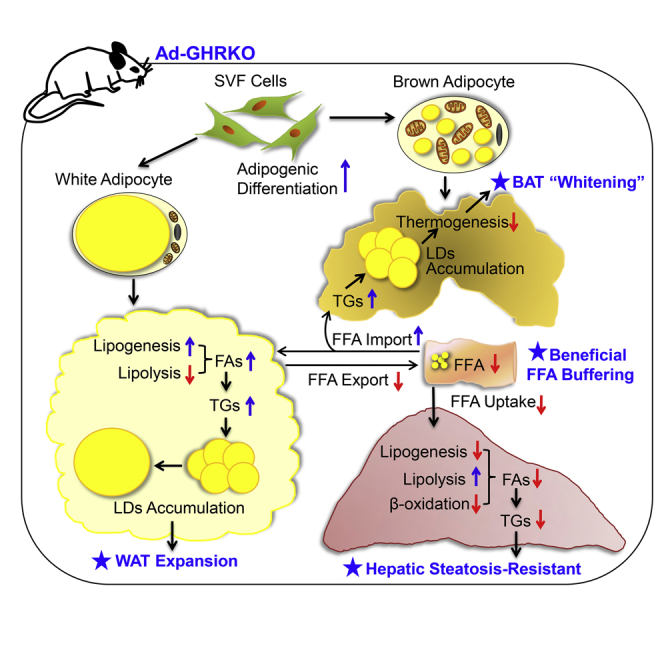
Summary
Growth hormone (GH) binds to its receptor (growth hormone receptor [GHR]) to exert its pleiotropic effects on growth and metabolism. Disrupted GH/GHR actions not only fail growth but also are involved in many metabolic disorders, as shown in murine models with global or tissue-specific Ghr deficiency and clinical observations. Here we constructed an adipose-specific Ghr knockout mouse model Ad-GHRKO and studied the metabolic adaptability of the mice when stressed by high-fat diet (HFD) or cold. We found that disruption of adipose Ghr accelerated dietary obesity but protected the liver from ectopic adiposity through free fatty acid trapping. The heat-producing brown adipose tissue burning and white adipose tissue browning induced by cold were slowed in the absence of adipose Ghr but were recovered after prolonged cold acclimation. We conclude that at the expense of excessive subcutaneous fat accumulation and lower emergent cold tolerance, down-tuning adipose GHR signaling emulates a healthy obesity situation which has metabolic advantages against HFD.
Subject Areas: Physiology, Cellular Physiology, Endocrinology, Diabetology
Graphical Abstract
Highlights
-
•
Adipose growth hormone receptor defect augments dietary obesity in mice
-
•
Lack of adipose growth hormone receptor prevents ectopic adiposity
-
•
Adipose growth hormone receptor defect benefits free fatty acid turnover
-
•
Adipose growth hormone receptor facilitates thermogenic response to cold
Physiology; Cellular Physiology; Endocrinology; Diabetology
Introduction
Growth hormone (GH), secreted from the anterior pituitary, has profound effects on the regulation of growth and development throughout the life of mammals (Moller and Jorgensen, 2009, Baumann, 2009). The biological response of GH is primarily dependent on ligand binding to its membrane receptor, GHR (Flores-Morales et al., 2006). Ghr is predominantly expressed in the liver, and is also abundant in the fat and muscle. Laron syndrome (LS) is a specific hereditary disease caused by mutations in the Ghr gene that leads to GH insensitivity, despite sufficient GH secretion. Patients with LS exhibit short stature, delayed puberty, and truncal obesity (Laron et al., 1966, Rosenbloom, 1999). On the other hand, individuals with LS have very low cancer risk and rarely develop diabetes, implying that Ghr is intimately involved in glucose and lipid metabolism (Guevara-Aguirre and Rosenbloom, 2015; Laron, 2015, Janecka et al., 2016). These phenotypes of LS are well reproduced in the Ghr gene-disrupted mouse model (GHR−/−) (Zhou et al., 1997, List et al., 2011). Going from LS to the other extreme, disturbance of glucose metabolism is one of the most common complications in patients with acromegaly with pituitary overproduction of GH. The prevalence and severity of diabetes in patients with acromegaly are considerably higher than those in other populations including people with high risk of diabetes (Dreval et al., 2014). Children with height at the upper end of gender- and age-specific scales were found to have greater adult adiposity and a higher risk of obesity onset than their shorter counterparts (Freedman et al., 2002, Stovitz et al., 2010). The unexpected uncoupling of obesity and type 2 diabetes observed in LS patients and related animal models prompts researchers to tease apart the exact role of adipose GHR in the multifaceted metabolic control.
The GHR−/− mice exhibit dwarfism, severe postnatal growth retardation, obesity, greatly decreased levels of serum insulin-like growth factor 1 (IGF-1), but elevated levels of GH, and longevity (List et al., 2011, Zhou et al., 1997, Berryman et al., 2006). Meanwhile, GHR−/− mice display enhanced insulin sensitivity and improved glucose homeostasis, even when challenged with high-fat (HF) diet (Coschigano et al., 2000, Coschigano et al., 2003, Dominici et al., 2000). Apart from the global Ghr-null models, tissue- and cell-specific Ghr knockout in liver (Fan et al., 2009, List et al., 2014), muscle (Vijayakumar et al., 2012a, Vijayakumar et al., 2012b, Mavalli et al., 2010, List et al., 2015), adipose tissues (List et al., 2013, List et al., 2019), macrophage (Lu et al., 2013), cardiac (Jara et al., 2016), and islet β cell (Wu et al., 2011) have been generated in mice, which have provided detailed information on physiological functions of GH signaling in local tissues. Most of the tissue-specific Ghr knockout (KO) mice showed similarities to GHR−/− mice (Wu et al., 2011, Vijayakumar et al., 2012a, Vijayakumar et al., 2012b, List et al., 2013, List et al., 2014, Fan et al., 2009, Sun and Bartke, 2014).
The adipose organ is composed of white and brown/beige adipocytes that are respective major sites of energy storage and cold-adapted thermogenesis. White adipose tissue (WAT) is predominately used to store excess energy in the form of triglycerides (TGs) and to provide free fatty acids (FFAs) under conditions of starvation or energy deficiency. In addition, WAT releases several important adipokines to regulate metabolic homeostasis. Cold exposure or β-adrenergic stimulation could induce browning of WAT, which results in a distinct type of fat cells called “beige adipocytes.” The increase of beige adipose has anti-obesity and anti-diabetic benefits (Giralt and Villarroya, 2013, Schrauwen et al., 2015). As a thermogenic organ in mammals, brown adipose tissue (BAT) is characterized by a high mitochondrial content to maintain normal body temperature and is involved in energy expenditure (Nicholls and Locke, 1984). The mitochondria within BAT are rich in uncoupling protein 1 (UCP1), which can dissipate chemical energy as heat through uncoupling oxidative respiration and ATP synthesis (Cohen and Spiegelman, 2015). Excess expansion of WAT, deficiency of thermogenic capacity, and low expression of Ucp1 gene in BAT have been linked to obesity and various metabolic diseases (Chathoth et al., 2018, Rui, 2017, Bonet et al., 2017, Bond and Ntambi, 2018).
The first adipose-specific deletion of Ghr mouse model (FaGHRKO) was reported by List et al., using adipocyte protein 2 (aP2) promoter as the Cre recombinase driver (List et al., 2013). Both GHR−/− and FaGHRKO mice displayed enlarged fat mass and adipocyte size (Zhou et al., 1997, List et al., 2013). The unexpected mild phenotypes of FaGHRKO, such as altered fat composition, lower circulating IGF-1, and leptin levels (List et al., 2013), were soon explained by the leaked activity of the aP2 promoter in many non-adipose cells types including macrophages and skeletal muscle (Wang et al., 2010, Lee et al., 2013). Utilization of different Cre mouse deletion strains and variance in diet schemes further confused the phenotype interpretation. Apart from the GHR−/− and FaGHRKO model, an adiponectin-Cre-driving adipocyte-specific GHRKO mice (AdGHRKO) was generated by List et al. during preparation of this study (List et al., 2019). The phenotypes of several other tissue-specific GHRKO mouse lines were exacerbated by HF diet (Lu et al., 2013, Wu et al., 2011), but both FaGHRKO and AdGHRKO did not try HF treatment. In the present work, we constructed an adiponectin-driven Cre transgenic mouse line (Ad-GHRKO) to attain targeted Ghr deletion in adipose. The high specificity and efficacy of the model provided an ideal tool to discern the contribution of adipose GH/GHR to the fat-related metabolic aberrance.
Excessive calorie intake from opulent eating has been fueling the epidemic of metabolic disorders. Therefore the leverage of adipose Ghr on susceptibility to dietary obesity and liver lesions was analyzed in this study with particular interest. Recently, GH's anti-obesity action has also been reported to be correlated with “browning” of inguinal white adipose (Nelson et al., 2018). Less effective adaptive thermogenesis is claimed to be the major cause of failure or the high recidivism rate of weight loss therapy (Dulloo and Schutz, 2015), in which GH interacts with neuroendocrine hormones and other metabolic and psychobehavioral factors to actively defend body energy storage. BAT thermogenesis in global GHRKO and Ames dwarf mice, the latter being depleted of GH/IGF-1 signaling and thyroid hormone function, were significantly enhanced (Darcy et al., 2016, Darcy and Bartke, 2017, Archana et al., 2011). However, the roles of GH in adipose thermogenesis had heavily interfered with systemic changes unrelated to GH action or alterations in multiple hormonal axes in these models. The functional roles of adipose Ghr control of adaptive thermogenesis remain unknown, which is the main focus of this study.
Results
Generation and Characterization of Ad-GHRKO Mice
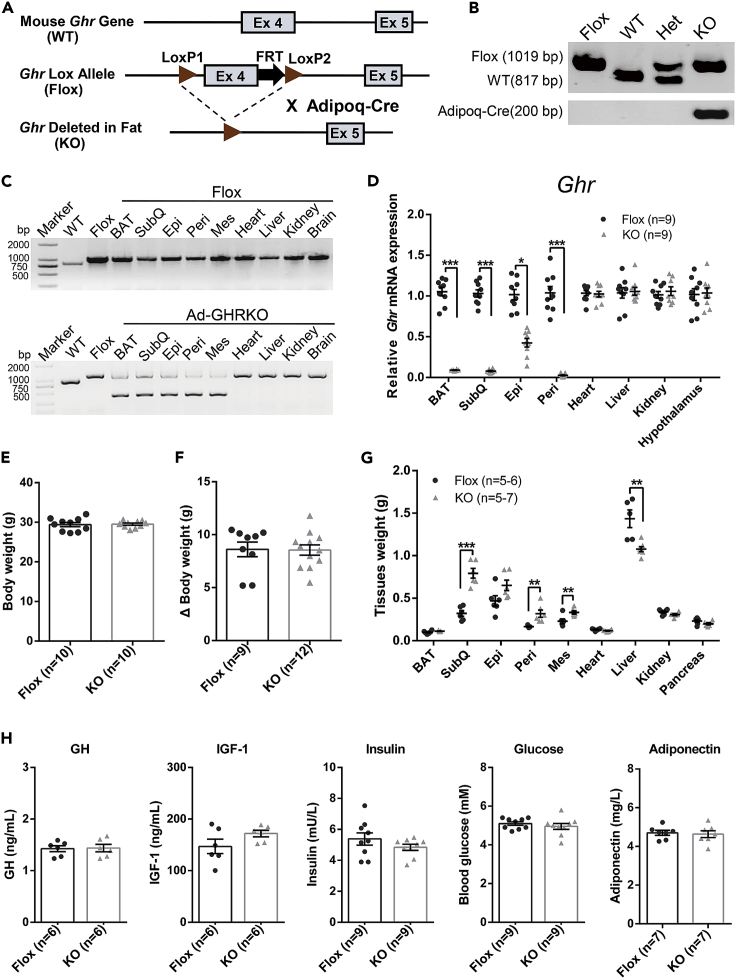
Beyond aP2 promoter, adiponectin (Adipoq) promoter serves as a second-generation adipose-targeting driver for gene engineering. We created an adipose-specific Ghr gene knockout (Ad-GHRKO) mouse model by crossing GHR-Floxed (Flox) mice with Adipoq-Cre mice (Figures 1A and 1B). The efficiency and specificity of Ghr deletion in the fat pads were validated at DNA and mRNA levels (Figures 1C and 1D). PCR genotyping with the primers listed in Table S1 showed that the Cre activity driven by the Adipoq promoter mediated adipocyte-specific Ghr disruption without affecting other tissues (Figure 1C). The mRNA expression level of Ghr was significantly and sufficiently decreased in most adipose tissues (Figure 1D).
Figure 1.
Generation and Characterization of Adipose-Specific Ghr Knockout Mice
(A) Schematic diagram of Ad-GHRKO mice construction.
(B) PCR genotyping of Flox and KO mice.
(C and D) PCR (C) and real-time PCR (D) analyses of Ghr expression level in various tissues. Note that a smaller deletion band due to Cre excision was detected only in adipose tissues of KO mice.
(E and F) The body weight (E) and increase in body weight (20 weeks versus 4 weeks) (F) of male Ad-GHRKO and Flox mice 20 weeks old.
(G) Tissues weight of male Ad-GHRKO and Flox mice 20 weeks old.
(H) Serum GH, IGF-1, insulin, blood glucose, and adiponectin levels of 20-week-old male Flox and Ad-GHRKO mice. WT, wild type; Flox, GHR-Floxed; KO, Ad-GHRKO; Het, heterozygote; BAT, brown adipose tissue; SubQ, subcutaneous white adipose tissue; Epi, epididymal white adipose tissue; Peri, perigonadal white adipose tissue; Mes, mesenteric white adipose tissue.
All values are presented as mean ± SEM. Statistical significance is shown as *p ≤ 0.05, **p ≤ 0.01, and ***p ≤ 0.001. See also Table S2.
To determine the effect of Ghr deficiency on body weight and composition, the body and organ weight of 20-week-old mice were measured. Unlike the FaGHRKO mice (List et al., 2013), no obvious differences in body weight and increase of body weight were observed between Ad-GHRKO and control mice in the rearing period (Figures 1E and 1F). Except for the BAT, all the different fat depots examined in Ad-GHRKO mice were heavier than the control (Figure 1G). Among them, the subcutaneous (subQ) WAT had the largest increase of weight, whereas the liver was markedly lighter and the weights of other tissues like the heart, kidney, and pancreas were slightly reduced in Ad-GHRKO mice (Figure 1G). We calculated the sum of the weight increase of the WAT fat pads and the weight decrease in the liver, heart, kidney, and pancreas. The results showed that the Ad-GHRKO mice had 0.996 ± 0.162 g weight gain in WAT, which was not equal to the 0.456 ± 0.194 g weight loss in other organs altogether. Among these organs, the liver was the only one displaying a significant decrease. Although other body parts such as muscles and bone were not measured, we concluded that the decreased liver weight was the principal, but not the only factor contributing to the unchanged body weight of Ad-GHRKO mice. Serological detection indicated that loss of Ghr in adipose tissues had little effect on circulating GH and IGF-1 levels in Ad-GHRKO mice (Figure 1H). Serum insulin, blood glucose levels, and adiponectin level also do not display any notable differences between 20-week-old Ad-GHRKO and Flox mice (Figure 1H).
The Absence of Adipose Ghr Exacerbated Diet-Induced Obesity but Improved Glucose Homeostasis
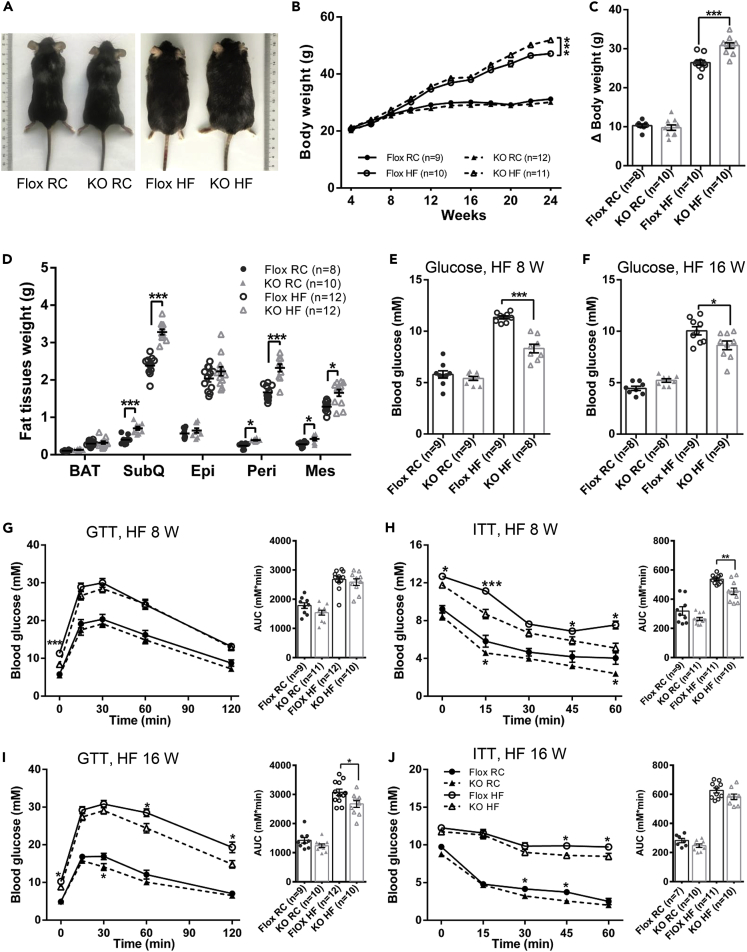
The weight gain of both Ad-GHRKO mice and the control littermates stayed in step with each other under regular chow, whereas an incrementally enlarged gap emanated between the two groups when fed with HF diet for 16 weeks (HF diet feeding was started from age 8 weeks) (Figures 2A–2C). Anatomical dissections revealed that the heavier body weight in Ad-GHRKO mice mainly originated from more weight gain in adipose tissues, especially the subcutaneous, perirenal, and mesenteric WAT (Figure 2D).
Figure 2.
Ad-GHRKO Exacerbates Dietary Obesity but Improves Glucose Homeostasis during HF Feeding
(A) Representative photographs of 24-week-old male Ad-GHRKO and Flox mice (HF fed for 16 weeks).
(B)The growth curves of Ad-GHRKO and Flox mice during RC or HF feeding.
(C) Increase in body weight (24 weeks versus 4 weeks) of 24-week-old male Ad-GHRKO and Flox mice (HF fed for 16 weeks).
(D) Fat tissue weight of 24-week-old male Ad-GHRKO and Flox mice (HF fed for 16 weeks).
(E and F) Fasting blood glucose levels of Ad-GHRKO and Flox mice fed with HF for 8 (E) and 16 weeks (F).
(G–J) Glucose tolerance test and insulin tolerance test of Ad-GHRKO and Flox mice fed with HF for 8 (G and H) and 16 weeks (I and J). Inset: The area under curves.
All values are presented as mean ± SEM. Statistical significance is shown as *p ≤ 0.05, **p ≤ 0.01, and ***p ≤ 0.001. See also Figures S1 and S5 and Table S2.
It has been reported that GHR−/− and AdGHRKO mice exhibited healthy adipose expansion with improved insulin sensitivity (Liu et al., 2004, Coschigano et al., 2003, Arum et al., 2014, List et al., 2019), whereas the basal glucose homeostasis of FaGHRKO mice was not changed (List et al., 2013). In good agreement with this, no significant difference was found in the serum glucose level in mice with or without adipose Ghr deletion, when fed with regular chow (RC). After loading with HF diet for 8 weeks, fasting blood glucose level in Ad-GHRKO mice was significantly lower than in Flox mice (KO 8.31 ± 1.18 mmol/L versus Flox 11.34 ± 0.44 mmol/L) (Figure 2E). Glucose tolerance test and insulin tolerance test assays revealed that Ad-GHRKO mice were more sensitive to insulin stimulation than control littermates after 8 weeks of HF diet (Figures 2G and 2H). With prolonged HF diet for 16 weeks, the fasted blood glucose level in Ad-GHRKO mice was still lower than that of the control mice (KO 8.63 ± 1.28 mmol/L versus Flox 10.26 ± 1.06 mmol/L) (Figure 2F), and Ad-GHRKO mice responded better to glucose challenge (Figure 2I) but no longer showed advantage in insulin sensitivity (Figure 2J). Overall, these results indicated that defective adipose GH signaling rescued, to a significant degree, the mice from hyperglycemia and insulin resistance imposed by oversupplied nutrients.
An Integration of Lipolysis, Lipogenesis, and Adipogenesis Magnified the HF-Induced Obesity and Limited FFA Liberation in Ad-GHRKO Mice
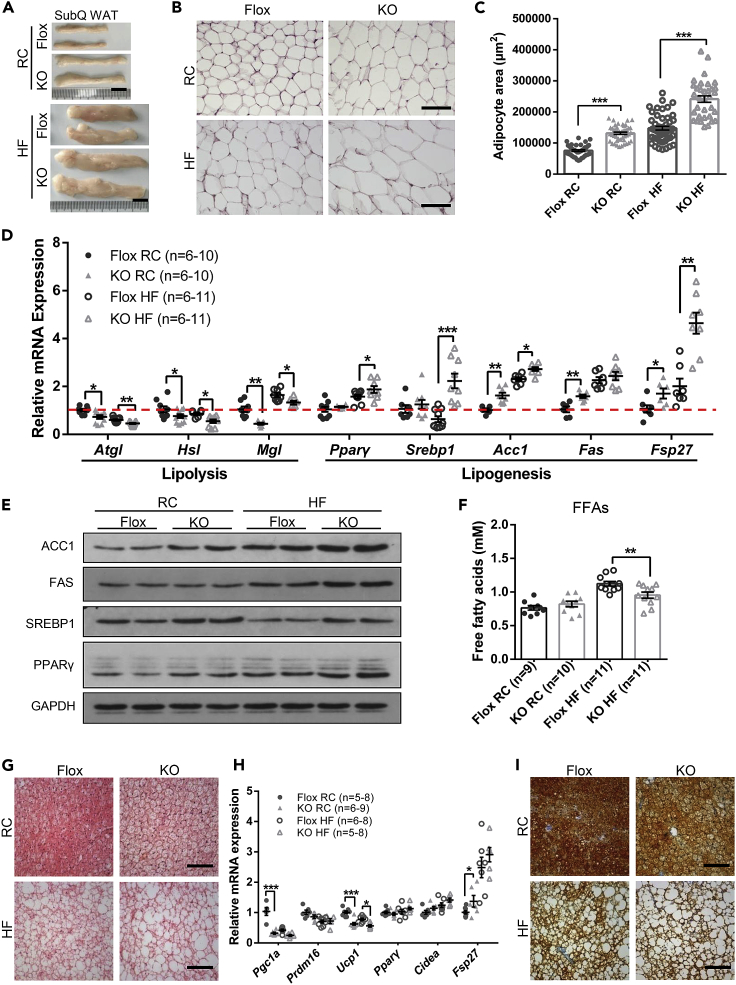
The obesity in GHR−/−, FaGHRKO, and AdGHRKO mice was attributed to fat expansion in both number and size of adipocytes (Zhou et al., 1997, Arum et al., 2014, List et al., 2013, List et al., 2019). As we expected, the subQ WATs of Ad-GHRKO mice were significantly larger than those of the control littermates when fed with RC, which was exaggerated by HF diet treatment (Figures 3A–3C). Furthermore, we examined the expression levels of lipid metabolism-related genes (Primers are listed in Table S1) and proteins. As expected, lipolysis-related genes (e.g., Atgl, Hsl, Mgl) were down-regulated, whereas genes involved in lipogenesis (e.g., Pparγ, Acc1, Fas) were up-regulated (Figure 3D) in subQ WAT of KO mice. The expression of Fsp27 gene, which encodes fat-specific protein 27 (Fsp27) and is an important positive regulator for fat droplet formation, was significantly higher in Ad-GHRKO mice than in the controls, especially when challenged with HF diet (Figure 3D). Consistently, the western blot results showed that deficiency of Ghr in adipose tissues dramatically enhanced the levels of ACC1, FAS, SREBP1, and PPARγ (Figure 3E). Although higher circulatory FFAs are commonly detected in obese individuals owing to expanded fat mass, serum FFA levels were comparable in the Ad-GHRKO versus control mice (Figure 3F). More intriguingly, despite the augmented obesity after HF diet, the FFA release of Ad-GHRKO mice was only mildly elevated by excessive feeding and was significantly lower than that of the HF-fed Flox control (Figure 3F). The data implied that lack of GHR in adipose improved local FFA recycling, which could provide a beneficial FFA buffering for systemic glucose metabolism and ectopic fat deposition in the liver.
Figure 3.
Ad-GHRKO Causes Lipid Accumulation in WAT and BAT
(A) Representative images of SubQ WAT from 24-week-old male Ad-GHRKO and Flox mice (HF fed for 16 weeks). Scale bar, 1 cm.
(B) H&E staining of SubQ WAT from 24-week-old male Ad-GHRKO and Flox mice (HF fed for 16 weeks). Scale bar, 100 μm.
(C) Quantification of adipocyte area of SubQ WAT.
(D) Relative expression levels of genes related to lipolysis and lipogenesis in SubQ WAT from 24-week-old male Ad-GHRKO and Flox mice (HF fed for 16 weeks).
(E) The levels of selected proteins in SubQ WAT of 24-week-old male Ad-GHRKO and Flox mice (HF fed for 16 weeks).
(F) Serum FFA levels of 24-week-old male Ad-GHRKO and Flox mice (HF fed for 16 weeks).
(G) BAT sections stained with H&E in 24-week-old male Ad-GHRKO and Flox mice (HF fed for 16 weeks). Scale bar, 100 μm.
(H) Relative expression levels of genes related to thermogenesis and lipogenesis in BAT of 24-week-old male Ad-GHRKO and Flox mice (HF fed for 16 weeks).
(I) Immunostaining of BAT sections with anti-UCP-1 antibody. Scale bar, 100 μm.
All values are presented as mean ± SEM. Statistical significance is shown as *p ≤ 0.05, **p ≤ 0.01, and ***p ≤ 0.001. See also Table S2.
Histology analysis showed that lipid droplet expansion and accumulation were displayed in BAT of Ad-GHRKO mice (Figure 3G). Compared with the Flox mice, the Ad-GHRKO mice had greatly increased expression of genes involved in fatty acid synthesis and lipid droplet accumulation (e.g., Ppar-γ, Cidea, and Fsp27) in BAT (Figure 3H). Meanwhile, the expression levels of thermogenic genes such as Pgc1α, Prdm16, and Ucp1 were significantly reduced in BAT by GHR deletion (Figure 3H). Consistent with the mRNA data, the staining of BAT UCP1 was significantly lower in Ad-GHRKO mice than in the controls, especially when challenged with HF diet (Figure 3I).
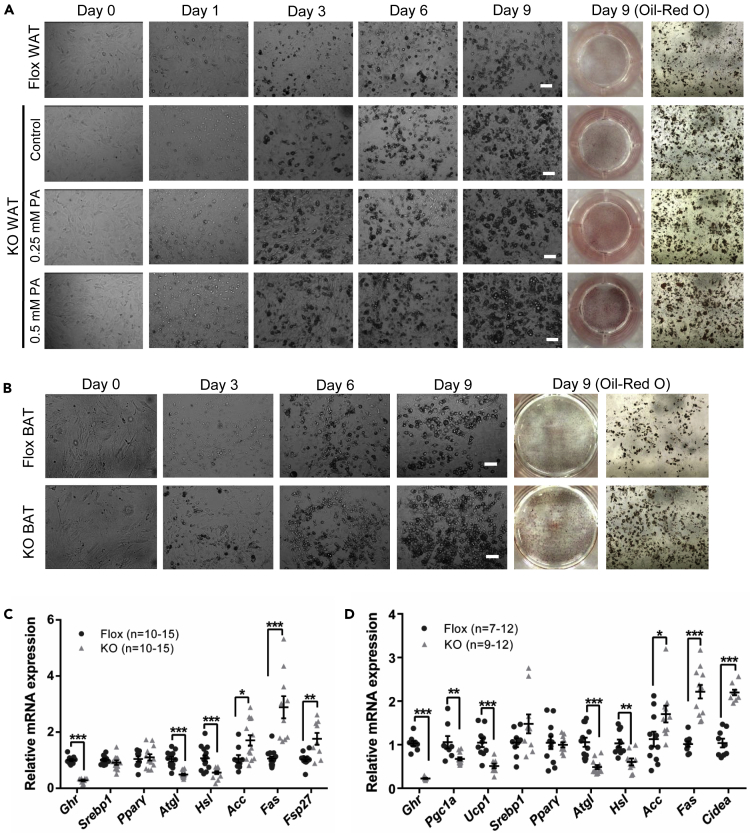
Not only adipocyte hypertrophy but also hyperplasia through activation of the adipogenic SVF cells contributes to obesity. SVF cells are a mixed cell population rich in pre-adipocytes, mesenchymal stem cells, and various inflammatory cells (Schipper et al., 2012, Riordan et al., 2009). The effect of Ghr disruption on adipocyte regeneration was determined by adipogenic differentiation of SVF cells separated from adipose tissues, in vitro. Consistent with in vivo experiments, the SVF cells isolated from WAT and BAT of KO mice showed higher differentiation potential (Figures 4A and 4B). Palmitic acid treatment, which simulated fatty acid influx, dose dependently increased the lipid droplets formation in the WAT SVF cells derived from Ad-GHRKO mice (Figure 4A). In addition, significant decreases in lipolysis gene (e.g., Atgl, Hsl) and increases in lipogenesis gene (e.g., Acc, Fas, Fsp27) expressions were observed in WAT SVF of Ad-GHTKO mice (Figure 4C). The results confirmed the participation of adipocyte differentiation in WAT expansion in adipose GHR-depleted mice. Meanwhile, SVF cells separated from BAT of Ad-GHRKO mice were more easily differentiated into adipocytes with lower expression levels of thermogenesis genes (e.g., Ucp1, Pgc1a) and lipolysis genes (e.g., Atgl, Hsl) and higher expression levels of lipogenesis genes (e.g., Acc, Fas, Cidea) than of the Flox mice (Figure 4D). Based on these results, we speculated that GHR could be required in the BAT thermogenic process, which is assessed in a later sector in the study.
Figure 4.
Deletion of Ghr in Adipocytes Promotes Adipogenic Differentiation of SVF Cells
(A) Induced adipogenic differentiation of SVF cells from subQ WAT of 20-week-old male Ad-GHRKO and Flox mice. Scale bar, 50 μm.
(B) Induced adipogenic differentiation of SVF cells from BAT of 20-week-old male Ad-GHRKO and Flox mice. Scale bar, 50 μm.
(C) Expression of Ghr and genes related to lipolysis and de novo lipogenesis in SVF cells from subQ WAT of 20-week-old male Ad-GHRKO and Flox mice.
(D) Expression of Ghr and genes related to thermogenesis, lipolysis, and de novo lipogenesis in SVF cells from BAT of 20-week-old male Ad-GHRKO and Flox mice.
All values are presented as mean ± SEM. Statistical significance is shown as *p ≤ 0.05, **p ≤ 0.01 and ***p ≤ 0.001.
Ad-GHRKO Mice Were More Resistant to HF-Induced Hepatic Steatosis
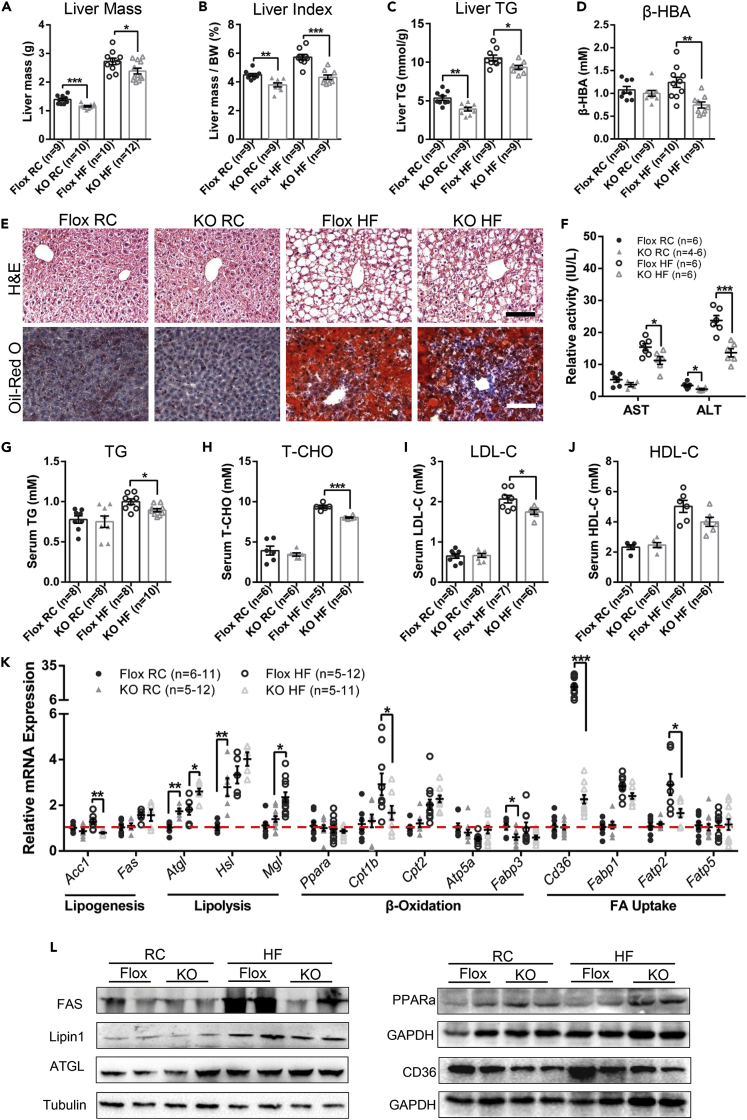
GH-stimulated hepatic secretion is the primary source of circulating IGF-1 (Sjogren et al., 1999). Serum levels of GH and IGF-1 have been shown to correlate with hepatic fat accumulation (Berryman et al., 2013). As a major site of lipogenesis and lipid oxidation, the liver is the central engine modulating whole-body metabolic homeostasis and is vulnerable to nutritional damage. We found that both the liver mass and liver index of Ad-GHRKO groups were significantly lower than those of the Flox mice, with or without HF diet stimuli (Figures 5A and 5B). Furthermore, deletion of Ghr in adipose brought down the liver TG contents significantly by 26.7% and 11.5% in mice fed with RC and HF for 16 weeks (Figure 5C), respectively. However, no apparent differences under RC condition were observed in hepatic histopathology by H&E and oil red O staining (Figure 5E). A significant increase of hepatic steatosis marked by vacuolated and lipid-laden hepatocytes was observed in both groups fed with HF for 16 weeks, but more manifest in the Flox mice than Ad-GHRKO mice (Figure 5E). The histopathologic changes and lipid deposit (Figure S1A, indicated by black arrows) occurred evidently in the liver of the Flox mice after only 8 weeks of HF feeding but not of the Ad-GHRKO mice (Figure S1A). Periodic acid-Schiff reaction of liver tissues showed that Ad-GHRKO mice have stronger glycogen synthesis capacity (Figure S1). The improvement of hepatic glycogen synthesis in Ad-GHRKO mice was conducive to the maintenance of blood glucose levels during HF feeding (Figures 2E and 2F). β-Hydroxybutyric acid (β-HBA) is the ketone body that indicates liver uptake of FFAs because it can only be produced from the mitochondrial β-oxidation of FFAs in the liver. Indeed, the serum β-HBA level in the HF-fed Ad-GHRKO mice was significantly lower than that in the Flox mice (Figure 5D). In conjunction with the lower FFA availability in Ad-GHRKO mice (Figure 3F), the results further demonstrated that an adipose FFA trapping is the mechanism underlying the hepatic protection against HF. The release of a variety of hepatic inflammation markers such as aspartate aminotransferase (AST) and alanine aminotransferase (ALT) is a biochemical sign indicating the loss of hepatocellular integrity. Compared with control littermates, a significant reduction in relative activity of both AST and ALT was observed in Ad-GHRKO mice fed with HF (Figure 5F). The influence of adipose Ghr deficiency on lipid profile was examined by measuring the serum triglyceride (TG), total cholesterol (T-CHO), high-density lipoprotein cholesterol (HDL-C), and low-density lipoprotein cholesterol (LDL-C) values. Despite the comparable baseline concentrations, the serum TG, T-CHO, and LDL-C in Ad-GHRKO groups were still significantly lower than in Flox mice under HF diet condition (Figures 5G–5J), confirming a suppressed development of hyperlipidemia in Ad-GHRKO mice.
Figure 5.
Ad-GHRKO Alleviates Hepatic Steatosis during HF Feeding
(A–C) Liver mass (A), liver index (liver weight/body weight %) (B), and liver triglyceride content (C) of 24-week-old male Ad-GHRKO and Flox mice (HF fed for 16 weeks).
(D) Serum β-HBA levels of 24-week-old male Ad-GHRKO and Flox mice (HF fed for 16 weeks).
(E) H&E and oil red O staining of liver sections of 24-week-old male Ad-GHRKO and Flox mice (HF fed for 16 weeks). Scale bar, 100 μm.
(F) Serum AST and ALT activity of 24-week-old male Ad-GHRKO and Flox mice (HF fed for 16 weeks).
(G–J) Serum TG (G), T-CHO (H), LDL-C (I), and HDL-C (J) levels of 24-week-old male Ad-GHRKO and Flox mice (HF fed for 16 weeks).
(K) Relative gene expression levels in livers of 24-week-old male Ad-GHRKO and Flox mice (HF fed for 16 weeks).
(L) Western blot analysis of proteins involved in de novo lipogenesis, lipolysis, β-oxidation, and FFA uptake in livers of 24-week-old male Ad-GHRKO and Flox mice (HF fed for 16 weeks).
All values are presented as mean ± SEM. Statistical significance is shown as *p ≤ 0.05, **p ≤ 0.01, and ***p ≤ 0.001. See also Figure S1 and Table S2.
In light of the findings of less lipid accumulation in the liver and a shrinking lipid and FFA reservoir in the circulation of Ad-GHRKO mice, we investigated whether the hepatic lipid turnover was directly altered. Compared with Flox mice under the same diet condition, the expression levels of lipogenic genes in the liver of Ad-GHRKO were slightly lower or even had no significant changes (e.g., Acc-1, Fas), whereas the expression of genes involved in lipolysis (e.g., Atgl, Hsl, Mgl) were markedly increased with statistical significance (Figure 5K). There were no significant differences in the expression of Pparα, Cpt2, and Atp5a. Down-regulation of Cpt1b and Fabp3 was detected in KO mice fed HF or RC diet (Figure 5K). We further found that the expression levels of genes related to fatty acid uptake, such as Cd36 and Fbtp2, were significantly lower in Ad-GHRKO than in Flox mice under HF diet condition (Figure 5K). In contrast to the Ad-GHRKO mice, the protein abundances of FAS and CD36 displayed a striking increase in Flox mice when fed with HF diet (Figure 5L). Meanwhile, the levels of PPARα and ATGL, responsible for fatty acid disposal and lipolysis, respectively, were up-regulated in Ad-GHRKO mice, especially when challenged with HF diet (Figure 5L). Phospholipids are the main components of lipid and are involved in adipogenesis. Lipin1 is known as a phosphatidic acid phosphohydrolase enzyme that catalyzes the conversion of phosphatidate to diacylglycerol in the cytoplasm (Han et al., 2006, Qi et al., 2017). Lipin1 is also expressed as a nuclear transcriptional coactivator to modulate the expression of other genes involved in lipid metabolism (Peterson et al., 2011). Up-regulation of Lipin 1 is implicated in hepatic steatosis. As we expected, the basal and HF-stimulated Lipin1 expression were higher in Flox mice than in Ad-GHRKO mice (Figure 5L). These results coalesced to demonstrate that the adipose-tissue-specific ablation of Ghr protected the liver from diet-induced steatosis via the sum of promoted lipolysis and dampened lipogenesis at both transcriptional and translational levels. Most importantly, the absence of adipose Ghr pathway trapped fatty acid locally in WAT and thereby restrained the fatty acid delivery necessary for lipid production in the liver.
Adipose Ghr Signaling Was Required in the Cold-Stimulated Adaptive Thermogenesis
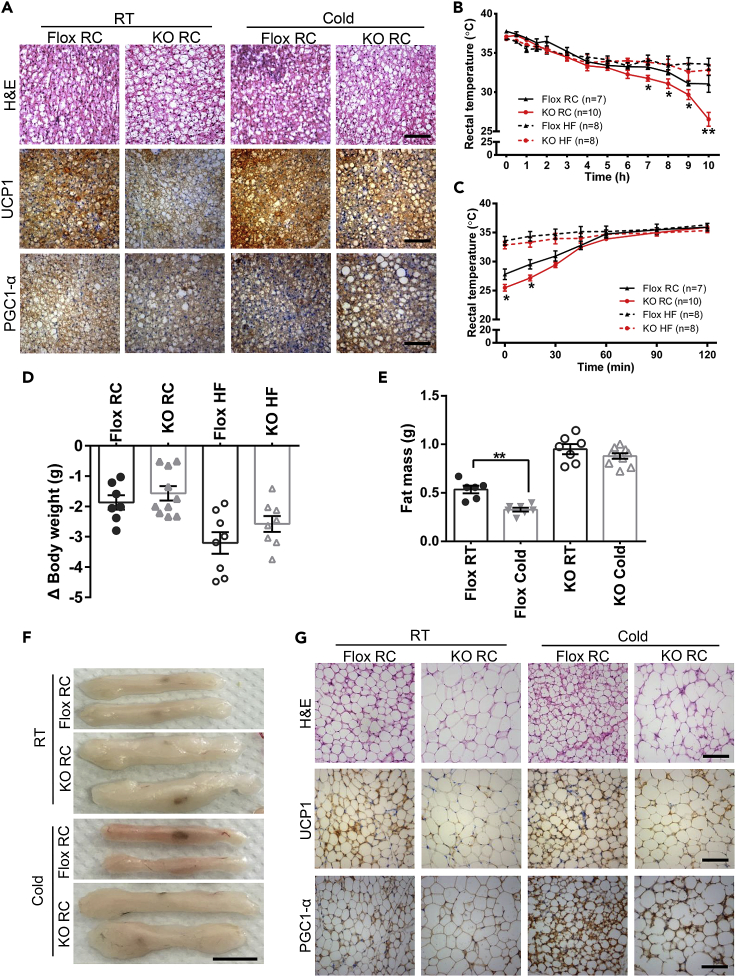
The high expression level of UCP1 is closely related to reduced body weight and increased insulin sensitivity (Kozak and Anunciado-Koza, 2008, Bond and Ntambi, 2018). PGC1α is a transcription co-activator related to mitochondrial function and adaptive thermogenesis (Puigserver et al., 1998). As we had observed above, BAT of Ad-GHRKO mice showed a “whitening” trend (Figure 3G). Besides, a down-regulation of thermogenic genes such as Pgc1α, Prdm16, and Ucp1 was detected in BAT tissue (Figure 3H) and BAT-originated SVF cells (Figure 4D) of Ad-GHRKO mice. Consistent with mRNA data, protein levels of UCP1 were significantly reduced in BAT of Ad-GHRKO mice, especially in HF-feeding condition (Figure 3I). H&E staining showed that cold exposure reduced the lipid accumulation in BAT of Ad-GHRKO mice, but qPCR and immunohostochemistry analysis demonstrated that the expression levels of UCP1 and PGC1α were still lower in BAT of KO mice than in Flox mice (Figures 6A and S2A). As UCP1 is the key mediator in adaptive thermogenesis, its down-regulation in Ad-GHRKO mice implied the potential involvement of GHR in energy expenditure and emergent heat generation.
Figure 6.
Ad-GHRKO Impairs Thermogenic Function and Significantly Inhibits Browning of SubQ WAT under Cold Stress
(A) H&E and Immunostaining of BAT from 16-week-old male Ad-GHRKO and Flox mice at room temperature or after 9-day cold stimulation. Scale bar, 100 μm.
(B) Body temperature of 16-week-old male Ad-GHRKO and Flox mice in the first day of cold stimulation.
(C) Body temperature recovery of 16-week-old male Ad-GHRKO and Flox mice at 25°C after first day of cold stimulation.
(D) Body weight reduction of 16-week-old Ad-GHRKO and Flox mice over 9-day cold stimulation.
(E) SubQ WAT mass of 16-week-old Ad-GHRKO and Flox mice over 9-day cold stimulation.
(F) Representative images of subQ WAT from 16-week-old male Ad-GHRKO and Flox mice at room temperature or after 9-day cold stimulation. Scale bar, 1 cm.
(G) H&E and Immunostaining of subQ WAT from 16-week-old male Ad-GHRKO and Flox mice at room temperature or after 9-day cold stimulation. Scale bar, 100 μm.
All values are presented as mean ± SEM. Statistical significance is shown as *p ≤ 0.05 and **p ≤ 0.01. See also Figures S2–S4.
To test the hypothesis, the mice were stressed under 4°C on a 10-/14-h cycle for 9 days and body temperature changes were recorded. On the first day of the experiment, the rectal temperature of RC-fed Ad-GHRKO mice dropped more sharply than that of the Flox mice during the cold exposure (KO 26.15 ± 2.34°C versus Flox 31.06 ± 2.86°C at the end of 10-h exposure) (Figure 6B). Consistently, the rectal temperatures of KO mice also recovered more slowly in room temperature after cold acclimation (Figure 6C). However, in the mouse groups fed with HF diet for 8 weeks, adipose Ghr knockout had no impact on the decrease and recovery rates of the rectal temperature (Figures 6B and 6C, dashed lines). The cold endurance of all four groups of mice, with deficient or intact adipose Ghr and regardless of the diet, had no significant difference in the fluctuations of body temperature from the second to the ninth day of cold induction (Figures S3 and S4). In addition, the Flox control lost more weight following cold stimulation than the Ad-GHRKO mice, under both HF and RC feeding conditions (Figure 6D). When subQ WAT mass was examined, the cold acclimation only caused statistically significant weight reduction in the Flox mice, not the Ad-GHRKO mice (Figure 6E). The browning capacity of WAT of Ad-GHRKO mice in response to cold stimulation for 9 days was also impaired as shown by the remarkable darkening of the subQ fat tissues of the Flox mice, but not the Ad-GHRKO mice (Figure 6F). Histological examination revealed that the cold-Flox-RC group had a new population of “beige” fat cells, which contain multiple small lipid droplets and high levels of UCP1 and PGC1α, which was not detected in the cold-KO-RC group (Figure 6G). Consistently, the expression of marker genes of beige and thermogenesis in subQ WAT (Figure S2B) and epi WAT (Figure S2C) were declined in Ad-GHRKO mice. These results confirmed the requirement of adipose GHR for emergent heat production and cold adaptation.
Discussion
GH is dubbed as an anti-obesity hormone for its actions of promoting lipolysis and inhibiting lipogenesis (Vijayakumar et al., 2010). Apart from the GHR−/− mice with global Ghr deletion and the FaGHRKO model, almost simultaneously and identical to our own design, an AdGHRKO model was generated by List et al. (List et al., 2013, List et al., 2019). These models not only represent a longitudinal technique updating but also have made valuable attempts to describe the pleiotropic role of adipose GH/GHR from different perspectives. Taking the great chance to compare the phenotypes of these four mouse lines (Table S2), we found that the basal metabolic parameters of our Ad-GHRKO mice were overly parallel to List et al.’s AdGHRKO mice. Metabolic alterations in adipose tissues and at the systemic level in both male and female AdGHRKO mice were reported, whereas sexual dimorphism was avoided in the present study by examining only male subjects. Also, we particularly focused on the response of Ad-GHRKO mice to the metabolic stress of HF feeding and cold acclimation, which was not studied in both FaGHRKO and AdGHRKO mice.
In agreement with the AdGHRKO and in contrast to the GHR−/− mice, the endocrine production of GH and IGF-1 were not intervened by adipose Ghr deletion in our Ad-GHRKO mice, and the serum glucose levels were not affected under RC feeding. In clinical and mouse model studies, GH-deficient individuals are more likely to develop insulin resistance and fatty liver (Xu et al., 2012, Berryman et al., 2010). A concurrence of excessive WAT fat deposition and reduction of liver weight was described in both AdGHRKO (List et al., 2019) and our Ad-GHRKO mice, which counteract each other to retain a balanced body weight. Lowered liver weight was mainly attributed to the reduction of TG content. The most striking characteristic of Ad-GHRKO mice was that this hepatic protection was dramatically accentuated by HF feeding. Loss of adipose Ghr exacerbated subcutaneous obesity but inhibited ectopic adiposity in the liver and muscle tissues, another preferred site of pathological fat deposition (Figure S5). The serum lipid index and liver function markers, such as AST and ALT, were correspondingly less affected by dietary overloading in mice lacking adipose Ghr. In the study of AdGHRKO mice, the causes of the inhibition of liver steatosis were postulated as declined serum insulin concentration and improved insulin sensitivity. However, differing from the AdGHRKO mice, the baseline insulin level was unchanged in our Ad-GHRKO model and the enhanced insulin sensitivity was only observed after the mice were subjected to HF. This implies that the insulin response may protect the liver from adverse environmental cues, but other mechanisms must be also operating.
The adipocyte-produced, insulin-sensitizing hormone adiponectin is inversely associated with many metabolic disorders. Circulating levels of adiponectin were elevated in GHR−/− mice (List et al., 2011, Lubbers et al., 2013) but decreased in the FaGHRKO and the AdGHRKO mice (List et al., 2013, List et al., 2019). However, the regulation of adiponectin to improve insulin sensitivity may be overridden by the divergent adipokine profile displayed in the AdGHRKO mice, as no impact of systemic glucose tolerance and insulin sensitivity was found. Interestingly, in our Ad-GHRKO mice, although adiponectin secretion was stable under RC (Figure 1H), it was more profoundly stimulated by HF diet in the Ad-GHRKO mice than in the control mice (Flox 5,758.4 ± 125.2 ng/mL versus KO 6,622.9 ± 703.6 ng/mL). The amplified expansion of adipocyte size (hypertrophy) and number (hyperplasia) might lead to elevated adiponectin secretion, which correlated with improved insulin sensitivity.
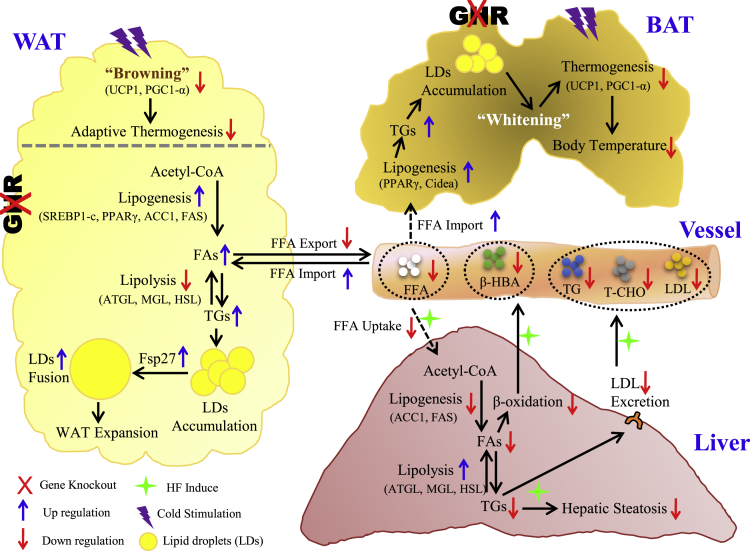
By analyzing the lipogenic and lipolytic factors at both genetic and protein levels, and the adipocyte differentiation of SVF cells in vitro, we found that GH/GHR acted as the master regulator of the comprehensive lipid-producing machinery. The absence of adipose Ghr overwhelmingly based WAT-dominant obesity in Ad-GHRKO mice. Losing GHR control, the privileged local fat production in adipose, especially in subQ WAT, limited FFA availability to the liver and muscle by depleting substrate supply. The significant decline of β-HBA synthesis further verified the lower level of FFA disposal in the liver of Ad-GHRKO mice. Suppressed FFA releasing into the circulation, accompanied by accelerated TG clearance and FFA uptake in adipose, is a phenomenon documented in some forms of obesity (Kalant et al., 2000). On the other hand, an impaired peripheral FFA trapping was supposed to be the etiology of insulin resistance, dyslipidemia, and liver diseases secondary to HIV lipodystrophy (van Wijk et al., 2005). We propose a mechanism of FFA trapping in adipose tissue to prevent ectopic adiposity as a good trade-off of excessive fat hoarding in adipose, where Ghr plays an instrumental part (Figure 7).
Figure 7.
Schematic Summary of the Local and Systemic Impacts of Adipose Ghr Deletion, in Response to Metabolic Stress
Disruption of Ghr in adipose tissue significantly increases de novo lipogenesis and facilitates the lipid droplet fusion in both WAT and BAT. Excessive local adiposity resulting from HF diet constitutes an adipose FFAs trapping effect that spares dietary liver damage via limited FFA availability and liver FFA uptake. Meanwhile, lacking adipose Ghr causes a less effective adaptive thermogenic response to acute cold stimulation, whereas the body temperature under chronic cold stress can be stabilized by thermal insulation from subcutaneous fat compensation.
The communication between adipose and liver, in respect of lipid metabolism and glucose homeostasis, has been investigated with different genetic engineering animal models. Some adipose-specific deletions of downstream post-receptor effectors of GH such as STAT5 in mice were studied and discussed (Johanna et al., 2011), but thus far the AdGHRKO and our Ad-GHRKO are the only mouse lines that underscored a clear-cut role of adipose GHR in fat draining from the liver to the adipose. The interorgan metabolic communication was also studied using genetically engineered animals lacking the key enzymes catalyzing lipolysis. The adipose-specific deletion of Hsl, rather than liver-specific Hsl deficiency, led to age-dependent lipodystrophy and liver steatosis (Xia et al., 2017), whereas intriguingly adipose-specific Atgl-KO mice had an opposite effect of reducing liver steatosis and improving hepatic insulin signaling (Schoiswohl et al., 2015). A similar liver protection from HF stress was reported in a systemic Mgl-KO mouse model, accompanied by appetite suppressing and decreased intestinal fat absorption (Yoshida et al., 2018).
The adaptive non-shivering thermogenesis mediated by UCP1-dependent activity in BAT is one of the facultative heat-producing strategies taken by mammals, especially in small animals, including rodents and infant humans, in response to limited nutrition supply or cold environment. Unlike the Ames dwarf mice and GHRKO mice, our Ad-GHRKO mice were less successful in maintaining normothermia, with declined gene expression and protein level of UCP1 and its regulator Pgc1α. WAT can acquire energy-burning potential by a “browning” process during which the white fat is converted into “beige.” Stimulations activation of WAT browning includes endogenous hormonal regulation and exogenous stress, typically cold. The extra energy expenditure raised from WAT browning is recently proposed to be anti-obesity (Cohen and Spiegelman, 2015, Giralt and Villarroya, 2013). Consistent with recent data indicating that GH is able to promote WAT browning through activation of STAT5 (Nelson et al., 2018), we found that Ghr ablation in adipose tissues was able to inhibit browning of subQ WAT under cold stimulation. In Ad-GHRKO mice, the contained heat dissipation by BAT and the inhibited WAT browning conspired to safeguard the fat storage attained from intensive energy intake. During the lipolysis processes, TGs from WAT are hydrolyzed into glycerol and FFAs. These FFAs then enter the mitochondria for lipid oxidation aimed to heat production. The low expression levels of lipolysis genes in WAT of Ad-GHRKO mice led to the decrease of circulating FFAs, which could restrict the calorie sources of KO mice. Vice versa, the redundant subQ white fat formed an insulation layer to assist Ad-GHRKO mice against cold, which might justify the transient impairment of temperature maintenance in Ad-GHRKO mice.
In summary, aided by the Ad-GHRKO mouse model, we were able to test the distinct roles of adipose GHR in response to metabolic stress represented by HF feeding and cold acclimation. Defective adipose Ghr action endowed the mice with healthy fat storage in WAT: trapping fat within adipose tissues spared the liver from steatosis. However, “healthy” obesity was developed at the expense of impaired cold endurance and WAT browning (Figure 7). For populations feeding on a diet densely packed with calories, the double dictation of adipose GHR in both the metabolic dysfunctions centered at liver damage and the therapeutic or cosmetic weight management is of far-reaching interest. More mechanistic investigation of adipokines and cross-organ FFA messaging in the setting of GHR manipulation, in particular, generation of a liver- and adipose-specific double GHRKO mouse model is our immediate endeavor.
Limitations of the Study
The most significant evidence provided by this study is that adipose deletion of Ghr inhibited ectopic adiposity, especially in the liver, with a trade-off of subcutaneous fat expansion. However, there are several limitations owing to constraints on methodology and the scope of the research. (1) The inherent problem of the flox/Cre mouse model we used for adipose-specific Ghr knockout is that it did not provide platforms to discern the regulations of GH in metabolism from organ development, which could be achieved by temporally controlled Ghr knockout in mature or young growing animals using, e.g., tamoxifen-inducible technique. (2) Despite high specificity, Cre recombination efficiency in our Cre mouse line was not ideal, which might arise from the less-sensitive floxed allele and the critical roles GHR plays in adipogenesis and potentially cell survival of adipocytes themselves. (3) Experiments could have been performed to tease apart the different contributions of GHR in subcutaneous (SAT) versus visceral fat (VAT) to liver metabolism, such as fat tissue from a certain depot in Ad-GHRKO mouse could be transplanted into control mouse and the results would tell us if GHR signalings in SAT and VAT cooperate or counteract each other in metabolic regulations.
Methods
All methods can be found in the accompanying Transparent Methods supplemental file.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (No. 81600668; No. 81471000l); the Ministry of Science and Technology of China (No. 2014DFA32120); and Research Project of Educational Commission of Liaoning, China (No.L2016023).
Author Contributions
Y.W. and L.R. conceived the study, designed the experiments, and wrote the manuscript. L.R. and X.W. performed experiments and collected and analyzed data. A.M., Y.L., and H.W. performed cold and cell experiments. J.W., J.S., and B.L. assisted with the qPCR and western Blot. M.G., D.W., W.G., and Y.L. performed animal breeding. R.J. and N.W. assisted with histological experiments. L.Z., L.H., X.C., B.L., and X.L. also designed the experiments and involved in discussion and writing the manuscript. L.Z., D.L., and X.L. provided language help. All authors reviewed the manuscript.
Declaration of Interests
The authors declare no competing interests.
Published: June 28, 2019
Footnotes
Supplemental Information can be found online at https://doi.org/10.1016/j.isci.2019.05.020.
Contributor Information
Bin Liang, Email: liangb@mail.kiz.ac.cn.
Xin Li, Email: xl15@nyu.edu.
Yingjie Wu, Email: yingjiewu@dmu.edu.cn.
Supplemental Information
References
- Archana V., Shoshana Y., Derek L.R. The intricate role of growth hormone in metabolism. Front. Endocrinol. 2011;2:1–11. doi: 10.3389/fendo.2011.00032. [DOI] [PMC free article] [PubMed] [Google Scholar]; Archana, V., Shoshana, Y. & Derek, L. R. 2011. The intricate role of growth hormone in metabolism. Front. Endocrinol., 2, 1-11. [DOI] [PMC free article] [PubMed]
- Arum O., Boparai R.K., Saleh J.K., Wang F., Dirks A.L., Turner J.G., Kopchick J.J., Liu J.L., Khardori R.K., Bartke A. Specific suppression of insulin sensitivity in growth hormone receptor gene-disrupted (GHR-KO) mice attenuates phenotypic features of slow aging. Aging Cell. 2014;13:981–1000. doi: 10.1111/acel.12262. [DOI] [PMC free article] [PubMed] [Google Scholar]; Arum, O., Boparai, R. K., Saleh, J. K., Wang, F., Dirks, A. L., Turner, J. G., Kopchick, J. J., Liu, J. L., Khardori, R. K. & Bartke, A. 2014. Specific suppression of insulin sensitivity in growth hormone receptor gene-disrupted (GHR-KO) mice attenuates phenotypic features of slow aging. Aging Cell, 13, 981-1000. [DOI] [PMC free article] [PubMed]
- Baumann G.P. Growth hormone isoforms. Growth Horm. IGF Res. 2009;19:333–340. doi: 10.1016/j.ghir.2009.04.011. [DOI] [PubMed] [Google Scholar]; Baumann, G. P. 2009. Growth hormone isoforms. Growth Horm. IGF Res., 19, 333-340. [DOI] [PubMed]
- Berryman D.E., List E.O., Kohn D.T., Coschigano K.T., Seeley R.J., Kopchick J.J. Effect of growth hormone on susceptibility to diet-induced obesity. Endocrinology. 2006;147:2801–2808. doi: 10.1210/en.2006-0086. [DOI] [PubMed] [Google Scholar]; Berryman, D. E., List, E. O., Kohn, D. T., Coschigano, K. T., Seeley, R. J. & Kopchick, J. J. 2006. Effect of growth hormone on susceptibility to diet-induced obesity. Endocrinology, 147, 2801-2808. [DOI] [PubMed]
- Berryman D.E., List E.O., Palmer A.J., Chung M.Y., Wright-Piekarski J., Lubbers E., O'connor P., Okada S., Kopchick J.J. Two-year body composition analyses of long-lived GHR null mice. J. Gerontol. A Biol. Sci. Med. Sci. 2010;65:31–40. doi: 10.1093/gerona/glp175. [DOI] [PMC free article] [PubMed] [Google Scholar]; Berryman, D. E., List, E. O., Palmer, A. J., Chung, M. Y., Wright-Piekarski, J., Lubbers, E., O'connor, P., Okada, S. & Kopchick, J. J. 2010. Two-year body composition analyses of long-lived GHR null mice. J. Gerontol. A Biol. Sci. Med. Sci., 65, 31-40. [DOI] [PMC free article] [PubMed]
- Berryman D.E., Glad C.A., List E.O., Johannsson G. The GH/IGF-1 axis in obesity: pathophysiology and therapeutic considerations. Nat. Rev. Endocrinol. 2013;9:346–356. doi: 10.1038/nrendo.2013.64. [DOI] [PubMed] [Google Scholar]; Berryman, D. E., Glad, C. A., List, E. O. & Johannsson, G. 2013. The GH/IGF-1 axis in obesity: pathophysiology and therapeutic considerations. Nat. Rev. Endocrinol., 9, 346-356. [DOI] [PubMed]
- Bond L.M., Ntambi J.M. UCP1 deficiency increases adipose tissue monounsaturated fatty acid synthesis and trafficking to the liver. J. Lipid Res. 2018;59:224–236. doi: 10.1194/jlr.M078469. [DOI] [PMC free article] [PubMed] [Google Scholar]; Bond LM & Ntambi JM.. 2018. UCP1 deficiency increases adipose tissue monounsaturated fatty acid synthesis and trafficking to the liver. J Lipid Res. , 59, 224-236. [DOI] [PMC free article] [PubMed]
- Bonet M.L., Mercader J., Palou P. A nutritional perspective on UCP1-dependent thermogenesis. Biochimie. 2017;134:99–117. doi: 10.1016/j.biochi.2016.12.014. [DOI] [PubMed] [Google Scholar]; Bonet ML, Mercader J & Palou P. 2017. A nutritional perspective on UCP1-dependent thermogenesis. Biochimie., 134, 99-117. [DOI] [PubMed]
- Chathoth S., Ismail M.H., Vatte C., Cyrus C., Al Ali Z., Ahmed K.A., Acharya S., Al Barqi A.M., Al Ali A. Association of Uncoupling Protein 1 (UCP1) gene polymorphism with obesity: a case-control study. BMC Med. Genet. 2018;19:203. doi: 10.1186/s12881-018-0715-5. [DOI] [PMC free article] [PubMed] [Google Scholar]; Chathoth S, Ismail MH, Vatte C, Cyrus C, AL Ali Z, Ahmed KA, Acharya S, Al Barqi AM & Al Ali A.. 2018. Association of Uncoupling Protein 1 (UCP1) gene polymorphism with obesity: a case-control study. BMC Med. Genet., 19, 203. [DOI] [PMC free article] [PubMed]
- Cohen P., Spiegelman B.M. Brown and beige fat: molecular parts of a thermogenic machine. Diabetes. 2015;64:2346–2351. doi: 10.2337/db15-0318. [DOI] [PMC free article] [PubMed] [Google Scholar]; Cohen, P. & Spiegelman, B. M. 2015a. Brown and beige fat: molecular parts of a thermogenic machine. Diabetes, 64, 2346-2351. [DOI] [PMC free article] [PubMed]
- Coschigano K.T., Clemmons D., Bellush L.L., Kopchick J.J. Assessment of growth parameters and life span of GHR/BP gene-disrupted mice. Endocrinology. 2000;141:2608–2613. doi: 10.1210/endo.141.7.7586. [DOI] [PubMed] [Google Scholar]; Coschigano, K. T., Clemmons, D., Bellush, L. L. & Kopchick, J. J. 2000. Assessment of growth parameters and life span of GHR/BP gene-disrupted mice. Endocrinology, 141, 2608-2613. [DOI] [PubMed]
- Coschigano K.T., Holland A.N., Riders M.E., List E.O., Flyvbjerg A., Kopchick J.J. Deletion, but not antagonism, of the mouse growth hormone receptor results in severely decreased body weights, insulin, and insulin-like growth factor I levels and increased life span. Endocrinology. 2003;144:3799–3810. doi: 10.1210/en.2003-0374. [DOI] [PubMed] [Google Scholar]; Coschigano, K. T., Holland, A. N., Riders, M. E., List, E. O., Flyvbjerg, A. & Kopchick, J. J. 2003. Deletion, but not antagonism, of the mouse growth hormone receptor results in severely decreased body weights, insulin, and insulin-like growth factor I levels and increased life span. Endocrinology, 144, 3799-3810. [DOI] [PubMed]
- Darcy J., Bartke A. Functionally enhanced brown adipose tissue in Ames dwarf mice. Adipocyte. 2017;6:62–67. doi: 10.1080/21623945.2016.1274470. [DOI] [PMC free article] [PubMed] [Google Scholar]; Darcy, J. & Bartke, A. 2017. Functionally enhanced brown adipose tissue in Ames dwarf mice. Adipocyte, 6, 62-67. [DOI] [PMC free article] [PubMed]
- Darcy J., Mcfadden S., Fang Y., Huber J.A., Zhang C., Sun L.Y., Bartke A. Brown adipose tissue function is enhanced in long-lived, male ames dwarf mice. Endocrinology. 2016;157:4744–4753. doi: 10.1210/en.2016-1593. [DOI] [PMC free article] [PubMed] [Google Scholar]; Darcy, J., Mcfadden, S., Fang, Y., Huber, J. A., Zhang, C., Sun, L. Y. & Bartke, A. 2016. Brown adipose tissue function is enhanced in long-lived, male ames dwarf mice. Endocrinology, 157, :4744-4753. [DOI] [PMC free article] [PubMed]
- Dominici F.P., Arostegui Diaz G., Bartke A., Kopchick J.J., Turyn D. Compensatory alterations of insulin signal transduction in liver of growth hormone receptor knockout mice. J. Endocrinol. 2000;166:579–590. doi: 10.1677/joe.0.1660579. [DOI] [PubMed] [Google Scholar]; Dominici, F. P., Arostegui DIAZ, G., Bartke, A., Kopchick, J. J. & Turyn, D. 2000. Compensatory alterations of insulin signal transduction in liver of growth hormone receptor knockout mice. J. Endocrinol., 166, 579-590. [DOI] [PubMed]
- Dreval A.V., Trigolosova I.V., Misnikova I.V., Kovalyova Y.A., Tishenina R.S., Barsukov I.A. Prevalence of diabetes mellitus in patients with acromegaly. Endocr. Connect. 2014;3:93–98. doi: 10.1530/EC-14-0021. [DOI] [PMC free article] [PubMed] [Google Scholar]; Dreval, A. V., Trigolosova, I. V., Misnikova, I. V., Kovalyova, Y. A., Tishenina, R. S. & Barsukov, I. A. 2014. Prevalence of diabetes mellitus in patients with acromegaly. Endocr. Connect.., 3, 93-98. [DOI] [PMC free article] [PubMed]
- Dulloo A.G., Schutz Y. Adaptive thermogenesis in resistance to obesity therapies: issues in quantifying thrifty energy expenditure phenotypes in humans. Curr. Obes. Rep. 2015;4:230–240. doi: 10.1007/s13679-015-0156-9. [DOI] [PubMed] [Google Scholar]; Dulloo, A. G. & Schutz, Y. 2015. Adaptive thermogenesis in resistance to obesity therapies: issues in quantifying thrifty energy expenditure phenotypes in humans.. Curr. Obes. Rep., 4, 230-240. [DOI] [PubMed]
- Fan Y., Menon R.K., Cohen P., Hwang D., Clemens T., Digirolamo D.J., Kopchick J.J., Le Roith D., Trucco M., Sperling M.A. Liver-specific deletion of the growth hormone receptor reveals essential role of growth hormone signaling in hepatic lipid metabolism. J. Biol. Chem. 2009;284:19937–19944. doi: 10.1074/jbc.M109.014308. [DOI] [PMC free article] [PubMed] [Google Scholar]; Fan, Y., Menon, R. K., Cohen, P., Hwang, D., Clemens, T., Digirolamo, D. J., Kopchick, J. J., Le Roith, D., Trucco, M. & Sperling, M. A. 2009. Liver-specific deletion of the growth hormone receptor reveals essential role of growth hormone signaling in hepatic lipid metabolism. J. Biol. Chem., 284, 19937-19944. [DOI] [PMC free article] [PubMed]
- Flores-Morales A., Greenhalgh C.J., Norstedt G., Rico-Bautista E. Negative regulation of growth hormone receptor signaling. Mol. Endocrinol. 2006;20:241–253. doi: 10.1210/me.2005-0170. [DOI] [PubMed] [Google Scholar]; Flores-Morales, A., Greenhalgh, C. J., Norstedt, G. & Rico-Bautista, E. 2006. Negative regulation of growth hormone receptor signaling. Mol. Endocrinol., 20, 241-253. [DOI] [PubMed]
- Freedman D.S., Khan L.K., Mei Z., Dietz W.H., Srinivasan S.R., Berenson G.S. Relation of childhood height to obesity among adults: the bogalusa heart study. Pediatrics. 2002;109:e23. doi: 10.1542/peds.109.2.e23. [DOI] [PubMed] [Google Scholar]; Freedman, D. S., Khan, L. K., Mei, Z., Dietz, W. H., Srinivasan, S. R. & Berenson, G. S. 2002. Relation of childhood height to obesity among adults: the bogalusa heart study.. Pediatrics, 109, e23-e23. [DOI] [PubMed]
- Giralt M., Villarroya F. White, brown, beige/brite: different adipose cells for different functions? Endocrinology. 2013;154:2992–3000. doi: 10.1210/en.2013-1403. [DOI] [PubMed] [Google Scholar]; Giralt, M. & Villarroya, F. 2013. White, brown, beige/brite: different adipose cells for different functions? Endocrinology, 154, 2992-3000. [DOI] [PubMed]
- Guevara-Aguirre J., Rosenbloom A.L. Obesity, diabetes and cancer: insight into the relationship from a cohort with growth hormone receptor deficiency. Diabetologia. 2015;58:37–42. doi: 10.1007/s00125-014-3397-3. [DOI] [PubMed] [Google Scholar]; Guevara-Aguirre J & Rosenbloom AL.. 2015. Obesity, diabetes and cancer: insight into the relationship from a cohort with growth hormone receptor deficiency. Diabetologia, 58, 37-42. [DOI] [PubMed]
- Han G.S., Wu W.I., Carman G.M. The Saccharomyces cerevisiae Lipin homolog is a Mg2+-dependent phosphatidate phosphatase enzyme. J. Biol. Chem. 2006;281:9210–9218. doi: 10.1074/jbc.M600425200. [DOI] [PMC free article] [PubMed] [Google Scholar]; Han, G. S., Wu, W. I. & Carman, G. M. 2006. The Saccharomyces cerevisiae Lipin homolog is a Mg2+-dependent phosphatidate phosphatase enzyme. J. Biol. Chem., 281, 9210-9218. [DOI] [PMC free article] [PubMed]
- Janecka A., Kołodziej-Rzepa M., Biesaga B. Clinical and molecular features of laron syndrome, a genetic disorder protecting from cancer. In Vivo. 2016;30:375–381. [PubMed] [Google Scholar]; Janecka A, Kołodziej-Rzepa M & Biesaga B. 2016. Clinical and molecular features of laron syndrome, a genetic disorder protecting from cancer. In Vivo, 30, 375-381. [PubMed]
- Jara A., Liu X., Sim D., Benner C.M., Duran-Ortiz S., Qian Y., List E.O., Berryman D.E., Kim J.K., Kopchick J.J. Cardiac-specific disruption of GH receptor alters glucose homeostasis while maintaining normal cardiac performance in adult male mice. Endocrinology. 2016;157:1929–1941. doi: 10.1210/en.2015-1686. [DOI] [PMC free article] [PubMed] [Google Scholar]; Jara A, Liu X, Sim D, Benner CM, Duran-Ortiz S, Qian Y, List EO, Berryman DE, Kim JK & Kopchick JJ, . 2016. Cardiac-specific disruption of GH receptor alters glucose homeostasis while maintaining normal cardiac performance in adult male mice. Endocrinology, 157, 1929-1941. [DOI] [PMC free article] [PubMed]
- Johanna L.B., Caroline N.N., Mayumi I., Lauren A.M., Linda M.K., Timothy R.M., Elizabeth E.P., Michael J.W. GH-dependent STAT5 signaling plays an important role in hepatic lipid metabolism. Endocrinology. 2011;152:181–192. doi: 10.1210/en.2010-0537. [DOI] [PubMed] [Google Scholar]; Johanna L.B., Caroline N.N., Mayumi, I., Lauren A.M., Linda M.K., Timothy R.M., Elizabeth E.P. & Michael J.W. 2011. GH-dependent STAT5 signaling plays an important role in hepatic lipid metabolism. Endocrinology, 152, 181-192. [DOI] [PubMed]
- Kalant D., Ph Lis S., Fielding B.A., Frayn K.N., Cianflone K., Sniderman A.D. Increased postprandial fatty acid trapping in subcutaneous adipose tissue in obese women. J. Lipid Res. 2000;41:1963–1968. [PubMed] [Google Scholar]; Kalant, D., Ph Lis, S., Fielding, B. A., Frayn, K. N., Cianflone, K. & Sniderman, A. D. 2000. Increased postprandial fatty acid trapping in subcutaneous adipose tissue in obese women.. J. Lipid Res., 41, 1963-1968. [PubMed]
- Kozak L.P., Anunciado-Koza R. UCP1: its involvement and utility in obesity. Int. J. Obes. (Lond.) 2008;32(Suppl 7):S32–S38. doi: 10.1038/ijo.2008.236. [DOI] [PMC free article] [PubMed] [Google Scholar]; Kozak, L. P. & Anunciado-Koza, R. 2008. UCP1: its involvement and utility in obesity. Int. J. Obes. (Lond.), 32 Suppl 7, S32-S38. [DOI] [PMC free article] [PubMed]
- Laron Z. Lessons from 50 years of study of laron syndrome. Endocr. Pract. 2015;21:1395–1402. doi: 10.4158/EP15939.RA. [DOI] [PubMed] [Google Scholar]; Laron, Z.. 2015. Lessons from 50 years of study of laron syndrome. Endocr Pract., 21, 1395-1402. [DOI] [PubMed]
- Laron Z., Pertzelan A., Mannheimer S. Genetic pituitary dwarfism with high serum concentation of growth hormone–a new inborn error of metabolism? Isr. J. Med. Sci. 1966;2:152–155. [PubMed] [Google Scholar]; Laron, Z., Pertzelan, A. & Mannheimer, S. 1966. Genetic pituitary dwarfism with high serum concentation of growth hormone-a new inborn error of metabolism? Isr. J. Med. Sci., 2, 152-155. [PubMed]
- Lee K.Y., Russell S.J., Ussar S., Boucher J., Vernochet C., Mori M.A., Smyth G., Rourk M., Cederquist C., Rosen E.D. Lessons on conditional gene targeting in mouse adipose tissue. Diabetes. 2013;62:864–874. doi: 10.2337/db12-1089. [DOI] [PMC free article] [PubMed] [Google Scholar]; Lee, K. Y., Russell, S. J., Ussar, S., Boucher, J., Vernochet, C., Mori, M. A., Smyth, G., Rourk, M., Cederquist, C., Rosen, E. D., Kahn, B. B. & Kahn, C. R. 2013. Lessons on conditional gene targeting in mouse adipose tissue. Diabetes, 62, 864-874. [DOI] [PMC free article] [PubMed]
- List E.O., Sackmann-Sala L., Berryman D.E., Funk K., Kelder B., Gosney E.S., Okada S., Ding J., Cruz-Topete D., Kopchick J.J. Endocrine parameters and phenotypes of the growth hormone receptor gene disrupted (GHR-/-) mouse. Endocr. Rev. 2011;32:356–386. doi: 10.1210/er.2010-0009. [DOI] [PMC free article] [PubMed] [Google Scholar]; List, E. O., Sackmann-Sala, L., Berryman, D. E., Funk, K., Kelder, B., Gosney, E. S., Okada, S., Ding, J., Cruz-Topete, D. & Kopchick, J. J. 2011. Endocrine parameters and phenotypes of the growth hormone receptor gene disrupted (GHR-/-) mouse. Endocr. Rev., 32, 356-386. [DOI] [PMC free article] [PubMed]
- List E.O., Berryman D.E., Funk K., Gosney E.S., Jara A., Kelder B., Wang X., Kutz L., Troike K., Lozier N. The role of GH in adipose tissue: lessons from adipose-specific GH receptor gene-disrupted mice. Mol. Endocrinol. 2013;27:524–535. doi: 10.1210/me.2012-1330. [DOI] [PMC free article] [PubMed] [Google Scholar]; List, E. O., Berryman, D. E., Funk, K., Gosney, E. S., Jara, A., Kelder, B., Wang, X., Kutz, L., Troike, K., Lozier, N., Mikula, V., Lubbers, E. R., Zhang, H., Vesel, C., Junnila, R. K., Frank, S. J., Masternak, M. M., Bartke, A. & Kopchick, J. J. 2013. The role of GH in adipose tissue: lessons from adipose-specific GH receptor gene-disrupted mice. Mol. Endocrinol., 27, 524-535. [DOI] [PMC free article] [PubMed]
- List E.O., Berryman D.E., Funk K., Jara A., Kelder B., Wang F., Stout M.B., Zhi X., Sun L., White T.A. Liver-specific GH receptor gene-disrupted (LiGHRKO) mice have decreased endocrine IGF-I, increased local IGF-I, and altered body size, body composition, and adipokine profiles. Endocrinology. 2014;155:1793–1805. doi: 10.1210/en.2013-2086. [DOI] [PMC free article] [PubMed] [Google Scholar]; List, E. O., Berryman, D. E., Funk, K., Jara, A., Kelder, B., Wang, F., Stout, M. B., Zhi, X., Sun, L., White, T. A., Lebrasseur, N. K., Pirtskhalava, T., Tchkonia, T., Jensen, E. A., Zhang, W., Masternak, M. M., Kirkland, J. L. 2014. Liver-specific GH receptor gene-disrupted (LiGHRKO) mice have decreased endocrine IGF-I, increased local IGF-I, and altered body size, body composition, and adipokine profiles. Endocrinology, 155, 1793-1805. [DOI] [PMC free article] [PubMed]
- List E.O., Berryman D.E., Ikeno Y., Hubbard G.B., Funk K., Comisford R., Young J.A., Stout M.B., Tchkonia T., Masternak M.M. Removal of growth hormone receptor (GHR) in muscle of male mice replicates some of the health benefits seen in global GHR-/- mice. Aging. 2015;7:500–512. doi: 10.18632/aging.100766. [DOI] [PMC free article] [PubMed] [Google Scholar]; List EO, Berryman DE, Ikeno Y, Hubbard GB, Funk K, Comisford R, Young JA, Stout MB, Tchkonia T, Masternak MM, Bartke A, Kirkland JL, Miller RA & JJ., K. 2015. Removal of growth hormone receptor (GHR) in muscle of male mice replicates some of the health benefits seen in global GHR-/- mice. Aging, 7, 500-512. [DOI] [PMC free article] [PubMed]
- List E.O., Berryman D.E., Buchman M., Parker C., Funk K., Bell S., Duran-Ortiz S., Qian Y., Young J.A., Wilson C. Adipocyte-specific GH receptor null (AdGHRKO) mice have enhanced insulin sensitivity with reduced liver triglycerides. Endocrinology. 2019;160:68–80. doi: 10.1210/en.2018-00850. [DOI] [PMC free article] [PubMed] [Google Scholar]; List E.O., Berryman D.E., Buchman M., Parker C., Funk K., Bell S., Duran-Ortiz S., Qian Y., Young J.A., Wilson C., Slyby J., Mckenna S., Jensen E.A. & J.J., K. 2019. Adipocyte-specific GH receptor null (AdGHRKO) mice have enhanced insulin sensitivity with reduced liver triglycerides. Endocrinology, 160, 68-80. [DOI] [PMC free article] [PubMed]
- Liu J.L., Coschigano K.T., Robertson K., Lipsett M., Guo Y., Kopchick J.J., Kumar U., Liu Y.L. Disruption of growth hormone receptor gene causes diminished pancreatic islet size and increased insulin sensitivity in mice. Am. J. Physiol. Endocrinol. Metab. 2004;287:E405–E413. doi: 10.1152/ajpendo.00423.2003. [DOI] [PubMed] [Google Scholar]; Liu, J. L., Coschigano, K. T., Robertson, K., Lipsett, M., Guo, Y., Kopchick, J. J., Kumar, U. & Liu, Y. L. 2004. Disruption of growth hormone receptor gene causes diminished pancreatic islet size and increased insulin sensitivity in mice. Am. J. Physiol. Endocrinol. Metab., 287, E405-E413. [DOI] [PubMed]
- Lu C., Kumar P.A., Sun J., Aggarwal A., Fan Y., Sperling M.A., Lumeng C.N., Menon R.K. Targeted deletion of growth hormone (GH) receptor in macrophage reveals novel osteopontin-mediated effects of GH on glucose homeostasis and insulin sensitivity in diet-induced obesity. J. Biol. Chem. 2013;288:15725–15735. doi: 10.1074/jbc.M113.460212. [DOI] [PMC free article] [PubMed] [Google Scholar]; Lu C, Kumar PA, Sun J, Aggarwal A, Fan Y, Sperling MA, Lumeng CN & RK, M. 2013. Targeted deletion of growth hormone (GH) receptor in macrophage reveals novel osteopontin-mediated effects of GH on glucose homeostasis and insulin sensitivity in diet-induced obesity. J. Biol. Chem. , 288, 15725-15735. [DOI] [PMC free article] [PubMed]
- Lubbers E.R., List E.O., Jara A., Sackman-Sala L., Cordoba-Chacon J., Gahete M.D., Kineman R.D., Boparai R., Bartke A., Kopchick J.J., Berryman D.E. Adiponectin in mice with altered GH action: links to insulin sensitivity and longevity? J. Endocrinol. 2013;216:363–374. doi: 10.1530/JOE-12-0505. [DOI] [PMC free article] [PubMed] [Google Scholar]; Lubbers, E. R., List, E. O., Jara, A., Sackman-Sala, L., Cordoba-Chacon, J., Gahete, M. D., Kineman, R. D., Boparai, R., Bartke, A., Kopchick, J. J. & Berryman, D. E. 2013. Adiponectin in mice with altered GH action: links to insulin sensitivity and longevity? J. Endocrinol., 216, 363-374. [DOI] [PMC free article] [PubMed]
- Mavalli M.D., Digirolamo D.J., Fan Y., Riddle R.C., Campbell K.S., Van Groen T. Distinct growth hormone receptor signaling modes regulate skeletal muscle development and insulin sensitivity in mice. J. Clin. Invest. 2010;120:4007–4020. doi: 10.1172/JCI42447. [DOI] [PMC free article] [PubMed] [Google Scholar]; Mavalli, M. D., Digirolamo, D. J., Fan, Y., Riddle, R. C., Campbell, K. S. & Van Groen, T. 2010. Distinct growth hormone receptor signaling modes regulate skeletal muscle development and insulin sensitivity in mice. J. Clin. Invest., 120, 4007-4020. [DOI] [PMC free article] [PubMed]
- Moller N., Jorgensen J.O. Effects of growth hormone on glucose, lipid, and protein metabolism in human subjects. Endocr. Rev. 2009;30:152–177. doi: 10.1210/er.2008-0027. [DOI] [PubMed] [Google Scholar]; Moller, N. & Jorgensen, J. O. 2009. Effects of growth hormone on glucose, lipid, and protein metabolism in human subjects. Endocr. Rev., 30, 152-177. [DOI] [PubMed]
- Nelson C.N., List E.O., Ieremia M., Constantin L., Chhabra Y., Kopchick J.J., Waters M.J. Growth hormone activated STAT5 is required for induction of beige fat in vivo. Growth Horm. IGF Res. 2018;2018:40–51. doi: 10.1016/j.ghir.2018.08.004. [DOI] [PubMed] [Google Scholar]; Nelson C.N., List E.O., Ieremia M., Constantin L., Chhabra Y., Kopchick J.J. & M.J., W. 2018. Growth hormone activated STAT5 is required for induction of beige fat in vivo. Growth Horm. IGF Res., 2018, 40-51. [DOI] [PubMed]
- Nicholls D.G., Locke R.M. Thermogenic mechanisms in brown fat. Physiol. Rev. 1984;64:1–64. doi: 10.1152/physrev.1984.64.1.1. [DOI] [PubMed] [Google Scholar]; Nicholls, D. G. & Locke, R. M. 1984. Thermogenic mechanisms in brown fat. Physiol. Rev., 64, 1-64. [DOI] [PubMed]
- Peterson T.R., Sengupta S.S., Harris T.E., Carmack A.E., Kang S.A., Balderas E., Guertin D.A., Madden K.L., Carpenter A.E., Finck B.N., Sabatini D.M. mTOR complex 1 regulates lipin 1 localization to control the SREBP pathway. Cell. 2011;146:408–420. doi: 10.1016/j.cell.2011.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]; Peterson, T. R., Sengupta, S. S., Harris, T. E., Carmack, A. E., Kang, S. A., Balderas, E., Guertin, D. A., Madden, K. L., Carpenter, A. E., Finck, B. N. & Sabatini, D. M. 2011. mTOR complex 1 regulates lipin 1 localization to control the SREBP pathway. Cell, 146, 408-420. [DOI] [PMC free article] [PubMed]
- Puigserver P., Wu Z., Park C.W., Graves R., Wright M., Spiegelman B.M. A cold-inducible coactivator of nuclear receptors linked to adaptive thermogenesis. Cell. 1998;92:829–839. doi: 10.1016/s0092-8674(00)81410-5. [DOI] [PubMed] [Google Scholar]; Puigserver, P., WU, Z., Park, C. W., Graves, R., Wright, M. & Spiegelman, B. M. 1998. A cold-inducible coactivator of nuclear receptors linked to adaptive thermogenesis. Cell, 92, 829-839. [DOI] [PubMed]
- Qi Y., Sun L., Yang H. Lipid droplet growth and adipocyte development: mechanistically distinct processes connected by phospholipids. Biochim. Biophys. Acta. 2017;1862:1273–1283. doi: 10.1016/j.bbalip.2017.06.016. [DOI] [PubMed] [Google Scholar]; Qi, Y., Sun, L. & Yang, H. 2017. Lipid droplet growth and adipocyte development: mechanistically distinct processes connected by phospholipids. Biochim. Biophys. Acta, 1862, 1273-1283. [DOI] [PubMed]
- Riordan N.H., Ichim T.E., Min W.P., Wang H., Solano F., Lara F., Alfaro M., Rodriguez J.P., Harman R.J., Patel A.N. Non-expanded adipose stromal vascular fraction cell therapy for multiple sclerosis. J. Transl. Med. 2009;7:29. doi: 10.1186/1479-5876-7-29. [DOI] [PMC free article] [PubMed] [Google Scholar]; Riordan, N. H., Ichim, T. E., Min, W. P., Wang, H., Solano, F., Lara, F., Alfaro, M., Rodriguez, J. P., Harman, R. J., Patel, A. N., Murphy, M. P., Lee, R. R. & Minev, B. 2009. Non-expanded adipose stromal vascular fraction cell therapy for multiple sclerosis. J. Transl. Med., 7, 29. [DOI] [PMC free article] [PubMed]
- Rosenbloom A.L. Growth hormone insensitivity: physiologic and genetic basis, phenotype, and treatment. J. Pediatr. 1999;135:280–289. doi: 10.1016/s0022-3476(99)70121-6. [DOI] [PubMed] [Google Scholar]; Rosenbloom, A. L. 1999. Growth hormone insensitivity: physiologic and genetic basis, phenotype, and treatment. J. Pediatr., 135, 280-289. [DOI] [PubMed]
- Rui L. Brown and beige adipose tissues in health and disease. Compr. Physiol. 2017;7:1281–1306. doi: 10.1002/cphy.c170001. [DOI] [PMC free article] [PubMed] [Google Scholar]; Rui, L. 2017. Brown and beige adipose tissues in health and disease Compr. Physiol., 7, 1281-1306. [DOI] [PMC free article] [PubMed]
- Schipper H.S., Prakken B., Kalkhoven E., Boes M. Adipose tissue-resident immune cells: key players in immunometabolism. Trends Endocrinol. Metab. 2012;23:407–415. doi: 10.1016/j.tem.2012.05.011. [DOI] [PubMed] [Google Scholar]; Schipper, H. S., Prakken, B., Kalkhoven, E. & Boes, M. 2012. Adipose tissue-resident immune cells: key players in immunometabolism. Trends Endocrinol. Metab., 23, 407-415. [DOI] [PubMed]
- Schoiswohl G., Stefanovic-Racic M., Menke M.N., Wills R.C., Surlow B.A., Basantani M.K., Sitnick M.T., Cai L., Yazbec K.C.F., Stolz D.B. Impact of reduced atgl-mediated adipocyte lipolysis on obesity-associated insulin resistance and inflammation in male mice. Endocrinology. 2015;156:3610–3624. doi: 10.1210/en.2015-1322. [DOI] [PMC free article] [PubMed] [Google Scholar]; Schoiswohl, G., Stefanovic-Racic, M., Menke, M. N., Wills, R. C., Surlow, B. A., Basantani, M. K., Sitnick, M. T., Cai, L., Yazbec, K. C. F., Stolz, D. B., Pulinilkunnil, T., O'Doherty, R. M. & Kershaw, E. E. 2015. Impact of reduced atgl-mediated adipocyte lipolysis on obesity-associated insulin resistance and inflammation in male mice. Endocrinology, 156, 3610-3624. [DOI] [PMC free article] [PubMed]
- Schrauwen P., Van Marken Lichtenbelt W.D., Spiegelman B.M. The future of brown adipose tissues in the treatment of type 2 diabetes. Diabetologia. 2015;58:1704–1707. doi: 10.1007/s00125-015-3611-y. [DOI] [PubMed] [Google Scholar]; Schrauwen P, Van Marken Lichtenbelt WD & BM., S. 2015. The future of brown adipose tissues in the treatment of type 2 diabetes. Diabetologia, 58, 1704-1707. [DOI] [PubMed]
- Sjogren K., Liu J.L., Blad K., Skrtic S., Vidal O., Wallenius V., Leroith D., Tornell J., Isaksson O.G., Jansson J.O., Ohlsson C. Liver-derived insulin-like growth factor I (IGF-I) is the principal source of IGF-I in blood but is not required for postnatal body growth in mice. Proc. Natl. Acad. Sci. U S A. 1999;96:7088–7092. doi: 10.1073/pnas.96.12.7088. [DOI] [PMC free article] [PubMed] [Google Scholar]; Sjogren, K., Liu, J. L., Blad, K., Skrtic, S., Vidal, O., Wallenius, V., Leroith, D., Tornell, J., Isaksson, O. G., Jansson, J. O. & Ohlsson, C. 1999. Liver-derived insulin-like growth factor I (IGF-I) is the principal source of IGF-I in blood but is not required for postnatal body growth in mice. Proc. Natl. Acad. Sci. U S A, 96, 7088-7092. [DOI] [PMC free article] [PubMed]
- Stovitz S.D., Hannan P.J., Lytle L.A., Demerath E.W., Pereira M.A., Himes J.H. Child height and the risk of young-adult obesity. Am. J. Prev. Med. 2010;38:0–77. doi: 10.1016/j.amepre.2009.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]; Stovitz, S. D., Hannan, P. J., Lytle, L. A., Demerath, E. W., Pereira, M. A. & Himes, J. H. 2010. Child height and the risk of young-adult obesity.Am. J. Prev. Med., 38, 0-77. [DOI] [PMC free article] [PubMed]
- Sun L.Y., Bartke A. Tissue-specific GHR knockout mice: metabolic phenotypes. Front. Endocrinol. (Lausanne) 2014;5:243. doi: 10.3389/fendo.2014.00243. [DOI] [PMC free article] [PubMed] [Google Scholar]; Sun, L. Y. & Bartke, A. 2014. Tissue-Specific GHR Knockout Mice: Metabolic Phenotypes. Front. Endocrinol. (Lausanne), 5, 243. [DOI] [PMC free article] [PubMed]
- Vijayakumar A., Novosyadlyy R., Wu Y., Yakar S., Leroith D. Biological effects of growth hormone on carbohydrate and lipid metabolism. Growth Horm. IGF Res. 2010;20:1–7. doi: 10.1016/j.ghir.2009.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]; Vijayakumar, A., Novosyadlyy, R., Wu, Y., Yakar, S. & Leroith, D. 2010. Biological effects of growth hormone on carbohydrate and lipid metabolism. Growth Horm. IGF Res. , 20, 1-7. [DOI] [PMC free article] [PubMed]
- Vijayakumar A., Wu Y., Buffin N.J., Li X., Sun H., Gordon R.E., Yakar S., Leroith D. Skeletal muscle growth hormone receptor signaling regulates basal, but not fasting-induced, lipid oxidation. PLoS One. 2012;7:e44777. doi: 10.1371/journal.pone.0044777. [DOI] [PMC free article] [PubMed] [Google Scholar]; Vijayakumar, A., Wu, Y., Buffin, N. J., Li, X., Sun, H., Gordon, R. E., Yakar, S. & Leroith, D. 2012a. Skeletal muscle growth hormone receptor signaling regulates basal, but not fasting-induced, lipid oxidation. PLoS One, 7, e44777. [DOI] [PMC free article] [PubMed]
- Vijayakumar A., Wu Y., Sun H., Li X., Jeddy Z., Liu C., Schwartz G.J., Yakar S., Leroith D. Targeted loss of GHR signaling in mouse skeletal muscle protects against high-fat diet-induced metabolic deterioration. Diabetes. 2012;61:94–103. doi: 10.2337/db11-0814. [DOI] [PMC free article] [PubMed] [Google Scholar]; Vijayakumar, A., Wu, Y., Sun, H., Li, X., Jeddy, Z., Liu, C., Schwartz, G. J., Yakar, S. & Leroith, D. 2012b. Targeted loss of GHR signaling in mouse skeletal muscle protects against high-fat diet-induced metabolic deterioration. Diabetes, 61, 94-103. [DOI] [PMC free article] [PubMed]
- Wang Z.V., Deng Y., Wang Q.A., Sun K., Scherer P.E. Identification and characterization of a promoter cassette conferring adipocyte-specific gene expression. Endocrinology. 2010;151:2933–2939. doi: 10.1210/en.2010-0136. [DOI] [PMC free article] [PubMed] [Google Scholar]; Wang, Z. V., Deng, Y., Wang, Q. A., Sun, K. & Scherer, P. E. 2010. Identification and characterization of a promoter cassette conferring adipocyte-specific gene expression. Endocrinology, 151, 2933-2939. [DOI] [PMC free article] [PubMed]
- van Wijk J.P., Cabezas M.C., De Koning E.J., Rabelink T.J., Van D.G.R., Hoepelman I.M. In vivo evidence of impaired peripheral fatty acid trapping in patients with human immunodeficiency virus-associated lipodystrophy. J. Clin. Endocrinol. Metab. 2005;90:3575–3582. doi: 10.1210/jc.2004-2343. [DOI] [PubMed] [Google Scholar]; van Wijk, J. P., Cabezas, M. C., De Koning, E. J., Rabelink, T. J., Van, D. G. R. & Hoepelman, I. M. 2005. In vivo evidence of impaired peripheral fatty acid trapping in patients with human immunodeficiency virus-associated lipodystrophy. J. Clin. Endocrinol. Metab., 90, 3575-82. [DOI] [PubMed]
- Wu Y., Liu C., Sun H., Vijayakumar A., Giglou P.R., Qiao R., Oppenheimer J., Yakar S., Leroith D. Growth hormone receptor regulates beta cell hyperplasia and glucose-stimulated insulin secretion in obese mice. J. Clin. Invest. 2011;121:2422–2426. doi: 10.1172/JCI45027. [DOI] [PMC free article] [PubMed] [Google Scholar]; Wu, Y., Liu, C., Sun, H., Vijayakumar, A., Giglou, P. R., Qiao, R., Oppenheimer, J., Yakar, S. & Leroith, D. 2011. Growth hormone receptor regulates beta cell hyperplasia and glucose-stimulated insulin secretion in obese mice. J. Clin. Invest., 121, 2422-2426. [DOI] [PMC free article] [PubMed]
- Xia B., Cai G.H., Yang H., Wang S.P., Mitchell G.A., Wu J.W. Adipose tissue deficiency of hormone-sensitive lipase causes fatty liver in mice. PLoS Genet. 2017;13:e1007110. doi: 10.1371/journal.pgen.1007110. [DOI] [PMC free article] [PubMed] [Google Scholar]; Xia, B., Cai, G. H., Yang, H., Wang, S. P., Mitchell, G. A. & Wu, J. W. 2017. Adipose tissue deficiency of hormone-sensitive lipase causes fatty liver in mice. PLoS Genet., 13, e1007110. [DOI] [PMC free article] [PubMed]
- Xu L., Xu C., Yu C., Miao M., Zhang X., Zhu Z., Ding X., Li Y. Association between serum growth hormone levels and nonalcoholic fatty liver disease: a cross-sectional study. PLoS One. 2012;7:e44136. doi: 10.1371/journal.pone.0044136. [DOI] [PMC free article] [PubMed] [Google Scholar]; Xu, L., Xu, C., C. Yu, M. Miao, X. Zhang, Zhu, Z., Ding, X. & Li, Y. 2012. Association between serum growth hormone levels and nonalcoholic fatty liver disease: a cross-sectional study. PLoS One, 7, e44136. [DOI] [PMC free article] [PubMed]
- Yoshida K., Kita Y., Tokuoka S.M., Hamano F., Yamazaki M., Sakimura K., Kano M., Shimizu T. Monoacylglycerol lipase deficiency affects diet-induced obesity, fat absorption, and feeding behavior in CB1 cannabinoid receptor-deficient mice. FASEB J. 2018;33:2484–2497. doi: 10.1096/fj.201801203R. [DOI] [PubMed] [Google Scholar]; Yoshida, K., Kita, Y., Tokuoka, S. M., Hamano, F., Yamazaki, M., Sakimura, K., Kano, M. & Shimizu, T. 2018. Monoacylglycerol lipase deficiency affects diet-induced obesity, fat absorption, and feeding behavior in CB1 cannabinoid receptor-deficient mice. FASEB J., 33:2484-2497. [DOI] [PubMed]
- Zhou Y., Xu B.C., Maheshwari H.G., He L., Reed M., Lozykowski M., Okada S., Cataldo L., Coschigamo K., Wagner T.E. A mammalian model for Laron syndrome produced by targeted disruption of the mouse growth hormone receptor/binding protein gene (the Laron mouse) Proc. Natl. Acad. Sci. U S A. 1997;94:13215–13220. doi: 10.1073/pnas.94.24.13215. [DOI] [PMC free article] [PubMed] [Google Scholar]; Zhou, Y., Xu, B. C., Maheshwari, H. G., He, L., Reed, M., Lozykowski, M., Okada, S., Cataldo, L., Coschigamo, K., Wagner, T. E., Baumann, G. & Kopchick, J. J. 1997. A mammalian model for Laron syndrome produced by targeted disruption of the mouse growth hormone receptor/binding protein gene (the Laron mouse). Proc. Natl. Acad. Sci. U S A, 94, 13215-13220. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.