Abstract
Women are at increased risk for developing depression and cardiovascular disease (CVD) across the lifespan and their comorbidity is associated with adverse outcomes that contribute significantly to rates of morbidity and mortality in women worldwide. Immune-system activity has been implicated in the etiology of both depression and CVD, but it is unclear how inflammation contributes to sex differences in this comorbidity. This narrative review provides an updated synthesis of research examining the association of inflammation with depression and CVD, and their comorbidity in women. Recent research provides evidence of pro-inflammatory states and sex differences associated with alterations in the hypothalamic–pituitary–adrenal axis, the renin–angiotensin–aldosterone system and the serotonin/kynurenine pathway, that likely contribute to the development of depression and CVD. Changes to inflammatory cytokines in relation to reproductive periods of hormonal fluctuation (i.e. the menstrual cycle, perinatal period and menopause) are highlighted and provide a greater understanding of the unique vulnerability women experience in developing both depressed mood and adverse cardiovascular events. Inflammatory biomarkers hold substantial promise when combined with a patient’s reproductive and mental health history to aid in the prediction, identification and treatment of the women most at risk for CVD and depression. However, more research is needed to improve our understanding of the mechanisms underlying inflammation in relation to their comorbidity, and how these findings can be translated to improve women’s health.
Keywords: cardiovascular diseases, depression, inflammation, menopause, menstrual cycle, pregnancy, sex differences, women
Introduction
A complex relationship exists between depression and cardiovascular disease (CVD), both of which are chronic conditions that have significant detrimental effects on women’s health. The burden of illness and prognosis is far worse in women with comorbid depression and CVD compared with either alone, despite advances in the recognition and treatment of these disorders.1 These conditions independently and concurrently are associated with staggering healthcare costs and are the top contributors to morbidity and mortality rates of women in developed countries.2
The comorbidity of these disorders arises as a result of a range of factors, including the activation of shared etiological pathways. Much of the recent research in these areas has focused on the role of the immune system and inflammation in both depression and CVD, though few studies have examined the comorbidity of these conditions and how women are affected during reproductive life events. The aim of this paper is to provide a narrative review of inflammatory states associated with alterations in biological systems and female reproductive life events that may contribute to the development of comorbid depression and CVD in women.
Depression disproportionally affects women worldwide at a rate that is nearly twofold greater than men, with sex differences emerging as early as adolescence.3 Depression in women is a significant risk factor for the development of CVD events (i.e. myocardial infarction, stroke, coronary heart disease, etc.) and cardiovascular mortality, and women who have experienced a cardiac-related event are more likely to be depressed than men.4–8 Despite a growing awareness of these issues in women, and initiatives aimed at improving outcomes in those with CVD and depression independently, more research is needed to assist in the prevention, identification and treatment of comorbid CVD and depression in women.
Evidence supports a bidirectional relationship between depression and CVD, whereby depression is a predictor for the development of CVD, and vice versa.9 Indeed, midlife patients with diagnosed major depressive disorder are twice as likely to have a cardiac event, defined as a fatal or nonfatal myocardial infarction, angina pectoris, congestive heart failure or cardiac arrhythmia.10 In women, coronary disease rates are strongly predicted by a diagnosis of depression over periods up to 20 years.11 Moreover, female patients are also more likely than males to experience depressive symptoms after diagnosis of acute myocardial infarction,12 and there is a nearly threefold greater risk for cardiac readmission in those who develop a depressive episode after an acute coronary syndrome event.13 Similarly, the first-ever onset of depression in patients following an acute coronary event significantly increases the risk of another cardiac-related event within 2 years.14 Despite these findings, the mechanisms contributing to the greater risk for developing both of these disorders in women are likely multifactorial and remain poorly understood.
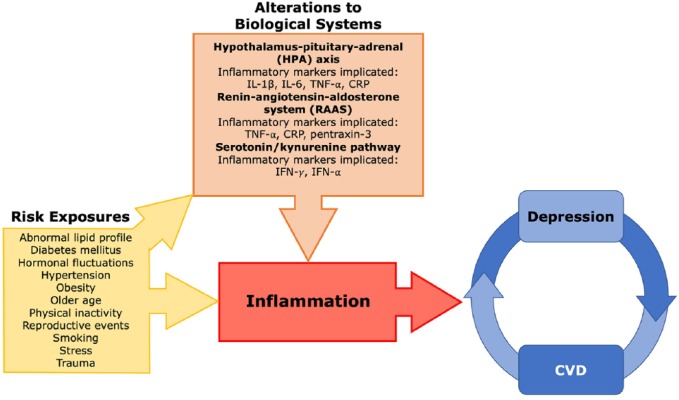
Immune-system dysregulation has been implicated in both the development of depression and CVD, highlighting the possibility of a common etiological basis. In fact, chronic low-grade inflammation has been hypothesized to contribute to the development of both disease states.9 Individuals with comorbid depression and CVD may also have imbalances in homeostatic regulation of different biological systems, with alterations observed in the hypothalamus–pituitary–adrenal (HPA) axis, renin–angiotensin–aldosterone system (RAAS), and serotonin/kynurenine pathways that are accompanied by inflammation and endothelial dysfunction.9 Disruption to these systems, in combination with risk factors and periods of hormonal fluctuation associated with reproductive events, is hypothesized to promote a state of inflammation in women that may contribute to the development and reinforcement of a cycle linking depression and CVD (Figure 1).
Figure 1.
An overview of the proposed mechanism by which inflammation promotes the development of both depression and cardiovascular disease in women.
Risk exposures promote the disruption of several biological systems, including the HPA axis, RAAS, and serotonin/kynurenine metabolism pathway, which leads to inflammatory changes. It is hypothesized that women experience a greater amount of risk exposures, and when coupled with altered biological systems, produces a heightened pro-inflammatory state that contributes to the development and reinforcement of a cycle linking depression and cardiovascular disease.
CRP, C-reactive protein; CVD, cardiovascular disease; HPA, hypothalamus–pituitary–adrenal; IFN, interferon; RAAS, renin–angiotensin–aldosterone system; TNF, tumor necrosis factor.
Here, we build on previous work in this area,15 with the first section of the review focusing on inflammation and sex differences associated with alterations of key biological systems (HPA axis, RAAS, and serotonin/kynurenine pathway) implicated in the pathogenesis of depression and CVD. The remainder of the review examines immune-system functioning during vulnerable reproductive periods surrounding menstruation, the perinatal period, and menopause, in order to better understand how these events might contribute to the significant depression and CVD comorbidity observed in women. Lastly, we conclude with a brief discussion on future directions within the field.
Inflammation in CVD and depression
Measuring inflammation
Inflammatory biomarkers are an important feature of immune dysregulation in depression and CVD. Several of the most studied markers of inflammation in both disease states include the interleukin (IL) cytokine group, specifically IL-1β, IL-6, IL-8, IL-10, and IL-12. Of particular interest is IL-6, which is produced at the site of inflammation in response to tissue injury and functions as both a pro- and anti-inflammatory cytokine responsible for regulating cellular processes.16 Tumor necrosis factor alpha (TNF-α) also plays an important role as an early mediator of the inflammatory response, and is responsible for the production and recruitment of pro-inflammatory cytokines.17 Within the endothelium, both TNF-α and IL-1β can upregulate IL-6 gene expression. IL-6 is also responsible for stimulating expression of the acute-phase protein C-reactive protein (CRP) and the production of cortisol via effects on the HPA axis.18 The pro-inflammatory cytokine, interferon gamma (IFN-γ), is another immune-response mediator that is primarily produced by activated T lymphocytes in response to inflammation.19
Measurement of inflammatory cytokine levels is not only an effective tool for generating an individual’s inflammatory profile and assessing immune-system activity, but also has the potential to be used as a readily available test for identifying individuals at risk of developing inflammatory-related conditions.
Evidence of inflammation in CVD and depression
Elevated levels of similar pro-inflammatory cytokines have been found in individuals with depression and in those with cardiovascular conditions. For example, a recent large meta-analysis reported that levels of IL-6 and CRP are higher in those with depression.20 These same inflammatory markers are also elevated in patients with coronary heart disease and in those with heart failure,21,22 though the sensitivity of CRP in heart failure has recently been called into question.22
Given the number of studies that have reported associations of inflammation with depression and CVD independently, it is surprising that so few have investigated inflammatory markers in relation to concurrent depression and CVD. There has been a report of increased CRP levels and IL-6 messenger ribonucleic acid expression in depressed coronary heart disease patients, compared with those with heart disease alone,23 raising the possibility that CRP and IL-6 could eventually serve as a useful risk marker for this comorbidity.
Sex differences: inflammation in CVD and depression
Well-documented sex differences in inflammation in the general population have been reported, including higher CRP levels in adult women that result from accelerated increases in CRP levels during late adolescence.24 Within the context of cardiovascular health, pro-inflammatory markers may help predict cardiac outcomes in females. Specifically, CRP was found to be a predictor of myocardial infarction, stroke and cardiovascular death in women.25–28 In healthy women with no history of CVD, higher levels of CRP and IL-6 are associated with the presence of other cardiovascular risk factors, such as high body mass index, blood pressure, and smoking status,29 suggesting that increases in cardiovascular risk in women may be accompanied by increases in inflammation.
When comparing cytokine levels in heart failure patients by sex, age also appears to play a significant role. Specifically, lower and more stable levels of TNF-α were reported in women with heart failure under the age of 50, which was followed by a sharp increase after this age.30 Furthermore, this pattern of age-related change in TNF-α in women differs from the linear increase observed in men, suggesting that cytokine secretion is affected by age and sex. The inflammatory change observed in women may be related to physiological and hormonal alterations that accompany reproductive life events, such as menopause. In line with this reasoning, the cardiovascular effect of the sex hormone estradiol, which declines during the menopausal transition, has been shown to vary based on menopausal stage and the extent of atherosclerosis present in arteries.31 Additional evidence from preclinical animal models suggests that the cardio-protective effects of estradiol are negated in cases of severe atherosclerosis.32 Cumulatively, these reports provide evidence for unique inflammatory and physiological states in women that vary across the lifespan, which change based on age, fluctuations of reproductive hormones, and atherosclerosis severity.
The inflammatory states associated with depression have also been shown to vary by sex. Pro-inflammatory cytokines IL-8 and IFN-γ are elevated in depressed females, while depression severity was found to positively correlate with levels of IL-1β and TNF-α in women.33 Higher levels of CRP are also found in women with severe depression;34 further supporting the existence of a close relationship between mood and inflammation in depressed women.
The relatively small number of studies in these areas have made it difficult to draw conclusions as to how inflammation could contribute to the development of depression and CVD in women; however, a larger number of studies have provided indirect evidence of the role of inflammation in this comorbidity. Heightened inflammation may arise as a result of alterations of other physiological systems, such as the HPA axis, RAAS and serotonin/kynurenine metabolic pathway, and so a closer examination of the inflammatory changes that occur following disruption to these systems may help elucidate the complex relationship linking inflammation to comorbid depression and CVD in women.
Stress and HPA axis
Chronic stress and communication between the neuroendocrine and immune systems may be one link between depression and CVD. Stress often accompanies and can precede comorbid depression and CVD in women. For example, older, single, and hospitalized non-White females receiving cardiovascular care were more likely to have depressive symptoms and experience greater stress.35
HPA axis and inflammation
Several inflammatory cytokines, specifically IL-1β, IL-6 and TNF-α,36 also have the ability to activate the HPA axis, and this modulation of stress response is greater in female mice models.37 Sex-specific regulation of the HPA axis and immune system is also found in relation to depressive symptoms in human participants; where females with higher depressive symptom severity had lower cortisol to high-sensitivity CRP (hsCRP) ratios, suggesting heightened inflammation and homeostatic dysregulation among these two systems.38 The connection between inflammation and stress can be further modified by early exposure to external factors, such as childhood maltreatment.
Childhood maltreatment and inflammation
One form of early-life exposure that has significant effects on developmental and health trajectories is childhood maltreatment. Some have argued that early-life exposure to adversity leads to low-grade, chronic inflammation via changes to the signaling mechanisms between the peripheral immune system and the brain.39 This altered state, which persists into adulthood, likely predisposes individuals to several poor health outcomes that are accompanied by high-risk behaviours such as cigarette smoking, alcohol consumption, substance abuse, obesity, and physical inactivity.40 These same behaviours often accompany depression and predispose risk for development of CVD, and may be involved in associations between childhood adversity and inflammation later in life.41
The immune system is also affected by adverse childhood experiences. Studies have repeatedly detected increased levels of IL-6, TNF-α and CRP associated with exposure to adversity in childhood.42 Elevated levels of CRP have also been described in depressed children and adults exposed to maltreatment during childhood, but not in depressed individuals with no history of childhood adversity.43,44 Therefore, inflammation could potentially mediate the effects of childhood abuse on mental health, with higher levels of immune system responses being linked to depression, and potentially increasing subsequent risk for CVD.
Sex differences: childhood adversity in CVD and depression
Individuals with depression and lower levels of education are more likely to report past exposure to childhood adversity (i.e. financial difficulties, family conflicts, etc.) and females who specifically experienced adverse childhood events were more likely to have depression,45 as well as coronary heart disease or cerebrovascular disease in adulthood.46 A sex-specific dose–response relationship has been demonstrated in the CVD literature, showing that women who reported more than two childhood adversities were at risk for developing heart disease or cardiovascular mortality three times greater than those with no exposure to childhood adversity.47
RAAS
Another system that plays a significant role in the inflammatory response is the RAAS, whose main function is blood pressure regulation.48 When dysfunctional, the RAAS appears to contribute to the comorbidity of CVD and depression in women. Following chronic activity, the RAAS becomes maladaptive, resulting in endothelial dysfunction and a pro-inflammatory milieu that advances the progression of atherosclerosis and leads to CVD development.
The RAAS is able to induce inflammation, which is a necessary function for maintaining homeostatic control following stress, insult or injury to the body.49 Specifically, these inflammatory effects appear to be mediated via the actions of angiotensin II and aldosterone. Angiotensin II acts on angiotensin II receptor type 1 (ATR1) to activate several downstream pathways involved in stress regulation, including the nuclear-factor kappa B (NF-κB) pathway, which leads to the secretion of several pro-inflammatory cytokines,50 and functions to stimulate the release of aldosterone from the adrenal cortex. Although less characterized, the angiotensin II receptor type 2 (ATR2) is also activated by angiotensin II and has been shown to produce neuroprotective effects and an increase in the anti-inflammatory cytokine IL-10 in rodents following ischemic stroke.51 The role of angiotensin II and aldosterone in mediating inflammation has led to their investigation as potential therapeutic targets for CVD and depression (see Pacurari et al.48 for a review of their role in vascular inflammation).
RAAS in CVD and depression
Angiotensin II and aldosterone are involved in the pathogenesis of CVD and its risk factors including hypertension, heart failure, coronary artery disease and metabolic syndrome.48,52 Treatment studies have best illustrated how the RAAS is implicated in CVD. Drugs blocking angiotensin function appear to decrease morbidity and mortality in patients with heart failure, coronary artery disease, myocardial infarction, and stroke,53–56 further establishing the involvement of the RAAS system with cardiovascular events.57 Beneficial effects from the addition of aldosterone antagonists to treatments for heart failure with reduced ejection fraction have also been reported.58
Though much of the research examining RAAS activity has focused on its relation to CVD risk and outcomes, recent research has implicated RAAS dysfunction in depression, as well.59 Findings from both human and nonhuman animal studies have linked aldosterone to depression. Depressed individuals have greater aldosterone levels when coupled with a social stressor,60 and individuals with primary aldosteronism, a condition where excessive amounts of aldosterone are produced, have increased rates of psychiatric problems like depression and anxiety.61 In animal models, rats treated with aldosterone also display depressive-like symptoms.62
Evidence connecting angiotensin II to depression primarily comes from preclinical studies. Genetically mutated mice missing the gene responsible for producing angiotensin demonstrate fewer depressive-like behaviors and antidepressant-like effects have been observed in mice treated with drugs that block the angiotensin-converting enzyme or the ATR1.49 Collectively, these reports from both human and animal models provide compelling evidence of the involvement of RAAS dysfunction with cardiovascular complications and depressed mood.
RAAS and inflammation
Even though it is known that the RAAS influences inflammation, there are few studies examining the association of RAAS hormones with inflammatory markers. Early reports have shown that angiotensin-receptor blockers or angiotensin-converting-enzyme inhibitors can modify levels of TNF-α and CRP,63 and more recently, aldosterone has been linked to vascular inflammatory markers such as pentraxin-3, an acute-phase protein.64 In a sample of patients with endocrine disorders, serum pentraxin-3 levels were negatively correlated with plasma aldosterone concentrations, suggesting that aldosterone may be a negative regulator of pentraxin-3.65 However, there is conflicting evidence regarding the influence of pentraxin-3 on CVD due to pentraxin-3 having both beneficial and detrimental cardiovascular effects that are context dependent.66
Sex differences: RAAS in CVD: case of preeclampsia
The unique case of preeclampsia, a condition characterized by high blood pressure in pregnancy, offers insights into how the RAAS and inflammation may be contributing to CVD risk in women. Dysfunction of the RAAS, in particular changes to angiotensin II, during pregnancy is implicated in preeclampsia.67 Inflammation (as measured by CRP levels) in pregnant women exhibiting altered utero–placental function was associated with an insertion/deletion polymorphism in the angiotensin-converting-enzyme gene responsible for the conversion of angiotensin II from angiotensin I.67 Lasting changes to endothelial function are also observed in postpartum women with a past history of preeclampsia who display enhanced vasoconstriction sensitivity to angiotensin II,68 suggesting that increased sensitivity to angiotensin II may contribute to persisting vasculature dysfunction and increase risk for future CVD.
To date, there have been no studies looking at the association of the immune system and RAAS dysfunction in women with comorbid depression and CVD. However, the above reports suggest a link between inflammation and the RAAS hormones aldosterone and angiotensin II, which are implicated in cardiovascular health and mood. Therefore, RAAS dysfunction may confer greater risk to subsets of women during reproductive events.
Serotonin/kynurenine pathway
Serotonin/kynurenine pathway and inflammation
Several pieces of evidence have pointed to the involvement of altered tryptophan metabolism in inflammation and the development of mood disorders. The amino acid tryptophan can be converted to the neurotransmitter serotonin by tryptophan hydroxylase, or kynurenine via the actions of indoleamine 2,3-dioxygenase (IDO) and tryptophan 2,3-dioxygenase (TDO). Several molecules such as cortisol can promote TDO, whereas the most potent activator of IDO expression is the pro-inflammatory cytokine IFN-γ.69 Under stress or increased inflammatory conditions, both IDO and TDO enhance activation of the kynurenine pathway. Activation of the kynurenine pathway following chronic inflammation is modified to increase the production of neurotoxic metabolites and release reactive oxygen species, resulting in lipid peroxidation and neurodegenerative brain changes.70,71
Serotonin/kynurenine pathway in CVD and depression
This type of inflammatory-mediated change to the kynurenine pathway appears to be involved in both CVD and mood disorders. Higher levels of serum kynurenine compared with tryptophan (KYN/TRP ratio), a measure of IDO activity, have been demonstrated in CVD patients, such as those with coronary heart disease72 and ischemic stroke,73 and provide evidence for altered kynurenine synthesis. Furthermore, the KYN/TRP ratio, as well as other kynurenine metabolites, are strongly associated with increased risk of poor outcomes following stroke74 and cardiovascular-related mortality in the general population.75
Depressed coronary heart disease patients show greater serum KYN/TRP ratio compared with those without depression,23 suggesting that depression coupled with CVD leads to increased activation of the kynurenine pathway. In the case of depression caused by immunotherapies, patients receiving high-dose IFN-α treatment experience greater amounts of depressive symptoms, which further lends support to the involvement of kynurenine pathway dysfunction in the emergence of depressive symptoms.71,76
The serotonin hypothesis of depression originally posited that mood changes occur as a result of serotonin deficiency, although it is now understood that a multitude of factors are implicated.77 Even though increased kynurenine activation may be associated with depression via decreased serotonin synthesis, accumulating evidence suggests that depressive symptoms may arise due to an imbalance of kynurenine metabolites, with the production of neurotoxic metabolites being favoured.78 A meta-analysis of the relationship between kynurenine metabolite levels in relation to mood disorders reported lower levels of kynurenine in unipolar major depression; yet the results with other metabolites, such as the KYN/TRP ratio, were inconclusive, however.79
Sex differences: serotonin/kynurenine pathway in CVD and depression
Inflammation could increase the risk of CVD and depression in women through altered serotonin/kynurenine synthesis. However, relatively few studies have investigated sex differences in tryptophan metabolism that are related to depression or CVD. A study conducted on young healthy adults found stronger evidence linking greater IDO activity with multiple atherosclerosis risk factors in females.80 When examining tryptophan metabolism in depressed patients, the KYN/TRP ratio was predictive of depressive symptoms in women only.81 Recently, a study was unable to find sex differences in the KYN/TRP ratio among depressed patients compared with healthy controls; though sex differences in the absolute levels of serum and cerebrospinal levels of tryptophan showed that women displayed reduced levels compared with men.82
Although disruption to the abovementioned biological systems are potential pathways by which inflammation may promote the development of both depression and CVD, additional CVD risk factors provide insight into sex differences and the increased comorbidity preponderance in women.
CVD risk factors in women
Well-characterized sex differences exist among traditional risk factors that contribute to CVD risk. In women, these factors include: older age, abnormal lipid profiles (i.e. elevated low-density lipoprotein cholesterol and triglycerides, and decreased high-density lipoprotein cholesterol), hypertension, diabetes mellitus, smoking, physical inactivity and obesity.83 Women with depression are also more likely to have CVD risk factors related to metabolic dysregulation,84 and many of these CVD factors also increase the risk for depression. A large body of evidence also supports the involvement of inflammatory processes with these traditional CVD risk factors.
Hypertension and inflammation
Hypertension is one CVD risk factor that affects a large proportion of postmenopausal women and is linked to CVD mortality.85,86 In a large-scale longitudinal study, women with hypertension and elevated hsCRP levels experienced a greater risk of developing a stroke compared with normotensive women,87 implicating both hypertension and inflammation in the development of stroke in women. Women are also vulnerable to the development of hypertension during other reproductive events, such as preeclampsia during pregnancy, which may be associated with lasting inflammatory changes. A systematic review and meta-analysis found that a majority of studies assessing CVD risk markers in complicated pregnancies reported higher levels of inflammatory biomarkers, such as IL-6, TNF-α, intracellular adhesion molecule, vascular cell adhesion molecule, and IL-10, among women following pregnancy with preeclampsia, but these did not reach statistical significance.88 It’s important to note that the meta-analysis was not performed for all markers due to the limited number of published studies, and those included in the quantitative analyses may have failed to reach significance due to small sample sizes.
Diabetes mellitus and inflammation
Diabetes, a metabolic disorder characterized by insulin resistance and high blood glucose levels, also appears to increase CVD risk to a greater degree in women than men.89 A large amount of evidence supports the role of chronic low-grade inflammation in the etiology of type 2 diabetes mellitus.90 A prospective study revealed that a heightened pro-inflammatory state in women, as evidenced by higher levels of CRP, IL-6 and TNF-α, is associated with an increased risk for developing type 2 diabetes mellitus.91 Women experience a unique risk for developing diabetes during pregnancy, known as gestational diabetes mellitus, which is also associated with immune system activation, resulting in increased levels of pro-inflammatory markers and decreased levels of anti-inflammatory markers.92
Obesity and inflammation
Obesity is another CVD risk factor that triggers a pro-inflammatory immune response that contributes to the increased risk women have for developing type 2 diabetes mellitus, preeclampsia, gestational diabetes mellitus, and depression.93 Several inflammatory markers, including CRP, IL-1β, and IL-6, are positively associated with body composition and measures of adipocyte size.94,95 In obese women, CRP and IL-10 levels are greater than nonobese controls, with the highest levels observed in those with comorbid type 2 diabetes mellitus,96 suggesting that there is a greater inflammatory response in women with more than one CVD risk factor. Additionally, depressed individuals are more likely to be obese, and obese women are more likely to experience depression.97 Inflammation may help to explain this relationship in women, as higher levels of TNF-α and leptin, a hormone linked to obesity, were reported in women with depressive symptoms.98
Physical inactivity and inflammation
Sedentary lifestyle is also often present in obese individuals and can further promote CVD development in women.99 Overweight women with the least amount of physical activity energy expenditure display greater hsCRP levels,100 suggesting that lack of physical activity contributes to a heightened inflammatory profile in overweight women. Furthermore, it is well known that increasing physical activity has beneficial effects in reducing inflammation101 and mild-to-moderate levels of depression in women.102 Taken together, several of the traditional risk factors that contribute to CVD risk in women are associated with a state of increased inflammation.
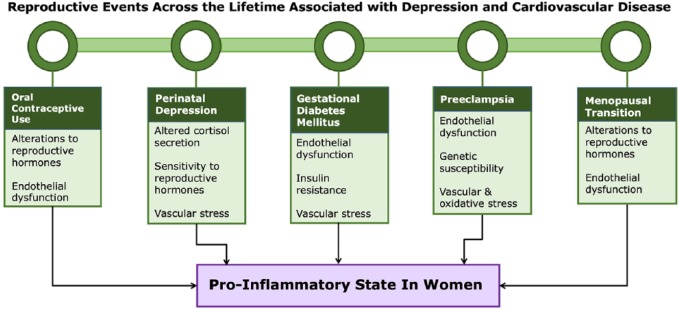
It is important to note that these CVD risk factors and altered physiological states are not unique to women, and therefore are not fully able to explain how inflammation may be contributing to depression and CVD comorbidity due to sex differences. To further our understanding of why women are at greater risk for developing comorbid depression and CVD, inflammatory processes and physiological features associated with female reproductive events and sex hormones will now be discussed (Figure 2).
Figure 2.
A summary of female-specific reproductive events and physiological features that contribute to a pro-inflammatory state in women.
It is hypothesized that alterations to circulating levels of sex hormones, endothelial dysfunction and exposure to vascular stress during unique reproductive events promote an inflammatory response in women that likely contribute to the development or exacerbation of depression and cardiovascular disease.
Sex hormones and inflammation
Inflammatory profiles in women appear to fluctuate based on reproductive events across the lifespan, with estradiol (17 β-estradiol) exposure playing a significant role. Estradiol is the primary sex hormone of the three endogenous estrogens (which also include estriol and estrone) that drives sexual development and regulates reproductive processes, such as menstruation in females.103 The influence of estradiol on the immune response includes alterations to cytokine secretion that varies based on immune cell type, estradiol concentrations and expression of estradiol receptors.104,105 Estrogens can induce an inflammatory reaction via their effects on neutrophils, likely via estrogen receptors α and β.106 In general, estradiol appears to be linked to suppression of pro-inflammatory cytokine production, such as reduced expression of IL-6107 and TNF-α,108 and increased production of anti-inflammatory cytokine IL-10,109 though effects on IL-10 levels change depending on location in the nervous system.110 Further complicating this relationship is estradiol dose, as higher concentrations are linked to anti-inflammatory responses, whereas low concentrations are associated with pro-inflammatory responses.105 Progesterone is another sex hormone that can bind to its receptors on a variety of immune cell types, but the effects are predominantly anti-inflammatory.105 Changes to the natural fluctuation of estrogen and progesterone during reproductive events in women may impact the immune system and subsequently trigger development of CVD and depression.
It has long been known that a woman’s reproductive history can alter their health trajectory. Earlier age of menarche and menopause has been consistently linked to higher CVD risk, including coronary heart disease, electrocardiogram abnormalities, and cardiovascular-related mortality.111–113 Limited research has been conducted examining inflammation related to age of menarche or menopause onset, but some evidence supports the involvement of inflammation in their timing. Increased levels of CRP and adiposity measures in female children at age 5 was correlated with decreased levels of sex hormone binding globulin, which predicted earlier menarche.114 Recently, it has been shown that earlier menopause onset is associated with higher CRP levels in postmenopausal women, with stronger associations noted for African-American women.115 Complications that arise during these pivotal time periods can reveal subclinical syndromes and provide insight into future risk of cardiovascular and other chronic diseases.116
Oral contraceptive use
Women may use oral contraceptives for a variety of reasons throughout their reproductive lifetime, including for preventing pregnancy, reducing abdominal menstrual pain, and as part of treatment for conditions such as polycystic ovarian syndrome (PCOS) and endometriosis.
Oral contraceptive use, CVD and depression
The use of certain oral contraceptive formulas may increase CVD risk in women. For example, combination oral contraceptives containing exogenous estrogen and progestin are linked to the development of CVD risk factors, such as hypertension,117 as well as thromboembolism and ischemic stroke118 (even though the overall risk in healthy women of reproductive age is relatively low119). Other formulations of oral contraceptives, specifically those containing progestin-only, are associated with reduced risk of CVD when compared with women taking the combination type.120 However, women with a history of using both the progestin-only and combined oral contraceptives had twice the odds of developing heart disease or stroke compared with women who only took the combined oral contraceptive.120
While there is no conclusive evidence supporting the link between oral contraceptive use and depression, there appears to be a small proportion of women who are vulnerable to developing negative mood following combination contraceptive use.121 In the case of premenstrual dysphoric disorder (PMDD), an oral contraceptive formula containing drosperinone, a form of progestin, and ethinyl estradiol has been shown to be clinically effective in treating premenstrual mood symptoms, such as depression.122 Therefore, it is possible that some women may be more susceptible to mood alterations following oral contraceptive use based on the type of progestin used.
Oral contraceptive use and inflammation
In terms of inflammation, a small amount of research has shown that oral contraceptive use in healthy young women is linked to increased CRP levels,123–125 though findings have been inconsistent.126 In nonhuman animal models, administration of combination oral contraceptives led to increased blood pressure and greater endothelial dysfunction associated with elevated circulating levels of CRP, uric acid and plasminogen-activator inhibitor 1 (PAI-1) pro-inflammatory mediators in female rats.127 Uric acid is related to several cardiometabolic risk factors, such as hypertension and diabetes, and can increase pro-inflammatory cytokine expression,128 whereas the protein PAI-1 is stimulated by several pro-inflammatory cytokines.129 In the case of women with PCOS, oral contraceptive use was associated with increased CRP, whereas no significant change in IL-6 or TNF-α levels was found, and PAI-1 levels tended to be decreased depending on the type of oral contraceptive used.130 Taken together, there is some evidence supporting altered inflammatory state in women taking oral contraception that may contribute to risk of depression and CVD, but more research is needed before conclusive findings may be drawn.
Perinatal depression
Perinatal depression and CVD
The perinatal period is a unique reproductive time characterized by hormonal and metabolic changes that put women at risk for developing psychiatric problems and cardiovascular events. Global pooled prevalence rates demonstrate that 11.9% of women experience perinatal depression.131 Similar rates of antenatal depression have been described across the gestational period, with higher rates observed in the second and third trimester.132,133
Pregnancy has been described as a ‘stress test’ that can reveal a woman’s underlying vascular health.116,134 Complications that arise during pregnancy, such as depression, preeclampsia, gestational diabetes and preterm delivery, can indicate women who have an increased risk for developing CVD or depression later in life.
Perinatal depression and inflammation
Vulnerable populations may experience alterations to the cytokine-glucocorticoid feedback circuit during the perinatal period, leading to decreased regulation of cytokine production and cortisol secretion, and increased depression susceptibility.135 One possible mechanism by which the immune system influences mood during pregnancy is through activation of toll-like receptors and NLRP3 inflammasome from immune placental cells, causing an inflammatory shift which may trigger depression in pregnancy.136
Increased levels of pro-inflammatory cytokines have been demonstrated in perinatal women with major depression, but the literature is mixed.137 One study investigating serum levels of inflammatory markers prior to delivery found lower levels of 40 markers of inflammation (including IL-10) linked to postpartum depression.138 However, another recent study found no association between cytokine levels and perinatal depression in plasma, but noted that higher levels of IL-1β, IL-23 and IL-33 in cerebrospinal fluid were associated with an increased odds of developing perinatal depression.139 A cross-sectional study found increased pro-inflammatory markers, specifically IL-6, IL-15 and chemokine ligand 3 (CCL3), in women with depressive symptoms during the third trimester, but these results varied based on ethnicity and timepoint examined during the perinatal period.140 In a sample of Swedish women, several peripheral inflammatory biomarkers, including IL-18, was increased in women with postpartum depression.141 The variability of findings observed among studies investigating inflammatory markers with perinatal depression likely reflects the different study designs employed and highlights the importance of timing and source of sample collection.
The protein PAI-1 may provide a plausible link for how inflammation is associated with perinatal depression and CVD risk. PAI-1 is secreted by various endothelial cells and its production is increased during pregnancy.142 An increase in several inflammatory cytokines, such as CRP, IL-1β, IL-6, and TNF-α, which may occur as a result of perinatal depression or HPA axis dysfunction, can lead to increased expression of PAI-1 and subsequently increase risk for the development of atherosclerosis and myocardial infarction.142
Based on the evidence described above, there is strong support for the involvement of pro-inflammatory cytokines with perinatal depression, but more research is needed to determine a reliable inflammatory profile associated with developing mood symptoms in the perinatal period. Perinatal depression may contribute to increased CVD risk in women through inflammatory changes that promote hypertension in the form of preeclampsia and the development of gestational diabetes, but this has yet to be directly tested.143,144 The following two sections will summarize the recent advances in the literature pertaining to depression and CVD risk in gestational diabetes and preeclampsia.
Gestational diabetes mellitus
Gestational diabetes mellitus, CVD and depression
A relationship exists between depression in pregnancy and gestational diabetes mellitus (GDM), as depression in the first trimester of pregnancy has been shown to predict the development of GDM.145 In the general population, depression and type 2 diabetes are comorbid conditions,146 with higher prevalence observed in females;147 however, there are conflicting reports on the directionality of the relationship between depression and GDM.148,149 Regardless, the association between depression and GDM may be explained by a shared pro-inflammatory state that results in the inability to produce enough insulin,150 and puts women at risk for adverse cardiovascular outcomes.123
GDM affects 9% of all pregnancies in North America, with even higher rates in the Middle East and North Africa.151 GDM increases risk for pregnancy complications and unfavourable outcomes, such as preeclampsia,152 and later development of type 2 diabetes and CVD, including acute myocardial infarction, stroke, and coronary artery disease.153 In uncomplicated pregnancies, insulin resistance increases midgestation, but women are able to retain normal blood glucose levels during this time through compensatory mechanisms that result in greater insulin secretion.154 Failure to adequately compensate the increase in insulin resistance leads to the development of gestational diabetes.
Gestational diabetes mellitus and inflammation
Multiple studies have shown altered immune cytokine levels associated with GDM, specifically increased levels of serum pro-inflammatory cytokines, IL-1β,155 IL-6,156,157 CRP,158 and TNF-α,159,160 and lowered levels of anti-inflammatory marker IL-10;157 however, cytokine levels in women with GDM vary based on time of sampling in pregnancy and not all results reached statistical significance. Altered secretion of cytokines from adipose cells, known as adipokines, are also implicated in GDM and play a role in promoting inflammation, insulin resistance and endothelial dysfunction.92 Even though these results have been replicated, a recent study failed to detect any noticeable cytokine differences among women with GDM or normal glucose tolerance pregnancies,161 possibly because the study was not adequately powered to detect differences between groups. It currently remains uncertain whether the associated inflammatory response is a causal factor or consequence of GDM. Even though the nature of this relationship has yet to be fully explored, the pro-inflammatory state associated with GDM may help explain the increased prevalence of comorbid depression in pregnancy and be a contributor toward CVD development later on.
Preeclampsia
During pregnancy, physiological changes take place to ensure that the woman’s body is able to meet the increased demands of a developing fetus. Preeclampsia is thought to develop as a result of several factors, including (a) abnormal vascular remodeling leading to placental ischemia, endothelial dysfunction and oxidative stress, (b) a systemic inflammatory response generated from toll-like receptors in the placenta, and (c) genetic vulnerability.162 A balance between pro-oxidative and antioxidative responses is required for regulating embryogenesis, whereby healthy women display increasing levels of oxidative stress that peak during the second trimester.144 It is hypothesized that disruption to this balance can promote an inflammatory response that may lead to the development of preeclampsia. Furthermore, exposure to factors contributing to cardiovascular risk prior to pregnancy may also contribute to inflammation and create a lowered threshold for managing vascular stress during pregnancy, which can result in preeclampsia.163
Preeclampsia, CVD and depression
Preeclampsia affects roughly 5–8% of pregnancies worldwide and is a significant contributor to maternal and fetal morbidity and mortality despite advancements in perinatal care.164,165 Preeclampsia is characterized by hypertension, edema and proteinuria, with normotensive women developing new-onset hypertension at around 20 weeks’ gestation.166 The adverse effects of preeclampsia extend beyond the perinatal period, putting women at greater risk for future adverse cardiovascular events, with risk levels that are proportional to preeclampsia severity.163 Preeclampsia is linked to CVD risk factors such as chronic hypertension, metabolic disorders, renal disease, as well as stroke in women, and preterm-born offspring with low birth weight.165 Even though the exact causal mechanism for preeclampsia is unknown, it is plausible that physiological alterations leading to a state of heightened inflammation may help explain why women are at risk for developing preeclampsia in pregnancy.
Depression may also contribute to increases in inflammation and oxidative stress associated with preeclampsia and CVD later in life in women.144 Depression or anxiety in early pregnancy is associated with a greater risk for developing preeclampsia,167 and a diagnosis of hypertensive disorder during pregnancy is associated with higher levels of postpartum depression.168 Furthermore, pregnant women taking antidepressants, especially serotonin–norepinephrine reuptake inhibitors (SNRIs) and tricyclic antidepressants (TCAs), are at increased risk of developing preeclampsia,169 but it has not yet been established if this association is due to these medications or is a result of depression itself. A recent review of the literature further provides support that antidepressant use in pregnancy is associated with moderately increased risk for both preeclampsia and gestational hypertension;170 however, methodological limitations and differences between studies reduce the ability to draw clear conclusions.
Preeclampsia and inflammation
The literature examining the association between preeclampsia and inflammatory biomarkers is mixed. Multiple recent studies have demonstrated increased serum levels of IL-1β,171,172 IL-6,172–175 IL-8,176 IL-10,172,173 CRP,172,175,177 TNF-α172,173,175 and IFN-γ174,178 in women with preeclampsia compared with women with normotensive pregnancies; however, these results remain inconclusive.179 From all of the inflammatory biomarkers investigated, CRP, IL-6, IL-8, and TNF-α hold the most promise for early identification of women with preeclampsia,180 yet there is not enough evidence supporting the prognostic ability of individual inflammatory markers in predicting the development of preeclampsia,172,179–181 which may be due to discrepancies among gestational timepoints and population studied. The combination of several biomarkers may afford greater predictive ability for identifying preeclampsia onset or prognosis, but more research is needed.
An increased susceptibility for developing preeclampsia during pregnancy may be in part due to genetic influences on the immune system. Several reports highlight the association of cytokine gene polymorphisms with preeclampsia, such as IFN-γ (A874T),174 IL-6 (G-174C),182 IL-10 (A-592C),183 and TNF-α (G-308A);184 however, these results are still inconclusive and may only be reflective of specific ethnic groups. A recent review of meta-analyses examining genetic risk factors for preeclampsia found strongest evidence for PAI-1 4G/5G polymorphism.185 Future studies using larger sample sizes are warranted.
Alterations to the endothelium prior to pregnancy may better explain the inflammatory response observed in women with preeclampsia.186 Plaque erosion and endothelium dysfunction more frequently develops in women and is associated with up to 44% of fatal coronary thrombosis events;187 however, this was observed in a small sample of older-aged women and may not be reflective of females in the general population. When the physiology and function of the vascular endothelium is altered, a pro-inflammatory response is activated, resulting in increased cytokine release, leukocyte adhesion and permeability of the endothelium.188 In the case of preeclampsia, increased macrophage activation is observed within the placenta of women.163 Alterations to the vascular system following preeclampsia may be long lasting, as one study found vascular dysfunction associated with women who had preeclampsia over 5 years previously, which correlated with measures of hsCRP.189 Enduring inflammatory states are also reported within women who developed preeclampsia, as increased levels of plasma CRP and IL-6 were found approximately 17 years after their first pregnancy.190 These results suggest long-lasting changes to a woman’s inflammatory state observed almost 2 decades after giving birth in those with a history of preeclampsia, which may contribute to the development of depression and CVD.
A complete understanding of the multifaceted relationship between depression, inflammation and preeclampsia requires further research. A major challenge with generalizing results from studies assessing inflammation in relation to perinatal mood has been the large heterogeneity of the samples studied, differences in instruments used to assess mood, and the varying timepoints at which mood and inflammatory state were measured.191
Menopausal transition
Menopausal transition, CVD and depression
The menopausal period is associated with significant hormonal change and increased prevalence of CVD risk factors in women.83 Women who experience natural or surgical menopause are at higher risk for experiencing a fatal cardiovascular event as compared with younger menstruating women,192 suggesting that the heightened risk may be related to age or hormonal change that accompanies this time period. Evidence that CVD risk in the menopausal period is associated with timing of hormonal exposure comes from prospective studies showing that women with a shorter reproductive life span, defined as the duration of time from age at menarche to age at menopause, experienced a higher risk of incident coronary heart disease or stroke after menopause.193 Furthermore, postmenopausal women display more dyslipidemia and hypertension than premenopausal women, which likely results from a combination of hormonal and age-related change.194,195
The menopausal transition and postmenopause periods are associated with greater vulnerability for developing mood symptoms and clinically diagnosed mood episodes.196 Age of menopausal onset, as well duration of the reproductive period, is associated with postmenopausal depression; whereby earlier-onset menopause was associated with higher risk.197 The impact of menopause on women’s mental and cardiovascular health may be partially explained by altered sex-hormone production.
The menopausal transition is marked by declining levels of estrogen, which have known neuro- and cardio-protective effects.198,199 Results from animal studies on older female rats provide evidence that the protective and anti-inflammatory effects of estradiol are likely age dependent.200 With older age and menopause, estradiol may no longer be able to exert vascular protection in women as a result of alterations to the estrogen receptor function and expression that occurs in combination with estrogen loss.201 In postmenopausal women, levels of estradiol, testosterone, and their ratio are associated with CVD incidence, with higher testosterone-to-estradiol ratio increasing risk for CVD, coronary heart disease and heart failure.202 These results suggest that a woman’s hormone profile following menopause may be indicative of CVD risk.
In addition to lower levels of estrogen, the menopausal period is associated with vascular alterations that significantly impact CVD risk. Across the life span, women display greater endothelial dysfunction postmenopause.203 Postmenopausal women with hypertension show evidence of systemic inflammation, via neutrophil count, associated with increased arterial stiffness,204 which provides support for the involvement of inflammation with altered endothelial function in postmenopausal women, leading to greater CVD risk.
Menopausal transition and inflammation
When examining the immune system in relation to menopause, changes to several inflammatory markers have been described and vary based on the stages of the menopausal transition. A 5-year longitudinal study investigating inflammatory markers in premenopausal women who entered the menopausal transition detected notable changes: increased serum IL-8 and soluble TNF-receptor 1 and 2 (sTNFR1 and sTNFR2, respectively), and decreased serum hsCRP.205 Another study found that women transitioning from pre- to postmenopause displayed an increase in the inflammatory marker glycoprotein acetyls, which has been linked to incident CVD in women in other studies,206 yet found no changes in CRP levels.207 A 10-year longitudinal study conducted on premenopausal women found that women who transitioned through menopause experienced elevations in the acute-phase protein haptoglobin, a marker indicative of heightened inflammation, but contrary to other reports, they found reduction in levels of the anti-inflammatory cytokine IL-8.208 Among nonobese postmenopausal women, increased levels of IL-8 and TNF-α, along with decreased levels of IL-1α and IL-3 have been reported.209 In sum, longitudinal studies assessing inflammatory markers across the menopausal period have reported differing results that likely reflect methodological disparities among the studies. Nonetheless, the majority of the results provide evidence for greater inflammation associated with the menopausal transition.
There is an increased prevalence of depressive symptoms in peri- and postmenopausal women,210 which may be related to altered immune-system activity during this time. In midlife-aged women during the menopausal transition, an increase in pro-inflammatory response was observed prior to development of first-onset depression,211 suggestive of a potential cascading effect. In postmenopausal women, higher CRP levels are found in those with clinical levels of depression and in those taking antidepressants,212 linking inflammatory status with depression severity in postmenopausal women. Levels of haptoglobin, which is previously shown to be increased in patients with depression and associated with CVD risk,213 were positively correlated with estradiol levels only in the group of perimenopausal women experiencing a first episode of depression and not taking antidepressants.214 These results suggest that first-ever-onset depression occurring in the menopausal period may reflect a distinct inflammatory profile, which is negatively influenced by estradiol in this population. Currently, there is not enough consistent evidence supporting the practicality of using one or more inflammatory biomarkers to predict depression during the menopausal transition.
Very few studies have examined the association of inflammatory markers with CVD risk in menopausal women. In a sample of postmenopausal women with higher-than-normal blood glucose levels, higher serum CRP levels were associated with insulin resistance,215 a physiological condition which promotes dyslipidemia and atherosclerosis development. A more recent study investigating CRP found a positive association with coronary artery calcification, a measure predictive of CVD among African-American midlife women only,216 which highlights the importance of considering ethnicity differences. Presence of low-grade inflammation has been shown associated with biomarkers of atherosclerosis risk in postmenopausal women; specifically, levels of IL-12 were positively associated with measures of very-low-density-lipoprotein cholesterol.217 These studies collectively show that increased inflammation during the menopausal transition and in postmenopause appears to be related to both depression and CVD risk, which is further complicated by the influence of estrogens, or lack thereof.
A summary of the evidence describing the inflammatory response in women according to reproductive events that increase risk for depression and CVD can be found in Table 1. Collectively, these reports suggest that increased pro-inflammatory cytokine and immune markers, as well as decreased anti-inflammatory cytokines in women across several reproductive events, are associated with hormonal and physiological change, and that these changes are likely involved in the development of CVD and depression.
Table 1.
Summary of recent results obtained from preclinical models, healthy populations and those with chronic diseases, such as depression or cardiovascular disease, during female-specific reproductive events.
| Reproductive event associated with greater risk for depression and CVD in women | Change to inflammatory markers | |
|---|---|---|
| Pro-inflammatory | Anti-inflammatory | |
| Oral contraceptive use | ↑ CRP, uric acid ↕ PAI-1 |
|
| Perinatal depression | ↑ IL-1β, IL-6, IL-15, IL-18, IL-23, IL-33, PAI-1, CCL3 | ↓ IL-10 |
| Gestational diabetes | ↑ IL-6, IL-12, CRP, TNF-α | ↓ IL-4, IL-10 |
| Preeclampsia | ↑ IL-1β, IL-6, IL-8, CRP, TNF-α, IFN-γ | ↑ IL-10 |
| Menopausal transition | ↑ sTNFR1, sTNFR2, glycoprotein acetyls, haptoglobin ↓ CRP, IL-1α, IL-3 ↕ IL-8 |
|
Changes to inflammatory markers vary depending on the population studied, type and timing of sample collection.
CCL3, chemokine ligand 3; CRP, C-reactive protein; CVD, cardiovascular disease; IFN-γ, interferon gamma; IL, interleukin; PAI-1, plasminogen-activator inhibitor 1; sTNFR1, soluble TNF-receptor 1; sTNFR2, soluble TNF-receptor 2; TNF-α, tumor necrosis factor alpha.
Future directions
Use of inflammatory markers in predicting CVD outcomes
Inflammatory markers may be helpful in predicting adverse health outcomes following a cardiac event. Increased levels of IL-6 and TNF-α following heart failure were associated with increased mortality risk among men and women.30 Similar results have been found in patients with coronary heart disease, where several adverse outcomes, including cardiovascular and all-cause mortality, myocardial infarction, heart failure hospitalization and cancer mortality, were linked to IL-6 levels.218 This has also been noted in women presenting with symptoms of ischemia, where higher IL-6 levels were predictive of heart-failure hospitalization and mortality.219 These results suggest the potential predictive value of IL-6 as an inflammatory marker for adverse outcomes in women with CVD.
Treatment strategies targeting inflammation
At the moment, there are not enough conclusive data to support the use of inflammatory biomarkers in guiding detection or treatment with pharmacotherapies aimed at reducing inflammation associated with depression or CVD within clinical practice.220 A current promising avenue for treating inflammation associated with depression and CVD is use of antidepressants and drugs with anti-inflammatory properties, specifically nonsteroidal anti-inflammatory drugs, omega-3 polyunsaturated fatty acids and statins,221 though treatment considerations must be made regarding their use.222 If dysregulated immune-system functioning plays a primary role in the etiology of these diseases and can be reliably identified, then treatment strategies targeting inflammation may be beneficial (to learn more about recent advances, see Jones and Patel220 and Zuzarte et al.221). With this promising potential comes the need for prospective, randomized-controlled-trial intervention studies in order to determine the effects of anti-inflammatories on long-term outcomes.
Inflammation and the gut microbiome
There is increasing interest in investigating the influence of the gut microbiome on pathophysiology and progression of psychiatric disorders and CVD.223,224 Cross-talk between microbiota and the sex hormones of the endocrine system, such as estrogens, may result in changes to inflammation,225 leading to increased susceptibility for developing depression.226 Furthermore, there is evidence supporting a link between gut-flora metabolism and CVD risk and pathogenesis.227,228 The influence of the gut microbiome on inflammation, mood and CVD risk adds to the complexity of the overall picture. Future research is needed to determine its role in women and in the development of comorbid depression and CVD. If closely associated, examination of the gut bacterial ecosystem may be beneficial as a diagnostic tool and the development of novel therapeutics targeting diet and changes to the gut flora.
Conclusion
An abundance of research has linked immune-system dysfunction to the development of depression and CVD, with evidence supporting a unique pathogenesis in women. However, there is a scarcity of studies assessing inflammatory profiles in women that also examine depressed mood and CVD simultaneously. Prospective studies focusing on reproductive histories of women are needed, as the majority of investigations completed to date are cross-sectional in design and have failed to consider the effects of sex hormones at several timepoints or focus on reproductive events. Studies conducted with larger female sample sizes will be helpful in determining whether subsets of women are particularly vulnerable to inflammatory changes associated with the development of these conditions.
The likelihood that a single inflammatory marker will be able to reliably predict onset of depression or CVD is unlikely, and future research should embrace investigations that examine multiple inflammatory biomarkers to examine their collective influence or those investigating the balance between pro- and anti-inflammatory states. Additionally, research within this field may help guide future treatment initiatives aimed at targeting inflammatory dysregulation in women with comorbid CVD and depression.
Several methodological limitations have made it difficult to assess sex differences associated with comorbid depression and CVD. The reduced number of female participants and lack of female-specific variables relating to reproductive health in past CVD research has contributed to the difficulty in investigating sex-specific factors that contribute to health outcomes in women.229 Many reports only examine samples composed primarily of males; therefore, it is difficult to determine whether the results generalize to females.
Overall, it appears that there is likely a subset of women who are more at risk and susceptible to inflammatory changes that in turn leads to the development and reinforcement of a cycle contributing to both depression and CVD. Heightened risk in women is apparent during periods of hormonal fluctuation, suggesting a strong interaction between inflammation and sex hormones. This model is multifaceted and the mechanistic pathways through which the immune system influences these disease states in women have yet to be fully elucidated. As research within this field progresses, it will undoubtedly continue to highlight and bring greater awareness of sex differences that contribute to the development of comorbid depression and CVD in women, improved treatments and superior outcomes in this population.
Footnotes
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest statement: The authors declare that there is no conflict of interest.
ORCID iDs: Gabriella F. Mattina  https://orcid.org/0000-0002-6205-4261
https://orcid.org/0000-0002-6205-4261
Meir Steiner  https://orcid.org/0000-0001-7838-2246
https://orcid.org/0000-0001-7838-2246
Contributor Information
Gabriella F. Mattina, Neuroscience Graduate Program, McMaster University, 1280 Main Street West, ON L8S 4L8, Canada.
Ryan J. Van Lieshout, Neuroscience Graduate Program, McMaster University, ON, Canada Department of Psychiatry and Behavioral Neurosciences, McMaster University, Hamilton, ON, Canada.
Meir Steiner, Women’s Health Concerns Clinic, St. Joseph’s Healthcare, Hamilton, ON, Canada; Department of Psychiatry and Behavioral Neurosciences, McMaster University, Hamilton, ON, Canada.
References
- 1. Mehta LS. Cardiovascular disease and depression in women. Heart Fail Clin 2011; 7: 39–45. [DOI] [PubMed] [Google Scholar]
- 2. Hajat C, Stein E. The global burden of multiple chronic conditions: a narrative review. Prev Med Reports 2018; 12: 284–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Salk RH, Hyde JS, Abramson LY. Gender differences in depression in representative national samples: Meta-analyses of diagnoses and symptoms. Psychol Bull 2017; 143: 783–822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Whang W, Kubzansky LD, Kawachi I, et al. Depression and risk of sudden cardiac death and coronary heart disease in women: results from the Nurses’ Health Study. J Am Coll Cardiol 2009; 53: 950–958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Smolderen KG, Strait KM, Dreyer RP, et al. Depressive symptoms in younger women and men with acute myocardial infarction: insights from the VIRGO study. J Am Heart Assoc 2015; 4(4): pii: e001424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ibeneme SC, Nwosu AON, Ibeneme GC, et al. Distribution of symptoms of post-stroke depression in relation to some characteristics of the vulnerable patients in socio-cultural context. Afr Health Sci 2017; 17: 70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Agarwal S, Presciutti A, Verma J, et al. Women have worse cognitive, functional, and psychiatric outcomes at hospital discharge after cardiac arrest. Resuscitation 2018; 125: 12–15. [DOI] [PubMed] [Google Scholar]
- 8. Möller-Leimkühler AM. Gender differences in cardiovascular disease and comorbid depression. Dialogues Clin Neurosci 2007; 9: 71–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Halaris A. Inflammation-associated co-morbidity between depression and cardiovascular disease. Cogn Affect Behav Neurosci 2017; 31: 45–70. [DOI] [PubMed] [Google Scholar]
- 10. Bremmer MA, Hoogendijk WJG, Deeg DJH, et al. Depression in older age is a risk factor for first ischemic cardiac events. Am J Geriatr Psychiatry 2006; 14: 523–530. [DOI] [PubMed] [Google Scholar]
- 11. O’Neil A, Fisher AJ, Kibbey KJ, et al. Depression is a risk factor for incident coronary heart disease in women: an 18-year longitudinal study. J Affect Disord 2016; 196: 117–124. [DOI] [PubMed] [Google Scholar]
- 12. AbuRuz ME, Al-Dweik G. Depressive symptoms and complications early after acute myocardial infarction: gender differences. Open Nurs J 2018; 12: 205–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Parker GB, Cvejic E, Vollmer-Conna U, et al. Depression and poor outcome after an acute coronary event: clarification of risk periods and mechanisms. Aust New Zeal J Psychiatry 2019; 53: 148–157. [DOI] [PubMed] [Google Scholar]
- 14. Ossola P, Gerra ML, De Panfilis C, et al. Anxiety, depression, and cardiac outcomes after a first diagnosis of acute coronary syndrome. Heal Psychol 2018; 37: 1115–1122. [DOI] [PubMed] [Google Scholar]
- 15. Wright L, Simpson W, Van Lieshout RJ, et al. Depression and cardiovascular disease in women: is there a common immunological basis? A theoretical synthesis. Ther Adv Cardiovasc Dis 2014; 8: 56–69. [DOI] [PubMed] [Google Scholar]
- 16. Tanaka T, Narazaki M, Kishimoto T. IL-6 in inflammation, immunity, and disease. Cold Spring Harb Perspect Biol 2014; 6: a016295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Parameswaran N, Patial S. Tumor necrosis factor-α signaling in macrophages. Crit Rev Eukaryot Gene Expr 2010; 20: 87–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Papanicolaou DA, Wilder RL, Manolagas SC, et al. The pathophysiologic roles of interleukin-6 in human disease. Ann Intern Med 1998; 128: 127–137. [DOI] [PubMed] [Google Scholar]
- 19. Schroecksnadel K, Frick B, Winkler C, et al. Crucial role of interferon-gamma and stimulated macrophages in cardiovascular disease. Curr Vasc Pharmacol 2006; 4: 205–213. [DOI] [PubMed] [Google Scholar]
- 20. Haapakoski R, Mathieu J, Ebmeier KP, et al. Cumulative meta-analysis of interleukins 6 and 1β, tumour necrosis factor α and C-reactive protein in patients with major depressive disorder. Brain Behav Immun 2015; 49: 206–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kaptoge S, Seshasai SRK, Gao P, et al. Inflammatory cytokines and risk of coronary heart disease: new prospective study and updated meta-analysis. Eur Heart J 2014; 35: 578–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. DuBrock HM, AbouEzzeddine OF, Redfield MM. High-sensitivity C-reactive protein in heart failure with preserved ejection fraction. PLoS One 2018; 13: e0201836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Nikkheslat N, Zunszain PA, Horowitz MA, et al. Insufficient glucocorticoid signaling and elevated inflammation in coronary heart disease patients with comorbid depression. Brain Behav Immun 2015; 48: 8–18. [DOI] [PubMed] [Google Scholar]
- 24. Shanahan L, Copeland WE, Worthman CM, et al. Sex-differentiated changes in C-reactive protein from ages 9 to 21: the contributions of BMI and physical/sexual maturation. Psychoneuroendocrinology 2013; 38: 2209–2217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wang A, Liu J, Li C, et al. Cumulative exposure to high-sensitivity C-reactive protein predicts the risk of cardiovascular disease. J Am Heart Assoc 2017; 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ridker PM, Rifai N, Rose L, et al. Comparison of C-reactive protein and low-density lipoprotein cholesterol levels in the prediction of first cardiovascular events. N Engl J Med 2002; 347: 1557–1565. [DOI] [PubMed] [Google Scholar]
- 27. Ridker PM, Buring JE, Shih J, et al. Prospective study of C-reactive protein and the risk of future cardiovascular events among apparently healthy women. Circulation 1998; 98: 731–733. [DOI] [PubMed] [Google Scholar]
- 28. Avan A, Tavakoly Sany SB, Ghayour-Mobarhan M, et al. Serum C-reactive protein in the prediction of cardiovascular diseases: overview of the latest clinical studies and public health practice. J Cell Physiol 2018; 233: 8508–8525. [DOI] [PubMed] [Google Scholar]
- 29. Bermudez EA, Rifai N, Buring J, et al. Interrelationships among circulating interleukin-6, C-reactive protein, and traditional cardiovascular risk factors in women. Arterioscler Thromb Vasc Biol 2002; 22: 1668–1673. [DOI] [PubMed] [Google Scholar]
- 30. Deswal A, Petersen NJ, Feldman AM, et al. Cytokines and cytokine receptors in advanced heart failure: an analysis of the cytokine database from the Vesnarinone trial (VEST). Circulation 2001; 103: 2055–2059. [DOI] [PubMed] [Google Scholar]
- 31. Clarkson TB. Estrogen effects on arteries vary with stage of reproductive life and extent of subclinical atherosclerosis progression. Menopause 2018; 25: 1262–1274. [DOI] [PubMed] [Google Scholar]
- 32. Hanke H, Kamenz J, Hanke S, et al. Effect of 17-beta estradiol on pre-existing atherosclerotic lesions: role of the endothelium. Atherosclerosis 1999; 147: 123–132. [DOI] [PubMed] [Google Scholar]
- 33. Birur B, Amrock EM, Shelton RC, et al. Sex differences in the peripheral immune system in patients with depression. Front Psychiatry 2017; 8: 108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Köhler-Forsberg O, Buttenschøn HN, Tansey KE, et al. Association between C-reactive protein (CRP) with depression symptom severity and specific depressive symptoms in major depression. Brain Behav Immun 2017; 62: 344–350. [DOI] [PubMed] [Google Scholar]
- 35. Dupre ME, Nelson A, Lynch SM, et al. Socioeconomic, psychosocial and behavioral characteristics of patients hospitalized with cardiovascular disease. Am J Med Sci 2017; 354: 565–572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Van der Meer MJ, Sweep CG, Rijnkels CE, et al. Acute stimulation of the hypothalamic-pituitary-adrenal axis by IL-1 beta, TNF alpha and IL-6: a dose response study. J Endocrinol Invest 1996; 19: 175–182. [DOI] [PubMed] [Google Scholar]
- 37. Bethin KE, Vogt SK, Muglia LJ. Interleukin-6 is an essential, corticotropin-releasing hormone-independent stimulator of the adrenal axis during immune system activation. Proc Natl Acad Sci U S A 2000; 97: 9317–9322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Suarez EC, Sundy JS, Erkanli A. Depressogenic vulnerability and gender-specific patterns of neuro-immune dysregulation: what the ratio of cortisol to C-reactive protein can tell us about loss of normal regulatory control. Brain Behav Immun 2015; 44: 137–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Nusslock R, Miller GE. Early-life adversity and physical and emotional health across the lifespan: a neuroimmune network hypothesis. Biol Psychiatry 2016; 80: 23–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Campbell JA, Walker RJ, Egede LE. Associations between adverse childhood experiences, high-risk behaviors, and morbidity in adulthood. Am J Prev Med 2016; 50: 344–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Li A, Tu MT, Sousa AC, et al. Early life adversity and C-reactive protein in diverse populations of older adults: a cross-sectional analysis from the International Mobility in Aging Study (IMIAS). BMC Geriatr 2015; 15: 102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Deighton S, Neville A, Pusch D, et al. Biomarkers of adverse childhood experiences: a scoping review. Psychiatry Res 2018; 269: 719–732. [DOI] [PubMed] [Google Scholar]
- 43. Danese A, Moffitt TE, Pariante CM, et al. Elevated inflammation levels in depressed adults with a history of childhood maltreatment. Arch Gen Psychiatry 2008; 65: 409–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Danese A, Caspi A, Williams B, et al. Biological embedding of stress through inflammation processes in childhood. Mol Psychiatry 2011; 16: 244–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Pirkola S, Isometsä E, Aro H, et al. Childhood adversities as risk factors for adult mental disorders. Soc Psychiatry Psychiatr Epidemiol 2005; 40: 769–777. [DOI] [PubMed] [Google Scholar]
- 46. Korkeila J, Vahtera J, Korkeila K, et al. Childhood adversities as predictors of incident coronary heart disease and cerebrovascular disease. Heart 2010; 96: 298–303. [DOI] [PubMed] [Google Scholar]
- 47. Garad Y, Maximova K, MacKinnon N, et al. Sex-specific differences in the association between childhood adversity and cardiovascular disease in adulthood: evidence from a national cohort study. Can J Cardiol 2017; 33: 1013–1019. [DOI] [PubMed] [Google Scholar]
- 48. Pacurari M, Kafoury R, Tchounwou PB, et al. The renin-angiotensin-aldosterone system in vascular inflammation and remodeling. Int J Inflam 2014; 2014: 689360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Vian J, Pereira C, Chavarria V, et al. The renin–angiotensin system: a possible new target for depression. BMC Med 2017; 15: 144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Liu T, Zhang L, Joo D, et al. NF-κB signaling in inflammation. Signal Transduct Target Ther 2017; 2: 17023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Fouda AY, Pillai B, Dhandapani KM, et al. Role of interleukin-10 in the neuroprotective effect of the angiotensin type 2 receptor agonist, compound 21, after ischemia/reperfusion injury. Eur J Pharmacol 2017; 799: 128–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Gaddam KK, Pimenta E, Husain S, et al. Aldosterone and cardiovascular disease. Curr Probl Cardiol 2009; 34: 51–84. [DOI] [PubMed] [Google Scholar]
- 53. CONSENSUS Trial Study Group. Effects of enalapril on mortality in severe congestive heart failure. N Engl J Med 1987; 316: 1429–1435. [DOI] [PubMed] [Google Scholar]
- 54. Fox KM; European Trial on Reduction of Cardiac Events With Perindopril in Stable Coronary Artery Disease Investigators. Efficacy of perindopril in reduction of cardiovascular events among patients with stable coronary artery disease: randomised, double-blind, placebo-controlled, multicentre trial (the EUROPA study). Lancet 2003; 362: 782–788. [DOI] [PubMed] [Google Scholar]
- 55. Pfeffer MA, Braunwald E, Moyé LA, et al. Effect of captopril on mortality and morbidity in patients with left ventricular dysfunction after myocardial infarction. N Engl J Med 1992; 327: 669–677. [DOI] [PubMed] [Google Scholar]
- 56. Yusuf S, Sleight P, Pogue J, et al. Effects of an angiotensin-converting–enzyme inhibitor, ramipril, on cardiovascular events in high-risk patients. N Engl J Med 2000; 342: 145–153. [DOI] [PubMed] [Google Scholar]
- 57. Cohn JN. Reducing cardiovascular risk by blockade of the renin-angiotensin-aldosterone system. Adv Ther 2007; 24: 1290–304. [DOI] [PubMed] [Google Scholar]
- 58. Nair A, Deswal A. Aldosterone receptor blockade in heart failure with preserved ejection fraction. Heart Fail Clin 2018; 14: 525–535. [DOI] [PubMed] [Google Scholar]
- 59. Murck H, Büttner M, Kircher T, et al. Genetic, molecular and clinical determinants for the involvement of aldosterone and its receptors in major depression. Nephron Physiol 2014; 128: 17–25. [DOI] [PubMed] [Google Scholar]
- 60. Häfner S, Baumert J, Emeny RT, et al. To live alone and to be depressed, an alarming combination for the renin–angiotensin–aldosterone-system (RAAS). Psychoneuroendocrinology 2012; 37: 230–237. [DOI] [PubMed] [Google Scholar]
- 61. Reincke M. Anxiety, depression, and impaired quality of life in primary aldosteronism: why we shouldn’t ignore it! J Clin Endocrinol Metab 2018; 103: 1–4. [DOI] [PubMed] [Google Scholar]
- 62. Hlavacova N, Wes PD, Ondrejcakova M, et al. Subchronic treatment with aldosterone induces depression-like behaviours and gene expression changes relevant to major depressive disorder. Int J Neuropsychopharmacol 2012; 15: 247–265. [DOI] [PubMed] [Google Scholar]
- 63. Ferrario CM, Strawn WB. Role of the renin-angiotensin-aldosterone system and proinflammatory mediators in cardiovascular disease. Am J Cardiol 2006; 98: 121–128. [DOI] [PubMed] [Google Scholar]
- 64. Shiraki A, Kotooka N, Komoda H, et al. Pentraxin-3 regulates the inflammatory activity of macrophages. Biochem Biophys Reports 2016; 5: 290–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Takashi Y, Koga M, Matsuzawa Y, et al. Circulating pentraxin 3 is positively associated with chronic hyperglycemia but negatively associated with plasma aldosterone concentration. PLoS One 2018; 13: e0196526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Fornai F, Carrizzo A, Forte M, et al. The inflammatory protein pentraxin 3 in cardiovascular disease. Immun Ageing 2016; 13: 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Häupl T, Zimmermann M, Kalus U, et al. Angiotensin converting enzyme intron 16 insertion/deletion genotype is associated with plasma C-reactive protein concentration in uteroplacental dysfunction. J Renin-Angiotensin-Aldosterone Syst 2015; 16: 422–427. [DOI] [PubMed] [Google Scholar]
- 68. Stanhewicz AE, Jandu S, Santhanam L, et al. Increased angiotensin II sensitivity contributes to microvascular dysfunction in women who have had preeclampsia. Hypertension 2017; 70: 382–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Mangge H, Stelzer I, Reininghaus EZ, et al. Disturbed tryptophan metabolism in cardiovascular disease. Curr Med Chem 2014; 21: 1931–1937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Wang Q, Liu D, Song P, et al. Tryptophan-kynurenine pathway is dysregulated in inflammation, and immune activation. Front Biosci (Landmark Ed) 2015; 20: 1116–1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Jeon SW, Kim Y-K. Inflammation-induced depression: its pathophysiology and therapeutic implications. J Neuroimmunol 2017; 313: 92–98. [DOI] [PubMed] [Google Scholar]
- 72. Wirleitner B, Rudzite V, Neurauter G, et al. Immune activation and degradation of tryptophan in coronary heart disease. Eur J Clin Invest 2003; 33: 550–554. [DOI] [PubMed] [Google Scholar]
- 73. Mo X, Pi L, Yang J, et al. Serum indoleamine 2,3-dioxygenase and kynurenine aminotransferase enzyme activity in patients with ischemic stroke. J Clin Neurosci 2014; 21: 482–486. [DOI] [PubMed] [Google Scholar]
- 74. Brouns R, Verkerk R, Aerts T, et al. The role of tryptophan catabolism along the kynurenine pathway in acute ischemic stroke. Neurochem Res 2010; 35: 1315–1322. [DOI] [PubMed] [Google Scholar]
- 75. Zuo H, Ueland PM, Ulvik A, et al. Plasma biomarkers of inflammation, the kynurenine pathway, and risks of all-cause, cancer, and cardiovascular disease mortality. Am J Epidemiol 2016; 183: 249–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Murakami Y, Ishibashi T, Tomita E, et al. Depressive symptoms as a side effect of interferon-α therapy induced by induction of indoleamine 2,3-dioxygenase 1. Sci Rep 2016; 6: 29920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Albert PR, Benkelfat C. The neurobiology of depression—revisiting the serotonin hypothesis. II. Genetic, epigenetic and clinical studies. Philos Trans R Soc Lond B Biol Sci 2013; 368: 20120535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Savitz J. Role of kynurenine metabolism pathway activation in major depressive disorders. Curr Top Behav Neurosci 2017; 31: 249–267. [DOI] [PubMed] [Google Scholar]
- 79. Arnone D, Saraykar S, Salem H, et al. Role of kynurenine pathway and its metabolites in mood disorders: a systematic review and meta-analysis of clinical studies. Neurosci Biobehav Rev 2018; 92: 477–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Pertovaara M, Raitala A, Juonala M, et al. Indoleamine 2,3-dioxygenase enzyme activity correlates with risk factors for atherosclerosis: the Cardiovascular Risk in Young Finns Study. Clin Exp Immunol 2007; 148: 106–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Elovainio M, Hurme M, Jokela M, et al. Indoleamine 2,3-dioxygenase activation and depressive symptoms: results from the Young Finns Study. Psychosom Med 2012; 74: 675–681. [DOI] [PubMed] [Google Scholar]
- 82. Hestad KA, Engedal K, Whist JE, et al. The relationships among tryptophan, kynurenine, indoleamine 2,3-dioxygenase, depression, and neuropsychological performance. Front Psychol 2017; 8: 1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Bairey Merz CN, Ramineni T, Leong D. Sex-specific risk factors for cardiovascular disease in women-making cardiovascular disease real. Curr Opin Cardiol 2018; 33: 1. [DOI] [PubMed] [Google Scholar]
- 84. Klakk H, Kristensen PL, Andersen LB, et al. Symptoms of depression in young adulthood is associated with unfavorable clinical- and behavioral cardiovascular disease risk factors. Prev Med Reports 2018; 11: 209–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Lima R, Wofford M, Reckelhoff JF. Hypertension in postmenopausal women. Curr Hypertens Rep 2012; 14: 254–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Wu C-Y, Hu H-Y, Chou Y-J, et al. High blood pressure and all-cause and cardiovascular disease mortalities in community-dwelling older adults. Medicine (Baltimore) 2015; 94: e2160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Jiménez MC, Rexrode KM, Kotler G, et al. Association between markers of inflammation and total stroke by hypertensive status among women. Am J Hypertens 2016; 29: 1117–1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Visser S, Hermes W, Ket JCF, et al. Systematic review and metaanalysis on nonclassic cardiovascular biomarkers after hypertensive pregnancy disorders. Am J Obstet Gynecol 2014; 211: 373.e1–9. [DOI] [PubMed] [Google Scholar]
- 89. Humphries KH, Izadnegahdar M, Sedlak T, et al. Sex differences in cardiovascular disease – impact on care and outcomes. Front Neuroendocrinol 2017; 46: 46–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Lontchi-Yimagou E, Sobngwi E, Matsha TE, et al. Diabetes mellitus and inflammation. Curr Diab Rep 2013; 13: 435–444. [DOI] [PubMed] [Google Scholar]
- 91. Hu FB, Meigs JB, Li TY, et al. Inflammatory markers and risk of developing type 2 diabetes in women. Diabetes 2004; 53: 693–700. [DOI] [PubMed] [Google Scholar]
- 92. Abell SK, De Courten B, Boyle JA, et al. Inflammatory and other biomarkers: role in pathophysiology and prediction of gestational diabetes mellitus. Int J Mol Sci 2015; 16: 13442–13473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Lyons CL, Kennedy EB, Roche HM. Metabolic inflammation-differential modulation by dietary constituents. Nutrients 2016; 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Bahceci M, Gokalp D, Bahceci S, et al. The correlation between adiposity and adiponectin, tumor necrosis factor alpha, interleukin-6 and high sensitivity C-reactive protein levels. Is adipocyte size associated with inflammation in adults? J Endocrinol Invest 2007; 30: 210–214. [DOI] [PubMed] [Google Scholar]
- 95. Marques-Vidal P, Bochud M, Bastardot F, et al. Association between inflammatory and obesity markers in a Swiss population-based sample (CoLaus Study). Obes Facts 2012; 5: 734–744. [DOI] [PubMed] [Google Scholar]
- 96. Janowska J, Chudek J, Olszanecka-Glinianowicz M, et al. Interdependencies among selected pro-inflammatory markers of endothelial dysfunction, C-peptide, anti-inflammatory interleukin-10 and glucose metabolism disturbance in obese women. Int J Med Sci 2016; 13: 490–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Steinig J, Nagl M, Linde K, et al. Antenatal and postnatal depression in women with obesity: a systematic review. Arch Womens Ment Health 2017; 20: 569–585. [DOI] [PubMed] [Google Scholar]
- 98. Webb M, Davies M, Ashra N, et al. The association between depressive symptoms and insulin resistance, inflammation and adiposity in men and women. PLoS One 2017; 12: e0187448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Barnes AS. Obesity and sedentary lifestyles: risk for cardiovascular disease in women. Texas Hear Inst J 2012; 39: 224–227. [PMC free article] [PubMed] [Google Scholar]
- 100. Lavoie M-E, Rabasa-Lhoret R, Doucet É, et al. Association between physical activity energy expenditure and inflammatory markers in sedentary overweight and obese women. Int J Obes 2010; 34: 1387–1395. [DOI] [PubMed] [Google Scholar]
- 101. You T, Arsenis NC, Disanzo BL, et al. Effects of exercise training on chronic inflammation in obesity. Sport Med 2013; 43: 243–256. [DOI] [PubMed] [Google Scholar]
- 102. Zhang J, Yen ST. Physical activity, gender difference, and depressive symptoms. Health Serv Res 2015; 50: 1550–1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Findlay JK, Liew SH, Simpson ER, et al. Estrogen signaling in the regulation of female reproductive functions. Handb Exp Pharmacol 2010; 29–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Straub RH. The complex role of estrogens in inflammation. Endocr Rev 2007; 28: 521–574. [DOI] [PubMed] [Google Scholar]
- 105. Klein SL, Flanagan KL. Sex differences in immune responses. Nat Rev Immunol 2016; 16: 626–638. [DOI] [PubMed] [Google Scholar]
- 106. Nowak K, Jabłońska E, Ratajczak-Wrona W. Neutrophils life under estrogenic and xenoestrogenic control. J Steroid Biochem Mol Biol. Epub ahead of print 28 October 2018. DOI: 10.1016/j.jsbmb.2018.10.015. [DOI] [PubMed] [Google Scholar]
- 107. Kassem M, Harris SA, Spelsberg TC, et al. Estrogen inhibits interleukin-6 production and gene expression in a human osteoblastic cell line with high levels of estrogen receptors. J Bone Miner Res 2009; 11: 193–199. [DOI] [PubMed] [Google Scholar]
- 108. An J, Ribeiro RC, Webb P, et al. Estradiol repression of tumor necrosis factor-alpha transcription requires estrogen receptor activation function-2 and is enhanced by coactivators. Proc Natl Acad Sci U S A 1999; 96: 15161–15166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Matalka KZ. The effect of estradiol, but not progesterone, on the production of cytokines in stimulated whole blood, is concentration-dependent. Neuro Endocrinol Lett 2003; 24: 185–191. [PubMed] [Google Scholar]
- 110. Shivers K-Y, Amador N, Abrams L, et al. Estrogen alters baseline and inflammatory-induced cytokine levels independent from hypothalamic-pituitary-adrenal axis activity. Cytokine 2015; 72: 121–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Qiu C, Chen H, Wen J, et al. Associations between age at menarche and menopause with cardiovascular disease, diabetes, and osteoporosis in Chinese women. J Clin Endocrinol Metab 2013; 98: 1612–1621. [DOI] [PubMed] [Google Scholar]
- 112. Chen X, Liu Y, Sun X, et al. Age at menarche and risk of all-cause and cardiovascular mortality: a systematic review and dose-response meta-analysis. Menopause 2018; 26: 1–7. [DOI] [PubMed] [Google Scholar]
- 113. Muka T, Oliver-Williams C, Kunutsor S, et al. Association of age at onset of menopause and time since onset of menopause with cardiovascular outcomes, intermediate vascular traits, and all-cause mortality. JAMA Cardiol 2016; 1: 767. [DOI] [PubMed] [Google Scholar]
- 114. Pinkney J, Streeter A, Hosking J, et al. Adiposity, chronic inflammation, and the prepubertal decline of sex hormone binding globulin in children: evidence for associations with the timing of puberty (Earlybird 58). J Clin Endocrinol Metab 2014; 99: 3224–3232. [DOI] [PubMed] [Google Scholar]
- 115. Nowakowski ACH, Graves KY. Does inflammation mediate relationships between racial identity and onset of menopause among US adults? J Racial Ethn Heal Disparities 2017; 4: 1128–1137. [DOI] [PubMed] [Google Scholar]
- 116. Rich-Edwards JW, McElrath TF, Karumanchi SA, et al. Breathing life into the lifecourse approach: pregnancy history and cardiovascular disease in women. Hypertens (Dallas, Tex 1979) 2010; 56: 331–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Plu-Bureau G, Hugon-Rodin J, Maitrot-Mantelet L, et al. Hormonal contraceptives and arterial disease: an epidemiological update. Best Pract Res Clin Endocrinol Metab 2013; 27: 35–45. [DOI] [PubMed] [Google Scholar]
- 118. Peragallo Urrutia R, Coeytaux RR, McBroom AJ, et al. Risk of acute thromboembolic events with oral contraceptive use. Obstet Gynecol 2013; 122: 380–389. [DOI] [PubMed] [Google Scholar]
- 119. Bassuk SS, Manson JE. Oral contraceptives and menopausal hormone therapy: relative and attributable risks of cardiovascular disease, cancer, and other health outcomes. Ann Epidemiol 2015; 25: 193–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Samson ME, Adams SA, Merchant AT, et al. Cardiovascular disease incidence among females in South Carolina by type of oral contraceptives, 2000–2013: a retrospective cohort study. Arch Gynecol Obstet 2016; 294: 991–997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Schaffir J, Worly BL, Gur TL. Combined hormonal contraception and its effects on mood: a critical review. Eur J Contracept Reprod Health Care 2016; 21: 347–355. [DOI] [PubMed] [Google Scholar]
- 122. Lopez LM, Kaptein AA, Helmerhorst FM. Oral contraceptives containing drospirenone for premenstrual syndrome. Cochrane Database Syst Rev 2012; CD006586. [DOI] [PubMed] [Google Scholar]
- 123. Cauci S, Di Santolo M, Culhane JF, et al. Effects of third-generation oral contraceptives on high-sensitivity C-reactive protein and homocysteine in young women. Obstet Gynecol 2008; 111: 857–864. [DOI] [PubMed] [Google Scholar]
- 124. Norouzi V, Seifi M, Fallah S, et al. Effect of oral contraceptive therapy on homocysteine and C-reactive protein levels in women: an observational study. Anadolu Kardiyol Derg 2011; 11: 698–702. [DOI] [PubMed] [Google Scholar]
- 125. Cauci S, Francescato MP, Curcio F. Combined oral contraceptives increase high-sensitivity C-reactive protein but not haptoglobin in female athletes. Sport Med 2017; 47: 175–185. [DOI] [PubMed] [Google Scholar]
- 126. Rios-Avila L, Coats B, Chi Y-Y, et al. Metabolite profile analysis reveals association of vitamin B-6 with metabolites related to one-carbon metabolism and tryptophan catabolism but not with biomarkers of inflammation in oral contraceptive users and reveals the effects of oral contraceptives on these processes. J Nutr 2015; 145: 87–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Olatunji LA, Seok Y-M, Igunnu A, et al. Combined oral contraceptive-induced hypertension is accompanied by endothelial dysfunction and upregulated intrarenal angiotensin II type 1 receptor gene expression. Naunyn Schmiedebergs Arch Pharmacol 2016; 389: 1147–1157. [DOI] [PubMed] [Google Scholar]
- 128. Lu W, Xu Y, Shao X, et al. Uric acid produces an inflammatory response through activation of NF-κB in the hypothalamus: implications for the pathogenesis of metabolic disorders. Sci Rep 2015; 5: 12144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Cesari M, Pahor M, Incalzi RA. Plasminogen activator inhibitor-1 (PAI-1): a key factor linking fibrinolysis and age-related subclinical and clinical conditions. Cardiovasc Ther 2010; 28: e72–e91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. De Medeiros Alves V, De Silva ACP, De Souza EVM, et al. Suicide attempt in mental disorders (MeDi): Association with 5-HTT, IL-10 and TNF-alpha polymorphisms. J Psychiatr Res 2017; 91: 36–46. [DOI] [PubMed] [Google Scholar]
- 131. Woody CA, Ferrari AJ, Siskind DJ, et al. A systematic review and meta-regression of the prevalence and incidence of perinatal depression. J Affect Disord 2017; 219: 86–92. [DOI] [PubMed] [Google Scholar]
- 132. Castro e Couto T, Cardoso MN, Brancaglion MYM, et al. Antenatal depression: prevalence and risk factor patterns across the gestational period. J Affect Disord 2016; 192: 70–75. [DOI] [PubMed] [Google Scholar]
- 133. Truijens SEM, Spek V, Van Son MJM, et al. Different patterns of depressive symptoms during pregnancy. Arch Womens Ment Health 2017; 20: 539–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Sattar N, Greer IA. Pregnancy complications and maternal cardiovascular risk: opportunities for intervention and screening? BMJ 2002; 325: 157–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Corwin EJ, Pajer K, Paul S, et al. Bidirectional psychoneuroimmune interactions in the early postpartum period influence risk of postpartum depression. Brain Behav Immun 2015; 49: 86–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Leff-Gelman P, Mancilla-Herrera I, Flores-Ramos M, et al. The immune system and the role of inflammation in perinatal depression. Neurosci Bull 2016; 32: 398–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Buglione-Corbett R, Deligiannidis KM, Leung K, et al. Expression of inflammatory markers in women with perinatal depressive symptoms. Arch Womens Ment Health 2018; 21: 671–679. [DOI] [PubMed] [Google Scholar]
- 138. Bränn E, Papadopoulos F, Fransson E, et al. Inflammatory markers in late pregnancy in association with postpartum depression—a nested case-control study. Psychoneuroendocrinology 2017; 79: 146–159. [DOI] [PubMed] [Google Scholar]
- 139. Miller ES, Sakowicz A, Roy A, et al. Plasma and cerebrospinal fluid inflammatory cytokines in perinatal depression. Am J Obstet Gynecol. Epub ahead of print 14 December 2018. DOI: 10.1016/j.ajog.2018.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Osborne LM, Yenokyan G, Fei K, et al. Innate immune activation and depressive and anxious symptoms across the peripartum: an exploratory study. Psychoneuroendocrinology 2019; 99: 80–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Bränn E, Fransson E, White RA, et al. Inflammatory markers in women with postpartum depressive symptoms. J Neurosci Res. Epub ahead of print 5 September 2018. DOI: 10.1002/jnr.24312. [DOI] [PubMed] [Google Scholar]
- 142. Savoy C, Van Lieshout RJ, Steiner M. Is plasminogen activator inhibitor-1 a physiological bottleneck bridging major depressive disorder and cardiovascular disease? Acta Physiol 2017; 219: 715–727. [DOI] [PubMed] [Google Scholar]
- 143. Robakis TK, Aasly L, Williams KE, et al. Roles of inflammation and depression in the development of gestational diabetes. Curr Behav Neurosci Reports 2017; 4: 369–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Nicholson L, Lecour S, Wedegärtner S, et al. Assessing perinatal depression as an indicator of risk for pregnancy-associated cardiovascular disease. Cardiovasc J Afr 2016; 27: 119–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Morrison C, McCook JG, Bailey BA. First trimester depression scores predict development of gestational diabetes mellitus in pregnant rural Appalachian women. J Psychosom Obstet Gynaecol 2016; 37: 21–25. [DOI] [PubMed] [Google Scholar]
- 146. Anderson RJ, Freedland KE, Clouse RE, et al. The prevalence of comorbid depression in adults with diabetes: a meta-analysis. Diabetes Care 2001; 24: 1069–1078. [DOI] [PubMed] [Google Scholar]
- 147. Huang C-J, Hsieh H-M, Tu H-P, et al. Major depressive disorder in patients with type 2 diabetes mellitus: prevalence and clinical characteristics. J Affect Disord 2018; 227: 141–148. [DOI] [PubMed] [Google Scholar]
- 148. Hinkle SN, Buck Louis GM, Rawal S, et al. A longitudinal study of depression and gestational diabetes in pregnancy and the postpartum period. Diabetologia 2016; 59: 2594–2602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Beka Q, Bowker SL, Savu A, et al. History of mood or anxiety disorders and risk of gestational diabetes mellitus in a population-based cohort. Diabet Med 2018; 35: 147–151. [DOI] [PubMed] [Google Scholar]
- 150. Robakis TK, Aasly L, Williams KE, et al. Roles of inflammation and depression in the development of gestational diabetes. Curr Behav Neurosci reports 2017; 4: 369–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Zhu Y, Zhang C. Prevalence of gestational diabetes and risk of progression to type 2 diabetes: a global perspective. Curr Diab Rep 2016; 16: 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Yogev Chen Hod, et al. Hyperglycemia and adverse pregnancy outcome (HAPO) study: preeclampsia. Am J Obstet Gynecol 2010; 202: 255.e1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Shah BR, Retnakaran R, Booth GL. Increased risk of cardiovascular disease in young women following gestational diabetes mellitus. Diabetes Care 2008; 31: 1668–1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Fasshauer M, Blüher M, Stumvoll M. Adipokines in gestational diabetes. Lancet Diabetes Endocrinol 2014; 2: 488–499. [DOI] [PubMed] [Google Scholar]
- 155. Vitoratos N, Valsamakis G, Mastorakos G, et al. Pre- and early post-partum adiponectin and interleukin-1beta levels in women with and without gestational diabetes. Hormones (Athens) 2008; 7: 230–236. [DOI] [PubMed] [Google Scholar]
- 156. Hassiakos D, Eleftheriades M, Papastefanou I, et al. Increased maternal serum interleukin-6 concentrations at 11 to 14 weeks of gestation in low risk pregnancies complicated with gestational diabetes mellitus: development of a prediction model. Horm Metab Res 2016; 48: 35–41. [DOI] [PubMed] [Google Scholar]
- 157. Kuzmicki M, Telejko B, Zonenberg A, et al. Circulating pro- and anti-inflammatory cytokines in polish women with gestational diabetes. Horm Metab Res 2008; 40: 556–560. [DOI] [PubMed] [Google Scholar]
- 158. Haidari F, Jalali M-T, Shahbazian N, et al. Comparison of serum levels of vitamin D and inflammatory markers between women with gestational diabetes mellitus and healthy pregnant control. J Fam Reprod Heal 2016; 10: 1–8. [PMC free article] [PubMed] [Google Scholar]
- 159. Gao X, Yang H, Zhao Y. Variations of tumor necrosis factor-alpha, leptin and adiponectin in mid-trimester of gestational diabetes mellitus. Chin Med J (Engl) 2008; 121: 701–705. [PubMed] [Google Scholar]
- 160. Moreli JB, Corrêa-Silva S, Damasceno DC, et al. Changes in the TNF-alpha/IL-10 ratio in hyperglycemia-associated pregnancies. Diabetes Res Clin Pract 2015; 107: 362–369. [DOI] [PubMed] [Google Scholar]
- 161. Eschler DC, Kulina G, Garcia-Ocana A, et al. Circulating levels of bone and inflammatory markers in gestational diabetes mellitus. Biores Open Access 2018; 7: 123–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. El-Sayed AAF. Preeclampsia: A review of the pathogenesis and possible management strategies based on its pathophysiological derangements. Taiwan J Obstet Gynecol 2017; 56: 593–598. [DOI] [PubMed] [Google Scholar]
- 163. De Jager SCA, Meeuwsen JAL, van Pijpen FM, et al. Preeclampsia and coronary plaque erosion: manifestations of endothelial dysfunction resulting in cardiovascular events in women. Eur J Pharmacol 2017; 816: 129–137. [DOI] [PubMed] [Google Scholar]
- 164. American College of Obstetricians and Gynecologists, Task Force on Hypertension in Pregnancy. Hypertension in pregnancy. Report of the American College of Obstetricians and Gynecologists’ Task Force on Hypertension in Pregnancy. Obstet Gynecol 2013; 122: 1122–1131. [DOI] [PubMed] [Google Scholar]
- 165. Jim B, Karumanchi SA. Preeclampsia: pathogenesis, prevention, and long-term complications. Semin Nephrol 2017; 37: 386–397. [DOI] [PubMed] [Google Scholar]
- 166. Al-Jameil N, Aziz Khan F, Fareed Khan M, et al. A brief overview of preeclampsia. J Clin Med Res 2014; 6: 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Qiu C, Williams MA, Calderon-Margalit R, et al. Preeclampsia risk in relation to maternal mood and anxiety disorders diagnosed before or during early pregnancy. Am J Hypertens 2009; 22: 397–402. [DOI] [PubMed] [Google Scholar]
- 168. Strapasson MR, Ferreira CF, Ramos JGL. Associations between postpartum depression and hypertensive disorders of pregnancy. Int J Gynecol Obstet 2018; 143: 367–373. [DOI] [PubMed] [Google Scholar]
- 169. Palmsten K, Setoguchi S, Margulis A V, et al. Elevated risk of preeclampsia in pregnant women with depression: depression or antidepressants? Am J Epidemiol 2012; 175: 988–997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170. Uguz F. Is there any association between use of antidepressants and preeclampsia or gestational hypertension? J Clin Psychopharmacol 2017; 37: 72–77. [DOI] [PubMed] [Google Scholar]
- 171. Taylor BD, Ness RB, Klebanoff MA, et al. First and second trimester immune biomarkers in preeclamptic and normotensive women. Pregnancy Hypertens An Int J Women’s Cardiovasc Heal 2016; 6: 388–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172. Ferguson KK, Meeker JD, McElrath TF, et al. Repeated measures of inflammation and oxidative stress biomarkers in preeclamptic and normotensive pregnancies. Am J Obstet Gynecol 2017; 216: 527.e1–527.e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173. Lau SY, Guild S-J, Barrett CJ, et al. Tumor necrosis factor-alpha, interleukin-6, and interleukin-10 levels are altered in preeclampsia: a systematic review and meta-analysis. Am J Reprod Immunol 2013; 70. [DOI] [PubMed] [Google Scholar]
- 174. Pinheiro MB, Gomes KB, Ronda CRSC, et al. Severe preeclampsia: association of genes polymorphisms and maternal cytokines production in Brazilian population. Cytokine 2015; 71: 232–237. [DOI] [PubMed] [Google Scholar]
- 175. Udenze I, Amadi C, Awolola N, et al. The role of cytokines as inflammatory mediators in preeclampsia. Pan Afr Med J 2015; 20: 219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176. Tosun M, Celik H, Avci B, et al. Maternal and umbilical serum levels of interleukin-6, interleukin-8, and tumor necrosis factor-alpha in normal pregnancies and in pregnancies complicated by preeclampsia. J Matern Fetal Neonatal Med 2010; 23: 880–886. [DOI] [PubMed] [Google Scholar]
- 177. Kashanian M, Aghbali F, Mahali N. Evaluation of the diagnostic value of the first-trimester maternal serum high-sensitivity C-reactive protein level for prediction of pre-eclampsia. J Obstet Gynaecol Res 2013; 39: 1549–1554. [DOI] [PubMed] [Google Scholar]
- 178. Yang Y, Su X, Xu W, et al. Interleukin-18 and interferon gamma levels in preeclampsia: a systematic review and meta-analysis. Am J Reprod Immunol 2014; 72: 504–514. [DOI] [PubMed] [Google Scholar]
- 179. Taylor BD, Tang G, Ness RB, et al. Mid-pregnancy circulating immune biomarkers in women with preeclampsia and normotensive controls. Pregnancy Hypertens An Int J Women’s Cardiovasc Heal 2016; 6: 72–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180. Black KD, Horowitz JA. Inflammatory markers and preeclampsia. Nurs Res 2018; 67: 242–251. [DOI] [PubMed] [Google Scholar]
- 181. Stubert J, Kleber T, Bolz M, et al. Acute-phase proteins in prediction of preeclampsia in patients with abnormal midtrimester uterine Doppler velocimetry. Arch Gynecol Obstet 2016; 294: 1151–1160. [DOI] [PubMed] [Google Scholar]
- 182. Sowmya S, Ramaiah A, Nallari P, et al. Role of IL-6 −174(G/C) promoter polymorphism in the etiology of early-onset preeclampsia. Inflamm Res 2015; 64: 433–439. [DOI] [PubMed] [Google Scholar]
- 183. Fan DM, Wang Y, Liu XL, et al. Polymorphisms in interleukin-6 and interleukin-10 may be associated with risk of preeclampsia. Genet Mol Res 2017; 16. [DOI] [PubMed] [Google Scholar]
- 184. Tavakkol Afshari Z, Rahimi HR, Ehteshamfar SM, et al. Tumor necrosis factor-α and interleukin-1-β polymorphisms in pre-eclampsia. Iran J Immunol 2016; 13: 309–316. [DOI] [PubMed] [Google Scholar]
- 185. Giannakou K, Evangelou E, Papatheodorou SI. Genetic and non-genetic risk factors for pre-eclampsia: umbrella review of systematic reviews and meta-analyses of observational studies. Ultrasound Obstet Gynecol 2018; 51: 720–730. [DOI] [PubMed] [Google Scholar]
- 186. Harmon AC, Cornelius DC, Amaral LM, et al. The role of inflammation in the pathology of preeclampsia. Clin Sci 2016; 130: 409–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187. Farb A, Burke AP, Tang AL, et al. Coronary plaque erosion without rupture into a lipid core. A frequent cause of coronary thrombosis in sudden coronary death. Circulation 1996; 93: 1354–1363. [DOI] [PubMed] [Google Scholar]
- 188. Hadi HAR, Carr CS, Al Suwaidi J. Endothelial dysfunction: cardiovascular risk factors, therapy, and outcome. Vasc Health Risk Manag 2005; 1: 183–198. [PMC free article] [PubMed] [Google Scholar]
- 189. Ciftci FC, Caliskan M, Ciftci O, et al. Impaired coronary microvascular function and increased intima-media thickness in preeclampsia. J Am Soc Hypertens 2014; 8: 820–826. [DOI] [PubMed] [Google Scholar]
- 190. Tanz LJ, Stuart JJ, Missmer SA, et al. Cardiovascular biomarkers in the years following pregnancies complicated by hypertensive disorders or delivered preterm. Pregnancy Hypertens 2018; 13: 14–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191. Osborne LM, Monk C. Perinatal depression—the fourth inflammatory morbidity of pregnancy? Theory and literature review. Psychoneuroendocrinology 2013; 38: 1929–1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192. Piskorz A, Brzostek T. Comparison of SCORE-predicted risk of death due to cardiovascular events in women before and after menopause. Menopausal Rev 2015; 3: 168–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193. Ley SH, Li Y, Tobias DK, et al. Duration of reproductive life span, age at menarche, and age at menopause are associated with risk of cardiovascular disease in women. J Am Heart Assoc 2017; 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194. De Kat AC, Dam V, Onland-Moret NC, et al. Unraveling the associations of age and menopause with cardiovascular risk factors in a large population-based study. BMC Med 2017; 15: 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195. Colafella KMM, Denton KM. Sex-specific differences in hypertension and associated cardiovascular disease. Nat Rev Nephrol 2018; 14: 185–201. [DOI] [PubMed] [Google Scholar]
- 196. Gordon JL, Girdler SS, Meltzer-Brody SE, et al. Ovarian hormone fluctuation, neurosteroids, and HPA axis dysregulation in perimenopausal depression: a novel heuristic model. Am J Psychiatry 2015; 172: 227–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197. Georgakis MK, Thomopoulos TP, Diamantaras A-A, et al. Association of age at menopause and duration of reproductive period with depression after menopause. JAMA Psychiatry 2016; 73: 139. [DOI] [PubMed] [Google Scholar]
- 198. Arevalo M-A, Azcoitia I, Garcia-Segura LM. The neuroprotective actions of oestradiol and oestrogen receptors. Nat Rev Neurosci 2015; 16: 17–29. [DOI] [PubMed] [Google Scholar]
- 199. Lagranha CJ, Silva TLA, Silva SCA, et al. Protective effects of estrogen against cardiovascular disease mediated via oxidative stress in the brain. Life Sci 2018; 192: 190–198. [DOI] [PubMed] [Google Scholar]
- 200. Bowling MR, Xing D, Kapadia A, et al. Estrogen effects on vascular inflammation are age dependent: role of estrogen receptors. Arterioscler Thromb Vasc Biol 2014; 34: 1477–1485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201. Pabbidi MR, Kuppusamy M, Didion SP, et al. Sex differences in the vascular function and related mechanisms: role of 17β-estradiol. Am J Physiol Circ Physiol 2018; 315: H1499–H1518. [DOI] [PubMed] [Google Scholar]
- 202. Zhao D, Guallar E, Ouyang P, et al. Endogenous sex hormones and incident cardiovascular disease in post-menopausal women. J Am Coll Cardiol 2018; 71: 2555–2566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203. Mathews L, Iantorno M, Schär M, et al. Coronary endothelial function is better in healthy premenopausal women than in healthy older postmenopausal women and men. PLoS One 2017; 12: e0186448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204. Angeli F, Angeli E, Ambrosio G, et al. Neutrophil count and ambulatory pulse pressure as predictors of cardiovascular adverse events in postmenopausal women with hypertension. Am J Hypertens 2011; 24: 591–598. [DOI] [PubMed] [Google Scholar]
- 205. Razmjou S, Bastard J-P, Doucet E, et al. Effect of the menopausal transition and physical activity energy expenditure on inflammatory markers. Menopause 2016; 23: 1330–1338. [DOI] [PubMed] [Google Scholar]
- 206. Akinkuolie AO, Buring JE, Ridker PM, et al. A novel protein glycan biomarker and future cardiovascular disease events. J Am Heart Assoc 2014; 3: e001221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207. Wang Q, Ferreira DLS, Nelson SM, et al. Metabolic characterization of menopause: cross-sectional and longitudinal evidence. BMC Med 2018; 16: 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208. Razmjou S, Abdulnour J, Bastard J-P, et al. Body composition, cardiometabolic risk factors, physical activity, and inflammatory markers in premenopausal women after a 10-year follow-up. Menopause 2018; 25: 89–97. [DOI] [PubMed] [Google Scholar]
- 209. July M, Faiz S, Yaqub A, et al. Role of adipokines and inflammatory markers in postmenopausal hypertension. Minerva Endocrinol 2018; 43: 101–108. [DOI] [PubMed] [Google Scholar]
- 210. Willi J, Ehlert U. Assessment of perimenopausal depression: a review. J Affect Disord 2019; 249: 216–222. [DOI] [PubMed] [Google Scholar]
- 211. Pasquali MA, Harlow BL, Soares CN, et al. A longitudinal study of neurotrophic, oxidative, and inflammatory markers in first-onset depression in midlife women. Eur Arch Psychiatry Clin Neurosci 2018; 268: 771–781. [DOI] [PubMed] [Google Scholar]
- 212. Ma Y, Balasubramanian R, Pagoto SL, et al. Relations of depressive symptoms and antidepressant use to body mass index and selected biomarkers for diabetes and cardiovascular disease. Am J Public Health 2013; 103: e34–e43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213. Liu R-H, Pan J-Q, Tang X-E, et al. The role of immune abnormality in depression and cardiovascular disease. J Geriatr Cardiol 2017; 14: 703–710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214. Karaoulanis SE, Rizouli KA, Rizoulis AA, et al. Lack of association of acute phase response proteins with hormone levels and antidepressant medication in perimenopausal depression. BMC Psychiatry 2014; 14: 164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215. Liu Z, Ho SC. The association of serum C-reactive protein, uric acid and magnesium with insulin resistance in Chinese postmenopausal women with prediabetes or early untreated diabetes. Maturitas 2011; 70: 176–181. [DOI] [PubMed] [Google Scholar]
- 216. Wang NC, Matthews KA, Barinas-Mitchell EJM, et al. Inflammatory/hemostatic biomarkers and coronary artery calcification in midlife women of African-American and White race/ethnicity. Menopause 2016; 23: 653–661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217. Pires AS, Souza VC, Paula RS, et al. Pro-inflammatory cytokines correlate with classical risk factors for atherosclerosis in the admixed Brazilian older women. Arch Gerontol Geriatr 2015; 60: 142–146. [DOI] [PubMed] [Google Scholar]
- 218. Held C, White HD, Stewart RAH, et al. Inflammatory biomarkers interleukin-6 and C-reactive protein and outcomes in stable coronary heart disease: experiences from the STABILITY (Stabilization of Atherosclerotic Plaque by Initiation of Darapladib Therapy) Trial. J Am Heart Assoc 2017; 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219. AlBadri A, Lai K, Wei J, et al. Inflammatory biomarkers as predictors of heart failure in women without obstructive coronary artery disease: a report from the NHLBI-sponsored Women’s Ischemia Syndrome Evaluation (WISE). PLoS One 2017; 12: e0177684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220. Jones D, Patel J. Therapeutic approaches targeting inflammation in cardiovascular disorders. Biology (Basel) 2018; 7: 49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221. Zuzarte P, Duong A, Figueira ML, et al. Current therapeutic approaches for targeting inflammation in depression and cardiovascular disease. Curr Drug Metab 2018; 19: 674–687. [DOI] [PubMed] [Google Scholar]
- 222. Varga Z, Sabzwari SRA, Vargova V. Cardiovascular risk of nonsteroidal anti-inflammatory drugs: an under-recognized public health issue. Cureus 2017; 9: e1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223. Mörkl S, Wagner-Skacel J, Lahousen T, et al. The role of nutrition and the gut-brain axis in psychiatry: a review of the literature. Neuropsychobiology 2018; 1–9. [DOI] [PubMed] [Google Scholar]
- 224. Yoshida N, Yamashita T, Hirata K-I. Gut microbiome and cardiovascular diseases. Dis (Basel, Switzerland) 2018; 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225. Rizzetto L, Fava F, Tuohy KM, et al. Connecting the immune system, systemic chronic inflammation and the gut microbiome: the role of sex. J Autoimmun 2018; 92: 12–34. [DOI] [PubMed] [Google Scholar]
- 226. Koopman M, El Aidy S; MIDtrauma consortium. Depressed gut? The microbiota-diet-inflammation trialogue in depression. Curr Opin Psychiatry 2017; 30: 369–377. [DOI] [PubMed] [Google Scholar]
- 227. Wang Z, Klipfell E, Bennett BJ, et al. Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature 2011; 472: 57–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228. Ahmadmehrabi S, Tang WHW. Gut microbiome and its role in cardiovascular diseases. Curr Opin Cardiol 2017; 32: 761–766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229. Ouyang P, Wenger NK, Taylor D, et al. Strategies and methods to study female-specific cardiovascular health and disease: a guide for clinical scientists. Biol Sex Differ 2016; 7: 19. [DOI] [PMC free article] [PubMed] [Google Scholar]