Abstract
Background
Hyperinsulinemia aggravates insulin resistance and cardio-vascular disease. How the insulinotropic glucagon-like peptide-1 receptor agonist liraglutide in a physiologic post-prandial setting may act on pancreatic alpha and beta-cell function in patients with coronary artery disease (CAD) and type 2 diabetes (T2DM) is less clear.
Methods
Insulin resistant patients with established CAD and newly diagnosed well-controlled T2DM were recruited to a placebo-controlled, cross-over trial with two treatment periods of 12 weeks and a 2 weeks wash-out period before and in-between. Treatment was liraglutide or placebo titrated from 0.6 mg q.d. to 1.8 mg q.d. within 4 weeks and metformin titrated from 500 mg b.i.d to 1000 mg b.i.d. within 4 weeks. Before and after intervention in both 12 weeks periods insulin, C-peptide, glucose, and glucagon were measured during a meal test. Beta-cell function derived from the oral glucose tolerance setting was calculated as changes in insulin secretion per unit changes in glucose concentration (Btotal) and whole-body insulin resistance using ISIcomposite.
Results
Liraglutide increased the disposition index [Btotal × ISIcomposite, by 40% (n = 24, p < 0.001)] compared to placebo. Post-prandial insulin and glucose was reduced by metformin in combination with liraglutide and differed, but not significantly different from placebo, moreover, glucagon concentration was unaffected. Additionally, insulin clearance tended to increase during liraglutide therapy (n = 26, p = 0.06).
Conclusions
The insulinotropic drug liraglutide may without increasing the insulin concentration reduce postprandial glucose but not glucagon excursions and improve beta-cell function in newly diagnosed and well-controlled T2DM.
Trial registration Clinicaltrials.gov ID: NCT01595789
Keywords: GLP1-receptor agonist, Diabetes mellitus type 2, Beta-cell function, Insulin sensitivity, Meal test, Insulin clearance, Glucagon
Background
The hyperglycemia in type 2 diabetes mellitus (T2DM) results from an imbalance between insulin secretion and insulin sensitivity [1] with impaired insulin action and an insufficient and delayed insulin response during meals as well as an inappropriate glucagon secretion [2]. Fasting as well as postprandial glucagon secretion increase progressively through the spectrum of impaired glucose tolerance to manifest T2DM [3]. The hyperglucagonemia is associated with hepatic insulin resistance [4] and an increased hepatic glucose production [5]. Elevated levels of non-esterified fatty acids (NEFA) resulting from adipose tissue insulin resistance may play a role in the development of peripheral as well as hepatic insulin resistance and may also impair beta-cell function in T2DM and in obese prediabetic individuals [6].
Metformin, which is recommended as first line therapy in patients with T2DM [7], has beneficial effects on HbA1c, body weight, cardiovascular mortality [7, 8] and insulin sensitivity [9]. However, metformin treatment has no effect on glucagon levels [10], whereas GLP-1 receptor agonists (GLP-1RA) have been suggested to inhibit glucagon secretion from alpha-cells [11, 12]. Furthermore, therapy with GLP1-RA is associated with a potentiation of glucose induced insulin secretion and a modest weight loss [13] and as a result it effectively reduces hyperglycemia in patients with T2DM [14]. This antihyperglycemic action of the GLP1-RA liraglutide is well-established in patients with longstanding not well-controlled diabetes [15–19]. The effect of GLP-1 receptor agonist on insulin sensitivity is still discussed [20–22], and its effect on insulin clearance is sparsely examined [23]. Newly diagnosed T2DM in patients with coronary artery disease (CAD) is associated with excess mortality [24]. Accordingly, it would be of interest how liraglutide may improve postprandial glycaemia and insulinemia in a population of newly diagnosed well-controlled T2DM subjects with CAD, particularly considering that liraglutide is insulinotropic.
The aims of the present study, therefore, were to evaluate effects of the GLP-1 RA liraglutide in combination with metformin on indices of alpha- and beta-cell function, insulin sensitivity and insulin clearance. This was evaluated using a mixed meal test in obese patients with newly diagnosed, well-controlled T2DM and high cardiovascular risk, a population in which efficacy of antidiabetic medication is essential and where several recent guidelines recommend GLP-1 RA, e.g. liraglutide as drug number 2 after metformin [7, 25].
Methods
Subjects
The inclusion criteria were stable coronary artery disease (CAD), body mass index (BMI) ≥ 25 kg/m2, age ≥ 18 and ≤ 85 years, and newly diagnosed (< 2 years) T2DM according to the criteria defined by the American Diabetes Association [26]. To be included, the patients were to be treated with diet, metformin or sulfonylurea alone or in combinations. All oral antidiabetic medications were stopped 2 weeks before baseline visit (Table 1). Exclusion criteria were, amongst others, previous treatment with a GLP-1RA or dipeptidyl peptidase-4 inhibitor. A comprehensive list of the exclusion criteria can be found elsewhere [27].
Table 1.
Baseline characteristics of the study population, in median (IQR)
| Variable | Units | Median | 25th Pctl | 75th Pctl | n |
|---|---|---|---|---|---|
| BMI | kg/m2 | 30.2 | 27.9 | 34.1 | 39 |
| Age | years | 64.0 | 58.0 | 68.0 | 39 |
| Weight | kg | 93.0 | 85.0 | 108.0 | 39 |
| HbA1ca | mmol/mol | 47 (6) | 39 | ||
| Fasting glucose | mmol/l | 5.3 | 5.0 | 6.2 | 39 |
| Fasting glucagona | pmol/l | 5.3 (3.5) | |||
| Fasting C-peptide | pmol/l | 1474 | 1006 | 1978 | 39 |
| Fasting insulin | pmol/l | 110 | 67 | 178 | 38 |
| Fasting NEFA | mmol/l | 0.392 | 0.298 | 0.455 | 39 |
| AUCglucose | mmol/l × 120min | 821.3 | 750.5 | 915.5 | 39 |
| AUCinsulin | pmol/l × 120min | 33,173 | 25,350 | 45,625 | 38 |
| AUCISR | pmol/kg | 969.5 | 811.1 | 1263.6 | 38 |
| AUCNEFA | mmol/l × 120min | 28.9 | 22.5 | 36.1 | 39 |
| HOMA-IR | 4.43 | 2.24 | 6.93 | 38 | |
| ISIComposite | l2/mg/microU | 3.2 | 2.03 | 4.62 | 38 |
| B-total | 2.54 | 1.61 | 3.2 | 38 | |
| MCRikg | l/kg/min | 0.029 | 0.023 | 0.036 | 37 |
| MCRitotal | l/min | 2.548 | 2.219 | 3.164 | 37 |
| DI | 8.06 | 5.71 | 12.91 | 37 | |
| IDRbasal | pmol/kg/min | 3.09 | 1.94 | 4.02 | 37 |
| HEXi | 0.30 | 0.22 | 0.36 | 37 | |
| Pre-study ADTb | n (%) | ||||
| Diet and lifestyle intervention | 24 (62) | ||||
| Metformin | 15 (38) | ||||
| Sulfonylurea | 1 (3) |
aMean (SD)
bAnti diabetic treatment
Design
The study was an investigator-initiated, double-blind, randomized, placebo-controlled, cross-over trial. Details of the design, participants and intervention have been described previously [27].
Patients meeting the inclusion criteria were included consecutively and study drugs in subject boxes were sequentially numbered with a unique code and randomized by computer in a 1:1 randomization ratio by Novo Nordisk A/S and allocation sequence was concealed until all participants had completed the study [27]. Enrollment and assignment of participants were done at Department of Cardiology, Copenhagen University Hospital, Bispebjerg, Denmark. Patients were recruited from May 2012 to October 2014.
Intervention
Liraglutide and metformin versus placebo and metformin in 12 plus 12 weeks with a 2-week wash-out period. The study period for each patient was 26 weeks and consisted of 4 major visits (at weeks 0, 12, 14 and 26); the wash-out was between weeks 12 and 14. Liraglutide dose was 1.8 mg once daily subcutaneously (titrated from 0.6 mg to 1.8 mg once daily within 4 weeks) and metformin 1 g twice daily orally (titrated from 500 mg twice daily to 1 g twice daily in 4 weeks) [27]. The last injection of liraglutide was in the morning on test days. Data collection was carried out in Department of Cardiology, Copenhagen University Hospital, Bispebjerg, Denmark.
Endpoints
Beta-cell function (as measured by disposition index), insulin sensitivity, insulin clearance and responses of glucose, insulin, C-peptide, glucagon and NEFA during a 2-h mixed meal tolerance test, respectively. Treatment effects were evaluated by comparing results from the visits at initiation and end of each treatment period.
Mixed meal test
A 375-g solid meal consisting of 60-g (46 E%) carbohydrates, 38-g (28 E%) protein and 16-g (26 E%) fat, equivalent to 550 kcal was consumed after an overnight fasting period of 10 h. Baseline blood samples were obtained at 10, 5 and 1 min before and 30, 60, 90 and 120 min after initiation of the test meal, which was to be finished within 15 min.
Assay
Glucose measurements were carried out using an Accu-Chek Inform II meter (Roche, Swiss). Coefficient of variance (CV) was ≤ 3.3% for glucose levels > 4.2 mmol/l. Plasma insulin and C-peptide concentrations (pmol/l) were determined by the enzyme-linked immunosorbent assay (ELISA) (Siemens Healthcare Diagnostics, LA, California, USA), for insulin with an intra-assay CV of 3.3–5.5% and an inter-assay CV of 4.1–7.3% and for C-peptide with an intra-assay CV of 1.7–2.3% and an inter-assay CV of 2.9–4.8%. Plasma NEFA (mmol/l) was measured by an enzymatic test (Wako Chemicals, Neuss, Germany) with a median intra-assay CV of 1.5% and a median inter-assay CV of 7.5%. Glucagon concentrations in plasma were measured after extraction of plasma with 70% ethanol (vol./vol., final concentration). The antibody employed (code no. 4305) is directed against the C-terminus of the glucagon molecule and therefore mainly measures glucagon of pancreatic origin [28]. Standards were human glucagon and tracer was monoiodinated human glucagon (both gifts from Novo Nordisk, Bagsværd, Denmark). Sensitivity and detection limit is below 1 pmol/l, intra-assay CV below 6% at 20–30 pmol/l, and recovery of standard, added to plasma before extraction, about 100% when corrected for losses inherent in the plasma extraction procedure [29].
Calculations
The composite measure of whole body insulin sensitivity (ISIComposite) [30] and the homeostasis model assessment of insulin resistance (HOMA-IR) [31] were determined as follows:
in which FPI is fasting plasma insulin (μU/ml), is mean plasma insulin, FPG is fasting plasma glucose (mg/dl) and is mean plasma glucose during meal test.
Prehepatic insulin secretion rates (ISR) (pmol/kg/min) were calculated from plasma C-peptide concentrations using the ISEC (Insulin SECretion) computer program [32]. This method is based upon the assumptions that C-peptide is not cleared by the liver and is co-secreted with insulin in equimolar amounts from the pancreas. The beta-cell response to changes in glucose during a meal test expresses the efficacy by which changes in plasma glucose concentrations stimulate insulin secretion. This relationship between changes in plasma glucose concentrations and ISR during the meal test was evaluated by cross-correlation analysis, and the slope of the regression lines (BTotal) is a measure of the change in insulin secretion per unit change in glucose concentration, i.e. beta cell sensitivity to glucose or beta-cell responsiveness. Beta-cell function was defined as the product of beta-cell responsiveness and insulin sensitivity, i.e. the disposition index (Di) [33], i.e. assuming a hyperbolic association between beta-cell responsiveness and insulin sensitivity
We tested for the hyperbolic law in the basal state and found a R2 = 0.81 suggesting that the disposition index may be valid to use in the present study.
The trapezoidal rule [34] was used to calculate the area under the curve (AUC) for ISR, insulin, glucose and NEFA concentrations. The integer of ISR (AUC − ISR0–120 min) represents the total amount of insulin secreted throughout the meal test while the integer of insulin concentration profiles during the meal test (AUC − insulin0–120 min) represents both insulin secretion and insulin clearance. Thus, the ratio of these integers adjusted for total body mass reflects insulin clearance (MCRi):
Plasma insulin levels are at steady-state in the basal period, therefore the posthepatic insulin delivery rate (IDRBasal) can be calculated as [35]:
The ratio of the IDRBasal to the ISRBasal is the fraction of insulin not extracted by the liver, therefore the hepatic extraction of insulin (HEXi) is calculated as [35]:
To evaluate the efficacy of insulin to suppress NEFA production [36] we divided ∆AUC − NEFA0–120 min by ∆AUC − insulin0–120 min, (denoted NEFAins).
Statistical analysis
Data are reported as median (IQR) or mean (SD). Students’ paired t-test was used for normally distributed data when comparing groups. In non-normally distributed data, Wilcoxon’s Signed Rank test was used. A two-sided value of p < 0.05 was considered statistically significant. Comprehensive details on the power calculation are published elsewhere [27]. Statistical analyses were performed with SAS 9.4 (SAS Institute Inc., Cary, North Carolina, USA). The study was approved by the Regional Committee on Biomedical Research Ethics of the Capital Region of Denmark and was carried out in accordance with the International Conference on Harmonization—Good Clinical Practice standards and informed consent was obtained from all participants. The study protocol was registered at Clinicaltrials.gov with ID: NCT01595789.
Results
Participants
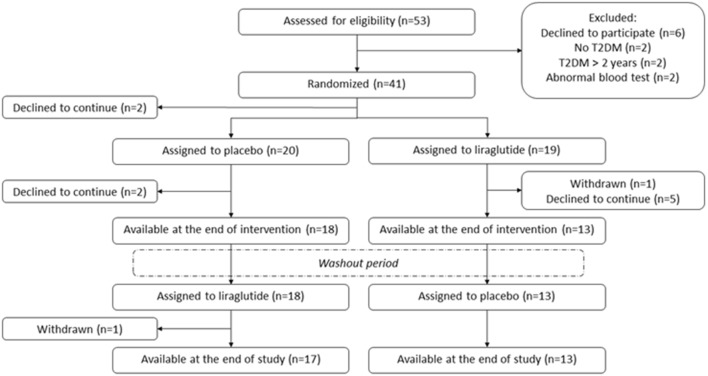
Of the 41 patients randomized, two patients declined to participate before first visit and nine patients discontinued the study (Fig. 1). Twenty-eight patients completed all study visits. Two patients treated with placebo in the first period could not attend visit 3 therefore data from visit 2 were carried forward and used as baseline for the second period. Thus, thirty participants were included in the paired analyses. Eliminating data from the two patients did not influence the main results. Baseline characteristics are found in Table 1. Calculations of indices were limited by missing values in a few patients, thereby leading to patients < 30 (Tables 2, 3). At baseline mean HbA1c was 47 [6] mmol/mol.
Fig. 1.
Screening, randomization and follow-up [39]
Table 2.
Indices of beta-cell function, insulin sensitivity and clearance
| Variable (unit) | Baseline | n | Placebo | n | p | Liraglutide | n | p | Difference | n | p |
|---|---|---|---|---|---|---|---|---|---|---|---|
| BTotal | 2.72 (2.00; 3.24) | 29 | − 0.14 (− 0.59; 0.37) | 28 | 0.16 | 0.2 (− 0.54; 0.79) | 25 | 0.42 | 0.25 (− 0.35; 0.93) | 24 | 0.24 |
| DI | 8.23 (5.87; 12.93) | 28 | 1.77 (− 1.83; 4.91) | 27 | 0.06 | 6.0 (2.78; 9.23) | 25 | < .0001 | 3.35 (− 0.51; 9.29) | 24 | 0.0005 |
| IDRbasal (pmol/kg/min) | 3.2 (2.18; 4.37) | 29 | − 0.28 (− 0.92; 0.25) | 28 | 0.1 | − 0.16 (− 1.13; 0.27) | 26 | 0.22 | 0.13 (− 1.01; 0.84) | 25 | 0.84 |
| ISIComposite (L2/mg/microU) | 3.15 (2.03; 4.5) | 30 | 0.73 (− 0.05; 2.31) | 29 | 0.0005 | 1.68 (0.57; 3.34) | 27 | < .0001 | 0.26 (− 0.81; 2.26) | 27 | 0.14 |
| HOMA-IR | 4.43 (2.75; 6.93) | 30 | − 0.74 (− 2.06; − 0.12) | 28 | 0.007 | − 1.32 (− 2.88; − 0.25) | 26 | 0.0003 | − 0.39 (− 1.98; 0.79) | 26 | 0.43 |
| MCRikg (ml/kg/min) | 29.35 (23.47; 35.07) | 29 | 1.65 (− 0.99; 4.38) | 28 | 0.08 | 3.56 (0.29; 7.02) | 27 | 0.005 | 2.92 (− 2.47; 6.55) | 26 | 0.06 |
| MCRitotal (L/min) | 2.54 (2.17; 3.33) | 29 | 0.11 (− 0.10; 0.36) | 28 | 0.15 | 0.27 (0.02; 0.37) | 26 | 0.02 | 0.25 (− 0.24; 0.49) | 25 | 0.13 |
| HEXi | 0.293 (0.208; 0.359) | 29 | 0.024 (− 0.089; 0.083) | 28 | 0.69 | 0.017 (− 0.073; 0.106) | 26 | 0.72 | 0.058 (− 0.137; 0.146) | 25 | 0.97 |
Units are mmol/l × 120min except for insulin secretion rate (ISR) measured i pmol/kg. Data reported as median (IQR)
Table 3.
Area under the curve (AUC) in median (IQR), units are mmol/l × 120min except ISR measured in pmol/kg
| Variable | Baseline | n | Placebo | n | p | Liraglutide | n | p | Difference | n | p |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Glucose | 804.3 (735.5; 911.3) | 31 | − 75.9 (− 143.5; − 29.0) | 29 | 0.0003 | − 105.6 (− 213.0; − 40.8) | 28 | < .0001 | − 44.8 (− 122.3; 33.8) | 27 | 0.09 |
| Insulin | 36,103 (25,435; 48,380) | 30 | − 8530 (− 14,630; 1915) | 29 | 0.006 | − 11,270 (− 19,382; − 1145) | 27 | 0.002 | − 2195 (− 7745; 4250) | 27 | 0.45 |
| ISR | 999 (840; 1281) | 30 | − 205 (− 360; − 32) | 29 | 0.001 | − 102 (− 260; 105) | 26 | 0.08 | 69 (− 25; 247) | 25 | 0.11 |
| NEFA | 29.1 (22.6; 37.9) | 31 | − 2.8 (− 7.5; 2.9) | 30 | 0.06 | 2.7 (− 5.5; 13.4) | 27 | 0.26 | 6.5 (− 5.7; 16.9) | 27 | 0.13 |
Alpha-cell function
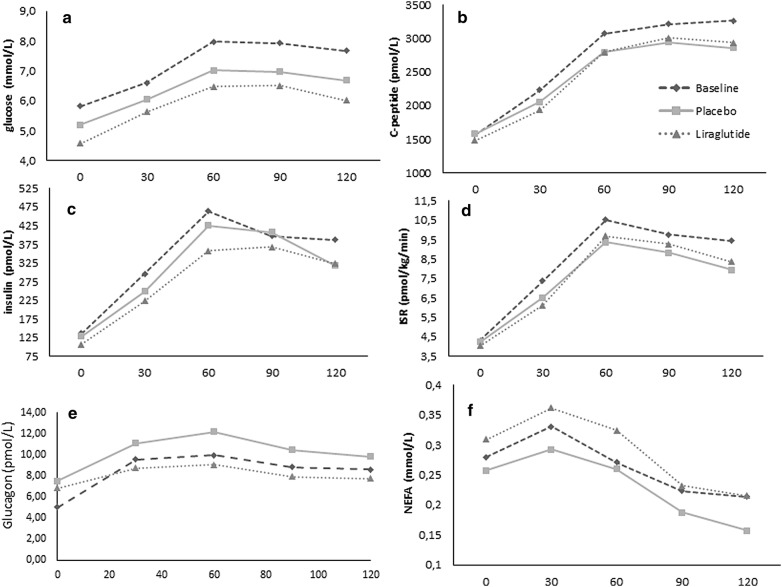
Baseline fasting p-glucagon was 5.3 (3.5) pmol/l. Both placebo and liraglutide treatment increased fasting glucagon levels to 7.5 (3.8) pmol/l (p < 0.001) and 6.8 (3.1) pmol/l (p < 0.002), respectively, but with no significant difference between periods (p < 0.7). AUCglucagon was not significantly changed by placebo treatment [1138 (663) to 1283 (637) pmol/l × 120min (p = 0.07)], and neither did liraglutide treatment affect AUCglucagon [992 (516) to 995 (417) pmol/l × 120min (p = 0.64)], and no difference between treatments was observed (p < 0.4). AUCinsulin/glucagon was reduced by placebo (p = 0.01) but not by liraglutide treatment (p < 0.06) and with no difference between treatment periods (p = 0.09) (Fig. 2).
Fig. 2.
Plasma levels of glucose (a), C-peptide (b), insulin (c), insulin secretion rates (d), glucagon (e) and NEFA (f) during meal test
Insulin sensitivity
ISI-composite (ISIcomp) was increased in both treatment periods: placebo; 0.73 (− 0.05 to 2.31) l2/mg/microU (p < 0.0005) and liraglutide; 1.68 (0.57 to 3.34) l2/mg/microU (p < 0.0001), with no significant differences between treatments (p = 0.14). HOMA-IR was reduced significantly in both placebo and the liraglutide period but with no differences between treatments (Table 2).
Beta-cell function and plasma glucose
AUCglucose was reduced in both placebo and liraglutide periods by − 76 (− 144 to − 29) mmol/l × 120min and − 106 (− 213 to − 41) mmol/l × 120min, p = 0.0003 vs. p < 0.0001, respectively, with no difference between treatments (Table 3 and Fig. 2). Insulin and C-peptide responses are depicted in Fig. 2. Analysis of AUC’s showed significant reductions of AUCinsulin in both placebo and liraglutide periods with no significant difference between treatments (p < 0.46) (Table 3). Additionally, the AUC insulin/glucose ratio was not increased by liraglutide treatment: − 50 (312) pmol/mmol (p = 0.4).
Basal insulin delivery rates (IDRbasal) were not affected by either treatment (Table 2). AUCISR was significantly reduced only in the placebo period, with no additional change observed with liraglutide treatment (Table 3). Paired analysis (n = 25) did not reveal significant differences in ISR between placebo and liraglutide treatment (Table 3). Beta-cell responsiveness (Btotal) did not differ between treatments (Table 2). Disposition index (DI) was improved in the liraglutide period; 6.0 (2.78 to 9.23), p < 0.0001, whereas placebo treatment did not affect DI significantly. Paired analysis revealed an effect of liraglutide therapy of 3.35 (− 0.51 to 9.29), p = 0.0005, improving baseline value by 40% (Table 2) towards a less diabetic state (Fig. 3). Of importance we observed a strong baseline hyperbolic association between ISIcomp and Btotal of R2 = 0.81, which comply with the data that was obtained in the original method study on this relationship using intravenous glucose defining the beta-cell function [37].
Fig. 3.
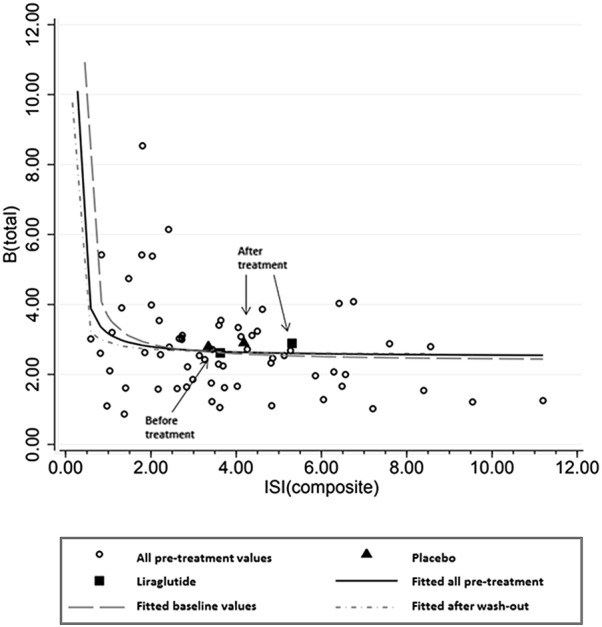
The hyperbolic relationship between Btotal and ISIComposite—Open circles represents all pretreatment values, i.e. baseline values combined with values after wash-out before beginning of period 2. The hyperbolic function is evident for both groups and for all pretreatment values. Fitted (all); R2 = 0.81, fitted (baseline):R2 = 0.8, fitted (washout); R2 = 0.83. Treatment effect is indicated by square and triangle (liraglutide vs. placebo), beginning and end of treatment are depicted by arrows
Insulin clearance
Baseline fasting insulin was inversely correlated to MCRikg (R2 = 0.58, p < 0.0001), MCRitotal (R2 = 0.33, p = 0.001) and HEXi (R2 = 0.31, p < 0.002). In the placebo period MCRikg was numerically but non-significantly increased but the combination of liraglutide and metformin increased MCRikg significantly by 3.56 (0.29 to 7.02) × 10−3 L/kg/min (p = 0.005) with a borderline difference between groups (p = 0.06) (Table 2). Improvement in ISIcomp was associated with reduction in MCRikg (R2 = 0.21, p = 0.02) and MCRitotal (R2 = 0.24, p = 0.01). There were no associations with HOMA-IR. Baseline HEXi was reduced as compared to normoglycemic subjects who usually clear app. 50% or more during the first pass of the liver [38]. These patients exhibit a HEXi of only 29% (21 to 36%) and liraglutide therapy had no effect on HEXi.
A subgroup analysis of the 24 patients with complete data set with respect to: Btotal, ISIcomp, DI, HOMA-IR, MCRikg/total and HEXi confirmed the results outlined previously. However, regarding MCRikg the effects of liraglutide and placebo were comparable to the analysis based on all patients, nevertheless, the placebo corrected difference reached statistical significance (p = 0.03).
NEFA
Baseline fasting NEFA did not correlate to weight, BMI or HOMA-IR. AUCNEFA did not change significantly in the placebo period in contrast to the liraglutide period in which AUCNEFA increased significantly, but the difference between the groups was not statistical different (p = 0.13) (Table 3). The amount of insulin required to suppress NEFA production (NEFAins) was not changed significantly in placebo period but was reduced in liraglutide period; − 470 (− 1790 to 10) nmol/pmol, p < 0.05 (Fig. 2).
Hba1c and body weight
HbA1c at 47 [6] mmol/mol was reduced by − 3.3 (6.51) mmol/mol, p < 0.01 and body weight at 93 (85 to 108) kg was reduced by − 2.7 (− 6.7 to − 0.6) kg, p = 0.0004, both corrected for placebo treatment.
Explanatory variables and carry-over effect
We found no correlations between weight loss and ISIcomp; placebo (R2 = 0.01; p > 0.7) and liraglutide (R2 = 0.06; p > 0.2) or weight loss and DI; placebo (R2 < 0.01; p > 0.7) and liraglutide (R2 = 0.08; p > 0.2). The variance of weight loss was not associated with baseline weight, BMI, age, sequence of treatment, and differences in treatment duration [39]. The presence of a possible carry-over effect was estimated using sum values by the t-test [40], but we did not find any significant carry-over effect between periods with respect to weight loss (p = 0.4) [39], DI (p = 0.4), Btotal (p = 0.1) or ISIcomp (p = 0.1).
Post-hoc power calculation
Power calculation was based on improvement of disposition index during i.v. glucose test as reported earlier [39]. In the present paper we report secondary outcomes, and post-hos power analysis were done. Given a power of 80% and a level of significance of p < 0.05 we would be able to detect an increase in Disposition index on > 40% with n = 34 patients in paired analysis. BTotal an increase on 20% with 28 patients, however, regarding ISIComposite we would need n = 134 patient for detecting the same change in paired analysis.
Compliance and safety
Use of the study drugs (metformin, liraglutide and placebo) was counted, and compliance to liraglutide/placebo and metformin was > 90% of prescribed dosages with no significant differences between treatment periods. Adverse event frequency was higher in the active treatment periods predominantly due to gastrointestinal side effects. Serious adverse events were observed in a total of 9 cases; 3 in the active period, 4 in the placebo period and 2 in the wash-out period. A detailed description of adverse events is published elsewhere [41].
Discussion
This meal test study of newly diagnosed well-controlled patients with T2DM and established CAD showed that liraglutide combined with metformin versus metformin (+placebo) significantly improved beta-cell function with a trend towards improved insulin sensitivity. Of note, the combination of the insulinotropic liraglutide and metformin reduced post-prandial insulin levels in face of a reduced glucose excursion, which is a new finding in this setting.
In this population we have already shown by use of an non-physiologic intravenous glucose tolerance test a significant improvement in hyperglycemia and a significant increase in insulin levels [39], which was expected as liraglutide is an insulin secretagogue [12].
However, in the present study we report data from a mixed meal test where we observed a reduction of insulin as well as glucose levels during this physiological test. The effect of liraglutide on beta-cell responsiveness (Btotal) was neutral. These finding might in part be explained by the very well-controlled hyperglycemia with a mean HbA1c of 47 mmol/mol and a baseline fasting plasma glucose on 5.3 mmol/l. Despite these findings the improvement in disposition index is substantial in the present study, just as seen in the non-physiological setting using intravenous glucose as beta-cell stimulation in combination with the so called “minimal model” in the same patients [39]. The novelty of the present physiologic study is that despite reduction of insulin levels, liraglutide (combined to metformin) retains its strong effect on beta-cell function (i.e. disposition index) in patients with well-controlled T2D and established CAD.
Furthermore, the combination therapy improved insulin clearance and insulin sensitivity, both hepatic and peripheral. In our setup we evaluated basal level of hepatic insulin extraction but did not find effect of liraglutide on this, however basal level was reduced compared to which was previously reported on glucose intolerant subjects [42]. Reduced hepatic extraction is the primary cause of high levels of circulating insulin after a glucose load [42] and in normoglycemic subjects hepatic extraction is suppressed up to 30% for ≤ 150 min following a glucose load [38] and 40–50% of secreted insulin is extracted during a standard meal [38]. A body weight loss of > 10 kg increased hepatic extraction of insulin [43] and the weight loss in our study was only < 3 kg.
A recent Japanese study used a mixed meal test and compared metformin and liraglutide as monotherapy in patients with T2DM, and was able to demonstrate improvements in beta-cell function (measured as disposition index) by liraglutide compared to no effect following metformin therapy [44]. An earlier acute study of 11 subjects with type 2 diabetes and with a HbA1c of 6.5 ± 0.6% a single dose of liraglutide revealed an increase in fasting ISR but post meal AUCISR was unaltered in contrast to AUCglucose which was markedly reduced, indicating an improved beta-cell function [45].
The effect on the alpha-cell function was unexpected; fasting glucagon levels increased during liraglutide and placebo treatments but neither treatment led to lower glucagon responses during meal test.
Data from the liraglutide effect and action in diabetes (LEAD) studies are conflicting with respect to the effect on fasting glucagon levels; in LEAD-3 a decrease of fasting glucagon was found but no effect was observed in LEAD-4 [15, 17]. The absent suppression of glucagon in the present study may indicate a somewhat different action of liraglutide on alpha-cell function compared to native GLP-1, which may relate to the duration of treatment, since a short term period of GLP-1-infusion inhibits glucagon secretion [46], whereas a long term infusion does not retain the same glucagon suppressive effect [20]. However, a previous study on patients with early stage T2DM revealed somewhat ambiguous results [47, 48], and it is suggested that attention must be paid to the performance of the assay used to measure glucagon, in order to obtain valid results [29]. Additionally, the observed differences in glucagon response can to some extend be caused by differences in the composition of the test meals [49] and whether a the oral challenge is a mixed meal or glucose [50]. It is indicated that postprandial glucagon levels are increased after at mixed meal as compared to glucose alone [50]. We therefore speculate that the present setting may implicate a somewhat different glucagon response compared to an oral glucose challenge or, alternatively that subjects with more advanced disease might reveal a different picture.
In T2DM the suppression of NEFA by insulin is diminished and increased NEFA levels impair insulin action and insulin secretion [1]. In the present study liraglutide did not change suppression of NEFA compared with metformin but less insulin was needed to suppress postprandial NEFA in the liraglutide arm.
The present results suggest that in well-controlled subjects with type 2 diabetes the positive effect of liraglutide treatment on beta-cell function is clinically relevant but the effect on alpha cell function is subtle. Additionally, the combination of liraglutide with metformin improves insulin sensitivity and clearance and no effect on post-meal hepatic extraction of insulin. However, it is emphasized that liraglutide may produce another metabolic response in patients with less well-controlled and more advanced diabetes patients, which limits the generalization of this study.
A limitation of the study was the relatively few samples and a short duration of the meal test, since longer protocols of longer duration including a higher number of samples will reveal a more detailed picture of early as well as late insulin secretion and alpha-cell function as well as NEFA metabolism in response to a meal test [51]. The disposition index calculated from the mixed meal test remains to be validated. The assumption is that the beta-cell adaption to ambient insulin resistance follows a hyperbolic law (y = 1/x), and that the product AIR glucose × Si (the disposition index), therefore, is constant in people with normal beta-cell function. The disposition index in subjects with type 2 diabetes is used to obtain a correct estimate of the beta-cell function it relation to the prevailing degree of insulin resistance of the individual. In the present study we tested for the hyperbolic law in the basal state and found a R2 = 0.81 suggesting that the disposition index may be valid. However, post hoc power analysis indicates that changes in some of the indices reported, especially regarding ISIComposite could flawed due to the study being underpowered. Nevertheless, this explorative study could indicate a more metabolic flexibility of liraglutide treatment than previously acknowledged. The data presented her warrants further experiments in a greater population.
The data presented here were obtained during a relatively short-term treatment period and demonstrated the effects of liraglutide in newly diagnosed patients. Data from real-world clinical practice indicates that the treatments effects we observed are similar to what was observed in clinical trials [52]. The present study aimed to reveal treatment effects on pathophysiological features of early T2DM and CAD, yet we speculate that effects in more advanced disease might reveal a different picture, which might add to our knowledge behind the long term positive cardiovascular profile of liraglutide [53]. In accordance with clinical trails the side-effect profile of liraglutide was relatively low and comprised mainly of gastrointestinal events, which often resolves within 4 weeks of therapy [41, 54]. The present study did not indicate pathophysiological pathways, which could indicate possible adverse long-term effects. Adverse out-come in our trial does not differ substantially from clinical trials [41, 54]. Additionally, recent data indicates that liraglutide treatment is not associated with an increased risk for pancreatitis or pancreatic cancer [55].
Conclusions
In patients with well-controlled T2DM and CAD liraglutide treatment significantly improved disposition index as a measure of beta-cell function but has no effect on alpha-cell function. Combined with metformin, liraglutide reduced ambient insulin levels and improved insulin sensitivity and insulin clearance in a physiologic meal test setting suggesting that liraglutide is as a flexible insulinotropic drug in these patients.
Acknowledgements
None.
Abbreviations
- T2DM
type 2 diabetes mellitus
- NEFA
non-esterified fatty acids
- GLP1-RA
glucagon-like peptide-1 receptor agonist
- CAD
coronary artery disease
- BMI
body mass index
- CV
coefficient of variance
- HOMA-IR
homeostasis model assessment of insulin resistance
- ISR
insulin secretion rates
- ISEC
Insulin SECretion (computer program)
- DI
disposition index
- AUC
area under the curve
- MCRi
insulin clearance
- IDR
insulin delivery rate
- HEXi
hepatic extraction of insulin
- IQR
interquartile range
- SD
standard deviation
- LEAD
liraglutide effect and action in diabetes
Authors’ contributions
CA: acquired data, performed the statistical analyses, interpreted data, and drafted and revised the manuscript for important intellectual content and approved the final version. PK, AJ, LRP: acquired data, interpreted data, and revised the manuscript for important intellectual content and approved the final version. OWN, OPK, SM: interpreted data and revised the manuscript for important intellectual content and approved the final version. MF, JJH: performed biochemical analysis, interpreted data, and revised the manuscript for important intellectual content and approved the final version. SBH, AS: conceived and designed the study, interpreted data, and revised the manuscript for important intellectual content and approved the final version. All authors read and approved the final manuscript.
Funding
The study was funded by Novo Nordisk with an unrestricted grant for investigator-initiated studies. Additional funding was provided by: The Danish Heart Foundation, The AP Møller Foundation, The Department of Internal Medicine, Copenhagen University Hospital, Amager, The Clinical Research Centre, Copenhagen University Hospital, Hvidovre and The Bispebjerg Hospital Research Foundation.
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Ethics approval and consent to participate
The study has been approved by the Regional Committee on Biomedical Research Ethics of the Capital Region of Denmark informed consent was obtained from all participants.
Consent for publication
Not applicable.
Competing interests
CA: Advisory board and lecture fees: Novo Nordisk. PK: none. AJ: none. LRP: share owner: Novo Nordisk. OWN: funding of educational and research tasks from ResMed and participated on advisory boards for Novartis. OPK: none. MF: none. JJH: none. SM: Advisory boards: AstraZeneca; Boehringer Ingelheim; Bristol-Meyers Squibb; Eli Lilly; Intarcia Therapeutics; Johnson & Johnson; Merck Sharp & Dohme; Novartis; Novo Nordisk; Sanofi Aventis. Lecture fees: AstraZeneca; Boehringer Ingelheim; Bristol-Meyers Squibb; Eli Lilly; Merck Sharp & Dohme; Novartis; Novo Nordisk; Sanofi Aventis. Research Grant Recipient: Novo Nordisk. AS: none. SBH: has received funding of educational and research tasks from Novo Nordisk, Abbott, Eli Lilly, Pfizer, Boehringer Ingelheim, Bristol-Meyers Squibb, and Merck Sharp & Dohme.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Christian Anholm, Phone: +45 20847285, Email: canholm@youmail.dk.
Preman Kumarathurai, Email: preman@kumarathurai.com.
Anders Jürs, Email: andersjurs@gmail.com.
Lene Rørholm Pedersen, Email: lrpd@regionsjaelland.dk.
Olav Wendelboe Nielsen, Email: own@dadlnet.dk.
Ole Peter Kristiansen, Email: ole.peter.kristiansen@regionh.dk.
Mogens Fenger, Email: mogens.fenger@regionh.dk.
Jens Juul Holst, Email: jjholst@sund.ku.dk.
Sten Madsbad, Email: sten.madsbad@regionh.dk.
Ahmad Sajadieh, Email: asajadieh@yahoo.com.
Steen Bendix Haugaard, Email: sbhau@dadlnet.dk.
References
- 1.Defronzo RA. Banting Lecture. From the triumvirate to the ominous octet: a new paradigm for the treatment of type 2 diabetes mellitus. Diabetes. 2009;58(4):773–795. doi: 10.2337/db09-9028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pratley RE, Weyer C. Progression from IGT to type 2 diabetes mellitus: the central role of impaired early insulin secretion. Curr Diab Rep. 2002;2(3):242–248. doi: 10.1007/s11892-002-0090-6. [DOI] [PubMed] [Google Scholar]
- 3.Knop FK, Aaboe K, Vilsbøll T, Vølund A, Holst JJ, Krarup T, et al. Impaired incretin effect and fasting hyperglucagonaemia characterizing type 2 diabetic subjects are early signs of dysmetabolism in obesity. Diabetes Obes Metab. 2012;14(6):500–510. doi: 10.1111/j.1463-1326.2011.01549.x. [DOI] [PubMed] [Google Scholar]
- 4.Baron AD, Schaeffer L, Shragg P, Kolterman OG. Role of hyperglucagonemia in maintenance of increased rates of hepatic glucose output in type II diabetics. Diabetes. 1987;36(3):274–283. doi: 10.2337/diab.36.3.274. [DOI] [PubMed] [Google Scholar]
- 5.Matsuda M, Defronzo RA, Glass L, Consoli A, Giordano M, Bressler P, et al. Glucagon dose-response curve for hepatic glucose production and glucose disposal in type 2 diabetic patients and normal individuals. Metabolism. 2002;51(9):1111–1119. doi: 10.1053/meta.2002.34700. [DOI] [PubMed] [Google Scholar]
- 6.Boden G. Interaction between free fatty acids and glucose metabolism. Curr Opin Clin Nutr Metab Care. 2002;5(5):545–549. doi: 10.1097/00075197-200209000-00014. [DOI] [PubMed] [Google Scholar]
- 7.Inzucchi SE, Bergenstal RM, Buse JB, Diamant M, Ferrannini E, Nauck M, et al. Management of hyperglycemia in type 2 diabetes: a patient-centered approach: position statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD) Diabetes Care. 2012;35(6):1364–1379. doi: 10.2337/dc12-0413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Maruthur NM, Tseng E, Hutfless S, Wilson LM, Suarez-Cuervo C, Berger Z, et al. Diabetes medications as monotherapy or metformin-based combination therapy for type 2 diabetes: a systematic review and meta-analysis. Ann Intern Med. 2016;164:740–751. doi: 10.7326/M15-2650. [DOI] [PubMed] [Google Scholar]
- 9.Vitale C, Mercuro G, Cornoldi A, Fini M, Volterrani M, Rosano GMC. Metformin improves endothelial function in patients with metabolic syndrome. J Intern Med. 2005;258(3):250–256. doi: 10.1111/j.1365-2796.2005.01531.x. [DOI] [PubMed] [Google Scholar]
- 10.Sun W, Zeng C, Liao L, Chen J, Wang Y. Comparison of acarbose and metformin therapy in newly diagnosed type 2 diabetic patients with overweight and/or obese. Curr Med Res Opin. 2016;32:1389–1396. doi: 10.1080/03007995.2016.1176013. [DOI] [PubMed] [Google Scholar]
- 11.Holst JJ. Glucagonlike peptide 1: a newly discovered gastrointestinal hormone. Gastroenterology. 1994;107(6):1848–1855. doi: 10.1016/0016-5085(94)90831-1. [DOI] [PubMed] [Google Scholar]
- 12.Meier JJ. GLP-1 receptor agonists for individualized treatment of type 2 diabetes mellitus. Nat Rev Endocrinol. 2012;8(12):728–742. doi: 10.1038/nrendo.2012.140. [DOI] [PubMed] [Google Scholar]
- 13.Vilsbøll T, Christensen M, Junker AE, Knop FK, Gluud LL. Effects of glucagon-like peptide-1 receptor agonists on weight loss: systematic review and meta-analyses of randomised controlled trials. BMJ. 2012;344:d7771. doi: 10.1136/bmj.d7771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Madsbad S, Schmitz O, Ranstam J, Jakobsen G, Matthews DR, NN2211-1310 International Study Group Improved glycemic control with no weight increase in patients with type 2 diabetes after once-daily treatment with the long-acting glucagon-like peptide 1 analog liraglutide (NN2211): a 12-week, double-blind, randomized, controlled trial. Diabetes Care. 2004;27(6):1335–1342. doi: 10.2337/diacare.27.6.1335. [DOI] [PubMed] [Google Scholar]
- 15.Zinman B, Gerich J, Buse JB, Lewin A, Schwartz S, Raskin P, et al. Efficacy and safety of the human glucagon-like peptide-1 analog liraglutide in combination with metformin and thiazolidinedione in patients with type 2 diabetes (LEAD-4 Met + TZD) Diabetes Care. 2009;32(7):1224–1230. doi: 10.2337/dc08-2124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Marre M, Shaw J, Brändle M, Bebakar WMW, Kamaruddin NA, Strand J, et al. Liraglutide, a once-daily human GLP-1 analogue, added to a sulphonylurea over 26 weeks produces greater improvements in glycaemic and weight control compared with adding rosiglitazone or placebo in subjects with Type 2 diabetes (LEAD-1 SU) Diabet Med. 2009;26(3):268–278. doi: 10.1111/j.1464-5491.2009.02666.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Garber A, Henry R, Ratner R, Garcia-Hernandez PA, Rodriguez-Pattzi H, Olvera-Alvarez I, et al. Liraglutide versus glimepiride monotherapy for type 2 diabetes (LEAD-3 Mono): a randomised, 52-week, phase III, double-blind, parallel-treatment trial. Lancet. 2009;373(9662):473–481. doi: 10.1016/S0140-6736(08)61246-5. [DOI] [PubMed] [Google Scholar]
- 18.Buse JB, Rosenstock J, Sesti G, Schmidt WE, Montanya E, Brett JH, et al. Liraglutide once a day versus exenatide twice a day for type 2 diabetes: a 26-week randomised, parallel-group, multinational, open-label trial (LEAD-6) Lancet. 2009;374(9683):39–47. doi: 10.1016/S0140-6736(09)60659-0. [DOI] [PubMed] [Google Scholar]
- 19.Nauck M, Frid A, Hermansen K, Shah NS, Tankova T, Mitha IH, et al. Efficacy and safety comparison of liraglutide, glimepiride, and placebo, all in combination with metformin, in type 2 diabetes: the LEAD (liraglutide effect and action in diabetes)-2 study. Diabetes Care. 2009;32(1):84–90. doi: 10.2337/dc08-1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zander M, Madsbad S, Madsen JL, Holst JJ. Effect of 6-week course of glucagon-like peptide 1 on glycaemic control, insulin sensitivity, and beta-cell function in type 2 diabetes: a parallel-group study. Lancet. 2002;359(9309):824–830. doi: 10.1016/S0140-6736(02)07952-7. [DOI] [PubMed] [Google Scholar]
- 21.Vilsbøll T, Brock B, Perrild H, Levin K, Lervang H-H, Kølendorf K, et al. Liraglutide, a once-daily human GLP-1 analogue, improves pancreatic B-cell function and arginine-stimulated insulin secretion during hyperglycaemia in patients with Type 2 diabetes mellitus. Diabet Med. 2008;25(2):152–156. doi: 10.1111/j.1464-5491.2007.02333.x. [DOI] [PubMed] [Google Scholar]
- 22.Jinnouchi H, Sugiyama S, Yoshida A, Hieshima K, Kurinami N, Suzuki T, et al. Liraglutide, a glucagon-like peptide-1 analog, increased insulin sensitivity assessed by hyperinsulinemic-euglycemic clamp examination in patients with uncontrolled type 2 diabetes mellitus. J Diabetes Res. 2015;2015:1–8. doi: 10.1155/2015/706416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim SH, Liu A, Ariel D, Abbasi F, Lamendola C, Grove K, et al. Pancreatic beta cell function following liraglutide-augmented weight loss in individuals with prediabetes: analysis of a randomised, placebo-controlled study. Diabetologia. 2014;57(3):455–462. doi: 10.1007/s00125-013-3134-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pararajasingam G, Høfsten DE, Løgstrup BB, Egstrup M, Henriksen FL, Hangaard J, et al. Newly detected abnormal glucose regulation and long-term prognosis after acute myocardial infarction: comparison of an oral glucose tolerance test and glycosylated haemoglobin A1c. Int J Cardiol. 2016;214:310–315. doi: 10.1016/j.ijcard.2016.03.199. [DOI] [PubMed] [Google Scholar]
- 25.Cheng AYY, Lau DCW. The Canadian Diabetes Association 2013 clinical practice guidelines-raising the bar and setting higher standards! Can J Diabetes. 2013;37(3):137–138. doi: 10.1016/j.jcjd.2013.04.005. [DOI] [PubMed] [Google Scholar]
- 26.Diabetes DOF. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2013;36(Suppl 1):S67–S74. doi: 10.2337/dc13-S067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Anholm C, Kumarathurai P, Klit MS, Kristiansen OP, Nielsen OW, Ladelund S, et al. Adding liraglutide to the backbone therapy of biguanide in patients with coronary artery disease and newly diagnosed type-2 diabetes (the AddHope2 study): a randomised controlled study protocol. BMJ Open. 2014;4(7):e005942. doi: 10.1136/bmjopen-2014-005942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Holst JJ. Evidence that enteroglucagon (II) is identical with the C-terminal sequence (residues 33–69) of glicentin. Biochem J. 1982;207(3):381–388. doi: 10.1042/bj2070381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Albrechtsen NJW, Hartmann B, Veedfald S, Idorn T, Feldt-rasmussen B, Knop FK, et al. Hyperglucagonaemia analysed by glucagon sandwich ELISA: nonspecific interference or truly elevated levels? Diabetologia. 2014;57(9):1919–1926. doi: 10.1007/s00125-014-3283-z. [DOI] [PubMed] [Google Scholar]
- 30.Matsuda M, DeFronzo RA. Insulin sensitivity indices obtained from oral glucose tolerance testing: comparison with the euglycemic insulin clamp. Diabetes Care. 1999;22(9):1462–1470. doi: 10.2337/diacare.22.9.1462. [DOI] [PubMed] [Google Scholar]
- 31.Matthews DR, Hosker JR, Rudenski AS, Naylor BA, Treacher DF, Turner RC, et al. Homeostasis model assessment: insulin resistance and fl-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28(7):412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 32.Hovorka R, Soons PA, Young MA. ISEC: a program to calculate insulin secretion. Comput Methods Programs Biomed. 1996;50(3):253–264. doi: 10.1016/0169-2607(96)01755-5. [DOI] [PubMed] [Google Scholar]
- 33.Bergman RN, Ader M, Huecking K, Van Citters G. Accurate Assessment of beta-cell function—the hyperbolic correction. Diabetes. 2002;51(Suppl 1):S212–S220. doi: 10.2337/diabetes.51.2007.S212. [DOI] [PubMed] [Google Scholar]
- 34.Allison DB, Paultre F, Maggio C, Mezzitis N, Pi-Sunyer FX. The use of areas under curves in diabetes research. Diabetes Care. 1995;18(2):245–250. doi: 10.2337/diacare.18.2.245. [DOI] [PubMed] [Google Scholar]
- 35.Polonsky KS, Given BD, Hirsch L, Shapiro ET, Tillil H, Beebe C, et al. Quantitative study of insulin secretion and clearance in normal and obese subjects. J Clin Invest. 1988;81(2):435–441. doi: 10.1172/JCI113338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Haugaard SB, Andersen O, Vølund A, Hansen BR, Iversen J, Andersen UB, et al. Beta-cell dysfunction and low insulin clearance in insulin-resistant human immunodeficiency virus (HIV)-infected patients with lipodystrophy. Clin Endocrinol. 2005;62(3):354–361. doi: 10.1111/j.1365-2265.2005.02223.x. [DOI] [PubMed] [Google Scholar]
- 37.Kahn SE, Prigeon RL, McCulloch DK, Boyko EJ, Bergman RN, Schwartz MW, et al. Quantification of the relationship between insulin sensitivity and beta-cell function in human subjects. Evidence for a hyperbolic function. Diabetes. 1993;42(11):1663–1672. doi: 10.2337/diab.42.11.1663. [DOI] [PubMed] [Google Scholar]
- 38.Campioni M, Toffolo G, Basu R, Rizza RA, Cobelli C. Minimal model assessment of hepatic insulin extraction during an oral test from standard insulin kinetic parameters. Am J Physiol Endocrinol Metab. 2009;297(4):E941–E948. doi: 10.1152/ajpendo.90842.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Anholm C, Kumarathurai P, Pedersen LR, Nielsen OW, Kristiansen OP, Fenger M, et al. Liraglutide effects on beta-cell, insulin sensitivity and glucose effectiveness in patients with stable coronary artery disease and newly diagnosed type 2 diabetes. Diabetes Obes Metab. 2017;19(6):850–857. doi: 10.1111/dom.12891. [DOI] [PubMed] [Google Scholar]
- 40.Senn S. Cross-over trials in clinical research. 2. Chichester: Wiley; 2002. [Google Scholar]
- 41.Kumarathurai P, Anholm C, Nielsen OW, Kristiansen OP, Mølvig J, Madsbad S, et al. Effects of the glucagon-like peptide-1 receptor agonist liraglutide on systolic function in patients with coronary artery disease and type 2 diabetes: a randomized double-blind placebo-controlled crossover study. Cardiovasc Diabetol. 2016;15(1):105. doi: 10.1186/s12933-016-0425-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bonora E, Zavaroni I, Coscelli C, Butturini U. Decreased hepatic insulin extraction in subjects with mild glucose intolerance. Metabolism. 1983;32(5):438–446. doi: 10.1016/0026-0495(83)90004-5. [DOI] [PubMed] [Google Scholar]
- 43.Viljanen APM, Iozzo P, Borra R, Kankaanpää M, Karmi A, Lautamäki R, et al. Effect of weight loss on liver free fatty acid uptake and hepatic insulin resistance. J Clin Endocrinol Metab. 2009;94(1):50–55. doi: 10.1210/jc.2008-1689. [DOI] [PubMed] [Google Scholar]
- 44.Tanaka K, Saisho Y, Manesso E, Tanaka M, Meguro S, Irie J, et al. Effects of liraglutide monotherapy on beta cell function and pancreatic enzymes compared with metformin in japanese overweight/obese patients with type 2 diabetes mellitus: a subpopulation analysis of the KIND-LM randomized trial. Clin Drug Investig. 2015;35(10):675–684. doi: 10.1007/s40261-015-0331-5. [DOI] [PubMed] [Google Scholar]
- 45.Juhl CB, Hollingdal M, Sturis J, Jakobsen G, Agersø H, Veldhuis J, et al. Bedtime administration of NN2211, a long-acting GLP-1 derivative, substantially reduces fasting and postprandial glycemia in type 2 diabetes. Diabetes. 2002;51(2):424–429. doi: 10.2337/diabetes.51.2.424. [DOI] [PubMed] [Google Scholar]
- 46.Nauck MA, Kleine N, Orskov C, Holst JJ, Willms B, Creutzfeldt W. Normalization of fasting hyperglycaemia by exogenous glucagon-like peptide 1 (7–36 amide) in type 2 (non-insulin-dependent) diabetic patients. Diabetologia. 1993;36(8):741–744. doi: 10.1007/BF00401145. [DOI] [PubMed] [Google Scholar]
- 47.Kramer CK, Zinman B, Choi H, Connelly PW, Retnakaran R. The impact of chronic liraglutide therapy on glucagon secretion in type 2 diabetes: insight From the LIBRA trial. J Clin Endocrinol Metab. 2015;100(10):3702–3709. doi: 10.1210/jc.2015-2725. [DOI] [PubMed] [Google Scholar]
- 48.Kramer CK, Zinman B, Choi H, Connelly PW, Retnakaran R. Impact of the glucagon assay when assessing the effect of chronic liraglutide therapy on glucagon secretion. J Clin Endocrinol Metab. 2017;102:2729–2733. doi: 10.1210/jc.2017-00928. [DOI] [PubMed] [Google Scholar]
- 49.Dandona P, Ghanim H, Abuaysheh S, Green K, Batra M, Dhindsa S, et al. Decreased insulin secretion and incretin concentrations and increased glucagon concentrations after a high-fat meal when compared with a high-fruit and -fiber meal. Am J Physiol Endocrinol Metab. 2015;308(3):E185–E191. doi: 10.1152/ajpendo.00275.2014. [DOI] [PubMed] [Google Scholar]
- 50.Alsalim W, Tura A, Pacini G, Omar B, Bizzotto R, Mari A, et al. Mixed meal ingestion diminishes glucose excursion in comparison with glucose ingestion via several adaptive mechanisms in people with and without type 2 diabetes. Diabetes Obes Metab. 2016;18(1):24–33. doi: 10.1111/dom.12570. [DOI] [PubMed] [Google Scholar]
- 51.Boston RC, Moate PJ. NEFA minimal model parameters estimated from the oral glucose tolerance test and the meal tolerance test. Am J Physiol Regul Integr Comp Physiol. 2008;295(2):R395–R403. doi: 10.1152/ajpregu.90317.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ostawal A, Mocevic E, Kragh N, Xu W. Clinical Effectiveness of liraglutide in type 2 diabetes treatment in the real-world setting: a systematic literature review. Diabetes Ther. 2016;7(3):411–438. doi: 10.1007/s13300-016-0180-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Marso SP, Daniels GH, Brown-Frandsen K, Kristensen P, Mann JFE, Nauck MA, et al. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2016;375(4):311–322. doi: 10.1056/NEJMoa1603827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Blonde L, Russell-Jones D. The safety and efficacy of liraglutide with or without oral antidiabetic drug therapy in type 2 diabetes: an overview of the LEAD 1–5 studies. Diabetes Obes Metab. 2009;11(Suppl 3):26–34. doi: 10.1111/j.1463-1326.2009.01075.x. [DOI] [PubMed] [Google Scholar]
- 55.Funch D, Mortimer K, Ziyadeh NJ, Seeger JD, Li L, Norman H, et al. Liraglutide use and evaluation of pancreatic outcomes in a US commercially insured population. Diabetes Obes Metab. 2019; (Epub ahead). [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.