Abstract
AIMS:
To conduct a meta-analysis of statin-associated type 2 diabetes mellitus (T2D) risk among randomized controlled trials (RCTs) and observational studies (OBSs), excluding studies conducted among secondary prevention populations.
METHODS:
Studies were identified by searching PubMed (1994-present) and EMBASE (1994-present). Articles had to meet the following criteria: 1) follow-up >one year; 2) >50% of participants free of clinically diagnosed ASCVD; 3) adult participants ≥30 years old; 4) reported statin-associated T2D effect estimates; and 5) quantified precision using 95% confidence interval. Data were pooled using random-effects model.
RESULTS:
We identified 23 studies (35% RCTs) of n=4,012,555 participants. OBS participants were on average younger (mean difference= 6.2 years) and had lower mean low-density lipoprotein cholesterol (LDL-C, mean difference=20.6 mg/dL) and mean fasting plasma glucose (mean difference=5.2 mg/dL) compared to RCT participants. There was little evidence for publication bias (P>0.1). However, evidence of heterogeneity was observed overall and among OBSs and RCTs (PCochran=<0.05). OBS designs, younger baseline mean ages, lower LDL-C concentrations, and high proportions of never or former smokers were significantly associated with increased statin-associated T2D risk.
CONCLUSIONS:
Potentially elevated statin-associated T2D risk in younger populations with lower LDL-C merits further investigation in light of evolving statin guidelines targeting primary prevention populations.
Keywords: Statin, type 2 diabetes mellitus, guidelines, low-density lipoprotein, prevention
I. Introduction
Hmg CoA reductase inhibitors, commonly known as statins, are the most widely prescribed class of medication used to reduce cardiovascular disease (CVD) risk and to treat elevated low density lipoprotein cholesterol (LDL-C).1–3 Changes in cholesterol treatment recommendations from the Third Adult Treatment Panel (ATP III) of the National Cholesterol Education Program guidelines to the American College of Cardiology/American Heart Association (ACC/AHA) 2013 guidelines increased the number of adults newly eligible for statin therapy for primary prevention by an estimated 10.4 million, with 80% of the increase attributable to individuals between the ages of 60–75.4
While the cardioprotective effect of statins are undeniable5, 6, experimental and observational research has also suggested that statins may lead to harm in lower risk individuals by increasing the risk of type 2 diabetes mellitus (T2D).7–11 Yet, most meta-analyses have combined primary and secondary prevention populations to examine statin associated T2D risk. Secondary prevention populations, however, include survivors of ASCVD whose mortality risk has been estimated to be five to six times higher than that of people of the same age who did not experience an ASCVD.12 Further, the risk of T2D may differ when used for primary vs. secondary prevention,13 complicating efforts to quantify statin-associated T2D risk in primary prevention populations. As primary prevention populations are most impacted by the statin-eligibility recommendations, additional research quantifying statin-associated T2D risk is needed.
In addition to combining primary and secondary populations, published meta-analyses of statin-associated T2D risk have also been restricted to either randomized controlled trials (RCTs) or observational studies (OBSs). Yet, meta-analyses that incorporate summary data from both study designs may take advantage of the internal validity of RCTs and the external validity of OBSs. This approach better reflects the existing evidence base, and may increase statistical power to investigate heterogeneity and expand upon past meta-analyses’ limited heterogeneity assessments.14, 15 Therefore, to estimate the effect of statins on T2D among populations most affected by changes to statin use guidelines and examine potential sources of heterogeneity, we performed a systematic review and meta-analysis of statin-associated T2D risk by synthesizing published data from RCTs and OBSs, excluding secondary prevention populations.
2. Materials and Methods
We followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines throughout the design, implementation, analysis, and reporting of this meta-analysis.16
2.1. DATA SOURCES
In consultations with a reference librarian (MW), studies of statin-associated T2D risk were identified by searching PubMed (1994-present) and EMBASE (1994-present) on 25 January 2018. Reference lists of articles were scanned, which included published RCT and OBS meta-analyses8–11 and all document types, languages, and publication dates. Consistent with previous systematic reviews and meta-analyses of RCT and OBSs examining statin-associated T2D risk8–11, the following free text and Medical Subject Headings terms were used: (Statin OR Statins OR Anticholesteremic Agents OR Anticholesteremic OR Hydroxymethylglutaryl-CoA Reductase Inhibitors) AND (Diabetes OR Diabetes Mellitus II OR Diabetes Mellitus Type II OR Diabetes Mellitus Type 2) AND (adverse effects OR adverse events) AND (cohort study OR case-control study OR trial).
2.2. STUDY SELECTION
Articles selected for the meta-analysis had to meet all of the following criteria: 1) follow-up >one year; 2) >50% of participants free of clinically diagnosed ASCVD; 3) adult participants ≥30 years old; 4) reported statin-associated T2D effect estimates using the odds ratio (OR), hazard ratio (HR), or risk ratio; and 5) quantified precision using the 95% confidence interval (CI) or included information to enable estimation.
2.3. DATA EXTRACTION AND EVALUATION
Citations were downloaded to an electronic reference manager (EndNote X7, Thomson Reuters17) and duplicates were removed. Two reviewers (JCE and AS) independently reviewed all titles and abstracts and extracted relevant information into tables. The tables were compared and disagreements were resolved by consulting the initial articles. For each study population in each article, the reviewers extracted statin-associated T2D relative risks (RR; primary endpoint), study design, mean length of follow-up time, sample size, year of publication, year of baseline, methods used to address for confounders (in OBSs only), type of effect estimate, methods used to measure and define T2D, residence of participants, proportion of population that is Caucasian, proportion of population taking statins, mean baseline characteristics (age, body mass index [BMI], low-density lipoprotein cholesterol [LDL-C] levels, glucose levels, systolic blood pressure [SBP] levels), baseline characteristics proportions (hypertensive, current smokers, ASCVD) and methodological qualities of each study (Supplemental Table 1). If information on study and participant characteristics of interest were not available, corresponding authors were contacted via email with one follow-up email sent two weeks after the initial inquiry and a final follow-up email sent two weeks thereafter.
2.4. QUALITY ASSESSMENT
PRISMA criteria were used to describe the quality of RCTs16 and assess potential for bias. Specifically, the following dimensions (agree or disagree) were evaluated: 1) adequate use of measures to conceal allocation (adequate through the use of randomization); 2) application of blinding (whether to the participant, care provider or outcome assessors); 3) proportion of participants lost to follow-up reported; and 4) whether the analysis followed the intention-to-treat principle.18
To assess quality of OBSs, articles were assigned scores using criteria consistent with past meta-analysis of cohort studies19 (agree or disagree): 1) Was a well-defined sample of participants identified?; 2) Were there clear definitions of statin use and 3) T2D?; 4) Was there information on baseline LDL-C levels and 5) fasting plasma glucose levels by treatment status?; and 6) Were differences in baseline factors accounted for?20
2.5. DATA SYNTHESIS
Publication bias was assessed using both qualitative and quantitative methods overall and by study design (OBS; RCT). First, funnel plot asymmetry was evaluated using a plot of each study-specific RR versus its precision.21 Second, p-values (α=0.1) for Begg and Mazumdar’s log rank test and Egger’s regression test were calculated to provide quantitative assessments of funnel plot assymetry.22, 23 Third, Duval and Tweedie’s non-parametric trim and fill imputation procedure was conducted to impute hypothetically missing results due to publication bias.24
Inter-study heterogeneity was assessed by Cochran’s Q test25 (α=0.126) and the I2 statistic, which is derived from Cochran’s Q test (I2 = 100x (Q − df)/Q).27 To further assess the extent of heterogeneity between studies, Galbraith plots were constructed displaying each study’s effect size divided by each study’s standard error (Z score) versus the inverse of each study’s standard error.28
Variation in the strength and precision of estimated RRs by study design (OBS; RCT) and across levels of the study and participant characteristics was assessed by estimating a summary random-effects RR within each study and participant characteristic using univariable random-effects meta-regression.29 We considered the following study and participant characteristics for interrogation via meta-regression: mean length of follow-up time, sample size, year of publication, year of baseline, methods used to address for confounders, type of effect estimate, methods used to measure and define T2D, residence of participants, proportion of population that is Caucasian, proportion of population taking statins, mean baseline characteristics (age, BMI, LDL-C, glucose levels, SBP levels), and baseline characteristics proportions (hypertensive, current smokers, ASCVD). All analyses were performed using STATA (College Station, TX).30
3. Results
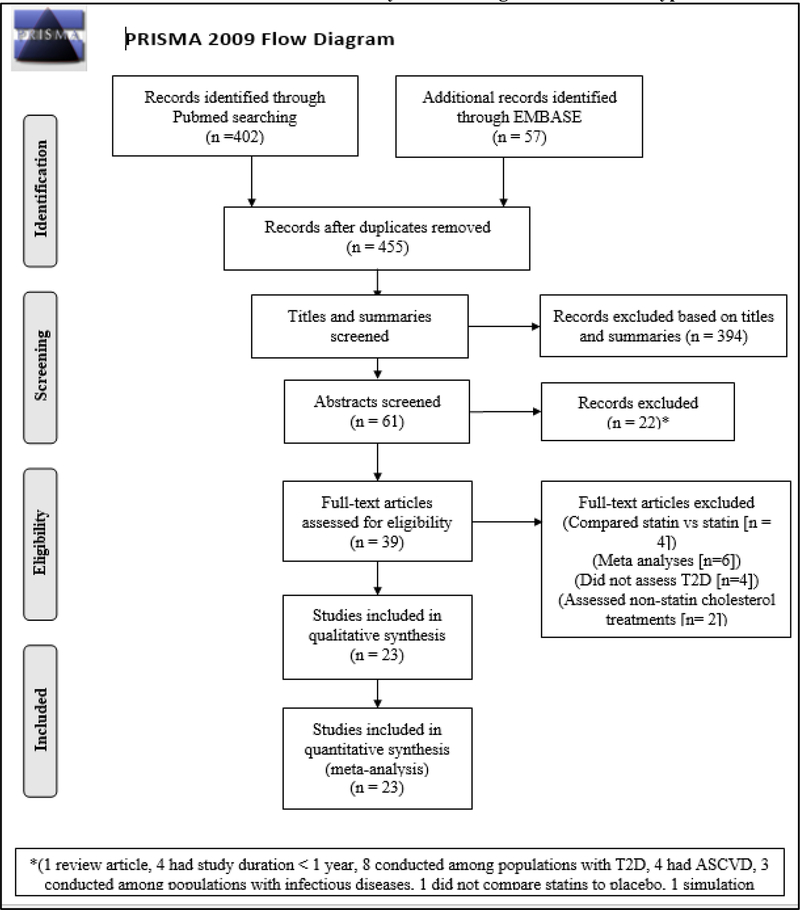
The systematic review identified a total of 459 candidate studies for screening (Figure 1). Of these studies, 23 (5%; eight RCTs and 15 OBSs) met the eligibility criteria for inclusion in the meta-analyses (see Supplement for article references). Eligible studies were conducted between 1998 and 2016, with OBSs on average being published more recently (mean publication date = 2012 vs 2005) and using more recent data (mean baseline study year = 2000 vs 1998) compared to RCTs (Tables 1 and 2). In contrast to RCTs, the mean length of follow-up time was longer (6.9 years vs 4.3 years), participants were more likely to be women (mean proportion of women = 51.5% vs 36%), more likely to be Caucasian (mean proportion Caucasian = 71.2% vs 63.6%), and less likely to be current smokers (mean proportion current smoker = 18% vs 24.5%) among OBSs.
Figure 1. Flow diagram of literature search to identify randomized controlled trials and observational studies for inclusion in meta-analysis examining statin-associated type 2 diabetes risk.
T2D = Type 2 diabetes
ASCVD = Atherosclerotic cardiovascular disease
Table 1.
Selected characteristics of interest among eight randomized controlled trials examining statin-associated type 2 diabetes risk.
| Study characteristics | Participant characteristics | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Studies | Mean length of follow-up (years) |
Study sample size |
Year of baseline data |
Method to define T2D |
% Women |
% Caucasian |
Mean age (years) |
Mean BMI (kg/m2) |
Mean LDL- C levels (mg/dL) |
Mean FPG Levels (mg/dL) |
| Downs (1998) | 5.2 | 6605 | 1993 | T2D diagnosis, T2D medication use, and FPG >126mg/dL |
15.1 | 89 | 58 | 26.7 | 150 | R |
| Freeman (2001) | 4.8 | 6595 | 1990 | T2D medication use, FPG >126mg/dL |
0.0 | 100 | 55 | 25.9 | 193.3 | 86.5 |
| Furberg (2002) | 4.8 | 10355 | 1994 | FPG >126mg/dL |
49 | 41 | 66.4 | 29.9 | 146 | 122.1 |
| Shepherd (2002) | 3.2 | 5804 | 1999 | T2D medication use, FPG >126mg/dL |
51.7 | 100 | 75.3 | 26.8 | 146.9 | 92.5 |
| Sever (2003) | 3.3 | 10341 | 1999 | FPG >126mg/dL |
18.8 | 94 | 63.2 | 28.7 | 131.5 | 111.7 |
| Nakamura (2006) | 5.3 | 7832 | 1999 | 12D diagnosis, T2D medication use, and FPG >126mg/dL |
68.0 | 0 | 59 | 23.9 | 156 | 109.1 |
| Ridker (2008) | 1.9 | 17802 | 2007 | T2D diagnosis, T2D medication use, and FPG >126mg/dL |
38.2 | 71.3 | 66 | 28.4 | 108 | 94 |
| Yusuf(2016) | 5.6* | 12705 | 2010 | T2D diagnosis, T2D medication use, and FPG >126mg/dL |
46.7 | 20 | 65.8 | 27.1 | 128.6 | 95.4* |
| 8 studies (1998–2016) | 4.3 | 9754.9 | 1998 | 35.9 | 64.4 | 63.6 | 27.2 | 145.0 | 101.6 | |
BMI = Body mass index
LDL-C = Low-density lipoprotein
FPG = Fasting plasma glucose
R = Data requested, but not available
= Median values
Table 2.
Selected characteristics of interest among 15 observational studies examining statin-associated type 2 diabetes risk.
| Study characteristics | Participant characteristics | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Studies | Type of OBS data |
Mean length of follow-up (years) |
Study sample size |
Year of Baseline data |
Method to define T2D |
% Women | % Caucasian | Mean age (years) |
Mean BMI (kg/m2) |
Mean LDL-C levels (mg/dL) |
Mean FPG levels (mg/dL) |
| Jick (2004) | Cohort | 11 | 2651 | 1996 | T2D diagnosis, T2D medication use |
49.1 | 100 | 59.2 | 27.6 | NA | NA |
| Culver (2012) | Cohort | 9 | 120173 | 1995 | Self-report of physician-diagnosed T2D |
100 | 83.7 | 63.2 | 27.8 | 120.4 | 94 |
| Wang (2012) | Insurance claims | 8 | 42060 | 2000 | T2D diagnosis, T2D medication use |
50 | 0 | 63 | NA | NA | NA |
| Danaei (2012) | Insurance claims | 9 | 285864 | 2000 | T2D diagnosis, T2D medication use |
55.5 | 100 | 63.2 | 27.7 | 135.9 | NA |
| Izzo (2013) | Cohort | 4.7 | 4750 | 1997 | T2D diagnosis, T2D medication use, and FPG >126mg/dL |
42.3 | 100 | 58.6 | 27.7 | 130.2 | 95.7 |
| Chen (2013) | Insurance claims | 2 | 11715 | 2004 | T2D diagnosis | 100 | 0 | 61.3 | NA | NA | NA |
| Currie (2013) | Cohort | 6 | 32086 | 2005 | T2D diagnosis, T2D medication use |
47 | 69.5 | 49.9 | R | R | R |
| Zaharan(2013) | Insurance claims | 8 | 1162911 | 2002 | T2D diagnosis, T2D medication use |
64 | 100 | R | R | R | R |
| Macedo (2014) | Cohort | 20 | 2016094 | 1989 | T2D diagnosis | 47 | 100 | 62.3 | NA | NA | NA |
| Bhattacharya(2014) | Cohort | 2 | 44047 | 2004 | T2D diagnosis | 55 | 73 | R | NA | NA | NA |
| Cederberg(2014) | Cohort | 5.9 | 8749 | 2010 | T2D diagnosis, T2D medication use, and FPG>126mg/dL |
0 | 100 | 57.1 | 26.8 | 130.2 | 103 |
| Mansi(2015) | Cohort | 10 | 6702 | 2004 | T2D diagnosis | 38.8 | NA | 53 | NA | 117 | NA |
| Radford(2015) | Cohort | 3 | 8853 | 1998 | T2D medication use, FPG>126 mg/dL |
26.4 | 100 | 48.2 | 26 | 122.8 | 95.2 |
| Olotu(2016) | Insurance claims | 1.3 | 106424 | 2003 | T2D diagnosis, T2D medication use |
48.9 | NA | 46.3 | NA | NA | NA |
| Rha(2016) | Insurance claims | 3 | 3398 | 2004 | T2D medication use, FPG>126 mg/dL |
48 | 0 | 60.3 | 24.5 | 114 | 94 |
| 15 studies (2004–2016) | 6.9 | 257098 | 2000 | 51.5 | 71.2 | 57.4 | 26.9 | 124.4 | 96.4 | ||
OBS = Observational study
BMI = Body mass index
LDL-C = Low-density lipoprotein
FPG = Fasting plasma glucose
R = Data requested, but not available
Participant characteristics also differed by study design. Participants in OBSs were on average younger (mean age = 57.4 years vs 63.6 years), had lower mean LDL-C levels at study baseline (124.4 mg/dL vs 145 mg/dL) and mean fasting plasma glucose levels (96.4 mg/dL vs 101.6 mg/dL) compared to participants enrolled in the RCTs (Tables 1 and 2 and Supplemental Tables 2 and 3). In addition, 50% of RCTs used a combination of physician diagnosis, T2D medication, and lab results to define T2D (compared to 13% of OBSs); while 53% of OBSs used physician diagnosis and T2D medication.
Quality assessment of RCTs showed >87% (7/8) of RCTs fulfilled the intention-to-treat, loss to follow-up, and randomization criteria; while two studies (25%) failed to adequately blind participants (Supplemental Figure 1). The quality evaluation among OBSs found >93% (14/15) of studies accounted for differences in baseline factors and clearly defined the sample, T2D, and statins use. However, >66% (10/15) of studies lacked information on baseline fasting glucose levels and >46% (7/15) of studies lacked information on LDL-C levels at baseline by treatment status (Supplemental Figure 2).
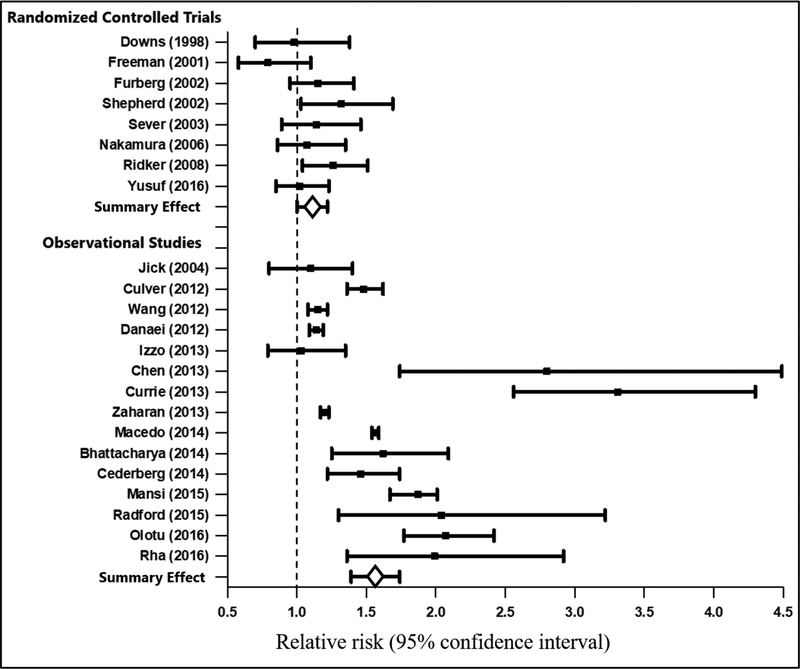
Among RCTs and OBSs, statin users had higher risk of incident T2D compared to nonstatin users, although the magnitude of effect was larger in OBSs (RR = 1.55 [95% CI: 1.39–1.74]) compared with RCTs (RR = 1.11 [95% CI: 1.00–1.22]) (Figure 2). Given the differences in the magnitude of effects, a summary effect is not reported. Funnel plots both overall and by study design suggested little evidence of publication bias (Supplemental Figures 3 and 4). This evidence is consistent with results from Begg’s and Egger’s tests ([RCTs: p-values =0.23 and 0.54] and [OBSs: p-values = 0.91 and 0.32]), but the “trim and fill” method imputed one hypothetically missing RCT (RR = 1.64) and three hypothetically missing OBSs with RRs <1.0. In contrast to RCTs where two studies reported effect estimates below the null (ie. RR < 1.0), all of the OBSs reported effect estimates above the null.
Figure 2.
Meta-analysis examining statin-associated type 2 diabetes risk stratified by study design.
Evidence of heterogeneity varied by study design. Galbraith plots for RCTs indicated that one (Supplemental Figures 5 and 6) Z score fell outside the 95% confidence bounds, evidence of heterogeneity that was consistent with the Cochran’s Q and I2 tests (I2= 27% [p-value = 0.21] and PCochrane = 0.04). Similarly, among OBSs, 47% of Z scores (7/15) fell outside the 95% confidence bounds, providing strong evidence of heterogeneity that was consistent with Cochran’s Q and I2 (I2 = 97.6% [p-value <0.01] and PCochrane <0.01).
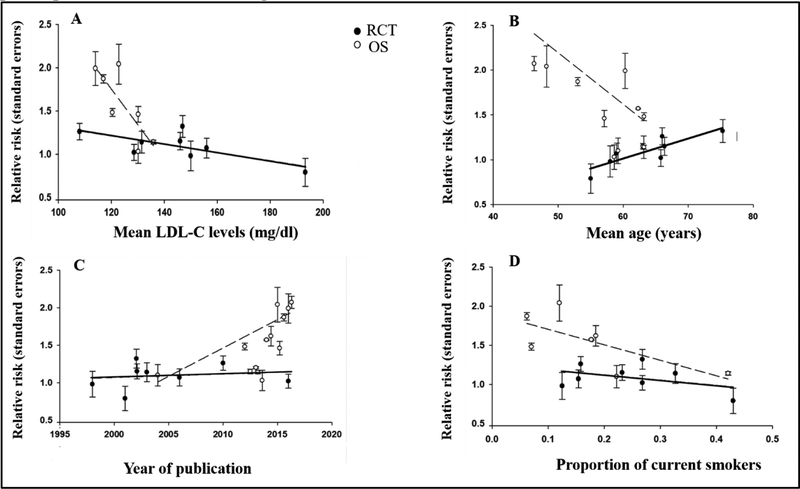
Analyses examining sources of heterogeneity overall and by study design demonstrated variations in effect estimates by study and participant characteristics (Figure 3 and Supplemental Tables 4–6). Overall, the association between statin use and T2D risk was stronger in OBSs compared to RCTs (RR = 1.45 [95% CI: 1.11–1.88]) and in studies published more recently (RR = 1.03 [1.00–1.06]). Comparing participant characteristics across study design, there was a decreased risk for T2D among, participants who were older (RR = 0.79 [95% CI: 0.63–0.98] per 10 year increase); smokers (RR = 0.27 [95% CI: 0.11–0.68] per 1% increase in the proportion of smokers); and had lower LDL-C levels (RR = 0.92 [95% CI: 0.87–0.97] per10mg/dL increase) (Figure 3 and Supplemental Table 4). Among OBSs, the association between statin use and risk of incident T2D was stronger among study populations from non-European countries and study populations that were younger, had fewer smokers, and lower LDL-C levels (Supplemental Table 5). No significant variability in effect estimates were found among RCTs (Supplemental Table 6).
Figure 3. Results from meta-regression analyses examining significant study and baseline participant characteristics among randomized controlled trials and observational studies.
A = Mean low-density lipoprotein cholesterol levels by statin associated type 2 diabetes risk
B = Mean age by statin associated type 2 diabetes risk
C = Year of publication by statin associated type 2 diabetes risk
D = Proportion of current smokers by statin associated type 2 diabetes risk
4. Discussion
Results of this meta-analysis, which are consistent with earlier studies synthesizing estimates of statin-associated T2D risk in primary and secondary prevention populations8, 11, suggest that statins have a moderate effect on T2D risk, increasing risk 11–55%. Yet, strong evidence of heterogeneity was observed, particularly with regard to participant age and baseline LDL-C level. Potential evidence of heterogeneity was not fully examined in earlier meta-analyses and merits further investigation in light of statin guidelines that target growing proportions of primary prevention populations, particularly populations with lower ASCVD risk profiles (e.g. individuals with ASCVD 10-year risk estimates <10%).
The current RCT meta-analysis findings are consistent with results from past meta-analyses conducted among primary and secondary prevention populations that reported T2D risks that were 9%−13% higher in participants randomized to statins compared to placebo.7–10 Interestingly, a prior US Preventive Services Task Force (USPSTF) meta-analysis among six primary prevention RCTs suggested an attenuated association (RR = 1.05 [95% CI: 0.91–1.20]). The USPSTF estimated effect is slightly smaller in size and less precise than our estimate of RR=1.11 (95% CI: 1.00–1.22), which included newly available data from the Heart Outcomes Prevention Evaluation (HOPE-3) trial (N= 12,705). Overall, the body of literature examining statin-associated T2D risk in RCTs suggests a modest relative effect that was consistent across primary and secondary populations.
Meta-analyses of OBSs indicated a similarly elevated statin-associated T2D risk, although the magnitude of effect was considerably higher (RR = 1.55 [95% CI: 1.39–1.74]), possibly reflecting differences in outcome measurement error, confounding, or source populations. For example, in contrast to RCTs, for which a majority included biomarkers (i.e. fasting plasma glucose) when measuring T2D, only four of fifteen OBS studies included biomarkers to measure T2D. Assessing biomarkers of T2D is important given the large burden of undiagnosed T2D in U.S. populations, as contemporary national estimates suggest that one in three adults with T2D are undiagnosed.31 Studies also suggest the potential for outcome measurement error to bias results towards the null32, which, if the case, would suggest that both RCT and OBSs underestimate T2D risk. Yet, use of fasting plasma glucose to define T2D was not a significant predictor of variation in statin-associated T2D risk although the small number of studies that measure fasting plasma glucose may have decreased our ability to detect an association. In addition, the potential for confounding may exist if factors associated with T2D diagnosis also were associated with statin prescription.33 For example, OBS participants prescribed statins may have been more likely to make and attend appointments with primary care physicians, increasing their chances of being clinically evaluated and obtaining a T2D diagnosis.34 However, studies using active comparators to evaluate statin-associated T2D risk reported that statin users had an even higher risk of T2D (RR =3.31 [95% CI: 2.56–4.30]) compared to new diclofenac users, even when both groups had similar chances of being evaluated.35 Ultimately, however, the results may not be combinable, not because of residual confounding bias, but because the target populations might differ. For instance, RCTs often exclude participants that demonstrate signs of drug intolerance before randomization, participants who may be more susceptible to developing T2D, and participants with relevant comorbidities.36, 37 Such exclusions may yield selected populations that are less prone to adverse drug reactions, including T2D, than population-based studies.38 Thus, the different nature of the studies and the different populations enrolled may simply produce estimates of statin-associated T2D risk that are generalizable to different target populations.39
One reason for a lack of widespread generalizability may be the presence of specific subpopulations who are particularly vulnerable to statin-associated T2D risk. For example, our observation of increased statin-associated T2D risk among studies with lower baseline mean LDL-C levels is consistent with evidence of an inverse association between LDL-C and T2D.40 Further, a recent meta-analysis among 34 RCTs found more intensive compared with less intensive LDL-C lowering therapy was associated with a greater reduction in risk of ASCVD mortality in populations with baseline LDL-C levels ≥ 100mg/dL, but not among populations with LDL-C levels < 100 mg/dL.41 Together, these results suggest that populations with the lowest estimated benefits of pharmacologically lowered LDL-C may also have the highest risk of T2D. However, our results remain somewhat tentative as 47% of OBSs included in the present meta-analysis either did not collect or report baseline LDL-C levels. In addition to missing baseline LDL-C levels, the majority of OBSs lacked baseline glucose levels. OBS populations that did not include fasting plasma glucose levels may have had elevated levels at baseline, potentially biasing the risk estimate for OBS towards a higher risk. However, a major risk factor for elevated fasting plasma glucose levels is BMI, and mean BMI estimates were similar between RCT and OBS (Tables 1 and 2). Meta-regression results examining heterogeneity by fasting plasma glucose levels also were not significant predictors of T2D risk.
The association between statins and T2D also varied by mean participant age. However, we had limited ability to fully interrogate the role of age, as the OBSs on average consisted of younger populations compared to RCTs and evidence of heterogeneity precluded pooling across study designs. Understanding the role age may play in the association between statins and T2D is important because age is a dominant factor when determining ASCVD risk and thus statin initiation. For example, in the U.S. nearly all men exceed the 7.5% ASCVD risk threshold for statin initiation in their mid to late 60s and nearly all women in their 70s, despite an otherwise optimal risk factor profile.42
Given the public health and clinical relevance of enumerating statin-associated risks and benefits, future studies specifically designed to accurately estimate statin-associated risk overall and in strata defined by baseline LDL-C and age, and possibly other plausible effect measure modifiers, are needed. Yet, such studies require careful consideration of design features. For example, five of fifteen OBSs were conducted using insurance claims databases, which may not capture participant baseline characteristics, including LDL-C. Contemporary, population-based cohort studies can provide validated baseline measurements on participant characteristics such as LDL-C or information on glucose levels and collect data on T2D incidence and medications. Yet, multi-year gaps between visits complicate the ability to precisely identify statin initiation and T2D diagnosis, although novel approaches that enable linkage with EMRs and claims data may offer opportunities to address some limitations.43 Other potential avenues include large biobanks linked to EMR (e.g. the UK Biobank), although low response rates may introduce additional sources of bias, the effect of which may be difficult to predict.44 In sum, these considerations suggest that comprehensively examining statin-associated T2D risk will continue to require multiple study designs, as the optimal design may not be feasible.
Despite many strengths, there are limitations that merit consideration. First, there were several studies that did not respond to repeated requests for additional study or participant characteristics. Obtaining missing estimates may have increased statistical power for heterogeneity investigations and allowed us to further examine potentially important characteristics such as age, LDL-C, fasting plasma glucose levels, or BMI. However, this study provides some of the first evidence of heterogeneity in statin-associated T2D risk, which may motivate future studies that address these limitations. Second, our investigation of heterogeneity leveraged aggregate rather than individual-participant data (IPD). Reliance on aggregate data reflected challenges associated with accessing, understanding, and analyzing separate datasets.45 Importantly, it has been suggested that an aggregate data meta-analysis is one of the first steps in conducting an IPD meta-analysis and can inform future IPD meta-analyses studies about the potential sources of heterogeneity that warrant examination.46 Third, our investigation of heterogeneity was limited by study and participant characteristics reported at large enough numbers to enable well-powered investigations. For instance, we were not able to obtain enough information to assess statin dose or type as a source of heterogeneity, although some degree of effect modification by dose on statin-associated T2D has been reported.47 However, we were able to assess statin use in multiple settings and compare populations under strict observation (i.e. RCT) with populations under conditions more generalizable to broader populations. Fourth, we were unable to investigate the role of statin adherence, although several studies attempted to exclude participants at risk of non-adherence.48 The effects of statin adherence are difficult to quantify, although failure to address medication non-adherence are long-described.49 Fifth, meta-regressions provide reliable results when at least 10 studies are included; however, our analysis among RCTs was limited to eight studies.29 Finally, our meta-analysis was limited to examining T2D, given the unavailability of studies examining statin-associated elevations in prediabetes risk or interval measures of glucose homeostasis. Future studies on these topics are warranted, given the association of fasting plasma glucose with elevated risk of cardiovascular disease.50
In conclusion, this meta-analysis adds to a growing body of literature statin-associated T2D risk. Findings highlight potentially increased statin-associated T2D risk in younger populations, and populations with lower LDL-C concentrations at study baseline. Taken together, these results help to inform risks of statin use at across CVD risk profiles and underscore the need for more research on statin-association T2D risk as guidelines continue to evolve.
Supplementary Material
Highlights.
Statin users had higher risk of incident type 2 diabetes compared to non-users
Statin-associated risk of diabetes higher among observational studies
Heterogeneity observed by study design and among observational studies
Younger ages and lower cholesterol levels associated with higher incident diabetes
Acknowledgements
Funding: This work was supported by National Heart, Lung, and Blood Institute training grant T32 predoctoral fellowship T32HL007055.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest
The authors declare no conflict of interest
References
- 1.Gu Q, Paulose-Ram R, Burt V and Kit B. Prescription cholesterol-lowering medication use in adults aged 40 and over: United States, 2003–2012 NCHS data brief, no 177. Hyattsville, MD: National Center for Health Statistics, US Department of Health and Human Services, CDC; 2014. 2015. [PubMed] [Google Scholar]
- 2.Kantor ED, Rehm CD, Haas JS, Chan AT and Giovannucci EL. Trends in prescription drug use among adults in the United States from 1999–2012. JAMA : the journal of the American Medical Association. 2015;314:1818–1830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Goff DC, Jr., Lloyd-Jones DM, Bennett G, Coady S, D’Agostino RB, Gibbons R Sr., Greenland P, Lackland DT, Levy D, O’Donnell CJ, Robinson JG, Schwartz JS, Shero ST, Smith SC Jr., Sorlie P, Stone NJ, Wilson PW and American College of Cardiology/American Heart Association Task Force on Practice G. 2013 ACC/AHA guideline on the assessment of cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Journal of the American College of Cardiology. 2014;63:2935–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stone NJ, Robinson JG, Lichtenstein AH, Bairey Merz CN, Blum CB, Eckel RH, Goldberg AC, Gordon D, Levy D, Lloyd-Jones DM, McBride P, Schwartz JS, Shero ST, Smith SC, Jr., Watson K, Wilson PW, Eddleman KM, Jarrett NM, LaBresh K, Nevo L, Wnek J, Anderson JL, Halperin JL, Albert NM, Bozkurt B, Brindis RG, Curtis LH, DeMets D, Hochman JS, Kovacs RJ, Ohman EM, Pressler SJ, Sellke FW, Shen WK, Smith SC Jr, Tomaselli GF and American College of Cardiology/American Heart Association Task Force on Practice G. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129:S1–45. [DOI] [PubMed] [Google Scholar]
- 5.Trialists CT. The effects of lowering LDL cholesterol with statin therapy in people at low risk of vascular disease: meta-analysis of individual data from 27 randomised trials. The Lancet. 2012;380:581–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Trialists CT. Efficacy and safety of LDL-lowering therapy among men and women: meta-analysis of individual data from 174 000 participants in 27 randomised trials. The Lancet. 2015;385:1397–1405. [DOI] [PubMed] [Google Scholar]
- 7.Rajpathak SN, Kumbhani DJ, Crandall J, Barzilai N, Alderman M and Ridker PM. Statin therapy and risk of developing type 2 diabetes: a meta-analysis. Diabetes care. 2009;32:1924–1929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sattar N, Preiss D, Murray HM, Welsh P, Buckley BM, de Craen AJ, Seshasai SRK, McMurray JJ, Freeman DJ and Jukema JW. Statins and risk of incident diabetes: a collaborative meta-analysis of randomised statin trials. The Lancet. 2010;375:735–742. [DOI] [PubMed] [Google Scholar]
- 9.Taylor F, Huffman MD, Macedo AF, Moore TH, Burke M, Davey Smith G, Ward K and Ebrahim S. Statins for the primary prevention of cardiovascular disease. The Cochrane Library. 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chou R, Tracy Dana M, Blazina I, Daeges M and Jeanne TL. Statins for Prevention of Cardiovascular Disease in Adults Evidence Report and Systematic Review for the US Preventive Services Task Force. JAMA : the journal of the American Medical Association. 2016;316:2008–2024. [DOI] [PubMed] [Google Scholar]
- 11.Casula M, Mozzanica F, Scotti L, Tragni E, Pirillo A, Corrao G and Catapano AL. Statin use and risk of new-onset diabetes: A meta-analysis of observational studies. Nutrition, Metabolism and Cardiovascular Diseases. 2017;27:396–406. [DOI] [PubMed] [Google Scholar]
- 12.Mendis S, Abegunde D, Yusuf S, Ebrahim S, Shaper G, Ghannem H and Shengelia B. WHO study on Prevention of REcurrences of Myocardial Infarction and StrokE (WHO-PREMISE). Bulletin of the World Health Organization. 2005;83:820–829. [PMC free article] [PubMed] [Google Scholar]
- 13.Lloyd-Jones DM, Leip EP, Larson MG, d’Agostino RB, Beiser A, Wilson PW, Wolf PA and Levy D. Prediction of lifetime risk for cardiovascular disease by risk factor burden at 50 years of age. Circulation. 2006;113:791–798. [DOI] [PubMed] [Google Scholar]
- 14.Shrier I, Boivin J- F, Steele RJ, Platt RW, Furlan A, Kakuma R, Brophy J and Rossignol M. Should meta-analyses of interventions include observational studies in addition to randomized controlled trials? A critical examination of underlying principles. American journal of epidemiology. 2007;166:1203–1209. [DOI] [PubMed] [Google Scholar]
- 15.Faraoni D and Schaefer ST. Randomized controlled trials vs. observational studies: why not just live together? BMC anesthesiology. 2016;16:102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moher D, Liberati A, Tetzlaff J and Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. International journal of surgery. 2010;8:336–341. [DOI] [PubMed] [Google Scholar]
- 17.Reuters T EndNote X7. Philadelphia: Thomson Reuters. 2013. [Google Scholar]
- 18.Guyatt G, Rennie D, Meade M and Cook D. Users’ guides to the medical literature: McGraw-Hill Medical:; 2015. [Google Scholar]
- 19.Wilson K, Gibson N, Willan A and Cook D. Effect of smoking cessation on mortality after myocardial infarction: meta-analysis of cohort studies. Archives of internal medicine. 2000;160:939–944. [DOI] [PubMed] [Google Scholar]
- 20.Austin PC. The relative ability of different propensity score methods to balance measured covariates between treated and untreated subjects in observational studies. Medical Decision Making. 2009;29:661–677. [DOI] [PubMed] [Google Scholar]
- 21.Sterne JA, Sutton AJ, Ioannidis JP, Terrin N, Jones DR, Lau J, Carpenter J, Rücker G, Harbord RM and Schmid CH. Recommendations for examining and interpreting funnel plot asymmetry in meta-analyses of randomised controlled trials. Bmj. 2011;343:d4002. [DOI] [PubMed] [Google Scholar]
- 22.Egger M, Smith GD, Schneider M and Minder C. Bias in meta-analysis detected by a simple, graphical test. Bmj. 1997;315:629–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Begg CB and Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994:1088–1101. [PubMed] [Google Scholar]
- 24.Duval S and Tweedie R. Trim and fill: a simple funnel‐plot–based method of testing and adjusting for publication bias in meta‐analysis. Biometrics. 2000;56:455–463. [DOI] [PubMed] [Google Scholar]
- 25.Cochran WG. The combination of estimates from different experiments. Biometrics. 1954;10:101–129. [Google Scholar]
- 26.Furlan AD, Pennick V, Bombardier C and van Tulder M. 2009 updated method guidelines for systematic reviews in the Cochrane Back Review Group. Spine. 2009;34:1929–1941. [DOI] [PubMed] [Google Scholar]
- 27.Higgins JP, Thompson SG, Deeks JJ and Altman DG. Measuring inconsistency in meta-analyses. BMJ: British Medical Journal. 2003;327:557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bax L, Ikeda N, Fukui N, Yaju Y, Tsuruta H and Moons KG. More than numbers: the power of graphs in meta-analysis. American journal of epidemiology. 2008;169:249–255. [DOI] [PubMed] [Google Scholar]
- 29.Thompson SG and Higgins JP. How should meta‐regression analyses be undertaken and interpreted? Statistics in medicine. 2002;21:1559–1573. [DOI] [PubMed] [Google Scholar]
- 30.StataCorp. Stata Statistical Software:Release 15. College Station, TX: StataCorp LLC. 2017. [Google Scholar]
- 31.Menke A, Casagrande S, Geiss L and Cowie CC. Prevalence of and trends in diabetes among adults in the United States, 1988–2012. JAMA : the journal of the American Medical Association. 2015;314:1021–1029. [DOI] [PubMed] [Google Scholar]
- 32.Magder LS and Hughes JP. Logistic regression when the outcome is measured with uncertainty. American journal of epidemiology. 1997;146:195–203. [DOI] [PubMed] [Google Scholar]
- 33.Lévesque LE, Hanley JA, Kezouh A and Suissa S. Problem of immortal time bias in cohort studies: example using statins for preventing progression of diabetes. Bmj. 2010;340:b5087. [DOI] [PubMed] [Google Scholar]
- 34.Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, Savović J, Schulz KF, Weeks L and Sterne JA. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. Bmj. 2011;343:d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Currie O, Mangin D, Williman J, McKinnon-Gee B and Bridgford P. The comparative risk of new-onset diabetes after prescription of drugs for cardiovascular risk prevention in primary care: a national cohort study. BMJ open. 2013;3:e003475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yola M and Lucien A. Evidence of the depletion of susceptibles effect in non-experimental pharmacoepidemiologic research. Journal of clinical epidemiology. 1994;47:731–737. [DOI] [PubMed] [Google Scholar]
- 37.Furberg CD, Wright JT, Davis BR, Cutler JA, Alderman M, Black H, Cushman W, Grimm R, Haywood LJ and Leenen F. Major outcomes in moderately hypercholesterolemic, hypertensive patients randomized to pravastatin vs usual care: the Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT-LLT). JAMA-Journal of the American Medical Association. 2002;288:2998–3007. [DOI] [PubMed] [Google Scholar]
- 38.Wilke R, Xu H, Denny J, Roden D, Krauss R, McCarty C, Davis R, Skaar T, Lamba J and Savova G. The emerging role of electronic medical records in pharmacogenomics. Clinical Pharmacology & Therapeutics. 2011;89:379–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stuart EA, Bradshaw CP and Leaf PJ. Assessing the generalizability of randomized trial results to target populations. Prevention Science. 2015;16:475–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Saleheen D, Rader DJ and Voight BF. Disentangling the Causal Association of Plasma Lipid Traits and Type 2 Diabetes Using Human Genetics. JAMA cardiology. 2016;1:631–633. [DOI] [PubMed] [Google Scholar]
- 41.Navarese EP, Robinson JG, Kowalewski M, Kołodziejczak M, Andreotti F, Bliden K, Tantry U, Kubica J, Raggi P and Gurbel PA. Association between baseline LDL-C level and total and cardiovascular mortality after LDL-C lowering: a systematic review and meta-analysis. JAMA : the journal of the American Medical Association. 2018;319:1566–1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bibbins-Domingo K, Grossman DC, Curry SJ, Davidson KW, Epling JW, García FA, Gillman MW, Kemper AR, Krist AH and Kurth AE. Statin use for the primary prevention of cardiovascular disease in adults: US Preventive Services Task Force recommendation statement. JAMA : the journal of the American Medical Association. 2016;316:1997–2007. [DOI] [PubMed] [Google Scholar]
- 43.Selby JV, Smith DH, Johnson ES, Raebel MA, Friedman GD and McFarland BH. Kaiser Permanente medical care program. Pharmacoepidemiology, Fourth Edition. 2005:241–259. [Google Scholar]
- 44.Lawlor DA. Commentary: Two-sample Mendelian randomization: opportunities and challenges. International journal of epidemiology. 2016;45:908–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Riley RD, Lambert PC and Abo-Zaid G. Meta-analysis of individual participant data: rationale, conduct, and reporting. Bmj. 2010;340:c221. [DOI] [PubMed] [Google Scholar]
- 46.Cooper H and Patall EA. The relative benefits of meta-analysis conducted with individual participant data versus aggregated data. Psychological methods. 2009;14:165. [DOI] [PubMed] [Google Scholar]
- 47.Preiss D, Seshasai SRK, Welsh P, Murphy SA, Ho JE, Waters DD, DeMicco DA, Barter P, Cannon CP and Sabatine MS. Risk of incident diabetes with intensive-dose compared with moderate-dose statin therapy: a meta-analysis. JAMA : the journal of the American Medical Association. 2011;305:2556–2564. [DOI] [PubMed] [Google Scholar]
- 48.Jick SS and Bradbury BD. Statins and newly diagnosed diabetes. British journal of clinical pharmacology. 2004;58:303–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sheiner LB and Rubin DB. Intention‐to‐treat analysis and the goals of clinical trials. Clinical Pharmacology & Therapeutics. 1995;57:6–15. [DOI] [PubMed] [Google Scholar]
- 50.Coutinho M, Gerstein HC, Wang Y and Yusuf S. The relationship between glucose and incident cardiovascular events. A metaregression analysis of published data from 20 studies of 95,783 individuals followed for 12.4 years. Diabetes care. 1999;22:233–240. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.