Abstract
Recent reports provide evidence for increased risk of substance use disorders (SUD) among patients with a history of early-life traumatic brain injury (TBI). Preclinical research utilizing animal models of TBI have identified injury-induced inflammation, blood-brain barrier permeability, and changes to synapses and neuronal networks within regions of the brain associated with the perception of reward. Importantly, these reward pathway networks are underdeveloped during childhood and adolescence, and early-life TBI pathology may interrupt ongoing maturation. As such, maladaptive changes induced by juvenile brain injury may underlie increased susceptibility to SUD. In this review, we describe the available clinical and preclinical evidence that identifies SUD as a persistent psychiatric consequence of pediatric neurotrauma by discussing 1) the incidence of early-life TBI, 2) how preclinical studies model TBI and SUD, 3) TBI-induced neuropathology and neuroinflammation in the corticostriatal regions of the brain, and 4) the link between childhood or adolescent TBI and addiction in adulthood. In summary, preclinical research utilizes an innovative combination of models of early-life TBI and SUD to recapitulate clinical features and to determine how TBI promotes a risk for the development of SUD. However, causal processes that link TBI and SUD remain unclear. Additional research to identify and therapeutically target underlying mechanisms of aberrant reward pathway development will provide a launching point for TBI and SUD treatment strategies.
Keywords: Early-life traumatic brain injury, neuroinflammation, substance use disorders, addiction, animal models of addiction
1. INTRODUCTION
Trauma is the leading cause of death among children and adolescents aged zero to 18 years old. This includes, but is not limited to shaken baby syndrome and falls in the first years of life, bicycle and transportation-related injuries in childhood, and sports and motor vehicle accidents during adolescence (Frieden, Houry, and Baldwin 2015). In fact, using the Healthcare Cost and Utilization Project’s National Emergency Department Sample and National Inpatient Sample, Taylor et al. reported that more than one million emergency department visits related to concussion or traumatic brain injury (TBI) among children (aged 0-4 and 4-14 years old) and adolescents/young adults (aged 15-24 years old) occurred in 2013 alone (Taylor et al. 2017). Notably, the number of TBI-related fatalities does not compare to the majority of TBI patients that survive and suffer lifelong consequences of their injuries. Non-fatal TBIs result in longer hospital stays, loss of productivity, extensive rehabilitation and patient care, which taken together contribute to the estimated economic cost of TBI being over $48 billion per year (Corso et al. 2006; Langlois, Rutland-Brown, and Wald 2006). Therefore, following efforts to increase awareness of TBI, recent research in the field of brain injury has identified physical, cognitive, emotional, and behavioral deficits as some of the adverse outcomes associated with pediatric TBI that persist into adulthood (Frieden, Houry, and Baldwin 2015; Haarbauer-Krupa et al. 2017). Prominent examples include the potentially life-long psychological consequences that can develop following a brain injury event. In addition to depression and anxiety, a link to substance abuse has been observed. Several studies have indicated that the younger the patient is at the time of injury and also increased severity of injury are associated with increased risk for problematic alcohol and drug use behavior and the development of substance use disorders (SUD) later in life (Corrigan et al. 2013; Whelan-Goodinson et al. 2009).
While SUD affects approximately 11% of the general population, the rate of SUD among TBI patients ranges between 37-66% (Corrigan 1995; Parry-Jones, Vaughan, and Cox 2006). Notably, data from the 2014 National Survey on Drug Use and Health demonstrates a significant increase in SUD rates from ages 12 to 18 (SAMHSA 2015). Therefore, adolescents represent a population at risk of both experiencing early-life TBI and SUD development with the potential for TBI and SUD comorbidities. Significant advancements in the understanding of the development of SUD among TBI patients can be attributed to the recent increase in interdisciplinary preclinical investigations between neurotrauma and addiction research fields. Molecular changes in the mesolimbic nuclei that occur after neurotrauma that may affect processing of drug euphoria and lead to increased risk for SUD following TBI. As such, animal models of TBI have identified chronic inflammation, blood-brain barrier permeability, and changes to synapses and neuronal networks within the regions of the brain associated with perception of reward that are distant from the initial area of impact (Merkel, Razmpour, et al. 2016; Merkel, Andrews, et al. 2016; Cannella et al. 2019).
This review will focus on key aspects of brain development that are affected when brain injury is experienced during the early stages of life that consequently could lead to an increased risk of SUD development. Thus, the following related topics will be discussed: the incidence of early-life TBI, how preclinical studies model TBI and SUD, TBI-induced neuropathology and neuroinflammation in the corticostriatal regions of the brain, and the link between TBI and addiction. First, epidemiological evidence identifying early-life TBI as an eminent health problem and how preclinical research can be used to help advance treatment approaches available to these patients will be addressed. Then, an overview of what is known regarding the ensuing pathology and inflammatory profile in brain regions anatomically distal from the initial area of impact that mediate the behavioral responses to rewarding stimuli will be provided. Finally, the association of TBI and increased vulnerability to develop SUD during adulthood will be discussed. Notably, this review will call attention to the need for further preclinical research to better understand the molecular underpinnings that contribute to addiction liabilities in the context of TBI; such knowledge will provide a launching pad for the investigation of novel treatment strategies for patients with comorbid TBI and SUD.
2. TBI and the young brain
A key finding by the CDC revealed that the incidence of TBI peaks across three developmental periods: infants aged 0-4, adolescents aged 15-19 (or youths aged 15-24), and adults over the age of 65 (Faul 2010; Taylor et al. 2017). Importantly, it is well established that many of the brain’s neuronal networks continue to undergo developmental changes well into adulthood. Therefore, the damage inflicted by TBI under the age of 19 can result in disruption of ongoing maturation and changes to ongoing development of specialized neuronal circuitry. Several studies have found that TBI-induced inflammation can persist for months or even years beyond when the initial injury occurs (Howell et al. 2018; Johnson et al. 2013). Yet, how chronic inflammation affects the developing brain remains poorly understood.
2.1. Clinical and epidemiological data on early-life TBI
Demographically TBIs are more common in males than females with some reports of ratios as high as 3:1, however both sexes follow similar age-related peaks in incidence, identifying those under the age of 19 at highest risk (Faul 2010; Taylor etal. 2017). The most common causes of TBI-related emergency visits from 2007 and 2013 were falls, being struck by or against an object, and car accidents (Taylor et al. 2017). Although the incidence of TBI and concussions during participation in contact sports during childhood and adolescence is difficult to quantify, the CDC reports the activities of children <19 years old most associated with early-life TBI related injuries are bicycling, football, basketball, soccer, and general playground activity.
Unlike more severe brain injuries, which typically results in loss of consciousness and observable neurological impairment that can be stratified using clinical guidelines such as the Glasgow Coma Scale (GCS), sports-related concussions often seen in child and adolescent athletes can occur in the absence of skull fracture or external bruising or bleeding, making accurate diagnosis of mild TBI a clinical challenge. Compounding the problem, 10%–20% of mild TBI patients continue to report detrimental symptoms for months and even years post-injury (Prince and Bruhns 2017). The extent and manifestation of psychiatric, cognitive, and affective disorders may present differently following childhood and adolescent TBI due to ongoing neural development. Therefore, standardized measures for outcomes unique to pediatric TBI also include academic performance, family, environmental, and daily-living skills, language proficiency, and social cognition (McCauley et al. 2012).
2.2. Preclinical studies (animal models of TBI)
Preclinical research offers valuable resources to investigate mechanisms underlying the link between early-life TBI and subsequent behavioral and molecular outcomes. The combination of interdisciplinary animal models offers many opportunities for novel experimental designs that can help to elucidate poorly understood pathologies and phenotypes seen clinically. Validated models of brain injury have been comprehensively described previously by our group as well as Xiong and colleagues (Merkel 2017; Xiong, Mahmood, and Chopp 2013). In brief, the four models widely used in research are the weight drop, fluid percussion injury (FPI), controlled cortical impact (CCI), and models of blast-induced injury. Each of these models have unique advantages and translational applications. The availability of multiple injury models grants preclinical researchers the opportunity to simulate both severity and type of TBI seen in patients. Notably, each of these models can be applied to juvenile and adolescent aged animals, with the advantage that studies can easily assess outcomes in adulthood that would otherwise require longitudinal clinical studies which are burdened by considerable expense and issues with patient retention.
Clinical studies have demonstrated that experiencing TBI early in life is associated with poorer behavioral outcomes later in life and that psychiatric disorders are more common after childhood TBI (Kennedy, Heron, and Munafo 2017; Max et al. 2015). Preclinical research can be used to recapitulate the psychological outcomes as a consequence of experiencing TBI early in life. For example, following administration of the CCI model of TBI, Washington et al. used a battery of behavioral assays to assess cognitive and emotional deficits including the elevated plus maze for evaluation of anxiety-like behavior, the forced swim test for depression-like phenotypes, and the Morris water maze to examine deficits in spatial learning and memory (Washington et al. 2012). Using juvenile exposure to a closed-head mild modification to the CCI, Rodriguez-Grande et al. also report long-term cognitive deficits including increased anxiety behavior assessed by the open-field test (Rodriguez-Grande et al. 2018). Several studies have reproduced social deficits observed after early-life TBI (Meadows et al. 2017; Ryan et al. 2018). For instance, Mychasiuk and colleagues recorded and analyzed play/fight behaviors following juvenile exposure to the weight drop TBI model and found that rats with a history of brain injury exhibited reductions in play behaviors (Mychasiuk et al. 2014). Similarly, utilizing a three-chamber apparatus to measure social interaction, Yu et al. reported that injured mice with a history of repeated, mild CCI injury demonstrated deficits in social interaction (Yu et al. 2017). Behavioral assays such as the five-choice serial reaction time task (SRTT) can also be combined with TBI models and used to characterize deficits in impulsivity which are commonly reported following TBI during childhood (Wade et al. 2017; Rhine et al. 2018). The findings of Mychasiuk as well as Hehar and colleagues showed that after juvenile weight drop exposure, inaccurate responding and reduced control of inhibitory responding in the SRTT task were higher among injured rats, indicative of increased impulsivity (Hehar et al. 2015; Mychasiuk et al. 2014).
Recent studies have begun to combine the prevailing models of TBI with assays that measure operant and drug-seeking behavior such as the conditioned place preference and intravenous self-administration (Merkel, Razmpour, et al. 2016; Vonder Haar et al. 2018). Therefore, pre-clinical models of early-life TBI represent a powerful tool that can be used to continue to study injury-induced susceptibility to psychiatric disorders seen in childhood TBI including depression, anxiety, conduct disorders, and drug addiction as causal mechanisms underlying this association remain unknown.
2.3. Development and maturation of the reward pathway
Cognitive, emotional, and behavioral deficits seen in humans and recapitulated by animal models of early-life TBI can be attributed to immaturity of brain systems within the prefrontal cortex (PFC) associated with executive functioning and higher-order thinking, changes induced by the injury itself, or both. In fact, adolescence is a developmental period classically known to be characterized by increases in sensationseeking, poor impulse-control, and deficient problem-solving (Walker et al. 2017; Williams et al. 2018). Anatomical and functional brain networks that connect PFC with mesolimbic nuclei such as the nucleus accumbens (NAc) and the ventral tegmental area (VTA) comprise the network affected by the pathophysiology of substance abuse, have been implicated in deficits of processing rewarding stimuli, and remain underdeveloped during adolescence (Hartley and Somerville 2015). Maturation of dopaminergic projections that innervate the PFC is associated with linear increases in white matter tract volume from childhood to adulthood (Walker et al. 2017; Naneix et al. 2012). White matter connectivity is associated with strengthening of neural signaling, executive control, and higher order cognitive functions (Schmithorst and Yuan 2010).
Likewise, grey matter maturation is another ongoing process during adolescence. However, gray matter volume is reported to peak earlier in life and begins to decline during adolescence due to processes of synaptic pruning (Tau and Peterson 2010; Gogtay et al. 2004). Neuronal synapse formation and elimination by pruning is an important aspect normal synaptic development (Paolicelli et al. 2011; Wake et al. 2013). Particularly within the reward pathway (PFC, NAc, VTA), we recently reported aberrant microglial engulfment of synaptic proteins and decreased synaptic spine density following adolescent TBI in mice (Cannella et al. 2019). Plasticity changes within the NAc during adolescence involves microglial-mediated synaptic pruning and maturation of dopaminergic signaling in response to reward-motivated behaviors. In adolescent rodents, Kopec et al. reported microglial involvement in changes in dopamine receptor expression in the NAc (Kopec et al. 2018). Therefore, when TBI occurs during adolescence, the dynamic maturation of anatomical and functional brain networks, such as the reward pathway, are interrupted. This likely contributes to cognitive and behavioral deficits such as SUD reported in patients with history of early-life TBI.
3. TBI neuropathology and neuroinflammation in the addiction pathway
When TBI occurs, the inflicted damage occurs at different stages. The first stage encompasses the initial impact and the damage occurs within seconds to minutes. During this time, there can be fracture of the skull, axonal shearing, disruption of the vasculature and cell death at the site of impact. This is known as the primary injury. Then, after minutes and up to several weeks following the initial injury, patients experience a secondary stage of injury (Walker and Tesco 2013). Metabolic changes begin to occur which can include an increase in the production of free radicals, oxidative stress, and inflammation. Secondary injury can initiate remodeling of the vasculature and programmed cell death by apoptosis and astrogliosis continues (Lozano et al. 2015; Lutton et al. 2017). Unfortunately, the damage does not stop there. Months and even years after the initial injury there can be changes in gene expression as well as alterations in ion channels and processes of cellular signaling. Even regenerative processes can alter the myelination and stability of neurocircuitry resulting in continued cell death and synaptic dysfunction (Johnson et al. 2013; Perez et al. 2016b). Importantly, these injury processes have recently been reported to occur not only at the initial site of injury, but also in distal brain nuclei such as the corticostriatal regions comprising the reward pathway (Merkel, Razmpour, et al. 2016).
3.1. Anatomical alterations following TBI in the maturing brain
Additional long-term consequences seen after pediatric brain injury include structural changes to brain regions beyond the area of impact. Damage to both grey and white matter in brain areas distal from the point of impact can affect ongoing maturation of neuronal networks being developed. Unfortunately, these structural deviations are often associated with long-term deficits since the changes do not occur or are not detectable immediately. Advances in neuroimaging technologies allow both clinical and preclinical researchers to visualize disruptions and alterations following early-life TBI and address adverse behaviors correlated with these gross anatomical differences. For example, magnetic resonance (MR) diffusion tensor imaging (DTI) is a neuroimaging technique that can be applied to both human TBI patients and preclinical animal models of TBI. DTI creates contrasted MR images of the brain based on the diffusion pattern of water along the brain’s white matter tracts.
In clinical studies of pediatric brain injury, DTI has been a useful imaging technique in identifying brain regions vulnerable to white matter damage. Specifically, Ryan and colleagues determined that in a population of 95 children, 53 of which suffered pediatric TBI, injury-induced microstructural damage to white matter and abnormalities in diffusion patterns in the corpus callosum (CC), middle and superior cerebellar peduncles, uncinate fasciculus, sagittal stratum, and dorsal cingulum were predictive of poorer social and cognitive performance at both 6- and 24-months post injury. Ultimately, these authors concluded that forces of acceleration and deceleration of childhood TBI lead to vulnerability of white matter tracts within fronto-temporal, limbic, and projection fibers (Ryan et al. 2018). Fronto-temporal and limbic projections are known to be involved in several cognitive processes including learning, memory, and emotional regulation, likely contributing to social and cognitive deficits that persist longterm following early-life TBI. Furthermore, in a study of young adults (mean age 22), Shah et al. tested the relationship between cognitive performance and DTI at 6 months post TBI. Their findings demonstrated asymmetrical white matter micro-tears, discontinuity, and ultimately decreased volume of the left ventral striatum in TBI patients, indicative of damage to deeper brain structures important for processing rewarding and reinforcing stimuli (Shah et al. 2012). In fact, white matter tracts known as the anterior commissure project directly through the NAc. Together, these results highlight the impact of TBI on brain regions distal from the area of impact. This research implies that damage at the impact and in these distal regions related to the pathophysiology of SUD contribute to long-term psychiatric disorders seen in patients with a history of early-life TBI. However, further clinical research that considers both the damage to the area directly insulted and projecting to areas of the brain that are associated with psychiatric disorders and TBI-related SUD, is critical.
Using DTI in mice, Rodriguez-Grande and colleagues found that distortions to white-matter tracts following exposure to juvenile mild CCI were not detectable at acute time points post injury, indicating their primary injury model was not severe enough to induce white matter alterations directly. However, their study reported breakdown and fragmentation of myelin as measured by increased quantification of myelin basic protein at 7 days post injury and significant white matter alteration in the CC 30 days post injury. Interestingly, white matter damage detected at later time points was correlated with long-term cognitive and anxiety behavioral deficits (Rodriguez-Grande et al. 2018). Yu et al. also used DTI in their animal model of repetitive mild TBI. This study looked at the chronic effects of repetitive injuries on white matter integrity by administering TBI in 8-week-old mice and examining longitudinal outcomes at 3, 6, and 42 days post injury. In addition to anisotropic alterations to the CC, their data also demonstrated white matter damage to cortical brain regions 6 weeks post injury. The cortical damage encapsulated the anterior cingulate cortex which is a brain region implicated in social behaviors. The chronic white matter changes were correlated with the functional deficits in social interaction as described above (Yu et al. 2017). These findings further support the notion that the vulnerability to structural damage induced by TBI is long-lasting and can be brain region dependent. As this area of research has not been fully explored, prospective preclinical research will continue to benefit from utilizing advances in neuroimaging techniques to better understand TBI pathology in deeper brain structures including the mesolimbic reward pathway of drug addiction.
3.2. Induction of inflammatory markers
A key secondary mechanism of TBI pathology is neuroinflammation (Walker and Tesco 2013). The neuroinflammatory response is a well-established consequence of TBI that can be robust and persist for years after a single injury (Johnson et al. 2013). The inflammatory response to the initial injury itself is well characterized by activation of glial cells at the area of impact. Microglia, the primary source for immune defense in the central nervous system (CNS), use phagocytic and cytotoxic mechanisms to maintain homeostasis during immune responses to injury conditions such as TBI. Recently, glial activation following early-life TBI has also been reported in distal brain regions including the PFC, NAc, and VTA (Merkel, Razmpour, et al. 2016; Merkel, Andrews, et al. 2016). Specifically, the presence of distinct pro-inflammatory genes upregulated in the NAc two weeks following adolescent CCI in mice was observed. Subsequent studies demonstrated that administration of an anti-inflammatory synthetic corticosteroid reduced microglial and astroglial activation in the NAc (Merkel, Andrews, et al. 2016). Similarly, at two weeks following adolescent CCI TBI, Karelina et al. found that treatment with minocycline, a tetracycline antibiotic commonly used to inhibit microglial activation, attenuated production of cytokines such as IL-1 β as well as microglial activation within the NAc (Karelina, Nicholson, and Weil 2018).
Prior to the peak activation of microglia, TBI stimulates the release of inflammatory cytokines and chemokines which then stimulate the infiltration of immune cells such as neutrophils and monocytes/macrophages (Beschorner et al. 2002; Loane and Byrnes 2010). The functional role of neutrophil and monocyte subtypes is not well understood, and long-term inflammatory cellular accumulation can be detrimental. Following inhibition of neutrophil elastase, a proteolytic enzyme secreted by activated neutrophils, Semple et al. demonstrated attenuated inflammatory profiles of the cortex and hippocampus as measured by decreased cell death and rescued cognitive behaviors after administration of CCI in juvenile mice (Semple et al. 2015). Recently, using flow cytometry to characterize infiltrating monocytes two weeks following CCI, we found that compared to the adult inflammatory response that resolved by 14 days after TBI, mice that experienced TBI during adolescence demonstrated persistent immune cell infiltration that was still markedly detectable at 28 days post injury both globally in the injured hemisphere as well as in isolated NAc regions (Cannella et al. 2019). Taken together, these results support the notion that persistent inflammation within the reward pathway induced by TBI may then contribute to increased drug-seeking behaviors seen in TBI-related SUD.
3.3. Blood-brain barrier hyperpermeability
Loss of blood-brain barrier (BBB) integrity is another hallmark pathological consequence of secondary injury after TBI (Lozano et al. 2015). Biomechanical forces and biochemical insults of TBI induce vascular disruptions which breach the neuronal microenvironment and foster the propagation of injury resulting in hyperpermeability, microhemorrhages, and rupture of capillaries. Age differences in indicators of BBB permeability after TBI, such as brain edema and water content, demonstrate more progressive pathology in patients who experience early-life TBI (Fukuda et al. 2012). Unresolved BBB damage can lead to continued and proliferative inflammation and put patients at prolonged risk for serious CNS consequences after the initial injury (Ichkova et al. 2017). Clinically, CT scans used to assess TBI severity typically present as normal in patients with mild TBI and concussions due to the limited ability for these scans to reveal ultrastructural damage to the vasculature in deeper brain regions (Gill et al.2018).
Preclinical studies utilize a plethora of techniques to quantify BBB hyperpermeability following TBI including extravasation of molecules that are too large to cross the BBB in the absence of damage. For example, Rodriguez-Grande et al. quantified the extravasation of immunoglobulin G (IgG) 24 hours post juvenile mild CCI and reported an increase in BBB permeability in the CC (Rodriguez-Grande et al. 2018). Similarly, we also observed BBB leakage in brain parenchyma 48 hours post adolescent CCI by immunohistochemical detection of fibrinogen, a plasma protein that does not cross the BBB under normal conditions (Lutton et al. 2017). The expression of tight junction protein (TJP) complexes that line cerebral vessels and form the physical barrier between the blood and the brain, has also been utilized as an indicator of BBB breakdown. Using CCI in juvenile rats, Badaut et al. found that the TJP claudin-5 exhibited a bi-phasic response that correlated with IgG extravasation. Specifically, they report a 21% decrease of expression of claudin-5 which coincided with peak IgG extravasation at 3 days post injury. However, at 7 days post injury, IgG extravasation normalized and was associated with a 39% increase in claudin-5 expression (Badaut et al. 2015). Although there are many techniques for quantifying damage to the BBB, more studies are needed to fully understand TBI induced BBB permeability. In this case, preclinical research identifying damage to vasculature in deeper brain regions would greatly increase translatability clinical studies of human TBI patients. Damage to brain vasculature and loss of BBB integrity can also be inferred from increased concentrations of proteins in peripheral blood that are typically expressed intracellularly by cells of the CNS. For example, in a study examining blood-based biomarkers for diagnostic efficacy in mild TBI, Gill and colleagues found that blood levels of CNS cellular proteins such as tau, glial fibrillary acidic protein, and neurofilament light chain, demonstrated good discriminatory power to detect MRI abnormalities even when CT scans were unable to detect ultrastructural damage to mild TBI and concussion patients (Gill et al. 2018). Preclinical and clinical research on BBB hyperpermeability in additional brain regions and long-term consequences is imperative.
3.4. Synaptic alterations
Acute neuronal cell death contributes to the known pathology of the primary stage of injury following TBI. Furthermore, chronic changes in neuronal morphology and synaptic characterization in the absence of cell death also occur after TBI (Perez et al. 2016a). Preclinical studies offer a distinct advantage of studying synaptic alterations following early-life TBI as cellular changes can only be evaluated post-mortem in clinical TBI cases. Using the FPI model of mild TBI in rats, Zhao et al. reported that in the absence of neuronal cell loss, TBI resulted in reduced spine density of pyramidal neurons in the infralimbic cortex of the PFC by golgi-cox analysis (Zhao et al. 2018). Comparably, Semple et al. evaluated morphological alterations of pyramidal neurons in the PFC and granule cells of the hippocampus and described reduced dendritic complexity associated with deficits in social interaction behaviors after pediatric CCI (Semple et al. 2017). In contrast, following juvenile exposure to the weight drop TBI model, Mychasiuk et al. demonstrated increased dendritic length, increased spine density, and overall greater complexity of neurons analyzed from the anterior cingulate region of the PFC. Whereas typically these neuronal characterizations would be considered neuroprotective, the authors describe maladaptive synaptic pruning during adolescent development as a consequence of brain injury in mice (Mychasiuk et al. 2015). Aberrant synaptic pruning has been linked to neurological dysfunction and seen in psychiatric disorders such as schizophrenia, autism, and SUD (Forsyth and Lewis 2017; Kim et al. 2017; Kogachi et al. 2017)
Hehar et al. examined morphology and synaptic alterations of medium spiny neurons in the NAc two weeks following the weight drop of mild TBI in juvenile rats and demonstrated injury-dependent reductions in both dendritic length and spine density associated with increased impulsivity behaviors (Hehar et al. 2015). Likewise, we measured changes in morphology and arborization of medium spiny neurons in the NAc two weeks following either adolescent or adult CCI TBI. We found that only moderate adolescent CCI induced significant reductions in dendritic length, dendritic complexity and arborization, and spine density compared to either mild CCI or adult CCI. Notably, we also reported increased spine length following adolescent CCI in the NAc, indicative of increased long, thin spines known to be easily excitable. These neuronal morphological changes in the NAc were associated with increased sensitivity to the rewarding effects of cocaine (Cannella et al. 2019).
3.5. Changes to the dopaminergic system
Dopamine (DA) is the primary CNS neurotransmitter that underlies reward-motivated behaviors. The DA pathway is comprised of anatomical and functional connectivity between dopamine neurons that project from the VTA and release DA into the NAc. In a clinical setting, Donnemiller and colleagues showed that even several months after the initial injury, adults with TBI demonstrated detectable deficits in striatal DA signaling using single-photon emission tomography imaging and radio-labeled tracers that bind to the DA transporter (DAT) (Donnemiller et al. 2000). The effect of brain injury on DA signaling has been implicated in the negative psychiatric sequelae afflicting TBI patients. Therefore, DA has been a targetable system with therapeutic potential for recovery. Treatment with DA agonists has shown improved executive cognitive function in humans (Kraus et al. 2005) and improved neuronal modulation in animals (Goldstein 1993).
Preclinically, several studies have demonstrated changes to the DA system in animal models of early-life TBI. For example, using fast-scan cyclic voltammetry, Chen et al. reported that adolescent rats exposed to the FPI model of brain injury demonstrated significant deficits in both DA release and reuptake within the NAc for up to two-weeks post TBI, with greater effects in the NAc core (Chen et al. 2017). These deficits normalized and returned to baseline DA levels at 4 weeks post injury suggestive of a temporal hypodopaminergic state following adolescent TBI. These authors expanded upon these findings in an additional study where they again found that DA release was suppressed by FPI and in the presence of nicotine desensitization, DA release remained proportionally suppressed after FPI (Chen et al. 2018). Taken together these results suggest drug sensitivity was affected by TBI-suppressed DA release and a reward response required a significantly stronger stimulus. Further, Karelina et al. also report a hypodopaminergic state as assessed by decreased quantification of tyrosine hydroxylase, the precursor enzyme in DA synthesis, in the VTA, and reduced expression of DAT in the NAc DR2 in the striatum following juvenile CCI that persisted into adulthood when analyzed 7 weeks post injury (Karelina, Gaier, and Weil 2017). The findings of these studies highlight the importance of considering deficits in DA release and reuptake in juvenile and pediatric TBI patients in order to assess long-term consequences and vulnerability to SUD mediated by dysfunction of the DA system. However, since the effects of TBI on the DA system are not fully understood, further research investigating TBI-induced DA dysfunction and increased risk for SUD development is needed
4. TBI and addiction
4.1. Clinical history of TBI and susceptibility to SUD
Several studies have demonstrated an increased risk for SUD in adults with a history of TBI (Whelan-Goodinson et al. 2009; Corrigan, Bogner, and Holloman 2012). For example, recent data from the 2012-2013 National Epidemiologic Survey on Alcohol and Related Conditions revealed that TBI was significantly associated with past year SUD (Vaughn et al. 2018). McHugo et al. assessed the rate and severity of TBI among 295 people in an outpatient mental health facility diagnosed with comorbid mental health disorders and SUD and found that 80% of these patients tested positive for a history of TBI according to the Ohio State University TBI Identification Method (McHugo et al. 2017). Additionally, in a study of electronic data of U.S. Military personnel, Miller etal. reported higher rates of comorbid addiction-related disorders among soldiers with history of mild TBI across all three periods post injury (1–30 days, 31–179 days, and >180 days post injury) (Miller etal. 2013).
Clinical studies have recently identified TBI experienced during childhood and/or adolescence as a risk factor for being vulnerable to problematic drug use (Corrigan et al. 2013; Hie et al. 2016). However, it is not known whether the specific age of injury (early childhood vs adolescence) can differentiate which population is at greatest risk of SUD development (see section 4.4.2 for more details). Furthermore, Kennedy et al. performed a longitudinal study of 14,541 patients recruited in England followed since birth and assessed the association of TBI with psychiatric symptoms at age 17. Their findings demonstrated an increased risk of problematic alcohol use in participants with a history of TBI compared to those who experienced an orthopedic injury or no injury (Kennedy, Heron, and Munafo 2017). In another longitudinal birth cohort study of 1,265 children by McKinlay et al., SUD assessed between ages 14-16 were significantly more prevalent in children with a history of being hospitalized for TBI before the age of 5 (McKinlay et al. 2009). Similarly, in a study of 636 inmates of the South Carolina Department of Corrections showed that those who had experienced their first TBI prior to age 13 reported a higher percentage of illicit drug use, compared to those that had a TBI after 13 or had no injury (Fishbein et al. 2016). While such longitudinal findings implicate early-life TBI in adverse neurodevelopmental changes that could then increase susceptibility to SUD, more research is needed to better understand the longterm effects of childhood and adolescent injury.
4.2. Preclinical evidence of SUD risk following early-life TBI
Although preclinical TBI research has advanced our understanding of the progression of brain injury pathology and its impact on various aspects of behavior, studies on how TBI affects addiction vulnerability are lacking. In fact, only a limited number of reports investigate assays of drug reward and reinforcement despite SUD being the third most frequent neuropsychiatric diagnoses among individuals with TBI (Whelan-Goodinson et al. 2009). Further, the majority of these studies have only examined SUD in the context of adult brain injury and have produced conflicting results. For example, using a two-bottle choice assay to quantify differences in alcohol consumption, Lim et al. report comparable rates of alcohol intake in rats exposed to a blast model of mild TBI compared to controls (Lim et al. 2015). By contrast, work by Mayeux et al. revealed augmented alcohol self-administration in adult rats following TBI induced by the FPI model (Mayeux et al. 2015). Recent work extends SUD after adult TBI research beyond the scope of alcohol and has tested the effects of brain injury on psychostimulant self-administration using cocaine. Specifically, Muelbl et al. analyzed cocaine self-administration following exposure to mild blast TBI and while they did not report differences in cocaine intake, the authors described cognitive deficits in operant learning in the injured rats compared to controls as measured by increased errors during self-administration acquisition (Muelbl et al. 2018). Although Muelbl et al. observed no distinctions in drug-seeking behavior in their brain injury model, a study by Vonder Haar et al. revealed significant increases in cocaine infusions in the selfadministration assay following both mild and severe CCI TBI (Vonder Haar et al. 2018).
While these studies corroborate the link of increased risk of SUD following TBI that occurs during adulthood, investigations that model early-life TBI are nearly absent from the literature. Studies by Weil et al. have assessed the impact of juvenile TBI on alcohol consumption later in life. Specifically, 21-day-old mice that suffered a closed-head injury demonstrated increased alcohol place preference in the conditioned place preference (CPP) assay and increased alcohol consumption quantified in the two-bottle choice test during adulthood (Weil et al. 2016). Karelina et al. expanded these findings by replicating the effect of juvenile TBI on increased alcohol intake which was attenuated by minocycline treatment (Karelina, Nicholson, and Weil 2018). While these two studies have demonstrated that juvenile TBI increases alcohol consumption, our previous report was the first to assess the effects of adolescent TBI on the rewarding effects of cocaine (Merkel, Razmpour, et al. 2016). Our recent work extends these findings and suggests that TBI during adolescence, a developmental period characterized by ongoing maturation of the reward pathway, increased sensitivity to the rewarding efficacy of a subthreshold dose of cocaine (Cannella et al. 2019). Collectively the preclinical literature investigating the link between TBI and SUD demonstrates that early-life brain injury exacerbates alcohol and cocaine addiction vulnerability. However, whether this phenomenon generalizes to other drugs of abuse is unknown as animal models have yet to investigate the effects of juvenile TBI on the rewarding effects of other substances such as opioids or marijuana. Further, studies so far have only tested SUD outcomes following a single TBI impact. Therefore, future studies may aim to investigate repetitive injury modeling, representative of multiple concussions as commonly experienced in adolescent participation in contact sports and subsequent vulnerability to SUD.
4.3. The possible mechanistic role of Orexin in TBI and SUD
Orexin is a CNS peptide that regulates arousal and is known to play a role in reward-motivated behaviors. Dysregulation of orexin systems has also been implicated in psychiatric disorders after TBI (Gentile et al. 2018; Harris, Wimmer, and Aston-Jones 2005; Martin-Fardon et al. 2018). For example, Baumann et al. found deficits in orexin levels measured by cerebral spinal fluid in patients with a history of TBI which was correlated with sleep-wake disturbances (Baumann et al. 2007; Baumann et al. 2005). Expanding upon these findings, Baumann et al. also evaluated post-mortem tissue from patients who did not survive severe brain injuries and found a 27% decrease in orexin neurons after TBI compared to control tissue (Baumann et al. 2009).
Alterations in sleep-wake behaviors, cognitive deficits, and reduced orexin neurons were also reported in preclinical studies by Skopin et al. one month following the FPI model of TBI in rats (Skopin et al. 2015). Thomasy et al. also found decreased wakefulness and fewer orexin-producing neurons that persisted for at least 15 days post TBI, and posited a role for chronic inflammation in orexin deficits after CCI in mice (Thomasy et al. 2017). The orexin system has also been considered for therapeutic efficacy in animal models of TBI. Following juvenile exposure to FPI, Lim et al. demonstrated that dietary supplement of branch-chained amino acids (BCAA) that directly modulate orexin neuron activity reinstated orexin neuron activation and improved deficits in wakefulness mice with mild brain injury (Lim et al. 2013). Elliott et al. expanded upon these findings, revealing that in mice with juvenile FPI, BCAA dietary modulation attenuated sleep-wake disturbances and restored orexin neuronal activity through synthesis of GABA (Elliott et al. 2018). Thus, given the anatomical relationship in that hypothalamic orexin neurons project to dopamine neurons in the VTA, the orexin system may be a promising targetable system to treat chronic psychiatric issues in TBI patients including risk for SUD (Fadel and Deutch 2002).
4.4. Confounding variables of TBI-related SUD
4.4.1. Sex as a biological variable underlying risk of SUD after early-life TBI
One potential factor that could influence the risk of SUD following early-life TBI and SUD susceptibility is biological sex. Unfortunately, TBI and the effect of sex is understudied and sex-dependent outcomes following TBI remain controversial. The studies discussed in this section highlight the need for further research to better understand how females are affected by TBI compared to males, particularly in the context of SUD vulnerability.
In the context of early-life TBI, sex-specific fluctuating hormones can only be considered as a potential contributing factor of TBI-related sex differences in outcomes after the onset of puberty, typically having occurred by age 13. For instance, using data from the National Trauma Data Bank Research Data Sets from 2007-2008, Ley et al. suggested that hormonal differences may contribute to sex differences in rates of mortality following isolated, moderate-to-severe TBI as they report lower mortality rates in postpubescent female TBI patients (aged 13-18 years old) compared to males, yet no difference in mortality rates between male and female pre-pubescent patients (aged 0-12 years old) patients (Ley et al. 2013). However, when the outcomes evaluated extend beyond rates of mortality and consider the development of long-lasting psychiatric disorders such as SUD, clinical studies report sex-specific effects but do not typically consider hormonal status at the time of early-life TBI. In a study of 9,299 students enrolled in grades 7 through 12, Ilie and colleagues found that among long-term consequences examined, adolescent males with a history of TBI were more likely to report daily nicotine use while adolescent females with a history of TBI were more likely to report increased alcohol use in the past year (llie et al. 2016). Similarly, in an assessment of neuropsychiatric outcomes among New Zealanders with a history of either moderate/severe childhood TBI (aged 0-17 years old), mild childhood TBI, or an orthopedic childhood injury, Scott et al. found that males and females displayed significant differences in internalizing versus externalizing behaviors such that males were more likely to report substance abuse/dependence (DSM-IV-TR criteria) and females reported higher rates of anxiety and depression, the effects of which were then increased further in those with a history of childhood TBI (Scott et al. 2015). It is important to note that psychosocial norms related to reporting symptoms of physical and mental health among females versus males may also influence sex differences in internalizing and externalizing behaviors observed in TBI and SUD populations.
The majority of the preclinical studies exploring the link between early-life TBI and SUD discussed above included the use of only male animals. Notably, preclinical studies that consider levels of fluctuating sex hormones in the extent that sex differences may mediate the risk of SUD after early-life TBI are also limited by animal age. For example, Weil et al found increase alcohol consumption in female mice with a history of juvenile CCI-TBI at post-natal day 21, prior to the age of onset of the estrous cycle (Weil et al. 2016). Our group has begun to assess estrous phase at the time of injury in adolescent female mice and determine the effect on increased vulnerability to cocaine conditioned preference. Unlike our observations in male mice discussed above, preliminary results suggest that after adolescent CCI-TBI, females do not demonstrate increased drug-seeking behaviors and that higher levels of estrogen and progesterone at the time of injury may be protective against augmented cocaine CPP shifts. The role of sex-specific hormones, particularly progesterone and estrogen, has been extensively investigated preclinically and has reproducibly exhibited neuroprotective effects following experimental TBI. Independently, the work of Roof et al., O’Connor et al., and Garcia-Estrada et al. each demonstrated that female hormones reduced brain edema and inflammation in animal models of contusion injury, diffuse axonal TBI, and penetrating brain injury, respectively (Roof, Duvdevani, and Stein 1993; O’Connor, Cernak, and Vink 2005; Garcia-Estrada et al. 1993). However, after being tested in phase 3 clinical trials in human TBI patients, progesterone failed to demonstrate an advantage in mortality rates compared to placebo and these trials were discontinued (Lu etal. 2016; Goldstein et al. 2017). Additional research investigating the influence of sex as a biological factor on outcomes following early-life TBI such as SUD vulnerability is critical, particularly among post-pubescent adolescent patients where fluctuating hormone levels can be taken into consideration. Further studies will improve the understanding and awareness of sex-specific vulnerabilities to TBI and interactions with persistent psychiatric problems like SUD and will advance individual strategies for treatment.
4.4.2. The role of age of injury in risk of SUD after TBI
Another factor that could potentially escalate SUD susceptibility after early-life TBI is the age at the time the initial injury occurs. As discussed above, early childhood brain development and function may represent a period of vulnerability to damage inflicted by TBI. Further, dynamic alterations in plasticity and strengthening of brain networks that mediate executive cognitive functions maturing throughout adolescence are likely also sensitive to external insults. A comprehensive review of how age of injury can be considered as a factor for increased risk of SUD or alcohol use disorder after TBI have been previously described by our group and Weil et al, respectively (Merkel 2017; Weil, Corrigan, and Karelina 2016). Yet, research continues to support the notion that age at the time of injury can mediate risk of SUD after early-life TBI. For example, McKinlay et al. extended their analysis of the birth cohort of children of the Christchurch Health and Development Study in New Zealand and found significant correlation between early childhood (age 0-5 years old) and adolescent (age 16-21 years old) TBI that required inpatient treatment and later alcohol and drug dependence (age 16-25 and 21-25, respectively) but no significant association if TBI occurred between ages 5-15 years old (McKinlay et al. 2014). Furthermore, Kennedy and colleagues also investigated the association between age of injury and psychiatric symptoms, substance use, and criminal behaviors assessed at 17 years old and found that childhood (age 0-11 years old) TBI was correlated with problematic cannabis use while adolescent (age 12-16 years old) TBI was correlated with problematic cannabis and alcohol use compared to controls who experienced age-matched orthopedic injuries (Kennedy, Heron, and Munafo 2017).
Adolescence is a developmental period in animal models of SUD that is associated with increased sensitivity to the rewarding effects of drugs of abuse. For example, although not in the context of TBI, Zakharova et al. found that adolescent male rats developed a significant cocaine CPP shift at lower cocaine doses than adult rats (Zakharova, Wade, and Izenwasser 2009). Badanich and colleagues also found that adolescent rats were more likely to establish CPP to subthreshold cocaine doses and that ontogenetic differences in DA release and reuptake, as measured by microdialysis, may account for age-specific sensitivities to the reinforcing properties of cocaine, and ultimately increased vulnerability to addiction during adolescence (Badanich, Adler, and Kirstein 2006). Similar to our findings, in a neurochemical brain injury model induced by administration of the dopaminergic neurotoxin 6-hydroxydopamine, Schenk and colleagues reported that lesioned rats reliably responded for the subthreshold intravenous doses of self-administered cocaine, suggestive of increased sensitivity to the rewarding effects of cocaine (Schenk et al. 1991). Our recent study is the first to report increased sensitivity to subthreshold doses of cocaine if CCI-TBI occurred during adolescence but not if CCI-TBI occurred during adulthood (Cannella et al. 2019).
Collectively, these studies suggest that age of injury is an important confounding variable to consider in the link between early-life TBI and SUD. Causal relationships between age of TBI and risk of problematic drug and alcohol use later in life is still unknown as several factors have been shown to characterize this risk. Thus, more specific details regarding age at the time TBI occurred rather than binary history of TBI or no history of TBI should be considered in future prospective and retrospective studies investigating the association of early-life TBI and SUD.
4.4.3. Intrinsic versus extrinsic factors that may influence the likelihood of TBI-related SUD
Both intrinsic factors, such as comorbid neuropsychiatric disorders, and extrinsic factors including the patient’s home and family environment exemplify additional confounding variables that can influence SUD development following early-life TBI. Studies that have assessed diagnosis of SUD after history of TBI typically adjust the analysis using confounding factors rather considering these as independent variables. For example, in an adult population, Vaughn et al. report a significant correlation of past year TBI with SUD after adjusting for age, sex, race/ethnicity, socioeconomic status (SES), education, and parental history of psychiatric disorders (Vaughn et al. 2018). In a population with childhood TBI, Kennedy and colleagues found TBI was associated with problematic alcohol use after adjusting for mother’s age and education at birth, social class, self-reported parenting style, and maternal drug use (Kennedy, Heron, and Munafo 2017). These findings imply that SUD risk is increased following TBI if such confounding variables are controlled for. However, intrinsic and extrinsic factors are important considerations that can directly influence long-term outcomes.
As mentioned above, the development of anxiety and depression represent the most common neuropsychiatric disorders that TBI patients experience, and similar to the general population, often account for comorbid diagnoses seen in TBI-SUD patients (Vaughn et al. 2018; Whelan-Goodinson et al. 2009). Just as TBI has been shown to compromise areas of the brain important for the processing of reward and drug-induced euphoria such as NAc, PFC, and VTA, childhood brain injury can also negatively impact areas involved in cognitive and emotional regulation linked to anxiety and depression such as the amygdala and hippocampus (Beauchamp et al. 2011). Risk of psychiatric disorders such anxiety and depression after early-life TBI is likely regulated by a complex combination of injury-induced anatomical alterations, neurochemical dysfunction, and psychosocial factors. Individuals diagnosed with mood disorders after experiencing TBI during childhood could turn to drugs of abuse as a maladaptive coping strategy to manage symptoms of underlying psychiatric disorders. For instance, Wills and Filer suggested that in attempts to alleviate depressed or anxious symptoms, adolescents may participate in substance use as a coping strategy (Wills and Filer 1996). However, even in adults, studies investigating the link between self-medicating to treat neuropsychiatric disorders and SUD development are limited and controversial. In a study of 494 hospitalized drug abusers, Weiss and colleagues reported the majority of patients used drugs to reduce symptoms of depression (Weiss, Griffin, and Mirin 1992); however, in a study of 70 patients undergoing methadone maintenance treatment, Hall and Queener failed to show an association between substance use as a means to cope with symptoms of anxiety and depression (Hall and Queener 2007).
Risk of Secondary Attention-Deficit/Hyperactivity Disorder (SADHD) has also been reported following early-life TBI (Narad et al. 2018). In a study of 200 children (aged 5-17 years old), Schachar and colleagues found that SADHD and symptoms of anxiety were significantly more frequent among children that experienced TBI at least 2 years prior and that the interaction of TBI and SADHD was predictive of deficits in measurements of impulsivity and response inhibition (Schachar et al. 2004). Impulsivity and response inhibition deficits have been extensively described as risk factors for the development of SUD (Lawrence et al. 2009; Groman, James, and Jentsch 2009; Kozak et al. 2018). Taken together, these findings suggest that individuals with a history of early-life TBI may be more likely to develop SUD as a consequence of confounding intrinsic factors such as comorbid neuropsychiatric disorders.
The extrinsic factor SES can financially regulate a patient’s access to innovative treatment approaches, state-of-the-art rehabilitation centers, and education about the care needed, which may then affect long-term outcomes. However, the support systems available to patients, particularly those that experience TBI during earlier childhood years, has shown a strong correlation with prognosis as several studies support the notion that the home environment has a significant effect on outcome following early-life TBI (Anderson etal. 2006; Wade etal. 2016; Yeates etal. 2010). Specifically, in a study of 550 SUD patients entered in an Alcohol-Drug Program, Felde and colleagues described extrinsic factors such as loss of a parent during childhood and childhood behavior problems (e.g. truancy, delinquency, vandalism, etc.) as strongly associated with a history of TBI (Felde, Westermeyer, and Thuras 2006). Likewise, in a study of 723 adolescent residents of the Missouri Division of Youth Services, Perron and Howard found that adolescents with a history of TBI were significantly more likely to have used drugs such as heroin, cocaine or crack cocaine, marijuana, and ecstasy than those without a history of TBI. Adolescents with TBI and SUD were also more likely than those without to report negative environmental experiences such as being hit by someone, having had someone use a weapon or force against them, and be attacked by someone who was otherwise trying to injure them (Perron and Howard 2008). Vaughn et al. also found significantly higher reporting of substance use, alcohol or drug abuse or dependence, violent victimization and witnessing violence among adjudicated adolescents with a history of TBI than those without (Vaughn et al. 2014).
Vulnerability to SUD following early-life TBI is multifactorial, and confounding variables, including but not limited to sex, age of injury, and intrinsic and extrinsic factors should be taken into consideration. Further preclinical and clinical research exploring risk of SUD after childhood or adolescent TBI is clearly needed and will benefit from controlling for confounding variables that might influence long-term psychiatric and behavioral outcomes after injury.
5. Summary and Future Perspectives
Substance abuse is a multifaceted brain disorder that has significant consequences on behavioral characteristics. A five-year study conducted by Columbia University reported that "40 million Americans age 12 and over meet the clinical criteria for addiction involving nicotine, alcohol or other drugs”. Worse yet is that “another 80 million Americans fall into the category of risky substance users” (Columbia 2012). As with any other health condition, it is important to understand the risk factors that contribute to the development of the addictive behavior. In this review, we have focused on one possible emerging risk factor that links a pre-adulthood history of TBI with increased vulnerability to substance use disorders. Thus, future investigations are needed to further explore the link between early-life TBI and SUD vulnerability. Findings from such studies will serve to increase awareness and education of TBI patients to the possibility of an increased risk for the development of SUD. Importantly, by understanding how TBI negatively affects the development of the reward pathway, we can develop innovate therapeutic strategies that help to restore normal function.
What then could be some broad areas of exploration that could provide crucial insight into how early-life TBI-induced neuropathology affects SUD vulnerability? First, we call attention to the need for studies to define whether addiction susceptibility after TBI also alters natural reward responses. For instance, would pre-adulthood TBI modify the responses to natural rewards such as those associated with eating, drinking, procreating, and nurturing? Secondly, in terms of addiction to illicit drugs, it is possible that the enhanced addiction phenotype seen (post-TBI) for the psychostimulant cocaine, differs from other stimulants like methamphetamine, synthetic designer drugs etc. Moreover, it is not known whether TBI may also affect other categories of drugs of abuse such as: hallucinogens, benzodiazepines, barbiturates, nicotine, and opiates. Third, addiction has phases that include: reinforcement, drive, incentive, tolerance, dependence, and relapse. Therefore, future studies could reveal how the stages of addiction are changed as a function of brain injury. Fourth, it is essential to recognize that TBI severity (i.e. mild, repeated TBI, moderate, and severe) must be considered in all the above, since severity affects acute and chronic pathologic responses that undoubtedly will have different behavioral manifestations. Fifth, a closer look into the changes in molecular and cellular pathways will aid in testing the notion that specific neuroinflammatory responses disrupt the neurocircuitry within the reward pathway which leads to aberrant neurotransmission such as that associated with the dopaminergic system.
Overall, it is the hope that scientific research into TBI and its link to SUD will: 1) help health care providers inform TBI patients about the risk for SUDs and 2) promote discoveries that can offer neuroprotection to this essential neural circuit, the reward pathway.
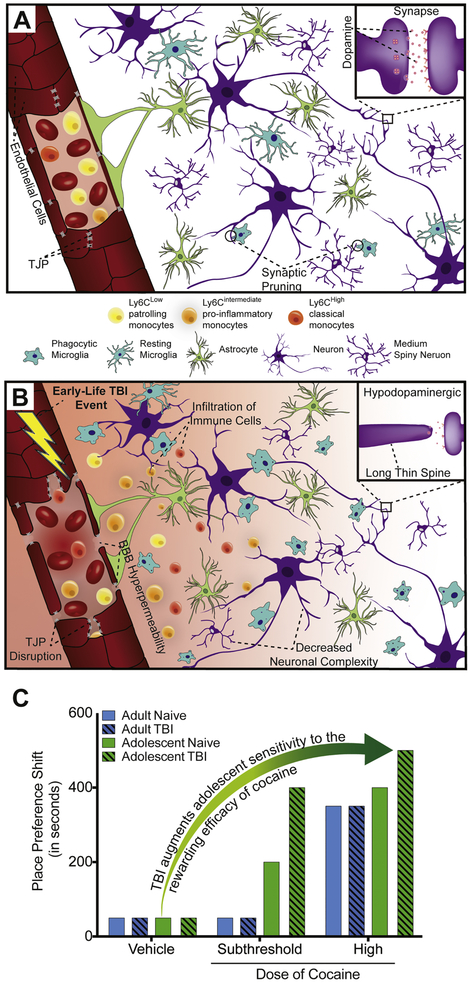
Figure 1.
Graphical representation of TBI-induced disruption of the neurovascular unit (NVU) within reward pathway nuclei and subsequent cellular and behavioral consequences linked to increased risk of substance use disorders. A: Under normal conditions, endothelial cells (red) lining the vasculature and expressing tight junction proteins (TJP) (grey) remain intact, with no indications of blood-brain-barrier (BBB) permeability, or immune cell infiltration (Yellow: Ly6C low/patrolling monocytes; Orange: Ly6C intermediate/pro-inflammatory monocytes; Red: Ly6C high/classical monocytes). Astrocytes (green) and microglia (blue) remain quiescently surveilling the environment, with some microglia involved in adolescent synaptic pruning of neuronal proteins (engulfed purple circles). Magnification shows the synapse of interneurons/medium spiny neurons residing in the NAc. Dopamine (pink) release mediates reward-motivated behaviors that underlie substance use disorders. B. Following early-life TBI, neuropathology includes endothelial activation, disruption of TJP, BBB hyperpermeability, and infiltration of immune cells with persistent Ly6C pro-inflammatory monocytes reported in adolescent brain injury. Activated microglia phagocytose neuronal proteins resulting in adverse changes to medium spiny neuron morphology and spine density. Magnification shows adolescent TBI-induced hypodopaminergic state and changes to spine length. Long thin spines are unstable and associated with dynamic changes and increased excitability. C. Proposed behavioral consequences seen in preclinical research models of early-life TBI and cocaine conditioned place preference. Adolescent animals show increased sensitivity to subthreshold doses of cocaine (green) compared to adults (blue). TBI during adulthood (blue, striped) augments the magnitude of CPP shift to high cocaine doses, which is further exacerbated in adolescent TBI (green, striped).
6. Acknowledgements
The authors acknowledge funding support from the National Institutes of Health/National Institute on Drug Abuse (NIH/NIDA) P30 DA013429-16 (SHR), R01DA046833 01 (SHR), T32 DA007237 (LAC), NIH/National Institute of Neurological Disorders and Stroke (NINDS), R01 NS086570-01 (SHR), and the PA-CURE program (Pennsylvania Department of Health).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERNCES
- Anderson VA, Catroppa C, Dudgeon P, Morse SA, Haritou F, and Rosenfeld JV. 2006. 'Understanding predictors of functional recovery and outcome 30 months following early childhood head injury', Neuropsychology. 20: 42–57. [DOI] [PubMed] [Google Scholar]
- Badanich KA, Adler KJ, and Kirstein CL. 2006. 'Adolescents differ from adults in cocaine conditioned place preference and cocaine-induced dopamine in the nucleus accumbens septi', Eur J Pharmacol, 550: 95–106. [DOI] [PubMed] [Google Scholar]
- Badaut J, Ajao DO, Sorensen DW, Fukuda AM, and Pellerin L. 2015. 'Caveolin expression changes in the neurovascular unit after juvenile traumatic brain injury: signs of blood-brain barrier healing?', Neuroscience, 285: 215–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumann CR, Bassetti CL, Valko PO, Haybaeck J, Keller M, Clark E, Stocker R, Tolnay M, and Scammell TE. 2009. 'Loss of hypocretin (orexin) neurons with traumatic brain injury', Ann Neurol, 66: 555–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumann CR, Stocker R, Imhof HG, Trentz O, Hersberger M, Mignot E, and Bassetti CL. 2005. 'Hypocretin-1 (orexin A) deficiency in acute traumatic brain injury', Neurology, 65: 147–9. [DOI] [PubMed] [Google Scholar]
- Baumann CR, Werth E, Stocker R, Ludwig S, and Bassetti CL. 2007. 'Sleep-wake disturbances 6 months after traumatic brain injury: a prospective study', Brain, 130: 1873–83. [DOI] [PubMed] [Google Scholar]
- Beauchamp MH, Ditchfield M, Maller JJ, Catroppa C, Godfrey C, Rosenfeld JV, Kean MJ, and Anderson VA. 2011. 'Hippocampus, amygdala and global brain changes 10 years after childhood traumatic brain injury', Int J Dev Neurosci, 29: 137–43. [DOI] [PubMed] [Google Scholar]
- Beschorner R, Nguyen TD, Gozalan F, Pedal I, Mattern R, Schluesener HJ, Meyermann R, and Schwab JM. 2002. 'CD14 expression by activated parenchymal microglia/macrophages and infiltrating monocytes following human traumatic brain injury', Acta Neuropathol, 103: 541–9. [DOI] [PubMed] [Google Scholar]
- Cannella LA, Andrews AM, Tran FH, Razmpour R, McGary HM, Collie C, Tsegaye T, Maynard M, Kaufman MJ, Rawls SM, and Ramirez SH. 2019. 'Experimental Traumatic Brain Injury during Adolescence Enhances Cocaine Rewarding Efficacy and Dysregulates Dopamine and Neuroimmune Systems in Brain Reward Substrates', J Neurotrauma, In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen YH, Huang EY, Kuo TT, Hoffer BJ, Miller J, Chou YC, and Chiang YH. 2017. 'Dopamine release in the nucleus accumbens is altered following traumatic brain injury', Neuroscience, 348: 180–90. [DOI] [PubMed] [Google Scholar]
- Chen YH, Kuo TT, Huang E. Yi-Kung, Chou YC, Chiang YH, Hoffer BJ, and Miller J. 2018. 'Effect of traumatic brain injury on nicotine-induced modulation of dopamine release in the striatum and nucleus accumbens shell', Oncotarget, 9: 10016–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Columbia, C.A.S.A. 2012. 'Addiction medicine: closing the gap between science and practice', New York: The National Center on Addiction and Substance Abuse (CASA) at Columbia University: pp.1–573. [Google Scholar]
- Corrigan JD 1995. 'Substance abuse as a mediating factor in outcome from traumatic brain injury', Arch Phys MedRehabil, 76: 302–9. [DOI] [PubMed] [Google Scholar]
- Corrigan JD, Bogner J, and Holloman C. 2012. 'Lifetime history of traumatic brain injury among persons with substance use disorders', Brain Inj, 26: 139–50. [DOI] [PubMed] [Google Scholar]
- Corrigan JD, Bogner J, Mellick D, Bushnik T, Dams-O'Connor K, Hammond FM, Hart T, and Kolakowsky-Hayner S. 2013. 'Prior history of traumatic brain injury among persons in the Traumatic Brain Injury Model Systems National Database', Arch Phys Med Rehabil, 94: 1940–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corso P, Finkelstein E, Miller T, Fiebelkorn I, and Zaloshnja E. 2006. 'Incidence and lifetime costs of injuries in the United States', Inj Prev, 12: 212–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donnemiller E, Brenneis C, Wissel J, Scherfler C, Poewe W, Riccabona G, and Wenning GK. 2000. 'Impaired dopaminergic neurotransmission in patients with traumatic brain injury: a SPECTstudy using 1231-beta-CIT and 1231-IBZM', Eur J Nucl Med, 27: 1410–4. [DOI] [PubMed] [Google Scholar]
- Elliott JE, De Luche SE, Churchill MJ, Moore C, Cohen AS, Meshul CK, and Lim MM. 2018. 'Dietary therapy restores glutamatergic input to orexin/hypocretin neurons after traumatic brain injury in mice', Sleep, 41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fadel J, and Deutch AY. 2002. 'Anatomical substrates of orexin-dopamine interactions: lateral hypothalamic projections to the ventral tegmental area', Neuroscience, 111: 379–87. [DOI] [PubMed] [Google Scholar]
- Faul M, Xu L, Wald MM, Coronado VG. 2010. “Traumatic Brain Injury in the United States: Emergency Department Visits, Hospitalizations and Deaths 2002–2006.” In. Atlanta, GA: National Center for Injury Prevention and Control, Centers Disease Control. [Google Scholar]
- Felde AB, Westermeyer J, and Thuras P. 2006. 'Co-morbid traumatic brain injury and substance use disorder: childhood predictors and adult correlates', Brain Inj, 20: 41–9. [DOI] [PubMed] [Google Scholar]
- Fishbein D, Dariotis JK, Ferguson PL, and Pickelsimer EE. 2016. 'Relationships Between Traumatic Brain Injury and Illicit Drug Use and Their Association With Aggression in Inmates', Int J Offender TherComp Criminol, 60: 575–97. [DOI] [PubMed] [Google Scholar]
- Forsyth JK, and Lewis DA. 2017. 'Mapping the Consequences of Impaired Synaptic Plasticity in Schizophrenia through Development: An Integrative Model for Diverse Clinical Features', Trends Cogn Sci, 21: 760–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frieden Thomas R., Houry Debra, and Baldwin Grant. 2015. “Report to Congress on Traumatic Brain Injury in the United States: Epidemiology and Rehabilitation” In Centers for Disease Control and Prevention. Atlanta, GA: National Center for Injury Prevention and Control; Division of Unintentional Injury Prevention. [Google Scholar]
- Fukuda AM, Pop V, Spagnoli D, Ashwal S, Obenaus A, and Badaut J. 2012. 'Delayed increase of astrocytic aquaporin 4 after juvenile traumatic brain injury: possible role in edema resolution?', Neuroscience, 222: 366–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Estrada J, Del Rio JA, Luquin S, Soriano E, and Garcia-Segura LM. 1993. 'Gonadal hormones down-regulate reactive gliosis and astrocyte proliferation after a penetrating brain injury', Brain Res, 628: 271–8. [DOI] [PubMed] [Google Scholar]
- Gentile TA, Simmons SJ, Barker DJ, Shaw JK, Espana RA, and Muschamp JW. 2018. 'Suvorexant, an orexin/hypocretin receptor antagonist, attenuates motivational and hedonic properties of cocaine'’, Addict Biol, 23: 247–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill J, Latour L, Diaz-Arrastia R, Motamedi V, Turtzo C, Shahim P, Mondello S, DeVoto C, Veras E, Hanlon D, Song L, and Jeromin A. 2018. 'Glial fibrillary acidic protein elevations relate to neuroimaging abnormalities after mild TBI', Neurology, 91: e1385–e89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gogtay N, Giedd JN, Lusk L, Hayashi KM, Greenstein D, Vaituzis AC, Nugent TF 3rd, Herman DH, Clasen LS, Toga AW, Rapoport JL, and Thompson PM. 2004. 'Dynamic mapping of human cortical development during childhood through early adulthood', Proc Natl Acad Sci USA, 101: 8174–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein FC, Caveney AF, Hertzberg VS, Silbergleit R, Yeatts SD, Palesch YY, Levin HS, and Wright DW. 2017. 'Very Early Administration of Progesterone Does Not Improve Neuropsychological Outcomes in Subjects with Moderate to Severe Traumatic Brain Injury', J Neurotrauma, 34: 115–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein LB 1993. 'Basic and clinical studies of pharmacologic effects on recovery from brain injury', J Neural Transplant Plast, 4: 175–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groman SM, James AS, and Jentsch JD. 2009. 'Poor response inhibition: at the nexus between substance abuse and attention deficit/hyperactivity disorder'’, Neurosci Biobehav Rev, 33: 690–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haarbauer-Krupa J, Ciccia A, Dodd J, Ettel D, Kurowski B, Lumba-Brown A, and Suskauer S. 2017. 'Service Delivery in the Healthcare and Educational Systems for Children Following Traumatic Brain Injury: Gaps in Care', J Head Trauma Rehabil, 32: 367–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall DH, and Queener JE. 2007. 'Self-medication hypothesis of substance use: testing Khantzian's updated theory', J Psychoactive Drugs, 39: 151–8. [DOI] [PubMed] [Google Scholar]
- Harris GC, Wimmer M, and Aston-Jones G. 2005. 'A role for lateral hypothalamic orexin neurons in reward seeking', Nature, 437: 556–9. [DOI] [PubMed] [Google Scholar]
- Hartley CA, and Somerville LH. 2015. 'The neuroscience of adolescent decision making', Curr Opin Behav Sci, 5: 108–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hehar H, Yeates K, Kolb B, Esser MJ, and Mychasiuk R. 2015. 'Impulsivity and Concussion in Juvenile Rats: Examining Molecular and Structural Aspects of the Frontostriatal Pathway', PLoS One, 10: e0139842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell DR, Zemek R, Brilliant AN, Mannix RC, Master CL, and Meehan WP 3rd. 2018. 'Identifying Persistent Postconcussion Symptom Risk in a Pediatric Sports Medicine Clinic', Am J Sports Med: 363546518796830. [DOI] [PubMed] [Google Scholar]
- Ichkova A, Rodriguez-Grande B, Bar C, Villega F, Konsman JP, and Badaut J. 2017. 'Vascular impairment as a pathological mechanism underlying long-lasting cognitive dysfunction after pediatric traumatic brain injury', Neurochem Int, 111: 93–102. [DOI] [PubMed] [Google Scholar]
- Ilie G, Mann RE, Boak A, Adlaf EM, Hamilton H, Asbridge M, Rehm J, and Cusimano MD. 2016. 'Cross-sectional examination of the association of co-occurring alcohol misuse and traumatic brain injury on mental health and conduct problems in adolescents in Ontario, Canada', BMJ Open, 6: e011824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson VE, Stewart JE, Begbie FD, Trojanowski JQ, Smith DH, and Stewart W. 2013. 'Inflammation and white matter degeneration persist for years after a single traumatic brain injury', Brain, 136: 28–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karelina K, Gaier KR, and Weil ZM. 2017. 'Traumatic brain injuries during development disrupt dopaminergic signaling', Exp Neurol, 297: 110–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karelina K, Nicholson S, and Weil ZM. 2018. 'Minocycline blocks traumatic brain injury-induced alcohol consumption and nucleus accumbens inflammation in adolescent male mice', Brain BehavImmun, 69: 532–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy E, Heron J, and Munafo M. 2017. 'Substance use, criminal behaviour and psychiatric symptoms following childhood traumatic brain injury: findings from the ALSPAC cohort', Eur ChildAdolesc Psychiatry, 26: 1197–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HJ, Cho MH, Shim WH, Kim JK, Jeon EY, Kim DH, and Yoon SY. 2017. 'Deficient autophagy in microglia impairs synaptic pruning and causes social behavioral defects', Mol Psychiatry, 22: 1576–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kogachi S, Chang L, Alicata D, Cunningham E, and Ernst T. 2017. 'Sex differences in impulsivity and brain morphometry in methamphetamine users', Brain Struct Funct, 222: 215–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopec AM, Smith CJ, Ayre NR, Sweat SC, and Bilbo SD. 2018. 'Microglial dopamine receptor elimination defines sex-specific nucleus accumbens development and social behavior in adolescent rats', Nat Commun, 9: 3769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak K, Lucatch AM, Lowe DJE, Balodis IM, MacKillop J, and George TP. 2017. 'The neurobiology of impulsivity and substance use disorders: implications for treatment', Ann N Y Acad Sci. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraus MF, Smith GS, Butters M, Donnell AJ, Dixon E, Yilong C, and Marion D. 2005. 'Effects of the dopaminergic agent and NMDA receptor antagonist amantadine on cognitive function, cerebral glucose metabolism and D2 receptor availability in chronic traumatic brain injury: a study using positron emission tomography (PET)', Brain Inj, 19: 471–9. [DOI] [PubMed] [Google Scholar]
- Langlois JA, Rutland-Brown W, and Wald MM. 2006. 'The epidemiology and impact of traumatic brain injury: a brief overview', J Head Trauma Rehabil, 21: 375–8. [DOI] [PubMed] [Google Scholar]
- Lawrence AJ, Luty J, Bogdan NA, Sahakian BJ, and Clark L. 2009. 'Impulsivity and response inhibition in alcohol dependence and problem gambling', Psychopharmacology (Berl), 207: 163–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ley EJ, Short SS, Liou DZ, Singer MB, Mirocha J, Melo N, Bukur M, and Salim A. 2013. 'Gender impacts mortality after traumatic brain injury in teenagers', J Trauma Acute Care Surg, 75: 682–6. [DOI] [PubMed] [Google Scholar]
- Lim MM, Elkind J, Xiong G, Galante R, Zhu J, Zhang L, Lian J, Rodin J, Kuzma NN, Pack AI, and Cohen AS. 2013. 'Dietary therapy mitigates persistent wake deficits caused by mild traumatic brain injury', Sci TranslMed, 5: 215ra173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim YW, Meyer NP, Shah AS, Budde MD, Stemper BD, and Olsen CM. 2015. 'Voluntary Alcohol Intake following Blast Exposure in a Rat Model of Mild Traumatic Brain Injury', PLoS One, 10: e0125130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loane DJ, and Byrnes KR. 2010. 'Role of microglia in neurotrauma', Neurotherapeutics, 7: 366–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lozano D, Gonzales-Portillo GS, Acosta S, de la Pena I, Tajiri N, Kaneko Y, and Borlongan CV. 2015. 'Neuroinflammatory responses to traumatic brain injury: etiology, clinical consequences, and therapeutic opportunities', Neuropsychiatr Dis Treat, 11: 97–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu XY, Sun H, Li QY, and Lu PS. 2016. 'Progesterone for Traumatic Brain Injury: A Meta-Analysis Review of Randomized Controlled Trials', World Neurosurg, 90: 199–210. [DOI] [PubMed] [Google Scholar]
- Lutton EM, Razmpour R, Andrews AM, Cannella LA, Son YJ, Shuvaev VV, Muzykantov VR, and Ramirez SH. 2017. 'Acute administration of catalase targeted to ICAM-1 attenuates neuropathology in experimental traumatic brain injury', Sci Rep, 7: 3846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin-Fardon R, Cauvi G, Kerr TM, and Weiss F. 2018. 'Differential role of hypothalamic orexin/hypocretin neurons in reward seeking motivated by cocaine versus palatable food', Addict Biol, 23: 6–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Max JE, Friedman K, Wilde EA, Bigler ED, Hanten G, Schachar RJ, Saunders AE, Dennis M, Ewing-Cobbs L, Chapman SB, Yang TT, and Levin HS. 2015. 'Psychiatric disorders in children and adolescents 24 months after mild traumatic brain injury', J Neuropsychiatry Clin Neurosci, 27: 112–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayeux JP, Teng SX, Katz PS, Gilpin NW, and Molina PE. 2015. 'Traumatic brain injury induces neuroinflammation and neuronal degeneration that is associated with escalated alcohol self-administration in rats', Behav Brain Res, 279: 22–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCauley SR, Wilde EA, Anderson VA, Bedell G, Beers SR, Campbell TF, Chapman SB, Ewing-Cobbs L, Gerring JP, Gioia GA, Levin HS, Michaud LJ, Prasad MR, Swaine BR, Turkstra LS, Wade SL, Yeates KO, and T. B. I. Outcomes Workgroup Pediatric. 2012. 'Recommendations for the use of common outcome measures in pediatric traumatic brain injury research', J Neurotrauma, 29: 678–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McHugo GJ, Krassenbaum S, Donley S, Corrigan JD, Bogner J, and Drake RE. 2017. 'The Prevalence of Traumatic Brain Injury Among People With Co-Occurring Mental Health and Substance Use Disorders', J Head Trauma Rehabil, 32: E65–E74. [DOI] [PubMed] [Google Scholar]
- McKinlay A, Corrigan J, Horwood LJ, and Fergusson DM. 2014. 'Substance abuse and criminal activities following traumatic brain injury in childhood, adolescence, and early adulthood', J Head Trauma Rehabil, 29: 498–506. [DOI] [PubMed] [Google Scholar]
- McKinlay A, Grace R, Horwood J, Fergusson D, and MacFarlane M. 2009. 'Adolescent psychiatric symptoms following preschool childhood mild traumatic brain injury: evidence from a birth cohort', J Head Trauma Rehabil, 24: 221–7. [DOI] [PubMed] [Google Scholar]
- Meadows EA, Owen Yeates K, Rubin KH, Taylor HG, Bigler ED, Dennis M, Gerhardt CA, Vannatta K, Stancin T, and Hoskinson KR. 2017. 'Rejection Sensitivity as a Moderator of Psychosocial Outcomes Following Pediatric Traumatic Brain Injury', J Int Neuropsychol Soc, 23: 451–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merkel SF, Andrews AM, Lutton EM, Razmpour R, Cannella LA, and Ramirez SH. 2016. 'Dexamethasone Attenuates the Enhanced Rewarding Effects of Cocaine Following Experimental Traumatic Brain Injury', Cell Transplantation, epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merkel SF, Cannella LA, Razmpour R, Lutton E, Raghupathi R, Rawls SM, & Ramirez SH 2017. 'Factors affecting increased risk for substance use disorders following traumatic brain injury: What we can learn from animal models', Neuroscience & Biobehavioral Reviews. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merkel SF, Razmpour R, Lutton EM, Tallarida CS, Heldt NA, Cannella LA, Persidsky Y, Rawls SM, and Ramirez SH. 2016. 'Adolescent Traumatic Brain Injury Induces Chronic Mesolimbic Neuroinflammation with Concurrent Enhancement in the Rewarding Effects of Cocaine in Mice during Adulthood', J Neurotrauma. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller SC, Baktash SH, Webb TS, Whitehead CR, Maynard C, Wells TS, Otte CN, and Gore RK. 2013. 'Risk for addiction-related disorders following mild traumatic brain injury in a large cohort of active-duty U.S. airmen', Am J Psychiatry, 170: 383–90. [DOI] [PubMed] [Google Scholar]
- Muelbl MJ, Slaker ML, Shah AS, Nawarawong NN, Gerndt CH, Budde MD, Stemper BD, and Olsen CM. 2018. 'Effects of Mild Blast Traumatic Brain Injury on Cognitive- and Addiction-Related Behaviors', Sci Rep, 8: 9941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mychasiuk R, Hehar H, Farran A, and Esser MJ. 2014. 'Mean girls: sex differences in the effects of mild traumatic brain injury on the social dynamics of juvenile rat play behaviour', Behav Brain Res, 259: 284–91. [DOI] [PubMed] [Google Scholar]
- Mychasiuk R, Hehar H, Ma I, Kolb B, and Esser MJ. 2015. 'The development of lasting impairments: a mild pediatric brain injury alters gene expression, dendritic morphology, and synaptic connectivity in the prefrontal cortex of rats', Neuroscience, 288: 145–55. [DOI] [PubMed] [Google Scholar]
- Naneix F, Marchand AR, Di Scala G, Pape JR, and Coutureau E. 2012. 'Parallel maturation of goal-directed behavior and dopaminergic systems during adolescence', JNeurosci, 32: 16223–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narad ME, Kennelly M, Zhang N, Wade SL, Yeates KO, Taylor HG, Epstein JN, and Kurowski BG. 2018. 'Secondary Attention-Deficit/Hyperactivity Disorder in Children and Adolescents 5 to 10 Years After Traumatic Brain Injury', JAMA Pediatr, 172: 437–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Connor CA, Cernak I, and Vink R. 2005. 'Both estrogen and progesterone attenuate edema formation following diffuse traumatic brain injury in rats', Brain Res, 1062: 171–4 [DOI] [PubMed] [Google Scholar]
- Paolicelli RC, Bolasco G, Pagani F, Maggi L, Scianni M, Panzanelli P, Giustetto M, Ferreira TA, Guiducci E, Dumas L, Ragozzino D, and Gross CT. 2011. 'Synaptic pruning by microglia is necessary for normal brain development', Science, 333: 1456–8. [DOI] [PubMed] [Google Scholar]
- Parry-Jones BL, Vaughan FL, and Cox WM. 2006. 'Traumatic brain injury and substance misuse: A systematic review of prevalence and outcomes research (1994–2004)', Neuropsychological Rehabilitation, 16: 537–60. [DOI] [PubMed] [Google Scholar]
- Perez EJ, Cepero ML, Perez SU, Coyle JT, Sick TJ, and Liebl DJ. 2016a. 'EphB3 signaling propagates synaptic dysfunction in the traumatic injured brain', Neurobiol Dis, 94: 73–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez EJ, Cepero ML, Perez SU, Coyle JT, Sick TJ, and Liebl DJ. 2016b. 'EphB3 signaling propagates synaptic dysfunction in the traumatic injured brain', Neurobiology of Disease, 94: 73–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perron BE, and Howard MO. 2008. 'Prevalence and correlates of traumatic brain injury among delinquent youths', Crim Behav Ment Health, 18: 243–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prince C, and Bruhns ME. 2017. 'Evaluation and Treatment of Mild Traumatic Brain Injury: The Role of Neuropsychology', Brain Sci, 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhine T, Cassedy A, Yeates KO, Taylor HG, Kirkwood MW, and Wade SL. 2018. 'Investigating the Connection Between Traumatic Brain Injury and Posttraumatic Stress Symptoms in Adolescents', J Head Trauma Rehabil, 33: 210–18. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Grande B, Obenaus A, Ichkova A, Aussudre J, Bessy T, Barse E, Hiba B, Catheline G, Barriere G, and Badaut J. 2018. 'Gliovascular changes precede white matter damage and long-term disorders in juvenile mild closed head injury', Glia. [DOI] [PubMed] [Google Scholar]
- Roof RL, Duvdevani R, and Stein DG. 1993. 'Gender influences outcome of brain injury: progesterone plays a protective role', Brain Res, 607: 333–6. [DOI] [PubMed] [Google Scholar]
- Ryan NP, Genc S, Beauchamp MH, Yeates KO, Hearps S, Catroppa C, Anderson VA, and Silk TJ. 2018. 'White matter microstructure predicts longitudinal social cognitive outcomes after paediatric traumatic brain injury: a diffusion tensor imaging study', Psychol Med, 48: 679–91. [DOI] [PubMed] [Google Scholar]
- SAMHSA, Center for Behavioral Health Statistics and Quality. 2015. “Behavioral health trands in the United States: Results from the 2014 National Survey on Drug Use and Health (HSS Publication No. SMA 15–4927, NSDUH Series H-50.” In. [Google Scholar]
- Schachar R, Levin HS, Max JE, Purvis K, and Chen S. 2004. 'Attention deficit hyperactivity disorder symptoms and response inhibition after closed head injury in children: do preinjury behavior and injury severity predict outcome?', Dev Neuropsychol, 25: 179–98. [DOI] [PubMed] [Google Scholar]
- Schenk S, Horger BA, Peltier R, and Shelton K. 1991. 'Supersensitivity to the reinforcing effects of cocaine following 6-hydroxydopamine lesions to the medial prefrontal cortex in rats', Brain Res, 543: 227–35. [DOI] [PubMed] [Google Scholar]
- Schmithorst VJ, and Yuan W. 2010. 'White matter development during adolescence as shown by diffusion MRI'’, Brain Cogn, 72: 16–25. [DOI] [PubMed] [Google Scholar]
- Scott C, McKinlay A, McLellan T, Britt E, Grace R, and MacFarlane M. 2015. 'A comparison of adult outcomes for males compared to females following pediatric traumatic brain injury', Neuropsychology, 29: 501–8. [DOI] [PubMed] [Google Scholar]
- Semple BD, Dixit S, Shultz SR, Boon WC, and O'Brien TJ. 2017. 'Sex-dependent changes in neuronal morphology and psychosocial behaviors after pediatric brain injury', Behav Brain Res, 319: 48–62. [DOI] [PubMed] [Google Scholar]
- Semple BD, Trivedi A, Gimlin K, and Noble-Haeusslein LJ. 2015. 'Neutrophil elastase mediates acute pathogenesis and is a determinant of long-term behavioral recovery after traumatic injury to the immature brain', Neurobiol Dis, 74: 263–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah S, Yallampalli R, Merkley TL, McCauley SR, Bigler ED, Macleod M, Chu Z, Li X, Troyanskaya M, Hunter JV, Levin HS, and Wilde EA. 2012. 'Diffusion tensor imaging and volumetric analysis of the ventral striatum in adults with traumatic brain injury', Brain Inj, 26: 201–10. [DOI] [PubMed] [Google Scholar]
- Skopin MD, Kabadi SV, Viechweg SS, Mong JA, and Faden AI. 2015. 'Chronic decrease in wakefulness and disruption of sleep-wake behavior after experimental traumatic brain injury', J Neurotrauma, 32: 289–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tau GZ, and Peterson BS. 2010. 'Normal development of brain circuits', Neuropsychopharmacology, 35: 147–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor CA, Bell JM, Breiding MJ, and Xu L. 2017. 'Traumatic Brain Injury-Related Emergency Department Visits, Hospitalizations, and Deaths - United States, 2007 and 2013', MMWR Surveill Summ, 66: 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomasy HE, Febinger HY, Ringgold KM, Gemma C, and Opp MR. 2017. 'Hypocretinergic and cholinergic contributions to sleep-wake disturbances in a mouse model of traumatic brain injury', Neurobiology of Sleep and Circadian Rhythms, 2: 71–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaughn MG, Salas-Wright CP, DelLisi M, and Perron B. 2014. 'Correlates of traumatic brain injury among juvenile offenders: a multi-site study', CrimBehav Merit Health, 24: 188–203. [DOI] [PubMed] [Google Scholar]
- Vaughn MG, Salas-Wright CP, John R, Holzer KJ, Qian Z, and Veeh C. 2018. 'Traumatic Brain Injury and Psychiatric Co-Morbidity in the United States', Psychiatr Q. [DOI] [PubMed] [Google Scholar]
- Vonder Haar C, Ferland JN, Kaur S, Riparip LK, Rosi S, and Winstanley CA. 2018. 'Cocaine self-administration is increased after frontal traumatic brain injury and associated with neuroinflammation', EurJ Neurosci. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wade SL, Cassedy AE, Fulks LE, Taylor HG, Stancin T, Kirkwood MW, Yeates KO, and Kurowski BG. 2017. 'Problem-Solving After Traumatic Brain Injury in Adolescence: Associations With Functional Outcomes', Arch Phys Med Rehabil, 98: 1614–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wade SL, Zhang N, Yeates KO, Stancin T, and Taylor HG. 2016. 'Social Environmental Moderators of Long-term Functional Outcomes of Early Childhood Brain Injury', JAMA Pediatr, 170: 343–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wake H, Moorhouse AJ, Miyamoto A, and Nabekura J. 2013. 'Microglia: actively surveying and shaping neuronal circuit structure and function', Trends Neurosci, 36: 209–17. [DOI] [PubMed] [Google Scholar]
- Walker DM, Bell MR, Flores C, Gulley JM, Willing J, and Paul MJ. 2017. 'Adolescence and Reward: Making Sense of Neural and Behavioral Changes Amid the Chaos', J Neurosci, 37: 10855–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker KR, and Tesco G. 2013. 'Molecular mechanisms of cognitive dysfunction following traumatic brain injury', Frontiers in Aging Neuroscience, 5: 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Washington PM, Forcelli PA, Wilkins T, Zapple DN, Parsadanian M, and Burns MP. 2012. 'The effect of injury severity on behavior: a phenotypic study of cognitive and emotional deficits after mild, moderate, and severe controlled cortical impact injury in mice', J Neurotrauma, 29: 2283–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weil ZM, Corrigan JD, and Karelina K. 2016. 'Alcohol abuse after traumatic brain injury: Experimental and clinical evidence', Neurosci BiobehavRev, 62: 89–99. [DOI] [PubMed] [Google Scholar]
- Weil ZM, Karelina K, Gaier KR, Corrigan TE, and Corrigan JD. 2016. 'Juvenile Traumatic Brain Injury Increases Alcohol Consumption and Reward in Female Mice', J Neurotrauma, 33: 895–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss RD, Griffin ML, and Mirin SM. 1992. 'Drug abuse as self-medication for depression: an empirical study', Am J Drug Alcohol Abuse, 18: 121–9. [DOI] [PubMed] [Google Scholar]
- Whelan-Goodinson R, Ponsford J, Johnston L, and Grant F. 2009. 'Psychiatric disorders following traumatic brain injury: their nature and frequency', J Head Trauma Rehabil, 24: 324–32. [DOI] [PubMed] [Google Scholar]
- Williams WH, Chitsabesan P, Fazel S, McMillan T, Hughes N, Parsonage M, and Tonks J. 2018. 'Traumatic brain injury: a potential cause of violent crime?', Lancet Psychiatry, 5: 836–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wills TA, and Filer M. 1996. 'Stress—Coping Model of Adolescent Substance Use', Advances in Clinical Child Psychology, 18. [Google Scholar]
- Xiong Y, Mahmood A, and Chopp M. 2013. 'Animal models of traumatic brain injury', Nat Rev Neurosci, 14: 128–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeates KO, Taylor HG, Walz NC, Stancin T, and Wade SL. 2010. 'The family environment as a moderator of psychosocial outcomes following traumatic brain injury in young children', Neuropsychology, 24: 345–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu F, Shukla DK, Armstrong RC, Marion CM, Radomski KL, Selwyn RG, and Dardzinski BJ. 2017. 'Repetitive Model of Mild Traumatic Brain Injury Produces Cortical Abnormalities Detectable by Magnetic Resonance Diffusion Imaging, Histopathology, and Behavior', J Neurotrauma, 34: 1364–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zakharova E, Wade D, and Izenwasser S. 2009. 'Sensitivity to cocaine conditioned reward depends on sex and age', Pharmacol Biochem Behav, 92: 131–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao J, Huynh J, Hylin MJ, O'Malley JJ, Perez A, Moore AN, and Dash PK. 2018. 'Mild Traumatic Brain Injury Reduces Spine Density of Projection Neurons in the Medial Prefrontal Cortex and Impairs Extinction of Contextual Fear Memory', J Neurotrauma, 35: 149–56. [DOI] [PMC free article] [PubMed] [Google Scholar]