Abstract
Objective
Clinical review on outcomes using burst spinal cord stimulation (SCS) in the treatment of chronic, intractable pain.
Design
Narrative clinical literature review conducted utilizing a priori search terms including key words for burst spinal cord stimulation. Synthesis and reporting of data from publications including an overview of comparative SCS outcomes.
Results
Burst SCS demonstrated greater pain relief over tonic stimulation in multiple studies, which included blinded, sham-controlled, randomized trials. Additionally, burst stimulation impacts multiple dimensions of pain, including somatic pain as well as emotional and psychological elements. Patient preference is weighted toward burst over tonic due to increased pain relief, a lack of paresthesias, and impression of change in condition.
Conclusion
Burst SCS has been shown to be both statistically and clinically superior to tonic stimulation and may provide additional benefits through different mechanisms of action. Further high-quality controlled studies are warranted to not only elucidate the basic mechanisms of burst SCS but also address how this unique stimulation signature/pattern may more adequately handle the multiple affective dimensions of pain in varying patient populations.
Keywords: Burst, Spinal Cord Stimulation, Outcomes, Clinical
Introduction
Despite of the immense burden that chronic pain has on patients, payers, and caregivers, it is rarely treated adequately and may even go untreated altogether [1,2]. Pharmacological treatments for neuropathic pain states are frequently ineffective [3], and opioid drugs, though effective in selective patients, may lead to harmful effect for both individuals [4] and society [5,6]. Much of the challenge in the development in new pharmacotherapies results from the lack of knowledge or translation of research from animal to human species [7,8].
The safety, efficacy, and cost-effectiveness of spinal cord stimulation have, in randomized controlled trials, been proven for failed back surgery syndrome (FBSS), complex regional pain syndrome (CRPS), and painful diabetic neuropathy (PDN) [9–11]. More than five decades of clinical experience with electrostimulation of the dorsal columns (and adjacent dorsal neural structures) for intractable pain conditions have established this treatment in the armamentarium of pain practitioners, anesthesiologists, and neurosurgeons [12–17]. Despite continuous improvement in the physical and functional aspects of implanted hardware and increased understanding of the limitations and possibilities of the treatment, the nature of the delivered stimulus to the spinal cord has remained unchanged for many years. The conventional paradigm of SCS is to elicit comfortable paresthesias in the painful area using a tonic stimulus pattern at low frequencies (typically 30–70 Hz). However, during the past decade, a number of novel stimulus paradigms have been introduced for clinical use, resulting in improved outcomes and reduction in unwanted side effects [13,18].
The concept of burst spinal cord stimulation (burst SCS), introduced in 2010 by DeRidder and colleagues [19], targets the dorsal columns in stimulus bursts comprised of five 1-ms pulses with an intraburst frequency of 500 Hz, delivered with a frequency of 40 Hz in a passive recharging paradigm to maintain charge balance across the electrical contacts (Figure 1) [18]. In contrast to other novel stimulus paradigms, burst SCS stems from original observations of thalamo-cortical firing patterns, which have the ability to strengthen synaptic connectivity [20,21]. It was originally applied to the auditory cortex in an attempt to treat tinnitus with transcranial magnetic stimulation resistant to tonic stimuli [22]. When the burst SCS pattern was electrically applied to the dorsal columns at adequate settings, it was effective at producing analgesia without the need for paresthesias [19,23,24]. Most recently, burst SCS was shown to result in statistically superior pain relief compared with tonic stimulation in a large prospective, randomized, controlled clinical trial [25].
Figure 1.
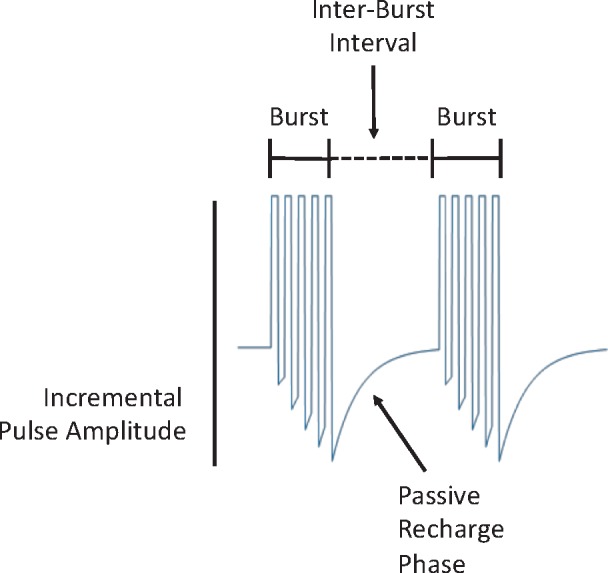
Burst stimulation pattern and waveform signature. Pulse trains are separated by an interburst interval of stimulation quiescence. Note the increasing pulse amplitude during the burst. During the interburst interval, a passive charge balance occurs that dissipates any charge imbalance that might occur across the electrodes.
Apart from affecting pain intensity, evidence suggests that burst SCS may impact important aspects of the chronic pain condition, such as pain vigilance, pain catastrophizing, and depression [18,26,27]. The purpose of this narrative review is to explore the available evidence for burst SCS spinal cord stimulation, providing a clinical perspective on the possible distinct therapy features, benefits, and limitations.
Methods
Literature Search Strategy
The reviewed literature was compiled by searching the MEDLINE and EMBASE databases using the search term “burst” combined with “dorsal column stimulation” or “spinal cord stimulation.” Google Scholar and the journal Neuromodulation were searched using the term “burst spinal cord stimulation.” Citation lists from contemporary reviews on spinal cord stimulation were also surveyed, and additional literature was added as appropriate.
Selection of Literature
Literature included both prospective and retrospective studies reporting clinical outcome measures when treating chronic pain using burst SCS, regardless of the underlying pain condition or length of treatment time. All included papers were peer reviewed, except two conference reports [28,29].
Articles were excluded on the following criteria: Indications other than pain of the trunk or limbs, nonhuman studies, published study protocols, technical reports with no outcome measurements, conference proceedings on smaller cohorts and treatments other than spinal cord stimulation. All authors participated in the selection process. Disagreement on selection of literature was resolved by consensus. Published papers were also graded by level of evidence utilizing standardized methods on a scale from level 1 (RCT) to 5 (Expert Opinion) [30,31].
In total, 20 papers were included in this review. All evaluated changes in pain intensity or pain quality with burst SCS (Table 1). Other secondary outcomes were reported and those varied between the studies.
Table 1.
Literature included in the narrative review and summary
| Reference | Study Type (LOE) | Sample Size | Diagnosis | Follow-up |
|---|---|---|---|---|
| Courtney et al. [32] (2015) |
|
22 | FBSS/radiculopathy/CRPS | Tonic at baseline, then burst for 7 and 14 d |
| De Ridder et al. [19] (2010) |
|
12 | Radiculopathy | On the table—1 h each |
| De Ridder et al. [24] (2013) |
|
15 | FBSS/neuropathy | 1 wk each |
| De Ridder et al. [33] (2015) |
|
102 | FBSS/PDN | 2 wk each |
| De Ridder et al. [34] (2015) |
|
49 | FBSS/PDN | 2 wk each |
| De Vos et al. [35] (2015) |
|
48 | FBSS/PDN/PR | 2 wk |
| Deer et al. [25] (2018) |
|
96 | FBSS/neuropathy/PR | 24 wk and 12 mo (12 mo used for pain) |
| Kinfe et al. [36] (2016) |
|
16 | FBSS | 3 mo |
| Kinfe et al. [37] (2017) |
|
12 | FBSS | 2 y |
| Kriek et al. [38] (2017) |
|
29 | CRPS | 2 wk each of burst, tonic (3 options), and placebo |
| Muhammad et al. [39] (2018) |
|
12 | FBSS | 15 mo (average 12–19) |
| Schu et al. [40] (2014) |
|
20 | FBSS | 2 wk each |
| Tjepkema-Cloostermans et al. [41] (2016) | RCT | 40 | FBSS/PR/neuropathy | 2 wk each |
| Bara et al. [42] (2013) |
|
29 | FBSS | 12 mo median |
| Colini-Baldeschi et al. [28] (2016) |
|
61 | Chronic low back and leg pain | 3, 6, 12 mo |
| Espinet et al. [43] (2014) |
|
22 | N/A | Tonic at baseline, then burst for 14 d |
| Kretzschmar et al. [44] (2017) | Retrospective cohort | 100 | N/A | 1 y |
| Kriek et al. [45] (2015) |
|
1 | CRPS | 2 y |
| Rasekhi et al. [46] (2018) |
|
1 | Neuropathy | N/A |
| Wahlstedt et al. [29] (2017) |
|
8 | Upper extremity pain | 4 mo |
CRPS = complex regional pain syndrome; PDN = painful diabetic neuropathy; FBSS = failed back surgery syndrome; LOE = level of evidence; PR = poor responder; RCT = randomized controlled trial.
Burst SCS Lead Placement, Contact Selection, and Stimulation Parameters
Lead Placement and Contact Selection
For years, the correct placement of SCS leads and electrodes was dependent upon generating a concordant overlap of paresthesia coverage in the area/region of pain. Conceptually, this technique targets the large-diameter fibers in the dorsal columns that in turn project to the anatomically correct pain processing neurons in the spinal cord. In general, before switching to burst SCS mode, primary lead placement is also mapped using paresthesia coverage with tonic stimulation.
This paradigm has changed slightly with the development of new stimulation parameters, such as with higher frequencies where paresthesias may not be perceived by the patient. With the introduction of 10-KHz stimulation frequencies, it has become possible to place SCS electrodes in anesthetized patients with a standardized, anatomically based procedure; this is not to assume that leads can simply be placed anywhere, as the physiologically relevant underlying neural structures must still be influenced by the electrical field. Similarly, preliminary results from a prospective, multicenter study have shown that anatomical lead placement near the T9-10 junction in patients suffering from FBSS and utilizing burst SCS can lead to subsequent pain relief [47]. These results showed visual analog scale (VAS) reductions of low back and leg pain from baselines of 79 mm and 60 mm to approximately 20–24 mm and 13–17 mm, respectively, at three months (N = 30). Follow-up at six months showed similar results. Importantly, reported pain reduction, using either paresthesia mapping or strict anatomical placement, did not differ. These results suggest that, like higher-frequency studies, anatomical lead placement with burst SCS might be effectively used near the T9-10 epidural space to treat low back and leg pain.
Although the literature shows pulse train activation patterns in alpha, beta, and theta ranges and beyond, the stimulation pattern utilized clinically by burst SCS is five pulses (1-msec duration) being delivered at 500 Hz at a 40-Hz burst frequency. Importantly, the system undergoes a passive recharge cycle at the end of the burst phase, as opposed to an active recharge phase, to provide charge balance [18]. Varying burst frequencies have been tested clinically; based on a preclinical study, clinical outcomes, and power consumption, an interburst frequency of 500 Hz was found to be preferential [48]. Similar studies have been completed in animal models, demonstrating frequency-dependent effects on pain processing neurons in the dorsal horn [49].
Pulse amplitudes used during burst stimulation generally are much lower than during tonic stimulation [25,35]. This is partly due to the higher frequency (reducing charge per pulse to accommodate increased total charge delivered) used during the burst period, as well as the 1-ms pulse width utilized. A recent substudy of the SUNBURST pivotal trial found that patients not only preferred lower-amplitude intensities, but also gained more pain relief at those settings [50]. This might seem counterintuitive compared with the dose–response relationship in low-frequency SCS (LF-SCS). Although it is not exactly clear why lower-intensity pulse amplitudes with burst SCS provide potentially better outcomes, conceptually, using the least amount of electrical “dose” needed helps both the battery use in the system and the potential to induce plastic adaptations by the nervous system. In an animal study published by Tang and colleagues, lower pulse amplitudes utilized in burst SCS were more effective in reducing neural responses to noxious pinch, reinforcing the concept of a therapeutic window [51]. Similarly, reductions in pulse amplitude showed a different response in LF-SCS, demonstrating a clear point of separation vs tonic stimulation with respect to pulse amplitude. There is increasing evidence that demonstrates a difference in the neurophysiologic responses to burst SCS [52]. These types of neural responses might allow a phasic approach to the use of burst SCS, further reducing the energy delivery requirements while maintaining clinical outcomes [53].
“Paresthesia-Free” Stimulation
Burst SCS was introduced to modulate the somatic nervous system without the need for the conventional paresthesia-dependent overlap as a means of analgesia [19]. As discussed previously, given the frequency of stimulation during the burst, the amplitude is reduced to a subperceptive level [19,24]. Although an in-depth discussion of the potential mechanisms underlying paresthesia-independent or paresthesia-free analgesia associated with burst SCS is beyond the scope of this review, multiple papers have highlighted this clinical observation and underlying neurophysiology [19,24,25,35,41]. Lower pulse amplitudes can partly explain the decreased somatic sensation of paresthesia, as can decreased sensitivity or conduction failure of orthodromically mediated action potentials in the dorsal column nuclei [18]. Schultheis et al. showed in a case series of six patients with chronic axial neck pain, cervicobrachialgia, and occipital neuralgia after neck surgery that pain relief could be achieved without significant overlap of paresthesias [54]. The latter findings, in conjunction with other studies, indicate that burst SCS may not only generate analgesia without paresthesias, but that the lead position utilized with burst SCS may not produce concordant paresthesias when low-frequency tonic stimulation is utilized. The implications for these findings are a potential difference in ideal or efficacious lead position when burst stimuli are used in a lead positioned with tonic stimulation. Moreover, it is consistent with different underlying mechanisms of analgesia between tonic and burst SCS.
Clinical Outcomes Using Burst SCS: Pain Relief
The first peer-reviewed evidence on burst SCS was published by De Ridder and colleagues in 2010 [19]. In this prospective controlled study, patients were exposed to both burst SCS and LF tonic stimulation (LF-SCS) in a randomized, crossover fashion. In patients with primarily FBSS and failed neck surgery syndrome (FNSS), with a mean follow-up at 20.5 months, effective long-term pain suppression was demonstrated, and evidence for paresthesia-free spinal cord stimulation with burst SCS was established.
De Ridder et al. followed up this study in 2013 with a first-of-its-kind prospective, randomized, sham-controlled clinical study [24]. During the trial phase, 15 patients diagnosed with FBSS were exposed to one-week periods of either tonic, burst SCS, or sham; the latter was accomplished by turning the stimulation amplitude to zero. Pain intensity scores demonstrated a statistically significant improvement of back, leg, and general pain with burst SCS as compared with sham. Interestingly, isolated back and leg pain intensities were not significantly different from tonic back and leg pain during tonic stimulation. However, combined general pain was significantly different between burst and tonic stimulation, possibly demonstrating that the proposed different central pain processing of pain with burst SCS is reflected in the perception of general pain intensity. Remarkably, burst SCS showed significant improvement over placebo for back, leg, and general pain. Indeed, central processing of pain as a cognitive construct has implicated both affective and somatic discriminatory brain areas as well as the connectivity between these regions [55,56].
In clinical practice, unresolved and often intractable low back and leg pain after spinal surgery (FBSS) [57] has served as the most common indication for SCS [12,58,59]. The published body of evidence for traditional tonic stimulation reflects this, as FBSS is the most thoroughly studied indication for SCS [58,60].
Multiple prospective and cohort-designed review studies have been published that demonstrate the clinical efficacy of burst SCS on FBSS, as well as a number of other low back pain conditions (Table 2) [14,18,26,33]. Schu and colleagues conducted a sham-controlled randomized trial comparing burst SCS with both placebo control and a continuous 500-Hz tonic paradigm in patients with FBSS already receiving LF-SCS—thus controlling for the higher frequency of stimulation without the interburst interval or the charge accumulation and passive discharge found in the burst SCS stimulation paradigm. The authors found that burst SCS demonstrated significantly better pain relief (P < 0.05) as compared with the other cohorts studied. The studies by Schu and DeRidder provide important and reliable data on burst SCS by utilizing sham controls, a study design element that is either not possible with LF-SCS or rarely attempted—in both cases, burst SCS was shown to be superior to sham. Courtney and colleagues published similar findings from a prospective multicenter study; subjects (N = 22) with a primary diagnosis of FBSS already receiving LF-SCS demonstrated superior pain relief when switched to burst SCS [32]. These findings are comparable to some of the original prospective data by DeRidder et al. [19] on FBSS patients showing superior “paresthesia-free” low back and limb pain relief in patients who had previously been treated with LF-SCS. Similar findings of significant pain relief when FBSS patients are provided with burst SCS have further supported these multiple observations [35,36,41,48].
Table 2.
Studies comparing burst SCS to other forms of spinal cord stimulation including low-frequency and high-frequency tonic stimulation
| Reference | Study Type | Sample Size | Diagnosis | Follow-up | Baseline Pain | Tonic | Burst SCS Pain | HF-SCS |
|---|---|---|---|---|---|---|---|---|
| Courtney et al. [32] (2015) | Prospective | 22 | FBSS/radiculopathy/CRPS | Tonic at baseline, then burst for 7 and 14 d | N/A | 54.0 (+ 19.8) | 28.3 (+ 17.3) | — |
| De Ridder et al. [24] (2013) | RCT | 15 | FBSS/neuropathy | 1 wk each |
|
|
|
— |
| De Ridder et al. [33] (2015) | Retrospective cohort | 102 | 2 wk each | 78 | 48.8 | 31.9 | — | |
| De Ridder [34] (2015) | Retrospective cohort | 49 | FBSS/PDN | 2 wk each | 78 (SD = 11.43) | 48 (SD = 27) | 36 (SD = 27) | — |
| De Vos et al [35] (2015) | RCT | 48 | FBSS/PDN/PR | 2 wk |
|
|
|
— |
| Deer et al [25] (2018) | RCT | 96 | FBSS/neuropathy/PR | 24 wk and 12 mo (12 mo used for pain) | 74.7 | 48.7 | 43.5 | — |
| Kinfe et al. [36] (2016) | Prospective | 16 | FBSS | 3 mo |
|
— |
|
|
| Kriek et al. [38] (2017) | RCT | 29 | CRPS | 2 wk each of burst, tonic (3 options), and placebo | 72.74 | 39.8 | 47.98 | — |
| Muhammad et al. [39] (2018) | Prospective | 12 | FBSS | 15 mo (average 12–19) |
|
— |
|
|
| Schu et al. [40] (2014) | RCT | 20 | FBSS | 2 wk each | N/A | 56 | 47 | — |
| Tjepkema-Cloostermans et al. [41] (2016) | RCT | 40 | FBSS/PR/neuropathy | 2 wk each | N/A | 52 | 42 | — |
| Espinet et al. [43] (2014) | Retrospective cohort | N/A | Tonic at baseline, then burst for 2 wk | N/A | 53.5 | 28.5 | — |
B-SCS = burst SCS; CRPS = complex regional pain syndrome; PDN = painful diabetic neuropathy; FBSS = failed back surgery syndrome; HF-SCS = high-frequency SCS; LOE = level of evidence; PR = poor responder; RCT = randomized controlled trial; SCS = spinal cord stimulation.
The SUNBURST trial, published in 2017, is the largest prospective, multicenter randomized controlled trial evaluating burst SCS to date [25]. This study was conducted to obtain Food and Drug Administration (FDA) approval and was therefore performed under strict FDA guidance. It was designed as a noninferiority crossover trial to compare burst SCS with control LF-SCS; subsequent superiority testing of secondary powered outcomes was also conducted, as outlined in the a priori statistical analysis plan.
After a successful LF-SCS trial stimulation and implant, subjects received, in randomized order, 12 weeks of either LF-SCS or burst SCS. After 12 weeks, subjects then crossed over to the other group. After 24 weeks, participants were allowed to use the stimulation mode of their choice, and subjects were followed until all subjects passed the one-year mark, with some subjects having two-year follow-up. FBSS and radiculopathies were the primary diagnoses of the study population, but pain conditions such as CRPS, degenerative spine disease, and postoperative pain were also included, thus reflecting the variability in SCS indications in everyday clinical settings. The primary outcome variable was difference in pain intensity between burst SCS and LF-SCS stimulation. The mean difference in pain intensity for general pain was 5.1 mm (VAS, 0–100 mm), resulting in burst SCS demonstrating both noninferiority and superiority compared with LF-SCS. The group difference in pain intensity was smaller in this study as compared with other trials reviewed comparing burst and tonic stimulation. However, it must be considered that only subjects proven to be responders to tonic stimulation were included in the trial, which potentially might disfavor burst SCS over tonic stimulation (enrich an LF-SCS responding population). Moreover, it is worth noting that despite the moderate difference in pain intensity between the stimulation modes, 70.8% of the study subjects preferred the burst stimulation mode over tonic stimulation. When questioned about the primary reason for preferring burst SCS, roughly half of the subjects answered superior pain relief, the other half lack of paresthesia. This potentially suggests that burst SCS targets other aspects of the pain or therapy experience, as indicated by earlier investigators demonstrating significant differences in pain vigilance and a possible difference in central processing of pain between burst SCS and LF-SCS [24].
The SUNBURST trial data point to clinically important relationships between stimulation amplitude, paresthesia, and analgesic effects for burst SCS, essentially a therapeutic window of optimal efficacy. Despite the fact that considerable burst SCS programming experience existed in Europe at the time the trial started in the United States, subjects were not programmed in the same standardized manner at the onset of SUNBURST. During the course of the study, there was a tendency toward higher stimulus amplitudes, and in a programming optimization substudy of 32 patients having completed the first 24 weeks of the SUNBURST trial, additional improvement and fewer paresthesias were observed after a lowering of stimulation amplitudes into the presumptive therapeutic window [50].
Burst SCS has also shown promising results in other pain patient populations, beyond low back pain and FBSS. De Vos and colleagues investigated the effectiveness in three cohorts: FBSS, poor responders (PRs) to tonic stimulation, and painful diabetic neuropathy [35]. Twelve patients with benefit from tonic stimulation for PDN were switched to burst SCS for two weeks, resulting in a 44% decrease in pain intensity from tonic baseline. The pain reduction in the PDN cohort surpassed both the FBSS and PR cohorts. Evidence for traditional tonic stimulation in PDN had previously been established in a multicenter randomized clinical trial [35], and despite methodological weaknesses such as short follow-up time in the burst SCS trial from the same author, evidence suggests that burst SCS is at least as effective as tonic stimulation in producing a substantial, clinically meaningful effect on pain in PDN, without paresthesias.
Burst SCS Regaining Pain Relief in Failed Tonic SCS
A number of studies have compared the analgesic efficacy of burst in patients with effective tonic therapy (Table 2), as well as salvage therapy with burst SCS in patients failing tonic therapy. Burst SCS may offer advantages over tonic stimulation, as the data suggest improved patient tolerance, increased function, and success in a subset of patients refractory to tonic SCS. De Vos et al. [35] tested burst SCS for two weeks in 48 patients with tonic stimulation for at least six months. The 48 patients were divided into three groups: 12 patients with painful diabetic neuropathy, 24 patients with FBSS who had significant pain reduction with tonic stimulation, and 12 patients with FBSS who did not benefit from tonic stimulation. After two weeks of burst SCS, pain scores were reduced significantly. Burst produced an additional pain reduction of 44% in PDN and 28% in the patients with FBSS who had already experienced pain reduction with tonic stimulation. On average, poor responders to tonic SCS reported decreased pain intensity by 23% when burst SCS was utilized. Although poor tonic responders seemed to benefit less, there was still a meaningful reduction in pain when compared with tonic SCS. Overall, burst SCS was able to produce an additional pain-relieving effect in almost 60% of the pain patients. In a separate study, DeRidder and colleagues reported that 62.5% of the patients not responding to LF-SCS did respond positively to burst SCS [34], similarly demonstrating that burst SCS can result in analgesia when LF-SCS fails.
Intuitively, it makes sense that a stimulation paradigm acting through different neurophysiologic mechanisms would be able to provide analgesia when a different stimulation mode has lost effect. Although it appears that burst SCS can provide pain relief for those patients who have failed tonic stimulation [26], further research is required to more fully elucidate this in multiple patient populations.
Studies Comparing Burst SCS with Higher-Frequency Paradigms
To the best of our knowledge, only three trials have compared burst SCS with another higher-frequency tonic stimulation mode (HF-SCS) (Table 2) [36,40,61,62]. HF-SCS therapy delivers tonic stimulation to the spinal cord at a frequency at or near 10 kHz. Kinfe et al. randomized 16 patients with FBSS and predominant low back pain to either HF-SCS or burst SCS and followed up at three months and 12 months [36,62]. Both treatments resulted in a robust therapeutic effect on back pain intensity and functional outcomes. The result was slightly in favor of burst SCS, but this result should be cautiously interpreted, considering the small sample size and single-center design. Schu and colleagues compared burst SCS with a 500-Hz tonic SCS stimulation pattern, thus matching the intraburst signal frequency. These authors found burst to provide better analgesia in patients with FBSS when compared with the 500-Hz group [40]. In patients suffering from CRPS, no clear preference for burst or other HF-SCS paradigms, including frequencies at 500 and 1200 Hz, could be demonstrated [61]. This suggests that there may be a difference in this patient population when compared with those suffering predominantly from low back pain.
Blinded, Sham-Controlled Studies Utilizing Burst SCS
Burst SCS has been studied in a total of 11 prospective studies (Table 1). Of these, four have also included sham/placebo controls (Table 3) [24,40,41,61]. This far exceeds any other cohort of placebo-controlled studies examining a specific stimulation frequency or pattern, where similar stimulation parameters might slightly vary between studies (including HF-SCS) [63,64]. In all but one study [41], burst SCS outperformed placebo control (sham stimulation); however, in that study, the “sham” stimulation was comprised of the stimulating amplitude being set at 0.1 mA, or as the authors termed it, the “low-burst” setting. This is in opposition with the other three studies, which set the device amplitude to 0 mA [24,27,61], and suggests that burst SCS may provide therapeutic benefit even at very low amplitudes. This is consistent with the concept that higher stimulation amplitudes, in general, may be less effective than lower amplitudes [50], with a lower dose–response curve “floor” than tonic stimulation. These consistent findings are in contrast to other placebo-controlled studies showing inconsistent and conflicting findings on differences between HF-SCS and sham stimulation [63,64].
Table 3.
Sham-controlled study outcomes utilizing burst SCS
| Reference | Sample Size | Diagnosis | Time Course, wk | Baseline Pain | Burst SCS Pain | Sham/Placebo |
|---|---|---|---|---|---|---|
| Kriek et al. [38] (2016) | 29 | CRPS | 2 | 72.74 | 47.98 | 63.7 |
| Schu et al. [40] (2014) | 20 | FBSS | 2 | N/A | 47 | 83 |
| Tjepkema-Cloostermans et al. [41] (2016) | 40 | FBSS/PR/neuropathy | 2 | N/A | 42 | 40 |
| De Ridder et al. [24] (2013) | 15 | FBSS/neuropathy | 1 |
|
|
|
CRPS = complex regional pain syndrome; FBSS = failed back surgery syndrome; PR = poor responder; SCS = spinal cord stimulation.
Emotional and Affective Component of Pain
The individual’s perception of pain is the result of a complex integration of sensory, emotional, and cognitive cerebral input. It is believed that the discriminative/sensory part of pain perception (e.g., exact location, character, intensity of pain) follows the lateral pathway via the anterior spinothalamic tract, which projects mainly to the S1 and S2 regions of the somatosensory cortex and the operculum of the insula. The affective/emotional part of pain perception follows the medial pathway via the lateral spinothalamic tract, which projects to the operculum part of the insula, anterior cingulate cortex (ACC), and other brain regions [18,23] The medial thalamic complex is believed to potentiate the ACC by burst firing patterns. Activation of the ACC is involved in processing the attention paid to pain [65] and the unpleasantness of pain perception [66,67]. This connection has been implicated in the evolution of acute to chronic pain as a result of low-frequency firing patterns within the spinothalamic–cingulate complex. This anatomic pathway might also facilitate pain-induced attention, memory, fear, and anxiety behaviors as a contextual basis for pain [18,68,69]. The mechanism of action article in this supplement, as well as other reviews, gives detailed information on the evidence gained over the years to support the possible clinical efficacy of burst SCS, as this simulation paradigm tends to act on the ACC [15,18]. Spinal, supraspinal, and neuro-immunological mechanisms of burst SCS might help explain its clinical effects on the affective component of pain perception.
We identified multiple studies describing the effects of burst SCS on validated clinical affective outcome parameters. In the Beck Depression Inventory (BDI), a validated questionnaire that looks at three domains of depression, the degree of symptoms is categorized as minimal, light, moderate/severe, or severe, with score ranges of 0–13, 14–19, 20–28, and 29–63, respectively. Four papers with a total of 128 patients used the BDI as a secondary outcome measure (Table 4). Unfortunately, the most comprehensive study used moderate/severe depression (BDI > 24) as an exclusion criterion [25]. For this reason, only patients with low BDI scores were included in this trial. Thus, the reported BDI scores in this pivotal study have no clinical meaning in further analysis. The smaller studies show a clinically meaningful reduction in BDI score for burst SCS compared with baseline measurements.
Table 4.
Published papers including the Beck Depression Inventory as an outcome measure and the results
| Reference | SCS Indication | No. | Follow-up, wk | Baseline BDI | Burst BDI | Comparator BDI |
|---|---|---|---|---|---|---|
| Deer et al. [25] | Mixed neuropathic pain | 100 | 52 | 10.1 (± 6) | 8.9 (± 7.6) | Tonic: 9.6 (± 7) |
| Kinfe et al. [36] (2016) | FBSS | 8 | 12 | 23.3 (± 2.1) | 13.5 (± 4.5) | Burst = HF-SCS |
| Kinfe et al. [37] (2017) | Refractory FBSS | 12 | 12 | 20.83 (± 3.56) | 10.92 (± 0.75) | Control 2.0 (± 0.5) |
| Muhammed et al. [62] (2017) | FBSS | 8 | 80 | 25.88 | 10.87 | NR |
BDI = Beck Depression Inventory; FBSS = failed back surgery syndrome; HF-SCS = high frequency SCS; NR = not reported; SCS = spinal cord stimulation.
The Pain Catastrophizing Scale (PCS) is a validated questionnaire that looks at three domains of pain catastrophizing: rumination, magnification, and helplessness. The maximum score is set at 52, with a total PCS score of 30 representing clinically relevant catastrophizing. A total PCS >30 results in a significant chance of prolonged unemployment and total disability for occupation-related activities. There is a strong correlation between high PCS scores and high BDI scores. Three papers with a total of 57 patients investigated PCS as a secondary outcome measure (Table 5). Results from multiple studies indicate that pain catastrophizing can be significantly reduced with burst SCS. Currently, it is unclear if the greater reductions in pain observed with burst SCS are a primary effect on the PCS; this could also result from direct neuromodulatory effects on supraspinal regions in the cerebrum. Further studies are needed to better elucidate the effects observed on the PCS and to better understand the cause and effect properties.
Table 5.
Published papers including the Pain Catastrophizing Scale as an outcome measure and the results
| Reference | SCS Indication | No. | Follow-up, wk | Baseline PCS | Burst PCS | Comparator PCS |
|---|---|---|---|---|---|---|
| Deer et al. [25] (2018) | Mixed neuropathic pain | 100 | 52 | NNR | NNR | Tonic = burst |
| Courtney et al. [32] (2015) | Mixed neuropathic pain | 22 | 2 | NR | 10.3 (± 9.9) | Tonic: 17.9 (± 12.9) |
| Schu et al. [40] (2014) | FBSS | 20 | 3 | 18.5 (± 13.9) | NR | NR |
| Van Havenbergh et al. [48] (2014) | FBSS | 15 | 4 | NNR | NNR |
|
FBSS = failed back surgery syndrome; NNR = difference reported, no absolute numbers; NR = not reported; PCS = Pain Catastrophizing Scale; SCS = spinal cord stimulation.
The insula and ACC in the brain play a pivotal role in attention to pain and may influence pain-related behavior [55,56]. Moreover, pain hypervigilance is associated with greater clinical pain severity and enhanced experimental pain sensitivity in chronic pain patients [70]. The Pain Vigilance and Awareness Questionnaire (PVAQ) is a validated questionnaire that scores two domains of attention to pain: actual attention and change in attention. The maximum score is set at 80. To support its validity, PVAQ scores are related to perception of bodily awareness and negatively correlated with the ability to ignore pain [71,72]. Thus, the amount of awareness or attention to pain is a contextually important factor in terms of how much pain a person perceives. Three papers with a total of 50 patients that studied the PVAQ as a secondary outcome measure were analyzed (Table 6). In two studies, there was a reduction of PVAQ reported. Although the reductions were modest, it is unclear how the results would differ in a cohort of de novo stimulation patients who had not undergone prior stimulation therapy where partial relief may have already been attained or the attentional context had already been altered.
Table 6.
Published papers including the Pain Vigilance and Awareness Questionnaire as an outcome measure and the results
| Reference | SCS Indication | No. | Follow-up, wk | Baseline PVAQ | Burst PVAQ | Comparator PVAQ | LOE |
|---|---|---|---|---|---|---|---|
| De Ridder [24] (2013) | FBSS | 15 | 3 | 34.5* | 31.4* | Tonic: 36* | 1b |
| Schu et al. [40] (2014) | FBSS | 20 | 3 | 35.4 (± 12.1) | NR | NR | 1c |
| Van Havenbergh et al. [48] (2014) | FBSS | 15 | 4 | NNR | NNR |
|
2b |
FBSS = failed back surgery syndrome; LOE = level of evidence; NNR = difference reported, no absolute numbers; NR = not reported; PVAQ = Pain Vigilance and Awareness Questionnaire; SCS = spinal cord stimulation.
Summation of the attention to pain and attention to change in pain.
Overall, clinical data suggest that burst SCS is able to alter the affective and emotional components of pain, probably by modulation of the brain regions involved in affective and emotional pain processing [23,24,73]. Psychological comorbidities such as depression and pain catastrophizing are viewed as negative predictors of a beneficial long-term outcome of SCS [74,75]. Novel stimulation paradigms such as burst SCS, due to proposed distinct differences in mode of action, may provide challenging patient populations with a better chance of success utilizing SCS therapy. As many of these outcomes are interrelated and covary with one another in a typical clinical setting, it will be interesting to see how burst SCS may directly influence the affective component of pain via modulation of centrally projecting pathways.
Explant Data
Lack or loss of efficacy is the most frequent reason for explant of neuromodulation systems [76–79]. This represents a tremendous burden for the patient, medical staff, and social care systems alike. In a multicenter retrospective study examining neuromodulation system explants (N = 352), Pope et al. found that 43.9% were explanted due to loss or lack of efficacy with tonic stimulation. The majority of the patients treated (45.7%) suffered from FBSS [76]. Explants after SCS were also recently analyzed by Jean Pierre Van Buyten et al. [77]. A retrospective chart review of 955 implants over a five-year time period from centers in three different countries were analyzed, and a mean explant rate of 7.9% annually was detected. The total number of unanticipated implanted pulse generator (IPG) explants was 180 (19%). Of these 180 explants, 94, or a little more than half, were due to inadequate pain relief; 14.2% of the patients utilizing HF-SCS (10 kHz) therapy were explanted due to inadequate pain relief. In patients treated with tonic SCS, the explant rate due to inadequate pain relief was 11.2% for the rechargeable system and only 6.9% for the nonrechargeable system [77]. In an eight-year follow-up of 234 patients with different pain pathologies, Hayek et al. found that 32 patients (13.7%) reported loss of efficacy, which was the most common reason for all explants (39%). The median time until explant was 19.6 months [79].
In an effort to decrease device explants and exiting from neuromodulation therapy, switching to burst SCS when tonic stimulation fails (and vice versa) has been shown to be a clinically valid approach [35]. Moreover, if pain relief has subsided due to adaption or accommodation of the nervous system, switching to a different stimulation paradigm with a different mechanism of action makes sense [18].
Conclusions
There is a high level of clinical evidence for the efficacy of burst SCS on pain intensity after one year of therapy in patients suffering from a variety of chronic pain conditions. Moreover, there are many blinded, sham-controlled RCTs indicating clinical superiority over both placebo and tonic SCS. In addition to high-quality data from controlled trials, there is a plethora of evidence from observational studies that demonstrates that burst SCS produces improvement, not only in pain intensity but over a range of other clinically meaningful patient-reported outcomes. Multiple sources of evidence suggest that burst SCS appears to interfere with the affective component of pain perception, indicating that unique changes in the central processing of pain are induced by this therapy.
The overall level of evidence in trials reviewed for this paper is variable, ranging from high-quality prospective trials with long follow-up periods to a number of low- to medium-quality trials. Randomized trials comparing different stimulation patterns powering for affective components of pain are needed to confirm the reported positive effects in prior reports. Although there are data on a variety of neuropathic pain conditions, future studies should also better focus on individual pain conditions, like FNSS, CRPS, peripheral neuropathies, postsurgical chronic pain, ischemic pain conditions, visceral pain, and central pain conditions (e.g., poststroke pain). A comprehensive means to approach this is bundling the existing data in an international database and running registry-based RCTs [80]. In this manner, new indications can be studied in real-world populations with a long follow-up period, gaining increasing clinical evidence levels.
It will be useful to best understand patient phenotypes that may best respond to burst SCS either before receiving any form of neuromodulation, or as a secondary attempt at regaining analgesia in patients losing efficacy with tonic or HF-SCS. Real-time pain intensity recording, validated questionnaires in affective pain perception, real-time functional outcome measures (e.g., pedometry and sleep quality), and standardized additional investigation (e.g., neuroimaging, neuro-electrophysiology, biometrics) may help in this process and push personalized pain therapies forward.
Acknowledgments
The authors thank Jeff Kramer, PhD, an independent scientist, for intellectual contributions and assistance in preparing the manuscript.
Funding sources: This manuscript was prepared with financial support from Abbott.
Conflicts of interest: Dr. Kirketeig has received educational fees from SJM/Abbott. Uppsala University Hospital has received an unrestricted research grant from Abbott. Dr. Schultheis is a consultant for Abbott. Dr. Zuidema is a consultant for Abbott and Medtronic. Dr. Hunter is a consultant for Abbott, Saluda, Nuvectra, and Flowonix and serves on the Medical Advisory Board for Vertiflex. Dr. Deer is a consultant for Abbott, Axonics, Nalu, Saluda, Vertos, and Vertiflex and has received research funding from Abbott, Saluda, Vertiflex, and Vertos. He has minority equity in Axonics, Bioness, Ethos, FLowonix, Saluda, Nalu, Cornerloc, Spinethera, Vertos, and Vertiflex.
Supplement sponsorship: This article appears as part of the supplement “Neuromodulation of the Spine and Nervous System” sponsored by Abbott.
References
- 1. Breivik H, Collett B, Ventafridda V, Cohen R, Gallacher D.. Survey of chronic pain in Europe: Prevalence, impact on daily life, and treatment. Eur J Pain 2006;104:287–333. [DOI] [PubMed] [Google Scholar]
- 2. Reid KJ, Harker J, Bala MM, et al. Epidemiology of chronic non-cancer pain in Europe: Narrative review of prevalence, pain treatments and pain impact. Curr Med Res Opin 2011;272:449–62. [DOI] [PubMed] [Google Scholar]
- 3. Finnerup NB. Translating basic research to pharmacological treatment of neuropathic pain. Scand J Pain 2017;13:169. [Google Scholar]
- 4. Dowell D, Noonan RK, Houry D.. Underlying factors in drug overdose deaths. JAMA 2017;31823:2295–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Barnett ML, Gray J, Zink A, Jena AB.. Coupling policymaking with evaluation - the case of the opioid crisis. N Engl J Med 2017;37724:2306–9. [DOI] [PubMed] [Google Scholar]
- 6. Volkow ND, Collins FS.. The role of science in the opioid crisis. N Engl J Med 2017;377:391–394. [DOI] [PubMed] [Google Scholar]
- 7. Colloca L, Ludman T, Bouhassira D, et al. Neuropathic pain. Nat Rev Dis Primers 2017;3: 17002. https://doi.org/10.1038/nrdp.2017.2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sommer C. Exploring pain pathophysiology in patients. Science 2016;3546312:588–92. [DOI] [PubMed] [Google Scholar]
- 9. Meier K. Spinal cord stimulation: Background and clinical application. Scand J Pain 2017;53:175–81. [DOI] [PubMed] [Google Scholar]
- 10. Kemler MA, Reulen JP, Barendse GA, et al. Impact of spinal cord stimulation on sensory characteristics in complex regional pain syndrome type I: A randomized trial. Anesthesiology 2001;951:72–80. [DOI] [PubMed] [Google Scholar]
- 11. Kumar K, Taylor RS, Jacques L, et al. Spinal cord stimulation versus conventional medical management for neuropathic pain: A multicentre randomised controlled trial in patients with failed back surgery syndrome. Pain 2007;132(1–2):179–88. [DOI] [PubMed] [Google Scholar]
- 12. Deer T, Pope J, Hayek S, et al. Neurostimulation for the treatment of axial back pain: A review of mechanisms, techniques, outcomes, and future advances. Neuromodulation 2014;17(Suppl 2):52–68. [DOI] [PubMed] [Google Scholar]
- 13. Chakravarthy K, Richter H, Christo PJ, Williams K, Guan Y.. Spinal cord stimulation for treating chronic pain: Reviewing preclinical and clinical data on paresthesia-free high-frequency therapy. Neuromodulation 2018;211:10–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Sdrulla AD, Guan Y, Raja SN.. Spinal cord stimulation: Clinical efficacy and potential mechanisms. Pain Pract 2018;188:1048–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Linderoth B, Foreman RD.. Conventional and novel spinal stimulation algorithms: Hypothetical mechanisms of action and comments on outcomes. Neuromodulation 2017;206:525–33. [DOI] [PubMed] [Google Scholar]
- 16. Waszak PM, Modric M, Paturej A, et al. Spinal cord stimulation in failed back surgery syndrome: Review of clinical use, quality of life and cost-effectiveness. Asian Spine J 2016;106:1195–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Verrills P, Sinclair C, Barnard A.. A review of spinal cord stimulation systems for chronic pain. J Pain Res 2016;9:481–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Chakravarthy K, Kent AR, Raza A, Xing F, Kinfe TM.. Burst spinal cord stimulation: Review of preclinical studies and comments on clinical outcomes. Neuromodulation 2018;215:431–9. [DOI] [PubMed] [Google Scholar]
- 19. De Ridder D, Vanneste S, Plazier M, van der Loo E, Menovsky T.. Burst spinal cord stimulation: Toward paresthesia-free pain suppression. Neurosurgery 2010;665:986–90. [DOI] [PubMed] [Google Scholar]
- 20. Sherman SM. Tonic and burst firing: Dual modes of thalamocortical relay. Trends Neurosci 2001;242:122–6. [DOI] [PubMed] [Google Scholar]
- 21. Swadlow HA, Gusev AG.. The impact of ‘bursting’ thalamic impulses at a neocortical synapse. Nat Neurosci 2001; 44: 402–8. [DOI] [PubMed] [Google Scholar]
- 22. De Ridder D, van der Loo E, Van der Kelen K, et al. Theta, alpha and beta burst transcranial magnetic stimulation: Brain modulation in tinnitus. Int J Med Sci 2007;45:237–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. De Ridder D, Vanneste S.. Burst and tonic spinal cord stimulation: Different and common brain mechanisms. Neuromodulation 2016;191:47–59. [DOI] [PubMed] [Google Scholar]
- 24. De Ridder D, Plazier M, Kamerling N, Menovsky T, Vanneste S.. Burst spinal cord stimulation for limb and back pain. World Neurosurg 2013;805:642–9, e1. [DOI] [PubMed] [Google Scholar]
- 25. Deer T, Slavin KV, Amirdelfan K, et al. Success using neuromodulation with BURST (SUNBURST) study: Results from a prospective, randomized controlled trial using a novel burst waveform. Neuromodulation 2018;211:56–66. [DOI] [PubMed] [Google Scholar]
- 26. Deer TR, Campos LW, Pope JE.. Evaluation of Abbott's BurstDR stimulation device for the treatment of chronic pain. Expert Rev Med Devices 2017;146:417–22. [DOI] [PubMed] [Google Scholar]
- 27. Ahmed S, Yearwood T, De Ridder D, Vanneste S.. Burst and high frequency stimulation: Underlying mechanism of action. Expert Rev Med Devices 2018;151:61–70. [DOI] [PubMed] [Google Scholar]
- 28. Colini-Baldeschi G, De Carolis G, Papa A, et al. Burst stimulation for chronic low back and leg pain. Pain Pract 2016;16:29. [Google Scholar]
- 29. Wahlstedt A, Leljevahl E, Venkatesan L, Agnesi F. Cervical burst spinal cord stimulation for upper limb chronic pain: A retrospective case series. Paper presented at: American Society of Regional Anesthesia and Pain Medicine. ASRA 16th Annual Pain Medicine Meeting; November 16–18, Lake Buena Vista, FL.
- 30. Guyatt GH, Oxman AD, Vist GE, et al. GRADE: An emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008;3367650:924–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Oxford Centre for Evidence-Based Medicine. 2009. Available at: https://www.cebm.net/index.aspx? o=5653 (accessed December 1, 2018).
- 32. Courtney P, Espinet A, Mitchell B, et al. Improved pain relief with burst spinal cord stimulation for two weeks in patients using tonic stimulation: Results from a small clinical study. Neuromodulation 2015;185:361–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. De Ridder D, Vanneste S, Plazier M, Vancamp T.. Mimicking the brain: Evaluation of St Jude Medical's Prodigy Chronic Pain System with Burst Technology. Expert Rev Med Devices 2015;122:143–50. [DOI] [PubMed] [Google Scholar]
- 34. De Ridder D, Lenders MW, De Vos CC, et al. A 2-center comparative study on tonic versus burst spinal cord stimulation: Amount of responders and amount of pain suppression. Clin J Pain 2015;315:433–7. [DOI] [PubMed] [Google Scholar]
- 35. De Vos CC, Bom MJ, Vanneste S, Lenders MW, de Ridder D.. Burst spinal cord stimulation evaluated in patients with failed back surgery syndrome and painful diabetic neuropathy. Neuromodulation 2014;172:152–9. [DOI] [PubMed] [Google Scholar]
- 36. Kinfe TM, Pintea B, Link C, et al. High frequency (10 kHz) or burst spinal cord stimulation in failed back surgery syndrome patients with predominant back pain: Preliminary data from a prospective observational study. Neuromodulation 2016;193:268–75. [DOI] [PubMed] [Google Scholar]
- 37. Kinfe TM, Muhammad S, Link C, et al. Burst spinal cord stimualtion increases peripheral antineuroinflammatory interleukin 10 levels in failed back surgery syndrome patients with predominant back pain. Neuromodulation. 2017;204:322–330. [DOI] [PubMed] [Google Scholar]
- 38. Kriek N, Groeneweg JG, Stronks DL, De Ridder D, Huygen FJPM.. Preferred frequencies and waveforms for spinal cord stimulation in patients with complex regional pain syndrome: a multicentre, double-blind, randomized and placebo-controlled crossover trial. Eur J Pain. 2017;213:507–519. [DOI] [PubMed] [Google Scholar]
- 39. Muhammad S, Chaudry SR, Yearwood TL, Krauss JK, Kinfe TM.. Changes in metabolic disorders associated peripheral cytokine/adipokine traffic in non-obese chronic back patients responsive to burst spinal cord stimulation. Neuromodulation. 2018;21:31–37. [DOI] [PubMed] [Google Scholar]
- 40. Schu S, Slotty PJ, Bara G, et al. A prospective, randomised, double-blind, placebo-controlled study to examine the effectiveness of burst spinal cord stimulation patterns for the treatment of failed back surgery syndrome. Neuromodulation 2014;175:443–50. [DOI] [PubMed] [Google Scholar]
- 41. Tjepkema-Cloostermans MC, De Vos CC, Wolters R, Dijkstra-Scholten C, Lenders MW.. Effect of burst stimulation evaluated in patients familiar with spinal cord stimulation. Neuromodulation 2016;195:492–7. [DOI] [PubMed] [Google Scholar]
- 42. Bara G, Schu S, Vesper J. First results of burst high frequency stimulation in failed FBSS stimulation patients: one year follow up. Paper presented at: 11th World Congress of the International Neuromodulation Society (INS), June 8-13 2013; Montreal, Quebec, Canada.
- 43. Espinet A, Courtney P, Mitchell B, et al. Burst spinal cord stimulation provides superior overall pain relief compared to tonic stimulation. Paper presented at: World Institute of Pain, May 7-10 2014; Maastrict, Netherlands.
- 44. Kretzschmar M, Vesper J, Van Havenbergh T, et al. Improved pain and psychosocial function with burst SCS: 1 year outcomes of a prospective study. Paper presented at: North American Neuromodulation Society (NANS), January 19-22 2017; Las Vegas, NV, USA.
- 45. Kriek N, Groeneweg G, Huygen FJPM.. Burst spinal cord stimulation in a patient with complex regional pain syndrome: a 2-year follow-up. Pain Pract. 2015;156:E59–E64. [DOI] [PubMed] [Google Scholar]
- 46. Rasekhi R, Babb D, Price C.. Neuromodulatory burst therapy for Agent Orange-induced peripheral neuropathy: a case report. A A Pract. 2018;107:165–7. [DOI] [PubMed] [Google Scholar]
- 47.Al-KAisy A, Baranidharan G, Palmisani S, et al. Paresthesia-mapping is not required for lead placements involving burst: Results of the prospective, randomized, double-blinded, crossover crisp study. Paper presented at 9th congress of the World Institute of Pain (WIP), May 9–12 2018, Dublin, Ireland. [Google Scholar]
- 48. Van Havenbergh T, Vancamp T, Van Looy P, Vanneste S, De Ridder D.. Spinal cord stimulation for the treatment of chronic back pain patients: 500-Hz vs. 1000-Hz burst stimulation. Neuromodulation 2015;181:9–12; discussion. [DOI] [PubMed] [Google Scholar]
- 49. Crosby ND, Goodman Keiser MD, Smith JR, Zeeman ME, Winkelstein BA.. Stimulation parameters define the effectiveness of burst spinal cord stimulation in a rat model of neuropathic pain. Neuromodulation 2015;181:1–8; discussion. [DOI] [PubMed] [Google Scholar]
- 50. Tavel E, Amirdelfan K, Phillips G, et al. Burst spinal cord stimulation programming optimization: Interim clinical outcomes for subjects programmed with a burst-specific programming strategy. Paper presented at: ASRA; November 17–19, 2016; San Diego, CA.
- 51. Tang R, Martinez M, Goodman-Keiser M, et al. Comparison of burst and tonic spinal cord stimulation on spinal neural processing in an animal model. Neuromodulation 2014;172:143–51. [DOI] [PubMed] [Google Scholar]
- 52. Falowski SM. An observational case series of spinal cord stimulation waveforms visualized on intraoperative neuromonitoring. Neuromodulation. 2019;22(2):219–228. [DOI] [PubMed] [Google Scholar]
- 53. Vesper J, Schu S, Slotty PJ, et al. BurstDR™ microdosing is as efficacious as standard BurstDR™ in treating chronic back and leg pain. Paper presented at: Annual Meeting of the North American Neuromodulation Society; January 11–14, 2018 Las Vegas, NV.
- 54. Schultheis BC, Bitter A, Haye O, Weidle PA A single center, retrospective review of burst SCS spinal cord stimulation responders for the treatment of failed neck surgery. Paper presented at: 12th Jahrestagung der Deutschen Gesellschaft für Neuromodulation; November 30 –December 2, 2017; Ratingen, Germany.
- 55. Kucyi A, Davis KD.. The neural code for pain: From single-cell electrophysiology to the dynamic pain connectome. Neuroscientist 2017;234:397–414. [DOI] [PubMed] [Google Scholar]
- 56. Kucyi A, Davis KD.. The dynamic pain connectome. Trends Neurosci 2015;382:86–95. [DOI] [PubMed] [Google Scholar]
- 57. Amirdelfan K, Webster L, Poree L, Sukul V, McRoberts P.. Treatment options for failed back surgery syndrome patients with refractory chronic pain: An evidence based approach. Spine (Phila Pa 1976) 2017;42(Suppl 14):S41–52. [DOI] [PubMed] [Google Scholar]
- 58. Kapural L, Peterson E, Provenzano DA, Staats P.. Clinical evidence for spinal cord stimulation for failed back surgery syndrome (FBSS): Systematic review. Spine (Phila Pa 1976) 2017;42(Suppl 14):S61–6. [DOI] [PubMed] [Google Scholar]
- 59. Deer TR, Mekhail N, Provenzano D, et al. The appropriate use of neurostimulation of the spinal cord and peripheral nervous system for the treatment of chronic pain and ischemic diseases: The Neuromodulation Appropriateness Consensus Committee. Neuromodulation 2014;176:515–50; discussion 50. [DOI] [PubMed] [Google Scholar]
- 60. Sitzman BT, Provenzano DA.. Best practices in spinal cord stimulation. Spine (Phila Pa 1976) 2017;42(Suppl 14):S67–71. [DOI] [PubMed] [Google Scholar]
- 61. Kriek N, Groeneweg JG, Stronks DL, de Ridder D, Huygen FJ.. Preferred frequencies and waveforms for spinal cord stimulation in patients with complex regional pain syndrome: A multicentre, double-blind, randomized and placebo-controlled crossover trial. Eur J Pain 2017;213:507–19. [DOI] [PubMed] [Google Scholar]
- 62. Muhammad S, Roeske S, Chaudhry SR, Kinfe TM.. Burst or high-frequency (10 kHz) spinal cord stimulation in failed back surgery syndrome patients with predominant back pain: One year comparative data. Neuromodulation 2017;207:661–7. [DOI] [PubMed] [Google Scholar]
- 63. Al-Kaisy A, Palmisani S, Pang D, et al. Prospective, randomized, sham-control, double blind, crossover trial of subthreshold spinal cord stimulation at various kilohertz frequencies in subjects suffering from failed back surgery syndrome (SCS frequency study). Neuromodulation 2018;215:457–465. [DOI] [PubMed] [Google Scholar]
- 64. Perruchoud C, Eldabe S, Batterham AM, et al. Analgesic efficacy of high-frequency spinal cord stimulation: A randomized double-blind placebo-controlled study. Neuromodulation 2013;164:363–9; discussion 9. [DOI] [PubMed] [Google Scholar]
- 65. Cohen RA, Kaplan RF, Moser DJ, Jenkins MA, Wilkinson H.. Impairments of attention after cingulotomy. Neurology 1999;534:819–24. [DOI] [PubMed] [Google Scholar]
- 66. Bushnell MC, Ceko M, Low LA.. Cognitive and emotional control of pain and its disruption in chronic pain. Nat Rev Neurosci 2013;147:502–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Rainville P, Duncan GH, Price DD, Carrier B, Bushnell MC.. Pain affect encoded in human anterior cingulate but not somatosensory cortex. Science 1997;2775328:968–71. [DOI] [PubMed] [Google Scholar]
- 68. Bai-Chuang SVB. Short-term synaptic plasticity in the nociceptive thalamic-anterior cingulate pathway. Molecular Pain 2009;5:51.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Shum FW, Wu LJ, Zhao MG, et al. Alteration of cingulate long-term plasticity and behavioral sensitization to inflammation by environmental enrichment. Learn Mem 2007;144:304–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Herbert MS, Goodin BR, Pero STt, et al. Pain hypervigilance is associated with greater clinical pain severity and enhanced experimental pain sensitivity among adults with symptomatic knee osteoarthritis. Ann Behav Med 2014;481:50–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Mc Cracken L. “Attention” to pain in persons with chronic pain: A behavioral approach. Behav Ther 1997;282:271–84. [Google Scholar]
- 72. McCracken LM. A contextual analysis of attention to chronic pain: What the patient does with their pain might be more important than their awareness or vigilance alone. J Pain 2007;83:230–6. [DOI] [PubMed] [Google Scholar]
- 73. Yearwood T, Falowski S, Venkatesan L, Vanneste S. Comparison of neural activity in chronic pain patients during tonic and burst spinal cord stimulation: A SUNBURST substudy. Paper presented at: NANS-NIC; June 25–29, 2016; Baltimore, MD.
- 74. Bendinger T, Plunkett N, Poole D, Turnbull D.. Psychological factors as outcome predictors for spinal cord stimulation. Neuromodulation 2015;186:465–71; discussion 71. [DOI] [PubMed] [Google Scholar]
- 75. Sparkes E, Raphael JH, Duarte RV, et al. A systematic literature review of psychological characteristics as determinants of outcome for spinal cord stimulation therapy. Pain 2010;1502:284–9. [DOI] [PubMed] [Google Scholar]
- 76. Pope JE, Deer TR, Falowski S, et al. Multicenter retrospective study of neurostimulation with exit of therapy by explant. Neuromodulation 2017;206:543–52. [DOI] [PubMed] [Google Scholar]
- 77. Van Buyten JP, Wille F, Smet I, et al. Therapy-related explants after spinal cord stimulation: Results of an international retrospective chart review study. Neuromodulation 2017;207:642–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Dupre DA, Tomycz N, Whiting D, Oh M.. Spinal cord stimulator explantation: Motives for removal of surgically placed paddle systems. Pain Pract 2018;184:500–4. [DOI] [PubMed] [Google Scholar]
- 79. Hayek SM, Veizi E, Hanes M.. Treatment-limiting complications of percutaneous spinal cord stimulator implants: A review of eight years of experience from an academic center database. Neuromodulation 2015;187:603–8; discussion 8–9. [DOI] [PubMed] [Google Scholar]
- 80. James S, Rao SV, Granger CB.. Registry-based randomized clinical trials–a new clinical trial paradigm. Nat Rev Cardiol 2015;125:312–6. [DOI] [PubMed] [Google Scholar]


