similar segmentation between operators.
Median HD values were below 1.3 mm for all methods of segmentations, which translated into a difference of 2 mL in volume according to the Bland-Altman plots. The outliers were mostly explained by the two aneurysms which contained a large amount of thrombus alongside the vascular wall. An additional analysis without these two thrombus-containing aneurysms revealed a median HD value of 1.1 mm, and a corresponding difference of 1 mL in volume. This means that the measurement error is 1–2 mL (depending on in- or exclusion of thrombus-containing aneurysms), and that a difference in ECAA volume should be at least 1–2 mL to allow detection with our volume measurement protocol. We consider this difference negligible for all aneurysms irrespective of their initial size, and believe this measurement error is small enough for our protocol to allow adequate detection of ECAA growth.
For this project we had to face several challenges. First, although we use a common definition for ECAA shape, this definition remains somewhat arbitrary, and mixed forms exist next to saccular and fusiform shapes. As our protocol required a different measurement approach for saccular and fusiform aneurysms, it was crucial to establish the ECAA morphology first and start the corresponding measurements thereafter. In addition, even though separate segmentations were reproducible with high ICC, DSC, and HD values (Table 1, Fig. 3), the distinction between ‘aneurysm sac’ and ‘vessel adjacent to aneurysm sac’ might be judged clinically irrelevant. Therefore, both volumes were added up to calculate aneurysm volume (method 2). Second, although significant thrombus formation within an ECAA is relatively rare, some ECAA do contain thrombus which is likely to challenge segmentation of the aneurysm sac (as can be seen in cases 3 and 7 with relatively low interoperator agreements). Therefore, in this stage, our protocol is only workable for ECAA which contain no or minimal thrombus, and future studies need to focus on better visualization techniques to enable volume measurements of the challenging ECAA with significant thrombus. Third, a thick slice 3.0 mm CTA scan is likely to be less accurate than a thin slice 0.6 mm CTA scan for volume measurements, and a discrepancy in slice thickness in two paired scans performed at different time points might introduce bias. Therefore, all CTA scans should have similar thin slice thickness in order to enable accurate conclusions about ECAA growth. Lastly, it is unknown how little changes in head posture during scanning might influence ECAA and ICA volumes. We hypothesize that 3D volume measurements are less sensitive to these postural changes than conventional 2D diameter measurements. Nevertheless, studies are advised to scan patients with their head in locked position to rule out confounding due to different head and neck postures.
Conclusion
This proposed protocol to measure ECAA volumes revealed excellent inter- and intraoperator reliability and agreement, as well as excellent similarity of the segmentations performed by two independent operators. Future volumetric assessments are recommended to be included in standardized imaging protocols, which should also focus on head and neck posture, and visualization of thrombus and vessel wall. These could be used as a step-up to (semi)automatic volumetric measurements to monitor patients with ECAA.
Supplementary information
Author Contributions
Evelien E. de Vries and Constance J.H.C.M. van Laarhoven contributed equally. E.V. and C.L. were responsible for data acquisition and manuscript writing. The expert panel consisted of G.B., C.H., J.H., E.V. and C.L. and as such designed the study protocol. H.K. and M.V. supervised the process of segmentation and the statistical analyses. All authors discussed the results and reviewed the manuscript.
Data Availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request. The in-house developed software is available for research partners on reasonable request.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
E. E. de Vries and C. J. H. C. M. van Laarhoven contributed equally.
Supplementary information
Supplementary information accompanies this paper at 10.1038/s41598-019-44553-0.
References
- 1.Welleweerd JC, et al. Management of Extracranial Carotid Artery Aneurysm. Eur. J. Vasc. Endovasc. Surg. 2015;50:141–147. doi: 10.1016/j.ejvs.2015.05.002. [DOI] [PubMed] [Google Scholar]
- 2.Pourier VEC, De Borst GJ. Which carotid artery aneurysms need to be treated (and how)? J. Cardiovasc. Surg. (Torino). 2016;57:152–157. [PubMed] [Google Scholar]
- 3.Pourier V. E. C., Welleweerd J. C., Kappelle L. J., Rinkel G. J. E., Ruigrok Y. M., Worp H. B., Lo T. H., Bots M. L., Moll F. L., de Borst G. J. Experience of a single center in the conservative approach of 20 consecutive cases of asymptomatic extracranial carotid artery aneurysms. European Journal of Neurology. 2018;25(10):1285–1289. doi: 10.1111/ene.13720. [DOI] [PubMed] [Google Scholar]
- 4.Chaikof EL, et al. The Society for Vascular Surgery practice guidelines on the care of patients with an abdominal aortic aneurysm. J. Vasc. Surg. 2018;67:2–77.e2. doi: 10.1016/j.jvs.2017.10.044. [DOI] [PubMed] [Google Scholar]
- 5.Riambau V, et al. Editor’s Choice – Management of Descending Thoracic Aorta Diseases: Clinical Practice Guidelines of the European Society for Vascular Surgery (ESVS) Eur. J. Vasc. Endovasc. Surg. 2017;53:4–52. doi: 10.1016/j.ejvs.2016.06.005. [DOI] [PubMed] [Google Scholar]
- 6.Wanhainen Anders, Verzini Fabio, Van Herzeele Isabelle, Allaire Eric, Bown Matthew, Cohnert Tina, Dick Florian, van Herwaarden Joost, Karkos Christos, Koelemay Mark, Kölbel Tilo, Loftus Ian, Mani Kevin, Melissano Germano, Powell Janet, Szeberin Zoltán, ESVS Guidelines Committee, de Borst Gert J., Chakfe Nabil, Debus Sebastian, Hinchliffe Rob, Kakkos Stavros, Koncar Igor, Kolh Philippe, Lindholt Jes S., de Vega Melina, Vermassen Frank, Document reviewers, Björck Martin, Cheng Stephen, Dalman Ronald, Davidovic Lazar, Donas Konstantinos, Earnshaw Jonothan, Eckstein Hans-Henning, Golledge Jonathan, Haulon Stephan, Mastracci Tara, Naylor Ross, Ricco Jean-Baptiste, Verhagen Hence. Editor's Choice – European Society for Vascular Surgery (ESVS) 2019 Clinical Practice Guidelines on the Management of Abdominal Aorto-iliac Artery Aneurysms. European Journal of Vascular and Endovascular Surgery. 2019;57(1):8–93. doi: 10.1016/j.ejvs.2018.09.020. [DOI] [PubMed] [Google Scholar]
- 7.Garg K, et al. Presentation and management of carotid artery aneurysms and pseudoaneurysms. J. Vasc. Surg. 2012;55:1618–1622. doi: 10.1016/j.jvs.2011.12.054. [DOI] [PubMed] [Google Scholar]
- 8.Nordanstig J, Gelin J, Jensen N, Osterberg K. National Experience with Extracranial Carotid Artery Aneurysms: Epidemiology, Surgical Treatment Strategy, and Treatment Outcome. Ann. Vasc. Surg. 2014;28:882–886. doi: 10.1016/j.avsg.2013.08.011. [DOI] [PubMed] [Google Scholar]
- 9.Welleweerd JC, et al. Rationale and design of the extracranial carotid artery aneurysm registry. J Cardiovasc Surg. 2015;56:1–27. doi: 10.23736/S0021-9509.16.08637-7. [DOI] [PubMed] [Google Scholar]
- 10.Kottner J, et al. Guidelines for Reporting Reliability and Agreement Studies (GRRAS) were proposed. J Clin Epidemiol. 2011;64:96–106. doi: 10.1016/j.jclinepi.2010.03.002. [DOI] [PubMed] [Google Scholar]
- 11.Dice LR. Measures of the Amount of Ecologic Association Between Species. Ecology. 1945;26:297–302. doi: 10.2307/1932409. [DOI] [Google Scholar]
- 12.Huttenlocher D, Klanderman G, Rucklidge W. Comparing images using the Hausdorff distance. IEEE Trans. Pattern Anal. Mach. Intell. 1993;15:850–863. doi: 10.1109/34.232073. [DOI] [Google Scholar]
- 13.Mendrik AM, et al. MRBrainS Challenge: Online Evaluation Framework for Brain Image Segmentation in 3T MRI Scans. Comput. Intell. Neurosci. 2015;2015:813696. doi: 10.1155/2015/813696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.van Keulen JW, van Prehn J, Prokop M, Moll FL, van Herwaarden JA. Potential value of aneurysm sac volume measurements in addition to diameter measurements after endovascular aneurysm repair. J. Endovasc. Ther. 2009;16:506–13. doi: 10.1583/09-2690.1. [DOI] [PubMed] [Google Scholar]
- 15.Schnitzbauer M, et al. CT after Endovascular Repair of Abdominal Aortic Aneurysms: Diagnostic Accuracy of Diameter Measurements for the Detection of Aneurysm Sac Enlargement. J. Vasc. Interv. Radiol. 2018;29:178–187.e3. doi: 10.1016/j.jvir.2017.09.012. [DOI] [PubMed] [Google Scholar]
- 16.Kontopodis N, et al. Advances in determining abdominal aortic aneurysm size and growth. World J. Radiol. 2016;8:148. doi: 10.4329/wjr.v8.i2.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dengler J, et al. Quantifying unruptured giant intracranial aneurysms by measuring diameter and volume—a comparative analysis of 69 cases. Acta Neurochir. (Wien). 2015;157:361–368. doi: 10.1007/s00701-014-2292-5. [DOI] [PubMed] [Google Scholar]
- 18.Ricco JB, Forbes TL. Trans-Atlantic Debate: External Diameter for Abdominal Aortic Aneurysm (AAA) Size Versus Volume. Eur. J. Vasc. Endovasc. Surg. 2013;46:1. doi: 10.1016/j.ejvs.2013.04.030. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request. The in-house developed software is available for research partners on reasonable request.


