Version Changes
Revised. Amendments from Version 3
In the version 4 of the manuscript, a sentence on the indoor/outdoor distribution of malaria vectors was rephrased in order to improve its readability by the reader. A typo “underestimated” was changed to “overestimated”. In the ‘Shift in vector-control intervention’ section, “16S ribosomal RNA genes” was changed to “mammalian mt16S ribosomal RNA genes”.
Abstract
Background: The Thailand-Myanmar borderland is an area endemic for malaria where transmission is low, seasonal and unstable. The epidemiology has been described but there is relatively few data on the entomological determinants of malaria transmission.
Methods: Entomological investigations were conducted during 24 months in four villages located in Kayin state, on the Myanmar side of the Thailand-Myanmar border. Anopheles mosquitoes were identified by morphology, and molecular assays were used in order to discriminate between closely related sibling species of malaria vectors. Plasmodium infection rate was determined using quantitative real-time PCR.
Results: The diversity of Anopheles mosquitoes was very high and multiple species were identified as malaria vectors. The intensity of human-vector contact (mean human-biting rate= 369 bites/person/month) compensates for the low infection rate in naturally infected populations of malaria vectors (mean sporozoite index= 0.04 and 0.17 % for P. falciparum and P. vivax respectively), yielding intermediary level of transmission intensity (mean entomological inoculation rate= 0.13 and 0.64 infective bites/person/month for P. falciparum and P. vivax, respectively). Only 36% of the infected mosquitoes were collected indoors between 09:00 pm and 05:00 am, suggesting that mosquito bed-nets would fail to prevent most of the infective bites in the study area.
Conclusion: This study provided a unique opportunity to describe the entomology of malaria in low transmission settings of Southeast Asia. Our data are important in the context of malaria elimination in the Greater Mekong Subregion.
Keywords: Anopheles, human biting rate, sporozoite index, entomological inoculation rate, parasite load, residual transmission, Plasmodium juxtanucleare, zoophagy, exophagy, hypnozoite reservoire
Introduction
Artemisinin resistance in Plasmodium falciparum has emerged and spread in the Greater Mekong Sub-region (GMS) 1, leading to the failure of several artemisinin-based combination therapies (ACTs) 2. Multi-drug resistant parasites spreading from Western Cambodia are responsible for a recent resurgence of the disease across the eastern part of the GMS 3. Meanwhile in Myanmar (western GMS), the incidence of clinical malaria cases has declined 4. In this area, dihydroartemisinin-piperaquine and artemether-lumefantrine remain effective against P. falciparum. It is therefore urgent to eliminate falciparum malaria in Myanmar, the main gateway to India and Bangladesh, before these two ACTs also fall to resistance.
Entomological aspects of malaria transmission are important in the context of elimination as they largely determine intervention design and outcome. For example, the interest of treating asymptomatic infections with mass drug administration or mass screening and treatment obviously depends on the contribution of asymptomatic carriers to the transmission 5, 6. Moreover, the efficacy of vector-control interventions such as mass distribution of long-lasting insecticide-impregnated mosquito bed-nets (LLINs) and indoor residual spraying (IRS) campaigns is greatly influenced by the ecology of malaria vectors 7– 9.
The transmission of P. falciparum is low, seasonal and unstable in Kayin state (Eastern Myanmar) 10. Some entomological surveys were conducted previously 11– 15, most of them on the Thai side of the Thailand-Myanmar border, where the transmission of P. falciparum is now interrupted. In this area, the primary vectors are Anopheles minimus ( s.s.) (Minimus Complex, Funestus Group), An. maculatus ( s.s.), An. sawadwongporni (Maculatus Group), An. dirus ( s.s.) and An. baimaii (Dirus Complex, Leucosphyrus Group) 11, 13, 15. Anopheles pseudowillmori (Maculatus Group) 16, An. aconitus ( s.s.) (Funestus Group) 17 and some members in the Annularis and Barbirostris Groups 13 also play a secondary role in the transmission ( Table 1). Numerous aspects of malaria vector ecology and biology have not been documented and the characteristics of the entomological indices are not known precisely 18.
Table 1. Vectorial status of the Anopheles species most frequently collected on the Thailand-Myanmar border.
| Group | Subgroup | Complex | Species | Vectorial status on the TMB |
|---|---|---|---|---|
| Annularis | An. annularis ( s.l.) | secondary vector | ||
| Barbirostris | An. barbirostris ( s.l.) | secondary vector † | ||
| Funestus | Minimus | Minimus | An. minimus ( s.s.) | primary vector |
| An. harrisoni | unknown | |||
| Aconitus | An. aconitus ( s.s.) | secondary vector | ||
| An. pampanai | unknown | |||
| An. varuna | unknown | |||
| Culicifacies | Culicifacies | An. culicifacies ( s.l.) | non vector ‡ | |
| Hyrcanus | An. hyrcanus ( s.l.) | non vector ‡ | ||
| Jamesii | An. jamesii ( s.l.) | non vector ‡ | ||
| Kochi | An. kochi | non vector | ||
| Leucosphyrus | Leucosphyrus | Dirus | An. dirus ( s.s.) | primary vector |
| An. baimaii | primary vector | |||
| An. cracens | unknown | |||
| An. nemophilous | unknown | |||
| An. scanloni | unknown | |||
| Maculatus | Maculatus | An. maculatus ( s.s.) | primary vector | |
| An. dravidicus | unknown | |||
| Sawadwongporni | An. sawadwongporni | primary vector | ||
| An. notanandai | unknown | |||
| An. rampae | unknown | |||
| Unclassified | An. willmori | unknown | ||
| An. pseudowillmori | secondary vector | |||
| Subpictus | An. subpictus ( s.l.) | non vector ‡ | ||
| Tessellatus | An. tessellatus | non vector ‡ |
† Plasmodium vivax ‡ Some species in these Groups are efficient malaria vectors elsewhere, although they were never found infected with human malaria parasites in the Thailand-Myanmar border area ( e.g. An. culicifacies A, An. sinensis, An. subpictus (s.s.), An. splendidus and An. tessellatus) 18, 21, 22.
Large-scale deployment of community-wide access to early diagnosis and treatments has paved the way for the elimination of falciparum malaria in Kayin state. Mass antimalarial drug administration campaigns were used as an accelerator to elimination in villages with submicroscopic reservoirs of asymptomatic malaria infections 6, 19, 20. The objective of this study was to describe the entomological determinants of malaria transmission in Kayin state in order to guide policy making for malaria elimination.
Methods
Study villages
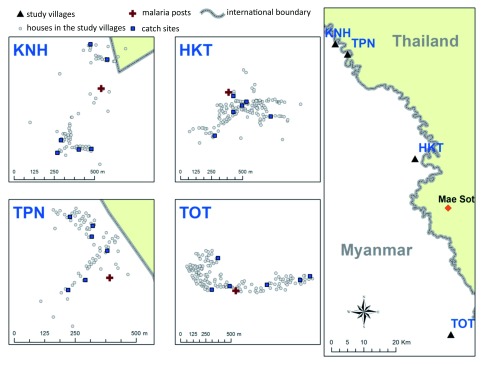
Four villages located in Kayin state were included in the study, namely HKT (16° 85 'N, 98° 47' E), KNH (17° 18 'N, 98° 24' E), TPN (17° 14' N, 98 ° 29' E) and TOT (16° 36' N, 98° 57' E) ( Figure 1). The demographics and baseline malaria epidemiology in the study villages were described previously 6, 15, 19. Briefly, the number of households at baseline was 160, 81, 138, and 69 in HKT, KNH, TOT and TPN respectively. The census population at baseline was 908, 349, 745 and 375 at HKT, KNH, TOT and TPN respectively. Residents were mainly farmers and forest workers. The prevalence of submicroscopic malaria at the beginning of the study ranged between 4 and 22% and between 19 and 33% for P. falciparum and P. vivax respectively.
Figure 1. Map of the study sites.
Intervention
Community-wide access to early diagnosis and treatments was deployed in all villages for the entire period of the study. Mass antimalarial drug administration campaigns with dihydroartemisinin-piperaquine and a single low dose of primaquine were repeated at monthly intervals for 3 months from 12 June to 24 August 2013 in KNH, from 27 May to 07 August 2013 in TOT, from 28 January to 29 March 2014 in TPN and from 01 April to 10 June 2014 in HKT. LLINs were also distributed to all villagers. The intervention and its impact on the parasitological and entomological indices of malaria were described into more details in Chaumeau et al. 6 and Landier et al. 19.
Entomological surveys
Villages were surveyed monthly from 2013 to 2015 for a total of 21 surveys per village. Entomological surveys consisted of five consecutive nights of collection from 06:00 pm to 06:00 am in five houses per village and on one cow, as described previously 15. There were two exceptions to the initial study design: the eleventh survey in TPN was cancelled and mosquitoes were collected for only two nights during the second survey in HKT ( Supplementary File 1, Table S1). In each village, five traditional houses were randomly selected for mosquito sampling using the human-landing catch (HLC) collection method. In each house, one mosquito collector sat indoors and one mosquito collector sat outdoors, yielding a total of 50 person-nights of collection per survey (25 person-nights indoors and 25 person-nights outdoors). Collectors were asked to collect every mosquito landing on their uncovered legs for 50 min per hour and allowed to rest for 10 min per hour. A cow-bait trap (CBT) was also set-up yielding an additional 5 cow-nights of collection. One cow was isolated from the herd and a 1m-wide mosquito net was fenced around the animal, 30cm above the ground level. One collector was asked to collect mosquitoes resting on the net for 50 min per hour and allowed to rest for 10 min. Mosquitoes were shipped at the Shoklo Malaria Research Unit (Mae Sot, Thailand) at the end of each night of collection.
Laboratory procedures for the processing of entomological samples
Mosquitoes were immediately identified at the genus level by morphology and Anopheles specimens were stored individually at -20°C in 1.5 mL plastic tubes containing silica gel. Anopheles mosquitoes were identified at the Group or Complex level using the key developed by Rattanarithikul et al. 23. Deoxyribonucleic acid (DNA) was extracted from head/thorax using a cetyltrimethyl ammonium bromide-based method described previously 24. Sibling species to the Funestus, Maculatus and Leucosphyrus Groups were discriminated using allele-specific polymerase chain reaction (AS-PCR) assays adapted from Garros et al. and Walton et al. 25– 27. In case AS-PCR yielded a negative result, identification at the species level was performed by sequencing the internal transcribed spacer 2 (ITS2) DNA marker using universal primers described by Beebe and Saul 28. DNA extracts were screened for the presence of Plasmodium sporozoites using a quantitative real-time PCR (qPCR) assay targeting 18S rRNA genes adapted from Mangold et al. 29. Specificity of the signal was confirmed in all positive samples by amplifying COX genes with primers described by Cunha et al. 30. In case the confirmation assay yielded a negative result, the PCR product of the screening assay was sequenced (BioSample accessions: SAMN09845988, SAMN09845989, SAMN09845990, SAMN09845991, SAMN09845992). The presence of Plasmodium oocysts in the abdomen of sporozoites-positive specimens was assessed using the same procedures. Abdomen were preserved at -20°C in 1.5 mL plastic tubes containing silica gel during the time between the screening of head/thorax for sporozoites and the processing of abdomen from sporozoite-positive specimens. The validation of the qPCR assays used for Plasmodium detection in this study was published elsewhere 24. Detailed laboratory procedures are presented in the Supplementary File 2.
Data analysis
HBR and CBR were defined as the number of mosquitoes collected divided by the number of person-nights or cow-nights respectively. Results were expressed as a number of bites/person/month or bites/cow/month. Sporozoite index (SI) was defined as the number of mosquitoes positive in qPCR Plasmodium divided by the total number of mosquitoes analyzed. Results were expressed as a percentage. Entomological inoculation rate (EIR) was defined as the number of specimens positive in qPCR Plasmodium divided by the corresponding number of person-nights of collection 31. The number of person-nights used for the calculation of EIR was multiplied by the proportion of collected mosquitoes that were analyzed with qPCR Plasmodium in order to take into account that not all mosquitoes were analysed by qPCR 6, 19. Results were expressed as a number of infective bites/person/month. The exophagy index (EI) was defined as the outdoor HBR divided by the sum of the indoor and outdoor HBRs. The cow-biting index (CBI) was defined as CBR divided by the sum of the HBR and the CBR. Confidence Intervals (CIs) for Poisson counts (HBR, CBR and EIR) were estimated using the exact method of the poisson.conf.int() function in the epitools package version 05-10 of R software version 3.3. Binomial CIs were estimated for proportions (SI, EI and CBI) using the exact method of the binom.confint() function in the binom package version 1.1-1 of R software version 3.3. Species-specific proportions were used to compute HBR, CBR, EI and CBI estimates at the species level if ≥30 samples in the corresponding Anopheles Group were genotyped for each value of the grouping variable. The grouping variables were the collection time (06:00 pm to 06:00 am), the collection method (HCL or CBT) or the location of the mosquito collector (indoors or outdoors). For example, the number of An. maculatus ( s.l.) collected by HLC indoors and outdoors was 2240 and 5041 respectively. The proportion of An. maculatus ( s.s.) in the indoor and outdoor populations was 0.34 (133/392) and 0.37 (404/1084) respectively. The predicted number of An. maculatus ( s.s.) collected by HLC indoors and outdoors was 762 and 1865 respectively. The EI estimate for An. maculatus ( s.s.) was therefore 0.71 (1865/2627).
Ethics approval
The protocol for mosquito collection and analysis has been approved by the Oxford Tropical Research Ethics Committee (1015–13, dated 29 Apr 2013) and by the Ethics Review Committee for Research Involving Human Research Subjects, Health Science Group, Chulalongkorn University (COA 154/2014). All participants provided their written consent to participate in this study. This consent procedure was approved by the ethics committees.
Results
Anopheles diversity
In total, 129228 Anopheles mosquitoes were collected during 4120 person-nights and 412 cow-nights of collection (63217 by HLC and 66011 by CBT). We report the occurrence of 10 Groups of Anopheles on the basis of morphological identification: Barbirostris, Hyrcanus ( Anopheles Subgenus, Myzorynchus Series), Annularis, Jamesii, Maculatus ( Cellia Subgenus, Neocellia Series), Funestus ( Cellia Subgenus, Myzomyia Series), Kochi, Leucosphyrus, Tessellatus ( Cellia Subgenus, Neomyzomyia Series) and Subpictus ( Cellia Subgenus, Pyretophorus Series). Anopheles karwari ( Cellia Subgenus, Neocellia Series) was also detected at a low frequency. Less than 5% (6010/129228) of the specimens could not be identified at the Group level because they were damaged (missing legs or wings).
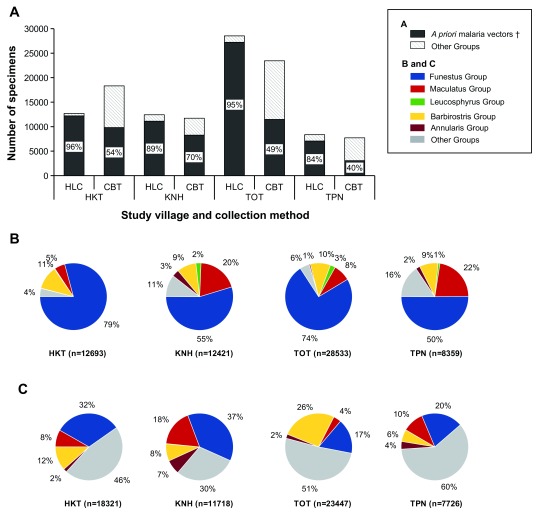
A priori malaria vectors in the Annularis, Barbirostris, Funestus, Leucosphyrus and Maculatus Groups ( i.e. Anopheles taxa that were previously reported to be infected with human malaria parasites in the Thailand-Myanmar border area) accounted for 84–96% and for 40–70% of the Anopheles mosquitoes collected by HLC and CBT collection methods respectively ( Figure 2). The abundance of malaria vectors was significantly different from one collection site to another in a given village, suggesting that local ecological factors are important drivers of exposure to malaria vectors ( Supplementary File 1, Figures S1–S4). The proportion of collection-night during which a given Anopheles taxa was collected varied between 3% and 75% in the Subpictus and Funestus Groups respectively ( Supplementary File 1, Figures S5–S6). The Funestus Group was the most abundant taxa during both the rainy and dry seasons (June to November and December to May, respectively). The Maculatus and Leucosphyrus Groups were mainly collected during the rainy season. The abundance of Annularis and Barbirostris Groups peaked during the transition period between the rainy and dry seasons, when the abundance of other groups decreased ( Supplementary File 1, Figures S7–S10).
Figure 2. Biodiversity of the Anopheles mosquitoes according to the collection method and study village.
A) Proportion of malaria vectors in Anopheles populations collected by human-landing catch (HLC) and cow bait trap (CBT) collection methods according to the village. B) Relative proportions of sensu lato malaria vectors collected by HLC according to the village. C) Relative proportions sensu lato malaria vectors collected by CBT according to the village. † A priori malaria vectors refers to Anopheles taxa that were previously reported to be infected with human malaria parasites in the Thailand-Myanmar border area, i.e. the Funestus, Maculatus, Leucosphyrus, Barbirostris and Annularis Groups 11– 17.
Anopheles minimus ( s.s.) was by far the most abundant species among the Funestus Group ( Table 2). Its sibling An. harrisoni and closely related members from the Aconitus and Culicifacies Subgroups represented <0.5% and 13% of the specimens from the Funestus populations collected by HLC and CBT, respectively. Aconitus and Culicifacies Subgroups represented up to 12% of the Funestus specimens in TPN ( Supplementary File 1, Table S2). Anopheles maculatus ( s.s.), An. sawadwongporni and An. pseudowillmori were the most abundant species in the Maculatus Group with proportion varying from 15 to 50%, 40 to 81% and 3.6 to 9% according to the village. Three species from the Dirus Complex accounted for >99% of the specimens in the Leucosphyrus Group, namely An. dirus ( s.s.) An baimaii and An. cracens. The proportion of An. dirus ( s.s.) and An baimaii in study villages varied from 60 to 97% and from 0 to 39%, respectively. The proportion of An. culicifacies B, An. maculatus ( s.s.) and An. cracens increased during the dry season whereas that of An. minimus ( s.s.), An. pseudowillmori and An. baimaii increased during the rainy season ( Supplementary File 1, Table S3).
Table 2. Molecular identification of sibling species among the Funestus, Maculatus and Leucosphyrus Groups.
| Group | Collection method
(% of collected specimen analyzed by PCR) |
Species | n/N | Relative proportion
estimate |
95%CI |
|---|---|---|---|---|---|
| Funestus | HLC (3294/42283=8%) | An. minimus ( s.s.) | 3277/3294 | 99.5 | 99.2-99.7 |
| An. aconitus ( s.s.) | 7/3294 | 0.2 | 0.1-0.4 | ||
| An. culicifacies B | 6/3294 | 0.2 | 0.1-0.4 | ||
| An. harrisoni | 2/3294 | 0.1 | 0-0.2 | ||
| An. varuna | 2/3294 | 0.1 | 0-0.2 | ||
| An. pampanai | 0/3294 | 0 | - | ||
| CBT (1543/15728=10%) | An. minimus ( s.s.) | 1342/1543 | 87 | 85.2-88.6 | |
| An. culicifacies B | 90/1543 | 5.8 | 4.7-7.1 | ||
| An. varuna | 59/1543 | 3.8 | 2.9-4.9 | ||
| An. aconitus ( s.s.) | 42/1543 | 2.7 | 2-3.7 | ||
| An. pampanai | 9/1543 | 0.6 | 0.3-1.1 | ||
| An. harrisoni | 1/1543 | 0.1 | 0-0.4 | ||
| Maculatus | HLC (1476/7281=20%) | An. sawadwongporni | 819/1476 | 55.5 | 52.9-58 |
| An. maculatus ( s.s.) | 537/1476 | 36.4 | 33.9-38.9 | ||
| An. pseudowillmori | 114/1476 | 7.7 | 6.4-9.2 | ||
| An. dravidicus | 6/1476 | 0.4 | 0.1-0.9 | ||
| CBT (1491/5239=28%) | An. sawadwongporni | 975/1491 | 65.4 | 62.9-67.8 | |
| An. maculatus ( s.s.) | 439/1491 | 29.4 | 27.1-31.8 | ||
| An. pseudowillmori | 74/1491 | 5 | 3.9-6.2 | ||
| An. dravidicus | 3/1491 | 0.2 | 0-0.6 | ||
| Leucosphyrus | HLC (856/1144=77%) | An. baimaii | 643/856 | 75.1 | 72.1-78 |
| An. dirus ( s.s.) | 205/856 | 23.9 | 21.1-27 | ||
| An. cracens | 5/856 | 0.6 | 0.2-1.4 | ||
| An. balabacensis | 3/856 | 0.4 | 0.1-1 | ||
| CBT (29/46=57%) | An. dirus ( s.s.) | 14/29 | 48.3 | 29.4-67.5 | |
| An. baimaii | 10/29 | 34.5 | 17.9-54.3 | ||
| An. cracens | 5/29 | 17.2 | 5.8-35.8 | ||
| An. balabacensis | 0/29 | 0 | - |
Malaria vectors
The contribution of six Groups of Anopheles to malaria transmission was determined by processing 56872 samples collected by HLC in qPCR Plasmodium. Human malaria parasites P. falciparum and P. vivax were detected in 106 Anopheles specimens that belonged to four Groups of Anopheles species (Funestus, Maculatus, Leucosphyrus and Barbirostris) ( Supplementary File 1, Table S4). Both P. falciparum and P. vivax were detected in the Funestus, Leucosphyrus and Maculatus Groups, whereas only P. vivax was detected in the Barbirostris Group. One specimen of the Funestus Group was co-infected with both P. falciparum and P. vivax. The Funestus Group was by far the most important taxa contributing to malaria transmission (Pf-EIR= 0.1 and Pv-EIR= 0.6 infective bites/person/month) followed by the Maculatus, Leucosphyrus and Barbirostris Groups ( Table 3). Due to the relatively low sample size analyzed in the Annularis and Subpictus Groups (747 and 126 respectively), it was not possible to rule out their contribution to malaria transmission.
Table 3. Entomological indices of malaria transmission presented per Group of Anopheles.
| Group | HBR
(b/p/m) |
Pf-SI
(%) |
Pv-SI
(%) |
Pf-EIR
(ib/p/m) |
Pv-EIR
(ib/p/m) |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n/N | Estimate | 95%CI | n/N | Estimate | 95%CI | n/N | Estimate | 95%CI | n/N | Estimate | 95%CI | n/N | Estimate | 95%CI | |
| Funestus | 42283/4120 | 307.9 | 305-310.8 | 13/41797 | 0.03 | 0.02-0.05 | 77/41797 | 0.18 | 0.15-0.05 | 13/4073 | 0.1 | 0.05-0.16 | 77/4073 | 0.57 | 0.45-0.71 |
| Maculatus | 7281/4120 | 53 | 51.8-54.2 | 4/7178 | 0.06 | 0.02-0.14 | 7/7178 | 0.1 | 0.04-0.14 | 4/4062 | 0.03 | 0.01-0.08 | 7/4062 | 0.05 | 0.02-0.11 |
| Leucosphyrus | 1144/4120 | 8.3 | 7.9-8.8 | 1/1107 | 0.09 | 0-0.5 | 3/1107 | 0.27 | 0.06-0.5 | 1/3987 | 0.01 | 0-0.04 | 3/3987 | 0.02 | 0-0.07 |
| Barbirostris | 6098/4120 | 44.4 | 43.3-45.5 | 0/5917 | 0 | 0-0.06 | 2/5917 | 0.03 | 0-0.06 | 0/3998 | 0 | 0-0.03 | 2/3998 | 0.02 | 0-0.05 |
| Annularis | 772/4120 | 5.6 | 5.2-6 | 0/747 | 0 | 0-0.49 | 0/747 | 0 | 0-0.49 | 0/3987 | 0 | 0-0.03 | 0/3987 | 0 | 0-0.03 |
| Subpictus | 144/4120 | 1 | 0.9-1.2 | 0/126 | 0 | 0-2.89 | 0/126 | 0 | 0-2.89 | 0/3605 | 0 | 0-0.03 | 0/3605 | 0 | 0-0.03 |
b/p/m: bites /person /month; CI: confidence interval; HBR: human-biting rate; ib/p/m: infective bites /person /month; n/N: value of the numerator and denominator in the calculation of the corresponding indice as per the definition given in the Methods section; Pf-EIR: P. falciparum entomological inoculation rate; Pv-EIR: P. vivax entomological inoculation rate; Pf-SI: P. falciparum sporozoite index; Pv-SI: P. vivax sporozoite index.
Plasmodium-infected mosquitoes were identified at the species level using molecular assays. Six formally named species were incriminated in malaria transmission: An. minimus ( s.s.), An. aconitus ( s.s.) (Funestus Group), An. maculatus ( s.s.), An. sawadwongporni (Maculatus Group), An. dirus ( s.s.) and An. baimaii (Leucosphyrus Group). Plasmodium vivax sporozoites were detected in all species whereas P. falciparum sporozoites were detected only in An. minimus ( s.s.), An. maculatus ( s.s.), An. sawadwongporni and An. dirus ( s.s.). Molecular identification at the species level was not possible for 6/106 positive samples because there was no DNA remaining.
Interestingly, the avian malaria P. juxtanucleare was detected in five specimens of the Funestus, Maculatus and Leucosphyrus Groups collected by HLC (two An. minimus ( s.s.), one An. baimaii and two undetermined species). In addition, 16% (3308/21013) of the specimens from the Funestus, Maculatus and Leucosphyrus Groups collected in the CBTs were screened for Plasmodium sporozoites. Plasmodium vivax sporozoites were detected in two An. minimus ( s.s.).
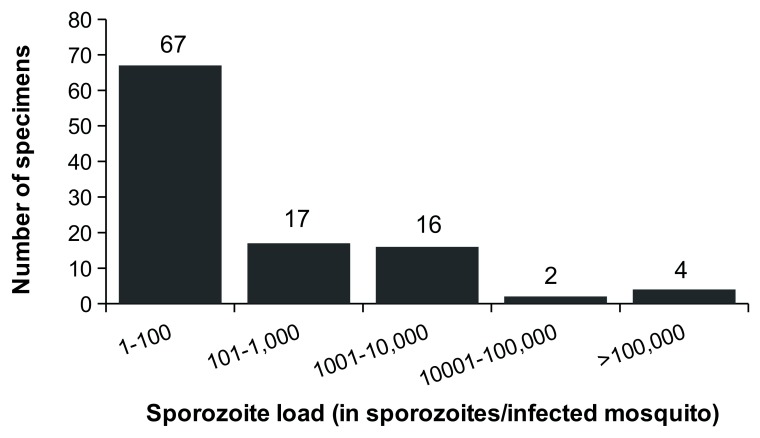
Quantitation of the sporozoite load was possible in 106/108 of the P. falciparum and P. vivax positive samples. Overall, 63% (67/106) of the infected specimens carried less than 100 sporozoites ( Figure 3). The geometric mean was 41 (95%CI= [14; 98]) and 162 (95%CI= [94; 167]) sporozoites /infected mosquito for P. falciparum and P. vivax respectively ( Supplementary File 1, Table S5). Anopheles maculatus ( s.l.) appeared to be infected with lower parasite loads compared to other anopheline species. The range of sporozoite loads was 6 to 9234 sporozoites for P. falciparum and 4 to 517500 sporozoites for P. vivax. Moreover, 81/108 abdomens from sporozoite-positive samples were screened for Plasmodium oocysts (remaining abdomens were lost or moldy). Plasmodium oocysts were detected in only 57% (46/81) of the sporozoites-positive specimens. Thirty-two out of the 35 sporozoites-positive oocysts-negative specimens carried less than 500 sporozoites in their salivary glands, suggesting that these specimens were infected with a low number of oocysts.
Figure 3. Frequency distribution of the sporozoite load in naturally infected malaria vectors.
Blood-seeking behaviour of Anopheles mosquitoes
Taking into account the whole dataset, the mean HBR of Anopheles mosquitoes was 460 bites/person/month (95%CI= [457; 463]) and the mean CBR was 4807 bites/cow/month (95%CI= [4770; 4843]). Mean HBR of primary malaria vectors varied from 2 to 306 bites/person/month in An. dirus ( s.s.) and An. minimus ( s.s.) respectively. Some primary malaria vectors were also frequently collected on CBT: Anopheles minimus ( s.s.), An. sawadwongporni and An. maculatus ( s.s.) had a mean CBR of 996, 249 and 112 bites/cow/month respectively ( Supplementary File 1, Figure S11). The data on secondary vectors ( e.g. Barbirostris and Annularis Groups) are more difficult to interpret because they probably represent a mix of several sibling species.
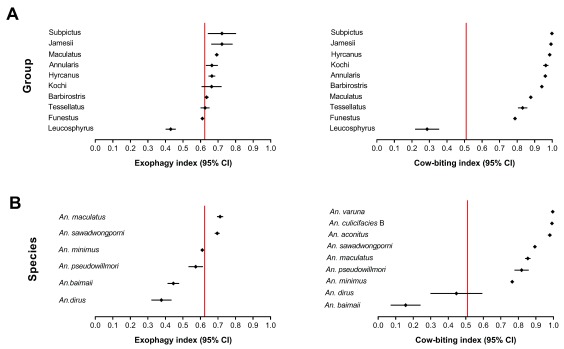
Leucosphyrus members were the most anthropophagic and endophagic species (mean CBI=0.16 and 0.44 and mean EI= 0.45 and 0.37 in An. baimaii and An. dirus ( s.s.) respectively). Other malaria vectors in the Funestus, Maculatus and Barbirostris Groups were more zoophagic and exophagic (mean CBI=0.75-0.95 and mean EI=0.60-0.75). All remaining species were strongly zoophagic and exophagic (mean CBI= 0.83-1.00 and mean EI= 0.63-0.83) ( Figure 4). Beyond zoophagy, malaria vectors appeared opportunistic in the choice of their blood meal source. Indeed, we have shown that Anopheles specimens collected by HLC can carry the avian malaria parasite P. juxtanucleare, and that Anopheles specimens collected on CBT can carry the human malaria parasite P. vivax ( i.e. Anopheles mosquitoes collected on a given host type can carry Plasmodium sporozoites acquired from a previous blood meal taken on a different host type). These findings clearly demonstrate that malaria vectors can feed alternatively on different blood sources during their life span.
Figure 4. Exophagy index (EI) and zoophagy index (CBI) of Anopheles mosquitoes.
A) EI and CBI are presented at the Group level. B) EI and CBI are presented at the species level. Vertical red lines show the mean value of the indice for the Anopheles spp. mosquitoes.
Anopheles mosquitoes exhibited an outdoor and/or early biting pattern ( Supplementary File 1, Figures S12 and S13). Some species were already active at 06:00 pm and/or at 06:00 am, suggesting that exposure to malaria vectors stretches out of the standard collection time. The proportion of malaria vectors (both Plasmodium-infected and uninfected specimens) collected indoors between 09:00 pm and 05:00 am was 29% (range= 15–48% according to the species). Conversely, 64% of the Plasmodium-infected specimens were collected either out of doors, or indoors before 09:00 pm and after 05:00 am ( Figure 5).
Figure 5. Distribution of infective bites according to the collection time and location.
π indoors, 9pm-5am: proportion of the Plasmodium-infected mosquitoes collected indoors between 09:00 pm and 05:00 am.
Entomological indices of malaria transmission
Only the Funestus, Maculatus and Leucosphyrus Groups were taken into account for the analysis of the entomological indices. Overall, mean HBR was 369 bites/person/month (95%CI= [366; 372]) and compensated for the low infection rate in these naturally infected populations of malaria vectors. Mean Pf-SI was 0.04% (95%CI= [0.02; 0.06]) and mean Pv-SI was 0.17% (95%CI= [0.14; 0.21]), yielding mean Pf-EIR of 0.13 (95%CI= [0.08; 0.21]) and mean Pv-EIR of 0.64 (95%CI= [0.51; 0.79]) infective bites/person/month ( Table 4). The transmission of P. falciparum was highly seasonal: the rainy season was associated with a 10-fold increase in Pf-EIR. In contrast, mean Pv-EIR was 0.52 (95%CI= [0.34; 0.75]) and 0.73 (95%CI= [0.55; 0.94]) infective bites/person/month during the rainy season and the dry season respectively ( Table 5). Only 6% (1/18) of the mosquitoes infected with P. falciparum sporozoites were detected during the dry season whereas 32% (28/87) of the mosquitoes infected with P. vivax sporozoites were detected during the dry season (two-sided Fisher’s exact test, p-value= 0.0218).
Table 4. Entomological indices presented per study village.
| Village | HBR
(bites/person/month) |
Pf-SI
(%) |
Pv-SI
(%) |
Pf-EIR
(infective bites/person/month) |
Pv-EIR
(infective bites/person/month) |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n/N | Estimate | 95%CI | n/N | Estimate | 95%CI | n/N | Estimate | 95%CI | n/N | Estimate | 95%CI | n/N | Estimate | 95%CI | |
| HKT | 10736/1020 | 316 | 310-322 | 5/10625 | 0.05 | 0.02-0.11 | 39/10625 | 0.37 | 0.26-0.5 | 5/1009.5 | 0.15 | 0.05-0.35 | 39/1009.5 | 1.16 | 0.82-1.58 |
| KNH | 9536/1050 | 272 | 267-278 | 4/9414 | 0.04 | 0.01-0.11 | 27/9414 | 0.29 | 0.19-0.42 | 4/1036.6 | 0.12 | 0.03-0.3 | 27/1036.6 | 0.78 | 0.51-1.14 |
| TOT | 24293/1050 | 694 | 685-703 | 6/24083 | 0.02 | 0.01-0.05 | 15/24083 | 0.06 | 0.03-0.1 | 6/1040.9 | 0.17 | 0.06-0.38 | 15/1040.9 | 0.43 | 0.24-0.71 |
| TPN | 6143/1000 | 184 | 180-189 | 3/5960 | 0.05 | 0.01-0.15 | 6/5960 | 0.1 | 0.04-0.22 | 3/970.2 | 0.09 | 0.02-0.27 | 6/970.2 | 0.19 | 0.07-0.4 |
| All
villages |
50708/4120 | 369 | 366-372 | 18/50082 | 0.04 | 0.02-0.06 | 87/50082 | 0.17 | 0.14-0.21 | 18/4069.1 | 0.13 | 0.08-0.21 | 87/4069.1 | 0.64 | 0.51-0.79 |
b/p/m: bites /person /month; CI: confidence interval; HBR: human-biting rate; ib/p/m: infective bites /person /month; n/N: value of the numerator and denominator in the calculation of the corresponding indice as per the definition given in the Methods section; Pf-EIR: P. falciparum entomological inoculation rate; Pv-EIR: P. vivax entomological inoculation rate; Pf-SI: P. falciparum sporozoite index; Pv-SI: P. vivax sporozoite index.
Table 5. Entomological indices presented per season.
| Season | HBR
(bites/person/month) |
Pf-SI
(%) |
Pv-SI
(%) |
Pf-EIR
(infective bites/person/month) |
Pv-EIR
(infective bites/person/month) |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n/N | Estimate | 95%CI | n/N | Estimate | 95%CI | n/N | Estimate | 95%CI | n/N | Estimate | 95%CI | n/N | Estimate | 95%CI | |
| Dry a | 39259/2470 | 477 | 472-482 | 17/38777 | 0.04 | 0.03-0.07 | 59/38777 | 0.15 | 0.12-0.2 | 17/2439.7 | 0.21 | 0.12-0.33 | 59/2439.7 | 0.73 | 0.55-0.94 |
| Rainy b | 11449/1650 | 208 | 204-212 | 1/11305 | 0.01 | 0-0.05 | 28/11305 | 0.25 | 0.16-0.36 | 1/1629.2 | 0.02 | 0-0.1 | 28/1629.2 | 0.52 | 0.34-0.75 |
| All
seasons |
50708/4120 | 369 | 366-372 | 18/50082 | 0.04 | 0.02-0.06 | 87/50082 | 0.17 | 0.14-0.21 | 18/4069.1 | 0.13 | 0.08-0.21 | 87/4069.1 | 0.64 | 0.51-0.79 |
a December to May b June to December b/p/m: bites /person /month; CI: confidence interval; HBR: human-biting rate; ib/p/m: infective bites /person /month; n/N: value of the numerator and denominator in the calculation of the corresponding indice as per the definition given in the Methods section; Pf-EIR: P. falciparum entomological inoculation rate; Pv-EIR: P. vivax entomological inoculation rate; Pf-SI: P. falciparum sporozoite index; Pv-SI: P. vivax sporozoite index.
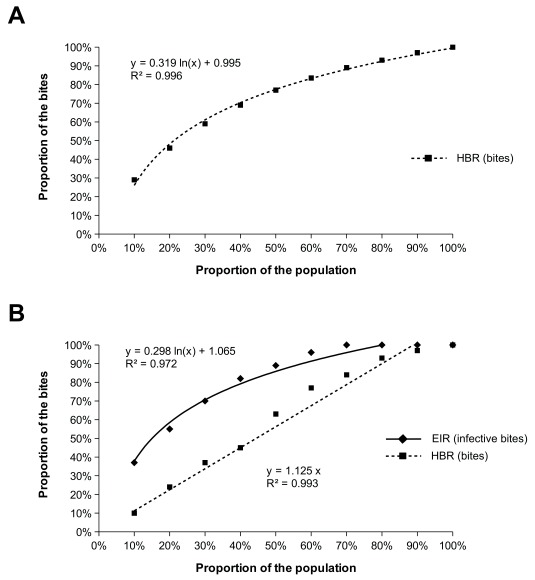
Average values of entomological indices concealed a high heterogeneity. When data were aggregated at the village and survey levels, mean HBR ranged from 13 to 2611 bites/person/month, mean Pf-EIR ranged from 0.00 to 3.05 infective bites/person/month and mean Pv-EIR ranged from 0.00 to 9.75 infective bites/person/month ( Supplementary File 1, Figure S7–S10). The lowest HBR measured on a single collector and during a single night of collection was 0 bites/night and the highest was 289 bites/night. When taking into account the entire follow-up, mean HBR measured on single collectors ranged from 66 to 1253 bites/person/month, mean Pf-EIR ranged from 0 to 0.86 infective bites/person/month and mean Pv-EIR ranged from 0 to 4.92 infective bites/person/month (100–105 collection nights/mosquito collector). The cumulative HBR and EIR measured in the cohort of mosquito collectors followed a logarithmic distribution: 20% of the collectors received 50% of the bites and 20% of the collectors received 50% of the infective bites. In contrast, 30% of the collectors did not receive any infective bites during the study. Interestingly, the cumulative HBR followed a linear trend when paired to EIR, suggesting that the heterogeneity in the distribution of infective bites was not explained by the mean of exposure to malaria vectors ( Figure 6).
Figure 6. Heterogeneous distribution of bites and infective bites among the cohort of mosquito collectors over the entire period of the study.
A) Cumulative distribution of the bites of malaria vectors among the cohort of mosquito collectors. B) Paired cumulative distribution of bites of malaria vectors and infective bites among the cohort of mosquito collectors.
Discussion
This study was a unique opportunity to document some entomological aspects of malaria transmission in low transmission settings of Southeast Asia. Our data are important in the context of malaria elimination locally in Kayin state, but also elsewhere in the Greater Mekong Subregion where malaria displays a similar transmission pattern.
The dynamics of entomological indices in an area of low, seasonal and unstable P. falciparum transmission setting
Our results confirm previous observations that primary malaria vectors in this area are An. minimus ( s.s.), An. maculatus ( s.s.), An. sawadwongporni, An. dirus ( s.s.) and An. baimaii, and that several other species also play a secondary role in the transmission. Infection rate is low in naturally infected populations of malaria vectors and balanced by the high biting-rate of malaria vectors 11, 15, yielding mean entomological inoculation rate of 1.6 and 7.7 infective bites/person/year for P. falciparum and P. vivax respectively. These EIR estimates were made in the context of falciparum malaria elimination (community wide access to early diagnosis and treatment, and mass drug administration). Therefore, baseline intensity of falciparum malaria transmission in hotspot villages in Kayin state is likely to be higher than that reported in this study 15. In contrast, Plasmodium vivax EIR estimates were higher than previously reported in the same area 11. This implies that new infections (primo-infections and reinfections) are more common than previously thought and probably frequently asymptomatic 32.
The transmission of P. falciparum was seasonal whereas infective bites of P. vivax occurred during both the dry and rainy seasons. The seasonality in P. falciparum transmission was only partially explained by the increase in malaria vector abundance during the rainy season when compared to the dry season. The longevity of malaria vectors was most likely to be the main factor driving the seasonality of P. falciparum transmission. During the dry season, the life expectancy of malaria vectors was probably too short for P. falciparum to complete its sporogonic cycle in the mosquito. During the rainy season, the longevity of malaria vectors may have increased and malaria vectors lived long enough for P. falciparum sporozoites to appear in the salivary glands 33, 34. For P. vivax, the duration of the sporogonic cycle was compatible with sporozoite detection throughout the year as this parasite develops faster than any other species in its mosquito vectors 35, 36. Relapsing vivax malaria parasites, vector competency and temperature are also potential factors that explain seasonal trends in malaria transmission.
Although 5% of the mosquitoes infected with Plasmodium sporozoites had >10,000 sporozoites in their salivary glands, the sporozoite load measured in these naturally infected malaria vectors was in average very low (60% of the infected specimens carried less than 100 sporozoites). This is consistent with previous attempts to quantify P. falciparum and P. vivax sporozoites in low transmission settings 24, 37– 39 and contrasts with the high sporozoite loads detected in Africa 40– 42. Considering a geometric mean of 162 sporozoites / P. vivax infected mosquito, a mean P. vivax EIR of 7.7 infective bites /person /year and that infected mosquitoes would transmit all their sporozoites, we estimated that each person would receive 1200 P. vivax sporozoites per year in average. Taking into account that not all sporozoites may be inoculated and that not all sporozoites may transform into hypnozoites, the reservoir of hypnozoite is likely to be small in most individuals exposed to the disease in this transmission setting.
The distribution of infective bites among the human population was highly heterogeneous. This pattern was not explained by the mean of exposure to malaria vectors as paired cumulative HBR and EIR did not follow the same trend. The study villages were hotspots of malaria transmission defined by the high prevalence of asymptomatic infection 19. This implies a substantial degree of premunition in asymptomatic carriers, i.e. the development of a protective immunity that maintains parasitaemia at sub-patent levels. The broad spectrum of EIRs measured in hotspot villages may explain why some people develop such a protective immunity and manage to control the infection.
Residual malaria transmission
The two broadly scalable vector-control interventions recommended by the World Health Organization for the control of malaria vectors are mass distribution of LLINs or, where appropriate, IRS campaigns 43. By definition, LLINs target malaria vectors seeking human blood, indoors and at a time when people are sleeping under mosquito nets. In order IRS to be effective, malaria vectors targeted by the intervention must also rest indoors, before or after a blood meal. However, this stereotypical blood seeking behavior applies only to a minority of the dominant malaria vectors worldwide 44. Several behavioral traits drive the refractoriness of malaria vectors to LLINs and IRS including (i) their ability to take blood meals from animals (zoophagy and opportunistic blood type selection), (ii) their tendency to rest and/or feed outdoors (exophily and exophagy) and (iii) their ability to feed before dawn and after dusk, at a time when people are not protected by LLINs or IRS intervention 9.
As previously reported, LLINs only have a limited efficacy in preventing human-vector contact and disease transmission in the Thailand-Myanmar border area. Somboon et al. evaluated the impact of mosquito bed nets impregnated with lambda-cyhalothrin using entomological endpoints in very similar transmission settings (Karen villages located on the Thai side of the border) 7. The authors reported that mosquito bed nets can prevent 36–78% of the human-vector contact according to the Anopheles species. Universal coverage with LLINs failed to reduce the abundance and longevity of malaria vectors, suggesting that this intervention had only a limited impact on the vectorial capacity. The impact of permethrin-impregnated mosquito bed nets was also evaluated in pregnant women and children living in refugee camps using epidemiological endpoints. The use of mosquito bed nets during pregnancy was associated with a significant reduction in the incidence of severe anaemia but not of malaria 45. At a time when EIR was higher, the use of mosquito bed nets in children was associated with a significant decrease of falciparum malaria incidence but no effect was observed on P. vivax 46. More recently, Smithuis et al. failed to observe an impact of LLINs among a cohort of 175 children followed for 10 months in Western Myanmar 47. This negative result was explained by the early and outdoor biting pattern of malaria vectors 48.
In this study, only 36% of the Plasmodium-infected mosquitoes were collected indoors between 09:00 pm and 05:00 am (when and where people are expected to sleep under a bed-net). This proportion might have been overestimated as malaria vectors were already active at 06:00 pm and/or at 06:00 am, suggesting that the exposure stretched out of the collection time. Accurate quantitation of the part of the transmission that LLINs fail to prevent would require collecting additional data on population movements and sleeping habits of people living in this area. Moreover, we have clearly demonstrated an opportunistic blood type selection in some vectors, i.e. that a given specimen is able to feed on several blood sources during successive gonotrophic cycles. This opportunism also appears as an important factor to explain why universal coverage with mosquito bed nets fails to affect the dynamic of anopheline populations and decrease vectorial capacity in the area 7. Consequently, the paradigm of residual transmission as experienced in high transmission settings of Africa does not apply to the Thailand-Myanmar border area and a drastic shift in vector-control interventions is required.
Shift in vector-control intervention
The design of effective intervention for the control of malaria vectors in Southeast Asia should take into account malaria vector ecology and transmission dynamics. In this study, we have shown that multiple vectors have different and complementary blood-seeking behaviours, making their control particularly difficult.
Veterinary approaches such as the injection of livestock with a slow-release formulation of endectocides 49, or the use of insecticide-treated mosquito nets fenced around cattle 50 may be an interesting strategy to decrease the vectorial capacity of some zoophilic and/or zoophagic malaria vectors (ex: An. minimus, An. maculatus and An. sawadwongporni). We have shown that malaria vectors can readily feed on a wide variety of blood types including human, cattle, pigs and birds. However, the diversity of blood sources and the relative proportion of blood meals taken on a given source remain to determine. In this regard, targeted sequencing of mammalian mt 16S ribosomal RNA genes detected in DNA extracts from blood-fed specimens is a promising tool for the determination of blood-meal sources in wild populations of malaria vectors 51.
The nature of Anopheles resting habitats is another important aspect of malaria vector ecology that can be targeted by residual insecticide spraying intervention. Resting habitats have been identified both indoors (ex: roof, wall, ceilings of houses and animal barns) and outdoors (ex: tree holes, rodent holes, dense bushes) 52. However, most mosquito species rest exclusively out of doors in natural settings, and only a relatively few species rest in man-made shelters 52. The size and importance of the exophilic population that commonly rest inside houses are typically overlooked because the sampling of outdoor-resting population is more difficult than the sampling of indoor-resting population. In Southeast Asia, most of the life cycle of Anopheles mosquitoes is likely to take place out of doors and malaria vectors are barely never found resting indoors 18. Therefore, the scope of residual insecticide spraying for the control of malaria vectors may be extended to outdoor applications.
Insecticide resistance may also represent an additional threat to the control of malaria vectors in this area. We have previously detected relatively low levels of resistances to deltamethrin and permethrin in populations of An. minimus ( s.l.) and An. maculatus ( s.l.) collected in the villages of the present study 53. Further investigations are needed in order to document the extent of pyrethroid resistance elsewhere in Kayin state and to evaluate the potential effectiveness of alternative class of insecticides such as carbamate (ex. bendiocarb), organophosphate (ex. malathion) or insect growth inhibitors (ex. pyriproxyfen).
Conclusion
This study highlights the importance of entomology in the context of malaria elimination in Kayin state. A drastic shift in vector-control strategy is required in order to address early and outdoor malaria transmission, and the modalities of vector-control should be retuned to address problematic specific to malaria elimination.
Data availability
The data is available upon request to the Mahidol Oxford Tropical Medicine Research Unit Data Access Committee ( Supplementary File 3; http://www.tropmedres.ac/data-sharing) and following the Mahidol Oxford Tropical Medicine Research Unit data access policy ( http://www.tropmedres.ac/_asset/file/data-sharing-policy-v1-0.pdf).
Acknowledgments
We thank all study participants and communities from Htoo Pyin Nyar, Tar Au Ta, Ka Nu Hta and Htee Kaw Taw villages for their participation and support to the study. SMRU is part of the Mahidol Oxford University Research Unit, supported by the Wellcome Trust of Great Britain.
Funding Statement
This work was supported by the Wellcome Trust [101148]; and the Bill and Melinda Gates Foundation [GH OPP 1081420]. Victor Chaumeau received a PhD scholarship by the Centre Hospitalier Universitaire de Montpellier (CHU Montpellier).
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
[version 4; peer review: 2 approved]
Supplementary material
Supplementary File 1. Supplemental data.
Supplementary File 3. Request form for Mahidol Oxford Tropical Medicine Research Unit Data Access Committee.
References
- 1. Imwong M, Suwannasin K, Kunasol C, et al. : The spread of artemisinin-resistant Plasmodium falciparum in the Greater Mekong subregion: a molecular epidemiology observational study. Lancet Infect Dis. 2017;17(5):491–7. 10.1016/S1473-3099(17)30048-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Phyo AP, Ashley EA, Anderson TJC, et al. : Declining Efficacy of Artemisinin Combination Therapy Against P. Falciparum Malaria on the Thai-Myanmar Border (2003-2013): The Role of Parasite Genetic Factors. Clin Infect Dis. 2016;63(6):784–91. 10.1093/cid/ciw388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Amato R, Pearson RD, Almagro-Garcia J, et al. : Origins of the current outbreak of multidrug-resistant malaria in southeast Asia: a retrospective genetic study. Lancet Infect Dis. 2018;18(3):337–45. 10.1016/S1473-3099(18)30068-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Murray CJ, Ortblad KF, Guinovart C, et al. : Global, regional, and national incidence and mortality for HIV, tuberculosis, and malaria during 1990-2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2014;384(9947):1005–1070. 10.1016/S0140-6736(14)60844-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. von Seidlein L: The failure of screening and treating as a malaria elimination strategy. PLoS Med. 2014;11(1):e1001595. 10.1371/journal.pmed.1001595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chaumeau V, Kajeechiwa L, Fustec B, et al. : Contribution of Asymptomatic Plasmodium Infections to the Transmission of Malaria in Kayin State, Myanmar. J Infect Dis. 2019;219(9):1499–1509. 10.1093/infdis/jiy686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Somboon P, Lines J, Aramrattana A, et al. : Entomological evaluation of community-wide use of lambdacyhalothrin-impregnated bed nets against malaria in a border area of north-west Thailand. Trans R Soc Trop Med Hyg. 1995;89(3):248–254. 10.1016/0035-9203(95)90525-1 [DOI] [PubMed] [Google Scholar]
- 8. Ismail IA, Notananda V, Schepens J: Studies on malaria and responses of Anopheles balabacensis balabacensis and Anopheles minimus to DDT residual spraying in Thailand. Acta Trop. 1975;32(3):206–31. 10.5169/seals-312088 [DOI] [PubMed] [Google Scholar]
- 9. Killeen GF: Characterizing, controlling and eliminating residual malaria transmission. Malar J. 2014;13:330. 10.1186/1475-2875-13-330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Luxemburger C, Thwai KL, White NJ, et al. : The epidemiology of malaria in a Karen population on the western border of Thailand. Trans R Soc Trop Med Hyg. 1996;90(2):105–111. 10.1016/S0035-9203(96)90102-9 [DOI] [PubMed] [Google Scholar]
- 11. Somboon P, Aramrattana A, Lines J, et al. : Entomological and epidemiological investigations of malaria transmission in relation to population movements in forest areas of north-west Thailand. Southeast Asian J Trop Med Public Health. 1998;29(1):3–9. [PubMed] [Google Scholar]
- 12. Ool TT, Storch V, Becker N: Review of the anopheline mosquitoes of Myanmar. J Vector Ecol. 2004;29(1):21–40. [PubMed] [Google Scholar]
- 13. Sriwichai P, Samung Y, Sumruayphol S, et al. : Natural human Plasmodium infections in major Anopheles mosquitoes in western Thailand. Parasit Vectors. 2016;9(1):17. 10.1186/s13071-016-1295-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Sriwichai P, Karl S, Samung Y, et al. : Imported Plasmodium falciparum and locally transmitted Plasmodium vivax: cross-border malaria transmission scenario in northwestern Thailand. Malar J. 2017;16(1):258. 10.1186/s12936-017-1900-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kwansomboon N, Chaumeau V, Kittiphanakun P, et al. : Vector bionomics and malaria transmission along the Thailand-Myanmar border: a baseline entomological survey. J Vector Ecol. 2017;42(1):84–93. 10.1111/jvec.12242 [DOI] [PubMed] [Google Scholar]
- 16. Green CA, Rattanarithikul R, Pongparit S, et al. : A newly-recognized vector of human malarial parasites in the Oriental region, Anopheles (Cellia) pseudowillmori (Theobald, 1910). Trans R Soc Trop Med Hyg. 1991;85(1):35–6. 10.1016/0035-9203(91)90143-M [DOI] [PubMed] [Google Scholar]
- 17. Gould D, Esah S, Pranith U: Relation of Anopheles aconitus to malaria transmission in the central plain of Thailand. Trans R Soc Trop Med Hyg. 1967;61(3):441–442. 10.1016/0035-9203(67)90024-7 [DOI] [Google Scholar]
- 18. Sinka ME, Bangs MJ, Manguin S, et al. : The dominant Anopheles vectors of human malaria in the Asia-Pacific region: occurrence data, distribution maps and bionomic précis. Parasit Vectors. 2011;4:89. 10.1186/1756-3305-4-89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Landier J, Kajeechiwa L, Thwin MM, et al. : Safety and effectiveness of mass drug administration to accelerate elimination of artemisinin-resistant falciparum malaria: A pilot trial in four villages of Eastern Myanmar [version 1; referees: 2 approved]. Wellcome Open Res. 2017;2:81. 10.12688/wellcomeopenres.12240.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Landier J, Parker DM, Thu AM, et al. : Effect of generalised access to early diagnosis and treatment and targeted mass drug administration on Plasmodium falciparum malaria in Eastern Myanmar: an observational study of a regional elimination programme. Lancet. 2018;391(10133):1916–26. 10.1016/S0140-6736(18)30792-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Amerasinghe FP, Amerasinghe PH, Peiris JS, et al. : Anopheline ecology and malaria infection during the irrigation development of an area of the Mahaweli Project, Sri Lanka. Am J Trop Med Hyg. 1991;45(2):226–35. 10.4269/ajtmh.1991.45.226 [DOI] [PubMed] [Google Scholar]
- 22. Ali N, Noreen S, Khan K, et al. : Population dynamics of mosquitoes and malaria vector incrimination in district Charsadda, Khyber Pakhtunkhwa (KP) Pakistan. Acta Trop. 2015;141(Pt A):25–31. 10.1016/j.actatropica.2014.08.020 [DOI] [PubMed] [Google Scholar]
- 23. Rattanarithikul R, Harrison BA, Harbach RE, et al. : Illustrated keys to the mosquitoes of Thailand. IV. Anopheles. Southeast Asian J Trop Med Public Health. 2006;37 Suppl 2:1–128. [PubMed] [Google Scholar]
- 24. Chaumeau V, Andolina C, Fustec B, et al. : Comparison of the Performances of Five Primer Sets for the Detection and Quantification of Plasmodium in Anopheline Vectors by Real-Time PCR. PLoS One. 2006;11(7):e0159160. 10.1371/journal.pone.0159160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Garros C, Koekemoer LL, Coetzee M, et al. : A single multiplex assay to identify major malaria vectors within the African Anopheles funestus and the Oriental An. minimus groups. Am J Trop Med Hyg. 2004;70(6):583–590. 10.4269/ajtmh.2004.70.583 [DOI] [PubMed] [Google Scholar]
- 26. Walton C, Somboon P, O'Loughlin SM, et al. : Genetic diversity and molecular identification of mosquito species in the Anopheles maculatus group using the ITS2 region of rDNA. Infect Genet Evol. 2007;7(1):93–102. 10.1016/j.meegid.2006.05.001 [DOI] [PubMed] [Google Scholar]
- 27. Walton C, Handley JM, Kuvangkadilok C, et al. : Identification of five species of the Anopheles dirus complex from Thailand, using allele-specific polymerase chain reaction. Med Vet Entomol. 1999;13(1):24–32. 10.1046/j.1365-2915.1999.00142.x [DOI] [PubMed] [Google Scholar]
- 28. Beebe NW, Saul A: Discrimination of all members of the Anopheles punctulatus complex by polymerase chain reaction--restriction fragment length polymorphism analysis. Am J Trop Med Hyg. 1995;53(5):478–481. 10.4269/ajtmh.1995.53.478 [DOI] [PubMed] [Google Scholar]
- 29. Mangold KA, Manson RU, Koay ES, et al. : Real-time PCR for detection and identification of Plasmodium spp. J Clin Microbiol. 2005;43(5):2435–2440. 10.1128/JCM.43.5.2435-2440.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Cunha MG, Medina TS, Oliveira SG, et al. : Development of a Polymerase Chain Reaction (PCR) method based on amplification of mitochondrial DNA to detect Plasmodium falciparum and Plasmodium vivax. Acta Trop. 2009;111(1):35–38. 10.1016/j.actatropica.2009.02.003 [DOI] [PubMed] [Google Scholar]
- 31. Drakeley C, Schellenberg D, Kihonda J, et al. : An estimation of the entomological inoculation rate for Ifakara: a semi-urban area in a region of intense malaria transmission in Tanzania. Trop Med Int Health. 2003;8(9):767–74. 10.1046/j.1365-3156.2003.01100.x [DOI] [PubMed] [Google Scholar]
- 32. Adekunle AI, Pinkevych M, McGready R, et al. : Modeling the dynamics of Plasmodium vivax infection and hypnozoite reactivation in vivo. PLoS Negl Trop Dis. 2015;9(3):e0003595. 10.1371/journal.pntd.0003595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ratanatham S, Upatham ES, Prasittisuk C, et al. : Bionomics of Anopheles minimus and its role in malaria transmission in Thailand. Southeast Asian J Trop Med Public Health. 1988;19(2):283–289. [PubMed] [Google Scholar]
- 34. Upatham ES, Prasittisuk C, Ratanatham S, et al. : Bionomics of Anopheles maculatus complex and their role in malaria transmission in Thailand. Southeast Asian J Trop Med Public Health. 1988;19(2):259–269. [PubMed] [Google Scholar]
- 35. Boyd M: Malariology. Saunders, Philadelphia,1949. Reference Source [Google Scholar]
- 36. Kasap H, Kasap M, Demirhan O, et al. : Development of Plasmodium vivax in Anopheles superpictus under experimental conditions. Am J Trop Med Hyg. 1987;37(2):241–245. 10.4269/ajtmh.1987.37.241 [DOI] [PubMed] [Google Scholar]
- 37. Baker EZ, Beier JC, Meek SR, et al. : Detection and quantification of Plasmodium falciparum and P. vivax infections in Thai-Kampuchean Anopheles (Diptera: Culicidae) by enzyme-linked immunosorbent assay. J Med Entomol. 1987;24(5):536–541. 10.1093/jmedent/24.5.536 [DOI] [PubMed] [Google Scholar]
- 38. Burkot TR, Graves PM, Cattan JA, et al. : The efficiency of sporozoite transmission in the human malarias, Plasmodium falciparum and P. vivax. Bull World Health Organ. 1987;65(3):375–380. [PMC free article] [PubMed] [Google Scholar]
- 39. Wirtz RA, Burkot TR, Graves PM, et al. : Field evaluation of enzyme-linked immunosorbent assays for Plasmodium falciparum and Plasmodium vivax sporozoites in mosquitoes (Diptera: Culicidae) from Papua New Guinea. J Med Entomol. 1987;24(4):433–437. 10.1093/jmedent/24.4.433 [DOI] [PubMed] [Google Scholar]
- 40. Kabiru EW, Mbogo CM, Muiruri SK, et al. : Sporozoite loads of naturally infected Anopheles in Kilifi District, Kenya. J Am Mosq Control Assoc. 1997;13(3):259–262. [PubMed] [Google Scholar]
- 41. Collins FH, Zavala F, Graves PM, et al. : First field trial of an immunoradiometric assay for the detection of malaria sporozoites in mosquitoes. Am J Trop Med Hyg. 1984;33(4):538–543. 10.4269/ajtmh.1984.33.538 [DOI] [PubMed] [Google Scholar]
- 42. Beier JC, Onyango FK, Koros JK, et al. : Quantitation of malaria sporozoites transmitted in vitro during salivation by wild Afrotropical Anopheles. Med Vet Entomol. 1991;5(1):71–79. 10.1111/j.1365-2915.1991.tb00523.x [DOI] [PubMed] [Google Scholar]
- 43. https://www.who.int/malaria/areas/vector_control/core_methods/en/ [Google Scholar]
- 44. Kiszewski A, Mellinger A, Spielman A, et al. : A global index representing the stability of malaria transmission. Am J Trop Med Hyg. 2004;70(5):486–498. 10.4269/ajtmh.2004.70.486 [DOI] [PubMed] [Google Scholar]
- 45. Dolan G, ter Kuile FO, Jacoutot V, et al. : Bed nets for the prevention of malaria and anaemia in pregnancy. Trans R Soc Trop Med Hyg. 1993;87(6):620–626. 10.1016/0035-9203(93)90262-O [DOI] [PubMed] [Google Scholar]
- 46. Luxemburger C, Perea WA, Delmas G, et al. : Permethrin-impregnated bed nets for the prevention of malaria in schoolchildren on the Thai-Burmese border. Trans R Soc Trop Med Hyg. 1994;88(2):155–159. 10.1016/0035-9203(94)90273-9 [DOI] [PubMed] [Google Scholar]
- 47. Smithuis FM, Kyaw MK, Phe UO, et al. : The effect of insecticide-treated bed nets on the incidence and prevalence of malaria in children in an area of unstable seasonal transmission in western Myanmar. Malar J. 2013;12:363. 10.1186/1475-2875-12-363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Smithuis FM, Kyaw MK, Phe UO, et al. : Entomological determinants of insecticide-treated bed net effectiveness in Western Myanmar. Malar J. 2013;12:364. 10.1186/1475-2875-12-364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Chaccour C, Killeen GF: Mind the gap: residual malaria transmission, veterinary endectocides and livestock as targets for malaria vector control. Malar J. 2016;15:24. 10.1186/s12936-015-1063-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Maia MF, Abonuusum A, Lorenz LM, et al. : The effect of deltamethrin-treated net fencing around cattle enclosures on outdoor-biting mosquitoes in Kumasi, Ghana. PLoS One. 2012;7(9):e45794. 10.1371/journal.pone.0045794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Logue K, Keven JB, Cannon MV, et al. : Unbiased Characterization of Anopheles Mosquito Blood Meals by Targeted High-Throughput Sequencing. PLoS Negl Trop Dis. 2016;10(3):e0004512. 10.1371/journal.pntd.0004512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Silver JB: Mosquito Ecology - Field Sampling Methods. Springer; Netherlands,2008. 10.1007/978-1-4020-6666-5 [DOI] [Google Scholar]
- 53. Chaumeau V, Cerqueira D, Zadrozny J, et al. : Insecticide resistance in malaria vectors along the Thailand-Myanmar border. Parasit Vectors. 2017;10(1):165. 10.1186/s13071-017-2102-z [DOI] [PMC free article] [PubMed] [Google Scholar]