Abstract
Cancer-associated fibrosis is a critical component of the tumor microenvironment (TME) which significantly impacts cancer behavior. However, there is significant controversy regarding fibrosis as a predominantly tumor promoting or tumor suppressing factor. Cells essential to the generation of tissue fibrosis such as fibroblasts and mesenchymal stem cells (MSCs) have dual phenotypes dependent upon their independence or association with cancer cells. Cancer-associated fibroblasts and cancer-associated mesenchymal stem cells have unique molecular profiles which facilitate cancer cell cross talk, influence extracellular matrix deposition, and direct the immune system to generate a pro-tumorigenic environment. In contrast, normal tissue fibroblasts and MSCs are important in restraining cancer initiation, influencing epithelial cell differentiation and limiting cancer cell invasion. We propose this apparent dichotomy of function is due to 1) cancer mediated stromal reprogramming, 2) tissue stromal source, 3) unique subtypes of fibrosis and 4) the impact of fibrosis on other TME elements. First, as cancer progresses, tumor cells influence their surrounding stroma to move from a cancer restraining phenotype into a cancer supportive role. Second, cancer has specific organ tropism thus stroma derived from preferred metastatic organs support growth while less preferred metastatic tissues do not. Third, there are subtypes of fibrosis which have unique function to support or inhibit cancer growth. Fourth, depleting fibrosis influences other TME components which drives the cancer response. Collectively, this review highlights the complexity of cancer associated fibrosis and supports a dual function of fibrosis which evolves during the continuum of cancer growth.
Cancer develops within a complex microenvironment critical to supporting tumor survival, growth and metastasis. This tumor microenvironment (TME) is composed of a web of vasculature, extracellular matrix (ECM), stromal cells, immune cells, and soluble signaling molecules which form a dynamic “organ” critical to the pathophysiology of cancer1. Within the TME, cancer-associated fibrosis has emerged as a critical regulator of cancer behavior. Indeed, fibrosis is a hallmark of cancer. Up to 20% of cancers are linked to chronic inflammation related fibrosis (either from infectious or autoimmune etiologies) including hepatocellular, gastric, esophageal, head and neck, colon, pancreatic, cervix and vulvar cancers2. The impact of fibrosis on cancer initiation, progression, metastasis and treatment outcomes have been increasingly studied however seemingly contradictory results leave the question unanswered: is fibrosis in cancer helpful or harmful? Perhaps fibrosis can be both helpful and harmful depending on the disease context. In this review, we will summarize our current understanding of the factors which drive tumor related fibrosis and how this fibrosis impacts cancer biology addressing evidence supporting fibrosis as a tumor restraining and tumor promoting factor and presenting a paradigm of a dual function of fibrosis in cancer.
Cellular sources of fibrosis:
Fibrosis is the formation of excess connective tissue causing stromal hardening and scar formation. Desmoplasia is another commonly used term which refers to the growth of benign fibrous tissue secondary to tissue injury such as cancer or infection. Below we introduce the main cellular mediators of fibrosis and desmoplasia: fibroblasts, mesenchymal stem cells, fibrocytes, and stellate cells.
Fibroblasts:
Fibroblasts are connective-tissue cells of mesenchymal origin. They are stromal cells which control tissue integrity. Fibroblasts maintain extracellular matrix (ECM) homeostasis through both deposition of ECM and secretion of matrix metalloproteinases (MMPs) to remodel the ECM. Fibroblasts also regulate adjacent epithelial cells directing epithelial proliferation and differentiation3–5. Further, fibroblasts moderate inflammation and aid in wound healing3,6. While alpha smooth muscle actin (αSMA), fibroblast activation protein (FAP), S100A4, vimentin, and platelet derived growth factor receptor-alpha (PDGFRα) are all expressed in fibroblasts, no one set of markers fully define these cells. This presents a challenge to delineate fibroblasts from other stromal cells and leads to significant heterogeneity within cells classified as ‘fibroblasts’7,8. Fibroblasts are considered the main effectors of fibrosis in both normal and pathologic settings. During inflammation, fibroblasts become “activated” and are referred to as myofibroblasts which are the main collagen producers in the body9. Fibroblasts associated with normal wound healing are phenotypically distinct from fibroblasts associated with cancer; Fibroblasts within the TME are referred to as cancer associated fibroblasts (CAFs) and they have a unique expression profile and function which significantly contributes to cancer-related fibrosis10–13. In contrast to normal fibroblasts, CAFs have increased autocrine signaling ability and proliferation tendencies14. CAFs are the major producer of ECM proteins within the TME thus drastically altering the physical properties of tumor stroma. The specific impact of CAFs on cancer biology will be discussed below.
Fibrocytes:
Fibrocytes are hematopoietic stem cell-derived fibroblast precursors implicated in chronic inflammation, fibrosis and wound healing15,16. Fibrocytes are monocyte-derived cells with features of both macrophages and fibroblasts expressing CD34, CD45, CD11b, αSMA and collagen I16,17. Normal or classic fibrocytes serve as antigen presenters, augment immune reactivity and mediate angiogenesis. In cancer, fibrocytes suppress the anti-tumor immune response acting as myeloid-derived suppressor cells16,18. Within the TME, fibrocytes secrete ECM components and acquire a contractile phenotype similar to that of CAFs and fibrocytes have been postulated as a hematopoietic source of CAFs thus they are a mediator of tumor-associated fibrosis19.
Mesenchymal stem cells (MSCs):
MSCs are non-hematopoietic, multipotent stromal cells capable of differentiating into stromal tissues including fibroblasts, adipocytes, osteocytes and chondrocytes. MSCs are an important source of fibroblast generation within the TME20–22. MSCs are known for their role in wound healing and as MSCs are found in virtually all tissues from the bone marrow to the eyelid, they may serve as ‘first responders’ to tissue injury. MSCs are also recruited to tissue in response to injury where they both modulate the immune response to dampen inflammation and aid in tissue repair through differentiation23. Similar to fibroblasts, the characterization of MSCs is challenging given the lack of one identifying cell surface marker however, the International Society for Cellular Therapy established minimal criteria for defining MSCs: 1) plastic adherent in standard culture conditions 2) express CD105, CD73, CD90 and lack expression of CD45, CD34, CD14, CD79a and HLA-DR 3) and must differentiate into at least two of the following: osteoblasts, adipocytes and chondroblasts24.
As tissue resident cells, MSCs are present within the TME of most cancers25–27. As with fibroblasts, normal tissue MSCs are phenotypically distinct from MSCs found within the TME28. MSCs within the TME are referred to as cancer educated or cancer-associated MSCs (CA-MSCs)29. CA-MSCs uniquely impact the TME compared to normal tissue derived MSCs and are critical players in tumor-associated fibrosis30.
Stellate cells:
Stellate cells have many similarities to MSCs. They are so closely related that they have been postulated to be a subtype of tissue specific MSCs31. Stellate cells reside within in the perisinusoidal space between hepatocytes and sinusoidal endothelial cells within the liver and the exocrine regions of the pancreas32. At rest, stellate cells serve as reservoirs of vitamin A. Hepatic stellate cells have progenitor cell characteristics with the capacity to differentiate into fibroblasts, endothelial cells and hepatocytes33. While no lineage tracing studies have been performed, stellate cells are thought to be the primary source of fibroblasts within the liver and possibly the pancreas thus they are likely to be important players in liver and pancreatic cancer related fibrosis34.
Role of fibrosis in Cancer Initiation:
Chronic inflammation results in fibrosis. As cancer is a disease of chronic inflammation mimicking a “non-healing wound,” similar mechanisms likely drive fibrosis in cancer. Indeed, chronic fibrosis predisposes to cancer initiation35. This has been reviewed elsewhere in detail36,37. Briefly, after tissue injury, wound healing occurs through a step-wise process of coagulation, inflammation, cell proliferation, inflammatory suppression, angiogenesis, and finally tissue remodeling37,38. In the setting of ongoing inflammatory stimulus, this cycle can either stall or be continuously activated leading to a chronic, non-healing wound. As a result, rather than normal, healthy remodeled tissue, a fibrotic phenotype eerges38. This fibrosis can then directly impact epithelial cell differentiation, epithelial mesenchymal transition, and epithelial proliferation3–5,39–41. Cancer mimics this process due to dysregulated cancer cell proliferation inducing chronic pro-inflammatory stimuli, altered immune infiltration, leaky vasculature and hypoxia ultimately creating a fibrotic TME36,37. The creation of this “non-healing wound” further drives the development of cancer associated fibrosis.
Treatment-related drivers of cancer fibrosis:
In addition to cancer induced chronic inflammation as a driver of fibrosis, cancer treatments also play an important role in creating the fibrotic TME. Organ fibrosis, most notably pulmonary fibrosis, is a known toxicity of multiple chemotherapeutic agents including bleomycin, gemcitabine, and methotrexate42. In vitro and in vivo studies demonstrate chemotherapy may promote an inflammatory and fibrotic microenvironment likely through tissue injury related to oxidative stress. Tissues exposed to chemotherapy undergo similar stages of wound healing including inflammation with influx of immune cells, followed by fibroblast activation and proliferation and remodeling which involves the accumulation and cross-linking of ECM43. The development of fibrosis in tissues treated with chemotherapy is widely reported in cancers including colorectal, prostate, breast, cervix, esophageal, ovarian and head and neck cancer44–47. Chemotherapy induced fibrosis may also be prognostic. For example, after neoadjuvant chemotherapy in rectal cancer, malignant cells are replaced by a fibroinflammatory milieu and increased fibrosis is correlated with worse outcomes48. Radiation therapy is also a known driver of fibrosis. Radiation generates hypoxia and results in immune system activation which drives a CAF phenotype49. This results in cellular damage and concludes with tissue inflammation and fibrosis generated by irradiated fibroblasts50. These irradiated fibroblasts secrete MMPs which cause cystic, disorganized growth of new epithelial cells51. Interestingly, desmoplastic unirradiated tissue is also thought to be inherently radio-resistant50. Likely related to both the impact on the cancer cell and the fibrotic response, disease that recurs within a previously irradiated field is extremely therapy resistant50. Thus, mainstays of cancer treatment may also enhance the development of tumor associated fibrosis.
Impact of fibrosis on cancer biology: Duality of function
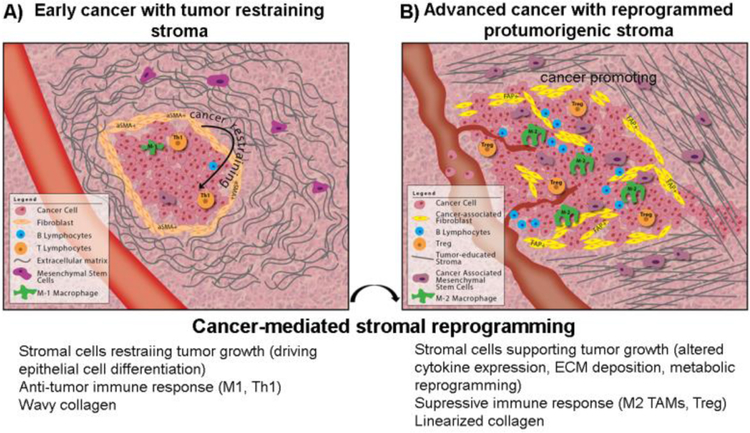
While long recognized as a key feature of the TME, the impact of fibrosis on cancer formation, growth and progression is controversial. Below we summarize the literature supporting pro and anti-tumorigenic roles of fibrosis in cancer initiation, growth and metastasis with figure 1 graphically summarizing this duality of function.
Figure 1.
Dual roles of fibrosis in cancer. A) Fibrosis acts to restrain cancer growth during cancer initiation however, after a process of cancer-mediated stromal reprogramming, B) fibrosis acts to enhance cancer growth with stiffened ECM, enhanced angiogenesis and suppressive immune response.
Fibrosis enhances cancer growth and progression:
Fibrosis has been reported to support cancer growth through a variety of mechanisms including direct cellular interactions, immune modulation and ECM remodeling. As a stromal progenitor cell, MSCs significantly impact the formation of the TME and are important mediators of fibrosis. After undergoing cancer stimulation or education, normal tissue MSCs are converted into CA-MSCs which subsequently enhance cancer cell proliferation, chemotherapy resistance, metastasis and immune evasion30,52–56. In ovarian cancer, CA-MSCs form a BMP4:HH positive feedback loop with cancer cells that enhances ovarian cancer growth, chemotherapy resistance and enriches the cancer stem-like cell pool (CSCs)30. CA-MSCs also secrete IL6 and LIF which play redundant roles in supporting ovarian cancer proliferation and highlight the multi-layered signaling between CA-MSCs and tumor cells30,56. In breast cancer, CA-MSCs communicate with cancer cells via exosomes to enhance the proliferation and metabolic activity of cancer cells57. In pancreatic cancer, chemotherapy educated CA-MSCs form a CXCL10: CXCR3 signaling loop with cancer cells to increase CSCs and enhance resistance to gemcitabine treatment58. CA-MSCs increase breast cancer cell mobility, invasiveness and dissemination via a CCL5:CCR5 signaling loop59. In a prostate cancer model, tumor derived CXCL16 induces the recruitment and differentiation of MSCs into CAFs which then secrete CXCL12 thus enhancing metastasis26. CA-MSCs increase ovarian cancer cell adherence and spread onto mesothelial cells leading to peritoneal metastasis60. Cancer cell: MSCs fusion events have also been reported and hybrid cells demonstrate enhanced metastatic capacity61.
MSCs are known to limit autoimmunity and suppress the inflammatory response. MSCs are considered “immune privileged” or “immune evasive” and multiple reports demonstrate an important role for CA-MSCs in cancer cell immune escape62. CA-MSCs resident in tumors are known to secrete immunosuppressive factors including prostaglandin E2 (PGE2), indoleamine 2,3-dioxygenase (IDO), nitric oxide (NO), IL-4, IL-5, IL-6 and TGFb. CA-MSCs also secrete soluble program death ligand 1 and 2 (sPD-L1 and sPD-L2) which suppress CD4+ T cells and enhance Treg formation63. In cervical cancer, CA-MSCs impair the anti-tumor response through the generation of extracellular adenosine (Ado) which downregulates the proliferation and activation of cytotoxic T lymphocytes in tumor islets64. In melanoma, CA-MSCs aid in immune evasion via increase iNOS expression facilitating the murine engraftment of B16 melanoma tumors65. In line with this, MSC produced NO suppresses T cell function in a model of graft-vs-host disease66. CA-MSCs also induce Tregs in breast cancer models via TFG-b signaling67 and recruit CD11b+Ly6c+ monocytes, F4/80+ macrophages and CD11b+Ly6g+ neutrophils via secretion of CCR2 in a mouse lymphoma model68. CA-MSCs also alter macrophage polarity enhancing M2 polarization and promoting angiogenesis in melanoma and ovarian cancer models69,70. Taken together, there is a strong body of evidence supporting the pro-tumorigenic, immunosuppressive role of CA-MSCs in cancer.
Stellate cells, which as noted above, are similar to MSCs and may represent tissue specific MSCs in the liver and pancreas, likewise have parallel pro-tumorigenic functions. Stellate cells enhance progression of hepatocellular carcinoma by increasing cancer cell proliferation, angiogenesis and immune suppression as well as ECM secretion71. Stellate cells enhance immune suppression in pancreatic cancer via sequestration of CD8+T cells and MDSC differentiation in an IL-6/STAT3 dependent manner72–74. It is important to note that potential heterogeneity amongst isolated stellate cells including the presence of differentiated fibroblasts was not addressed in these studies.
Fibrocytes have also been reported to support cancer. Fibrocytes enhance melanoma lung metastasis via recruitment of monocytes to the pre-metastatic niche75. Fibrocytes also enhance the proportion of CSCs and increase resistance to anti-angiogenic therapy in mesothelioma and lung cancer models75–78.
MSCs, stellate cells and fibrocytes are all important sources of fibroblasts. Considerable evidence implicates CAFs and CAF driven fibrosis in the promotion of cancer. Compared to normal tissue fibroblasts, CAFs have a unique secretome characterized by pro-inflammatory proteins, growth factors, angiogenic factors and altered ECM. A CAF transcriptomic profile found in squamous cell, breast and pancreatic cancer and characterized by CXCL2, IL6, IL-1b, CXCL5, and TGFb upregulation correlates with tumor growth, macrophage recruitment and neovascularization79. CAF secretion of growth factors including hepatocyte growth factor (HGF)80, fibroblast growth factor (FGF)81, and PDGFα enhance breast, ovarian and lung cancer proliferation82. CAFs also enhance angiogenesis and metastasis via VEGF secretion83 and alteration of ECM regulators including tenascin C (TN-C)84,85, MMPs, Ras homolog member A (RhoA), Rho-associated protein kinase (ROCK), and myosin II (MyoII)86,87 leading to increased stromal stiffness and altered mechanotransductive pathways84–86,88–93. For example, CAFs via caveolin1 expression induce Rho- and force-dependent contraction, matrix alignment, and microenvironment stiffening leading to enhanced tumor invasion and metastatic potential in melanoma and breast cancer92. Similar findings have been reported across multiple cancer types including colon, prostate, pancreatic, ovarian and gastric cancers80,84,90,93–97.
CAFs are also known to direct both the innate and adaptive immune systems. CAF secretion of TGFb and PGE2 decreases NK cell produced interferon gamma (INFg) and alters the NK cell phenotype98,99. Through secretion of CXCL12 and CCL2, CAFs recruit and polarize macrophages to a M2 immunosuppressive phenotype in prostate cancer100. In melanoma, colon, hepatocellular, breast, and lung cancer, CAFs enhance the recruitment of MDSCs101–103. CAFs also promote Treg cells within the TME via TGFb and IDO secretion104. IDO is an important driver of immune tolerance by regulating NK cells, T regs and MDSCs likely through starving the TME of tryptophan and increasing the tryptophan-derived metabolite kynurenine105. CAF secretion of VEGF, beyond its role in promoting angiogenesis, is also immunomodulatory inhibiting dendritic cell maturation, increasing MDSC cells and directly inducing T reg cell proliferation106,107. In a mouse model of colon cancer, VEGF enhances PD-1 leading to CD8+ T cell exhaustion. Targeting VEGF reverses PD-1 expression enhancing the anti-tumor immune response108.
CAFs also promote cancer via metabolic mechanisms. Metabolic coupling of cancer cells and CAFs have been demonstrated in multiple cancer types including prostate, pancreatic, breast, ovarian, lung, and leukemias109,110. Metabolically reprogrammed CAFs decrease isocitrate dehydrogenase 3a (IDH3a) leading to decreases in α-ketoglutarate thus stabilizing HIF1α promoting glycolysis even under normoxic conditions111. Aerobic glycolysis in CAFs provide lactate to cancer cells via the monocarboxylate transporter 4 (MCT4) thus supporting anabolic metabolism in cancer cells112,113. This “lactate shuttle” is critical for cancer cell survival and growth114. CAFs have also been reported to provide critical amino acids such as glutamine and lipids to tumor cells as further mechanisms to support cancer growth115,116.
In addition to cellular contributions, ECM desmoplasia is also implicated in cancer progression. A densely desmoplastic TME promotes tumor growth by mechanically altering changes in blood flow resulting in notable tumor hypoxia, decreased drug delivery, and decreased immune infiltration ultimately increasing resistance to chemotherapy, radiation, antiangiogenic and immunotherapy117–122. Also, the increased stiffness and change from wavy to linear collagen arrangement directs tumor cell intravasation and enhances metastasis123–125. For example, in breast cancer, tumor cells migrate along linearized, stiff collagen fibers to facilitate metastasis. This has also been demonstrated in other cancers such as hepatocellular carcinoma126.
Fibrosis is important in not only established tumor sites but also in the creation of a premetastatic niche. In vivo models demonstrate increased fibronectin expression in the stroma of future metastatic sites127. This premetastatic change in stroma is associated with bone marrow derived cell recruitment and primes future metastatic sites with increased angiogenesis prior to the establishment of metastatic disease127. In a colon cancer model, tissue inhibitor of metalloproteinases (TIMP-1) increased the formation of a pre-metastatic niche within the liver where CAF related factors such as SDF-1, fibronectin, TFGb and S100A4 were all elevated128. Interestingly, TIMP-1 is a reported activator of CAFs therefore implying that creation of the pre-metastatic niche may be dependent on CAF function129. Lysyl oxidase (LOX), an enzyme responsible for collagen crosslinking associated with fibrosis and primary tumor growth, is also elevated at pre-metastatic sites prior to the arrival of disseminated tumor cells. LOX crosslinks collagen IV, drives the recruitment of CD11b+ myeloid cells and bone marrow derived cells, alters ECM patterns, increases angiogenesis and facilitates malignant cell recruitment into the pre-metastatic niche130.
Fibrosis limits cancer growth and progression:
In contrast to the data discussed above, there is a body of evidence which argues that tumor-related fibrosis restrains cancer initiation, proliferation and metastasis. In models utilizing normal stromal cells, both fibroblasts and MSCs have been reported to inhibit cancer growth131,132. These results are complicated by the heterogenous source of normal stromal cells used (bone marrow vs. resident tissue derived) which impacts the effect of stromal cells on tumor growth27,55. Ganciclovir-induced ablation of αSMA-thymidine kinase (TK) expressing fibroblasts during the formation of premalignant pancreatic intraepithelial neoplasia (PanIN) lesions or early carcinoma stages leads to more aggressive tumors and decreased mouse survival. Interestingly, enhanced immune suppression was noted with increased CD4+Foxp3+ Tregs in the αSMA depleted tumors133.
Investigations into a critical fibrosis signaling pathway, hedgehog (HH), also supports a restraining function of stromal cells in some cancers. Tumor cell derived HH signals in a paracrine fashion to adjacent stroma to drive fibrosis. This has been well documented in pancreatic, colon and bladder cancer. Interestingly, epithelial HH deletion in a mouse model of pancreatic cancer initiation (Pdx1-Cre;KrasLSL-G12D/+;p53fl/+;Rosa26LSL-YFP/+ (PKCY) model) decreases stromal content but results in more aggressive, poorly differentiated and highly vascular tumors134. Similarly, genetic and pharmacologic HH inhibition accelerates the development of premalignant PanIN lesions and promotes the progression of PanIN into invasive pancreatic cancer. Deletion of Shh in the murine pancreatic epithelium in KCS mice (Ptfla-Cre:KrasG12D:Shhfl/fl) enhanced the formation of PanIN lesions135. Additionally, HH agonists induce stromal hyperplasia but decrease epithelial proliferation likewise suggesting stromal desmoplasia plays a restraining role in cancer initiation135. Elegant mouse models with genetic disruption of tumor to stroma paracrine HH signaling via stromal knockout of the HH receptor, SMO, enhance the development of bladder cancer further supporting the importance of stromal HH signaling in restraining cancer growth136. This is consistent with a critical role for HH signaling in normal epithelial differentiation akin to its role during development. Interestingly, knockdown of two of the three known HH coreceptors (GAS1 and BOC) in fibroblasts enhances the ability of fibroblasts to support pancreatic cancer while knockout of all three (GAS1, BOC and CDON) prevents fibroblasts from supporting cancer growth137. A pilot study of Vismodegib (a SMO inhibitor) with gemcitabine in pancreatic cancer demonstrated decreased fibrosis in paired pre vs post-treatment biopsy specimens in 45% of evaluable patients but overall median fibrosis score was unchanged138. In other cancers, pharmacologic inhibition of HH is associated with a stromal dependent reversal of chemotherapy resistance30,139–141. Collectively this work highlights the complexity of stromal HH signaling and implicates a dose-specific role of HH in cancer promotion137. As embryonic patterning is directed by HH gradients during development, a nuanced effect where varying levels of HH induce different phenotypes in cancer is perhaps not surprising142,143.
Therapeutic Targeting of Fibrosis in Cancer:
Highlighting the importance of CAFs in cancer, genetic, pharmacologic and immunologic targeting of specific subsets of CAFs dramatically impacts tumor growth. As mentioned above, there is not one specific marker to define fibroblasts but αSMA, FAP and S100A4, while likely marking phenotypically distinct subsets of fibroblasts (discussed further below), are often used to identify fibroblasts. A S100A4 knockout (KO) mouse demonstrates significant decreases in breast cancer initiation and metastasis which is restored with co-growth of tumor cells with S100A4 positive fibroblasts144. Targeting FAP+ fibroblasts with an antibody conjugate inhibits tumor growth and leads to complete regression in xenografts of lung, pancreas and head and neck cancer145. Chimeric antigen receptor (CAR) T cell therapy designed against FAP+ fibroblasts alone or in combination with CAR T cells against a tumor antigen leads to a significant survival advantage in a mouse lung cancer model146 and in other solid tumor models147,148. Additionally, depletion of FAP via genetic KO or pharmacologic inhibition decreases lung and colon cancer growth149. Selective depletion of FAP+ CAFs via expression of the human diphtheria toxin receptor in FAP+ cells followed by diphtheria toxin administration enhances anti-tumor immunity and induces synergistic effects with anti-PDL1 checkpoint therapy in pancreatic cancer150. However, given the evidence discussed above of a dichotomous role of tumor associated fibrosis both inhibiting and promoting cancer, caution needs to be taken when considering therapeutic approaches to target tumor stroma. Clinical trials using HH inhibitors have failed to demonstrate benefit in colon cancer151. Further, while there are concerns related to the trial design and patient population chosen, a phase II trial in pancreatic cancer was halted early due to concern for inferior outcomes in the HH inhibitor arm152–154. This may relate to the specific role of HH in the pancreas, the dose dependency of HH signaling or unanticipated effects of stromal depletion such as infiltration of immunoinhibitory cells as noted during stromal targeting in murine models of pancreatic cancer133. Moving forward, it will be important to understand the tissue/tumor specificity of stromal effects and the impact of stromal targeting on all aspects of the TME when designing future clinical trials. Given the preponderance of data for fibrosis as a therapeutic target, despite the negative experience with HH inhibitors, we believe fibrosis and the desmoplastic stroma remains a potential therapeutic target for cancer.
Conclusions, the duality of fibrosis in cancer:
Clearly fibrosis and the cellular drivers of fibrosis are important in cancer biology but how are the seemingly discordant findings that fibrosis enhances and inhibits tumor growth to be reconciled? One potential reason for these differing conclusions may arise from generalizing results from studies focused on tumor initiation or early stage tumors and advanced metastatic cancer. Tumor associated stroma in metastatic disease is likely significantly different than stroma found at the primary site or within pre-malignant lesions. Broadly concluding that fibrosis supports or inhibits cancer is an over simplification. In actuality, the effect of fibrosis is context dependent and likely both inhibits and supports cancer under certain conditions. A recurring theme within cancer stromal research is the duality of function of most stromal cells. As described in both MSCs and fibroblasts, stromal cells within normal tissue behave differently than their tumor educated counterparts. The tumor “educates” normal stromal cells converting them into cancer promoting cells. Fibroblasts become activated to a CAF phenotype155. MSCs become reprogrammed into CA-MSCs55. Evolutionarily, mesenchymal derived stromal cells direct epithelial differentiation and are critical to maintaining appropriate tissue structure hence their importance in wound healing. Indeed, the normal function of these stromal cells is to prevent pathologic states such as tumor growth. Thus, depending on the state of the stromal cells (normal vs cancer educated), divergent roles in tumor growth (suppression vs enhancement) are expected.
Additionally, the source of stromal cells likely dramatically alters their impact on cancer behavior. Each cancer has its own pattern of metastasis indicating disease specific tissue tropism. For example, while genetically similar cancers, triple negative breast cancer and high grade serous ovarian cancer have distinct metastatic patterns with breast cancer frequently metastasizing to the bone while ovarian cancer colonizes the abdomen and rarely metastasizes to bone. This may indicate a tissue specific ability of cancer cells to convert normal stroma into cancer supporting stroma. We recently demonstrated ovarian cancer converts normal omental and ovary derived MSCs into pro-tumorigenic CA-MSCs but fails to convert bone marrow derived MSCs (BM-MSCs) into ovarian cancer supporting CA-MSCs. In contrast, breast cancer cells functionally convert BM-MSCs into breast cancer supporting CA-MSCs. This work highlights the importance of tissue source in the formation of cancer promoting CA-MSCs55. Further, reports of BM-MSCs enhancing prostate and breast cancer growth (cancers which frequently metastasize to bone) but inhibiting ovarian cancer growth (which rarely metastasizes to bone) is consistent with a tissue specific capacity of stromal cells to support cancer growth. This may explain much of the divergent results depending on the tissue source of the stromal cells studied59,132,156.
Also, as the timing and spatial magnitude of cancer reprogramming is unknown, stroma found within in situ or pre-malignant lesions may not have undergone cancer education and may still maintain a normal stromal cell phenotype functioning to restrain tumor growth. In this situation, depleting normal stromal cells or blocking their critical signaling pathways will enhance tumorigenesis. As malignancy progresses, cancer cells eventual convert normal stromal cells into pro-tumorigenic cells and may in fact utilize the same epithelial to stromal signaling loops initially used to restrain cancer growth to now drive cancer progression. For example, a BMP4/HH signaling loop which in early bladder cancer restrains bladder cancer progression, enhances tumor growth and chemotherapy resistance in late stage ovarian cancer30,136. Further, as discussed above, TGFb is an important mediator of fibroblast signaling. A mouse model with a dominant-negative type II TGFbR in the mammary epithelium (effectively preventing epithelial response to stromal TGFb signaling) develops spontaneous in situ carcinoma indicating TGFb exerts an inhibitory role in the development of breast cancer. However, once established, there is marked suppression of tumor invasion supporting a dual function of stromal TGFb acting as a tumor suppressor during cancer initiation but enhancing malignant progression once carcinoma has developed157. Additionally, studies of RhoA supports this dual function of fibroblasts in cancer growth. RhoA is critical to CAF function including the formation of focal adhesions, F-actin stress fibers influencing contractile properties and expression of fibroblast markers such as αSMA11,158,159. Knockout of RhoA in normal fibroblasts decreases their ability to inhibit tumor initiation and induces a pro-tumorigenic phenotype enhancing engraftment and growth of prostate cancer xenografts87. As αSMA is regulated by the Rho GTPase signaling pathway88,159–161, investigators noted the expected loss of αSMA expression in RhoA KO fibroblasts however there was no disruption of FAP expression. Indeed, the remaining FAP+ fibroblasts enhanced prostate cancer growth.
Building on these findings, another potential reason for the differing function of fibroblasts in cancer is the existence of subsets of fibroblasts which have divergent roles in cancer. Most notably, fibroblast activation protein (FAP) and alpha smooth muscle actin (αSMA) may delineate two such subsets. Fibroblasts with high FAP expression may be particularly important drivers of fibrosis and poor outcomes89,148. In pancreatic cancer, FAP high fibroblasts are associated with worse outcomes162,163 while αSMA high fibroblasts are associated with improved outcomes133. FAP is expressed on the majority of CAFs and only a portion of these co-express αSMA12,164. In the RhoA KO mouse detailed above, the fibroblast phenotypic switch leading to tumor promotion is accompanied by loss of αSMA but retention of FAP+ cells. Similarly, in the αSMA-TK ganciclovir ablation mouse model of pancreatic cancer which demonstrated more aggressive cancer with decreased mouse survival, it is interesting to note that FAP+ CAFs remained present133. Targeting FAP+ cells using genetic knockout150,165, chimeric receptor T cells and vaccine strategies145–147,166,167 inhibited tumor growth in lymphoma, melanoma, lung, pancreatic, breast, and colon cancer models. Thus, αSMA+ and FAP+ stromal cells may differentially regulate tumorigenesis.
Finally, it is important to recognize the complex heterogeneity within the TME and that alterations in one compartment may have unintended effects in another. For example, targeting fibroblasts alone may remove a stromal barrier allowing immune infiltration but it is not clear if this immune infiltration will be pro or anti-tumorigenic as cancers have multi-layered approaches to hiding from the immune system. If the stromal barrier is removed but only immune-suppressive cells enter the TME, the overall outcome will be worse. However, if stromal targeting agents are combined with immune checkpoint inhibition, the net effect of removal of the stromal barrier may be beneficial. For example, in the αSMA-TK ganciclovir ablation mouse model of pancreatic cancer, the growth promotion effect of fibroblast depletion was reversed by using an anti-CTLA4 inhibitor133. It is possible that a similar approach combining stromal targeting with a HH inhibitor with immune modulation with an immune checkpoint inhibitor may demonstrate improved responses and that the lack of efficacy noted in the HH clinical trials may be due to targeting only half of the problem.
Overall, recognition of the TME as a vital contributor to cancer biology has yielded important insights on the importance as well as the complexity of fibrosis in tumor growth. The role of tumor-associated fibrosis is not one dimensional but dynamic, seeming to evolve during cancer progression and impacts multiple aspects of cancer biology. Targeting this fibrosis is an appealing approach to improving cancer outcomes. However detailed mechanistic studies are vital to understand the impact of such therapies within each specific disease context with emphasis on the stage of disease, subtype of fibroblasts affected and compensatory changes within the TME which will drive cancer response.
Acknowledgments:
L. Coffman and R. Buckanovich are supported by the Hillman Cancer Center and Magee Women’s Research Institute. L. Coffman is supported by NIH K08 CA211362. R. Buckanovich is supported by NIH R01 CA211913. All authors have read the journal’s authorship agreement and policy on disclosure of potential conflicts of interest. All authors have reviewed and approved this manuscript. All authors attest they have no conflicts of interest to disclose.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Bibliography
- 1.Bhome R, Bullock MD, Al Saihati HA, et al. A top-down view of the tumor microenvironment: structure, cells and signaling. Front. Cell Dev. Biol 2015;3:33. doi: 10.3389/fcell.2015.00033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aggarwal BB, Vijayalekshmi RV, Sung B. Targeting inflammatory pathways for prevention and therapy of cancer: short-term friend, long-term foe. Clin. Cancer Res 2009;15(2):425–430. doi: 10.1158/1078-0432.CCR-08-0149. [DOI] [PubMed] [Google Scholar]
- 3.Chang HY, Chi J-T, Dudoit S, et al. Diversity, topographic differentiation, and positional memory in human fibroblasts. Proc. Natl. Acad. Sci. USA 2002;99(20):12877–12882. doi: 10.1073/pnas.162488599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Göke M, Kanai M, Podolsky DK. Intestinal fibroblasts regulate intestinal epithelial cell proliferation via hepatocyte growth factor. Am. J. Physiol 1998;274(5 Pt 1):G809–18. [DOI] [PubMed] [Google Scholar]
- 5.Okazaki M, Yoshimura K, Suzuki Y, Harii K. Effects of subepithelial fibroblasts on epithelial differentiation in human skin and oral mucosa: heterotypically recombined organotypic culture model. Plast. Reconstr. Surg 2003;112(3):784–792. doi: 10.1097/01.PRS.0000069710.48139.4E. [DOI] [PubMed] [Google Scholar]
- 6.Parsonage G, Filer AD, Haworth O, et al. A stromal address code defined by fibroblasts. Trends Immunol 2005;26(3):150–156. doi: 10.1016/j.it.2004.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sugimoto H, Mundel TM, Kieran MW, Kalluri R. Identification of fibroblast heterogeneity in the tumor microenvironment. Cancer Biol. Ther 2006;5(12):1640–1646. doi: 10.4161/cbt.5.12.3354. [DOI] [PubMed] [Google Scholar]
- 8.Endale M, Ahlfeld S, Bao E, et al. Temporal, spatial, and phenotypical changes of PDGFRα expressing fibroblasts during late lung development. Dev. Biol 2017;425(2):161–175. doi: 10.1016/j.ydbio.2017.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Phan SH. Biology of fibroblasts and myofibroblasts. Proc Am Thorac Soc 2008;5(3):334–337. doi: 10.1513/pats.200708-146DR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Albrengues J, Bertero T, Grasset E, et al. Epigenetic switch drives the conversion of fibroblasts into proinvasive cancer-associated fibroblasts. Nat. Commun 2015;6:10204. doi: 10.1038/ncomms10204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bozóky B, Savchenko A, Csermely P, et al. Novel signatures of cancer-associated fibroblasts. Int. J. Cancer 2013;133(2):286–293. doi: 10.1002/ijc.28035. [DOI] [PubMed] [Google Scholar]
- 12.Kalluri R, Zeisberg M. Fibroblasts in cancer. Nat. Rev. Cancer 2006;6(5):392–401. doi: 10.1038/nrc1877. [DOI] [PubMed] [Google Scholar]
- 13.LeBleu VS, Kalluri R. A peek into cancer-associated fibroblasts: origins, functions and translational impact. Dis. Model. Mech 2018;11(4). doi: 10.1242/dmm.029447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kalluri R The biology and function of fibroblasts in cancer. Nat. Rev. Cancer 2016;16(9):582–598. doi: 10.1038/nrc.2016.73. [DOI] [PubMed] [Google Scholar]
- 15.Zhang H, Maric I, DiPrima MJ, et al. Fibrocytes represent a novel MDSC subset circulating in patients with metastatic cancer. Blood 2013;122(7):1105–1113. doi: 10.1182/blood-2012-08-449413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Reilkoff RA, Bucala R, Herzog EL. Fibrocytes: emerging effector cells in chronic inflammation. Nat. Rev. Immunol 2011;11(6):427–435. doi: 10.1038/nri2990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Suga H, Rennert RC, Rodrigues M, et al. Tracking the elusive fibrocyte: identification and characterization of collagen-producing hematopoietic lineage cells during murine wound healing. Stem Cells 2014;32(5):1347–1360. doi: 10.1002/stem.1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moeller A, Gilpin SE, Ask K, et al. Circulating fibrocytes are an indicator of poor prognosis in idiopathic pulmonary fibrosis. Am. J. Respir. Crit. Care Med 2009;179(7):588–594. doi: 10.1164/rccm.200810-1534OC. [DOI] [PubMed] [Google Scholar]
- 19.McDonald LT, Russell DL, Kelly RR, et al. Hematopoietic stem cell-derived cancer-associated fibroblasts are novel contributors to the pro-tumorigenic microenvironment. Neoplasia 2015;17(5):434–448. doi: 10.1016/j.neo.2015.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee CH, Shah B, Moioli EK, Mao JJ. CTGF directs fibroblast differentiation from human mesenchymal stem/stromal cells and defines connective tissue healing in a rodent injury model. J. Clin. Invest 2010;120(9):3340–3349. doi: 10.1172/JCI43230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Quante M, Tu SP, Tomita H, et al. Bone marrow-derived myofibroblasts contribute to the mesenchymal stem cell niche and promote tumor growth. Cancer Cell 2011;19(2):257–272. doi: 10.1016/j.ccr.2011.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Spaeth EL, Dembinski JL, Sasser AK, et al. Mesenchymal stem cell transition to tumor-associated fibroblasts contributes to fibrovascular network expansion and tumor progression. PLoS One 2009;4(4):e4992. doi: 10.1371/journal.pone.0004992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jiang D, Scharffetter-Kochanek K. Mesenchymal stem cells in wound repair, tissue homeostasis, and aging In: Geiger H, Jasper H, Florian MC, eds. Stem Cell Aging: Mechanisms, Consequences, Rejuvenation Vienna: Springer Vienna; 2015:287–318. doi: 10.1007/978-3-7091-1232-8_14. [DOI] [Google Scholar]
- 24.Dominici M, Le Blanc K, Mueller I, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 2006;8(4):315–317. doi: 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
- 25.da Silva Meirelles L, Chagastelles PC, Nardi NB. Mesenchymal stem cells reside in virtually all post-natal organs and tissues. J. Cell Sci 2006;119(Pt 11):2204–2213. doi: 10.1242/jcs.02932. [DOI] [PubMed] [Google Scholar]
- 26.Jung Y, Kim JK, Shiozawa Y, et al. Recruitment of mesenchymal stem cells into prostate tumours promotes metastasis. Nat. Commun 2013;4:1795. doi: 10.1038/ncomms2766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee H-Y, Hong I-S. Double-edged sword of mesenchymal stem cells: Cancer-promoting versus therapeutic potential. Cancer Sci 2017;108(10):1939–1946. doi: 10.1111/cas.13334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Waterman RS, Tomchuck SL, Henkle SL, Betancourt AM. A new mesenchymal stem cell (MSC) paradigm: polarization into a pro-inflammatory MSC1 or an Immunosuppressive MSC2 phenotype. PLoS One 2010;5(4):e10088. doi: 10.1371/journal.pone.0010088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McLean K, Gong Y, Choi Y, et al. Human ovarian carcinoma–associated mesenchymal stem cells regulate cancer stem cells and tumorigenesis via altered BMP production. J. Clin. Invest 2011;121(8):3206–3219. doi: 10.1172/JCI45273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Coffman LG, Choi Y-J, McLean K, Allen BL, di Magliano MP, Buckanovich RJ. Human carcinoma-associated mesenchymal stem cells promote ovarian cancer chemotherapy resistance via a BMP4/HH signaling loop. Oncotarget 2016;7(6):6916–6932. doi: 10.18632/oncotarget.6870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kordes C, Sawitza I, Götze S, Herebian D, Häussinger D. Stellate cells are mesenchymal stem cells. Eur J Med Res 2014;19(Suppl 1):S6. doi: 10.1186/2047-783X-19-S1-S6. [DOI] [Google Scholar]
- 32.Phillips P Pancreatic stellate cells and fibrosis In: Grippo PJ, Munshi HG, eds. Pancreatic Cancer and Tumor Microenvironment Trivandrum (India): Transworld Research Network; 2012. [PubMed] [Google Scholar]
- 33.Kordes C, Sawitza I, Müller-Marbach A, et al. CD133+ hepatic stellate cells are progenitor cells. Biochem. Biophys. Res. Commun 2007;352(2):410–417. doi: 10.1016/j.bbrc.2006.11.029. [DOI] [PubMed] [Google Scholar]
- 34.Zhang C-Y, Yuan W-G, He P, Lei J-H, Wang C-X. Liver fibrosis and hepatic stellate cells: Etiology, pathological hallmarks and therapeutic targets. World J. Gastroenterol 2016;22(48):10512–10522. doi: 10.3748/wjg.v22.i48.10512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rybinski B, Franco-Barraza J, Cukierman E. The wound healing, chronic fibrosis, and cancer progression triad. Physiol. Genomics 2014;46(7):223–244. doi: 10.1152/physiolgenomics.00158.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dvorak HF. Tumors: wounds that do not heal-redux. Cancer Immunol Res 2015;3(1):1–11. doi: 10.1158/2326-6066.CIR-14-0209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schäfer M, Werner S. Cancer as an overhealing wound: an old hypothesis revisited. Nat. Rev. Mol. Cell Biol 2008;9(8):628–638. doi: 10.1038/nrm2455. [DOI] [PubMed] [Google Scholar]
- 38.Gurtner GC, Werner S, Barrandon Y, Longaker MT. Wound repair and regeneration. Nature 2008;453(7193):314–321. doi: 10.1038/nature07039. [DOI] [PubMed] [Google Scholar]
- 39.Wang X, Zhang W, Sun X, Lin Y, Chen W. Cancer-associated fibroblasts induce epithelial-mesenchymal transition through secreted cytokines in endometrial cancer cells. Oncol. Lett 2018;15(4):5694–5702. doi: 10.3892/ol.2018.8000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.López-Novoa JM, Nieto MA. Inflammation and EMT: an alliance towards organ fibrosis and cancer progression. EMBO Mol. Med 2009;1(6–7):303–314. doi: 10.1002/emmm.200900043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Krtolica A, Parrinello S, Lockett S, Desprez PY, Campisi J. Senescent fibroblasts promote epithelial cell growth and tumorigenesis: a link between cancer and aging. Proc. Natl. Acad. Sci. USA 2001;98(21):12072–12077. doi: 10.1073/pnas.211053698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Torrisi JM, Schwartz LH, Gollub MJ, Ginsberg MS, Bosl GJ, Hricak H. CT findings of chemotherapy-induced toxicity: what radiologists need to know about the clinical and radiologic manifestations of chemotherapy toxicity. Radiology 2011;258(1):41–56. doi: 10.1148/radiol.10092129. [DOI] [PubMed] [Google Scholar]
- 43.Mancini ML, Sonis ST. Mechanisms of cellular fibrosis associated with cancer regimen-related toxicities. Front. Pharmacol 2014;5:51. doi: 10.3389/fphar.2014.00051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lotti F, Jarrar AM, Pai RK, et al. Chemotherapy activates cancer-associated fibroblasts to maintain colorectal cancer-initiating cells by IL-17A. J. Exp. Med 2013;210(13):2851–2872. doi: 10.1084/jem.20131195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.O‟Neil M, Damjanov I. Histopathology of Colorectal Cancer after Neoadjuvant Chemoradiation Therapy. Open Pathol. J 2009;3(2):91–98. doi: 10.2174/1874375700903020091. [DOI] [Google Scholar]
- 46.Tiwana KK, Nibhoria S, Kaur M, Monga T, Gupta R. Postchemotherapy histopathological evaluation of ovarian carcinoma: a 40-case study. Chemother. Res. Pract 2015;2015:197871. doi: 10.1155/2015/197871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sethi D, Sen R, Parshad S, Khetarpal S, Garg M, Sen J. Histopathologic changes following neoadjuvant chemotherapy in various malignancies. Int J Appl Basic Med Res 2012;2(2):111–116. doi: 10.4103/2229-516X.106353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Verset L, Tommelein J, Moles Lopez X, et al. Impact of neoadjuvant therapy on cancer-associated fibroblasts in rectal cancer. Radiother. Oncol 2015;116(3):449–454. doi: 10.1016/j.radonc.2015.05.007. [DOI] [PubMed] [Google Scholar]
- 49.Wynn TA. Cellular and molecular mechanisms of fibrosis. J. Pathol 2008;214(2):199–210. doi: 10.1002/path.2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Barker HE, Paget JTE, Khan AA, Harrington KJ. The tumour microenvironment after radiotherapy: mechanisms of resistance and recurrence. Nat. Rev. Cancer 2015;15(7):409–425. doi: 10.1038/nrc3958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tsai KKC, Chuang EY-Y, Little JB, Yuan Z-M. Cellular mechanisms for low-dose ionizing radiation-induced perturbation of the breast tissue microenvironment. Cancer Res 2005;65(15):6734–6744. doi: 10.1158/0008-5472.CAN-05-0703. [DOI] [PubMed] [Google Scholar]
- 52.Takigawa H, Kitadai Y, Shinagawa K, et al. Mesenchymal Stem Cells Induce Epithelial to Mesenchymal Transition in Colon Cancer Cells through Direct Cell-to-Cell Contact. Neoplasia 2017;19(5):429–438. doi: 10.1016/j.neo.2017.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Saito K, Sakaguchi M, Maruyama S, et al. Stromal mesenchymal stem cells facilitate pancreatic cancer progression by regulating specific secretory molecules through mutual cellular interaction. J. Cancer 2018;9(16):2916–2929. doi: 10.7150/jca.24415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Dabbah M, Attar-Schneider O, Tartakover Matalon S, et al. Microvesicles derived from normal and multiple myeloma bone marrow mesenchymal stem cells differentially modulate myeloma cells‟ phenotype and translation initiation. Carcinogenesis 2017;38(7):708–716. doi: 10.1093/carcin/bgx045. [DOI] [PubMed] [Google Scholar]
- 55.Coffman LG, Pearson AT, Frisbie LG, et al. Ovarian Carcinoma-Associated Mesenchymal Stem Cells Arise from Tissue-Specific Normal Stroma. Stem Cells 2018. doi: 10.1002/stem.2932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.McLean K, Tan L, Bolland DE, et al. Leukemia inhibitory factor functions in parallel with interleukin-6 to promote ovarian cancer growth. Oncogene 2018. doi: 10.1038/s41388-018-0523-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Maffey A, Storini C, Diceglie C, et al. Mesenchymal stem cells from tumor microenvironment favour breast cancer stem cell proliferation, cancerogenic and metastatic potential, via ionotropic purinergic signalling. Sci. Rep 2017;7(1):13162. doi: 10.1038/s41598-017-13460-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Timaner M, Letko-Khait N, Kotsofruk R, et al. Therapy-Educated Mesenchymal Stem Cells Enrich for Tumor-Initiating Cells. Cancer Res 2018;78(5):1253–1265. doi: 10.1158/0008-5472.CAN-17-1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Karnoub AE, Dash AB, Vo AP, et al. Mesenchymal stem cells within tumour stroma promote breast cancer metastasis. Nature 2007;449(7162):557–563. doi: 10.1038/nature06188. [DOI] [PubMed] [Google Scholar]
- 60.Yang H, Zheng Y, Zhang Y, Cao Z, Jiang Y. Mesenchymal stem cells derived from multiple myeloma patients protect against chemotherapy through autophagy-dependent activation of NF-κB signaling. Leuk. Res 2017;60:82–88. doi: 10.1016/j.leukres.2017.07.002. [DOI] [PubMed] [Google Scholar]
- 61.Melzer C, von der Ohe J, Hass R. Enhanced metastatic capacity of breast cancer cells after interaction and hybrid formation with mesenchymal stroma/stem cells (MSC). Cell Commun. Signal 2018;16(1):2. doi: 10.1186/s12964-018-0215-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Faiella W, Atoui R. Immunotolerant properties of mesenchymal stem cells: updated review. Stem Cells Int 2016;2016:1859567. doi: 10.1155/2016/1859567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Davies LC, Heldring N, Kadri N, Le Blanc K. Mesenchymal Stromal Cell Secretion of Programmed Death-1 Ligands Regulates T Cell Mediated Immunosuppression. Stem Cells 2017;35(3):766–776. doi: 10.1002/stem.2509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.de Lourdes Mora-García M, García-Rocha R, Morales-Ramírez O, et al. Mesenchymal stromal cells derived from cervical cancer produce high amounts of adenosine to suppress cytotoxic T lymphocyte functions. J. Transl. Med 2016;14(1):302. doi: 10.1186/s12967-016-1057-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Han Z, Tian Z, Lv G, et al. Immunosuppressive effect of bone marrow-derived mesenchymal stem cells in inflammatory microenvironment favours the growth of B16 melanoma cells. J. Cell Mol. Med 2011;15(11):2343–2352. doi: 10.1111/j.1582-4934.2010.01215.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ren G, Zhang L, Zhao X, et al. Mesenchymal stem cell-mediated immunosuppression occurs via concerted action of chemokines and nitric oxide. Cell Stem Cell 2008;2(2):141–150. doi: 10.1016/j.stem.2007.11.014. [DOI] [PubMed] [Google Scholar]
- 67.Patel SA, Meyer JR, Greco SJ, Corcoran KE, Bryan M, Rameshwar P. Mesenchymal stem cells protect breast cancer cells through regulatory T cells: role of mesenchymal stem cell-derived TGF-beta. J. Immunol 2010;184(10):5885–5894. doi: 10.4049/jimmunol.0903143. [DOI] [PubMed] [Google Scholar]
- 68.Ren G, Zhao X, Wang Y, et al. CCR2-dependent recruitment of macrophages by tumor-educated mesenchymal stromal cells promotes tumor development and is mimicked by TNFα. Cell Stem Cell 2012;11(6):812–824. doi: 10.1016/j.stem.2012.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yamada K, Uchiyama A, Uehara A, et al. MFG-E8 Drives Melanoma Growth by Stimulating Mesenchymal Stromal Cell-Induced Angiogenesis and M2 Polarization of Tumor-Associated Macrophages. Cancer Res 2016;76(14):4283–4292. doi: 10.1158/0008-5472.CAN-15-2812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Mathew E, Brannon AL, Del Vecchio A, et al. Mesenchymal stem cells promote pancreatic tumor growth by inducing alternative polarization of macrophages. Neoplasia 2016;18(3):142–151. doi: 10.1016/j.neo.2016.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Santamato A, Fransvea E, Dituri F, et al. Hepatic stellate cells stimulate HCC cell migration via laminin-5 production. Clin. Sci 2011;121(4):159–168. doi: 10.1042/CS20110002. [DOI] [PubMed] [Google Scholar]
- 72.Zhao W, Zhang L, Yin Z, et al. Activated hepatic stellate cells promote hepatocellular carcinoma development in immunocompetent mice. Int. J. Cancer 2011;129(11):2651–2661. doi: 10.1002/ijc.25920. [DOI] [PubMed] [Google Scholar]
- 73.Ene-Obong A, Clear AJ, Watt J, et al. Activated pancreatic stellate cells sequester CD8+ T cells to reduce their infiltration of the juxtatumoral compartment of pancreatic ductal adenocarcinoma. Gastroenterology 2013;145(5):1121–1132. doi: 10.1053/j.gastro.2013.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Mace TA, Ameen Z, Collins A, et al. Pancreatic cancer-associated stellate cells promote differentiation of myeloid-derived suppressor cells in a STAT3-dependent manner. Cancer Res 2013;73(10):3007–3018. doi: 10.1158/0008-5472.CAN-12-4601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.van Deventer HW, Palmieri DA, Wu QP, McCook EC, Serody JS. Circulating fibrocytes prepare the lung for cancer metastasis by recruiting Ly-6C+ monocytes via CCL2. J. Immunol 2013;190(9):4861–4867. doi: 10.4049/jimmunol.1202857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Mitsuhashi A, Goto H, Saijo A, et al. Fibrocyte-like cells mediate acquired resistance to anti-angiogenic therapy with bevacizumab. Nat. Commun 2015;6:8792. doi: 10.1038/ncomms9792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Goto H, Nishioka Y. Fibrocytes: A Novel Stromal Cells to Regulate Resistance to Anti-Angiogenic Therapy and Cancer Progression. Int. J. Mol. Sci 2017;19(1). doi: 10.3390/ijms19010098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Saijo A, Goto H, Nakano M, et al. Bone marrow-derived fibrocytes promote stem cell-like properties of lung cancer cells. Cancer Lett 2018;421:17–27. doi: 10.1016/j.canlet.2018.02.016. [DOI] [PubMed] [Google Scholar]
- 79.Erez N, Truitt M, Olson P, Arron ST, Hanahan D. Cancer-Associated Fibroblasts Are Activated in Incipient Neoplasia to Orchestrate Tumor-Promoting Inflammation in an NF-kappaB-Dependent Manner. Cancer Cell 2010;17(2):135–147. doi: 10.1016/j.ccr.2009.12.041. [DOI] [PubMed] [Google Scholar]
- 80.Tyan S-W, Kuo W-H, Huang C-K, et al. Breast cancer cells induce cancer-associated fibroblasts to secrete hepatocyte growth factor to enhance breast tumorigenesis. PLoS One 2011;6(1):e15313. doi: 10.1371/journal.pone.0015313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Sun Y, Fan X, Zhang Q, Shi X, Xu G, Zou C. Cancer-associated fibroblasts secrete FGF-1 to promote ovarian proliferation, migration, and invasion through the activation of FGF-1/FGFR4 signaling. Tumour Biol 2017;39(7):1010428317712592. doi: 10.1177/1010428317712592. [DOI] [PubMed] [Google Scholar]
- 82.Laing N, McDermott B, Wen S, et al. Inhibition of platelet-derived growth factor receptor α by MEDI-575 reduces tumor growth and stromal fibroblast content in a model of non-small cell lung cancer. Mol. Pharmacol 2013;83(6):1247–1256. doi: 10.1124/mol.112.084079. [DOI] [PubMed] [Google Scholar]
- 83.Ito TK, Ishii G, Chiba H, Ochiai A. The VEGF angiogenic switch of fibroblasts is regulated by MMP-7 from cancer cells. Oncogene 2007;26(51):7194–7203. doi: 10.1038/sj.onc.1210535. [DOI] [PubMed] [Google Scholar]
- 84.Zhuang J, Lu Q, Shen B, et al. TGFβ1 secreted by cancer-associated fibroblasts induces epithelial-mesenchymal transition of bladder cancer cells through lncRNA-ZEB2NAT. Sci. Rep 2015;5:11924. doi: 10.1038/srep11924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.O’Connell JT, Sugimoto H, Cooke VG, et al. VEGF-A and Tenascin-C produced by S100A4+ stromal cells are important for metastatic colonization. Proc. Natl. Acad. Sci. USA 2011;108(38):16002–16007. doi: 10.1073/pnas.1109493108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Erdogan B, Webb DJ. Cancer-associated fibroblasts modulate growth factor signaling and extracellular matrix remodeling to regulate tumor metastasis. Biochem. Soc. Trans 2017;45(1):229–236. doi: 10.1042/BST20160387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Alkasalias T, Alexeyenko A, Hennig K, et al. RhoA knockout fibroblasts lose tumor-inhibitory capacity in vitro and promote tumor growth in vivo. Proc. Natl. Acad. Sci. USA 2017;114(8):E1413–E1421. doi: 10.1073/pnas.1621161114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Huang X, Yang N, Fiore VF, et al. Matrix stiffness-induced myofibroblast differentiation is mediated by intrinsic mechanotransduction. Am. J. Respir. Cell Mol. Biol 2012;47(3):340–348. doi: 10.1165/rcmb.2012-0050OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Lo A, Li C-P, Buza EL, et al. Fibroblast activation protein augments progression and metastasis of pancreatic ductal adenocarcinoma. JCI Insight 2017;2(19). doi: 10.1172/jci.insight.92232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Calon A, Espinet E, Palomo-Ponce S, et al. Dependency of colorectal cancer on a TGF-β-driven program in stromal cells for metastasis initiation. Cancer Cell 2012;22(5):571–584. doi: 10.1016/j.ccr.2012.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Mezawa Y, Orimo A. The roles of tumor- and metastasis-promoting carcinoma-associated fibroblasts in human carcinomas. Cell Tissue Res 2016;365(3):675–689. doi: 10.1007/s00441-016-2471-1. [DOI] [PubMed] [Google Scholar]
- 92.Goetz JG, Minguet S, Navarro-Lérida I, et al. Biomechanical remodeling of the microenvironment by stromal caveolin-1 favors tumor invasion and metastasis. Cell 2011;146(1):148–163. doi: 10.1016/j.cell.2011.05.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Tang D, Gao J, Wang S, et al. Cancer-associated fibroblasts promote angiogenesis in gastric cancer through galectin-1 expression. Tumour Biol 2016;37(2):1889–1899. doi: 10.1007/s13277-015-3942-9. [DOI] [PubMed] [Google Scholar]
- 94.Shimao Y, Nabeshima K, Inoue T, Koono M. Role of fibroblasts in HGF/SF-induced cohort migration of human colorectal carcinoma cells: fibroblasts stimulate migration associated with increased fibronectin production via upregulated TGF-beta1. Int. J. Cancer 1999;82(3):449–458. doi:. [DOI] [PubMed] [Google Scholar]
- 95.Orimo A, Gupta PB, Sgroi DC, et al. Stromal fibroblasts present in invasive human breast carcinomas promote tumor growth and angiogenesis through elevated SDF-1/CXCL12 secretion. Cell 2005;121(3):335–348. doi: 10.1016/j.cell.2005.02.034. [DOI] [PubMed] [Google Scholar]
- 96.Yeung T-L, Leung CS, Mok SC. CAF reprogramming inhibits ovarian cancer progression. Cell Cycle 2014;13(24):3783–3784. doi: 10.4161/15384101.2014.988106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Cioni B, Nevedomskaya E, Melis MHM, et al. Loss of androgen receptor signaling in prostate cancer-associated fibroblasts (CAFs) promotes CCL2- and CXCL8-mediated cancer cell migration. Mol. Oncol 2018;12(8):1308–1323. doi: 10.1002/1878-0261.12327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Laouar Y, Sutterwala FS, Gorelik L, Flavell RA. Transforming growth factor-beta controls T helper type 1 cell development through regulation of natural killer cell interferon-gamma. Nat. Immunol 2005;6(6):600–607. doi: 10.1038/ni1197. [DOI] [PubMed] [Google Scholar]
- 99.Balsamo M, Scordamaglia F, Pietra G, et al. Melanoma-associated fibroblasts modulate NK cell phenotype and antitumor cytotoxicity. Proc. Natl. Acad. Sci. USA 2009;106(49):20847–20852. doi: 10.1073/pnas.0906481106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Comito G, Giannoni E, Segura CP, et al. Cancer-associated fibroblasts and M2-polarized macrophages synergize during prostate carcinoma progression. Oncogene 2014;33(19):2423–2431. doi: 10.1038/onc.2013.191. [DOI] [PubMed] [Google Scholar]
- 101.Tommelein J, Verset L, Boterberg T, Demetter P, Bracke M, De Wever O. Cancer-associated fibroblasts connect metastasis-promoting communication in colorectal cancer. Front. Oncol 2015;5:63. doi: 10.3389/fonc.2015.00063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Yang X, Lin Y, Shi Y, et al. FAP Promotes Immunosuppression by Cancer-Associated Fibroblasts in the Tumor Microenvironment via STAT3-CCL2 Signaling. Cancer Res 2016;76(14):4124–4135. doi: 10.1158/0008-5472.CAN-15-2973. [DOI] [PubMed] [Google Scholar]
- 103.Kumar V, Donthireddy L, Marvel D, et al. Cancer-Associated Fibroblasts Neutralize the Anti-tumor Effect of CSF1 Receptor Blockade by Inducing PMN-MDSC Infiltration of Tumors. Cancer Cell 2017;32(5):654–668.e5. doi: 10.1016/j.ccell.2017.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Kakarla S, Song X-T, Gottschalk S. Cancer-associated fibroblasts as targets for immunotherapy. Immunotherapy 2012;4(11):1129–1138. doi: 10.2217/imt.12.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Bilir C, Sarisozen C. Indoleamine 2,3-dioxygenase (IDO): Only an enzyme or a checkpoint controller? Journal of Oncological Sciences 2017;3(2):52–56. doi: 10.1016/j.jons.2017.04.001. [DOI] [Google Scholar]
- 106.Terme M, Pernot S, Marcheteau E, et al. VEGFA-VEGFR pathway blockade inhibits tumor-induced regulatory T-cell proliferation in colorectal cancer. Cancer Res 2013;73(2):539–549. doi: 10.1158/0008-5472.CAN-12-2325. [DOI] [PubMed] [Google Scholar]
- 107.Gabrilovich DI, Chen HL, Girgis KR, et al. Production of vascular endothelial growth factor by human tumors inhibits the functional maturation of dendritic cells. Nat. Med 1996;2(10):1096–1103. doi: 10.1038/nm1096-1096. [DOI] [PubMed] [Google Scholar]
- 108.Voron T, Colussi O, Marcheteau E, et al. VEGF-A modulates expression of inhibitory checkpoints on CD8+ T cells in tumors. J. Exp. Med 2015;212(2):139–148. doi: 10.1084/jem.20140559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Whitaker-Menezes D, Martinez-Outschoorn UE, Lin Z, et al. Evidence for a stromalepithelial “lactate shuttle” in human tumors: MCT4 is a marker of oxidative stress in cancer-associated fibroblasts. Cell Cycle 2011;10(11):1772–1783. doi: 10.4161/cc.10.11.15659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Lopes-Coelho F, Gouveia-Fernandes S, Serpa J. Metabolic cooperation between cancer and non-cancerous stromal cells is pivotal in cancer progression. Tumour Biol 2018;40(2):1010428318756203. doi: 10.1177/1010428318756203. [DOI] [PubMed] [Google Scholar]
- 111.Zhang D, Wang Y, Shi Z, et al. Metabolic reprogramming of cancer-associated fibroblasts by IDH3α downregulation. Cell Rep 2015;10(8):1335–1348. doi: 10.1016/j.celrep.2015.02.006. [DOI] [PubMed] [Google Scholar]
- 112.Fiaschi T, Marini A, Giannoni E, et al. Reciprocal metabolic reprogramming through lactate shuttle coordinately influences tumor-stroma interplay. Cancer Res 2012;72(19):5130–5140. doi: 10.1158/0008-5472.CAN-12-1949. [DOI] [PubMed] [Google Scholar]
- 113.Lisanti MP, Martinez-Outschoorn UE, Sotgia F. Oncogenes induce the cancer-associated fibroblast phenotype: metabolic symbiosis and “fibroblast addiction” are new therapeutic targets for drug discovery. Cell Cycle 2013;12(17):2723–2732. doi: 10.4161/cc.25695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Martinez-Outschoorn UE, Curry JM, Ko Y-H, et al. Oncogenes and inflammation rewire host energy metabolism in the tumor microenvironment: RAS and NFκB target stromal MCT4. Cell Cycle 2013;12(16):2580–2597. doi: 10.4161/cc.25510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Yang L, Achreja A, Yeung T-L, et al. Targeting Stromal Glutamine Synthetase in Tumors Disrupts Tumor Microenvironment-Regulated Cancer Cell Growth. Cell Metab 2016;24(5):685–700. doi: 10.1016/j.cmet.2016.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Santi A, Caselli A, Ranaldi F, et al. Cancer associated fibroblasts transfer lipids and proteins to cancer cells through cargo vesicles supporting tumor growth. Biochim. Biophys. Acta 2015;1853(12):3211–3223. doi: 10.1016/j.bbamcr.2015.09.013. [DOI] [PubMed] [Google Scholar]
- 117.Provenzano PP, Cuevas C, Chang AE, Goel VK, Von Hoff DD, Hingorani SR. Enzymatic targeting of the stroma ablates physical barriers to treatment of pancreatic ductal adenocarcinoma. Cancer Cell 2012;21(3):418–429. doi: 10.1016/j.ccr.2012.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Jacobetz MA, Chan DS, Neesse A, et al. Hyaluronan impairs vascular function and drug delivery in a mouse model of pancreatic cancer. Gut 2013;62(1):112–120. doi: 10.1136/gutjnl-2012-302529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Mantoni TS, Lunardi S, Al-Assar O, Masamune A, Brunner TB. Pancreatic stellate cells radioprotect pancreatic cancer cells through β1-integrin signaling. Cancer Res 2011;71(10):3453–3458. doi: 10.1158/0008-5472.CAN-10-1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Smith NR, Baker D, Farren M, et al. Tumor stromal architecture can define the intrinsic tumor response to VEGF-targeted therapy. Clin. Cancer Res 2013;19(24):6943–6956. doi: 10.1158/1078-0432.CCR-13-1637. [DOI] [PubMed] [Google Scholar]
- 121.Salmon H, Franciszkiewicz K, Damotte D, et al. Matrix architecture defines the preferential localization and migration of T cells into the stroma of human lung tumors. J. Clin. Invest 2012;122(3):899–910. doi: 10.1172/JCI45817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Beatty GL, Chiorean EG, Fishman MP, et al. CD40 agonists alter tumor stroma and show efficacy against pancreatic carcinoma in mice and humans. Science 2011;331(6024):1612–1616. doi: 10.1126/science.1198443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Han W, Chen S, Yuan W, et al. Oriented collagen fibers direct tumor cell intravasation. Proc. Natl. Acad. Sci. USA 2016;113(40):11208–11213. doi: 10.1073/pnas.1610347113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Levental KR, Yu H, Kass L, et al. Matrix crosslinking forces tumor progression by enhancing integrin signaling. Cell 2009;139(5):891–906. doi: 10.1016/j.cell.2009.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Filipe EC, Chitty JL, Cox TR. Charting the unexplored extracellular matrix in cancer. Int J Exp Pathol 2018;99(2):58–76. doi: 10.1111/iep.12269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Fang M, Yuan J, Peng C, Li Y. Collagen as a double-edged sword in tumor progression. Tumour Biol 2014;35(4):2871–2882. doi: 10.1007/s13277-013-1511-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Kaplan RN, Rafii S, Lyden D. Preparing the “soil”: the premetastatic niche. Cancer Res 2006;66(23):11089–11093. doi: 10.1158/0008-5472.CAN-06-2407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Seubert B, Grünwald B, Kobuch J, et al. Tissue inhibitor of metalloproteinases (TIMP)-1 creates a premetastatic niche in the liver through SDF-1/CXCR4-dependent neutrophil recruitment in mice. Hepatology 2015;61(1):238–248. doi: 10.1002/hep.27378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Song T, Dou C, Jia Y, Tu K, Zheng X. TIMP-1 activated carcinoma-associated fibroblasts inhibit tumor apoptosis by activating SDF1/CXCR4 signaling in hepatocellular carcinoma. Oncotarget 2015;6(14):12061–12079. doi: 10.18632/oncotarget.3616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Erler JT, Bennewith KL, Cox TR, et al. Hypoxia-induced lysyl oxidase is a critical mediator of bone marrow cell recruitment to form the premetastatic niche. Cancer Cell 2009;15(1):35–44. doi: 10.1016/j.ccr.2008.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Alkasalias T, Flaberg E, Kashuba V, et al. Inhibition of tumor cell proliferation and motility by fibroblasts is both contact and soluble factor dependent. Proc. Natl. Acad. Sci. USA 2014;111(48):17188–17193. doi: 10.1073/pnas.1419554111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Bruno S, Collino F, Deregibus MC, Grange C, Tetta C, Camussi G. Microvesicles derived from human bone marrow mesenchymal stem cells inhibit tumor growth. Stem Cells Dev 2013;22(5):758–771. doi: 10.1089/scd.2012.0304. [DOI] [PubMed] [Google Scholar]
- 133.Özdemir BC, Pentcheva-Hoang T, Carstens JL, et al. Depletion of carcinoma-associated fibroblasts and fibrosis induces immunosuppression and accelerates pancreas cancer with reduced survival. Cancer Cell 2014;25(6):719–734. doi: 10.1016/j.ccr.2014.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Rhim AD, Oberstein PE, Thomas DH, et al. Stromal elements act to restrain, rather than support, pancreatic ductal adenocarcinoma. Cancer Cell 2014;25(6):735–747. doi: 10.1016/j.ccr.2014.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Lee JJ, Perera RM, Wang H, et al. Stromal response to Hedgehog signaling restrains pancreatic cancer progression. Proc. Natl. Acad. Sci. USA 2014;111(30):E3091–100. doi: 10.1073/pnas.1411679111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Shin K, Lim A, Zhao C, et al. Hedgehog signaling restrains bladder cancer progression by eliciting stromal production of urothelial differentiation factors. Cancer Cell 2014;26(4):521–533. doi: 10.1016/j.ccell.2014.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Mathew E, Zhang Y, Holtz AM, et al. Dosage-dependent regulation of pancreatic cancer growth and angiogenesis by hedgehog signaling. Cell Rep 2014;9(2):484–494. doi: 10.1016/j.celrep.2014.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Kim EJ, Sahai V, Abel EV, et al. Pilot clinical trial of hedgehog pathway inhibitor GDC-0449 (vismodegib) in combination with gemcitabine in patients with metastatic pancreatic adenocarcinoma. Clin. Cancer Res 2014;20(23):5937–5945. doi: 10.1158/1078-0432.CCR-14-1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Steg AD, Katre AA, Bevis KS, et al. Smoothened antagonists reverse taxane resistance in ovarian cancer. Mol. Cancer Ther 2012;11(7):1587–1597. doi: 10.1158/1535-7163.MCT-11-1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Chai F, Zhou J, Chen C, et al. The Hedgehog inhibitor cyclopamine antagonizes chemoresistance of breast cancer cells. Onco. Targets. Ther 2013;6:1643–1647. doi: 10.2147/OTT.S51914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.McCann CK, Growdon WB, Kulkarni-Datar K, et al. Inhibition of Hedgehog signaling antagonizes serous ovarian cancer growth in a primary xenograft model. PLoS One 2011;6(11):e28077. doi: 10.1371/journal.pone.0028077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Briscoe J, Thérond PP. The mechanisms of Hedgehog signalling and its roles in development and disease. Nat. Rev. Mol. Cell Biol 2013;14(7):416–429. doi: 10.1038/nrm3598. [DOI] [PubMed] [Google Scholar]
- 143.Tickle C, Barker H. The Sonic hedgehog gradient in the developing limb. Wiley Interdiscip Rev Dev Biol 2013;2(2):275–290. doi: 10.1002/wdev.70. [DOI] [PubMed] [Google Scholar]
- 144.Grum-Schwensen B, Klingelhofer J, Berg CH, et al. Suppression of tumor development and metastasis formation in mice lacking the S100A4(mts1) gene. Cancer Res 2005;65(9):3772–3780. doi: 10.1158/0008-5472.CAN-04-4510. [DOI] [PubMed] [Google Scholar]
- 145.Ostermann E, Garin-Chesa P, Heider KH, et al. Effective immunoconjugate therapy in cancer models targeting a serine protease of tumor fibroblasts. Clin. Cancer Res 2008;14(14):4584–4592. doi: 10.1158/1078-0432.CCR-07-5211. [DOI] [PubMed] [Google Scholar]
- 146.Kakarla S, Chow KKH, Mata M, et al. Antitumor effects of chimeric receptor engineered human T cells directed to tumor stroma. Mol. Ther 2013;21(8):1611–1620. doi: 10.1038/mt.2013.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Wang L-CS, Lo A, Scholler J, et al. Targeting fibroblast activation protein in tumor stroma with chimeric antigen receptor T cells can inhibit tumor growth and augment host immunity without severe toxicity. Cancer Immunol Res 2014;2(2):154–166. doi: 10.1158/2326-6066.CIR-13-0027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Lo A, Wang L-CS, Scholler J, et al. Tumor-Promoting Desmoplasia Is Disrupted by Depleting FAP-Expressing Stromal Cells. Cancer Res 2015;75(14):2800–2810. doi: 10.1158/0008-5472.CAN-14-3041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Santos AM, Jung J, Aziz N, Kissil JL, Puré E. Targeting fibroblast activation protein inhibits tumor stromagenesis and growth in mice. J. Clin. Invest 2009;119(12):3613–3625. doi: 10.1172/JCI38988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Feig C, Jones JO, Kraman M, et al. Targeting CXCL12 from FAP-expressing carcinoma-associated fibroblasts synergizes with anti-PD-L1 immunotherapy in pancreatic cancer. Proc. Natl. Acad. Sci. USA 2013;110(50):20212–20217. doi: 10.1073/pnas.1320318110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Berlin J, Bendell JC, Hart LL, et al. A randomized phase II trial of vismodegib versus placebo with FOLFOX or FOLFIRI and bevacizumab in patients with previously untreated metastatic colorectal cancer. Clin. Cancer Res 2013;19(1):258–267. doi: 10.1158/1078-0432.CCR-12-1800. [DOI] [PubMed] [Google Scholar]
- 152.De Jesus-Acosta A O’Dwyer PJ, Ramanathan RK. A phase II study of vismodegib, a hedgehog (Hh) pathway inhibitor, combined with gemcitabine and nab-paclitaxel (nab-P) in patients (pts) with untreated metastatic …. 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Infinity Reports Update from Phase 2 Study of Saridegib Plus Gemcitabine in Patients with Metastatic Pancreatic Cancer | Business Wire Available at: https://www.businesswire.com/news/home/20120127005146/en/Infinity-Reports-Update-Phase-2-Study-Saridegib#.UxAvFfRdVxV. Accessed December 8, 2018.
- 154.Catenacci DVT, Junttila MR, Karrison T, et al. Randomized phase ib/ii study of gemcitabine plus placebo or vismodegib, a hedgehog pathway inhibitor, in patients with metastatic pancreatic cancer. J. Clin. Oncol 2015;33(36):4284–4292. doi: 10.1200/JCO.2015.62.8719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Olumi AF, Grossfeld GD, Hayward SW, Carroll PR, Tlsty TD, Cunha GR. Carcinoma-associated fibroblasts direct tumor progression of initiated human prostatic epithelium. Cancer Res 1999;59(19):5002–5011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Ye H, Cheng J, Tang Y, et al. Human bone marrow-derived mesenchymal stem cells produced TGFbeta contributes to progression and metastasis of prostate cancer. Cancer Invest 2012;30(7):513–518. doi: 10.3109/07357907.2012.692171. [DOI] [PubMed] [Google Scholar]
- 157.Gorska AE, Jensen RA, Shyr Y, Aakre ME, Bhowmick NA, Moses HL. Transgenic mice expressing a dominant-negative mutant type II transforming growth factor-beta receptor exhibit impaired mammary development and enhanced mammary tumor formation. Am. J. Pathol 2003;163(4):1539–1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Arthur WT, Noren NK, Burridge K. Regulation of Rho family GTPases by cell-cell and cell-matrix adhesion. Biol. Res 2002;35(2):239–246. [DOI] [PubMed] [Google Scholar]
- 159.Zhao X-H, Laschinger C, Arora P, Szászi K, Kapus A, McCulloch CA. Force activates smooth muscle alpha-actin promoter activity through the Rho signaling pathway. J. Cell Sci 2007;120(Pt 10):1801–1809. doi: 10.1242/jcs.001586. [DOI] [PubMed] [Google Scholar]
- 160.Mullin BH, Mamotte C, Prince RL, Wilson SG. Influence of ARHGEF3 and RHOA knockdown on ACTA2 and other genes in osteoblasts and osteoclasts. PLoS One 2014;9(5):e98116. doi: 10.1371/journal.pone.0098116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Zhou Y, Huang X, Hecker L, et al. Inhibition of mechanosensitive signaling in myofibroblasts ameliorates experimental pulmonary fibrosis. J. Clin. Invest 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Liu F, Qi L, Liu B, et al. Fibroblast activation protein overexpression and clinical implications in solid tumors: a meta-analysis. PLoS One 2015;10(3):e0116683. doi: 10.1371/journal.pone.0116683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Kawase T, Yasui Y, Nishina S, et al. Fibroblast activation protein-α-expressing fibroblasts promote the progression of pancreatic ductal adenocarcinoma. BMC Gastroenterol 2015;15:109. doi: 10.1186/s12876-015-0340-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Jacob M, Chang L, Puré E. Fibroblast activation protein in remodeling tissues. Curr. Mol. Med 2012;12(10):1220–1243. [DOI] [PubMed] [Google Scholar]
- 165.Kraman M, Bambrough PJ, Arnold JN, et al. Suppression of antitumor immunity by stromal cells expressing fibroblast activation protein-alpha. Science 2010;330(6005):827–830. doi: 10.1126/science.1195300. [DOI] [PubMed] [Google Scholar]
- 166.Loeffler M, Krüger JA, Niethammer AG, Reisfeld RA. Targeting tumor-associated fibroblasts improves cancer chemotherapy by increasing intratumoral drug uptake. J. Clin. Invest 2006;116(7):1955–1962. doi: 10.1172/JCI26532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Lee J, Fassnacht M, Nair S, Boczkowski D, Gilboa E. Tumor immunotherapy targeting fibroblast activation protein, a product expressed in tumor-associated fibroblasts. Cancer Res 2005;65(23):11156–11163. doi: 10.1158/0008-5472.CAN-05-2805. [DOI] [PubMed] [Google Scholar]