ABSTRACT
Background
The preventable premature mortality achievable by improvement in dietary quality at a global level is unclear.
Objective
The aim of this study was to assess dietary quality globally, and to quantify the potential global impact of improving dietary quality on population health.
Methods
We applied the Alternate Healthy Eating Index (AHEI, potential range 0–100) to a global dietary database to assess dietary quality among adults in 190 countries/territories. The relation of AHEI score to risks of major chronic disease was estimated from 2 large cohorts of men and women for whom many repeated dietary assessments during up to 30 years were available. We calculated the preventable premature deaths achievable by shifting from current national diets to a reference healthy diet.
Results
The global mean AHEI score in 2017 was 49.5 for males and 50.5 for females. Large differences between current and target intakes existed for whole grains, sodium, long-chain n–3 polyunsaturated fats, polyunsaturated fats, and fruits. From 1990 to 2017, the global mean AHEI score increased modestly from 45.4 to 50.0. Diet quality varied substantially across the world. Coastal Mediterranean nations, the Caribbean region, and Eastern Asia (except China and Mongolia) had a higher AHEI score, whereas Central Asia, the South Pacific, and Eastern and Northern Europe had a lower score. An improvement in dietary quality from the current global diet to the reference healthy diet could prevent >11 million premature deaths, ∼24% of total deaths in 2017. These included 1.6 million cancer deaths, 3.9 million coronary artery disease deaths, 1.0 million stroke deaths, 1.7 million respiratory disease deaths, 0.4 million neurodegenerative disease deaths, 0.5 million kidney disease deaths, 0.6 million diabetes deaths, and 1.2 million digestive disease deaths.
Conclusions
Global dietary quality is slowly improving, but remains far from optimal and varies across countries. Improvements in dietary quality have the potential to reduce mortality rates substantially.
Keywords: diet, chronic disease, global health, mortality, nutrition
Introduction
Adopting a healthy dietary pattern reduces morbidity and mortality from major chronic diseases (1). Several reports from the Global Burden of Disease (GBD) Study ranked unhealthful diet among the top contributors to the burdens of disease and death globally (2). However, these reports aggregated the disease burden associated with multiple individual dietary factors, essentially assuming independent contributions of each dietary factor and specific diseases, thus not accounting for the complex relationships among subcomponents of diet or the effects of all dietary factors on all diseases. An alternative, complementary approach to evaluate the overall healthfulness of a diet and account for the complex interrelations among individual dietary components is to calculate a score or an index summarizing an individual's adherence to multiple evidence-based dietary criteria (3). We developed the Alternate Healthy Eating Index (AHEI) in 2002 and updated it in 2010 based on the best available evidence on diet and health (4). The AHEI uniquely includes as a component trans fat (formed during the partial hydrogenation of vegetable oils), which has been robustly related to a high risk of cardiovascular disease (5). The AHEI also encompasses key components of healthful diets, including higher consumption of plant sources of fats, fish, nuts and legumes, whole grains, fruit, and vegetables, and lower consumption of red meat, sugar-sweetened beverages, and sodium. Previous studies have validated the AHEI as a strong predictor of major chronic disease (4, 6), mortality (6–8), and biomarkers of major chronic disease (9) in many populations in the United States, Europe, and Asia with diverse racial/ethnic backgrounds and different dietary habits (6). In previous work, we used the AHEI to evaluate dietary quality in the United States and found a modest improvement from 1999 through 2010 based on NHANES data (10). At the global level, Imamura et al. (11) applied 2 dietary scores similar to the AHEI to evaluate dietary quality and their time trends nationally and regionally.
A key input in the calculation of disease burden attributable to a dietary factor is the estimated biological effect, e.g., multivariable-adjusted risk ratio (RR) of a health outcome, for a specific dietary factor. The GBD Study and similar investigations derived the estimated biological effects through meta-analyses of observational cohort studies and a few dietary trials (2, 12). A majority of the studies included in the meta-analyses were limited by methodologic issues, such as single dietary measurement at baseline, lack of assessment of usual dietary intake, or short follow-up. These limitations would yield misclassification, and hence tend to underestimate the biological effects of dietary factors. In addition, although prior meta-analyses have estimated the biological effects of diet related to well-studied disease endpoints such as cardiovascular disease and type 2 diabetes, recent studies have identified potential effects of diet on additional chronic diseases such as respiratory and neurodegenerative diseases (13, 14). By applying the RRs of major chronic disease, total and cause-specific mortality per unit increase of the AHEI from the Nurses’ Health Study (NHS) (15) and the Health Professionals’ Follow-Up Study (HPFS) (16), we previously estimated that an 8.3-point increase in the AHEI from 1999 through 2012 has prevented 1.1 million premature deaths in the United States (17). These estimated biological effects of dietary quality from the NHS and the HPFS are likely to represent particularly valid evidence for the relation between long-term dietary intake and health outcomes because of the unique features of these cohorts, including extensively validated questionnaires for measuring usual dietary intake, many repeated and detailed measurements of diet and covariates, a continuously updated food composition database that accounts for changes in food processing including partial hydrogenation of plant oils, follow-up for up to 30 years, and large sample size (18). Confounding is likely to be better controlled in the 2 cohorts than in most studies because of the use of repeated measures of potentially confounding variables and the relatively homogenous socioeconomic status (SES) of the study populations due to their similar education levels and occupations. After careful adjustment for potential confounding variables, the estimated biological effects from the NHS and the HPFS represent the underlying physiologic mechanisms and are reasonably generalizable to a broader population in the world. The large number of deaths (33,903 total deaths) and incident cases of major chronic diseases accrued in the NHS and the HPFS enable the effects of diet on several specific causes of death that were not analyzed in the GBD project to be estimated, including deaths due to neurodegenerative, respiratory, and digestive diseases.
Although the quality of diet in different countries and regions has been previously reported (11, 19), the disease burden attributable to a potential improvement in dietary quality at a global level has only been analyzed based on specific dietary factors (20–22), but not based on adherence to a dietary pattern that summarizes the overall healthfulness of diet. We therefore applied the biological effects of dietary quality estimated from 2 large cohort studies, the NHS and the HPFS, to quantify the potential global impact of improving overall dietary quality on population health.
Methods
AHEI and reference healthy diet
We applied the AHEI to assess the dietary quality in different countries/territories around the world. For this study, we included 10 out of 11 components of the original AHEI (alcohol intake was excluded): fruits, vegetables, nuts and legumes, whole grains, red/processed meat, sugar-sweetened beverages, PUFAs [predominantly n–6 (ω-6) PUFAs], long-chain n-3 PUFAs (mainly from seafood), trans fat, and sodium. The 10-dimensional AHEI ranged from 0 (nonadherence) to 100 (perfect adherence); each of the components was scored from 0 to 10 (Supplemental Table 1). For fruits, vegetables, whole grains, nuts and legumes, long-chain n–3 PUFAs, and PUFAs, a higher score indicated higher intake. For trans fat, sugar-sweetened beverages, red/processed meat, and sodium, a higher score indicated lower intake. A reference healthy diet from the EAT-Lancet Commission Report (23), modified slightly to align with available dietary data, was used as the target dietary pattern.
Global dietary intakes
We used data from the GBD 2017 as input in the calculation of AHEI scores for 190 countries/territories in 1990 and 2017. The 190 countries/territories accounted for 99.8% of the global population in 2017. The GBD 2017 compiled dietary data from multiple sources including nationally and subnationally representative nutrition surveys, household budget surveys, accounts of national sales, and FAO Food Balance Sheets, and Supply and Utilization Accounts. To estimate trans fat intake, the GBD 2017 additionally used data on availability of hydrogenated vegetable oil in packaged foods. To make these dietary data more comparable, the GBD Study considered 24-h diet recall as the gold standard method for assessing population intake level, and adjusted dietary data from other sources accordingly. Dietary intake data measured by 24-h recall were available in 67 countries/territories. The estimated sodium intake incorporated data of urinary sodium excretion from studies with 24-h urine collections in 44 countries/territories. Because we aimed to estimate preventable deaths due to major chronic disease, we restricted the analysis to adults aged ≥25 y. The detailed methods for identification of data sources, and data extraction and analysis for diet in the GBD Study, can be found in the online supplementary data and elsewhere (2).
Estimated biological effects of dietary quality
We estimated the biological effects of dietary quality, i.e., multivariable-adjusted sex-specific RRs per 10 units of the AHEI for the risk of major chronic diseases (including cancer, coronary artery disease, stroke, type 2 diabetes, respiratory, neurodegenerative, kidney and digestive system diseases, and other causes except injury and infection), as well as total mortality (excluding deaths due to injury and infection) from the NHS (15) and the HPFS (16). Dietary information was collected with validated semiquantitative FFQs every 2–4 y (24). We assessed incidence of both fatal and nonfatal outcomes for type 2 diabetes, coronary artery disease, and stroke to improve statistical precision and minimize reverse causation; for the other causes of death we used only mortality data. For this analysis, we assumed no biological effects of dietary quality on mortality due to injury and infection, because the types of infectious disease vary greatly across countries/territories. Detailed information on the estimation of biological effects in the NHS and the HPFS can be found in the Supplemental Methods.
Global mortality data
We used the GBD 2017 mortality database to calculate current and potentially preventable deaths in each country/territory. This database has been compiled based on multiple data sources, including vital registration data, subnational verbal autopsy studies, and surveys and surveillance systems for specific causes. The database contained data for 264 causes of death by sex for 23 age groups in 195 countries/territories in 2017 as described previously (25). The quality of mortality data in the GBD Study varied by countries/territories. The GBD Study rated the quality of mortality data with scores from 0 (lowest quality) to 5 (highest quality) based on completeness of vital registration, percentage of deaths coded to causes that cannot be true underlying causes of death, detail of the cause list and age groups, and time periods covered. The GBD 2016 reported that 103 out of the 195 countries/territories have a score of data quality >2 (25). Based on the International Classification of Diseases 9 and 10, we grouped the causes of death into 10 major categories, including cancer, coronary artery disease, stroke, respiratory disease, neurodegenerative disease, kidney disease, diabetes, digestive system disease, injury and infection. The remaining causes of deaths were grouped into an “other causes” category.
Statistical analysis
In the NHS and the HPFS, the AHEI was calculated as the cumulative average up to the start of each 2-y follow-up interval to best represent long-term dietary intake and dampen within-person variation. Person-years of follow-up were calculated from baseline to the earliest of time of death, loss to follow-up, or the end of follow-up. To calculate the sex-specific HRs (hereafter referred to as RRs), we applied Cox proportional hazards models that included AHEI as an exposure variable and cause-specific incidence and mortality as outcomes, and simultaneously adjusted for potentially confounding variables (see footnotes of Supplemental Table 2). Because smoking is a strong confounding factor for the association between AHEI and respiratory disease mortality, we estimated the RRs on respiratory disease mortality among noncurrent smokers only to minimize residual confounding due to smoking. We also calculated the RRs with further adjustment for BMI, which could be considered as a mediating rather than a confounding variable.
We first applied the AHEI scoring criteria to the dietary database in the GBD 2017 to calculate the sex-specific mean AHEI score and its component scores in each country/territory. We then calculated the global mean score as an average of national AHEI scores in 1990 and in 2017, weighted by the population statistics in the corresponding year. We calculated the population-attributable fraction (PAF) attributable to suboptimal dietary quality by comparing the present distribution of the AHEI score to the reference distribution of the AHEI score for each sex and cause of death according to the following formula (26):
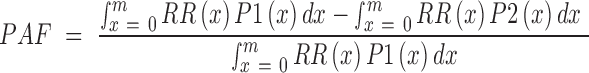 |
(1) |
where:
PAF is the population attributable fraction,
RR(x) is the RR at AHEI level of x, i.e., the estimated biological effects of AHEI from the NHS and the HPFS
P1(x) is the population distribution of AHEI
P2(x) is the reference distribution of AHEI
m is the maximum exposure level
The numbers of cause-specific deaths attributable to the potential improvement in dietary quality were calculated by multiplying the cause-specific PAF by the number of deaths due to that cause in each country/territory. We calculated the numbers of total preventable deaths in each country/territory in 2 ways. First, we summed up the preventable deaths across the 11 major categories of causes of death to derive the numbers of total preventable deaths. This accounted for the observed heterogeneity in distributions in causes of death across countries/territories. Second, for comparison, we applied the cohort-derived sex-specific RRs for total death (deaths due to injury and infection excluded) to calculate the PAF in a country/territory, and then calculated the numbers of preventable total deaths by multiplying the PAF by the number of total deaths (deaths due to injury and infection excluded) in that country/territory. The preventable total and cause-specific deaths at a global level were calculated by summing up the deaths across different countries/territories. The PAFs for total mortality in each country/territory and globally were then calculated as the percentage of preventable total deaths. In a sensitivity analysis, we conducted the same calculations of PAF and preventable deaths based on the use of RRs with additional adjustment for BMI. To calculate the uncertainty of the PAF, we used Monte Carlo simulations to take 1000 draws from the distribution of AHEI scores and the RRs of the AHEI simultaneously, propagating the uncertainty in dietary data and estimated biological effects of the AHEI into the final estimates (26, 27). All the analyses were conducted with SAS version 9.4 (SAS Institute) and R software version 3.5.0 (R Foundation for Statistical Computing).
Results
Global dietary quality
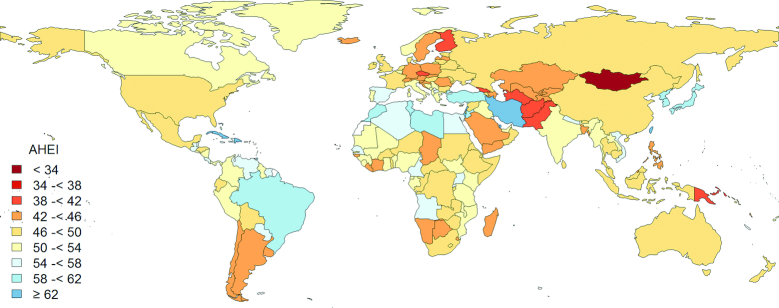
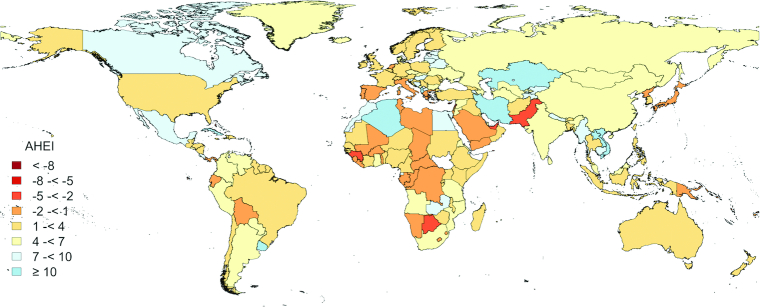
In 2017, the global means of the AHEI score were 49.5 for male adults and 50.5 for female adults out of a maximum score of 100 (Table 1). We observed particularly low AHEI component scores (where 10 is the highest score) for whole grains (AHEI = 1.3), sodium (AHEI = 1.8), long-chain n–3 PUFAs (AHEI = 2.2), PUFAs (AHEI = 2.8), and fruit (AHEI = 3.6), suggesting large differences between current intake and optimal level. The reference healthy diet was scored 94.0 based on the AHEI criteria. From 1990 to 2017, the global mean of the AHEI score increased modestly from 45.4 to 50.0. This improvement was largely due to increased consumption of fruit and vegetables, long-chain n–3 PUFAs, and total PUFAs, as well as decreased intake of trans fat. Figure 1 shows substantial geographic variation in diet quality across 190 countries/territories in 2017. Bermuda (AHEI = 67.9), Dominican Republic (AHEI = 66.6), Israel (AHEI = 65.8), Taiwan Province of China (AHEI = 65.6), Cuba (AHEI = 64.8), and Iran (AHEI = 63.0) were among the countries/territories with a high AHEI score (Supplemental Table 3). The coastal countries/territories around the Mediterranean Sea and those in the Caribbean region, and Eastern Asia (except China and Mongolia) had generally higher AHEI scores (Figure 1), which was mainly attributed to their diets being high in vegetables and nuts/legumes, but low in red/processed meat and sugar-sweetened beverages. Higher fruit consumption was another feature of the healthy diets in the Caribbean region (Supplemental Table 3). Several low- and middle-income (LMI) economies, such as Haiti, Rwanda, and Uganda, had relatively high AHEI scores. Low consumption of unhealthy components of the AHEI, including red/processed meat, sugar-sweetened beverages, and trans fat, rather than high consumption of healthy dietary components, contributed substantially to the high dietary quality in these LMI countries/territories. The lowest AHEI scores were observed in Mongolia (AHEI = 34.0), Afghanistan (AHEI = 39.5), Czech Republic (AHEI = 40.2), Georgia (AHEI = 40.5), and Pakistan (AHEI = 40.8). The countries/territories in Central Asia, the South Pacific region, and Eastern and Northern Europe had very low consumptions of fruit, whole grains, nuts/legumes, PUFAs, and long-chain n–3 PUFAs, and very high sodium intake, and were rated among those with the lowest AHEI scores. Most countries/territories showed improvements in dietary quality from 1990 to 2017 (Figure 2 and Supplemental Figure 1). In addition to several countries/territories in Africa and the South Pacific region, several Mediterranean countries/territories, including Cyprus, Italy, Malta, and Portugal, were among those with decreases in the AHEI over time. These decreases in AHEI were largely driven by reduced consumption of fruits, nuts, and legumes, and increased consumption of sodium (Supplemental Tables 3 and 4).
TABLE 1.
Targets for the healthy reference diet and the mean dietary intakes and AHEI scores in men and women aged ≥25 y in 190 countries/territories in 2017 and 19901
| Reference healthy diet2 | 2017 | 1990 | |||||
|---|---|---|---|---|---|---|---|
| Men | Women | Both sexes | Men | Women | Both sexes | ||
| Total AHEI3 | 94.0 | 49.5 | 50.5 | 50.0 | 45.3 | 45.6 | 45.4 |
| Vegetables | |||||||
| Intake, g/d | 300 | 197 | 183 | 190 | 134 | 125 | 129 |
| AHEI | 9.2 | 5.9 | 5.5 | 5.7 | 4.1 | 3.8 | 3.9 |
| Fruit | |||||||
| Intake, g/d | 200 | 87.0 | 99.5 | 93.4 | 58.2 | 68.1 | 63.2 |
| AHEI | 7.7 | 3.3 | 3.8 | 3.6 | 2.2 | 2.6 | 2.4 |
| Whole grains | |||||||
| Intake, g/d | 232 | 29.9 | 28.0 | 28.9 | 28.1 | 26.2 | 27.1 |
| AHEI | 10.0 | 1.2 | 1.4 | 1.3 | 1.2 | 1.3 | 1.2 |
| Sugar-sweetened beverages | |||||||
| Intake, g/d | 0 | 58.4 | 41.5 | 49.8 | 55.9 | 41.5 | 48.7 |
| AHEI | 10.0 | 7.6 | 8.2 | 7.9 | 8.1 | 8.4 | 8.2 |
| Nuts and legumes | |||||||
| Intake, g/d | 125 | 54.3 | 44.3 | 49.2 | 44.2 | 37.3 | 40.7 |
| AHEI | 10.0 | 7.3 | 6.7 | 7.0 | 7.2 | 6.5 | 6.8 |
| Red/processed meats | |||||||
| Intake, g/d | 14 | 36.6 | 24.3 | 30.4 | 32.6 | 22.7 | 27.6 |
| AHEI | 9.1 | 7.6 | 8.4 | 8.0 | 7.8 | 8.5 | 8.2 |
| Trans fat | |||||||
| Intake, % of energy | 0 | 0.4 | 0.5 | 0.5 | 0.6 | 0.7 | 0.7 |
| AHEI | 10.0 | 9.7 | 9.5 | 9.6 | 9.3 | 9.0 | 9.2 |
| Long-chain n–3 PUFAs | |||||||
| Intake, mg/d | 250 | 105.0 | 90.4 | 97.6 | 66.7 | 60.0 | 63.3 |
| AHEI | 10.0 | 2.3 | 2.0 | 2.2 | 1.7 | 1.6 | 1.7 |
| PUFAs | |||||||
| Intake, % of energy | 10 | 4.3 | 4.2 | 4.3 | 3.3 | 3.3 | 3.3 |
| AHEI | 10.0 | 2.9 | 2.8 | 2.8 | 1.9 | 2.0 | 2.0 |
| Sodium | |||||||
| Intake, mg/d | 2300 | 5792 | 5334 | 5560 | 5846 | 5731 | 5788 |
| AHEI | 8.0 | 1.7 | 2.0 | 1.8 | 1.7 | 1.8 | 1.8 |
Values are means of dietary intake and the AHEI calculated based on dietary data in 190 countries/territories in the GBD Study 2017. AHEI, Alternate Healthy Eating Index; GBD, Global Burden of Disease.
The reference healthy diet was modified slightly from the target dietary pattern in the EAT-Lancet Commission Report (23).
The AHEI score ranged from 0 (nonadherence) to 100 (perfect adherence); each of the components was scored from 0 to 10. For fruits, vegetables, whole grains, nuts and legumes, long-chain n–3 PUFAs, and PUFAs, a higher score indicated higher intake. For trans fat, sugar-sweetened beverages, red/processed meat, and sodium, a higher score indicated lower intake.
FIGURE 1.
Geographic distribution of AHEI scores in men and women aged ≥25 y in 190 countries/territories in 2017. White areas indicate that dietary data were not available. The AHEI scores range from 0 (non adherence) to 100 (perfect adherence). AHEI, Alternate Healthy Eating Index.
Figure 2.
Geographic distribution of changes in AHEI scores from 1990 through 2017 in men and women ≥25 y in 190 countries/territories. White areas indicate that dietary data were not available. The AHEI scores range from 0 (nonadherence) to 100 (perfect adherence). AHEI, Alternate Healthy Eating Index.
Potentially preventable deaths attributable to improvement in dietary quality
We estimated that improvements in dietary quality from current diet to the reference healthy diet could prevent 11.6 million premature deaths annually, accounting for 23.6% of total deaths in 2017 (Table 2). Supplemental Table 5 shows preventable deaths attributable to suboptimal dietary quality at country/territory level. The potential improvement in dietary quality could lead to 22.8% fewer premature deaths in men and 24.6% in women. Notably, when we applied the RRs for total death (deaths due to injury and infection excluded) to the estimate of PAF instead of using the RRs for cause-specific mortality or incident chronic diseases, the PAFs for total mortality (25.5%) and total preventable deaths (12.5 million) were similar. The largest number of preventable deaths was due to coronary artery disease: ∼2 million fewer deaths in men and ∼1.9 million fewer deaths in women. About 1 in 3 deaths due to coronary artery disease (PAF = 35.8%) could be prevented by a global improvement in dietary quality from current national diets to the reference healthy diet. The improvement in dietary quality could prevent about 1 in 6 premature deaths due to cancer (PAF = 16.4%) and stroke (PAF = 17.1%). Improvement in dietary quality is projected to yield ∼1 million preventable deaths due to respiratory disease in women and ∼0.7 million in men. In the sensitivity analysis that applied the RRs of AHEI with further adjustment for BMI, the estimated PAFs and preventable deaths were similar to those in the main analysis (Supplemental Table 6).
TABLE 2.
Numbers of preventable deaths and percentage reductions in deaths as a result of improvements in AHEI scores from the current diet to the reference healthy diet in men and women aged ≥25 y in 190 countries/territories in 20171
| Men | Women | Both sexes | |
|---|---|---|---|
| Total deaths (Summation of cause-specific deaths)2 | |||
| Preventable deaths | 6,071,032 (2,412,523, 8,965,512) | 5,522,247 (2,360,919, 8,017,118) | 11,593,279 (4,773,442, 16,982,629) |
| PAF, % | 22.8 (9.1, 33.7) | 24.6 (10.5, 35.7) | 23.6 (9.7, 34.6) |
| Total deaths (Calculated based on RRs of total | |||
| mortality)3 | |||
| Preventable deaths | 6,369,653 (5,082,373, 7,543,363) | 6,120,230 (5,081,667, 7,073,697) | 12,489,883 (10,164,040, 14,617,060) |
| PAF, % | 23.9 (19.1, 28.3) | 27.3 (22.6, 31.5) | 25.5 (20.7, 29.8) |
| Cause-specific deaths | |||
| Cancer | |||
| Preventable deaths | 1,031,548 (333,972, 1,641,670) | 551,585 (79,850, 970,589) | 1,583,133 (413,822, 2,612,260) |
| PAF, % | 18.3 (5.9, 29.1) | 13.6 (2.0, 24.0) | 16.4 (4.3, 27.0) |
| Coronary artery disease | |||
| Preventable deaths | 2,015,386 (1,408,313, 2,542,409) | 1,876,413 (1,237,383, 2,417,194) | 3,891,799 (2,645,697, 4,959,603) |
| PAF, % | 34.7 (24.2, 43.8) | 37.0 (24.4, 47.7) | 35.8 (24.3, 45.6) |
| Stroke | |||
| Preventable deaths | 353,474 (−381,158, 947,220) | 685,945 (201,983, 1,092,552) | 1,039,420 (−179,176, 2,039,772) |
| PAF, % | 11.3 (−12.1, 30.2) | 23.1 (6.8, 36.8) | 17.0 (−2.9, 33.4) |
| Respiratory disease | |||
| Preventable deaths | 710,420 (174,376, 1,108,400) | 958,753 (540,219, 1,239,757) | 1,669,173 (714,595, 2,348,156) |
| PAF, % | 33.2 (8.1, 51.8) | 55.5 (31.3, 71.7) | 43.1 (18.5, 60.7) |
| Neurodegenerative disease | |||
| Preventable deaths | 361,334 (130,880, 542,682) | 34,642 (−380,672, 382,265) | 395,975 (−249,792, 924,947) |
| PAF, % | 34.0 (12.3, 51.1) | 1.9 (−21.2, 21.2) | 13.8 (−8.7, 32.3) |
| Kidney disease | |||
| Preventable deaths | 247,448 (−47,308, 429,402) | 279,764 (−1,046, 436,642) | 527,212 (−48,354, 866,044) |
| PAF, % | 39.1 (−7.5, 67.9) | 49.7 (−0.2, 77.6) | 44.1 (−4.0, 72.5) |
| Diabetes | |||
| Preventable deaths | 274,212 (206,259, 332,458) | 293,277 (239,950, 340,646) | 567,489 (446,209, 673,104) |
| PAF, % | 42.0 (31.6, 51.0) | 41.7 (34.1, 48.4) | 41.8 (32.9, 49.6) |
| Digestive system disease | |||
| Preventable deaths | 790,088 (412,985, 1,039,195) | 455,382 (186,584, 638,129) | 1,245,471 (599,569, 1,677,324) |
| PAF, % | 57.0 (29.8, 75.0) | 50.2 (20.6, 70.3) | 54.3 (26.2, 73.2) |
| Injury (assumed) | |||
| Preventable deaths | 0 | 0 | 0 |
| PAF, % | 0 | 0 | 0 |
| Infection (assumed) | |||
| Preventable deaths | 0 | 0 | 0 |
| PAF, % | 0 | 0 | 0 |
| Other causes | |||
| Preventable deaths | 287,123 (174,204 - 382,075) | 386,486 (256,668 - 499,345) | 673,608 (430,872 - 881,419) |
| PAF, % | 38.0 (23.0, 50.5) | 35.1 (23.3, 45.4) | 36.3 (23.2, 47.5) |
Values were PAFs (95% CIs) calculated based on comparison in the AHEI scores between global diet in 2017 and the reference healthy diet modified slightly from the target dietary pattern in the EAT-Lancet Commission Report (23). AHEI, Alternate Healthy Eating Index; ; PAF, population attributable fraction; RR, risk ratio.
Total preventable deaths were calculated by summing up all the preventable cause-specific deaths. PAFs were calculated as the percentage of preventable total deaths in total deaths, including those due to infection and injury in the denominator.
PAFs for total mortality were calculated based on the biological effects (RRs) for total deaths (deaths due to injury and infection excluded) from the NHS and the HPFS. Preventable total deaths were calculated by multiplying the total deaths (deaths due to injury and infection excluded) by the PAFs. The final PAFs for total mortality were calculated as the percentage of preventable total deaths in total deaths, including those due to infection and injury in the denominator.
Discussion
We evaluated the quality of diet in 190 countries/territories by applying the AHEI, a dietary quality score summarizing 10 key components of healthy eating, to updated global dietary data. Overall global dietary quality improved modestly from 1990 to 2017, but remained far from optimal and varied substantially across the world. We estimated that a potential improvement in dietary quality from current national diets to a healthy reference diet could prevent >11 million premature deaths annually, ∼25% of total deaths among adults worldwide in 2017.
Our finding that dietary quality varied substantially across countries/territories corroborates the data for 187 countries/territories in Imamura et al. (11) and the report from the Prospective Urban Rural Epidemiology Study for 17 countries (19). Dietary quality in different countries and regions is determined by many interrelated factors at local levels, including traditional food patterns, local food availability, industrialization of food supply system, and food and agricultural policies. Although originally developed in a US population, the AHEI has predicted health outcomes in many countries and regions and has a documented ability to capture dietary patterns in geographically and socioeconomically diverse populations around the world (6). The AHEI rated many coastal countries around the Mediterranean Sea, such as Turkey, Lebanon, and Morocco, as those with the highest dietary quality. Populations in this region consume dietary patterns influenced by the traditional Mediterranean diet (28), even though industrialization of foods has modified the traditional dietary patterns in this region, (29) as indicated by the declines in diet quality in Italy and Portugal and other countries between 1990 and 2017. The healthfulness of the Mediterranean diet was first proposed by Keys (30) and colleagues because populations in the Mediterranean region had long life expectancy and low rates of many chronic diseases, such as coronary artery disease and certain cancers, despite their limited access to medical services. Subsequent prospective cohort studies (31–34) and controlled clinical trials (35, 36) provided strong evidence supporting these benefits of the Mediterranean diet. Several LMI countries were also among those with the highest AHEI scores, which may be explained by both their traditional eating habits and less industrialized food supply system. These countries/territories with high dietary quality had very low consumption of sugary beverages and trans fat, suggesting limited access to processed foods and a less industrialized food system, which was consistent with the report by Imamura et al. (11) of an inverse association between income level and an index scoring the intakes of unhealthy dietary factors in 187 countries/territories. Although these LMI countries have low rates of chronic diseases, some still have high rates of undernutrition in children, presenting a challenge to address the latter while still retaining the positive aspects of traditional diets.
Despite their high sodium intake, several Eastern Asian countries/territories, including Taiwan Province of China, Japan, and the Republic of Korea, were rated as those with relatively high AHEI scores, suggesting their successes in preserving their traditional diets high in vegetables, nuts/legumes, and PUFAs from either vegetable oil or fish and low in red/processed meat, as well as nutrition education efforts (37–40). The relatively high AHEI score in the Democratic People's Republic of Korea may partially reflect sustained shortage of food, particularly animal-sourced foods, during the past decades, as well as less certainty given limited FAO estimates and direct dietary data from this nation (41). As the most populous country in Eastern Asia, China was not among those with a high AHEI score. Compared with Japan and Republic of Korea, China had higher consumption of red/processed meat and lower consumptions of nuts/legumes and PUFAs, which was consistent with a previous report that China has experienced a dramatic shift in dietary patterns, from a traditional diet high in minimally processed plant-based foods to a more Westernized diet since the early 1980s (42). In addition, sodium intake, largely from added condiments, in China remained the highest in Eastern Asia (43, 44). In addition to their low consumption of minimally processed plant-sourced foods and vegetable oils and high consumption of red meat, very high consumption of sugar-sweetened beverage was a major contributor to the low dietary quality in several countries/territories in the Scandinavian region, Eastern Europe (e.g., Czech Republic and Poland), Chile, and Argentina, which was consistent with previous findings from cross-national comparison studies on sugar-sweetened beverages (45, 46). Of note, Canada, the United States, and many countries/territories in Latin America also showed very high intake of sugary beverages in addition to their high sodium intake and low intake of minimally processed plant-sourced foods. The dietary patterns in this region were characterized by a wide variety of processed and low-nutrient-density foods and sugar-sweetened beverages produced by food-supply systems that are dominated by large food processors and restaurant chains (47–49).
Food availability and traditional dietary habits in several countries/territories in Central Asia, such as Mongolia, Turkmenistan, and Afghanistan, may largely explain their low dietary scores, characterized by very low consumption of fruits and vegetables, legumes, nuts, and whole grains, as well as high sodium intake. The extreme continental climate in this region has shaped a traditional diet where red meat and dairy products are the main sources of energy, and there is limited access to fresh plant-sourced foods (50). Political factors, such as domestic and international conflicts, and economic crises, have also influenced food production and trade (51), and may be responsible for the worsening dietary quality over time in countries/territories such as Cyprus and Lebanon.
Our modeling analysis suggested that >11 million premature deaths could be prevented by an improvement in dietary quality. Importantly, our study expanded estimates of preventable deaths to traditionally understudied causes, such as respiratory, neurodegenerative, and digestive diseases, providing a more comprehensive estimate of the potential disease burden attributable to poor dietary quality. In comparison to our findings, Springmann et al. (52) estimated that adoption of balanced diets including predominantly minimally processed plant-based foods, including the reference flexitarian-style diet used in our analysis as well as pescatarian, vegetarian, and vegan dietary patterns, could lead to estimated reductions in premature mortality by 19–22% in 2030. Notably, the inclusion of reductions in underweight, overweight, and obesity accounted for a proportion of the decrease in premature mortality in Springmann et al.’s analysis, whereas our analysis did not include reductions in energy intake or benefits of weight loss as it focused on changes in dietary quality alone. Our estimate of preventable deaths is similar to that of the GBD collaborators (53), calculated from RRs obtained from published meta-analyses and separate criteria for each dietary component.
The substantial reductions in premature deaths, ∼25% of total deaths globally, provide strong justification for prioritizing healthy diets in the global agenda and governmental policymaking for the prevention of chronic disease. Various strategic documents from the WHO and the FAO, including the Rome Declaration on Nutrition (54), the Global Action Plan for the Prevention and Control of Noncommunicable Diseases 2013–2020 (55), and the Fiscal Policies for Diet and the Prevention of Noncommunicable Diseases (56), have recommended a set of policy options and strategies to promote healthy eating. These recommendations particularly encouraged governments to consider the use of legislative and economic tools aimed at changing the environment to foster and support industry's production of, and consumers’ healthy choices about, food (57). At national and global levels, the continued development and regular updating of dietary guidelines that incorporate the newest scientific evidence on diet and health is one important step for guiding educational and policy actions (58). In addition, quality standards, taxes and subsidies, healthcare reform, government feeding programs, and procurement standards in schools and worksites are important means of creating environments and incentives to promote behavioral changes and improve access to healthy dietary choices. The role of government actions in eliminating trans fats in the United States and several other countries has set a successful precedent. In our previous study, we estimated that about half of the improvement in dietary quality in the United States from 1999 to 2012 was contributed by the gradual elimination of trans fat (17), which has already contributed to significant reductions in the rate of cardiovascular disease (59). Recently, the WHO released the REPLACE action package, providing countries and regions with actionable tools to accelerate the elimination of industrially produced trans fats from the global food supply (60), which will lead to a substantial reduction in disease burden. The role of government actions was also exemplified by the nation-level taxation on sugary beverages and low-nutrient-density foods pioneered by the Mexican government. After the implementation of a specific excise tax of ∼10% on sugary beverages and an ad valorem sales tax of 8% on nonessential foods in 2014, Mexico has seen a 6% decrease in the purchases of taxed beverages (49) and a 5.1% decrease in the purchases of taxed foods (48) in 1 y. Notably, most of these policies have focused on reducing additives or nutrients with adverse effects, rather than increasing the supply of and demand for less processed, healthful foods such as fruits, vegetables, legumes, nuts, and PUFA-rich oils. It is critical to align agricultural policy with nutrition policy at global and country levels via an array of policy arrangements including economic incentives and regulatory actions (61). The global agricultural system after the end of World War II focused more on addressing hunger and the supply of basic starchy staples, animal-sourced foods, and cash crops such as sugar cane and palm oil (62). Because diet-related chronic diseases have become global epidemics, this system must be transformed to deliver affordable, healthful, and equitable diets globally, but at the same time also address environmental sustainability (52). The tremendous direct and indirect costs of these diet-related chronic diseases must also be considered and incorporated into policy decisions, including costs for families, governments, and private businesses.
Our study has several strengths. First, we calculated the dietary quality based on updated global dietary data from the GBD Study. Although these data are inevitably imperfect, they do describe a global pattern that is consistent with other knowledge (11). Second, the biological effects of dietary quality estimated from the NHS and the HPFS have unique advantages because of the extensive serial assessment of diet, which reduces measurement error; extended follow-up, which increases power and allows assessment of development of chronic diseases; the ability to adjust for multiple major risk factors and the homogeneous SES of participants, which reduce residual confounding; and a large number of deaths and incident cases of disease, which enabled our calculations of preventable deaths to include traditionally understudied causes. Importantly, we separately evaluated estimated biological effects and attributable mortality due to cause-specific mortality compared with total mortality. The concordance in these findings provides a measure of internal validation of the methods and results. In addition, the RRs estimated from the NHS and the HPFS are reasonably valid and generalizable estimates of the biological effects of diet in the calculations of PAFs and preventable deaths attributable to unhealthy diet in a world population. First, previous studies found little evidence that the RRs for an overall healthy diet pattern and their major components differ substantially across racial or ethnic groups (6, 63). Second, the RRs from populations who have consumed Westernized/industrialized diets for many decades will likely better predict the long-term effects of these diets when adopted worldwide. Through the use of these RRs, this analysis also emphasizes that the best time to adopt a healthier diet is now, not when chronic diseases become global epidemics several decades from now. Third, it is clear that the populations of LMI countries are susceptible to an unhealthy diet characterized by a low AHEI score because ecologic data suggest that those counties are rapidly catching up with the United States in diet-related chronic diseases, and in many cases are actually surpassing US rates for diabetes (64). Fourth, confounding by SES is minimal in our estimates of diet-disease etiologic effects because the study populations in the NHS and HPFS where these effects were derived were homogeneous in income, education, and occupation, whereas the potential for confounding by SES is especially high in analyses of diet-disease relationships in LMI countries, where higher incomes are strongly related to diet, especially intake of animal-sourced foods.
Our findings should be interpreted in the context of several limitations. First, we assumed casual relationships between AHEI score and major chronic diseases in the calculation of PAFs even though the biological effects of the AHEI were calculated from 2 observational studies. However, the components of the AHEI were selected because of strong evidence based on replicated epidemiologic and randomized controlled feeding studies (4), and the PREDIMED Trial demonstrated effects of a similar healthy dietary pattern on many chronic diseases analyzed in our study (65–67). Second, the GBD Study dietary data include relatively few individual-level dietary surveys, and rely on data from FAO food-balance sheets and industry sources to account for the incomplete data for some regions. In addition, the dietary assessments used in surveys across the world and FFQs used in the cohorts to estimate the biological effects inevitably incorporate some measurement error. Although other efforts are ongoing to improve the data quality available to the GBD and other global studies (68), sampling errors in the GBD Study dietary data were incorporated into the uncertainty calculations. Random measurement errors in FFQ-measured dietary intake in the NHS and HPFS cohorts would tend to underestimate the biological effects and thus the percentage of premature deaths preventable by improvements in diet. Third, we cannot rule out the possibility of residual confounding when estimating the biological effects of dietary quality in the observational cohort studies. However, our prior work suggests that, compared with findings from controlled dietary trials, the magnitude of such confounding is unlikely to be large (69). Fourth, the study populations in the NHS and HPFS were homogeneous in education and occupation, and ∼94% were of European background. The limited diversity, although minimizing confounding, might limit the generalizability of our estimates of the biological effects of diet. However, previous studies found little evidence that the biological effects of an overall healthy diet pattern (6) and their major components (63) differ substantially across racial or ethnic groups, even though the distribution of dietary factors differ greatly and were incorporated into our analyses. Fifth, our disease burden calculations were based on a counterfactual approach, rather than estimating the effects of any specific intervention, in which changes in diet and disease rates over an unspecified period of time would be incorporated. We assumed no effect of dietary quality on mortality due to infectious disease, and included all the deaths due to infectious disease in the denominator in the calculation of PAF. Thus, our PAFs will tend to be underestimates because healthy diets may prevent infectious disease (70). Sixth, we did not apply age-specific biological effects of diet. However, the mean age at death the NHS (74.4 y) was similar to approximate mean age at death in women in the GBD mortality database (74.3 y), while the mean age at death in the HPFS (79.9 y) was older than the approximate mean age at death in men in the GBD database (69.8 y), which could lead to conservative estimates of the PAFs and preventable premature deaths because relative risks for modifiable variables generally decline with age (71). Seventh, the current study included 10 out of 11 AHEI components and excluded alcohol intake in the calculation of dietary quality scores due to the unavailability of alcohol data in the GBD Study and the complexity of the health effects of alcohol consumption (72). Lastly, although the AHEI was validated as a strong predictor of major chronic disease risk in populations from the United States, Europe, Australia, and China, large cohort studies in other parts of the world, including Africa and South America, are desirable to evaluate further the validity of the dietary quality score.
In conclusion, the global quality of diet is far from optimal and varies greatly across countries/territories. We estimate that a potential change from current national diets to a healthy dietary pattern could prevent >11 million premature deaths annually. Given recent regulatory successes in beginning to address healthy eating, such as elimination of trans fat and taxation on sugary beverages, further policy initiatives and actions are needed to also increase healthful foods and reduce the burden of major chronic disease.
Supplementary Material
Acknowledgments
The authors’ responsibilities were as follows—DDW and WCW: designed the research; DDW, YL and WCW: conducted the research; DDW and YL: analyzed the data; DDW, YL, AA, MS, DM, MJS, FBH, CJLM, and WCW: wrote the paper; WCW: had primary responsibility for final content; and all authors read and approved the final manuscript.
Notes
The Nurses’ Health Study was supported by research grants UM1 CA186107, P01 CA87969, R01 CA49449, R01 HL034594, R01 HL60712 and R01 HL088521 and the Health Professionals’ Follow-Up Study was supported by research grants UM1 CA167552 and R01 HL35464 from the NIH. DDW was supported by research grant K99 DK119412 from the NIH. MS was supported by the Wellcome Trust, Our Planet Our Health (Livestock, Environment and People—LEAP), award 205212/Z/16/Z. DM was supported by research grant R01 HL130735 from the NIH.
Author disclosures: YL reported research support from the California Walnut Commission outside the submitted work. DM reported research funding from the NIH and the Bill and Melinda Gates Foundation, and (all outside the submitted work) personal fees from GOED, Nutrition Impact, Pollock Communications, Bunge, Indigo Agriculture, Amarin, Acasti Pharma, Cleveland Clinic Foundation, Danone, and America's Test Kitchen; scientific advisory board, Elysium Health (with stock options), Omada Health, and DayTwo; and chapter royalties from UpToDate. FH reported research funding from the NIH; research support from the California Walnut Commission; and lecture fees from Standard Process and Metagenics (all outside the submitted work). DDW, AA, MS, MJS, CJLM, and WCW: no conflicts of interest.
Supplemental Tables 1–6, Supplemental Methods, and Supplemental Figure 1 are available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/jn/.
Abbreviations used: AHEI, Alternate Healthy Eating Index; GBD, Global Burden of Disease; HPFS, Health Professionals’ Follow-Up Study; LMI, low- and middle-income; NHS, Nurses’ Health Study; PAF, population-attributable fraction; SES, socioeconomic status.
References
- 1. Willett WC, Stampfer MJ. Current evidence on healthy eating. Annu Rev Public Health. 2013;34:77–95. [DOI] [PubMed] [Google Scholar]
- 2. Gakidou E, Afshin A, Abajobir AA, Abate KH, Abbafati C, Abbas KM, Abd-Allah F, Abdulle AM, Abera SF, Aboyans V et al.. Global, regional, and national comparative risk assessment of 84 behavioural, environmental and occupational, and metabolic risks or clusters of risks, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet. 2017;390:1345–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hu FB. Dietary pattern analysis: a new direction in nutritional epidemiology. Curr Opin Lipidol. 2002;13:3–9. [DOI] [PubMed] [Google Scholar]
- 4. Chiuve SE, Fung TT, Rimm EB, Hu FB, McCullough ML, Wang M, Stampfer MJ, Willett WC. Alternative dietary indices both strongly predict risk of chronic disease. J Nutr. 2012;142:1009–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Mozaffarian D, Katan MB, Ascherio A, Stampfer MJ, Willett WC. Trans fatty acids and cardiovascular disease. N Engl J Med. 2006;354:1601–13. [DOI] [PubMed] [Google Scholar]
- 6. Schwingshackl L, Bogensberger B, Hoffmann G. Diet quality as assessed by the Healthy Eating Index, Alternate Healthy Eating Index, Dietary Approaches to Stop Hypertension Score, and Health Outcomes: an updated systematic review and meta-analysis of cohort studies. J Acad Nutr Diet. 2018;118:74–100. e11. [DOI] [PubMed] [Google Scholar]
- 7. Belin RJ, Greenland P, Allison M, Martin L, Shikany JM, Larson J, Tinker L, Howard BV, Lloyd-Jones D, Van Horn L. Diet quality and the risk of cardiovascular disease: the Women's Health Initiative (WHI). Am J Clin Nutr. 2011;94:49–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sotos-Prieto M, Bhupathiraju SN, Mattei J, Fung TT, Li Y, Pan A, Willett WC, Rimm EB, Hu FB. Association of changes in diet quality with total and cause-specific mortality. N Eng J Med. 2017;377:143–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Fung TT, McCullough ML, Newby PK, Manson JE, Meigs JB, Rifai N, Willett WC, Hu FB. Diet-quality scores and plasma concentrations of markers of inflammation and endothelial dysfunction. Am J Clin Nutr. 2005;82:163–73. [DOI] [PubMed] [Google Scholar]
- 10. Wang DD, Leung CW, Li Y, Ding EL, Chiuve SE, Hu FB, Willett WC. Trends in dietary quality among adults in the United States, 1999 through 2010. JAMA Intern Med. 2014;174:1587–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Imamura F, Micha R, Khatibzadeh S, Fahimi S, Shi P, Powles J, Mozaffarian D. Dietary quality among men and women in 187 countries in 1990 and 2010: a systematic assessment. Lancet Glob Health. 2015;3:e132–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Mokdad AH, Ballestros K, Echko M, Glenn S, Olsen HE, Mullany E, Lee A, Khan AR, Ahmadi A, Ferrari AJ et al.. The state of US health, 1990–2016: burden of diseases, injuries, and risk factors among US states. JAMA. 2018;319:1444–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Varraso R, Chiuve SE, Fung TT, Barr RG, Hu FB, Willett WC, Camargo CA. Alternate Healthy Eating Index 2010 and risk of chronic obstructive pulmonary disease among US women and men: prospective study. BMJ. 2015;350:h286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gao X, Chen H, Fung TT, Logroscino G, Schwarzschild MA, Hu FB, Ascherio A. Prospective study of dietary pattern and risk of Parkinson disease. Am J Clin Nutr. 2007;86:1486–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. The Nurses' Health Study [Internet]. Harvard Medical School. [cited September 24, 2018], 2000,; Available from:http://www.nurseshealthstudy.org [Google Scholar]
- 16. The Health Professionals Follow-Up Study [Internet]. Harvard T.H. Chan School of Public Health. [cited September 24, 2015], 1985, Available from: https://www.hsph.harvard.edu/hpfs/. [Google Scholar]
- 17. Wang DD, Li Y, Chiuve SE, Hu FB, Willett WC. Improvements in US diet helped reduce disease burden and lower premature deaths, 1999–2012; overall diet remains poor. Health Aff (Millwood). 2015;34:1916–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Liese AD, Krebs-Smith SM, Subar AF, George SM, Harmon BE, Neuhouser ML, Boushey CJ, Schap TE, Reedy J. The Dietary Patterns Methods Project: synthesis of findings across cohorts and relevance to dietary guidance. J Nutr. 2015;145:393–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Teo K, Lear S, Islam S, Mony P, Dehghan M, Li W, Rosengren A, Lopez-Jaramillo P, Diaz R, Oliveira G et al.. Prevalence of a healthy lifestyle among individuals with cardiovascular disease in high-, middle- and low-income countries: the Prospective Urban Rural Epidemiology (PURE) study. JAMA. 2013;309:1613–21. [DOI] [PubMed] [Google Scholar]
- 20. Springmann M, Mason-D'Croz D, Robinson S, Garnett T, Godfray HC, Gollin D, Rayner M, Ballon P, Scarborough P. Global and regional health effects of future food production under climate change: a modelling study. Lancet. 2016;387:1937–46. [DOI] [PubMed] [Google Scholar]
- 21. Mozaffarian D, Fahimi S, Singh GM, Micha R, Khatibzadeh S, Engell RE, Lim S, Danaei G, Ezzati M, Powles J. Global sodium consumption and death from cardiovascular causes. N Eng J Med. 2014;371:624–34. [DOI] [PubMed] [Google Scholar]
- 22. GBD 2016 Risk Factors Collaborators. Global, regional, and national comparative risk assessment of 84 behavioural, environmental and occupational, and metabolic risks or clusters of risks, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet. 2017;390:1345–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Willett W, Rockström J, Loken B, Springmann M, Lang T, Vermeulen S, Garnett T, Tilman D, DeClerck F, Wood A, Jonell M. Food in the Anthropocene: the EAT-Lancet Commission on healthy diets from sustainable food systems. Lancet. 2019;393:447–92. [DOI] [PubMed] [Google Scholar]
- 24. Willett W. Food frequency methods; Reproducibility and validity of food-frequency questionnaires. In: Nutritional epidemiology, 3rd ed. Oxford University Press; 2013. chap 5 and 6. [Google Scholar]
- 25. GBD 2016 Causes of Death Collaborators. Global, regional, and national age-sex specific mortality for 264 causes of death, 1980–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet. 2017;390:1151–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lim SS, Vos T, Flaxman AD, Danaei G, Shibuya K, Adair-Rohani H, Amann M, Anderson HR, Andrews KG, Aryee M et al.. A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2224–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Micha R, Peñalvo JL, Cudhea F, Imamura F, Rehm CD, Mozaffarian D. Association between dietary factors and mortality from heart disease, stroke, and type 2 diabetes in the United States. JAMA. 2017;317:912–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Willett WC, Sacks F, Trichopoulou A, Drescher G, Ferro-Luzzi A, Helsing E, Trichopoulos D. Mediterranean diet pyramid: a cultural model for healthy eating. Am J Clin Nutr. 1995;61:1402S–6S. [DOI] [PubMed] [Google Scholar]
- 29. Denoth F, Scalese M, Siciliano V, Di Renzo L, De Lorenzo A, Molinaro S. Clustering eating habits: frequent consumption of different dietary patterns among the Italian general population in the association with obesity, physical activity, sociocultural characteristics and psychological factors. Eat Weight Disord. 2016;21:257–68. [DOI] [PubMed] [Google Scholar]
- 30. Keys A. Seven countries. A multivariate analysis of death and coronary heart disease. Harvard University Press; 1980. [Google Scholar]
- 31. Trichopoulou A, Costacou T, Bamia C, Trichopoulos D. Adherence to a Mediterranean diet and survival in a Greek population. N Eng J Med. 2003;348:2599–608. [DOI] [PubMed] [Google Scholar]
- 32. Willett WC. The Mediterranean diet: science and practice. Public Health Nutr. 2006;9:105–10. [DOI] [PubMed] [Google Scholar]
- 33. Sofi F, Abbate R, Gensini GF, Casini A. Accruing evidence on benefits of adherence to the Mediterranean diet on health: an updated systematic review and meta-analysis. Am J Clin Nutr. 2010;92:1189–96. [DOI] [PubMed] [Google Scholar]
- 34. Fung TT, Rexrode KM, Mantzoros CS, Manson JE, Willett WC, Hu FB. Mediterranean diet and incidence of and mortality from coronary heart disease and stroke in women. Circulation. 2009;119:1093–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. De Lorgeril M, Salen P, Martin J-L, Monjaud I, Delaye J, Mamelle N. Mediterranean diet, traditional risk factors, and the rate of cardiovascular complications after myocardial infarction: final report of the Lyon Diet Heart Study. Circulation. 1999;99:779–85. [DOI] [PubMed] [Google Scholar]
- 36. Estruch R, Ros E, Salas-Salvado J, Covas MI, Corella D, Aros F, Gomez-Gracia E, Ruiz-Gutierrez V, Fiol M, Lapetra J et al.. Primary prevention of cardiovascular disease with a Mediterranean diet supplemented with extra-virgin olive oil or nuts. N Engl J Med. 2018;378:e34. [DOI] [PubMed] [Google Scholar]
- 37. Tzeng MS. From dietary guidelines to daily food guide: the Taiwanese experience. Asia Pac J Clin Nutr. 2008;17 Suppl 1:59–62. [PubMed] [Google Scholar]
- 38. Adachi M. Theories of nutrition education and promotion in Japan: enactment of the “Food Education Basic Law”. Asia Pac J Clin Nutr. 2008;17:180–4. [PubMed] [Google Scholar]
- 39. Miyoshi M, Tsuboyama-Kasaoka N, Nishi N. School-based “Shokuiku” program in Japan: application to nutrition education in Asian countries. Asia Pac J Clin Nutr. 2012;21:159–62. [PubMed] [Google Scholar]
- 40. Park HK. Nutrition policy in South Korea. Asia Pac J Clin Nutr. 2008;17 Suppl 1:343–5. [PubMed] [Google Scholar]
- 41. Kim T. Strategizing aid: US-China food aid relations to North Korea in the 1990 s. Int Relat Asia-Pacific. 2011;12:41–70. [Google Scholar]
- 42. Li Y, Wang DD, Ley SH, Howard AG, He Y, Lu Y, Danaei G, Hu FB. Potential impact of time trend of life-style factors on cardiovascular disease burden in China. J Am Coll Cardiol. 2016;68:818–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Anderson CA, Appel LJ, Okuda N, Brown IJ, Chan Q, Zhao L, Ueshima H, Kesteloot H, Miura K, Curb JD. Dietary sources of sodium in China, Japan, the United Kingdom, and the United States, women and men aged 40 to 59 years: the INTERMAP study. J Am Diet Assoc. 2010;110:736–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Du S, Neiman A, Batis C, Wang H, Zhang B, Zhang J, Popkin BM. Understanding the patterns and trends of sodium intake, potassium intake, and sodium to potassium ratio and their effect on hypertension in China. Am J Clin Nutr. 2013;99:334–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Naska A, Bountziouka V, Trichopoulou A. Soft drinks: time trends and correlates in twenty-four European countries. A cross-national study using the DAFNE (Data Food Networking) databank. Public Health Nutr. 2010;13:1346–55. [DOI] [PubMed] [Google Scholar]
- 46. Singh GM, Micha R, Khatibzadeh S, Shi P, Lim S, Andrews KG, Engell RE, Ezzati M, Mozaffarian DNutrition GBoD, et al., Nutrition GBoD Global, regional, and national consumption of sugar-sweetened beverages, fruit juices, and milk: a systematic assessment of beverage intake in 187 countries. PLoS One. 2015;10:e0124845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Popkin BM, Reardon T. Obesity and the food system transformation in Latin America. Obes Rev. 2018;19:1028–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Batis C, Rivera JA, Popkin BM, Taillie LS. First-year evaluation of Mexico's tax on nonessential energy-dense foods: an observational study. PLoS Med. 2016;13:e1002057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Colchero MA, Popkin BM, Rivera JA, Ng SW. Beverage purchases from stores in Mexico under the excise tax on sugar sweetened beverages: observational study. BMJ. 2016;352:h6704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Dugee O, Khor GL, Lye MS, Luvsannyam L, Janchiv O, Jamyan B, Esa N. Association of major dietary patterns with obesity risk among Mongolian men and women. Asia Pac J Clin Nutr. 2009;18:433–40. [PubMed] [Google Scholar]
- 51. Messer E, Cohen MJ, Breaking the links between conflict and hunger redux. World Medical & Health Policy, 2015;7:211–11. [Google Scholar]
- 52. Springmann M, Wiebe K, Mason-D'Croz D, Sulser TB, Rayner M, Scarborough P. Health and nutritional aspects of sustainable diet strategies and their association with environmental impacts: a global modelling analysis with country-level detail. Lancet Planet Health. 2018;2:e451–e61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Global Burden of Disease 2017 Diet Collaborators. Health effects of dietary risks in 195 countries: findings from the Global Burden of Diseases study 2017. Lancet. 2019(in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Food and Agriculture Organization of the United Nations and World Health Organization. [Internet].Rome declaration on nutrition. 2014, Available from:http://www.fao.org/3/a-ml542e.pdf. [Google Scholar]
- 55. World Health Organization. Global action plan for the prevention and control of noncommunicable diseases 2013–2020. Geneva: World Health Organization; 2013. [Google Scholar]
- 56. World Health Organization. Fiscal policies for diet and the prevention of noncommunicable diseases. Geneva: World Health Organization; 2016. [Google Scholar]
- 57. Mozaffarian D, Angell SY, Lang T, Rivera JA. Role of government policy in nutrition-barriers to and opportunities for healthier eating. BMJ. 2018;361:k2426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Satija A, Yu E, Willett WC, Hu FB. Understanding nutritional epidemiology and its role in policy. Adv Nutr. 2015;6:5–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Brandt EJ, Myerson R, Perraillon MC, Polonsky TS. Hospital admissions for myocardial infarction and stroke before and after the trans-fatty acid restrictions in New York. JAMA Cardiol. 2017;2:627–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Ghebreyesus TA, Frieden TR.. REPLACE: a roadmap to make the world trans fat free by 2023. Lancet. 2018;391:1978–80. [DOI] [PubMed] [Google Scholar]
- 61. Anand SS, Hawkes C, de Souza RJ, Mente A, Dehghan M, Nugent R, Zulyniak MA, Weis T, Bernstein AM, Krauss RM et al.. Food consumption and its impact on cardiovascular disease: importance of solutions focused on the globalized food system: a report from the workshop convened by the World Heart Federation. J Am Coll Cardiol. 2015;66:1590–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Mozaffarian D, Rosenberg I, Uauy R. History of modern nutrition science-implications for current research, dietary guidelines, and food policy. BMJ. 2018;361:k2392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Rhee JJ, Mattei J, Hughes MD, Hu FB, Willett WC. Dietary diabetes risk reduction score, race and ethnicity, and risk of type 2 diabetes in women. Diabetes Care. 2015;38:596–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Zheng Y, Ley SH, Hu FB. Global aetiology and epidemiology of type 2 diabetes mellitus and its complications. Nat Rev Endocrinol. 2018;14:88–98. [DOI] [PubMed] [Google Scholar]
- 65. Estruch R, Ros E, Salas-Salvadó J, Covas M-I, Corella D, Arós F, Gómez-Gracia E, Ruiz-Gutiérrez V, Fiol M, Lapetra J. Primary prevention of cardiovascular disease with a mediterranean diet supplemented with extra-virgin olive oil or nuts. N Engl J Med. 2018;378:e34. [DOI] [PubMed] [Google Scholar]
- 66. Salas-Salvadó J, Bulló M, Estruch R, Ros E, Covas M-I, Ibarrola-Jurado N, Corella D, Arós F, Gómez-Gracia E, Ruiz-Gutiérrez V. Prevention of diabetes with Mediterranean diets: a subgroup analysis of a randomized trial. Ann Intern Med. 2014;160:1–10. [DOI] [PubMed] [Google Scholar]
- 67. Toledo E, Salas-Salvadó J, Donat-Vargas C, Buil-Cosiales P, Estruch R, Ros E, Corella D, Fitó M, Hu FB, Arós F. Mediterranean diet and invasive breast cancer risk among women at high cardiovascular risk in the PREDIMED trial: a randomized clinical trial. JAMA Intern Med. 2015;175:1752–60. [DOI] [PubMed] [Google Scholar]
- 68. Global dietary database [Internet]. Gerald J and Dorothy R Friedman School of Nutrition Science and Policy at Tufts University. 2018. [cited January 21, 2019]. Available from: https://www.globaldietarydatabase.org/. [Google Scholar]
- 69. Micha R, Shulkin ML, Peñalvo JL, Khatibzadeh S, Singh GM, Rao M, Fahimi S, Powles J, Mozaffarian D. Etiologic effects and optimal intakes of foods and nutrients for risk of cardiovascular diseases and diabetes: systematic reviews and meta-analyses from the Nutrition and Chronic Diseases Expert Group (NutriCoDE). PLoS One. 2017;12:e0175149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Katona P, Katona-Apte J. The Interaction between nutrition and infection. Clin Infect Dis. 2008;46:1582–8. [DOI] [PubMed] [Google Scholar]
- 71. Singh GM, Danaei G, Farzadfar F, Stevens GA, Woodward M, Wormser D, Kaptoge S, Whitlock G, Qiao Q, Lewington S. The age-specific quantitative effects of metabolic risk factors on cardiovascular diseases and diabetes: a pooled analysis. PloS One. 2013;8:e65174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Griswold MG, Fullman N, Hawley C, Arian N, Zimsen SR, Tymeson HD, Venkateswaran V, Tapp AD, Forouzanfar MH, Salama JS. Alcohol use and burden for 195 countries and territories, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet. 2018;392:1015–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.