Abstract
Tendon injuries are common and can dramatically impair patient mobility and productivity, resulting in a significant socioeconomic burden and reduced quality of life. Because the tendon healing process results in the formation of a fibrotic scar, injured tendons never regain the mechanical strength of the uninjured tendon, leading to frequent re-injury. Many tendons are also prone to the development of peritendinous adhesions and excess scar formation, which further reduce tendon function and lead to chronic complications. Despite this, there are currently no treatments that adequately improve the tendon healing process due in part to a lack of information regarding the contributions of various cell types to tendon healing and how their activity may be modulated for therapeutic value. In this review, we summarize recent efforts to identify and characterize the distinct cell populations involved at each stage of tendon healing. In addition, we examine the mechanisms through which different cell populations contribute to the fibrotic response to tendon injury, and how these responses can be affected by systemic factors and co-morbidities. We then discuss gaps in our current understanding of tendon fibrosis and highlight how new technologies and research areas are shedding light on this clinically important and intractable challenge. A better understanding of the complex cellular environment during tendon healing is crucial to the development of new therapies to prevent fibrosis and promote tissue regeneration.
Introduction
Tendon injuries are among the most common orthopedic conditions, and unsatisfactory healing has an enormous impact on the quality of life of affected patients. In 2013, musculoskeletal diagnoses accounted for 19.2% of all medical diagnoses, resulting in over nine million procedures involving tendons or joint structures performed in the United States (1). Because tendons are critical for normal mobility, these injuries can dramatically affect patient productivity; in 2017, major tears or traumatic injuries to tendon, ligaments, and muscles alone affected nearly half a million full time employees (2). Injuries requiring surgical intervention are particularly common in the Achilles tendon (18 per 100,000 person-years) (3), rotator cuff (131 per 100,000 person-years) (4), and flexor tendons of the hand (33.2 per 100,000 person-years) (5, 6). Recovery from tendon injuries is slow and often requires extensive rehabilitation, which results in a median 50 lost work days per injury (2). Combined, the number of tendon injuries, the cost of associated surgeries and rehabilitation, and the subsequent loss of productivity have a significant socioeconomic impact that is expected to increase in coming years.
In addition to the high frequency of tendon injuries, the burden is further compounded by the fibrotic nature of healing to which tendons are prone. Rather than regeneration of the native tendon structure following injury, tendons heal with deposition of excessive, disorganized extracellular matrix (ECM). The reason for this insufficient healing response is often attributed to the tendon’s relatively low cellularity and vascularity compared to other tissues (7, 8). The resulting scar provides some level of tissue stability; however, it lacks the mechanical integrity of the original tissue, and therefore the risk of re-injury is high (9). Moreover, fibrotic changes to the tendon ECM following injury are thought to be one factor in the subsequent development of chronic, degenerative tendinopathies (10-12).
Despite the rate at which tendon injuries occur, and the substantial associated morbidity, there are no consensus treatments to restore native function to the damaged tissue. While fibrotic healing is largely conserved among all tendons, healing in certain tendons is further complicated due to complex anatomical constraints. For example, satisfactory healing of intrasynovial tendons, such as the flexor tendons of the hand, is particularly challenging due to the formation of fibrous adhesions between the tendon, sheath and surrounding tissues which can further limit mobility (13). Though advances in surgical and rehabilitation protocols have greatly improved outcomes, 30 – 40% of patients still develop function-limiting adhesions following primary flexor tendon repair (14, 15). This high complication rate strongly suggests a need for biological, pharmacological or tissue-engineering augmentation of the healing process to modulate fibrosis. Thus, the basis of recent efforts have been two-fold: 1) promoting true regeneration of tendon tissue rather than the normal fibrotic scar formation and 2) limiting the development of peritendinous adhesions in patients and injury types in which they are likely to occur.
A more thorough understanding of the basic mechanisms that drive tendon healing is crucial to the development of treatments that improve the normal healing response and prevent peritendinous fibrosis. To date, various animal models of tendon injury, surgical repair, and rehabilitation have been instrumental in furthering our knowledge of the tendon healing process (reviewed extensively in (16), (17), and (18)). Canine models been used to demonstrate that early, active mobilization following repair reduced the formation of peritendinous adhesions and improved the ultimate strength of injured compared to immobilization following flexor tendon injury (19, 20). The canine flexor tendon repair model has also been used to identify improved suture techniques (21, 22) and evaluate various biologics for tendon repair (23, 24). Rodent models, most notably mouse and rat, have been used to study the healing process in various tendons following injury both with and without surgical repair. These models are attractive as they allow investigators to evaluate the response to a variety of injury types including full (25, 26) and partial transection (27, 28), window defect (29, 30), and collagenase injection (31). Rodent models have also shown the negative effects of mechanical unloading following injury (32, 33). More recently, mouse models in particular have emerged as powerful tools due to the availability of genetic tools that allow the labeling and tracing of specific cell populations during tendon injury and repair (25, 27, 34), as well as the ability to perform loss or gain of function experiments in vivo.
Though our knowledge of tissue-level tendon biology has expanded greatly in recent years, there is still a paucity of information regarding the contributions of various cell types to tendon healing which greatly impairs the design of new, targeted therapeutics to treat fibrotic tendon healing and promote improved tissue regeneration. In this review, we describe recent efforts to identify and characterize the individual tendon cell populations involved at each stage of the tendon healing process and highlight potential roles for these cells in development of pathologic scar formation. We then discuss how modulation of cellular functions and alterations in the cellular milieu, due to systemic factors such as diabetes and aging, may alter the fibrotic response. Importantly, the terms ‘tendon’ and ‘ligament’ are often used interchangeably due to some similarities in structure and function. Given that there is certainly some overlap between the cellular response to injury between these tissues, our understanding of the tendon healing process may be informed by ligament studies. Work by Chamberlain et al., for example demonstrates a similar inflammatory cell profile following acute injury in ligament as described below for tendon (35, 36). However, in the interest of clarity and accuracy, we have focused this review specifically on the tendon. Finally, we outline gaps in our current understanding of tendon fibrosis by drawing on what is known about fibrotic processes in other tissues and discuss important areas for future research.
Tendon structure and resident cell niches
Tendons are responsible for transmitting the forces generated by muscle contraction to bone, facilitating movement. In doing so, the tendon must withstand immense loads. Though the exact composition of the tissue varies depending on the particular tendon, anatomic location, and species (37), all tendons are characterized by a highly organized, anisotropic ECM composed predominantly of collagen type I with smaller amounts of collagen type III (38, 39). Individual collagen fibrils are bundled together to form fascicles (40, 41). In humans and other large animals, groups of fascicles are further bound together by a thin layer of connective tissue called the endotenon (39, 42). The endotenon permits independent gliding between fascicles and provides a path for blood vessels and nerves to travel deep into the tendon parenchyma, supplying nutrition and innervation to the tissue (43). Finally, groups of fascicles are bound by the epitenon, a fibrous layer that is contiguous with the endotenon and serves to bind the fascicles together into a single encapsulated tendon (39). In contrast, mouse tendons lack a defined endotenon and are instead comprised of individual fascicles surrounded by a single-cell thick epitenon/basement membrane (44). Some tendons, like the flexor tendons of the hand, are further encased in a synovial sheath that provides lubrication, allowing tendons to slide more easily between tight anatomical gaps or around bony prominences that would otherwise produce damaging friction (39, 45). Others, like the Achilles tendon, are not surrounded by a true tendon sheath and are instead covered by a sheet of loose connective tissue referred to as the paratenon (39). The tendon sheath and paratenon serve analogous functions in protecting the tendon from surrounding tissues as it moves and in providing a passage for blood vessels and nerves that supply and innervate the tissue (46, 47).
In addition to providing mechanical strength to tendons, the organized structure of the matrix provides a number of unique niches for resident tendon cell populations. The predominant cell type in tendons are the tenocytes (also called tendon fibroblasts). Tenocytes are located between the collagen fibrils, with elongated nuclei oriented along the axis of tension (39). Traditionally, tenocytes have been treated as a homogenous population that express common tendon marker genes including the transcription factor scleraxis (Scx), the transmembrane glycoprotein tenomodulin (Tnmd), and the tendon matrix protein collagen type I (Col1) (48, 49). In recent years however, due to the use of various reporter mouse strains, evidence has begun to emerge suggesting that there are a number of distinct resident cell populations located both within the tendon parenchyma and along the periphery of the tissue whose contributions to homeostasis and repair are only beginning to be fully appreciated.
Tendon healing
Like many other tissues, tendon healing follows a typical wound healing response that occurs in three broad, overlapping phases: a short inflammatory phase (lasting days), a fibroblastic/proliferative phase (weeks), and a prolonged remodeling phase (months to years) (50). The exact duration and timing of each phase is highly dependent on the organism, tendon location, injury type, treatment regimen, and the presence of comorbidities (51-53). Furthermore, tendon healing is complex and occurs through the contributions of both intrinsic and extrinsic cell populations (54). Intrinsic healing is driven by cells derived from the tendon parenchyma and epitenon/endotenon (55), whereas extrinsic healing is attributed to cells recruited into the injury area from outside the tendon itself (e.g. circulating cells, cells from neighboring tissues including the paratenon and sheath, etc.) (56, 57). Both sources of cells are important, however conditions that promote increased contribution from extrinsic cell populations are thought to result in greater scar tissue and adhesion formation compared to those that promote intrinsic healing (58). The importance of understanding the activity of intrinsic vs. extrinsic cells in tendon healing is highlighted by studies of tendon healing during embryonic development. The difference between the two healing paradigms is thought to be due to decreased contribution from extrinsic sources (inflammatory cells, paratenon/sheath cells, bone marrow-derived cells, etc.) and increased activity from intrinsic tendon cells in the embryo. While the adult tendon heals by the formation of a mechanically inferior, fibrous scar, injured fetal tendons heal by scar-less regeneration (59). Importantly, conservation of scar-less, more regenerative healing is conserved during the early neonatal period (26, 59, 60).
While the overall progression of tendon healing is relatively well understood, less is known about the origin and function of cells involved at each stage. What is known of the identity and behavior of these specific cell populations during each phase of tendon healing and their potential contributions to pathologic fibrosis are discussed in further detail below.
Inflammatory phase
Immediately following acute injury, platelet-derived cytokine signaling results in increased vascular permeability and the recruitment of circulating inflammatory cells to the injury site. Phagocytic neutrophils appear within minutes of the initial injury, followed shortly by the arrival monocytes/macrophages (61, 62). When neutrophils were depleted in vivo using a Ly6C/Ly6G antibody in a model of mouse Achilles tendon repair, there was a significant increase in the number of F4/80+ macrophages, suggesting that neutrophils help regulate macrophage recruitment (63). The concentration of neutrophils rapidly declines after 24 hours and macrophages become the predominant cell population at the injury site (61).
Macrophages themselves are a dynamic cell population that can reversibly polarize into several subtypes depending on the particular cytokine, matrix, and cellular environment to which they are exposed (64, 65). As such, macrophages participate in nearly every aspect of the normal healing response, though it is also well established that aberrant macrophage activity is a main driver of fibrosis in a variety of tissues (66-68). In tendon, macrophage depletion with liposomal clodronate resulted in decreased scar tissue, which was attributed to decreased production of the pro-fibrotic cytokine transforming growth factor (TGF)-β (69, 70). Additionally, several studies have reported that a decrease in macrophage content resulted in lower levels of cell proliferation, reducing the overall numbers of matrix producing fibroblasts (63, 69). Macrophages are often divided into two polarization groups: classically activated (M1) or alternatively activated (M2). M1 macrophages are traditionally associated with a pro-inflammatory response, while M2 macrophages are generally considered to be anti-inflammatory and to contribute to ECM deposition and remodeling (71). Interestingly, more recent studies have indicated that M2 macrophages can also be pro-fibrotic. For instance, increased prevalence of M2 macrophages is associated with the development of lung fibrosis (72), and decreasing the accumulation of M2 macrophages has been shown to reduce fibrosis in a mouse model of idiopathic lung fibrosis (73). Despite these important and often opposing roles for macrophages in wound healing, little is known about macrophage polarization in tendon following injury. Lin et al. reported that an elevated M1:M2 ratio correlated with an increase in aberrant ECM deposition following injury, implicating M1 macrophages as a driver of fibrosis in tendon healing(74). However, as in other tissues, M2 macrophages can also be pro-fibrotic in tendon (75, 76). In addition to circulating macrophages recruited following injury, most tissues contain subpopulations of tissue resident macrophages (77). There is conflicting evidence regarding the presence of macrophages in normal tendon; Dakin et al. reported no macrophages present in normal equine flexor tendon (76)whereas Sugg et al. found both M1 and M2 macrophages present in the endotenon, but not the tendon parenchyma, of uninjured rat Achilles tendons (78). In any case, there is no definitive characterization of these macrophages as tissue resident macrophages and therefore it is impossible to determine whether they are in fact bona fide tendon resident macrophages and not circulating blood monocytes. Given the important roles of resident macrophage populations in the homeostasis and repair of other tissues, it is reasonable to assume that they may be present and perform similar functions in tendon. Future studies to clarify the origin of the macrophage populations present during tendon repair, and the roles of discrete populations in the development of fibrosis, are clearly needed.
In addition to neutrophils and macrophages, other inflammatory cell types have been shown to localize to the tendon during healing. T helper cells are found in the tendon 3 days following injury and are thought to drive fibronectin production by migrating fibroblasts (79). Mast cells have also been detected following tendon injury, but their functions in the healing process have not been evaluated (80). Studies that have evaluated the cells present during the inflammatory phase are summarized in Table 1.
Table 1:
Inflammatory cells present during tendon healing
| Cell Type | Marker (detection method) | Time points evaluated post-injury |
Model | Reference |
|---|---|---|---|---|
| Neutrophils | Anti-neutrophil (IHC/IF) | 1*, 3, 7, 14, 28 days | AT collagenase injection (Rat) | (61) |
| Myeloperoxidase assay | 1*, 3, 7 days | AT transection and repair (Mouse) | (63) | |
| Ly6G (IHC/IF) | 1*, 3*, 5, 7, 10, 14, 21, 28, 56, 84, 112 days | FT partial laceration (Mouse) | (62) | |
| Macrophages | F4/80 (IHC/IF) | 1, 3, 7* days | AT transection and repair (Mouse) | (63) |
| F4/80 (IHC/IF) | 1, 3, 5, 7, 10, 14, 21*, 28, 56, 84, 112 days | FT partial laceration (Mouse) | (62) | |
| F4/80 (qPCR) | 3, 7, 10, 14, 21*, 28 days | FT transection and repair (Mouse) | (75) | |
| F4/80 (IHC/IF) | 3*, 7*, 14 days | AT transection and repair (Mouse) | (69) | |
| CD14 (IHC/IF) | 3*, 7, 14 days | FT crush injury (Rat) | (79) | |
| M1 Macrophages | CD68/ED1 (IHC/IF) | 1*, 3, 7, 14, 28 days | AT collagenase injection (Rat) | (61) |
| CD86 qPCR (IHC/IF) | 3, 7, 10, 14, 21*, 28 days | FT transection and repair (Mouse) | (75) | |
| M2 Macrophages | CD163/ED2 (IHC/IF) | 1, 3, 7, 14, 28* days | AT collagenase injection (Rat) | (61) |
| Arginase1 (qPCR) | 3*, 7*, 10, 14, 21, 28 days | FT transection and repair (Mouse) | (75) | |
| CD206 (qPCR) | 3, 7, 10, 14, 21*, 28 days | FT transection and repair (Mouse) | (75) | |
| T-cells | CD2/CD4/CD8 (IHC/IF) | 3*, 7, 14 days | FT crush injury (Rat) | (79) |
| Mast Cells | Tryptase (IHC/IF) | 3, 6, 21*, and 42 days | FT transection and repair (Rabbit) | (80) |
Timepoint at which the number of the indicated cell type peaked. IHC/IF= Immunohistochemistry/Immunofluorescence, qPCR= quantitative polymerase chain reaction, AT= Achilles tendon, FT= flexor tendon.
It is worth noting that a transient inflammatory phase is required for proper tendon healing to occur. Administration of common non-steroidal anti-inflammatory drugs (NSAIDs) immediately following patellar tendon or rotator cuff injury and repair has been shown to decrease tendon mechanical properties in rat models, indicating that decreasing early inflammation can significantly alter the quality of the subsequent healing response (81-83). In vitro studies have further demonstrated that exposing tenocytes to NSAIDs decreases both their proliferation rate and the production of matrix proteins (84, 85). Several recent reports have found that applying adipose-derived mesenchymal stem cell sheets combined with various protenogenic growth factors including connective tissue growth factor (CTGF) and bone morphogenic protein-12 (BMP-12) to flexor tendon repairs increased the presence of M2 macrophages and decreased the expression of pro-fibrotic genes in a canine model (24, 86, 87). Together, these studies suggest the development of therapies that can modulate the inflammatory phase to promote regenerative healing are preferable to those that result in complete inflammatory blockade.
Fibroblastic/proliferative phase
The architects of the fibroblastic/proliferative phase are fibroblasts derived from both intrinsic and extrinsic sources (88). During this second stage of tendon healing, various fibroblast populations are recruited to the injury site and begin to create a provisional matrix (granulation tissue) composed mainly of collagen type III, which restores partial tissue continuity and provides a scaffold for the migration of subsequently recruited cells into the wound area. Very little is known about the identity, sequence of arrival, and ultimate contributions of the individual fibroblast subsets present during this phase of tendon healing. Initially, cells recruited from the bone marrow are present in the scar tissue during tendon healing (57, 89). Chemotactic signaling from a number of important growth factors, including TGF-β family members (28), platelet-derived growth factor BB (PDGF-BB) (90, 91), insulin-like growth factor-1 (IGF-1) (92), and basic fibroblast growth factor (bFGF) (93) are thought to stimulate the proliferation and migration of various fibroblast populations, with the majority of the early healing response appearing to originate from cells derived from the epitenon and not from the tendon body itself. Shortly following injury, epitenon cells adjacent to the injury site begin to proliferate and form a thickened epitenon layer in both canine and murine models (29, 94, 95). A similar proliferative response is seen in the endotenon in an equine collagenase-induced flexor tendon injury model, however the identity of these cells and how they contribute to the healing response has yet to be determined (92). During both murine flexor and patellar tendon healing, cells derived from the epitenon/paratenon appear to migrate into the injury area (29, 95). Taylor et al. demonstrated that the murine tendon epitenon is positive for the basal lamina-related protein laminin during homeostasis (44), and Gelberman et al. found that epitenon cells express elevated levels of fibronectin compared to the tendon body shortly following injury (94). Interestingly, both fibronectin and laminin staining are also found in the granulation tissue of healing tendons, indicating that these outer cells are in fact involved in the early healing process (26, 44, 96). In vitro, epitenon-derived cells have been shown to proliferate faster, migrate quicker, display increased expression of progenitor cell markers, and differentiate more readily into myofibroblasts compared to cells from within the tendon parenchyma, with some groups suggesting that some or all of these peripheral cells may represent a tendon stem/progenitor cell (TSPC) population (97-99). Besides the epitenon, potential TSPC niches have also been reported within the tendon fascicles (100, 101) and surrounding the tendon vascular (102, 103). In apparent contrast to the positive attributes normally associated with stem cells, the presence of putative TSPCs during healing has been reported to contribute to scar formation, suggesting that the activity of these cells may in fact have a negative effect on tendon healing (102).
Less is known about how the fibroblastic cells located within the tendon body respond to injury, or their potential contribution to the development of pathologic fibrosis. In an equine collagenase-induced model of tendon injury, tenocytes located within the collagen fibrils adjacent to the injury site exhibited increased expression of collagen types I and III, IGF-1, and TGF-β compared to normal tendon, indicating that they are responsive to changes in their local environment, though their response is delayed compared to cells from the endo- or epitenon (92). Whether tenocyte activity is stimulated due to alterations in the mechanical environment following injury or through paracrine signaling mechanisms is unknown.
Tracing the activity of Scx-expressing cells in mouse models has provided perhaps the best insight to the fate of tenocytes following injury, though the process is still not well understood. Scx is expressed by the majority of adult tenocytes in the tendon body, but is not expressed by epitenon or paratenon cells, during homeostasis (28, 95, 104). The localization of Scx+ cells during healing is controversial and likely context dependent, with differential results observed between different models of healing. Studies using a patellar window defect (29), partial Achilles transection (28), and complete flexor tendon transection and repair (95) demonstrate localization of these cells within the scar tissue. In contrast, complete transection without repair of the Achilles tendon demonstrates a lack of Scx+ cells in the bridging scar tissue (26). Moreover, Scx+ tenocytes located within the tendon body adjacent to the injury site do not appear to proliferate following injury in adults (26, 28, 29). As to the origin of Scx+ cells, Dyment et al. suggested that the Scx+ cells located within the scar tissue may be derived from migrating epitenon cells that turn on Scx expression following injury (29), however there is also evidence that Scx-lineage cells migrate to the injury site from within the tendon parenchyma (95). Combined, these studies indicate that the localization of Scx-expressing cells is likely tendon-, context-, and time-dependent.
In addition to Scx, we have recently shown that the small calcium binding protein S100a4 is expressed by many tendon cells during homeostasis, and both S100a4+ and S100a4-lineage cells are found within the bridging scar tissue during tendon healing (105, 106). Functionally, depletion of proliferating S100a4+ cells impairs restoration of mechanical properties following injury. In contrast, knock-down of S100a4 expression promotes regenerative tendon healing, including improvements in both range of motion and mechanical properties. Furthermore, these mice healing with decreases in macrophage and myofibroblast content, consistent with a more regenerative environment (106). However, the origin(s) of S100a4+ cells during healing, and the specific contributions of intrinsic vs. extrinsically derived S100a4+ cells are unclear.
Remodeling phase
The remodeling phase is typically characterized by a dramatic decrease in both the vascularity and cellularity generated during the first two stages as the granulation tissue matures into a scar. In more regenerative tissues such as bone, this provisional scar is eventually replaced by organ-specific cell populations and matrix that restore the properties of the uninjured tissue . In contrast, adult tendons lack the inherent ability to fully regenerate damaged tissue and the fibrovascular scar generated during the initial healing phases is never fully replaced. The reason for this deficit in tendon is unknown, though it is thought to be due to an inability of the tendon cells to effectively remodel the provisional collagen III-rich matrix into the highly cross-linked, collagen type I matrix of the original tendon. Work by Sakabe et al. demonstrated that knockdown of Scx expression in vivo resulted in decreased deposition of ECM components and incomplete remodeling of type III collagen into type I collagen, suggesting that Scx+ cells play a critical, though insufficient, role in both matrix deposition and remodeling during this final stage of healing (28).
In addition to an inadequate response from native tendon cells, the presence of extrinsic cells may also contribute to the lack of tissue remodeling in tendon. Prolonged myofibroblast activity is a critical driver of fibrosis in many organs including kidney (107), lung (108), and liver (109). Myofibroblasts play a critical role in matrix remodeling during the typical wound healing process, first appearing during the fibroblastic phase where they begin to synthesize and contract the matrix that will eventually supplant the granulation tissue and form the fibrous scar (110). The origins of myofibroblasts in wound healing is unclear, however they are thought to arise by a number of mechanisms that include the activation of resident fibroblasts or pericytes by pro-inflammatory cytokines such as TGF-β1 and interleukin-1β (IL-1β), the transformation of epithelial cells through a process called epithelial-to-mesenchymal transition, or from the recruitment of bone marrow-derived circulating cells into the injury site (111). Fully differentiated myofibroblasts are defined by the expression of alpha smooth muscle actin (αSMA). In tendon, numerous studies have shown that αSMA+ cells are prevalent during healing (26, 29, 80, 112-114), though their origin and function is still debated. In murine patellar tendon, more than 65% of αSMA-lineage cells expressed Scx two weeks following injury (114). In a converse experiment, very few Scx-lineage cells expressed αSMA two weeks post-repair in murine flexor tendons (95). This suggests that Scx-lineage cells are not the primary source of αSMA+ cells in healing tendon but that αSMA-lineage cells can express Scx in response to injury. We have recently shown that a subset of S100a4-lineage cells become αSMA+ following tendon injury while actively expressing S100a4+ cells do not co-express αSMA, indicating that some S100a4-lineage cells turn off S100a4 during the transition to myofibroblasts following tendon repair (106). Moreover, myofibroblastic cells have been noted in the scar tissue of naturally occurring equine flexor tendon injuries along with substantially increased levels of collagen type III (112), suggesting that these cells are somehow involved in maintaining the scar tissue, whether it is by actively producing the fibrotic ECM or by inhibiting the migration of other cell types. Understanding the origins, function, and regulatory components of myofibroblast activity in tendon healing will therefore be crucial to our ability to prevent fibrosis in tendon.
Factors that impact tendon healing
Further complicating an already challenging healing process, a number of factors put patients at elevated risk for developing tendon disorders, including age, activity level, body mass index, and the presence of co-morbidities (115-118). There also appear to be differences between the tendon healing response in males vs females: female patients experience more symptoms and a greater loss of function following surgical repair of Achilles tendon rupture compared to male patients (119), and ovariectomized rats show impaired Achilles tendon healing suggesting an effect of ovarian hormones on the healing process (120). Complicating factors also frequently arise due to pathological changes in the ECM or cellular composition of the affected tendon. In patients with type II diabetes mellitus (T2DM), for instance, tendon disorders are common because of alterations in the tendon ECM that occur in the presence of hyperglycemia (121). Elevated cholesterol levels put patients at significantly increased risk for rotator cuff tear (122) and the development of tendon xanthomas (lipid-filled nodules within the tendon ECM) that can result in Achilles tendon pain and/or rupture (123). Smoking is also reported to affect tendon composition: the Achilles and patellar tendons of patients who smoke were found to be thinner and stiffer than non-smoking patients, potentially predisposing these patients to the development of tendon disorders (124). Moreover, alterations to the vascularity associated with smoking have been correlated with increased risk for rotator cuff tear (125, 126). In many cases, how these changes in tendon ECM affect the behavior and function of tendon cells is unknown.
Aging in particular is associated with an increased risk for the development of tendon disorders (127, 128), and aging further impedes the healing process following injury (116). Patients over the age 60 are at a two-fold higher risk of rotator cuff tear, are three times more likely to suffer a massive tear, compared to younger patients (129), and have less satisfactory Achilles tendon healing following rupture (130.). Though there is no consensus on the mechanism or the extent of the changes, aged tendons are thought to have differences in mechanical properties compared to young tendons (131). Aging has also been shown to alter the composition of the equine tendon ECM (132, 133). Additionally, both tenocytes and TSPC become less proliferative as tendons age, though the degree to which this occurs is highly tendon-specific (134-136). Changes in the mechanical properties coupled with a concomitant decrease in cellularity with age is thought to be the major reason why aged tendons injury more frequently and fail to heal adequately.
In addition to changes to the tendon ECM and cellular milieu, patients with systemic comorbidities that that amplify the inflammatory response during healing are at elevated risk for the development of pathologic fibrosis following tendon injury. Obese patients and those with T2DM have increased levels of circulating pro-inflammatory cytokines at baseline (137, 138), and both conditions are associated with biased macrophage polarization towards the pro-inflammatory M1 phenotype compared to non-obese and non-diabetic patients (139, 140). Fibrotic tendon disorders and complications during tendon healing are common in both obese and/or T2DM patients. Similarly, in obese/T2DM rodents, flexor tendons heal with increased fibrosis and significantly impaired mechanical properties (75, 141, 142). This poor healing response is associated with increased expression of M1 macrophage markers in addition to increased and sustained expression of M2 macrophage markers throughout healing in obese/T2DM mice compared to controls (75). Though the reason for poor tendon healing in obese and/or diabetic patients is likely multifactorial, these studies further imply that inflammation is a critical driver of tendon fibrosis.
Perspectives
The cellular landscape of tendon healing is complex and varied, consisting of both intrinsic and extrinsic populations that contribute to the healing response in tendon. Because our knowledge of these cell populations and their contributions to both normal and pathologic tendon healing is in its infancy, there are a number of significant hurdles that need to be addressed before new therapies can be brought to the clinic. Most importantly, while recent work has demonstrated great heterogeneity of the resident tendon cell environment, the precise identity, including lineage and fate, of these cells are unknown, as are the potentially disparate functional roles of these sub-populations. Moreover, though the use of rodent models has allowed us to identify and track tendon cell populations present during tendon homeostasis and healing, the degree to which these populations and their temporal/spatial function are conserved in humans is unknown. There are also a number of differences between the human and rodent immune systems, including the timing and duration of the healing response and important differences in the cell populations affected by various drugs (143, 144). The relationship between rodent models and human tendon healing is an important issue that that will need to be addressed in future studies and prior to clinical translation. The relationship and potential overlap between the sub-populations identified by different laboratories using divergent rodent models also remains to be determined. There is still little understanding of how different sub-populations of cells may change during different stages of tendon healing or in response to comorbidities, and there are likely to be undiscovered minor cell populations due to the reliance on single markers/genetic tracing and bulk sequencing tools. The advent of high throughput single cell RNA-sequencing (scRNA) technologies has led to the identification of previously unknown cell populations in relatively well-characterized tissues such as heart (145) and liver (146). Importantly, scRNA has revealed the heterogeneity and plasticity of the cells that have traditionally been seen as homogenous and static (147, 148). Application of scRNA technology to tendon will accelerate our ability to identify and characterize the various tendon cell populations. In addition, given the differential effects of fibrotic healing on tendon-type, it will be important to understand potential differences in cell populations and responses between tendons. Use of scRNA could facilitate comparative studies, allowing us to identify cells and processes which are common to all tendons, and those which may be specific to certain tendons. This knowledge would greatly enhance our ability to identify cellular and molecular targets for preventing tendon fibrosis.
Though it has been shown that many different cell types including various fibroblast populations, myofibroblasts, and macrophages are present during tendon healing, there is almost no information available about the interactions between these cell populations. Understanding these relationships between cell types is crucial as paracrine interactions between cells have been shown to drive fibrosis in other tissues. In both liver and idiopathic lung fibrosis, macrophage-derived S100a4 has been shown to prompt the conversion of resident cells into αSMA+ myofibroblasts, increasing the degree of organ fibrosis (149, 150). Inhibition of S100a4 signaling can attenuate or even reverse fibrotic progression, indicating S100a4 as a therapeutic target in these tissues (149, 150). Both S100a4 expressing cells and αSMA+ myofibroblasts are present during tendon healing, though the relationship between these cells or how their respective activities may affect the behavior of resident tendon cells is only beginning to be understood. A recent in vitro study by Stolk et al. demonstrated for the first time that co-culturing tenocytes and macrophages can alter the behavior of both cell types, and that cytokine signaling by tenocytes can drive macrophage polarization (151). Additional studies of this kind are clearly warranted in order to further delineate the relationships between cell types known to be present in tendon and to identify potential points at which these interactions may be modulated for therapeutic value.
Complicating the development of new treatment modalities for tendon injuries is the fact that, as previously discussed, normal tendon healing and the development of pathologic fibrosis share a number of common signaling pathways that are active at different points during healing. Broad pharmacological inhibition intended to improve one aspect of tendon healing therefore often negatively affects another. Prolonged TGF-β1 signaling, for instance, is a major driver of fibrosis in many tissues (152). In tendon, inhibition of TGF-β1 during the inflammatory phase has been shown to decrease the formation of peritendinous adhesions following flexor tendon injury and repair (153, 154); however, TGF-β1 is also critically involved in promoting cell proliferation and collagen deposition during the fibroblastic/proliferative and remodeling phases. Complete inhibition of TGF-β signaling through genetic knockout of SMAD3, a downstream effector of TGF-β signaling, in mice resulted in significantly decreased adhesion formation but also led to decreased strength of flexor tendons following repair (155). In contrast, knockdown of SMAD3 using antisense oligonucleotides immediately following repair using the same injury model decreased peritendinous adhesions but did not alter the mechanical properties of the tendon (156). Similarly, recent work by Freeberg et al. found that genetic knockout of plasminogen activator inhibitor 1(PAI-1), a protease suppressor regulated by of TGF-β signaling, reduces the formation of peritendinous adhesions without compromising the mechanical integrity of the repair (157). As these studies demonstrate, successful therapies for tendon fibrosis will therefore require not only knowledge of the cells and pathways involved, but also a more sophisticated understanding of the specific downstream effectors and the timing at which pharmacologic therapies should be administered. Though many gaps in the fundamental understanding of tendon cell biology during healing still remain, this is also a period of immense potential for the tendon community to leverage cutting-edge techniques to define the cellular environment and inform identification of therapeutic candidates to promote more regenerative tendon healing.
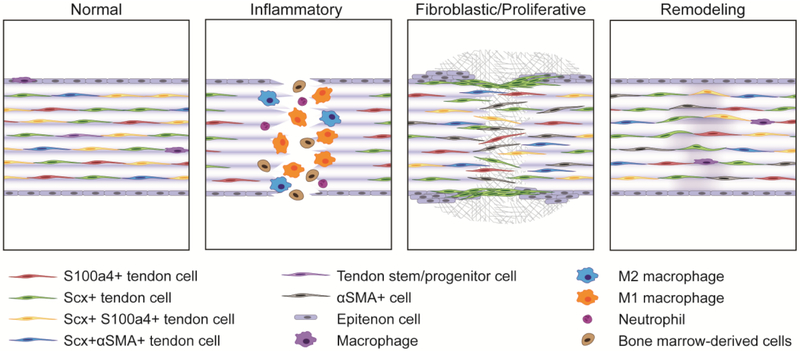
Figure 1. Illustration of the various cell populations during the phases of tendon healing.
Tendons contain a number of resident tendon cell sub-populations during homeostasis, including scleraxis (Scx)+ cells, S100a4+ cells, Scx+S100a4+ cells, and Scx+ alpha smooth muscle actin (αSMA)+ cells. Macrophages have also been reported in normal tendon, along with the presence of a tendon/stem progenitor population. There are likely additional resident cell sub-populations that have yet to be identified, and the relationships between those identified here (with respect to lineage or origin) are currently unknown. Shortly following injury, resident tendon cells directly adjacent to the injury site appear to undergo apoptosis. Neutrophils arrive, followed shortly by both M1 and M2 macrophages, as well as other bone-marrow derived cells whose identity and fate are currently unknown. During the fibroblastic/proliferative phase, epitenon cells close to the injury proliferate, and Scx+ cells that appear to originate from the epitenon migrate into the scar area, forming an organized bridging tissue between the two opposing ends of the tendon while S100a4+ and αSMA+ cells are found throughout the scar tissue. S100a4+ and αSMA+ cells during this phase appear to be derived from both intrinsic as well as extrinsic sources and likely represent heterogenous populations. Of the healing phases, the remodeling phase is the least characterized due to significant differences in the duration between tendons and injury models. During this phase, tissue continuity is restored, however the scar tissue remains disorganized compared to native tendon. It is currently unknown whether the phenotypes and relative abundance of tendon cell sub-populations remain altered or return to their pre-injury states and to what extent these potential changes in cell population may affect tendon function.
Table 2:
Effects of changes in cell phenotype on tendon function
| Alteration | Result | Model | Reference |
|---|---|---|---|
| Neutrophil depletion | Increased macrophages (F4/80) | AT transection and repair (Mouse) | (63) |
| Macrophage depletion | Increased ultimate tensile strength | AT transection and repair (Mouse) | (69) |
| Increased tensile strength and stiffness | Tendon graft in a bone tunnel(Rat) | (70) | |
| Increased/prolonged presence of macrophages | Increased collagen production/fibrosis, reduced tensile strength | FT transection and repair (T2DM Mouse) | (75) |
| Matrix disorganization | Naturally occurring tendon injury (Horse) | (76) | |
| S100a4+ cell depletion | Decreased tensile strength and stiffness | FT transection and repair (Mouse) | (106) |
Acknowledgements
All authors have disclosed that there are no potential conflicts of interest, and all authors have read the journal's policy on conflicts of interest, and the journal's authorship agreement. All authors have reviewed and approved this manuscript. This work was supported in part by NIH/NIAMS K01AR068386 and R01AR073169 (to AEL).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.United States Bone and Joint Initiative: The burden of musculoskeletal diseases in the United States (BMUS). Rosemont, IL: Available from: http://www.boneandjointburden.org. [Google Scholar]
- 2.Bureau of Labor Statistics. Available from: https://www.bls.gov/.
- 3.Raikin SM, Garras DN, Krapchev PV. Achilles tendon injuries in a united states population. Foot Ankle Int. 2013; 34: 475–80. [DOI] [PubMed] [Google Scholar]
- 4.Paloneva J, Lepola V, Äärimaa V, et al. Increasing incidence of rotator cuff repairs--a nationwide registry study in finland. BMC Musculoskelet Disord. 2015; 16: 189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.de Jong JP, Nguyen JT, Sonnema AJ, et al. The incidence of acute traumatic tendon injuries in the hand and wrist: A 10-year population-based study. Clin Orthop Surg. 2014; 6: 196–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Glaviano NR, Kew M, Hart JM, et al. Demographic and epidemiological trends in patellofemoral pain. Int J Sports Phys Ther. 2015; 10: 281–90. [PMC free article] [PubMed] [Google Scholar]
- 7.Whalen WP. Utilization of scar tissue in bridging tendon defects. Ann Surg. 1951; 133: 567–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tempfer H, Traweger A Tendon vasculature in health and disease. Front Physiol. 2015; 6: 330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Miyashita H, Ochi M, Ikuta Y Histological and biomechanical observations of the rabbit patellar tendon after removal of its central one-third. Arch Orthop Trauma Surg. 1997; 116: 454–62. [DOI] [PubMed] [Google Scholar]
- 10.Abate M, Silbernagel KG, Siljeholm C, et al. Pathogenesis of tendinopathies: Inflammation or degeneration? Arthritis Res Ther. 2009; 11: 235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Astrom M, Rausing A Chronic Achilles tendinopathy. A survey of surgical and histopathologic findings. Clin Orthop Relat Res. 1995: 151–64. [PubMed] [Google Scholar]
- 12.Longo UG, Franceschi F, Ruzzini L, et al. Histopathology of the supraspinatus tendon in rotator cuff tears. Am J Sports Med. 2008; 36: 533–8. [DOI] [PubMed] [Google Scholar]
- 13.Watson M The determinants of flexor tendon fibrosis following trauma: An experimental study in rabbits. Hand. 1978; 10: 150–3. [DOI] [PubMed] [Google Scholar]
- 14.Strickland JW. The scientific basis for advances in flexor tendon surgery. J Hand Ther. 2005; 18: 94–110. [DOI] [PubMed] [Google Scholar]
- 15.Aydin A, Topalan M, Mezdegi A, et al. Single-stage flexor tendoplasty in the treatment of flexor tendon injuries. Acta Orthop Traumatol Turc. 2004; 38: 54–9. [PubMed] [Google Scholar]
- 16.Bottagisio M, Lovati AB. A review on animal models and treatments for the reconstruction of Achilles and flexor tendons. J Mater Sci Mater Med. 2017; 28: 45. [DOI] [PubMed] [Google Scholar]
- 17.Hast MW, Zuskov A, Soslowsky LJ. The role of animal models in tendon research. Bone Joint Res. 2014; 3: 193–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thomopoulos S, Parks WC, Rifkin DB, et al. Mechanisms of tendon injury and repair. J Orthop Res. 2015; 33: 832–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Woo SL, Gelberman RH, Cobb NG, et al. The importance of controlled passive mobilization on flexor tendon healing. A biomechanical study. Acta Orthop Scand. 1981; 52: 615–22. [DOI] [PubMed] [Google Scholar]
- 20.Wada A, Kubota H, Miyanishi K, et al. Comparison of postoperative early active mobilization and immobilization in vivo utilising a four-strand flexor tendon repair. J Hand Surg Br. 2001; 26: 301–6. [DOI] [PubMed] [Google Scholar]
- 21.Winters SC, Gelberman RH, Woo SL, et al. The effects of multiple-strand suture methods on the strength and excursion of repaired intrasynovial flexor tendons: A biomechanical study in dogs. J Hand Surg Am. 1998; 23: 97–104. [DOI] [PubMed] [Google Scholar]
- 22.Kormpakis I, Linderman SW, Thomopoulos S, et al. Enhanced zone ii flexor tendon repair through a new half hitch loop suture configuration. PLoS One. 2016; 11: e0153822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Linderman SW, Shen H, Yoneda S, et al. Effect of connective tissue growth factor delivered via porous sutures on the proliferative stage of intrasynovial tendon repair. J Orthop Res. 2018; 36: 2052–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shen H, Jayaram R, Yoneda S, et al. The effect of adipose-derived stem cell sheets and CTGF on early flexor tendon healing in a canine model. Sci Rep. 2018; 8: 11078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ackerman JE, Loiselle AE. Murine flexor tendon injury and repair surgery. J Vis Exp. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Howell K, Chien C, Bell R, et al. Novel model of tendon regeneration reveals distinct cell mechanisms underlying regenerative and fibrotic tendon healing. Sci Rep. 2017; 7: 45238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lemmon EA, Locke RC, Szostek AK, et al. Partial-width injuries of the rat rotator cuff heal with fibrosis. Connect Tissue Res. 2018; 59: 437–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sakabe T, Sakai K, Maeda T, et al. Transcription factor scleraxis vitally contributes to progenitor lineage direction in wound healing of adult tendon in mice. J Biol Chem. 2018; 293: 5766–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dyment NA, Liu CF, Kazemi N, et al. The paratenon contributes to scleraxis-expressing cells during patellar tendon healing. PLoS One. 2013; 8: e59944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rui YF, Lui PP, Rolf CG, et al. Expression of chondro-osteogenic BMPs in clinical samples of patellar tendinopathy. Knee Surg Sports Traumatol Arthrosc. 2012; 20: 1409–17. [DOI] [PubMed] [Google Scholar]
- 31.Wang PH, Luh JJ, Chen WS, et al. In vivo photoacoustic micro-imaging of microvascular changes for Achilles tendon injury on a mouse model. Biomed Opt Express. 2011; 2: 1462–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Eliasson P, Andersson T, Aspenberg P Influence of a single loading episode on gene expression in healing rat Achilles tendons. J Appl Physiol (1985). 2012; 112: 279–88. [DOI] [PubMed] [Google Scholar]
- 33.Killian ML, Cavinatto L, Shah SA, et al. The effects of chronic unloading and gap formation on tendon-to-bone healing in a rat model of massive rotator cuff tears. J Orthop Res. 2014; 32: 439–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Beason DP, Kuntz AF, Hsu JE, et al. Development and evaluation of multiple tendon injury models in the mouse. J Biomech. 2012; 45: 1550–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chamberlain CS, Crowley E, Vanderby R The spatio-temporal dynamics of ligament healing. Wound Repair Regen. 2009; 17: 206–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chamberlain CS, Brounts SH, Sterken DG, et al. Gene profiling of the rat medial collateral ligament during early healing using microarray analysis. J Appl Physiol (1985). 2011; 111: 552–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Franchi M, Trire A, Quaranta M, et al. Collagen structure of tendon relates to function. Scientific World Journal. 2007; 7: 404–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Biewener A Tendons and ligaments: Structure, mechanical behavior and biological function In: Fratzl P, editor. Collagen: Structure and Mechanics. Boston, MA: Springer US; 2008. p. 269–84. [Google Scholar]
- 39.Kannus P Structure of the tendon connective tissue. Scand J Med Sci Sports. 2000; 10: 312–20. [DOI] [PubMed] [Google Scholar]
- 40.Gelse K, Poschl E, Aigner T Collagens-structure, function, and biosynthesis. Adv Drug Deliv Rev. 2003; 55: 1531–46. [DOI] [PubMed] [Google Scholar]
- 41.Thorpe CT, Screen HR. Tendon structure and composition. Adv Exp Med Biol. 2016; 920: 3–10. [DOI] [PubMed] [Google Scholar]
- 42.Ali OJ, Comerford EJ, Clegg PD, et al. Variations during ageing in the threedimensional anatomical arrangement of fascicles within the equine superficial digital flexor tendon. Eur Cell Mater. 2018; 35: 87–102. [DOI] [PubMed] [Google Scholar]
- 43.Thorpe CT, Godinho MSC, Riley GP, et al. The interfascicular matrix enables fascicle sliding and recovery in tendon, and behaves more elastically in energy storing tendons. J Mech Behav Biomed Mater. 2015; 52: 85–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Taylor SH, Al-Youha S, Van Agtmael T, et al. Tendon is covered by a basement membrane epithelium that is required for cell retention and the prevention of adhesion formation. PLoS One. 2011; 6: e16337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hartmann. The tendon sheaths and synovial bursae of the foot. 1981; 1: 247–69. [PubMed] [Google Scholar]
- 46.Ahmed IM, Lagopoulos M, McConnell P, et al. Blood supply of the Achilles tendon. J Orthop Res. 1998; 16: 591–6. [DOI] [PubMed] [Google Scholar]
- 47.Benjamin M, Ralphs JR. Tendons and ligaments- an overview. Histol Histopathol. 1997; 12: 1135–44. [PubMed] [Google Scholar]
- 48.Schweitzer R, Chyung JH, Murtaugh LC, et al. Analysis of the tendon cell fate using scleraxis, a specific marker for tendons and ligaments. Development. 2001; 128: 3855–66. [DOI] [PubMed] [Google Scholar]
- 49.Brandau O, Meindl A, Fassler R, et al. A novel gene, tendin, is strongly expressed in tendons and ligaments and shows high homology with chondromodulin-I. Dev Dyn. 2001; 221: 72–80. [DOI] [PubMed] [Google Scholar]
- 50.Voleti PB, Buckley MR, Soslowsky LJ. Tendon healing: Repair and regeneration. Annu Rev Biomed Eng. 2012; 14: 47–71. [DOI] [PubMed] [Google Scholar]
- 51.Carpenter JE, Hankenson KD. Animal models of tendon and ligament injuries for tissue engineering applications. Biomaterials. 2004; 25: 1715–22. [DOI] [PubMed] [Google Scholar]
- 52.Sandrey M Acute and chronic tendon injuries: Factors affecting the healing response and treatment. J Sport Rehabil. 2003; 12: 70–91. [Google Scholar]
- 53.Hope M, Saxby TS. Tendon healing. Foot Ankle Clin. 2007; 12: 553–67. [DOI] [PubMed] [Google Scholar]
- 54.Koob TJ, Summers AP. Tendon- bridging the gap. Comp Biochem Physiol A Mol Integr Physiol. 2002; 133: 905–9. [DOI] [PubMed] [Google Scholar]
- 55.Garner WL, McDonald JA, Koo M, et al. Identification of the collagen-producing cells in healing flexor tendons. Plast Reconstr Surg. 1989; 83: 875–9. [DOI] [PubMed] [Google Scholar]
- 56.Wang ED. Tendon repair. J Hand Ther. 1998; 11: 105–10. [DOI] [PubMed] [Google Scholar]
- 57.Loiselle AE, Frisch BJ, Wolenski M, et al. Bone marrow-derived matrix metalloproteinase-9 is associated with fibrous adhesion formation after murine flexor tendon injury. PLoS One. 2012; 7: e40602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Strickland JW. Flexor tendons: Acute injuries In: Green DHR, Pedersen W editor. Green's operative hand surgery. New York, USA: Churchill Livingstone; 1999. p. 1851–97. [Google Scholar]
- 59.Beredjiklian PK, Favata M, Cartmell JS, et al. Regenerative versus reparative healing in tendon: A study of biomechanical and histological properties in fetal sheep. Ann Biomed Eng. 2003; 31: 1143–52. [DOI] [PubMed] [Google Scholar]
- 60.Tang QM, Chen JL, Shen WL, et al. Fetal and adult fibroblasts display intrinsic differences in tendon tissue engineering and regeneration. Sci Rep. 2014; 4: 5515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Marsolais D, Cote CH, Frenette J Neutrophils and macrophages accumulate sequentially following Achilles tendon injury. J Orthop Res. 2001; 19: 1203–9. [DOI] [PubMed] [Google Scholar]
- 62.Wong JK, Lui YH, Kapacee Z, et al. The cellular biology of flexor tendon adhesion formation: An old problem in a new paradigm. Am J Pathol. 2009; 175: 1938–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Godbout C, Bilodeau R, Van Rooijen N, et al. Transient neutropenia increases macrophage accumulation and cell proliferation but does not improve repair following intratendinous rupture of Achilles tendon. J Orthop Res. 2010; 28: 1084–91. [DOI] [PubMed] [Google Scholar]
- 64.Martinez FO, Gordon S The M1 and M2 paradigm of macrophage activation: Time for reassessment. F1000Prime Rep. 2014; 6: 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wang N, Liang H, Zen K Molecular mechanisms that influence the macrophage M1-M2 polarization balance. Front Immunol. 2014; 5: 614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lech M, Anders HJ. Macrophages and fibrosis: How resident and infiltrating mononuclear phagocytes orchestrate all phases of tissue injury and repair. Biochim Biophys Acta. 2013; 1832: 989–97. [DOI] [PubMed] [Google Scholar]
- 67.Wynn TA, Vannella KM. Macrophages in tissue repair, regeneration, and fibrosis. Immunity. 2016; 44: 450–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Braga TT, Agudelo JS, Camara NO. Macrophages during the fibrotic process: M2 as friend and foe. Front Immunol. 2015; 6: 602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.de la Durantaye M, Piette AB, van Rooijen N, et al. Macrophage depletion reduces cell proliferation and extracellular matrix accumulation but increases the ultimate tensile strength of injured Achilles tendons. J Orthop Res. 2014; 32: 279–85. [DOI] [PubMed] [Google Scholar]
- 70.Hays PL, Kawamura S, Deng XH, et al. The role of macrophages in early healing of a tendon graft in a bone tunnel. J Bone Joint Surg Am. 2008; 90: 565–79. [DOI] [PubMed] [Google Scholar]
- 71.Das A, Sinha M, Datta S, et al. Monocyte and macrophage plasticity in tissue repair and regeneration. Am J Pathol. 2015; 185: 2596–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Gibbons MA, MacKinnon AC, Ramachandran P, et al. Ly6chi monocytes direct alternatively activated profibrotic macrophage regulation of lung fibrosis. Am J Respir Crit Care Med. 2011; 184: 569–81. [DOI] [PubMed] [Google Scholar]
- 73.Murray LA, Chen Q, Kramer MS, et al. TGF-beta driven lung fibrosis is macrophage dependent and blocked by serum amyloid P. Int J Biochem Cell Biol. 2011; 43: 154–62. [DOI] [PubMed] [Google Scholar]
- 74.Lin D, Alberton P, Caceres MD, et al. Tenomodulin is essential for prevention of adipocyte accumulation and fibrovascular scar formation during early tendon healing. Cell Death Dis. 2017; 8: e3116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ackerman JE, Geary MB, Orner CA, et al. Obesity/type II diabetes alters macrophage polarization resulting in a fibrotic tendon healing response. PLoS One. 2017; 12: e0181127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Dakin SG, Werling D, Hibbert A, et al. Macrophage sub-populations and the lipoxin a4 receptor implicate active inflammation during equine tendon repair. PLoS One. 2012; 7: e32333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Davies LC, Jenkins SJ, Allen JE, et al. Tissue-resident macrophages. Nature Immunology. 2013; 14: 986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Sugg KB, Lubardic J, Gumucio JP, et al. Changes in macrophage phenotype and induction of epithelial-to-mesenchymal transition genes following acute Achilles tenotomy and repair. J Orthop Res. 2014; 32: 944–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wojciak B, Crossan JF. The accumulation of inflammatory cells in synovial sheath and epitenon during adhesion formation in healing rat flexor tendons. Clin Exp Immunol. 1993; 93: 108–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Berglund ME, Hildebrand KA, Zhang M, et al. Neuropeptide, mast cell, and myofibroblast expression after rabbit deep flexor tendon repair. J Hand Surg Am. 2010; 35: 1842–9. [DOI] [PubMed] [Google Scholar]
- 81.Connizzo BK, Yannascoli SM, Tucker JJ, et al. The detrimental effects of systemic ibuprofen delivery on tendon healing are time-dependent. Clin Orthop Relat Res. 2014; 472: 2433–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Virchenko O, Skoglund B, Aspenberg P Parecoxib impairs early tendon repair but improves later remodeling. Am J Sports Med. 2004; 32: 1743–7. [DOI] [PubMed] [Google Scholar]
- 83.Ferry ST, Dahners LE, Afshari HM, et al. The effects of common anti-inflammatory drugs on the healing rat patellar tendon. Am J Sports Med. 2007; 35: 1326–33. [DOI] [PubMed] [Google Scholar]
- 84.Tsai WC, Tang FT, Hsu CC, et al. Ibuprofen inhibition of tendon cell proliferation and upregulation of the cyclin kinase inhibitor p21CIP1. J Orthop Res. 2004; 22: 586–91. [DOI] [PubMed] [Google Scholar]
- 85.Riley GP, Cox M, Harrall RL, et al. Inhibition of tendon cell proliferation and matrix glycosaminoglycan synthesis by non-steroidal anti-inflammatory drugs in vitro. J Hand Surg Br. 2001; 26: 224–8. [DOI] [PubMed] [Google Scholar]
- 86.Shen H, Kormpakis I, Havlioglu N, et al. The effect of mesenchymal stromal cell sheets on the inflammatory stage of flexor tendon healing. Stem Cell Res Ther. 2016; 7: 144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Gelberman RH, Linderman SW, Jayaram R, et al. Combined administration of ASCx and BMP-12 promotes an M2 macrophage phenotype and enhances tendon healing. Clin Orthop Relat Res. 2017; 475: 2318–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Juneja SC, Schwarz EM, O'Keefe RJ, et al. Cellular and molecular factors in flexor tendon repair and adhesions: A histological and gene expression analysis. Connect Tissue Res. 2013; 54: 218–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kajikawa Y, Morihara T, Watanabe N, et al. GFP chimeric models exhibited a biphasic pattern of mesenchymal cell invasion in tendon healing. J Cell Physiol. 2007; 210: 684–91. [DOI] [PubMed] [Google Scholar]
- 90.Thomopoulos S, Das R, Silva MJ, et al. Enhanced flexor tendon healing through controlled delivery of PDGF-BB. J Orthop Res. 2009; 27: 1209–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Thomopoulos S, Harwood FL, Silva MJ, et al. Effect of several growth factors on canine flexor tendon fibroblast proliferation and collagen synthesis in vitro. J Hand Surg Am. 2005; 30: 441–7. [DOI] [PubMed] [Google Scholar]
- 92.Dahlgren LA, Mohammed HO, Nixon AJ. Temporal expression of growth factors and matrix molecules in healing tendon lesions. J Orthop Res. 2005; 23: 84–92. [DOI] [PubMed] [Google Scholar]
- 93.Thomopoulos S, Kim HM, Das R, et al. The effects of exogenous basic fibroblast growth factor on intrasynovial flexor tendon healing in a canine model. J Bone Joint Surg Am. 2010; 92: 2285–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Gelberman RH, Steinberg D, Amiel D, et al. Fibroblast chemotaxis after tendon repair. J Hand Surg Am. 1991; 16: 686–93. [DOI] [PubMed] [Google Scholar]
- 95.Best KT, Loiselle A Scleraxis lineage cells contribute to organized bridging tissue during tendon healing, and identifies subpopulations of resident tendon cells. bioRxiv. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Williams IF, McCullagh KG, Silver IA. The distribution of types I and III collagen and fibronectin in the healing equine tendon. Connect Tissue Res. 1984; 12: 211–27. [DOI] [PubMed] [Google Scholar]
- 97.Khan U, Edwards JC, McGrouther DA. Patterns of cellular activation after tendon injury. J Hand Surg Br. 1996; 21: 813–20. [DOI] [PubMed] [Google Scholar]
- 98.Cadby JA, Buehler E, Godbout C, et al. Differences between the cell populations from the peritenon and the tendon core with regard to their potential implication in tendon repair. PLoS One. 2014; 9: e92474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Tan Q, Lui PP, Lee YW. In vivo identity of tendon stem cells and the roles of stem cells in tendon healing. Stem Cells Dev. 2013; 22: 3128–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Bi Y, Ehirchiou D, Kilts TM, et al. Identification of tendon stem/progenitor cells and the role of the extracellular matrix in their niche. Nat Med. 2007; 13: 1219–27. [DOI] [PubMed] [Google Scholar]
- 101.Salingcarnboriboon R, Yoshitake H, Tsuji K, et al. Establishment of tendon-derived cell lines exhibiting pluripotent mesenchymal stem cell-like property. Exp Cell Res. 2003; 287: 289–300. [DOI] [PubMed] [Google Scholar]
- 102.Xu W, Sun Y, Zhang J, et al. Perivascular-derived stem cells with neural crest characteristics are involved in tendon repair. Stem Cells Dev. 2015; 24: 857–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Tempfer H, Wagner A, Gehwolf R, et al. Perivascular cells of the supraspinatus tendon express both tendon- and stem cell-related markers. Histochem Cell Biol. 2009; 131: 733–41. [DOI] [PubMed] [Google Scholar]
- 104.Pryce BA, Brent AE, Murchison ND, et al. Generation of transgenic tendon reporters, ScxGFP and ScxAP, using regulatory elements of the scleraxis gene. Dev Dyn. 2007; 236: 1677–82. [DOI] [PubMed] [Google Scholar]
- 105.Ackerman JE, Best KT, O'Keefe RJ, et al. Deletion of EP4 in s100a4-lineage cells reduces scar tissue formation during early but not later stages of tendon healing. Sci Rep. 2017; 7: 8658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Ackerman JE, SV, Best KT, Knapp E, Loiselle AE. Cell-non-autonomous functions of s100a4 drive fibrotic tendon healing. bioRxiv. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Meran S, Steadman R Fibroblasts and myofibroblasts in renal fibrosis. Int J Exp Pathol. 2011; 92: 158–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Phan SH. The myofibroblast in pulmonary fibrosis. Chest. 2002; 122: 286S–9S. [DOI] [PubMed] [Google Scholar]
- 109.Iwaisako K, Jiang C, Zhang M, et al. Origin of myofibroblasts in the fibrotic liver in mice. Proc Natl Acad Sci USA. 2014; 111: E3297–E305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Darby IA, Laverdet B, Bonté F, et al. Fibroblasts and myofibroblasts in wound healing. Clin Cosmet Investig Dermatol. 2014; 7: 301–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Hu B, Phan SH. Myofibroblasts. Curr Opin Rheumatol. 2013; 25: 71–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Williams IF, Heaton A, McCullagh KG. Cell morphology and collagen types in equine tendon scar. Res Vet Sci. 1980; 28: 302–10. [PubMed] [Google Scholar]
- 113.Weiler A, Unterhauser FN, Bail HJ, et al. Alpha-smooth muscle actin is expressed by fibroblastic cells of the ovine anterior cruciate ligament and its free tendon graft during remodeling. J Orthop Res. 2002; 20: 310–7. [DOI] [PubMed] [Google Scholar]
- 114.Dyment NA, Hagiwara Y, Matthews BG, et al. Lineage tracing of resident tendon progenitor cells during growth and natural healing. PLoS One. 2014; 9: e96113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Zhou B, Zhou Y, Tang K An overview of structure, mechanical properties, and treatment for age-related tendinopathy. J Nutr Health Aging. 2014; 18: 441–8. [DOI] [PubMed] [Google Scholar]
- 116.Ackerman JE, Bah I, Jonason JH, et al. Aging does not alter tendon mechanical properties during homeostasis, but does impair flexor tendon healing. J Orthop Res. 2017; 35: 2716–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Franceschi F, Papalia R, Paciotti M, et al. Obesity as a risk factor for tendinopathy: A systematic review. Int J Endocrinol. 2014; 2014: 670262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.O'Neill S, Watson PJ, Barry S A delphi study of risk factors for Achilles tendinopathy-opinions of world tendon experts. Int J Sports Phys Ther. 2016; 11: 684–97. [PMC free article] [PubMed] [Google Scholar]
- 119.Silbernagel KG, Brorsson A, Olsson N, et al. Sex differences in outcome after an acute Achilles tendon rupture. Orthop J Sports Med. 2015; 3: 2325967115586768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Fryhofer GW, Freedman BR, Hillin CD, et al. Postinjury biomechanics of Achilles tendon vary by Sex and hormone status. J Appl Physiol (1985). 2016; 121: 1106–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Snedeker JG, Gautieri A The role of collagen crosslinks in ageing and diabetes - the good, the bad, and the ugly. Muscles Ligaments Tendons J. 2014; 4: 303–8. [PMC free article] [PubMed] [Google Scholar]
- 122.Abboud JA, Kim JS. The effect of hypercholesterolemia on rotator cuff disease. Clin Orthop Relat Res. 2010; 468: 1493–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Tsouli SG, Kiortsis DN, Argyropoulou MI, et al. Pathogenesis, detection and treatment of Achilles tendon xanthomas. Eur J Clin Invest. 2005; 35: 236–44. [DOI] [PubMed] [Google Scholar]
- 124.Agladioglu K, Akkaya N, Gungor HR, et al. Effects of cigarette smoking on elastographic strain ratio measurements of patellar and Achilles tendons. J Ultrasound Med. 2016; 35: 2431–8. [DOI] [PubMed] [Google Scholar]
- 125.Baumgarten KM, Gerlach D, Galatz LM, et al. Cigarette smoking increases the risk for rotator cuff tears. Clin Orthop Relat Res. 2010; 468: 1534–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Carbone S, Gumina S, Arceri V, et al. The impact of preoperative smoking habit on rotator cuff tear: Cigarette smoking influences rotator cuff tear sizes. J Shoulder Elbow Surg. 2012; 21: 56–60. [DOI] [PubMed] [Google Scholar]
- 127.Albers IS, Zwerver J, Diercks RL, et al. Incidence and prevalence of lower extremity tendinopathy in a dutch general practice population: A cross sectional study. BMC Musculoskelet Disord. 2016; 17: 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.de Jonge S, van den Berg C, de Vos RJ, et al. Incidence of midportion Achilles tendinopathy in the general population. Br J Sports Med. 2011; 45: 1026–8. [DOI] [PubMed] [Google Scholar]
- 129.Gumina S, Carbone S, Campagna V, et al. The impact of aging on rotator cuff tear size. Musculoskelet Surg. 2013; 97 Suppl 1: 69–72. [DOI] [PubMed] [Google Scholar]
- 130.Nestorson J, Movin T, Moller M, et al. Function after Achilles tendon rupture in the elderly: 25 patients older than 65 years followed for 3 years. Acta Orthop Scand. 2000; 71: 64–8. [DOI] [PubMed] [Google Scholar]
- 131.Svensson RB, Heinemeier KM, Couppe C, et al. Effect of aging and exercise on the tendon. J Appl Physiol (1985). 2016; 121: 1237–46. [DOI] [PubMed] [Google Scholar]
- 132.Peffers MJ, Thorpe CT, Collins JA, et al. Proteomic analysis reveals age-related changes in tendon matrix composition, with age- and injury-specific matrix fragmentation. J Biol Chem. 2014; 289: 25867–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Birch HL, Bailey JV, Bailey AJ, et al. Age-related changes to the molecular and cellular components of equine flexor tendons. Equine Vet J. 1999; 31: 391–6. [DOI] [PubMed] [Google Scholar]
- 134.Tsai WC, Chang HN, Yu TY, et al. Decreased proliferation of aging tenocytes is associated with down-regulation of cellular senescence-inhibited gene and up-regulation of p27. J Orthop Res. 2011; 29: 1598–603. [DOI] [PubMed] [Google Scholar]
- 135.Zhou Z, Akinbiyi T, Xu L, et al. Tendon-derived stem/progenitor cell aging: Defective self-renewal and altered fate. Aging Cell. 2010; 9: 911–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Kohler J, Popov C, Klotz B, et al. Uncovering the cellular and molecular changes in tendon stem/progenitor cells attributed to tendon aging and degeneration. Aging Cell. 2013; 12: 988–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Schmidt FM, Weschenfelder J, Sander C, et al. Inflammatory cytokines in general and central obesity and modulating effects of physical activity. PLoS One. 2015; 10: e0121971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Devaraj S, Dasu MR, Jialal I Diabetes is a proinflammatory state: A translational perspective. Expert Rev Endocrinol Metab. 2010; 5: 19–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Lumeng CN, Bodzin JL, Saltiel AR. Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J Clin Invest. 2007; 117: 175–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Reddy MA, Chen Z, Park JT, et al. Regulation of inflammatory phenotype in macrophages by a diabetes-induced long noncoding RNA. Diabetes. 2014; 63: 4249–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Ahmed AS, Schizas N, Li J, et al. Type 2 diabetes impairs tendon repair after injury in a rat model. J Appl Physiol (1985). 2012; 113: 1784–91. [DOI] [PubMed] [Google Scholar]
- 142.David MA, Jones KH, Inzana JA, et al. Tendon repair is compromised in a high fat diet-induced mouse model of obesity and type 2 diabetes. PLoS One. 2014; 9: e91234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Haley PJ. Species differences in the structure and function of the immune system. Toxicology. 2003; 188: 49–71. [DOI] [PubMed] [Google Scholar]
- 144.Seok J, Warren HS, Cuenca AG, et al. Genomic responses in mouse models poorly mimic human inflammatory diseases. Proc Natl Acad Sci USA 2013; 110: 3507–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Skelly DA, Squiers GT, McLellan MA, et al. Single-cell transcriptional profiling reveals cellular diversity and intercommunication in the mouse heart. Cell Reports. 2018; 22: 600–10. [DOI] [PubMed] [Google Scholar]
- 146.MacParland SA, Liu JC, Ma X-Z, et al. Single cell RNA sequencing of human liver reveals distinct intrahepatic macrophage populations. Nature Communications. 2018; 9: 4383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Cho DS, Doles JD. Single cell transcriptome analysis of muscle satellite cells reveals widespread transcriptional heterogeneity. Gene. 2017; 636: 54–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Tritschler S, Theis FJ, Lickert H, et al. Systematic single-cell analysis provides new insights into heterogeneity and plasticity of the pancreas. Molecular Metabolism. 2017; 6: 974–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Chen L, Li J, Zhang J, et al. S100a4 promotes liver fibrosis via activation of hepatic stellate cells. J Hepatol. 2015; 62: 156–64. [DOI] [PubMed] [Google Scholar]
- 150.Li Y, Bao J, Bian Y, et al. S100a4(+) macrophages are necessary for pulmonary fibrosis by activating lung fibroblasts. Front Immunol. 2018; 9: 1776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Stolk M, Klatte-Schulz F, Schmock A, et al. New insights into tenocyte-immune cell interplay in an in vitro model of inflammation. Sci Rep. 2017; 7: 9801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Meng XM, Nikolic-Paterson DJ, Lan HY. TGF-beta: The master regulator of fibrosis. Nat Rev Nephrol. 2016; 12: 325–38. [DOI] [PubMed] [Google Scholar]
- 153.Chang J, Thunder R, Most D, et al. Studies in flexor tendon wound healing: Neutralizing antibody to TGF-beta1 increases postoperative range of motion. Plast Reconstr Surg. 2000; 105: 148–55. [DOI] [PubMed] [Google Scholar]
- 154.Jorgensen HG, McLellan SD, Crossan JF, et al. Neutralisation of TGF beta or binding of VLA-4 to fibronectin prevents rat tendon adhesion following transection. Cytokine. 2005; 30: 195–202. [DOI] [PubMed] [Google Scholar]
- 155.Katzel EB, Wolenski M, Loiselle AE, et al. Impact of SMAD3 loss of function on scarring and adhesion formation during tendon healing. J Orthop Res. 2011; 29: 684–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Loiselle AE, Yukata K, Geary MB, et al. Development of antisense oligonucleotide (ASO) technology against TGF-beta signaling to prevent scarring during flexor tendon repair. J Orthop Res. 2015; 33: 859–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Freeberg MAT, Farhat YM, Easa A, et al. Serpine1 knockdown enhances mmp activity after flexor tendon injury in mice: Implications for adhesions therapy. Sci Rep. 2018; 8: 5810. [DOI] [PMC free article] [PubMed] [Google Scholar]