Abstract
Background
White matter hyperintensities (WMH) are frequently observed on T2-weighted brain magnetic resonance imaging studies of healthy older adults and have been linked with impairments in balance, gait, and cognition. Nonetheless, few studies have investigated the longitudinal effects of comorbid WMH on cognition and motor function in Parkinson's disease.
Methods
The Lesion Segmentation Tool for Statistical Parametric Mapping was used to obtain total lesion volume and map regional WMH probabilities in 29 PD and 42 control participants at two study visits 18 months apart. Both cross-sectional and longitudinal comparisons were made between composite scores in the domains of executive function, memory, and language, and Unified Parkinson's Disease Rating Scale (UPDRS) scores.
Results
We found no difference between disease and control groups in total WMH volume or progression during the study. Greater regional and global WMH at baseline was more strongly associated with lower executive function in PD subjects than in controls. Increased regional WMH was also more strongly associated with impaired memory performance in PD relative to controls. Longitudinally, no associations between cognitive change and total or regional WMH progression were detected in either group. A positive relationship between baseline regional WMH and total UPDRS scores was present in the control group, but not PD. However, greater WMH increase was associated with a greater increase in UPDRS motor sub-scores in PD.
Conclusions
These findings suggest that although PD patients do not experience greater mean WMH load than normal aged adults, comorbid WMH do exacerbate cognitive and motor symptoms in PD.
Keywords: Aged, Cognitive impairment, Magnetic resonance imaging, Neuroimaging, Parkinson's disease/pathology*, White matter hyperintensities
Highlights
-
•
PD patients did not demonstrate greater white matter lesion burden at baseline.
-
•
PD patients did not have increased white matter lesion accumulation over 18 months.
-
•
Lesion burden was related to lower executive function and memory in PD patients.
-
•
Greater total lesion accumulation was associated to worsening motor scores in PD.
1. Introduction
White matter hyperintensities (WMH) are areas of high signal intensity on T2-weighted fluid attenuated inversion recovery (T2FLAIR) magnetic resonance imaging (MRI) sequences. WMH increase in frequency and volume with age and are most densely distributed in the deep cerebral white matter (WM) and periventricular regions, progressing from discrete to confluent lesions (de Leeuw et al., 2001; Murray et al., 2010; Yamawaki et al., 2015). Pathological and longitudinal observations, as well as the presence of reduced blood flow in normal appearing WM prior to the formation of WMH (Bernbaum et al., 2015) suggests an ischemic etiology (Hachinski et al., 1987; Wardlaw et al., 2015). It is hypothesized that ultimately an interplay of ischemic, inflammatory, and protein deposition processes contribute to the formation and progression of these lesions. Consequent microglial and endothelial activation, as well as associated blood brain barrier breakdown, may initiate a neuropathological cascade that contributes to neurodegeneration (Shechter and Schwartz, 2013). Hypertension, loss of cerebral autoregulation, and blood pressure (BP) fluctuations have generally been associated with WMH (Mok and Kim, 2015). The clinical consequences of WMH have been debated over the years, but emerging evidence suggests that WMH, along with enlargement of the perivascular spaces, represent a continuum of age- and cerebrovascular-related changes that affect brain function. The relationships between WMH and executive dysfunction, global cognitive impairment, rigidity, falls, and gait and balance disorders in non-Parkinson's disease elderly are well documented (Baezner et al., 2008; Baloh et al., 1995; Camarda et al., 2018; Gunning-Dixon and Raz, 2000; Kerber et al., 1998; Murray et al., 2010; Novak et al., 2009; Prins et al., 2005; Rosano et al., 2007; Tullberg et al., 2004).
Parkinson's disease (PD) is a multi-system disorder that shares some of the cognitive and motor dysfunctions associated with high WMH burden in the non-PD elderly. However, there is lack of consensus about the extent to which comorbid WMH contribute to clinical features in Parkinson's patients. Although early studies reported a greater burden of WMH in PD (Piccini et al., 1995; Stern et al., 1989), later studies suggested not (Acharya et al., 2007; Dalaker et al., 2009a, Dalaker et al., 2009b). Associations between greater burden of WMH in PD patients and axial symptomatology, falls, and the postural instability gait difficulty variant of the disease have been reported (Bohnen and Albin, 2011; Bohnen et al., 2011; Lee et al., 2009). However, these relationships haven't been found in other cohorts (Herman et al., 2013; Sławek et al., 2010). Dysautonomia, a frequent accompaniment of PD, manifested as both orthostatic hypotension and supine hypertension, has been reported to be correlated with WMH in PD in some studies (Oh et al., 2013). Reports disagree regarding the relationship between WMH load and cognitive impairment in PD, and whether WMH load predicts the development of dementia (Burton et al., 2006; Choi et al., 2010; Dalaker et al., 2009a, Dalaker et al., 2009b; González-Redondo et al., 2012; Haugarvoll et al., 2005; Kandiah et al., 2013; Lee et al., 2010; Lee and Lee, 2016; Mak et al., 2015; Santangelo et al., 2010; Sunwoo et al., 2014; Sławek et al., 2008, Sławek et al., 2010, Sławek et al., 2013).
Differences in methodology may explain some of the inconsistent findings in studies on WMH. While automated techniques to measure WMH lesion volume have been developed more recently (Bohnen et al., 2011; de Boer et al., 2009), many previous investigations used semi-quantitative visual rating scales (Brant-Zawadzki et al., 1985; Fazekas et al., 1987; Longstreth et al., 1996; Scheltens et al., 1993). While automated techniques avoid inter-rater variance, the anatomic location of WMH with respect to eloquent connections may not be taken into account. The approach taken in the present study attempts to balance the benefits of both techniques by performing automated total lesion volume quantification as well as voxel-wise analyses of regional relationships based on lesion probability maps (LPMs) generated using the Lesion Segmentation Tool (LST; Schmidt et al., 2012) available for Statistical Parametric Mapping (SPM).
Considering the paucity of longitudinal investigations, as well as the divergence of cross-sectional results regarding the impact of comorbid WMH on cognitive function in PD, the primary aim of this study was to evaluate the relationship between total and regional WMH and cognition in PD, both cross-sectionally and longitudinally. We hypothesized that WMH would adversely affect cerebral reserve, thus producing greater cognitive effects in PD subjects versus controls.
2. Methods
2.1. Study participants
Participants were recruited into the V.A.-sponsored Longitudinal MRI in Parkinson's Disease (LMPD) study through the William S. Middleton V.A. and University of Wisconsin neurology clinics, as well as the Wisconsin Alzheimer's Disease Research Center (ADRC). While a few controls were relatives of PD participants, the majority were participants in ongoing longitudinal studies run by the Wisconsin ADRC, recruited into the LMPD study as controls under a resource use agreement. Participants were screened for significant cognitive impairment or dementia using the Mini-Mental State Examination (MMSE; Folstein et al., 1975; O'Bryant et al., 2008), and those scoring <27/30 were excluded. Additional exclusion criteria included a medical history of major/active cardiovascular, cerebrovascular, or peripheral vascular disease. Participants completed two study visits 18 months apart, each consisting of a clinical and neuropsychological assessment battery, brain MRI, orthostatic blood pressure measurements, and Unified Parkinson's Disease Rating Scale (UPDRS; Fahn and Elton, 1987) scoring by a movement disorders neurologist (C.G.). The UPDRS is an extensive rating scale that consists of sections for (1) mentation, behavior, and mood, (2) self-evaluation of activities of daily living, (3) clinician evaluation of motor function, (4) complications of therapy, (5) modified Hoehn and Yahr staging, and (6) the Schwab and England activities of daily living scale (Fahn and Elton, 1987). PD participants completed study procedures off anti-Parkinson medications for 12–18 h.
Orthostatic blood pressure was collected as an index of dysautonomia, a frequent symptom of PD that has been associated with WMH (Oh et al., 2013). Blood pressure (BP) was measured using an automated cuff, after participants had been supine in the MRI scanner for 1 h, and again after 3 min standing. A drop in systolic blood pressure of ≥20 mmHg between supine and standing positions was considered indicative of orthostatic hypotension (OH). Mean arterial pressure, supine and standing, was calculated as follows: MAP = (Systolic BP + 2*Diastolic BP)/3.
Of the 85 participants who completed the baseline visit, 71 (29 PD and 42 controls) completed the 18-month follow-up visit with good quality clinical and imaging data and were included in the analyses. Informed consent was obtained from all study participants, and all study procedures were approved by the University of Wisconsin Institutional Review Board and the Research and Development committee of the William S. Middleton Memorial Veterans Hospital. All procedures were in accordance with the Helsinki Declaration of 1975.
2.2. Neuropsychological assessments
Neuropsychological testing was conducted immediately prior to brain MRI, with PD participants in the off-medication state. The cognitive battery was designed to evaluate performance within the domains of executive function, memory, and language and included category (Goodglass and Kaplan, 1972; Rosen, 1980) and phonemic fluency (C-F-L form; Benton and Hamsher, 1976) tests, Trail Making Tests A and B (Reitan and Wolfson, 1993), Wisconsin Card Sort Test – 64 (WCST-64; Grant and Berg, 1948; Heaton et al., 1993), Hopkins Verbal Learning Test (HVLT; Benedict et al., 1998; Brandt, 1991), and Boston Naming Test (BNT; Kaplan et al., 1983). The Wide Range Achievement Test – Fourth Edition (WRAT-IV) reading test (Wilkinson and Robertson, 2006) was used in lieu of years of formal education, as an index of quality of educational attainment (Manly et al., 2002; Rohit et al., 2007; Sayegh et al., 2014).
To improve the stability of measures within cognitive domains, individual task scores were combined to create composite measures for executive function, memory, and language. To accomplish this, subject- and visit-specific individual task scores (x) were scaled according to a cognitively normal baseline frame of reference using the baseline control group mean (μc) and standard deviation (σc) to generate a z-score as follows: [z-score = (x-μc)/σc]. Individual z-scores were then combined as follows: Executive composite = [(Category Fluency + WCST-64 categories completed – {Trails Part B – Trails Part A time})/3]; Memory composite = [(HVLT delayed recall + recognition discrimination index)/2]; Language composite = [(BNT + Phonemic Fluency)/2]. Change within each measure was calculated by taking the difference between baseline and follow-up z-scores. In the case of language, only baseline and follow-up phonemic fluency scores were included in this calculation in cases where raw BNT change was less than the reliable change index of 6 (Sachs et al., 2012).
Due to the known relationship between depression and cognitive impairment in both PD and non-PD elderly (Morimoto et al., 2014; Tröster et al., 1995), the Geriatric Depression Scale (GDS-30; Yesavage et al., 1982) was obtained to investigate whether groups differed based on depression status, and thus, if our analyses required correction for the confounding effect depression may have on group comparisons of cognitive performance. In both PD and non-PD elderly, major depressive disorder (MDD) can be indicated by a GDS score of 9 or greater (Schrag et al., 2007; Yesavage et al., 1982). Based on this criterion, 7 PD and 3 control participants met GDS cutoff for depression at baseline. The ratio of participants with depression did not differ between groups (p > .05, via Fisher's Exact Test), and therefore, the use of depression status as a covariate was not indicated.
2.3. Brain image acquisition
Brain MRIs were acquired on a GE 750 Discovery 3 T MRI system (General Electric Healthcare, Waukesha, WI) with an eight-channel phased array head coil. The same scanner and sequences were used at the baseline and follow-up visits. For WMH segmentation, two structural sequences, a high resolution 3D brain volumetric (BRAVO) T1-weighted inversion prepared sequence of inversion time (TI) = 450 ms, repetition time (TR) = 8.2 ms, echo time (TE) = 3.2 ms, flip angle = 12°, acquisition matrix = 256 × 256, field of view (FOV) = 256 mm, and slice thickness = 1.0 mm collected in the axial plane, and a 3D T2-weighted fluid-attenuated inversion recovery (T2FLAIR) sequence in the sagittal plane of TR = 6000 ms, TE = 124 ms, TI = 1867 ms, flip angle = 90°, acquisition matrix = 256 × 256, FOV = 256 mm, and slice thickness = 2.0 mm, no gap, yielding a 1 × 1 × 2 mm voxel resolution, were acquired.
2.4. Intracranial volume calculation
Intracranial volume (ICV; mL) was calculated for the purpose of scaling WMH volume to head size from T1-weighted images according to the reverse brain masking method (Keihaninejad et al., 2010).
2.5. White matter hyperintensity segmentation
Total volume of WMH was calculated from each subject's baseline and follow-up images using the Lesion Segmentation Tool, version 2.0.15 (LST; Schmidt et al., 2012) for SPM12, which has been used previously by our group (Birdsill et al., 2014; Pozorski et al., 2018). During this process, lesions are seeded based on spatial and intensity probabilities from the T1 images and hyperintense outliers on T2FLAIR images. The initial threshold was set at 0.3 to create a conservative binary lesion map, from which a growth algorithm (Schmidt et al., 2012) enlarges these seeds to grow lesion probability maps (LPMs) along hyperintense T2FLAIR voxels. The LPMs were then visually inspected against the T2FLAIR images to ensure accurate capture of WMHs.
2.6. Longitudinal mapping
To ensure congruence between the LPMs at each time point, the longitudinal LST pipeline was then employed (Schmidt et al., 2012; https://www.applied-statistics.de/lst.html). The pipeline proceeds by comparing all consecutive time points (T2FLAIR images and lesion probability maps) in an iterative manner, determining if changes in lesion structure are significant or due to “natural variations of the T2FLAIR signal.” In areas originally labeled as lesions by the LGA at either time point, non-significant differences of the T2FLAIR signal are labeled as lesions in both probability maps. Conversely, significantly different T2FLAIR signals between time points are labeled as lesions only in the respective time point. Therefore, lesion structure change is only considered significant in regions where T2FLAIR signal is significantly different between timepoints, and thus, the longitudinal LST pipeline produces “corrected” LPMs that may differ from the original maps segmented based on a single time point. Then, each participant's corrected baseline LPM was subtracted from the corresponding follow-up LPM to create lesion change maps. Subsequently, the baseline LPM, lesion change maps, and T2FLAIR images were normalized to Montreal Neurological Institute (MNI) standard space by applying the deformation field produced by coregistering the baseline T1 image to standard space. Both baseline and change LPMs were smoothed with an 8-mm Gaussian Kernel. All LPMs were visually inspected against their respective T2FLAIR images for accuracy following each step.
To compare total WMH burden between groups and to evaluate its association with clinical measures, subject- and visit-specific total lesion volumes (TLVs; mL) were extracted from corrected LPMs generated using the longitudinal LST pipeline (Schmidt et al., 2012). TLVs were divided by the corresponding ICV and multiplied by 100 to convert them into a WMH ratio (WMHr; Birdsill et al., 2014; Pozorski et al., 2018) in units of percent ICV. Since the WMHr variable showed significant skewedness, it was log transformed and added to a constant of 1 to produce uniformly positive values (Birdsill et al., 2014). The resulting value of WMH [log10(WMHr) + 1] was used for analysis. To calculate within-subject WMH change, total baseline WMH was subtracted from follow-up.
2.7. Statistics
2.7.1. Cross-sectional analyses of baseline data
Statistical analysis was completed using International Business Machine's Statistical Program for the Social Sciences (IBM SPSS; Version 22.0). Comparisons between PD and control groups were carried out using two-tailed independent samples t-tests, Mann-Whitney U tests for non-normally distributed data, determined via Shapiro-Wilk tests, and one-way analysis of covariance (ANCOVA). Dichotomous variables such as sex were evaluated using Fisher's exact test.
Group differences in the relationship between WMH and cognitive measures or UPDRS scores were evaluated in regression models with the cognitive index or UPDRS score as the dependent variable, and group (PD versus control), WMH, and group-by-WMH as explanatory terms. Non-significant between-group regressions were followed up with post hoc, within-group regressions to detect situations in which a similar, significant relationship between WMH and the cognitive variable was present in both groups, leading to a non-significant interaction effect. Age, sex, and education were included as covariates for all tests. Supine MAP was also included as a covariate due to the known relationship of WMH to blood pressure, as well as previous reports of an additional relationship between WMH and supine BP in PD (Oh et al., 2013). In addition, smoking has been found to be related with increased WMH burden (Habes et al., 2016; Power et al., 2015); therefore, pack-years (pack-years = average number of packs smoked per day * years smoked) was also included as a covariate.
Significance was determined according to the Holm-Bonferroni procedure. Planned comparisons, including group comparisons of total baseline WMH burden and between-group regression results, were considered significant at p < .05, as they were the primary study outcome and should be evaluated at the most sensitive level (Holm, 1979). Post hoc comparisons, including within-group regressions, were corrected for multiple comparisons according to the Holm-Bonferroni procedure at an alpha of 0.05.
Voxel-wise analyses of the normalized LPMs were conducted in SPM12. An independent samples t-test was performed to investigate group differences in regional WMH burden. Regression models were used to evaluate where the relationship between WMH and cognition differed by group: WMH probability maps were modeled as the dependent variable and group, cognitive index, and group-by-cognitive index interaction terms were the independent variables. Age, sex, education, supine MAP, and pack-years were included as covariates. In the case of a null result, within-group regressions were conducted to detect similar relationships between WMH and cognition in both groups. To restrict the analyses to WM, a mask produced by binarizing the ICBM Tissue Probability Atlas WM map at a threshold of 0.3 was applied as an explicit mask. To control for multiple comparisons, Monte Carlo simulations with 10,000 iterations were performed using Analysis of Functional NeuroImages (AFNI; Version 18.1.18 3dClustSim; Cox, 1996; Saad and Reynolds, 2012) to compute the voxel-probability and minimum cluster-extent threshold needed to obtain a 0.05 familywise alpha with the original uncorrected per-voxel threshold of p < .001. A minimum cluster-extent of 300 contiguous voxels was determined to be significant (p < .05, cluster-corrected). The Johns Hopkins University (JHU) ICBM DTI-81 WM labels atlas (Hua et al., 2008; Mori et al., 2005; Wakana et al., 2007) and MRI Atlas of Human White Matter (Oishi et al., 2011) were utilized to identify regions of association.
2.7.2. Longitudinal comparisons
Relationships between total WMH volume change (WMHΔ) and change in UPDRS scores or neuropsychological measures were evaluated using similar regression models to those used for the cross-sectional data, substituting WMH volume change and clinical change as the variables of interest, while controlling for age at follow-up, sex, education, time between study visits (years), supine MAP (averaged across the two visits), and pack-years. Similar to the cross-sectional data, planned comparisons were significant at p < .05 and post hoc comparisons were corrected for multiple comparisons according to the Holm-Bonferroni procedure with an alpha = 0.05.
As with the cross-sectional data, voxel-wise analyses were conducted in SPM12, with LPM change maps as the dependent outcome and neuropsychological change scores as the explanatory variables. Age, sex, education, supine MAP, time between visits, and pack-years were included as covariates. Results were restricted to WM with a minimum cluster-extent of 300 contiguous voxels considered significant (p < .05, cluster-corrected).
3. Results
3.1. Demographics and group characteristics
Demographic and disease characteristics of the PD and control groups are summarized in Table 1. Age, sex, quality of education (WRAT-IV), and time between study visits did not differ between groups. PD participants' mean age at diagnosis was 62.8 ± 9.2 years, with an average disease duration of 3.8 ± 3.3 years at baseline. All but two of the PD participants were taking anti-Parkinson medications (dopamine agonists, levodopa, amantadine, and/or monoamine oxidase inhibitors) at the time of study enrollment. As expected, PD participants had higher scores on the total and motor sub-section of the UPDRS at baseline, and significantly greater increases on both measures longitudinally. In terms of cerebrovascular risk factors, PD participants had higher MAP in both supine and standing positions at baseline, as well as higher standing MAP averaged across the two study visits. However, average body mass index and the proportion of participants with a diagnosis of hypertension, coronary artery disease, peripheral artery disease, and controlled diabetes did not differ between groups, while a greater proportion of control participants had a history of smoking. The proportion of current smokers did not differ between groups, nor did the average pack-years for participants with a history of tobacco use.
Table 1.
Participant characteristics.
| Control (N = 42) | PD (N = 29) | p-Value | |
|---|---|---|---|
| Age (years) Baseline | 66.4 ± 7.7 | 66.6 ± 9.7 | 0.946 |
| Time between visits (years) | 1.5 ± 0.1 | 1.5 ± 0.1 | 0.159 |
| Sex (Male/Female) | 30/12 | 24/5 | 0.397a |
| WRAT-IVBaseline | 62.2 ± 3.7 | 61.6 ± 5.3 | 0.765b |
| GDS > 9Baseline | 3/39 | 7/22 | 0.079a |
| Standing MAP (mmHg) Baseline | 102.9 ± 13.0 | 111.3 ± 13.2 | 0.010⁎ |
| Supine MAP (mmHg) Baseline | 96.9 ± 10.5 | 103.9 ± 13.2 | 0.016⁎ |
| Standing MAP (mmHg) Average | 103.4 ± 12.6 | 109.9 ± 12.3 | 0.033⁎ |
| Supine MAP (mmHg) Average | 98.5 ± 11.0 | 103.3 ± 12.6 | 0.090 |
| Hypertension (yes/no) | 8/34 | 10/19 | 0.171a |
| Orthostatic hypotension (yes/no) | 8/34 | 6/23 | 1.000a |
| Coronary artery disease (yes/no) | 2/40 | 0/29 | 0.510a |
| Peripheral vascular disease (yes/no) | 0/40 | 0/29 | 1.000a |
| History of smoking (yes/no) | 26/16 | 9/20 | 0.016⁎,a |
| Current smoker (yes/no) | 2/40 | 0/29 | 0.510a |
| Pack-years of ever smokers Baseline | 12.3 ± 13.0 | 13.7 ± 10.7 | 0.540b |
| Body mass index (kg/m2) | 27.0 ± 4.2 | 26.6 ± 4.1 | 0.683 |
| Adult onset diabetes (yes/no) | 2/40 | 1/28 | 1.000a |
| Age at diagnosis (years) | N/A | 62.8 ± 9.2 | N/A |
| Side of onset (R/L/S) | N/A | 16/10/3 | N/A |
| Disease duration (years) | N/A | 3.8 ± 3.3 | N/A |
| UPDRS total Baseline | 3.2 ± 3.3 | 35.1 ± 14.4 | <0.001⁎, b |
| UPDRS motor sub-score Baseline | 1.5 ± 1.7 | 20.5 ± 11.0 | <0.001⁎, b |
| UPDRS total ∆ | 0.8 ± 2.8 | 6.8 ± 11.3 | 0.021⁎, b |
| UPDRS motor sub-score ∆ | 0.3 ± 1.7 | 3.3 ± 6.8 | 0.024⁎, b |
Both baseline values (mean ± SD) and change (∆) over the 18-month interval are shown.
P-values for between group comparisons were derived via two-tailed independent samples t-test unless noted.
Abbreviations: GDS; Geriatric Depression Scale; L, left; MAP, mean arterial pressure; MMSE, Mini-Mental State Examination; N/A, not applicable; PD, Parkinson's disease; R, right; S, symmetrical; UPDRS, Unified Parkinson's Disease Rating Scale; WRAT-IV, Wide Range Achievement Test – Fourth Edition word reading sub scale.
Fisher's Exact Probability test.
Mann-Whitney U test.
P-values <.05 were considered significant.
3.2. Neuropsychological test performance
Mean baseline neuropsychological test scores and change in performance during the 18-month study are summarized in Table 2. In comparison to the control baseline, the PD group had significantly lower performance on composite measures of both executive function and memory. PD subjects had greater decline in executive function, as well as a trend toward greater decline in memory performance during the study. Groups did not differ in baseline or change in language performance.
Table 2.
Neuropsychological composite scores.
| Control (N = 42) | PD (N = 29) | p-value | |
|---|---|---|---|
| Executive Baseline | 0.1 ± 0.6 | −0.7 ± 1.1 | 0.003⁎,b |
| Executive ∆ | 0.0 ± 0.5 | −0.4 ± 0.9 | 0.045⁎ |
| Memory Baseline | 0.1 ± 0.8 | −0.9 ± 1.4 | 0.002⁎,b |
| Memory ∆ | 0.3 ± 0.7 | −0.2 ± 0.9 | 0.057b |
| Language Baseline | 0.1 ± 0.8 | −0.2 ± 0.9 | 0.231 |
| Language ∆ | 0.1 ± 0.7 | 0.1 ± 0.9 | 0.794 |
Composite z-scores at baseline (mean ± SD) and change (∆) over the 18-month interval are shown.
P-values for between group comparisons were derived via two-tailed independent samples t-test unless noted.
Mann-Whitney U test.
P-values <.05 were considered significant.
3.3. Total WMH volume
Total WMH did not differ by group at baseline when controlling for age, sex, education, supine MAP, and pack-years (Table 3). The results of regression analyses testing for differential group associations between total WMH burden at baseline and cognitive scores are summarized in Table 4, while post-hoc regressions results are available in Supplemental Table 1. There was a significant group-by-WMH interaction for executive function – higher WMH was related to lower executive performance in both groups, but the relationship was stronger in PD. There was a trend toward a greater degree of memory function impairment with increasing WMH volume in PD compared to controls (p = .085), but no relationship between WMH and language performance nor UPDRS scores in either group at baseline.
Table 3.
Total WMH volume comparison.
| Control (N = 42) | PD (N = 29) | p-value | |
|---|---|---|---|
| WMH Baseline | 0.984 ± 0.260 | 1.054 ± 0.317 | 0.517 |
| WMH ∆ | 0.015 ± 0.046 | 0.024 ± 0.061 | 0.707 |
Both baseline WMH (mean ± SD) and change (∆) over the 18-month interval are shown.
P-values for between group comparisons were derived via one-way ANCOVA controlling for age, sex, education, supine MAP, and pack-years. Time between visits was also included as a covariate when comparing WMH change over the study interval.
Abbreviations: PD, Parkinson's disease; WMH, total white matter hyperintensity volume.
P-values <.05 were considered significant.
Table 4.
Differential associations between cognitive or UPDRS scores and WMH in PD versus control groups.⁎
| Task | Group interaction (N = 71) |
|
|---|---|---|
| β | p-Value | |
| Executive Baseline | −0.775 | 0.026⁎ |
| Executive Δ | −0.102 | 0.559 |
| Memory Baseline | −0.811 | 0.085 |
| Memory Δ | −0.161 | 0.405 |
| Language Baseline | −0.018 | 0.969 |
| Language Δ | −0.254 | 0.194 |
| UPDRS total Baseline | −0.088 | 0.737 |
| UPDRS total ∆ | 0.243 | 0.135 |
| UPDRS motor sub-score Baseline | 0.169 | 0.588 |
| UPDRS motor sub-score ∆ | 0.421 | 0.010⁎ |
Summary of results of regression analyses designed to evaluate differences between PD and control groups in the relationship between the cognitive index and WMH burden at baseline and over the study interval (Δ). The standardized regression coefficient (β) for the interaction term and its associated p-value are shown.
P-values <.05 were considered significant.
3.4. Longitudinal WMH volume change
Total WMH volume change over the 18-month study did not differ between groups when controlling for age, sex, education, average supine MAP, time between visit, and pack-years (Table 3). No associations between cognitive change and WMHΔ were detected (Table 4 & Supplemental Table 1). There was a significant interaction effect for group-by-WMHΔ on UPDRS motor sub-score change – greater WMH accumulation was associated with increased UPDRS motor sub-scores over the 18-month period in the PD but not the control group (β = 0.423, p = .010).
3.5. Cross-sectional voxel-wise analyses
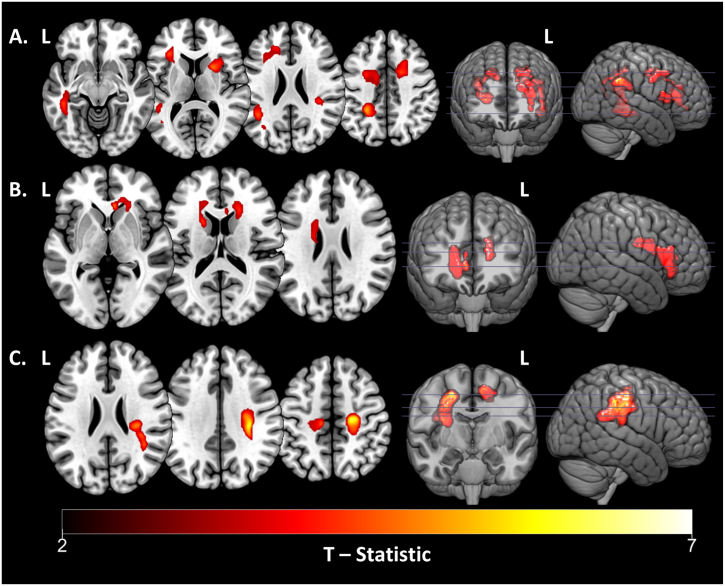
The results of voxel-wise linear regression analyses of baseline WMH probability maps are summarized in Table 5 and visualized in Fig. 1. There was no group difference in the spatial distribution of WMH at baseline when controlling for age, sex, education, supine MAP, and pack years. The PD group showed a stronger relationship (significant interaction term) between lower baseline executive function and greater WMH probabilities within the WM of the right angular gyrus, left superior parietal lobule, left angular gyrus, left sagittal stratum, right anterior limb of the internal capsule, left inferior frontal gyrus, left anterior corona radiata, right cingulum, and right superior and middle frontal gyri compared to controls (Fig. 1A). Similarly, there was a more significant association between lower memory performance and higher WMH probabilities within the right genu of the corpus callosum, right anterior corona radiata, left anterior limb of the internal capsule, and left superior and anterior corona radiata in PD subjects compared with controls (Fig. 1B). No differential associations were observed between groups in regards to the spatial distribution of WMH probabilities and baseline language composite measures or UPDRS scores.
Table 5.
Baseline voxel-wise results for within- and between-group analyses.
| Task | Relationship Direction | MNI Coordinates |
Peak T | Cluster Extent | Location | ||
|---|---|---|---|---|---|---|---|
| x | Y | Z | |||||
| Group interaction, N = 71 | |||||||
| Executive Function Composite | Negative Interaction | 38 | −46 | 32 | 5.73 | 310 | R. angular gyrus WM |
| −26 | −44 | 42 | 5.20 | 1475 | L. sup. parietal lobule WM, L. angular gyrus WM, L. sagittal stratum | ||
| −42 | −50 | 24 | 4.42 | ||||
| −44 | −36 | −10 | 4.33 | ||||
| 24 | 10 | 10 | 4.74 | 305 | R. anterior limb of internal capsule | ||
| −32 | 28 | 10 | 4.43 | 655 | L. inferior frontal gyrus WM, L. anterior corona radiata |
||
| −32 | 32 | 2 | 3.97 | ||||
| −18 | 34 | 24 | 3.83 | ||||
| 12 | −2 | 36 | 4.36 | 444 | R. cingulum, R. sup. Frontal gyrus WM | ||
| 18 | 4 | 42 | 4.33 | ||||
| 22 | 14 | 42 | 4.01 | ||||
| −26 | 0 | 42 | 4.08 | 338 | R. middle frontal gyrus WM, R. superior frontal gyrus WM | ||
| −14 | −8 | 44 | 3.74 | ||||
| −24 | −8 | 46 | 3.55 | ||||
| Memory Composite | Negative Interaction | 6 | 28 | 0 | 4.90 | 618 | R. genu of corpus callosum, R. anterior corona radiata |
| 20 | 24 | 14 | 4.12 | ||||
| 6 | 22 | 14 | 3.80 | ||||
| −20 | 10 | 18 | 4.23 | 407 | L. anterior limb of internal capsule, L. sup. Corona radiata, L. anterior corona radiata | ||
| −20 | −6 | 26 | 3.81 | ||||
| −18 | 30 | 16 | 3.56 | ||||
| Control Group, N = 42 (Post hoc) | |||||||
| UPDRS Total Score | Positive Relationship | 22 | −18 | 48 | 6.20 | 1401 | R. precentral gyrus WM, R. sup. Long. fasciculus |
| 32 | −24 | 34 | 6.03 | ||||
| 34 | −34 | 30 | 4.94 | ||||
| −12 | −20 | 52 | 5.12 | 313 | L. precentral gyrus WM, L. postcentral gyrus WM | ||
| −18 | −34 | 44 | 4.25 | ||||
| −26 | −22 | 50 | 3.54 | ||||
The results of voxel-wise regressions testing for associations between baseline regional WMH probabilities and neuropsychological measures or UPDRS scores, while controlling for age, sex, WRAT-IV, and supine MAP. Significant results of the interaction effects are shown in the upper panels, while post hoc within-group analyses are shown in the lower panels. Contiguous clusters of 300 or more voxels were significant (p < .05, cluster-corrected).
Abbreviations: Ant, anterior; L, left; Long, longitudinal; MNI, Montreal Neurological Institute; R, right; Sup, superior; UPDRS, Unified Parkinson's Disease Rating Scale; WM, white matter.
Fig. 1.
Cross-sectional voxel-wise results. Voxel-wise analyses of baseline WMH probability maps showing areas of significant group-by-task interaction effect for greater WMH probability and lower task performance within PD subjects relative to controls for A, executive function; and B, memory. C. Results of post hoc within group regression, displaying regions of significant relationship between higher WMH probability and higher total UPDRS scores in the control group at baseline. Peak T-statistics (red-yellow) for result clusters (p < .05, cluster-corrected) are shown. Result clusters are overlaid on the “mni152_2009_256” MNI space brain template using MRIcroGL (http://www.mccauslandcenter.sc.edu/mricrogl/). Magnitude (peak T-statistics), MNI coordinates, and anatomical locations of result clusters are given in Table 5.
Abbreviations: L, left; MNI, Montreal Neurological Institute; PD, Parkinson's disease; UPDRS, Unified Parkinson's Disease Rating Scale; WMH, white matter hyperintensities.
Post hoc within group analyses revealed that in controls, there was a positive association between total UPDRS scores and WMH within white matter of the bilateral precentral gyri, right superior longitudinal fasciculus, and left postcentral gyrus (Fig. 1C). There was no cross-sectional relationship between regional WMH and UPDRS scores in PD.
3.6. Longitudinal voxel-wise analyses
There was no detectable difference between disease and control groups in the rate of accumulation of WMH over the 18-month study. In addition, there were no significant regional associations between spatial WMH progression and cognitive or UPDRS score change in either group.
4. Discussion
This longitudinal MRI study used an automated WM lesion segmentation tool to compare baseline total and regional WMH burden between 29 PD patients and 42 age-matched controls, as well as WMH change over an 18-month period. In addition, we examined the degree of association between WMH and measures of cognition and motor function. These analyses revealed several findings: First, total WMH volume at baseline and change over 18 months did not differ between PD and control groups, despite some differences in risk factors such as MAP and smoking history. Second, baseline WMH volume was significantly associated with lower executive function performance in PD patients, compared to controls. In the voxel-wise analyses, impaired executive function in PD was associated with bilateral frontal and parietal subcortical WMH, whereas impaired memory was associated with higher WMH probability in genu of corpus callosum and adjacent WM. We did not detect effects of WMH accumulation on cognitive change over 18 months. We hypothesize that these findings may represent a limitation of our relatively small sample size, and from the slow rate of WMH accumulation, as well as the patchy, random spatial distribution of WMH formation.
We did observe associations between WMH and UPDRS scores. In the control group, greater WMH within the bilateral precentral and left postcentral gyri white matter was associated with higher (more abnormal) UPDRS scores at baseline. In PD subjects, greater longitudinal increase in total WMH volume was associated with greater increases in UPDRS motor sub-scores.
Regarding whether Parkinson's patients have a higher average incidence of WMH than controls, this study is in agreement with the majority of recent reports, which suggest no disease-specific difference in WMH volume in comparison to normal aging. An initial study by Piccini et al. (1995), using a quantitative visual rating scale (Fazekas et al., 1987), reported a higher frequency and extent of periventricular hyperintensities in a group of 102 non-demented PD patients when compared to 68 sex- and age-matched controls, especially within PD patients of a high disease progression index. Later, a large cross-sectional study comparing total and regional WMH burden between 163 newly diagnosed, drug-naïve PD patients and 102 age-matched controls, found no difference in the total volume or spatial distribution of WMH, nor between groups when split into 102 normal controls, 30 PD patients with mild cognitive impairment (MCI), and 133 non-MCI PD (Dalaker et al., 2009b). While few longitudinal studies have evaluated the rate of WMH progression in PD, a 1-year MRI study (Burton et al., 2006) of 14 dementia with Lewy bodies (DLB), 13 Parkinson's disease with dementia (PDD), 23 Alzheimer's disease (AD), and 33 age-matched adults reported no significant difference in the rate of WMH change between groups. Disparities in these reports may in part be due to the use of manual semi-quantitative visual rating scales, in comparison to the semi- and fully-automated quantification techniques currently available. Taken together with these larger studies, the present study supports the conclusion that PD patients do not on average experience a greater volume or rate of WMH progression relative to otherwise healthy aging adults, especially when quantified using automated techniques.
Although cerebral WM changes are common in aging populations, the degree to which they are associated with cognitive and motor symptoms varies between individuals, and may be mediated by protective factors that influence cerebral reserve such as education (Blume et al., 2017) and cardiorespiratory fitness (Johnson et al., 2012; Vesperman et al., 2018). Parkinson's disease, which causes underlying dysfunction of frontal-striatal circuits, represents a preexisting strike against cognitive reserve. This could be the reason we found significantly stronger relationships between total WMH and both executive function and memory performance in the PD group relative to the control group. In addition, the voxel-wise results demonstrated the critical WM changes occupy diffuse subcortical regions neuroanatomically related to executive processing (bilateral frontal and parietal subcortical WM). With respect to memory function, the voxel-wise analysis suggested that this effect was localized to the genu of corpus callosum, anterior limb of the internal capsule, and anterior and superior corona radiata. While these regions are not always considered to be specifically associated with memory encoding, disruption of the Papez circuit has been linked with impairment in mnemonic functions, and more precisely, spatial and episodic memory dysfunction (Aggleton and Brown, 1999; Papez, 1995; Vertes et al., 2001), which is commensurate with our findings.
Whether WMH influence cognitive decline in PD remains controversial. In relatively large cross-sectional studies, PD patients with dementia and MCI have been found to have a larger average burden of WMH than their non-impaired counterparts (Kandiah et al., 2013; Lee et al., 2010). In a voxel-wise analysis of 90 non-demented PD patients, lower scores on both the Frontal Assessment Battery and Category Fluency Test were related to increased periventricular WMH probabilities (Mak et al., 2015). Two multi-center studies from the Norwegian ParkWest project found WMH volume to be negatively correlated with attention/executive function in both PD and control groups (Dalaker et al., 2009b), and negatively correlated with memory in PD (Dalaker et al., 2009a) – results similar to the present study. However, these relationships did not remain significant in regression analyses controlling for other relevant explanatory variables (Dalaker et al., 2009a, Dalaker et al., 2009b). In regards to the impact of comorbid WMH on cognition in PD, our findings provide support for the exacerbation of executive function and memory decline in PD relative to otherwise healthy aging adults.
Few studies have evaluated the cognitive effects of WMH longitudinally in PD. One study reported no association between changes in measures of global cognition (MMSE and the Cambridge Cognitive Examination) and rates of WMH change in 13 PDD patients (Burton et al., 2006); another that used the Scheltens rating scale (Scheltens et al., 1993) to compare WMH burden between 39 PD non-MCI, 46 PD-MCI, and 26 PDD (González-Redondo et al., 2012), reported no difference in total WMH burden between the three groups at baseline, but noted that periventricular WMH progression at the 30-month follow-up was associated with conversion to dementia. In another study in which baseline WMH volume was quantified using an automated method and conversion rates of 46 PD non-MCI and 65 PD-MCI were tracked over 24 months (Sunwoo et al., 2014), WMH burden at baseline was a predictor of conversion from PD-MCI to PDD (Sunwoo et al., 2014). Conversely, in a retrospective study of 132 PD patients, Lee and Lee (2016) reported that the ability to predict conversion to PDD with baseline WMH burden was attenuated when adjusted for confounders and could be more reliably predicted based on postural instability. Longitudinal analyses in the present study suggest that decline in executive function and memory in PD is independent of total and regional WMH progression; however, the small sample size and patchy nature of lesion accumulation may have limited our study's power.
In terms of symptom severity and motor abnormalities, this study demonstrated two notable relationships with WMH. First, higher total UPDRS scores in controls were associated with higher WMH probability within white matter of bilateral precentral gyri, left postcentral gyrus, and right superior longitudinal fasciculus. These results are commensurate with growing evidence linking impairments in balance, gait, mood, and cognition with WMH burden in otherwise healthy aging adults (Baezner et al., 2008; Baloh et al., 1995; Camarda et al., 2018; de Leeuw et al., 2001; Gunning-Dixon and Raz, 2000; Kerber et al., 1998; Novak et al., 2009; Prins et al., 2005; Rosano et al., 2007; Tullberg et al., 2004; Yamawaki et al., 2015). Second, within the PD group, our findings that greater WMH volume change predicted a greater increase in UPDRS motor scores are consistent with previous cross-sectional associations with higher progression index (Piccini et al., 1995), and a previous report of correlation between the sum of gait, posture, and postural stability UPDRS items and WMH burden in multiple subcortical regions (Murray et al., 2010).
While greater WMH accumulation over the study interval was associated with higher (worsening) UPDRS motor sub-scores in the PD group, it is somewhat surprising that baseline WMH burden was not related with either UPDRS total nor motor scores in PD, as it was in controls. We suspect that in PD, UPDRS scores are largely determined by severity of symptoms related to Parkinson's pathology rather than symptoms related to WMH, whereas in controls, WMH are associated with subtle motor symptoms and depression, as has been supported by other studies (Baezner et al., 2008; Murray et al., 2010; Yamawaki et al., 2015). Perhaps this allows for the detection of more subtle manifestations of WMH burden in normal aging adults that may be “masked” by the more pronounced symptoms of the disease process in PD sufferers, and thus, explain the observed association in controls, and lack thereof, in patients.
5. Limitations
The sample size for this study was relatively small, which limited the statistical power of the analyses and our ability to detect differences in WMH progression over time. In addition, due to patient withdrawal (health or personal complications), participant attrition between study visits was greater in the PD group than the control group, which may have limited the representation of PD patients with more rapid motor progression, cognitive decline, or WMH accumulation over the study interval. Furthermore, the 18-month study duration may have been too short to allow for measurable differences in regional WMH progression, and thus, account for the lack of observed differences in WMH accumulation between groups and the absence of relationships among cognitive change and alterations in the spatial distribution of WMH over the course of the study. It's also worth noting that there were 3 MRI scanner software updates over the course of the study. While there was no significant difference in WMH burden or progression based on software version in the present study, this is an additional detail that might be crucial in maintaining constancy in future longitudinal studies. Finally, despite including pack-years as a covariate in our analyses, the increased prevalence of smokers in the control group may have artificially increased the WMH burden in this sample, and consequently, obscured the true group difference in lesion load and progression.
6. Conclusion
In conclusion, the present study is one of the first to investigate both total and regional WMH burden in PD, cross-sectionally and longitudinally. Together with evidence from previous investigations, our findings suggest that WMH volume and distribution does not differ between PD patients and otherwise healthy aging adults, but can provide a “second hit” on PD patients with compromised cognitive reserve, making the emergence of cognitive, behavioral, and motor deficits more likely in the PD population than in healthy controls. The relationship between memory encoding deficits and WMH in PD, demonstrated in the present study and suggested by previous studies (Dalaker), is intriguing. Although the neuroanatomic causes of executive dysfunction in PD can be modeled on the basis of frontostriatal circuit dysfunction, which could be further disrupted by the accumulation of subcortical WMH, the cause of memory deficits in PD is less well-understood. Future longitudinal studies in PD are needed to confirm these findings and would benefit from the voxel-wise resolution of our methodology; however, a large sample size and extended study duration are recommended.
Role of funding source
This work was supported by Merit Review Award 101CX000555 (Gallagher, PI) from the US Department of Veterans Affairs Clinical Sciences Research and Development. The authors would also like to acknowledge the support provided by the University of Wisconsin Alzheimer's Disease Research Center Clinical Core (NIH grant P50 AG033514; Asthana, PI) for subject recruitment and the Neuroimaging Core for study design and data analysis as well as the facilities and resources at the Geriatric Research, Education, and Clinical Centers (GRECC) of the William S. Middleton Memorial Veterans Hospital, Madison, WI. The content is solely the responsibility of the authors and does neither represent the official views of the US Department of Veteran Affairs, nor the US Government, nor the National Institutes of Health. Importantly, the authors wish to thank our volunteers, without whom this work would not be possible.
Funding source
United States Department of Veterans Affairs, Clinical Sciences R&D Merit Review Award Number 101CX000555; University of Wisconsin Alzheimer's Disease Research Center Clinical Core, Grant/Award Number: P50 AG033514.
Conflicts of interest
The authors declare no conflicts of interest.
Disclosures of interest
Authors Vincent Pozorski, Jennifer M. Oh, Ozioma Okonkwo, Stephanie Krislov, Amy Barzgari, Frances Theisen, Jitka Sojkova, Barbara B. Bendlin, Sterling C. Johnson, Catherine L. Gallagher declare no conflicts of interest.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.nicl.2019.101870.
Appendix A. Supplementary data
Supplementary material
References
- Acharya H.J., Bouchard T.P., Emery D.J., Camicioli R.M. Axial signs and magnetic resonance imaging correlates in Parkinson's disease. Can. J. Neurol. Sci. 2007;34(1):56–61. doi: 10.1017/s0317167100005795. [DOI] [PubMed] [Google Scholar]
- Aggleton J.P., Brown M.W. Episodic memory, amnesia, and the hippocampal-anterior thalamic axis. Behav. Brain Sci. 1999;22(3):425–444. (discussion 444-489) [PubMed] [Google Scholar]
- Baezner H., Blahak C., Poggesi A., Pantoni L., Inzitari D., Chabriat H.…Group, L. S Association of gait and balance disorders with age-related white matter changes: the LADIS study. Neurology. 2008;70(12):935–942. doi: 10.1212/01.wnl.0000305959.46197.e6. [DOI] [PubMed] [Google Scholar]
- Baloh R.W., Yue Q., Socotch T.M., Jacobson K.M. White matter lesions and disequilibrium in older people. I. Case-control comparison. Arch. Neurol. 1995;52(10):970–974. doi: 10.1001/archneur.1995.00540340062013. [DOI] [PubMed] [Google Scholar]
- Benedict R.H.B., Schretlen D., Groninger L., Brandt J. Hopkins verbal learning test revised: normative data and analysis of inter-form and test-retest reliability. Clin. Neuropsychol. 1998;12(1):43–55. [Google Scholar]
- Benton A., Hamsher K. 1976. Multilingual Aphasia Examination Manual. [Google Scholar]
- Bernbaum M., Menon B.K., Fick G., Smith E.E., Goyal M., Frayne R., Coutts S.B. Reduced blood flow in normal white matter predicts development of leukoaraiosis. J. Cereb. Blood Flow Metab. 2015;35(10):1610–1615. doi: 10.1038/jcbfm.2015.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birdsill A.C., Koscik R.L., Jonaitis E.M., Johnson S.C., Okonkwo O.C., Hermann B.P.…Bendlin B.B. Regional white matter hyperintensities: aging, Alzheimer's disease risk, and cognitive function. Neurobiol. Aging. 2014;35(4):769–776. doi: 10.1016/j.neurobiolaging.2013.10.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blume J., Rothenfusser E., Schlaier J., Bogdahn U., Lange M. Educational attainment and motor burden in advanced Parkinson's disease - the emerging role of education in motor reserve. J. Neurol. Sci. 2017;381:141–143. doi: 10.1016/j.jns.2017.08.3241. [DOI] [PubMed] [Google Scholar]
- de Boer R., Vrooman H.A., van der Lijn F., Vernooij M.W., Ikram M.A., van der Lugt A.…Niessen W.J. White matter lesion extension to automatic brain tissue segmentation on MRI. Neuroimage. 2009;45(4):1151–1161. doi: 10.1016/j.neuroimage.2009.01.011. [DOI] [PubMed] [Google Scholar]
- Bohnen N.I., Albin R.L. White matter lesions in Parkinson disease. Nat. Rev. Neurol. 2011;7(4):229–236. doi: 10.1038/nrneurol.2011.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohnen N.I., Müller M.L., Zarzhevsky N., Koeppe R.A., Bogan C.W., Kilbourn M.R.…Albin R.L. Leucoaraiosis, nigrostriatal denervation and motor symptoms in Parkinson's disease. Brain. 2011;134:2358–2365. doi: 10.1093/brain/awr139. Pt 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandt J. The Hopkins verbal learning test: development of a new memory test with six equivalent forms. Clin. Neuropsychol. 1991;5(2):125–142. [Google Scholar]
- Brant-Zawadzki M., Fein G., Van Dyke C., Kiernan R., Davenport L., de Groot J. MR imaging of the aging brain: patchy white-matter lesions and dementia. AJNR Am. J. Neuroradiol. 1985;6(5):675–682. [PMC free article] [PubMed] [Google Scholar]
- Burton E.J., McKeith I.G., Burn D.J., Firbank M.J., O'Brien J.T. Progression of white matter hyperintensities in Alzheimer disease, dementia with lewy bodies, and Parkinson disease dementia: a comparison with normal aging. Am. J. Geriatr. Psychiatry. 2006;14(10):842–849. doi: 10.1097/01.JGP.0000236596.56982.1c. [DOI] [PubMed] [Google Scholar]
- Camarda C., Torelli P., Pipia C., Battaglini I., Azzarello D., Rosano R.…Camarda R. Association between cerebral Small vessel disease, measures of brain atrophy and mild Parkinsonian signs in neurologically and cognitively healthy subjects aged 45-84 years: a cross-sectional study. Curr. Alzheimer Res. 2018 doi: 10.2174/1567205015666180702111110. [DOI] [PubMed] [Google Scholar]
- Choi S.A., Evidente V.G., Caviness J.N., Shill H.A., Sabbagh M.N., Connor D.J.…Beach T.G. Are there differences in cerebral white matter lesion burdens between Parkinson's disease patients with or without dementia? Acta Neuropathol. 2010;119(1):147–149. doi: 10.1007/s00401-009-0620-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox R.W. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput. Biomed. Res. 1996;29(3):162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- Dalaker T.O., Larsen J.P., Bergsland N., Beyer M.K., Alves G., Dwyer M.G.…Zivadinov R. Brain atrophy and white matter hyperintensities in early Parkinson's disease(a) Mov. Disord. 2009;24(15):2233–2241. doi: 10.1002/mds.22754. [DOI] [PubMed] [Google Scholar]
- Dalaker T.O., Larsen J.P., Dwyer M.G., Aarsland D., Beyer M.K., Alves G.…Zivadinov R. White matter hyperintensities do not impact cognitive function in patients with newly diagnosed Parkinson's disease. Neuroimage. 2009;47(4):2083–2089. doi: 10.1016/j.neuroimage.2009.06.020. [DOI] [PubMed] [Google Scholar]
- Fahn S., Elton R. UPDRS Development Committee. Unified Parkinson's Disease Rating Scale. In: Fahn S., Marsden C.D., Calne D.B., Goldstein M., editors. Recent Developments in Parkinson's Disease. Vol. 2. Macmillan Health Care Information; Florham Park, NJ: 1987. pp. 153–163. [Google Scholar]
- Fazekas F., Chawluk J.B., Alavi A., Hurtig H.I., Zimmerman R.A. MR signal abnormalities at 1.5 T in Alzheimer's dementia and normal aging. AJR Am. J. Roentgenol. 1987;149(2):351–356. doi: 10.2214/ajr.149.2.351. [DOI] [PubMed] [Google Scholar]
- Folstein M.F., Folstein S.E., McHugh P.R. "Mini-mental state". A practical method for grading the cognitive state of patients for the clinician. J. Psychiatr. Res. 1975;12(3):189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- González-Redondo R., Toledo J., Clavero P., Lamet I., García-García D., García-Eulate R.…Rodríguez-Oroz M.C. The impact of silent vascular brain burden in cognitive impairment in Parkinson's disease. Eur. J. Neurol. 2012;19(8):1100–1107. doi: 10.1111/j.1468-1331.2012.03682.x. [DOI] [PubMed] [Google Scholar]
- Goodglass H., Kaplan E. Lea & Febiger; Philadelphia: 1972. Assessment of Aphasia and Related Disorders. [Google Scholar]
- Grant D.A., Berg E. A behavioral analysis of degree of reinforcement and ease of shifting to new responses in a Weigl-type card-sorting problem. J. Exp. Psychol. 1948;38(4):404–411. doi: 10.1037/h0059831. [DOI] [PubMed] [Google Scholar]
- Gunning-Dixon F.M., Raz N. The cognitive correlates of white matter abnormalities in normal aging: a quantitative review. Neuropsychology. 2000;14(2):224–232. doi: 10.1037//0894-4105.14.2.224. [DOI] [PubMed] [Google Scholar]
- Habes M., Erus G., Toledo J.B., Zhang T., Bryan N., Launer L.J.…Davatzikos C. White matter hyperintensities and imaging patterns of brain ageing in the general population. Brain. 2016;139(Pt 4):1164–1179. doi: 10.1093/brain/aww008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hachinski V.C., Potter P., Merskey H. Leuko-araiosis. Arch. Neurol. 1987;44(1):21–23. doi: 10.1001/archneur.1987.00520130013009. [DOI] [PubMed] [Google Scholar]
- Haugarvoll K., Aarsland D., Wentzel-Larsen T., Larsen J.P. The influence of cerebrovascular risk factors on incident dementia in patients with Parkinson's disease. Acta Neurol. Scand. 2005;112(6):386–390. doi: 10.1111/j.1600-0404.2005.00389.x. [DOI] [PubMed] [Google Scholar]
- Heaton R.K., Chelune G.J., Talley J.L., Kay G.G., Curtiss G. FL: Psychological Assessment Resources; Odessa: 1993. Wisconsin Card Sorting Test Manual: Revised and Expanded. [Google Scholar]
- Herman T., Rosenberg-Katz K., Jacob Y., Auriel E., Gurevich T., Giladi N., Hausdorff J.M. White matter hyperintensities in Parkinson's disease: do they explain the disparity between the postural instability gait difficulty and tremor dominant subtypes? PLoS One. 2013;8(1) doi: 10.1371/journal.pone.0055193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holm S. A simple sequential rejective multiple test procedure. Scand. J. Stat. 1979;6:65–70. [Google Scholar]
- Hua K., Zhang J., Wakana S., Jiang H., Li X., Reich D.S.…Mori S. Tract probability maps in stereotaxic spaces: analyses of white matter anatomy and tract-specific quantification. Neuroimage. 2008;39(1):336–347. doi: 10.1016/j.neuroimage.2007.07.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson N.F., Kim C., Clasey J.L., Bailey A., Gold B.T. Cardiorespiratory fitness is positively correlated with cerebral white matter integrity in healthy seniors. Neuroimage. 2012;59(2):1514–1523. doi: 10.1016/j.neuroimage.2011.08.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandiah N., Mak E., Ng A., Huang S., Au W.L., Sitoh Y.Y., Tan L.C. Cerebral white matter hyperintensity in Parkinson's disease: a major risk factor for mild cognitive impairment. Parkinsonism Relat. Disord. 2013;19(7):680–683. doi: 10.1016/j.parkreldis.2013.03.008. [DOI] [PubMed] [Google Scholar]
- Kaplan E., Goodglass H., Brand S. Lea & Febiger; Philadelphia: 1983. Boston naming test. [Google Scholar]
- Keihaninejad S., Heckemann R.A., Fagiolo G., Symms M.R., Hajnal J.V., Hammers A., Initiative A.s.D.N. A robust method to estimate the intracranial volume across MRI field strengths (1.5T and 3T) Neuroimage. 2010;50(4):1427–1437. doi: 10.1016/j.neuroimage.2010.01.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerber K.A., Enrietto J.A., Jacobson K.M., Baloh R.W. Disequilibrium in older people: a prospective study. Neurology. 1998;51(2):574–580. doi: 10.1212/wnl.51.2.574. [DOI] [PubMed] [Google Scholar]
- Lee S.J., Lee D.G. The cross-sectional and longitudinal relationships between white matter hyperintensities and dementia in patients with Parkinson's disease: a retrospective analysis of 132 patients in a single center. Arch. Gerontol. Geriatr. 2016;62:133–137. doi: 10.1016/j.archger.2015.10.006. [DOI] [PubMed] [Google Scholar]
- Lee S.J., Kim J.S., Lee K.S., An J.Y., Kim W., Kim Y.I.…Jung S.L. The severity of leukoaraiosis correlates with the clinical phenotype of Parkinson's disease. Arch. Gerontol. Geriatr. 2009;49(2):255–259. doi: 10.1016/j.archger.2008.09.005. [DOI] [PubMed] [Google Scholar]
- Lee S.J., Kim J.S., Yoo J.Y., Song I.U., Kim B.S., Jung S.L.…Lee K.S. Influence of white matter hyperintensities on the cognition of patients with Parkinson disease. Alzheimer Dis. Assoc. Disord. 2010;24(3):227–233. doi: 10.1097/WAD.0b013e3181d71a13. [DOI] [PubMed] [Google Scholar]
- de Leeuw F.E., de Groot J.C., Achten E., Oudkerk M., Ramos L.M., Heijboer R.…Breteler M.M. Prevalence of cerebral white matter lesions in elderly people: a population based magnetic resonance imaging study. The Rotterdam Scan Study. J. Neurol. Neurosurg. Psychiatry. 2001;70(1):9–14. doi: 10.1136/jnnp.70.1.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longstreth W.T., Manolio T.A., Arnold A., Burke G.L., Bryan N., Jungreis C.A.…Fried L. Clinical correlates of white matter findings on cranial magnetic resonance imaging of 3301 elderly people. The Cardiovascular Health Study. Stroke. 1996;27(8):1274–1282. doi: 10.1161/01.str.27.8.1274. [DOI] [PubMed] [Google Scholar]
- Mak E., Dwyer M.G., Ramasamy D.P., Au W.L., Tan L.C., Zivadinov R., Kandiah N. White matter hyperintensities and mild cognitive impairment in Parkinson's disease. J. Neuroimaging. 2015;25(5):754–760. doi: 10.1111/jon.12230. [DOI] [PubMed] [Google Scholar]
- Manly J.J., Jacobs D.M., Touradji P., Small S.A., Stern Y. Reading level attenuates differences in neuropsychological test performance between African American and White elders. J. Int. Neuropsychol. Soc. 2002;8(3):341–348. doi: 10.1017/s1355617702813157. [DOI] [PubMed] [Google Scholar]
- Mok V., Kim J.S. Prevention and Management of Cerebral Small Vessel Disease. J Stroke. 2015;17(2):111–122. doi: 10.5853/jos.2015.17.2.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori S., Wakana S., Nagae-Poetscher L.M., van Zijl P.C. Elsevier; Amsterdam, The Netherlands: 2005. MRI Atlas of Human White Matter. [Google Scholar]
- Morimoto S.S., Kanellopoulos D., Alexopoulos G.S. Cognitive impairment in depressed older adults: implications for prognosis and treatment. Psychiatr. Ann. 2014;44(3):138–142. doi: 10.3928/00485713-20140306-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray M.E., Senjem M.L., Petersen R.C., Hollman J.H., Preboske G.M., Weigand S.D.…Jack C.R. Functional impact of white matter hyperintensities in cognitively normal elderly subjects. Arch. Neurol. 2010;67(11):1379–1385. doi: 10.1001/archneurol.2010.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novak V., Haertle M., Zhao P., Hu K., Munshi M., Novak P.…Alsop D. White matter hyperintensities and dynamics of postural control. Magn. Reson. Imaging. 2009;27(6):752–759. doi: 10.1016/j.mri.2009.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Bryant S.E., Humphreys J.D., Smith G.E., Ivnik R.J., Graff-Radford N.R., Petersen R.C., Lucas J.A. Detecting dementia with the mini-mental state examination in highly educated individuals. Arch. Neurol. 2008;65(7):963–967. doi: 10.1001/archneur.65.7.963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh Y.S., Kim J.S., Lee K.S. Orthostatic and supine blood pressures are associated with white matter hyperintensities in Parkinson disease. J. Mov. Disord. 2013;6(2):23–27. doi: 10.14802/jmd.13006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oishi K., Faria A., Van Zijl P., Mori S. 2 ed. Elsevier/Academic Press; 2011. MRI Atlas of Human White Matter. [Google Scholar]
- Papez J.W. A proposed mechanism of emotion. 1937. J. Neuropsychiatr. Clin. Neurosci. 1995;7(1):103–112. doi: 10.1176/jnp.7.1.103. [DOI] [PubMed] [Google Scholar]
- Piccini P., Pavese N., Canapicchi R., Paoli C., Del Dotto P., Puglioli M.…Bonuccelli U. White matter hyperintensities in Parkinson's disease. Clinical correlations. Arch. Neurol. 1995;52(2):191–194. doi: 10.1001/archneur.1995.00540260097023. [DOI] [PubMed] [Google Scholar]
- Power M.C., Deal J.A., Sharrett A.R., Jack C.R., Knopman D., Mosley T.H., Gottesman R.F. Smoking and white matter hyperintensity progression: the ARIC-MRI study. Neurology. 2015;84(8):841–848. doi: 10.1212/WNL.0000000000001283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pozorski V., Oh J.M., Adluru N., Merluzzi A.P., Theisen F., Okonkwo O.…Gallagher C.L. Longitudinal white matter microstructural change in Parkinson's disease. Hum. Brain Mapp. 2018 doi: 10.1002/hbm.24239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prins N.D., van Dijk E.J., den Heijer T., Vermeer S.E., Jolles J., Koudstaal P.J.…Breteler M.M. Cerebral small-vessel disease and decline in information processing speed, executive function and memory. Brain. 2005;128:2034–2041. doi: 10.1093/brain/awh553. Pt 9. [DOI] [PubMed] [Google Scholar]
- Reitan R.M., Wolfson D. In: The Halstead-Reitan neuropsychological test battery: theory and clinical interpretation. 2nd ed. Tucson S., editor. Neuropsychology Press; Arizona: 1993. [Google Scholar]
- Rohit M., Levine A., Hinkin C., Abramyan S., Saxton E., Valdes-Sueiras M., Singer E. Education correction using years in school or reading grade-level equivalent? Comparing the accuracy of two methods in diagnosing HIV-associated neurocognitive impairment. J. Int. Neuropsychol. Soc. 2007;13(3):462–470. doi: 10.1017/S1355617707070506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosano C., Brach J., Studenski S., Longstreth W.T., Newman A.B. Gait variability is associated with subclinical brain vascular abnormalities in high-functioning older adults. Neuroepidemiology. 2007;29(3–4):193–200. doi: 10.1159/000111582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen W.G. Verbal fluency in aging and dementia. J. Clin. Neuropsychol. 1980;2:135–146. [Google Scholar]
- Saad Z.S., Reynolds R.C. SUMA. Neuroimage. 2012;62(2):768–773. doi: 10.1016/j.neuroimage.2011.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sachs B.C., Lucas J.A., Smith G.E., Ivnik R.J., Petersen R.C., Graff-Radford N.R., Pedraza O. Reliable change on the Boston naming test. J. Int. Neuropsychol. Soc. 2012;18(2):375–378. doi: 10.1017/S1355617711001810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santangelo G., Vitale C., Trojano L., De Gaspari D., Bilo L., Antonini A., Barone P. Differential neuropsychological profiles in Parkinsonian patients with or without vascular lesions. Mov. Disord. 2010;25(1):50–56. doi: 10.1002/mds.22893. [DOI] [PubMed] [Google Scholar]
- Sayegh P., Arentoft A., Thaler N.S., Dean A.C., Thames A.D. Quality of education predicts performance on the wide range achievement test-4th edition word Reading subtest. Arch. Clin. Neuropsychol. 2014;29(8):731–736. doi: 10.1093/arclin/acu059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheltens P., Barkhof F., Leys D., Pruvo J.P., Nauta J.J., Vermersch P.…Valk J. A semiquantative rating scale for the assessment of signal hyperintensities on magnetic resonance imaging. J. Neurol. Sci. 1993;114(1):7–12. doi: 10.1016/0022-510x(93)90041-v. [DOI] [PubMed] [Google Scholar]
- Schmidt P., Gaser C., Arsic M., Buck D., Förschler A., Berthele A.…Mühlau M. An automated tool for detection of FLAIR-hyperintense white-matter lesions in multiple sclerosis. Neuroimage. 2012;59(4):3774–3783. doi: 10.1016/j.neuroimage.2011.11.032. [DOI] [PubMed] [Google Scholar]
- Schrag A., Barone P., Brown R.G., Leentjens A.F., McDonald W.M., Starkstein S.…Goetz C.G. Depression rating scales in Parkinson's disease: critique and recommendations. Mov. Disord. 2007;22(8):1077–1092. doi: 10.1002/mds.21333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shechter R., Schwartz M. CNS sterile injury: just another wound healing? Trends Mol. Med. 2013;19(3):135–143. doi: 10.1016/j.molmed.2012.11.007. [DOI] [PubMed] [Google Scholar]
- Sławek J., Wieczorek D., Derejko M., Dubaniewicz M., Brockhuis B., Sitek E.…Lass P. The influence of vascular risk factors and white matter hyperintensities on the degree of cognitive impairment in Parkinson's disease. Neurol. Neurochir. Pol. 2008;42(6):505–512. [PubMed] [Google Scholar]
- Sławek J., Wieczorek D., Derejko M., Dubaniewicz M., Brockhuis B., Sitek E.…Lass P. Vascular risk factors do not contribute to motor and cognitive impairment in Parkinson's disease. Parkinsonism Relat. Disord. 2010;16(1):73–74. doi: 10.1016/j.parkreldis.2009.07.012. author reply 75-76. [DOI] [PubMed] [Google Scholar]
- Sławek J., Roszmann A., Robowski P., Dubaniewicz M., Sitek E.J., Honczarenko K.…Białecka M. The impact of MRI white matter hyperintensities on dementia in Parkinson's disease in relation to the homocysteine level and other vascular risk factors. Neurodegener. Dis. 2013;12(1):1–12. doi: 10.1159/000338610. [DOI] [PubMed] [Google Scholar]
- Stern M.B., Braffman B.H., Skolnick B.E., Hurtig H.I., Grossman R.I. Magnetic resonance imaging in Parkinson's disease and parkinsonian syndromes. Neurology. 1989;39(11):1524–1526. doi: 10.1212/wnl.39.11.1524. [DOI] [PubMed] [Google Scholar]
- Sunwoo M.K., Jeon S., Ham J.H., Hong J.Y., Lee J.E., Lee J.M.…Lee P.H. The burden of white matter hyperintensities is a predictor of progressive mild cognitive impairment in patients with Parkinson's disease. Eur. J. Neurol. 2014;21(6):922–e950. doi: 10.1111/ene.12412. [DOI] [PubMed] [Google Scholar]
- Tröster A.I., Paolo A.M., Lyons K.E., Glatt S.L., Hubble J.P., Koller W.C. The influence of depression on cognition in Parkinson's disease: a pattern of impairment distinguishable from Alzheimer's disease. Neurology. 1995;45(4):672–676. doi: 10.1212/wnl.45.4.672. [DOI] [PubMed] [Google Scholar]
- Tullberg M., Fletcher E., DeCarli C., Mungas D., Reed B.R., Harvey D.J.…Jagust W.J. White matter lesions impair frontal lobe function regardless of their location. Neurology. 2004;63(2):246–253. doi: 10.1212/01.wnl.0000130530.55104.b5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vertes R.P., Albo Z., Viana Di Prisco G. Theta-rhythmically firing neurons in the anterior thalamus: implications for mnemonic functions of Papez's circuit. Neuroscience. 2001;104(3):619–625. doi: 10.1016/s0306-4522(01)00131-2. [DOI] [PubMed] [Google Scholar]
- Vesperman C.J., Pozorski V., Dougherty R.J., Law L.L., Boots E., Oh J.M.…Okonkwo O.C. Cardiorespiratory fitness attenuates age-associated aggregation of white matter hyperintensities in an at-risk cohort. Alzheimers Res. Ther. 2018;10(1):97. doi: 10.1186/s13195-018-0429-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakana S., Caprihan A., Panzenboeck M.M., Fallon J.H., Perry M., Gollub R.L.…Mori S. Reproducibility of quantitative tractography methods applied to cerebral white matter. Neuroimage. 2007;36(3):630–644. doi: 10.1016/j.neuroimage.2007.02.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wardlaw J.M., Valdés Hernández M.C., Muñoz-Maniega S. What are white matter hyperintensities made of? Relevance to vascular cognitive impairment. J. Am. Heart Assoc. 2015;4(6) doi: 10.1161/JAHA.114.001140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson G.S., Robertson G.J. 4th ed. Psychological Assessment Resources; Lutz, FL: 2006. Wide range Achievement Test. [Google Scholar]
- Yamawaki M., Wada-Isoe K., Yamamoto M., Nakashita S., Uemura Y., Takahashi Y.…Nakashima K. Association of cerebral white matter lesions with cognitive function and mood in Japanese elderly people: a population-based study. Brain Behav. 2015;5(3) doi: 10.1002/brb3.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yesavage J.A., Brink T.L., Rose T.L., Lum O., Huang V., Adey M., Leirer V.O. Development and validation of a geriatric depression screening scale: a preliminary report. J. Psychiatr. Res. 1982;17(1):37–49. doi: 10.1016/0022-3956(82)90033-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material