Abstract
Objective
To describe first episodes of bacterial cholangitis complicating autosomal dominant polycystic kidney disease (ADPKD) and autosomal dominant polycystic liver disease (ADPLD) and to identify risk factors for cholangitis episodes among patients with ADPKD-associated polycystic liver disease (PLD).
Patients and Methods
We searched the electronic medical records at our tertiary referral center for episodes of cholangitis in patients with ADPKD or ADPLD from January 1, 1996, through June 30, 2017. Cases were categorized as suspected or definite cholangitis by expert review. Clinical, laboratory, and radiologic data were manually abstracted. A nested case-control study was conducted to investigate risk factors for cholangitis in patients with ADPKD.
Results
We identified 29 cases of definite or suspected cholangitis complicating PLD (24 with ADPKD-associated PLD and 5 with ADPLD). Among patients with definite cholangitis in ADPKD-associated PLD (n=19) vs ADPLD (n=4), the mean ± SD age was 62.4±12.2 vs 55.1±8.6 years, and 9 (47.4%) vs 0 (0%), respectively, were male. The odds of gallstones (odds ratio [OR], 21.6; 95% CI, 3.17-927; P<.001), prior cholecystectomy (OR, 12.2; 95% CI, 1.59-552; P=.008), duodenal diverticulum (OR, 13.5; 95% CI, 2.44 to not estimable; P=.004), type 2 diabetes mellitus (OR, 6.41; 95% CI, 1.01 to not estimable; P=.05), prior endoscopic retrograde cholangiopancreatography (OR, 14.0; 95% CI, 1.80-631; P=.005), and prior kidney transplant (OR, 8.06; 95% CI, 1.72-76.0; P=.004) were higher in patients with ADPKD-associated PLD with definite cholangitis compared to controls.
Conclusion
Gallstones, prior cholecystectomy, duodenal diverticulosis, type 2 diabetes mellitus, prior endoscopic retrograde cholangiopancreatography, and prior kidney transplant constituted risk factors for cholangitis among patients with ADPKD-associated PLD.
Abbreviations and Acronyms: ADPKD, autosomal dominant polycystic kidney disease; ADPLD, autosomal dominant polycystic liver disease; ALP, alkaline phosphatase; ALT, alanine aminotransferase; AST, aspartate aminotransferase; CT, computed tomography; ERCP, endoscopic retrograde cholangiopancreatography; ICD-9, International Classification of Diseases,Ninth Revision; ICD-10, International Classification of Diseases,Tenth Revision; MCR, Mayo Clinic, Rochester, MN; MRI, magnetic resonance imaging; OR, odds ratio; PET, positron emission tomography; PLD, polycystic liver disease; T2DM, type 2 diabetes mellitus
Polycystic liver disease (PLD) is characterized by the presence of more than 20 liver parenchymal cysts1 and is seen as part or all of the phenotype of 2 inherited disorders, autosomal dominant polycystic kidney disease (ADPKD) and autosomal dominant polycystic liver disease (ADPLD). The liver is the most frequent extrarenal site of disease involvement in ADPKD; prevalence of hepatic cysts as identified by magnetic resonance imaging (MRI) was 58% in 15- to 24-year-olds, 85% in 25- to 34-year-olds, and 94% in 35- to 46-year-olds.2 Autosomal dominant polycystic liver disease is a genetically distinct and rarer disease characterized by few or absent renal cysts that predominantly affects females.3, 4
Autosomal recessive polycystic kidney disease is associated with nonobstructive intrahepatic bile duct dilatation (Caroli disease) and recurrent cholangitis.5 Cholangitis has not been recognized traditionally as a consequence of PLD, with descriptions in ADPKD limited to case reports.6, 7, 8, 9 Alterations in extrahepatic bile duct diameter, despite a similar prevalence of gallstones to non-ADPKD controls, are recognized as part of the phenotype of ADPKD.10 Hospitalization rates in the United Kingdom for biliary tract disease were higher in patients with ADPKD than in non-ADPKD controls (relative risk, 2.24; 95% CI, 2.16-2.33).11 Hospitalization for cholangitis in Taiwan during 2000-2010 was higher in patients with ADPKD than in non-ADPKD controls (hazard ratio, 2.41; 95% CI, 1.93-3.01).12
We aimed to describe the presentation and outcomes of first episodes of bacterial cholangitis complicating ADPKD and ADPLD at our tertiary referral center and to identify risk factors for cholangitis in ADPKD-associated PLD.
Patients and Methods
Study Population
The study was approved by the Institutional Review Board at Mayo Clinic, Rochester, Minnesota (MCR). The Advanced Cohort Explorer program was used to search the Mayo Clinic electronic medical records from January 1, 1996, through June 30, 2017, for potentially eligible cases of cholangitis complicating ADPKD or ADPLD. Searching via the Advanced Cohort Explorer tool was conducted using 2015 International Classification of Diseases, Ninth Revision (ICD-9) codes and 2017 International Classification of Diseases, Tenth Revision (ICD-10) codes and text searching of key terms. Patients with ICD diagnosis codes for both cystic kidney (Q61.0-Q61.9 [ICD-10] and 753.1 [ICD-9]) or cystic liver (Q44.6 [ICD-10] and 751.62 [ICD-9]) disease and cholangitis (K83.0-K83.9 [ICD-10] and 576.1 [ICD-9]) were retrieved. Patients with the combination of “polycystic kidney disease,” “polycystic liver disease,” “PKD,” “PLD,” “ADPKD,” or “ADPLD” plus one or more key search terms such as cholangitis, cholangitis and bacteremia, cholangitis and sepsis, biliary sepsis, biliary infection, gram-negative bacteremia, sepsis, and bacteremia in their electronic medical records were also retrieved.
Each retrieved case was independently reviewed for features suggestive of bacterial cholangitis and for the presence of ADPKD or ADPLD (W.P.M.). Cases with one or more of the following features were considered suspicious for cholangitis and were selected for further review: abrupt onset of systemic symptoms (fevers/chills), right upper quadrant pain, jaundice, liver biochemistry derangements, biliary dilatation on imaging, and absence of a clear alternative source of infection other than the biliary tract.
Diagnosis of ADPKD and ADPLD
Diagnosis of ADPKD was based on family history and radiologic criteria according to the modified Pei criteria.13 Diagnosis of ADPLD was based on family history and radiologic criteria (presence of ≥20 hepatic cysts).3 Diagnosis of ADPKD and ADPLD among cholangitis cases was assigned on a case-by-case basis after independent review of patients’ radiologic findings and family history by an experienced physician (P.S.K., M.C.H.).
Diagnosis of Cholangitis
Because patients with ADPKD or ADPLD have a high rate of biliary dilatation in the absence of biliary tract infection or obstruction,10 specificity of conventional diagnostic criteria for the diagnosis of cholangitis is diminished. We employed the following diagnostic criteria and assigned 2 levels of certainty to the diagnosis of cholangitis: (1) definite cholangitis—(a) positive peripheral blood culture results with transient elevations in liver biochemistry values (>1.5 times the upper limit of normal for alkaline phosphatase [ALP], aspartate aminotransferase [AST], alanine aminotransferase [ALT], or total bilirubin) and absence of an alternative source of infection or (b) systemic symptoms (fevers/chills) with transient elevations in liver biochemistry results (>1.5 times the upper limit of normal for ALP, AST, ALT, or total bilirubin) and absence of an alternative source of infection; (2) suspected cholangitis—systemic symptoms (fevers/chills) with or without positive peripheral blood culture results in the presence of normal liver biochemistry values and the absence of an alternative source of infection (including kidney or liver cyst infection). The Tokyo Guidelines 2018 consensus criteria for cholangitis diagnosis were also applied to the final study cohort to determine the accuracy of assigned cholangitis diagnoses.14 To minimize the impact of early iatrogenic cholangitis on study findings, first cholangitis episodes occurring 14 or fewer days after liver biopsy, liver surgery, or endoscopic retrograde cholangiopancreatography (ERCP) were excluded from analysis.
Data Collection
Patients admitted to the hospital at MCR with definite or suspected cholangitis were considered inpatient cholangitis cases. Patients who were admitted to the hospital locally with episodes of cholangitis during the course of outpatient follow-up for ADPKD or ADPLD at MCR were considered outpatient cholangitis cases. Although less inpatient data were available for the outpatient cholangitis group, sufficient information necessary to make the diagnosis of definite or suspected cholangitis according to the aforementioned criteria was available. Clinical, laboratory, and radiologic data were manually abstracted from the Mayo Clinic electronic medical records, and liver volumes and bile duct diameters were measured for the cholangitis cases and controls (Supplemental Appendix 1, available online at http://mcpiqojournal.org).
Nested Case-Control Analysis
We conducted a nested case-control study to investigate risk factors for first cholangitis episode in patients with ADPKD. Patients with ADPLD were excluded from this analysis. Patients with definite cholangitis in the setting of ADPKD-associated PLD (n=19) were considered cases; 5 patients with suspected cholangitis in the setting of ADPKD-associated PLD were excluded. Patients with ADPKD-associated PLD in whom cholangitis did not develop were identified from the ADPKD registry at MCR as potential controls. This registry is composed of patients with ADPKD as identified by ICD-9 (753.1) and ICD-10 (Q61.0-61.9) billing codes. Patients entered into the registry were reviewed and confirmed to have ADPKD (Z.E.Z.).
For each patient with ADPKD-associated PLD who had cholangitis, 2 patients without cholangitis who were seen at MCR both before and after the case’s first cholangitis episode were randomly selected as controls. Medical records were reviewed to confirm that these individuals did not have cholangitis (W.P.M.). The matching criteria chosen were: (1) date of birth ± 5 years and (2) sex. The index date for cases and controls was defined as the date of diagnosis of first cholangitis in the cases.
Statistical Analyses
Patient characteristics were summarized and reported using mean ± SD or median (interquartile range) for continuous variables and frequency (percentage) for categorical variables. Among cases, comparisons between PLD disease type and cholangitis type were evaluated using equal variance t tests and Wilcoxon rank sum tests for numerical and ordinal variables and χ2 and Fisher exact tests for categorical variables. Odds ratios and P values were calculated using conditional logistic regression models adjusting for matching when comparing cases and controls in patients with ADPKD and associated PLD. All calculated P values were 2-sided, and P≤.05 was considered statistically significant. Analyses were performed using SAS statistical software, version 9.4 (SAS Institute).
Results
We identified 745 cases of possible bacterial cholangitis complicating ADPKD or ADPLD. Four patients were excluded because of refusal of Minnesota research authorization, and the remaining patients’ medical records were retrieved and reviewed (W.P.M.). Among these cases, 362 were excluded because they did not have ADPKD or ADPLD, and a further 310 cases were excluded because they did not have features suspicious for cholangitis. Among the remaining cases, patients were also excluded for presentation to Mayo Clinic sites other than MCR (n=26).
After these exclusions, 43 cases highly suspicious for cholangitis arising in patients with ADPKD or ADPLD were selected for independent review by 2 clinicians experienced in the diagnosis of cholangitis in patients with PLD (P.S.K., M.C.H.). Among these cases, 14 patients were removed from the final cohort because of uncertainty regarding the diagnosis of ADPKD/ADPLD (n=4), iatrogenic cholangitis (defined as first cholangitis episode occurring ≤14 days after liver biopsy, liver surgery, or ERCP [n=5]), infected hepatic cysts rather than cholangitis (n=2), secondary sclerosing cholangitis rather than bacterial cholangitis (n=1), no convincing evidence for cholangitis (n=1), and autosomal recessive polycystic kidney disease rather than ADPKD (n=1). Specifically, for iatrogenic cholangitis cases, 1 patient was excluded for cholangitis arising in the setting of hemobilia after liver biopsy, 2 for obstructed bile duct stents after ERCP, and 2 after hepatectomy complicated by bile leak. A flowchart summarizing selection of the final study cohort is presented in Supplemental Figure 1 (available online at http://mcpiqojournal.org).
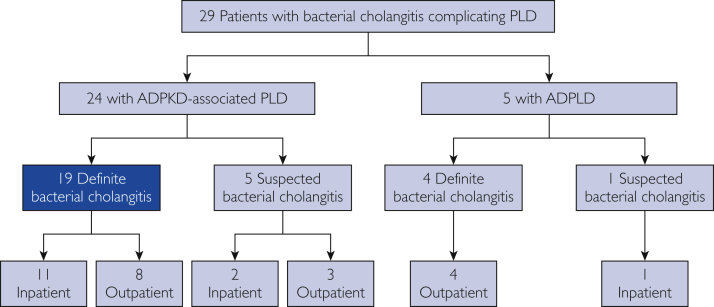
As outlined in the Figure, there were 29 cases retained in the final cohort (24 with ADPKD-associated PLD and 5 with ADPLD). Among patients with ADPKD-associated PLD, there were 19 definite cases of cholangitis and 5 suspected cases of cholangitis based on clinical review and application of the diagnostic criteria for cholangitis in patients with PLD; among patients with ADPLD, 4 definite cases of cholangitis and 1 suspected case of cholangitis were identified. In patients with APDKD-associated PLD, there were 13 definite or suspected cases of cholangitis for which inpatient data during the cholangitis episode were available at MCR, while there were 11 patients who presented to MCR in the outpatient setting after inpatient hospitalization with definite or suspected cholangitis at another institution. In patients with ADPLD, there was 1 case of suspected cholangitis for which inpatient data during the episode were available at MCR, while 4 definite cases of cholangitis presented to MCR in the outpatient setting after inpatient hospitalization at another institution. When the Tokyo Guidelines 2018 consensus criteria14 for the diagnosis of cholangitis were applied to the final cohort of cholangitis cases (n=29), 21 were classified as definite (17 ADPKD and 4 ADPLD) and 8 were classified as suspected (7 ADPKD and 1 ADPLD) cholangitis. Given the lack of specificity of these criteria in the setting of PLD, final classification of the study cohort was based on our diagnostic criteria for cholangitis outlined previously.
Figure.
Overview of cholangitis cases occurring in patients with polycystic liver disease (PLD). ADPKD = autosomal dominant polycystic kidney disease; ADPLD = autosomal dominant polycystic liver disease.
Baseline characteristics of patients with ADPKD and ADPLD at the time of the first cholangitis episode are presented in Table 1. Males comprised 54.2% (13 of 24) and 20.0% (1 of 5) of patients with definite or suspected cholangitis in the ADPKD and ADPLD groups, respectively (P=.33). Age and ethnicity were similar between the ADPKD and ADPLD subgroups (P=.68 and P>.99, respectively). Among patients with definite cholangitis in ADPKD-associated PLD (n=19) vs ADPLD (n=4), the mean ± SD age was 62.4 ± 12.2 vs 55.1 ± 8.6 years, and 9 (47.4%) vs 0 (0%), respectively, were male. Genetic mutations were identified in 5 of 24 patients with ADPKD (20.8%) (truncating PKD1 [for expansion of gene symbols, use search tool at www.genenames.org] mutation in 3 and nontruncating PKD1 mutation in 2) and 2 of 5 patients with ADPLD (40.0%) (both with PRKCSH mutation). Among patients with ERCP before the first cholangitis episode, 6 of 8 patients with ADPKD-associated PLD (75.0%) and 1 of 2 patients with ADPLD (50.0%) had a sphincterotomy performed at the time of ERCP (P>.99). Sphincterotomy indications for these 7 individuals are presented in Supplemental Table 1 (available online at http://mcpiqojournal.org). There were no significant differences observed in the number of ERCPs performed before the development of the first cholangitis episode or antibiotic therapy during the first cholangitis episode between the ADPKD and ADPLD subgroups (P=.53). Although there was a higher rate of blood culture positivity in the ADPKD group compared with the ADPLD group, results were not statistically significant (P=.05). In particular, there was a higher rate of bloodstream infections with gram-negative bacilli in patients with ADPKD (68.2% [15 of 22 patients]) compared with ADPLD (20.0% [1 of 5 patients]). More patients in the ADPKD group underwent kidney transplant before the first cholangitis episode than in the ADPLD group (14 of 24 [58.3%] vs 0 of 5 [0%], respectively; P=.04). Among the 14 patients in the ADPKD group with a kidney transplant, median time from first kidney transplant to first cholangitis episode in patients with ADPKD was 13.3 years (interquartile range, 7.2-16.1 years). No patients with cholangitis in either the ADPKD or ADPLD subgroups underwent liver transplant prior to index date (results not shown).
Table 1.
Baseline Characteristics of First Cholangitis Episode Occurring in Patients With ADPKD-Associated PLD and ADPLD, Stratified by Definite and Suspected Diagnosis of Cholangitisa
| Variable | ADPKD-associated PLD (n=24) |
ADPLD (n=5) |
Total (n=29) |
||||
|---|---|---|---|---|---|---|---|
| Definite (n=19) | Suspected (n=5) | Definite (n=4) | Suspected (n=1) | ADPKD with PLD (n=24) | ADPLD (n=5) | P valueb | |
| Demographic characteristics | |||||||
| Age (y) at first cholangitis episode, mean ± SD | 62.4±12.2 | 53.9±9.3 | 55.1±8.6 | 70.9 | 60.6±12.0 | 58.2±10.3 | .68 |
| Male, No. (%) | 9 (47.4) | 4 (80.0) | 0 (0.0) | 1 (100.0) | 13 (54.2) | 1 (20.0) | .33 |
| White, No. (%) | 17 (89.5) | 3 (60.0) | 4 (100.0) | 1 (100.0) | 20 (83.3) | 5 (100.0) | >.99 |
| Patients with ERCP before first cholangitis episode | n=7 | n=1 | n=2 | n=0 | n=8 | n=2 | |
| No. of ERCP episodes, No. (%) | .53 | ||||||
| 1 | 5 (71.4) | 1 (100.0) | 1 (50.0) | 0 (0) | 6 (75.0) | 1 (50.0) | |
| 2 | 1 (14.3) | 0 (0.0) | 1 (50.0) | 0 (0) | 1 (12.5) | 1 (50.0) | |
| 3 | 1 (14.3) | 0 (0.0) | 0 (0.0) | 0 (0) | 1 (12.5) | 0 (0.0) | |
| Time between ERCP and cholangitis episodes (y),c median (IQR) | 0.6 (0.1-2.8) | 1.4 | 1.9 (0.9-2.9) | 0 (0) | 1.0 (0.2-2.5) | 1.9 (0.9-2.9) | .51 |
| Sphincterotomy,d No. (%) | 6 (85.7) | 0 (0.0) | 1 (50.0) | 0 (0) | 6 (75.0) | 1 (50.0) | >.99 |
| Gallstone extraction,d No. (%) | 2 (28.6) | 0 (0.0) | 0 (0.0) | 0 (0) | 2 (25.0) | 0 (0.0) | >.99 |
| Sludge removal,d No. (%) | 1 (14.3) | 0 (0.0) | 0 (0.0) | 0 (0) | 1 (12.5) | 0 (0.0) | >.99 |
| Plastic biliary stent,d No. (%) | 2 (28.6) | 1 (100.0) | 1 (50.0) | 0 (0) | 3 (37.5) | 1 (50.0) | >.99 |
| Microbiology | n=17 | n=5 | n=4 | n=1 | n=22 | n=5 | |
| Blood culture,e No. (%) | .05 | ||||||
| Gram-negative bacilli | 12 (70.6) | 3 (60.0) | 1 (25.0) | 0 (0.0) | 15 (68.2) | 1 (20.0) | |
| Gram-positive cocci | 0 (0.0) | 0 (0.0) | 1 (25.0) | 0 (0.0) | 0 (0.0) | 1 (20.0) | |
| Negative | 5 (29.4) | 2 (40.0) | 2 (50.0) | 1 (100.0) | 7 (31.8) | 3 (60.0) | |
| Antibiotic therapy | n=13 | n=3 | n=3 | n=1 | n=16 | n=4 | |
| Antibiotic treatment,f No. (%) | .87 | ||||||
| Monobactam | 1 (7.7) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (6.3) | 0 (0.0) | |
| Fluoroquinolone | 6 (46.2) | 1 (33.3) | 1 (33.3) | 1 (100.0) | 7 (43.8) | 2 (50.0) | |
| Carbapenem | 3 (23.1) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 3 (18.8) | 0 (0.0) | |
| Penicillin–β-lactamase inhibitor | 2 (15.4) | 2 (66.7) | 2 (66.7) | 0 (0.0) | 4 (25.0) | 2 (50.0) | |
| Glycopeptide | 1 (7.7) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (6.3) | 0 (0.0) | |
| Duration of antibiotic use (d), median (IQR) | 14.0 (10.0-17.0) | 14.0 (14.0-28.0) | 19.0 (10.0-28.0) | 10.0 | 14.0 (12.0-19.0) | 10.0 (10.0-28.0) | .82 |
| ERCP treatment | n=19 | n=5 | n=4 | n=1 | n=24 | n=5 | |
| Patients with ERCP for treatment of first cholangitis episode,g No. (%) | 10 (52.6) | 4 (80.0) | 1 (25.0) | 0 (0.0) | 14 (58.3) | 1 (20.0) | .17 |
| ERCP with gallstone removal | 7 (36.8) | 3 (60.0) | 0 (0.0) | 0 (0.0) | 10 (41.7) | 0 (0.0) | .13 |
| ERCP with sludge removal | 5 (26.3) | 1 (20.0) | 0 (0.0) | 0 (0.0) | 6 (25.0) | 0 (0.0) | .55 |
| ERCP with plastic biliary stenting | 5 (26.3) | 2 (40.0) | 0 (0.0) | 0 (0.0) | 7 (29.2) | 0 (0.0) | .30 |
ADPKD = autosomal dominant polycystic kidney disease; ADPLD = autosomal dominant polycystic liver disease; ERCP = endoscopic retrograde cholangiopancreatography; IQR = interquartile range; PLD = polycystic liver disease.
P values were derived using equal variance t tests and Wilcoxon rank sum tests for continuous variables and χ2 tests and Fisher exact tests for categorical variables.
Time between last ERCP before first cholangitis episode and first cholangitis episode.
Before first cholangitis episode.
Gram-negative bacilli: Klebsiella spp (n=5), Klebsiella oxytoca (n=2), Klebsiella aerogenes (n=1), Klebsiella pneumoniae (n=1), Escherichia coli (n=5), Acinetobacter spp (n=1), gram-negative bacillus without further speciation (n=1). Gram-positive cocci: Enterococcus faecium (n=1). No multidrug-resistant organisms isolated.
Antibiotic treatment: piperacillin-tazobactam (n=6), ciprofloxacin (n=5), levofloxacin (n=4), ertapenem (n=2), imipenem (n=1), aztreonam (n=1), vancomycin (n=1).
ERCPs performed at or within 6 months after first cholangitis episode were considered to be performed for treatment of cholangitis.
Risk factors for onset of the first cholangitis episode in patients with ADPKD-associated PLD are presented in Table 2. The odds of gallstones (73.7% [14 of 19 patients] vs 15.8% [6 of 38 controls]; OR, 21.6; 95% CI, 3.17-927; P<.001), prior cholecystectomy (47.4% [9 of 19 patients] vs 13.2% [5 of 38 controls]; OR, 12.2; 95% CI, 1.59-552; P=.008), duodenal diverticulosis (27.8% [5 of 18 patients] vs 0% [0 of 38 controls]; OR, 13.5; 95% CI, 2.44 to not estimable; P=.004), type 2 diabetes mellitus (T2DM) (21.1% [4 of 19 patients] vs 5.3% [2 of 38 controls]; OR, 6.4; 95% CI, 1.01 to not estimable; P=.05), prior ERCP (36.8% [7 of 19 patients] vs 2.6% [1 of 38 controls]; OR, 14.0; 95% CI, 1.80-631; P=.005), and previous kidney transplant (68.4% [13 of 19 patients] vs 23.7% [9 of 38 controls]; OR, 8.06; 95% CI, 1.72-76.0, P=.004) were higher in patients with cholangitis compared to controls. Duodenal diverticula were present in 5 individuals with definite cholangitis, all with ADPKD (Supplemental Table 2, available online at http://mcpiqojournal.org). Duodenal diverticula were visible by abdominal cross-sectional imaging in all cases (4 on computed tomography [CT] and 1 on MRI). Four of the 5 individuals with duodenal diverticula underwent ERCP; the diverticula were visible by ERCP in each of these cases. Representative images of risk factors for cholangitis in patients with PLD in this cohort are presented in Supplemental Figure 2 (available online at http://mcpiqojournal.org).
Table 2.
Characteristics of Definite Cases of Cholangitis and Controls Without Cholangitis in ADPKD-Associated PLDa
| Variable | Cases (n=19) | Controls (n=38) | OR (95% CI)b | P valueb |
|---|---|---|---|---|
| Demographic characteristics | ||||
| Age (y),c mean ± SD | 62.4±12.2 | 62.0±11.3 | ||
| Male,c No. (%) | 9 (47.4) | 18 (47.4) | ||
| White, No. (%) | 17 (89.5) | 36 (94.7) | 0.50 (0.04-6.90) | .82 |
| Comorbiditiesd | ||||
| Hypertension, No. (%) | 11 (57.9) | 34 (89.5) | 0.14 (0.01-0.75) | .02 |
| Type 2 diabetes mellitus, No. (%) | 4 (21.1) | 2 (5.3) | 6.41 (1.01-NE) | .05 |
| HD, No. (%) | 1 (5.3) | 5 (13.2) | 0.35 (0.006-3.89) | .66 |
| Gallstones, No. (%) | 14 (73.7) | 6 (15.8) | 21.6 (3.17-927) | <.001 |
| Duodenal diverticulum, No. (%) | 5/18 (27.8) | 0 (0.0) | 13.5 (2.44-NE) | .004 |
| Surgical proceduresd | ||||
| Kidney transplant, No. (%) | 13 (68.4) | 9 (23.7) | 8.06 (1.72-76.0) | .004 |
| Living donor, No. (%) | 6 (46.2) | 7 (77.8) | ||
| Deceased donor, No. (%) | 6 (46.2) | 2 (22.2) | ||
| Unknown donor status, No. (%) | 1 (7.7) | 0 (0.0) | ||
| Nephrectomy, No. (%) | 5 (26.3) | 5 (13.2) | 3.07 (0.4-34.5) | .35 |
| ERCP procedure, No. (%) | 7 (36.8) | 1 (2.6) | 14.0 (1.80-631) | .005 |
| Cholecystectomy, No. (%) | 9 (47.4) | 5 (13.2) | 12.2 (1.59-552) | .008 |
| Liver biopsy, No. (%) | 2 (10.5) | 1 (2.6) | 4.00 (0.21-236) | .52 |
| Cyst fenestration, No. (%) | 1 (5.3) | 6 (15.8) | 0.29 (0.006-2.83) | .48 |
| Hepatic resection, No. (%) | 1 (5.3) | 3 (7.9) | 0.59 (0.008-13.4) | >.99 |
| Imaging characteristicse | n=17 | n=31 | ||
| Liver volume (mL),f mean ± SD | 2609±1117 | 2499±1438 | 1.01 (0.97-1.06) | .64 |
| Height-adjusted liver volume (mL/m),g mean ± SD | 1515±655 | 1471±848 | 1.02 (0.94-1.10) | .69 |
| Bile duct diameters | n=11 | n=22 | ||
| Average extrahepatic duct diameter (mm),h mean ± SD | 8.2±2.0 | 5.1±0.9 | 4.78 (0.75-30.5) | .10 |
| Average intrahepatic duct diameter (mm),i mean ± SD | 6.2±2.7 | 4.0±1.3 | NE | NE |
ADPKD = autosomal dominant polycystic kidney disease; ERCP = endoscopic retrograde cholangiopancreatography; HD = hemodialysis; NE = not estimable; OR = odds ratio; PLD = polycystic liver disease.
Odds ratios and P values were derived from conditional logistic regression models.
Cases and controls were matched by date of birth and sex, so no P values are reported for these variables.
Comorbidities and surgical procedures were considered if they occurred before the index date. Index date refers to date of diagnosis of first episode of cholangitis among cases.
Imaging was obtained between 3 years before and up to 3 years after index date.
OR per 100 mL.
OR per 100 mL/m.
Average of common hepatic, upper common bile duct, and lower common bile duct diameters.
Average of left and right intrahepatic duct diameters.
Six of 7 patients (85.7%) who had ERCP before the first cholangitis episode had sphincterotomy performed at the time of ERCP, and 1 of 1 controls (100%) who had ERCP had sphincterotomy performed at the time of ERCP. Only 57.9% of cholangitis cases (11 of 19 patients) had hypertension before the first cholangitis episode, compared with 89.5% of age- and sex-matched controls (34 of 38) (OR, 0.14; 95% CI, 0.01-0.75; P=.02).
Liver volumes were measured in 17 of 19 cases (89.5%) and 31 of 38 controls (81.6%) who had suitable imaging available. Magnetic resonance imaging was used for liver volume measurements in 5 of 17 cases (29.4%) and 16 of 31 controls (51.6%), while CT imaging was used for 12 cases (70.6%) and 15 controls (48.4%). Mean ± SD unadjusted (2609±1117 vs 2499±1438 mL; P=.64) and height-adjusted (1515±655 vs 1471±848 mL/m; P=.69) liver volumes were similar between cholangitis cases and controls, respectively.
Available imaging for measurement of bile duct diameters included contrast-enhanced CT (5 of 33 [15.2%]), contrast-enhanced MRI (3 of 33 [9.1%]), contrast-enhanced MRI and non–contrast-enhanced CT (1 of 33 [3.03%]), non–contrast-enhanced CT (9 of 33 [27.3%]), non–contrast-enhanced MRI (10 of 33 [30.3%]), and non–contrast-enhanced MRI and non–contrast-enhanced CT (5 of 33 [15.2%]). Average extrahepatic bile duct diameter was not significantly different between cholangitis cases and controls (8.2±2.0 vs 5.1±0.9 mm; OR, 4.78; 95% CI, 0.75-30.5; P=.10).
Characteristics of first episode of definite cholangitis in ADPKD-associated PLD among 11 inpatients who presented to MCR are presented in Table 3. All patients presented with systemic symptoms, but none presented with clinical evidence of jaundice. A majority of patients presented with elevated white blood cell count or C-reactive protein level (8 of 11 [72.7%]) and had evidence of gallstones (8 of 11 [72.7%]) or biliary dilatation (6 of 11 [54.5%]) on imaging. A cholestatic pattern of liver biochemistry results was observed at first presentation for the group as a whole (mean direct bilirubin, 1.2±1.3 mg/dL [to convert values to μmol/L, multiply by 17.104]; mean ALP, 223.1±109.6 IU/L [to convert values to μkat/L, multiply by 0.0167), with a milder degree of transaminitis evident (mean AST, 161.2±220.1 IU/L [to convert values to μkat/L, multiply by 0.01667]; mean ALT, 118.3±121.3 IU/L [to convert values to μkat/L, multiply by 0.0167]). Impaired hepatic synthetic and kidney function were present at initial presentation for the group as a whole, with mean total bilirubin elevated at 1.6±1.4 mg/dL, mean international normalized ratio elevated at 1.5±0.5, mean albumin at the lower limit of normal at 3.5±0.8 g/dL (to convert to g/L, multiply by 10), and mean serum creatinine elevated at 1.6±0.8 mg/dL (to convert to μmol/L, multiply by 88.4).
Table 3.
| Presentation | No. of patients | Value |
|---|---|---|
| Symptoms/signs | ||
| Fever/chills, No. (%) | 11 | 11 (100.0) |
| Jaundice, No. (%) | 11 | 0 (0.00) |
| Imaging | ||
| Biliary dilatation, No. (%) | 11 | 6 (54.5) |
| Gallstones, No. (%) | 11 | 8 (72.7) |
| CKD stage, No. (%) | 11 | |
| 1 | 0 (0.00) | |
| 2 | 2 (18.2) | |
| 3A | 3 (27.3) | |
| 3B | 4 (36.4) | |
| 4 | 1 (9.1) | |
| 5 | 1 (9.1) | |
| Laboratory valuesc | ||
| Elevated WBC/CRP, No. (%) | 11 | 8 (72.7) |
| Total bilirubin (mg/dL) | 10 | 1.6±1.4 |
| Direct bilirubin (mg/dL) | 8 | 1.2±1.3 |
| Lactate (mmol/L) | 6 | 1.6±1.2 |
| INR | 7 | 1.5±0.5 |
| Creatinine (mg/dL) | 11 | 1.6±0.8 |
| Albumin (g/dL) | 9 | 3.5±0.8 |
| ALP (IU/L) | 11 | 223.1±109.6 |
| AST (IU/L) | 10 | 161.2±220.1 |
| ALT (2IU/L) | 9 | 118.3±121.3 |
ADPKD = autosomal dominant polycystic kidney disease; ALP = alkaline phosphatase; ALT = alanine aminotransferase; AST = aspartate aminotransferase; CKD = chronic kidney disease; CRP = C-reactive protein; INR = international normalized ratio; PLD = polycystic liver disease; WBC = white blood cell count.
SI conversion factors: To convert total and direct bilirubin values to μmol/L, multiply by 17.104; to convert creatinine value to μmol/L, multiply by 88.4; to convert albumin value to g/L, multiply by 10; to convert ALP and ALT values to μkat/L, multiply by 0.0167; to convert AST value to μkat/L, multiply by 0.01667.
Laboratory values are presented as mean ± SD unless indicated otherwise.
Positron emission tomography (PET)–CT was performed at the time of first presentation with cholangitis for 3 individuals: 2 cases of definite cholangitis (July 2007 and July 2015, respectively) and 1 case of suspected cholangitis (September 2015). PET-CT revealed evidence of cholangitis in both definite cases of cholangitis. For the suspected cholangitis case, PET-CT revealed evidence of peripheral fluorodeoxyglucose activity in the posterior segment of the right lower lobe. Subsequent hepatic cyst aspiration yielded negative results in this individual, while clinical features suggestive of cholangitis including elevated liver enzyme levels were present. Additionally, in one individual with definite cholangitis, PET-CT performed on recurrent presentation with similar symptoms 6 months later (July 2017) revealed evidence of cholangitis.
Discussion
In this study, impaired hepatic synthetic and renal function was common at first cholangitis presentation in patients with ADPKD. The former is not commonly attributable to PLD15 and so may represent hepatic synthetic dysfunction due to severe biliary infection. Patients with negative results on blood cultures comprised 31.8% and 60.0% of first cholangitis episodes in patients with ADPKD-associated PLD and ADPLD, respectively. This finding was similar to that in the cohort presented by Judge et al,11 in which approximately 50% of biliary infections were culture-negative. Additionally, median duration of antibiotic use for first cholangitis episode was 14 days and 10 days in patients with ADPKD and ADPLD, respectively, similar to acute cholangitis treatment guidelines16 and less than the typical duration of parenteral antimicrobial therapy for hepatic cyst infections.17
Among the ADPKD cases, gallstones and prior cholecystectomy were identified as risk factors for first cholangitis episode. The identification of prior cholecystectomy as a risk factor for cholangitis was unexpected and possibly explained by a residual risk of cholangitis in gallstone formers following cholecystectomy (due to retained common bile duct gallstones, the prevalence of which has been estimated at 1.8% in a Korean population who had cholecystectomy performed for symptomatic cholelithiasis18).
Duodenal diverticula increased risk for cholangitis in patients with ADPKD-associated PLD. Colonic diverticulosis is a recognized extrarenal manifestation in patients with end-stage renal disease related to ADPKD,19 although the association between ADPKD and extracolonic diverticulosis is less certain. In a series of 8 cases of duodenal diverticula arising in patients with ADPKD, 6 had development of biliary complications.20 Two-thirds of duodenal diverticula arise near the ampulla of Vater in the second part of the duodenum,21 where they have the potential to result in common bile duct dilatation and obstruction. Duodenal diverticula may also be associated with a higher rate of common bile duct stone formation.22 Duodenal diverticula can make cannulation of the common bile duct at ERCP more challenging,20 which may increase risk of cholangitis. However, only 2 of 5 individuals with duodenal diverticula in our study had ERCP performed before the onset of the first cholangitis episode, and the timing of ERCP was remote from cholangitis onset (804 and 153 days previously, respectively). Thus, duodenal diverticula appear to increase risk of cholangitis independently of ERCP in individuals with ADPKD-associated PLD.
Some patients who underwent sphincterotomy before development of cholangitis lacked a clear indication for this procedure. In our experience, it is common for sphincterotomy to be performed when patients with ADPKD present with fever and sources of infection other than the biliary tract are not readily apparent. The high prevalence of extrahepatic ductal dilatation in patients with ADPKD10 may erroneously implicate the biliary tract as a source of infection in this setting. Once sphincterotomy is performed, we postulate that a nidus for infection develops through reflux of intestinal bacteria into the biliary tree, something that is exacerbated by extrinsic compression of the biliary tree in the setting of PLD.23 Prior kidney transplant was also a risk factor for first cholangitis episode, likely explained by the accompanying immunosuppressed state increasing risk for intra-abdominal infection.24 Similarly, increased cholangitis risk in patients with ADPKD and T2DM in our cohort may reflect impaired innate and adaptive aspects of immunity related to hyperglycemia.25
We present a small cohort of individuals with definite and suspected cholangitis episodes complicating PLD. The small sample number of definite cholangitis episodes complicating APDKD-associated PLD (19) resulted in limited statistical power in the identification of risk factors for first cholangitis episode. However, cholangitis is a relatively rare complication in PLD. This is the first study to describe presentation and management of cholangitis in the PLD population and to identify risk factors for cholangitis in patients with ADPKD. It was not possible to accurately quantify the number of cholangitis episodes per patient or the number of patients treated with antimicrobial prophylaxis through retrospective medical record review. PET-CT imaging was performed with increasing frequency at presentation with first cholangitis episode toward the latter end of the study period (once in 2007 and twice in 2015), reflecting increasing availability of this imaging modality at our institution. This factor may have increased the yield of cholangitis diagnoses toward the latter end of the study period, although its impact on selection of our final study cohort was small given that it confirmed the diagnosis of cholangitis in just 2 individuals.
Conclusion
Gallstones, prior cholecystectomy, duodenal diverticulosis, T2DM, prior ERCP, and kidney transplant were identified as risk factors for cholangitis among patients with ADPKD-associated PLD in this cohort. A clear indication for sphincterotomy at ERCP, when performed before the first cholangitis episode, was lacking in some. Given the high prevalence of extrahepatic bile duct dilatation in the absence of biliary infection in patients with ADPKD, one should carefully consider the need for sphincterotomy at the time of ERCP in patients with ADPKD presenting with fever.
Footnotes
Potential Competing Interests: Dr Kremers has received research funding from AstraZeneca, Biogen, and Roche. The other authors report no competing interests.
Supplemental Online Material
Supplemental material can be found online at http://mcpiqojournal.org. Supplemental material attached to journal articles has not been edited, and the authors take responsibility for the accuracy of all data.
References
- 1.Drenth J.P., Chrispijn M., Nagorney D.M., Kamath P.S., Torres V.E. Medical and surgical treatment options for polycystic liver disease. Hepatology. 2010;52(6):2223–2230. doi: 10.1002/hep.24036. [DOI] [PubMed] [Google Scholar]
- 2.Bae K.T., Zhu F., Chapman A.B., et al. Consortium for Radiologic Imaging Studies of Polycystic Kidney Disease (CRISP). Magnetic resonance imaging evaluation of hepatic cysts in early autosomal-dominant polycystic kidney disease: the Consortium for Radiologic Imaging Studies of Polycystic Kidney Disease cohort. Clin J Am Soc Nephrol. 2006;1(1):64–69. doi: 10.2215/CJN.00080605. [DOI] [PubMed] [Google Scholar]
- 3.Qian Q., Li A., King B.F., et al. Clinical profile of autosomal dominant polycystic liver disease. Hepatology. 2003;37(1):164–171. doi: 10.1053/jhep.2003.50006. [DOI] [PubMed] [Google Scholar]
- 4.Hoevenaren I.A., Wester R., Schrier R.W., et al. Polycystic liver: clinical characteristics of patients with isolated polycystic liver disease compared with patients with polycystic liver and autosomal dominant polycystic kidney disease. Liver Int. 2008;28(2):264–270. doi: 10.1111/j.1478-3231.2007.01595.x. [DOI] [PubMed] [Google Scholar]
- 5.Yonem O., Bayraktar Y. Clinical characteristics of Caroli’s syndrome. World J Gastroenterol. 2007;13(13):1934–1937. doi: 10.3748/wjg.v13.i13.1934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Deutsch E. Congenital cystic disease of the kidneys and liver complicated by cholangitis. Gastroenterology. 1953;23(1):92–96. [PubMed] [Google Scholar]
- 7.Yazdanpanah K., Manouchehri N., Hosseinzadeh E., Emami M.H., Karami M., Sarrami A.H. Recurrent acute pancreatitis and cholangitis in a patient with autosomal dominant polycystic kidney disease. Int J Prev Med. 2013;4(2):233–236. [PMC free article] [PubMed] [Google Scholar]
- 8.Mahadeva S., Bux S.I., Goh K.-L. Endoscopic decompression may not relieve biliary obstruction caused by polycystic liver disease: report of a case from Malaysia. Dig Endosc. 2003;15(3):216–218. [Google Scholar]
- 9.Figurelli H.G., Laurie T., Chi K.D. Therapeutic challenge of ERCP for biliary decompression in a patient with autosomal dominant polycystic kidney disease and polycystic liver disease and pancreaticoduodenectomy anatomy. Gastrointest Endosc. 2012;75(4):896–897. doi: 10.1016/j.gie.2011.11.015. [DOI] [PubMed] [Google Scholar]
- 10.Ishikawa I., Chikamoto E., Nakamura M., Asaka M., Tomosugi N., Yuri T. High incidence of common bile duct dilatation in autosomal dominant polycystic kidney disease patients. Am J Kidney Dis. 1996;27(3):321–326. doi: 10.1016/s0272-6386(96)90353-4. [DOI] [PubMed] [Google Scholar]
- 11.Judge P.K., Harper C.H.S., Storey B.C., et al. Biliary tract and liver complications in polycystic kidney disease. J Am Soc Nephrol. 2017;28(9):2738–2748. doi: 10.1681/ASN.2017010084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huang S.-T., Chuang Y.-W., Yu T.-M., Lin C.-L., Jeng L.-B. Hepatointestinal complications in polycystic kidney disease. Oncotarget. 2017;8(46):80971–80980. doi: 10.18632/oncotarget.20901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pei Y., Obaji J., Dupuis A., et al. Unified criteria for ultrasonographic diagnosis of ADPKD. J Am Soc Nephrol. 2009;20(1):205–212. doi: 10.1681/ASN.2008050507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kiriyama S., Kozaka K., Takada T., et al. Tokyo Guidelines 2018: diagnostic criteria and severity grading of acute cholangitis (with videos) J Hepatobiliary Pancreat Sci. 2018;25(1):17–30. doi: 10.1002/jhbp.512. [DOI] [PubMed] [Google Scholar]
- 15.Hogan M.C., Abebe K., Torres V.E., et al. Liver involvement in early autosomal-dominant polycystic kidney disease. Clin Gastroenterol Hepatol. 2015;13(1):155–164.e6. doi: 10.1016/j.cgh.2014.07.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Solomkin J.S., Mazuski J.E., Bradley J.S., et al. Diagnosis and management of complicated intra-abdominal infection in adults and children: guidelines by the Surgical Infection Society and the Infectious Diseases Society of America. Surg Infect (Larchmt) 2010;11(1):79–109. doi: 10.1089/sur.2009.9930. [DOI] [PubMed] [Google Scholar]
- 17.Telenti A., Torres V.E., Gross J.B., Jr., Van Scoy R.E., Brown M.L., Hattery R.R. Hepatic cyst infection in autosomal dominant polycystic kidney disease. Mayo Clin Proc. 1990;65(7):933–942. doi: 10.1016/s0025-6196(12)65154-4. [DOI] [PubMed] [Google Scholar]
- 18.Lee D., Ahn Y.J., Lee H.W., Chung J.K., Jung I.M. Prevalence and characteristics of clinically significant retained common bile duct stones after laparoscopic cholecystectomy for symptomatic cholelithiasis. Ann Surg Treat Res. 2016;91(5):239–246. doi: 10.4174/astr.2016.91.5.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Scheff R.T., Zuckerman G., Harter H., Delmez J., Koehler R. Diverticular disease in patients with chronic renal failure due to polycystic kidney disease. Ann Intern Med. 1980;92(2, pt 1):202–204. doi: 10.7326/0003-4819-92-2-202. [DOI] [PubMed] [Google Scholar]
- 20.Kumar S., Adeva M., King B.F., Kamath P.S., Torres V.E. Duodenal diverticulosis in autosomal dominant polycystic kidney disease. Nephrol Dial Transplant. 2006;21(12):3576–3578. doi: 10.1093/ndt/gfl405. [DOI] [PubMed] [Google Scholar]
- 21.Perdikakis E., Chryssou E.G., Karantanas A. Diagnosis of periampullary duodenal diverticula: the value of new imaging techniques. Ann Gastroenterol. 2011;24(3):192–199. [PMC free article] [PubMed] [Google Scholar]
- 22.Leivonen M.K., Halttunen J.A., Kivilaakso E.O. Duodenal diverticulum at endoscopic retrograde cholangiopancreatography, analysis of 123 patients. Hepatogastroenterology. 1996;43(10):961–966. [PubMed] [Google Scholar]
- 23.Macutkiewicz C., Plastow R., Chrispijn M., et al. Complications arising in simple and polycystic liver cysts. World J Hepatol. 2012;4(12):406–411. doi: 10.4254/wjh.v4.i12.406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kritikos A., Manuel O. Bloodstream infections after solid-organ transplantation. Virulence. 2016;7(3):329–340. doi: 10.1080/21505594.2016.1139279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schuetz P., Castro P., Shapiro N.I. Diabetes and sepsis: preclinical findings and clinical relevance. Diabetes Care. 2011;34(3):771–778. doi: 10.2337/dc10-1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.