Abstract
Background
The discovery of non-steroidal anti-estrogens created a new group of medicines looking for an application. However, at the time cytotoxic chemotherapy was the modality of choice to treat all cancers. Anti-estrogens were orphan drugs until 1971 with the passing of the National Cancer Act. This enabled laboratory innovations to aid patient care.
Methods
This article traces the strategic application of tamoxifen to treat breast cancer by targeting the estrogen receptor (ER), deploying long-term adjuvant tamoxifen therapy, and becoming the first chemo-preventive for any cancer. Laboratory discoveries from the University of Wisconsin Clinical Cancer Center are described that addressed a broad range of biological issues with tamoxifen. These translated to improvements in clinical care.
Results
Tamoxifen was studied extensively at Wisconsin in the 1980s, for the development of acquired resistance to long-term therapy. Additionally, the long-term metabolism of tamoxifen and regulation of growth factors were studied. A concern with tamoxifen use for chemoprevention was that an anti-estrogen would increase bone loss and atherosclerosis. Laboratory studies with tamoxifen and keoxifene (subsequently named raloxifene) demonstrated that “non-steroidal anti-estrogens” maintained bone density. This translated into successful clinical trials with tamoxifen at Wisconsin. However, tamoxifen also increased endometrial cancer growth. This discovery in the laboratory translated into changes in clinical care. SERMs were born at Wisconsin.
Conclusions
There are now five FDA approved SERMs all with discovery origins at Wisconsin. Women’s health was revolutionized, as SERMs have the ability to treat multiple diseases by switching on or off target sites around a woman’s body. (250)
The successful treatment of childhood leukemia during the 1960s became the evidence-based catalyst to develop a lexicon of chemotherapies to cure all cancers, including breast cancer. The treatment of breast cancer evolved from radical surgery and radiation to incorporate combination cytotoxic chemotherapy. Finally, the new science of bone marrow transplantation, perfected in the treatment of childhood leukemia, was evaluated unsuccessfully in breast cancer.
To put the war against cancer into the words of General George Patton “if everyone in the room is thinking the same way, then somebody is not thinking.” Nevertheless, a body of evidence, using a failed contraceptive tamoxifen targeted to estrogen receptor (ER) positive breast cancer, would serve as a foundation for all future progress in precision or targeted therapy.
The origins and initial development of tamoxifen will not be described here as the story has been described previously(1). Tamoxifen is an active agent in the treatment of metastatic breast cancer(2) but the key to the success of tamoxifen, as a pioneering anti-cancer agent, was the translational research strategy proven by subsequent clinical trials. During the 1970s, initially at the Worcester Foundation for Experimental Biology, and continued at the University of Leeds, the principles for the treatment and prevention of breast cancer with tamoxifen were established.
1. Targeting only patients with ER positive breast cancers(3), 2. Deploying long-term (>5 years) adjuvant tamoxifen therapy(4, 5) and 3. The discovery (6) of the potential of tamoxifen as the first chemo preventive for breast cancer, in high-risk women. Following rigorous randomized clinical trials(7), each of these translational treatment principles is now FDA approved. This path to clinical progress in the treatment and prevention of breast cancer illustrates it is not the availability of a therapeutic agent alone that creates an environment for success, but it is how the new agent is applied strategically for clinical care.
A move to the University of Wisconsin, Madison, to establish a Tamoxifen Team (1980–1995), proved to be an ideal opportunity for research and clinical translation. The University of Wisconsin was unique as it had two NCI funded Cancer Centers: the world famous McArdle laboratory and the newly built Clinical Cancer Center. Additionally, Jack Gorski of ER fame(8), was in the Department of Biochemistry. This university environment, along with the support of the founding director of the Wisconsin Clinical Cancer Center, Harold Rusch, the new director Paul P. Carbone, and the head of the Breast Cancer Research and Treatment Program, Doug Tormey (Fig. 1) would be the place that changed healthcare dramatically with the discovery and development of SERMs.
Figure 1.
University of Wisconsin Clinical Cancer Center faculty involved in hormone action and breast cancer therapy. Back row left to right: Dr. Gerald C. Mueller, Dr. Jack Gorski, Dr. V Craig Jordan and Dr. Douglas C. Tormey; seated left to right: Dr. Harold Rusch and Dr. Paul P. Carbone. This photograph was taken in 1984 just prior to the satellite symposium entitled “Estrogen and Antiestrogen Action: Basic and Clinical Aspects” hosted by the Cancer center in Madison as part of the events surrounding the 7th International Congress of Endocrinology in Montreal Canada. The proceeding were published by the University of Wisconsin Press as a book under the title Estrogren/Anti-estrogen Action and Breast Cancer Therapy edited by V. Craig Jordan.
Translational research in breast cancer at the University of Wisconsin Comprehensive Cancer Center (1980–1993)
The expanding clinical applications of tamoxifen demanded parallel laboratory studies to exam pharmacology and toxicology. There was little information about a) the mechanism of acquired resistance to tamoxifen, b) how antiestrogens function to block estrogen action at a target gene or regulate breast cancer cell replication and c) the long-term metabolic stability of tamoxifen.
a). Acquired resistance to anti-hormone therapy.
Multiple approaches were employed to study acquired resistance to tamoxifen and estrogen deprivation in breast cancer once aromatase inhibitors were developed. This knowledge was critical for safety and to determine how to develop second line treatments.
The role of growth factor cell signaling was a “hot topic” during the 1980s. It was possible that tumor cell growth factors could play a role in undermining the effectiveness of tamoxifen to control tumor cell growth. Two ideas were popular. Tamoxifen could increase levels of Transforming Growth Factor beta, which prevented the growth of adjacent ER negative tumor cells (9). However testing the theory in vivo in athymic mice did not support the hypothesis (10). An alternate proposition was the role of growth factors to subvert the action of tamoxifen through “growth factor cross talk” at the tumor cell membrane. Studies demonstrated (11–13) that paracrine mechanisms for growth factors subverted the anti-tumor actions of tamoxifen and reduced progesterone receptor synthesis. This latter observation was significant as the breast tumor progesterone receptor is a marker for good prognosis.
One interesting and important result of a study of growth factor regulation was the unanticipated finding that synthetic progestins in oral contraceptives, that were 19nor testosterone derivatives, were also estrogenic for the growth of breast cancer (14–17).
Tamoxifen treatment of athymic mice implanted with MCF7 breast tumor cells, to replicate long-term adjuvant therapy, resulted in a surprise. Marco Gottardis (a PhD student) discovered that the growth of the ER positive tumors occurred despite tamoxifen treatment. Subsequent studies, re-transplanting the tamoxifen resistant tumors into a new generation of athymic mice or athymic rats demonstrated that the tumors grew only with estrogen or tamoxifen (18, 19). This was not autonomous tumor growth. These data were important for the development of the pure antiestrogen fulvestrant. The Wisconsin research program tested the first pure antiestrogen successfully in this animal model (20). Subsequent clinical studies demonstrated that either an aromatase inhibitor or fulvestrant should be used as second line treatment for tamoxifen failure in metastatic breast cancer (21).
In the 1980’s/1990’s, tamoxifen stimulated tumors could not be grown in cell culture. As a result, tumor re-transplantation into successive generations of tamoxifen treated athymic mice was the only way to preserve the tamoxifen resistant phenotype. This methodology would result in another discovery. The plan was to document growth factor tumor cell regulation for estrogen or tamoxifen stimulated growth. Were the same growth factors being activated by estrogen or tamoxifen? Unexpectedly Doug Wolf, another PhD student was the first to discover(22) that physiological estrogen would cause rapid tumor regression of MCF7 tumors exposed to five years of tamoxifen in vivo. These data were confirmed(23) and resulted in the new biology of estrogen induced apoptosis(24) that has been used to explain the reason why 1. Estrogen replacement therapy given to women over the age of 60 results in a decreased incidence of breast cancer(25) and 2. Why women treated with five years of tamoxifen continue to have a decreasing recurrence rate after tamoxifen is stopped. It is proposed(26) that a woman’s own estrogen is killing micro-metastasis with acquired resistance to tamoxifen.
With the advent of aromatase inhibitors for the treatment of breast cancer, long-term estrogen deprivation studies were conducted on T47D and MCF7 ER positive breast cancer cell lines (27, 28). Two mechanisms of ER regulations were discovered (29). The T47D cells lost the ER during long-term estrogen deprivation (30, 31). MCF7 cells increase ER levels and became autonomous for growth(28). Clonal selection resulted in MCF7 5C cells (32), where estradiol triggers apoptosis within 7 days (33) and MCF72A where the process takes 14 days (34). These cells were used extensively to study the mechanism of estrogen-induced apoptosis. Clinical confirmation of the value of estrogen treatment following exhaustive endocrine therapy (35) validated the translational research model at Wisconsin.
b). An estrogen receptor model of antiestrogen action.
The discovery that tamoxifen was hydroxylated in the 4 position to an anti-estrogen with high affinity for ER (36, 37) not only provided important knowledge in medicinal chemistry (38, 39) to synthesize future non-steroidal antiestrogens, but also created a valuable reagent to be made radio labeled for studies in vivo (40) and in vitro (41–44) with interactions at the ER. The first model cell culture system in vitro of estradiol-stimulated prolactin synthesis in isolated cell from the mouse pituitary gland(45) was an important innovation to address the mechanics of estrogen and anti-estrogen action at the ER. The model in vitro avoided concerns about metabolism in vivo. Extensive structure function relationship studies classified ligands as antagonist, partial agonist and agonist (46–50) based on structure. This investigation at an estrogen responsive gene was subsequently expanded to map the structure function relationships of breast cancer cell replication (51–53). The model developed was referred to as the “crocodile model” as the anti-estrogenic sidechain was predicted to prevent “the jaws of the crocodile” from closing (54). The antiestrogenic sidechain was predicted to interact with a region referred to as the “anti-estrogenic region” (55). This region was subsequently identified as asp351 that was found to be mutated to asp351tyr in a tamoxifen stimulated tumor line (56, 57). Most importantly, it was demonstrated that this specific natural mutation could convert raloxifene an antiestrogen to an estrogen. This scientific detective work was accomplished by, isolation of the asp351tyr cDNA (57), creation of the first stable transfectants of ER in ER negative breast cancer cells(58, 59), and finally the demonstration using a TGF alpha target that asp351tyr can convert raloxifene into an estrogen(60, 61). These pharmacologic data complimented the subsequent xray-crystalography of both the raloxifene and tamoxifen ER complexes(62, 63). The antiestrogenic side chain interacts with asp351. Today it is known that asp351 is an essential amino acid necessary to close the unoccupied mutant ERs noted in aromatase resistant breast cancer(64). This story illustrates how basic science deciphers the mechanics of how the ER complex functions in clinical situations. The validity of knowledge comes from multiple investigations, discovery and then “rediscovery.”
c). The long-term metabolic stability of tamoxifen and patient endocrinology.
The major advantage of the Wisconsin Cancer Center was a mandatory serum collection process for all patients attending a Cancer Center Clinic. These serial samples allowed not only monitoring of tamoxifen and metabolites (65, 66) but also circulating hormone levels in pre and postmenopausal patients (67, 68). Tamoxifen and metabolites were monitored over 5 years of adjuvant tamoxifen therapy (69) and subsequently over 10 years (70). Little was known of the metabolism of tamoxifen but almost immediately a new metabolite of tamoxifen metabolite Y was discovered (66, 71). Other investigators studying the metabolism of the related antiestrogen toremifene discovered an equivalent metabolite. Today this SERM is marketed for treating dyspareunia, in post-menopausal women (72).
Most importantly comparative studies of circulating tamoxifen and metabolites were completed in laboratory animals and athymic mice (73) and compared with circulating patient levels (74, 75).
The discovery and proposed applications of SERMs
A series of simultaneous investigations at Wisconsin created a database that resulted in the new science of Selective Estrogen Receptor Modulation (SERM). It was known that tamoxifen could prevent the growth of human breast cancer cells in athymic mice but the mouse uterus was stimulated grow (76). The same metabolites of tamoxifen accumulated in the human tumor and the mouse uterus so it was proposed that the drug receptor complex is perceived as either “a stimulatory or inhibitory signal in the different target tissues of different species” (76). Simultaneously it was discovered (77) that tamoxifen and raloxifene preserved bone density in ovariectomized rats similar to that observed with estradiol. This was a completely counter intuitive result as “antiestrogens” were expected to decrease bone density. Tamoxifen and raloxifene were both found to inhibit rat mammary carcinogenesis (78). Subsequent studies by others confirmed the bone sparing properties of tamoxifen and raloxifene (79, 80).
The use of long-term adjuvant tamoxifen raised a safety question about the effects of tamoxifen in the human uterus and endometrial cancer. Nothing was known. To address this issue Marco Gottardis conducted a pivotal experiment using athymic mice bitransplanted with ER positive breast cancer cells or an ER positive human endometrial carcinoma (81). Tamoxifen blocked estrogen stimulated breast cancer growth but endometrial cancers grew dramatically. The presentation of these data at a symposium in Italy attracted considerable interest from the clinical community (82, 83). It was subsequently found that long-term tamoxifen therapy produced a small but significant increase in the incidence of endometrial cancer in tamoxifen treated patients (84). These data caused changes in clinical care but also restricted the use of tamoxifen to patients with breast cancer or at high risk for developing breast cancer.
Based on these laboratory data it was concluded, “We have obtained valuable clinical information about this group of drugs that can be applied to other disease states. Research does not travel in straight lines and observations in one field of science often become major discoveries in another. Important clues have been garnered about the effects of tamoxifen on bone and lipids so it is possible that derivatives could find targeted applications to retard osteoporosis or atherosclerosis. The ubiquitous applications of novel compounds to prevent disease associated with the progressive changes after menopause may, as a side effect, significantly retard the development of breast cancer. The targeted population would be post-menopausal women in general, thereby avoiding the requirement to select a high risk group to prevent breast cancer”(85).
Eli Lilly abandoned work on keoxifene (raloxifene) but clinical studies went forward with adjuvant tamoxifen at Wisconsin. Tamoxifen maintained bone density in post-menopausal patients (86), and decreased low-density lipoprotein cholesterol (87, 88). However, the immerging toxicology of tamoxifen with endometrial cancer (89) and the induction of rat liver carcinogenesis (90–93) required a safer compound to implement the aforementioned strategy (85) to treat multiple diseases in women with a single medicine.
Eli Lilly confirmed the Wisconsin bone data (80) and advanced the stated blue print for women’s health (85) with clinical trials to prevent osteoporosis. Their study demonstrated that raloxifene reduces fractures in post-menopausal women and produces a dramatic decrease in the incidence of ER positive breast cancer (94). A subsequent clinical trial referred to as the Study of Tamoxifen And Raloxifene (STAR) demonstrated that both SERMs produced a decrease in breast cancer in high-risk women but raloxifene had fewer side effects than tamoxifen (95, 96). There are now five FDA approved SERMs on the market with discovery origins back to studies at Wisconsin: toremifene (97, 98), raloxifene (77, 78), bazedoxifene (99), ospemiphene (66), also tamoxifen (5). There is one additional compound lasofoxifene that is a miracle of medicinal chemistry (100). Unlike any of the other SERMs lasofoxifene is extremely potent; it is used at 0.5mg daily compared with 60mg daily used for raloxifene to prevent osteoporosis.
Lasofoxifene, unlike the other SERMs decreases coronary heart disease and strokes. There is a decrease in breast and endometrial cancer. However, like other SERMs there is a small but significant increase thromboembolic events.
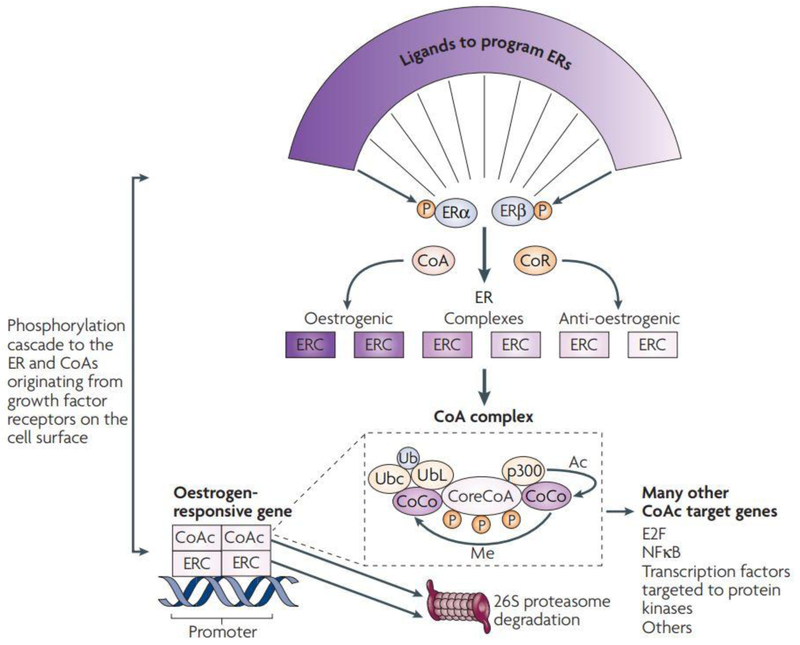
The SERM saga originating at the University of Leeds and the University of Wisconsin is an example of the value of Comprehensive Cancer Centers to take ideas from the laboratory to aid patients with multiple diseases. Selective Estrogen Receptor Modulation is a unique mechanism not anticipated. Only by examining a broad range of estrogen target tissues, cell culture models, and animal models focused in a comparison with human disease in one laboratory could the principles be deciphered correctly. Today, there is a clear understanding of the molecular mechanisms of SERM action in different target tissues. Fig. 2. It is possible that in the future new selective modulators of other members of the nuclear receptor super family can be modulated to treat diseases previously believed to be impossible. (Words: 2444)
Figure 2.
Molecular networks potentially influence the expression of SERM action in a target tissue. The shape of the ligands that bind to the oestrogen receptor (ERs) α and β programs the complex to become an estrogenic or anti-estrogenic signal. The context of the ER complex (ERC) can influence the expression of the response through the number of co-repressors (CoR) or co-activators (CoA). In simple terms, a site with few CoAs or high levels of CoRs might be a dominant anti-estrogenic site. However, the expression of estrogenic action is not simply the binding of the receptor complex to the promoter of the estrogen-responsive gene, but a dynamic process of CoA complex assembly and destruction. A core CoA, for example, steroid receptor coactivator protein 39 (SRC3), and the ERC are influenced by phosphorylation cascades that phosphorylate target sites on both complexes. The core CoA then assembles an activated multiprotein complex containing specific co-co-activators (CoCo) that might include p300, each of which has a specific enzymatic activity to be activated later. The CoA complex (CoAc) bind to the ERC at the estrogen-responsive gene promoter to switch on transcription. The CoCo proteins then perform methylation (Me) or acetylation (Ac) to activate dissociation of the complex. Simultaneously, ubiquitylated and destroyed in the 26S proteasome. Therefore, a regimented cycle of assembly, activation and destruction occurs on the basis of the preprogrammed ER complex. However, the co-activator, specifically SRC3, has ubiquitous action and can further modulate or amplify the ligand-activated trigger through many modulating genes that can consolidate and increase the stimulatory response of the ERC in a tissue. Therefore, the target tissue programmed to express a spectrum of responses between full estrogen action and anti-oestrogen action on the basis of the shape of the ligand and the sophistication of the tissue-modulating network. NFκB, nuclear factor κB. With permission from Nature Publishing Group, Jordan VC, Chemoprevention of Breast Cancer with Selective Estrogen Receptor Modulators, Nature Reviews Cancer, Volume 7:46–53, 2007.
Figure 4.
The Wisconsin Tamoxifen Team in the mid 1980’s. Front row left to right Simon Robinson (Post Doc from the University of Leeds), Wade Welshons (Assistant Scientist from Jack Gorski’s laboratory), Chris Parker (Estrogen Receptor Laboratory), Peter Ravdin (Medical Oncology Fellow), Rich Koch (Technician). Center (back row), Marco Gottardis, PhD Student, Right Mike Wolf (estrogen receptor laboratory), Richard Bain’s (technician metabolite analysis), Eric Phelps (summer student), Pat Mortons (Medical Illustrator for the hospital).
Synopsis:
Tamoxifen, a failed contraceptive, became a pioneering breast cancer medicine. Raloxifene (originally keoxifene), a failed breast cancer drug, became the first multifunctional Selective Estrogen Receptor Modulator (SERMs) to prevent osteoporosis and breast cancer. Multiple SERMs have revolutionized women’s health. (39 words)
Acknowledgements:
This article is dedicated to members of the University of Wisconsin Tamoxifen Team. The question can be asked what was achieved for the Team members during 1980 – 1995. My PhD student Anna C. Riegel (née/Tate) and I arrived in 1980 to build a research program that did not exist. During the 15 years, the Tamoxifen Team never exceeded two dozen students and staff. In 1983, I inherited the Directorship of the steroid receptor laboratory for Southern Wisconsin with its six staff. This was a daunting prospect so I called my mentor the late Dr. Bill McGuire in San Antonio to express my uncertainty at the challenge ahead. He explained that I was looking at this incorrectly. “This was an opportunity” and I should treat it as such. He was absolutely correct. During the whole of this period, I had stable funding with NIH grants and pharmaceutical contracts as well as philanthropic donations to the laboratory. I was promoted to Professor of Human Oncology and Pharmacology in 1985 and appointed the Director of Breast Cancer Research and Treatment Program at the University of Wisconsin Comprehensive Cancer Center in 1987. Ten - PhD students successfully received their degrees and publications flowed: refereed research papers (146), invited refereed reviews (22), editorials (11), book chapters (91), books edited (2), international meetings organized (2). Members of the Wisconsin Tamoxifen Team are show in Figures 3 – 5. I would like to thank the benefactors of the Dallas/Ft. Worth Living Legend Chair of Cancer Research, George and Barbara Bush Endowment for Cancer Research and the CCSG (P30-CA16672 (Peter Pisters)).
Figure 3.
The ladies of the UWCCC Tamoxifen Team in 1983. Far left Ethel Cormier and far right Anna T. Riegel (née Tate) both PhD students. In the center is Barbara Gosden (deceased) who spent two years in the laboratory following her MSc degree at the University of Leeds in 1979 with Anna Riegel. Mara E. Lieberman (deceased) between Barbara and Anna, joined my Team in 1981 from Jack Gorski’s Laboratory. This article is dedicated to their memory. Both were outstanding practical scientists. Mara recruited Rich Koch who performed hundreds of experiments using the isolated pituitary gland cells assay from immature mice to perform structure function relationships of SERMs that bound to the ER.
Figure 5.
The Wisconsin Tamoxifen Team in 1991 at the Swan Hotel, Orlando, Florida. Front Row (left to right), Professor Y Iino, who visited for a year in the 1980s and subsequently ensured that the other Japanese Physicians would join my later Tamoxifen Teams). M-Wei Jeng (PhD student) and S-Y Jeng. Back row near American Flag Doug Wolf (PhD Student), John Pink (PhD Student), Craig Jordan (estrogen receptor laboratory), Delinda Mauel (radioimmuno assay), Sue Langan-Fahy (tamoxifen assays), Simon Robinson (post doc). The meeting on Long Term Tamoxifen Treatment for Breast Cancer lasted for 3 days over the 4th of July. The University of Wisconsin published the book “Long Term Tamoxifen Treatment for Breast Cancer ed. V Craig Jordan in 1994.”
Footnotes
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
Conflicts of Interest – none.
References
- 1.Jordan VC, Tamoxifen as the first targeted long-term adjuvant therapy for breast cancer. Endocr Relat Cancer 21, R235–246 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cole MP, Jones CT, Todd ID, A new anti-oestrogenic agent in late breast cancer. An early clinical appraisal of ICI46474. Br J Cancer 25, 270–275 (1971). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jordan VC, Koerner S, Tamoxifen (ICI 46,474) and the human carcinoma 8S oestrogen receptor. Eur J Cancer 11, 205–206 (1975). [DOI] [PubMed] [Google Scholar]
- 4.Jordan VC, Allen KE, Evaluation of the antitumour activity of the nonsteroidal antioestrogen monohydroxytamoxifen in the DMBA-induced rat mammary carcinoma model. Eur J Cancer 16, 239–251 (1980). [DOI] [PubMed] [Google Scholar]
- 5.Jordan VC, Allen KE, Dix CJ, Pharmacology of tamoxifen in laboratory animals. Cancer Treat Rep 64, 745–759 (1980). [PubMed] [Google Scholar]
- 6.Jordan VC, Effect of tamoxifen (ICI 46,474) on initiation and growth of DMBA-induced rat mammary carcinomata. Eur J Cancer 12, 419–424 (1976). [DOI] [PubMed] [Google Scholar]
- 7.Tamoxifen for early breast cancer: an overview of the randomised trials. Early Breast Cancer Trialists’ Collaborative Group. Lancet 351, 1451–1467 (1998). [PubMed] [Google Scholar]
- 8.Gorski J, Toft D, Shyamala G, Smith D, Notides A, Hormone receptors: studies on the interaction of estrogen with the uterus. Recent Prog Horm Res 24, 45–80 (1968). [DOI] [PubMed] [Google Scholar]
- 9.Knabbe C et al. , Evidence that transforming growth factor-beta is a hormonally regulated negative growth factor in human breast cancer cells. Cell 48, 417–428 (1987). [DOI] [PubMed] [Google Scholar]
- 10.Robinson SP, Jordan VC, Antiestrogenic action of toremifene on hormone-dependent, -independent, and heterogeneous breast tumor growth in the athymic mouse. Cancer Res 49, 1758–1762 (1989). [PubMed] [Google Scholar]
- 11.Cormier EM, Jordan VC, Contrasting ability of antiestrogens to inhibit MCF-7 growth stimulated by estradiol or epidermal growth factor. Eur J Cancer Clin Oncol 25, 57–63 (1989). [DOI] [PubMed] [Google Scholar]
- 12.Cormier EM, Wolf MF, Jordan VC, Decrease in estradiol-stimulated progesterone receptor production in MCF-7 cells by epidermal growth factor and possible clinical implication for paracrine-regulated breast cancer growth. Cancer Res 49, 576–580 (1989). [PubMed] [Google Scholar]
- 13.Robinson SP, Jordan VC, The paracrine stimulation of MCF-7 cells by MDAMB-231 cells: possible role in antiestrogen failure. Eur J Cancer Clin Oncol 25, 493–497 (1989). [DOI] [PubMed] [Google Scholar]
- 14.Catherino WH, Jeng MH, Jordan VC, Norgestrel and gestodene stimulate breast cancer cell growth through an oestrogen receptor mediated mechanism. Br J Cancer 67, 945–952 (1993). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jeng MH, Jordan VC, Growth stimulation and differential regulation of transforming growth factor-beta 1 (TGF beta 1), TGF beta 2, and TGF beta 3 messenger RNA levels by norethindrone in MCF-7 human breast cancer cells. Mol Endocrinol 5, 1120–1128 (1991). [DOI] [PubMed] [Google Scholar]
- 16.Jeng MH, Langan-Fahey SM, Jordan VC, Estrogenic actions of RU486 in hormone-responsive MCF-7 human breast cancer cells. Endocrinology 132, 2622–2630 (1993). [DOI] [PubMed] [Google Scholar]
- 17.Jeng MH, Parker CJ, Jordan VC, Estrogenic potential of progestins in oral contraceptives to stimulate human breast cancer cell proliferation. Cancer Res 52, 6539–6546 (1992). [PubMed] [Google Scholar]
- 18.Gottardis MM, Jordan VC, Development of tamoxifen-stimulated growth of MCF-7 tumors in athymic mice after long-term antiestrogen administration. Cancer Res 48, 5183–5187 (1988). [PubMed] [Google Scholar]
- 19.Gottardis MM, Wagner RJ, Borden EC, Jordan VC, Differential ability of antiestrogens to stimulate breast cancer cell (MCF-7) growth in vivo and in vitro. Cancer Res 49, 4765–4769 (1989). [PubMed] [Google Scholar]
- 20.Gottardis MM, Jiang SY, Jeng MH, Jordan VC, Inhibition of tamoxifen-stimulated growth of an MCF-7 tumor variant in athymic mice by novel steroidal antiestrogens. Cancer Res 49, 4090–4093 (1989). [PubMed] [Google Scholar]
- 21.Osborne CK et al. , Double-blind, randomized trial comparing the efficacy and tolerability of fulvestrant versus anastrozole in postmenopausal women with advanced breast cancer progressing on prior endocrine therapy: results of a North American trial. J Clin Oncol 20, 3386–3395 (2002). [DOI] [PubMed] [Google Scholar]
- 22.Wolf DM, Jordan VC, A laboratory model to explain the survival advantage observed in patients taking adjuvant tamoxifen therapy. Recent Results Cancer Res 127, 23–33 (1993). [DOI] [PubMed] [Google Scholar]
- 23.Yao K et al. , Antitumor action of physiological estradiol on tamoxifen-stimulated breast tumors grown in athymic mice. Clin Cancer Res 6, 2028–2036 (2000). [PubMed] [Google Scholar]
- 24.Jordan VC, The new biology of estrogen-induced apoptosis applied to treat and prevent breast cancer. Endocr Relat Cancer 22, R1–31 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Abderrahman B, Jordan VC, The modulation of estrogen-induced apoptosis as an interpretation of the women’s health initiative trials. Expert Rev Endocrinol Metab 11, 81–86 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jordan VC, Linking estrogen-induced apoptosis with decreases in mortality following long-term adjuvant tamoxifen therapy. J Natl Cancer Inst 106, (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Murphy CS, Meisner LF, Wu SQ, Jordan VC, Short- and long-term estrogen deprivation of T47D human breast cancer cells in culture. Eur J Cancer Clin Oncol 25, 1777–1788 (1989). [DOI] [PubMed] [Google Scholar]
- 28.Welshons WV, Jordan VC, Adaptation of estrogen-dependent MCF-7 cells to low estrogen (phenol red-free) culture. Eur J Cancer Clin Oncol 23, 1935–1939 (1987). [DOI] [PubMed] [Google Scholar]
- 29.Pink JJ, Jordan VC, Models of estrogen receptor regulation by estrogens and antiestrogens in breast cancer cell lines. Cancer Res 56, 2321–2330 (1996). [PubMed] [Google Scholar]
- 30.Murphy CS, Pink JJ, Jordan VC, Characterization of a receptor-negative, hormone-nonresponsive clone derived from a T47D human breast cancer cell line kept under estrogen-free conditions. Cancer Res 50, 7285–7292 (1990). [PubMed] [Google Scholar]
- 31.Pink JJ, Bilimoria MM, Assikis J, Jordan VC, Irreversible loss of the oestrogen receptor in T47D breast cancer cells following prolonged oestrogen deprivation. Br J Cancer 74, 1227–1236 (1996). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jiang SY, Wolf DM, Yingling JM, Chang C, Jordan VC, An estrogen receptor positive MCF-7 clone that is resistant to antiestrogens and estradiol. Cell Endocrinol 90, 77–86 (1992). [DOI] [PubMed] [Google Scholar]
- 33.Lewis JS, Osipo C, Meeke K, Jordan VC, Estrogen-induced apoptosis in a breast cancer model resistant to long-term estrogen withdrawal. J Steroid Biochem Mol Biol 94, 131–141 (2005). [DOI] [PubMed] [Google Scholar]
- 34.Ariazi EA et al. , Estrogen induces apoptosis in estrogen deprivation-resistant breast cancer through stress responses as identified by global gene expression across time. Proc Natl Acad Sci U S A 108, 18879–18886 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ellis MJ et al. , Lower-dose vs high-dose oral estradiol therapy of hormone receptor-positive, aromatase inhibitor-resistant advanced breast cancer: a phase 2 randomized study. JAMA 302, 774–780 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Allen KE, Clark ER, Jordan VC, Evidence for the metabolic activation of non-steroidal antioestrogens: a study of structure-activity relationships. Br J Pharmacol 71, 83–91 (1980). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jordan VC, Collins MM, Rowsby L, Prestwich G, A monohydroxylated metabolite of tamoxifen with potent antioestrogenic activity. J Endocrinol 75, 305–316 (1977). [DOI] [PubMed] [Google Scholar]
- 38.Jordan VC, Antiestrogens and selective estrogen receptor modulators as multifunctional medicines. 1. Receptor interactions. J Med Chem 46, 883–908 (2003). [DOI] [PubMed] [Google Scholar]
- 39.Jordan VC, Antiestrogens and selective estrogen receptor modulators as multifunctional medicines. 2. Clinical considerations and new agents. J Med Chem 46, 1081–1111 (2003). [DOI] [PubMed] [Google Scholar]
- 40.Jordan VC, Bowser-Finn RA, Binding of [3H]monohydroxytamoxifen by immature rat tissues in vivo. Endocrinology 110, 1281–1291 (1982). [DOI] [PubMed] [Google Scholar]
- 41.Tate AC, DeSombre ER, Greene GL, Jensen EV, Jordan VC, Interaction of [3H] estradiol - and [3H] monohydroxytamoxifen-estrogen receptor complexes with a monoclonal antibody. Breast Cancer Res Treat 3, 267–277 (1983). [DOI] [PubMed] [Google Scholar]
- 42.Tate AC, Greene GL, DeSombre ER, Jensen EV, Jordan VC, Differences between estrogen- and antiestrogen-estrogen receptor complexes from human breast tumors identified with an antibody raised against the estrogen receptor. Cancer Res 44, 1012–1018 (1984). [PubMed] [Google Scholar]
- 43.Tate AC, Jordan VC, Nuclear [3H]4-hydroxytamoxifen (4-OHTAM)- and [3H]estradiol (E2)-estrogen receptor complexes in the MCF-7 breast cancer and GH3 pituitary tumor cell lines. Mol Cell Endocrinol 36, 211–219 (1984). [DOI] [PubMed] [Google Scholar]
- 44.Tate AC, Lieberman ME, Jordan VC, The inhibition of prolactin synthesis in GH3 rat pituitary tumor cells by monohydroxytamoxifen is associated with changes in the properties of the estrogen receptor. J Steroid Biochem 20, 391–395 (1984). [DOI] [PubMed] [Google Scholar]
- 45.Lieberman ME, Maurer RA, Gorski J, Estrogen control of prolactin synthesis in vitro. Proc Natl Acad Sci U S A 75, 5946–5949 (1978). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jordan VC, Koch R, Langan S, McCague R, Ligand interaction at the estrogen receptor to program antiestrogen action: a study with nonsteroidal compounds in vitro. Endocrinology 122, 1449–1454 (1988). [DOI] [PubMed] [Google Scholar]
- 47.Jordan VC, Koch R, Mittal S, Schneider MR, Oestrogenic and antioestrogenic actions in a series of triphenylbut-1-enes: modulation of prolactin synthesis in vitro. Br J Pharmacol 87, 217–223 (1986). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jordan VC, Lieberman ME, Estrogen-stimulated prolactin synthesis in vitro. Classification of agonist, partial agonist, and antagonist actions based on structure. Mol Pharmacol 26, 279–285 (1984). [PubMed] [Google Scholar]
- 49.Jordan VC et al. , Structural requirements for the pharmacological activity of nonsteroidal antiestrogens in vitro. Mol Pharmacol 26, 272–278 (1984). [PubMed] [Google Scholar]
- 50.Lieberman ME, Jordan VC, Fritsch M, Santos MA, Gorski J, Direct and reversible inhibition of estradiol-stimulated prolactin synthesis by antiestrogens in vitro. J Biol Chem 258, 4734–4740 (1983). [PubMed] [Google Scholar]
- 51.Murphy CS, Jordan VC, Structural components necessary for the antiestrogenic activity of tamoxifen. J Steroid Biochem 34, 407–411 (1989). [DOI] [PubMed] [Google Scholar]
- 52.Murphy CS, Langan-Fahey SM, McCague R, Jordan VC, Structure-function relationships of hydroxylated metabolites of tamoxifen that control the proliferation of estrogen-responsive T47D breast cancer cells in vitro. Mol Pharmacol 38, 737–743 (1990). [PubMed] [Google Scholar]
- 53.Murphy CS, Parker CJ, McCague R, Jordan VC, Structure-activity relationships of nonisomerizable derivatives of tamoxifen: importance of hydroxyl group and side chain positioning for biological activity. Mol Pharmacol 39, 421–428 (1991). [PubMed] [Google Scholar]
- 54.Jordan VC, Laboratory models of breast cancer to aid the elucidation of antiestrogen action. J Lab Clin Med 109, 267–277 (1987). [PubMed] [Google Scholar]
- 55.Lieberman ME, Gorski J, Jordan VC, An estrogen receptor model to describe the regulation of prolactin synthesis by antiestrogens in vitro. J Biol Chem 258, 4741–4745 (1983). [PubMed] [Google Scholar]
- 56.Wolf DM, Jordan VC, Characterization of tamoxifen stimulated MCF-7 tumor variants grown in athymic mice. Breast Cancer Res Treat 31, 117–127 (1994). [DOI] [PubMed] [Google Scholar]
- 57.Wolf DM, Jordan VC, The estrogen receptor from a tamoxifen stimulated MCF-7 tumor variant contains a point mutation in the ligand binding domain. Breast Cancer Res Treat 31, 129–138 (1994). [DOI] [PubMed] [Google Scholar]
- 58.Catherino WH, Wolf DM, Jordan VC, A naturally occurring estrogen receptor mutation results in increased estrogenicity of a tamoxifen analog. Mol Endocrinol 9, 1053–1063 (1995). [DOI] [PubMed] [Google Scholar]
- 59.Jiang SY, Jordan VC, Growth regulation of estrogen receptor-negative breast cancer cells transfected with complementary DNAs for estrogen receptor. J Natl Cancer Inst 84, 580–591 (1992). [DOI] [PubMed] [Google Scholar]
- 60.Levenson AS, Catherino WH, Jordan VC, Estrogenic activity is increased for an antiestrogen by a natural mutation of the estrogen receptor. J Steroid Biochem Mol Biol 60, 261–268 (1997). [DOI] [PubMed] [Google Scholar]
- 61.Levenson AS, Jordan VC, The key to the antiestrogenic mechanism of raloxifene is amino acid 351 (aspartate) in the estrogen receptor. Cancer Res 58, 1872–1875 (1998). [PubMed] [Google Scholar]
- 62.Brzozowski AM et al. , Molecular basis of agonism and antagonism in the oestrogen receptor. Nature 389, 753–758 (1997). [DOI] [PubMed] [Google Scholar]
- 63.Shiau AK et al. , The structural basis of estrogen receptor/coactivator recognition and the antagonism of this interaction by tamoxifen. Cell 95, 927–937 (1998). [DOI] [PubMed] [Google Scholar]
- 64.Fanning SW et al. , Estrogen receptor alpha somatic mutations Y537S and D538G confer breast cancer endocrine resistance by stabilizing the activating function-2 binding conformation. Elife 5, (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Brown RR, Bain R, Jordan VC, Determination of tamoxifen and metabolites in human serum by high-performance liquid chromatography with post-column fluorescence activation. J Chromatogr 272, 351–358 (1983). [DOI] [PubMed] [Google Scholar]
- 66.Jordan VC, Bain RR, Brown RR, Gosden B, Santos MA, Determination and pharmacology of a new hydroxylated metabolite of tamoxifen observed in patient sera during therapy for advanced breast cancer. Cancer Res 43, 1446–1450 (1983). [PubMed] [Google Scholar]
- 67.Jordan VC, Fritz NF, Langan-Fahey S, Thompson M, Tormey DC, Alteration of endocrine parameters in premenopausal women with breast cancer during long-term adjuvant therapy with tamoxifen as the single agent. J Natl Cancer Inst 83, 1488–1491 (1991). [DOI] [PubMed] [Google Scholar]
- 68.Ravdin PM, Fritz NF, Tormey DC, Jordan VC, Endocrine status of premenopausal node-positive breast cancer patients following adjuvant chemotherapy and long-term tamoxifen. Cancer Res 48, 1026–1029 (1988). [PubMed] [Google Scholar]
- 69.Tormey DC, Jordan VC, Long-term tamoxifen adjuvant therapy in node-positive breast cancer: a metabolic and pilot clinical study. Breast Cancer Res Treat 4, 297–302 (1984). [DOI] [PubMed] [Google Scholar]
- 70.Langan-Fahey SM, Tormey DC, Jordan VC, Tamoxifen metabolites in patients on long-term adjuvant therapy for breast cancer. Eur J Cancer 26, 883–888 (1990). [DOI] [PubMed] [Google Scholar]
- 71.Bain RR, Jordan VC, Identification of a new metabolite of tamoxifen in patient serum during breast cancer therapy. Biochem Pharmacol 32, 373–375 (1983). [DOI] [PubMed] [Google Scholar]
- 72.Archer DF et al. , Efficacy and safety of ospemifene in postmenopausal women with moderate-to-severe vaginal dryness: a phase 3, randomized, double-blind, placebo-controlled, multicenter trial. Menopause, (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wolf DM, Langan-Fahey SM, Parker CJ, McCague R, Jordan VC, Investigation of the mechanism of tamoxifen-stimulated breast tumor growth with nonisomerizable analogues of tamoxifen and metabolites. J Natl Cancer Inst 85, 806–812 (1993). [DOI] [PubMed] [Google Scholar]
- 74.Robinson SP, Langan-Fahey SM, Johnson DA, Jordan VC, Metabolites, pharmacodynamics, and pharmacokinetics of tamoxifen in rats and mice compared to the breast cancer patient. Drug Metab Dispos 19, 36–43 (1991). [PubMed] [Google Scholar]
- 75.Robinson SP, Langan-Fahey SM, Jordan VC, Implications of tamoxifen metabolism in the athymic mouse for the study of antitumor effects upon human breast cancer xenografts. Eur J Cancer Clin Oncol 25, 1769–1776 (1989). [DOI] [PubMed] [Google Scholar]
- 76.Jordan VC, Robinson SP, Species-specific pharmacology of antiestrogens: role of metabolism. Fed Proc 46, 1870–1874 (1987). [PubMed] [Google Scholar]
- 77.Jordan VC, Phelps E, Lindgren JU, Effects of anti-estrogens on bone in castrated and intact female rats. Breast Cancer Res Treat 10, 31–35 (1987). [DOI] [PubMed] [Google Scholar]
- 78.Gottardis MM, Jordan VC, Antitumor actions of keoxifene and tamoxifen in the N-nitrosomethylurea-induced rat mammary carcinoma model. Cancer Res 47, 4020–4024 (1987). [PubMed] [Google Scholar]
- 79.Turner RT, Wakley GK, Hannon KS, Bell NH, Tamoxifen prevents the skeletal effects of ovarian hormone deficiency in rats. J Bone Miner Res 2, 449–456 (1987). [DOI] [PubMed] [Google Scholar]
- 80.Black LJ et al. , Raloxifene (LY139481 HCI) prevents bone loss and reduces serum cholesterol without causing uterine hypertrophy in ovariectomized rats. J Clin Invest 93, 63–69 (1994). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Gottardis MM, Robinson SP, Satyaswaroop PG, Jordan VC, Contrasting actions of tamoxifen on endometrial and breast tumor growth in the athymic mouse. Cancer Res 48, 812–815 (1988). [PubMed] [Google Scholar]
- 82.Jordan VC, Tamoxifen and endometrial cancer. Lancet 2, 1019 (1988). [DOI] [PubMed] [Google Scholar]
- 83.Jordan VC, Tamoxifen and endometrial cancer. Lancet 1, 733–734 (1989). [DOI] [PubMed] [Google Scholar]
- 84.Fornander T et al. , Adjuvant tamoxifen in early breast cancer: occurrence of new primary cancers. Lancet 1, 117–120 (1989). [DOI] [PubMed] [Google Scholar]
- 85.Lerner LJ, Jordan VC, Development of antiestrogens and their use in breast cancer: eighth Cain memorial award lecture. Cancer Res 50, 4177–4189 (1990). [PubMed] [Google Scholar]
- 86.Love RR et al. , Effects of tamoxifen on bone mineral density in postmenopausal women with breast cancer. N Engl J Med 326, 852–856 (1992). [DOI] [PubMed] [Google Scholar]
- 87.Love RR et al. , Effects of tamoxifen therapy on lipid and lipoprotein levels in postmenopausal patients with node-negative breast cancer. J Natl Cancer Inst 82, 1327–1332 (1990). [DOI] [PubMed] [Google Scholar]
- 88.Love RR et al. , Effects of tamoxifen on cardiovascular risk factors in postmenopausal women. Ann Intern Med 115, 860–864 (1991). [DOI] [PubMed] [Google Scholar]
- 89.Jordan VC, Morrow M, Should clinicians be concerned about the carcinogenic potential of tamoxifen? Eur J Cancer 30A, 1714–1721 (1994). [DOI] [PubMed] [Google Scholar]
- 90.Dragan VP, Vaughan J, Jordan VC, Pitot HC, Comparison of the effects of tamoxifen and toremifene on liver and kidney tumor promotion in female rats. Carcinogenesis 16, 2733–2741 (1995). [DOI] [PubMed] [Google Scholar]
- 91.Dragan YP et al. , The effect of tamoxifen and two of its non-isomerizable fixed-ring analogs on multistage rat hepatocarcinogenesis. Carcinogenesis 17, 585–594 (1996). [DOI] [PubMed] [Google Scholar]
- 92.Dragan YP et al. , Studies of tamoxifen as a promoter of hepatocarcinogenesis in female Fischer F344 rats. Breast Cancer Res Treat 31, 11–25 (1994). [DOI] [PubMed] [Google Scholar]
- 93.Nuwaysir EF, Dragan YP, Jefcoate CR, Jordan VC, Pitot HC, Effects of tamoxifen administration on the expression of xenobiotic metabolizing enzymes in rat liver. Cancer Res 55, 1780–1786 (1995). [PubMed] [Google Scholar]
- 94.Cummings SR et al. , The effect of raloxifene on risk of breast cancer in postmenopausal women: results from the MORE randomized trial. Multiple Outcomes of Raloxifene Evaluation. JAMA 281, 2189–2197 (1999). [DOI] [PubMed] [Google Scholar]
- 95.Vogel VG et al. , Effects of tamoxifen vs raloxifene on the risk of developing invasive breast cancer and other disease outcomes: the NSABP Study of Tamoxifen and Raloxifene (STAR) P-2 trial. JAMA 295, 2727–2741 (2006). [DOI] [PubMed] [Google Scholar]
- 96.Vogel VG et al. , Update of the National Surgical Adjuvant Breast and Bowel Project Study of Tamoxifen and Raloxifene (STAR) P-2 Trial: Preventing breast cancer. Cancer Prev Res (Phila) 3, 696–706 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Robinson SP, Mauel DA, Jordan VC, Antitumor actions of toremifene in the 7,12-dimethylbenzanthracene (DMBA)-induced rat mammary tumor model. Eur J Cancer Clin Oncol 24, 1817–1821 (1988). [DOI] [PubMed] [Google Scholar]
- 98.Robinson SP, Parker CJ, Jordan VC, Preclinical studies with toremifene as an antitumor agent. Breast Cancer Res Treat 16 Suppl, S9–17 (1990). [DOI] [PubMed] [Google Scholar]
- 99.Robinson SP, Koch R, Jordan VC, In vitro estrogenic actions in rat and human cells of hydroxylated derivatives of D16726 (zindoxifene), an agent with known antimammary cancer activity in vivo. Cancer Res 48, 784–787 (1988). [PubMed] [Google Scholar]
- 100.Cummings SR et al. , Lasofoxifene in postmenopausal women with osteoporosis. N Engl J Med 362, 686–696 (2010). [DOI] [PubMed] [Google Scholar]