Abstract
Extrachromosomal circular DNAs (eccDNAs) are circular DNAs that are originated from chromosomes but independent from chromosomal DNA. The eccDNAs are commonly found in various tissues and cell types, and in both normal and diseased conditions. Due to highly heterogeneous origins and widely spread in nearly all eukaryotes, the eccDNAs are believed to reflect the genome’s plasticity and instability. With assistance of next generation sequencing, more eccDNAs have been characterized at molecular level. Recently, eccDNAs are reported as cell-free DNAs in circulating system. Importantly, these circulating eccDNAs has shown some evidence with disease associations, suggesting their potential utility as new type of biomarkers for disease detection, treatment assessment and progress surveillance. However, many challenges need to be addressed before implementing the eccDNAs as new source of genetic materials for liquid biopsy.
1. Introduction
Chromosomes are the classical vehicles of DNA. The genomes of eukaryote cells are believed to be stable inside of the nucleus. However, the existence of eccDNAs, the extrachromosomally located and circularly formed DNAs, indicates the plasticity and instability of the genome. From a broader definition, the eccDNAs can be classified as organelle (like the mitochondrial DNA) and non-organelle eccDNAs. The latter is widely spread in eukaryotes in different types of cells and more recently found in circulating system as cell-free eccDNAs in both physiological and pathological conditions. In this article, we will summarize new findings on the non-organelle eccDNAs. We will discuss their potential roles in disease development, their potential applications and technical challenges as new nucleic acid markers for liquid biopsy.
2. Overview of eccDNAs
The eccDNAs are universally spread in eukaryotes, including plants (1, 2), yeasts (3, 4) and mammalians (5–7). Contrast to the more stable linear DNA on the chromosomes, the eccDNAs are extrachromosomally located, unstable, dynamic, and heterogeneously originated. They have been described in various terms including circular extrachromosomal DNA (8), extrachromosomal circles of DNA (7), and covalently closed DNA circles (9). To unify the nomenclature, Moller et al. has proposed to use extrachromosomal circular DNAs (eccDNAs) to describe all circular DNAs in eukaryotes (6). The eccDNAs have different sizes, including large circular DNAs such as episomes (10, 11) and double minute chromosomes (DMs) (12, 13), and the smaller circular molecules such as the small polydispersed DNA (spcDNAs) (14, 15) and microDNAs (5, 16). Episomes (from hundreds of kilobases to 1 megabase, submicroscopic) are considered as precursors of DMs (>1 megabase, microscopically visible). Both episomes and DMs are vehicles of extra-chromosomal gene amplification. They usually contain amplified oncogenes (like c-MYC) and drug resistance genes (like DHFR) that provide growth advantages to tumor and drug-resistance cells (17–19). spcDNAs, characterized decades ago, are a few hundred bases to a few kilobases long, containing either repetitive or unique sequences and are closely related chromosome instability (14, 20, 21). microDNAs, a new class of eccDNAs, are recently characterized in mouse, chicken and human cells. They are predominantly in 200–400 bases long and in either single- or double-stranded DNA forms (5, 16). The microDNAs are heterogeneously originated and spread throughout the whole genome but their distribution in the genome tend to enrich in genic regions (5, 16, 22). For example, microDNAs are highly enriched in exons, 5’ and 3’ UTRs, regions of higher GC content and DNase hypersensitivity sites (5, 16, 22). Additionally, the microDNAs has shown lineage specificity in human ovarian and prostate cancer cell lines (16) and cell type specificity in human fibroblast cells and granulocytes (23). Most recently, kilobase-sized eccDNAs were detected and characterized from healthy human muscle and blood samples. Half of these eccDNAs carry genes or gene fragments. Transcripts of eccDNAs were also detected, suggesting that the eccDNAs may not be by-product of nucleotide metabolism but rather have functional roles (6).
EccDNAs are generated from the chromosomes mediated by heterogeneous mechanisms, which can be classified as recombination-dependent and replication-dependent mechanisms. For the recombination-dependent mechanism, DNA double strand breaks are repaired by homologous recombination (24–26), non-homologous end joining and microhomology-mediated end joining (3, 6, 16, 27). The circular DNA are formed by looping out or being excised from the genomes. For the replication-dependent mechanism, there is single or double stranded DNA breakage because of replication fork stalling, replication fork collapse or replication slippage. Then pathways such as break-induced replication, microhomology-mediated break-induced replication, fork stalling and template switching, and mismatch repair (16) may be used to form circular DNAs. In addition, transcription-associated DNA damage (R-loops) was also proposed to produce microDNAs (16). Further molecular structure analysis and eccDNA generation in cell line/animal models with deficiency in specific pathway may facilitate understanding of the underlying mechanisms.
3. Methods for identification and enrichment of eccDNAs
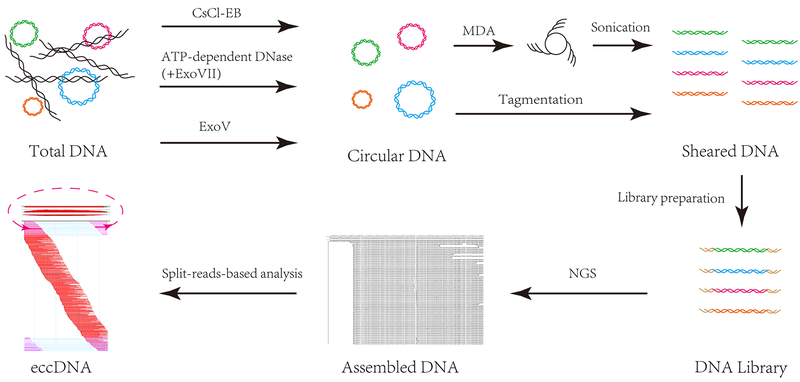
Multiple methods have been evaluated to identify and analyze the biological and molecular characteristics of eccDNAs. Electron microscopy has been applied to study the ultra-structure, size and double/single strand status (5, 16, 28, 29). At molecular level, 2D gel electrophoresis along with probe hybridization seems effective to detect, quantify and characterize eccDNAs in Drosophila, Xenopus, rodents and humans (14, 30–34). With rapid advances in high-throughput DNA sequencing, eccDNAs have been characterized at a highly precise level (35). Commonly used procedure includes eccDNA enrichment and circular DNA analysis. The enrichment is involved in exonuclease digestion (such as Plasmid-Safe-ATP-dependent DNase and/or exonuclease V) to remove linear DNA and multiple displacement amplification (MDA) to preferentially amplify circular DNAs (1, 3, 5, 6, 16, 27, 36–38). This method has been proved to be effective for eccDNA detection and enrichment, especially when the initial DNA amount is very limited, for example the cell-free DNA (cfDNA) in the circulating system (27, 37). Other reported methods for eccDNA enrichment include multiple rounds of extensive exonuclease V digestion and chloride ethidium bromide density gradient centrifugation. The latter method is used in a most recent study and claims to be less biased and with less artifacts (23). For sequencing library preparation, the amplified products or the enriched circular DNAs need to be fragmented using either the physical methods like sonication or chemical fragmentation such as NexteraXT Tagmentase. Sequencing data analysis requires a customized algorithm to identify split reads. Mapping of the split reads has to be inconsistent, i.e. first part of sequence read being mapped to 3’ end while second part of the split read being mapped to 5’ end of a defined genomic region (27). This entire procedure is illustrated in Figure 1. Although this strategy is very effective for small eccDNAs (like microDNA and spcDNA) it remains difficult to extract the entire circular DNA for the large DMs, for which alternative methods like microdissection (13), array-CGH (39) or direct sequencing of the total DNA (40) can be applied, as sequences on DMs are highly amplified. As the quantity of cfDNAs obtained from body fluids is very limited, when using cfDNAs as starting material, eccDNA enrichment method may need further improvement to increase sensitivity.
Figure 1. eccDNA enrichment, library preparation and sequence analysis.
To enrich eccDNAs, total DNA extracted from tissue/cells/body fluids will be digested with exonucleases, such as Plasmid-Safe-ATP-dependent-DNase or Exo V, to remove the linear DNA or centrifugated using CsCl-EB method targeting for circular DNAs. The resultant circular DNA will undergo MDA to further enrich eccDNAs. Alternatively, under the circumstance that there is enough initial DNA and complete removal of linear DNA, the circular DNA may be directly used without MDA procedure. DNA will be fragmented by sonication or fragmentase, followed by sequencing library preparation and next generation sequencing. To extract non-organelle eccDNA sequence, mtDNA sequences will be removed in silico. Customized algorithms, based on split reads (pink sequence in the figure), will be applied to extract eccDNA sequences. The typical eccDNAs, shown in the figure, have sharp boundaries formed by split reads. One part of a split read is mapped to the 5’ end of the eccDNA and the other part is mapped to the 3’ end. The arrow shows the direction of the ligation of eccDNAs ends.
4. Implications of eccDNAs in human diseases and treatments
EccDNAs are produced in both physiological and pathological conditions. For eccDNAs in normal cells, like the eccDNAs containing transposable elements and the extrachromosomal ribosomal DNA circles in yeast (4, 41, 42), their presence may be related to genome plasticity during development and aging (25). In pathological conditions, eccDNAs are found to play roles in human diseases and disease treatments, especially for larger eccDNAs (episomes and DMs) with their functions being better characterized. For example, DMs, exclusively found in tumor cells (13) and drug-resistance cells (43), significantly elevate the active gene transcripts to promote tumor progression. Because of their self-replication and uneven segregation, DMs are related to tumor cell heterogeneity and adaptation (44). In a recent study, extrachromosomal gene amplification was proved to drive tumor evolution and heterogeneity, with higher efficiency than the intrachromosomal gene amplification (40). In T-cell acute lymphoblystic leukemia (ALL) patients, NUP214-ABL1 fusion gene amplification in form of episomes, acting as an activated tyrosine kinase, may define a subgroup of T-ALL patients who benefit from imatinib treatment (45–47).
As to the smaller eccDNAs, their functions are less known. It is believed that the small eccDNA may be related to chromosomal instability and genome adaptation to cancer and other disease circumstances. Elevated spcDNA level was observed in Hela tumor cell line and colon cancer tissues, fibroblasts derived from Fanconi’s Anemia (FA) patients and under treatment of cancer initiation agents but not detected in genetically stable normal human fibroblasts (14). 85-fold increase of spcDNA was reported in skin-derived fibroblast of FA patients compared to normal controls (17). In angiofibroma cells derived from patients with tuberous sclerosis, the spcDNA quantity was also greatly increased when compared to skin cells from these patients or healthy donors (20). Length and components of spcDNAs showed difference between disease and normal controls. Additionally, more larger spcDNAs were observed in the angiofibroma cells from patients with tuberous sclerosis than normal fibroblast cells (21). The mean length of spcDNA was significantly greater in the FA than in the control cells (17). Tel-spcDNA (spcDNAs with extensive telomeric repeats) levels were higher in transformed cells and enhanced by carcinogen treatments in rodent cells (18). Furthermore, Alu, LINE-1 (L1) and telomeric sequences were present more frequently in tumor cell lines than in normal lymphocytes (48). Finally, spcDNAs were reported as structure for gene amplification (instead of DMs or episomes) to amplify the PIP exons and intron in breast cancer (49). These results suggest that spcDNA sequences (including the repetitive sequences) may play roles in chromosomal rearrangements and genomic instability.
For another group of small eccDNAs, microDNAs, knowledge on their function and disease association is rather limited. A study has shown that tumor specimens contain longer eccDNAs than normal lung tissues. Consistently, the circulating eccDNAs were longer in lung cancer patients collected prior to surgical resection of the tumor than in the same patient after surgery (37). Another study revealed that methotrexate (MTX)-treated human lymphoblastoid cells lines (LCLs) generated higher numbers of unique microDNA entities compared to non-treated cells. L-asparaginase (ASP)- and MTX-treated LCLs have longer microDNAs than non-treated cells (22). The increase in number and length under the malignant and drug treatment conditions seem to be consistent between the microDNA and spcDNAs.
In conclusion, eccDNAs are a group of extrachromosomal circular elements that reflect plasticity and dynamics of the genome. eccDNAs may contribute to the physiological cell development, aging, adaptive evolution, and cancerous genome instability.
5. Cell-free eccDNAs and their potential applications
Due to non-invasive and highly informative nature, liquid biopsy by testing tumor or fetus component in body fluids has made great progress in recent years (50, 51). There are three main components for liquid biopsy including cfDNA, circulating tumor cells (CTC) and exosomes. Currently, clinical applications of liquid biopsy are limited to the fetal cfDNA in maternal blood for detection of chromosome disorders, and CTCs and tumor-derived cfDNA (circulating tumor DNA, ctDNA) for tumor detection, relapse prediction and therapeutic evaluation (52, 53).
With identification of the eccDNAs in mammalian cells and tissues, researchers are working to detect and characterize the cell-free eccDNAs in circulating system. It is believed that the larger eccDNAs are basically located inside of the nucleus while the smaller ones spread in nucleus, cytoplasm, and extracellular sites. Recent findings have shown highly abundant smaller eccDNAs in the form of cfDNAs in human circulation system in both healthy and cancerous conditions (27, 37). Consistent with the origin features of eccDNAs in tissues and cell lines, the cell-free eccDNAs also showed some distribution preference in the genome, such as UTRs, exons, DNase-hypersensitivity sites and histone markers-enriched regions (27, 37). By comparing the sizes of microDNAs in circulation, studies have found that cell-free microDNAs have different sizes under different conditions. Lung cancer and ovarian cancer patients have longer circulating eccDNAs in pre- than in post-tumor resection (37). ctDNA in cancer patients are frequently shorter than normal cfDNAs, suggesting that size selection can improve the detection of ctDNA (6, 54). Similarly, size selection of eccDNAs, possibly in silico during the analysis, may help to enrich more disease-originated eccDNAs for molecular characterization.
Extracellular DNA (exDNA) is the DNA component exported and assembled with other proteins to form the DNA-containing extracellular structure (neutrophil extracellular traps (NET) on the surface of cells (17). Elevated amount of exDNA component was found in inflammatory cells and tumor cells. Recently, exDNAs were proved to play important roles for cancer cell invasion and metastasis. Treatment of cells using DNase I significantly reduced the invasion and metastatic capability of tumor cells (55). Circulating exDNA is basically a component of circulating cfDNA. Increased circulating exDNA in human blood is observed in metastatic cancers. Treatment of Lewis lung carcinoma (LLC)-bearing mice using DNase I inhibited the metastasis of tumor cells and altered the profile of circulating exDNA (56). Together, these data suggest that exDNAs on cell surface and in the circulation may have a function of mediating the metastasis of tumor cells. Although exDNA is not necessary to be the circular formed DNA, it is reasonable to speculate that either extracellular eccDNAs or circulating eccDNAs are a part of the functional exDNAs.
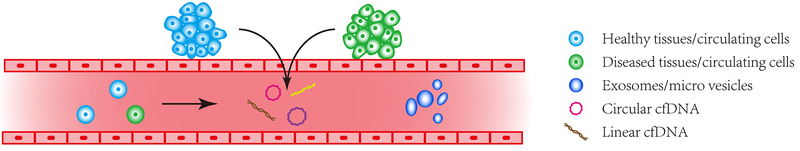
Biological origins of eccDNAs have not been fully illustrated. However, it is likely that, similar to the linear cfDNA, eccDNAs may be released by cell secretion, apoptosis (22), necrosis, cell lysis and rupture of micronuclei in either normal or diseased tissues (37), as well as from direct lysis of the circulating cells (Figure 2). A recent study showed that chromosome instability from errors during chromosome segregation may create micronuclei whose rupture released DNA into cytosol (cytosolic DNA) and activated cGAS-STING (cyclic GMP-AMP synthase-stimulator of interferon genes) cytosolic DNA-sensing pathway to drive their metastasis (57). eccDNAs may also be a component of these functional cytosolic DNA and actively released by tumor or diseased cells into the cytoplasm and extracellular matrix to influence the cells themselves, cells nearby and distant cells as well.
Figure 2.
Possible components in circulation system for liquid biopsy. In circulation system, such as blood, both the classical linear cell-free DNA and the new disclosed circular DNAs (circulating eccDNAs) along with circulating cells and exosomes are main source of materials for liquid biopsy-based biomarker study. eccDNAs may be released or actively produced from both healthy and diseased cells or directly from the lysis of circulating cells.
In addition to their size and origin features, compared to linear cfDNAs, eccDNAs have other characteristics: eccDNAs in blood are longer than the linear cfDNAs that are normally 160–170 bp in size (37); eccDNAs are more stable than linear cfDNAs as the circularly formed DNAs are more resistant to nuclease. Additionally, eccDNAs (such as microDNAs) are lineage-specific in human ovarian/prostate cancer cell lines (16) and cell type specific in human fibroblasts and granulocytes (23). Repetitive sequences in mouse eccDNAs are tissue-specific and demonstrated age-related changes (58). Together, these data suggest that eccDNAs, with their size and sequence characteristics in different conditions, may serve as new markers for disease prediction and treatment evaluation.
6. Challenges and future directions
Cell-free eccDNAs have been observed in circulation system and their presence has been proposed as potential biomarker. However, more work is needed to fully understand their molecular characteristics and functional roles. To date, many challenges exist for the potential clinical applications. For example, a recent study compared the sequences of cell-free eccDNAs at the nucleotide level and found that only a small fraction of eccDNAs from a total of 20 thousands of eccDNA molecules shared the same sequence (same origins and breakpoints) in technical replicates, suggesting extreme diversity of eccDNA origin (27). The high number of eccDNAs with unique sequences is a significant problem for biomarker discovery because the most sequences are extremely rare and the sequence diversity requires a huge amount of sequencing data to capture the rare event. To solve this problem, future study may first identify hotspots of eccDNA origination under different disease status and then perform sequence capture for targeted region analysis. Furthermore, current studies are limited to plasma and serum samples only. Other body fluids such as urine and saliva remain to be tested.
In addition, biological roles of most eccDNAs are largely unknown. So far, well studied eccDNAs are limited to large-sized and oncogene-carrying DMs and episomes. It remains unclear why cells generate a wide variety of eccDNAs, what are their biological roles in cell functions, and what sequence features are disease-related. Although smaller eccDNAs (spcDNAs and microDNAs) are associated with chromosome instability, there is no direct evidence showing regulatory role of the eccDNAs. As exDNAs promote tumor cell invasion and migration and the DNase I removal of circulating exDNAs suppresses tumor metastasis (55–57), it is reasonable to hypothesize that eccDNAs may be a part of exDNAs and directly participate in regulating tumor cell invasion and metastasis. Further, circle RNAs have been identified as important regulatory molecules (59). Because microDNA sequences are enriched in UTRs, histone markers and DNase hypersensitivity sites, is it possible that the eccDNAs also play an important regulatory role?
Furthermore, the existence and function of eccDNA transcripts still need to be addressed. A study showed that circular DNA excised from the genome could be further transcribed to generate regulatory RNAs in Paramecium (60). More recently, a study molecularly characterized kilobase-sized eccDNAs in human tissues and identified RNA transcripts identical to the eccDNA junction sequences, strongly suggesting that some of the kilobase-sized eccDNAs are transcribed (6). Instead of being excluded from the nucleus, a part of eccDNAs will remain inside the nucleus and have gene products from eccDNA transcripts which may alter the phenotype of cells. Clearly, in addition to by-products of chromosome recombination and nucleotide metabolism, eccDNAs may have functional roles as active gene transcripts and gene expression regulators (35). Therefore, it is important to further characterize biological functions and cellular influence of these eccDNAs and their transcripts in future research.
Finally, technology improvement in eccDNA enrichment and data analysis is also needed. Currently, there is no gold standard for eccDNA analysis. Since approaches used in eccDNA enrichment and data analysis may significantly influence results, standardization of this procedure is necessary to compare different studies. To make more reproducible results, multiple factors should be considered during eccDNA analysis. First, standard analytical algorithm is needed. A computer program that can effectively extract eccDNA sequences and characterize their molecular features will significantly improve accuracy and efficiency. The program should be able to report chromosome coordinates of breakpoints, sizes of DNA fragments, and counts of circular molecules. Second, standard eccDNA enrichment is needed. Highly efficient enrichment with minimum contamination from other genomic DNA (such as mitochondrial DNA) will increase target sequence reads and save sequencing cost. When using exonuclease to enrich eccDNAs, input DNA before exonuclease digestion may be used to control digestion efficiency. To test enrichment efficiency, a plasmid DNA may be used as spike-in control. Third, standard library preparation is needed. The eccDNAs are extremely heterogeneous and can be either single- or double-stranded. Common library preparation captures double-strand DNA only. For cfDNA, MDA is often used to increase sensitivity. However, this procedure may also generate amplification bias. Therefore, technical replicates are often needed. The technical replicates can also be used to test reproducibility of this procedure. Fourth, use of control is needed. Ideally, the known circular DNAs with different sizes should be embedded in each step to control efficiency of eccDNA extraction and enrichment and avoid library preparation bias. When analyzing diseased conditions, healthy DNA may be used as control. Additionally, there is no knowledge on potential difference between eccDNAs derived from living cells and from dead cells. Successfully addressing these questions will have significant impact on our understanding of eccDNA biology and may pave the way toward eccDNA-based clinical applications.
Key points.
eccDNAs are widely spread in eukaryotes in both normal and diseased conditions and believed to be associated with genome instability and evolution.
Cell-free eccDNAs were recently reported in circulating system.
Due to their relative stability and their association with human diseases, eccDNAs have been proposed as potential biomarkers for liquid biopsy.
Funding
This study was partially supported by National Institute of Health (R01CA212097) to LW, National Natural Science Foundation of China (NSFC) (project#81301752) and University Nursing Program for Young Scholars with Creative Talents in Heilongjiang Province (project# UNPYSCT-2017056) to JZ.
Footnotes
Conflict of interest The authors JZ, SC, FZ and LW disclose no potential conflicts of interest.
References
- 1.Lanciano S, Carpentier MC, Llauro C, Jobet E, Robakowska-Hyzorek D, Lasserre E, et al. Sequencing the extrachromosomal circular mobilome reveals retrotransposon activity in plants. PLoS genetics. 2017;13(2):e1006630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Koo DH, Molin WT, Saski CA, Jiang J, Putta K, Jugulam M, et al. Extrachromosomal circular DNA-based amplification and transmission of herbicide resistance in crop weed Amaranthus palmeri. Proc Natl Acad Sci U S A. 2018;115(13):3332–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Moller HD, Parsons L, Jorgensen TS, Botstein D, Regenberg B. Extrachromosomal circular DNA is common in yeast. Proc Natl Acad Sci U S A. 2015;112(24):E3114–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Moller HD, Larsen CE, Parsons L, Hansen AJ, Regenberg B, Mourier T. Formation of Extrachromosomal Circular DNA from Long Terminal Repeats of Retrotransposons in Saccharomyces cerevisiae. G3 (Bethesda). 2015;6(2):453–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shibata Y, Kumar P, Layer R, Willcox S, Gagan JR, Griffith JD, et al. Extrachromosomal microDNAs and chromosomal microdeletions in normal tissues. Science. 2012;336(6077):82–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Moller HD, Mohiyuddin M, Prada-Luengo I, Sailani MR, Halling JF, Plomgaard P, et al. Circular DNA elements of chromosomal origin are common in healthy human somatic tissue. Nat Commun. 2018;9(1):1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Paulsen T, Kumar P, Koseoglu MM, Dutta A. Discoveries of Extrachromosomal Circles of DNA in Normal and Tumor Cells. Trends Genet. 2018;34(4):270–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Alsford NS, Navarro M, Jamnadass HR, Dunbar H, Ackroyd M, Murphy NB, et al. The identification of circular extrachromosomal DNA in the nuclear genome of Trypanosoma brucei. Mol Microbiol. 2003;47(2):277–89. [DOI] [PubMed] [Google Scholar]
- 9.Shore D, Langowski J, Baldwin RL. DNA flexibility studied by covalent closure of short fragments into circles. Proc Natl Acad Sci U S A. 1981;78(8):4833–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Von Hoff DD, Needham-VanDevanter DR, Yucel J, Windle BE, Wahl GM. Amplified human MYC oncogenes localized to replicating submicroscopic circular DNA molecules. Proceedings of the National Academy of Sciences of the United States of America. 1988;85(13):4804–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ruiz JC, Choi KH, von Hoff DD, Roninson IB, Wahl GM. Autonomously replicating episomes contain mdr1 genes in a multidrug-resistant human cell line. Molecular and cellular biology. 1989;9(1):109–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Albertson DG. Gene amplification in cancer. Trends Genet. 2006;22(8):447–55. [DOI] [PubMed] [Google Scholar]
- 13.Zhu J, Yu Y, Meng X, Fan Y, Zhang Y, Zhou C, et al. De novo-generated small palindromes are characteristic of amplicon boundary junction of double minutes. Int J Cancer. 2013;133(4):797–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cohen S, Regev A, Lavi S. Small polydispersed circular DNA (spcDNA) in human cells: association with genomic instability. Oncogene. 1997;14(8):977–85. [DOI] [PubMed] [Google Scholar]
- 15.Kunisada T, Yamagishi H. Sequence organization of repetitive sequences enriched in small polydisperse circular DNAs from HeLa cells. J Mol Biol. 1987;198(4):557–65. [DOI] [PubMed] [Google Scholar]
- 16.Dillon LW, Kumar P, Shibata Y, Wang YH, Willcox S, Griffith JD, et al. Production of Extrachromosomal MicroDNAs Is Linked to Mismatch Repair Pathways and Transcriptional Activity. Cell Rep. 2015;11(11):1749–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Motejlek K, Schindler D, Assum G, Krone W. Increased amount and contour length distribution of small polydisperse circular DNA (spcDNA) in Fanconi anemia. Mutat Res. 1993;293(3):205–14. [DOI] [PubMed] [Google Scholar]
- 18.Regev A, Cohen S, Cohen E, Bar-Am I, Lavi S. Telomeric repeats on small polydisperse circular DNA (spcDNA) and genomic instability. Oncogene. 1998;17(26):3455–61. [DOI] [PubMed] [Google Scholar]
- 19.Windle B, Draper BW, Yin YX, O’Gorman S, Wahl GM. A central role for chromosome breakage in gene amplification, deletion formation, and amplicon integration. Genes Dev. 1991;5(2):160–74. [DOI] [PubMed] [Google Scholar]
- 20.Neidlinger C, Assum G, Krone W, Dietrich C, Hochsattel R, Klotz G. Increased amounts of small polydisperse circular DNA (spcDNA) in angiofibroma-derived cell cultures from patients with tuberous sclerosis (TS). Hum Genet. 1988;79(3):286–8. [DOI] [PubMed] [Google Scholar]
- 21.Motejlek K, Assum G, Krone W, Kleinschmidt AK. The size of small polydisperse circular DNA (spcDNA) in angiofibroma-derived cell cultures from patients with tuberous sclerosis (TSC) differs from that in fibroblasts. Hum Genet. 1991;87(1):6–10. [DOI] [PubMed] [Google Scholar]
- 22.Mehanna P, Gagne V, Lajoie M, Spinella JF, St-Onge P, Sinnett D, et al. Characterization of the microDNA through the response to chemotherapeutics in lymphoblastoid cell lines. PLoS One. 2017;12(9):e0184365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shoura MJ, Gabdank I, Hansen L, Merker J, Gotlib J, Levene SD, et al. Intricate and Cell Type-Specific Populations of Endogenous Circular DNA (eccDNA) in Caenorhabditis elegans and Homo sapiens. G3 (Bethesda). 2017;7(10):3295–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Moller HD, Larsen CE, Parsons L, Hansen AJ, Regenberg B, Mourier T. Formation of Extrachromosomal Circular DNA from Long Terminal Repeats of Retrotransposons in Saccharomyces cerevisiae. G3 (Bethesda). 2016;6(2):453–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gaubatz JW. Extrachromosomal circular DNAs and genomic sequence plasticity in eukaryotic cells. Mutat Res. 1990;237(5–6):271–92. [DOI] [PubMed] [Google Scholar]
- 26.Jorgensen TS, Xu Z, Hansen MA, Sorensen SJ, Hansen LH. Hundreds of circular novel plasmids and DNA elements identified in a rat cecum metamobilome. PLoS One. 2014;9(2):e87924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhu J, Zhang F, Du M, Zhang P, Fu S, Wang L. Molecular characterization of cell-free eccDNAs in human plasma. Sci Rep. 2017;7(1):10968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yamagishi YKNOYYTKH. Extrachromosomal Circular DNA from Nuclear Fraction of Higher Plants. Plant & cell physiology. 1985;26(7):1401–9. [Google Scholar]
- 29.Diaz-Lara A, Gent DH, Martin RR. Identification of Extrachromosomal Circular DNA in Hop via Rolling Circle Amplification. Cytogenet Genome Res. 2016;148(2–3):237–40. [DOI] [PubMed] [Google Scholar]
- 30.Cohen S, Yacobi K, Segal D. Extrachromosomal circular DNA of tandemly repeated genomic sequences in Drosophila. Genome Res. 2003;13(6A):1133–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cohen S, Houben A, Segal D. Extrachromosomal circular DNA derived from tandemly repeated genomic sequences in plants. Plant J. 2008;53(6):1027–34. [DOI] [PubMed] [Google Scholar]
- 32.Cohen S, Lavi S. Induction of circles of heterogeneous sizes in carcinogen-treated cells: two-dimensional gel analysis of circular DNA molecules. Molecular and cellular biology. 1996;16(5):2002–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cohen S, Mechali M. A novel cell-free system reveals a mechanism of circular DNA formation from tandem repeats. Nucleic acids research. 2001;29(12):2542–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cohen S, Menut S, Mechali M. Regulated formation of extrachromosomal circular DNA molecules during development in Xenopus laevis. Molecular and cellular biology. 1999;19(10):6682–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Reon BJ, Dutta A. Biological Processes Discovered by High-Throughput Sequencing. Am J Pathol. 2016;186(4):722–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yamagishi H, Tsuda T, Fujimoto S, Toda M, Kato K, Maekawa Y, et al. Purification of small polydisperse circular DNA of eukaryotic cells by use of ATP-dependent deoxyribonuclease. Gene. 1983;26(2–3):317–21. [DOI] [PubMed] [Google Scholar]
- 37.Kumar P, Dillon LW, Shibata Y, Jazaeri AA, Jones DR, Dutta A. Normal and Cancerous Tissues Release Extrachromosomal Circular DNA (eccDNA) into the Circulation. Mol Cancer Res. 2017;15(9):1197–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Moller HD, Bojsen RK, Tachibana C, Parsons L, Botstein D, Regenberg B. Genome-wide Purification of Extrachromosomal Circular DNA from Eukaryotic Cells. J Vis Exp. 2016(110). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ji W, Bian Z, Yu Y, Yuan C, Liu Y, Yu L, et al. Expulsion of micronuclei containing amplified genes contributes to a decrease in double minute chromosomes from malignant tumor cells. Int J Cancer. 2014;134(6):1279–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Turner KM, Deshpande V, Beyter D, Koga T, Rusert J, Lee C, et al. Extrachromosomal oncogene amplification drives tumour evolution and genetic heterogeneity. Nature. 2017;543(7643):122–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mourier T Transposable elements and circular DNAs. Mobile genetic elements. 2016;6(6):e1240748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sinclair DA, Guarente L. Extrachromosomal rDNA circles--a cause of aging in yeast. Cell. 1997;91(7):1033–42. [DOI] [PubMed] [Google Scholar]
- 43.Meng X, Qi X, Guo H, Cai M, Li C, Zhu J, et al. Novel role for non-homologous end joining in the formation of double minutes in methotrexate-resistant colon cancer cells. J Med Genet. 2015;52(2):135–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.deCarvalho AC, Kim H, Poisson LM, Winn ME, Mueller C, Cherba D, et al. Discordant inheritance of chromosomal and extrachromosomal DNA elements contributes to dynamic disease evolution in glioblastoma. Nat Genet. 2018;50(5):708–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Stergianou K, Fox C, Russell NH. Fusion of NUP214 to ABL1 on amplified episomes in T-ALL--implications for treatment. Leukemia. 2005;19(9):1680–1. [DOI] [PubMed] [Google Scholar]
- 46.Graux C, Cools J, Melotte C, Quentmeier H, Ferrando A, Levine R, et al. Fusion of NUP214 to ABL1 on amplified episomes in T-cell acute lymphoblastic leukemia. Nature genetics. 2004;36(10):1084–9. [DOI] [PubMed] [Google Scholar]
- 47.Rowley JD, Le Beau MM, Rabbitts TH. Chromosomal translocations and genome rearrangements in cancer: Springer; 2015. [Google Scholar]
- 48.Schmidt H, Taubert H, Lange H, Kriese K, Schmitt WD, Hoffmann S, et al. Small polydispersed circular DNA contains strains of mobile genetic elements and occurs more frequently in permanent cell lines of malignant tumors than in normal lymphocytes. Oncol Rep. 2009;22(2):393–400. [PubMed] [Google Scholar]
- 49.Autiero M, Camarca A, Ciullo M, Debily MA, El Marhomy S, Pasquinelli R, et al. Intragenic amplification and formation of extrachromosomal small circular DNA molecules from the PIP gene on chromosome 7 in primary breast carcinomas. Int J Cancer. 2002;99(3):370–7. [DOI] [PubMed] [Google Scholar]
- 50.Crowley E, Di Nicolantonio F, Loupakis F, Bardelli A. Liquid biopsy: monitoring cancer-genetics in the blood. Nat Rev Clin Oncol. 2013;10(8):472–84. [DOI] [PubMed] [Google Scholar]
- 51.Alix-Panabieres C, Pantel K. Clinical Applications of Circulating Tumor Cells and Circulating Tumor DNA as Liquid Biopsy. Cancer discovery. 2016;6(5):479–91. [DOI] [PubMed] [Google Scholar]
- 52.Ashoor G, Syngelaki A, Wagner M, Birdir C, Nicolaides KH. Chromosome-selective sequencing of maternal plasma cell-free DNA for first-trimester detection of trisomy 21 and trisomy 18. American journal of obstetrics and gynecology. 2012;206(4):322 e1–5. [DOI] [PubMed] [Google Scholar]
- 53.Heitzer E, Auer M, Ulz P, Geigl JB, Speicher MR. Circulating tumor cells and DNA as liquid biopsies. Genome medicine. 2013;5(8):73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Florent Mouliere AMP, Dineika Chandrananda, Moore Elizabeth, Morris James, Smith Christopher G., Teodora Goranova, Heider Katrin, Mair Richard, Anna Supernat, Ioannis Gounaris, Susana Ros, Wan Jonathan C. M., Mercedes Jimenez-Linan, Davina Gale, Brindle Kevin, Massie Charles E., Parkinson Christine A., Brenton James D., Rosenfeld Nitzan. Selecting Short DNA Fragments In Plasma Improves Detection Of Circulating Tumour DNA. bioRxiv. 2017. [Google Scholar]
- 55.Wen F, Shen A, Choi A, Gerner EW, Shi J. Extracellular DNA in pancreatic cancer promotes cell invasion and metastasis. Cancer research. 2013;73(14):4256–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Alekseeva LA, Mironova NL, Brenner EV, Kurilshikov AM, Patutina OA, Zenkova MA. Alteration of the exDNA profile in blood serum of LLC-bearing mice under the decrease of tumour invasion potential by bovine pancreatic DNase I treatment. PLoS One. 2017;12(2):e0171988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bakhoum SF, Ngo B, Laughney AM, Cavallo JA, Murphy CJ, Ly P, et al. Chromosomal instability drives metastasis through a cytosolic DNA response. Nature. 2018;553(7689):467–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gaubatz JW, Flores SC. Tissue-specific and age-related variations in repetitive sequences of mouse extrachromosomal circular DNAs. Mutat Res. 1990;237(1):29–36. [DOI] [PubMed] [Google Scholar]
- 59.Greene J, Baird AM, Brady L, Lim M, Gray SG, McDermott R, et al. Circular RNAs: Biogenesis, Function and Role in Human Diseases. Frontiers in molecular biosciences. 2017;4:38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Allen SE, Hug I, Pabian S, Rzeszutek I, Hoehener C, Nowacki M. Circular Concatemers of Ultra-Short DNA Segments Produce Regulatory RNAs. Cell. 2017;168(6):990–9 e7. [DOI] [PMC free article] [PubMed] [Google Scholar]