Abstract
Background
Population-based estimates of the long-term risk of loco-regional recurrence and distant metastases of breast cancer (BRC) patients are scant, as most published studies used hospital-based cohorts or participants of clinical trials. This work aims to extend available knowledge by providing population-based long-term estimates of the cumulative risk of BRC recurrence up to 10 years after diagnosis.
Methods
Data from the population-based Saarland Cancer Registry were used and included 9359 female patients with primary invasive BRC diagnosed between 1999 and 2009. Estimates of the cumulative incidence (CI) of BRC recurrence were derived for patients who had received local surgery with free resection margins by type of recurrence and stratified by age, tumor characteristics and major treatment options, taking into account mortality from any cause as a competing risk.
Results
The 10-year CI of BRC recurrence was 16%. For loco-regional recurrence and distant metastases alone it was 8 and 11%, respectively. The estimates showed substantial variation and were particularly increased if tumors were advanced (T1/2N+ 23%, T3/4N0 24%, T3/4N+ 34%), of high grade (23%), or of ‘HER2/neu positive’ (28%) or ‘triple negative’ subtype (23%), respectively.
Conclusions
The derived estimates reflect the risk of ‘real world’ patients and may therefore extend available knowledge. These data are thus of great relevance for clinicians, their patients and researchers. The study likewise demonstrated the usefulness of cancer registries for a population-based monitoring of the effectiveness of cancer care in terms of disease recurrence as a major treatment related outcome measure.
Electronic supplementary material
The online version of this article (10.1186/s12885-019-5710-5) contains supplementary material, which is available to authorized users.
Keywords: Invasive breast cancer, Loco-regional recurrence, Distant metastases, Cumulative incidence, Cancer registry, Population-based, Germany
Background
Breast cancer (BRC) is the most common invasive cancer in women worldwide [1] and the overall prognosis of BRC patients in industrialized countries is favorable [2, 3]. However, significant proportions of patients suffer from recurrence, e.g. a trial from the US cited in clinical practice guidelines (CPG) reported occurrence of loco-regional recurrence and distant metastases over 10 years in one out of every six patients and in one out of every three patients who had been diagnosed between 1975 and 1994, respectively [4]. BRC recurrence may occur in the ipsilateral breast or chest wall after surgery, regional lymph nodes (including ipsilateral axillary, infraclavicular, internal mammary, or supraclavicular nodes), as well as distant sites and organs.
Factors associated with increased risk of loco-regional recurrence include young age at diagnosis, advanced tumor size, involvement of regional lymph nodes, high grade, vascular invasion, and omitting an indicated adjuvant radiotherapy [5]. Surrogate definitions based on immunohistochemical measurements of the expression of hormone receptors (HR), human epidermal growth factor receptor 2 (HER2/neu), and other markers such as the Ki-67 antigen are used to classify BRC and correlate well with genetically different BRC subtypes [6, 7], which are associated with the risk of recurrence and outcome in addition to classic prognostic factors [8, 9].
Treatment options of potentially curable loco-regional recurrence include complete surgical resection of the recurrent tumor, radiotherapy and systemic treatment based on histological examination of the cancerous tissue and re-staging. Occurring distant metastases are generally treated with palliative intent and therapy includes systemic treatment, radiotherapy or resection of metastases. Any treatment of recurrent BRC should be based on an interdisciplinary approach [10–12].
Data on the long-term risk of BRC recurrence which were derived from population-based samples of patients are scant, as the vast majority of published studies used selected patient samples such as hospital-based cohorts (e.g. [7, 13–15]) or participants of clinical trials (e.g. [4, 16, 17]). Furthermore, most studies were restricted to 5 years of follow-up only. This study from Germany aims to overcome these shortcomings by providing long-term estimates of the cumulative risk of loco-regional recurrence and distant metastases up to 10 years after diagnosis according to age, tumor characteristics and major treatment options derived from a population-based sample of BRC patients who had received surgery with free resection margins.
Methods
Data from the population-based Saarland Cancer Registry (CR) were used and included records of 9359 female patients with primary invasive BRC (ICD-10 code: C50) diagnosed between 1999 and 2009. Saarland is a federal state in Southwest Germany with a population of approximately 1.02 million people in 2009. The CR is in operation since 1968 and its case ascertainment is regularly estimated to be almost complete (e.g. [18, 19]). In Saarland, cancer reporting is mandatory by law. The CR obtains cancer notifications from hospitals, radiotherapy departments, outpatient clinics, screening programs, and doctors in private practice as well as reports from pathology laboratories. In addition to notifications of newly diagnosed cancers, the CR obtains notifications of recurrent cancer from the above listed sources on the occasion of their diagnosis and treatment. The vital status of the patients is regularly ascertained using death certificates from local health authorities and records from population registries. The Saarland CR applies the rules of recording multiple primaries as recommended by the International Association of Cancer Registries [20]. Loco-regional recurrences and distant metastases of a tumor are recorded, if stated as such in a case summary or a pathology report. Accordingly, tumors of the contralateral breast and multiple primary tumors of the breast were not considered as cancer recurrences.
The following classifications of socio-demographic and tumor characteristics as well as administered treatments were used in the analyses: age at diagnosis (< 70, ≥ 70 years), clinical stage (T1/2N0, T1/2N+, T3/4N0, T3/4N+, M1), morphologic type of tumor (invasive ductal, invasive lobular, mixed type, other), histopathologic grade (G1, G2, G3/4), HR expression (positive (including mixed: either estrogen or progesterone receptor positive), negative), HER2/neu expression (positive (including borderline results with further examinations), negative), definitive local surgery (breast conserving surgery (BCS), mastectomy, none), provision of radiotherapy (yes, none), chemotherapy (yes, none), hormonal (anti-estrogen) therapy (yes, none) and targeted treatment (TT) with monoclonal antibodies (yes, none), residual tumor after surgery (no residual tumor (R0), microscopic or macroscopic residual tumor (R1/2)), and source of registration (notification at lifetime, death certificate only). Further details on the data collection may be found elsewhere [21]. Information on the HR status and HER2/neu expression was extracted from available pathology reports and case summaries. HR status was based on immunohistochemistry. According to CPGs, expression of estrogen and progesterone receptors was considered, if more than 10% of cancerous cells showed immunohistochemical nuclear staining (for two tumors, biochemistry was used for quantitative measurements) [22, 23]. HER2/neu positivity was based on immunohistochemical tests with score 3+ or in 28 cases with unclear results after fluorescence or chromogenic in situ hybridization [22, 23]. Based on the available measurements of HR expression and HER2/neu expression, tumors were classified as ‘HR positive’, ‘HER2/neu positive’ (HER2/neu positive, but HR negative) and ‘triple negative’ (both HR and HER2/neu negative) [12, 24]. It has been shown, that these immunohistochemical definitions largely describe the major intrinsic BRC subtypes ‘luminal like’, ‘HER2/neu like’ and ‘basal like’, respectively [6, 7].
During the period of diagnosis, the national CPG recommended BCS followed by radiotherapy or mastectomy for early stage BRC or mastectomy followed by radiotherapy for locally advanced tumors (guidelines recommended surgery with clear resection margins), sentinel node biopsy or dissection of regional lymph nodes for proper staging, adjuvant chemotherapy for patients with involved lymph nodes or HR negative tumors, hormonal treatment for patients with HR positive tumors and TT with monoclonal antibodies for patients with HER2/neu expressed tumors. In case of advanced tumors, systemic treatment was recommended prior to surgery and radiotherapy. For elderly patients, a treatment comparable to younger patients was recommended taking altered organ functions and co-morbidity into account [25].
Descriptive analyses were used to summarize the patients with regard to age and tumor characteristics. The study sample included all BRC patients without distant metastases at the time of diagnosis. A summary of the provided local and systemic treatments was derived. For the analysis of the risk of recurrence, information on its type (loco-regional recurrence, distant metastases), date of first occurrence and date of death was used.
Survival observations were right censored, if no event was observed until end of follow-up (31 December 2014, at the latest) and survival times between diagnosis and first occurrence of local recurrence, distant metastases, death, or end of follow-up were derived. The analyses of the risk of BRC recurrence included patients who received local surgery with free resection margins (R0) without any BRC recurrence within 3 months and for whom follow-up was available (n = 5311, 62% of the study sample). This 3 month cutoff period was chosen to allow for any changes in the staging during diagnostic work-up or initial treatment to avoid misclassification of such changes as recurrences. Five- and 10-year estimates of the cumulative incidence (CI) were derived by type of recurrence and stratified by age, tumor characteristics and major treatment options, taking into account mortality from any cause as a competing risk. The used estimator of the CI is based on a generalization of the Kaplan-Meier estimator and quantifies the probability that the event under study will occur before any specified time in the presence of competing risks [26, 27]. Along with point estimates, 95% confidence intervals of the CI were derived [28]. The R Language and Environment for Statistical Computing (release 3.1.3) [29] and the cmprsk extension package [30] were used for data preparation, statistical analyses and visualization. P-values of a two sided test of the equality of CI curves across subsamples [30] were derived and considered as a statistically significant result, if < 0.05.
Results
Table 1 presents the distribution of tumor characteristics of the patients. Overall, 56% had a T1/2N0 tumor, 69% had an invasive ductal carcinoma, and 64% had a tumor of intermediate grade. Of the tumors, 84 and 25% showed expression of HR and HER2/neu, respectively. Mean age at diagnosis was 62.7 years, 2.4% of the patients presented with bilateral BRC and mean observation time was 10.3 years. Among elderly patients, higher proportions of tumors with advanced stage, HR expression and without HER2/neu expression were observed.
Table 1.
Tumor characteristics of female patients with primary invasive breast cancer overall and by age
| Category | Overall | < 70 years | ≥ 70 years | ||||
|---|---|---|---|---|---|---|---|
| N | % a | N | % a | N | % a | ||
| Overall | 9359 | 6138 | 65.6 | 3221 | 34.4 | ||
| Clinical stage | M1 | 658 | 7.0 | 377 | 6.1 | 281 | 8.7 |
| Death certificate only notification | 183 | 2.0 | 36 | 0.6 | 147 | 4.6 | |
| Study sample | 8518 | 91.0 | 5725 | 93.3 | 2793 | 86.7 | |
| Laterality | available | 8508 | 99.9 | 5716 | 99.8 | 2792 | 100.0 |
| unilateral | 8306 | 97.6 | 5600 | 98.0 | 2706 | 96.9 | |
| synchronous bilateralb | 202 | 2.4 | 116 | 2.0 | 86 | 3.1 | |
| Clinical stage | available | 7447 | 87.4 | 5312 | 92.8 | 2135 | 76.4 |
| T1/2N0 | 4132 | 55.5 | 3011 | 56.7 | 1121 | 52.5 | |
| T1/2N+ | 2379 | 31.9 | 1787 | 33.6 | 592 | 27.7 | |
| T3/4N0 | 205 | 2.8 | 111 | 2.1 | 94 | 4.4 | |
| T3/4N+ | 731 | 9.8 | 403 | 7.6 | 328 | 15.4 | |
| Microscopically verified | 8482 | 99.6 | 5723 | 100.0 | 2759 | 98.8 | |
| Morphology | available | 8477 | 99.5 | 5720 | 99.9 | 2757 | 98.7 |
| invasive ductal | 5883 | 69.4 | 4053 | 70.9 | 1830 | 66.4 | |
| invasive lobular | 1190 | 14.0 | 773 | 13.5 | 417 | 15.1 | |
| mixed type | 654 | 7.7 | 465 | 8.1 | 189 | 6.9 | |
| other | 750 | 8.8 | 429 | 7.5 | 321 | 11.6 | |
| Histopathologic grade | available | 8216 | 96.5 | 5564 | 97.2 | 2652 | 95.0 |
| 1 | 610 | 7.4 | 405 | 7.3 | 205 | 7.7 | |
| 2 | 5232 | 63.7 | 3458 | 62.1 | 1774 | 66.9 | |
| 3/4 | 2374 | 28.9 | 1701 | 30.6 | 673 | 25.4 | |
| HR expression | available | 7424 | 87.2 | 5048 | 88.2 | 2376 | 85.1 |
| positivec | 6235 | 84.0 | 4152 | 82.3 | 2083 | 87.7 | |
| negative | 1189 | 16.0 | 896 | 17.7 | 293 | 12.3 | |
| HER2/neu expression | available | 5938 | 69.7 | 4051 | 70.8 | 1887 | 67.6 |
| positived | 1466 | 24.7 | 1054 | 26.0 | 412 | 21.8 | |
| negative | 4472 | 75.3 | 2997 | 74.0 | 1475 | 78.2 | |
| Subtype based on immunohistochemical surrogates | HR and HER2/neu status available | 7197 | 84.5 | 4883 | 85.3 | 2314 | 82.8 |
| ‚HR positive‘ | 6235 | 86.6 | 4152 | 85.0 | 2083 | 90.0 | |
| ‚HER2/neu positive‘e | 368 | 5.1 | 278 | 5.7 | 90 | 3.9 | |
| ‚triple negative’f | 594 | 8.3 | 453 | 9.3 | 141 | 6.1 | |
| Follow-up available | 8380 | 98.4 | 5634 | 98.4 | 2746 | 98.3 | |
| years | years | years | |||||
| Mean observation time | 10.3 | 10.4 | 10.3 | ||||
The overall study sample includes all breast cancer patients (ICD-10 code: C50) from Saarland diagnosed between 1999 and 2009. T, N = T and N category of TNM classification, HR = hormone receptor, HER2/neu = human epidermal growth factor receptor 2. afigures printed in normal text represent numbers and proportions of the overall cohort or study sample, figures printed in italic text represent proportions of cases of the respective sample with available information, btumors were classified as synchronous bilateral if the time interval between their detection was ≤3 months, cincludes tumors with mixed HR expression (either estrogen or progesterone receptor positive), dincludes tumors with borderline expression of HER2/neu, eHER2/neu positive, but HR negative, fboth HR negative and HER2/neu negative
Table 2 shows the provision of local and systemic treatments. Overall, 64 and 35% of the patients received BCS and mastectomy, respectively. Among patients with T1/2N0 and T1/2N+ tumors, 71 and 73% received both surgery and radiotherapy. Of the patients with T3/4 tumors, 46% received mastectomy followed by radiotherapy. After local surgery, 95% of the patients had free resection margins (R0). Of the patients with nodal positive or HR negative tumors, 70% received chemotherapy and 85% of the patients with HR positive tumors received hormonal treatment. Of the patients with HER2/neu positive tumors, 25% received TT. The provision of treatments varied markedly by age. Elderly patients received mastectomy more often and recommended radiotherapy, chemotherapy and TT less often.
Table 2.
Provision of local and systemic treatments to breast cancer patients
| Category/treatment | Sample | Overall | < 70 years | ≥ 70 years | |||
|---|---|---|---|---|---|---|---|
| N | % a | N | % a | N | % a | ||
| Overall | study sample (8518) | 8518 | 5725 | 2793 | |||
| Information on local treatment available | study sample (8518) | 6634 | 77.9 | 4548 | 79.4 | 2086 | 74.7 |
| BCS | patients with information on surgery (6634) | 4245 | 64.0 | 3225 | 70.9 | 1020 | 48.9 |
| Mastectomy | 2290 | 34.5 | 1300 | 28.6 | 990 | 47.5 | |
| None | 99 | 1.5 | 23 | 0.5 | 76 | 3.6 | |
| BCS | patients with T1/2N0 tumors and information on surgery (3435) | 244 | 7.1 | 134 | 5.4 | 110 | 11.7 |
| BCS + RT | 2427 | 70.7 | 1925 | 77.3 | 502 | 53.2 | |
| Mastectomy | 754 | 22.0 | 427 | 17.1 | 327 | 34.6 | |
| BCS or mastectomy + RT | patients with T1/2N+ tumors and information on surgery (1995) | 1452 | 72.8 | 1162 | 78.2 | 290 | 57.0 |
| Mastectomy + RT | patients with T3/4 tumors and information on surgery (755) | 350 | 46.4 | 232 | 55.6 | 118 | 34.9 |
| Residual status available | patients with surgery (6535) | 5709 | 87.4 | 3972 | 87.8 | 1737 | 86.4 |
| R0 | 5409 | 94.7 | 3773 | 95.0 | 1636 | 94.2 | |
| R1/2 | 300 | 5.3 | 199 | 5.0 | 101 | 5.8 | |
| CT information available | study sample (8518) | 6941 | 81.5 | 4961 | 86.7 | 1980 | 70.9 |
| CT | patients with N+ or HR negative tumors and information on CT usage (3472) | 2429 | 70.0 | 2171 | 84.9 | 258 | 28.2 |
| HT information available | study sample (8518) | 6740 | 79.1 | 4709 | 82.3 | 2031 | 72.7 |
| HT | patients with HR positive tumors b and information on HT usage (5122) | 4356 | 85.0 | 3031 | 85.4 | 1325 | 84.3 |
| TT information available | study sample (8518) | 4786 | 56.2 | 3375 | 59.0 | 1411 | 50.5 |
| TT | patients with HER2/neu positive tumors c and information on TT usage (839) | 208 | 24.8 | 183 | 29.3 | 25 | 11.7 |
The study sample includes female patients with primary invasive breast cancer (ICD-10 code: C50) without distant metastases from Saarland diagnosed between 1999 and 2009. BCS breast conserving surgery, RT radiotherapy, T, N T and N category of TNM classification, CT chemotherapy, HR hormone receptor, HT hormonal (anti-estrogen) treatment, TT targeted treatment (trastuzumab), HER2/neu human epidermal growth factor receptor 2. afigures printed in normal text represent numbers and proportions of the study sample, figures printed in italic text represent proportions of cases of the respective sample with available information, bincludes tumors with mixed HR expression (either estrogen or progesterone receptor positive), cincludes tumors with borderline expression of HER2/neu
Table 3 presents the 5- and 10-year CI of cancer recurrence (both loco-regional recurrence and distant metastases) of BRC patients with local R0 resection by age, tumor characteristics and provided recommended treatments. Overall 5- and 10-year CI of BRC recurrence was 10 and 16%, respectively (unless otherwise stated, the 10-year estimate of the CI will be reported subsequently). The CI was higher of patients aged < 70 years (17% vs. 13%; p < 0.001). It was 9% in patients with T1/2N0 tumors, 23 and 24% in patients with T1/2N+ and T3/4N0 tumors and 34% in patients with T3/4N+ tumors, respectively (p < 0.001). It was 6, 14 and 23% in patients with low, intermediate and high grade carcinomas (p < 0.001). Little variation of the CI was observed by tumor morphology. The CI of cancer recurrence of patients with tumors of the subtype ‘HR positive’ was approximately half compared to the CI of patients with subtype ‘HER2/neu positive’ and ‘triple negative’ tumors (14% vs. 28 and 26%, p < 0.001), respectively. The overall 10-year risk of BRC recurrence of patients with and without HR expression was 27 and 14%, respectively (p < 0.001, the risk did not vary significantly between patients with tumors showing expression of estrogen or progesterone receptors, data not shown). The overall 10-year risk of BRC recurrence of patients with a HER2/neu positive tumor was 22%, varying between 28% among those with HR negative tumor and 20% among those with a HR positive tumor (p < 0.001, data not shown).
Table 3.
Five- and 10-year cumulative incidence of cancer recurrence of breast cancer patients with local R0 resection by age, tumor characteristics and provided recommended treatments
| Category/treatment | N | 5-year CI | 10-year CI | p-value | |||
|---|---|---|---|---|---|---|---|
| PE | 95% CI | PE | 95% CI | ||||
| Overall | 5311 | 10.4 | [9.6, 11.3] | 15.9 | [14.8, 17.0] | ||
| Age | < 70 years | 3714 | 11.2 | [10.2, 12.3] | 17.2 | [16.0, 18.6] | < 0.001 |
| ≥ 70 years | 1597 | 8.7 | [7.4, 10.2] | 12.6 | [11.0, 14.5] | ||
| Clinical stage | T1/2N0 | 2918 | 5.6 | [4.8, 6.5] | 9.4 | [8.3, 10.7] | < 0.001 |
| T1/2N+ | 1613 | 14.8 | [13.1, 16.6] | 23.1 | [20.9, 25.5] | ||
| T3/4N0 | 124 | 16.1 | [10.8, 24.2] | 23.5 | [16.7, 33.1] | ||
| T3/4N+ | 409 | 26.9 | [22.9, 31.6] | 34.3 | [29.8, 39.6] | ||
| missing | 247 | 9.0 | [6.0, 13.4] | 9.9 | [6.7, 14.5] | ||
| Morphology | invasive ductal | 3690 | 10.9 | [10.0, 12.0] | 16.2 | [14.9, 17.5] | 0.075 |
| invasive lobular | 758 | 9.7 | [7.8, 12.0] | 17.8 | [15.0, 21.1] | ||
| mixed type | 459 | 9.0 | [6.7, 12.0] | 14.3 | [11.3, 18.2] | ||
| other | 404 | 9.0 | [6.6, 12.3] | 11.1 | [8.3, 14.9] | ||
| Histopathologic grade | 1 | 447 | 2.7 | [1.5, 4.7] | 5.9 | [3.9, 8.9] | < 0.001 |
| 2 | 3332 | 8.5 | [7.6, 9.5] | 13.9 | [12.6, 15.2] | ||
| 3/4 | 1497 | 16.8 | [15.0, 18.8] | 23.0 | [20.9, 25.4] | ||
| missing | 35 | 17.6 | [8.4, 36.8] | 21.8 | [11.0, 43.1] | ||
| HR expression | positivea | 4132 | 8.3 | [7.5, 9.2] | 14.0 | [12.9, 15.2] | < 0.001 |
| negative | 775 | 22.8 | [20.0, 26.0] | 27.1 | [24.0, 30.5] | ||
| missing | 404 | 9.0 | [6.6, 12.3] | 12.6 | [9.4, 16.8] | ||
| HER2/neu expression | positiveb | 972 | 13.3 | [11.3, 15.6] | 21.7 | [18.9, 24.8] | < 0.001 |
| negative | 2941 | 9.6 | [8.6, 10.7] | 14.0 | [12.7, 15.5] | ||
| missing | 1398 | 10.2 | [8.7, 11.9] | 15.6 | [13.8, 17.7] | ||
| Subtype based on immunohistochemical surrogates | ‚HR positive‘ | 4132 | 8.3 | [7.5, 9.2] | 14.0 | [12.9, 15.2] | < 0.001 |
| ‚HER2/neu positive‘c | 234 | 22.8 | [18.0, 28.9] | 28.0 | [22.4, 34.9] | ||
| ‚triple negative‘d | 376 | 23.2 | [19.3, 27.9] | 25.6 | [21.4, 30.5] | ||
| missing | 569 | 12.7 | [10.3, 15.8] | 17.8 | [14.7, 21.6] | ||
| BCS | T1/2N0 | 198 | 5.6 | [3.1, 10.0] | 7.7 | [4.4, 13.4] | |
| BCS + RT | T1/2N0 | 2114 | 4.6 | [3.8, 5.6] | 8.4 | [7.2, 9.8] | |
| Mastectomy | T1/2N0 | 606 | 9.1 | [7.1, 11.8] | 13.5 | [10.9, 16.7] | |
| (BCS + RT or mastectomy) + HT | T1/2N0 HR positivea | 1749 | 3.9 | [3.1, 4.9] | 8.2 | [6.9, 9.8] | |
| (BCS + RT or mastectomy) + CT | T1/2N0 HR negative | 277 | 12.3 | [9.0, 16.8] | 15.5 | [11.6, 20.6] | |
| (BCS + RT or mastectomy) + HT (if HR positive) + CT (if HR negative) + TT | T1/2N0 HER2/neu positive | 73 | 2.7 | [0.7, 10.9] | 2.7 | [0.7, 10.9] | |
| (BCS or mastectomy) + RT + CT + HT | T1/2N+ HR positivea | 554 | 12.0 | [9.6, 15.1] | 19.5 | [16.1, 23.7] | |
| (BCS or mastectomy) + RT + CT | T1/2N+ HR negative | 156 | 28.4 | [22.1, 36.5] | 36.9 | [29.6, 46.0] | |
| (BCS or mastectomy) + RT + CT + HT (if HR positive) + TT | T1/2N+ HER2/neu positive | 51 | 19.6 | [11.2, 34.4] | 21.9 | [12.9, 37.2] | |
| MAST + RT + CT + HT | T3/4 HR positivea | 88 | 26.1 | [18.4, 37.2] | 34.0 | [24.7, 46.9] | |
| MAST + RT + CT | T3/4 HR negative | 50 | 48.0 | [35.8, 64.3] | 52.2 | [39.8, 68.5] | |
The sample includes female patients with primary invasive breast cancer (ICD-10 code: C50) without distant metastases from Saarland diagnosed between 1999 and 2009. CI cumulative incidence, PE point estimate of CI, 95% CI 95% confidence interval of PE, T, N T and N category of TNM classification, HR hormone receptor, HER2/neu human epidermal growth factor receptor 2, BCS breast conserving surgery, RT radiotherapy, CT chemotherapy, HT hormonal (anti-estrogen) treatment, TT targeted therapy (trastuzumab). aincludes tumors with mixed HR expression (either estrogen or progesterone receptor positive), bincludes tumors with borderline expression of HER2/neu, cHER2/neu positive, but HR negative, dboth HR negative and HER2/neu negative
The 10-year CI of BRC recurrence of patients with T1/2N0 tumors was 8% if they had received BCS, but 14% if they had received a mastectomy. Patients with T1/2N0 tumors who had undergone BCS followed by radiation therapy (RT) or mastectomy had a CI of 8% if HR positive tumors were treated with hormonal therapy and of 16% if HR negative tumors were treated with chemotherapy, respectively. Patients with T1/2N+ tumors who had received BCS or mastectomy followed by RT had a CI of 20% if having a HR positive tumor and treated with both chemotherapy and hormonal therapy and of 37% if having a HR negative tumor and treated with chemotherapy alone, respectively. Patients with locally advanced T3/4 tumors who had received mastectomy, radiotherapy and chemotherapy had a CI of 52% if the tumors were HR negative, but if the tumors were HR positive and the patients additionally had received hormonal therapy, the CI was 34%.
Patients with HER2/neu positive T1/2N0 tumors had a 10-year risk of tumor recurrence of 3% if they received trastuzumab (N = 73) compared to 11% if they did not receive trastuzumab (N = 159) in addition to other recommended local and systemic treatments (p = 0.074). The overall 10-year risk of BRC recurrence of patients with T1/2N+ tumors showing HER2/neu expression was 22% if they received (N = 51) and 32% if they did not receive (N = 73) TT (p = 0.862, data not shown). The patients of the entire study sample and those included in the analysis of the risk of BRC recurrence strongly resembled both in terms of age and tumor characteristics (Table 4).
Table 4.
Distribution of age and tumor characteristics of breast cancer patients of the study sample (N = 8511) and those included in the analysis of the risk of BRC recurrence (N = 5311)
| Category | Study sample | Patients with a local R0 resection, available follow-up and without recurrence within 3 months | |||
|---|---|---|---|---|---|
| N | % a | N | % a | ||
| Overall | 8518 | 5311 | |||
| Age | < 70 years | 5725 | 67.2 | 3714 | 69.9 |
| ≥ 70 years | 2793 | 32.8 | 1597 | 30.1 | |
| Laterality | available | 8508 | 99.9 | 5307 | 99.9 |
| unilateral | 8306 | 97.6 | 5198 | 97.9 | |
| synchronous bilateral b | 202 | 2.4 | 109 | 2.1 | |
| Clinical stage | available | 7447 | 87.4 | 5064 | 95.3 |
| T1/2N0 | 4132 | 55.5 | 2918 | 57.6 | |
| T1/2N+ | 2379 | 31.9 | 1613 | 31.9 | |
| T3/4N0 | 205 | 2.8 | 124 | 2.4 | |
| T3/4N+ | 731 | 9.8 | 409 | 8.1 | |
| Microscopically verified | 8482 | 99.6 | 5311 | 100.0 | |
| Morphology | available | 8477 | 99.5 | 5311 | 100.0 |
| invasive ductal | 5883 | 69.4 | 3690 | 69.5 | |
| invasive lobular | 1190 | 14.0 | 758 | 14.3 | |
| mixed type | 654 | 7.7 | 459 | 8.6 | |
| other | 750 | 8.8 | 404 | 7.6 | |
| Histopathologic grade | available | 8216 | 96.5 | 5376 | 99.3 |
| 1 | 610 | 7.4 | 447 | 8.5 | |
| 2 | 5232 | 63.7 | 3332 | 63.2 | |
| 3/4 | 2374 | 28.9 | 1497 | 28.4 | |
| HR expression | available | 7424 | 87.2 | 4907 | 92.4 |
| positive c | 6235 | 84.0 | 4132 | 84.2 | |
| negative | 1189 | 16.0 | 775 | 15.8 | |
| HER2/neu expression | available | 5938 | 69.7 | 3913 | 73.7 |
| positive d | 1466 | 24.7 | 972 | 24.8 | |
| negative | 4472 | 75.3 | 2941 | 75.2 | |
| Subtype based on immunohistochemical surrogates | HR and HER2/neu status available | 7197 | 84.5 | 4742 | 89.3 |
| ’HR positive‘ | 6235 | 86.6 | 4132 | 87.1 | |
| ‚HER2/neu positive‘e | 368 | 5.1 | 234 | 4.9 | |
| ‚triple negative‘f | 594 | 8.3 | 376 | 7.9 | |
| Follow-up available | 8380 | 98.4 | 5311 | 100.0 | |
| years | years | ||||
| Mean observation time | 10.3 | 10.2 | |||
The sample includes female patients with primary invasive breast cancer (BRC) (ICD-10 code: C50) without distant metastases from Saarland diagnosed between 1999 and 2009. T, N T and N category of TNM classification, HR hormone receptor, HER2/neu human epidermal growth factor receptor 2. a figures printed in normal text represent numbers and proportions of the study sample and of patients with a local R0 resection who were included in the analysis of the risk of BRC recurrence, figures printed in italic text represent proportions of patients of the respective sample with available information, b tumors were classified as synchronous bilateral if the time interval between their detection was ≤3 months, c includes tumors with mixed HR expression (either estrogen or progesterone receptor positive), d includes tumors with borderline expression of HER2/neu, e HER2/neu positive, but HR negative, f both HR negative and HER2/neu negative
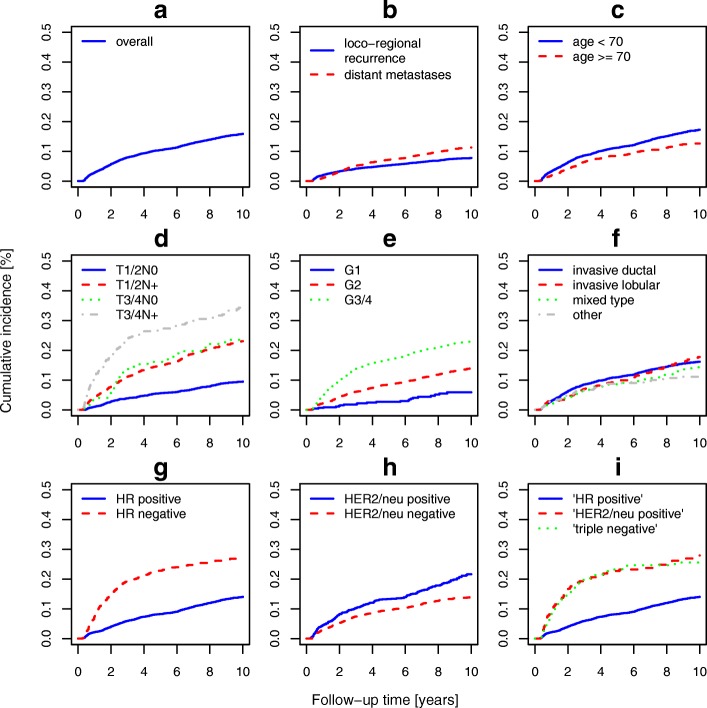
Tables 5 and 6 present estimates of the 5- and 10-year CI by type of recurrence. The overall 10-year CI of loco-regional recurrence and distant metastases after surgery with free resection margins was 8 and 11%, respectively. The CI of loco-regional recurrence and distant metastases of patients with small and localized tumors was of similar size. However, if regional lymph nodes were involved, the risk of distant metastases increased disproportionally, i.e. the 10-year CI of loco-regional recurrence and distant metastases of patients with T1/2 tumors with positive lymph nodes was 10 and 18%, respectively. The overall 10-year risk of metastasis to distant sites of patients who did not have clear resection margins after definitive surgery was 27% and thus 2.4-fold increased compared to those with free resection margins (p < 0.001, data not shown). Figure 1 depicts curves of 10-year CI of BRC recurrence by type of recurrence, age, and tumor characteristics.
Table 5.
Five- and 10-year cumulative incidence of loco-regional recurrence of breast cancer patients with local R0 resection by age, tumor characteristics and provided recommended treatments
| Category/treatment | N | 5-year CI | 10-year CI | p-value | |||
|---|---|---|---|---|---|---|---|
| PE | 95% CI | PE | 95% CI | ||||
| Overall | 5311 | 5.4 | [4.8, 6.0] | 7.8 | [7.0, 8.6] | ||
| Age | < 70 years | 3714 | 5.7 | [5.0, 6.5] | 8.5 | [7.6, 9.5] | 0.007 |
| ≥ 70 years | 1597 | 4.5 | [3.6, 5.6] | 6.1 | [4.9, 7.4] | ||
| Clinical stage | T1/2N0 | 2918 | 2.8 | [2.3, 3.5] | 5.1 | [4.3, 6.1] | < 0.001 |
| T1/2N+ | 1613 | 7.3 | [6.1, 8.7] | 10.3 | [8.8, 12.0] | ||
| T3/4N0 | 124 | 8.9 | [5.0, 15.7] | 13.0 | [8.0, 21.1] | ||
| T3/4N+ | 409 | 13.9 | [10.9, 17.7] | 15.1 | [12.0, 19.0] | ||
| missing | 247 | 6.9 | [4.4, 11.0] | 7.8 | [5.1, 12.1] | ||
| Morphology | invasive ductal | 3690 | 5.7 | [5.0, 6.5] | 8.1 | [7.2, 9.1] | 0.395 |
| invasive lobular | 758 | 4.5 | [3.2, 6.3] | 8.0 | [6.2, 10.5] | ||
| mixed type | 459 | 4.1 | [2.7, 6.4] | 6.0 | [4.1, 8.7] | ||
| other | 404 | 5.5 | [3.7, 8.3] | 6.5 | [4.4, 9.5] | ||
| Histopathologic grade | 1 | 447 | 1.3 | [0.6, 3.0] | 3.3 | [1.9, 5.9] | < 0.001 |
| 2 | 3332 | 4.3 | [3.6, 5.0] | 6.6 | [5.8, 7.6] | ||
| 3/4 | 1497 | 8.8 | [7.5, 10.3] | 11.4 | [9.9, 13.3] | ||
| missing | 35 | 11.4 | [4.5, 29.2] | 15.5 | [6.7, 36.0] | ||
| HR expression | positivea | 4132 | 4.1 | [3.6, 4.8] | 6.6 | [5.8, 7.4] | < 0.001 |
| negative | 775 | 12.4 | [10.3, 15.0] | 15.2 | [12.8, 18.1] | ||
| missing | 404 | 4.2 | [2.7, 6.8] | 5.2 | [3.4, 8.0] | ||
| HER2/neu expression | positiveb | 972 | 7.1 | [5.7, 8.9] | 10.8 | [8.8, 13.2] | 0.004 |
| negative | 2941 | 4.9 | [4.2, 5.7] | 6.9 | [6.0, 8.0] | ||
| missing | 1398 | 5.1 | [4.1, 6.4] | 7.6 | [6.3, 9.2] | ||
| Subtype based on immunohistochemical surrogates | ‘HR positive‘ | 4132 | 4.1 | [3.6, 4.8] | 6.6 | [5.8, 7.4] | < 0.001 |
| ‚HER2/neu positive‘c | 234 | 12.9 | [9.2, 18.0] | 16.2 | [11.8, 22.3] | ||
| ‘triple negative‘d | 376 | 12.0 | [9.1, 15.8] | 13.8 | [10.6, 17.9] | ||
| missing | 569 | 6.7 | [4.9, 9.1] | 9.1 | [6.9, 11.9] | ||
| BCS | T1/2N0 | 198 | 3.6 | [1.7, 7.4] | 4.9 | [2.3, 10.2] | |
| BCS + RT | T1/2N0 | 2114 | 2.1 | [1.6, 2.9] | 4.4 | [3.5, 5.5] | |
| Mastectomy | T1/2N0 | 606 | 4.8 | [3.4, 6.9] | 7.9 | [5.9, 10.5] | |
| (BCS + RT or mastectomy) + HT | T1/2N0 HR positive a | 1749 | 1.7 | [1.2, 2.4] | 4.3 | [3.3, 5.5] | |
| (BCS + RT or mastectomy) + CT | T1/2N0 HR negative | 277 | 6.5 | [4.2, 10.2] | 9.3 | [6.3, 13.7] | |
| (BCS + RT or mastectomy) + HT (if HR positive) + CT (if HR negative) + TT | T1/2N0 HER2/neu positive | 73 | 0.0 | – | 0.0 | – | |
| (BCS or mastectomy) + RT + CT + HT | T1/2N+ HR positive a | 554 | 5.8 | [4.2, 8.2] | 8.3 | [6.2, 11.2] | |
| (BCS or mastectomy) + RT + CT | T1/2N+ HR negative | 156 | 12.9 | [8.5, 19.4] | 16.5 | [11.3, 24.1] | |
| (BCS or mastectomy) + RT + CT + HT (if HR positive) + TT | T1/2N+ HER2/neu positive | 51 | 9.8 | [4.2, 22,7] | 9.8 | [4.2, 22,7] | |
| MAST + RT + CT + HT | T3/4 HR positive a | 88 | 17.0 | [10.7, 27.1] | 17.0 | [10.7, 27.1] | |
| MAST + RT + CT | T3/4 HR negative | 50 | 22.0 | [13.0, 37.3] | 26.2 | [16.3, 42.1] | |
The sample includes female patients with primary invasive breast cancer (ICD-10 code: C50) without distant metastases from Saarland diagnosed between 1999 and 2009. CI cumulative incidence, PE point estimate of CI, 95% CI 95% confidence interval of PE, T, N T and N category of TNM classification, HR hormone receptor, HER2/neu human epidermal growth factor receptor 2, BCS breast conserving surgery, RT radiotherapy, CT chemotherapy, HT hormonal (anti-estrogen) treatment, TT targeted treatment (trastuzumab). aincludes tumors with mixed HR expression (either estrogen or progesterone receptor positive), bincludes tumors with borderline expression of HER2/neu, cHER2/neu positive, but HR negative, dboth HR negative and HER2/neu negative
Table 6.
Five- and 10-year cumulative incidence of distant metastases of breast cancer patients with local R0 resection by age, tumor characteristics and provided recommended treatments
| Category/treatment | N | 5-year CI | 10-year CI | p-value | |||
|---|---|---|---|---|---|---|---|
| PE | 95% CI | PE | 95% CI | ||||
| Overall | 5311 | 7.2 | [6.5, 7.9] | 11.3 | [10.4, 12.3] | ||
| Age | < 70 years | 3714 | 7.8 | [7.0, 8.7] | 12.5 | [11.4, 13.7] | < 0.001 |
| ≥ 70 years | 1597 | 5.8 | [4.7, 7.0] | 8.6 | [7.2, 10.2] | ||
| Clinical stage | T1/2N0 | 2918 | 3.6 | [3.0, 4.4] | 6.0 | [5.1, 7.0] | < 0.001 |
| T1/2N+ | 1613 | 10.8 | [9.4, 12.4] | 17.5 | [15.6, 19.7] | ||
| T3/4N0 | 124 | 11.3 | [6.9, 18.6] | 16.6 | [10.9, 25.4] | ||
| T3/4N+ | 409 | 19.6 | [16.1, 23.8] | 27.3 | [23.0, 32.4] | ||
| missing | 247 | 3.3 | [1.6, 6.5] | 4.3 | [2.3, 7.9] | ||
| Morphology | invasive ductal | 3690 | 7.6 | [6.8, 8.5] | 11.5 | [10.4, 12.7] | 0.085 |
| invasive lobular | 758 | 6.6 | [5.1, 8.7] | 12.4 | [10.1, 15.4] | ||
| mixed type | 459 | 6.3 | [4.5, 9.0] | 11.3 | [8.6, 15.0] | ||
| other | 404 | 5.3 | [3.5, 8.0] | 7.0 | [4.8, 10.2] | ||
| Histopathologic grade | 1 | 447 | 1.4 | [0.6, 3.0] | 3.9 | [2.3, 6.6] | < 0.001 |
| 2 | 3332 | 5.8 | [5.0, 6.6] | 9.8 | [8.7, 10.9] | ||
| 3/4 | 1497 | 12.1 | [10.5, 13.8] | 17.0 | [15.1, 19.1] | ||
| missing | 35 | 9.0 | [3.0, 26.9] | 9.0 | [3.0, 26.9] | ||
| HR expression | positive a | 4132 | 5.6 | [5.0, 6.4] | 10.0 | [9.0, 11.0] | < 0.001 |
| negative | 775 | 15.9 | [13.5, 18.7] | 19.0 | [16.4, 22.1] | ||
| missing | 404 | 6.3 | [4.3, 9.1] | 9.8 | [7.0, 13.8] | ||
| HER2/neu expression | positive b | 972 | 9.1 | [7.4, 11.1] | 15.1 | [12.8, 17.8] | 0.001 |
| negative | 2941 | 6.6 | [5.7, 7.5] | 9.8 | [8.7, 11.0] | ||
| missing | 1398 | 7.2 | [6.0, 8.7] | 11.6 | [9.9, 13.5] | ||
| Subtype based on immunohistochemical surrogates | ’HR positive‘ | 4132 | 5.6 | [5.0, 6.4] | 10.0 | [9.0, 11.0] | < 0.001 |
| ‚HER2/neu positive‘c | 234 | 15.9 | [11.8, 21.4] | 19.4 | [14.7, 25.5] | ||
| ‚triple negative‘d | 376 | 16.3 | [12.9, 20.5] | 18.7 | [15.0, 23.2] | ||
| missing | 569 | 8.9 | [6.8, 11.5] | 12.6 | [10.0, 15.9] | ||
| BCS | T1/2N0 | 198 | 3.1 | [1.4, 6.7] | 4.4 | [2.2, 8.7] | |
| BCS + RT | T1/2N0 | 2114 | 3.0 | [2.4, 3.9] | 5.4 | [4.5, 6.6] | |
| Mastectomy | T1/2N0 | 606 | 5.8 | [4.2, 8.0] | 8.2 | [6.2, 10.8] | |
| (BCS + RT or mastectomy) + HT | T1/2N0 HR positive a | 1749 | 2.7 | [2.0, 3.6] | 5.2 | [4.2, 6.5] | |
| (BCS + RT or mastectomy) + CT | T1/2N0 HR negative | 277 | 7.9 | [5.3, 11.9] | 9.2 | [6.3, 13.4] | |
| (BCS + RT or mastectomy) + HT (if HR positive) + CT (if HR negative) + TT | T1/2N0 HER2/neu positive | 73 | 2.7 | [0.7, 10.9] | 2.7 | [0.7, 10.9] | |
| (BCS or mastectomy) + RT + CT + HT | T1/2N+ HR positive a | 554 | 8.6 | [6.5, 11.3] | 15.4 | [12.2, 19.3] | |
| (BCS or mastectomy) + RT + CT | T1/2N+ HR negative | 156 | 23.2 | [17.4, 31.0] | 30.7 | [23.9, 39.4] | |
| (BCS or mastectomy) + RT + CT + HT (if HR positive) + TT | T1/2N+ HER2/neu positive | 51 | 13.7 | [6.8, 27.5] | 16.0 | [8.4, 30.5] | |
| MAST + RT + CT + HT | T3/4 HR positive a | 88 | 14.8 | [8.9, 24.5] | 24.1 | [15.8, 36.8] | |
| MAST + RT + CT | T3/4 HR negative | 50 | 40.0 | [28.3, 56.5] | 40.0 | [28.3, 56.5] | |
The sample includes female patients with primary invasive breast cancer (ICD-10 code: C50) without distant metastases from Saarland diagnosed between 1999 and 2009. CI cumulative incidence, PE point estimate of CI, 95% CI 95% confidence interval of PE, T, N T and N category of TNM classification, HR hormone receptor, HER2/neu human epidermal growth factor receptor 2, BCS breast conserving surgery, RT radiotherapy, CT chemotherapy, HT hormonal (anti-estrogen) treatment, TT targeted treatment (trastuzumab). aincludes tumors with mixed HR expression (either estrogen or progesterone receptor positive), bincludes tumors with borderline expression of HER2/neu, cHER2/neu positive, but HR negative, dboth HR negative and HER2/neu negative
Fig. 1.
Ten-year cumulative incidence curves of cancer recurrence of female patients with primary invasive breast cancer. Cumulative incidence of cancer recurrence of breast cancer patients (ICD-10 code: C50) without distant metastases from Saarland after local R0 resection and diagnosed between 1999 and 2009 overall (subfigure a), by type of recurrence (b), age (c), clinical stage (d), histopathologic grade (e), morphologic type (f), hormone receptor expression (g), HER2/neu expression (h), and subtype based on immunohistochemical surrogates (i). T, N T and N category of TNM classification, HR hormone receptor, HER2/neu human epidermal growth factor receptor 2
Discussion
This study derived population-based long-term estimates of the risk of cancer recurrence of BRC patients from Germany who had received local surgery with free resection margins (R0) up to 10 years after diagnosis. Overall 5- and 10-year CI of BRC recurrence was 10 and 16%. The 10-year CI of loco-regional recurrence and distant metastases was 8 and 11%, respectively. The derived estimates showed substantial variation and were particularly increased if tumors were locally or regionally advanced, of high grade, or classified as subtype ‘HER2/neu positive’ (without HR expression) or ‘triple negative’.
To date, studies of the risk of loco-regional recurrence and distant metastases using population-based samples of BRC patients are scant. Published CPGs such as the European Society for Medical Oncology clinical practice guidelines [24], the American Society of Breast Surgeons consensus guideline [31], and the German interdisciplinary S3 guidelines [12] mostly refer to studies which used hospital-based cohorts [7, 13–15] or participants of clinical trials [4, 32]. Furthermore, several of these studies included patients who had been diagnosed decades ago (e. g. two studies from the US reported a 5-year risk of local recurrence of 8% of BRC patients who had been treated with lumpectomy and radiotherapy between 1968 and 1984 [13] or a 10-year risk of loco-regional recurrence and distant metastases of 17 and 35% of BRC patients who had been treated between 1975 and 1994 with mastectomy, chemotherapy and with or without hormonal therapy, respectively [4], and a more recently published study from the Netherlands reported a 5-year risk of BRC recurrence of 29 and 11% of patients with T1 tumors aged ≤40 and > 40 who had received BCS and radiotherapy between 1984 and 1997, respectively [15]). Therefore, the results of these studies may be outdated and not representative for more recently treated patients. Studies including participants of clinical trials (e.g. [4, 32]) may have even less external validity, as trial enrollees are often highly selected and therefore little representative of the overall population of BRC patients [33, 34].
To systematically identify registry-based studies presenting results of more recently diagnosed patients, the PubMed database was searched using the terms ‘breast cancer’, ‘recurrence’, and ‘registry’. Few studies from the Netherlands [35–39], the US [40], Canada [41], Italy [42] could be identified upon careful reading. However, several issues hampered the comparison of these studies’ findings with the results presented in this article. First, selective referral to participating clinics may have limited the applicability of the results derived from hospital-based cohorts (e.g. [7, 35, 40, 43–45]) to unselected populations of BRC patients. Second, some population-based studies investigated patient samples with special characteristics (e.g. patients with T1/2N0 basal like BRC subtype [36] or patients aged ≤35 years who received post-mastectomy radiation [41]), and corresponding subgroups of patients could not be analyzed in this study due to small patient numbers or missing data items. Third, some published population-based studies used ‘classical’ Kaplan-Meier estimators, reported proportions, or did not mention whether competing risks had been taken into account (e.g. [7, 37, 39, 40]).
A population-based study from the Netherlands reported similar overall 5-year risks of loco-regional recurrence and distant metastases of 4 and 9% of BRC patients who received surgery between 2003 and 2008 and who had a comparable stage distribution [37]. A recently published study from Denmark which linked data from different registries reported 5-year CI of BRC recurrence and bone and visceral metastases of 18, 2 and 5%, respectively, in a sample of 23,478 patients with regional or stage II or III BRC [46]. Patients of the current study with regional or stage II or III BRC who were overall comparable to the Danish sample had a 5-year CI of BRC recurrence and distant metastases of 16 and 11%, respectively (data not shown). In both studies the overall risk of BRC recurrence was comparable. The somewhat lower risk of distant metastases derived in the Danish study could have resulted from both a possible underestimation of metastases [46] and a somewhat higher proportion of patients with involved lymph nodes in the current study. Another recently published study from Italy used population-based samples of BRC patients and reported comparable overall proportions of patients with tumors with HR and HER2/neu expression and accordingly, similar proportions of luminal (subtypes A, B, and HER2/neu), HER2/neu like and basal like BRC of 83, 7, and 11%, respectively [42]. Observed 5-year risks of BRC recurrence of the Italian patients with luminal A, HER2/neu and basal like tumors of 7, 22, and 20% were quite comparable to this study’s results. A summary of the aforementioned and cited studies is provided as Additional file 1.
This study included virtually all women residing in the federal state of Saarland who had been diagnosed with primary invasive BRC between 1999 and 2009 [18, 19]. The derived estimates of BRC recurrence given in this work therefore reflect the risk of ‘real world’ patients and the effectiveness of cancer care provided in a routine setting. This study intended to investigate the risk of BRC recurrence of patients who had received surgery with free resection margins as a recommended local treatment [23, 25, 47]. The overall proportion of patients with involved resection margins after definitive surgery was 5% and these patients had a 2.4-fold increased risk of a metastatic spread of their tumor compared to patients with free resection margins.
Preceding analyses of the implementation of guideline recommendations in the study sample revealed that the proportion of patients (with available information on HER2/neu expression of their tumor) who received trastuzumab rose from 2 to 47% between 2000 and 2009 [48]. Thus, the observed findings of a 4.2-fold and a 1.5-fold increased risk of BRC recurrence of patients with early T1/2N0 and T1/2N+ BRC who had not received TT in addition to recommended local and other systemic treatments may be indicative for the effectiveness of trastuzumab therapy on a population level, even though the limited sample size and the observational design of this registry-based study must be taken into account.
One of the studies cited in the text and listed in Additional file 1 provided estimates of the effect of TT on the risk of BRC recurrence. Patients from The Netherlands with HER2/neu expressed tumors who had been treated with trastuzumab conveyed a 50% risk reduction of loco-regional BRC recurrence in the first 5 years after treatment compared to untreated patients [37]. The observed absolute risks of local and regional recurrence in that study were 2 and 1% among patients treated with trastuzumab and 4 and 3% among those without. In the current study, 5-year risk estimates of loco-regional recurrence of 6% among patients with and 7% among patients without trastuzumab treatment were observed in a patient sample which additionally encompassed patients with neoadjuvant systemic treatments.
The shapes of the curves of CI of BRC recurrence point to an initially higher hazard of patients with HR negative tumors which decreases over time and an initially lower but constant hazard of patients with HR positive tumors. Additional analyses confirmed these patterns and revealed a slightly higher hazard of BRC recurrence among patients with HR positive tumors starting 6 or 7 years after diagnosis (data not shown), which has been observed in participants of several clinical trials [23].
Recent studies have shown fear of recurrence to be highly prevalent among BRC survivors [49], and that many BRC patients overestimate their risk of cancer recurrence [50]. For these reasons, the presented results may extend available knowledge and have great relevance for clinicians, their patients and researchers. Furthermore, this study derived estimates of the long-term risk of BRC recurrence up to 10 years after diagnosis. To the best of the knowledge of the authors, so far only one study from the Netherlands had reported population-based long-term estimates of BRC recurrence of such an extended follow-up period [39]. This study has further strengths. First, the size of the study sample allowed to deriving detailed estimates by tumor characteristics and major treatment options. Second, the use of multiple sources of information ensured a high completeness and validity of the available data [48], i.e. detailed information of characteristics such as clinical stage, histopathologic grade, and subtype were available for 87, 97 and 85% of the tumors, respectively. The registration model with different sources of information and statutory provisions on the events to trigger cancer notifications, the interdisciplinary approach of the treatment of patients with recurrent BRC [22, 23] and the fact that recurrent and metastatic BRC often turns into a chronic condition maximized the likelihood of a registration of cancer recurrence and thus an almost complete follow-up of the patients with regard to these outcomes. Since the mid-1990s, the annual number of notifications of recurrent BRC to the Saarland CR was rather stable and ranged between 192 and 255, respectively.
However, there are also weaknesses that require careful consideration. Detailed data on administered local surgery and radiotherapy, chemotherapy, hormonal treatment and TT were available for 78, 82, 79, and 56% of the patients, respectively. Trastuzumab became available for treatment in the calendar period when the patients of this study received primary treatment. Therefore, the proportion of patients with information whether or not a TT was administered was lower compared to the other treatments. Despite this, the sample of patients used for the analyses showed great similarity with the study sample and was large enough to obtain estimates of the CI of BRC recurrence with sufficient precision for most of the clinically relevant subgroups of patients. As the majority of patients had received a guideline adherent treatment [48] and additional information, e.g. on co-morbidity of the patients was lacking, no comparative analyses of the risk of recurrence of patients with and without guideline adherent treatment have been carried out except for patients with early stage BRC showing HER2/neu expression. Furthermore, additional information on histopathological tumor characteristics which affect the risk of recurrence such as vessel invasion or prognostic marker Ki-67 [51] have not been collected or have come into routine use very recently only. The CR did not collect information of the staging examinations of individual patients. Major implications of different staging over time or an increasing sensitivity and improved accuracy of diagnostic procedures seem unlikely, as the observed proportion of patients with metastatic BRC at the time of diagnosis remained rather constant over time.
Based on recommendations of the National Cancer Plan from 2008, the majority of German federal states have meanwhile extended the existing population-based CRs to comprehensive CRs [52]. The collection of the data used in this study including cancer recurrence as outcome anticipated the items of the common and mandatory basic dataset which is now basis for data collection of comprehensive CRs in Germany. This common catalogue of data items, passive registration with defined events to trigger mandatory notifications to CRs and a sustainable funding hopefully prove to be suitable for collecting high quality CR data in Germany for reporting cancer burden, for monitoring the quality of cancer care and for oncologic research [52]. A recently published article which demonstrated the lack of reliable data on cancer recurrence after primary cancer treatment in the US and an accompanying editorial argued for the collection of such data by population-based CRs [53, 54]. The availability of population-based data of the long-term risk of cancer recurrence up to 10 years after diagnosis and derived from a much larger population of BRC patients from Germany is still some way off.
Conclusions
The presented findings of this study may extend available knowledge of the long-term risk of cancer recurrence of an unselected population of BRC patients from a central European region who received cancer care in a routine setting. The study further demonstrated the usefulness of CRs for a population-based monitoring of the effectiveness of cancer care in terms of disease recurrence as a major treatment related outcome measure.
Additional file
Cited studies of the risk of cancer recurrence of breast cancer patients. (PDF 27 kb)
Acknowledgements
Not applicable.
Funding
This work was supported in part by the German Cancer Aid (Deutsche Krebshilfe) (grant numbers 70–3166-Br5, 108257 and 108761). The German Cancer Aid had no role in the design, the data collection, the analysis, the interpretation of the results, the writing of the manuscript, or in the decision to submit the manuscript.
Availability of data and materials
The access to the data used during this study is subject to state provisions.
Abbreviations
- BCS
Breast conserving surgery
- BRC
Breast cancer
- CI
Cumulative incidence
- CPG
Clinical practice guidelines
- CR
Cancer registry
- HER2/neu
Human epidermal growth factor receptor 2
- HR
Hormone receptor
- ICD-10
International Classification of Diseases, 10th Revision
- RT
Radiation therapy
- T, N, M
Categories of the UICC TNM staging classification
- TT
Targeted treatment
Authors’ contributions
This study was designed by BH, CS and HB. BH prepared and managed the dataset and analyzed the data. All authors provided interpretation of the results. BH drafted the manuscript and CS, JCR, ES and HB critically reviewed and revised the draft. All authors have approved the final version of the manuscript.
Ethics approval and consent to participate
No informed consent of the patients was necessary for this study. The data were collected according to state provisions (Saarland Cancer Registration Act) for the purpose of monitoring cancer burden, care and outcomes and provided and used for this study according to the respective legal regulations.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Cancer Today http://gco.iarc.fr/today. Accessed 23, Jan 2018.
- 2.Sant M, Chirlaque Lopez MD, Agresti R, Sanchez Perez MJ, Holleczek B, Bielska-Lasota M, Dimitrova N, Innos K, Katalinic A, Langseth H, et al. Survival of women with cancers of breast and genital organs in Europe 1999-2007: results of the EUROCARE-5 study. Eur J Cancer. 2015;51:2191–2205. doi: 10.1016/j.ejca.2015.07.022. [DOI] [PubMed] [Google Scholar]
- 3.Allemani C, Weir HK, Carreira H, Harewood R, Spika D, Wang XS, Bannon F, Ahn JV, Johnson CJ, Bonaventure A, et al. Global surveillance of cancer survival 1995-2009: analysis of individual data for 25,676,887 patients from 279 population-based registries in 67 countries (CONCORD-2) Lancet. 2015;385:977–1010. doi: 10.1016/S0140-6736(14)62038-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Katz A, Strom EA, Buchholz TA, Theriault R, Singletary SE, McNeese MD. The influence of pathologic tumor characteristics on locoregional recurrence rates following mastectomy. Int J Radiat Oncol Biol Phys. 2001;50:735–742. doi: 10.1016/S0360-3016(01)01500-0. [DOI] [PubMed] [Google Scholar]
- 5.Wallgren A, Bonetti M, Gelber RD, Goldhirsch A, Castiglione-Gertsch M, Holmberg SB, Lindtner J, Thurlimann B, Fey M, Werner ID, et al. Risk factors for locoregional recurrence among breast cancer patients: results from international breast Cancer study group trials I through VII. J Clin Oncol. 2003;21:1205–1213. doi: 10.1200/JCO.2003.03.130. [DOI] [PubMed] [Google Scholar]
- 6.Livasy CA, Karaca G, Nanda R, Tretiakova MS, Olopade OI, Moore DT, Perou CM. Phenotypic evaluation of the basal-like subtype of invasive breast carcinoma. Mod Pathol. 2006;19:264–271. doi: 10.1038/modpathol.3800528. [DOI] [PubMed] [Google Scholar]
- 7.Park S, Koo JS, Kim MS, Park HS, Lee JS, Kim SI, Park BW. Characteristics and outcomes according to molecular subtypes of breast cancer as classified by a panel of four biomarkers using immunohistochemistry. Breast. 2012;21:50–57. doi: 10.1016/j.breast.2011.07.008. [DOI] [PubMed] [Google Scholar]
- 8.Lowery AJ, Kell MR, Glynn RW, Kerin MJ, Sweeney KJ. Locoregional recurrence after breast cancer surgery: a systematic review by receptor phenotype. Breast Cancer Res Treat. 2012;133:831–841. doi: 10.1007/s10549-011-1891-6. [DOI] [PubMed] [Google Scholar]
- 9.Ribelles N, Perez-Villa L, Jerez JM, Pajares B, Vicioso L, Jimenez B, de Luque V, Franco L, Gallego E, Marquez A, et al. Pattern of recurrence of early breast cancer is different according to intrinsic subtype and proliferation index. Breast Cancer Res. 2013;15:R98. doi: 10.1186/bcr3559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Thill M, Liedtke C, Solomayer EF, Muller V, Janni W, Schmidt M. AGO recommendations for the diagnosis and treatment of patients with advanced and metastatic breast Cancer: update 2017. Breast Care (Basel) 2017;12:184–191. doi: 10.1159/000477576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liedtke C, Thill M, Jackisch C, Thomssen C, Muller V, Janni W. AGO recommendations for the diagnosis and treatment of patients with early breast Cancer: update 2017. Breast Care (Basel) 2017;12:172–183. doi: 10.1159/000477575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.[Interdisciplinary S3 guidelines for the diagnosis, treatment and follow-up care of breast cancer] S3-Leitlinie Früherkennung, Diagnose, Therapie und Nachsorge des Mammakarzinoms, Kurzversion 4.0 , 2017, AWMF Registernummer: 032-045OL https://www.leitlinienprogramm-onkologie.de/fileadmin/user_upload/Downloads/Leitlinien/Mammakarzinom_4_0/Version_4.0/LL_Mammakarzinom_Kurzversion_4.0.pdf. Accessed 20 May 2019.
- 13.Haffty BG, Fischer D, Beinfield M, McKhann C. Prognosis following local recurrence in the conservatively treated breast cancer patient. Int J Radiat Oncol Biol Phys. 1991;21:293–298. doi: 10.1016/0360-3016(91)90774-X. [DOI] [PubMed] [Google Scholar]
- 14.Karabali-Dalamaga S, Souhami RL, O'Higgins NJ, Soumilas A, Clark CG. Natural history and prognosis of recurrent breast cancer. Br Med J. 1978;2:730–733. doi: 10.1136/bmj.2.6139.730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jobsen JJ, van der Palen J, Meerwaldt JH. The impact of age on local control in women with pT1 breast cancer treated with conservative surgery and radiation therapy. Eur J Cancer. 2001;37:1820–1827. doi: 10.1016/S0959-8049(01)00173-3. [DOI] [PubMed] [Google Scholar]
- 16.Saphner T, Tormey DC, Gray R. Annual hazard rates of recurrence for breast cancer after primary therapy. J Clin Oncol. 1996;14:2738–2746. doi: 10.1200/JCO.1996.14.10.2738. [DOI] [PubMed] [Google Scholar]
- 17.Demicheli R, Miceli R, Brambilla C, Ferrari L, Moliterni A, Zambetti M, Valagussa P, Bonadonna G. Comparative analysis of breast cancer recurrence risk for patients receiving or not receiving adjuvant cyclophosphamide, methotrexate, fluorouracil (CMF). Data supporting the occurrence of 'cures. Breast Cancer Res Treat. 1999;53:209–215. doi: 10.1023/A:1006134702484. [DOI] [PubMed] [Google Scholar]
- 18.Robert Koch Institute. Association of Population-based Cancer Registries in Germany . Cancer in Germany 2007/2008. Incidence and trends. 8. Berlin: Robert Koch Institute; 2012. [Google Scholar]
- 19.Bertz J, Hentschel S, Hundsdörfer G, Kaatsch P, Katalinic A, Lehnert M, Schön D, Stegmaier C, Ziegler H: [Cancer in Germany. Forth revised, updated edition]. Saarbrücken: Association of Population-Based Cancer Registries in Germany in cooperation with the Robert Koch Institute; 2004.
- 20.International Agency for Research on Cancer (ed.): International rules for multiple primary cancers. ICD-O third edition. Internal report 2004/02. Lyon: IARC; 2004.
- 21.Holleczek B, Jansen L, Brenner H. Breast cancer survival in Germany: a population-based high resolution study from Saarland. PLoS One. 2013;8:e70680. doi: 10.1371/journal.pone.0070680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.German Cancer Society . German Society for Gynecology and Obstetrics (eds.): Interdisciplinary S3 Guidelines for the Diagnosis, Treatment and Follow-up Care of Breast Cancer. München: Zuckschwerdt; 2008. [Google Scholar]
- 23.Colleoni M, Sun Z, Price KN, Karlsson P, Forbes JF, Thurlimann B, Gianni L, Castiglione M, Gelber RD, Coates AS, et al. Annual Hazard rates of recurrence for breast Cancer during 24 years of follow-up: results from the international breast Cancer study group trials I to V. J Clin Oncol. 2016;34:927–935. doi: 10.1200/JCO.2015.62.3504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Senkus E, Kyriakides S, Ohno S, Penault-Llorca F, Poortmans P, Rutgers E, Zackrisson S, Cardoso F. Primary breast cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2015;26(Suppl 5):v8–30. doi: 10.1093/annonc/mdv298. [DOI] [PubMed] [Google Scholar]
- 25.Wockel A, Kreienberg R. First revision of the German S3 guideline 'Diagnosis, therapy, and follow-up of breast Cancer. Breast Care (Basel) 2008;3:82–86. doi: 10.1159/000127509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kalbfleisch JD, Prentice RL. The statistical analysis of failure time data. New York: John Wiley & Sons; 1980. [Google Scholar]
- 27.Aalen O. Nonparametric estimation of partial transition probabilities in multiple decrement models. Ann Stat. 1978;6:534–545. doi: 10.1214/aos/1176344198. [DOI] [Google Scholar]
- 28.Bie O, Borgan O, Liestøl K. Confidence intervals and confidence bands for the cumulative Hazard rate function and their small sample properties. Scand J Stat. 1987;14:221–233. [Google Scholar]
- 29.R Development Core . Team: R: a language and environment for statistical computing. Vienna: R Foundation for Statistical Computing; 2015. [Google Scholar]
- 30.Gray RJ. A class of K-sample tests for comparing the cumulative incidence of a competing risk. Ann Stat. 1988;16:1141–1154. doi: 10.1214/aos/1176350951. [DOI] [Google Scholar]
- 31.Consensus Guideline on Breast Cancer Lumpectomy Margins https://www.breastsurgeons.org/docs/statements/Consensus-Guideline-on-Breast-Cancer-Lumpectomy-Margins.pdf. Accessed 20 May 2019.
- 32.Darby S, McGale P, Correa C, Taylor C, Arriagada R, Clarke M, Cutter D, Davies C, Ewertz M, Godwin J, et al. Effect of radiotherapy after breast-conserving surgery on 10-year recurrence and 15-year breast cancer death: meta-analysis of individual patient data for 10,801 women in 17 randomised trials. Lancet. 2011;378:1707–1716. doi: 10.1016/S0140-6736(11)61629-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Al-Refaie WB, Vickers SM, Zhong W, Parsons H, Rothenberger D, Habermann EB. Cancer trials versus the real world in the United States. Ann Surg. 2011;254:438–442. doi: 10.1097/SLA.0b013e31822a7047. [DOI] [PubMed] [Google Scholar]
- 34.Simon MS, Du W, Flaherty L, Philip PA, Lorusso P, Miree C, Smith D, Brown DR. Factors associated with breast cancer clinical trials participation and enrollment at a large academic medical center. J Clin Oncol. 2004;22:2046–2052. doi: 10.1200/JCO.2004.03.005. [DOI] [PubMed] [Google Scholar]
- 35.van der Heiden-van der Loo M, Siesling S, Wouters MW, van Dalen T, Rutgers EJ, Peeters PH. The value of Ipsilateral breast tumor recurrence as a quality Indicator: hospital variation in the Netherlands. Ann Surg Oncol. 2015;22(Suppl 3):S522–S528. doi: 10.1245/s10434-015-4626-9. [DOI] [PubMed] [Google Scholar]
- 36.van Roozendaal LM, Smit LHM, Duijsens G, de Vries B, Siesling S, Lobbes MBI, de Boer M, de Wilt JHW, Smidt ML. Risk of regional recurrence in triple-negative breast cancer patients: a Dutch cohort study. Breast Cancer Res Treat. 2016;156:465–472. doi: 10.1007/s10549-016-3757-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Aalders KC, van Bommel AC, van Dalen T, Sonke GS, van Diest PJ, Boersma LJ, van der Heiden-van der Loo M. Contemporary risks of local and regional recurrence and contralateral breast cancer in patients treated for primary breast cancer. Eur J Cancer. 2016;63:118–126. doi: 10.1016/j.ejca.2016.05.010. [DOI] [PubMed] [Google Scholar]
- 38.Aalders KC, Postma EL, Strobbe LJ, van der Heiden-van der Loo M, Sonke GS, Boersma LJ, van Diest PJ, Siesling S, van Dalen T. Contemporary Locoregional recurrence rates in young patients with early-stage breast Cancer. J Clin Oncol. 2016;34:2107–2114. doi: 10.1200/JCO.2015.64.3536. [DOI] [PubMed] [Google Scholar]
- 39.Geurts YM, Witteveen A, Bretveld R, Poortmans PM, Sonke GS, Strobbe LJA, Siesling S. Patterns and predictors of first and subsequent recurrence in women with early breast cancer. Breast Cancer Res Treat. 2017;165:709–720. doi: 10.1007/s10549-017-4340-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fehrenbacher L, Capra AM, Quesenberry CP, Jr, Fulton R, Shiraz P, Habel LA. Distant invasive breast cancer recurrence risk in human epidermal growth factor receptor 2-positive T1a and T1b node-negative localized breast cancer diagnosed from 2000 to 2006: a cohort from an integrated health care delivery system. J Clin Oncol. 2014;32:2151–2158. doi: 10.1200/JCO.2013.52.0858. [DOI] [PubMed] [Google Scholar]
- 41.Quan ML, Osman F, McCready D, Fernandes K, Sutradhar R, Paszat L. Postmastectomy radiation and recurrence patterns in breast cancer patients younger than age 35 years: a population-based cohort. Ann Surg Oncol. 2014;21:395–400. doi: 10.1245/s10434-013-3319-5. [DOI] [PubMed] [Google Scholar]
- 42.Minicozzi P, Bella F, Toss A, Giacomin A, Fusco M, Zarcone M, Tumino R, Falcini F, Cesaraccio R, Candela G, et al. Relative and disease-free survival for breast cancer in relation to subtype: a population-based study. J Cancer Res Clin Oncol. 2013;139:1569–1577. doi: 10.1007/s00432-013-1478-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Corradini S, Bauerfeind I, Belka C, Braun M, Combs SE, Eckel R, Harbeck N, Holzel D, Kiechle M, Niyazi M, et al. Trends in use and outcome of postoperative radiotherapy following mastectomy: a population-based study. Radiother Oncol. 2017;122:2–10. doi: 10.1016/j.radonc.2016.08.018. [DOI] [PubMed] [Google Scholar]
- 44.Corradini S, Niyazi M, Niemoeller OM, Li M, Roeder F, Eckel R, Schubert-Fritschle G, Scheithauer HR, Harbeck N, Engel J, et al. Adjuvant radiotherapy after breast conserving surgery - a comparative effectiveness research study. Radiother Oncol. 2015;114:28–34. doi: 10.1016/j.radonc.2014.08.027. [DOI] [PubMed] [Google Scholar]
- 45.Radosa JC, Eaton A, Stempel M, Khander A, Liedtke C, Solomayer EF, Karsten M, Pilewskie M, Morrow M, King TA. Evaluation of local and distant recurrence patterns in patients with triple-negative breast Cancer according to age. Ann Surg Oncol. 2017;24:698–704. doi: 10.1245/s10434-016-5631-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cronin-Fenton D, Kjaersgaard A, Norgaard M, Amelio J, Liede A, Hernandez RK, Sorensen HT. Breast cancer recurrence, bone metastases, and visceral metastases in women with stage II and III breast cancer in Denmark. Breast Cancer Res Treat. 2018;167:517–528. doi: 10.1007/s10549-017-4510-3. [DOI] [PubMed] [Google Scholar]
- 47.Goldhirsch A, Glick JH, Gelber RD, Senn HJ. Meeting highlights: international consensus panel on the treatment of primary breast Cancer. J Natl Cancer Inst. 1998;90:1601–1608. doi: 10.1093/jnci/90.21.1601. [DOI] [PubMed] [Google Scholar]
- 48.Holleczek B, Brenner H. Provision of breast cancer care and survival in Germany - results from a population-based high resolution study from Saarland. BMC Cancer. 2014;14:757. doi: 10.1186/1471-2407-14-757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Koch L, Bertram H, Eberle A, Holleczek B, Schmid-Hopfner S, Waldmann A, Zeissig SR, Brenner H, Arndt V. Fear of recurrence in long-term breast cancer survivors-still an issue. Results on prevalence, determinants, and the association with quality of life and depression from the Cancer survivorship-a multi-regional population-based study. Psychooncology. 2014;23:547–554. doi: 10.1002/pon.3452. [DOI] [PubMed] [Google Scholar]
- 50.Hawley ST, Janz NK, Griffith KA, Jagsi R, Friese CR, Kurian AW, Hamilton AS, Ward KC, Morrow M, Wallner LP, et al. Recurrence risk perception and quality of life following treatment of breast cancer. Breast Cancer Res Treat. 2017;161:557–565. doi: 10.1007/s10549-016-4082-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.de Azambuja E, Cardoso F, de Castro G, Jr, Colozza M, Mano MS, Durbecq V, Sotiriou C, Larsimont D, Piccart-Gebhart MJ, Paesmans M. Ki-67 as prognostic marker in early breast cancer: a meta-analysis of published studies involving 12,155 patients. Br J Cancer. 2007;96:1504–1513. doi: 10.1038/sj.bjc.6603756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Holleczek B, Katalinic A. Toward a comprehensive cancer registration in Germany. Eur J Cancer Prev. 2017;26:S132–S138. doi: 10.1097/CEJ.0000000000000388. [DOI] [PubMed] [Google Scholar]
- 53.In H, Bilimoria KY, Stewart AK, Wroblewski KE, Posner MC, Talamonti MS, Winchester DP. Cancer recurrence: an important but missing variable in national cancer registries. Ann Surg Oncol. 2014;21:1520–1529. doi: 10.1245/s10434-014-3516-x. [DOI] [PubMed] [Google Scholar]
- 54.Pezzi CM. Big data and clinical research in oncology: the good, the bad, the challenges, and the opportunities. Ann Surg Oncol. 2014;21:1506–1507. doi: 10.1245/s10434-014-3519-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Cited studies of the risk of cancer recurrence of breast cancer patients. (PDF 27 kb)
Data Availability Statement
The access to the data used during this study is subject to state provisions.