Abstract
Purpose
An important, unmet clinical need is for cost-effective, reliable, easy-to-use, and portable retinal photography to evaluate preventable causes of vision loss in children. This study presents the feasibility of a novel smartphone-based retinal imaging device tailored to imaging the pediatric fundus.
Methods
Several modifications for children were made to our previous device, including a child-friendly 3D printed housing of animals, attention-grabbing targets, enhanced image stitching, and video-recording capabilities. Retinal photographs were obtained in children undergoing routine dilated eye examination. Experienced masked retina-specialist graders determined photograph quality and made diagnoses based on the images, which were compared to the treating clinician's diagnosis.
Results
Dilated fundus photographs were acquired in 43 patients with a mean age of 6.7 years. The diagnoses included retinoblastoma, Coats' disease, commotio retinae, and optic nerve hypoplasia, among others. Mean time to acquire five standard photographs totaling 90-degree field of vision was 2.3 ± 1.1 minutes. Patients rated their experience of image acquisition favorably, with a Likert score of 4.6 ± 0.8 out of 5. There was 96% agreement between image-based diagnosis and the treating clinician's diagnosis.
Conclusions
We report a handheld smartphone-based device with modifications tailored for wide-field fundus photography in pediatric patients that can rapidly acquire fundus photos while being well-tolerated.
Translational Relevance
Advances in handheld smartphone-based fundus photography devices decrease the technical barrier for image acquisition in children and may potentially increase access to ophthalmic care in communities with limited resources.
Keywords: portable imaging, fundus photography, smartphone, pediatric retina, wide-field
Introduction
Fundus photography is an immensely valuable tool for disease documentation, treatment monitoring, and patient education. Although traditional fundus cameras acquire high-resolution images of the retina, major limitations of these systems include their bulky and stationary nature, and the need for patients to be cooperative and to be able to maintain an upright seated position during acquisition. These challenges are especially important in children, who are often too young, distractible, and physically small to sit reliably at a tabletop machine. Our goal is to implement refined instrumentation and technological advances that transform fundus photography into a comfortable experience for children. This approach may minimize patient distress and improve accessibility of noninvasive retinal photography in the pediatric patient population.
The ideal device for pediatric fundus photography would be handheld, portable, easy to use, and child friendly. Significant interest already exists in developing portable retinal imaging devices, given their potential in the primary care setting for screening common eye diseases such as diabetic retinopathy.1,2 The Pictor Plus (Volk Optical, Mentor, OH) is an FDA-approved, commercially available portable system that allows both nonmydriatic and mydriatic imaging of the retina and shows utility in determining the presence of optic disc edema3 and diabetic retinopathy.4,5
Smartphone-based photography is an especially attractive approach to retinal imaging as it drastically reduces equipment cost while capitalizing on the high-quality camera and computational capacity of smartphones. Two studies in India evaluated the feasibility and utility of a “fundus on phone” camera system for diabetic retinopathy screening and found high sensitivity and specificity for detecting referral-warranted diabetic retinopathy.6,7
Mobile devices are commonplace in healthcare, and more than 80% of healthcare workers (nurses, doctors) own smartphones such as an iPhone and are familiar with its use.3,4 Furthermore, previous spectral irradiance studies have demonstrated the safety of fundus photography using an iPhone.5 The most basic and cost-effective device involves coupling a +20 diopter (D) lens to a smartphone camera as an indirect ophthalmoscope. With the patient in a supine position, a 20-D lens is held in front of the eye with one hand, and with the other hand, the smartphone's continuous flash is used to both illuminate the retina and capture a video of the fundus.6–10 Several variations on this concept exist and place the smartphone and a condensing lens at a fixed distance apart, with their optical axes aligned to form a digital indirect ophthalmoscope.11–13 Although cost effective, this method requires an operator skilled in indirect ophthalmoscopy. To circumvent this barrier, DigiSight (San Francisco, CA) created a housing for the iPhone and attached a +20-D lens at a fixed distance from the iPhone's camera to allow easier smartphone-based indirect ophthalmoscopy. In contrast, two commercially available devices, the D-Eye (D-Eye, Truckee, CA) and the iExaminer (Welch Allyn, Skaneateles Falls, NY), allow digital direct ophthalmoscopy by connecting either a portable optical device or the panoptic ophthalmoscope to the smartphone to capture images of the retina. While optically simpler, direct ophthalmoscopy offers smaller fields of view that may not be sufficient for all clinical applications. Additionally, smartphone-based fundus photography tools include the InView (Volk Optical, Bengaluru, India) and Fundus On Phone (Remidio, Bengaluru, India) that allow approximately 50-degrees of retinal imaging.
We have previously reported on a compact smartphone-based retinal camera, the Ocular CellScope. The system comprises a mobile phone, a housing that contains the illumination and collection optics, and an integrated phone holder that ensures alignment of the optics with the camera on the phone.14 The device captures a 50-degree field of view of the retina in a single fundus image, and multiple images can be stitched together to generate a wide-field montage photograph of the retina. In the current study, we made several important design modifications to the Ocular CellScope, referred to here as the RetinaScope, which permits a more comfortable imaging experience for children and facilitates rapid acquisition of high-quality photographs of the retina. We show the feasibility of acquiring diagnostic-quality fundus photographs in children (from newborns to adolescents) in a variety of clinical settings, including the outpatient pediatric ophthalmology clinic, emergency room, inpatient ward, and during examination under anesthesia. To our knowledge, this is the first report of wide-field portable smartphone-based fundus photography in children.
Methods
Patient Recruitment and Imaging
Study participants were recruited at the University of Michigan, Kellogg Eye Center and the ophthalmology consultation service at the University of Michigan Hospital and C.S. Mott Children's Hospital (Ann Arbor, MI), in accordance with the University of Michigan Institutional Review Board Committee. The study adhered to the tenets of the Declaration of Helsinki (ClinicalTrials.gov Identifier NCT03076697). Patients younger than 18 years old were included in this study, and enrollment was conducted from May 3, 2017, to January 30, 2018.
All patients in the study underwent a dilated fundus examination by an experienced ophthalmologist as part of a comprehensive ocular examination during their routine clinical care. Following clinical examination, the patient's parents or legal guardian provided informed consent to acquire further images using our device, the RetinaScope. Imaging was performed either in the examination chair or at the bedside, depending on the location of the clinical visit. The examination locations included the outpatient pediatric ophthalmology clinic at the Kellogg Eye Center (Ann Arbor, MI), the C.S. Mott Children's Hospital emergency room, the pediatric inpatient ward, or the operating room during an examination under anesthesia. Two ophthalmology residents (TPP and TNK) and a medical student (GY) acquired the images. For each eye, standard five fields of view images were captured: central, nasal, temporal, inferior, and superior. If the child was too uncooperative or too young to follow directions, a video of the fundus was recorded and later analyzed for extraction of best-quality fundus stills. A good-quality still-frame image needed to be in focus, have minimal glare, and be devoid of nonretinal artifacts that can be introduced by the patient's shifting gaze or from holding the RetinaScope too far away from the eye. The working distance of RetinaScope is approximately 2 cm. The demographic data, clinical findings, and diagnoses of all participants were recorded in a Health Insurance Portability and Accountability Act (HIPAA)–compliant format.
At the conclusion of the study, patients or their parents completed a brief questionnaire on their imaging experience consisting of the following questions (Table 1):
Table 1.
Survey of Patient Experience With the RetinaScope Using a 5-Point Likert Scale, Where 1 = Strongly Disagree and 5 = Strongly Agree
| Average Score (n = 24) |
Standard Deviation |
|
| The length of time for image acquisition was appropriate. | 4.6 | 1.1 |
| The camera flash was not too bright. | 4.3 | 0.8 |
| The device appeared friendly. | 4.5 | 0.9 |
| Overall experience was positive, and I would use the device again. | 4.7 | 1.2 |
Was the length of time for image acquisition appropriate?
Was the brightness of the camera flash tolerable?
Did the device appear child friendly?
Would you choose to have fundus photos taken using the handheld device in the future?
For each question, subjects assigned a score on a 5-point Likert scale, with 1 = strongly disagree, 2 = disagree, 3 = neutral, 4 = agree, 5 = strongly agree. The average and standard deviation scores for each question were computed.
Handheld Mydriatic Fundus Photography in Children
Several revisions to our previously published device14 were made to allow easier imaging.15 These adaptations do not change the safety parameters of the device. Briefly, the optics and electronics component housing were updated to make it more compact and easy to operate with one hand. The device weighs approximately 313 g and consists of a 3D-printed plastic housing containing optics for illuminating and imaging the retina using the camera of a smartphone (iPhone 5s; Apple, Cupertino, CA). The device was powered by a rechargeable lithium battery (Fig. 1A, B). The retina was imaged through a 54-D ophthalmic lens (OI54-A; Ocular Instruments, Bellevue, WA) that forms an intermediate image that was then relayed by a 20-mm focal length achromatic lens (47661; Edmund Optics, Barrington, NJ) to the camera of an iPhone 5s. The autofocus feature of the iPhone camera was used to correct for refractive error.
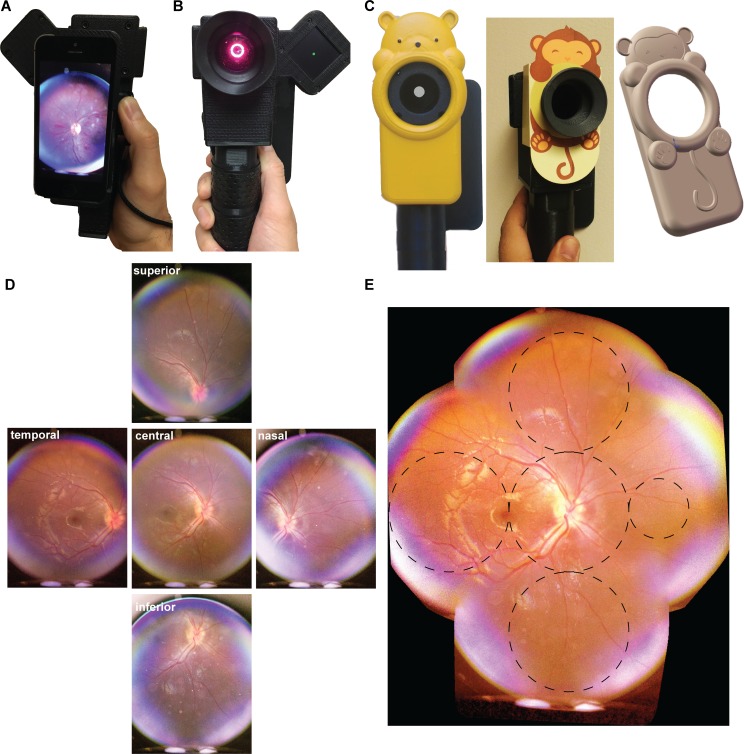
Figure 1.
Smartphone-based pediatric retina fundus camera. (A, B) Front and back view of the device. The device is designed for easy single-handed operation. A detachable magnetic light-emitting diode (LED) display can be mounted on either side of the device and provides a green dot as a fixation target. (C) Custom-made 3D printed and 2D cardboard cutouts designed and created in the shape of child-friendly cartoon and animal characters house the RetinaScope. These designs make the unit more appealing to children and retain their cooperation and attention for a better image quality during a more sustained period of image acquisition time. (D) Each individual image is approximately 50 degrees wide, and by translating the fixation target through a series of positions, five overlapping images are captured with the patient looking in the cardinal directions: primary gaze, up gaze, down gaze, right gaze, and left gaze. (E) These five images are automatically merged together on the smartphone to generate a wide-field 90-degree image of the retina. Nonoverlapping circles that subtend 30 degrees of retina are overlaid on the photograph to approximate the field of view; smaller circle subtends 15 degrees of retinal area.
One of the biggest challenges in indirect ophthalmoscopy and fundus photography in children is poor cooperation due to photophobia when a strong light source is shone into the dilated eye. To improve patient comfort, we used a low-intensity red light to survey and focus on the retina. A reduced white-light flash was then used to capture a fundus image. The red-focusing light-emitting diodes (LEDs) are continuous source while in use (CW) and the white LED flash is an 80-ms duration pulse. The light hazards were assessed based on the ISO 15004-2:2007 standard for optical radiation safety for ophthalmic instruments and were reported in our previous publication.15 Briefly, the red LED produces 0.67 mW at the cornea and illuminates 0.1 cm2 of the cornea and 1 cm2 of the retina when used at 100% (typically we use 50% intensity for pediatric application). The white LED flash can produce up to 19.4-mW peak power with a pulse duration of 80 ms. Typically, we use reduced power for children, ranging from 10% to 40%, depending on the degree of retinal pigmentation. The power of the flash can be controlled via the iPhone app. These levels are more than 100× below the ISO 15004 §5.4 safety limits for Group 1 CW instruments and 25× below the safety limit of pulsed instruments. A magnetically attachable LED fixation screen was attached to the side of the device and used in some examinations to direct gaze and bring different regions of the imaged retina into view. Each photograph captured approximately 50 degrees of the retina with a resolution of 52.3 pixels per retinal degree (Fig. 1D). In previous work, we used a custom-coded algorithm to align and stitch images on the iPhone to generate a wide-field composite photograph. This algorithm was not robust against imaging artifacts and required a high percentage of overlap to generate a montage. In the current work, we integrated a commercially available library for image stitching, which provides more consistent and accurate composite photograph (Fig. 1E). Image stitching can be a challenging task that requires finding points of correspondence between a set of images, aligning the images via translation or rotational transformations, and blending the edges to generate a seamless montage or mosaic. Commercially available software such as i2k Retina (DualAlign LLC, Clifton Park, NY) solves this problem by using an algorithm employing dual-bootstrap iterative closest-point registration.16 We compiled the i2k retina libraries for the iOS operating system and integrated the dual-bootstrap algorithm into our imaging workflow to allow fast and accurate mosaicism directly on the iPhone.
We further improved the exterior appearance of the device to make it more appealing and child friendly. Older children were familiar with smartphone technology and were generally enthusiastic in allowing photographs of their retina to be taken using an iPhone. For younger children, we created several 3D-printed cases in the shape of animals (Dimension SST 1200es 3D printer; Stratasys, Eden Prairie, MN) and custom painted the cases in bright colors in order to disguise the camera-housing elements and attract and maintain the children's attention during image acquisition (Fig. 1C). Children who were too young to follow commands or maintain fixation required a different approach. We built a video acquisition module in our device that allows continuous video recording of the fundus as the child spontaneously shifted gaze. The videos were acquired at 30 frames per second, and typical duration ranged from 1 to 2 minutes. The user was then able to quickly scan through the video and select useful frames for image stitching on the iPhone.
Image Grading
A subset of images and videos (n = 20) comprising a combination of normal images (n = 5) and retinal pathologies (n = 15) were graded by two masked experienced retina specialists (VSD, PL). For patients with who had a video recording, graders were presented with both the unedited video and a montage generated from still frames. Audio was removed from the video recording to eliminate bias. Graders were provided with knowledge of the patient's age, gender, and presenting complaint (e.g., a 10-year-old male with blurred vision). The images were presented in a randomized order. The graders assessed the photograph image quality for making a clinical diagnosis and provided the most likely clinical diagnosis based on the clinical context, the patient's demographic information, and the clinical photographs. The graders had no prior knowledge of the types of retinal pathology that they would be shown. The graders assessed the overall photograph quality (excellent, acceptable, and not gradable) using the following criteria: a photograph was considered excellent if it was in focus and the entire posterior pole was visualized. For consistency, posterior pole is defined as the retina between the optic disc and the temporal arcades and includes the macula and the optic nerve head. A photograph was considered acceptable if it was overexposed, underexposed, or out of focus but adequate to determine the presence or absence of pathology. An ungradable photograph was one in which the image was out of focus or obscured by glare or motion artifact. This grading scheme was adapted from the National Health Service (United Kingdom) Diabetic Grading Forms, which have been used in prior studies of various eye diseases.17–19 If a diagnosis could not be made, the grader provided a detailed description of the photograph. The agreement between the two graders and agreement between a grader and clinical diagnosis were computed as the primary outcome measure of the study.
Statistical Analysis
Statistical analysis was performed using software (JMP version 13; SAS Institute, Cary, NC). Intergrader Cohen's κ statistic and percent agreements were calculated. Images were first graded as either being normal or abnormal (i.e., with retinal pathology) by two masked graders. The sensitivity and specificity of detecting the presence of pathology were calculated for the graders using clinical examination as the ground truth. This endpoint was used because the presence of pathology is cause for a decision to refer to an ophthalmologist in a telemedicine model, which is the most common application of this technology. If an image showed pathology, the graders provided a diagnosis (such as optic disc edema or optic nerve hypoplasia), and the percent agreement between image-based diagnosis and clinical examination-based diagnosis was computed. A χ2 test was used to determine if there was a difference in the grader's ability to arrive at diagnosis based on whether the images were acquired in a clinic setting (awake patient) or during examination under anesthesia.
Results
The primary outcome measure of this study was to determine whether a handheld smartphone-based fundus photography device can acquire quality photographs of diagnostic value in children. Secondary outcome measurements included time to image acquisition and patient comfort during image acquisition.
A total of 43 patients (24 males and 19 females) were recruited in this study (Table 2). The average age of the cohort was 6.7 years (range 6 weeks to 18 years). There were 37 patients aged 0 to 5 years old, four patients aged 5 to 12 years old, and two patients older than 12 years of age. The youngest patient imaged in the clinic setting was 6 weeks old (n = 1), and the youngest patient imaged during examination under anesthesia was 4 months old (n = 2). Of the 43 patients, fundus photographs were acquired in 12 (28%) patients during an examination under anesthesia, 14 (33%) at the bedside during an inpatient/emergency room consult, and 17 (40%) during routine pediatric ophthalmology outpatient clinic visits.
Table 2.
Patient Demographics
| Participants |
Number (%) |
Average Age, y |
Age Range |
Standard Deviation, Age |
| Patients | 43 | 6.7 | 6 wk–18 y | 4.6 y |
| Males | 24 (56) | 7.3 | 6 wk–18 y | 4.5 y |
| Females | 19 (44) | 5.9 | 6 mo–18 y | 4.7 y |
| Clinic setting | 17 (40) | 8.7 | 6 wk–18 y | 7.2 y |
| Examination under anesthesia | 12 (28) | 3.2 | 4 mo–7 y | 2.1 y |
| Inpatient/emergency room consultation | 14 (33) | 8.5 | 4–13 y | 2.9 y |
Patient Experience With Imaging
Images were acquired by two ophthalmology residents and a medical student. One resident and the medical student had no prior training in acquiring fundus photography. The photographers received a 5-minute tutorial on the use of the device and practiced taking photographs on each other under mydriatic conditions.
The average time to acquire a standard five-field photograph of each eye was 2.3 ± 1.1 minute. With increasing user experience, time to acquire photographs decreased, and it took an average of five imaging sessions before the users consistently were able to complete an imaging session in approximately 2 minutes. Six patients, all of whom were younger than 5 years of age, required video recording and subsequent extraction of still-frame photographs. Image alignment of five standard photographs, each with pixel size 3264 × 2448, occurred within 60 seconds on an iPhone 5s to generate a wide-field montage of the retina. When using a video recording, users spent an additional 2 to 5 minutes in selecting still-frame photographs for image alignment. Image quality as assessed by the two masked graders was 5% and 10% as not gradable; 70% and 60% as acceptable, and 25% and 30% as excellent. There was no correlation between image quality and the photographer. A total of two images (one eye of each of two patients) were deemed not gradable. One was of an 11 year old with Coats' disease who had a total retinal detachment with subretinal cholesterol deposits. The RetinaScope was unable to clearly focus on the anteriorly displaced retinal detachment, and reflections from subretinal deposits degraded image quality. The other ungradable image was of a 7-year-old boy with microphthalmia and persistent hyperplastic primary vitreous who had marked corneal and lens media opacity.
Patients or their parents/legal guardians were asked four survey questions at the end of the imaging session to assess their experience with the RetinaScope (Table 1). Patients undergoing examination under anesthesia were excluded from this survey, and the remaining 24 patients agreed to partake in the survey. Of the 24 surveys, 10 were completed by patient's legal guardian, and the remainder completed by the patient. The legal guardian completed the survey when the patient was too young or apprehensive to provide answers. Overall, patients rated their experience with the RetinaScope very favorably. On a 5-point Likert scale, where 1 = strongly disagree and 5 = strongly agree, average responses to surveyed questions were as follows: (1) The length of time for image acquisition was appropriate: mean score 4.6 ± 1.1; (2) The camera flash was not too bright: mean score 4.3 ± 0.8; (3) The device appeared friendly: mean score 4.5 ± 0.9; (4) Overall experience was positive: mean score 4.7 ± 1.2. There was no difference in score ratings whether the patient or legal guardian completed the survey (t-test, P > 0.05).
Fundus Photography With RetinaScope
Most current smartphone-based devices for fundus photography can image the posterior pole but have limited ability to image the midperiphery, which is of great clinical utility. We attached an LED fixation screen to the RetinaScope to direct the patient's gaze in five cardinal positions in order to facilitate imaging of the peripheral retina (Fig. 1). The images were then aligned and directly stitched together on the iPhone to generate approximately an 80- to 90-degree montage of the retina (Fig. 1E). The total degrees of the retina imaged were approximated by assuming 15 degrees of retina from the fovea to the center of the optic disc; nonoverlapping circles of radius equal to the distance between fovea and center of the optic disc were placed on the montage image (Fig. 1E, dashed-line circles). The diameter of each circle covers 30 degrees of the retina so the extent of photographed retina is approximately the number of nonoverlapping circles multiplied by 30 degrees, which is approximately 80 to 90 degrees for RetinaScope. In Figure 1E, the larger dashed circles subtend 30 degrees of retina, and the smaller circle subtends 15 degrees of retina. The montage image covers approximately 90 degrees of retina in the vertical axis and 80 degrees in the horizontal axis.
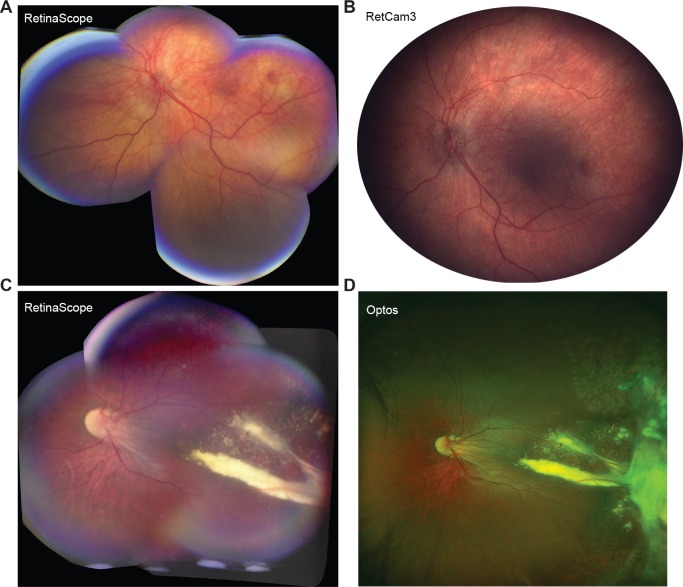
Image quality and the extent of peripheral retina that could be photographed with RetinaScope were compared with the most up-to-date commercially available devices, such as the RetCam3 (Clarity Medical Systems, Pleasanton, CA) and Optos 200Tx (Optos, Inc., Marlborough, MA) (Fig. 2). The image used in comparing RetinaScope to RetCam3 was acquired in a 6-month-old patient with optic nerve hypoplasia during examination under anesthesia. The image comparing RetinaScope to Optos was acquired in an awake 13-year-old patient during the same clinic visit. Although the RetCam3 enables wide-field imaging of the retina (130-degree view), it requires that a coupling agent and a dedicated wide-field lens be applied directly onto the ocular surface for image acquisition. Because of this contact system, the RetCam is mostly limited to use in children undergoing examination under anesthesia or who are too small to resist examination. Compared to the RetCam, the RetinaScope was able to image a similar surface area of the retina and capture diagnostic details of the posterior pole and midperiphery in a noncontact fashion (Fig. 2A, B). Older children who were able to sit at the slit lamp underwent ultra-widefield 200-degree retinal photography with the Optos 200Tx machine. The Optos 200Tx system uses scanning laser ophthalmoscopy to generate a high-resolution, ultra-wide-field image of the retina. In both instances, RetinaScope was able to capture important diagnostic details up to the retinal midperiphery (Fig. 2C, D). The Optos image was able to capture peripheral retinal details such as prior laser scars and areas of retinal fibrosis, which were not imaged with RetinaScope.
Figure 2.
A side-by-side image demonstrating the field of view when comparing the RetinaScope image with the RetCam3 and with the Optos. (A) Montage image of left optic nerve hypoplasia in a 6-month-old male acquired with the RetinaScope. (B) Same patient as in A imaged with the RetCam3. (C) Coats' disease with temporal exudates in an 18-year-old male imaged with the RetinaScope and image stitching. (D) The same patient as in C imaged with the Optos demonstrates similar temporal exudates but also more peripheral findings, such as fibrosis and peripheral laser scars.
Diagnostic Quality of Images
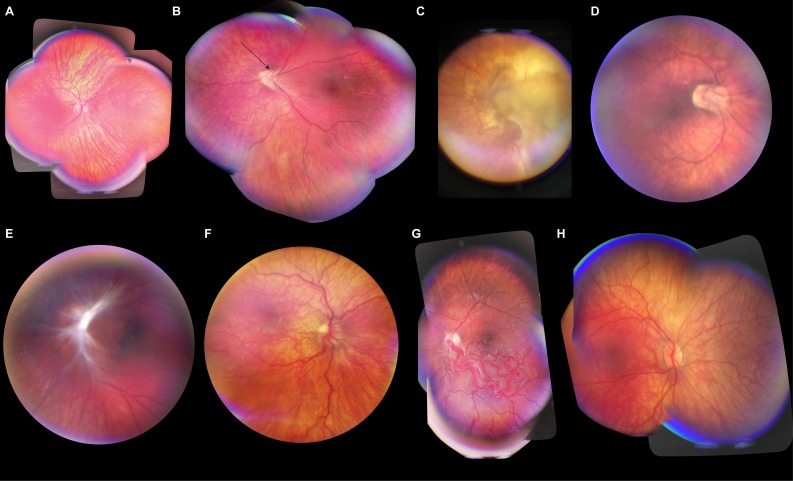
Patients with both normal fundi and posterior segment pathologies were imaged with the RetinaScope under mydriatic conditions. Of the 43 patients, eight did not have posterior segment pathology. Clinical diagnoses included retinoblastoma, Coats' disease, retinopathy of prematurity, nonaccidental trauma, optic nerve hypoplasia, and commotio retinae, among others. The full list of diagnoses is listed in Table 3. Representative photographs of retinal pathology acquired with the RetinaScope are shown in Figure 3. The youngest patient that was imaged in an awake clinic setting was a 6-week-old male with optic disc coloboma.
Table 3.
Posterior Segment Pathologies Imaged With the RetinaScope
| Posterior Segment Pathology |
Patients, n |
| Retinoblastoma | 6 |
| Coats' disease | 3 |
| Familial exudative vitreoretinopathy | 3 |
| Optic disc edema | 3 |
| Retinopathy of prematurity | 3 |
| Optic disc coloboma | 2 |
| Optic nerve hypoplasia/dysplasia | 2 |
| Commotio retinae | 1 |
| Nonaccidental trauma | 1 |
| Ocular albinism | 1 |
| Optic disc pit | 1 |
| Combined hamartoma of the retinal pigment epithelium and retina | 1 |
| Persistent hyperplastic primary vitreous | 1 |
| Racemose angiomatosis | 1 |
| Radiation retinopathy | 1 |
| Terson's syndrome | 1 |
Figure 3.
Representative diagnostic-quality photographs of retinal abnormalities acquired using the RetinaScope in pediatric patients. All images were acquired in a clinic setting unless otherwise noted. A montage image was created for all patients; a 50-degree photograph is presented to highlight retinal pathology. (A) Ocular albinism with foveal hypoplasia in an 11-year-old female. (B) Regressed retinopathy of prematurity (ROP) with abnormal vascular loop (arrow) in a 9-year-old female. (C) Untreated endophytic retinoblastoma in a 5-month-old male; image acquired during examination under anesthesia. (D) Regressed ROP in a 9-year-old male. (E) Combined hamartoma of the retinal pigment epithelium and retina overlying the left optic disc in a 6-year-old male. This is a still-frame image extracted from a video recording. (F) Optic disc dysplasia in a 4-year-old male. (G) Racemose angiomatosis in a 5-year-old male with Wyburn-Mason syndrome; image acquired during examination under anesthesia. (H) Cutis marmorata telangiectasia congenita in a 3-year-old female. Montage was generated from still-frame images extracted from a video recording.
The graders had strong agreement on the presence or absence of posterior segment pathology (κ = 0.93, 95% CI = 0.89–0.97). Grader A had 100% agreement and grader B had 93% agreement between the image-based diagnosis and the final clinical diagnosis. Grader B misdiagnosed a video of retinoblastoma with vitreous seeding as Toxocara and misdiagnosed Coats' disease as familial exudative vitreoretinopathy. Both graders correctly identified images with retinal pathology as being abnormal (100% sensitivity for each grader). The graders occasionally falsely interpreted a normal retinal image as having vascular sheathing, vessel attenuation, or retinal edema. Specificity of determining normal retinal photographs were 85% and 80% for graders A and B, respectively. A χ2 test was performed, and no relationship was found between the imaging setting (i.e., examination under anesthesia versus clinic or bedside setting) and the ability of the grader to arrive at the diagnosis using images acquired with RetinaScope, χ2 (2, N = 43), P > 0.05.
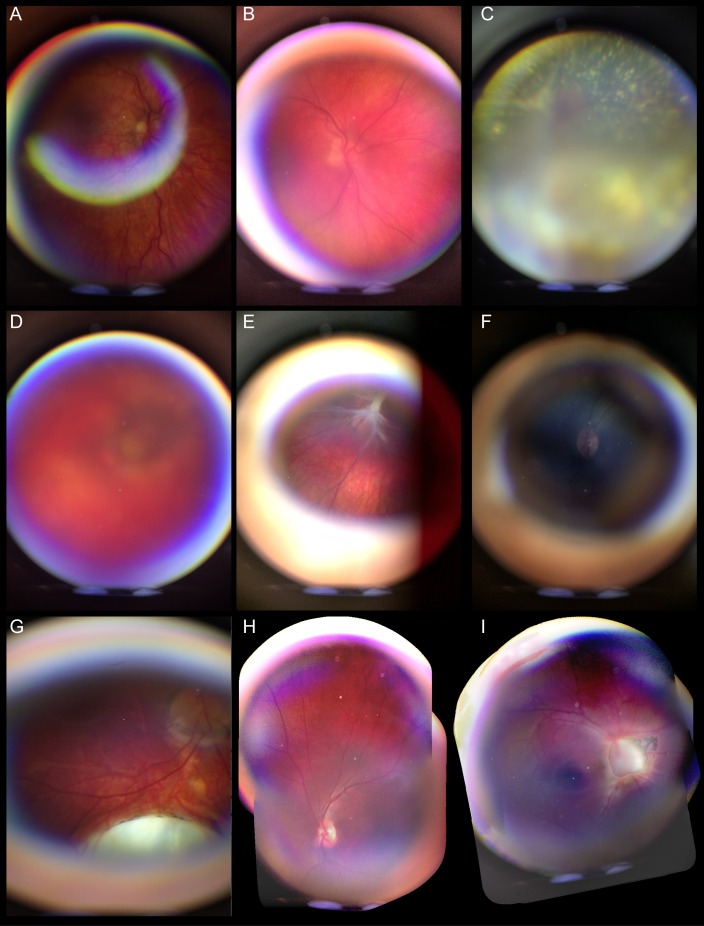
Glare, motion artifact, and poor focus can degrade the quality of any imaging technology. Figure 4 illustrates some of the imaging artifacts that can be introduced with RetinaScope, in addition to an example of retinal photograph in an undilated patient (Fig. 4F).
Figure 4.
Representative photographs of imaging artifacts, poor-quality images, and failure in image montaging. (A) Glare artifact resulting from reflections from the anterior ocular surface. (B) Out of focus image. (C) Out of focus image in a patient with Coats' disease with total retinal detachment and subretinal cholesterol deposits. (D) Poor-quality image in a patient with microphthalmia with media opacity. (E) Still-frame image from a video recording showing a motion artifact resulting from shifting gaze; compare with Figure 3E for a good-quality still-frame image from the same video recording. (F) Nonmydriatic photograph acquired with RetinaScope. (G) Still-frame image from a video recording in which RetinaScope was held too far from the eye, resulting in peripheral ring of artifact and smaller field of view of the retina. (H, I) Two examples of failed image montage as a result of insufficient overlap between individual photographs.
Discussion
Retinal examination in children is challenging, even for experienced ophthalmologists. Fundus photography can be especially difficult in young children who are unable to sit still at a tabletop machine or maintain fixation. We demonstrate child-friendly smartphone-based fundus imaging by employing several helpful features. We find that child-friendly and attractive housing for the device is well-tolerated for imaging younger children. We leverage the smartphone's camera and computational abilities to perform multi-image acquisition and a wide-field montage fully on the phone. Additionally, video recording of the retina is used to capture images in younger, less cooperative patients.
To our knowledge, this is the first report of diagnostic-quality fundus photography using a fully handheld device in awake children as young as 6 weeks old. Prior work demonstrated nonmydriatic fundus photography of the optic nerve in children; however, the authors cited poor image quality and difficulty with image acquisition in children younger than 3 years old.20 The RetinaScope has a 50-degree field of view, but by imaging the retina from five fields (central, nasal, temporal, superior, inferior), we were able to generate a montage of approximately 90 degrees.
The goal of this study was to determine the feasibility of acquiring diagnostic-quality photographs in children using a smartphone-based retinal camera. There was excellent agreement between image-based diagnosis and clinical diagnosis as determined by the graders, who were able to identify the presence of retinal pathology with 100% sensitivity. Specificity was lower, most notably where graders misinterpreted internal limiting membrane sheen or reflections as vascular sheathing or attenuation. These results are amenable to a disease-screening model, where excellent sensitivity is critical and maximizing specificity improves the safe ophthalmic triage of patients.
A limitation of our device is the need to dilate the pupil for good-quality wide-field imaging. The optical design of RetinaScope is such that illumination light from LEDs passes through several optical elements (an aspheric condenser lens, diffuser, polarizer, and an annular mask) to form an annulus with a 4.8-mm inner diameter and 9.6-mm outer diameter at the surface of the cornea. As the light traverses through the eye, it becomes defocused and uniformly illuminates the retina. Given the size of the entrance beam, pupil size of at least 5 mm is needed for good-quality imaging. In addition, our device does not have internal movable optics, and hence the range of fine focus is limited to that of the smartphone's internal camera. The entire device can certainly be moved closer or farther away from the patient's eye for improved imaging. Although we were able to acquire in-focus images of the retina in most cases, large retinal detachment or endophytic retinal tumors could not be imaged in focus with the system. Reflections from calcified retinoblastoma or subretinal deposits also degraded image quality. Our study also did not include patients with aphakia, and it is unclear whether image quality would be further degraded in children with large refractive errors. Additional limitations of this study include its small sample size and the possibility of nongeneralizability of sensitivity and specificity results. To our knowledge, this is the first report of smartphone-based, portable fundus photography in awake children, and future work will determine the utility of this device to screen for common retinal diseases in a larger, outpatient clinical setting.
In summary, handheld fundus photography devices such as the RetinaScope significantly decrease the technical barrier for image acquisition in children and may potentially increase access to ophthalmic care in communities with limited resources. One potential application of RetinaScope may be in telemedicine-based screening for pediatric eye diseases such as diabetic retinopathy in children and adolescents. Type 1 diabetes mellitus (T1DM) is one of the most common metabolic disorders in children, with an estimated prevalence of 1 per 300 in the United States, and accounts for >85% of all diabetes cases in patients <20 years of age worldwide.21 Data on the epidemiology of diabetic retinopathy in youths is limited, but a recent study found that 6% of youths (aged <20 years) with T1DM and 9% with type 2 diabetes had diabetic retinopathy.22 Guidelines recommend that screening for diabetic retinopathy should begin about 4 to 5 years after the diagnosis of diabetes once the patient is older than 8 to 9 years of age. Unfortunately, many children with diabetes do not receive the guideline-recommended eye screenings. A recent retrospective study of 5453 patients with T1DM aged 21 years or younger found that only 65% of patients underwent an eye examination by 6 years after initial diagnosis of diabetes.23 Rates of eye screenings are even lower for racial minorities and patients from less-affluent families. We may be able to improve the implementation of guidelines for eye screening among youths with diabetes by providing retinal photography in the pediatrician's or pediatric endocrinologist's clinics.24 The images can be acquired in the primary care setting and may be interpreted in either an automated fashion or via a remote grading site for referral-warranted diabetic retinopathy. Future work will explore the feasibility of incorporating the RetinaScope in the workflow of an outpatient primary care clinic for diabetic retinopathy screening. Smartphone-based fundus photography devices may provide a cost-effective and portable alternative to screen for vision-threatening diseases in children.
Acknowledgments
The authors thank Yuxin (Horatio) Han (Cohu Lab, LLC, Boston, MA), Daniel Morgan and David Kim (Co-Works, Rhode Island School of Design, Providence, RI) for their work in creating innovative and child-friendly cases for the RetinaScope. Jason Ardell contributed to smartphone app development, and DualAlign LLC (Clifton Park, NJ) provided C++ libraries for image stitching.
Supported by the Knights Templar Eye Foundation Career-Starter Research Grant (TPP, TNK, YMP), the University of Michigan Translational Research and Commercialization for Life Sciences (TNK, YMP), the University of Michigan Center for Entrepreneurship Dean's Engineering Translational Prototype Research Fund (TNK, YMP), the QB3 Bridging the Gap Award from the Rogers Family Foundation (DAF), the Bakar Fellows Award (DAF), the Chan-Zuckerberg Biohub Investigator award (DAF), the National Eye Institute Grants 1K08EY027458 (YMP) and 4K12EY022299 (YMP), the University of Michigan Department of Ophthalmology and Visual Sciences, and unrestricted departmental support from Research to Prevent Blindness.
DAF is a cofounder of CellScope, Inc., a company commercializing a cellphone-based otoscope, and holds shares in CellScope, Inc. DAF and TNK are inventors on U.S. patents and related applications pertaining to a Retinal CellScope Apparatus.
Disclosure: T.P. Patel, None; T.N. Kim, P; G. Yu, None; V.S. Dedania, None; P. Lieu, None; C.X. Qian, None; C.G. Besirli, None; H. Demirci, None; T. Margolis, P; D.A. Fletcher, CellScope Inc. (I, S) P; Y.M. Paulus, None
References
- 1.Micheletti JM, Hendrick AM, Khan FN, Ziemer DC, Pasquel FJ. Current and next generation portable screening devices for diabetic retinopathy. J Diabetes Sci Technol. 2016;10:295–300. doi: 10.1177/1932296816629158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bolster NM, Giardini ME, Bastawrous A. The diabetic retinopathy screening workflow: potential for smartphone imaging. J Diabetes Sci Technol. 2015;10:318–324. doi: 10.1177/1932296815617969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Teichman JC, Sher JH, Ahmed II. From iPhone to eyePhone: a technique for photodocumentation. Can J Ophthalmol. 2011;46:284–286. doi: 10.1016/j.jcjo.2011.05.016. [DOI] [PubMed] [Google Scholar]
- 4.Ventola CL. Mobile devices and apps for health care professionals: uses and benefits. PT. 2014;39:356–364. [PMC free article] [PubMed] [Google Scholar]
- 5.Hong SC, Wynn-Williams G, Wilson G. Safety of iPhone retinal photography. J Med Eng Technol. 2017;41:165–169. doi: 10.1080/03091902.2016.1264491. [DOI] [PubMed] [Google Scholar]
- 6.Ademola-Popoola DS, Olatunji VA. Retinal imaging with smartphone. Niger J Clin Pract. 2017;20:341–345. doi: 10.4103/1119-3077.201428. [DOI] [PubMed] [Google Scholar]
- 7.Lord RK, Shah VA, San Filippo AN, Krishna R. Novel uses of smartphones in ophthalmology. Ophthalmology. 2010;117;:1274–1274.e3. doi: 10.1016/j.ophtha.2010.01.001. [DOI] [PubMed] [Google Scholar]
- 8.Bastawrous A. Smartphone fundoscopy. Ophthalmology. 2012;119:432–433.e432. doi: 10.1016/j.ophtha.2011.11.014. author reply 433. [DOI] [PubMed] [Google Scholar]
- 9.Haddock LJ, Kim DY, Mukai S. Simple, inexpensive technique for high-quality smartphone fundus photography in human and animal eyes. J Ophthalmol. 2013. 518479. [DOI] [PMC free article] [PubMed]
- 10.Nazari Khanamiri H, Nakatsuka A, El-Annan J. Smartphone fundus photography. J Vis Exp. 2017;125:e55958. doi: 10.3791/55958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Raju B, Raju NS, Akkara JD, Pathengay A. Do it yourself smartphone fundus camera—DIYretCAM. Indian J Ophthalmol. 2016;64:663–667. doi: 10.4103/0301-4738.194325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sharma A, Subramaniam SD, Ramachandran KI, Lakshmikanthan C, Krishna S, Sundaramoorthy SK. Smartphone-based fundus camera device (MII Ret Cam) and technique with ability to image peripheral retina. Eur J Ophthalmol. 2016;26:142–144. doi: 10.5301/ejo.5000663. [DOI] [PubMed] [Google Scholar]
- 13.Ludwig CA, Murthy SI, Pappuru RR, Jais A, Myung DJ, Chang RT. A novel smartphone ophthalmic imaging adapter: user feasibility studies in Hyderabad, India. Indian J Ophthalmol. 2016;64:191–200. doi: 10.4103/0301-4738.181742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Maamari RN, Keenan JD, Fletcher DA, Margolis TP. A mobile phone-based retinal camera for portable wide field imaging. Br J Ophthalmol. 2014;98:438–441. doi: 10.1136/bjophthalmol-2013-303797. [DOI] [PubMed] [Google Scholar]
- 15.Kim TN, Myers F, Reber C, et al. A smartphone-based tool for rapid, portable, and automated wide-field retinal imaging. Trans Vis Sci Tech. 2018;7:21. doi: 10.1167/tvst.7.5.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stewart CV, Tsai CL, Roysam B. The dual-bootstrap iterative closest point algorithm with application to retinal image registration. IEEE Trans Med Imaging. 2003;22:1379–1394. doi: 10.1109/TMI.2003.819276. [DOI] [PubMed] [Google Scholar]
- 17.Davila JR, Sengupta SS, Niziol LM, et al. Predictors of photographic quality with a handheld nonmydriatic fundus camera used for screening of vision-threatening diabetic retinopathy. Ophthalmologica. 2017;238:89–99. doi: 10.1159/000475773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sengupta S, Sindal MD, Besirli CG, et al. Screening for vision-threatening diabetic retinopathy in South India: comparing portable non-mydriatic and standard fundus cameras and clinical exam. Eye (Lond) 2018;32:375–383. doi: 10.1038/eye.2017.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Woodward MA, Bavinger JC, Amin S, et al. Telemedicine for ophthalmic consultation services: use of a portable device and layering information for graders. J Telemed Telecare. 2017;23:365–370. doi: 10.1177/1357633X16634544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Toffoli D, Bruce BB, Lamirel C, Henderson AD, Newman NJ, Biousse V. Feasibility and quality of nonmydriatic fundus photography in children. J AAPOS. 2011;15:567–572. doi: 10.1016/j.jaapos.2011.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Maahs DM, West NA, Lawrence JM, Mayer-Davis EJ. Epidemiology of type 1 diabetes. Endocrinol Metab Clin North Am. 2010;39:481–497. doi: 10.1016/j.ecl.2010.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dabelea D, Mayer-Davis EJ, Saydah S, et al. Prevalence of type 1 and type 2 diabetes among children and adolescents from 2001 to 2009. JAMA. 2014;311:778–1786. doi: 10.1001/jama.2014.3201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang SY, Andrews CA, Gardner TW, Wood M, Singer K, Stein JD. Ophthalmic screening patterns among youths with diabetes enrolled in a large US managed care network. JAMA Ophthalmol. 2017;135:432–438. doi: 10.1001/jamaophthalmol.2017.0089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Garg S. Diabetic retinopathy screening with telemedicine: a potential strategy to engage our youth. JAMA Ophthalmol. 2017;135:438–439. doi: 10.1001/jamaophthalmol.2017.0150. [DOI] [PubMed] [Google Scholar]