Abstract
The Head Positioning in Acute Stroke Trial (HeadPoST) is a pragmatic, international, cluster crossover randomized trial of 11,093 patients with acute stroke assigned to a lying-flat (0°) or sitting-up (head elevated ≥30°) position. This post-hoc analysis aimed to determine the association between BPV and outcomes for patients from a wide range of international clinical settings and how the association was modified by randomized head position. BPV was defined according to standard criteria with the key parameter considered the coefficient of variation (CV) of systolic BP (SBP) over 24 hours. Outcome was ordinal 90-day modified Rankin Scale (mRS) score. The association was analyzed by ordinal, logistic regression, hierarchical, mixed models with fixed intervention (lying-flat vs. sitting-up), and fixed period, random cluster, and random cluster-period, effects. 9,156 (8,324 AIS and 817 ICH; mean age 68.1 years; 39.2% women) were included in the analysis. CV of SBP had a significant linear association with unfavorable shift of mRS at 90 days (adjusted odds ratio [OR] 1.06, 95% confidence interval [CI] 1.02-1.11; P=0.01). There was no heterogeneity of the association by randomized head positioning. In addition, CV of diastolic BP (DBP) (1.08, 1.03-1.12; P=0.001) over 24 hours post stroke, was significantly associated with 3-month poor outcome. The association was more apparent in sitting-up position (1.12, 1.06-1.19) compared with lying-flat position (1.03, 0.98-1.09) (P interaction = 0.005). BPV was associated with adverse stroke outcome, the magnitude of the association was greater with sitting-up head positioning in terms of DBP variability.
Clinical Trial Registration
Keywords: head position, blood pressure, stroke, intracerebral hemorrhage, acute stroke outcome
Introduction
The importance of specialized stroke units is evidenced by randomized trials demonstrating reductions in death and disability caused by prevention of secondary complications of stroke [1]. In acute stroke syndromes, elevated blood pressures (BP) have been a particular focus, as post-stroke hypertension precipitates secondary stroke complications [2]. More recently, BP variability (BPV), using widely applied measures such as standard deviation (SD) or coefficient of variation (CV), and measured early after acute ischemic stroke (AIS), intracerebral hemorrhage (ICH) or transient ischemic attack (TIA), has been shown to be associated with poor functional outcome [3–5].
Traditionally, AIS patients have been nursed in elevated positions (≥30°) due to concern over aspiration risk and propensity to reduce raised intracranial pressure (ICP) [6]. However, it is conceivable that head positioning may impact on BPV, with data from healthy individuals and AIS patients (in affected hemispheres) showing significant changes in cerebral hemodynamic parameters during head position changes of varying tilt [7,8].
The Head Positioning in Acute Stroke Trial (HeadPoST) was designed to evaluate whether outcomes for unselected acute stroke patients nursed lying-flat (i.e. fully supine with back horizontal and face upwards or to the side) were improved compared to those in a sitting-up position with head elevated to at least 30 degrees [9]. The study showed no significant difference in modified Rankin Scale (mRS) scores at 90 days between the head position groups initiated early after presentation and maintained for 24 hours. Accordingly, the HeadPoST results suggest any changes in cerebral blood flow based on head position initiated within 24 hours are insufficient to reduce the neurological deficit associated with acute stroke pathologies [9].
However, the potential impact of head positioning and any associated hemodynamic changes in relation to lying-flat (increased cerebral blood flow and oxygenation) and sitting-up (potential reduction in ICP in large hemispheric AIS) on the predictive potential of BPV on outcome have yet to be considered. Herein, we report the predictive potential of BPV parameters on functional outcome in a post-hoc individual patient analysis of the HeadPoST study.
Materials and methods
Patients
HeadPoST was an international, multicenter, cluster-randomized, crossover, open trial with blinded outcome evaluation, conducted at 114 hospitals in nine countries [9]. The trial was designed to compare the effects of the lying-flat with the sitting-up position, initiated soon after stroke and maintained for 24 hours after the onset of acute stroke, full details of which are outlined elsewhere [9,10].
Patients with a clinical diagnosis of acute stroke, including AIS and ICH, were included in order to facilitate consecutive patient recruitment. Patients were excluded if the clinician-investigator deemed either head position futile based on compliance or if a TIA was diagnosed. Other reasons for exclusion were refusal to participate in the intervention and/or follow-up, or any contraindication to either head position. Patients were assigned a head position according to the randomization cluster as soon as was feasible after admission to hospital and they were encouraged to strictly maintain this position for the next 24 hours. Interruption to the assigned head position was permitted, for three non-consecutive periods of less than 30 minutes, to permit eating, drinking and toileting, should this not have been possible in the assigned position.
The appropriate ethics committee at each participating center approved the study protocol. A senior executive officer at each center acted as a ‘guardian’ and provided institutional consent for this low-risk intervention to be implemented consecutively as part of routine nursing care in each cluster. Written informed consent was then sought from all patients or approved surrogates for ongoing assessments and data collection.
Procedures
Key demographic and clinical characteristics were recorded at the time of enrollment, including stroke severity measured using the National Institutes of Health stroke scale (NIHSS) at baseline, 24 hours, and at day 7 (or earlier, on discharge from hospital). A 24-hour bed-side diary was maintained by the treating clinical nurses to record vital signs, lowest oxygen saturation and interruptions in head positioning. Follow-up data were collected at 7 days (or at hospital discharge if before 7 days) unless death occurred earlier by independent outcome assessors blind to group allocation. Data included final diagnosis, repeat NIHSS score and assessment of functioning using the mRS, a standard disability scale with categorical scores ranging from 0 to 6 (0 indicates, no symptoms at all; 1, no clinically significant disability despite symptoms; 2, slight disability; 3, moderate disability requiring some help; 4, moderately severe disability requiring assistance with daily living; 5, severe disability, bed-bound, and incontinent; and 6, death).
The primary clinical outcome was the ordinal mRS scores at 90 days. BP was measured with a casual cuff at 4 hourly intervals during the first 24 hours post-stroke and BPV was calculated from all available BP measurements using BPV measures with demonstrable independence of mean SBP on an individual patient level. The parameters included were mean, standard deviation (SD), coefficient of variation (CV) and variation independent of mean (VIM, a transformation of SD that is defined to be uncorrelated with mean levels) as well as diastolic BPV over 24 hours (Table I in Supplementary Files). There was an expectation that limited variation would exist in the standard BP measurement equipment used across different hospitals and countries. Coefficient of Variation (CV) [CV=SD/mean] was derived from these parameters. CV of SBP was categorized into 5 equal groups (quintiles), using the lowest fifth as the reference group.
Statistical analysis
We used ordinal, logistic regression, hierarchical, mixed models with fixed intervention (lying-flat vs. sitting-up), and fixed period, random cluster, and random cluster-period, effects to assess the associations. The multivariable model was adjusted for country, prestroke mRS score, age, sex, baseline NIHSS score, and history of heart disease, stroke or diabetes mellitus, or hypertension and prior antiplatelet therapy. We also investigated whether the associations were different between groups by AIS etiology, stroke sub-type and head position by adding an interaction term to the adjusted statistical models. In study analyses, two-sided P values are reported and P<0·05 was considered statistically significant. The SAS version 9.3 (SAS Institute, Cary, NC) was used for all analyses.
Results
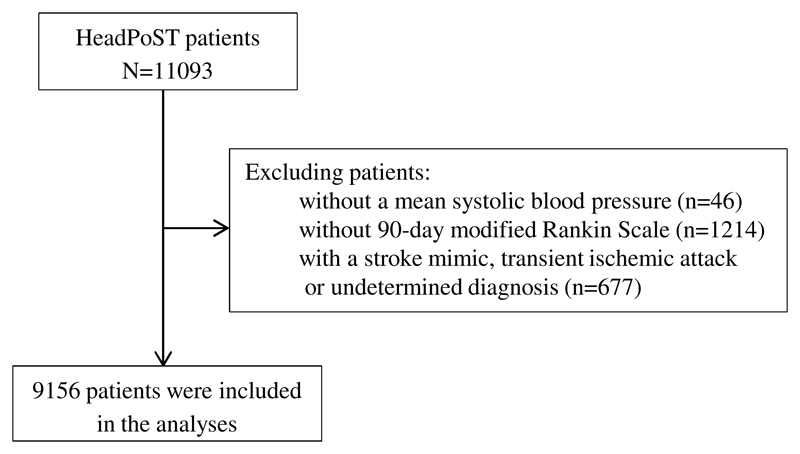
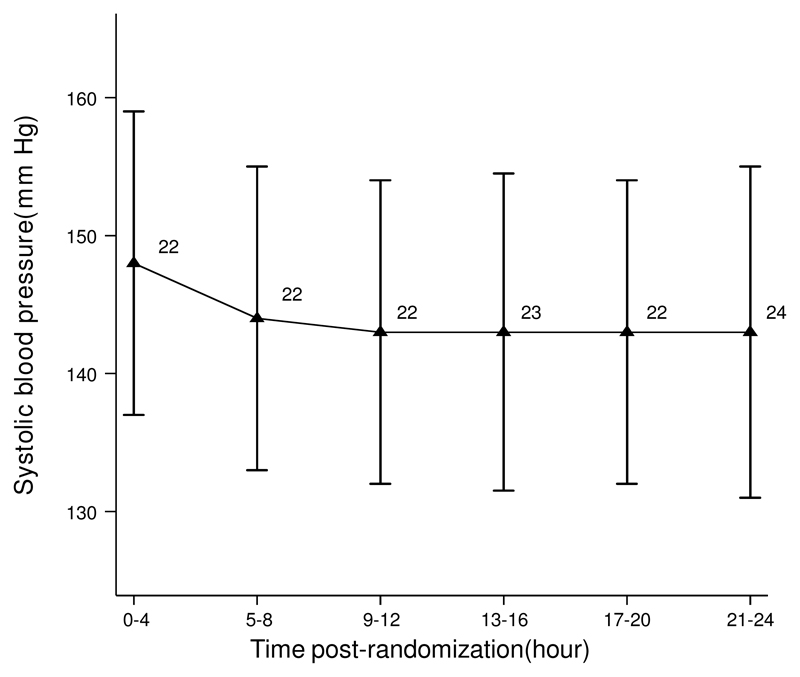
The HeadPoST trial included 11,093 patients (39.9% female) whose mean age was 68 years; patients without a mean systolic BP (46), without 90-day mRS (1,214) and those with a stroke mimic, TIA or undeterminated diagnosis (677) were excluded. 9,156 patients (82.5%) had a complete set of 4-hourly BP measurements recorded over the first 24 hours, on which subsequent analyses were undertaken. The study procedures are summarised in a flowchart (Fig.1). Those excluded at baseline were more likely to be female, have a shorter time between onset and intervention, less likely to have known hypertension and less likely to be taking aspirin (Table II in Supplementary Files). Mean age of the participants was 68.1 years, 39.2% were female, 91.1% had AIS, and 8.9% had ICH. The median pretreatment NIHSS score was 4 (interquartile range, 2 to 9). Time from stroke onset to commencement of head positioning was 14 hours (interquartile range, 5 to 38). Other baseline characteristics are presented in Table 1. Mean systolic BP was 156±28mmHg and diastolic BP 87±17mmHg, with mean systolic BP showed a steady fall over the first 24 hours following randomization (Fig. 2). Baseline characteristics by randomized treatment demonstrated higher incidence of coronary artery disease and heart failure in the sitting-up group (Table III in Supplementary Files). The acute values for systolic BPV demonstrated a mean SBP of 145±18.9mmHg, SD of 11±7.5mmHg and CV of 8±4.4mmHg (Table IV in Supplementary Files). For diastolic BPV, SD was 8±4.2mmHg and CV 9±5.3mmHg (Table IV in Supplementary Files).
Fig. 1. Flowchart of the study procedures.
Table 1. Baseline characteristics of the 9,156 patients with acute stroke and 4-hourly blood pressure parameters for 24 hours included in the analysis.
| Overall (N=9156) | |
|---|---|
| Socio-demographic characteristics | |
| Female sex | 3587 (39.2) |
| Age (years) | 68.1 (13.5) |
| Region of Recruitment | |
| Australia and UK | 3383 (36.9) |
| South America | 802 (8.8) |
| China (incl. Taiwan) | 4281 (46.8) |
| India and Sri Lanka | 690 (7.5) |
| Time from stroke onset to commencing intervention (hrs) | 15.0 (5.0 – 38.0) |
| Time from admission to commencing intervention (hrs) | 8.0 (2.0 – 29.0) |
| Stroke type§ | |
| AIS | 8324 (91.1) |
| ICH | 817 (8.9) |
| Pre-stroke mRS of 0 | 5595 (61.1) |
| NIHSS at admission | 4.0 (2.0 – 9.0) |
| GCS score on arrival | 15.0 (14.0 – 15.0) |
| Vital signs and laboratory results | |
| Systolic BP (mmHg) | 155.5 (27.5) |
| Diastolic BP (mmHg) | 86.7 (16.6) |
| Heart Rate (bpm) | 76.0 (68.0 – 84.0) |
| Glucose (mmol/l) | 5.5 (4.9 - 6.1) |
| Serum Creatinine (mmol/l) | 75.0 (63.0 – 90.0) |
| Medical History | |
| Previous stroke | 2164 (23.7) |
| Coronary artery disease | 1238 (13.6) |
| Atrial fibrillation | 960 (10.6) |
| Heart Failure | 325 (3.6) |
| Hypertension | 5981 (65.5) |
| Diabetes Mellitus | 2235 (24.5) |
| Current Smoker | 1799 (19.8) |
| Medications at time of admission | |
| Aspirin | 4092 (44.7) |
| Other antiplatelet agent | 1836 (20.1) |
| Anticoagulant | 779 (8.5) |
| Swallow screen on admission | 7155 (78.2) |
| Swallow assessment on admission | 3341 (36.5) |
Data are n (%), mean (standard deviation) or median (interquartile range).
AIS: acute ischemic stroke; ICH: intracerebral hemorrhage; mRS: modified Rankin score; NIHSS: National Institutes of Health stroke scale; GCS: Glasgow coma scale; BP: blood pressure.
Reported by clinician investigator from brain imaging and other investigations on hospital discharge.
Fig. 2. Mean and SD of systolic blood pressure over time.
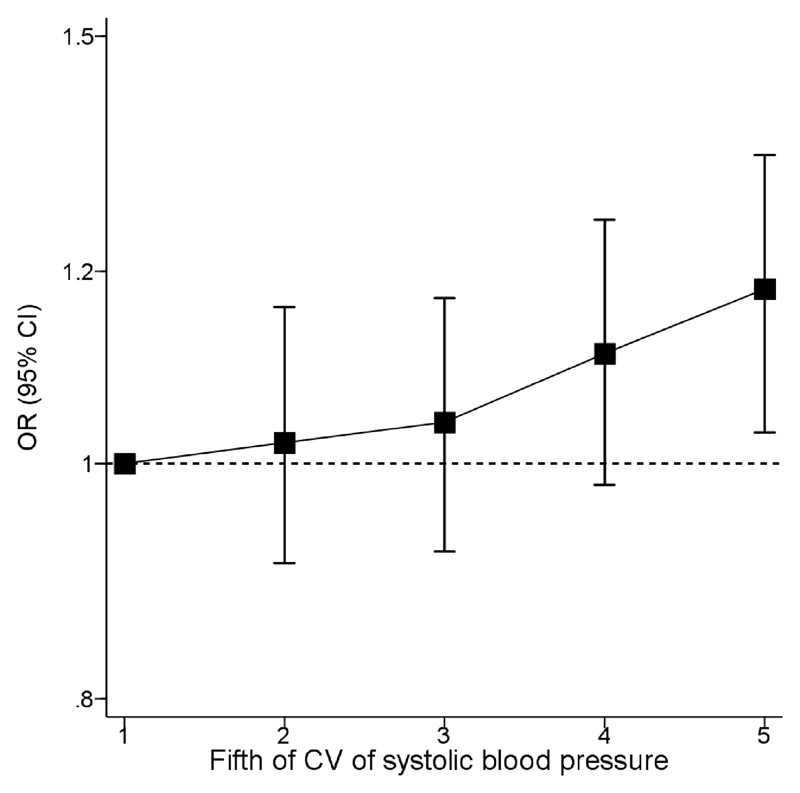
Overall, increased systolic BPV parameters were all associated with unfavorable shift of the mRS at 90 days except VIM (Table 2). Systolic BPV assessed using CV demonstrated a significant linear association with unfavorable shift of the mRS at 90 days (odds ratio [OR] effect of 1 SD increment of CV, 1.06 [95% confidence interval] 1.02-1.10; P=0.0065 (Table 2). Mean systolic BP (1.21 [1.16-1.25]; P<0.0001) and SD of systolic BP (1.10 [1.05-1.15]; P<0.0001) both demonstrated significant associations with unfavorable shift in mRS at 90 days. There was no heterogeneity of the association by randomized head positioning. The trend in mean systolic BP and SD of systolic BP demonstrated a progressive downward trend from 0-12 hours and a plateau from 12-24 hours (Fig. 2). A positive trend was seen for association of fifths of CV of SBP and unfavorable shift on the mRS at 90 days (Fig. 3). The P-value of proportional odds assumption for the model of CV and ordinal outcome was 0.0008. The trend demonstrated a consistent risk relationship between differing levels of the ordinal outcome measure. This was demonstrated by mRS 0-3 vs. 4-6 (1.07 [1.01-1.13); P=0.026), mRS 0-4 vs. 5-6 (1.10 [1.03-1.17]; P=0.004) and mRS 0-5 vs. 6 (1.14 [1.06-1.22]; P=0.001); showing an increasing association of CV of systolic BP with outcome.
Table 2. Association of SBP variability with unfavorable shift of the mRS at 90 days.
| Univariate analysis | Multivariate Model | |||
|---|---|---|---|---|
| OR(95%CI) | P value | OR(95%CI) | P value | |
| Mean | 1.26(1.21-1.31) | <0.0001 | 1.21(1.16-1.25) | <0.0001 |
| MIN | 1.18(1.14-1.23) | <0.0001 | 1.15(1.11-1.20) | <0.0001 |
| MAX | 1.28(1.23-1.33) | <0.0001 | 1.21(1.16-1.26) | <0.0001 |
| SD | 1.17(1.12-1.23) | <0.0001 | 1.10(1.05-1.15) | <0.0001 |
| CV | 1.11(1.06-1.15) | <0.0001 | 1.06(1.02-1.10) | 0.0065 |
| ARV | 1.17(1.11-1.22) | <0.0001 | 1.12(1.06-1.17) | <0.0001 |
| RSD | 1.13(1.08-1.18) | <0.0001 | 1.08(1.04-1.13) | 0.0002 |
| VIM | 1.08(1.04-1.13) | <0.0001 | 1.04(1.00-1.08) | 0.0533 |
CV: coefficient of variation; ARV: average absolute difference between successive BP measurements; RSD: residual SD; VIM: variation independent of mean.
Data are presented as odds ratio [OR] (95% confidence intervals [95%CI]) for per 1 unit increment of systolic blood pressure [SBP] variability (i.e. for SD, the unit is 1 SD of SD; for CV, the unit is 1 SD of CV; for ARV, the unit is 1 SD of ARV; for RSD, the unit is 1 SD of RSD; and for VIM, the unit is 1 SD of VIM).
Multivariable Model: Adjusted for country, prestroke mRS score, sex, baseline NIHSS score, and history of heart disease, stroke, diabetes mellitus, or hypertension, prior antiplatelet therapy
Fig. 3. Association between fifths of coefficient of variation of systolic blood pressure and unfavorable shift of mRS at 90 days.
Footnote: Association between fifths of CV of systolic blood pressure at baseline and unfavorable shift of mRS at 90 days
With respect to head position, there were no significant changes in systolic BP over 24 hours by randomized intervention (lying-flat or sitting-up) (Fig. I in the Supplementary Files). Mean systolic BP was higher in ICH (149±19) patients compared to AIS (144±19) at successive 4-hourly intervals over the initial 24-hour period (P<0.0001) (Fig. II in the Supplementary Files). The subgroup analysis by AIS etiology, stroke subtype and head position did not demonstrate any significant associations with adverse outcome from greater systolic BPV (Table 3).
Table 3. Multivariate subgroup analysis by AIS etiology, stroke sub-type, head position and reperfusion therapy.
| Multivariable analysis | |||
|---|---|---|---|
| AIS | OR(95%CI) | P interaction | |
| Large artery occlusion due to significant extracranial internal carotid atheroma | N=654 | 1.05(0.90-1.23) | 0.333 |
| Large artery occlusion due to significant intracranial cerebral atheroma | N=2004 | 1.03(0.94-1.13) | |
| Small vessel or perforator lacunar disease | N=2580 | 1.06(0.98-1.16) | |
| Others | N=3094 | 1.08(1.01-1.16) | |
| Stroke subtype | |||
| AIS | N=8338 | 1.06(1.01-1.11) | 0.908 |
| ICH | N=818 | 1.03(0.94-1.13) | |
| Head position | |||
| Lying flat | N=4375 | 1.04(0.98-1.10) | 0.120 |
| Sitting up | N=4781 | 1.08(1.02-1.15) | |
| Reperfusion therapy | |||
| No | N=7183 | 1.05(1.00-1.10) | 0.139 |
| Yes | N=1115 | 1.04(0.93-1.17) |
Data are presented as odds ratio [OR] (95% confidence intervals [95%CI]) for per 1 unit increment of systolic blood pressure [SBP] variability. AIS: acute ischemic stroke; ICH: intracerebral hemorrhage.
Adjusted covariates include covariates of country, prestroke mRS score, sex, baseline NIHSS score, and history of heart disease, stroke, diabetes mellitus, or hypertension, and prior antiplatelet therapy
Lastly, increased mean diastolic BP (1.08 [1.03-1.12]; P=0.001) and mean arterial pressure (1.06 [1.01-1.10]; P=0.009) were associated with unfavorable shift of the mRS at 90 days. (Table V in the Supplementary Files). In addition, CV of diastolic BP (DBP) (1.08, 1.03-1.12; P=0.001) over 24 hours post stroke, was significantly associated with 3-month poor outcome (Table V in Supplementary Files). The association was more apparent in sitting-up position (1.12, 1.06-1.19) compared with lying-flat position (1.03, 0.98-1.09) (P interaction = 0.005). Among patients without AF, mean SBP (1.17 [1.12-1.22]; P<0.0001), SD (1.05 [1.01-1.09]; P<0.017), ARV (1.06 [1.01-1.12]; P<0.015), DBP (1.01 [1.00-1.02]; P<0.004) and pulse pressure (1.02 [1.01-1.03]; P<0.001) were associated with death or disability at 90 days (Table VI in Supplementary Files).
Discussion
Firstly, these post-hoc analyses of the large multicenter HeadPoST trial demonstrated that increased BPV is associated with poor outcome from stroke as determined by unfavorable shift in mRS at 90 days. Secondly, there was no interaction of AIS etiology, stroke sub-type, head-position or use of reperfusion therapy with the predictive effects of BPV on outcome. Lastly, head position change did not influence systolic BP over the 24-hour period post stroke.
The HeadPoST study design provided an opportunity to test the predictive significance of BPV across the broad range of patients with acute stroke. The paucity of randomized data examining head position, and central and peripheral hemodynamics are also apparent. Furthermore, there are no randomized data to date examining head position and BPV parameters specifically. BPV is an important measure as it provides information on systemic hemodynamics and can be used for risk assessment. Challenges exist around beat-to-beat BPV measurements and translation into clinical practice.
BPV was assessed from multiple readings taken at various time points after hospital admission for acute stroke [11]. While heterogeneity exists across the methods of assessment and BPV metrics used in reporting [11], SD correlates well with mean BP levels and the number of readings and time period do not generally affect the magnitude of short-term BPV, though mean BP should always be adjusted for as a consequence of this relationship [11]. Furthermore, this analysis describes the minimum criteria for reporting as determined by a recent systematic review and meta-analysis (timing, number of BP measurements, duration of BP monitoring, and a low computational complexity BPV metric) [11]. Importantly, mean SBP, SD and ARV are crucial confounders as they are often correlated with systolic BPV [12,13]. ARV was developed as a measure to overcome deficiencies in the commonly used SD and was largely targeted for use when sampling BP from prolonged ambulatory BP devices as opposed to casual cuff measurements. Therefore, the focus on measures of BPV that are not closely correlated with mean SBP, CV and VIM, is key. CV is the most appropriate index of BPV within this clinical context as it is largely independent of mean SBP at an individual patient level. This study demonstrated CV to be highly predictive of an unfavorable outcome as demonstrated by association of worsening fifths of CV with poorer mRS scores. Interestingly, this study demonstrated DBP variability was also independently associated with outcome after multivariable adjustment. Though the effect size was small, this is of interest both pathophysiologically and clinically, particularly as sitting up conferred a greater magnitude of DBP variability. This appears to be the first time this has been reported, perhaps largely potentiated by the primary focus of prior work on SBP variability. Nevertheless, this finding warrants further observation in mechanistic studies of central and peripheral blood pressure changes during alteration in head positioning.
Overall, the data from this study do provide support for validation of targeted strategies aimed at improving BPV independent of head position [14]. Currently there are a lack of data to support a prognostic benefit, although there have been calls for randomized controlled trials of interventions to reduce BPV [15]. The basis of such recommendations is in part also due to the effect of pre-stroke anti-hypertensive therapies on certain BPV parameters, with those on beta-blocker therapy being reported as having higher SD and VIM but not CV of SBP at 2 weeks [15]. This heterogeneity could explain the borderline VIM result as compared to other parameters, particularly as 65.5% of the cohort had a diagnosis of hypertension with wide variation in anti-hypertensive agent use being expected in part due to the geographical variation of study participants.
A key strength of this study is the consecutive unselected recruitment strategy, which provided information on a mixture of unstratified stroke pathologies independent of vascular imaging. This is crucial, as uncertainty remained due to discordance between findings from post-hoc analyses of INTERACT2 [3] (only ICH patients), compared with the Controlling Hypertension and Hypotension Immediately Post Stroke (CHHIPS) [16] and the Continue or Stop Post-Stroke Antihypertensives Collaboration Study (COSSACS) [17] (which were largely AIS patients). The findings of this study support those from INTERACT2 where greater systolic BPV was associated with a poor outcome in acute stroke due to ICH, though do support those from the mixed CHHIPS and COSSACS patient cohorts where no significant association was found. The differing study design and timing of BPV assessments may account for the concordance of the findings of this analysis with the INTERACT2 BPV analysis and consequent discordance with CHHIPS AND COSSACS BPV analysis [15]. Firstly, this study and INTERACT2 included a comparable number of ICH patients for whom arguably BPV demonstrates greater relevance from a prognostic perspective based on underlying mechanisms precipitating neuropathological deterioration. In addition, in the INTERACT2 BPV analysis both diastolic BP and mean arterial pressure appeared to confer some value in predicting prognosis at 30 days, which was confirmed in the present study. Secondly, the BPV data from INTERACT2 were gathered in the immediate aftermath of hyperacute stroke (within 6 hours) as opposed to in the acute period in CHHIPS AND COSSACS (36-48 hours).
Thirdly, as stated in the results section, those excluded were more likely to be female, have a shorter time between onset and intervention, less likely to have known hypertension and less likely to be taking aspirin. The reasons for exclusion included a lack of mean systolic BP, 90-day mRS or a final diagnosis that was indeterminate, stroke mimic, or TIA. The impact of missing data on the analyses is unclear though it could be argued that these individuals are healthier and hence the results could have provided an overestimation. However, should they have had a diagnosis of stroke, the shorter time between onset and intervention may have provided more robust BPV data in the hyperacute post-stroke period. Furthermore, sex differences do exist in severity of strokes and survival, and therefore the increased likelihood of exclusion of females may have attributed to a milder stroke population as stroke is often more severe in a female population [18]. Importantly, further analyses of patients without AF (Table VI in Supplementary Files), showed significant associations between pulse pressure and outcome. These findings suggest that the presence of AF confounds any effect pulse pressure has on outcome. These findings support those of the Standard Medical Management in Secondary Prevention of Ischemic Stroke in China (SMART) study that demonstrated pulse pressure was associated with poor stroke outcome in those over 60 years of age without AF [19].
A key limitation is the heterogeneity posed by assessment of BPV from standard isolated measurements as opposed to beat-to-beat assessment. However, BP readings were taken according to standard practice guidelines in participating hospitals, which are likely to be in accordance with national guidelines in respect of device, positioning and degree of acceptable measurement error. In addition, the inclusion of patients with atrial fibrillation presents a risk of excess unquantifiable variation during BPV assessments as compared to those in sinus rhythm [20].
The HeadPoST study generally included strokes of mild to moderate severity (median NIHSS of 4) and most people recruited had a 90-day mRS of 0-1 [9]. However despite patients presenting late and the intervention being delivered within 24 hours of admission as opposed to onset, findings of the study were consistent across all categorical scores on the NIHSS at 7 days [9]. The ability to assess the impact of BPV on early neurological deterioration is therefore limited, though data presented in this analysis provide 90-day prognostic information despite this. Prior work demonstrating systolic BPV as a cause for poorer neurological outcome post AIS showed increased systolic BPV was associated with large lesion core volume, proximal vessel occlusion and good collaterals [21]. Arguably this study was not representative of this population, who often fail or are not eligible for reperfusion therapies, though it provides prognostic information about BPV metrics in less severe strokes. Therefore, there is inadequate power to examine associations in specific etiological subtypes of ischemic stroke. Nonetheless, all other data, derived from observational and clinical trial settings, suggest consistency in risk factors and prognostic variables across such subtypes.
This secondary analysis did not assess the BP lowering efficacy of certain anti-hypertensive agents and the consequent efficacy in preventing adverse cardiovascular outcomes as a consequence of uncontrolled BPV. Finally, the limited number of patients with large artery occlusions, with or without mechanical thrombectomy intervention, limits generalizability to those individuals often at the peaks of systolic BP values, though the inclusion of those with ICH provides some perspectives on stroke severity and extremes of BP. Further work examining BPV metrics in a more severe subset of AIS patients is warranted including those undergoing mechanical reperfusion interventions.
In conclusion, increased BPV is associated with poor outcome from stroke as determined by unfavorable shift in mRS at 90 days. Head position has no influence on BPV in the largest randomized study of nursing care following acute stroke to date.
Supplementary Material
Summary Table.
What is known about this topic?
Blood pressure variability (BPV) has been shown to be associated with poor functional outcomes in acute ischemic stroke (AIS) and acute intracerebral hemorrhage (ICH).
Traditionally, AIS patients have been nursed in elevated positions (≥30°) due to concern over aspiration risk and propensity to reduce raised intracranial pressure.
However, disability outcomes do not differ between acute stroke patients nursed in a lying-flat position as compared to a head elevated position.
No studies have assessed the relationship between BPV and outcome following adjustment for head position.
What this study adds?
This sub-study assessed the predictive potential of BPV on stroke outcomes considering the interaction with head positioning and any associated hemodynamic changes in relation to lying-flat (increased cerebral blood flow and oxygenation) and sitting-up (potential reduction in ICP in large hemispheric AIS).
Systolic BPV was associated with unfavorable shift of the mRS at 90 days.
AIS etiology, stroke sub-type, head-position or use of reperfusion therapy did not interact with the predictive effects of systolic BPV on stroke outcome.
Diastolic BP was associated with unfavorable shift of the mRS at 90 days with a greater magnitude of effect of sitting-up head positioning on DBP variability and outcome.
Acknowledgements
None.
Funding
The study is supported by a research grant (1066966) from the National Health and Medical Research Council of Australia. The sponsors had no role in the study design, data collection, data analysis, data interpretation or writing of the report. All authors had full access to the study data. The corresponding author had final responsibility for the decision to submit the paper for publication.
Sources of support
JSM: Dunhill Medical Trust Research Training Fellow (RTF97/0117). PML: Research grants from The George Institute for Global Health, grants from Clínica Alemana de Santiago, during the conduct of the study; non-financial support from Boehringer Ingelheim, grants and personal fees from Bayer, grants and personal fees from AstraZeneca, grants from CONICYT, outside the submitted work. TJM: British Heart Foundation Clinical Research Training Fellow. HA: Lecture fees from Bayer, Daiichi-Sankyo and Takeda. MLH: NHMRC Career Development Fellowship Level 2 APP 1141328. OMPN: Research grants from CNPq and Brazilian Ministry of Health. THL: Research grant from MOST, BMRP and CMRP, Taiwan. CSA: Advisory Panel fees from Astra Zeneca and Amgen, speaking at seminars for Takeda China; research grant from Takeda China. TGR: NIHR Senior Investigator.
Footnotes
Disclosures
JSM: Dunhill Medical Trust Research Training Fellow (RTF97/0117). PML: Research grants from The George Institute for Global Health, grants from Clínica Alemana de Santiago, during the conduct of the study; non-financial support from Boehringer Ingelheim, grants and personal fees from Bayer, grants and personal fees from AstraZeneca, grants from CONICYT, outside the submitted work. TJM: British Heart Foundation Clinical Research Training Fellow. HA: Lecture fees from Bayer, Daiichi-Sankyo and Takeda. MLH: NHMRC Career Development Fellowship Level 2 APP 1141328. OMPN: Research grants from CNPq and Brazilian Ministry of Health. THL: Research grant from MOST, BMRP and CMRP, Taiwan. CSA: Advisory Panel fees from Astra Zeneca and Amgen, speaking at seminars for Takeda China; research grant from Takeda China. TGR: NIHR Senior Investigator.
Conflicts of interest
The authors declare no conflicts of interest.
References
- 1.How do stroke units improve patient outcomes? A collaborative systematic review of the randomized trials. Stroke Unit Trialists Collaboration. Stroke. 1997;28:2139–2144. doi: 10.1161/01.str.28.11.2139. [DOI] [PubMed] [Google Scholar]
- 2.Appiah KO, Minhas JS, Robinson TG. Managing high blood pressure during acute ischemic stroke and intracerebral hemorrhage. Curr Opin Neurol. 2018;31:8–13. doi: 10.1097/WCO.0000000000000508. [DOI] [PubMed] [Google Scholar]
- 3.Manning L, Hirakawa Y, Arima H, Wang X, Chalmers J, Wang J, et al. Blood pressure variability and outcome after acute intracerebral haemorrhage: a post-hoc analysis of INTERACT2, a randomised controlled trial. Lancet Neurol. 2014;13:364–373. doi: 10.1016/S1474-4422(14)70018-3. [DOI] [PubMed] [Google Scholar]
- 4.Zhang Y, Wang H, Xu K, Wang P, Li X, Zhao J, et al. Ambulatory blood pressure variability within the first 24 hours after admission and outcomes of acute ischemic stroke. J Am Soc Hypertens. 2018;12:195–203. doi: 10.1016/j.jash.2017.12.012. [DOI] [PubMed] [Google Scholar]
- 5.Webb AJS, Mazzucco S, Li L, Rothwell PM. Prognostic Significance of Blood Pressure Variability on Beat-to-Beat Monitoring After Transient Ischemic Attack and Stroke. Stroke. 2018;49:62–67. doi: 10.1161/STROKEAHA.117.019107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kenning JA, Toutant SM, Saunders RL. Upright patient positioning in the management of intracranial hypertension. Surg Neurol. 1981;15:148–152. doi: 10.1016/0090-3019(81)90037-9. [DOI] [PubMed] [Google Scholar]
- 7.Aries MJ, Elting JW, Stewart R, De Keyser J, Kremer B, Vroomen P. Cerebral blood flow velocity changes during upright positioning in bed after acute stroke: an observational study. BMJ Open. 2013;3 doi: 10.1136/bmjopen-2013-002960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lam MY, Haunton VJ, Robinson TG, Panerai RB. Does gradual change in head positioning affect cerebrovascular physiology? Physiol Rep. 2018;6 doi: 10.14814/phy2.13603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Anderson CS, Arima H, Lavados P, Billot L, Hackett ML, Olavarria VV, et al. Cluster-Randomized, Crossover Trial of Head Positioning in Acute Stroke. N Engl J Med. 2017;376:2437–2447. doi: 10.1056/NEJMoa1615715. [DOI] [PubMed] [Google Scholar]
- 10.Munoz-Venturelli P, Arima H, Lavados P, Brunser A, Peng B, Cui L, et al. Head Position in Stroke Trial (HeadPoST)--sitting-up vs lying-flat positioning of patients with acute stroke: study protocol for a cluster randomised controlled trial. Trials. 2015;16 doi: 10.1186/s13063-015-0767-1. 256-015-0767-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Veloudi P, Sharman JE. Methodological factors affecting quantification of blood pressure variability: a scoping review. J Hypertens. 2018;36:711–719. doi: 10.1097/HJH.0000000000001606. [DOI] [PubMed] [Google Scholar]
- 12.Mena L, Pintos S, Queipo NV, Aizpurua JA, Maestre G, Sulbaran T. A reliable index for the prognostic significance of blood pressure variability. Journal Hypertens. 2005;23:505–511. doi: 10.1097/01.hjh.0000160205.81652.5a. [DOI] [PubMed] [Google Scholar]
- 13.Parati G, Stergiou GS, Dolan E, Bilo G. Blood pressure variability: clinical relevance and application. J Clin Hypertens. 2018;20:1133–1137. doi: 10.1111/jch.13304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wojner-Alexander AW, Garami Z, Chernyshev OY, Alexandrov AV. Heads down: flat positioning improves blood flow velocity in acute ischemic stroke. Neurology. 2005;64:1354–1357. doi: 10.1212/01.WNL.0000158284.41705.A5. [DOI] [PubMed] [Google Scholar]
- 15.Manning LS, Mistri AK, Potter J, Rothwell PM, Robinson TG. Short-term blood pressure variability in acute stroke: post hoc analysis of the controlling hypertension and hypotension immediately post stroke and continue or stop post-stroke antihypertensives collaborative study trials. Stroke. 2015;46:1518–1524. doi: 10.1161/STROKEAHA.115.009078. [DOI] [PubMed] [Google Scholar]
- 16.Potter JF, Robinson TG, Ford GA, Mistri A, James M, Chernova J, et al. Controlling hypertension and hypotension immediately post-stroke (CHHIPS): a randomised, placebo-controlled, double-blind pilot trial. Lancet Neurol. 2009;8:48–56. doi: 10.1016/S1474-4422(08)70263-1. [DOI] [PubMed] [Google Scholar]
- 17.Robinson TG, Potter JF, Ford GA, Bulpitt CJ, Chernova J, Jagger C, et al. Effects of antihypertensive treatment after acute stroke in the Continue or Stop Post-Stroke Antihypertensives Collaborative Study (COSSACS): a prospective, randomised, open, blinded-endpoint trial. Lancet Neurol. 2010;9:767–775. doi: 10.1016/S1474-4422(10)70163-0. [DOI] [PubMed] [Google Scholar]
- 18.Dehlendorff C, Andersen KK, Olsen TS. Sex disparaties in stroke: women have more severe strokes but better survival than men. J Am Heart Assoc. 2015;4:e001967. doi: 10.1161/JAHA.115.001967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Su N, Zhai FF, Ni J, Zhou LX, Yao M, Peng B, Zhu YC, Cui LY. Pulse pressure within 3 months after ischemic stroke is associated with long-term stroke outcomes. Am J Hypertens. 2017;30:1189–1195. doi: 10.1093/ajh/hpx121. [DOI] [PubMed] [Google Scholar]
- 20.Olbers J, Gille A, Ljungman P, Rosenqvist M, Ostergren J, Witt N. High beat-to-beat blood pressure variability in atrial fibrillation compared to sinus rhythm. Blood Press. 2018:1–7. doi: 10.1080/08037051.2018.1436400. [DOI] [PubMed] [Google Scholar]
- 21.de Havenon A, Bennett A, Stoddard GJ, Smith G, Chung L, O'Donnell S, et al. Determinants of the impact of blood pressure variability on neurological outcome after acute ischaemic stroke. Stroke Vasc Neurol. 2017;2:1–6. doi: 10.1136/svn-2016-000057. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.