Abstract
Context
Chronic hyperglycemia worsens skeletal muscle insulin resistance and β-cell function. However, the effect of sustained physiologic hyperglycemia on hepatic insulin sensitivity is not clear.
Objective
To examine the effect of sustained physiologic hyperglycemia (similar to that observed in patients with type 2 diabetes) on endogenous (primarily reflecting hepatic) glucose production (EGP) in healthy individuals.
Design
Volunteers participated in a three-step hyperinsulinemic (10, 20, 40 mU/m2 per minute) euglycemic clamp before and after a 48-hour glucose infusion to increase plasma glucose concentration by ∼40 mg/dL above baseline. EGP was measured with 3-3H-glucose before and after chronic glucose infusion.
Participants
Sixteen persons with normal glucose tolerance [eight with and eight without a family history (FH) of diabetes] participated in the study.
Main outcome measure
EGP.
Results
Basal EGP increased following 48 hours of glucose infusion (from a mean ± SEM of 2.04 ± 0.08 to 3.06 ± 0.29 mg/kgffm⋅ min; P < 0.005). The hepatic insulin resistance index (basal EGP × fasting plasma insulin) markedly increased following glucose infusion (20.1 ± 1.8 to 51.7 ± 6.6; P < 0.005) in both FH+ and FH− subjects.
Conclusion
Sustained physiologic hyperglycemia for as little as 48 hours increased the rate of basal hepatic glucose production and induced hepatic insulin resistance in health persons with normal glucose tolerance, providing evidence for the role of glucotoxicity in the increase in hepatic glucose production in type 2 diabetes.
This study examined the effect of mild physiologic hyperglycemia (40 mg/dL) for 48 hours in healthy glucose-tolerant participants with and without FH of type 2 diabetes.
Postabsorptive hyperglycemia primarily reflects increased endogenous (hepatic) glucose production (EGP) and is a characteristic feature of type 2 diabetes with elevated fasting plasma glucose concentrations (>140 to 160 mg/dL) (1–5). Glycogenolysis and gluconeogenesis contribute approximately equally to endogenous glucose production in normal glucose-tolerant persons (6–8). In patients with poorly controlled type 2 diabetes mellitus (T2DM), postabsorptive hyperglycemia primarily results from elevated hepatic gluconeogenesis (8–12). Although an absolute increase in EGP may not be observed in diabetic patients with mild fasting hyperglycemia, hepatic insulin resistance is evident by the impaired suppression of EGP by insulin (5, 13–15). In prediabetic individuals, elevated gluconeogenesis in the fasting state and impaired suppression of both gluconeogenesis and glycogenolysis by insulin (16) indicates that these metabolic defects are present early in the natural history of T2DM.
Chronic hyperglycemia exacerbates insulin resistance in skeletal muscle, and normalization of the plasma glucose concentration leads to improved skeletal muscle glucose uptake (17–19). In animal models of T2DM, correction of hyperglycemia normalizes hepatic insulin sensitivity (17, 20, 21). Multiple factors have been suggested to contribute to the development of hepatic insulin resistance, including lipotoxicity (22, 23) and glucotoxicity (24, 25), in T2DM. Increased hexosamine levels have been proposed as a possible mechanism responsible for hepatic insulin resistance (26, 27). Consistent with this, glucosamine infusion induces hepatic insulin resistance (28), and in diabetic animal models, lowering the blood glucose concentration by pharmacologic intervention improves hepatic insulin resistance (17, 18). In cultured hepatocytes, both glucose and glucosamine (26, 29) upregulate glucose-6-phosphatase via O-glycosylation of FoxO1 (26, 28, 30). These studies in rodents suggest that glucotoxicity plays an important role in the development of hepatic insulin resistance.
Although acute hyperglycemia is known to suppress endogenous glucose production (31–33), the effect of chronic hyperglycemia on hepatic insulin sensitivity in humans has not previously been examined. In the current study we examined the effect of sustained physiologic hyperglycemia, as seen in individuals with mild T2DM, on basal hepatic glucose production and suppression of hepatic glucose production (HGP) by insulin in persons with normal glucose tolerance with and without a family history (FH) of T2DM. Individuals with a positive FH of diabetes were included because we previously showed that they are predisposed to the adverse effects of metabolic signals known to contribute to the development of T2DM, specifically lipotoxicity (34).
Participants and Methods
Participants
Persons with NGT and FH of T2DM (n = 8) and no FH of T2DM (n = 8) participated in this study. Their clinical characteristics are shown in Table 1. No participant was taking any medication known to affect glucose metabolism. All participants were in good general health as determined by medical history, physical exam, screening blood tests, and electrocardiography. Body weight was stable (±3 pounds) in all participants for at least 3 months before the study, and no participant had engaged in an excessively heavy exercise program. Positive FH was defined as at least two first-degree family members with T2DM. All studies were performed on the Bartter Research Unit (BRU), South Texas Veterans Health Care System, Audie L. Murphy Hospital, San Antonio, Texas.
Table 1.
Baseline Characteristics in Participants With NGT Without and With FH of Diabetes
| Characteristic | No FH of Diabetes (n = 8) | FH of Diabetes (n = 8) | P Value |
|---|---|---|---|
| Men/women, n/n | 5/3 | 4/4 | NS |
| Age, y | 47 ± 4 | 42 ± 3 | NS |
| BMI, kg/m2 | 27 ± 1 | 25.5 ± 1.1 | NS |
| FFM, kg | 54 ± 6.4 | 48 ± 4.3 | NS |
| Hemoglobin A1c, % | 5.4 ± 0.1 | 5.5 ± 0.1 | NS |
| FPG, mg/dL | 95 ± 4 | 94 ± 5 | NS |
| 2-h glucose OGTT, mg/dL | 110 ± 7 | 128 ± 9 | NS |
Unless otherwise noted, data represent the mean ±SEM. BMI, body mass index; FFM, fat free mass; FPG, fasting plasma glucose concentration; NS, not significant; OGTT, oral glucose tolerance test.
All participants underwent a 75-g oral glucose tolerance test to document the presence of normal glucose tolerance according to American Diabetes Association criteria. Blood samples were drawn at −30, −15, and 0 minutes and every 15 minutes thereafter for 2 hours for determination of plasma glucose, insulin, C-peptide, and free fatty acid (FFA) concentrations. Body weight (nearest 0.1 kg on digital scale) (Health-O-Meter Inc., Bridgeview, IL) and height (nearest 1 cm) were recorded. Total body fat content and percentage body fat were measured by dual-energy x-ray absorptiometry (4500 Hologic, Bedford, MA).
Experimental protocol
All participants underwent a baseline three-step euglycemic insulin clamp (10, 20, and 40 mU/m2⋅ min) to raise plasma insulin by ∼20, 40, and 80 μU/mL (35). Each step lasted for 90 minutes. The insulin clamp was performed at 7 am after a ∼10-hour overnight fast. At 10 pm on the evening before the insulin clamp, the first six participants recruited into the study drank 2H2O, 1.67 g/kg body water, in divided doses over 1 hour to quantitate gluconeogenesis. At 7 am, participants reported to the BRU; a prime (40 μCi) continuous (0.4 μCi/min) infusion of 3-3H-glucose was started and continued throughout the 270-minute study to quantitate HGP. Blood samples for determination of C5 and C2 deuterated glucose enrichment were collected at −120, 0, 90, 180, and 270 minutes. Plasma samples for determination of plasma titrated glucose specific activity were obtained at −30, −20, −10, −5, and 0 minutes and every 10 to 15 minutes during the three-step hyperinsulinemic euglycemic clamp to calculate the rate of hepatic glucose production, percentage HGP from gluconeogenesis, and total gluconeogenesis rate (6, 9–11, 36). Participants who received 2H2O returned to the BRU in ∼6 weeks (allowing for washout of 2H2O) and received a variable glucose infusion to raise the plasma glucose concentration by ∼40 mg/dL for 48 hours. Participants who did not receive 2H2O returned to the BRU within 3 to 5 days for 48-hour glucose infusion. On the morning of day 3, the three-step euglycemic insulin clamp with 3-3H-glucose and 2H2O was repeated exactly as described above. The cold glucose infusion was stopped at 6 am on the morning of day 3, and the plasma glucose concentration was allowed to drop spontaneously to the fasting level. At 8 am, the three-step euglycemic insulin clamp was repeated.
Analytical measurements
Plasma glucose concentration was determined by the glucose oxidase reaction (Glucose Oxidase Analyzer; Beckman, Fullerton, CA), plasma insulin and C-peptide concentrations were measured by radioimmunoassay (Coat A Coat; Diagnostic Products, Los Angeles, CA), and plasma glucagon concentration was measured by radioimmunoassay (Linco Research, St. Charles, MO). Plasma 3-3H-glucose radioactivity was measured in Somogyi precipitates. Enrichment of deuterium in carbons 2 and 5 of plasma glucose was measured as previously described (6, 9–11, 36).
Statistical analysis
Statistical analyses were done with SPSS software version 2.2 (IBM, Armonk, NY). Data are presented as mean ± SEM. Comparison of variables before and after 48 hours of glucose infusion was performed with ANOVA and Bonferroni post hoc correction. Correlation analysis was done with Pearson correlation coefficient. P values < 0.05 were considered to indicate statistically significant differences.
Calculations
Under steady-state postabsorptive conditions, the basal rate of endogenous glucose appearance equals the 3-3H-glucose infusion rate divided by steady-state plasma titrated glucose specific activity. During the insulin clamp, non–steady-state conditions for 3-3H-glucose specific activity prevail and the rate of glucose appearance was calculated with the Steele equation (37). The rate of residual EGP during the insulin clamp was calculated by subtracting the exogenous glucose infusion rate from the tracer-derived rate of glucose appearance. The insulin-stimulated rate of total body glucose disposal (TGD) was calculated by adding the rate of residual EGP to the exogenous glucose infusion rate. Rates of gluconeogenesis were calculated by multiplying the plasma C5/C2 glucose ratio by the rate of EGP. Glycogenolysis was calculated by subtracting the rate of gluconeogenesis from the rate of EGP (6, 9–11, 36).
Results
Plasma glucose, insulin, C-peptide, and glucagon concentrations
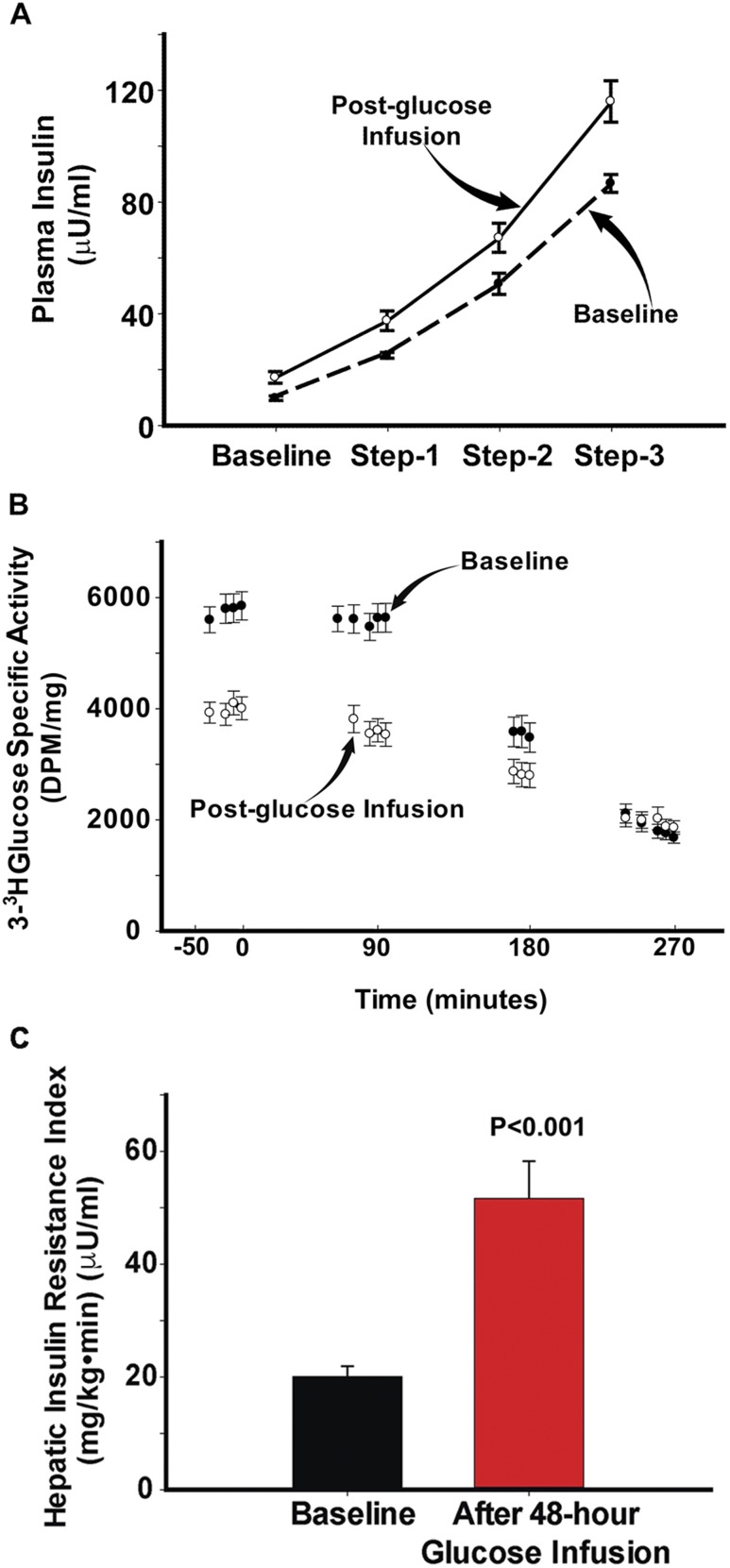
Because the results in the FH− and FH+ groups are superimposable, we have combined the results for ease of presentation. Mean fasting glucose concentration was 97 ± 2 mg/dL; during the 48-hour glucose infusion, the plasma glucose concentration was maintained at 136 ± 4 mg/dL. The plasma insulin concentration during the 48-hour glucose infusion progressively increased from 10 ± 1 μU/mL to 68 ± 7 μU/mL (P < 0.005). Before the start of the repeat euglycemic insulin clamp, after 48 hours of sustained hyperglycemia, the plasma glucose concentration (135 ± 5 mg/dL) was allowed to return to the fasting level (96 ± 3 mg/dL), at which time the plasma insulin concentration remained higher (17 ± 2 μU/mL; P < 0.05) than on the day of the baseline insulin clamp. Of note, plasma glucose and insulin concentrations and plasma 3-3H-glucose specific activity were at steady state before the start of the insulin clamp. Fasting plasma C-peptide levels followed a similar trend (2.8 ± 0.3 vs 1.86 ± 0.2 ng/mL; P < 0.05) to those of insulin during the 48-hour glucose infusion. The steady-state plasma insulin concentrations during the three insulin clamp steps were higher following 48 hours of glucose infusion (Fig. 1A).
Figure 1.
(A) Plasma insulin concentration and before and during the three insulin clamp steps performed before (baseline) and after 48 h of glucose infusion. (B) HGP during the three insulin clamp steps performed before and after 48 h of glucose infusion. (C) Basal hepatic insulin resistance index (basal HGP × fasting plasma insulin concentration) before (baseline) and after 48 h of glucose infusion.
Plasma glucose and insulin concentrations and metabolic clearance rate of insulin during the insulin clamp
The steady-state plasma insulin (Fig. 1A) and glucose concentrations during the three steps of the baseline insulin clamp were 25 ± 1, 51 ± 4, and 87 ± 3 μU/mL and 97 ± 3, 98 ± 2, and 99 ± 2 mg/dL, respectively. During the repeat insulin clamp performed after 48 hours of glucose infusion, the steady-state plasma insulin and glucose concentrations were 37 ± 3, 67 ± 5, and 116 ± 7 μU/mL and 99 ± 2, 96 ± 2, and 97 ± 3 mg/dL. The increment in plasma insulin concentration during the three steps of the euglycemic insulin clamp performed before and after 48 hours of glucose infusion was significantly higher during the 40 mU/m2 ⋅ min clamp step. The metabolic clearance rates of insulin during the three insulin clamp steps (10, 20, and 40 mU/m2 ⋅ min) performed at baseline were 82 ± 5, 66 ± 4, and 65 ± 3 mL/m2 ⋅ min, respectively. Following 48 hours of sustained hyperglycemia, the metabolic clearance rates of insulin were 71 ± 9 (P = not significant), 60 ± 7 (P = not significant), and 49 ± 4 (P < 0.05 vs baseline) mL/m2 ⋅ min.
TGD rate
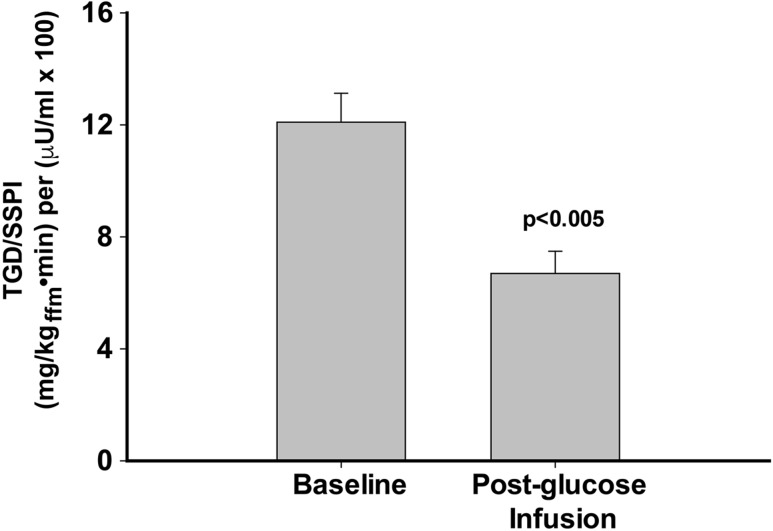
The rate of TGD during each of the three insulin clamp steps (10, 20, and 40 mU/m2 ⋅ min) was similar between FH+ and FH− participants before and after 48 hours of glucose infusion. The rate of glucose disposal divided by the steady-state plasma insulin concentration during the 40 mU/m2 ⋅ min insulin clamp step (Fig. 2) was significantly reduced (6.71 ± 0.79 vs 12.1 ± 1.04 mg/kgffm · min per μU/mL; P < 0.005). Results were similar when the rate of glucose disposal was divided by the increment in steady-state plasma insulin concentration. During the 40 mU/m2 ⋅ min insulin clamp step, TGD/Δ steady-state plasma insulin concentration was 8.1 ± 0.9 vs 14.7 ± 1.2 mg/kgffm · min per μU/mL (P < 0.005).
Figure 2.
Insulin sensitivity (TGD/steady-state plasma insulin concentration) during the 40 mU/m2•min euglycemic insulin clamp performed before and after 48 h of glucose infusion. SSPI, steady-state plasma insulin.
EGP
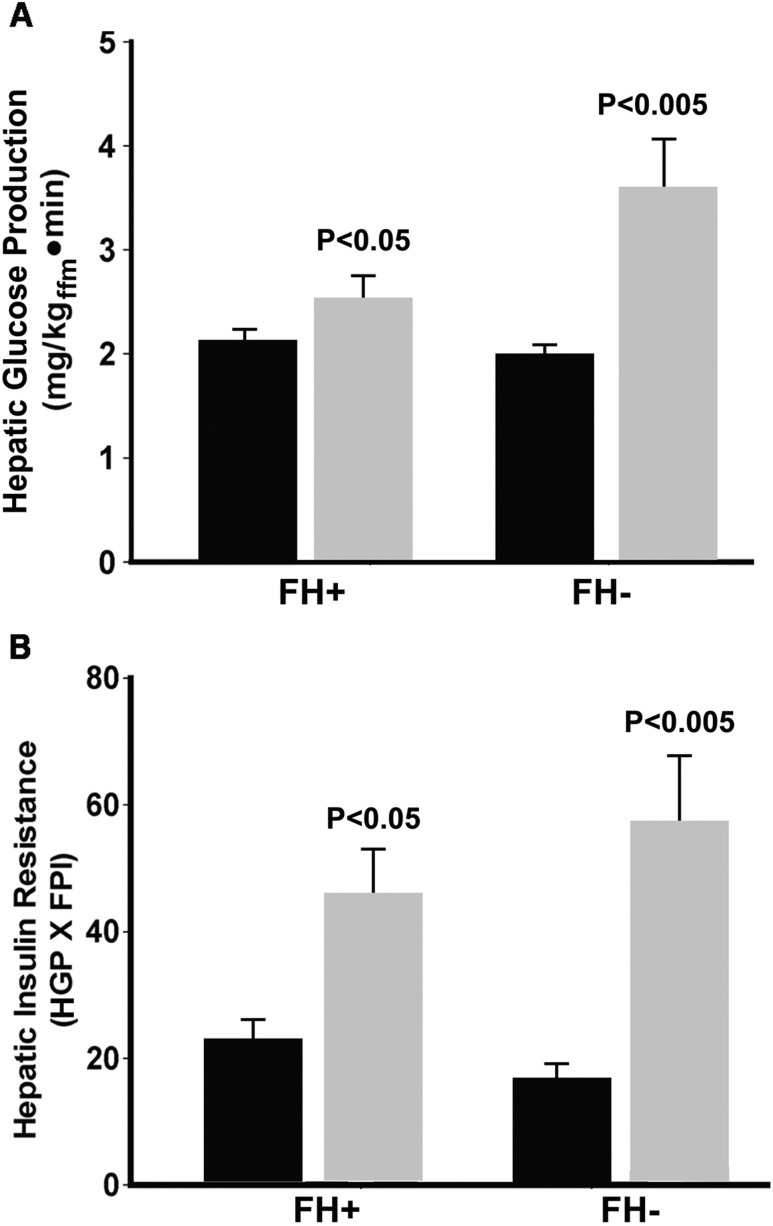
The basal rate of EGP was similar in FH+ and FH− participants during the baseline insulin clamp study. Following 48 hours of glucose infusion, the basal rate of EGP increased similarly and significantly in the FH− group (from 1.97 ± 0.1 to 3.5 ± 0.5 mg/kg ⋅ min; P < 0.01) and FH+ group (from 2.1 ± 0.1 to 2.55 ± 0.25 mg/kg ⋅ min; P < 0.05) (Fig. 3A) and in both groups combined (2.04 ± 0.08 to 3.06 ± 0.29 mg/kg · min per minute; P < 0.005) (Fig. 1C). EGP declined progressively during each step of the euglycemic insulin clamp, with impaired suppression (P < 0.01) observed during the 10 mU/m2 ⋅ min insulin clamp step (Fig. 1B). Because the fasting plasma insulin concentration was significantly higher following 48 hours of glucose infusion, the hepatic insulin resistance index (basal EGP × fasting plasma insulin) increased markedly following glucose infusion in the FH− (16.8 ± 2.2 to 54.7±11; P < 0.001) and FH+ (23 ± 2.5 to 46 ± 7.6; P < 0.05) groups (Fig. 3B) and in both groups combined (20.1 ± 1.8 vs 51.7 ± 6.6; P < 0.001) (Fig. 1C).
Figure 3.
(A) Basal hepatic glucose production during the baseline study (black bars) and during the study performed after 48 h of glucose infusion (gray bars) in the FH+ and FH− participants. (B) Basal hepatic insulin resistance index (basal HGP × fasting plasma insulin concentration) before (black bars) and after 48 h of (gray bars) glucose infusion in the FH+ and FH− participants. dpm, disintegrations per minute; FPI, fasting plasma insulin.
Gluconeogenesis and glycogenolysis
The basal rate of gluconeogenesis was measured by multiplying the ratio of C5/C2 in plasma glucose by the basal rate of EGP in six participants. Following 48 hours of glucose infusion, the C5/C2 ratio tended to decrease from 0.54 ± 0.07 to 0.45 ± 0.04 (P = not significant) and the basal rate of gluconeogenesis (5.58 ± 1.8 vs 5.78 ± 2.06 µmol/kg ⋅ min) did not change, whereas the basal rate of glycogenolysis increased significantly (8.02 ± 1.7 to 10.5 ± 1.3 µmol/kg ⋅ min; P < 0.05). Thus, the increase in EGP and hepatic insulin resistance primarily resulted from impaired suppression of glycogenolysis.
Plasma FFA and glucagon concentrations
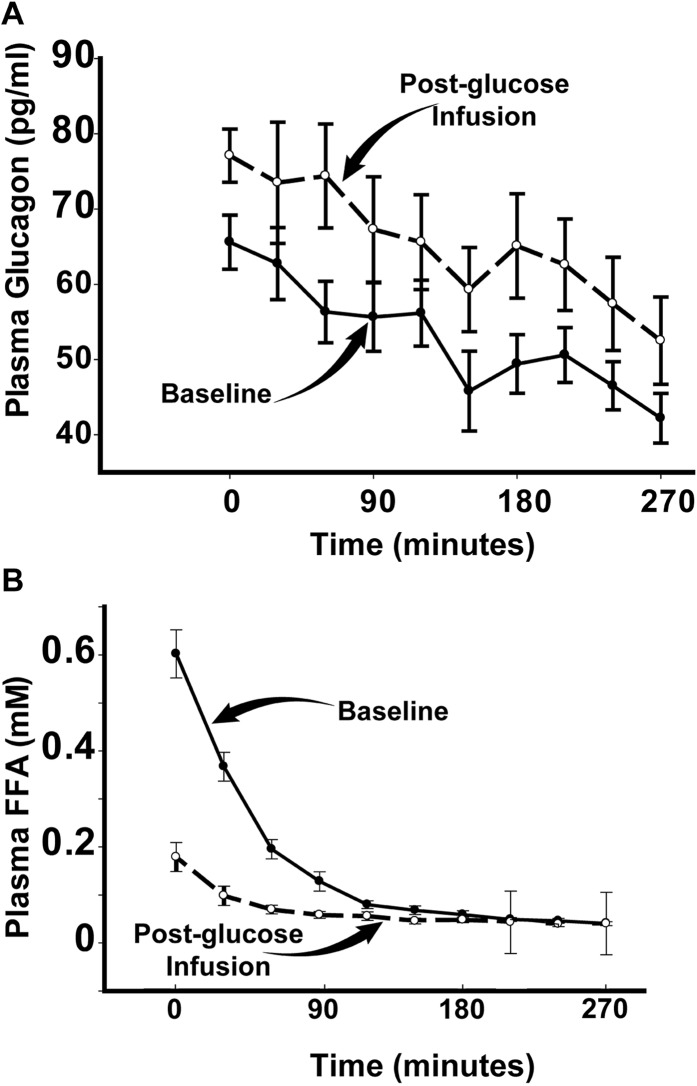
To gain insight into the mechanisms responsible for the development hepatic insulin resistance following 48 hours of glucose infusion, we measured plasma FFA and glucagon concentrations. Baseline plasma glucagon concentration tended to be higher following 48 hours of glucose infusion (64 ± 3 vs 77 ± 8 pg/mL; P = 0.25) (Fig. 4), whereas the product of plasma glucagon and insulin concentrations was markedly increased (540 ± 56 vs 1336 ± 262; P = 0.004), indicating that the suppressive effect of insulin on glucagon secretion was impaired. Baseline plasma FFA concentration was markedly reduced following 48 hours of glucose infusion and suppressed to a similar magnitude during each of the three insulin clamp steps (Fig. 4).
Figure 4.
(A) Plasma glucagon concentration and (B) plasma FFA concentration before and after 48 h of glucose infusion.
Plasma lactate, alanine, and glycerol
Plasma lactate, alanine, and glycerol are key gluconeogenic substrates. Although the plasma FFA was markedly suppressed following 48 hours of glucose infusion, there was no difference in plasma glycerol concentration (13.4 ± 1.2 vs 13.3 ± 2.2 mg/L; P = not significant). Following 48 hours of glucose infusion, there were small increases in both the plasma lactate (1.05 ± 0.05 vs 1.48 ± 0.07 mM; P < 0.05) and alanine (2.38 ± 0.3 vs 3.32 ± 0.3 mM; P = 0.03) concentrations.
Discussion
The present results demonstrate that mild sustained physiologic hyperglycemia for only 48 hours results in severe hepatic insulin resistance and an increase in the basal rate of endogenous (primarily reflects hepatic) glucose production. This observation has important clinical implications and indicates that mild persistent hyperglycemia aggravates hepatic insulin resistance and can exacerbate fasting hyperglycemia in patients with T2DM.
Studies from our laboratory (1–3) and others (4, 5, 8, 10, 15) have shown that fasting hyperglycemia (plasma glucose > 140 mg/dL) primarily results from hepatic insulin resistance and an elevated basal rate of hepatic glucose production. An acute rise in plasma glucose concentration suppresses hepatic glucose production in healthy individuals (32, 38). In contrast, the present results show that 48 hours of sustained physiologic hyperglycemia in individuals with NGT causes marked hepatic insulin resistance, resulting in an increased rate of basal hepatic glucose production and impaired suppression of HGP by insulin (Fig. 3). The major difference between the previous studies (32, 38) and the current study is the duration of hyperglycemia. In the previous studies (32, 38), hyperglycemia was maintained for only 6 hours, whereas in the current study hyperglycemia was maintained for 48 hours. We did not examine the effect of acute hyperglycemia on EGP, so the present findings do not contradict these prior results. In healthy individuals, acute hyperglycemia-induced suppression of EGP has been attributed, in part, to the glucose-induced reduction in plasma FFA concentration, secondary to suppression of lipolysis (39, 40). In the current study, as expected, the plasma FFA concentration declined markedly following 48 hours of glucose infusion. This, if anything, would minimize (not increase) the severity of glucose-induced hepatic insulin resistance.
In individuals with NGT, approximately half of HGP is derived from glycogenolysis and half from gluconeogenesis (6–9, 11). In patients with T2DM, the increased basal rate of HGP following an overnight fast primarily is derived from an elevated rate of hepatic gluconeogenesis (8–11). In the current study, the percentage of glucose derived from gluconeogenesis (ratio of C5/C2 in glucose) was slightly, although not significantly, decreased after 48 hours of glucose infusion. When multiplied by the rate of basal HGP, the absolute rate of gluconeogenesis was not significantly changed. Thus, from a quantitative standpoint the increased rate of glycogenolysis was the primary factor responsible for the increased basal rate of HGP.
With respect to gluconeogenesis, substrate delivery to the liver has been shown to be an important determinant of hepatic gluconeogenesis (7). In the current study, circulating levels of alanine and lactate increased slightly following 48 hours of glucose infusion, whereas plasma glycerol concentration was unchanged. Although the plasma alanine and lactate levels were increased following 48 hours of glucose infusion, the increase was small and, more importantly, the rate of gluconeogenesis was not increased. FFAs are not a precursor for glucose, but elevated plasma FFA levels can stimulate hepatic gluconeogenesis by activating enzymes involved in gluconeogenesis and stimulating glycogenolysis (22, 41). However, in the current study plasma FFA levels were markedly decreased following glucose infusion for 48 hours.
Increased hexosamine flux induces insulin resistance in muscle, adipocytes, and liver (27) by increasing intracellular levels of uridine diphosphate-N-acetylglucosamine, which subsequently is O-linked to serine/threonine residues of cytosolic proteins (42). This has been referred to as glucotoxicity (43). Although not measured in the current study, TRIB3 (44) has been suggested to be involved in glucose-mediated muscle insulin resistance. In rodent models of T2DM, correction of hyperglycemia with a renal glucose transport inhibitor restores normal hepatic insulin sensitivity, reduces the elevated basal rate of hepatic gluconeogenesis, and inhibits glucose-6-phosphatase (17). Conversely, glucosamine infusion is normal rats induces hepatic insulin resistance and upregulates glucose-6-phosphatase (45). In cultured hepatocytes, both glucose and glucosamine (26, 29) upregulate glucose-6-phosphatase via O-glycosylation of FOxO1 (26). When dephosphorylated, FOxO1 translocates to the nucleus and induces the transcription of key gluconeogenic genes that encode for PEPCK and glucose-6-phosphatase (45). Thus, increased hexosamine flux, as a result of chronic hyperglycemia, could induce insulin resistance via the FOxO1 pathway. Proof of this pathogenic mechanism would require liver biopsy before and after 48 hours of glucose infusion, and this is not possible in humans.
Studies in rodents have demonstrated that sustained glucose infusion to create a state of physiologic hyperglycemia (plasma glucose concentration ∼250 mg/dL) for 4 days resulted in >90% suppression of HGP (46). However, continued glucose infusion during days 5 to 7 was associated with a marked and progressive rise in HGP, which was associated with an increase in plasma glucagon concentration. Thus, increased α-cell glucagon secretion is a manifestation of glucotoxicity and contributes to the rise in HGP. In the current study the basal plasma glucagon concentration was increased by 20% (P = 0.20) after 48 hours of glucose infusion. Given that the fasting plasma insulin concentration was increased following glucose infusion, the elevated plasma glucagon levels suggest resistance to the inhibitory effect of insulin on plasma glucagon. Consistent with this, the product of the plasma glucagon and plasma insulin concentrations was increased 2.5-fold following 48 hours of glucose infusion. Thus, increased plasma glucagon levels could have contributed, in part, to the increase in basal rate of HGP and impaired suppression during the euglycemic insulin clamp.
Although not measured in the current study, one would expect that 48 hours of glucose infusion would increase hepatic glycogen content (47, 48) because glucose acutely binds to and activates glycogen synthase and inhibits glycogen phosphorylase (13, 49). However, a chronic increase in hepatic glycogen concentration inhibits glycogen synthase and activates glycogen phosphorylase (50). Therefore, when glucose is infused for 48 hours, one could hypothesize that an increase in hepatic glycogen concentration could activate glycogen phosphorylase and accelerate the rate of glycogenolysis; this could partly account for the present observations.
During the 48 hours of glucose infusion, insulin secretion was stimulated and the plasma insulin concentration increased significantly. We previously have shown that chronic hyperinsulinemia, although maintaining euglycemia, can induce hepatic, as well as peripheral tissue, insulin resistance (51, 52). Insulin-induced insulin resistance is mediated via downregulation of the insulin signaling pathway (IR/IRS-1,2/PI3K/Akt) (53), and this could have contributed to the hepatic insulin resistance and elevated rate of basal HGP and impaired suppression of HGP by insulin (Fig. 3). With regard to this, it should be noted that in patients with T2DM, combined hyperglycemia plus hyperinsulinemia commonly coexist, especially early in the natural history of T2DM, when β-cell function is still preserved (1).
Although not the primary purpose of the current study, 48 hours of sustained hyperglycemia was sufficient to induce peripheral tissue (primarily reflects muscle) insulin resistance. Thus, during the 40 mU/m2 ⋅ min insulin clamp step (Fig. 2), insulin sensitivity (TGD/steady-state plasma insulin concentration) was reduced by 37%.
Although we hypothesized that participants with NGT who have a positive FH of diabetes might be more susceptible to the deleterious effects of chronic hyperglycemia on hepatic insulin sensitivity, induction of hepatic insulin resistance and the increase in basal rate of HGP in FH+ participants was similar to that in participants without FH of diabetes.
In summary, sustained physiologic hyperglycemia (glucose level of ∼40 mg/dL) for only 48 hours induced marked hepatic insulin resistance in participants with NGT with and without FH of diabetes.
Acknowledgments
Financial Support: This work was supported by National Institutes of Health grants DK-24092-34 (R.A.D.) and DK-29953-36 (R.B.). D.T.'s salary is supported by the South Texas Veterans Health Care System, Audie Murphy Division.
Author Contributions: All authors contributed to performing the study. The initial draft of the manuscript was prepared by D.T., subsequently revised by R.A.D., and then reviewed and revised by all authors.
Disclosure Summary: The authors have nothing to disclose.
Glossary
Abbreviations:
- BRU
Bartter Research Unit
- EGP
endogenous glucose production
- FFA
free fatty acid
- FH
family history
- HGP
hepatic glucose production
- NGT
normal glucose tolerance
- T2DM
type 2 diabetes mellitus
- TGD
total body glucose disposal
References
- 1. Defronzo RA. Banting Lecture. From the triumvirate to the ominous octet: a new paradigm for the treatment of type 2 diabetes mellitus. Diabetes. 2009;58(4):773–795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. DeFronzo RA. Lilly lecture 1987. The triumvirate: beta-cell, muscle, liver. A collusion responsible for NIDDM. Diabetes. 1988;37(6):667–687. [DOI] [PubMed] [Google Scholar]
- 3. DeFronzo RA, Ferrannini E, Simonson DC. Fasting hyperglycemia in non-insulin-dependent diabetes mellitus: contributions of excessive hepatic glucose production and impaired tissue glucose uptake. Metabolism. 1989;38(4):387–395. [DOI] [PubMed] [Google Scholar]
- 4. Basu R, Schwenk WF, Rizza RA. Both fasting glucose production and disappearance are abnormal in people with “mild” and “severe” type 2 diabetes. Am J Physiol Endocrinol Metab. 2004;287(1):E55–E62. [DOI] [PubMed] [Google Scholar]
- 5. Tripathy D, Eriksson KF, Orho-Melander M, Fredriksson J, Ahlqvist G, Groop L. Parallel manifestation of insulin resistance and beta cell decompensation is compatible with a common defect in Type 2 diabetes. Diabetologia. 2004;47(5):782–793. [DOI] [PubMed] [Google Scholar]
- 6. Landau BR, Wahren J, Chandramouli V, Schumann WC, Ekberg K, Kalhan SC. Contributions of gluconeogenesis to glucose production in the fasted state. J Clin Invest. 1996;98(2):378–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cherrington AD, Stevenson RW, Steiner KE, Davis MA, Myers SR, Adkins BA, Abumrad NN, Williams PE. Insulin, glucagon, and glucose as regulators of hepatic glucose uptake and production in vivo. Diabetes Metab Rev. 1987;3(1):307–332. [DOI] [PubMed] [Google Scholar]
- 8. Cline GW, Rothman DL, Magnusson I, Katz LD, Shulman GI. 13C-nuclear magnetic resonance spectroscopy studies of hepatic glucose metabolism in normal subjects and subjects with insulin-dependent diabetes mellitus. J Clin Invest. 1994;94(6):2369–2376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Adkins A, Basu R, Persson M, Dicke B, Shah P, Vella A, Schwenk WF, Rizza R. Higher insulin concentrations are required to suppress gluconeogenesis than glycogenolysis in nondiabetic humans. Diabetes. 2003;52(9):2213–2220. [DOI] [PubMed] [Google Scholar]
- 10. Basu R, Chandramouli V, Dicke B, Landau B, Rizza R. Obesity and type 2 diabetes impair insulin-induced suppression of glycogenolysis as well as gluconeogenesis. Diabetes. 2005;54(7):1942–1948. [DOI] [PubMed] [Google Scholar]
- 11. Gastaldelli A, Toschi E, Pettiti M, Frascerra S, Quiñones-Galvan A, Sironi AM, Natali A, Ferrannini E. Effect of physiological hyperinsulinemia on gluconeogenesis in nondiabetic subjects and in type 2 diabetic patients. Diabetes. 2001;50(8):1807–1812. [DOI] [PubMed] [Google Scholar]
- 12. Magnusson I, Rothman DL, Katz LD, Shulman RG, Shulman GI. Increased rate of gluconeogenesis in type II diabetes mellitus. A 13C nuclear magnetic resonance study. J Clin Invest. 1992;90(4):1323–1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Petersen KF, Laurent D, Rothman DL, Cline GW, Shulman GI. Mechanism by which glucose and insulin inhibit net hepatic glycogenolysis in humans. J Clin Invest. 1998;101(6):1203–1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Abdul-Ghani MA, Jenkinson CP, Richardson DK, Tripathy D, DeFronzo RA. Insulin secretion and action in subjects with impaired fasting glucose and impaired glucose tolerance: results from the Veterans Administration Genetic Epidemiology Study. Diabetes. 2006;55(5):1430–1435. [DOI] [PubMed] [Google Scholar]
- 15. Jeng CY, Sheu WH, Fuh MM, Chen YD, Reaven GM. Relationship between hepatic glucose production and fasting plasma glucose concentration in patients with NIDDM. Diabetes. 1994;43(12):1440–1444. [DOI] [PubMed] [Google Scholar]
- 16. Basu R, Barosa C, Jones J, Dube S, Carter R, Basu A, Rizza RA. Pathogenesis of prediabetes: role of the liver in isolated fasting hyperglycemia and combined fasting and postprandial hyperglycemia. J Clin Endocrinol Metab. 2013;98(3):E409–E417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Rossetti L, Smith D, Shulman GI, Papachristou D, DeFronzo RA. Correction of hyperglycemia with phlorizin normalizes tissue sensitivity to insulin in diabetic rats. J Clin Invest. 1987;79(5):1510–1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Merovci A, Mari A, Solis-Herrera C, Xiong J, Daniele G, Chavez-Velazquez A, Tripathy D, Urban McCarthy S, Abdul-Ghani M, DeFronzo RA. Dapagliflozin lowers plasma glucose concentration and improves β-cell function. J Clin Endocrinol Metab. 2015;100(5):1927–1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Friedman JE, Dohm GL, Leggett-Frazier N, Elton CW, Tapscott EB, Pories WP, Caro JF. Restoration of insulin responsiveness in skeletal muscle of morbidly obese patients after weight loss. Effect on muscle glucose transport and glucose transporter GLUT4. J Clin Invest. 1992;89(2):701–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. O’Brien TP, Jenkins EC, Estes SK, Castaneda AV, Ueta K, Farmer TD, Puglisi AE, Swift LL, Printz RL, Shiota M. Correcting postprandial hyperglycemia in zucker diabetic fatty rats with an sglt2 inhibitor restores glucose effectiveness in the liver and reduces insulin resistance in skeletal muscle. Diabetes. 2017;66(5):1172–1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Petersen KF, Dufour S, Befroy D, Lehrke M, Hendler RE, Shulman GI. Reversal of nonalcoholic hepatic steatosis, hepatic insulin resistance, and hyperglycemia by moderate weight reduction in patients with type 2 diabetes. Diabetes.2005:54(3):603.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Boden G, Cheung P, Stein TP, Kresge K, Mozzoli M. FFA cause hepatic insulin resistance by inhibiting insulin suppression of glycogenolysis. Am J Physiol Endocrinol Metab. 2002;283(1):E12–E19. [DOI] [PubMed] [Google Scholar]
- 23. DeFronzo RA. Dysfunctional fat cells, lipotoxicity and type 2 diabetes. Int J Clin Pract Suppl. 2004;58(143):9–21. [DOI] [PubMed] [Google Scholar]
- 24. Dentin R, Hedrick S, Xie J, Yates J III, Montminy M. Hepatic glucose sensing via the CREB coactivator CRTC2. Science. 2008;319(5868):1402–1405. [DOI] [PubMed] [Google Scholar]
- 25. Veerababu G, Tang J, Hoffman RT, Daniels MC, Hebert LF Jr, Crook ED, Cooksey RC, McClain DA. Overexpression of glutamine: fructose-6-phosphate amidotransferase in the liver of transgenic mice results in enhanced glycogen storage, hyperlipidemia, obesity, and impaired glucose tolerance. Diabetes. 2000;49(12):2070–2078. [DOI] [PubMed] [Google Scholar]
- 26. Kuo M, Zilberfarb V, Gangneux N, Christeff N, Issad T. O-glycosylation of FoxO1 increases its transcriptional activity towards the glucose 6-phosphatase gene. FEBS Lett. 2008;582(5):829–834. [DOI] [PubMed] [Google Scholar]
- 27. Yki-Jarvinen H, McClain DA Glucotoxicity. In: DeFronzo RA, Ferrannini E, Zimmet P, Alberti KGMM, eds. International Textbook of Diabetes Mellitus 4th ed. Oxford, United Kingdom: Wiley Blackwell; 2015:. 413–425. [Google Scholar]
- 28. Buse MG. Hexosamines, insulin resistance, and the complications of diabetes: current status. Am J Physiol Endocrinol Metab. 2006;290(1):E1–E8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Massillon D, Barzilai N, Chen W, Hu M, Rossetti L. Glucose regulates in vivo glucose-6-phosphatase gene expression in the liver of diabetic rats. J Biol Chem. 1996;271(17):9871–9874. [DOI] [PubMed] [Google Scholar]
- 30. Sage AT, Walter LA, Shi Y, Khan MI, Kaneto H, Capretta A, Werstuck GH. Hexosamine biosynthesis pathway flux promotes endoplasmic reticulum stress, lipid accumulation, and inflammatory gene expression in hepatic cells. Am J Physiol Endocrinol Metab. 2010;298(3):E499–E511. [DOI] [PubMed] [Google Scholar]
- 31. Bell PM, Firth RG, Rizza RA. Effects of hyperglycemia on glucose production and utilization in humans. Measurement with [23H]-, [33H]-, and [614C]glucose. Diabetes. 1986;35(6):642–648. [DOI] [PubMed] [Google Scholar]
- 32. DeFronzo RA, Ferrannini E, Hendler R, Felig P, Wahren J. Regulation of splanchnic and peripheral glucose uptake by insulin and hyperglycemia in man. Diabetes. 1983;32(1):35–45. [DOI] [PubMed] [Google Scholar]
- 33. Mevorach M, Giacca A, Aharon Y, Hawkins M, Shamoon H, Rossetti L. Regulation of endogenous glucose production by glucose per se is impaired in type 2 diabetes mellitus. J Clin Invest. 1998;102(4):744–753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kashyap S, Belfort R, Gastaldelli A, Pratipanawatr T, Berria R, Pratipanawatr W, Bajaj M, Mandarino L, DeFronzo R, Cusi K. A sustained increase in plasma free fatty acids impairs insulin secretion in nondiabetic subjects genetically predisposed to develop type 2 diabetes. Diabetes. 2003;52(10):2461–2474. [DOI] [PubMed] [Google Scholar]
- 35. DeFronzo RA, Tobin JD, Andres R. Glucose clamp technique: a method for quantifying insulin secretion and resistance. Am J Physiol. 1979;237(3):E214–E223. [DOI] [PubMed] [Google Scholar]
- 36. Basu R, Chandramouli V, Dicke B, Landau BR, Rizza RA. Plasma C5 glucose-to-2H2O ratio does not provide an accurate assessment of gluconeogenesis during hyperinsulinemic-euglycemic clamps in either nondiabetic or diabetic humans. Diabetes. 2008;57(7):1800–1804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Steele R, Wall JS, De Bodo RC, Altszuler N. Measurement of size and turnover rate of body glucose pool by the isotope dilution method. Am J Physiol. 1956;187(1):15–24. [DOI] [PubMed] [Google Scholar]
- 38. Liljenquist JE, Mueller GL, Cherrington AD, Perry JM, Rabinowitz D. Hyperglycemia per se (insulin and glucagon withdrawn) can inhibit hepatic glucose production in man. J Clin Endocrinol Metab. 1979;48(1):171–175. [DOI] [PubMed] [Google Scholar]
- 39. Park KS, Rhee BD, Lee KU, Lee HK, Koh CS, Min HK. Hyperglycemia per se can reduce plasma free fatty acid and glycerol levels in the acutely insulin-deficient dog. Metabolism. 1990;39(6):595–597. [DOI] [PubMed] [Google Scholar]
- 40. Staehr P, Hother-Nielsen O, Landau BR, Chandramouli V, Holst JJ, Beck-Nielsen H. Effects of free fatty acids per se on glucose production, gluconeogenesis, and glycogenolysis. Diabetes. 2003;52(2):260–267. [DOI] [PubMed] [Google Scholar]
- 41. Kehlenbrink S, Tonelli J, Koppaka S, Chandramouli V, Hawkins M, Kishore P. Inhibiting gluconeogenesis prevents fatty acid-induced increases in endogenous glucose production. Am J Physiol Endocrinol Metab. 2009;297(1):E165–E173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Haltiwanger RS, Blomberg MA, Hart GW. Glycosylation of nuclear and cytoplasmic proteins. Purification and characterization of a uridine diphospho-N-acetylglucosamine:polypeptide beta-N-acetylglucosaminyltransferase. J Biol Chem. 1992;267(13):9005–9013. [PubMed] [Google Scholar]
- 43. Rossetti L, Giaccari A, DeFronzo RA. Glucose toxicity. Diabetes Care. 1990;13(6):610–630. [DOI] [PubMed] [Google Scholar]
- 44. Zhang W, Liu J, Tian L, Liu Q, Fu Y, Garvey WT. TRIB3 mediates glucose-induced insulin resistance via a mechanism that requires the hexosamine biosynthetic pathway. Diabetes. 2013;62(12):4192–4200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Matsuzaki H, Ichino A, Hayashi T, Yamamoto T, Kikkawa U. Regulation of intracellular localization and transcriptional activity of FOXO4 by protein kinase B through phosphorylation at the motif sites conserved among the FOXO family. J Biochem. 2005;138(4):485–491. [DOI] [PubMed] [Google Scholar]
- 46. Jamison RA, Stark R, Dong J, Yonemitsu S, Zhang D, Shulman GI, Kibbey RG. Hyperglucagonemia precedes a decline in insulin secretion and causes hyperglycemia in chronically glucose-infused rats. Am J Physiol Endocrinol Metab. 2011;301(6):E1174–E1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Shulman GI, DeFronzo RA, Rossetti L. Differential effect of hyperglycemia and hyperinsulinemia on pathways of hepatic glycogen repletion. Am J Physiol. 1991;260(5 Pt 1):E731–E735. [DOI] [PubMed] [Google Scholar]
- 48. Cardin S, Emshwiller M, Jackson PA, Snead WL, Hastings J, Edgerton DS, Cherrington AD. Portal glucose infusion increases hepatic glycogen deposition in conscious unrestrained rats. J Appl Physiol (1985). 1999;87(4):1470–1475. [DOI] [PubMed] [Google Scholar]
- 49. Edgerton DS, Cardin S, Neal D, Farmer B, Lautz M, Pan C, Cherrington AD. Effects of hyperglycemia on hepatic gluconeogenic flux during glycogen phosphorylase inhibition in the conscious dog. Am J Physiol Endocrinol Metab. 2004;286(4):E510–E522. [DOI] [PubMed] [Google Scholar]
- 50. Winnick JJ, An Z, Kraft G, Ramnanan CJ, Irimia JM, Smith M, Lautz M, Roach PJ, Cherrington AD. Liver glycogen loading dampens glycogen synthesis seen in response to either hyperinsulinemia or intraportal glucose infusion. Diabetes. 2013;62(1):96–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Del Prato S, Leonetti F, Simonson DC, Sheehan P, Matsuda M, DeFronzo RA. Effect of sustained physiologic hyperinsulinaemia and hyperglycaemia on insulin secretion and insulin sensitivity in man. Diabetologia. 1994;37(10):1025–1035. [DOI] [PubMed] [Google Scholar]
- 52. Koopmans SJ, Kushwaha RS, DeFronzo RA. Chronic physiologic hyperinsulinemia impairs suppression of plasma free fatty acids and increases de novo lipogenesis but does not cause dyslipidemia in conscious normal rats. Metabolism. 1999;48(3):330–337. [DOI] [PubMed] [Google Scholar]
- 53. Catalano KJ, Maddux BA, Szary J, Youngren JF, Goldfine ID, Schaufele F. Insulin resistance induced by hyperinsulinemia coincides with a persistent alteration at the insulin receptor tyrosine kinase domain. PLoS One. 2014;9(9):e108693. [DOI] [PMC free article] [PubMed] [Google Scholar]